Abstract
Background:
Postoperative myocardial injury (PMI) is associated with short- and long-term mortality. The incidence of PMI in very old patients is currently unknown. There is currently neither known effective prophylaxis nor a uniform strategy for the elderly with PMI.
Objective:
To share our 10 years of experience in the comprehensive management of PMI after non-cardiac surgery in patients aged ≥ 80 years.
Methods:
In this case series, we retrospectively collected and assessed the 2,984 cases aged ≥ 80 years who accepted non-cardiac surgery from 2011 to 2021 at the second Medical Center, Chinese PLA General Hospital. The incidence, risk factors, management strategy, and prognosis of surgical patients with PMI were analyzed.
Results:
A total of 2,984 patients met our inclusion criteria. The overall incidence of PMI was 14%. In multivariable analysis, coronary artery disease, chronic heart failure, and hypotension were independently associated with the development of PMI. The patients with PMI were at a higher risk of death (OR, 2.69; 95% CI, 1.78–3.65). They were more likely to have received low molecular heparin, anti-plantlet therapy, beta-blocker, early coronary angiography, and statin than patients without PMI. The 30-day (0.96% vs. 0.35%; OR 3.46; 95% CI, 1.49–7.98; P < 0.001) and 1-year mortality (5.37% vs. 2.60%; OR 2.35; 95% CI, 1.12–6.53; P < 0.001) was significantly higher in patients with PMI compared with those without PMI.
Conclusions:
The incidence of PMI in very old patients was high. The PMI is associated with an increased risk of 30 days and 1-year mortality. These patients can benefit from intensification of assessment and individualized care of multi-morbidities during the perioperative period. Especially cardiovascular medical treatments, such as antiplatelet, anticoagulation, β-blockers, and statins are very important for patients with PMI.
Introduction
As the population ages, the rate of surgical procedures in the older population is rising. Aged patients, especially those over 80 years, are more likely to suffer from comorbidities, such as coronary heart disease, hypertension, diabetes, polypharmacy, and decline of organ function. Thus, to ensure surgical safety, the perioperative management team is of great significance. The department of geriatric comprehensive surgery of the author's team is composed of geriatric physicians, who have been engaged in geriatric perioperative management for 35 years. We have established a co-management care model by geriatricians and surgeons, ensuring the perioperative safety of geriatric surgical patients and accumulating rich clinical experience.
Considering the advancing age and co-morbid status of the surgical patient cohort, the incidence of postoperative cardiac complications will likely continue to rise in the aging population. Postoperative myocardial injury (PMI) after non-cardiac surgery continues to pose challenges to geriatric physicians with regards to a comprehensive diagnostic and management approach. According to the VISION study, so far, the largest prospective study performed in non-cardiac surgery (1), PMI has been identified as troponin elevation within 30 days after non-cardiac surgery, which is associated with an increased risk of mortality and major vascular complications at 30 days and up to 2 years after non-cardiac surgery (1–5). Recent studies have shown that more than 85% of PMIs are missed in clinical practice when relying on the reporting of typical symptoms. Therefore, PMIs can only be reliably detected with perioperative screening using sensitive or high-sensitivity cardiac troponin T/I measurements (6). The estimated incidence of myocardial injury after non-cardiac surgery based on data from 44 high quality and prospective studies, which routinely measured troponin after surgery, is 19.5% (7). However, the incidence of PMI in old patients is currently unknown. At present, there are few reports on myocardial injury after non-cardiac surgery in people over 80 years old and there is still a lack of treatment strategy for PMI. We, therefore, aimed to investigate the incidence of PMI in old patients, as well 30-day and 365-day mortality, and to summarize the clinical experience of the department for 10 years, to provide a reference for the prevention and treatment of PMI.
Methods
Study Population
Patients in this study were drawn from the Second Medical Center, Chinese PLA General Hospital. We included consecutive patients undergoing non-cardiac surgery who were at least 80 years of age and had a troponin measurement within the first 24 h and 3 days after surgery between January 1, 2011, and January 1, 2021.
Data Collection
Data were collected from electronic health records (EHR) from our hospital. The variables collected included demographics, laboratory measurements, troponin I concentrations, disease diagnosis, comorbidities, procedures, and outcomes. Comorbidities were extracted using International Classification of Disease-Revision 9/10 billing codes for atrial fibrillation (AF), asthma, coronary artery disease (CAD), cancer, chronic kidney disease (CKD), chronic obstructive pulmonary, diabetes (DM), heart failure (HF), and hypertension (HTN). Troponin T concentrations were assessed and the reference level for normal is <0.03 ng/ml. The clinical follow-up data were obtained using direct contact with the patients, their referring physicians, and family physicians.
Evaluation of PMI
The PMI was defined as a peak plasma troponin T concentration of 0.03 ng/ml or greater than resulted from myocardial ischemia within 3 days after surgery. The pre-operative assessment was carried out by physicians.
Individualizing Risk Assessment and Care in Patients With Associated Conditions and Comorbidities
Trained research personnel reviewed the charts to obtain information on potential preoperative predictors of major perioperative complications by using standardized definitions. The Revised Cardiac Risk Index (RCRI) was calculated in all patients to estimate cardiovascular risk. The RCRI has six predictors of risk as to the following: creatinine ≥2 mg/dl, heart failure, insulin-dependent diabetes mellitus, intrathoracic, intra-abdominal, or suprainguinal vascular surgery, history of cerebrovascular accident or Transient Ischaemic Attack (TIA), and ischemic heart disease. Each predictor is worth 1 point. Patients with more predictors of risk would have a higher risk.
Postoperative pulmonary complications were evaluated by using a seven-variable risk assessment tool Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT). The ARISCAT score included seven independent risk factors: low preoperative arterial oxygen saturation, acute respiratory infection during the previous month, age, preoperative anemia, upper abdominal or intrathoracic surgery, surgical duration of at least 2 h, and emergency surgery (8).
Hypotension was defined as a mean arterial pressure (MAP) below 65 mm Hg for at least 1 min (9).
