Abstract
Subclinical alterations in cardiac structure and function include a variety of abnormal phenotypes of recognized adverse prognostic values, such as left ventricular hypertrophy (LVH), concentric remodeling, systolic/diastolic dysfunction, left atrial dilatation, and alterations of LV geometry. The excess cardiovascular risk associated with these markers has been documented in multiple clinical settings, such as the general population, hypertensive cohorts, patients with coronary heart disease, diabetes mellitus, chronic heart failure, and chronic kidney disease. On the contrary, the value of aortic root (AR) and ascending aortic diameter in predicting cardiovascular outcomes and all-cause mortality in populations free from overt aortic pathology is still debated. The present review, aimed at pointing out the prognostic implications of thoracic aortic dimensions in populations free from known connective and aortic diseases, suggests that available evidence supporting an association between aortic diameter and cardiovascular events, and all-cause mortality is based on the limited number of studies, conducted with different imaging techniques and definition of the aortic phenotype.
Introduction
Subclinical target organ damage (TOD) refers to the structural and functional alterations of the cardiovascular system associated with unhealthy risk factors, among which systemic hypertension stands out (1, 2). Asymptomatic alterations of the cardiovascular system reflect an intermediate step in the disease continuum linking hypertension and coexistent risk factors, such as dyslipidemia, obesity, and diabetes mellitus, to non-fatal and fatal cardiovascular events (3). A large body of evidence supports the view that subclinical TOD simultaneously occurs in the heart, brain, eye, kidney, and peripheral arteries presumably because cardiac and vascular tissues are similarly exposed to hemodynamic, neural, and hormonal stimuli operating in hypertension (4, 5).
Hypertensive heart disease represents one of the most important manifestations of subclinical TOD due to its high prevalence, possibly related to the early onset of this adverse phenotype in the natural history of hypertension and to its prognostic significance independent of traditional risk factors, including office blood pressure (BP) levels (6, 7). Furthermore, reversal of cardiac TOD, as assessed by echocardiography (i.e., left ventricular mass reduction), at the difference from other markers, such as carotid intima–media thickness and ankle–brachial index, has been consistently reported to be a reliable indicator of the protective effects of non-pharmacological and pharmacological antihypertensive therapy (8, 9). Echocardiographic left ventricular hypertrophy (LVH) is widely recognized as a key biomarker of hypertensive heart disease and a powerful predictor of cardiovascular morbidity and mortality in hypertensive patients as well as in different clinical settings, such as members of the general population, patients with coronary heart disease, diabetes mellitus, chronic heart failure (HF), and kidney disease (10–13). It should be underlined, however, that cardiac TOD, in addition to LVH, includes other important markers, namely LV geometry alterations, left atrial size, and aortic root (AR) dilatation as well as systolic/diastolic dysfunction that, alone or in association with LVH, may improve cardiovascular risk stratification (14). The independent role of concentric LV geometry, atrial dilatation, and systolic and diastolic dysfunction in predicting cardiovascular outcomes has been proven, with only some exceptions, by several studies carried out in patients with hypertension and in general population-based samples (15, 16). On the contrary, evidence on the prognostic value of AR and ascending aortic diameter in populations free from known aortic pathological conditions is very scanty. Consequently, the hypertension guidelines did not include aortic diameter among the markers of cardiac TOD useful for the evaluation of hypertensive heart disease (2). Therefore, this article is aimed at pointing out available evidence on the prognostic implications of thoracic aortic dimensions after excluding from the review specific clinical conditions, such as connective diseases (i.e., Marfan’s syndrome and Ehlers–Danlos syndrome) and aortic aneurysms.
Methods
This article was prepared in accordance with the Narrative Review Checklist (available at http://dx.doi.org/10.21037/jtd-20-2728). The medical literature was reviewed to identify all articles evaluating the relationship between AR and aortic ascending diameter with incident cardiovascular events and mortality. A computerized search was performed using Pub-Med, OVID, EMBASE, and Cochrane library databases from inception up to December 31st 2021. Studies were identified by using the following search terms: “aortic root,” “ascending aorta,” “vascular damage,” “echocardiography,” “computed tomography,” “cardiovascular events,” “cardiovascular prognosis,” and “mortality.” Checks of the reference lists of selected papers and pertinent reviews complemented the electronic search. Data were examined and extracted by three independent investigators (EG, CC, and MT).
Results
The first literature search identified 4,320 papers. After the initial screening of titles and abstracts, 4,130 studies were excluded as they were not related to the topic. Therefore, 190 studies were reviewed; of these, 101 did not report data on incident non-fatal or fatal cardiovascular events or all-cause mortality and 41 on AR diameter or ascending aorta data, 33 were review, commentary, editorial articles, and 6 were excluded for miscellaneous reasons. A total of 9 studies, including participants without underlying known aortic pathologies (i.e., aneurysms) or connective diseases and containing sufficient clinical and cardiac imaging data, were included in the final review (17–25) (Figure 1). The Newcastle–Ottawa Score, used for assessing the quality of the studies, ranged from 7 to 9, and the mean score was 7.8. Therefore, no study was excluded based on its limited quality.
