Abstract
Background and aims:
Early detection of mortality after percutaneous coronary intervention (PCI) is crucial, whereas most risk prediction models are based on outdated cohorts before the year 2000. This study aimed to establish a nomogram predicting 30-day mortality after PCI.
Materials and methods:
In total, 10,444 patients undergoing PCI in National Center for Cardiovascular Diseases in China were enrolled to establish a nomogram to predict 30-day mortality after PCI. The nomogram was generated by incorporating parameters selected by logistic regression with the stepwise backward method.
Results:
Five features were selected to build the nomogram, including age, male sex, cardiac dysfunction, STEMI, and TIMI 0–2 after PCI. The performance of the nomogram was evaluated, and the area under the curves (AUC) was 0.881 (95% CI: 0.8–0.961). Our nomogram exhibited better performance than a previous risk model (AUC = 0.7, 95% CI: 0.586–0.813) established by Brener et al. The survival curve successfully stratified the patients above and below the median score of 4.
Conclusion:
A novel nomogram for predicting 30-day mortality was established in unselected patients undergoing PCI, which may help risk stratification in clinical practice.
Introduction
Annually, percutaneous coronary intervention (PCI) has been widely used in millions of patients with ischemic heart diseases to save lives and improve the quality of life around the world (1), and the number of patients undergoing PCI is still increasing. However, mortality still exists after PCI, especially in high-risk patients (2). Therefore, it is valuable to develop a risk-prediction model which can help doctors identify patients who are at high-risk and select suitable treatment strategies.
Approximately two-thirds of death after PCI occurs within 30 days after the procedure (3). Therefore, evaluating 30-day mortality after PCI may help identify events related to PCI (4–6). In previous studies, several prediction models have been established to predict the mortality of patients undergoing PCI. However, most of them are based on outdated cohorts in the 1990s (5, 7, 8). In the past two decades, with the progression in intervention strategy, stent technology, and pharmacological therapy, the outcomes of patients undergoing PCI have improved considerably. Therefore, an updated risk prediction model is also needed. Recently, Brener et al. established a novel risk score predicting 30-day mortality after PCI (called Brener’s score hereafter) (9). Brener’s score was derived from 21 randomized PCI trials and showed a good concordance index value of 0.848 for predicting 30-day mortality after PCI. The Brener’s score filled the blank of the risk score predicting 30-day mortality after PCI in the last 15 years. However, Brener’s score lacks data on cardiac function and renal function, which were closely related to mortality after PCI.
Nomogram is a medical tool calculating the risk of a particular clinical outcome for each individual in a graphic manner. It has been applied in risk prediction for cancer, stroke, and surgery (10–12). However, there is no nomogram for predicting the risk of 30-day mortality after PCI. Therefore, this study aimed to establish a novel nomogram for predicting 30-day mortality from a large-scale PCI cohort.
Materials and methods
Study population
Our original cohort included 10,724 consecutive patients undergoing PCI at the National Center for Cardiovascular Diseases in China (Fuwai Hospital, Beijing, China) between January 2013 and December 2013. The clinical data were collected prospectively. Patients with age <18 and without left ventricular ejection fraction (LVEF) data were excluded. Finally, we enrolled 10,444 patients. All patients had written informed consent. The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the hospital.
Endpoint and definitions
The primary endpoint was all-cause mortality within 30 days after PCI. Left ventricular function was evaluated by echocardiography before the PCI procedure. Types of stents were recorded in the PCI procedure. Cardiac dysfunction was defined as LVEF <50% (13), and renal insufficiency was defined as the estimated Glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 (14). Patients were followed up at a regular interval.
Statistical analysis
Continuous variables were compared by the Student’s t-test, and categorical variables were compared by the chi-square test. Variables in Brener’s model and traditional risk factors were candidates for univariable logistic analysis. Variables with a p-value < 0.1 in the univariable logistic analysis were selected for subsequent stepwise backward multivariable logistic analysis, and variables with a p-value < 0.05 were retained in the multivariable logistic regression. Then, the nomogram was established based on the results of the multivariable analysis, and the coefficient of each variable was converted into a 0–100-point scale. The area under the curves (AUC) was internally validated by bootstrapping with 1,000 resamples. Calibration plots were generated to evaluate the consistency between nomogram-predicting probability and actual probability. Also, the calibration plots were generated through bootstrapping with 1,000-fold resamples. The AUC was calculated to evaluate the performance of the predictive model. The AUC values between 0.7 and 0.8 are considered moderate, and the AUC values higher than 0.8 are considered good. The survival probability was observed by the Kaplan–Meier analysis. The p-value < 0.05 was considered to be statistically significant. The data were analyzed with SPSS version 24.0 (Chicago, IL, United States) and R statistical software version 4.0.31.
Results
Baseline characteristics
The baseline characteristics of the patients are shown in Table 1. Among 10,444 patients in our study, the patients who died were significantly older than the living patients and were more likely to be women and smokers (p < 0.001). Patients with renal insufficiency, cardiac dysfunction, STEMI, prior MI, TIMI flow 0–1 before PCI, TIMI flow 0–2 after PCI, unstable angina, and second-generation DES implantation had a higher probability of death (all p < 0.05). Other characteristics had no significant difference.
TABLE 1
| Alive (n = 10419) | Death (n = 25) | P-value | |
| Age | 58.3 (10.3) | 69.5 (10.9) | < 0.001 |
| Men | 8044 (77.2) | 9 (36.0) | < 0.001 |
| Cardiac dysfunction | 693 (6.7) | 8 (32.0) | < 0.001 |
| Renal insufficiency | 284 (2.7) | 3 (12.0) | 0.026 |
| Diabetes mellitus | 3165 (30.4) | 4 (16.0) | 0.179 |
| Smoking | 5950 (57.1) | 8 (32.0) | 0.02 |
| Hypertension | 6701 (64.3) | 16 (64.0) | 1 |
| Hyperlipidemia | 7033 (67.5) | 13 (52.0) | 0.15 |
| Prior PCI | 2568 (24.6) | 7 (28.0) | 0.876 |
| Prior CABG | 1110 (10.7) | 5 (20.0) | 0.235 |
| Prior Cerebrovascular disease | 428 (4.1) | 1 (4.0) | 1 |
| Prior MI | 3765 (36.1) | 17 (68.0) | 0.002 |
| ACS | 6498 (62.4) | 17 (68.0) | 0.708 |
| Unstable angina | 4496 (43.2) | 2 (8.0) | 0.001 |
| NSTEMI | 469 (4.5) | 2 (8.0) | 0.719 |
| STEMI | 1533 (14.7) | 13 (52.0) | < 0.001 |
| Stable coronary artery disease | 3921 (37.6) | 8 (32.0) | 0.708 |
| Left main artery disease | 650 (6.2) | 3 (12.0) | 0.438 |
| LM-LAD | 7546 (72.4) | 16 (64.0) | 0.473 |
| Multivessel disease | 7840 (75.2) | 20 (80.0) | 0.75 |
| TIMI flow 0–1 before PCI | 2240 (21.5) | 10 (40.0) | 0.045 |
| TIMI flow 0–2 after PCI | 366 (3.5) | 6 (24.0) | < 0.001 |
| First-generation DES | 995 (9.5) | 2 (8.0) | 2 |
| Second-generation DES | 8945 (85.9) | 17 (68.0) | 0.023 |
| Lesion length | 29.0 (19.3) | 31.6 (16.9) | 0.497 |
Baseline characteristics.