Major bleeding was defined using modified International Society on Thrombosis and Haemostasis (ISTH) criteria to include any one of the following: (1) fatal bleeding, (2) bleeding into a critical area or organ, (3) fall in hemoglobin of ≥ 2 g/dl over 24 hours, (4) transfusion of two or more units of whole blood or packed red blood cells over 24 h, and in surgical patients, and (5) surgical site bleeding that required a second intervention (10).
We excluded patients who were incorrectly screened (<80 years, <24-h hospital stays), had their surgery canceled, had cardiac surgery or MI within 14 days before surgery. For the analysis addressing 30-day and 1-year mortality, we only included every patient once at first enrollment.
Statistical Analysis
Descriptive analyses were performed by troponin T levels stratified into normal and elevated groups (more than the upper limit of normal, >0.03 ng/ml). We used troponin measurements within 3 days after the operation. If multiple troponin measurements were available within 24 h, the patient's highest measurement was used. Categorical variables were reported as total count and percentage of patients. Continuous no troponin laboratory values were reported as median and interquartile range. The characteristics of patients who did and did not develop PMI were compared using the Chi-square test for categorical variables and the Student's t-test for continuous variables. Multivariable logistic regression analysis was used to estimate the independent predictors of myocardial injury after non-cardiac surgery. We report the 30-day mortality and other important 30-day outcomes (non-fatal cardiac arrest, congestive heart failure, stroke, and composite of major events) for patients with and without PMI. To assess the effects of PMI on outcomes, we conducted a survival analysis with the dependent variable of time to mortality, setting the time of days after the operation.
Results
Study Cohort and Patient Characteristics
A total of 2,984 surgeries met our inclusion criteria. The baseline characteristics of the surgical patients who did and did not develop PMI are reported in Table 1. All the patients underwent troponin measurement every day within 3 days after the operation. Approximately 50% of PMI occurs on the day of surgery, and 18% on the second postoperative day. The mean [±standard deviation (SD)] age of the patients was 83.7 ± 8.25 years. The majority of participants were classified as either ASA class 2 (70.38%) or ASA class 3 (23.39%) before surgery. More often, patients with PMI had coronary artery disease, atrial fibrillation, peripheral artery disease, prior stroke, diabetes mellitus, chronic obstructive pulmonary disease (COPD), active tumor disease, and had a higher ARISCAT score than patients who did not develop PMI.
Table 1
| All Patients (n = 2,984) | PMI (n = 418) | No PMI (n = 2,566) | P-Value | |
|---|---|---|---|---|
| Age | 83.7 ± 8.25 | 83.5 ± 8.15 | 83.9 ± 8.30 | 0.371 |
| Sex, male | 2,730 (91) | 356 (85.17) | 1,143 (89.09) | 0.102 |
| ASA | ||||
| I | 118 (3.95) | 10 (2.39) | 108 (4.21) | |
| II | 2,100 (70.38) | 256 (6.12) | 1,844 (71.86) | |
| III | 698 (23.39) | 76 (18.18) | 622 (24.24) | |
| IV | 68 (2.28) | 14 (3.34) | 54 (2.10) | 0.423 |
| ≥4 MET | 1,910 (64.0) | 272 (65.07) | 1,638 (63.83) | 0.730 |
| Clinical covariates | ||||
| Body mass index, kg/m2 | 26.4 ± 2.5 | 26.9 ± 2.9 | 25.8 ± 2.1 | 0.745 |
| ACE inhibitor or ARB use | 1,064 (35.66) | 168 (40.19) | 896 (34.92) | 0.140 |
| Statin use | 1,478 (49.53) | 216 (51.67) | 1,182 (46.06) | 0.000 |
| Comorbidities | ||||
| Coronary artery Disease |
972 (32.57) | 310 (74.16) | 662 (25.80) | 0.000 |
| Prior myocardial Infarction |
394 (13.20) | 178 (42.58) | 216 (8.42) | 0.000 |
| Chronic heart failure | 828 (27.75) | 206 (49.28) | 622 (24.24) | 0.000 |
| Chronic kidney disease | 858 (28.75) | 140 (33.49) | 718 (27.98) | 0.103 |
| Atrial fibrillation | 326 (10.92) | 166 (39.71) | 160 (6.23) | 0.000 |
| Peripheral artery Disease |
1,182 (39.61) | 372 (88.99) | 810 (31.57) | 0.000 |
| Hypertension | 1,534 (51.41) | 216 (51.67) | 1,318 (51.36) | 0.934 |
| Prior stroke/TIA | 1,304 (43.70) | 218 (52.15) | 1,086 (42.32) | 0.008 |
| Diabetes mellitus | 1,268 (42.50) | 226 (54.07) | 1,042 (40.60) | 0.000 |
| COPD | 1,058 (35.46) | 236 (56.46) | 822 (32.03) | 0.000 |
| Asthma | 254 (8.51) | 36 (8.61) | 218 (8.50) | 0.955 |
| ARISCACT score | 28 ± 8 | 32 ± 5 | 26 ± 7 | 0.035 |
| Active tumor disease | 1,274 (42.69) | 108 (25.84) | 1,166 (45.44) | 0.000 |
| RCRI class | ||||
| I | 210 (7.04) | 40 (9.57) | 170 (6.62) | |