FIGURE 1
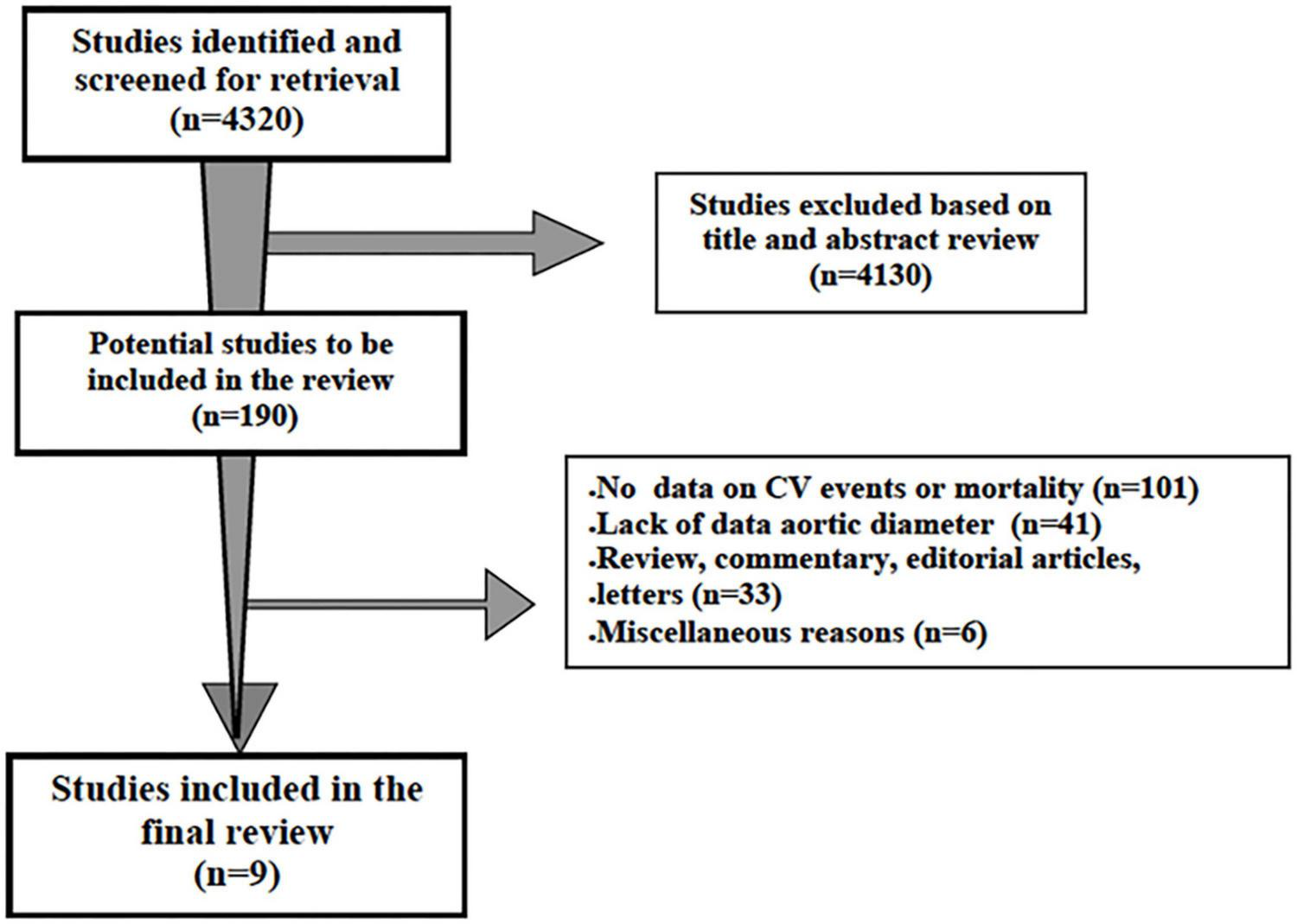
Schematic flowchart for the selection of studies.
Characteristics of the Studies
On the whole, 39,969 individuals were included in 9 studies (sample size ranging from 423 to 10,410 participants), performed in three continental areas (Europe = 4, North America = 4, Asia = 1).
Table 1 shows demographic and clinical characteristics of patients of selected studies, such as sample size, setting, mean age, prevalence of men, duration of follow-up, pre-specified outcomes of interest, and their association with baseline AR and ascending aortic diameter.
