Abstract
Background:
Antithrombotic therapy for patients with atrial fibrillation undergoing percutaneous coronary intervention is facing major treatment problems in clinical practice.
Methods:
We firstly conducted a Bayesian network meta-analysis to study the safety and efficacy of different antithrombotic regimens. Only randomized controlled trials from PubMed, Web of Science, Cochrane Central Register of Controlled Trials, Embase, and China National Knowledge Infrastructure were included in our study. The Bayesian random-effects model was used in this study. The primary safety and efficacy outcomes were major bleeding according to the criteria of Thrombolysis In Myocardial Infarction (TIMI) and trial-defined major adverse cardiovascular events, respectively. The secondary safety outcomes were combined TIMI major and minor bleeding, trial-defined primary bleeding events, and intracranial hemorrhage. The secondary efficacy outcomes were all-cause or cardiovascular mortality, myocardial infarction, stroke, stent thrombosis, and hospitalization.
Results:
Total of 11,532 patients from the five randomized controlled trials were analyzed, of whom 8,426 were male. Compared with vitamin K antagonist (VKA) plus P2Y12 inhibitor, the odds ratios (95% credible intervals) for TIMI major bleeding were 1.70 (0.77–3.80) for VKA plus dual antiplatelet therapy (DAPT), 1.20 (0.30–4.60) for rivaroxaban plus P2Y12 inhibitor, 1.00 (0.25–3.90) for rivaroxaban plus DAPT, 0.76 (0.21–2.80) for dabigatran plus P2Y12 inhibitor, 0.71 (0.25–2.10) for apixaban plus P2Y12 inhibitor, 1.40 (0.52–3.80) for apixaban plus DAPT, and 1.00 (0.27–4.00) for edoxaban plus P2Y12 inhibitor. For trial-defined major adverse cardiovascular events, compared with VKA plus P2Y12 inhibitor, the odds ratios (95% credible intervals) were 1.10 (0.61–2.00) for VKA plus DAPT, 1.20 (0.45–3.70) for rivaroxaban plus P2Y12 inhibitor, 1.10 (0.38–3.20) for rivaroxaban plus DAPT, 1.10 (0.43–3.10) for dabigatran plus P2Y12 inhibitor, 1.00 (0.47–2.20) for apixaban plus P2Y12 inhibitor, 0.99 (0.46–2.20) for apixaban plus DAPT, and 1.20 (0.43–3.40) for edoxaban plus P2Y12 inhibitor. Apixaban plus P2Y12 inhibitor was the highest-ranking of safety outcomes and VKA plus P2Y12 inhibitor was the highest-ranking of efficacy outcomes other than trial-defined major adverse cardiovascular events.
Conclusion:
Apixaban plus P2Y12 inhibitor seems to be linked with fewer bleeding complications while retaining antithrombotic efficacy. Moreover, for most efficacy indicators, the ranking of VKA plus P2Y12 inhibitor is still very high.
Systematic Review Registration:
[www.crd.york.ac.uk/prospero/], identifier [CRD42020149894].
Introduction
In the past 50 years, cardiovascular disease brings a huge burden to countries all over the world, not only economically, although great progress has been made in the prevention and management (1). The vigorous development of coronary intervention technology has reduced the mortality of ischemic heart disease to a very low level, but antithrombotic drugs, usually aspirin and a P2Y12 inhibitor dual antiplatelet therapy (DAPT), are needed after operation to reduce the ischemic events (2). We all know that patients with atrial fibrillation (AF) also need antithrombotic therapy, whether vitamin K antagonists (VKA) or non-vitamin K antagonist oral anticoagulants (NOAC), to prevent stroke (3). Such antithrombotic strategies for AF patients undergoing percutaneous coronary intervention (PCI) require the constant balance of the risks of bleeding and ischemia (4), creating difficulties for clinicians. Theoretically, in this special population, the use of triple therapy (DAPT and VKA/NOAC) may provide better cardiovascular benefit, but this limited benefit may be offset by higher bleeding risk, and discontinuation of aspirin may lead to higher rates of stent thrombosis and ischemic events (5, 6).
Many high-quality randomized controlled trials (RCTs) have compared the safety and efficacy of different antithrombotic regimens in patients with AF undergoing PCI (7–11). Lopes et al. summarized these high-quality RCTs and found that an NOAC plus a P2Y12 inhibitor without aspirin may be the most preferred antithrombotic regimen option and the favorable treatment option for most patients with AF undergoing PCI (12). Although these findings give us great enlightenment, there is still no best answer to which NOAC is the best and most dominant. Here, we conducted a Bayesian network meta-analysis which allowed simultaneous comparisons of multiple antithrombotic strategies to finally provide the latest and comprehensive evidence of antithrombotic therapy for this special population.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). We developed a protocol and registered on PROSPERO (CRD42020149894).
Search Strategy and Selection Criteria
Two reviewers searched PubMed, Web of Science, Cochrane Central Register of Controlled Trials, Embase, and China National Knowledge Infrastructure for relevant published studies before 30 September 2021, without any language restrictions. We used keywords related to atrial fibrillation, AF, percutaneous coronary intervention, PCI, acute coronary syndrome, ACS, NOAC, VKA, antithrombosis, and antiplatelets. We only included RCTs that compared different antithrombotic strategies in patients with AF undergoing PCI.
The inclusion criteria of our study included (1) RCTs with at least two comparators; (2) study patients were AF, including previous, persistent, permanent, or paroxysmal AF, and had undergone PCI; (3) patients were prescribed with anticoagulation combined with antiplatelet therapy; (4) major bleeding and major adverse cardiovascular events (MACE) were reported; and (5) follow-up was at least 6 months. Our exclusion criteria included observational studies, registry data, ongoing trials without results, editorials, case series, and duplicate studies (14). Previous systematic review and meta-analysis studies were screened for eligible studies.
After removing all duplicates, two reviewers independently screened titles and abstracts followed by full-text for those potentially eligible. Additionally, a third reviewer made final decisions in contested judgments.
Outcomes
The primary safety and efficacy outcomes were major bleeding according to the criteria of Thrombolysis In Myocardial Infarction (TIMI) and trial-defined MACE, respectively. Trial-defined MACE was usually defined as a combination of either all-cause or cardiovascular mortality, myocardial infarction (MI), stroke, and stent thrombosis. The secondary safety outcomes were combined TIMI major and minor bleeding, trial-defined primary bleeding events, and intracranial hemorrhage. TIMI major bleeding was defined as any symptomatic intracranial hemorrhage, or clinically overt signs of hemorrhage (including imaging) associated with a drop in hemoglobin of ≥5 g/dL (or when the hemoglobin concentration is not available, an absolute drop in hematocrit of ≥15%). TIMI minor bleeding was defined as any clinically overt sign of hemorrhage (including imaging) that is associated with a fall in hemoglobin concentration of 3 to <5 g/dL (or, when hemoglobin concentration is not available, a fall in hematocrit of 9 to <15%). The secondary efficacy outcomes were all-cause or cardiovascular mortality, MI, stroke, stent thrombosis, and hospitalization.
Data Extraction and Quality Assessment
Two reviewers independently extracted the following data from the eligible RCTs: Study design, baseline characteristics, interventions, and outcomes. Any contradiction was settled through consensus. All data were cross-checked. Two reviewers then used the Cochrane Collaboration tool to assess the quality of the included studies independently (15). The final author made final decisions under contradictory circumstances. Finally, all the extracted data were stored in the pre-designed excel spreadsheet.
Data Synthesis and Analysis
We conducted a Bayesian random-effects network meta-analysis model to compare multiple regimens at the same time. We estimated odds ratios (ORs) and the associated 95% credible intervals (CrIs) for the treatment effects of the two regimens. All analyses were carried out using the gemtc package (version 1.0–1) (16) and the rjags package (version 4–11) (17) in R (version 4.0.3). We used the default setting of the package, including non-informative prior distributions, with four parallel chains. We used trace plots and Gelman–Rubin diagnostic statistics to check the convergence of Markov Chain Monte Carlo (MCMC) for all model parameters.
