Abstract
Objectives:
The relationship between cancer and subclinical atherosclerosis has always been the focus of people's attention. We conducted a systematic review and meta-analysis by evaluating the effects of cancer on functional and structural markers of subclinical atherosclerosis:intima-media thickness (IMT), pulse wave velocity (PWV), and flow-mediated vasodilation (FMD).
Methods:
A comprehensive and systematic literature search was conducted on the internet. Sensitivity analysis, publication bias, standard mean difference (SMD), corresponding 95% confidence interval (95% CI), and subgroup analysis were performed for all relevant research indicators in the retrieved literature.
Results:
Forty-six studies were included, including 3,729 cancer patients and 2,404 healthy controls. Cancer patients had significantly thicker IMT [SMD (95%CI) = 0.290 (0.069 to 0.511), P = 0.010] and higher PWV [SMD (95%CI) = 0.392 (0.136 to 0.647), P = 0.003] compared with healthy controls. There was no significant difference in FMD [SMD (95% CI) = −0.192 (−0.527 to 0.144), P > 0.05). After subgrouping by age, male proportion, and treatment, the analysis results of IMT ≥ 50 years old, PWV and FMD < 50 years old, male proportion ≥50%, chemotherapy group, IMT and PWV radiotherapy group, and PWV endocrine therapy group were statistically significant (P < 0.05). There were no significant differences in other subgroup analyses, overall sensitivity analysis, and publication bias (p < 0.05).
Conclusions:
Cancer may promote subclinical atherosclerosis, and change the functional and structural markers of subclinical atherosclerosis such as IMT and PWV. Early intervention and prevention should be pursued.
Introduction
The latest global cancer burden data for 2020 released by the World Health Organization's International Agency for Research on Cancer (IARC) shows that there were 19.29 million new cancer cases and 9.96 million cancer deaths worldwide in 2020. Cancer has become a common disease with high mortality and morbidity worldwide (1, 2), but with the progress of cancer treatment, the 5-year survival rate of cancer patients is getting higher and higher, and cancer has now become a chronic disease (2). At present, many studies show that cancer patients die from cardiovascular diseases in addition to cancer recurrence (3–6). The incidence of atherosclerosis in cancer patients is very high (4), which may be related to the systemic inflammatory response and impaired vascular endothelial function caused by the mechanism of cancer cell infection and corresponding chemoradiotherapy. Cancer patients all need to suffer from this change whether caused by simple cancer infection or its treatment. However, early detection and timely intervention can avoid the occurrence of atherosclerosis and even cardiovascular accidents.
Intima-media thickness (IMT), flow-mediated vasodilation (FMD), and pulse wave conduction velocity (PWV) are considered markers of atherosclerosis and provide important information about artery function and structure (7). These three markers have been widely used to evaluate subclinical atherosclerosis in order to improve prognosis and timely individualized treatment. Some studies have shown increased IMT, PWV, and reduced FMD in cancer patients compared to healthy controls (8–11). Conversely, some studies have produced inconsistent results (12–14). Currently, it is still controversial that cancer causes subclinical atherosclerosis and increased risk of cardiovascular disease, therefore we conducted this meta-analysis to further determine the relationship between cancer and subclinical atherosclerosis.
Since the available results are controversial, we conducted this meta-analysis to further evaluate differences in IMT, PWV, and FMD caused by cancer and to clarify the association between cancer and subclinical atherosclerosis.
Article Types
Systematic Review articles. Our systematic reviews conform to the reporting guideline PRISMA.
Manuscript Formatting
Materials and Methods
Publication Search
We searched the databases of PubMed, Web of Science, and Medline for articles concerning the relationship among the effects of cancer infection or related treatments on functional and structural markers of the vasculature (IMT, FMD, and PWV). The articles published between the earliest available dates to 5 August 2021 were applied. The key terms were as follows: “IMT” or “intima media thickness” and cancer or “flow-mediated dilatation” or “FMD” and cancer or “PWV” or “pulse wave velocity” and cancer.
Study Selection
Articles searched through the above retrieval methods were further screened using the following criteria. Figure 1 shows the filtering process of the studies.
Figure 1
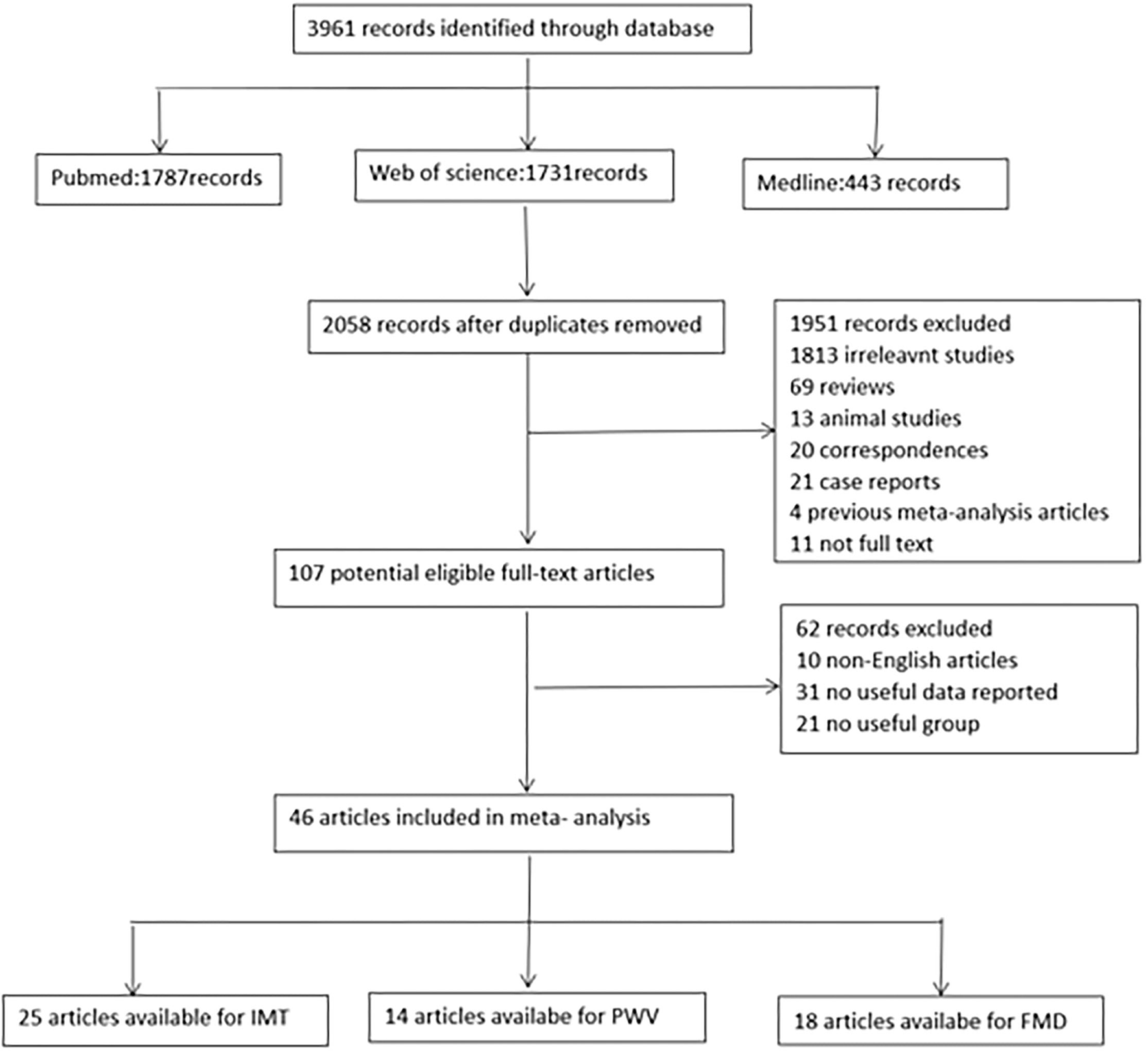
Flow chart for identification of studies. FMD indicates flow-mediated vasodilation; IMT, intima media thickness; PWV, pulse wave velocity.