TABLE 1
| References | Sample size (n) | Setting | Age (years) | Men% | Duration of FU | Outcomes | Main findings (and imaging tool) |
| Gardin et al. (17) | 3,933 | Elderly free of CVD | 73 ± 6 | 42 | 10 years | Incident MI, CHF, Stroke, all-cause Mortality | Absolute ARD was predictive of incident CHF, stroke, CVD mortality, and all-cause mortality, but not of incident MI. (M-mode 2D guided Echocardiography). |
| Lai et al. (18) | 1,851 | General population | 58 ± 10 | 44 | 12 years | Incident all-cause death | ARD indexed to BSA was predictive of all-cause death in participants < 65 years (M-mode 2D guided Echocardiography). |
| Gondrie et al. (19) | 10,410 | Population without history of CVD | 63 (40–96) | 60 | 17 months | Incident CV events | Ascending aortic diameter was associated to increased risk of CV events (Computed Tomography) |
| Lam et al. (20) | 6,493 | General population | 56 ± 14 | 46 | 8 years | Incident HF | ARD was predictive of incident HF. (M-mode 2D guided Echocardiography) |
| Cuspidi et al. (21) | 1,860 | General population | 50 ± 14 | 50 | 12 years | Incident CV events | ARD indexed to height were predictive of non-fatal and fatal CV events. (M-mode 2D guided Echocardiography) |
| Kamimura et al. (22) | 3,108 | Community-based black cohort | 56 ± 12 | 31 | 8 years | Incident CV events, all-cause mortality | ARD, ARD indexed to BSA and height were predictive of non-fatal and fatal CV events and all-cause mortality (2D Echocardiography). |
| Qazi et al. (23) | 3,318 | General population | 49 ± 10 | 51 | 9 years | Incident CV events | Ascending aortic diameter was not associated with excess of fatal and non-fatal CV events. (Computed Tomography). |
| Canciello et al. (24) | 8,573 | Hypertensive cohort | 53 ± 12 | 58 | 4 years | Incident CV events | ARD indexed to height was independent predictor of CV events (2D Echocardiography). |
| Leone et al. (25) | 423 | Hypertensive cohort | 53 ± 13 | 78 | 7 years | Incident CV events | Ascending aortic diameter was independent predictor of CV events. (2D Echocardiography). |
Summary of longitudinal studies that addressed the relationship aortic root and ascending aortic diameter with cardiovascular prognosis and/or all-cause death.
ARD, aortic root diameter; BSA, body surface area; CHF, chronic heart failure; CVD, cardiovascular disease; FU, follow-up; MI, myocardial infarction. Data are presented as absolute numbers, percentage, mean ± SD, Inter quartile range.
The mean age range was 49–73 years (17, 23); 51% of participants were men. The majority of studies included free-living members of the general population, two studies were carried out in hypertensive cohorts (24, 25) and one study in patients undergoing chest computed tomography (CT) for non-cardiovascular indications (19). The duration of the follow-up period ranged from 17 months (19) to 12 years (18, 21).
Figure 2 provides a flow-chart targeting, the association of AR and ascending aortic diameter with the outcomes of interest in the 9 studies included in the review.
FIGURE 2
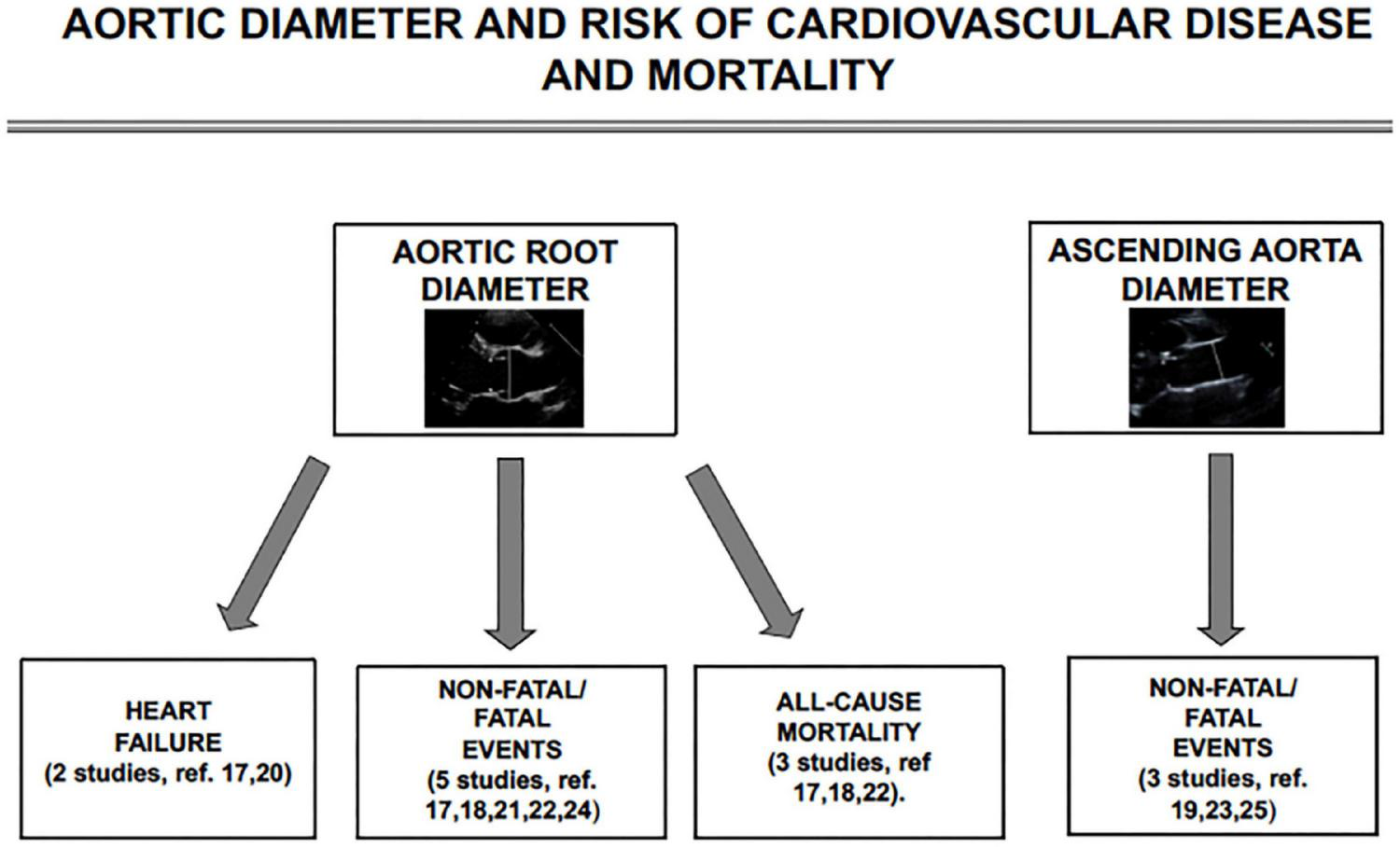
Schematic flow-chart for studies targeting the association of aortic root and ascending aortic diameter with incident heart failure, non-fatal cardiovascular events, and all-cause mortality.
Imaging Procedures and Main Findings
Two different cardiac imaging methods were employed in the studies included in the present review: echocardiography (n = 7) and CT (n = 2). Echocardiographic studies were performed according to recommendations of major contemporary guidelines. AR diameter was measured in the parasternal long-axis view at the level of Valsalva’s sinuses in six echocardiographic studies (17, 18, 20–22, 24). The diameter of ascending aorta was the vascular phenotype of interest in both CT studies (19, 23) and in one of the echocardiographic studies (25).
All-Cause Mortality