We calculated the rank probabilities (probability of a regimen being the best, second best, or worst for an outcome) and the surface under the cumulative ranking (SUCRA) to evaluate and rank each regimen with respect to each safety and efficacy outcome. A simple numerical summary, the supplement of the graphical display of cumulative ranking, was used to estimate the SUCRA line for each regime. SUCRA would be 1 when a regimen is certain to be the best and 0 when a regimen is certain to be the worst (18).
We assessed statistical evidence of inconsistency defined as the difference between direct and indirect comparisons of treatment effects using the node-splitting method (19).
Results
Search Results
Our database search yielded 1,486 unique records. Among them, 1,461 records were considered irrelevant according to the title and abstract screening. A total of 25 records were assessed in full text for eligibility. Of those, five RCTs [WOEST (7), PIONEER AF-PCI (8), RE-DUAL PCI (9), AUGUSTUS (10), and ENTRUST-AF PCI (11)] met the inclusion criteria (Figure 1). Among these RCTs, eight regimens (VKA plus P2Y12 inhibitor, VKA plus DAPT, rivaroxaban plus P2Y12 inhibitor, rivaroxaban plus DAPT, dabigatran plus P2Y12 inhibitor, apixaban plus P2Y12 inhibitor, apixaban plus DAPT, and edoxaban plus P2Y12 inhibitor) were evaluated.
FIGURE 1
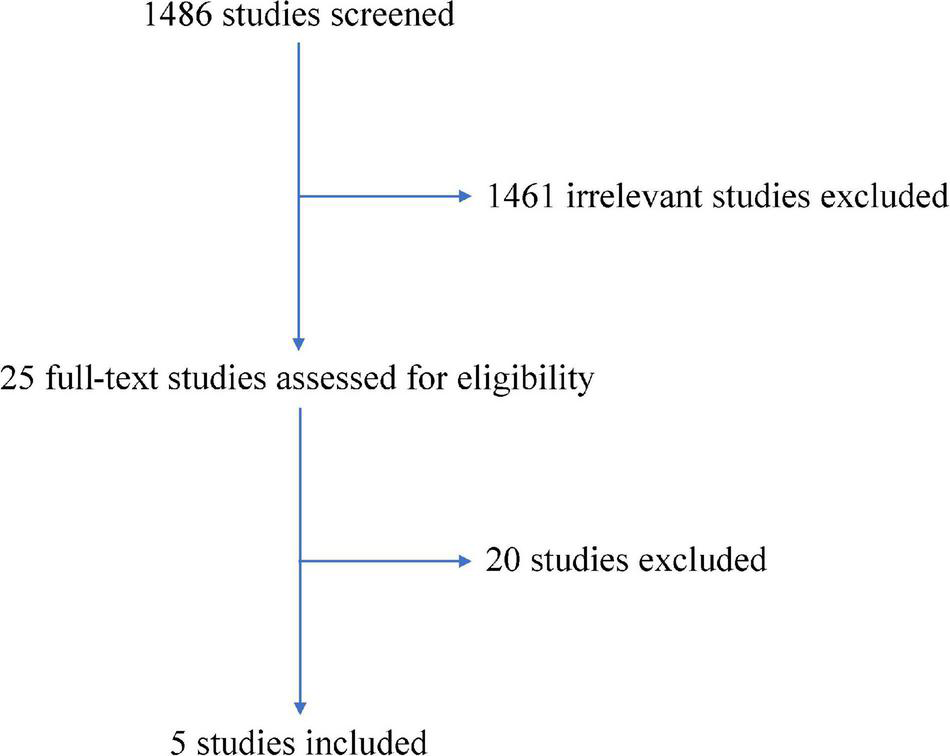
Flowchart of literature review.
Study and Patient Characteristics
A total of 11,532 patients from the five trials were analyzed, of whom 8,426 were male. Study and patient characteristics are shown in Tables 1, 2, respectively. Except for AUGUSTUS, the follow-up time of other RCTs was more than 1 year. All RCTs were open-label, and the outcomes assessment was blinded (Supplementary Table 1).
TABLE 1
| WOEST | PIONEER AF-PCI | RE-DUAL PCI | AUGUSTUS | ENTRUST-AF PCI | |
| NCT00769938 | NCT01830543 | NCT02164864 | NCT02415400 | NCT02866175 | |
| Follow-up | 12 months | 12 months | 14 months | 6 months | 12 months |
| Sample size | 573 | 2,124 | 2,725 | 4,614 | 1,506 |
| Enrollment | November 2008 to November 2011 | May 2013 to July 2015 | July 2014 to October 2016 | September 2015 to April 2018 | February 2017 to May 2018 |
| Design | Open-label, randomized, controlled trial at 15 sites in the Netherlands and Belgium | International, multicenter, randomized, open-label trial at 431 sites | International, multicenter, randomized, open-label trial at 414 sites in 41 countries | Prospective, multicenter, two-by-two factorial, randomized clinical trial at 492 sites in 33 countries | Randomized, multicenter, open-label, non-inferiority phase 3b trial at 186 sites in 18 countries |
| Intervention | Double therapy: OAC and clopidogrel Triple therapy: aspirin, OAC, and clopidogrel | Group 1: low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor for 12 months Group 2: very-low-dose rivaroxaban (2.5 mg twice daily) plus DAPT for 1, 6, or 12 months Group 3: VKA plus DAPT for 1, 6, or 12 months | Dual therapy: dabigatran (110 or 150 mg twice daily) plus a P2Y12 inhibitor (clopidogrel or ticagrelor) Triple therapy: warfarin plus DAPT (for 1–3 months) | Dual therapy: apixaban (5 mg twice daily) or VKA and a P2Y12 inhibitor for 6 months Triple therapy: apixaban or VKA and DAPT for 6 months | Dual therapy: edoxaban (60 mg once daily) plus a P2Y12 inhibitor for 12 months Triple therapy: VKA and DAPT |
| Inclusion criteria | A long-term indication for oral anticoagulation treatment (until at least 1 year after the study); a severe coronary lesion with indication for PCI; and age 18–80 years | Documented atrial fibrillation that occurred within 1 year before screening; patients with documented atrial fibrillation that occurred more than 1 year before screening were also eligible if the participant had been receiving oral anticoagulation for atrial fibrillation for the 3 months immediately preceding the index PCI | Men and women who were at least 18 years of age were eligible for inclusion in the trial if they had non-valvular atrial fibrillation and had successfully undergone PCI with a bare-metal or drug-eluting stent within the previous 120 h. Non-valvular atrial fibrillation could be paroxysmal, persistent, or permanent, but it could not be secondary to a reversible disorder unless long-term treatment with an oral anticoagulant was anticipated. Patients who had been receiving treatment with an oral anticoagulant before PCI and those who had not received oral anticoagulation were eligible | An age of at least 18 years; previous, persistent, permanent, or paroxysmal atrial fibrillation and planned long-term use of an oral anticoagulant; recent acute coronary syndrome or PCI; and planned use of a P2Y12 inhibitor for at least 6 months | Aged at least 18 years, and had a successful PCI for stable coronary artery disease or acute coronary syndrome |
| Exclusion criteria | A history of intracranial bleeding; cardiogenic shock; contra indication to use of aspirin, clopidogrel, or both; peptic ulcer in the previous 6 months; thrombocytopenia; major bleeding in the past 12 months; and pregnancy | A history of stroke or transient ischemic attack, clinically significant gastrointestinal bleeding within 12 months before randomization, a calculated creatinine clearance of less than 30 ml per minute, anemia of an unknown cause with a hemoglobin concentration of less than 10 g per deciliter, or any other condition known to increase the risk of bleeding | The presence of bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major coexisting conditions | Patients who were using anticoagulation for other conditions were not eligible. Other key exclusion criteria were severe renal insufficiency, a history of intracranial hemorrhage, recent or planned coronary-artery bypass graft surgery, coagulopathy or ongoing bleeding, and contraindication to a VKA, apixaban, all P2Y12 inhibitors, or aspirin | Patients with non-valvular atrial fibrillation not secondary to a reversible disorder were included and patients with mechanical heart valves, moderate-to-severe mitral stenosis, end-stage renal disease, and other major comorbidities were excluded |
| Outcomes | The primary endpoint was the occurrence of any bleeding episode during 1-year follow-up. The composite secondary endpoint of death, myocardial infarction, stroke, target-vessel revascularization, and stent thrombosis | The primary safety end point was the occurrence of clinically significant bleeding. Secondary end points included the incidence of each component of the primary safety end point, as well as the following efficacy end points: the occurrence of a major adverse cardiovascular event, each component of the major adverse cardiovascular event end point, and stent thrombosis | The primary end point was a major or clinically relevant non-major bleeding event. A main secondary end point was a composite efficacy end point of thromboembolic events, death, or unplanned revascularization. Other secondary end points included a combined end point of thromboembolic events or death, as well as the individual thromboembolic events and definite stent thrombosis | The primary outcome was major or clinically relevant non-major bleeding. Secondary outcomes included death or hospitalization and a composite of ischemic events | The primary endpoint was a composite of major or clinically relevant non-major bleeding. The main efficacy outcome was the composite of cardiovascular death, stroke, systemic embolic events, myocardial infarction, and definite stent thrombosis |
Study characteristics.