Inclusion Criteria
-
The study should provide IMT, PWV, and/or FMD values.
-
The study should contain at least 2 independent groups or subgroups, 1 diagnosed with cancer and the other included healthy control subjects.
Exclusion Criteria
-
Find duplicate papers and keep one.
-
Take out reviews, case reports, letters, animal studies, and previous meta-analysis articles.
-
The study retrieved by abbreviations was not the markers of the vasculature.
-
No papers available in full.
-
The study was not published in English.
Data Extraction and Management
The data of the studies after the inclusion criteria and exclusion criteria were searched and extracted by two authors, describing in a standard format. In the screening process, they check each other out and come up with a consistent conclusion. The basic information to be collected is as follows: author, country, region, year, study design, site measured, sample size, mean age, the proportion of male, proportion of hypertension, proportion of smokers, proportion of patients with diabetes, the proportion of patients with hypertension, mean body mass index, mean systolic blood pressure, mean diastolic blood pressure, mean fasting blood glucose, mean total cholesterol, mean triglyceride, mean high-density lipoprotein, mean low-density lipoprotein, inflammatory markers, and the values of IMT, PWV, and FMD.
Data and Statistical Analysis
All of the study outcomes (values of IMT, PWV, and FMD) were transformed into mean and SD forms, which were performed using STATA 12.0 software (Stata Corp. College Station, TX, USA). Considering that the study values were measured on different sites, we calculated the standardized mean differences (SMDs) and 95% confidence intervals (95% CIs). Statistical heterogeneity between studies was assessed with the value of I2. If I2 was more than 50%, a fixed-effects model was considered (15). If I2 was no more than 50%, a random-effects model was applied. In the meantime, the sub-group analysis was used for potential confounders (mean age and male percentage). And univariate meta-regression analysis for the covariates (sample size, age, sex, region, and site measured) was also conducted. In addition, the sensitivity analysis was performed by removing studies one by one to assess the impact of each study on the overall outcome (16). Using funnel plots, the Begg model and Egger test evaluated qualitatively publication bias.
Results
Characteristics of Identified Studies
The search process is presented in the flow chart (Figure 1). In total, 3,961 articles were identified. According to the inclusion and exclusion criteria, 46 articles were finally applied in the meta-analysis, of which 25 could be used for IMT,14 studies included PWV, and 18 studies reported FMD values (8–14, 17–55) (Supplementary MaterialsSupplementary Tables S1–3). The articles were selected from different regions. Nine of the articles are from Asia, 22 are from Europe, 12 are from North America and two are from South America. They were published from 1991 to 2021. Their average age ranged from 9.5 to 70.6, and the proportion of men ranged from 0 to 100 percent. Their treatment methods are complex and varied, including radiotherapy, chemotherapy, endocrine therapy, and mixed treatment or early untreated treatment. In addition, different articles measured IMT, PWV, and FMD at different anatomical sites. More clinical characteristics included in the study, such as smokers, BMI, blood pressure, fasting glucose, and lipid ratio, are shown in Supplementary Table S4 of the online Supplementary Materials table.
The Relationship of Cancer and IMT
After analyzing the 25 articles comprising 1,927 cancer patients and 1,077 healthy controls collected, we concluded that the IMT of patients with cancer was significantly increased than that of controls without cancer [SMD (95% CI) = 0.290 (0.069–0.511), P = 0.010] (Table 1; Figure 2). Because I2 = 85.9% and P < 0.001, which represented significant heterogeneity, the random-effects model was conducted in the meta-analysis. In terms of sub-group analyses, there was a thicker IMT in the following subgroups: age ≥50 years [SMD (95% CI = 0.451 (0.020, 0.883), P = 0.040], male ratio ≥ 50% [SMD (95% CI) = 0.451 (0.020, 0.883), P = 0.011], radiotherapy group [SMD (95% CI) = 0.713 (0.418, 1.008), P < 0.001], and chemotherapy group [SMD (95% CI) = 0.795(0.108, 1.481), P = 0.023] (Table 1). There was no significant change in the SMD (95%CI) by taking out studies one by one (Supplementary MaterialsSupplementary Table S5). Beyond that, the results of the Begg test (P = 0.834) and Egger test (P = 0.566) didn't show significant publication bias (Table 2). Analysis of subgroup, analysis of sensitivity, and meta-regression (Supplementary MaterialsSupplementary Table S8) failed to explain the source of heterogeneity.
Table 1
| Variables | Group | N | SMD(95%CI) | p | I 2(%) | Phetero |
|---|---|---|---|---|---|---|
| IMT | ||||||
| All | 25 | 0.290 (0.069 to 0.511) | 0.010 | 85.9 | <0.001 | |
| Age, years | <50 | 16 | 0.202 (−0.050 to 0.455) | 0.116 | 84.0 | <0.001 |
| ≥50 | 9 | 0.451 (0.020 to 0.883) | 0.040 | 87.5 | <0.001 | |
| Male, % | <50 | 13 | 0.171 (−0.156 to 0.497) | 0.306 | 85.1 | <0.001 |
| ≥50 | 12 | 0.417 (0.097 to 0.738) | 0.011 | 87.5 | <0.001 | |
| Therapy | Radiotherapy | 12 | 0.713 (0.418 to 1.008) | <0.001 | 76.6 | <0.001 |
| Chemotherapy | 6 | 0.795 (0.108 to 1.481) | 0.023 | 91.9 | <0.001 | |
| Endocrine therapy | 3 | −0.350 (−0.742 to 0.043) | 0.081 | 47.6 | 0.149 | |
| Other | 4 | 0.018 (−0.112 to 0.148) | 0.786 | 0 | 0.628 | |
| PWV | ||||||
| All | 14 | 0.392 (0.136 to 0.647) | 0.003 | 77.0 | <0.001 | |
| Age, years | <50 | 5 | 0.448 (0.195 to 0.702) | 0.001 | 34.0 | 0.195 |
| ≥50 | 9 | 0.346 (−0.035 to 0.727) | 0.075 | 84.2 | <0.001 | |
| Male, % | <50 | 7 | 0.406 (−0.075 to 0.888) | 0.098 | 83.2 | <0.001 |
| ≥50 | 7 | 0.350 (0.088 to 0.612) | 0.009 | 63.9 | 0.011 | |
| Therapy | Radiotherapy | 3 | 0.704 (0.325 to 1.083) | <0.001 | 66.9 | 0.049 |
| Chemotherapy | 5 | 0.463 (0.020 to 0.905) | 0.040 | 73.0 | 0.005 | |
| Endocrine therapy | 1 | 1.081 (0.220 to 1.943) | 0.014 | - | - | |
| Other | 5 | 0.068(−0.287 to 0.423) | 0.706 | 64.8 | 0.023 | |
| FMD | ||||||
| All | 18 | −0.192 (−0.527 to 0.144) | 0.263 | 88.2 | <0.001 | |
| Age, years | <50 | 9 | −0.520 (−0.915 to −0.125) | 0.010 | 89.4 | <0.001 |
| ≥50 | 9 | 0.214 (−0.444 to 0.872) | 0.523 | 85.6 | <0.001 | |
| Male, % | <50 | 13 | 0.099 (−0.374 to 0.572) | 0.681 | 84.1 | <0.001 |
| ≥50 | 5 | −0.797 (−1.351 to −0.242) | 0.005 | 93.5 | <0.001 | |
| Therapy | Radiotherapy | 2 | 1.638 (−0.554 to 3.831) | 0.143 | 90.4 | 0.001 |
| Chemotherapy | 7 | −0.853 (−1.682 to −0.023) | 0.044 | 91.4 | <0.001 | |
| Endocrine therapy | 3 | −0.028 (−0.658 to 0.601) | 0.930 | 67.3 | 0.047 | |
| Other | 6 | −0.085 (−0.209 to 0.038) | 0.176 | 0 | 0.997 |
IMT, PWV, and FMD between patients with cancer and controls without cancer in overall and sub-group meta-analyses by age and female ratio.