Variables are shown as mean (standard deviation, SD) or n (%). CABG, coronary artery bypass grafting; ACS, acute coronary syndrome; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; LM-LAD, left main coronary artery disease and left anterior descending artery disease; DES, drug-eluting stent; TIMI, thrombolysis in myocardial infarction. Cardiac dysfunction was defined as LVEF <50%. Renal insufficiency was defined as estimated Glomerular filtration rate (eGFR) <30 ml/min/1.73 m2.
Predictors of 30-day mortality after percutaneous coronary intervention
The results of the univariable logistic analysis are shown in Table 2. Variables with a p-value < 0.1 in the univariable logistic regression analysis, including age, male sex, prior MI, STEMI, smoking, TIMI flow 0–1 before PCI, TIMI flow 0–2 after PCI, second generation drug-eluting stent (DES), renal insufficiency, and cardiac dysfunction were selected for subsequent stepwise backward multivariable logistic analysis; variables with p < 0.05 were retained in the multivariable logistic regression (Table 2). Therefore, age, male sex, STEMI, TIMI flow 0–2 after PCI, and cardiac dysfunction were selected to formulate a nomogram (Figure 1 and Table 3). The average AUC generated by bootstrap resampling was 0.884. There was a good agreement between the predicted and the observed 30-day mortality risk (Figures 2, 3). The Hosmer–Lemeshow test also demonstrated that the model was a good fit (X2 = 10.605, p = 0.225). The distribution of the risk scores of the population were shown in Figure 4.
TABLE 2
| Univariable | Multivariable | ||||
|
|
|
||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | Coefficient | |
| Age | 1.12 (1.08–1.18) | <0.001 | 1.1 (1.05–1.15) | <0.001 | 0.0924 |
| Men | 0.17 (0.07–0.38) | <0.001 | 0.22 (0.09–0.52) | 0.001 | −1.5214 |
| BMI | 0.97 (0.85–1.09) | 0.58 | |||
| Diabetes mellitus | 0.44 (0.15–1.27) | 0.13 | |||
| Hypertension | 0.99 (0.44–2.23) | 0.97 | |||
| Hyperlipidemia | 0.52 (0.24–1.14) | 0.1 | |||
| Prior PCI | 1.19 (0.5–2.85) | 0.7 | |||
| Prior CABG | 2.1 (0.79–5.6) | 0.14 | |||
| Prior Cerebrovascular disease | 0.97 (0.13–7.21) | 0.98 | |||
| Prior MI | 3.76 (1.62–8.71) | <0.001 | |||
| STEMI | 6.28 (2.86–13.79) | <0.001 | 5.72 (2.44–13.38) | <0.001 | 1.7431 |
| NSTEMI | 1.84 (0.43–7.85) | 0.41 | |||
| ACS | 1.28 (0.55–2.97) | 0.56 | |||
| Smoking | 0.35 (0.15–0.82) | 0.02 | |||
| LM-LAD | 0.68 (0.3–1.53) | 0.35 | |||
| Multivessel disease | 1.32 (0.49–3.51) | 0.58 | |||
| TIMI flow 0–1 before PCI | 2.43 (1.09–5.43) | 0.03 | |||
| TIMI flow 0–2 after PCI | 8.67 (3.44–21.85) | <0.001 | 8.45 (3.22–22.17) | <0.001 | 2.1344 |
| First-generation DES | 0.82 (0.19–3.5) | 0.79 | |||
| Second-generation DES | 0.35 (0.15–0.81) | 0.01 | |||
| Renal insufficiency | 4.87 (1.45–16.35) | 0.01 | |||
| Lesion Length | 1.01 (0.99–1.02) | 0.49 | |||
| Cardiac dysfunction | 6.6 (2.84–15.36) | <0.001 | 3.02 (1.2–7.62) | 0.019 | 1.1066 |
Logistic regression model for 30-day mortality.
Variables are shown as mean (standard deviation, SD) or n (%). PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; ACS, acute coronary syndrome; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; LM-LAD, left main artery disease and left anterior descending artery disease; DES, drug-eluting stent; TIMI, thrombolysis in myocardial infarction. Cardiac dysfunction was defined as LVEF <50%. Renal insufficiency was defined as estimated Glomerular filtration rate (eGFR) <30 ml/min/1.73 m2.
