Abstract
Objectives:
To explore the associations between different types and doses of statins and adverse events in secondary prevention of cardiovascular disease.
Methods:
We searched PubMed, Embase, and Cochrane databases for randomized controlled trials that compared statins with non-statin controls or different types or doses of statins. The primary outcomes included muscle condition, transaminase elevations, renal insufficiency, gastrointestinal discomfort, cancer, new onset or exacerbation of diabetes, cognitive impairment, and eye condition. We also analyzed myocardial infarction (MI), stroke, death from cardiovascular diseases (CVD), and all-cause death as the secondary outcomes to compare the potential harms with the benefits of statins. We conducted pairwise meta-analyses to calculate the odds ratio (OR) and 95% confidence intervals (CIs) for each outcome. Network meta-analyses were performed to compare the adverse effects of different statins. An Emax model was used to examine the dose-response relationships of the adverse effects of each statin.
Results:
Forty-seven trials involving 107,752 participants were enrolled and followed up for 4.05 years. Compared with non-statin control, statins were associated with an increased risk of transaminase elevations [OR 1.62 (95% CI 1.20 to 2.18)]. Statins decreased the risk of MI [OR 0.66 (95% CI 0.61 to 0.71), P < 0.001], stroke [OR 0.78 (95% CI 0.72 to 0.84), P < 0.001], death from CVD [OR 0.77 (95% CI 0.72 to 0.83), P < 0.001] and all-cause death [OR 0.83 (95% CI 0.79 to 0.88), P < 0.001]. Atorvastatin showed a higher risk of transaminase elevations than non-statin control [OR 4.0 (95% CI 2.2 to 7.6)], pravastatin [OR 3.49 (95% CI 1.77 to 6.92)] and simvastatin [OR 2.77 (95% CI 1.31 to 5.09)], respectively. Compared with atorvastatin, simvastatin was associated with a lower risk of muscle problems [OR 0.70 (95% CI 0.55 to 0.90)], while rosuvastatin showed a higher risk [OR 1.75 (95% CI 1.17 to 2.61)]. An Emax dose-response relationship was identified for the effect of atorvastatin on transaminase elevations.
Conclusion:
Statins were associated with increased risks of transaminases elevations in secondary prevention. Our study provides the ranking probabilities of statins that can help clinicians make optimal decisions when there is not enough literature to refer to.
Systematic review registration:
[https://www.crd.york.ac.uk/prospero/], identifier [CRD42021285161].
Introduction
Statins are widely used in clinical practice and recommended as first-line treatment for atherosclerotic cardiovascular diseases (ASCVD) (1). However, various adverse events documented in clinical trials were considered statin-related, such as muscle problems and elevated hepatic transaminase (2, 3). Although other types of lipid-lowering agents are available (e.g., ezetimibe, niacin, PCSK9), so far there are no published trials with any of these new drugs in patients who are intolerant to statins. Physicians often face the dilemma of choosing optimal statins with the best efficacy and least adverse effects. Although previous study has reported comparative effectiveness and safety of statins as a class and of specific statins (4) in primary prevention in which usually a lower-intensity or dose was use, current guideline recommends that patients should be treated with the maximum-appropriate intensity of a statin that does not cause adverse effects for patients with ASCVD (5). In addition, although high-intensity statin therapy has been shown to reduce ASCVD events better than moderate or lower-intensity statin therapy (5), it is also associated with a greater risk of statin-induced adverse events (6). Therefore, it is of great necessity to assess the association of adverse events with the types and doses of statins in patients with ASCVD. The hypothesis suggested that the types and doses of statins may be related to different adverse reactions (7). Current suggestions on the type and dose of statins are based on their lipid-lowering effect, without considering the varying adverse reactions of different schemes. Understanding the relationship between the types and doses of statins and specific adverse events can help clinicians make appropriate choices. Therefore, we systematically reviewed randomized controlled trials (RCTs) in secondary prevention to evaluate the associations between statins and adverse events, and to explore how the associations vary by type and dose of statin.
Methods
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (8). The study protocol was registered on PROSPERO (CRD42021285161).
Search methods and resources
Studies were searched from PubMed, Embase and the Cochrane Database from their inception to October 2021. Also, we checked previous systematic reviews of clinical trials of statins to avoid omission. Supplementary Table 1 shows the detailed search strategies.