VKA, vitamin K antagonist (warfarin or phenprocoumon, a target international normalization ratio of 2.0–3.0); DAPT, dual antiplatelet therapy (aspirin a P2Y12 inhibitor (clopidogrel, ticagrelor, or prasugrel).
TABLE 2
| WOEST |
PIONEER AF-PCI |
RE-DUAL PCI |
AUGUSTUS |
ENTRUST-AF PCI |
||||||||||
| VKA + P2Y12 inhibitor |
VKA + DAPT |
Rivaroxaban + P2Y12 inhibitor |
Rivaroxaban + DAPT |
VKA + DAPT |
Dabigatran + P2Y12 inhibitor |
Dabigatran + P2Y12 inhibitor |
VKA + DAPT |
Apixaban + DAPT |
Apixaban + P2Y12 inhibitor |
VKA + DAPT |
VKA + P2Y12 inhibitor |
Edoxaban + P2Y12 inhibitor |
VKA + DAPT |
|
| Sample size | 279 | 284 | 709 | 709 | 706 | 981 | 763 | 981 | 1,153 | 1,153 | 1,154 | 1,154 | 751 | 755 |
| Age | 70 | 70 | 70 | 70 | 70 | 72 | 69 | 72 | 71 | 70 | 71 | 71 | 69 | 70 |
| Male | 214 | 234 | 528 | 535 | 518 | 710 | 529 | 750 | 796 | 841 | 815 | 826 | 557 | 563 |
| Diabetes, % | 24.3 | 25.4 | 28.8 | 28.1 | 31.3 | 36.9 | 34.1 | 37.9 | 37.1 | 35.9 | 35.9 | 36.6 | 34.5 | 34.2 |
| History, % | ||||||||||||||
| MI | 34.3 | 35.2 | 19.7 | 25.4 | 22.2 | 24.2 | 25,4 | 27,3 | – | – | – | – | 25 | 23.4 |
| PCI | 30.8 | 35.6 | – | – | – | 33.3 | 31.3 | 35.4 | – | – | – | – | 25 | 23.4 |
| CABG | 20.1 | 26.1 | – | – | – | 9.9 | 10.4 | 11.3 | – | – | – | – | 6.1 | 6.5 |
| GB | 5 | 4.9 | 1 | 1.3 | 0.7 | – | – | – | – | – | – | – | – | – |
| CHA2DS2-VASc score, % | ||||||||||||||
| <3 | – | – | 26.7 | 23.7 | 20.8 | 23.4 | 32.4 | 19.7 | 20.8 | 21.5 | 20.8 | 19.1 | – | – |
| >2 | – | – | 73.3 | 76.3 | 79.2 | 76.6 | 67.6 | 80.3 | 79.2 | 78.5 | 79.2 | 80.9 | – | – |
| HAS-BLED score, % | ||||||||||||||
| <3 | – | – | 27.6 | 32 | 29.5 | 33.3 | 40.5 | 29.4 | 50.5 | 51.7 | 50.9 | 39.6 | 31.8 | 27 |
| >2 | – | – | 72.3 | 68 | 70.5 | 66.8 | 59.5 | 70.6 | 49.5 | 48.3 | 49.1 | 50.4 | 62.2 | 66.6 |
| Arterial access, % | ||||||||||||||
| Radial | 26.5 | 25 | – | – | – | 63 | 65.8 | 62.3 | – | – | – | – | 77.5 | 80.5 |
| Femoral | 73.1 | 73.2 | – | – | – | 36.6 | 33 | 36.8 | – | – | – | – | 22.4 | 19.3 |
| Stent type, % | ||||||||||||||
| None | 1.8 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – | – | – |
| Bare metal | 31.9 | 30.3 | 32.6 | 31.2 | 31.8 | 15.2 | 16.1 | 13.6 | – | – | – | – | – | – |
| Drug eluting | 64.9 | 64.4 | 65.4 | 66.8 | 66.5 | 82.1 | 81.5 | 84.6 | – | – | – | – | – | – |
| Both | 1.1 | 3.9 | 2 | 2.9 | 1.7 | 1.9 | 1.3 | 1.2 | – | – | – | – | – | – |
| Other | – | – | – | – | – | 0.8 | 1 | 0.5 | – | – | – | – | – | – |
| Creatinine clearance, mL/min/1.73 m2 | – | – | 78.3 | 77.5 | 80.7 | 76.3 | 83.7 | 75.4 | 78.5 | 79.4 | 78.7 | 80 | 71.8 | 71.7 |
| Type of index event, % | ||||||||||||||
| NSTEMI | – | – | 18.5 | 18.3 | 17.8 | 20.7 | 23.5 | 21 | – | – | – | – | 21.7 | 20.8 |
| STEMI | – | – | 12.3 | 13.8 | 10.7 | 14.7 | 14.9 | 14.6 | – | – | – | – | 17.7 | 17.5 |
| UA | – | – | 20.7 | 21.1 | 23.7 | 19.9 | 16.5 | 16.9 | – | – | – | – | 14.9 | 16.3 |
| Type of AF | ||||||||||||||
| Persistent | – | – | 20.7 | 20.6 | 21.1 | 17.7 | 17.3 | 18.2 | – | – | – | – | 18.6 | 19.3 |
| Permanent | – | – | 37.4 | 33.6 | 34.5 | 32.6 | 32.8 | 32.4 | – | – | – | – | 27.8 | 33.1 |
| Paroxysmal | – | – | 42.8 | 46.1 | 44.4 | 49.6 | 49.8 | 49.4 | – | – | – | – | 53.5 | 47.4 |
Patient characteristics.
VKA, vitamin K antagonists; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; GB, gastrointestinal bleeding; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction; UA, unstable angina; AF, atrial fibrillation.
Structure of Network Meta-Analysis
We compared the eight treatment regimens at the same time: VKA plus P2Y12 inhibitor, VKA plus DAPT, rivaroxaban plus P2Y12 inhibitor, rivaroxaban plus DAPT, dabigatran plus P2Y12 inhibitor, apixaban plus P2Y12 inhibitor, apixaban plus DAPT, and edoxaban plus P2Y12 inhibitor (Figure 2). We hypothesized that the eight treatment regimens had comparable safety and efficacy. We set VKA plus P2Y12 inhibitor as a reference.