The results are in bold if p < 0.05.
N, number; SMD, standard mean difference; CI, confidence interval; Phetero, pvalue of heterogenicity; IMT, intima-media thickness; PWV, pulse wave velocity; FMD, flow-mediated vasodilation.
Figure 2
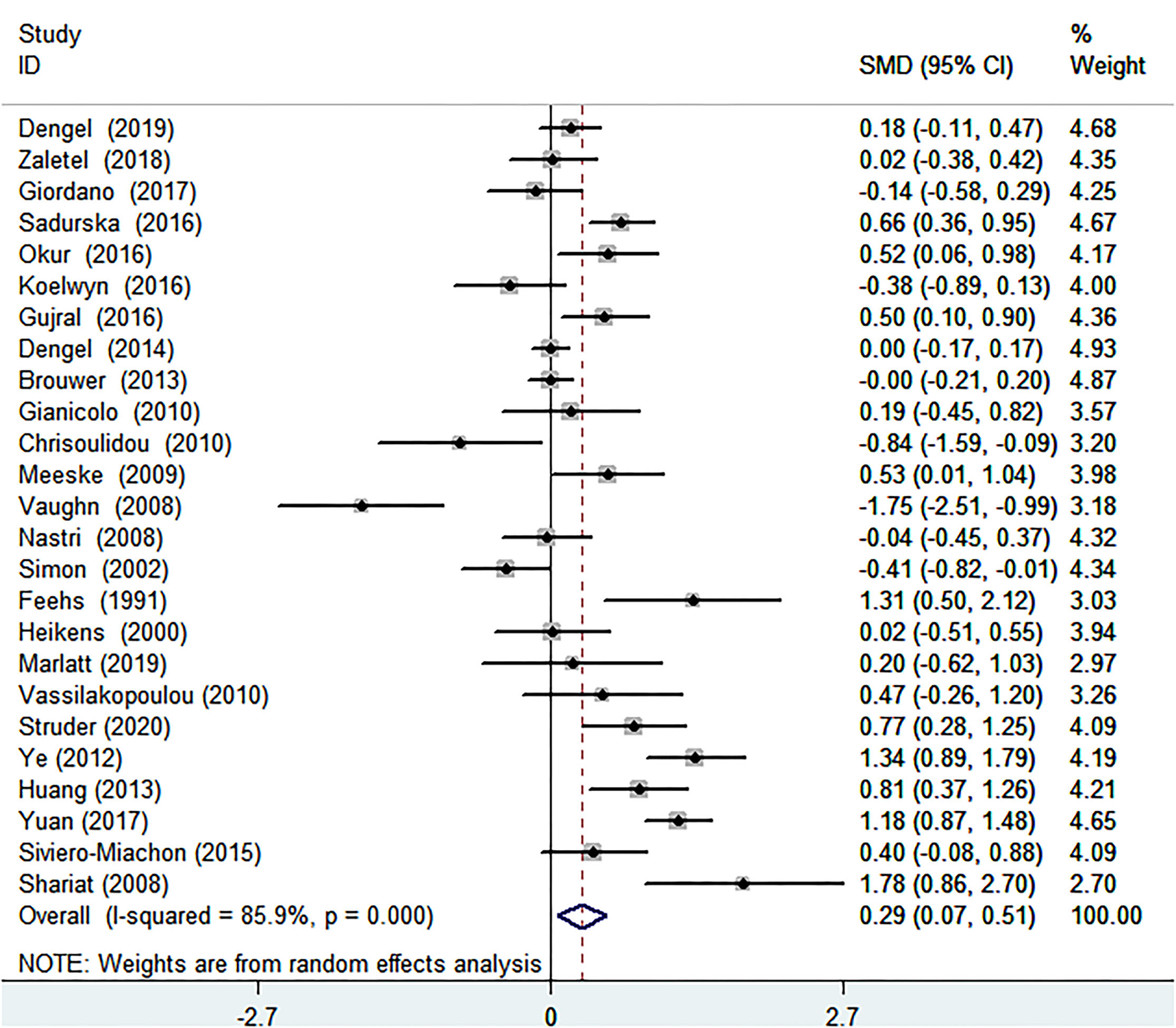
Forest plot of intima-media thickness in patients with cancer and controls without cancer. SMD, standard mean difference; CI, confidence interval.
Table 2
| Begg Test | Egger Test | |||
|---|---|---|---|---|
| Z Value | P-Value | t Value | P-Value | |
| IMT | 0.21 | 0.834 | 0.58 | 0.566 |
| PWV | 0.33 | 0.743 | −0.69 | 0.501 |
| FMD | 0.00 | 1.000 | −0.08 | 0.935 |
Publication bias evaluated by Begg's and Egger's tests.
IMT, intima-media thickness; PWV, pulse wave velocity; FMD, flow-mediated vasodilation.
The Relationship of Cancer and PWV
The conclusion that PWV was visibly increased in patients with cancer was suggested by comparing the summarized data of patients with cancer and healthy controls, SMD of 0.392 (95% CI = 0.136–0.647, P = 0.003) (Table 1; Figure 3). There was significant heterogeneity, I2 = 77.0%. In terms of sub-group analyses, there was a higher PWV values in the following subgroups: age <50 years [SMD (95% CI = 0.448 (0.195, 0.702), p = 0.001), male ratio ≥ 50% [SMD (95% CI) = 0.350 (0.088, 0.612), P = 0.009], radiotherapy group [SMD (95% CI) = 0.704 (0.325, 1.083), P < 0.001], chemotherapy group [SMD (95% CI) = 0.463 (0.020, 0.905), P = 0.040], and endocrine therapy group [SMD (95% CI) = 1.081 (0.220, 1.943), P = 0.014] (Table 1). There was no obvious publication bias from the results of the Begg test (P = 0.743) and Egger test (P = 0.501) (Table 2). There was no significant change in the SMD (95%CI) by taking out studies one by one (Supplementary MaterialsSupplementary Table S6). Moreover, analysis of subgroup, analysis of sensitivity, and meta-regression (Supplementary MaterialsSupplementary Table S8) failed to explain the source of heterogeneity.