Selection of studies
Eligible studies were RCTs in patients with established ASCVD [i.e., coronary heart disease (CHD), peripheral artery disease, or cerebrovascular disease (5)], which compared statins with non-statin controls or compared different types or dosages of statins and reported at least one primary outcome of interest. Statin treatments were monotherapy or add-on treatment to routine care or non-drug treatments (e.g., diet or exercise). Non-statin controls included placebo, no treatment, and routine care. We also included studies involving > 60% of patients with established ASCVD to avoid the loss of large trials with a small proportion of patients without ASCVD. In instances where subgroup data for ASCVD patients was unpublished, the authors were contacted to request the data. Studies that enrolled < 100 patients (to exclude small studies with unreliable hazard ratios) or lasted for < 4 weeks of intervention were excluded (9). The eligibility criteria were detailly described in Supplementary Table 2. Two reviewers (XW and JL) independently screened titles and abstracts of all items and identified eligible trials. Discrepancies were resolved by consensus.
Study outcomes
The primary outcomes were reported adverse events in previous clinical trials, including muscle condition, transaminase elevations, renal insufficiency, gastrointestinal discomfort, cancer, new-onset or exacerbation of diabetes, cognitive impairment, and eye condition. Muscle condition included self-reported muscle symptoms (i.e., myalgia, muscle weakness, and other non-specified muscle discomforts) and clinically confirmed muscle disorders (i.e., myopathy and rhabdomyolysis) (10). Transaminase elevations referred to incidence of elevations in serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Renal insufficiency included any decline in renal function, the presence of proteinuria, and other diagnosed renal disorders. Gastrointestinal discomfort included nausea, vomiting, dyspepsia, constipation, abdominal pain and other symptoms related to the digestive system. Cancer referred to the incidence of any cancer, excluding non-melanoma skin cancer. New onset or exacerbation of diabetes (type 2 diabetes), cognitive impairment, and eye conditions were defined as the diagnoses in the original trials. We also analyzed myocardial infarction (MI), stroke, death from cardiovascular diseases (CVD), and all-cause death as the secondary outcomes to compare the potential harms with the benefits of statins.
Data extraction and quality assessment
Two reviewers (XW and JL) independently extracted the information on study design, characteristics of participants, interventions, controls, outcome measurements, details relevant to the risk of bias and the quality of the evidence. The risk of bias in individual studies was evaluated by the Cochrane risk of bias tool (11, 12). The quality of evidence for each outcome in the pairwise meta-analyses and significant results in the network meta-analyses were evaluated based on the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) process (12, 13). Any discrepancy that appeared during the data extraction or evaluation process was resolved through discussion.
Statistical analysis
Pairwise meta-analyses were conducted to compare the effect of statins and non-statin controls for each outcome. Heterogeneity among individual studies was assessed with the Q test and quantified with the I2 statistic (14). When no significant heterogeneity was detected (P > 0.05 for the Q test and I2 < 50%), a fixed-effects model was employed to calculate pooled odds ratios (OR) with 95% confidence intervals (CIs); otherwise, a random-effects model was used (15). Publication bias was examined by the Harbord test of the symmetry of funnel plots. The robustness of the pooled results was tested by leave-one-out influence analysis (16). For sensitivity analyses, studies with a non-double-blind design were excluded to examine the effect of placebo. Because the evidence showed that Asians were less tolerant to statins (17), we further excluded studies on Asian populations to investigate the influence of race/ethnicity. For further sensitivity analysis, we excluded studies or individuals with transaminase elevation < 3 times the upper limit of normal (ULN) for the outcome of transaminase elevations because the rise in serum concentration of transaminase to more than three times the ULN was often considered to be a mark of liver dysfunction. In addition, the random-effects model was used for all outcomes as the further sensitivity analysis.
We performed a network meta-analysis to compare the adverse effects between different types of statins and non-statin controls by the Bayesian method (18). A random-effects model was employed to calculate the pooled OR and 95% CI instead of a fixed-effects model because the former measure provided more conservative results (19). As an alternative method for inconsistency assessment in network meta-analysis, node-splitting analysis was used to evaluate the consistency of data (20). P value of node-splitting analysis > 0.05 indicates no significant inconsistency. We calculated the ranking probabilities for each treatment’s efficacy. Probability values were reported using surface under the cumulative ranking (SUCRA) values. A higher SUCRA value indicated a better outcome for that intervention (21).
