Abstract
Background:
Here we aimed to analyze changes in the outcomes of atrial fibrillation (AF) catheter ablation (AFCA) during the coronavirus disease 2019 (COVID-19) pandemic and examine the relationship between rhythm outcomes and the stringency of government social distancing measures.
Methods:
We included 453 patients who underwent de novo AFCA between May 2018 and October 2019 (pre-COVID-19 era) and 601 between November 2019 and April 2021 (COVID-19 era). The primary outcome was late recurrence, defined as any episode of AF or atrial tachycardia documented after a 3-month blanking period. A multivariable Cox regression analysis was performed to estimate the relative hazards of AF recurrence in the two eras.
Results:
In the study population (24.3% women; median age, 60 years), 660 (62.6%) patients had paroxysmal AF. Among those with paroxysmal AF, the late recurrence rate was significantly lower in the COVID-19 era than in the pre-COVID-19 era [9.4% vs. 17.0%, respectively, log-rank P = 0.004; adjusted hazard ratio (HR) 0.56, 95% confidence interval (CI) 0.35–0.90] during a median follow-up of 11 months. In patients with persistent AF, the late recurrence rate did not significantly differ between the pre-COVID-19 and COVID-19 era groups (18.9% vs. 21.5%, respectively; log-rank P = 0.523; adjusted HR 0.84, 95% CI 0.47–1.53) during the median follow-up of 11 months.
Conclusion:
A decrease in AF recurrence after catheter ablation was observed in patients with paroxysmal AF during the COVID-19 outbreak, whereas no change was observed in those with persistent AF.
Introduction
Severe acute respiratory syndrome coronavirus-2, which causes coronavirus disease 2019 (COVID-19), has affected over 2 million people worldwide (1). As a result, many countries have implemented public health restrictions to mitigate its spread. In Korea, non-pharmaceutical interventions (NPIs), including compulsory mask-wearing, social distancing, and enhanced screening and testing, were implemented in February 2020, the early phase of the outbreak (Supplementary Figure 1A) (2).
Electrophysiologic issues, including arrhythmias or device-related issues, have been increasingly recognized as a manifestation of COVID-19. While the need for services from electrophysiology laboratories continues to increase, a recent consensus paper recommended canceling or postponing elective cases during the pandemic (3). However, little is known about the impact of the COVID-19 pandemic and associated public health restrictions on clinical outcomes of catheter ablation for atrial fibrillation (AF).
Methods
Study Population
This single-center retrospective observational study aimed to analyze changes in the outcomes of AF catheter ablation (AFCA) during the COVID-19 pandemic and examine the relationship between rhythm outcomes and the stringency of government social distancing measures. We included 453 consecutive patients who underwent de novo AFCA between May 2018 and October 2019 (18 months of the pre-COVID-19 era) and 601 between November 2019 and April 2021 (18 months of the COVID-19 era) at Severance Cardiovascular Hospital, a tertiary referral center in the Republic of Korea (Supplementary Figures 1B,C). The study protocol adhered to the Declaration of Helsinki and was approved by our institutional review board. Written informed consent was obtained from all patients (ClinicalTrials.gov: NCT02138695). The exclusion criteria were: (1) permanent AF refractory to electrical cardioversion; (2) AF with valvular disease ≥ grade 2; (3) a previous cardiac surgery with concomitant AF surgery or AFCA; and (4) empirical extra-pulmonary vein (PV) ablations other than the typical circumferential PV isolation. All antiarrhythmic drugs (AADs) were discontinued for at least five half-lives, and amiodarone was stopped at least 4 weeks before the procedure.
Echocardiographic Evaluation
All patients underwent transthoracic echocardiography (Sonos 5500, Philips Medical System, Andover, MA or Vivid 7, GE Vingmed Ultrasound, Horten, Norway) prior to their ablation. Chamber size, left ventricular ejection fraction, transmitral Doppler flow velocity, and the ratio of early diastolic peak mitral inflow velocity to early diastolic mitral annular velocity (E/Em) were acquired according to the American Society of Echocardiography guidelines (4).
