Abstract
Objectives:
Low cardiac output syndrome (LCOS) is a serious complication after cardiac surgery. Despite scientific interest in LCOS, there is no uniform definition used in current research and clinicians cannot properly compare different study findings. We aimed to collect the LCOS definitions used in literature and subsequently applied the definitions obtained to existing data to estimate their effect on the intraoperative LCOS incidences in adults, children and infants.
Design:
This is a literature review, followed by a retrospective cohort study.
Setting:
This is a single-institutional study from a university hospital in the Netherlands.
Participants:
Patients from all ages undergoing cardiac surgery with cardiopulmonary bypass between June 2011 and August 2018.
Interventions:
We obtained different definitions of LCOS used in the literature and applied these to data obtained from an anesthesia information management system to estimate intraoperative incidences of LCOS. We compared intraoperative incidences of LCOS in different populations based on age (infants, children and adults).
Measurements and main results:
The literature search identified 262 LCOS definitions, that were applied to intraoperative data from 7,366 patients. Using the 10 most frequently published LCOS definitions, the obtained incidence estimates ranged from 0.4 to 82% in infants, from 0.6 to 56% in children and from 1.5 to 91% in adults.
Conclusion:
There is an important variety in definitions used to describe LCOS. When applied to data obtained from clinical care, these different definitions resulted in large distribution of intraoperative LCOS incidence rates. We therefore advocate for standardization of the LCOS definition to improve clinical understanding and enable adequate comparison of outcomes and treatment effects both in daily care and in research.
Introduction
Low cardiac output syndrome (LCOS) is a frequently occurring complication after cardiac surgery. LCOS is characterized by an inadequate cardiac pump function resulting in reduced oxygen delivery and tissue hypoxia, in both adults and children (1). Clinicians may refer to LCOS for a symptomatic state that ranges from mild myocardial stunning to severe cardiogenic shock with the need for mechanical ventricular assistance. The reported incidence of LCOS varies from 2 to 27% in the adult population (2–8). In the pediatric population reported incidences are between 17 and 67% (9–11). Most studies describe the occurrence of LCOS, considering the associated morbidity (renal and pulmonary failure, stroke, myocardial infarction, sepsis, and a prolonged length of stay), mortality (up to 38%) and, therefore, increased healthcare costs (2–4, 6, 8, 12).
In order to properly address the features of LCOS that make it a potentially serious complication and to reduce its occurrence and seriousness, it is of crucial importance to study the syndrome. However, despite the obvious interest in LCOS from a clinical perspective, researchers do not use uniform criteria based on specific thresholds to describe the syndrome (definition) (13, 14). Several therapies have been evaluated for their effect on LCOS and compared in meta-analyses, however LCOS definitions differ among studies varying from the temporary use of a single vasoactive agent to counteract “stunning” to the requirement of mechanical support (15, 16). The comparison of study findings without the standardization of the LCOS definition including the use of uniform criteria (predefined thresholds) therefore is hampered.
We hypothesized that the variety in operational definitions of LCOS at least partly explains the wide range in reported incidences of LCOS. The primary aim of this study was to evaluate the variety of LCOS definitions described in literature among adult and pediatric cardiac surgery populations and subsequently to examine to what extent these different definitions affect the incidence of intraoperative LCOS.
Methods
Design and conduct of the study
In this study, we combined a literature review approach with a retrospective cohort study. A study protocol was not published nor registered. The literature review was used to extract LCOS definitions. Subsequently, we applied the definitions found to a retrospective intraoperative cohort, to study the effects of the different definitions on the estimated incidence of LCOS. The cohort included cardiac surgery with cardiopulmonary bypass patients from all ages, i.e., both children and adults.
Review of the literature
The following literature search was performed in the PubMed database (17) on August 24th, 2020:
(((((Low cardiac output syndrome[Title/Abstract]) OR LCOS[Title/Abstract])) AND (((((((((Surger*) OR Operation*) OR Surgical procedure*) OR operative surgical procedure*) OR operative procedure*)) AND ((heart) OR cardiac))) OR ((((“Heart”[Mesh]) AND “Surgical Procedures, Operative”[Mesh])) OR “Cardiac Surgical Procedures”[Mesh])))).
We excluded articles when the full text was not available, those written in non-English language, those including a non-human study population and duplicate papers. We also excluded articles without a definition of LCOS (for example where LCOS was not a main outcome), systematic reviews, case reports, editorials, author's opinions and letters to the editor. We did not exclude articles based on publication year. The remaining articles were reviewed, the definitions of LCOS were extracted and categorized. We classified the articles on the following items: study population (adult/pediatric/both/questionnaires completed by pediatric Intensive Care Unit (PICU) professionals), reproducibility and scope. Definitions included “inotropes,” “cardiovascular mechanical support,” “acidosis,” “cardiac pump function,” “blood pressure,” “clinical signs of hypoperfusion,” “saturation,” “pulmonary capillary wedge pressure,” “renal replacement therapy,” “hemodynamic instable,” “cardiac arrest,” “death,” and “others.” Definitions were listed as reproducible, when they had cut-off values in their definition and when they did not use vague terms without further explanation, such as “a situation, in which circulation and organ perfusion is barely maintained” (18). Detailed information about selection, data extraction, and scoring is provided in Supplementary material 1. Screening, selection and data extraction was done by author AS and in case of uncertainty discussed with KL.
Retrospective cohort study
We listed the 10 most frequently published LCOS definitions and used these to determine the intraoperative incidence of LCOS according to these definitions. The study data were collected from the University Medical Center Utrecht (UMCU; The Netherlands) and the Wilhelmina Children's hospital, which is part of the UMCU. The UMCU is a tertiary referral hospital for pediatric and adult cardiac surgery. The study population included patients of all ages who underwent cardiac surgery with cardiopulmonary bypass (CPB) between June 2011 and August 2018. Our center performs the full range (Basic Aristotle score 1.5–15) of congenital cardiac surgery, including neonatal Norwood procedures, with an average Basic Aristotle score of 7.06 (SD 2.97). We did not use postoperative intensive care data as the AIMS and intensive care databases were not connected, nor similarly constructed. The Medical Ethics Review committee reviewed the study protocol and waived the need for patient consent (WAG/rgj/18/022047).
We collected the following variables: age, gender, weight, height, type of surgery, surgical urgency, duration of CPB, vital parameters, laboratory tests, inotropic drugs administration and in-hospital mortality. Patient characteristics, laboratory tests and in-hospital mortality were obtained from the Electronic Medical Record system (HIX 5.2, ChipSoft, Amsterdam, The Netherlands). Intraoperative data were obtained from the anesthesia information management system (AIMS) database (Anstat, Carepoint, Ede, The Netherlands).The first postoperative laboratory test results of lactate, arterial pH, arterial oxygen saturation, mixed venous oxygen saturation and central venous oxygen saturation were used. Variables extracted from the AIMS database were stored as median per minute values during the intraoperative period. These variables included intraoperative vital signs, anesthesia ventilator data and data on inotropic use. For the vital parameters and inotropic drugs, we used the mean, timeframes, or limits defined otherwise of all variables stored in the AIMS after the patients were weaned from the CPB. We used the 50th percentile of the national growth charts to estimate the height of children, because the documentation of this variable was unreliable (19). We considered other missing data to occur under the “missing not at random” condition as cardiac vascular monitoring and treatment is initiated based on clinical indication. Therefore, no further missing data assumptions were made.