| II | 1,792 (60.05) | 308 (73.68) | 1,484 (57.83) | |
| III | 727 (24.40) | 60 (14.35) | 668 (26.03) | |
| IV | 74 (2.48) | 10 (2.39) | 64 (2.49) | |
| V | 1 (0) | 0 | 0 | 0.000 |
| Elective surgery | 2,506 (77.28) | 246 (58.85) | 2,060 (80.28) | 0.000 |
| Emergency surgery, ≤ 24 h |
428 (14.34) | 116 (27.75) | 312 (12.16) | 0.000 |
| Urgent surgery, >24 h |
250 (8.38) | 56 (13.40) | 194 (7.56) | 0.316 |
| Type of Surgery | ||||
| General Surgery | 542 (18.16) | 125 (29.9) | 417 (16.25) | |
| Thoracic | 379 (12.70) | 35 (8.37) | 344 (13.41) | |
| Hepatobiliary surgery | 217 (7.27) | 26 (6.22) | 191 (7.44) | |
| Orthopedic | 326 (10.92) | 43 (10.28) | 283 (11.03) | |
| cerebral surgery | 119 (3.99) | 30 (7.18) | 89 (3.47) | |
| Urology | 988 (33.11) | 128 (30.62) | 860 (33.52) | |
| Other | 413 (13.84) | 31 (7.41) | 382 (14.89) | 0.000 |
| Laboratory values | ||||
| Hemoglobin, g/dl | 121.30 ± 18.97 | 122.88 (17.63) | 121.04 (19.17) | 0.193 |
| Lymphocyte, % | 0.19 ± 0.10 | 0.19 (0.10) | 0.19 (0.09) | 0.541 |
| APTT | 36.62 ± 6.31 | 36.42 (5.69) | 36.66 (6.41) | 0.616 |
| D-dimer, mg/ml | 2.31 ± 2.56 | 2.33 (2.48) | 2.31 (2.58) | 0.903 |
| C-reactive protein, mg/l | 3.39 (4.76) | 3.37 (4.99) | 3.40 (4.72) | 0.942 |
| Creatine kinase, U/l | 87.1 (121.74) | 76.23 (87.05) | 88.9 (126.45) | 0.175 |
| Bilirubin | 17.92 ± 2.87 | 18.12 (3.47) | 17.89 (1.54) | 0.953 |
| CK-MB | 20.83 ± 6.24 | 26.45 ± 7.39 | 19.5 ± 4.5 | 0.019 |
| Myoglobin | 80.26 ± 31.21 | 203.67 ± 34.39 | 84.65 ± 20.47 | 0.027 |
| Lactate dehydrogenase, U/l | 2.38 ± 0.81 | 2.35 (0.82) | 2.40 (0.80) | 0.924 |
| Creatinine, mg/dl | 80.81 ± 33.88 | 77.86 ± 32.91 | 81.29 ± 34.02 | 0.765 |
| Albumin, g/dl | 33.9 ± 4.80 | 32.93 (5.19) | 34.90 (4.74) | 0.698 |
| Sodium, mEq/l | 139.15 ± 3.89 | 139.47 ± 3.57 | 139.10 ± 3.94 | 0.919 |
| BNP | 343.21 ± 28.7 | 647.96 (18.01) | 321.28 (34.78) | 0.043 |
| Tachycardia (heart rate >100 beats/min) | 1,012 (33.91) | 218 (52.15) | 794 (30.94) | 0.261 |
| Hypotension (MAP <65 mm Hg) | 538 (18.03) | 124 (29.66) | 414 (16.13) | 0.000 |
| SBP >160 mm Hg | 946 (31.70) | 184 (44.02) | 762 (29.70) | 0.000 |
Baseline characteristics.
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, American Society of Anesthesiologists; BNP, COPD, chronic obstructive pulmonary disease; MAP, mean artery pressure; METS, metabolic Equivalent of Task; RCRI revised cardiac risk index; SBP, systolic blood pressure.
Myocardial Injury and Clinical Outcomes After Surgery
The overall incidence of PMI was 418/2,984 (14%). In clinical presentation, the ECG changes within 7 days in patients experiencing a PMI were shown in Table 2. Among the surgical patients who suffered PMI, 156 (37.32%) patients had palpitations and forty (9.57%) patients had clinical ischemic symptoms. The most common ischemic ECG findings were T wave inversion (32.54%) and ST-segment depression (24.4%). Twenty patients were with ECG findings of LBBB and the mortality at 30 days was as high as 10%. In multivariable analyses, coronary artery disease [adjusted OR (aOR) 1.92 95% CI (1.43–2.69)], chronic heart failure [aOR 2.16 95% CI (1.48–4.15), and hypotension [aOR 2.79 95% CI (1.88–4.20) were independently associated with the development of PMI (Table 3).
Table 2
| Prevalence n (%) | Mortality at 30 days n (%) | |
|---|---|---|
| Ischemic symptoms | 40(9.57) | 0 |
| Dyspnea | 34(8.13) | 0 |
| Typical chest pain | 18(4.31) | 1(5.5) |
| Atypical, but still possible ischemic symptoms |
78(18.66) | 0 |
| Palpitations | 156(37.32) | 0 |
| Nausea and vomiting | 44(10.53) | 0 |
| Edema | 30(7.18) | 0 |
| New ECG findings | ||
| ST-segment elevation | 40(9.57) | 3(7.5) |
| ST-segment depression | 102(24.40) | 0 |
| T-wave inversion | 136(32.54) | 0 |
| New pathological Q waves | 28(6.70) | 0 |
| LBBB | 20(4.78) | 2(10) |
| Any ischemic ECG changes | 320(76.56) | 0 |
| PMI fulfilling additional criteria for spontaneous AMI | 48(11.48) | 2(8.33) |
Clinical presentation, ECG changes within 7 days in patients experiencing a postoperative myocardial injury (PMI).
AMI, acute myocardial infarction; LBBB, left bundle branch block.
Table 3
| Variable | Adjusted OR (95% CI) |
|---|---|
| Coronary artery disease | 1.92 (1.43–2.69) |
| Hypertension | 1.06 (0.77–1.45) |
| Diabetes Mellitus | 1.16 (0.82–1.63) |
| COPD | 1.04 (0.89–1.50) |
| Cancer | 0.65 (0.41–1.09) |
| Hypotension | 2.79 (1.88–4.20) |
| Chronic heart failure | 2.16 (1.48–4.15) |
| Atrial fibrillation | 0.70 (0.35–1.41) |
| renal insufficiency | 2.05(1.60–3.78) |
Multivariable predictors of myocardial injury after non-cardiac surgery.
COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Patients who suffered from PMI were at higher risk of death (OR, 2.69; 95% CI, 1.78–3.65; P < 0.001), non-fatal cardiac arrest (OR,4.52; 95% CI, 2.63–7.64; P < 0.001), congestive heart failure (OR, 10.25; 95% CI, 7.86–13.40; P < 0.001), and stroke (OR, 3.06; 95% CI, 2.62–4.31; P < 0.001) compared with patients who did not develop PMI (Table 4). The overall mortality was 3.15% (Figure 1). The 30-day and 1-year mortality for surgical patients with and without PMI was shown in Figures 2, 3. The 30-day (0.96 vs.0.35%; OR 3.46; 95% CI, 1.49–7.98; P < 0.001) and 1-year mortalities (5.37 vs. 2.60%; OR 2.35; 95% CI, 1.12–6.53; P < 0.001) were significantly higher in patients who developed PMI compared with those who did not develop PMI.
Table 4
| Outcomes | No PMI (n = 2,566, %) | PMI (n = 418, %) | Unadjusted OR (95% CI) |
|---|---|---|---|
| Mortality | 9 (0.35) | 4 (0.96) | 2.69 (1.78–3.65) |
| Nonfatal cardiac arrest | 8 (0.31) | 8 (1.9) | 4.52 (2.63–7.64) |
| Congestive heart failure | 52 (2.03) | 42 (10.05) | 10.25 (7.86–13.40) |
| Stroke | 31 (1.2) | 16 (3.83) | 3.06 (2.62–4.31) |
| Composite of major events | 90 (3.50) | 61 (14.59) | 4.15 (2.76–7.56) |
Thirty-day outcomes.
OR, odds ratio.
Figure 1
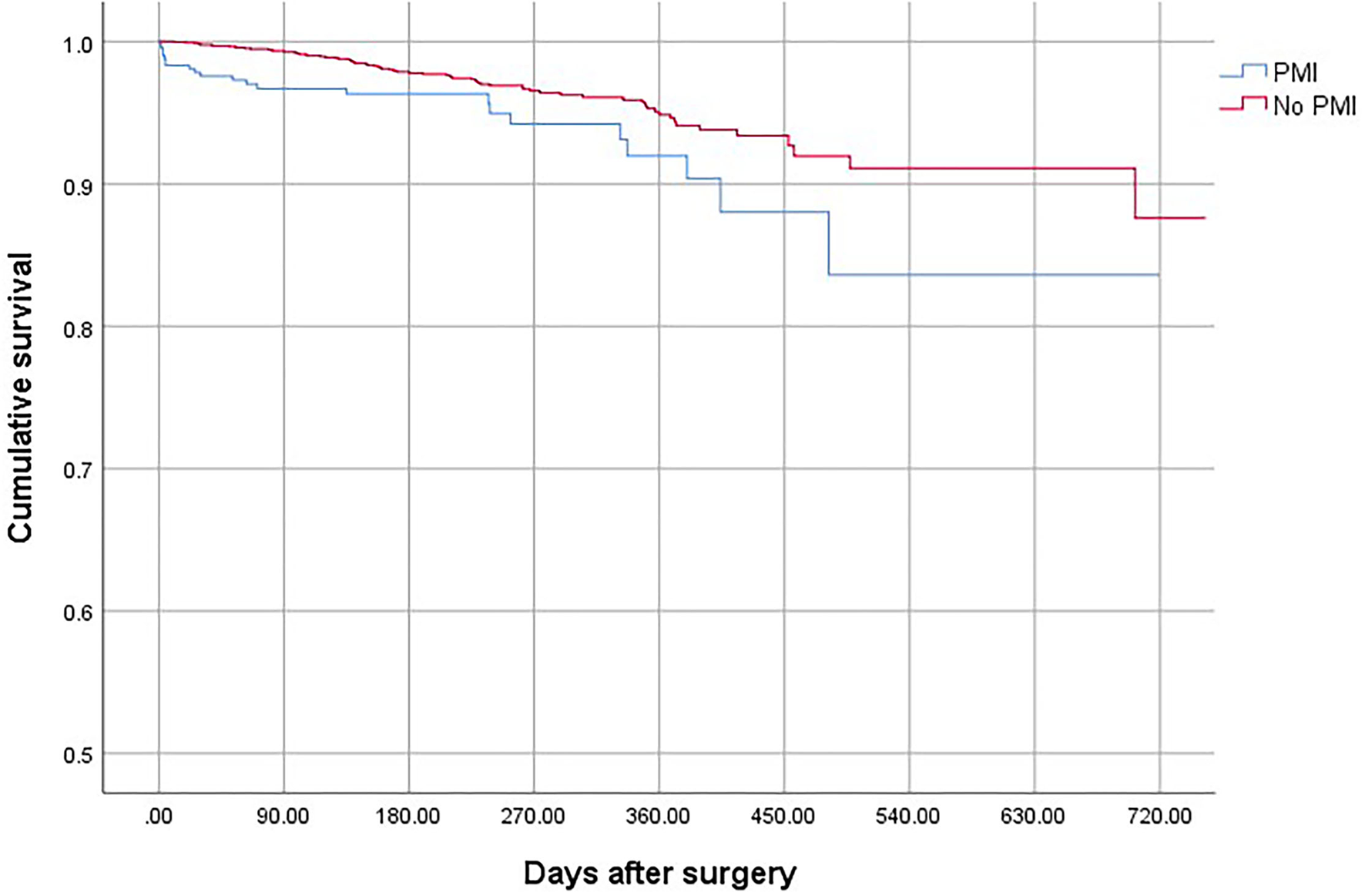
The overall mortality of the patients with and without PMI.