Figure 3
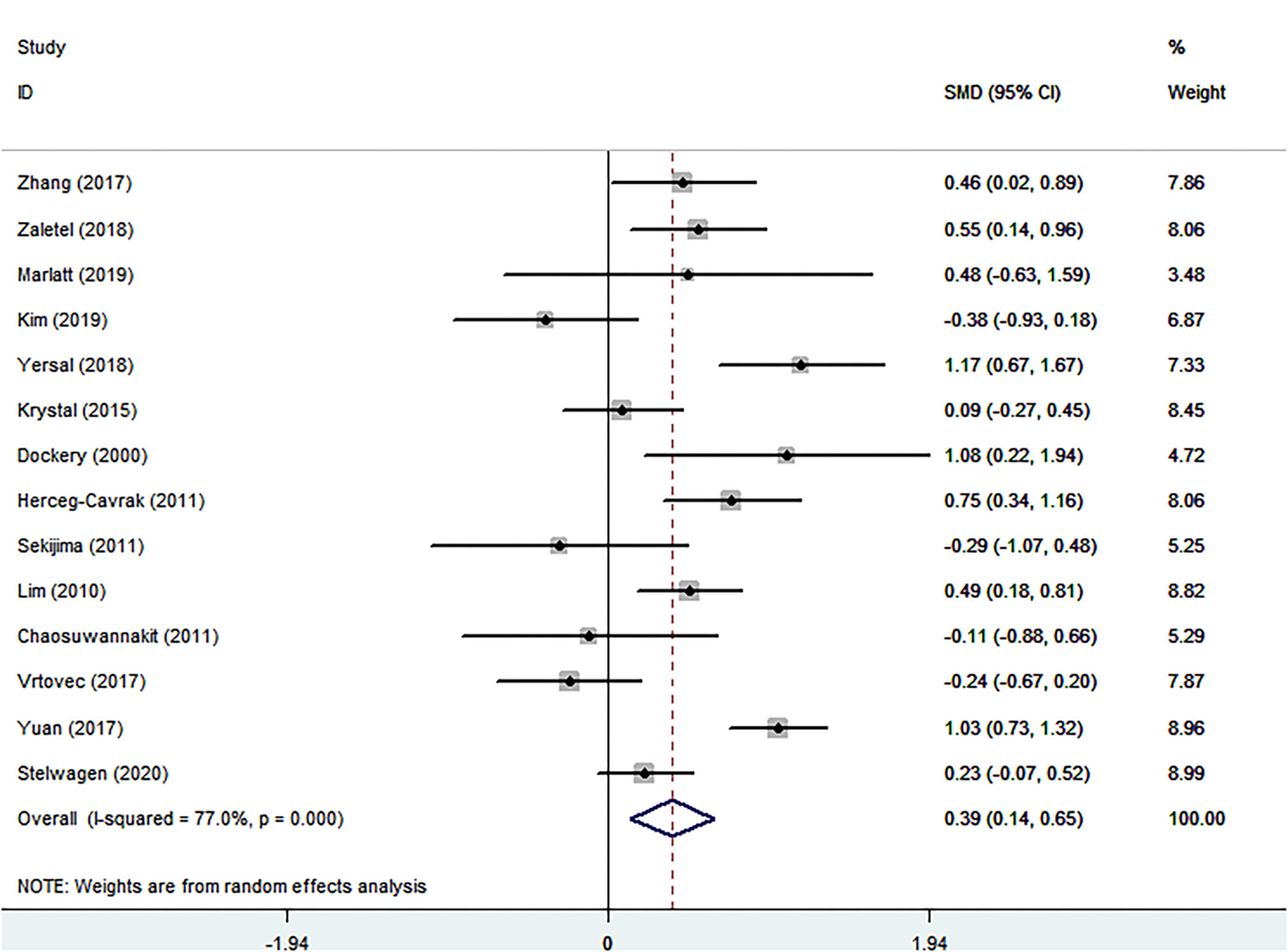
Forest plot of pulse wave velocity in patients with cancer and controls without cancer. SMD, standard mean difference; CI, confidence interval.
The Relationship of Cancer and FMD
Eighteen eligible studies were finally identified, involving 1,074 cancer patients and 672 healthy controls.The SMD of −0.192 (95% CI = −0.527 to 0.144, P = 0.263) was no significance (Table 1; Figure 4). The I2 statistic was 88.2%. In terms of sub-group analyses, there was a lower FMD in the following subgroups: age <50 years [SMD (95% CI) = −0.520 (−0.915, −0.125), P = 0.010], male ratio ≥ 50% [SMD (95% CI) = −0.797(−1.351, −0.242), p = 0.005], and chemotherapy group [SMD (95% CI) = −0.853 (−1.682, −0.023), P = 0.044] (Table 1). The Begg test (P = 0.743) and Egger test (P = 0.501) was no obvious publication bias (Table 2). There was no significant change in the SMD (95%CI) by taking out studies one by one (Supplementary MaterialsSupplementary Table S7). Analysis of subgroup, analysis of sensitivity, and meta-regression (Supplementary MaterialsSupplementary Table S8) failed to explain the cause of meaningless.
Figure 4
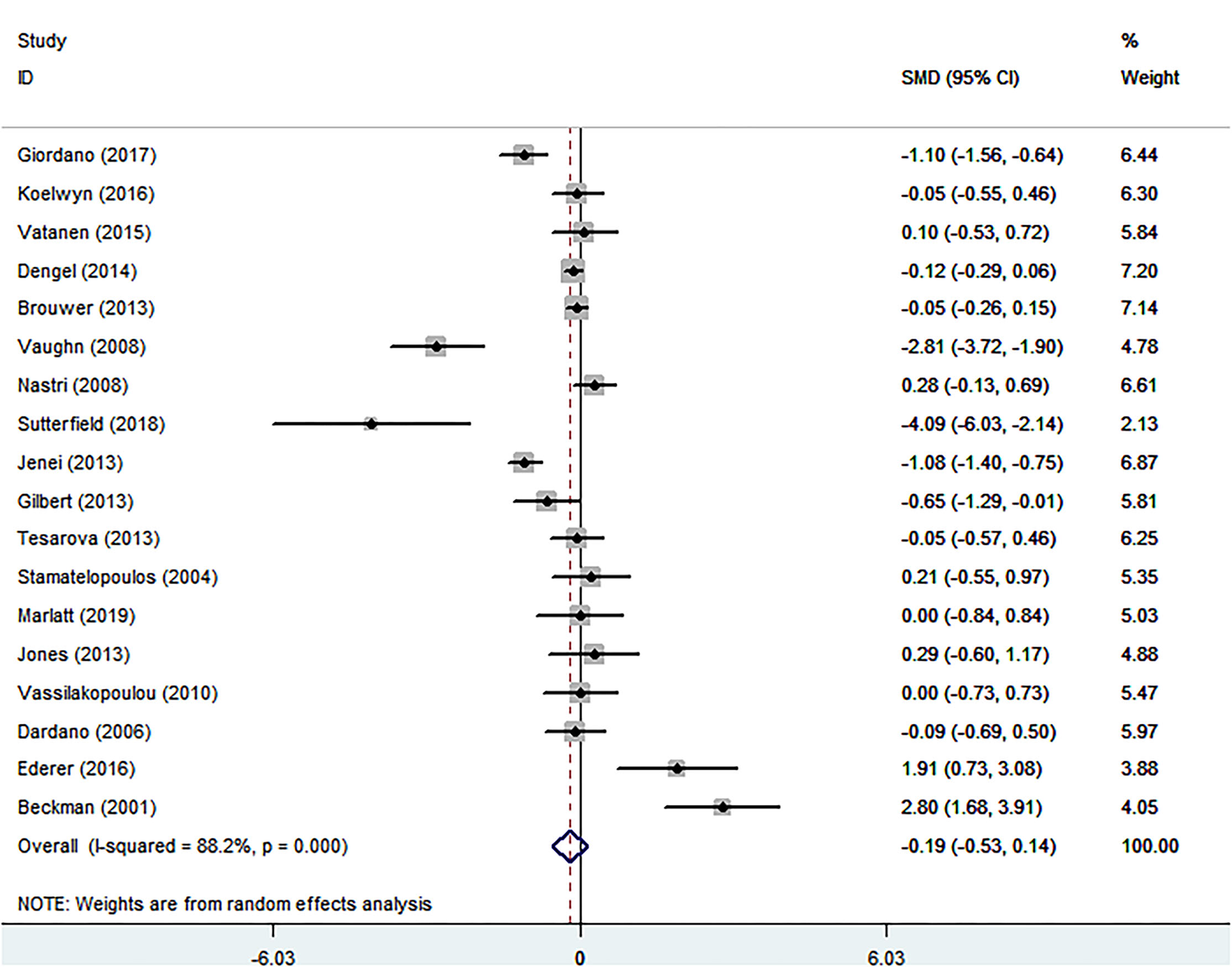
Forest plot of flow-mediated vasodilation in patients with cancer and controls without cancer. SMD, standard mean difference; CI, confidence interval.