The primary outcome was the difference in incidence of LCOS. We applied the 10 most frequently reported LCOS definitions to the data obtained to determine the incidences. To calculate LCOS incidences, we used the total population as the denominator (i.e., the patients with and without missing data) to prevent biased estimates due to selective missingness of data. When LCOS definitions were built with “OR” condition statements (e.g., “the use of inotropes OR mechanical support”), patient data were considered missing only if all parts of the statement could not be filled in (e.g., in this case, patient data were only considered missing if there was no data available on the use of inotropes AND no data on mechanical support).
As secondary outcome, we compared incidences between adults (≥18 years), children (>6 months and <18 years) and infants (≤6 months). We chose 6 months as cut-off point between infants and children, because maturation of the human heart is completed at 6 months of age, anatomically (remodeling of pulmonary blood flow and closure of the foramen ovale, ductus venosus and ductus arteriosus), histologically (growth of mitochondria numbers, myofibrils numbers and sarcomere volume and development the sarcoplasmic reticulum) and physiologically (increasing the coronary oxygen supply and preload due to a decreased heartrate) (20).
Statistical analysis
The statistical analysis was performed with R-studio software, version 1.1.456 (21). Continuous variables are presented as means ± standard deviations (SD) or when skewness or kurtosis was observed, as medians with interquartile ranges (IQR). Categorical variables are presented as proportions.
Results
Literature search
The literature search identified 964 records that were handled as presented in Figure 1. Ultimately, 250 articles were included (Supplementary material 2). In five of these articles multiple definitions were used (5 (15), 5 (9), 2 (22), 3 (23) and 2 (24), respectively), initially resulting in a total of 262 definitions.
Figure 1
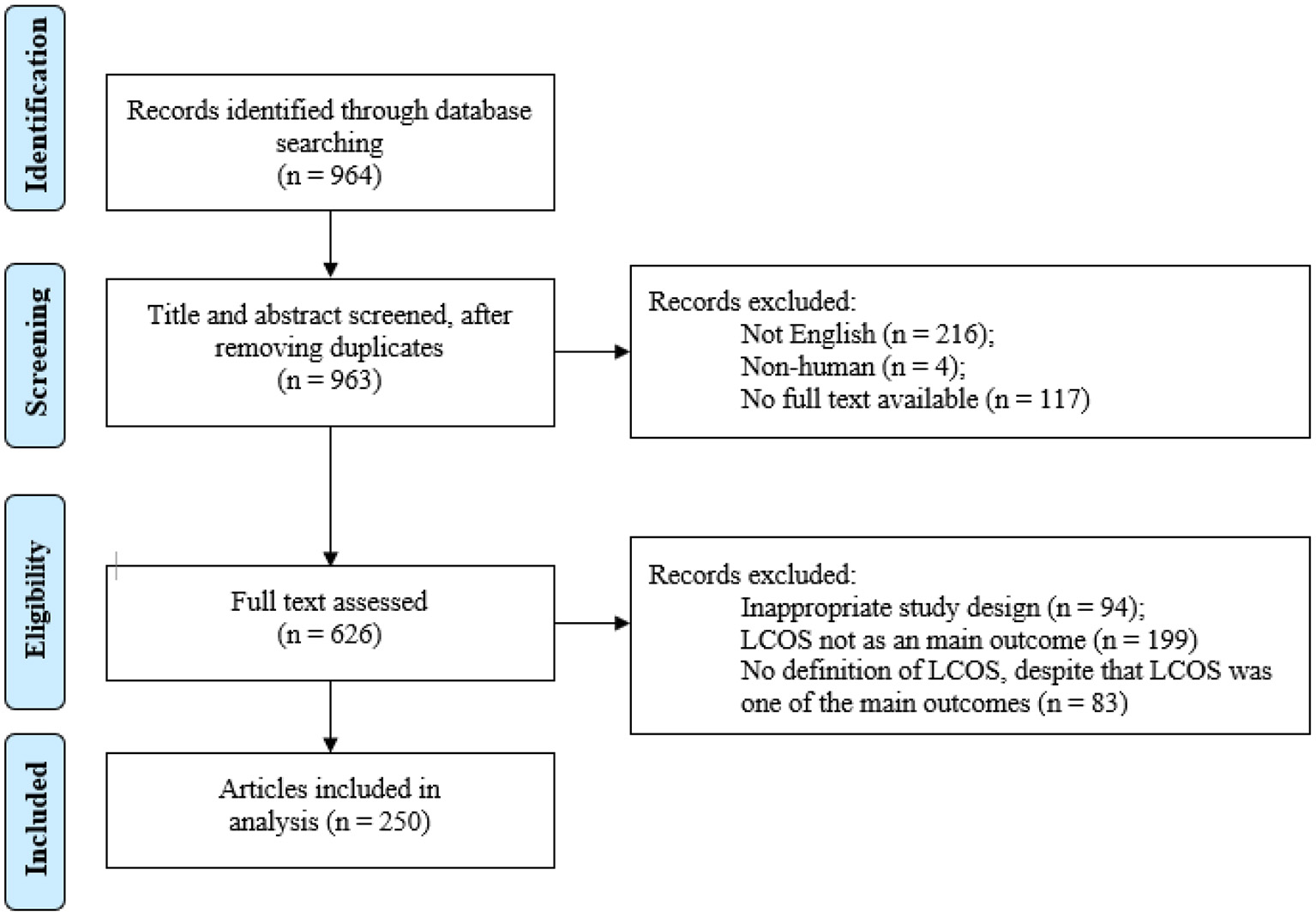
Literature search. LCOS, low cardiac output syndrome.
Of the 262 included definitions, 177 (68%) focused on adult surgery, 80 (31%) focused on pediatric surgery and 5 (2%) used information gained by questionnaires completed by pediatric ICU professionals as study population. Of the 262 definitions, 175 definitions (67%) were reproducible, i.e., definitions were clearly described and used cut-off values, and 87 definitions (33%) were not. Twelve items were repeatedly used within the LCOS definitions, namely: the use of inotropes; mechanical support; metabolic acidosis; cardiac pump function; blood pressure; clinical signs of hypoperfusion; saturation; pulmonary capillary wedge pressure (PCWP); renal replacement therapy; clinical judgement; cardiac arrest and death (Table 1). The definition of LCOS in studies among adults more often included need for mechanical support and cardiac pump function, while definitions used in pediatric studies more often included metabolic acidosis and clinical signs of hypoperfusion (Table 1).
