Abstract
Aims:
The efficacy and safety of sacubitril/valsartan for patients with heart failure with preserved ejection fraction (HFpEF) are controversial. Hence, the primary objective of the study was to evaluate the efficacy and safety of sacubitril/valsartan treatment for patients with HFpEF.
Methods and results:
We used the PubMed, Embase, and Web of Science databases to search for randomized controlled trials of sacubitril–valsartan in patients with HFpEF. Three studies, involving a total of 7,663 patients, were eligible for inclusion. Sacubitril–valsartan reduced the risk of hospitalization for heart failure (HF) [odds ratio (OR): 0.78; 95% CI: 0.70–0.88; p < 0.0001] and the incidence of worsening renal function [risk ratio (RR): 0.79, p = 0.002] among patients with HFpEF in the three trials, but there was no significant reduction in all-cause mortality (0.99, 95% CI: 0.84–1.15; p = 0.86) or cardiovascular mortality (0.95, 95% CI: 0.78–1.15; p = 0.16). Moreover, sacubitril/valsartan was associated with an increased risk of symptomatic hypotension (RR: 1.44; p < 0.00001) and angioedema (RR: 2.66; p < 0.04); there was no difference for decreasing the incidence of hyperkalemia (RR: 0.89; p = 0.11).
Conclusion:
Compared with valsartan or individualized medical therapy (IMT), sacubitril/valsartan significantly decreased the risk of hospitalization for HF and reduced the incidence of renal dysfunction.
Introduction
Heart failure (HF) is a clinical syndrome caused by structural or functional cardiac abnormalities and manifests as an increase in internal pressure or a decrease in cardiac output. Due to increases in the size of the aging population and the incidence of risk factors, the prevalence of heart failure has been on the rise as well (1). To date, almost half of the 5 million patients with heart failure in the United States display heart failure with preserved ejection fraction (HFpEF) (2). The pathophysiological mechanism of HFpEF includes left ventricular (LV) structure and remodeling, LV diastolic limitations, and LV systolic limitations. Currently, however, no effective treatment drug exists (3).
Sacubitril/valsartan is the first new angiotensin receptor neprilysin inhibitor for treating hypertension and heart failure (4); such drugs treat HFpEF by blocking a profibrotic/prohypertrophic mechanism and stimulating an antifibrotic/antihypertrophic mechanism. Compared with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), sacubitril/valsartan can increase the levels of many vasoactive peptides, especially natriuretic peptides (NPs), which have powerful effects on sodium, fluid balance, and vascular diastolic function by inhibiting the renin–angiotensin–aldosterone system (RAAS), reducing the sympathetic nervous system activity, and exerting antiproliferative and anti-muscle hypertrophy effects (5).
Kuno et al. (6) demonstrated that treatment with sacubitril/valsartan can reduce the risk of hospitalization for HF, but the results of the PARAGON-HF trial did not reach a similar conclusion (7). The use of sacubitril/valsartan for patients with HFpEF is still controversial. Thus, we conducted a systematic review to evaluate the efficacy and safety of sacubitril/valsartan treatment for patients with HFpEF.
Methods
Protocol registration
We registered the protocol for this systematic review with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020207370).
Data sources and search strategy
We searched for articles in the PubMed, Embase, and Web of Science databases through 13 December 2021 using the following search terms: “heart failure with preserved ejection fraction” or “heart failure with normal ejection fraction” or “diastolic heart failure” or “diastolic dysfunction” or “preserved cardiac function heart failure” or “HFpEF” and “sacubitril valsartan” or “sacubitril/valsartan” or “lcz696” or “sacubitril” or “entresto” In addition, we reviewed the corresponding reference lists of the retrieved articles to avoid missing any relevant studies. The meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (8).
Selection criteria
Eligible studies had to meet the following inclusion criteria: (1) the enrolled participants had HFpEF (LVEF ≥ 45%); (2) the study design was a randomized controlled trial (RCT) of the treatment group (sacubitril/valsartan) and the control group; and (3) the trial provided primary outcome data (cardiovascular mortality, all-cause mortality, hospitalization for HF, and main adverse events, such as symptomatic hypotension, worsening renal function, hyperkalemia, and angioedema).
The exclusion criteria were as follows: (1) duplicated trials; (2) studies, such as systematic reviews, comments, case reports, conference abstracts, and editorials; and (3) RCTs that did not involve humans. The details are shown in Table 1.
Table 1
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Patient population | HFpEF defined as LVEF ≥ 45% | Not HFpEF |
| Intervention/comparator | Sacubitril/valsartan and control group | Other drugs vs. control group |
| Outcome | “All-cause mortality”, “cardiovascular causes”, “hospitalization for HF”, “symptomatic hypotension”, “worsening renal function”, “hyperkalemia”, “angioedema” | No “all-cause mortality”, “cardiovascular causes”, “hospitalization for HF”, “symptomatic hypotension”, “worsening renal function”, “hyperkalemia”, and “angioedema” outcomes reported |
| Study design | RCT | Not-RCTs: systemic reviews, comments, case reports, conference abstracts, editorials, and not in human |
| Language | English | Non-English language publications |
Inclusion and exclusion criteria.
HFpEF, heart failure with preserved ejection fraction; HF, heart failure; LVEF, left ventricular ejection fraction; RCT, randomized controlled trial.
Data extraction and quality assessment
YW and ZH independently extracted data and assessed the quality of the studies from the electronic database. The relevant data we extracted included the following: the baseline characteristics of the trials, interventions, comparisons, sample size, the medication used, and follow-up duration. The outcomes included death from any cause, death from cardiovascular causes, hospitalization for HF, symptomatic hypotension, renal dysfunction, hyperkalemia, and angioedema. Disagreements were resolved by discussion with a third author (W. Q. H.).
Risk of bias assessment
The methodological quality of the three included RCTs was assessed by using the Cochrane Collaboration Risk of Bias Tool (Review Manager 5.4.1), which included the following sections: selection, performance, detection, attrition, reporting, and other biases. The results are shown in Supplementary Figure S1.