FIGURE 2
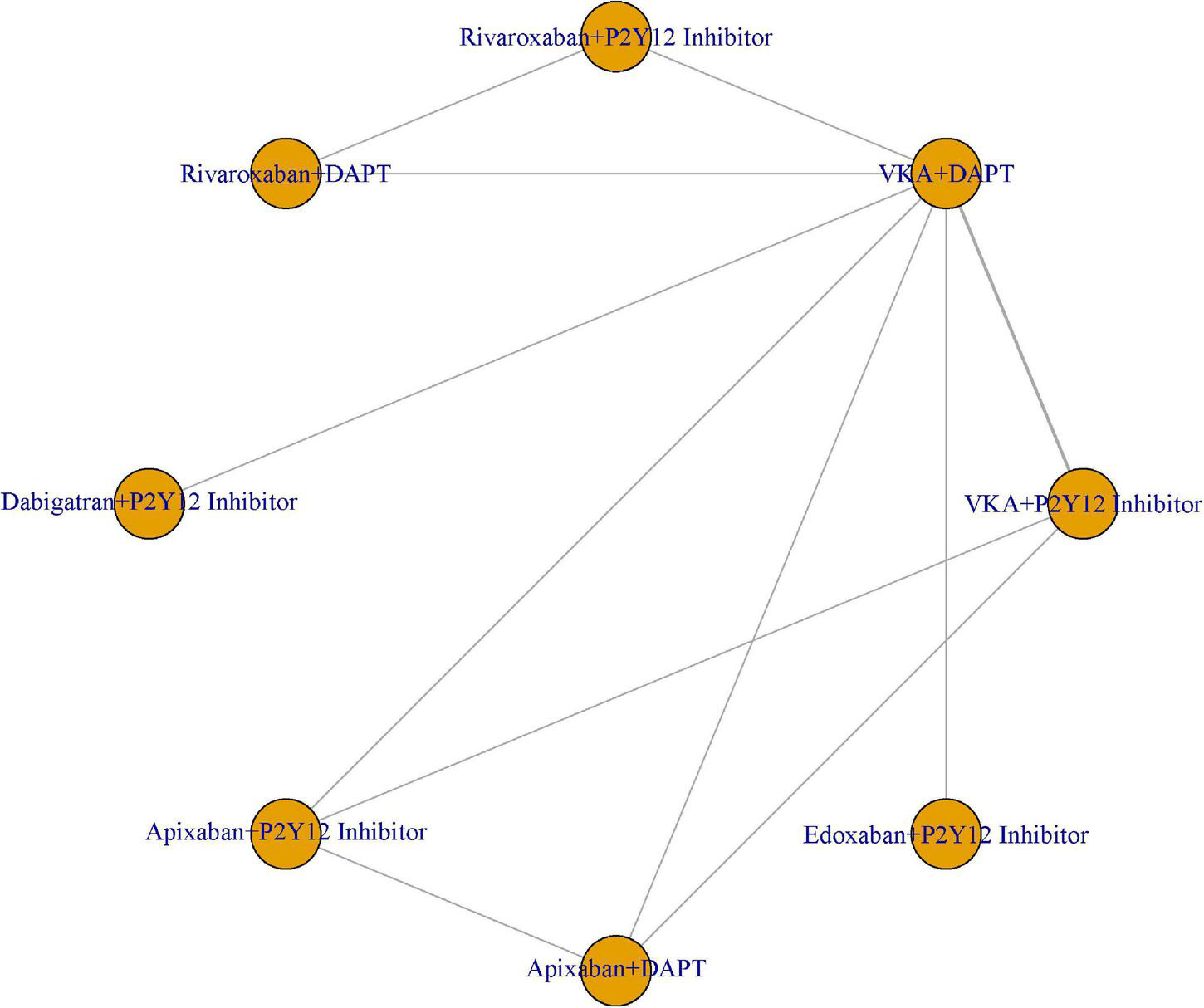
Network of eight antithrombotic treatment regimens.
Safety Outcomes
Compared with the ORs for VKA plus P2Y12 inhibitor, those for all safety outcomes were lower for apixaban plus P2Y12 inhibitor with no significances (Figures 3A–D). Compared with VKA plus P2Y12 inhibitor, the ORs for TIMI major bleeding were 1.70 (95% CrI, 0.77–3.80) for VKA plus DAPT, 1.20 (95% CrI, 0.30–4.60) for rivaroxaban plus P2Y12 inhibitor, 1.00 (95% CrI, 0.25–3.90) for rivaroxaban plus DAPT, 0.76 (95% CrI, 0.21–2.80) for dabigatran plus P2Y12 inhibitor, 0.71 (95% CrI, 0.25–2.10) for apixaban plus P2Y12 inhibitor, 1.40 (95% CrI, 0.52–3.80) for apixaban plus DAPT, and 1.00 (95% CrI, 0.27–4.00) for edoxaban plus P2Y12 inhibitor (Figure 3A). For combined TIMI major and minor bleeding, compared with VKA plus P2Y12 inhibitor, the ORs were 2.10 (95% CrI, 0.86–5.30) for VKA plus DAPT, 1.30 (95% CrI, 0.26–6.60) for rivaroxaban plus P2Y12 inhibitor, 1.10 (95% CrI, 0.23–5.80) for rivaroxaban plus DAPT, 0.92 (95% CrI, 0.20–4.40) for dabigatran plus P2Y12 inhibitor, 0.69 (95% CrI, 0.21–2.30) for apixaban plus P2Y12 inhibitor, 1.40 (95% CrI, 0.44–4.70) for apixaban plus DAPT, and 1.80 (95% CrI, 0.38–8.20) for edoxaban plus P2Y12 inhibitor (Figure 3B). For trial-defined primary bleeding events, compared with VKA plus P2Y12 inhibitor, the ORs were 2.40 (95% CrI, 0.90–6.80) for VKA plus DAPT, 1.40 (95% CrI, 0.25–8.40) for rivaroxaban plus P2Y12 inhibitor, 1.50 (95% CrI, 0.27–9.20) for rivaroxaban plus DAPT, 1.40 (95% CrI, 0.25–8.10) for dabigatran plus P2Y12 inhibitor, 0.75 (95% CrI, 0.19–2.90) for apixaban plus P2Y12 inhibitor, 1.50 (95% CrI, 0.39–5.80) for apixaban plus DAPT, and 2.00 (95% CrI, 0.35–12.00) for edoxaban plus P2Y12 inhibitor (Figure 3C). For intracranial hemorrhage, compared with VKA plus P2Y12 inhibitor, the ORs were 0.64 (95% CrI, 0.12–3.40) for VKA plus DAPT, 0.25 (95% CrI, 0.01–4.20) for rivaroxaban plus P2Y12 inhibitor, 0.25 (95% CrI, 0.01–4.30) for rivaroxaban plus DAPT, 0.13 (95% CrI, 0.01–4.30) for dabigatran plus P2Y12 inhibitor, 0.10 (95% CrI, 0.00–1.30) for apixaban plus P2Y12 inhibitor, 0.52 (95% CrI, 0.06–4.50) for apixaban plus DAPT, and 0.27 (95% CrI, 0.02–4.40) for edoxaban plus P2Y12 inhibitor (Figure 3D).