Table 1
| Definitions in a study with an adult study population, n (%) | Definitions in a study with a pediatric study population, n (%) | Definitions in a study where PICU workers were interviewed, n (%) | |
|---|---|---|---|
| No. of definitions | 177 (100) | 80 (100) | 5 (100) |
| Reproducible | 135 (76) | 39 (49) | 1 (20) |
| Inotropes | 126 (71) | 49 (61) | 3 (60) |
| Duration of the use of inotropes | 69 (39) | 7 (8.8) | 0 (0.0) |
| Type of inotropes | 45 (25) | 7 (8.8) | 0 (0.0) |
| VIS | 2 (1.1) | 13 (16) | 0 (0.0) |
| Number of used inotropes | 13 (7.3) | 19 (24) | 3 (60) |
| Mechanical support | 94 (53) | 16 (20) | 0 |
| ECMO | 11 (6.2) | 6 (7.5) | 0 |
| IABP | 80 (45) | 2 (2.5) | 0 |
| VAD | 5 (2.8) | 2 (2.5) | 0 |
| Not specified | 14 (7.9) | 8 (10) | 0 |
| Metabolic acidosis | 11 (6.2) | 51 (64) | 3 (60) |
| High lactate | 7 (4.0) | 38 (48) | 1 (20) |
| High base difference | 0 | 13 (16) | 0 |
| Low pH | 0 | 4 (5.0) | 0 |
| Low bicarbonate | 0 | 3 (3.8) | 0 |
| Cardiac pump function | 115 (65) | 16 (20) | 0 |
| Low CI | 114 (64) | 9 (11) | 0 |
| Low left ventricle ejection fraction | 1 (0.6) | 6 (7.5 | 0 |
| Blood pressure | 70 (40) | 20 (25) | 1 (20) |
| Low systolic blood pressure | 58 (33) | 7 (8.8) | 0 |
| Low mean arterial pressure | 5 (2.8) | 4 (5.0) | 0 |
| Low central venous pressure | 4 (2.3) | 2 (2.5) | 0 |
| High systemic vascular resistance | 2 (1.1) | 1 (1.3) | 0 |
| Clinical signs of hypoperfusion | 32 (18) | 47 (59) | 5 (100) |
| Oliguria | 20 (11) | 43 (54) | 2 (40) |
| Tachycardia | 4 (2.3) | 33 (41) | 3 (60) |
| Cold extremities | 16 (9.0) | 35 (44) | 2 (40) |
| Altered mental state | 10 (5.6) | 2 (2.5) | 0 |
| Clammy skin | 4 (2.3) | 0 | 0 |
| Others | 0 (0.0) | 3 (3.8) | 0 |
| Decreased oxygen saturation | 11 (6.2) | 28 (35) | 1 (20) |
| High difference between arterial and venous saturation | 0 | 22 (28) | 1 (20) |
| Low arterial oxygen pressure | 3 (1.7) | 0 | 0 |
| Low venous oxygen saturation | 9 (5.1) | 10 (13) | 0 |
| PCWP | 16 (9.0) | 0 | 0 |
| Renal replacement therapy | 2 (1.1) | 1 (1.3) | 0 |
| Clinical judgement | 9 (5.1) | 1 (1.3) | 0 |
| Cardiac arrest | 0 | 22 (28) | 1 (20) |
| Death | 2 (1.1) | 6 (7.5) | 0 |
| Other | 9 (5.1) | 9 (11) | 0 |
Variables used in low cardiac output syndrome definitions.
PICU, pediatric intensive care unit; VIS, vasoactive-inotropic score; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; VAD, ventricle assistant device; CI, cardiac index; PCWP, pulmonary capillary wedge pressure.
The need of inotropes was used in the definition in 71 and 61% of the articles including adult and pediatric cardiac surgery patients, respectively. There were four different ways in which inotropes were used in definitions, namely: (1) the duration of inotrope administration; (2) the specific inotropic drug used (epinephrine, norepinephrine, dopamine, dobutamine and milrinone); (3) the Vasoactive-Inotropic Score (VIS), a formula that quantifies the amount of cardiovascular support (25, 26); and (4) the number of used inotropes (Table 1). In studies involving the adult population, the cut-off value for the duration of inotrope administration in LCOS definitions was shorter (median 2.0 h) than for the studies involving the pediatric population (median 24.0 h). Compared to the pediatric population, in studies including adults, the inotropic drug was more often specified (25 vs. 9%) and VIS and the number of inotropes used were less likely used in the definition (1 vs 16%, and 7 vs 24%, respectively).
Cohort study
Between June 2011 and August 2018, 7,366 patients underwent cardiac surgery with cardiopulmonary bypass. The cohort included 5,934 (80.6%) adults, 690 (9.4%) children and 742 (10.1%) infants (Table 2). In all groups, there were more males than females. In adults, coronary artery bypass grafting (CABG) was the most frequently performed procedure. Repairs for atrial septal defects and ventricle septal defects were performed most frequently in the pediatric and infant group, respectively. Adults and infants had a higher in-hospital mortality rate than children: 3.1, 3.0, and 0.7%, respectively.
Table 2
| Adults (N = 5,934) | Children (N = 690) | Infants (N = 742) | |
|---|---|---|---|
| Male sex, n (%) | 4,136 (70) | 368 (53) | 432 (58) |
| Age, median (IQR) | 66 (56–73) years | 3.0 (1.0–9.0) years | 2.0 (0.0–4.0) months |
| Body surface area, median (IQR) | 1.96 (1.8–2.1) | 0.8 (0.5–1.1) | 0.3 (0.2–0.3) |
| Urgency of surgery, n(%) | |||
| Elective | 5,383 (91) | 677 (98) | 710 (96) |
| Emergency | 551 (9.3) | 13 (1.9) | 32 (4.3) |
| Reoperation, n (%)* | 255 (4.3) | 90 (13) | 121 (16) |
| Type of surgery, n (%) ** | CABG 2,462 (41.5) | ASD surgery 135 (19.6) | Combined congenital surgery 160 (21.6) |
| AV surgery 584 (9.8) | VSD surgery 87 (12.6) | VSD surgery 118 (15.9) | |
| Combined CABG and AV surgery 475 (8.0) | Combined congenital surgery 82 (11.9) | Tetralogy of Fallot 91 (12.3) | |
| MV surgery 304 (5.1) | PV surgery 74 (10.7) | Arterial switch operation 62 (8.4) | |
| Thoracic aortic surgery 201 (3.4) | Repair of anomalous pulmonary venous connection 71 (10.3) | Repair of anomalous pulmonary venous connection 54 (7.3) | |
| Duration of operation (min), median (IQR) | 282 (239–351) | 247 (197–310) | 282 (230–337) |
| Length of CPB (min), median (IQR) | 107 (78–158) | 68 (48–114) | 113 (72–147) |
| Need of MCS, n (%) | 316 (5.3) | 4 (0.6) | 3 (0.4) |
| 30-day mortality, n (%) | 182 (3.1) | 5 (0.7) | 22 (3.0) |
Patient demographics and baseline characteristics.
Reoperation was defined as any patient, who had more than one cardiac surgery in our institution between June 2011 and August 2018.
In this table we only show the five most frequently performed procedures.
IQR, interquartile range; CABG, coronary artery bypass grafting; AV, aortic valve; MV, mitral valve; ASD, atrial septum defect; VSD, ventricle septum defect; PV, pulmonary valve; min, minutes; CPB, cardiopulmonary bypass; MCS, mechanical circulatory support.
Incidences of low cardiac output syndrome
We used the complete cohort (5,934 adults, 690 children, 742 infants) as denominator within the LCOS incidence calculation for the 10 most frequently published definitions. Tables 3, 4 show the number of patients available to count the LCOS cases in the numerator. We were unable to calculate the incidences for all definitions due to missing not at random (MNAR) data. As an example, children and infants did not receive invasive cardiac output monitoring (such devices are not intended nor validated for pediatric use) and no extracorporeal circulation devices like ventricular assist or intra-aortic balloon counterpulsation (IABP) devices.