FIGURE 1
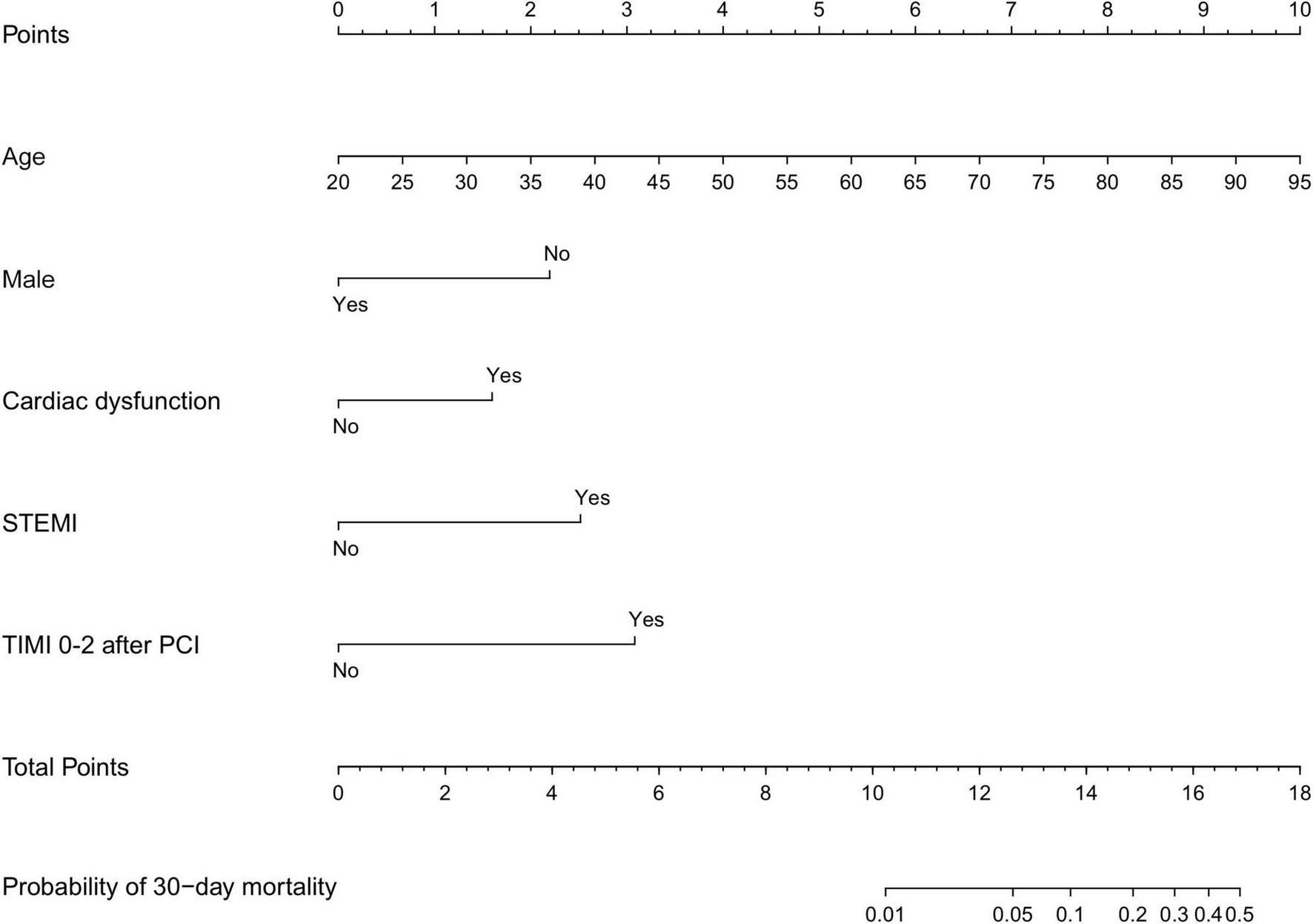
Nomogram scores of 30-day mortality. Nomogram for predicting the probability of 30-day mortality after PCI. Points were assigned for age, male sex, cardiac dysfunction, STEMI, and TIMI 0–2 after PCI by drawing a line upward from the corresponding values to the “points line”. The “total points” are calculated as the sum of the individual score of each of the five variables included in the nomogram. STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; PCI, percutaneous coronary intervention. Cardiac dysfunction was defined as LVEF <50%.
TABLE 3
| Age (years) | Score | Sex | Score | ||
| 20 | 0 | Male | 0 | ||
| 25 | 1 | Female | 2 | ||
|
|
|
||||
| 30 | 1 | Cardiac dysfunction | Score | ||
|
|
|
||||
| 35 | 2 | No | 0 | ||
| 40 | 3 | Yes | 2 | ||
|
|
|
||||
| 45 | 3 | STEMI | Score | ||
|
|
|
||||
| 50 | 4 | No | 0 | ||
| 55 | 5 | Yes | 3 | ||
|
|
|
||||
| 60 | 5 | TIMI 0–2 after PCI | Score | ||
|
|
|
||||
| 65 | 6 | No | 0 | ||
| 70 | 7 | Yes | 3 | ||
| 75 | 7 | ||||
| 80 | 8 | ||||
| 85 | 9 | ||||
| 90 | 9 | ||||
| 95 | 10 | ||||
Risk-prediction nomogram.
STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; PCI, percutaneous coronary intervention. Cardiac dysfunction was defined as LVEF <50%.