A model-based meta-analysis method that fitted the dose-specific effects from a network meta-analysis to an Emax dose-response model was employed to examine the dose-response relationship of the adverse effects of individual statins (22, 23). The key parameter Emax represents the asymptotic maximum drug effect, and ED50 means the dose that produces half of the maximum effect (24). Posterior means and 95% CI of the model parameters were estimated by the Bayesian approach (22). We analyzed outcomes using the non-statin control as a reference and ranked different statins with SUCRA probabilities based on the dose-response relationship.
All statistical tests had a two-tailed significance level of P ≤ 0.05. Analyses were performed in R version 4.0.1 with meta, metafor, gemtc, rjags, and MBNMAdose packages.
Results
Our searches identified 11,973 citations (11,869 from database searches and 104 from previous meta-analyses). Finally, after assessing the full text, forty-seven eligible studies (25–71) were included (Figure 1). Supplementary Table 3 presents the list of studies excluded after assessing the full text and reasons for exclusion.
FIGURE 1
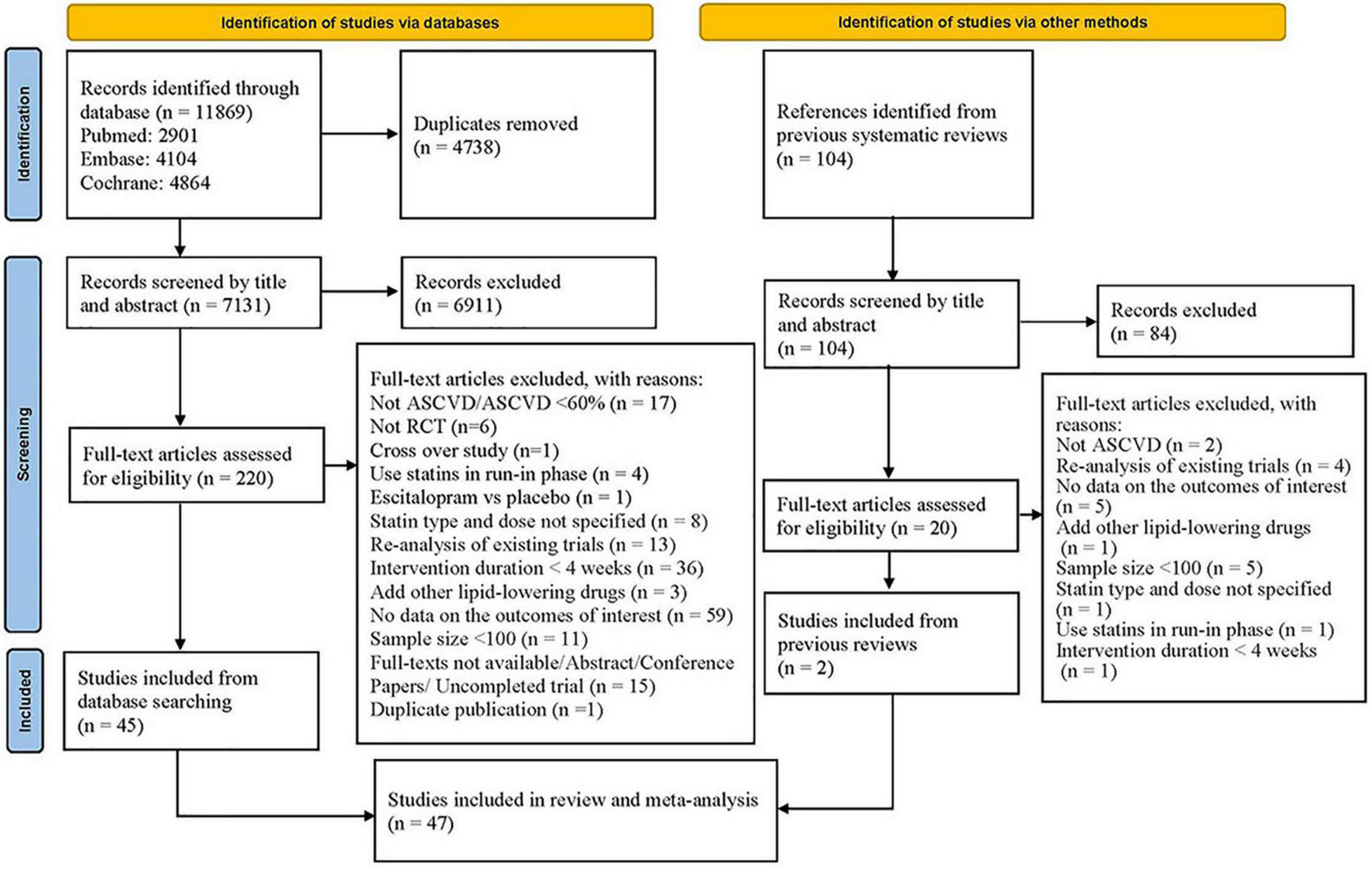
Flowchart of study selection.