Electrophysiological Mapping and Radiofrequency Catheter Ablation
Intracardiac electrograms were recorded using a Prucka CardioLab Electrophysiology system (General Electric Medical Systems, Inc., Milwaukee, WI, United States). Three-dimensional electroanatomic mapping (NavX, St. Jude Medical, Inc., Minnetonka, MN, United States; CARTO, Biosense-Webster, Inc., Diamond Bar, CA, United States) was performed using a circumferential PV mapping catheter (Lasso, Biosense-Webster Inc.) through a long sheath (Schwartz left 1, St. Jude Medical, Inc.). Transseptal punctures were performed, and multiview pulmonary venograms were obtained. The details of the AFCA technique were described previously (5, 6). All patients underwent circumferential PV isolation (CPVI) during the de novo procedure. Two-thirds of the patients (62.1%) underwent the creation of a cavotricuspid isthmus block during the de novo procedure. Systemic anticoagulation was achieved with intravenous heparin to maintain an activated clotting time of 350–400 s during the procedure. After completion of the protocol-based ablation, the procedure was completed when no recurrence of AF was observed within 10 min after cardioversion with isoproterenol infusion (5–10 μg/min depending on β-blocker use, target sinus heart rate, 120 bpm) (6). Complications were defined according to the 2017 HRS (Heart Rhythm Society)/EHRA (European Heart Rhythm Association)/APHRS (Asia Pacific Heart Rhythm Society)/SOLAECE (Latin American Society of Cardiac Stimulation and Electrophysiology) expert consensus (7). Detailed definitions of the complications have been described previously (8).
Follow-Up and Atrial Fibrillation Recurrence
We discharged patients not taking AADs except for those who had recurrent extra-PV triggers after the AFCA procedure, symptomatic frequent atrial premature beats, non-sustained atrial tachycardia, or an early recurrence of AF on telemetry during the admission period. Electrocardiography was performed for all patients visiting the outpatient clinic 1, 3, 6, and 12 months after AFCA and every 6 months thereafter or whenever symptoms developed. Twenty-four-hour Holter recordings were performed at 3, 6, and 12 months and every 6 months thereafter. Patients who reported episodes of palpitations suggestive of arrhythmia recurrence underwent Holter monitoring or event monitoring recordings.
The primary outcome was late recurrence defined as any episode of AF or atrial tachycardia (AT) lasting at least 30 s after a 3-month blanking period. Early recurrence was defined as any documentation of AF or AT recurrence on ECG within the 3-month blanking period. Follow-up lasted up to January 31, 2020 for the pre-COVID-19 era group and July 31, 2021, for the COVID-19 era group with equal follow-up durations for the groups with a minimum follow-up of 3 months (Supplementary Figure 1B).
Statistical Analysis
Continuous variables are summarized as median (interquartile range), while categorical variables are summarized as frequencies (percentages). A Kaplan–Meier analysis with the log-rank test was used to calculate AF recurrence-free survival over time across groups. Multivariable Cox regression analysis was performed to estimate the relative hazards of AF recurrence. The following variables were adjusted: age, sex, duration of AF, body mass index, CHA2DS2-VASc, medical history, antiarrhythmic drug use, alcohol use, echocardiographic parameters, an inflammatory marker, ablation lesion set, and follow-up duration (variables in Table 1). The proportional hazards assumption was tested based on Schoenfeld residuals (9).