FIGURE 2
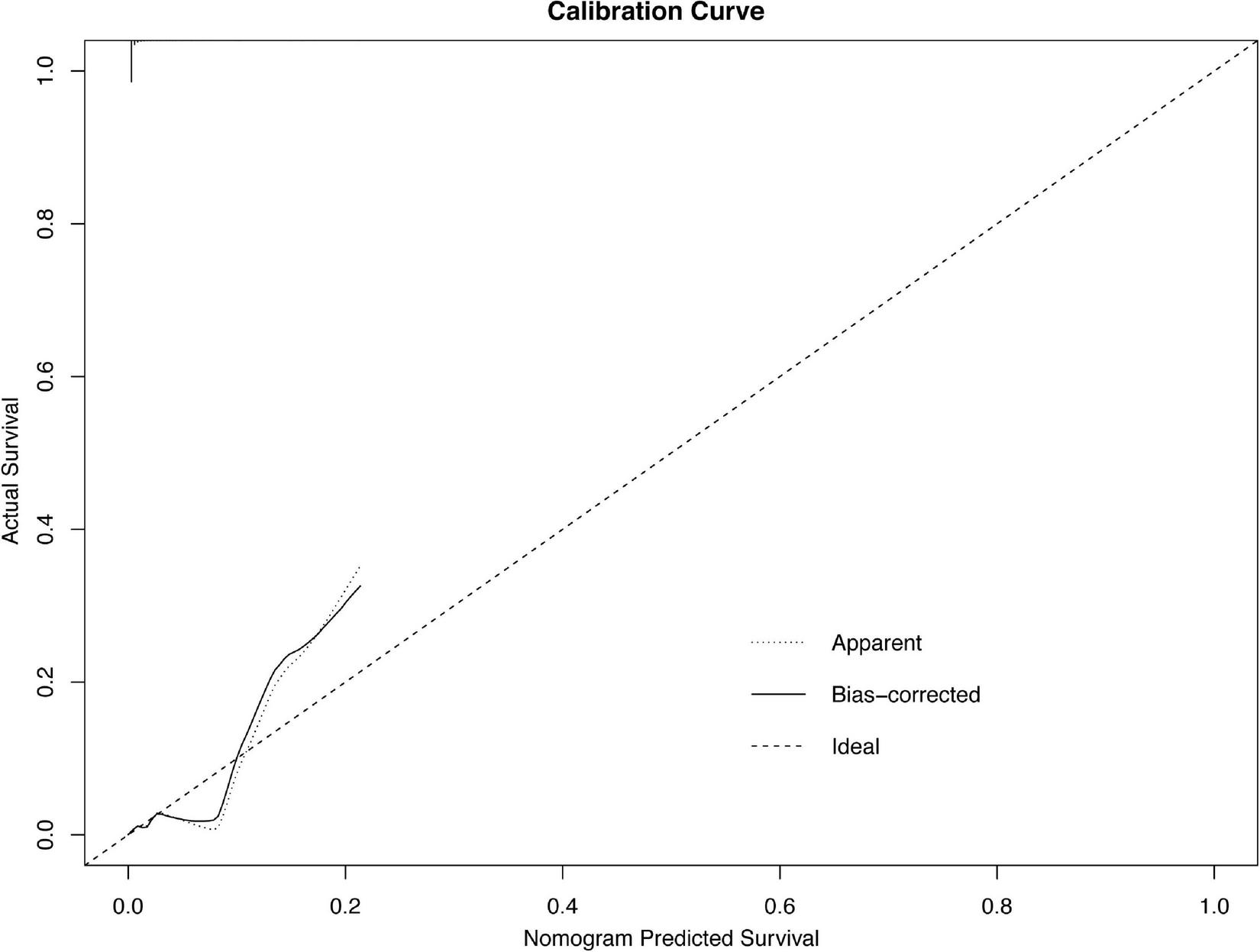
Calibration plots of the nomogram.
FIGURE 3
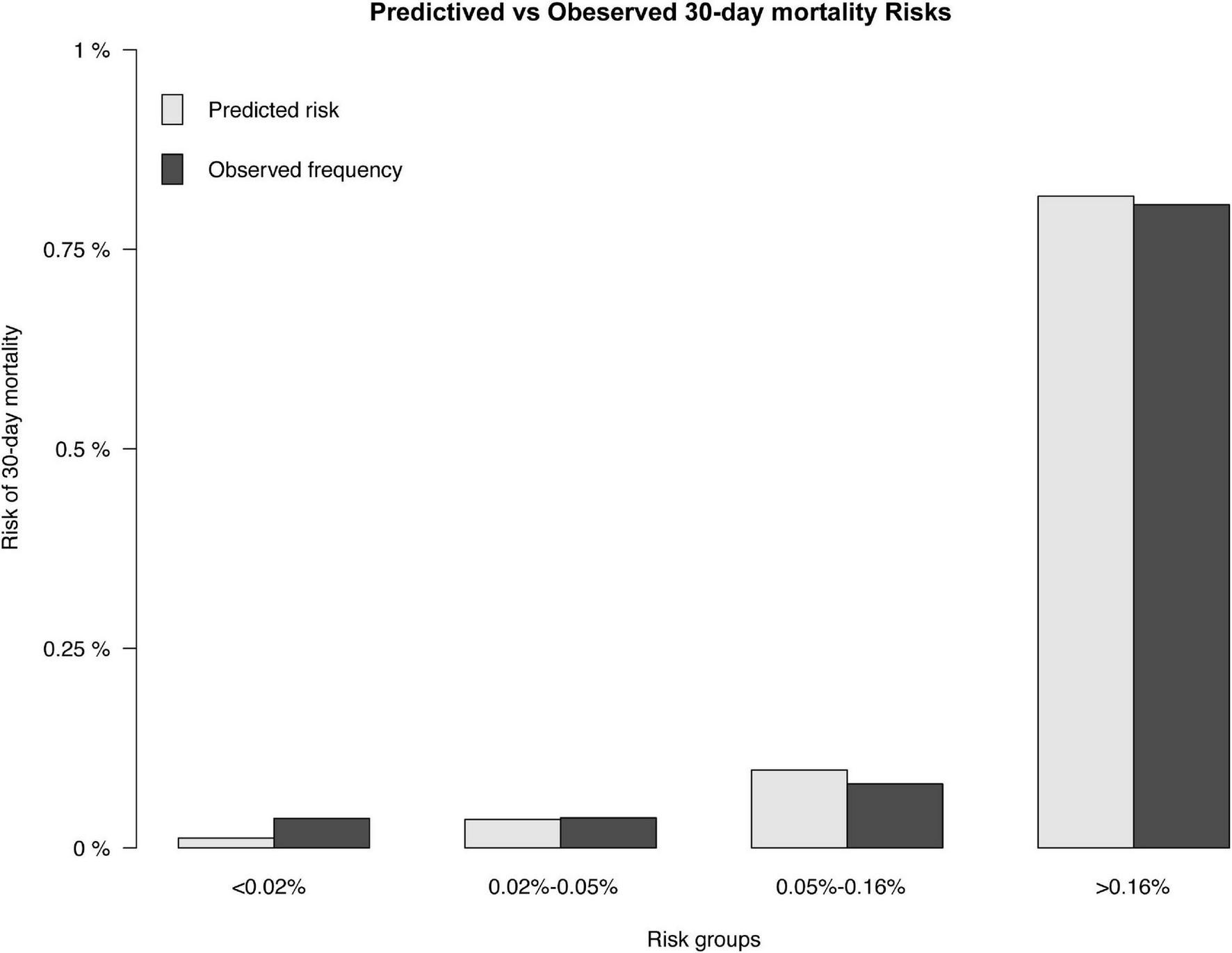
Predicted and observed 30-day mortality risk. The predicted (gray) and observed (black) risk of 30-day mortality for patients following PCI.