Study characteristics
A total of 107,752 participants were enrolled and followed up for a mean of 4.05 years. The mean age of all participants was 62 years old, and 77% were men. The target populations in the included studies were various. Thirty-five trials enrolled patients with CHD, and other trials enrolled patients with cerebrovascular disease (4 studies) and ASCVD (2 studies), or ≥ 60% ASCVD (6 studies), respectively. Eighteen studies compared statins with non-statin controls that included placebo (14 studies) and no treatment (4 studies). Seven types of statins were evaluated: atorvastatin (29 studies), fluvastatin (3 studies), lovastatin (1 study), pitavastatin (2 studies), pravastatin (10 studies), rosuvastatin (8 studies), and simvastatin (12 studies). The characteristics of the included studies are shown in Table 1.
TABLE 1
| Author, year | No of participants | Country | Study duration | Study population | Mean age | Proportion of men (%) | Statin treatment (dose, mg/day) | Comparator |
| MARS, (25) | 247 | United States | 2.2 years | CHD | 58 | 91 | Lovastatin (80) | Placebo |
| Oxford Cholesterol, (26) | 621 | United Kingdom | 44 months | ASCVD > 60% | 63 | 85 | Simvastatin (20/40) | Placebo |
| 4S, (27) | 4,444 | Scandinavia | 5.4 years | CHD | 51% ≥ 60 year | 82 | Simvastatin 20 | Placebo |
| PLAC I, (28) | 408 | United Kingdom, United States, Canada | 3 years | CHD | 57 | 38 | Pravastatin (40) | Placebo |
| CARE, (29) | 4,159 | United States, Canada | 5 years | MI | 59 | 86 | Pravastatin (40) | Placebo |
| LIPID, (30) | 9,014 | Australia, New Zealand | 6.1 years | MI/unstable angina | 62 | 83 | Pravastatin (40) | Placebo |
| TARGET TANGIBLE, (31) | 2,856 | Germany | 3.5 months | CHD | 61 | 63 | Atorvastatin (10–40), Simvastatin (10–40) | Different statin types |
| FLARE, (32) | 834 | Europe | 10 months | PTCA | 61 | 83 | Fluvastatin (80) | Placebo |
| MIRACL, (33) | 3,086 | Europe, North America, South Africa and Australasia | 4 months | MI/unstable angina | 65 | 65 | Atorvastatin (80) | Placebo |
| Karalis et al., (34) | 1,595 | United States | 1.5 months | ASCVD > 60% | 61.5 | 62 | Atorvastatin (10/80), Simvastatin (20/80) | Different statin types and doses |
| LIPS, (35) | 1,677 | Europe, Canada and Brazil | 3.9 years | Stable or unstable angina | 60 | 84 | Fluvastatin (80) | Placebo |
| HPS, (36) | 20,536 | United Kingdom | 5 years | ASCVD > 60% | Not mentioned | 75 | Simvastatin (40) | Placebo |
| 3T, (37) | 1,093 | Denmark, Finland, Iceland, Norway, and Sweden | 13 months | CHD | 63 | 75 | Atorvastatin (20–40), Simvastatin (20–40) | Different statin types |
| REVERSAL, (38) | 654 | United States | 4.5 months | CHD | 56 | 72 | Pravastatin (40), Atorvastatin (80) | Different statin types |
| Schwartz et al., (39) | 383 | United States and Canada | 4.5 months | ASCVD | 62 | 61 | Rosuvastatin (5–80), Atorvastatin (10–80) | Different statin types |
| PROVE IT–TIMI 22, (40) | 4,162 | 349 sites in eight countries | 2 years | ACS | 58 | 78 | Pravastatin (40), Atorvastatin (80) | Different statin types |
| JUST, (41) | 299 | Japan | 2 years | CHD | 59 | 77 | Simvastatin (10) | No treatment |
| IDEAL, (42) | 8,888 | Northern Europe | 4.8 years | MI | 62 | 81 | Atorvastatin (80), Simvastatin (20) | Different statin types |
| TNT, (43) | 10,001 | 14 countries worldwide | 4.9 years | CHD | 61 | 81 | Atorvastatin (10/80) | Different statin doses |
| ATHEROMA, (44) | 361 | Japan | 3 years | CHD | 59 | 83 | Pravastatin (10–20) | No treatment |