FIGURE 3
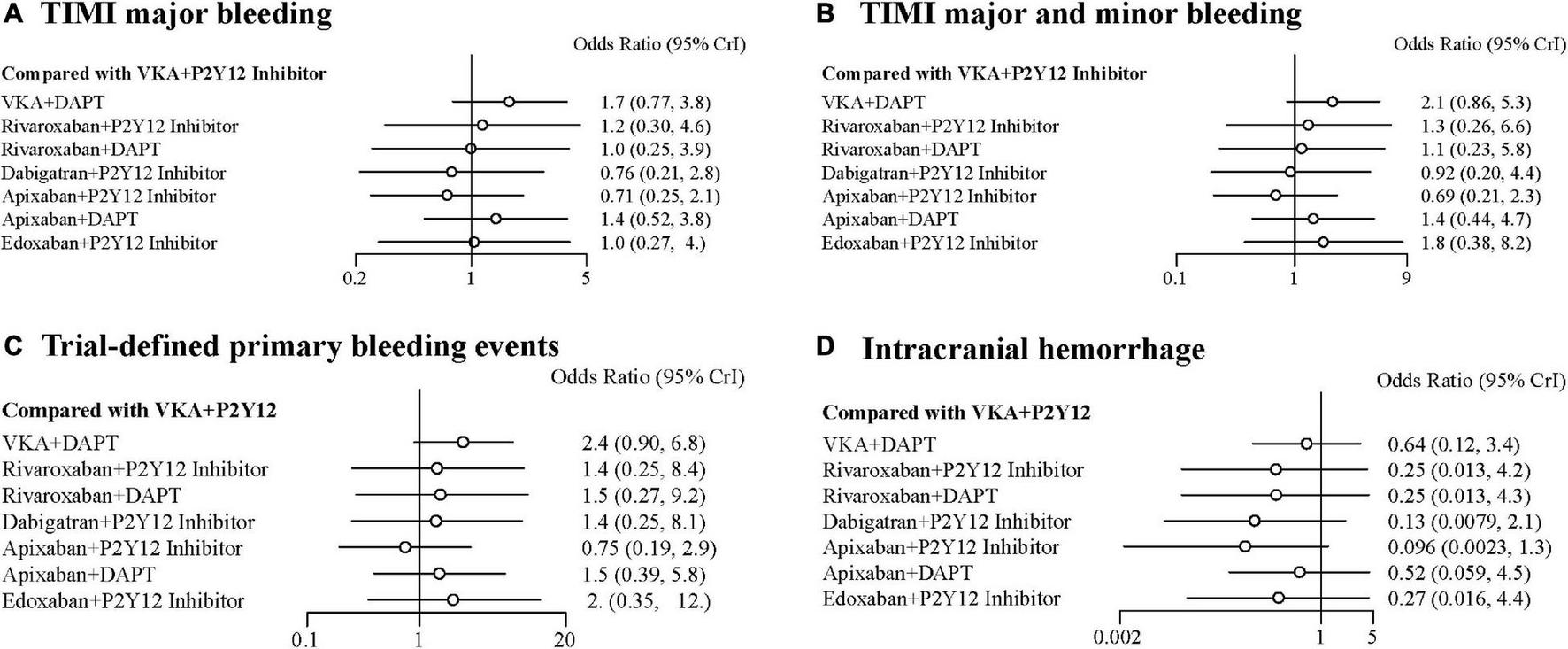
Forest plots for safety outcomes. (A) Thrombolysis In Myocardial Infarction (TIMI) major bleeding. (B) TIMI major and minor bleeding. (C) Trial-defined primary bleeding events. (D) Intracranial hemorrhage.
Efficacy Outcomes
There were no statistical differences among the antithrombotic regimens in trial-defined MACE, all-cause or cardiovascular mortality, MI, stroke, stent thrombosis, and hospitalization (Figures 4A–G). For trial-defined MACE, compared with VKA plus P2Y12 inhibitor, the ORs were 1.10 (95% CrI, 0.61–2.00) for VKA plus DAPT, 1.20 (95% CrI, 0.45–3.70) for rivaroxaban plus P2Y12 inhibitor, 1.10 (95% CrI, 0.38–3.20) for rivaroxaban plus DAPT, 1.10 (95% CrI, 0.43–3.10) for dabigatran plus P2Y12 inhibitor, 1.00 (95% CrI, 0.47–2.20) for apixaban plus P2Y12 inhibitor, 0.99 (95% CrI, 0.46–2.20) for apixaban plus DAPT, and 1.20 (95% CrI, 0.43–3.40) for edoxaban plus P2Y12 inhibitor (Figure 4A). For all-cause mortality, compared with VKA plus P2Y12 inhibitor, the ORs were 1.30 (95% CrI, 0.52–3.80) for VKA plus DAPT, 1.60 (95% CrI, 0.31–10.00) for rivaroxaban plus P2Y12 inhibitor, 1.70 (95% CrI, 0.32–11.00) for rivaroxaban plus DAPT, 1.30 (95% CrI, 0.27–7.40) for dabigatran plus P2Y12 inhibitor, 1.20 (95% CrI, 0.36–4.40) for apixaban plus P2Y12 inhibitor, 1.20 (95% CrI, 0.35–4.30) for apixaban plus DAPT, and 1.60 (95% CrI, 0.33–9.50) for edoxaban plus P2Y12 inhibitor (Figure 4B). For cardiovascular mortality, compared with VKA plus P2Y12 inhibitor, the ORs were 1.30 (95% CrI, 0.53–3.80) for VKA plus DAPT, 1.60 (95% CrI, 0.31–10.00) for rivaroxaban plus P2Y12 inhibitor, 1.70 (95% CrI, 0.33–11.00) for rivaroxaban plus DAPT, 1.30 (95% CrI, 0.28–7.40) for dabigatran plus P2Y12 inhibitor, 1.20 (95% CrI, 0.36–4.40) for apixaban plus P2Y12 inhibitor, 1.20 (95% CrI, 0.35–4.30) for apixaban plus DAPT, and 1.70 (95% CrI, 0.35–9.40) for edoxaban plus P2Y12 inhibitor (Figure 4C). For MI, compared with VKA plus P2Y12 inhibitor, the ORs were 1.30 (95% CrI, 0.52–3.80) for VKA plus DAPT, 1.60 (95% CrI, 0.31–10.00) for rivaroxaban plus P2Y12 inhibitor, 1.70 (95% CrI, 0.32–10.00) for rivaroxaban plus DAPT, 1.30 (95% CrI, 0.27–7.30) for dabigatran plus P2Y12 inhibitor, 1.20 (95% CrI, 0.36–4.40) for apixaban plus P2Y12 inhibitor, 1.20 (95% CrI, 0.35–4.30) for apixaban plus DAPT, and 1.60 (95% CrI, 0.34–9.40) for edoxaban plus P2Y12 inhibitor (Figure 4D). The details of stroke, stent thrombosis, and hospitalization are shown in Figures 4E–G.
FIGURE 4
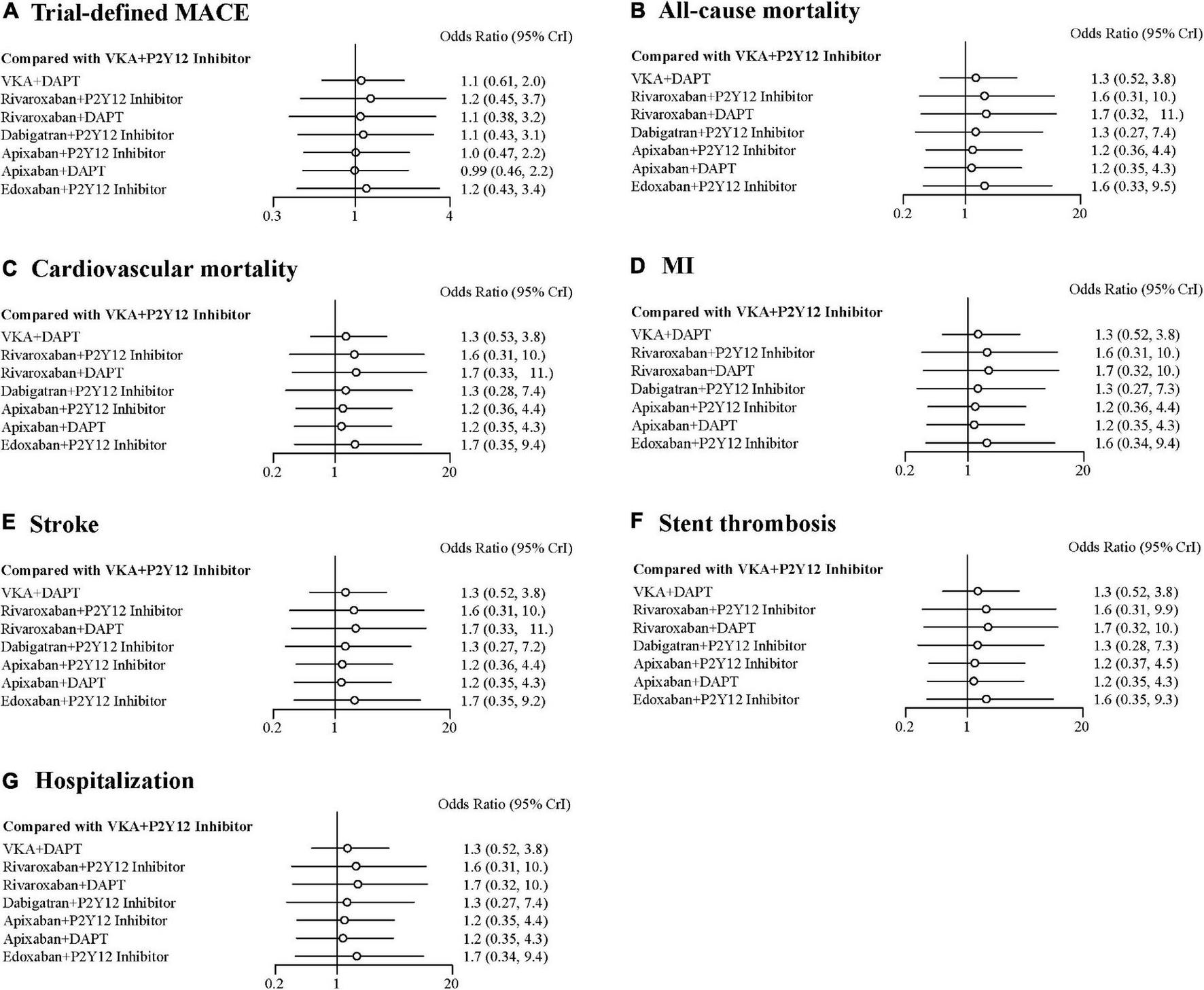
Forest plots for efficacy outcomes. (A) Trial-defined major adverse cardiovascular events (MACE). (B) All-cause mortality. (C) Cardiovascular mortality. (D) Myocardial infarction (MI). (E) Stroke. (F) Stent thrombosis. (G) Hospitalization.