Discussion
To provide evidence for the impact of cancer on arterial structure and function (IMT, PWV, and FMD) in relation to subclinical atherosclerosis, we pooled data from 46 full-text articles and conducted a meta-analysis of available data from 3,729 cancer patients and 2,404 healthy controls. The results showed that cancer patients had more severe impairment of vascular structure and function, with thicker IMT and higher PWV than healthy subjects. The carotid artery is generally selected for IMT measurement, which can be obtained by ultrasound examination. The new ultrasound technology–ultrafast pulse wave velocity–can accurately and quickly obtain the carotid PWV. Subgroup analysis was performed to exclude the effects of age and sex on vascular structure and function. In addition, by analyzing and comparing different treatment methods in subgroups, it can be concluded that chemotherapy has a significant effect on functional and structural markers of subclinical atherosclerosis, such as IMT, PWV, and FMD, leading to show increased IMT, PWV, and reduced FMD. Radiotherapy also has a significant effect on IMT and PWV, which can cause an increase in IMT and PWV. Due to the relatively small sample size of the endocrine therapy group, its efficacy needs to be further determined. According to the results of the meta-analysis, cancer can promote the progression of atherosclerosis, damage vascular structure, and function, and increase the occurrence of cardiovascular risk. So, early detection and timely treatment can greatly improve the patients' quality of life.
More cancer patients die from cardiovascular disease than from other causes (56). At present, subclinical atherosclerosis in tumor patients has always been the focus of attention, but the specific pathophysiological mechanism is still unclear. The progression of atherosclerosis is closely related to inflammatory response and immune dysregulation after cancer cell infection (57). Chronic persistent systemic inflammation can lead to loss of confluent luminal elastin layer and activation of glycoprotein in confluence lumen of the vascular wall, resulting in subcutaneous aggregation of low-density lipoprotein and increased circulating cholesterol in the body, thus triggering the expression of endothelial adhesion molecules and chemokine secretion, platelet-derived chemokine deposition and intimal immune cell infiltration (58–60). Besides, the multidirectional inflammatory factor MIF is closely related to LDL and can promote the occurrence and development of atherosclerosis. And many studies have confirmed the expression of MIF in various cancers (61–64).
Of course, antitumor drugs and chemoradiotherapy are also key causes of atherosclerosis. Radiotherapy is the key step leading to vascular injury. The vascular endothelium is sensitive to radiation, and its pathophysiological basis is direct damage to the vascular wall (65–68). Radiotherapy induces endothelial dysfunction, increases capillary permeability, activates inflammation, and leads to intimal hyperplasia, collagen formation and deposition, and fibrosis, which is conducive to the formation of atherosclerotic plaques (69). Testicular cancer patients who received chemotherapy were reported to have a 1.6 times higher risk of dying from cardiovascular disease 10 years after treatment than the general population (70). Cisplatin-based chemotherapy directly impairs vascular structure and function, increasing early cardiovascular events and vascular age at the time of treatment, but symptoms do not appear until many years have accumulated (71–74). Antimetabolites, antimicrobials, and tyrosine kinase inhibitors are chemotherapeutic agents with a high risk of atherosclerosis.
Cancer and atherosclerosis share common risk factors, such as age, smoking, a high-sugar, high-fat diet, and a sedentary lifestyle (75). Cancer patients often have metabolic abnormalities, especially glucose metabolism and lipoprotein abnormalities (76). The proliferation of cancer cells and the apoptosis of normal cells disrupt homeostasis. Dyshomeostasis leads to the development of atherosclerosis (77).
Men with cancer in our study were more likely to develop atherosclerosis than women. This may be related to higher levels of estrogen in women, which limits inflammation, increases nitric oxide production, and inhibits endothelial cell apoptosis (78). This finding is consistent with previous reports (79).
Our meta-analysis has several limitations. First, only English articles are selected for analysis. Secondly, the source of heterogeneity between studies has not been identified. Multivariate logistic regression on a larger sample is needed in the future to find the source of heterogeneity. Thirdly, some included studies lacked basic information, failed to conduct covariate analysis and adjustment for cardiovascular risk factors such as hypertension, diabetes, hyperlipidemia and smoking, failed to analyze the relationship between IMT, PWV, FMD and cholesterol level, failed to analyze inflammatory markers and other risk factors in cancer and health control databases, failed to show the association of the time point of the data was collected upon cancer diagnosis and treatment, and failed to judge whether the changes in IMT, PWV and FMD are cumulative responses over a long period of time after cancer treatment or short-term acute responses. Fourthly, the inclusion study excluded people who had already experienced a cardiovascular accident, and the cancer population in the study had a shorter follow-up period, so no association could be drawn between IMT, PWV, FMD and actual CAD event complications. Finally, the number of studies was insufficient and the sample size was small, leading to the failure to distinguish different types of cancer and to conduct subgroup analysis before and after treatment.
In conclusion, cancer promotes subclinical atherosclerosis, destroys functional and structural markers of blood vessels, and leads to higher IMT and PWV. The measurement of PWV and IMT values can be easily and quickly obtained by ultrasonic technology. Increased attention, early assessment, and timely prevention of atherosclerosis progression can reduce the incidence of cardiovascular accidents in cancer survivors.