Three of the nine studies that analyzed the relationship between aortic diameter and all-cause mortality (17, 18, 22) found that the baseline values of absolute AR diameter (17) and indexed diameter (18, 22) were independent predictors of this fatal outcome. It is worth noting, however, that in the study by Lai et al. (18), this association persisted, after the adjustment for confounders, only in participants = 65 years of age.
Non-fatal and Fatal Cardiovascular Events
Two out of three studies (21, 24) found that AR diameter indexed to BSA or height predicted non-fatal and fatal cardiovascular events, regardless of traditional risk factors. This was not the case, however, in the PAMELA study in which the association of AR diameter with incident cardiovascular events lost its significance when LV mass index was included in the multivariate analysis (21). Studies targeting ascending aortic diameter (19, 23, 25) showed non-univocal findings. Among the participants from the Framingham offspring and third-generation cohorts, the enlarged ascending aorta was not significantly associated with cardiovascular events (23). In one of the two studies documenting an excess risk of cardiovascular events related to the enlargement of ascending aorta diameter, the adjustment for confounding factors was limited to age and sex.
Heart Failure
Among the individuals belonging to Cardiovascular Health Study (CHS), an increased AR diameter was found to be an independent predictor of incident HF in men after the adjustment for several confounders, including electrocardiographic LV mass (17). The Framingham Heart Study researchers reported a positive association between AR diameter with incident chronic HF; this relationship, however, lost statistical significance after the adjustment for echocardiographic LV mass in addition to clinical risk factors (20).
Discussion
The progressive arterial remodeling related to age represents a key mechanism in the pathogenesis of cardiovascular disease (26, 27). For many decades, numerous imaging-based and post-mortem studies have shown an association between the aging process and dilatation of the thoracic and abdominal aorta (28, 29). Age-related dilation of the aorta due to long-term exposure to cardiovascular risk factors, such as hypertension, metabolic disorders, sleep apnea syndrome, and smoking, has been related to structural changes in the aortic wall, such as calcification, collagen deposition, elastin fractures, and reduced elastin content (30). Although elevated BP levels tend to increase aortic wall stress, the contribution of BP to aortic dilatation appears to be substantially lower compared to other factors, such as age, gender, and body size measures (31). This view has been supported by cross-sectional studies targeting AR dilatation and carried out in the general population and in the hypertensive setting. Prevalence rates of AR dilatation in the PAMELA population (n = 1,860) varied from 5.6% (AR/BSA) to 9.6% (AR/height), the men/women ratio being approximately 1.1 with both criteria (21). A meta-analysis of eight studies, including a pooled population of 10,791 hypertensive patients, documented that the overall prevalence of AR dilatation was 9.1%, quite similar to the PAMELA study (32).
It should be noted, however, that available evidence regarding dynamic changes over time in AR diameter in the community and in hypertensive cohorts suggests somewhat different conclusions from cross-sectional studies. Indeed, among the participants to the PAMELA study, the incidence of new AR dilatation over the 10-year follow-up period ranged from 3.4% (AR/BSA) to 4.4% (AR/height) (33). In difference, the Campania Salute Network study, including 4,856 hypertensive patients, showed that as many as 366 participants (11%) with normal AR diameter at baseline developed AR dilatation during a follow-up of 6 years (34).