Ranking of Antithrombotic Regimens
The performance of the tested regimens was visualized in a two-dimensional forest plot of ORs (Figure 5). Apixaban plus P2Y12 inhibitor was the highest-ranking of safety outcomes (SUCRA values of TIMI major bleeding, combined TIMI major and minor bleeding, trial-defined primary bleeding events, and intracranial hemorrhage were 0.331850, 0.469975, 0.539425, and 0.4321875, respectively) (Figure 6). Apixaban plus DAPT was the highest-ranking of trial-defined MACE (SUCRA value was 0.180525) (Figure 7A). VKA plus P2Y12 inhibitor was the highest-ranking of all-cause mortality, cardiovascular mortality, MI, stroke, stent thrombosis, and hospitalization (SUCRA values were 0.2439375, 0.2508375, 0.2455375, 0.24465, 0.24215, and 0.23865, respectively) (Figures 7B–G). All fitted models were well converged (Supplementary Figure 1) and we found no evidence of statistical inconsistency in our Bayesian network meta-analysis (Supplementary Figure 2).
FIGURE 5
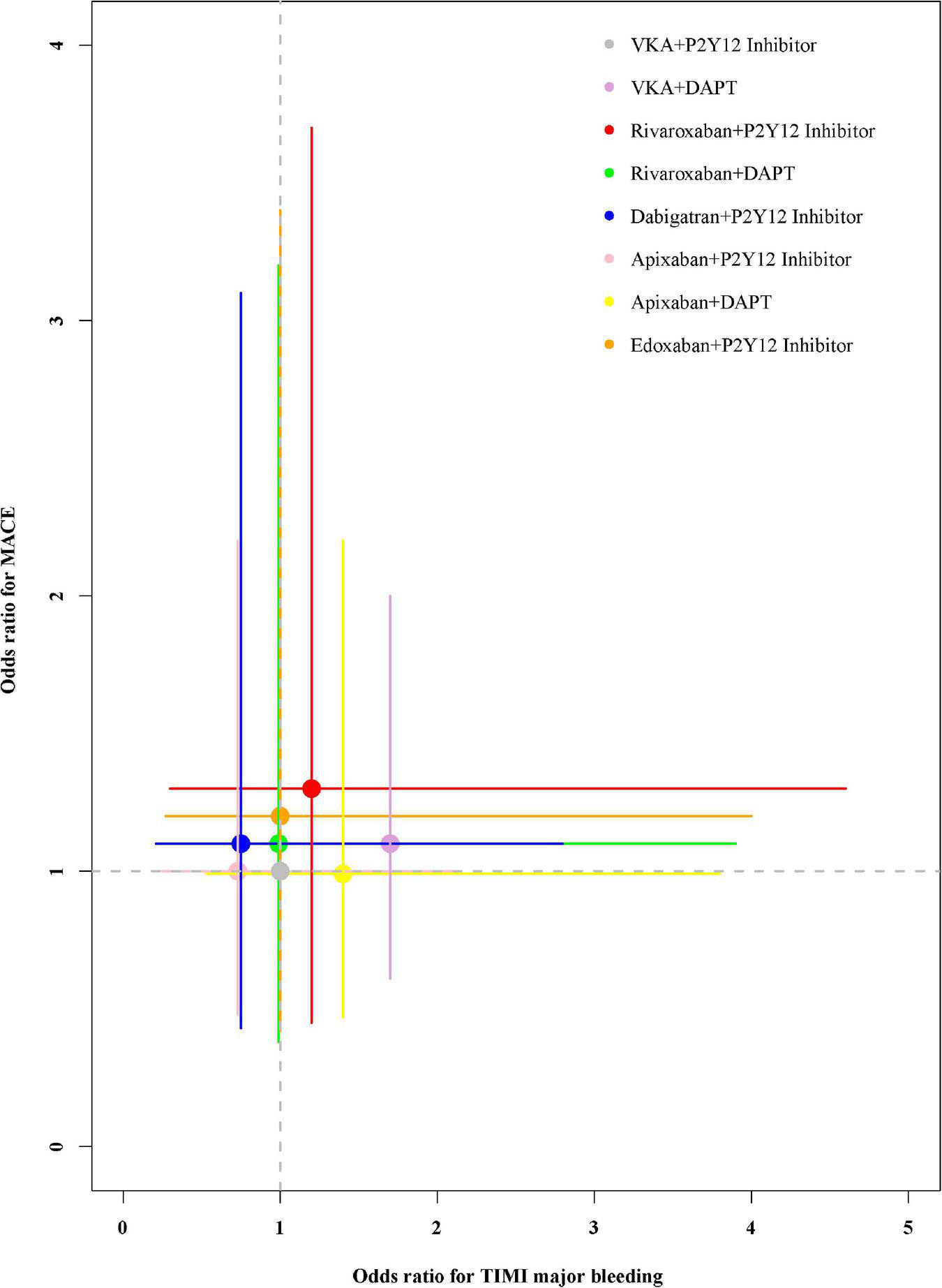
Odds ratios for TIMI major bleeding and trial-defined MACE.