Figure 2
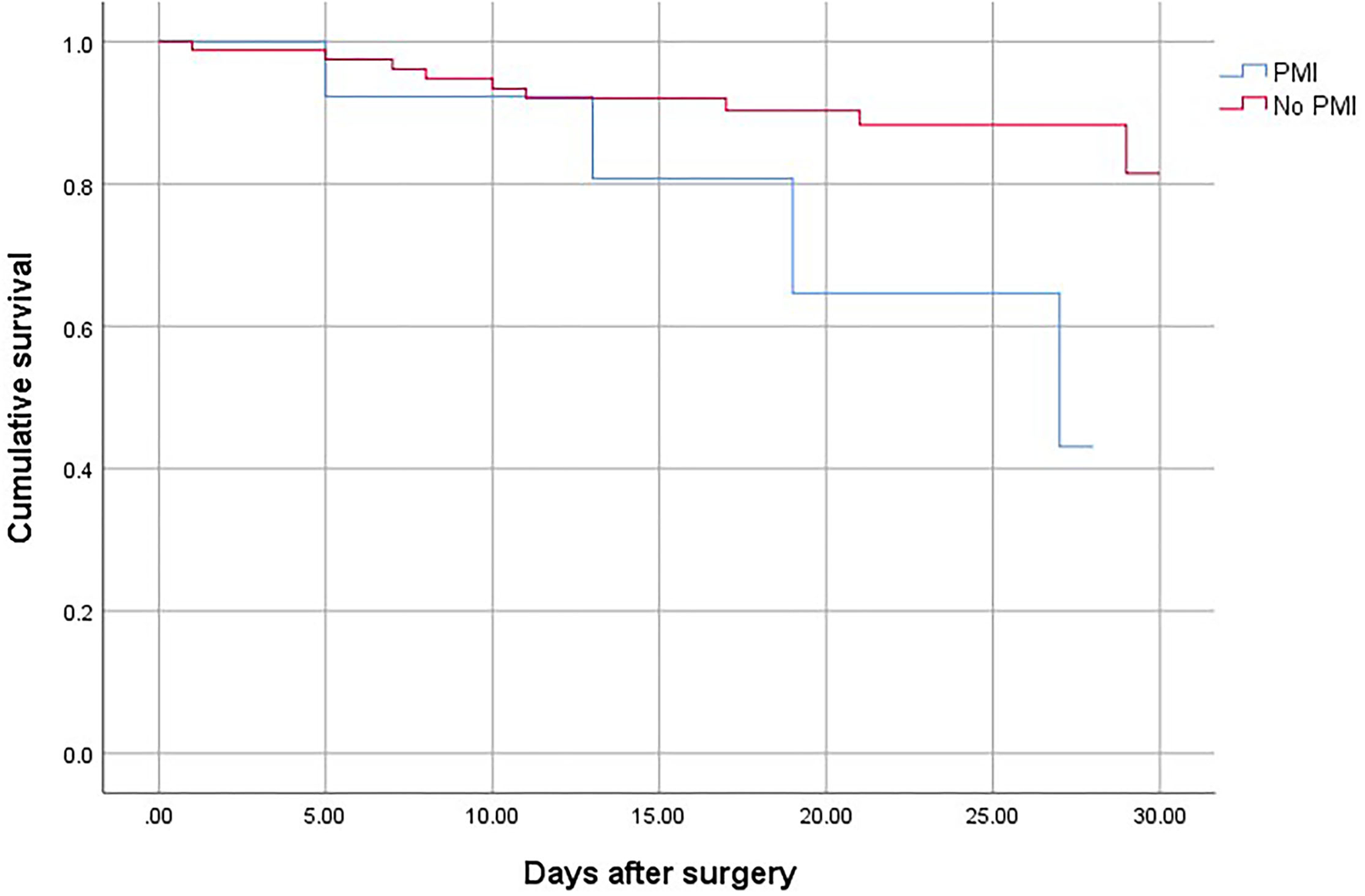
The 30-day mortality for surgical patients with and without PMI.
Figure 3
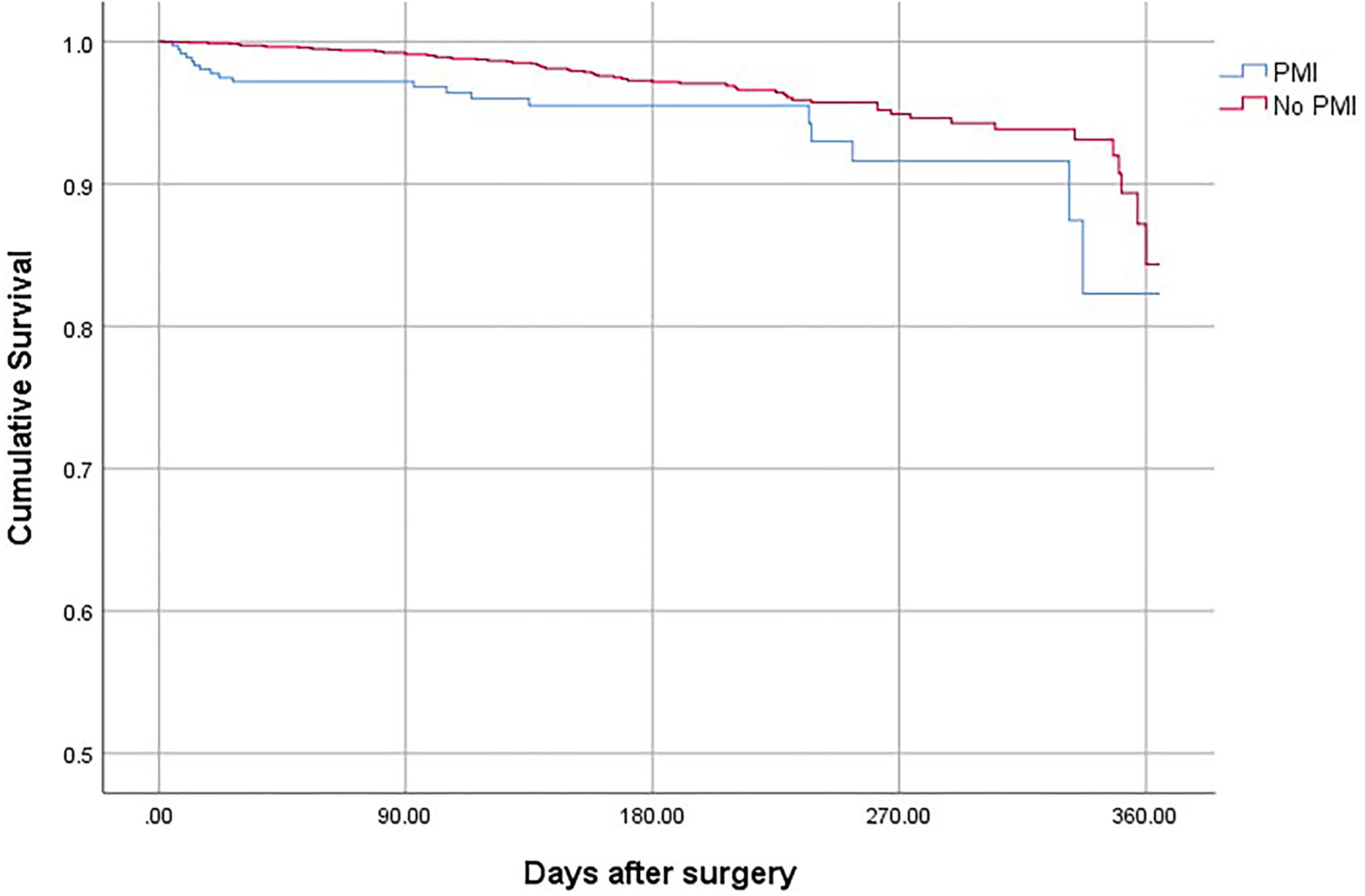
The 1-year mortality for surgical patients with and without PMI.