TABLE 1
| Variables | Paroxysmal AF (n = 660) |
Persistent AF (n = 394) |
||||||
| Overall (n = 660) | Pre COVID-19 era (n = 318) | COVID-19 era (n = 342) | P-value | Overall (n = 394) | Pre COVID-19 era (n = 135) | COVID-19 era (n = 259) | P-value | |
| Age, years | 60 (52–67) | 59 (51–66) | 61 (54–68) | 0.018 | 61 (53–67) | 58 (50–65) | 61 (55–68) | 0.001 |
| Female, n (%) | 179 (27.1) | 84 (26.4) | 95 (27.8) | 0.760 | 77 (19.5) | 27 (20.0) | 50 (19.3) | 0.975 |
| AF duration, months | 15 (7–36) | 14 (7–36) | 15 (7–36) | 0.628 | 20 (9–48) | 19 (9–47) | 20 (9–48) | 0.746 |
| BMI, kg/m2 | 24.6 (22.9–26.7) | 24.5 (22.8–26.6) | 24.7 (23.1–26.7) | 0.562 | 25.3 (23.3–27.4) | 25.7 (23.7–27.5) | 25.2 (23.0–27.2) | 0.092 |
| CHA2DS2-VASc score | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.140 | 2 (1–2.75) | 2 (1–2) | 2 (1–3) | 0.019 |
| Comorbidities, n (%) | ||||||||
| Heart failure | 54 (8.2) | 22 (6.9) | 32 (9.4) | 0.317 | 102 (25.9) | 32 (23.7) | 70 (27.0) | 0.553 |
| Hypertension | 298 (45.2) | 150 (47.2) | 148 (43.3) | 0.354 | 208 (52.8) | 65 (48.1) | 143 (55.2) | 0.220 |
| Diabetes mellitus | 92 (13.9) | 47 (14.8) | 45 (13.2) | 0.625 | 75 (19.0) | 27 (20.0) | 48 (18.5) | 0.828 |
| Stroke | 53 (8.0) | 19 (6.0) | 34 (9.9) | 0.084 | 37 (9.4) | 10 (7.4) | 27 (10.4) | 0.428 |
| TIA | 6 (0.9) | 4 (1.3) | 2 (0.6) | 0.617 | 1 (0.3) | 0 (0.0) | 1 (0.4) | 1.000 |
| Vascular disease | 20 (3.0) | 10 (3.1) | 10 (2.9) | 1.000 | 24 (6.1) | 3 (2.2) | 21 (8.1) | 0.036 |
| Current drinking, n (%) | 158 (23.9) | 93 (29.2) | 65 (19.0) | 0.003 | 121 (30.7) | 46 (34.1) | 75 (29.0) | 0.352 |
| Total alcohol intake per week in current drinkers, g | 51.8 (17.7–148.9) | 64.8 (19.4–155.4) | 51.8 (15.5–103.6) | 0.155 | 77.7 (17.7–207.2) | 90.7 (24.2–207.2) | 77.7 (17.8–155.4) | 0.544 |
| Drinking frequency per week | 1.5 (0.9–2.5) | 1.5 (1.0–3.0) | 1.0 (0.8–2.0) | 0.190 | 2.0 (1.0–3.0) | 2.0 (1.0–3.5) | 1.5 (1.0–3.0) | 0.375 |
| AAD use prior to the ablation, n (%) | ||||||||
| Class Ic | 378 (57.3) | 189 (59.4) | 189 (55.3) | 0.316 | 158 (40.1) | 51 (37.8) | 107 (41.3) | 0.568 |
| Class III | 306 (46.4) | 142 (44.7) | 164 (48.0) | 0.441 | 264 (67.0) | 98 (72.6) | 166 (64.1) | 0.112 |
| Echocardiographic parameters | ||||||||
| LA dimension, mm | 39 (35–43) | 38 (35–43) | 39 (36–43) | 0.331 | 43 (39–46) | 44 (40–48) | 43 (39–45) | 0.011 |
| LV ejection fraction,% | 65 (61–69) | 65 (61–69) | 65 (62–69) | 0.448 | 62 (57–66) | 61 (56–65) | 62 (58–67) | 0.030 |
| E/Em | 9.1 (7.4–11.8) | 9.0 (7.2–11.1) | 9.3 (7.6–12.3) | 0.133 | 9.0 (7.3–11.5) | 8.4 (7.4–10.6) | 9.4 (7.4–12.0) | 0.009 |
| hsCRP, mg/dL | 0.60 (0.30–1.10) | 0.70 (0.40–1.37) | 0.50 (0.20–1.00) | < 0.001 | 0.70 (0.40–1.35) | 0.80 (0.60–1.72) | 0.60 (0.20–1.20) | < 0.001 |
| CPVI, n (%) | 660 (100.0) | 318 (100.0) | 342 (100.0) | 1.000 | 394 (100.0) | 135 (100.0) | 259 (100.0) | 1.000 |
| CTI, n (%) | 399 (60.5) | 206 (64.8) | 193 (56.4) | 0.035 | 256 (65.0) | 111 (82.2) | 145 (56.0) | < 0.001 |
| Follow-up duration, months | 11 (6–15) | 11 (7–15) | 12 (6–15) | 0.898 | 11 (6–15) | 11 (7–15) | 11 (6–14) | 0.609 |
Baseline characteristics of patients with paroxysmal and persistent atrial fibrillation undergoing catheter ablation.