In understanding the prognostic role of aortic diameter, the following important questions need to be carefully considered: (I) is its predictive meaning independent of traditional risk factors and, more importantly, of other parameters of LV structure and function, namely LV mass index?; (II) do sex and age influence the relationship between aortic diameter and outcomes?; (III) is aortic dilatation an independent correlate of both cardiovascular and non-cardiovascular mortality?
Regarding the first question, eight of the nine studies that found a positive relationship between aortic diameter and cardiovascular outcomes provided statistical data adjusted for several key confounders; only in one study the adjustment was limited to age and gender (23). It is worth noting, however, that inclusion of LV mass in statistical models abolished the prognostic significance of aortic diameter in predicting HF (20), non-fatal and fatal cardiovascular events (21), and all-cause mortality in hypertensive patients on anti-hypertensive medications (17). In contrast, two Italian studies carried out in patients referred to specialist hypertension centers showed that AR and ascending aorta diameter were independent predictors of cardiovascular events regardless of LVH and other common confounders (24, 25).
Only a few studies performed subgroup analyses stratified by gender (17, 18) and age (18). The CHS, based on a bi-racial sample of the general population, including 3,993 elderly without overt cardiovascular disease, reported that an enlarged AR diameter was associated with a greater risk for incident HF in men (HR: 1.47; p = 0.014), but not in women (17). No gender differences were found for other outcomes, such as stroke, cardiovascular mortality, and all-cause mortality.
In the Chin–Shan Community Cardiovascular Cohort, the association between AR dilatation and non-cardiovascular death was found in adults < 65 years, but not in older participants, without differences in the analysis stratified by sex (18).
Evidence on the adverse impact of aortic dilation on all-cause mortality is based on two general population-based samples (18, 22) and on a subgroup of treated hypertensive elderly people belonging to CHS (17). As previously mentioned, however, it should be underlined that adjustment for LV mass abolished the significance of this relationship in the CHS cohort (17) and that the Jackson Heart Study did not include this echocardiographic parameter among the confounding factors (22).
As for cardiovascular events, no specific evidence is available about the predictive role of aortic dilatation on cardiovascular mortality. In fact, most studies examined exclusively a composite of non-fatal and fatal stroke, coronary events, and HF requiring hospitalization (18, 19, 21–25). Additional events, such as transient ischemic attacks, atrial fibrillation, cardioverter-defibrillator implants, and surgery involving major aorta branches, have been included in the composite outcome in some studies, but not in others. The CHS, the only study providing separate data on cardiac and cerebrovascular outcomes, showed that aortic dilatation predicted an increased risk for stroke and HF (in men) but not for myocardial infarction (17). As for HF, the Framingham Heart Study focused on this specific outcome showed that participants with a greater AR diameter experienced a higher risk of incident HF over an 8-year period of follow-up (18). As the association of AR diameter with incident HF was rendered non-significant after the adjustment for LV mass, a possible interpretation of the link between aortic dilation and HF is that LV mass mediates the progression to HF in presence of AR remodeling. In this regard, numerous studies have shown an independent association between LVH and aortic dilatation assessed with different imaging techniques, in several clinical settings, such as hypertensive patients, elderly individuals, and patients with aortic aneurysms (35–38). These observations suggest that alterations of the aortic wall structure/function associated with dilatation may contribute to increased LV afterload, an important factor leading to LVH. Thus, the dilatation of the most proximal arterial segment (i.e., AR and ascending tract), in addition to being a known risk factor for aortic dissection, can be considered a sign of TOD paralleling other cardiac markers of established prognostic value (39, 40). In particular, the association between aortic dilatation and LVH emphasizes the role of combined arterial–ventricular remodeling in the progression of cardiovascular continuum. Findings from the general population and hypertensive cohorts showed that the incidence of cardiovascular events was significantly increased when changes in LV structure were paralleled by those in aortic dimension and the fully adjusted risk of cardiovascular events was markedly greater in individuals with LVH and aortic dilatation than in their counterparts with LVH alone (21, 24).
It should be remarked that the mechanisms underlying aortic dilation are extremely complex and related to the interplay of adverse hemodynamic and non-hemodynamic factors, such as wall stress, inflammatory processes, altered regulation of growth factors, activation of the sympathetic nervous system, and imbalance between proteases and corresponding inhibitors resulting in degradation and fragmentation of extracellular matrix (41–44). The pathophysiological mechanisms linking aortic dilation to cardiovascular events must be considered mostly hypothetical. There are several pathophysiological mechanisms in the relationship between aortic dilatation and CV events that may be considered. Tissue remodeling of the aortic wall (i.e., reduction in elastin fiber, and increased collagen and calcium deposition) resulting in increased arterial stiffness may contribute to the relationship between aortic diameter and cardiovascular events. Furthermore, it has been speculated that combined aortic and ventricular remodeling have a pivotal role in the pathogenesis of HF. However, proximal aortic dilation may be considered as a marker of the impact of multiple cardiovascular risk factors of established prognostic significance rather than a mediator of cardiac, cerebrovascular events, and all-cause mortality.