FIGURE 6
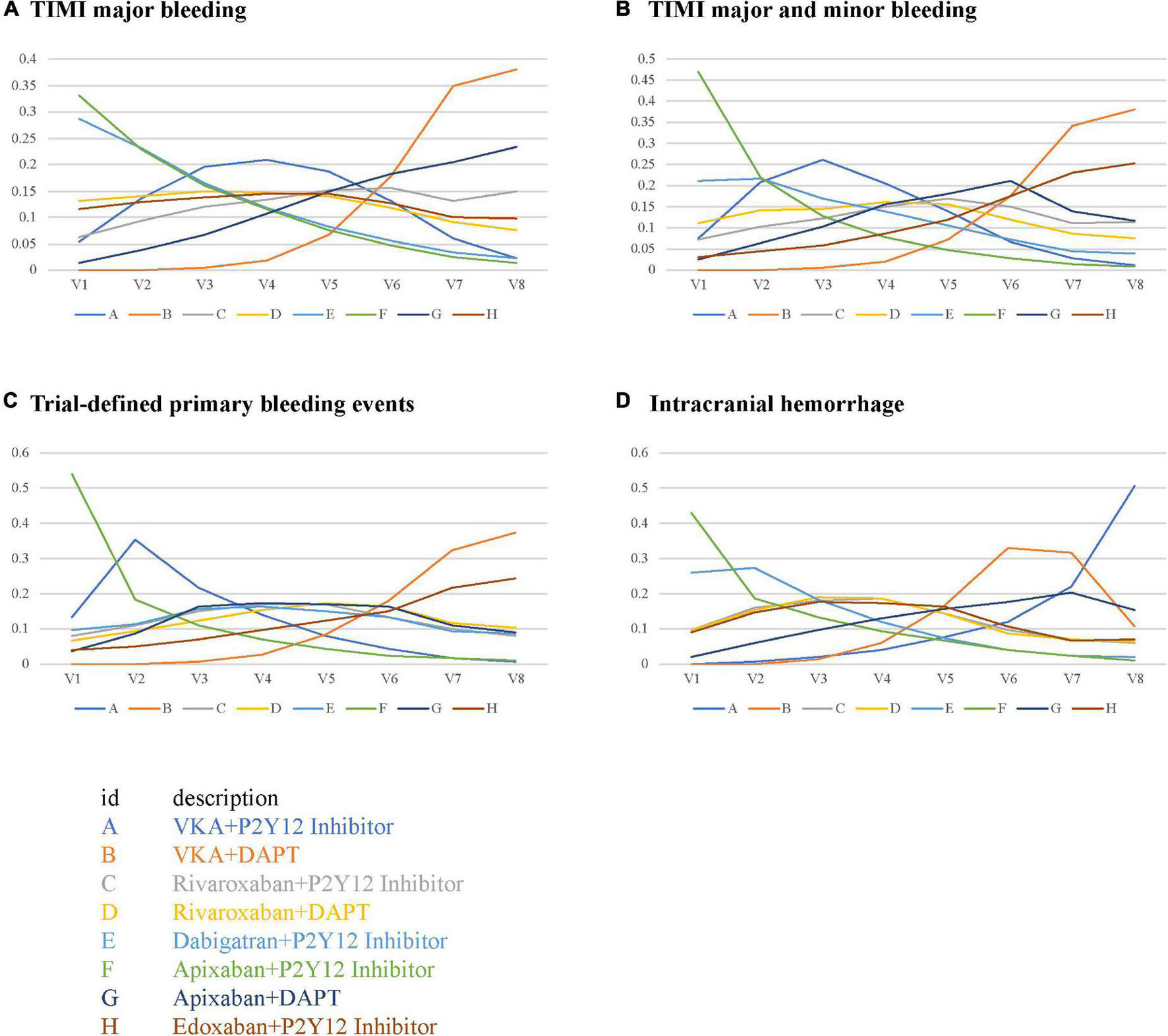
Rank probabilities for safety outcomes. (A) TIMI major bleeding. (B) TIMI major and minor bleeding. (C) Trial-defined primary bleeding events. (D) Intracranial hemorrhage.
FIGURE 7
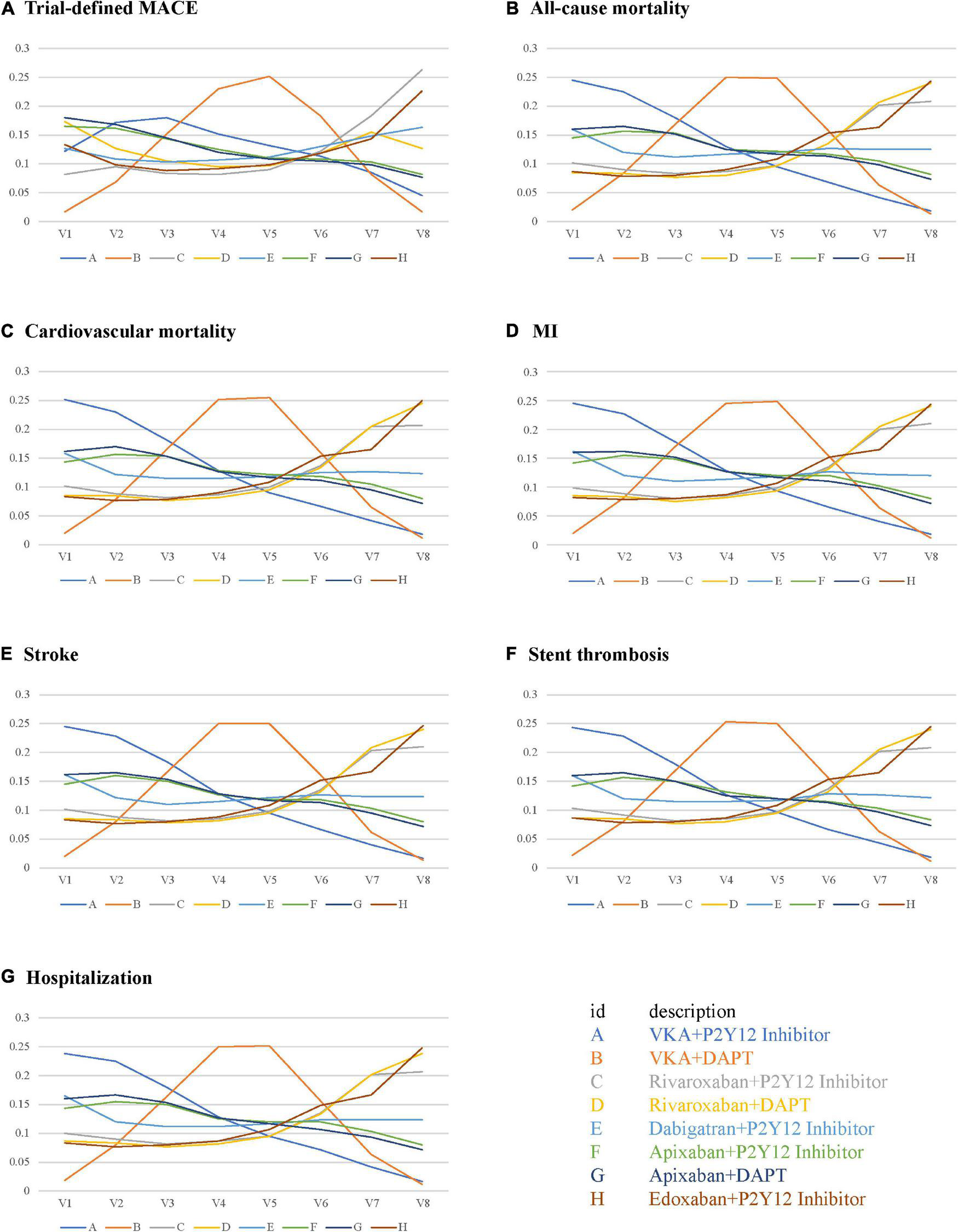
Rank probabilities for efficacy outcomes. (A) Trial-defined MACE. (B) All-cause mortality. (C) Cardiovascular mortality. (D) MI. (E) Stroke. (F) Stent thrombosis. (G) Hospitalization.
Discussion
Antithrombotic drugs are widely used treatments for patients with AF undergoing PCI worldwide. In this comprehensive Bayesian network meta-analysis of five multicenter RCTs, we investigated the safety and efficacy profile of eight antithrombotic regimens in 11,532 AF patients undergoing PCI. Apixaban plus P2Y12 inhibitor seems to reduce bleeding complications while maintaining antithrombotic efficacy. Moreover, for most efficacy indicators, VKA plus P2Y12 inhibitor ranking is still very high. The summary of this study indicated that discontinuing aspirin seems to be feasible clinically, which is consistent with a previous study (12).
Most guideline recommendations in cardiology are based on low-quality evidence, and the field of antithrombotic therapy for AF undergoing PCI is no exception. In the past few years, we have been hovering directly between dual antiplatelet therapy and three antiplatelet therapy (20). Here, to our knowledge, we determined the specific drug composition for the first time. This is due to the publication of high-quality evidence, which allows us to more objectively evaluate the risk of bleeding and ischemia.
ACTIVE W indicated that oral anticoagulation therapy is superior to clopidogrel plus aspirin for the prevention of vascular events in patients with AF at high risk of stroke, especially in those already taking oral anticoagulation therapy (21). The prognosis is unsatisfactory in warfarin-treated stented patients, and warfarin plus aspirin increased the risk of stent thrombosis (22). Oral anticoagulants exert antiplatelet effects to prevent stroke and recurrent MI, and further synergize through P2Y12 inhibition (23, 24). Therefore, oral anticoagulants plus P2Y12 inhibition may be appropriate for the vast majority of patients with AF undergoing PCI. NOACs simplify long-term anticoagulation therapy by promoting optimal thromboembolic protection and reducing bleeding complications (20). Our results of rank probabilities and SUCRA indicated that apixaban plus P2Y12 inhibitor had the least TIMI major bleeding, combined TIMI major and minor bleeding, trial-defined primary bleeding events, and intracranial hemorrhage, indicating apixaban plus P2Y12 inhibitor is the safest, and that VKA plus P2Y12 inhibitor had the least all-cause mortality, cardiovascular mortality, MI, stroke, stent thrombosis, and hospitalization, indicating VKA plus P2Y12 inhibitor is the most effective.