Statistical analysis
Review Manager Version 5.4.1 was used to analyze the data. The efficacy and safety outcomes were measured as dichotomous outcome variables and compared between the sacubitril–valsartan group and the control group. The pooled odds ratio (OR) or risk ratio (RR) and the corresponding 95% confidence interval (CI) were calculated in the comparative analyses. We assessed heterogeneity by using the I2 test and Cochran's χ2 test. The total variation in the studies was described by the I2 statistic, which reflected heterogeneity. When the heterogeneity test result was I2 < 50% and p > 0.10, we used a fixed-effects model for data analysis; I2 > 50% or a corresponding p-value < 0.10 indicated statistical heterogeneity among the studies that needed further analysis. All p-values were two-tailed, with statistical significance indicated at 0.05 and CIs reported at the 95% level. When I2 was >45%, a sensitivity analysis was further performed by sequentially deleting each study and reanalyzing the datasets of all remaining studies.
Results
Description of the study selection process and study characteristics
A detailed flowchart of the study selection is presented in Figure 1. Ultimately, three double-blind RCTs that involved a total of 7,663 patients were included in our study (Figure 1). The baseline characteristics of the included studies are shown in Table 2 and include follow-up duration, left ventricular ejection fraction (LVEF), primary efficacy outcomes, and key adverse events.
Figure 1
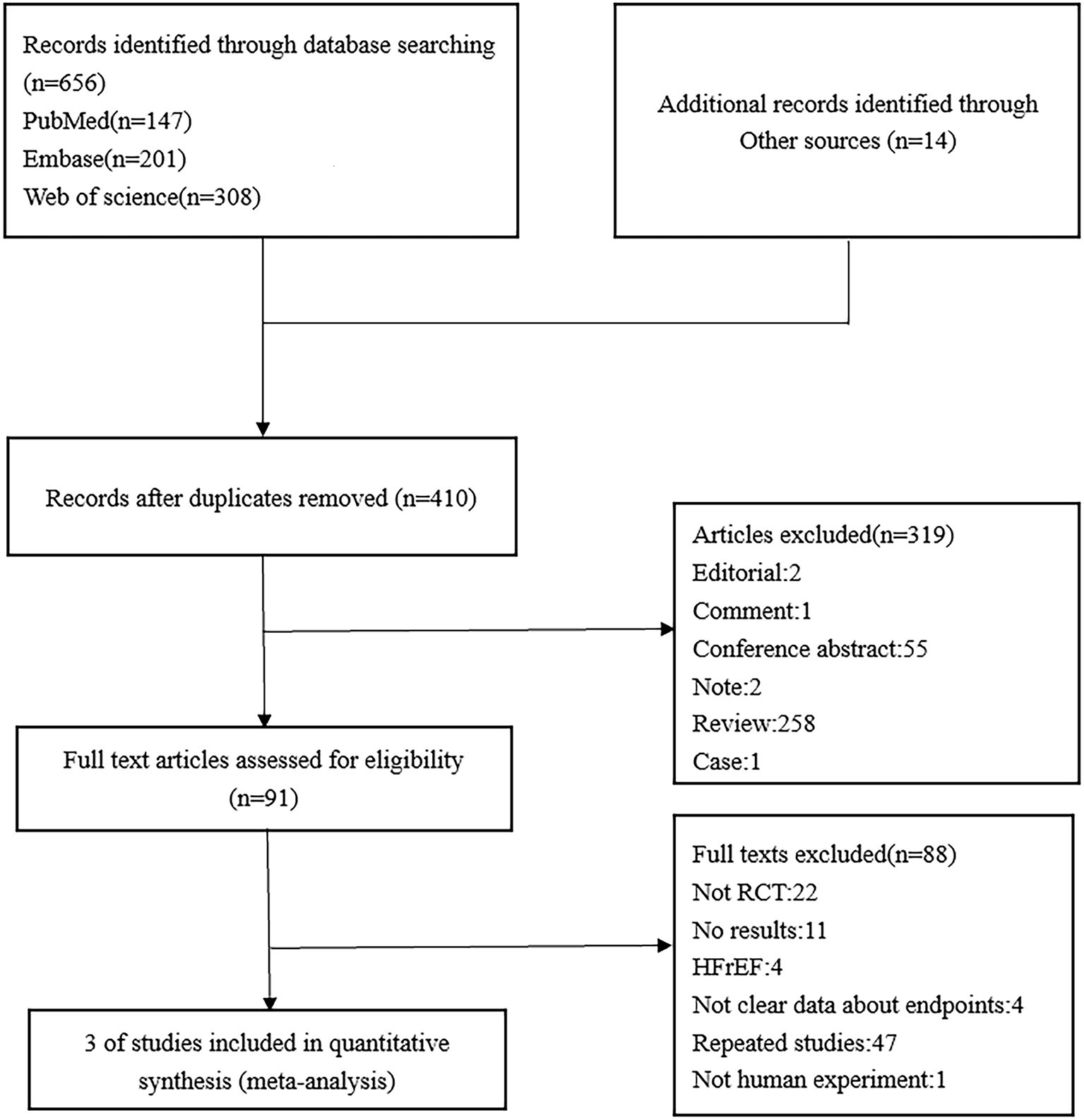
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram of the study selection process for the meta-analysis.