Table 3
| Definitions* | Appearance of definition in articles n (%) | Articles concerning adults [A], children [C] or both [AC] | |
|---|---|---|---|
| 1 | Cardiac index < 2.0 L/min/M2 | 16 (5.9) (27–42) | [AC] |
| 2 | Duration of inotropic use for >30 min with specified types of inotropes to maintain a systolic blood pressure >90 mmHg*; or the use of IABP; or a cardiac index < 2.2 L/min/M2 | 13 (4.8) (2–4, 8, 43–51) | [A] |
| 3 | Duration of inotropic use for >30 min to maintain a systolic blood pressure 90 mmHg*; or the use of IABP; or cardiac index < 2.2 L/min/M2 | 9 (3.3) (52–60) | [A] |
| 4 | The use of more than one inotropic drug and a lactate >2.0 mmol/L | 6 (2.2) (9, 15) | [C] |
| 5 | Duration of inotropic support for >24 h; or a cardiac index < 2.0 L/min/M2** | 6 (2.2) (6, 61–65) | [A] |
| Metabolic acidosis (a lactate >2.0 or base difference >4.0) with clinical signs of hypoperfusion (tachycardia >90/min* or oliguria 0.5 ml/kg/h); or an arterial-venous saturation difference >30% with clinical signs of hypoperfusion (tachycardia >90/min* or oliguria 0.5 ml/kg/h); or a cardiac arrest | 6 (2.2) (66–71) | [C] | |
| 7 | Cardiac index < 2.0 L/min/M2 and an increased PCWP | 6 (2.2) (12, 72–75) | [A] |
| 8 | Cardiac index < 2.0 L/min/M2 despite the use of inotropes (not further specified); or the use of IABP | 5 (1.8) (76–80) | [A] |
| 9 | The use of inotropes (not further specified); or mechanical support (ECMO, IABP or VAD) | 5 (1.8) (81–85) | [A] |
| 10 | Mechanical support (ECMO, IABP or VAD) | 5 (1.8) (86–90) | [A] |
Top 10 most published definitions of low cardiac output syndrome.
If articles used different cut-off values, e.g., cardiac index cut-off values ranched from 1.75 till 3.0, we present the median cut-off value.
IABP, intra-aortic balloon pump; PCWP, pulmonary capillary wedge pressure; ECMO, extracorporeal membrane oxygenation; VAD, ventricle assistant device.
Table 4
| Top 10 LCOS definitions (see Table 3 for definitions) | Adults ( N = 5,934) | Children ( N = 690) | Infants ( N = 742) | |||
|---|---|---|---|---|---|---|
| No. of patients without missing data | Incidence LCOS in % | No. of patients without missing data | Incidence LCOS in % | No. of patients without missing data | Incidence LCOS in % | |
| 1 | 400 | 1.5 | 0 | – | 0 | – |
| 2* | 5,667 | 8.3 | 516 | 23.8 | 534 | 30.1 |
| 3* | 5,667 | 31.5 | 516 | 27.4 | 534 | 32.3 |
| 4 | 2,049 | 12.3 | 688 | 5.1 | 739 | 16.3 |
| 5** | – | – | – | – | – | – |
| 6* | 3,365 | 13.7 | 542 | 6.4 | 571 | 14.4 |
| 7 | 0 | – | 0 | – | 0 | – |
| 8 | 414 | 1.8 | 0 | – | 0 | – |
| 9 | 5,934 | 91.0 | 690 | 55.7 | 742 | 82.2 |
| 10 | 5,934 | 4.5 | 690 | 0.6 | 742 | 0.4 |
Top 10 most published definitions of Low Cardiac Output Syndrome (LCOS) and corresponding incidence rates in an intraoperative cohort with adults, children and infants.
For systolic hypotension and tachycardia we used for children and infants the p-values (91, 92). The p5 for systolic hypotension and p90 for tachycardia.
Intensive care follow-up data was not available (e.g., maximum duration of inotropic support). Hence, we could not determine the incidence of LCOS using this definition.
LCOS, low cardiac output syndrome.
In all three groups (adults, children and infants), applying the definition “The need of inotropic useORmechanical support (IABP, VAD or ECMO)” resulted in the highest LCOS incidence: 91.0% in adults, 55.7% in children and 82.2% in infants, respectively (Table 4). The definition “Cardiac index <2.0 L/min/M2” resulted in the lowest incidence in adults (1.5%). The definition “The need for mechanical support” resulted in the lowest incidences in children and infants, respectively 0.6 and 0.4%. Definitions without missing data were “the need of inotropic use or mechanical support” and “the need of mechanical support.”
Discussion
This study summarized different criteria used for the definition of LCOS described in literature and subsequently estimated the incidence of LCOS immediately after surgery by applying these definitions to a large patient cohort. We found 171 different definitions and using the 10 most frequently reported ones resulted in an estimated incidence of intraoperative LCOS ranging from 1.5%−91% and 0.6%−56% in adults and in children, respectively. To the best of our knowledge, this is the first article to focus on the description and use of different LCOS definitions.
Low cardiac output syndrome, caused by an inadequate cardiac pump function, is a serious complication after cardiac surgery with high morbidity and mortality (2–4, 6, 8, 12). Far-reaching scientific interest in different kinds of interventions and their effect on LCOS have resulted in numerous publications on the subject. Many studies used the incidence of LCOS as a primary outcome. Although the criteria used to define LCOS were reported in most articles, these were frequently not reproducible (34%) and most articles did not explain why specific criteria were chosen. This resulted in the use of pluriform criteria to define the syndrome. In our study, we found a striking total of 171 different variations to define LCOS. Furthermore, we noticed that definitions used also greatly differed between the adult and pediatric populations. Our study demonstrated that the definition of LCOS is a very important explanatory determinant for the reported incidence of LCOS.
Currently, there is no uniform definition of LCOS, despite the presumed importance that clinicians use the same language and the generalizability of future scientific evaluations of new therapies. Therefore, we question whether LCOS should be used to describe an inadequate cardiac pump function after surgery without taking the necessary step toward uniformity. A uniform definition ensures that there will be fewer reasoning errors, misunderstandings, unnecessary controversies or problems in comparing scientific results. We argue that a good definition should be reproducible, generally valid among different populations and measurements should be as less invasive as possible (minimizing the risk of side effects). Abstract concepts, vague terminology and measurements without cut-off values result in unreproducible definitions with an immediate effect on the incidence rate of a certain outcome like LCOS. From our literature review, 34% of the definitions were not reproducible, due to lack of cut-off values or vague terminology. None reproducible criteria hamper generalizability of findings and re-evaluation of study results. Furthermore, definitions should preferably not rely on invasive monitoring technology that is only used in high risk populations, especially if there are good alternatives without those consequences. For example, the pulmonary capillary wedge pressure was frequently used in the definitions of LCOS (10% of the articles with an adult cardiac population). A Pulmonary Artery Catheter gives an inherent risk of mechanical, thrombotic, and infectious complications (93, 94) and is therefore only used in complex cardiac cases. These practical issues bias the incidence of LCOS as a significant part of the study population never adheres to the criteria because PCWP or CI were not routinely measured. Also, PCWP and CI were not measured in children because of technical impossibilities and the unavailability of validated monitoring equipment for pediatric use.
Another interesting finding of this study is the apparent difference between definitions used in adults and children. These differences elicit the question whether we are describing two different disease entities with maybe also a different biochemical origin. For LCOS in adults, studies used the items “mechanical support” and “cardiac pump function” more often, whereas in children, studies frequently used the items “metabolic acidosis” and “clinical signs of hypoperfusion.” Although it needs no explanation that pediatric patients differ from adult cardiac surgery patients, in both settings a valid definition for post-bypass inadequate cardiac pump function or LCOS is valuable and may contribute to the generalizability of scientific work.