Values are presented as median (interquartile range) or n (%).
AAD, antiarrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CPVI, circumferential pulmonary vein isolation; CTI, cavotricuspid isthmus; E/Em, ratio of the peak mitral flow velocity of the early rapid filling to the early diastolic velocity of the mitral annulus; hsCRP, high sensitive C-reactive protein; LA, left atrium; LV, left ventricle; TIA, transient ischemic attack.
A two-sided P-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using R version 4.0.2 software (The R Foundation).1
Results
In the study population (24.3% women; median age, 60 years), 660 (62.6%) had paroxysmal AF. Patients ablated in the COVID-19 era tended to be older and more frequently had a history of stroke than those in the pre-COVID-19 era group (Table 1). The proportion of current drinkers was lower in the COVID-19 era than in the pre COVID-19 era (23.3% vs. 30.3%, respectively; P = 0.009) (Figure 1A) whereas there were no differences in the amount of weekly alcohol intake (Figure 1B) and the frequency of drinking (Figure 1C) between the groups. Procedural complication rates did not differ between the pre-COVID-19 era (2.4%) and COVID-19 era (3.2%) groups (P = 0.606). There were no differences in the compliances to Holter monitoring between the pre-COVID-19 era and COVID-19 era groups (Table 2). Among those with paroxysmal AF, the rate of late recurrence was significantly lower in those ablated in the COVID-19 era than in those ablated in the pre-COVID-19 era (9.4% vs. 17.0%, respectively; P = 0.005) during a median follow-up of 11 months (Table 2). The cumulative incidence of late recurrence at 1 year of follow-up was significantly lower in the COVID-19 era group (9.8%) than in the pre-COVID-19 era group (19.2%; log-rank P = 0.004) (Figure 2A). In multivariable Cox regression, the patients ablated in the COVID-19 era were at a lower risk of recurrence than those ablated in the pre-COVID-19 era [adjusted hazard ratio (HR) 0.56; 95% confidence interval (CI), 0.35–0.90]. In patients with persistent AF, the recurrence rate did not significantly differ between the pre-COVID-19 and COVID-19 eras (18.9% vs. 21.5%, respectively; P = 0.636) during the median follow-up of 11 months (Table 2). There was no difference in the cumulative incidence of recurrence at 1 year of follow-up between the two eras (26.4% in the pre-COVID-19 era vs. 22.1% in the COVID-19 era; log-rank P = 0.523) (Figure 2B). Risk of late recurrence did not differ between the two eras in multivariable Cox regression (adjusted HR 0.84; 95% CI, 0.47–1.53).