Table 2
| PARALLAX-HF Pieske 2021 | PARAGON-HF Solomon 2019 | PARAMOUNT Solomon 2012 | ||||
|---|---|---|---|---|---|---|
| Group | Sac/Val (n = 1,281) | IMT (n = 1,285) | Sac/Val (n = 2,407) | Valsartan (n = 2,389) | Sac/Val (n = 149) | Valsartan (n = 152) |
| Follow-up duration | 12 weeks | 8 months | 36 weeks | |||
| Age, years | 72.9 ± 8.4 | 72.4 ± 8.6 | 72.7 ± 8.3 | 72.8 ± 8.5 | 70.9 ± 9.4 | 71.2 ± 8.9 |
| Female (%) | 638 (49.8) | 627 (48.8) | 1,241 (51.6) | 1,238 (51.8) | 85 (57) | 85 (56) |
| White race (%) | 1,112 (86.8) | 1,117 (86.9) | 1,963 (81.6) | 1,944 (81.4) | NA | NA |
| NYHA class (%) | ||||||
| I | 1 (0.1) | 4 (0.3) | 73 (3.0) | 64 (2.7) | 1 (1) | 1 (1) |
| II | 858 (67) | 876 (68.2) | 1,866 (77.5) | 1,840 (77.0) | 120 (81) | 119 (78) |
| III | 416 (32.5) | 401 (31.2) | 458 (19.0) | 474 (19.8) | 28 (19) | 32 (21) |
| IV | 5 (0.4) | 4 (0.3) | 8 (0.3) | 11 (0.5) | ||
| LVEF (%) | 56.7 ± 8.3 | 56.2 ± 8.0 | 57.6 ± 7.8 | 57.5 ± 8.0 | 58 ± 7.3 | 58 ± 8.1 |
| Heart rate, beats/min | NA | NA | 70.6 ± 12.3 | 70.3 ± 12.2 | 69 ± 12 | 70 ± 14 |
| Systolic blood pressure, mmHg | 132.6 ± 13.9 | 134.2 ± 14.5 | 130.5 ± 15.6 | 130.6 ± 15.3 | 137.1 ± 11.2* | 135.7 ± 14.2* |
| Body mass index | 30.6 ± 5.0 | 30.5 ± 4.8 | 30.2 ± 4.9 | 30.3 ± 5.1 | 30.1 ± 5.5 | 29.8 ± 6.1 |
| Scr, mg/dl | 1.1 ± 0.3 | 1.1 ± 0.3 | ||||
| GFR, ml/min/1.73 m2 | 62.5 ± 20.2 | 62.7 ± 19.6 | 63 ± 19 | 62 ± 19 | 67 ± 19.4 | 64 ± 21.3 |
| Potassium, mmol/L | NA | NA | NA | NA | NA | NA |
| Medications at baseline (%) | ||||||
| ACE inhibitors | 1,115 (87.1) | 1,124 (87.5) | 2,074 (86.2) | 2,065 (86.4) | 83 (56) | 80 (53) |
| ARBs | 57 (38) | 62 (41) | ||||
| Diuretics | 1,277 (99.8) | 1,282 (99.8) | 2,294 (95.3) | 2,291 (95.9) | 149 (100) | 152 (100) |
| Beta-blockers | 1,071 (83.7) | 1,066 (83) | 1,922 (79.9) | 1,899 (79.5) | 117 (79) | 121 (80) |
| Aldosterone antagonists | 419 (32.7) | 392 (30.5) | 592 (24.6) | 647 (27.1) | 28 (19) | 35 (23) |
| SGLT-2 inhibitors | 34 (2.7) | 26 (2.0) | NA | NA | NA | NA |
Baseline characteristics of RCTs.
Sac/Val, sacubitril–valsartan; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; Scr, serum creatinine; GFR, glomerular filtration rate; ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; RCTs, randomized controlled trials.
The sample mean and standard deviation (SD) were estimated from the sample size, median, and interquartile range (IQR) through the special website (http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html).
Primary efficacy outcomes
All three trials included in the meta-analysis reported the primary outcome. The estimated results of the primary efficacy outcomes of death from all causes, death from cardiovascular causes, and hospitalization for HF are presented in Figure 2.
Figure 2
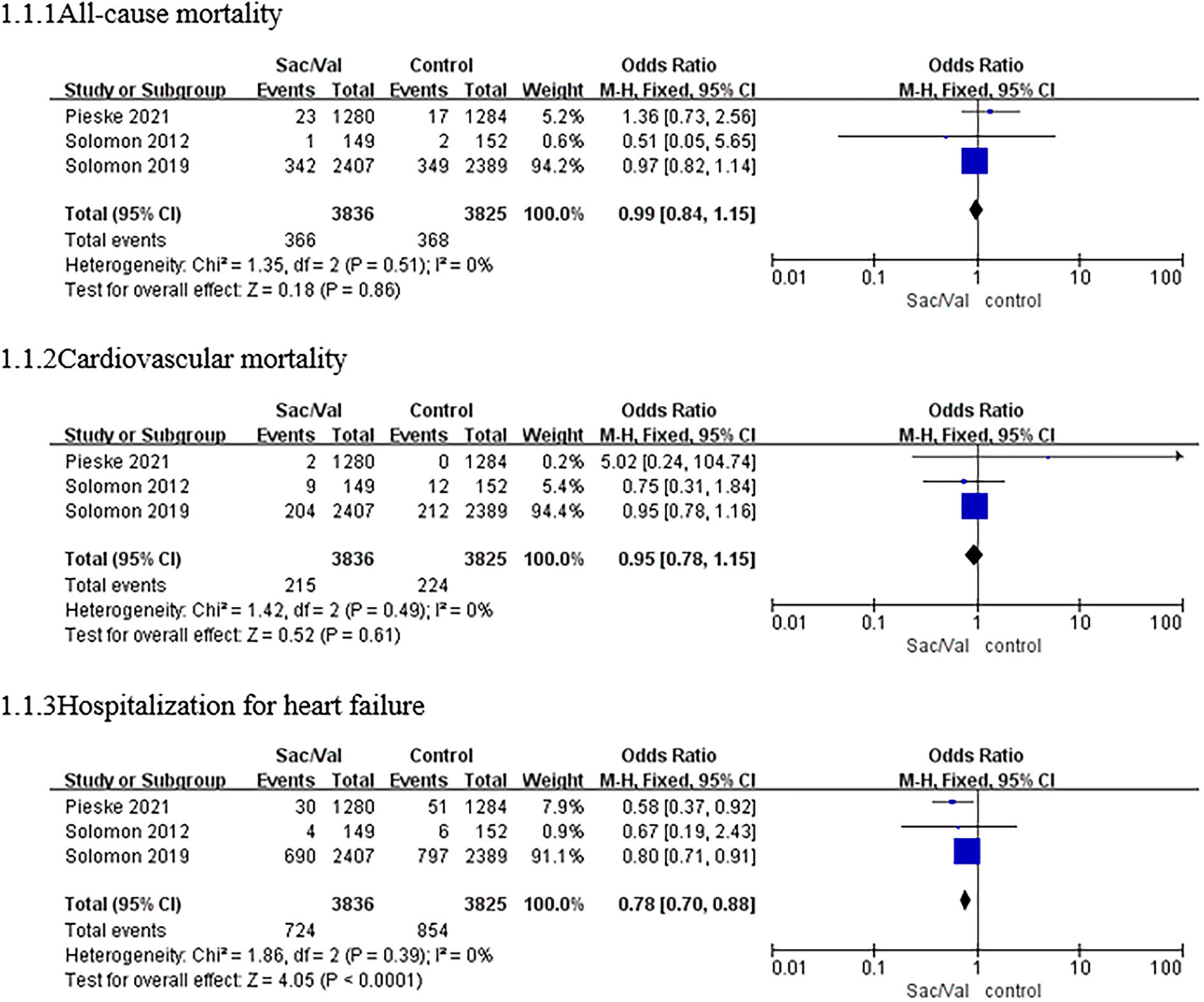
A forest plot of the effective outcomes of all-cause mortality, cardiovascular mortality, and hospitalization for HF in different patients with HFpEF. HF, heart failure; HFpEF, heart failure with preserved ejection fraction; Sac/Val, sacubitril/valsartan.