FIGURE 4
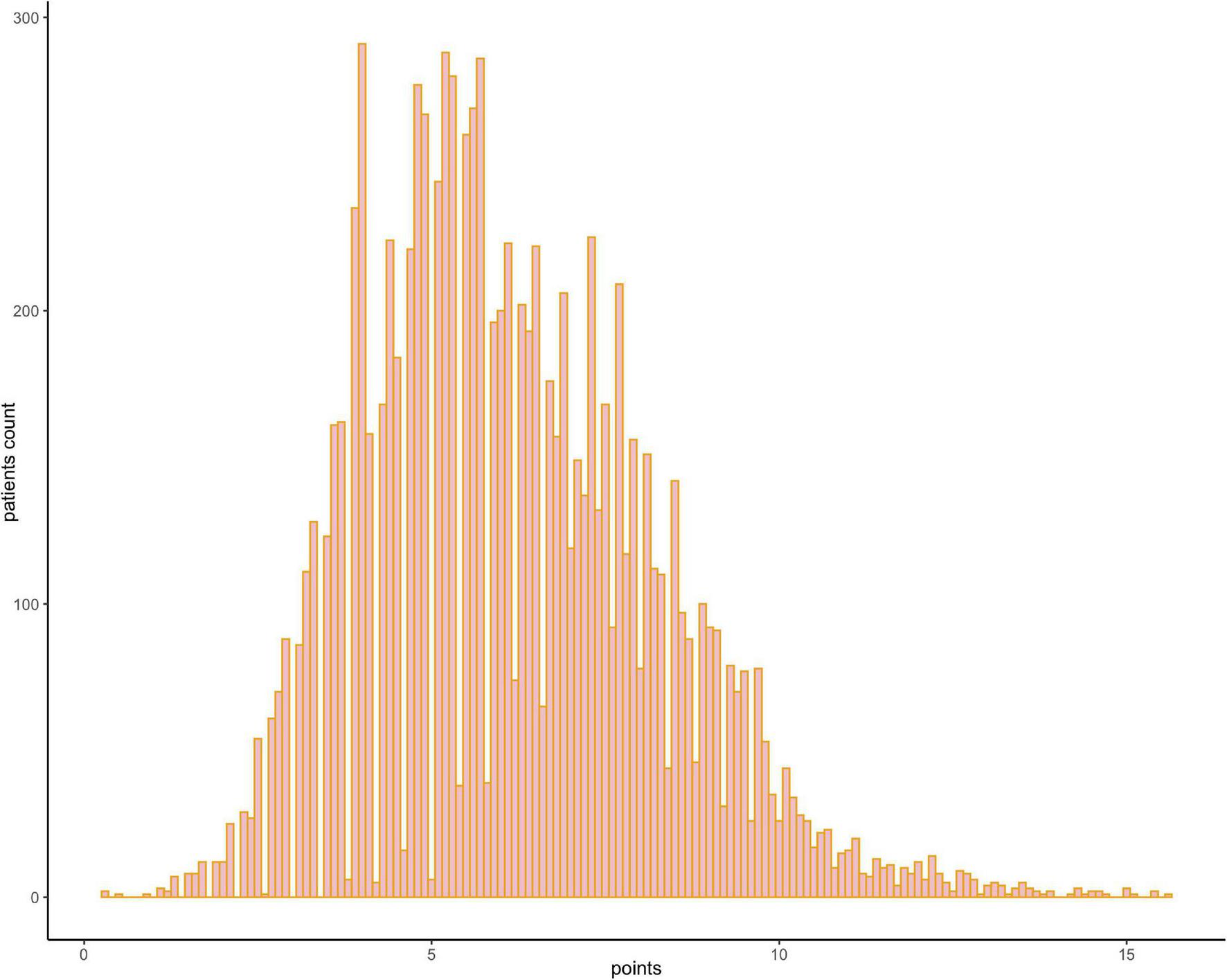
Frequency distribution bar graph of the cohort. The distribution of the risk scores of the population.
The nomogram had a good performance in predicting 30-day mortality after PCI, with an AUC of 0.881 (95% CI: 0.8–0.961), and the nomogram showed a better predictive performance than Brener’s model with an AUC of 0.7 (95% CI: 0.586–0.813) (Figure 5).
FIGURE 5
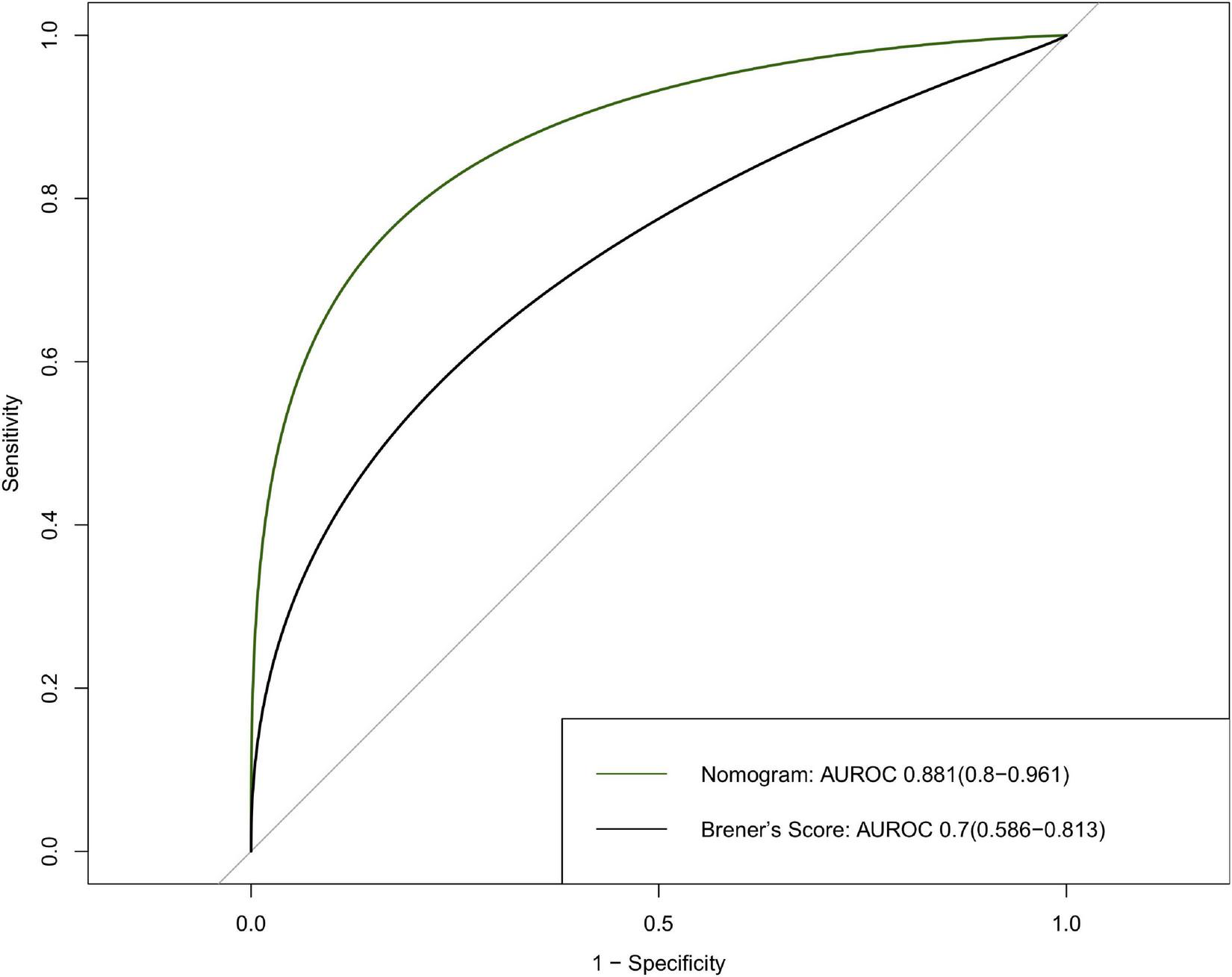
Discrimination of the 30-day mortality of derivation cohort. ROC curves of the nomogram and Brener’s risk model predicting 30-day mortality with corresponding AUC values. ROC, receiver operating characteristic; AUC, area under curve.