In conclusion, current evidence supporting an association between aortic diameter and cardiovascular events as well as all-cause mortality in populations without overt vascular pathology is based on the limited number of studies, conducted in different settings (i.e., elderly individuals, free-living members of the general population, and hypertensive cohorts) with different imaging techniques (i.e., echocardiography and CT), based on different definitions of the aortic phenotype of interest (i.e., AR diameter or ascending aorta diameter), a wide range of follow-up duration (i.e., 12–144 months) and heterogeneous primary outcomes (i.e., cardiovascular events, HF, and total mortality) (45). Furthermore, an accumulating amount of evidence suggests that a single measurement of aortic diameter, is an unreliable indicator of vascular damage of prognostic significance, especially when this parameter is not indexed by body size (17, 19, 23, 24, 46). Therefore, further studies using more homogeneous methods, populations, and outcomes are still needed.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
MT: writing and reviewing. EG and CS: methodology and statistics. SC: searching the literature. CC: writing, methodology, statics, reviewing, and revising. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer RF declared a shared affiliation with one of the author CC to the handling editor at the time of review.
References
1.
Whelton PK Carey RM Aronow WS Casey DE Jr Collins KJ Dennison Himmelfarb C et al 2017 ACC/AHA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension. (2018) 71:e13–115. 10.1161/HYP.0000000000000065
2.
Williams B Mancia G Spiering W AgabitiRosei E Azizi M Burnier M et al 2018 ESC/ESH Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. (2018) 36: 2284–309.
3.
Ribeiro AB . Angiotensin II antagonists–therapeutic benefits spanning thecardiovascular disease continuum from hypertension to heart failure and diabetic nephropathy.Curr Med Res Opin. (2006) 22:1–16. 10.1185/030079905X75041
4.
Cuspidi C Mancia G Ambrosioni E Pessina A Trimarco B Zanchetti A et al Left ventricular and carotid structure in untreated, uncomplicated essential hypertension: results from the assessment prognostic risk observational survey (APROS). J Hum Hypertens. (2004) 18:891–6. 10.1038/sj.jhh.1001759
5.
Bruno RM Cartoni G Stea F Armenia S Bianchini E Buralli S et al Carotid and aortic stiffness in essential hypertension and their relation with target organ damage: the CATOD study. J Hypertens. (2017) 35:310–8. 10.1097/HJH.0000000000001167
6.
Cuspidi C Sala C Tadic M Gherbesi E Grassi G . Mancia GPre-hypertension and subclinical cardiac damage: a meta-analysis of echocardiographic studies.Int J Cardiol. (2018) 270:302–30. 10.1016/j.ijcard.2018.06.031
7.
Mancusi C Angeli F Verdecchia P Poltronieri C de Simone G Reboldi G . Echocardiography in low-risk hypertensive patients.J Am Heart Assoc. (2019) 8:e013497. 10.1161/JAHA.119.013497
8.
Triantafyllidi H Benas D Schoinas A Birmpa D Trivilou P Varytimiadi E et al Hypertension-mediated organ damage regression associates with blood pressure variability improvement three years after successful treatment initiation in essential hypertension. J Clin Hypertens (Greenwich). (2021) 23:1150–8. 10.1111/jch.14209
9.
Cuspidi C Tadic M Sala C Quarti-Trevano F Gherbesi E Mancia G et al Left ventricular mass reduction and hypertrophy regression following renal artery revascularization: a meta-analysis. J Hypertens. (2021) 39:4–11. 10.1097/HJH.0000000000002586
10.
Pareek M Vaduganathan M Bhatt DL Leósdóttir M Olsen MH . Prognostic implications of fasting plasma glucose in subjects with echocardiographic abnormalities.Int J Cardiol. (2017) 241:423–9. 10.1016/j.ijcard.2017.01.133
11.
Lundorff I Modin D Mogelvang R GodskJørgensen P Schnohr P Gislason G et al Echocardiographic predictors of cardiovascular morbidity and mortality in women from the general population. Eur Heart J Cardiovasc Imaging. (2021) 22:1026–34. 10.1093/ehjci/jeaa167
12.
Guzik BM McCallum L Zmudka K Guzik TJ Dominiczak AF Padmanabhan S . Echocardiography predictors of survival in hypertensive patientswith left ventricular hypertrophy.Am J Hypertens. (2021) 34:636–44. 10.1093/ajh/hpaa194
13.
East MA Jollis JG Nelson CL Marks D Peterson ED . The influence of left ventricular hypertrophy on survival in patients with coronary artery disease: do race and gender matter?J Am Coll Cardiol. (2003) 41:949–54. 10.1016/s0735-1097(02)03006-1
14.