Our study certainly has some limitations. First, our literature review was executed in PubMed and other databases were not searched for, and we thus might have missed relevant publications, and we did not publish nor register the study protocol. Second, this is retrospective review of a single-center experience and so this implicates that we could not track down the reasons behind inotropic use, mechanical support and others. We also had to assume the association between the therapeutic interventions and vital parameters measured which might limit generalizability. Third, we did not have any information about observations like cold extremities or altered state of mind, as we used intraoperative data and these were uncommonly observed and documented in the AIMS. As a result, we expressed the clinical signs of hypoperfusion solely with tachycardia and oliguria. Fourth, we had missing data for some of the items in the LCOS definitions that were time dependent. We only used intra-operative data, collected from our AIMS and no postoperative follow-up data, while the peak incidence of LCOS is expected 6–12 h after cardiac surgery. For that reason also, we were unable to calculate the incidence of LCOS in one of the 10 most frequently published definitions, that used inotropic support for 24 h after cardiac surgery as criterion. These limitions may cause under- and/or overestimations of the incidence of LCOS. However, because our primary outcome was the difference in incidence of LCOS using different definitions, we still report these numbers to show the effect different definitions have on the incidence. Finally, most definitions are also applicable during postoperative admission. We did not collect data about decreased cardiac indices or decreased systolic blood pressures at the ICU, which may be considered as a major limitation of our study. However, this study should not be used as a reference for LCOS occurrence after cardiac surgery, because we mainly aimed to illustrate the effect of the absence of a uniform LCOS definition in daily practice. Our study results could additionally serve to compare different age groups.
We suggest that consensus should be reached about a reproducible and practical LCOS definition within and between the international scientific societies. Prospective research that evaluates this universal LCOS definition would help to understand the features and occurrence of the syndrome in adults, children and infants. Furthermore, studies (95) in which LCOS is precursor for poor outcome, would enable the use of LCOS as a useful surrogate endpoint.
Conclusion
This study collected different definitions of LCOS and evaluated how they influenced estimations of the intraoperative incidence of LCOS in adults, children and infants. From the 171 different kind of definitions found, we used the 10 most frequently published and applied these to a large sized cohort including patients from all ages. We calculated LCOS incidence estimates ranging form 0.4 to 91%. We would like to advocate for standardization of the LCOS definition to improve clinical understanding and enable adequate comparison of outcomes and treatment effects both in daily care and in research.
Funding
WK is supported by the R. Fraser Elliott Chair in Cardiac Anesthesia (Toronto, ON, Canada).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AS and KvL contributed to conception and design of the study, performed the statistical analysis and wrote the first draft of the manuscript. LvW designed and completed the database for the retrospective cohort study. WvK contributed with a thorough review and rewriting of the manuscript draft. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.926957/full#supplementary-material
- LCOS
low cardiac output syndrome
- PICU
pediatric intensive care unit
- AIMS
anesthesia information management system
- UMCU
University Medical Center Utrecht
- CPB
cardiopulmonary bypass
- SD
standard deviation
- IQR
interquartile range
- PCWP
pulmonary capillary wedge pressure
- ECMO
extracorporeal membrane oxygenation
- IABP
intra-aortic balloon pump
- VAD
ventricle assistant device
- CI
cardiac index
- VIS
vasoactive-inotropic score
- CABG
coronary artery bypass grafting
- AV
aortic valve
- MV
mitral valve
- ASD
atrial septum defect
- VSD
ventricle septum defect
- PV
pulmonary valve
- min
minutes
- MNAR
missing not at random
- ICU
intensive care unit.
Abbreviations
References
1.
Lomivorotov VV Efremov SM Kirov MY Fominskiy EV Karaskov AM . Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. (2017) 31:291–308. 10.1053/j.jvca.2016.05.029
2.
Maganti M Badiwala M Sheikh A Scully H Feindel C David TE et al . Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg. (2010) 140:790–6. 10.1016/j.jtcvs.2009.11.022
3.
Algarni KD Maganti M Yau TM . Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. (2011) 92:1678–84. 10.1016/j.athoracsur.2011.06.017
4.
Rao V Ivanov J Weisel RD Ikonomidis JS Christakis GT David TE . Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. (1996) 112:38–51. 10.1016/S0022-5223(96)70176-9
5.
Osawa EA Rhodes A Landoni G Galas FRBG Fukushima JT Park CHL et al . Effect of perioperative goal-directed hemodynamic resuscitation therapy on outcomes following cardiac surgery: a randomized clinical trial and systematic review. Crit Care Med. (2016) 44:724–33.
6.
Hogue CW Sundt T Barzilai B Schecthman KB Dávila-Román VG . Cardiac and neurologic complications identify risks for mortality for both men and women undergoing coronary artery bypass graft surgery. Anesthesiology. (2001) 95:1074–8. 10.1097/00000542-200111000-00008
7.
Algarni KD Weisel RD Caldarone CA Maganti M Tsang K Yau TM . Microplegia during coronary artery bypass grafting was associated with less low cardiac output syndrome: a propensity-matched comparison. Ann Thorac Surg. (2013) 95:1532–8. 10.1016/j.athoracsur.2012.09.056
8.
Maganti MD Rao V Borger MA Ivanov J David TE . Predictors of low cardiac output syndrome after isolated aortic valve surgery. Circulation. (2005) 112(9 Suppl) I448–52. 10.1161/CIRCULATIONAHA.104.526087
9.
Flores S Cooper DS Opoka AM Iliopoulos I Pluckebaum S Alder MN et al . Characterization of the glucocorticoid receptor in children undergoing cardiac surgery. Pediatr Crit Care Med. (2018) 19:705–12. 10.1097/PCC.0000000000001572
10.
Cavigelli-Brunner A Hug MI Dave H Baenziger O Buerki C Bettex D et al . Prevention of low cardiac output syndrome after pediatric cardiac surgery: a double-blind randomized clinical pilot study comparing dobutamine and milrinone. Pediatr Crit Care Med. (2018) 19:619–25. 10.1097/PCC.0000000000001533
11.
Busro PW Romolo H Sastroasmoro S Rachmat J Sadikin M Santoso A et al . Role of terminal warm blood cardioplegia in complex congenital heart surgery. Asian Cardiovasc Thorac Ann. (2018) 26:196–202. 10.1177/0218492318759105
12.
Michalopoulos A Tzelepis G Pavlides G Kriaras J Dafni U Geroulanos S . Determinants of duration of ICU stay after coronary artery bypass graft surgery. Br J Anaesth. (1996) 77:208–12. 10.1093/bja/77.2.208
13.
Hummel J Rücker G Stiller B . Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease. Cochrane Database Syst Rev. (2017) 8:CD011312. 10.1002/14651858.CD011312.pub3
14.
Burkhardt BEU Rücker G Stiller B . Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease. Cochrane Database Syst Rev. (2015) 2015:CD009515. 10.1002/14651858.CD009515.pub2
15.
Iliopoulos I Alder MN Cooper DS Villarreal EG Loomba R Sahay RD et al . Pre-operative neutrophil-lymphocyte ratio predicts low cardiac output in children after cardiac surgery. Cardiol Young. (2020) 30:521–5. 10.1017/S1047951120000487
16.
Manso PH Carmona F Dal-Pizzol F Petronilho F Cardoso F Castro M et al . Oxidative stress markers are not associated with outcomes after pediatric heart surgery. Paediatr Anaesth. (2013) 23:188–94. 10.1111/pan.12040
17.
US National Library of Medicine National Institutes of Health . PubMed - NCBI.gov.
18.
Ok YJ Lim JY Jung SH . Critical illness-related corticosteroid insufficiency in patients with low cardiac output syndrome after cardiac surgery. Korean J Thorac Cardiovasc Surg. (2018) 51:109–13. 10.5090/kjtcs.2018.51.2.109
19.