Perioperative Medical Therapy
Patients were prescribed with low molecular heparin in 18.13% of cases, antiplatelet therapy in 30.7% of cases, oral anticoagulation in 9.42%, an angiotensin-converting enzyme (ACE) inhibitor in 19.7%, a statin in 34.75%, and a beta-blocker in 11.16%. As shown in Table 5, patients who developed PMI were more likely to have received low molecular heparin, anti-plantlet therapy, beta-blocker, early coronary angiography, and statin than patients who did not develop PMI. There were no differences in the prescribing frequency of Angiotensin-converting-enzyme inhibitors (ACEI)/ angiotensin II receptor blockers (ARB) , oral anticoagulation. Overall, low molecular heparin was prescribed in 31.05% (130/418) of PMI cases. Dalteparin sodium 2,500 IU was injected subcutaneously every 12 h. The incidence of major bleeding was 15.38% (20/130) in the entire group. However, four (3.07%, 4/130) patients, who received low molecular heparin therapy, died of massive hemorrhage of the gastrointestinal tract, all of them suffered from acute myocardial infarction.
Table 5
| All patients (N = 2,984, %) | PMI (n = 418, %) | No PMI (n = 2,566, %) | |
|---|---|---|---|
| Low molecular heparin | 541 (18.13) | 130 (31.05) | 411 (16.02) |
| Anti-platelet therapy | 916 (30.70) | 326(y77.99) | 590 (22.99) |
| beta-blocker | 333 (11.16) | 192 (45.93) | 141 (5.49) |
| ACEI/ARB | 588 (19.7) | 75 (17.94) | 513 (19.99) |
| Oral Anticoagulation | 281 (9.42) | 50 (11.96) | 231 (9) |
| Early coronary angiography | 34 (1.14) | 21 (5.02) | 13 (0.55 ) |
| Statin | 1,037 (34.75) | 293 (70.10) | 744 (28.99) |
| DAPT | 270 (9.05) | 142 (33.97) | 128 (4.98) |
| Combinations | |||
| No Aspirin or Statin | 378 (12.67) | 121 (28.94) | 257 (10.01) |
| APT + Statin | 677 (22.69) | 292 (69.85) | 385 (15.00) |
| DAPT + Statin | 103 (3.45) | 26 (6.22) | 77 (3.00) |
Perioperative medical therapy in patients with and without PIM.
APT, anti-platelet therapy; DAPT, dual anti-platelet therapy; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitors.
Discussion
Principal Findings
In this retrospective study of 2,984 patients 80 years of age or older undergoing non-cardiac surgery, the incidence of PMI was 14%, which was higher than that seen in a large national database (8%) (3). The 30-day mortality was 0.43%, which was much lower than that seen in other studies (11, 12). The observed 30-day mortality after surgery ranges between 1 and 10% (3, 12–17).
Approximately 50% of PMI occurs on the day of surgery, and 18% on the second postoperative day. Only 22% of patients with PMI fulfilled the universal definition of myocardial infarction. A minority (9.57%) of patients suffering PMI experienced an ischemic feature. Any ischemic ECG changes were presented in 76.56% of patients with PMI. Therefore, nearly 90% of PMI would, probably, have gone undetected without systematic troponin and ECG monitoring after surgery. Patients with PMI had a greater burden of cardiovascular events than patients without PMI. In comprehensive elevations of cardiac troponin in combination with ischemic symptoms, ECG changes are helpful to identify PMI.
Perioperative Myocardial Infarction/Injury
The combination of aging and multi-comorbidities, such as cardiovascular disorders or renal insufficiency, is the principal factor increasing surgical stress in older patients. The elderly patient who undergoes surgery has decreased ability to compensate and maintain homeostasis when the body is stressed. The perioperative management of old patients with multiple conditions is a great challenge for hospital physicians.
The perioperative risk of patients is markedly increased due to the cardiac condition itself and co-morbidities. In the present study, multivariable analyses showed that coronary artery disease, chronic heart failure, and hypotension were independently associated with the development of PMI. Co-management model by surgeons and geriatricians is helpful with perioperative management of cardiovascular complications and has the potential to improve outcomes. In our study, the incidence of PMI was higher and the 30-day mortality was lower than that seen in other studies (11, 12). A system review showed orthogeriatric collaboration to improve mortality after hip repair (18). One recent meta-analysis demonstrated that orthogeriatric care models reduced length of stay, in-hospital mortality, 1-year mortality, and delirium of patients with hip fracture and may reduce complications and cost (19).