The heterogeneity test results showed no significant heterogeneity among the three studies (p = 0.51, I2 = 0%); thus, the meta-analysis was carried out using a fixed-effects model. Regarding the outcome of all-cause mortality, the pooled OR based on three studies was 0.99 (95% CI: 0.84–1.15, p = 0.86). The OR of cardiovascular mortality based on three studies was 0.95 (95% CI: 0.78–1.15, p = 0.16; p = 0.49 for heterogeneity, I2 = 0%). There were no significant differences in all-cause mortality or cardiovascular mortality among the patients with HFpEF between the sacubitril–valsartan group and the control group (Figure 2).
For the risk of hospitalization for HF, no significant heterogeneity was observed among the three studies (p = 0.39, I2 = 0%), and a fixed-effects model was used. Compared with valsartan or individualized medical therapy (IMT), sacubitril–valsartan reduced the composite risk of hospitalization for HF by 22% based on the three studies, and the pooled OR was 0.78 (95% CI: 0.70–0.88, p < 0.0001; Figure 2).
Adverse events of interest
Symptomatic hypotension
Regarding this adverse event, compared with valsartan or IMT, sacubitril–valsartan led to a higher risk of symptomatic hypotension in all three trials with a pooled RR of 1.44 (95% CI: 1.25–1.66, p < 0.00001; p = 0.29 for heterogeneity, I2 = 19%; Figure 3).
Figure 3
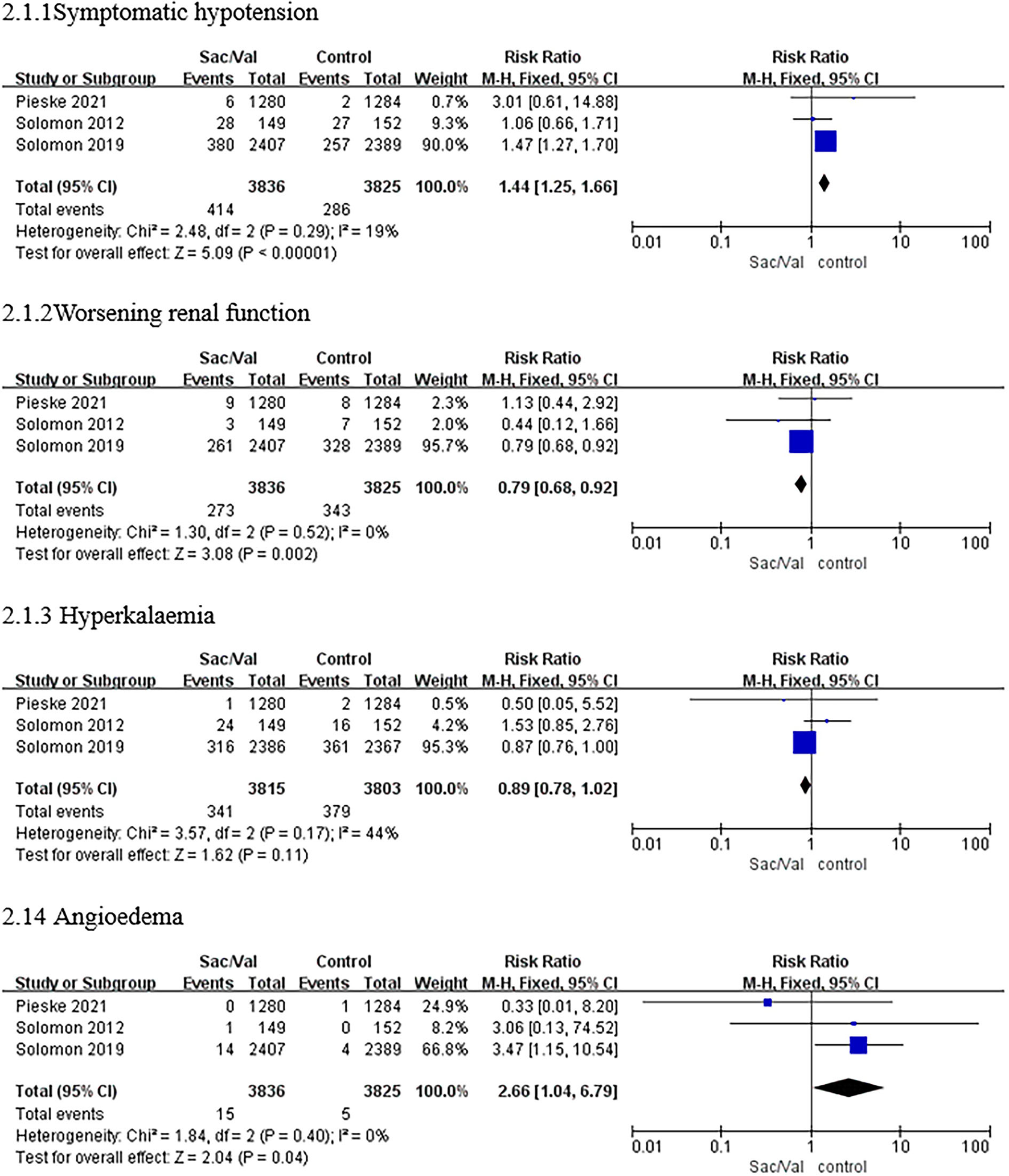
A forest plot of the safety outcomes of symptomatic hypotension, worsening renal function, hyperkalemia (≥5.5 mmol/L), and angioedema. Worsening renal function was defined as a decrease in estimated glomerular filtration rate (eGFR) ≥35% or an increase in serum creatinine ≥0.5 mg/dl from baseline and a decrease in eGFR ≥25% from baseline or serum creatinine >2.5 mg/dl. Sac/Val, sacubitril/valsartan; eGFR, estimated glomerular filtration rate.