Funding
This article was funded by the Jiangxi Provincial Department of Science and Technology key research and development program general project (20203BBGL73196) and the Natural Science Foundation of Jiangxi Province (20212BAB206040).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YD and DS contributed to the conception or design of the work. WZ, YD, and ZL contributed to the acquisition, analysis, or interpretation of data for the work. YD drafted the manuscript. DS, LC, and ZL critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.849538/full#supplementary-material
References
1.
Farzin L Shamsipur M . Recent advances in design of electrochemical affinity biosensors for low level detection of cancer protein biomarkers using nanomaterial-assisted signal enhancement strategies. J Pharm Biomed Anal. (2018) 147:185–210. 10.1016/j.jpba.2017.07.042
2.
Fiorica F Stefanelli A Pascale G Fisichella R . Elderly gastrointestinal cancer patients and radiochemotherapy: a review. Clin Ter. (2014) 165:57–61. 10.7471/CT.2014.1662
3.
Afifi AM Saad AM Al-Husseini MJ Elmehrath AO Northfelt DW Sonbol MB . Causes of death after breast cancer diagnosis: a US population-based analysis. Cancer Apr 1. (2020) 126:1559–67. 10.1002/cncr.32648
4.
Oh C-M Lee D Kong H-J Lee S Won Y-J Jung K-W et al . Causes of death among cancer patients in the era of cancer survivorship in Korea: attention to the suicide and cardiovascular mortality. Cancer Med. (2020) 9:1741–52. 10.1002/cam4.2813
5.
Nguyen-Nielsen M Møller H Tjønneland A Borre M . Causes of death in men with prostate cancer: results from the danish prostate cancer registry (DAPROCAdata). Cancer Epidemiol. (2019) 59:249–57. 10.1016/j.canep.2019.02.017
6.
Xie S-H Chen H Lagergren J . Causes of death in patients diagnosed with gastric adenocarcinoma in Sweden, 1970-2014: a population-based study. Cancer Sci. (2020) 111:2451–9. 10.1111/cas.14441
7.
Stein JH Korcarz CE Hurst RT Lonn E Kendall CB Mohler ER et al . Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. (2008) 21:93–111. 10.1016/j.echo.2007.11.011
8.
Shariat M Alias NAA Biswal BM . Radiation effects on the intima-media thickness of the common carotid artery in post-radiotherapy patients with head and neck malignancy. Postgrad Med J. (2008) 84:609–12. 10.1136/pgmj.2008.068569
9.
Yuan C Wu VW Yip SP Kwong DL Ying M . Ultrasound evaluation of carotid atherosclerosis in post-radiotherapy nasopharyngeal carcinoma patients, type 2 diabetics, and healthy controls. Ultraschall in der Medizin. (2017) 38:190–7. 10.1055/s-0034-1399293
10.
Dockery F Rajkumar C Agarwal S Waxman J Bulpitt CJ . Androgen deprivation in males is associated with decreased central arterial compliance and reduced central systolic blood pressure. J Hum Hypertens. (2000) 14:395–7. 10.1038/sj.jhh.1001028
11.
Giordano P Muggeo P Delvecchio M Carbonara S Romano A Altomare M et al . Endothelial dysfunction and cardiovascular risk factors in childhood acute lymphoblastic leukemia survivors. Int J Cardiol. (2017) 228:621–7. 10.1016/j.ijcard.2016.11.025
12.
Chrisoulidou A Pazaitou-Panayiotou K Georgiou E Boudina M Lytras K Iakovou I et al . The hypothalamic-pituitary-adrenal axis in women with differentiated thyroid cancer. Endocr Res. (2010) 35:137–43. 10.3109/07435800.2010.496760
13.
Sekijima T Tanabe A Maruoka R Fujishiro N Yu S Fujiwara S et al . Impact of platinum-based chemotherapy on the progression of atherosclerosis. Climacteric. (2011) 14:31–40. 10.3109/13697137.2010.522278
14.
Vatanen A Sarkola T Ojala TH Turanlahti M Jahnukainen T Saarinen-Pihkala UM et al . Radiotherapy-related arterial intima thickening and plaque formation in childhood cancer survivors detected with very-high resolution ultrasound during young adulthood. Pediatr Blood Cancer. (2015) 62:2000–6. 10.1002/pbc.25616
15.
DerSimonian R Laird N . Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. 10.1016/0197-2456(86)90046-2
16.
Bai R Zhang Y Liu W Ma C Chen X Yang J et al . The relationship of ankylosing spondylitis and subclinical atherosclerosis: a systemic review and meta-analysis. Angiology. (2019) 70:492–500. 10.1177/0003319718814309
17.
Beckman JA Thakore A Kalinowski BH Harris JR Creager MA . Radiation therapy impairs endothelium-dependent vasodilation in humans. J Am Coll Cardiol. (2001) 37:761–5. 10.1016/S0735-1097(00)01190-6
18.
Brouwer CAJ Postma A Hooimeijer HLH Smit AJ Vonk JM van Roon AM et al . Endothelial damage in long-term survivors of childhood cancer. J Clin Oncol. (2013) 31:3906–13. 10.1200/JCO.2012.46.6086
19.
Chaosuwannakit N D'Agostino R Hamilton CA Lane KS Ntim WO Lawrence J et al . Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. (2010) 28:166–72. 10.1200/JCO.2009.23.8527
20.
Dardano A Ghiadoni L Plantinga Y Caraccio N Bemi A Duranti E et al . Recombinant human thyrotropin reduces endothelium-dependent vasodilation in patients monitored for differentiated thyroid carcinoma. J Clin Endocrinol Metab. (2006) 91:4175–8. 10.1210/jc.2006-0440
21.
Dengel DR Kelly AS Zhang L Hodges JS Baker KS Steinberger J . Signs of early sub-clinical atherosclerosis in childhood cancer survivors. Pediatr Blood Cancer. (2014) 61:532–7. 10.1002/pbc.24829
22.
Dengel DR Kelly AS Zhang L Wang Q Hodges JS Steinberger J et al . Vascular Structure and Function in Cancer Survivors after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. (2019) 25:151–6. 10.1016/j.bbmt.2018.08.005
23.
Ederer AK Didier KD Reiter LK Brown M Hardy R Caldwell J et al . Influence of Adjuvant Therapy in Cancer Survivors on Endothelial Function and Skeletal Muscle Deoxygenation. PLoS ONE. (2016) 11:e0147691. 10.1371/journal.pone.0147691
24.
Feehs RS McGuirt WF Bond MG Strickland HL Craven TE Hiltbrand JB . Irradiation. A significant risk factor for carotid atherosclerosis. Arch Otolaryngol Head Neck Surg. (1991) 117:1135–7. 10.1001/archotol.1991.01870220083014
25.
Gianicolo ME Gianicolo EAL Tramacere F Andreassi MG Portaluri M . Effects of external irradiation of the neck region on intima media thickness of the common carotid artery. Cardiovasc Ultrasound. (2010) 8:8. 10.1186/1476-7120-8-8
26.
Gilbert SE Tew GA Bourke L Winter EM Rosario DJ . Assessment of endothelial dysfunction by flow-mediated dilatation in men on long-term androgen deprivation therapy for prostate cancer. Exp Physiol. (2013) 98:1401–10. 10.1113/expphysiol.2013.073353
27.
Gujral DM Shah BN Chahal NS Bhattacharyya S Senior R Harrington KJ et al . Arterial Stiffness as a Biomarker of Radiation-Induced Carotid Atherosclerosis. Angiology. (2016) 67:266–71. 10.1177/0003319715589520
28.