Talma H Schönbeck Y Bakker B Hirasing RA van Buuren S . Groeidiagrammen 2010: Nederlandse Jongens 0 - 4 Jaar; Nederlandse Meisjes 0–4 Jaar;Nederlandse Jongens 1 - 21 Jaar;NederlandseMeisjes 1 - 21 Jaar. Leiden: TNOKwaliteit van Leven (2011). p. 42–64.
20.
Baum V YKSDd . Cardiovascular physiology. In:DavisPCladisF, eds. Smith's Anesthesia for Infants and Children, 9th ed. St. Louis, MO: Elsevier (2017), p. 73–89.
21.
RStudio Team . RStudio: Integrated development for R.Boston, MA: RStudio Team (2015).
22.
Ellenberger C Sologashvili T Cikirikcioglu M Verdon G Diaper J Cassina T et al . Risk factors of postcardiotomy ventricular dysfunction in moderate-to-high risk patients undergoing open-heart surgery. Ann Card Anaesth. (2017) 20:287–96. 10.4103/aca.ACA_60_17
23.
Flores S Fitzgerald MR Iliopoulos I Daily JA Rodriguez M Nelson DP et al . An international survey of corticosteroid use for the management of low cardiac output syndrome. Pediatr Crit Care Med. (2017) 18:630–7. 10.1097/PCC.0000000000001180
24.
Pérez-Navero JL de la Torre-Aguilar MJ Ibarra de la Rosa I Gil-Campos M Gómez-Guzmán E Merino-Cejas C et al . Cardiac biomarkers of low cardiac output syndrome in the postoperative period after congenital heart disease surgery in children. Rev Esp Cardiol. (2017) 70:267–74. 10.1016/j.rec.2016.09.011
25.
Gaies MG Jeffries HE Niebler RA Pasquali SK Donohue JE Yu S et al . Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. (2014) 15:529–37. 10.1097/PCC.0000000000000153
26.
Yamazaki Y Oba K Matsui Y Morimoto Y . Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth. (2018) 32:167–73. 10.1007/s00540-018-2447-2
27.
Nguyen LS Squara P Amour J Carbognani D Bouabdallah K Thierry S et al . Intravenous ivabradine versus placebo in patients with low cardiac output syndrome treated by dobutamine after elective coronary artery bypass surgery: a phase 2 exploratory randomized controlled trial. Crit Care. (2018) 22:193. 10.1186/s13054-018-2124-8
28.
Guo Y He S Wang T Chen Z Shu Y . Comparison of modified total leaflet preservation, posterior leaflet preservation, and no leaflet preservation techniques in mitral valve replacement - a retrospective study. J Cardiothorac Surg. (2019) 14:102. 10.1186/s13019-019-0918-7
29.
Jha AK Hittalmani SK . Septic shock in low-cardiac-output patients with heart and lung transplantation: diagnosis and management dilemma. J Cardiothorac Vasc Anesth. (2017) 31:1389–96. 10.1053/j.jvca.2016.11.003
30.
Chandler HK Kirsch R . Management of the low cardiac output syndrome following surgery for congenital heart disease. Curr Cardiol Rev. (2016) 12:107–11. 10.2174/1573403X12666151119164647
31.
Gist KM Goldstein SL Joy MS Vinks AA . Milrinone dosing issues in critically ill children with kidney injury: a review. J Cardiovasc Pharmacol. (2016) 67:175–81. 10.1097/FJC.0000000000000327
32.
Zhang J Lang Y Guo L Song X Shu L Su G et al . Preventive use of intra-aortic balloon pump in patients undergoing high-risk coronary artery bypass grafting: a retrospective study. Med Sci Monit. (2015) 21:855–60. 10.12659/MSM.893021
33.
Sharma P Malhotra A Gandhi S Garg P Bishnoi A Gandhi H . Preoperative levosimendan in ischemic mitral valve repair. Asian Cardiovasc Thorac Ann. (2014) 22:539–45. 10.1177/0218492313499352
34.
Barile L Landoni G Pieri M Ruggeri L Maj G Nigro Neto C et al . Cardiac index assessment by the pressure recording analytic method in critically ill unstable patients after cardiac surgery. J Cardiothorac Vasc Anesth. (2013) 27:1108–13. 10.1053/j.jvca.2013.02.016
35.
Börgermann J Hakim K Renner A Parsa A Aboud A Becker T et al . Clampless off-pump versus conventional coronary artery revascularization: a propensity score analysis of 788 patients. Circulation. (2012) 126(11 Suppl 1):S176–82. 10.1161/CIRCULATIONAHA.111.084285
36.
Biancari F Mikkola R Heikkinen J Lahtinen J Kettunen U Juvonen T . Individual surgeon's impact on the risk of re-exploration for excessive bleeding after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. (2012) 26:550–6. 10.1053/j.jvca.2012.02.009
37.
Howell NJ Ashrafian H Drury NE Ranasinghe AM Contractor H Isackson H et al . Glucose-insulin-potassium reduces the incidence of low cardiac output episodes after aortic valve replacement for aortic stenosis in patients with left ventricular hypertrophy: results from the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) trial. Circulation. (2011) 123:170–7. 10.1161/CIRCULATIONAHA.110.945170
38.
Zangrillo A Maj G Monaco F Scandroglio AM Nuzzi M Plumari V et al . Cardiac index validation using the pressure recording analytic method in unstable patients. J Cardiothorac Vasc Anesth. (2010) 24:265–9. 10.1053/j.jvca.2009.09.019
39.
Remadi JP Rakotoarivelo Z Marticho P Benamar A . Prospective randomized study comparing coronary artery bypass grafting with the new mini-extracorporeal circulation Jostra System or with a standard cardiopulmonary bypass. Am Heart J. (2006) 151:198.e1–7. 10.1016/j.ahj.2005.03.067
40.
Raja SG Haider Z Ahmad M . Predictors of gastrointestinal complications after conventional and beating heart coronary surgery. Surgeon. (2003) 1:221–8. 10.1016/S1479-666X(03)80021-5
41.
Tritapepe L Voci P Cogliati AA Pasotti E Papalia U Menichetti A . Successful weaning from cardiopulmonary bypass with central venous prostaglandin E1 and left atrial norepinephrine infusion in patients with acute pulmonary hypertension. Crit Care Med. (1999) 27:2180–3. 10.1097/00003246-199910000-00018
42.
Ruokonen E Takala J Kari A . Regional blood flow and oxygen transport in patients with the low cardiac output syndrome after cardiac surgery. Crit Care Med. (1993) 21:1304–11. 10.1097/00003246-199309000-00012
43.
Ak K Demirbaş E Ataş H Birkan Y Herz FA . 2017 undefined. Results of pericardiectomy for constrictive pericarditis. New York, NY: Springer. Available online at: https://link.springer.com/article/10.1007/s00059-016-4436-2
44.
Ansley DM Raedschelders K Choi PT Wang B Cook RC Chen DDY . Propofol cardioprotection for on-pump aortocoronary bypass surgery in patients with type 2 diabetes mellitus (PRO-TECT II): a phase 2 randomized-controlled trial. Can J Anaesth. (2016) 63:442–53. 10.1007/s12630-015-0580-z
45.
Kunt AS Andac MH . Decrease of total antioxidative capacity in developed low cardiac output syndrome. Oxid Med Cell Longev. (2012) 2012:356301. 10.1155/2012/356301
46.
Tsai YT Lin FY Lai CH Lin YC Lin CY Tsai CS . On-pump beating-heart coronary artery bypass provides efficacious short- and long-term outcomes in hemodialysis patients. Nephrol Dial Transplant. (2012) 27:2059–65. 10.1093/ndt/gfr536
47.