Worsening renal function
As shown in Figure 3, the treatment with sacubitril–valsartan was related to a significant reduction in the incidence of worsening renal function with a pooled RR of 0.79 (95% CI: 0.68–0.92, p = 0.002; p = 0.52 for heterogeneity, I2 = 0%).
Hyperkalemia
Regarding hyperkalemia, there was no significant heterogeneity in the incidence between the sacubitril/valsartan group and the control group in all trials (p = 0.17, I2 = 44%), as shown in Figure 3. Furthermore, sacubitril–valsartan led to a numerically higher risk of hyperkalemia than valsartan or IMT with a pooled RR of 0.89 (95% CI: 0.78–1.02, p = 0.11; Figure 3).
Angioedema
The results showed that patients receiving sacubitril/valsartan had a higher risk of angioedema in all three trials, with a pooled RR of 2.66 (CI: 1.04–6.79, p < 0.04; p = 0.40 for heterogeneity, I2 = 0%; Figure 3).
Discussion
The present meta-analysis, which involved 7,663 patients, is the first to provide composite evidence of the efficacy and safety of sacubitril/valsartan in patients with HFpEF by pooling data from relevant RCTs. All of the studies included in this meta-analysis were randomized, controlled, and double-blind multicenter clinical trials. The results suggest that compared with valsartan or IMT, sacubitril/valsartan showed a significant advantage in reducing the rate of hospitalization for hazard ratio (HR). However, there was no obvious difference in the reduction in all-cause mortality or in the rate of death from cardiovascular diseases. Sacubitril/valsartan increased the risk of hypotension and angioedema, but it could reduce the incidence of worsening renal function. At the same time, the occurrence of hyperkalemia was numerically higher, but not statistically significant, in the sacubitril/valsartan group than in the control group.
The PARAMOUNT study (9) was a prospective study that compared the treatment of patients with HFpEF. The results showed that the level of N-terminal pro-brain natriuretic peptide (NT-proBNP), a key biomarker used in HF diagnosis to reflect the severity of heart failure (10, 11), in patients with HFpEF was significantly lower in the treatment group than in the valsartan group after 12 weeks of treatment (12). After 36 weeks of treatment, the left atrial volume in the treatment group was significantly lower than that in the control group, and the cardiac function in the treatment group was significantly better than that in the control group. Notably, the left atrial volume and dimension were significantly reduced in patients with HFpEF. The PARALLAX trial had similar results at 12 weeks (ratio 0.84, 95% CI: 0.80–0.88, p < 0.0001) (13). Left atrial volume is a biomarker of cardiac diastolic function (14). In addition, the Kansas City Cardiopathy Questionnaire (KCCQ) score was significantly lower than that of the control group. Studies have confirmed that sacubitril/valsartan has a certain therapeutic effect on patients with HFpEF and is beneficial for inhibiting ventricular remodeling.
The PARAGON-HF was a randomized, double-blind, and active-controlled trial (15). The results of the PARAGON trial (7) did not indicate a statistically significant difference but revealed that compared with valsartan, sacubitril/valsartan reduced the risk of experiencing the major endpoints (cardiovascular death and total hospitalization for HF) by 13% (RR: 0.87, 95% CI: 0.75–1.01, p = 0.06). Notably, the subgroup analysis in this trial showed that sacubitril/valsartan had a beneficial effect on patients with an LVEF between 45 and 57% (RR: 0.78, 95% CI: 0.64–0.95). This could be because these patients have systolic dysfunction, and sacubitril/valsartan has the same physiologic effects in these patients as it does in those with heart failure with reduced ejection fraction (HFrEF). For the secondary endpoints, sacubitril/valsartan significantly improved the New York Heart Association (NYHA) grade by 45% compared with valsartan (OR: 1.45, 95% CI: 1.13–1.86). Similarly, compared with the valsartan group, the sacubitril/valsartan group had a higher percentage of patients with a KCCQ clinical summary score of 5 or higher (33.0 vs. 29.6%). In addition, the incidence of the compound renal endpoint in the sacubitril/valsartan group was significantly reduced compared with that in the valsartan group (HR: 0.50, 95% CI: 0.33–0.77). The reasons for the negative results of the PARAGON trial (7) may be as follows: most patients were treated with ACEIs or ARBs before participating in the trial or the main endpoints of observation were different. Another study showed that the occurrence of HFpEF is heterogeneous, and this result was consistent with the PARAGON trial (12). In the PARAGON trial (7), the subgroup analysis showed that the two pre-specified subgroups—those with LVEF ≤ 57% and women—received significant benefits: the risk of the primary endpoints decreased by 27% in women and by 22% in the low LVEF group (16), demonstrating that sacubitril/valsartan may benefit high-risk patients with HFpEF (17). Based on these results, patients with HFpEF with structural heart disease and volume overload may be more sensitive to the sacubitril/valsartan treatment. In the PARALLAX trial (13), hospitalization for HF or the compound endpoint of all-cause mortality or heart failure for hospitalization was lower in the sacubitril–valsartan group than in the IMT group. In the PARALLAX trial (13), sacubitril/valsartan did not show superiority over valsartan in terms of the KCCQ score (13), which may be related to the severity of the patient's condition and insufficient treatment time.