FIGURE 1
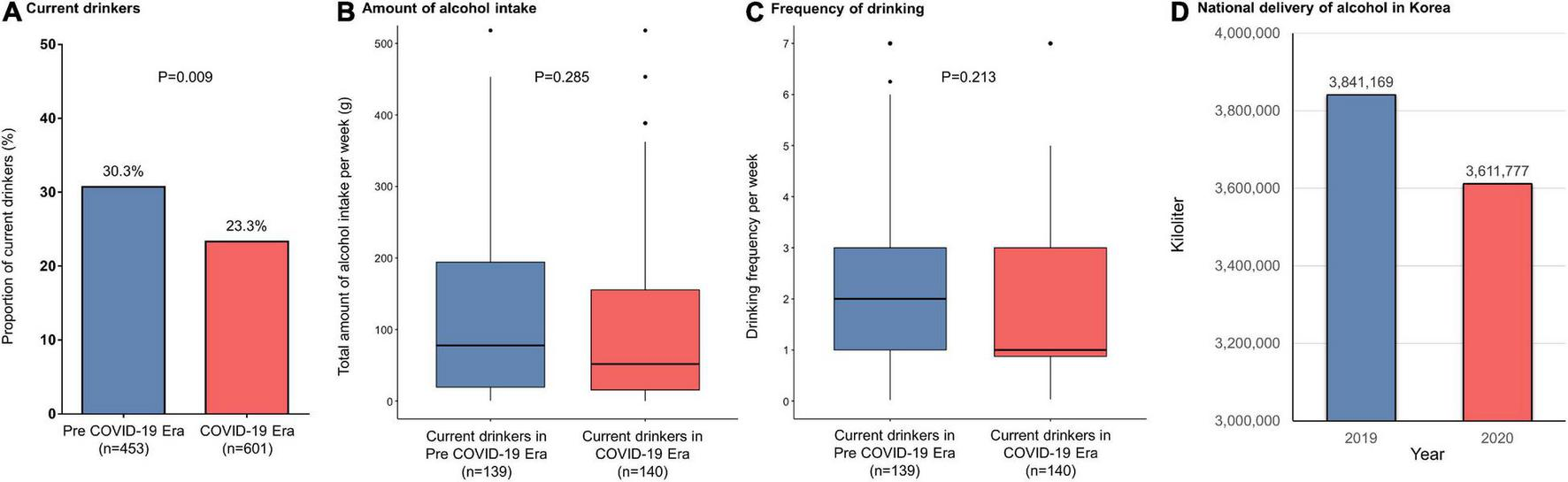
(A) Proportion of current drinkers, (B) total alcohol intake per week, (C) frequency of drinking per week in atrial fibrillation patients undergoing catheter ablation, and (D) amounts of national alcohol deliveries in Korea. COVID-19, coronavirus disease 2019.
TABLE 2
| Paroxysmal AF (n = 660) |
Persistent AF (n = 394) |
|||||
| Pre COVID-19 era | COVID-19 era | P-value | Pre COVID-19 era | COVID-19 era | P-value | |
| (n = 318) | (n = 342) | (n = 135) | (n = 259) | |||
| Follow-up months | 11 (7–15) | 12 (6–15) | 0.898 | 11 (7–15) | 11 (6–14) | 0.609 |
| Compliant to Holter monitoring | 235 (73.9) | 235 (68.7) | 0.166 | 90 (66.7) | 145 (56.0) | 0.052 |
| Post-ablation medication | ||||||
| ACEi, or ARB, n (%) | 105 (33.0) | 100 (29.2) | 0.335 | 43 (31.9) | 110 (42.5) | 0.052 |
| Beta blocker, n (%) | 134 (42.1) | 146 (42.7) | 0.949 | 79 (58.5) | 154 (59.5) | 0.942 |
| Statin, n (%) | 115 (36.2) | 144 (42.1) | 0.138 | 53 (39.3) | 123 (47.5) | 0.146 |
| AAD use | ||||||
| AADs at discharge, n (%) | 93 (29.2) | 76 (22.2) | 0.048 | 70 (51.9) | 95 (36.7) | 0.005 |
| AADs after 3 months, n (%) | 116 (36.5) | 87 (27.0) | 0.013 | 84 (62.2) | 106 (45.1) | 0.002 |
| AADs at final follow-up, n (%) | 92 (28.9) | 80 (25.2) | 0.326 | 65 (48.1) | 109 (47.4) | 0.975 |
| Early recurrence, n (%) | 80 (25.2) | 58 (17.0) | 0.013 | 73 (54.1) | 129 (49.8) | 0.485 |
| Recurrence type AF, n (% in early recur) | 72 (90.0) | 54 (93.1) | 0.739 | 68 (93.2) | 123 (95.3) | 0.735 |
| Recurrence type AT, n (% in early recur) | 8 (10.0) | 4 (6.9) | 5 (6.8) | 6 (4.7) | ||
| Late recurrence, n (%) | 54 (17.0) | 32 (9.4) | 0.005 | 29 (21.5) | 49 (18.9) | 0.636 |
| Recurrence type AF, n (% in recur) | 48 (88.9) | 29 (90.6) | 1.000 | 28 (96.6) | 48 (98.0) | 1.000 |
| Recurrence type AT, n (% in recur) | 6 (11.1) | 3 (9.4) | 1 (3.4) | 1 (2.0) | ||
| Cardioversion, n (% in recur) | 10 (18.5) | 1 (3.1) | 0.083 | 12 (41.4) | 12 (25.0) | 0.211 |
Clinical rhythm outcomes.