The PMI is a complex syndrome with multiple different underlying etiologies, including type 1 myocardial infarction (T1MI), and emphasis on the causal relationship of plaque disruption with coronary atherothrombosis; while in type 2 myocardial infarction (T2MI), settings with oxygen demand and supply imbalance are unrelated to acute coronary atherothrombosis (6). The PMIs, after non-cardiac surgery, are largely considered type 2 infarction. The OPTIMUS and Coronary CTA VISION Studies showed that, at most, a quarter to a third of PMIs are due to thrombosis, whereas at least two-thirds to three-quarters are likely due to supply-demand mismatch (20, 21). In the present study, 22% of patients with PMI fulfilled the universal definition of myocardial infarction. A patient with a history of previous coronary artery disease merits special attention as it is a strong predictor of PMI. Given the super old age and higher prevalence of concomitant coronary artery disease in a series of patients with PMI, we suggest consideration of non-invasive evaluation for ischemia if appropriate. The risk and benefit of coronary interventions should be taken into account more cautiously in patients, who are at risk of bleeding shortly after surgery, because withdrawing antiplatelet therapy may lead to in-stent thrombosis (22, 23).
Another issue of perioperative risk is bleeding (24). Of note, 31.05% of patients with PMI received therapy of low molecular weight heparin (LMWH). The median duration of postoperative LMWH administration was 7 days. The incidence of major bleeding was 15.38% and 30-day mortality was 3.07%. Management of anticoagulation during invasive procedures varies widely and remains challenging and controversial. Based on these findings, it was concluded that LMWH should be used to decrease the risk of thromboembolic events without increasing the risk of bleeding. The risks of bleeding for any surgical procedure must be weighed against the benefit of remaining on anticoagulants on a case-by-case basis.
The optimal management of patients with PMI remains an area of the ongoing investigation. A multidisciplinary approach and expert guidance on the assessment and medical management of multi-morbidity, pump flow control, hemodynamic monitoring, anticoagulation strategies, infection, and bleeding prevention strategies are considered important. Appropriate use of intravenous fluids to maintain fluid and electrolyte balance is important for normal organ function after surgery. Optimal perioperative medical therapy should be adopted for the prevention and management of PMI, including antiplatelet agents, β-Blocker (25–29), statins (12, 30–34), ACE inhibitors, and anticoagulants. These patients can benefit from intensification of assessment and medical management of multi-morbidity during the perioperative period (35).
Strengths and Weaknesses of Our Study
The main strength of the present study is that it includes a large sample of surgical patients aged over 80 years, which allowed us to demonstrate the incidence and prognosis of the PMI diagnosis in elderly surgical patients, to show the advantage of the co-management model by geriatricians and surgeons in mortality. There are several limitations of this study. First, this study was a retrospective study, excluding patients without troponin T measurement, thus, leading to a remarkable risk of selection bias. Second, the effects of troponin levels on outcomes were not analyzed. Third, the subjects were from a single health center and most of them were male.
Conclusion
The incidence of PMI in the very old surgical patients was relatively high. The PMI is associated with an increased risk of 30-day and 1-year mortality. The treatment strategy is firstly focused on the risk factors and guideline-directed medical therapy. Cardiovascular medical treatments, such as antiplatelets, β-blockers, statins, and angiotensin-converting enzyme inhibitors, have shown improved outcomes in patients with PMI. The surgical risk of bleeding should be considered before using antiplatelets and anticoagulation.
Funding
This work was supported by the Military Healthcare Fund 19BJZ35.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Chinese PLA General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LG, LC, and BW: conceptualization and methodology. LG, JH, and LC: investigation and writing—original draft. LG, LC, and BW: writing—review and English editing. LG, RW, and RC: supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Writing Committee for the VSI Devereaux PJ Biccard BM Sigamani A Xavier D Chan MTV . Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. (2017) 317:1642–51. 10.1001/jama.2017.4360
2.
Lee TH Marcantonio ER Mangione CM Thomas EJ Polanczyk CA Cook EF et al . Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. (1999) 100:1043–9. 10.1161/01.CIR.100.10.1043
3.
Botto F Alonso-Coello P Chan MT Villar JC Xavier D Srinathan S et al . Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. (2014) 120:564–78. 10.1097/ALN.0000000000000113
4.
Archan S Fleisher LA . From creatine kinase-MB to troponin: the adoption of a new standard. Anesthesiology. (2010) 112:1005–12. 10.1097/ALN.0b013e3181d31fa8
5.
Puelacher C Lurati Buse G Seeberger D Sazgary L Marbot S Lampart A et al . Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. (2018) 137:1221–32. 10.1161/CIRCULATIONAHA.117.030114
6.
Thygesen K Alpert JS Jaffe AS Chaitman BR Bax JJ Morrow DA et al . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. 10.1016/j.jacc.2018.08.1038
7.
Smilowitz NR Redel-Traub G Hausvater A Armanious A Nicholson J Puelacher C et al . Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. (2019) 27:267–73. 10.1097/CRD.0000000000000254
8.
Canet J Gallart L Gomar C Paluzie G Valles J Castillo J et al . Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. (2010) 113:1338–50. 10.1097/ALN.0b013e3181fc6e0a
9.
Wijnberge M Geerts BF Hol L Lemmers N Mulder MP Berge P et al . Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. JAMA. (2020) 323:1052–60. 10.1001/jama.2020.0592
10.
Pishko AM Lefler DS Gimotty P Paydary K Fardin S Arepally GM et al . The risk of major bleeding in patients with suspected heparin-induced thrombocytopenia. J Thromb Haemost. (2019) 17:1956–65. 10.1111/jth.14587
11.
Puelacher C Lurati-Buse G Singeisen H Dang M Cuculi F Muller C . Perioperative myocardial infarction/injury after noncardiac surgery. Swiss Med Wkly. (2015) 145:w14219. 10.4414/smw.2015.14219
12.
Devereaux PJ Xavier D Pogue J Guyatt G Sigamani A Garutti I et al . Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. (2011) 154:523–8. 10.7326/0003-4819-154-8-201104190-00003
13.