In the PARAGON-HF study (7) and the PARALLAX study (18, 19), there was a higher risk of symptomatic hypotension caused by sacubitril/valsartan. This result is consistent with this meta-analysis; sacubitril/valsartan increased the incidence of hypotension with an RR of 2.66 (p = 0.04) compared with enalapril or valsartan. This finding coincides with previous studies (20–22). In the TITRATION study (23), patients with lower systolic blood pressure were successfully treated by gradual titration, which suggested that patients with lower systolic blood pressure can also use sacubitril/valsartan. Although hypotension can cause insufficient blood perfusion in the kidneys, causing kidney damage, previous studies (24, 25) found that sacubitril/valsartan could protect renal function, which was similar to our outcome that treatment with sacubitril/valsartan could protect renal function to prevent deterioration. Rubattu found that sacubitril/valsartan was more effective in reducing cardiovascular risk in rats with chronic kidney disease than valsartan alone (26). The UK HARP-III experiment showed that sacubitril/valsartan and irbesartan had similar effects on renal function after 12 months of follow-up (27). Damman conducted further studies that had similar results (28). Moreover, the results of the PARAMOUNT (9) and PARAGON-HF (7) studies showed that patients in the sacubitril/valsartan group had more beneficial effects on kidney function than those in the valsartan (ARB) group. The main mechanisms of renal protection by sacubitril/valsartan are as follows: (A) direct action on the kidney, inhibiting water and sodium reabsorption in the proximal and distal nephrons (the inhibition of proximal sodium and potassium exchange, distal sodium chloride exchange, and sodium channels in the collecting tubule) (29); (B) indirectly inhibits the release or action of other vasoconstrictors (renin, vasopressin, aldosterone, and norepinephrine), resulting in natriuretic and diuretic effects (30); and (C) inhibits inflammation and oxidative stress, delays glomerulosclerosis, etc (31, 32). Consequently, sacubitril/valsartan has more beneficial effects on renal function than ACEIs or ARBs in patients with HF. Concerning the incidence of hyperkalemia, there was no difference between the two groups (95% CI: 0.78–1.02, p = 0.11), which was consistent with previous studies (33). Although the risk of angioedema was low in the included studies, the pooled analysis indicated that sacubitril/valsartan led to a higher risk of angioedema (CI: 1.04–6.79, p < 0.04). However, the occurrence of angioedema is known to be related to the inhibition of bradykinin degradation (34), and sacubitril/valsartan does not inhibit ACE or aminopeptidase P, so it does not increase the risk of angioedema (4, 35). The reason for the result needs further research.
Of note, although no medical therapy has been shown to reduce all-cause or cardiovascular death in HFpEF trials, according to the latest AHA/ACC/HFSA heart failure guidelines (2022) (36), a number of drugs other than sacubitril/valsartan are recommended for treating HFpEF. Despite a lack of strong evidence, diuretics have been used in HFpEF to reduce symptoms due to volume overload (37). Renin–angiotensin–aldosterone system (RAAS) inhibitors and mineralocorticoid receptor antagonists have a well-established role in HFrEF but have been less effective in HFpEF, possibly because the RAAS plays a less prominent pathophysiological role as LVEF increases (38). Trials of ACEI and ARB use in HFpEF have not shown a significant reduction in all-cause or cardiovascular death, but these drugs may have reduced the risk of HF hospitalization (39–41). The TOPCAT trial (42) found no overall benefit in the primary composite outcome of cardiovascular death or hospitalization for heart failure. However, spironolactone reduced the risk of HF hospitalization in both the TOPCAT and TOPCAT-Americas subgroups, which could be linked to improved diastolic function in patients with HFpEF. In the EMPEROR-Preserved trial (43), a sodium-glucose cotransporter-2 inhibitor (SGLT-2i; empagliflozin) reduced the risk of composite cardiovascular death or total HF hospitalization by 21% in patients with HF with LVEF > 40%, driven primarily by a significant reduction in the HF hospitalization of 29% (no significant reduction in cardiovascular death [hazard ratio (HR), 0.91; 95% CI: 0.76–1.0]), with no benefit for all-cause mortality. In addition, the SOLOIST-WHF trial (44) showed that both patients with HFpEF and HFrEF had a reduced risk of endpoint events (cardiovascular death and HF hospitalizations; LVEF < 50% subgroup: HR = 0.72; LVEF ≥ 50% subgroup: HR = 0.48), which was driven by the reduction in HF hospitalizations. Sacubitril/valsartan and empagliflozin, two new drugs in the field of heart failure, have some similarities and differences in the clinical trials associated with them (Table 3). In the EMPEROR-Preserved subgroup analysis, patients with LVEF > 60% received no benefit. This result was similar to that in the subgroup of patients with LVEF > 57% in the PARAGON trial (RR, 1.00; 95% CI: 0.81–1.23). In terms of safety outcome events, while both drugs resulted in symptomatic hypotension, patients taking empagliflozin were more prone to volume depletion and urinary tract infections (45), while those taking sacubitril/valsartan were more susceptible to hyperkalemia and angioedema. Therefore, when assessing cardiac function and making treatment decisions for patients with heart failure, we should not simply use LVEF as the reference indicator; rather, treatment should be individualized.
Table 3
| Outcome | PARAGON-HF 2019 (sacubitril/valsartan) | EMPEROR-preserved 2021 (Empagliflozin) | SOLOIST-WHF 2021 (Sotagliflozin) |
|---|---|---|---|
| Total hospitalizations for heart failure and death from cardiovascular causes | RR, 0.87 (0.75–1.01) | HR, 0.79 (0.69, 0.9) | HR, 0.67 (0.52, 0.85) |
| Hospitalizations for heart failure | RR, 0.85 (0.72–1.00) | HR, 0.71 (0.60, 0.83) | HR, 0.64 (0.49, 0.83) |
| Death from cardiovascular causes | HR, 0.95 (0.79–1.16) | HR, 0.91 (0.76, 1.09) | HR, 0.84 (0.58, 1.22) |
| Death from any cause | HR, 0.97 (0.84–1.13) | HR, 1.00 (0.87, 1.15) | HR, 0.82 (0.59, 1.14) |
The difference between the end-point of the PARAGON trial and of SGLT2i trials.
Limitations
There were some limitations to our study. First, because only three RCTs were included in our work, the sample size of this systematic review was too small, and we could not produce a funnel plot. Second, HFpEF was defined as LVEF ≥ 50%, but the studies included in our work defined HFpEF as LVEF ≥ 45%. Third, unpublished data or articles published not in English were excluded. Fourth, we used data that were not published but were available on ClinicalTrials.gov. Fifth, for meaningful conclusions from the meta-analysis of randomized trials, we used secondary outcomes, not primary outcomes. Sixth, there were many mixed factors that may have caused bias. For example, the comparator drug was different among the three trials included in our work. Finally, the majority of the sample in our work was from the PARAGON-HF trial, which may lead to sample imbalance and affect the results of our work. Thus, further studies are needed to assess more potential clinical benefits of sacubitril/valsartan in patients with HFpEF.