AAD, antiarrhythmic drug; ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; AT, atrial tachycardia; COVID-19, coronavirus disease 2019.
FIGURE 2
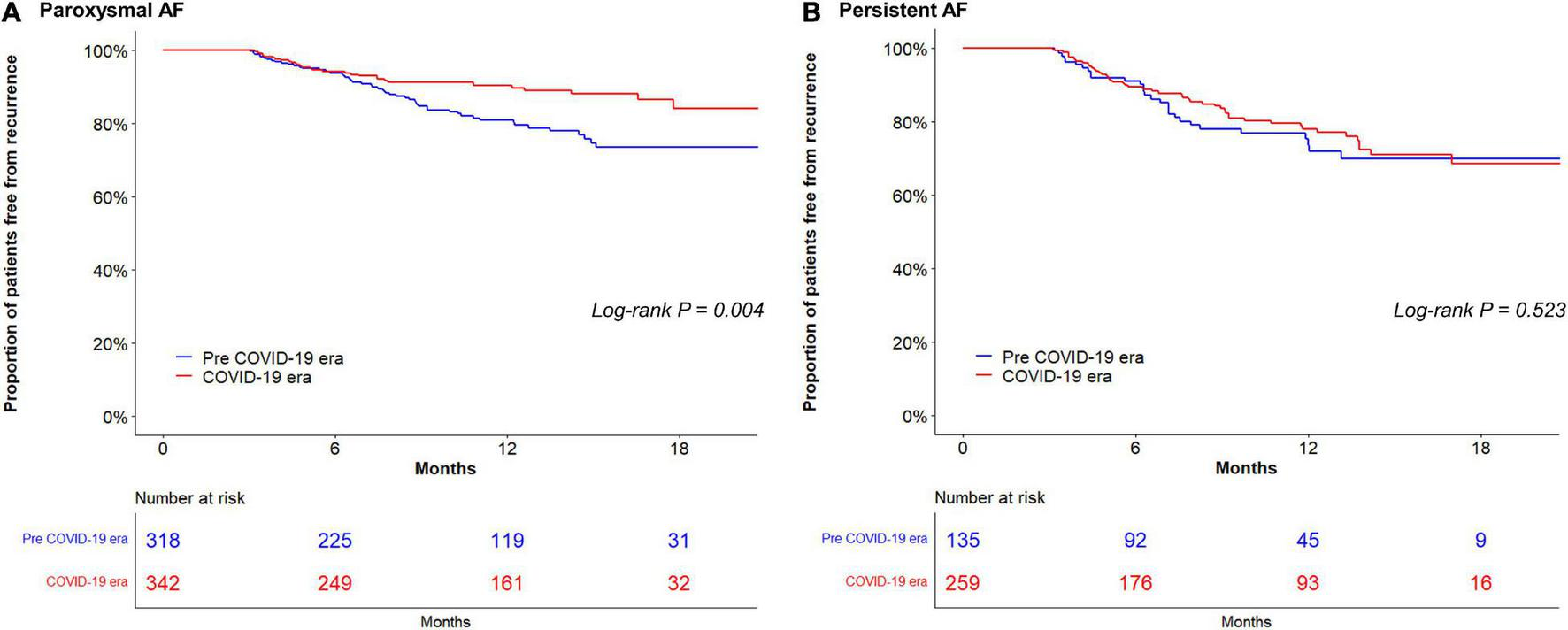
Kaplan–Meier analysis of atrial fibrillation: (AF) recurrence-free survival in paroxysmal AF (A); and in persistent AF (B). AF, atrial fibrillation; COVID-19, coronavirus disease 2019.