Heikens J Ubbink MC van der Pal HP Bakker PJ Fliers E Smilde TJ et al . Long term survivors of childhood brain cancer have an increased risk for cardiovascular disease. Cancer. (2000) 88:2116–21. 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1
29.
Herceg-Cavrak V Ahel V Batinica M Matec L Kardos D . Increased arterial stiffness in children treated with anthracyclines for malignant disease. Coll Antropol. (2011) 35:389–95. 10.2753/CSA0009-4625430404
30.
Huang T-L Hsu H-C Chen H-C Lin H-C Chien C-Y Fang F-M et al . Long-term effects on carotid intima-media thickness after radiotherapy in patients with nasopharyngeal carcinoma. Radiation oncology. (2013) 8:261. 10.1186/1748-717X-8-261
31.
Jenei Z Bárdi E Magyar MT Horváth A Paragh G Kiss C . Anthracycline causes impaired vascular endothelial function and aortic stiffness in long term survivors of childhood cancer. Pathol Oncol Res. (2013) 19:375–83. 10.1007/s12253-012-9589-6
32.
Jones LW Fels DR West M Allen JD Broadwater G Barry WT et al . Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res. (2013) 6:925–37. 10.1158/1940-6207.CAPR-12-0416
33.
Kim HJ Cho EJ Kwak MH Eom BW Yoon HM Cho S-J et al . Effect of gastrectomy on blood pressure in early gastric cancer survivors with hypertension. Support Care Cancer. (2019) 27:2237–45. 10.1007/s00520-018-4491-8
34.
Koelwyn GJ Lewis NC Ellard SL Jones LW Gelinas JC Rolf JD et al . Ventricular-arterial coupling in breast cancer patients after treatment with anthracycline-containing adjuvant chemotherapy. Oncologist. (2016) 21:141–9. 10.1634/theoncologist.2015-0352
35.
Krystal JI Reppucci M Mayr T Fish JD Sethna C . Arterial stiffness in childhood cancer survivors. Pediatr Blood Cancer Oct. (2015) 62:1832–7. 10.1002/pbc.25547
36.
Lim YJ Kwack WG Lee YS Hahm KB Kim YK . Increased pulse wave velocity reflecting arterial stiffness in patients with colorectal adenomas. J Clin Biochem Nutr Nov. (2010) 47:261–5. 10.3164/jcbn.10-70
37.
Marlatt KL Steinberger J Rudser KD Dengel DR Sadak KT Lee JL et al . The effect of atorvastatin on vascular function and structure in young adult survivors of childhood cancer: a randomized, placebo-controlled pilot clinical trial. J Adolesc Young Adult Oncol. (2019) 8:442–50. 10.1089/jayao.2017.0075
38.
Meeske KA Siegel SE Gilsanz V Bernstein L Nelson MB Sposto R et al . Premature carotid artery disease in pediatric cancer survivors treated with neck irradiation. Pediatr Blood Cancer. (2009) 53:615–21. 10.1002/pbc.22111
39.
Nastri CO Martins WP Ferriani RA Filho FM Dos Reis FJC . Sonographic evaluation of endothelial function in letrozole and tamoxifen users. Maturitas. (2008) 61:340–4. 10.1016/j.maturitas.2008.09.019
40.
Okur A Karadeniz C Özhan Oktar S Pinarli FG Aral A Oguz A . Assessment of brachial artery reactivity, carotid intima-media thickness, and adhesion molecules in pediatric solid tumor patients treated with anthracyclines. Pediatr Hematol Oncol. (2016) 33:178–85. 10.3109/08880018.2016.1146375
41.
Sadurska E Brodzisz A Zaucha-Prazmo A Kowalczyk J . The estimation of intima-media thickness and cardiovascular risk factors in young survivors of childhood cancer. J Pediatr Hematol Oncol. (2016) 38:549–54. 10.1097/MPH.0000000000000513
42.
Simon T Boutouyrie P Simon JM Laloux B Tournigand C Tropeano AI et al . Influence of tamoxifen on carotid intima-media thickness in postmenopausal women. Circulation. (2002) 106:2925–9. 10.1161/01.CIR.0000041044.93571.CA
43.
Siviero-Miachon AA Spinola-Castro AM de Martino Lee ML de Castro Monteiro CM de Camargo Carvalho AC Calixto AR et al . Subcutaneous adipose tissue plays a beneficial effect on subclinical atherosclerosis in young survivors of acute lymphocytic leukemia. Vasc Health Risk Manag. (2015) 11:479–88. 10.2147/VHRM.S86883
44.
Stamatelopoulos KS Lekakis JP Poulakaki NA Papamichael CM Venetsanou K Aznaouridis K et al . Tamoxifen improves endothelial function and reduces carotid intima-media thickness in postmenopausal women. Am Heart J. (2004) 147:1093–9. 10.1016/j.ahj.2003.12.029
45.
Stelwagen J Lubberts S Steggink LC Steursma G Kruyt LM Donkerbroek JW et al . Vascular aging in long-term survivors of testicular cancer more than 20 years after treatment with cisplatin-based chemotherapy. Br J Cancer. (2020) 123:1599–607. 10.1038/s41416-020-01049-3
46.
Strüder D Hellwig S Rennau H van Bonn S Schraven SP Mlynski R et al . Screening for irradiation vasculopathy by intima-media thickness sonography in head and neck cancer patients. Eur Arch Otorhinolaryngol. (2021) 278:2017–26. 10.1007/s00405-020-06301-3
47.
Sutterfield SL Caldwell JT Post HK Lovoy GM Banister HR Ade CJ . Lower cutaneous microvascular reactivity in adult cancer patients receiving chemotherapy. J Appl Physiol. (2018) 125:1141–9. 10.1152/japplphysiol.00394.2018
48.
Tesarova P Kalousova M Zima T Suchanek M Malikova I Kvasnicka J et al . Endotelial activation and flow-mediated vasodilation in young patients with breast cancer. Neoplasma. (2013) 60:690–7. 10.4149/neo_2013_088
49.
Vassilakopoulou M Mountzios G Papamechael C Protogerou AD Aznaouridis K Katsichti P et al . Paclitaxel chemotherapy and vascular toxicity as assessed by flow-mediated and nitrate-mediated vasodilatation. Vascul Pharmacol. (2010) 53:115–21. 10.1016/j.vph.2010.05.002
50.
Vaughn DJ Palmer SC Carver JR Jacobs LA Mohler ER . Cardiovascular risk in long-term survivors of testicular cancer. Cancer. (2008) 112:1949–53. 10.1002/cncr.23389
51.
Vrtovec M Anzic A Zupan IP Zaletel K Blinc A . Carotid artery stiffness, digital endothelial function, and coronary calcium in patients with essential thrombocytosis, free of overt atherosclerotic disease. Radiol Oncol. (2017) 51:203–10. 10.1515/raon-2017-0006
52.
Ye J Rong X Xiang Y Xing Y Tang Y A . study of radiation-induced cerebral vascular injury in nasopharyngeal carcinoma patients with radiation-induced temporal lobe necrosis. PLoS ONE. (2012) 7:e42890. 10.1371/journal.pone.0042890
53.