Spiliotopoulos K Maganti M Brister S Rao V . Changing pattern of reoperative coronary artery bypass grafting: a 20-year study. Ann Thorac Surg. (2011) 92:40–7. 10.1016/j.athoracsur.2011.03.104
48.
Maganti M Brister SJ Yau TM Collins S Badiwala M Rao V . Changing trends in emergency coronary bypass surgery. J Thorac Cardiovasc Surg. (2011) 142:816–22. 10.1016/j.jtcvs.2011.01.021
49.
Nicolini F Beghi C Barbieri F Secchi P Agostinelli A Fragnito C et al . Aortic valve replacement in octogenarians: analysis of risk factors for early and late mortality. J Heart Valve Dis. (2010) 19:615–22.
50.
Nicolini F Fragnito C Molardi A Agostinelli A Campodonico R Spaggiari I et al . Heart surgery in patients on chronic dialysis: is there still room for improvement in early and long-term outcome?Heart Vessels. (2011) 26:46–54. 10.1007/s00380-010-0024-1
51.
Açil T Türköz R Açil M Sezgin AT Baltali M Gülcan Ö et al . Value of prolonged QRS duration as a predictor of low cardiac output syndrome in patients with impaired left ventricular systolic function who undergo isolated coronary artery bypass grafting. Am J Cardiol. (2006) 98:1357–62. 10.1016/j.amjcard.2006.06.031
52.
Rzucidło-Resil J Plicner D Gackowski A Kapelak B Stoliński J . Impact of the mechanism of mitral regurgitation on clinical outcomes in patients after mitral valve surgery. Kardiol Pol. (2019) 77:525–34. 10.5603/KP.a2019.0043
53.
Zhao K Zhang Y Li J Cui Q Zhao R Chen W et al . Modified glucose-insulin-potassium regimen provides cardioprotection with improved tissue perfusion in patients undergoing cardiopulmonary bypass surgery. J Am Heart Assoc. (2020) 9:e012376. 10.1161/JAHA.119.012376
54.
Yuan X Li B Yang Y Wang H Sun H Song Y et al . Surgical results and pathological analysis of cardiac fibroma in the adolescent and the adult. J Card Surg. (2020) 35:1912–9. 10.1111/jocs.14790
55.
Anantasit N Boyd JH Russell JA Fjell CD Lichtenstein SV . Walley KR Prolonged QTc affects short-term and long-term outcomes in patients with normal left ventricular function undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2014) 147:1627–33. 10.1016/j.jtcvs.2013.11.043
56.
Rosu C Laflamme M Perrault-Hébert C Carrier M Perrault LP . Decreased incidence of low output syndrome with a switch from tepid to cold continuous minimally diluted blood cardioplegia in isolated coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. (2012) 15:655–60. 10.1093/icvts/ivs294
57.
Kim H. seon Kim KB Hwang HY Chang HW Park KJ . Subxiphoid incisional hernia development after coronary artery bypass grafting. Korean J Thorac Cardiovasc Surg. (2012) 45:161–5. 10.5090/kjtcs.2012.45.3.161
58.
Borger MA Seeburger J Walther T Borger F Rastan A Doenst T et al . Effect of preoperative statin therapy on patients undergoing isolated and combined valvular heart surgery. Ann Thorac Surg. (2010) 89:773–80. 10.1016/j.athoracsur.2009.12.001
59.
Miceli A Fiorani B Danesi TH Melina G Sinatra R . Prophylactic intra-aortic balloon pump in high-risk patients undergoing coronary artery bypass grafting: a propensity score analysis. Interact Cardiovasc Thorac Surg. (2009) 9:291–4. 10.1510/icvts.2008.196105
60.
Rao V Ivanov J Weisel RD Cohen G Borger MA Mickle DAG . Lactate release during reperfusion predicts low cardiac output syndrome after coronary bypass surgery. Ann Thorac Surg. (2001) 71:1925–30. 10.1016/S0003-4975(01)02634-0
61.
Lio A Bovio E Nicolò F Saitto G Scafuri A Bassano C et al . Influence of body mass index on outcomes of patients undergoing surgery for acute aortic dissection: a propensity-matched analysis. Tex Heart Inst J. (2019) 46:7–13. 10.14503/THIJ-17-6365
62.
García-Fuster R Estevez V Gil O Cánovas S Martínez-Leon J . Mitral valve replacement in rheumatic patients: effects of chordal preservation. Ann Thorac Surg. (2008) 86:472–81. 10.1016/j.athoracsur.2008.04.046
63.
Hogue CW Palin CA Kailasam R Lawton JS Nassief A Dávila-Román VG et al . C-reactive protein levels and atrial fibrillation after cardiac surgery in women. Ann Thorac Surg. (2006) 82:97–102. 10.1016/j.athoracsur.2006.02.043
64.
Hogue CW de Wet CJ Schechtman KB Dávila-Román VG . The importance of prior stroke for the adjusted risk of neurologic injury after cardiac surgery for women and men. Anesthesiology. (2003) 98:823–9. 10.1097/00000542-200304000-00006
65.
Hogue CW Murphy SF Schechtman KB Dávila-Román VG . Risk factors for early or delayed stroke after cardiac surgery. Circulation. (1999) 100:642–7. 10.1161/01.CIR.100.6.642
66.
Nordness MJ Westrick AC Chen H Clay MA . Identification of Low cardiac output syndrome at the bedside: a pediatric cardiac intensive care unit survey. Crit Care Nurse. (2019) 39:e1–7. 10.4037/ccn2019794
67.
Favia I Rizza A Garisto C Haiberger R di Chiara L Romagnoli S et al . Cardiac index assessment by the pressure recording analytical method in infants after paediatric cardiac surgery: a pilot retrospective study. Interact Cardiovasc Thorac Surg. (2016) 23:919–23. 10.1093/icvts/ivw251
68.
Pagowska-Klimek I Swierzko AS Michalski M Głowacka E Szala-Pozdziej A Sokołowska A et al . Activation of the lectin pathway of complement by cardiopulmonary bypass contributes to the development of systemic inflammatory response syndrome after paediatric cardiac surgery. Clin Exp Immunol. (2016) 184:257–63. 10.1111/cei.12763
69.
Pagowska-Klimek I Swierzko AS Michalski M Moll M Szala-Pozdziej A Sokołowska A et al . Mannose-binding lectin (MBL) insufficiency protects against the development of systemic inflammatory response after pediatric cardiac surgery. Immunobiology. (2016) 221:175–81. 10.1016/j.imbio.2015.09.010
70.
Bailey JM Hoffman TM Wessel DL Nelson DP Atz AM Chang AC et al . A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn. (2004) 31:43–59. 10.1023/B:JOPA.0000029488.45177.48
71.
Hoffman TM Wernovsky G Atz AM Bailey JM Akbary A Kocsis JF et al . Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics. Am Heart J. (2002) 143:15–21. 10.1067/mhj.2002.120305
72.
Michalopoulos A Stavridis G Geroulanos S . Severe sepsis in cardiac surgical patients. Eur J Surg. (1998) 164:217–22. 10.1080/110241598750004670
73.
Böhrer H Schmidt H Motsch J Gust R Bach A Martin E . Gastric intramucosal pH: a predictor of survival in cardiac surgery patients with low cardiac output?J Cardiothorac Vasc Anesth. (1997) 11:184–6. 10.1016/S1053-0770(97)90211-1
74.