The discrimination of the nomogram was also assessed by Kaplan–Meier analysis (Figure 6). The patients were stratified by the median of the risk score (score = 4) of our cohort. A total of 5,188 (49.67%) patients were stratified into the low-risk group and 5,256 (50.33%) patients were stratified into the high-risk group. The cumulative probability of the occurrence of 30-day mortality was significantly higher in patients with scores higher than 4 (p < 0.0001). The decision curve analysis (DCA) demonstrated that the nomogram predicting 30-day mortality confers a net benefit over the “full treatment” and “no treatment” strategies in our cohort (Figure 7).
FIGURE 6
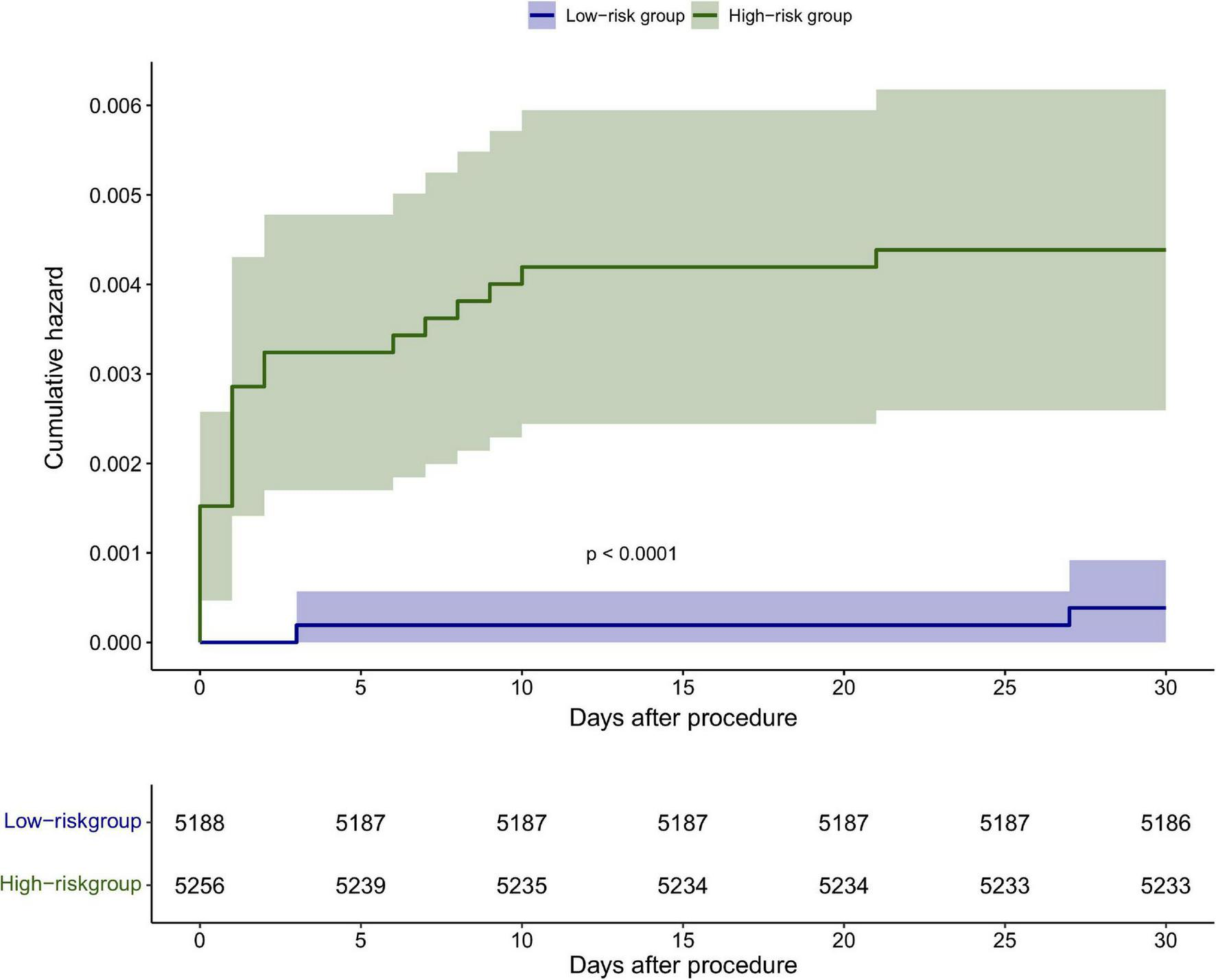
Kaplan–Meier analysis of the 30-day mortality based on the median score <4 and ≥4 in the cohort.