Beattie WS Karkouti K Tait G Steel A Yip P McCluskey S et al . Use of clinically based troponin underestimates the cardiac injury in non-cardiac surgery: a single-centre cohort study in 51,701 consecutive patients. Can J Anaesth. (2012) 59:1013–22. 10.1007/s12630-012-9782-9
14.
van Waes JA Nathoe HM de Graaff JC Kemperman H de Borst GJ Peelen LM et al . Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. (2013) 127:2264–71. 10.1161/CIRCULATIONAHA.113.002128
15.
Ashton CM Petersen NJ Wray NP Kiefe CI Dunn JK Wu L et al . The incidence of perioperative myocardial infarction in men undergoing noncardiac surgery. Ann Intern Med. (1993) 118:504–10. 10.7326/0003-4819-118-7-199304010-00004
16.
Badner NH Knill RL Brown JE Novick TV Gelb AW . Myocardial infarction after noncardiac surgery. Anesthesiology. (1998) 88:572–8. 10.1097/00000542-199803000-00005
17.
Mangano DT Browner WS Hollenberg M London MJ Tubau JF Tateo IM . Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The study of perioperative ischemia research group. N Engl J Med. (1990) 323:1781–8. 10.1056/NEJM199012273232601
18.
Grigoryan KV Javedan H Rudolph JL . Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. (2014) 28:e49–55. 10.1097/BOT.0b013e3182a5a045
19.
Van Heghe A Mordant G Dupont J Dejaeger M Laurent MR Gielen E . Effects of orthogeriatric care models on outcomes of hip fracture patients: a systematic review and meta-analysis. Calcif Tissue Int. (2021) 110:162–84. 10.1007/s00223-021-00913-5
20.
Helwani MA Amin A Lavigne P Rao S Oesterreich S Samaha E et al . Etiology of acute coronary syndrome after noncardiac surgery. Anesthesiology. (2018) 128:1084–91. 10.1097/ALN.0000000000002107
21.
Sheth T Natarajan MK Hsieh V Valettas N Rokoss M Mehta S et al . Incidence of thrombosis in perioperative and non-operative myocardial infarction. Br J Anaesth. (2018) 120:725–33. 10.1016/j.bja.2017.11.063
22.
Sheth T Chan M Butler C Chow B Tandon V Nagele P et al . Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: prospective cohort study. BMJ. (2015) 350:h1907. 10.1136/bmj.h1907
23.
Devereaux PJ Szczeklik W . Myocardial injury after non-cardiac surgery: diagnosis and management. Eur Heart J. (2020) 41:3083–91. 10.1093/eurheartj/ehz301
24.
Godier A Dincq AS Martin AC Radu A Leblanc I Antona M et al . Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J. (2017) 38:2431–9. 10.1093/eurheartj/ehx403
25.
Levine GN Bates ER Bittl JA Brindis RG Fihn SD Fleisher LA et al . 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. (2016) 134:e123–55. 10.1161/CIR.0000000000000404
26.
Fleisher LA Fleischmann KE Auerbach AD Barnason SA Beckman JA Bozkurt B et al . 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 130:2215–45. 10.1161/CIR.0000000000000105
27.
Feringa HH Bax JJ Boersma E Kertai MD Meij SH Galal W et al . High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. (2006) 114:I344–9. 10.1161/CIRCULATIONAHA.105.000463
28.
Group PS Devereaux PJ Yang H Yusuf S Guyatt G Leslie K et al . Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. (2008) 371:1839–47. 10.1016/S0140-6736(08)60601-7
29.
Jorgensen ME Sanders RD Kober L Mehta K Torp-Pedersen C Hlatky MA et al . Beta-blocker subtype and risks of perioperative adverse events following non-cardiac surgery: a nationwide cohort study. Eur Heart J. (2017) 38:2421–8. 10.1093/eurheartj/ehx214
30.
Kristensen SD Knuuti J Saraste A Anker S Botker HE Hert SD et al . 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. (2014) 35:2383–431. 10.1093/eurheartj/ehu282
31.
Task Force M Montalescot G Sechtem U Achenbach S Andreotti F Arden C et al . 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. (2013) 34:2949–3003. 10.1093/eurheartj/eht296
32.
Winchester DE Wen X Xie L Bavry AA . Evidence of pre-procedural statin therapy a meta-analysis of randomized trials. J Am Coll Cardiol. (2010) 56:1099−109. 10.1016/j.jacc.2010.04.023
33.
Foucrier A Rodseth R Aissaoui M Ibanes C Goarin JP Landais P et al . The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. (2014) 119:1053–63. 10.1213/ANE.0000000000000302
34.
Park J Kim J Lee SH Lee JH Min JJ Kwon JH et al . Postoperative statin treatment may be associated with improved mortality in patients with myocardial injury after noncardiac surgery. Sci Rep. (2020) 10:11616. 10.1038/s41598-020-68511-3
35.
Duceppe E Parlow J MacDonald P Lyons K McMullen M Srinathan S et al . Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. (2017) 33:17–32. 10.1016/j.cjca.2016.09.008
Summary
Keywords
postoperative myocardial injury, non-cardiac surgery, comanagement care model, management strategy, prognosis
Citation
Gao L, Chen L, Wang B, He J, Liu C, Wang R and Cheng R (2022) Management of Postoperative Myocardial Injury After Non-cardiac Surgery in Patients Aged ≥ 80 Years: Our 10 Years' Experience. Front. Cardiovasc. Med. 9:869243. doi: 10.3389/fcvm.2022.869243
Received
08 February 2022
Accepted
16 March 2022
Published
13 April 2022
Volume
9 - 2022
Edited by
Paolo Emilio Puddu, Université de Caen Normandie, France
Reviewed by
Mauro Chiarito, Humanitas University, Italy; Vladimir Uspenskiy, Almazov National Medical Research Centre, Russia
Updates
Copyright
© 2022 Gao, Chen, Wang, He, Liu, Wang and Cheng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Wang wangrong6969@126.comRui Cheng chengrui20212021@163.com
†These authors have contributed equally to this work
This article was submitted to Cardiovascular Epidemiology and Prevention, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.