Conclusion
In conclusion, the existing evidence shows that sacubitril/valsartan not only is effective in the treatment of heart failure but also has a protective effect on kidney function. Although there was no significant reduction in all-cause mortality or cardiovascular mortality, patients treated with sacubitril/valsartan could obtain the benefits of a reduction in hospitalizations for HF and prevention of renal functional deterioration. Moreover, compared with valsartan or IMT, sacubitril/valsartan could increase the risk of symptomatic hypotension and angioedema but did not differ in the elevation of serum potassium. Compared with ACEIs or ARBs, sacubitril/valsartan is a better choice, but it is best to monitor blood pressure and renal function during treatment.
Funding
This work was supported by the Provincial Plans-Social Development Areas—Major Projects of Jiangxi Province (No. 20161ACG70012) and the incubation project of the National Natural Science Foundation of the Second Affiliated Hospital of Nanchang University (No. 2021YNFY2021).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YW and ZH reviewed the articles, performed the meta-analysis and wrote the manuscript. SW, LF, and YS were responsible for the statistical analysis. ZY provided editing assistance. YP and WQ designed and revised the manuscript. All authors have reviewed and agreed on this information before submission.
Acknowledgments
The authors thank the teacher in the Imaging Department of the Second Affiliated Hospital of Nanchang University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.897423/full#supplementary-material
- RCTs
randomized controlled trials
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HF
heart failure
- LV
left ventricular
- NPs
natriuretic peptides
- RAAS
renin–angiotensin–aldosterone system
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- IMT
individualized medical therapy
- LVEF
left ventricular ejection fraction
- OR
odds ratio
- RR
risk ratio
- HR
hazard ratio
- Cl
confidence interval
- NT-pro BNP
N-terminal pro-brain natriuretic peptide
- NYHA
New York Heart Association
- KCCQ
The Kansas City Cardiomyopathy Questionnaire
- ACC
American College of Cardiology.
Abbreviations
References
1.
Metra M Teerlink JR . Heart failure. Lancet. (2017) 390:1981–95. 10.1016/S0140-6736(17)31071-1
2.
Gazewood JD Turner PL . Heart failure with preserved ejection fraction: diagnosis and management. Am Fam Physician. (2017) 96:582–8. 10.1016/j.amjmed.2016.12.031
3.
Ma C Luo H Fan L Liu X Gao C . Heart failure with preserved ejection fraction: an update on pathophysiology, diagnosis, treatment, and prognosis. Braz J Med Biol Res. (2020) 53:e9646. 10.1590/1414-431x20209646
4.
Gu J Noe A Chandra P Al-Fayoumi S Ligueros-Saylan M Sarangapani R et al . Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. (2010) 50:401–14. 10.1177/0091270009343932
5.
Gori M D'Elia E Senni M . Sacubitril/valsartan therapeutic strategy in HFpEF: clinical insights and perspectives. Int J Cardiol. (2019) 281:158–65. 10.1016/j.ijcard.2018.06.060
6.
Kuno T Ueyama H Fujisaki T Briasouli A Takagi H Briasoulis A . Meta-analysis evaluating the effects of renin-angiotensin-aldosterone system blockade on outcomes of heart failure with preserved ejection fraction. Am J Cardiol. (2020) 125:1187–93. 10.1016/j.amjcard.2020.01.009
7.
Solomon SD McMurray JJV Anand IS Ge J Lam CSP Maggioni AP et al . Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381:1609–20. 10.1056/NEJMoa1908655
8.
Moher D Liberati A Tetzlaff J Altman DG . PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097
9.
Solomon SD Zile M Pieske B Voors A Shah A Kraigher-Krainer E et al . The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. (2012) 380:1387–95. 10.1016/S0140-6736(12)61227-6
10.
Maries L Manitiu I . Diagnostic and prognostic values of B-type natriuretic peptides (BNP) and N-terminal fragment brain natriuretic peptides (NT-pro-BNP). Cardiovasc J Afr. (2013) 24:286–9. 10.5830/CVJA-2013-055
11.
Cocco G Jerie P . Assessing the benefits of natriuretic peptides-guided therapy in chronic heart failure. Cardiol J. (2015) 22:5–11. 10.5603/CJ.a2014.0041
12.
Shah SJ Katz DH Selvaraj S Burke MA Yancy CW Gheorghiade M et al . Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. (2015) 131:269–79. 10.1161/CIRCULATIONAHA.114.010637
13.
Pieske B Wachter R Shah SJ Baldridge A Szeczoedy P Ibram G et al . Effect of Sacubitril/Valsartan vs. standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. (2021) 326:1919–29. 10.1001/jama.2021.18463
14.
Thomas L Marwick TH Popescu BA Donal E Badano LP . Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1961–77. 10.1016/j.jacc.2019.01.059
15.
Solomon SD Rizkala AR Gong J Wang W Anand IS Ge J et al . Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail. (2017) 5:471–82. 10.1016/j.jchf.2017.04.013
16.
Del Buono MG Bonaventura A Vecchie A Wohlford GF Dixon DL Van Tassel BW et al . Sacubitril–valsartan for the treatment of heart failure: time for a paragon?J Cardiovasc Pharmacol. (2020) 75:105–7. 10.1097/FJC.0000000000000782
17.
Del Buono MG Iannaccone G Scacciavillani R Carbone S Camilli M Niccoli G et al . Heart failure with preserved ejection fraction diagnosis and treatment: an updated review of the evidence. Prog Cardiovasc Dis. (2020) 63:570–84. 10.1016/j.pcad.2020.04.011
18.
Nct . A Randomized, Double-Blind Controlled Study Comparing LCZ696 to Medical Therapy for Comorbidities in HFpEF Patients. (2017). Available online at: https://clinicaltrials.gov/show/NCT03066804 (accessed February 28, 2017).
19.
Wachter R Shah SJ Cowie MR Szecsödy P Shi V Ibram G et al . Angiotensin receptor neprilysin inhibition vs. individualized RAAS blockade: design and rationale of the PARALLAX trial. ESC Heart Fail. (2020) 7:856–64. 10.1002/ehf2.12694
20.
Zheng L Xia B Zhang X Zhao Y . A meta-analysis on the effect and safety of LCZ696 in the treatment of hypertension. Cardiol Res Pract. (2021) 2021:8867578. 10.1155/2021/8867578
21.