This is a study-level meta-analysis without obtaining individual patient data, which has well-known inherent limitations. In addition, due to the limited number of studies (less than 10), we were unable to identify potential publication bias (25). Third, our primary efficacy outcome is trial-defined MACE, but because each RCT defines MACE differently, our conclusion about trial-defined MACE may not be entirely credible. We concluded here that apixaban plus DAPT had the highest SUCRA value than others, which may be inconsistent with our previous knowledge. Moreover, our results are primarily on the clopidogrel-based therapy (most patients received this P2Y12 inhibitor), therefore, whether the use of strategies to identify poor-responders or the use of alternative P2Y12 inhibitors may reduce the risk of thrombus while retaining the bleeding benefit remains to be investigated. Finally, future studies focused on individual patient-level data analyses, whether trial-specific or pooled, may help to further refine which patients would benefit most from longer-term apixaban plus P2Y12 inhibitor consumption.
Conclusion
Apixaban plus P2Y12 inhibitor seems to be linked with fewer bleeding complications while retaining antithrombotic efficacy. Moreover, the ranking of VKA plus P2Y12 inhibitor is highest for most efficacy indicators.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
BL and NG designed the research. BL wrote the manuscript. NG revised the manuscript. All authors acquired and analyzed the data, interpreted the results, and read and approved the final manuscript.
Funding
This work was partly supported by Nanjing Municipal Health Science and Technology Development Special Fund (ZKX21060) and Jiangsu Leading Talent Project of Traditional Chinese Medicine (Jiangsu TCM 2018 No. 4).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.832164/full#supplementary-material
Supplementary Figure 1Model diagnostics under the assumption of evidence consistency. (A) TIMI major bleeding. (B) TIMI major and minor bleeding. (C) Trial-defined primary bleeding events. (D) Intracranial hemorrhage. (E) Trial-defined MACE. (F) All-cause mortality. (G) Cardiovascular mortality. (H) MI. (I) Stroke. (J) Stent thrombosis. (K) Hospitalization.
Supplementary Figure 2Checking the assumption of evidence inconsistency.
References
1.
Liang B Zhao Y-X Zhang X-X Liao H-L Gu N . Reappraisal on pharmacological and mechanical treatments of heart failure.Cardiovasc Diabetol. (2020) 19:55. 10.1186/s12933-020-01024-5
2.
Knuuti J Wijns W Saraste A Capodanno D Barbato E Funck-Brentano C et al 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. 10.1093/eurheartj/ehz425
3.
Liang B Zhao Y-X Gu N . Letter by Liang et al regarding article, “Oral anticoagulation in asian patients with atrial fibrillation and a history of intracranial hemorrhage”.Stroke. (2020) 51:e111. 10.1161/STROKEAHA.120.029434
4.
January CT Wann LS Calkins H Chen LY Cigarroa JE Cleveland JC et al 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–e51. 10.1161/CIR.0000000000000665
5.
Gwyn JCV Thomas MR Kirchhof P . Triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a viewpoint.Eur Heart J Cardiovasc Pharmacother. (2017) 3:157–62. 10.1093/ehjcvp/pvx002
6.
Capodanno D Huber K Mehran R Lip GYH Faxon DP Granger CB et al Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74:83–99. 10.1016/j.jacc.2019.05.016
7.
Dewilde WJM Oirbans T Verheugt FWA Kelder JC De Smet BJGL Herrman J-P et al Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet (Lond Engl). (2013) 381:1107–15. 10.1016/S0140-6736(12)62177-1
8.
Gibson CM Mehran R Bode C Halperin J Verheugt FW Wildgoose P et al Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. (2016) 375:2423–34. 10.1056/NEJMoa1611594
9.
Cannon CP Bhatt DL Oldgren J Lip GYH Ellis SG Kimura T et al Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. (2017) 377:1513–24. 10.1056/NEJMoa1708454
10.
Lopes RD Heizer G Aronson R Vora AN Massaro T Mehran R et al Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. (2019) 380:1509–24. 10.1056/NEJMoa1817083
11.
Vranckx P Valgimigli M Eckardt L Tijssen J Lewalter T Gargiulo G et al Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet (Lond Engl). (2019) 394:1335–43. 10.1016/S0140-6736(19)31872-0
12.
Lopes RD Hong H Harskamp RE Bhatt DL Mehran R Cannon CP et al Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol. (2020) 5:582–9. 10.1001/jamacardio.2019.6175
13.
Hutton B Salanti G Caldwell DM Chaimani A Schmid CH Cameron C et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. 10.7326/M14-2385
14.
Liang B Liang Y Zhao L-Z Zhao Y-X Gu N . Rivaroxaban for cancer-associated venous thromboembolism.Sci Prog. (2021) 104:368504211012160. 10.1177/00368504211012160
15.
Higgins JPT Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:889–93.
16.
van Valkenhoef G Lu G de Brock B Hillege H Ades AE Welton NJ . Automating network meta-analysis.Res Synth Methods. (2012) 3:285–99. 10.1002/jrsm.1054
17.
Lunn D Spiegelhalter D Thomas A Best N . The BUGS project: evolution, critique and future directions.Stat Med. (2009) 28:3049–67. 10.1002/sim.3680
18.
Salanti G Ades AE Ioannidis JPA . Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial.J Clin Epidemiol. (2011) 64:163–71. 10.1016/j.jclinepi.2010.03.016
19.
Dias S Welton NJ Caldwell DM Ades AE . Checking consistency in mixed treatment comparison meta-analysis.Stat Med. (2010) 29:932–44. 10.1002/sim.3767
20.
Lopes RD Hong H Harskamp RE Bhatt DL Mehran R Cannon CP et al Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis of randomized controlled trials. JAMA Cardiol. (2019) 4:747–55. 10.1001/jamacardio.2019.1880
21.
Connolly S Pogue J Hart R Pfeffer M Hohnloser S Chrolavicius S et al Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. (2006) 367:1903–12. 10.1016/s0140-6736(06)68845-4
22.
Karjalainen PP Porela P Ylitalo A Vikman S Nyman K Vaittinen M-A et al Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. (2007) 28:726–32. 10.1093/eurheartj/ehl488
23.
Nakase T Moroi J Ishikawa T . Anti-inflammatory and antiplatelet effects of non-vitamin K antagonist oral anticoagulants in acute phase of ischemic stroke patients.Clin Transl Med. (2018) 7:e2. 10.1186/s40169-017-0179-9
24.
Perzborn E Heitmeier S Laux V . Effects of rivaroxaban on platelet activation and platelet-coagulation pathway interaction: in vitro and in vivo studies.J Cardiovasc Pharmacol Ther. (2015) 20:554–62. 10.1177/1074248415578172
25.
Sterne JAC Sutton AJ Ioannidis JPA Terrin N Jones DR Lau J et al Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. 10.1136/bmj.d4002
Summary
Keywords
antithrombotic regimens, atrial fibrillation, percutaneous coronary intervention, VKA, NOAC, safety and efficacy
Citation
Liang B, Zhu Y-C and Gu N (2022) Comparative Safety and Efficacy of Eight Antithrombotic Regimens for Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 9:832164. doi: 10.3389/fcvm.2022.832164
Received
09 December 2021
Accepted
21 February 2022
Published
21 March 2022
Volume
9 - 2022
Edited by
Ji Huang, Capital Medical University, China
Reviewed by
Hui-Ling Liao, Southwest Medical University, China; Liang Rui, Kunming Medical University, China; Jaroslaw Zalewski, Jagiellonian University Medical College, Poland
Updates
Copyright
© 2022 Liang, Zhu and Gu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Gu, guning@njucm.edu.cn
This article was submitted to Coronary Artery Disease, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.