Discussion
The reasons for the association of the pandemic situation and public restrictions with a lower recurrence rate after AFCA in paroxysmal AF patients are unclear. After the implementation of NPIs in Korea, the monthly drinking rate, indicating the proportion of citizens who drink at least once a month for the past year, decreased from 59.9% in 2019 to 54.7% in 2020, the lowest value in the last 15 years (10). The Korean nationwide liquor delivery decreased from 3,841,169 kl in 2019 to 3,611,777 kl in 2020 (Figure 1D) (11). Takahashi et al. reported that alcohol reduction was associated with a 37% lower risk of recurrence after AFCA (12). In particular, the risk almost halved in those with paroxysmal AF (12). The proportion of current drinkers among patients with paroxysmal AF in this study was significantly higher in the pre-COVID-19 era (29.2%) than in the COVID-19 era (19.0%; P = 0.003), whereas there was no significant difference between 34.1% in the pre-COVID-19 era and 29.0% in the COVID-19 era among patients with persistent AF (P = 0.352). High-sensitivity C-reactive protein levels were also lower among patients undergoing AFCA in the COVID-19 era than those in the pre-COVID-19 era. Importantly, up-regulation of inflammatory biomarkers has been shown to be a valid predictor for AF recurrence (13, 14), and inflammation is known to alter atrial electrophysiology and structure to increase vulnerability to AF (15). Thus, changes in alcohol habits and systemic inflammation during the period of COVID-19 pandemic and associated social distancing might partly explain the results of this study.
This retrospective observational cohort study was performed at a single center and included patients using strict selection criteria for AF ablation; hence, our findings cannot be used to establish causal relationships. Although the follow-up period of this study was designed to enable a 3-month blanking period in all patients and to equalize follow-up durations between groups, there might be a discrepancy depending on the timing at which procedures were performed during the inclusion period. However, there were no differences in the follow-up durations between patients in the pre-COVID-19 and COVID-19 groups. In the pandemic period, the Korean medical system was under normal operation, and all elective AFCA procedures proceeded in the same manner as that before the pandemic without significant delay. Among the patients included this study, there were no differences in the compliances to Holter monitoring between the pre-COVID-19 era and COVID-19 era groups. In both paroxysmal AF and persistent AF patients, the uses of AADs at discharge and at 3 months of follow-up were more frequently observed in the pre-COVID-19 era than in the COVID-19 era, whereas there were no differences at the time of final follow-up. This difference might impact the outcomes.
Conclusion
This study reveals comparable outcomes of AFCA performed during the COVID-19 pandemic vs. the pre-pandemic period. Rather, a striking decrease in AF recurrence after catheter ablation was observed in patients with paroxysmal AF during the COVID-19 outbreak, whereas no change was observed in those with persistent AF.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board at Yonsei University Health System. The patients/participants provided their written informed consent to participate in this study.
Author contributions
H-NP contributed to the conception and design of the work, acquisition of data, and critical revision of the manuscript. DK contributed to the conception and design of the work, interpretation of data, and drafting of the manuscript. HTY, T-HK, J-SU, BJ, and M-HL contributed to the conception and design of the work and acquisition data. H-NP attested that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by a grant (HI21C0011) from the Ministry of Health and Welfare and a grant (NRF-2020R1A2B5B01001695) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF) which was funded by the Ministry of Science, ICT and Future Planning (MSIP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.836288/full#supplementary-material
Footnotes
References
1.