| SPARCL, (45) | 4,731 | 205 centers worldwide | 4.9 years | Stroke or TIA | 63 | 60 | Atorvastatin (80) | Placebo |
| SOLAR, (46) | 1,621 | United States | 3 months | ASCVD > 60% | 62 | 58 | Rosuvastatin (10–20), Atorvastatin (10–20), Simvastatin (20–40) | Different statin types |
| ARIANE, (47) | 844 | France | 3 months | ASCVD > 60% | 63 | 76 | Atorvastatin (10), Rosuvastatin (10) | Different statin types |
| Yun et al., (48) | 155 | South Korea | 10 months | ACS/stroke | 63 | 60 | Rosuvastatin (10), Atorvastatin (20) | Different statin types |
| Yu et al., (49) | 112 | China | 6.5 months | CHD | 66 | 82 | Atorvastatin (10/80) | Different statin doses |
| SAGE, (50) | 891 | 192 sites worldwide in 16 countries | 1 year | CHD | 72 | 70 | Atorvastatin (80), Pravastatin (40) | Different statin types |
| CAP, (51) | 340 | Canada and Europe | 6.5 months | CHD | 63 | 83 | Atorvastatin (10/80) | Different statin doses |
| JAPAN-ACS, (52) | 296 | Japan | 1 year | ACS + PCI | 63 | 82 | Pitavastatin (4), Atorvastatin (20) | Different statin types |
| Zhao et al., (53) | 164 | China | 2 months | Unstable angina | 71 | 65 | Atorvastatin (20/80) | Different statin doses |
| SPACE ROCKET, (54) | 1,263 | United Kingdom | 3 months | MI | 62 | 79 | Simvastatin (40), Rosuvastatin (10) | Different statin types |
| Mok et al., (55) | 227 | United Kingdom | 2 years | MCA stenosis | 63 | 34 | Simvastatin (20) | Placebo |
| CENTAURUS, (56) | 829 | Belgium, Canada, Estonia, France, Greece, Hungary, Ireland, Italy, Portugal, Spain and Tunisia | 3 months | NSTEACS | 60 | 75 | Rosuvastatin (20); Atorvastatin (80) | Different statin types |
| FACS, (57) | 156 | Czechia | 1 month | ACS | 62 | 68 | Fluvastatin (80) | Placebo |
| SEARCH, (58) | 12,064 | United Kingdom | 6.7 years | MI | 64 | 83 | Simvastatin (20/80) | Different statin doses |
| LUNAR, (59) | 799 | United States, Costa Rica and Panama | 3 months | ACS | 53 | 76 | Rosuvastatin (20/40), Atorvastatin (80) | Different statin types and doses |
| TRUTH, (60) | 154 | Japan | 8 months | Stable/unstable angina + PCI | 67 | 83 | Pitavastatin (4), Pravastatin (20) | Different statin types |
| CURE-ACS, (61) | 173 | India | 3 months | ACS | 56 | 82 | Atorvastatin (40/80) | Different statin doses |
| PACT, (62) | 3,408 | Australia, Poland, Southeast Asian | 1 month | MI/unstable angina | Not mentioned | 76 | Pravastatin (20–40) | Placebo |
| Zhou et al., (63) | 112 | China | 13 months | AICAS | 63 | 68 | Atorvastatin (10/20/40) | Different statin doses |
| Khurana et al., (64) | 100 | India | 1 month | ACS | Not mentioned | Not mentioned | Atorvastatin (40), Rosuvastatin (20) | Different statin types |
| J-STARS, (65) | 1,565 | Japan | 4.9 years | Non-cardioembolic ischemic stroke | 66 | 69 | Pravastatin (10) | No treatment |
| Liu et al., (66) | 591 | China | 1 year | ACS + PCI | 62 | 49 | Atorvastatin (20/40) | Different statin doses |
| Priti et al., (67) | 1,027 | India | 1 month | STEMI | 57 | 74 | Atorvastatin (10/80) | Different statin doses |
| ACTIVE, (68) | 173 | United States, Canada | 1 year | CABG | 69 | 82 | Atorvastatin (10/80) | Different statin doses |
| Liu et al., (69) | 265 | China | 1 year | STEMI + PCI | 59 | 72 | Atorvastatin (20/40) | Different statin doses |
| Wang et al., (70) | 162 | China | 1 year | MI | 57 | 72 | Atorvastatin (20) | No treatment |
| Kim et al., (71) | 249 | South Korea | 3 months | CHD, PAD, TIA, stroke | 63 | 81 | Atorvastatin (10/20) | Different statin doses |
Characteristics of included studies.