Yersal Ö Eryilmaz U Akdam H Meydan N Barutca S . Arterial stiffness in breast cancer patients treated with anthracycline and trastuzumab-based regimens. Cardiol Res Pract. (2018) 2018:5352914. 10.1155/2018/5352914
54.
Zaletel LZ Popit M Zaletel M . Is Carotid stiffness a possible surrogate for stroke in long-term survivors of childhood cancer after neck radiotherapy?Radiol Oncol. (2018) 52:136–42. 10.2478/raon-2018-0006
55.
Zhang Z Luo R Tan B Qian J Duan Y Wang N et al . Carotid artery stiffness evaluated early by wave intensity in normal left ventricular function in post-radiotherapy patients with nasopharyngeal carcinoma. J Med Ultrason. (2001) 45:301–6. 10.1007/s10396-017-0817-2
56.
Henson KE Reulen RC Winter DL Bright CJ Fidler MM Frobisher C et al . Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 years of age: the teenage and young adult cancer survivor study. Circulation. (2016) 134:1519–31. 10.1161/CIRCULATIONAHA.116.022514
57.
Puri R Nissen SE Libby P Shao M Ballantyne CM Barter PJ et al . C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. (2013) 128:2395–403. 10.1161/CIRCULATIONAHA.113.004243
58.
Kwon GP Schroeder JL Amar MJ Remaley AT Balaban RS . Contribution of macromolecular structure to the retention of low-density lipoprotein at arterial branch points. Circulation. (2008) 117:2919–27. 10.1161/CIRCULATIONAHA.107.754614
59.
Hansson GK Hermansson A . The immune system in atherosclerosis. Nat Immunol. (2011) 12:204–12. 10.1038/ni.2001
60.
Weber C Noels H . Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. (2011) 17:1410–22. 10.1038/nm.2538
61.
Hauser S Kaminski A Syring I Holdenrieder S Dieckmann K-P Muller SC et al . Evaluation of serum biomarkers (FGF-2, HGF, MIF and PTN) in patients with testicular germ cell cancer. In vivo. (2019) 33:1935–40. 10.21873/invivo.11688
62.
Avalos-Navarro G Muñoz-Valle JF Daneri-Navarro A Quintero-Ramos A Franco-Topete RA Morán-Mendoza AdJ et al . Circulating soluble levels of MIF in women with breast cancer in the molecular subtypes: relationship with Th17 cytokine profile. Clin Exp Med. (2019) 19:385–91. 10.1007/s10238-019-00559-6
63.
Klemke L De Oliveira T Witt D Winkler N Bohnenberger H Bucala R et al . Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer. Cell Death Dis. (2021) 12:155. 10.1038/s41419-021-03426-z
64.
Jäger B Klatt D Plappert L Golpon H Lienenklaus S Barbosa PD et al . CXCR4/MIF axis amplifies tumor growth and epithelial-mesenchymal interaction in non-small cell lung cancer. Cell Signal. (2020) 73:109672. 10.1016/j.cellsig.2020.109672
65.
Milliat F François A Tamarat R Benderitter M . [Role of endothelium in radiation-induced normal tissue damages]. Annales de cardiologie et d'angeiologie. (2008) 57:139–48. 10.1016/j.ancard.2008.02.015
66.
Gaugler MH Squiban C Mouthon MA Gourmelon P van der Meeren A . Irradiation enhances the support of haemopoietic cell transmigration, proliferation and differentiation by endothelial cells. Br J Haematol. (2001) 113:940–50. 10.1046/j.1365-2141.2001.02852.x
67.
van Kleef E Verheij M Oussoren Y Dewit L Stewart F . In vitro and in vivo expression of endothelial von Willebrand factor and leukocyte accumulation after fractionated irradiation. Radiat Res. (2000) 154:375–81. 10.1667/0033-7587(2000)1540375:ivaive2.0.co;2
68.
Soucy KG Lim HK Benjo A Santhanam L Ryoo S Shoukas AA et al . Single exposure gamma-irradiation amplifies xanthine oxidase activity and induces endothelial dysfunction in rat aorta. Radiat Environ Biophys. (2007) 46:179–86. 10.1007/s00411-006-0090-z
69.
Raposeiras Roubín S Cordero A . The Two-way Relationship Between Cancer and Atherosclerosis. Revista espanola de cardiologia. (2019) 72:487–94. 10.1016/j.rec.2018.12.010
70.
Lauritsen J Hansen MK Bandak M Kreiberg MB Skøtt JW Wagner T et al . Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol. (2020) 38:584–92. 10.1200/JCO.19.01180
71.
Feldman DR Schaffer WL Steingart RM . Late cardiovascular toxicity following chemotherapy for germ cell tumors. J Natl Compr Canc Netw. (2012) 10:537–44. 10.6004/jnccn.2012.0051
72.
Lubberts S Meijer C Demaria M Gietema JA . Early ageing after cytotoxic treatment for testicular cancer and cellular senescence: time to act. Crit Rev Oncol Hematol. (2020) 151:102963. 10.1016/j.critrevonc.2020.102963
73.
Yeh ETH Bickford CL . Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. (2009) 53:2231–47. 10.1016/j.jacc.2009.02.050
74.
Aichberger KJ Herndlhofer S Schernthaner G-H Schillinger M Mitterbauer-Hohendanner G Sillaber C et al . Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. (2011) 86:533–9. 10.1002/ajh.22037
75.
Cordero A Fácila L García-Carrilero M Gunturiz C Montagud V Núñez J . Breakfast habits in patients hospitalized for acute coronary syndrome. Revista espanola de cardiologia. (2015) 68:814–5. 10.1016/j.rec.2015.05.007
76.
de Haas EC Oosting SF Lefrandt JD Wolffenbuttel BH Sleijfer DT Gietema JA . The metabolic syndrome in cancer survivors. Lancet Oncol. (2010) 11:193–203. 10.1016/S1470-2045(09)70287-6
77.
Narula J Nakano M Virmani R Kolodgie FD Petersen R Newcomb R et al . Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. (2013) 61:1041–51. 10.1016/j.jacc.2012.10.054
78.
Spyridopoulos I Sullivan AB Kearney M Isner JM Losordo DW . Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis. Estradiol as a survival factor Circulation. (1997) 95:1505–14. 10.1161/01.CIR.95.6.1505
79.
Sun D Wu Y Yuan Y Wang Y Liu W Yang J . Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure? Meta-analysis of the markers of arterial structure and function. Atherosclerosis. (2015) 242:109–16. 10.1016/j.atherosclerosis.2015.06.059
Summary
Keywords
cancer, intima-media thickness, pulse wave velocity, flow-mediated vasodilation, systematic review and meta-analysis
Citation
Diao Y, Liu Z, Chen L, Zhang W and Sun D (2022) The Relationship Between Cancer and Functional and Structural Markers of Subclinical Atherosclerosis: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:849538. doi: 10.3389/fcvm.2022.849538
Received
11 January 2022
Accepted
11 April 2022
Published
04 May 2022
Volume
9 - 2022
Edited by
Daniel Petrovič, University of Ljubljana, Slovenia
Reviewed by
Hong Jin, Karolinska Institutet (KI), Sweden; Emin Grbic, University of Tuzla, Bosnia and Herzegovina
Updates
Copyright
© 2022 Diao, Liu, Chen, Zhang and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chen 1727237899@qq.comDandan Sun dan_101912@163.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Atherosclerosis and Vascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.