George M Lehot JJ Bastien O Durand PG Estanove S . Comparison of cardiovascular effects of dobutamine and enoximone in treatment of low cardiac output syndrome after valvular surgery–preliminary results. J Cardiothorac Anesth. (1989) 3(5 Suppl 1):12. 10.1016/0888-6296(89)90755-2
75.
Roberts AJ Spies SM Lichtenthal PR Moran JM Sanders JH Michaelis LL . Changes in left ventricular performance related to perioperative myocardial infarction in coronary artery bypass graft surgery. Ann Thorac Surg. (1983) 35:516–24. 10.1016/S0003-4975(10)60425-0
76.
Christenson JT Schmuziger M Simonet F . Reoperative coronary artery bypass procedures: risk factors for early mortality and late survival. Eur J Cardiothorac Surg. (1997) 11:129–33. 10.1016/S1010-7940(96)01030-5
77.
Prifti E Bonacchi M Frati G Giunti G Proietti P Leacche M et al . Beating heart myocardial revascularization on extracorporeal circulation in patients with end-stage coronary artery disease. Cardiovasc Surg. (2001) 9:608–14. 10.1016/S0967-2109(01)00092-8
78.
Prifti E Bonacchi M Giunti G Frati G Proietti P Leacche M et al . Does on-pump/beating-heart coronary artery bypass grafting offer better outcome in end-stage coronary artery disease patients?J Card Surg. (2000) 15:403–10. 10.1111/j.1540-8191.2000.tb01300.x
79.
Lahtinen J Biancari F Ala-Kokko T Rainio P Salmela E Pokela R et al . Pulmonary artery blood temperature at admission to the intensive care unit is predictive of outcome after on-pump coronary artery bypass surgery. Scand Cardiovasc J. (2004) 38:104–12. 10.1080/14017430410028500
80.
Erkut B Dag O Kaygin MA Senocak M Limandal HK Arslan U et al . On-pump beating-heart versus conventional coronary artery bypass grafting for revascularization in patients with severe left ventricular dysfunction: early outcomes. Can J Surg. (2013) 56:398–404. 10.1503/cjs.018412
81.
Formica F Mariani S D'Alessandro S Singh G di Mauro M Cerrito MG et al . Does additional coronary artery bypass grafting to aortic valve replacement in elderly patients affect the early and long-term outcome?Heart Vessels. (2020) 35:487–501. 10.1007/s00380-019-01519-6
82.
Burgos LM Gil Ramírez A Seoane L Espinoza J Furmento JF Costabel JP et al . Is the Obesity paradox in cardiac surgery really a myth? Effect of body mass index on early and late clinical outcomes. J Cardiothorac Vasc Anesth. (2021) 35:492–8. 10.1053/j.jvca.2020.03.051
83.
Biancari F Onorati F Mariscalco G de Feo M Messina A Santarpino G et al . First-time, isolated surgical aortic valve replacement after prior coronary artery bypass surgery: results from the RECORD multicenter registry. J Card Surg. (2014) 29:450–4. 10.1111/jocs.12365
84.
Thielmann M Massoudy P Neuhäuser M Knipp S Kamler M Piotrowski J et al . Prognostic value of preoperative cardiac troponin I in patients with non-ST-segment elevation acute coronary syndromes undergoing coronary artery bypass surgery. Chest. (2005) 128:3526–36. 10.1016/S0012-3692(15)52926-7
85.
Ranucci M Frigiola A Menicanti L Ditta A Boncilli A Brozzi S . Postoperative antithrombin levels and outcome in cardiac operations. Crit Care Med. (2005) 33:355–60. 10.1097/01.CCM.0000153409.55645.58
86.
Kim BJ Kim YS Kim HJ Ju MH Kim JB Jung SH et al . Concomitant mitral valve surgery in patients with moderate ischemic mitral regurgitation undergoing coronary artery bypass grafting. J Thorac Dis. (2018) 10:3632–42. 10.21037/jtd.2018.05.148
87.
Mourad F Cleve N Nowak J Wendt D Sander A Demircioglu E et al . Long-term single-center outcomes of patients with chronic renal dialysis undergoing cardiac surgery. Ann Thorac Surg. (2020) 109:1442–8. 10.1016/j.athoracsur.2019.08.042
88.
Lee WY Yoo JS Kim JB Jung SH Choo SJ Chung CH et al . Outcomes of open surgical repair of descending thoracic aortic disease. Korean J Thorac Cardiovasc Surg. (2014) 47:255–61. 10.5090/kjtcs.2014.47.3.255
89.
Yoo JS Kim JB Jung SH Choo SJ Chung CH Lee JW . Surgical repair of descending thoracic and thoracoabdominal aortic aneurysm involving the distal arch: open proximal anastomosis under deep hypothermia versus arch clamping technique. J Thorac Cardiovasc Surg. (2014) 148:2101–7. 10.1016/j.jtcvs.2014.06.068
90.
Yoo JS Kim JB Joo Y Lee WY Jung SH Choo SJ et al . Deep hypothermic circulatory arrest versus non-deep hypothermic beating heart strategy in descending thoracic or thoracoabdominal aortic surgery. Eur J Cardiothorac Surg. (2014) 46:678–84. 10.1093/ejcts/ezu053
91.
Fleming S Thompson M Stevens R Heneghan C Plüddemann A Maconochie I et al . Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet. (2011) 377:1011–8. 10.1016/S0140-6736(10)62226-X
92.
Rosner B Cook N Portman R Daniels S Falkner B . Determination of blood pressure percentiles in normal-weight children: Some methodological issues. Am J Epidemiol. (2008) 167:653–66. 10.1093/AJE/KWM348
93.
Chiang Y Hosseinian L Rhee A Itagaki S Cavallaro P Chikwe J . Questionable benefit of the pulmonary artery catheter after cardiac surgery in high-risk patients. J Cardiothorac Vasc Anesth. (2015) 29:76–81. 10.1053/j.jvca.2014.07.017
94.
Judge O Ji F Fleming N Liu H . Current use of the pulmonary artery catheter in cardiac surgery: a survey study. J Cardiothorac Vasc Anesth. (2015) 29:69–75. 10.1053/j.jvca.2014.07.016
95.
Ulate KP Yanay O Jeffries H Baden H di Gennaro JL Zimmerman J . An elevated low cardiac output syndrome score is associated with morbidity in infants after congenital heart surgery. Pediatr Crit Care Med. (2017) 18:26–33. 10.1097/PCC.0000000000000979
Summary
Keywords
low cardiac output syndrome, complication, definitions, incidence, cardiac surgery, LCOS
Citation
Schoonen A, van Klei WA, van Wolfswinkel L and van Loon K (2022) Definitions of low cardiac output syndrome after cardiac surgery and their effect on the incidence of intraoperative LCOS: A literature review and cohort study. Front. Cardiovasc. Med. 9:926957. doi: 10.3389/fcvm.2022.926957
Received
23 April 2022
Accepted
29 August 2022
Published
29 September 2022
Volume
9 - 2022
Edited by
Luregn J. Schlapbach, University Children's Hospital Zurich, Switzerland
Reviewed by
Siva Namachivayam, Melbourne Children's Campus, Australia; Janet Fiona Kelly-Geyer, University Children's Hospital Zurich, Switzerland; Katie Moynihan, Boston Children's Hospital and Harvard Medical School, United States
Updates
Copyright
© 2022 Schoonen, van Klei, van Wolfswinkel and van Loon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Schoonen annaschoonen2@gmail.com
This article was submitted to Heart Surgery, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.