ACS, acute coronary syndrome; AICAS, atherosclerotic intracranial arterial stenosis; ASCVD, atherosclerotic cardiovascular disease; CABG, coronary artery bypass graft; CHD, coronary heart disease; MCA, middle cerebral artery; MI, myocardial infarction; NSTEACS, non-ST-elevation acute coronary syndrome; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PTCA, Percutaneous transluminal coronary angioplasty; STEMI, ST segment elevation myocardial infarction; TIA, transient ischemic attack.
Risk of bias and quality of evidence
The overall risk of bias was rated as low or unclear in most studies (Figure 2). Twenty-six trials were double-blinded, and three did not state the blinding of participants and personnel. Seventeen trials were graded high risk regarding blind owing to the open-label design. The risk of bias in individual studies was described in Supplementary Table 4.
FIGURE 2
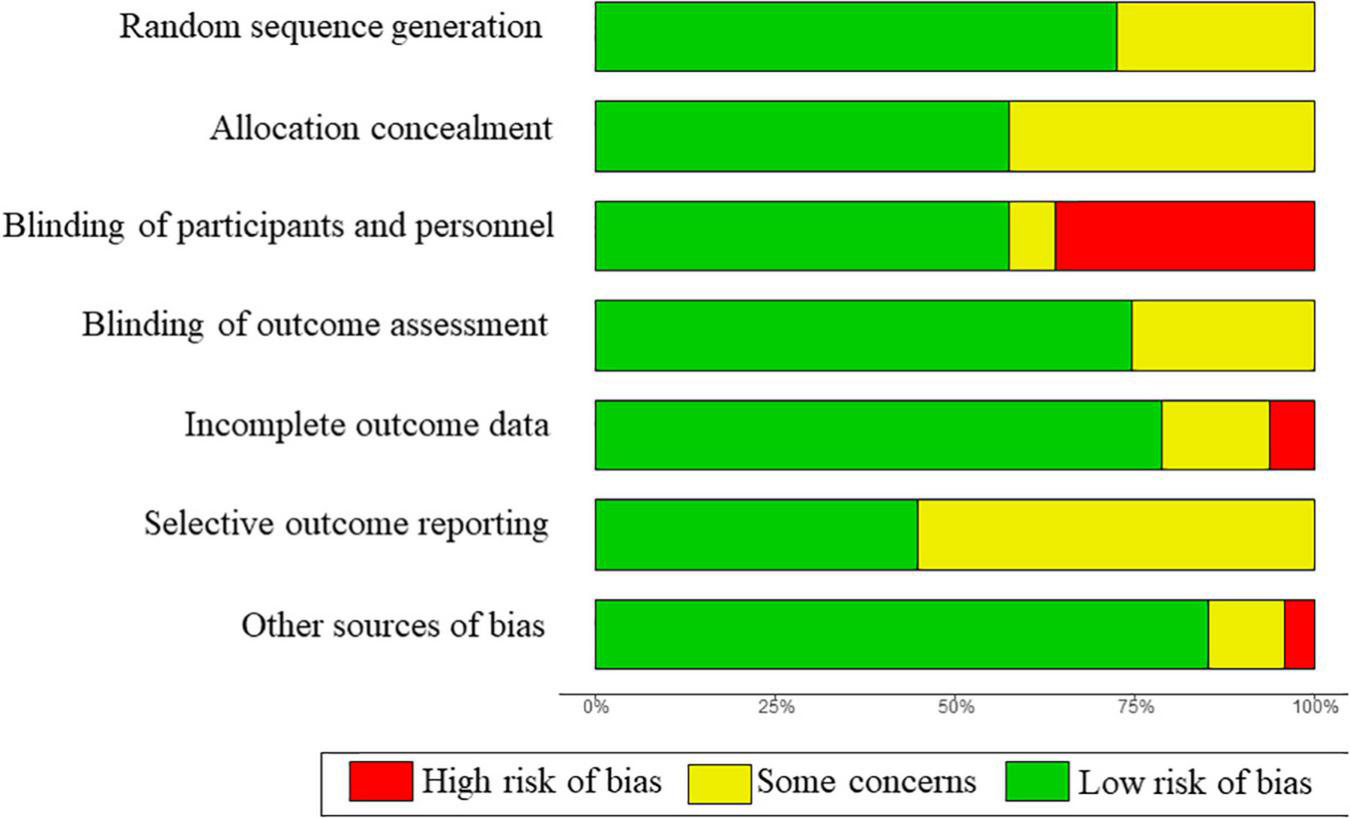
Summary of risk of bias across all included studies.