Cameli M Lembo M Sciaccaluga C Bandera F Ciccone MM D’Andrea A et al Working groups of echocardiography and arterial hypertension of Italian society of cardiology (SIC) Identification of cardiac organ damage in arterial hypertension: insights by echocardiography for a comprehensive assessment. J Hypertens. (2020) 38:588–98. 10.1097/HJH.0000000000002323
15.
Vasan RS Urbina EM Jin L Xanthakis V . Prognostic significance of echocardiographic measures of cardiac remodeling in the community.CurrCardiol Rep. (2021) 23:86. 10.1007/s11886-021-01512-4
16.
Tadic M Cuspidi C Bombelli M Grassi G . Hypertensive heart disease beyond left ventricular hypertrophy: are we ready for echocardiographic strain evaluation in everyday clinical practice?J Hypertens. (2018) 36:744–53. 10.1097/HJH.0000000000001632
17.
Gardin JM Arnold AM Polak J Jackson S Smith V Gottdiener J . Usefulness of aortic root dimension in persons > 65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the cardiovascular health study).Am J Cardiol. (2006) 97:270–5. 10.1016/j.amjcard.2005.08.039
18.
Lai CL Chien KL Hsu HC Su TC Chen MF Lee YT . Aortic root dimension as an independent predictor for all-cause death in adults <65 years of age (from the Chin-Shan community cardiovascular cohort study).Echocardiography. (2010) 27:487–95. 10.1111/j.1540-8175.2009.01072.x
19.
Gondrie MJ van der Graaf Y Jacobs PC Buckens SC Mali WP Providi Study Group. The prognostic value of vascular diameter measurements on routine chest computed tomography in patients not referred for cardiovascular indications. J Comput Assist Tomogr. (2011) 35:734–41. 10.1097/RCT.0b013e318231824a
20.
Lam CSP Gona P Larson MG Aragam J Lee DS Mitchell GF et al Aortic root remodeling and risk of heart failure in the Framingham heart study. J Am Coll Cardiol Heart Fail. (2013) 1:79–83. 10.1016/j.jchf.2012.10.003
21.
Cuspidi C Facchetti R Bombelli M Re A Cairo M Sala C et al Aortic root diameter and risk of cardiovascular events in a general population: data from the PAMELA study. J Hypertens. (2014) 32:1879–87. 10.1097/HJH.0000000000000264
22.
Kamimura D Suzuki T Musani SK Hall ME Samdarshi TE Correa A et al Increased proximal aortic diameter is associated with risk of cardiovascular events and all-cause mortality in blacks the Jackson heart study. Am Heart Assoc. (2017) 6:e005005. 10.1161/JAHA.116.005005
23.
Qazi S Massaro JM Chuang ML D’Agostino RB Sr. Hoffmann U O’Donnell CJ . Increased aortic diameters on multidetector computed tomographic scan are independent predictors of incident adverse cardiovascular events: the Framingham heart study.Circ Cardiovasc Imaging. (2017) 10:e006776. 10.1161/CIRCIMAGING.117.006776
24.
Canciello G Mancusi C Losi MA Izzo R Trimarco B de Simone G et al Aortic root dilatation is associated with incident cardiovascular events in a population of treated hypertensive patients: the Campania salute network. Am J Hypertens. (2018) 31:1317–23. 10.1093/ajh/hpy113
25.
Leone D Airale L Bernardi S Mingrone G Astarita A Cesareo M et al Prognostic role of the ascending aorta dilatation in patients with arterial hypertension. J Hypertens. (2021) 39:1163–9. 10.1097/HJH.0000000000002752
26.
Chirinos JA Segers P Hughes T Townsend R . Large-artery stiffness in health and disease: JACC State-of-the-Art Review.J Am Coll Cardiol. (2019) 74:1237–63. 10.1016/j.jacc.2019.07.012
27.
Kim SH Monticone RE McGraw KR Wang M . Age-associated proinflammatory elastic fiber remodeling in large arteries.Mech Ageing Dev. (2021) 196:111490. 10.1016/j.mad.2021.111490
28.
Sawabe M Hamamatsu A Chida K Mieno MN Ozawa T . Age is a major pathobiological determinant of aortic dilatation: a large autopsy study of community deaths.J AtherosclerThromb. (2011) 18:157–65. 10.5551/jat.6528
29.
Matsumura Y Ochi Y Wada M Hirakawa D Yamanaka S Kamioka M et al Usefulness of screening for abdominal aortic aneurysm during transthoracic echocardiography in women 50 years of age. Am J Cardiol. (2018) 122:2147–50. 10.1016/j.amjcard.2018.08.050
30.
Wang D Xu JZ Kang YY Zhang W Hu LX Wang JG . Aortic root diameterin hypertensive patients with various stages of obstructive sleep apnea.Am J Hypertens. (2021) 18:hab167.
31.
Palmieri V Bella JN Arnett DK Roman MJ Oberman A Kitzman DW et al Aortic root dilatation at sinuses of Valsalva and aortic regurgitation in hypertensive and normotensive subjects: the hypertension genetic epidemiology network study. Hypertension. (2001) 37:1229–35. 10.1161/01.hyp.37.5.1229
32.