World Health Organization [WHO]. Coronavirus Disease (COVID-19) Situation Reports. (2021). Available online at: https://covid19.who.int/WHO-COVID-19-global-data.csv (accessed October 23, 2021).
2.
Huh K Jung J Hong J Kim M Ahn JG Kim JH et al Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. (2021) 72:e184–91. 10.1093/cid/ciaa1682
3.
Lakkireddy DR Chung MK Gopinathannair R Patton KK Gluckman TJ Turagam M et al Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. (2020) 141:e823–31. 10.1161/CIRCULATIONAHA.120.047063
4.
Nagueh SF Smiseth OA Appleton CP Byrd BF III Dokainish H Edvardsen T et al Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. 10.1016/j.echo.2016.01.011
5.
Kim D Shim J Kim YG Yu HT Kim TH Uhm JS et al Malnutrition and risk of procedural complications in patients with atrial fibrillation undergoing catheter ablation. Front Cardiovasc Med. (2021) 8:736042.
6.
Kim D Hwang T Kim M Yu HT Kim TH Uhm JS et al Extra-pulmonary vein triggers at de novo and the repeat atrial fibrillation catheter ablation. Front Cardiovasc Med. (2021) 8:759967. 10.3389/fcvm.2021.759967
7.
Calkins H Hindricks G Cappato R Kim YH Saad EB Aguinaga L et al 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2018) 20:e1–160. 10.1093/europace/eux274
8.
Lee JH Yu HT Kwon OS Han HJ Kim TH Uhm JS et al Atrial wall thickness and risk of hemopericardium in elderly women after catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2021) 14:e009368. 10.1161/CIRCEP.120.009368
9.
Grambsch PM Therneau TM . Proportional hazards tests and diagnostics based on weighted residuals.Biometrika. (1994) 81:515–26. 10.1093/biomet/81.3.515
10.
Korea Disease Control and Prevention Agency. Community Health Survey Reports 2020. (2021). Available online at: https://chs.kdca.go.kr/chs/stats/statsMain.do (accessed December 10, 2021).
11.
Statistics Korea. E-National Index. (2021). Available online at: https://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=2824 (accessed December 10, 2021).
12.
Takahashi Y Nitta J Kobori A Sakamoto Y Nagata Y Tanimoto K et al Alcohol consumption reduction and clinical outcomes of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2021) 14:e009770. 10.1161/CIRCEP.121.009770
13.
Korodi S Toganel R Benedek T Hodas R Chitu M Ratiu M et al Impact of inflammation-mediated myocardial fibrosis on the risk of recurrence after successful ablation of atrial fibrillation - the FIBRO-RISK study: protocol for a non-randomized clinical trial. Medicine. (2019) 98:e14504. 10.1097/MD.0000000000014504
14.
Richter B Gwechenberger M Socas A Zorn G Albinni S Marx M et al Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol. (2012) 101:217–25. 10.1007/s00392-011-0383-3
15.
Zhou X Dudley SC Jr. Evidence for inflammation as a driver of atrial fibrillation. Front Cardiovasc Med. (2020) 7:62. 10.3389/fcvm.2020.00062
Summary
Keywords
atrial fibrillation, COVID-19, pandemic, catheter ablation, rhythm outcome
Citation
Kim D, Yu HT, Kim T-H, Uhm J-S, Joung B, Lee M-H and Pak H-N (2022) Impact of the COVID-19 Pandemic and Public Restrictions on Outcomes After Catheter Ablation of Atrial Fibrillation. Front. Cardiovasc. Med. 9:836288. doi: 10.3389/fcvm.2022.836288
Received
15 December 2021
Accepted
02 March 2022
Published
24 March 2022
Volume
9 - 2022
Edited by
Junbeom Park, Ewha Womans Medical Center, South Korea
Reviewed by
François Regoli, University of Zurich, Switzerland; Alexis Hermida, University Hospital Center (CHU) of Amiens, France
Updates
Copyright
© 2022 Kim, Yu, Kim, Uhm, Joung, Lee and Pak.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Nam Pak, hnpak@yuhs.ac
This article was submitted to Cardiac Rhythmology, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.