In pairwise meta-analyses, the quality of evidence for comparisons between statins and non-statin controls for cancer, MI, stroke, death from CVD, and all-cause death was rated as high, with evidence for the muscle condition, transaminase elevations, and gastrointestinal discomfort rated as moderate (Supplementary Table 5). In network meta-analyses, the quality of evidence for significant results was rated as high or moderate for transaminase elevations, and low for cancer (Supplementary Table 6).
Results from systematic reviews
The rare number of studies prevented us from performing meta-analyses for the outcomes of new-onset or exacerbation of diabetes, cognitive impairment, and eye conditions. Only three of the included studies reported the new onset or exacerbation of diabetes. The Oxford Cholesterol study (26) reported 0, 1, 0 case of instability of control of diabetes in the simvastatin 40 mg (n = 206), 20 mg (n = 208) and placebo group (n = 207), respectively. The ATHEROMA study (44) documented 9 and 5 cases in the pravastatin group (n = 182) and no-treatment group (n = 179), respectively. Only the SEARCH study (58) reported the new-onset diabetes, of which 625 and 587 cases occurred in 80 mg (n = 6,031) and 20 mg (n = 6,033) simvastatin group, respectively. Only the HPS study (36) reported 2,434 and 2,484 cases of cognitive impairments in the simvastatin group (n = 10,269) and the placebo group (n = 10,267), respectively. Only two studies reported the eye conditions. The J-STARS study (64) reported 9 and 7 cases of colon polyp in the pravastatin group (n = 780) and no-treatment group (n = 785), respectively. The Oxford Cholesterol study (26) reported 2 and 0 cases of visual deterioration or eye-watering in the simvastatin group (n = 414) and placebo group (n = 207), respectively.
Pairwise meta-analyses for primary and secondary outcomes
Eighteen studies that compared statins with non-statin controls were included in pairwise meta-analyses. We found no significant heterogeneity between individual studies and used a fixed-effects model for most outcomes, except for transaminase elevations and renal insufficiency where random-effects models were adopted due to the significant heterogeneity (P < 0.01; I2 = 69%; 95% CI 45% to 83%, and P = 0.14; I2 = 55%; 95% CI 0% to 89%, respectively) (Figure 3 and Supplementary Figure 1).
FIGURE 3
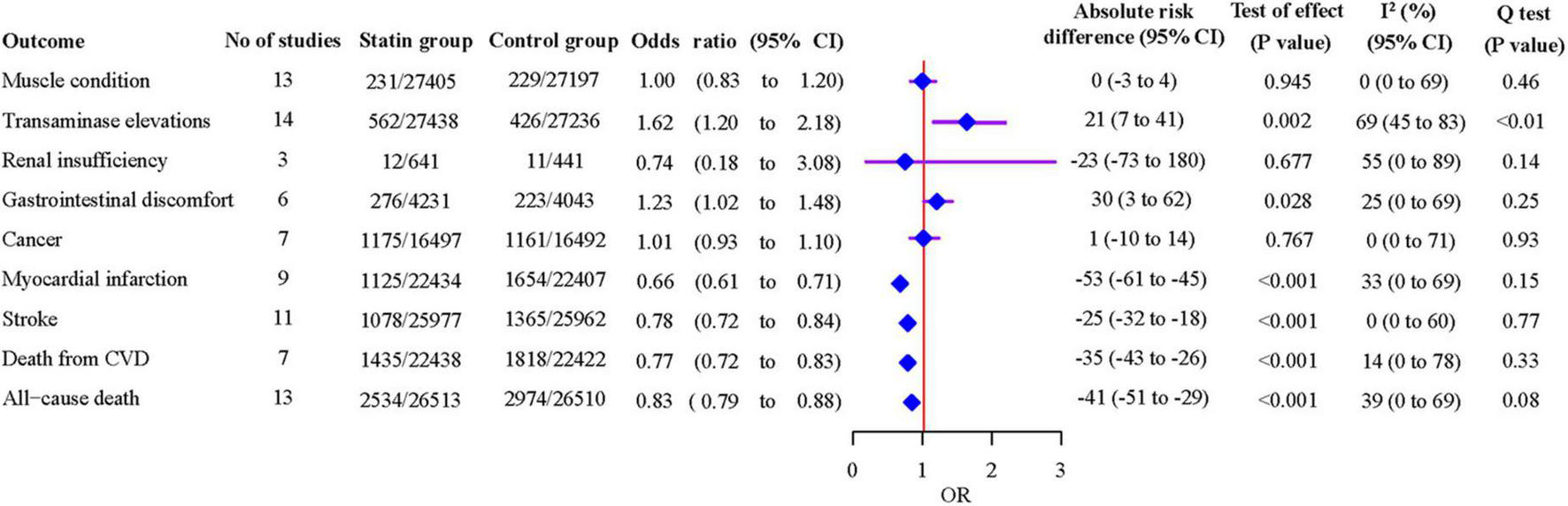
Associations of statins with safety and efficacy outcomes from pairwise meta-analyses.