Williams B Cockcroft JR Kario K Zappe DH Brunel PC Wang Q et al . Effects of Sacubitril/Valsartan vs. olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. (2017) 69:411–20. 10.1161/HYPERTENSIONAHA.116.08556
22.
Malik AH Aronow WS . Efficacy of Sacubitril/Valsartan in hypertension. Am J Ther. (2019) 29:e322–33. 10.1097/MJT.0000000000000925
23.
Senni M McMurray JJV Wachter R McIntyre HF Anand IS Duino V et al . Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail. (2018) 20:491–500. 10.1002/ejhf.1054
24.
Armentaro G D'Arrigo G Magurno M Toscani AF Condoleo V Miceli S et al . Impact of Sacubitril/Valsartan on clinical and echocardiographic parameters in heart failure patients with reduced ejection fraction: data from a real life 2-year follow-up study. Front Pharmacol. (2021) 12:733475. 10.3389/fphar.2021.733475
25.
Spannella F Giulietti F Filipponi A Sarzani R . Effect of sacubitril/valsartan on renal function: a systematic review and meta-analysis of randomized controlled trials. ESC Heart Fail. (2020) 7:3487–96. 10.1002/ehf2.13002
26.
Rubattu S Cotugno M Forte M Stanzione R Bianchi F Madonna M et al . Effects of dual angiotensin type 1 receptor/neprilysin inhibition vs. angiotensin type 1 receptor inhibition on target organ injury in the stroke-prone spontaneously hypertensive rat. J Hypertens. (2018) 36:1902–14. 10.1097/HJH.0000000000001762
27.
Haynes R Judge PK Staplin N Herrington WG Storey BC Bethel A et al . Effects of sacubitril/valsartan vs. irbesartan in patients with chronic kidney disease. Circulation. (2018) 138:1505–14. 10.1161/CIRCULATIONAHA.118.034818
28.
Damman K Gori M Claggett B Jhund PS Senni M Lefkowitz MP et al . Renal effects and associated outcomes during Angiotensin Neprilysin inhibition in heart failure. JACC Heart Fail. (2018) 6:489–98. 10.1016/j.jchf.2018.02.004
29.
Volpe M Carnovali M Mastromarino V . The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci. (2016) 130:57–77. 10.1042/CS20150469
30.
Wong PC Guo J Zhang A . The renal and cardiovascular effects of natriuretic peptides. Adv Physiol Educ. (2017) 41:179–85. 10.1152/advan.00177.2016
31.
Suematsu Y Jing W Nunes A Kashyap ML Khazaeli M Vaziri ND et al . LCZ696 (Sacubitril/Valsartan), an angiotensin-receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease. J Card Fail. (2018) 24:266–75. 10.1016/j.cardfail.2017.12.010
32.
Habibi J Aroor AR Das NA Manrique-Acevedo CM Johnson MS Hayden MR et al . The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc Diabetol. (2019) 18:40. 10.1186/s12933-019-0847-8
33.
Zhang H Huang T Shen W Xu X Yang P Zhu D et al . Efficacy and safety of sacubitril–valsartan in heart failure: a meta-analysis of randomized controlled trials. ESC Heart Fail. (2020) 7:3841–50. 10.1002/ehf2.12974
34.
Fryer RM Segreti J Banfor PN Widomski DL Backes BJ Lin CW et al . Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. (2008) 153:947–55. 10.1038/sj.bjp.0707641
35.
Hegde LG Yu C Renner T Thibodeaux H Armstrong SR Park T et al . Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J Cardiovasc Pharmacol. (2011) 57:495–504. 10.1097/FJC.0b013e318210fc7e
36.
Heidenreich PA Bozkurt B Aguilar D Allen LA Byun JJ Colvin MM et al . 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 145:e895–e1032. 10.1161/CIR.0000000000001073
37.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131. 10.1002/ejhf.2333
38.
Dewan P Jackson A Lam CSP Pfeffer MA Zannad F Pitt B et al . Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail. (2020) 22:898–901. 10.1002/ejhf.1776
39.
Yusuf S Pfeffer MA Swedberg K Granger CB Held P McMurray JJ et al . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. (2003) 362:777–81. 10.1016/S0140-6736(03)14285-7
40.
Cleland JG Tendera M Adamus J Freemantle N Polonski L Taylor J et al . The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. (2006) 27:2338–45. 10.1093/eurheartj/ehl250
41.
Massie BM Carson PE McMurray JJ Komajda M McKelvie R Zile MR et al . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. (2008) 359:2456–67. 10.1056/NEJMoa0805450
42.
Pitt B Pfeffer MA Assmann SF Boineau R Anand IS Claggett B et al . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370:1383–92. 10.1056/NEJMoa1313731
43.
Anker SD Butler J Filippatos G Ferreira JP Bocchi E Böhm M et al . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. 10.1056/NEJMoa2107038
44.
Bhatt DL Szarek M Steg PG Cannon CP Leiter LA McGuire DK et al . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384:117–28. 10.1056/NEJMoa2030183
45.
Lu Y Li F Fan Y Yang Y Chen M Xi J . Effect of SGLT-2 inhibitors on cardiovascular outcomes in heart failure patients: a meta-analysis of randomized controlled trials. Eur J Intern Med. (2021) 87:20–8. 10.1016/j.ejim.2021.03.020
Summary
Keywords
sacubitril/valsartan, LCZ696, heart failure, heart failure with preserved ejection fraction (HFpEF), meta-analysis
Citation
Yu W, Zhang H, Shen W, Luo F, Yang S, Gan L, Zhao Y, Yang P and Wu Q (2022) Efficacy and safety of sacubitril/valsartan on heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 9:897423. doi: 10.3389/fcvm.2022.897423
Received
16 March 2022
Accepted
15 August 2022
Published
08 September 2022
Volume
9 - 2022
Edited by
Peter Moritz Becher, University Medical Center Hamburg-Eppendorf, Germany
Reviewed by
Edoardo Sciatti, Local Social Health Agency Garda, Italy; Daniele Masarone, Azienda Ospedaliera dei Colli, Italy
Updates
Copyright
© 2022 Yu, Zhang, Shen, Luo, Yang, Gan, Zhao, Yang and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pingping Yang pingpingyang177@163.comQinghua Wu ncwqh@163.com
†These authors have contributed equally to this work
This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.