FIGURE 7
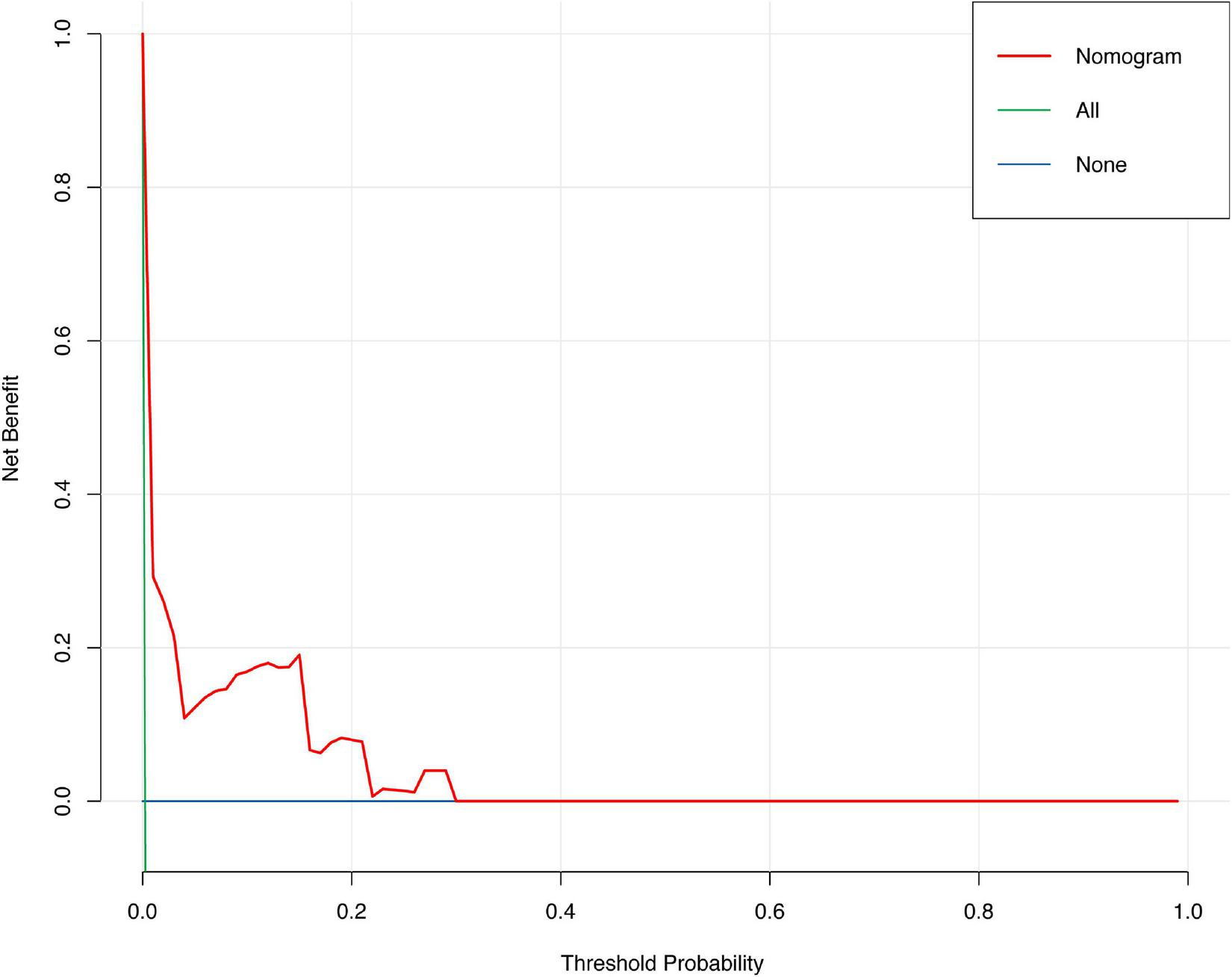
Decision carve analysis of the nomogram predicting 30-day mortality after PCI.
Discussion
In the present study, we established a nomogram for predicting 30-day mortality after PCI in a large-scale, real-world PCI cohort. The nomogram shows excellent discrimination and calibration, which may help clinicians in risk prediction.
To our knowledge, this study firstly used a nomogram to predict the 30-day mortality of patients undergoing PCI. Nomogram is a graphic and easy-to-use calculation instrument which could make an individualized prediction of 30-day mortality after PCI. This nomogram incorporates clinical variables and angiographic variables: age, sex, cardiac dysfunction, STEMI, and TIMI flow after PCI. Previous scoring systems rarely incorporated angiographic variables (6, 15, 16).
In Brener’s risk model, cardiac function and renal function were not included due to the lack of data (9). However, in previous studies, cardiac function and renal insufficiency are independent risk factors of 30-day mortality after PCI (3, 17–19), and both are included in several risk scores, such as the CADILLAC score (17), the New York State Risk Score (20), and Mayo Clinic Risk Score (5). To improve the predictive value of the risk score, left ventricular ejection fraction and renal function are added to our analysis. Finally, cardiac dysfunction was selected to incorporate the nomogram. Compared to Brener’s score, this nomogram exhibited an enhanced 30-day mortality predictive value in Asian patients (0.7 vs. 0.881). Among the total patients undergoing PCI with a score of <4, 0.03% died within 30 days; while for those with scores of ≥4, 0.36% died within 30 days after PCI. The results showed that the risk of mortality increased as the sum of scores increased.
Previous studies established several risk models for predicting 30-day mortality after PCI, including the New York State risk score (20), the British Columbia PCI risk score (6), the ALPHA score (21), and the Mayo Clinic risk score (5); all of these scores focused on the European or American population. Our study is the first risk model aimed to predict the 30-day mortality of Asian patients undergoing PCI.
In conclusion, we established a graphic nomogram to predict 30-day mortality in patients who underwent PCI based on a large-scale real-world cohort study, and our nomogram exhibited better performance than Brener’s score. This nomogram would be a good tool to help doctors identify patients with high risk and make better clinical decisions.
Statements
Data availability statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the Institutional Review Board of the Fuwai Hospital, Beijing, China. Written informed consent was obtained from all participants for their participation in this study.