Covella M Milan A Totaro S Cuspidi C Re A Rabbia F et al Echocardiographic aortic root dilatation in hypertensive patients: a systematic review and meta-analysis. J Hypertens. (2014) 32:1928–35. 10.1097/HJH.0000000000000286
33.
Cuspidi C Facchetti R Quarti-Trevano F Dell’Oro R Tadic M Mancia G et al Incident aortic root dilatation in the general population: findings from the Pamela study. J Hypertens. (2022) 40:544–52. 10.1097/HJH.0000000000003047
34.
Canciello G Mancusi C Izzo R Morisco C Strisciuglio T Barbato E et al Determinants of aortic root dilatation over time in patients with essential hypertension: the Campania salute network. Eur J Prev Cardiol. (2020) 12:2047487320931630.
35.
Bella JN Wachtell K Boman K Palmieri V Papademetriou V Gerdts E et al Relation of left ventricular geometry and function to aortic root dilatation in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Am J Cardiol. (2002) 89:337–41. 10.1016/s0002-9149(01)02238-x
36.
Rayner BL Goodman H Opie LH . The chest radiograph. A useful investigation in the evaluation of hypertensive patients.Am J Hypertens. (2004) 17:507–10. 10.1016/j.amjhyper.2004.02.012
37.
Cuspidi C Meani S Fusi V Valerio C Sala C Zanchetti A . Prevalence and correlates of aortic root dilatation in patients with essential hypertension relationship with cardiac and extra-cardiac organ damage.J Hypertens. (2006) 24:573–80. 10.1097/01.hjh.0000209992.48928.1f
38.
Iarussi D Caruso A Galderisi M Covino FE Dialetto G Bossone E et al Associations of left ventricular hypertrophy and aortic dilation in patients with acute thoracic aortic dissection. Angiology. (2001) 52:447–55. 10.1177/000331970105200702
39.
Shirali AS Bischoff MS Lin HM Oife I Lookstein R Griepp RB et al Predicting the risk for acute type B aortic dissection in hypertensive patients using anatomic variables. J Am Coll Cardiol Img. (2013) 36:349–57. 10.1016/j.jcmg.2012.07.018
40.
Liu LY Yun CH Kuo JY Lai YH Sung KT Yuan PJ et al Aortic root remodeling as an indicator for diastolic dysfunction and normative ranges in asians: comparison and validation with multidetector computed tomography. Diagnostics (Basel). (2020) 10:712. 10.3390/diagnostics10090712
41.
Wang Y Wu B dong L Wang C Wang X Shu X . Circulating matrix metalloproteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis.Heart Vessels. (2016) 31:189–97. 10.1007/s00380-014-0593-5
42.
Fujita D Preiss L Aizawa K Asch F Eagle K Suzuki T et al Circulating interleukin-6 (IL-6) levels are associated with aortic dimensions in genetic aortic conditions. PLoS One. (2019) 14:e0214084. 10.1371/journal.pone.0214084
43.
van Dorst DCH de Wagenaar NP van der Pluijm I Roos-Hesselink JW Essers J Danser AHJ . Transforming growth factor-beta and the renin-angiotensin system in syndromic thoracic aortic aneurysms: implications for treatment.Cardiovasc Drugs Ther. (2021) 35:1233–52. 10.1007/s10557-020-07116-4
44.
Wortmann M Peters AS Erhart P Körfer D Böckler D Dihlmann S . Inflammasomes in the pathophysiology of aortic disease.Cells. (2021) 10:2433. 10.3390/cells10092433
45.
Fernandes LP Barreto ATF Neto MG Câmara EJN Durães AR Roever L et al Prognostic power of conventional echocardiography in individuals without history of cardiovascular diseases: a systematic review and meta-analysis. Clinics (Sao Paulo). (2021) 76:e2754. 10.6061/clinics/2021/e2754
46.
Girardi LN Lau C Gambardella I . Aortic dimensions as predictors of adverse events.J Thorac Cardiovasc Surg. (2021) 161:1193–7. 10.1016/j.jtcvs.2020.06.137
Summary
Keywords
cardiovascular events, mortality, echocardiography, computed tomography, aortic diameter
Citation
Tadic M, Gherbesi E, Sala C, Carugo S and Cuspidi C (2022) Is Thoracic Aortic Diameter an Independent Predictor of Cardiovascular Disease and Mortality? A Narrative Review. Front. Cardiovasc. Med. 9:867026. doi: 10.3389/fcvm.2022.867026
Received
31 January 2022
Accepted
04 April 2022
Published
29 April 2022
Volume
9 - 2022
Edited by
Gian Marco Rosa, San Martino Hospital (IRCCS), Italy
Reviewed by
Mona Mostafa Rayan, Ain Shams University, Egypt; Rita Facchetti, University of Milano-Bicocca, Italy; Yuling Zhang, Sun Yat-sen Memorial Hospital, China
Updates
Copyright
© 2022 Tadic, Gherbesi, Sala, Carugo and Cuspidi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marijana Tadic, marijana_tadic@hotmail.com; marijana.tadic82@gmail.com
This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.