Author contributions
JS, YL, and WW participated in the research design. JS, YL, JC, JY, JW, and JG performed the data analysis and interpretation. JS and YL drafted the manuscript. Y-DT edited the manuscript and supervised the studies. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2020YFC2004705), National Natural Science Foundation of China (81825003, 91957123, 81800327, and 81900272), Beijing Nova Program (Z201100006820002) from Beijing Municipal Science & Technology Commission, Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases from Chinese Academy of Medical Sciences (2021RU003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- PCI
percutaneous coronary intervention
- DES
drug-eluting stents
- LAD
left anterior descending coronary artery
- LM
left main coronary artery
- TIMI
Thrombolysis in Myocardial Infarction
- STEMI
ST-segment elevation myocardial infarction.
Footnotes
References
1.
Benjamin EJ Virani SS Callaway CW Chamberlain AM Chang AR Cheng S et al Heart disease and stroke statistics—2018 update: a report from the American heart association. Circulation. (2018) 137:558. 10.1161/CIR.0000000000000558
2.
Castro-Dominguez YS Wang Y Minges KE McNamara RL Spertus JA Dehmer GJ et al Predicting in-hospital mortality in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. (2021) 78:216–29. 10.1016/j.jacc.2021.04.067
3.
Serruys PW Morice M-C Kappetein AP Colombo A Holmes DR Mack MJ et al Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. (2009) 360:961–72. 10.1056/NEJMoa0804626
4.
Holmes DR Berger PB Garratt KN Mathew V Bell MR Barsness GW et al Application of the New York State PTCA mortality model in patients undergoing stent implantation. Circulation. (2000) 102:517–22.
5.
Singh M Rihal CS Lennon RJ Spertus J Rumsfeld JS Holmes DR . Bedside estimation of risk from percutaneous coronary intervention: the new mayo clinic risk scores.Mayo Clinic Proc. (2007) 82:701–8. 10.4065/82.6.701
6.
Hamburger JN Walsh SJ Khurana R Ding L Gao M Humphries KH et al Percutaneous coronary intervention and 30-day mortality: the British Columbia PCI risk score. Cathet Cardiovasc Intervent. (2009) 74:377–85. 10.1002/ccd.22151
7.
Wu C Hannan EL Walford G Ambrose JA Holmes DR King SB et al A risk score to predict in-hospital mortality for percutaneous coronary interventions. J Am Coll Cardiol. (2006) 47:654–60. 10.1016/j.jacc.2005.09.071
8.
Moscucci M Kline-Rogers E Share D O’Donnell M Maxwell-Eward A Meengs WL et al Simple bedside additive tool for prediction of in-hospital mortality after percutaneous coronary interventions. Circulation. (2001) 104:263–8.
9.
Brener SJ Leon MB Serruys PW Smits PC von Birgelen C Mehran R et al Derivation and external validation of a novel risk score for prediction of 30-day mortality after percutaneous coronary intervention. EuroIntervention. (2019) 15:e551–7. 10.4244/EIJ-D-19-00262
10.
Cappellari M Turcato G Forlivesi S Zivelonghi C Bovi P Bonetti B et al STARTING-SICH nomogram to predict symptomatic intracerebral hemorrhage after intravenous thrombolysis for stroke. Stroke. (2018) 49:397–404. 10.1161/STROKEAHA.117.018427
11.
Jehi L Yardi R Chagin K Tassi L Russo GL Worrell G et al Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. (2015) 14:283–90. 10.1016/S1474-4422(14)70325-4
12.
Kim Y Margonis GA Prescott JD Tran TB Postlewait LM Maithel SK et al Nomograms to predict recurrence-free and overall survival after curative resection of adrenocortical carcinoma. JAMA Surg. (2016) 151:365–73. 10.1001/jamasurg.2015.4516
13.
Zhang L Wang F Wang L Wang W Liu B Liu J et al Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. (2012) 379:815–22. 10.1016/S0140-6736(12)60033-6
14.
Lang RM Badano LP Mor-Avi V Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–71. 10.1093/ehjci/jev014
15.
Klein LW Block P Brindis RG McKay CR McCallister BD Wolk M et al Percutaneous coronary interventions in octogenarians in the American college of cardiology–national cardiovascular data registry. J Am Coll Cardiol. (2002) 40:394–402. 10.1016/S0735-1097(02)01992-7
16.
Brennan JM Curtis JP Dai D Fitzgerald S Khandelwal AK Spertus JA et al Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention. JACC. (2013) 6:790–9. 10.1016/j.jcin.2013.03.020
17.
Halkin A Singh M Nikolsky E Grines CL Tcheng JE Garcia E et al Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. (2005) 45:1397–405. 10.1016/j.jacc.2005.01.041
18.
Rab T Wilson H . Public reporting of mortality after PCI in cardiac arrest and cardiogenic shock.JACC. (2016) 9:496–8. 10.1016/j.jcin.2015.12.006
19.
Matsushita K Coresh J Sang Y Chalmers J Fox C Guallar E et al Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diab Endocrinol. (2015) 3:514–25. 10.1016/S2213-8587(15)00040-6
20.
Hannan EL Farrell LS Walford G Jacobs AK Berger PB Holmes DR et al The New York State risk score for predicting in-hospital/30-day mortality following percutaneous coronary intervention. JACC. (2013) 6:614–22. 10.1016/j.jcin.2013.02.015
21.
Hizoh I Gulyas Z Domokos D Banhegyi G Majoros Z Major L et al A novel risk model including vascular access site for predicting 30-day mortality after primary PCI: the ALPHA score. Cardiovasc Revasculariz Med. (2017) 18:33–9. 10.1016/j.carrev.2016.10.002
Summary
Keywords
nomogram, risk prediction, percutaneous coronary intervention, 30-day mortality, model establishment
Citation
Song J, Liu Y, Wang W, Chen J, Yang J, Wen J, Gao J, Shao C and Tang Y-D (2022) A nomogram predicting 30-day mortality in patients undergoing percutaneous coronary intervention. Front. Cardiovasc. Med. 9:897020. doi: 10.3389/fcvm.2022.897020
Received
15 March 2022
Accepted
25 July 2022
Published
17 August 2022
Volume
9 - 2022
Edited by
Stefania Triunfo, University of Milan, Italy
Reviewed by
Fabrizio D’Ascenzo, University of Turin, Italy; Lingyao Kong, The First Affiliated Hospital of Zhengzhou University, China
Updates
Copyright
© 2022 Song, Liu, Wang, Chen, Yang, Wen, Gao, Shao and Tang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Da Tang, tangyida@bjmu.edu.cn
†These authors have contributed equally to this work
This article was submitted to Cardiovascular Epidemiology and Prevention, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.