As shown in Figure 3, statins were associated with an increased risk of transaminase elevations [14 studies, OR 1.62 (95% CI 1.20 to 2.18), P = 0.002], and gastrointestinal discomfort [6 studies, OR 1.23 (95% CI 1.02 to 1.48), P = 0.028]. Statins decreased the risk of MI [9 studies, OR 0.66 (95% CI 0.61 to 0.71), P < 0.001], stroke [11 studies, OR 0.78 (95% CI 0.72 to 0.84), P < 0.001], death from CVD [7 studies, OR 0.77 (95% CI 0.72 to 0.83), P < 0.001] and all-cause death [13 studies, OR 0.83 (95% CI 0.79 to 0.88), P < 0.001]. The leave-one-out influence analyses showed that the associations between statins and muscle condition, transaminase elevations, renal insufficiency, cancer, MI, stroke, death from CVD and all-cause death were not determined by any individual study (Supplementary Figure 2). The association with gastrointestinal discomfort was determined by the SPARCL trial (45), which reported diarrhea.
Compared with non-statin control, statins were estimated to induce 21 (7–41) more events of transaminase elevations, 30 (3–62) more events of gastrointestinal discomfort per 10,000 patients treated for a year (Figure 3). On the other hand, statins were estimated to prevent 53 (45–61) myocardial infarctions, 25 (18–32) strokes, 35 (26–43) deaths from CVD, and 41 (29–51) all-cause deaths per 10,000 patients treated for a year. The event rate per 10,000 patients throughout the duration of the studies is shown in Table 2. The absolute excess risk of the observed adverse effects of statins is smaller than the beneficial effects of statins on major cardiovascular events and all-cause death.
TABLE 2
| Statin | Muscle condition | Transaminase elevations | Renal insufficiency | Gastrointestinal discomfort | Cancer |
| Atorvastatin | 1.02 (0.58 to 1.88) | 19.72 (5.54 to 164.95) | 0.87 (0.28 to 3.81) | 1.34 (0.82 to 2.94) | 0.65 (0.25 to 2.80) |
| Fluvastatin | 1.21 (0.45 to 4.71) | 3.70 (1.15 to 669.11) | / | 1.26 (0.52 to 3.14) | 0.92 (0.15 to 3.60) |
| Lovastatin | / | 1.48 (0.24 to 640.74) | / | / | 1.09 (0.30 to 2.72) |
| Pravastatin | 0.85 (0.33 to 1.58) | 1.40 (0.82 to 19,174.20) | / | 1.49 (0.62 to 3.89) | 1.09 (0.38 to 4.44) |
| Pitavastatin | / | 3.57 (0.63 to 1,314.21) | / | / | 1.29 (0.37 to 5.58) |
| Rosuvastatin | 0.93 (0.38 to 2.31) | 2.80 (0.98 to 287.30) | 0.86 (0.20 to 3.46) | 1.21 (0.35 to 6.09) | / |
| Simvastatin | / | 7.42 (0.85 to 1,671.29) | 0.93 (0.32 to 3.76) | 0.41 (0.22 to 0.89) | 0.98 (0.07 to 2.03) |
Estimated maximum adverse effects of individual statins from Emax dose-response models*.
Emax, asymptotic maximum drug effect. *The maximum odds ratio (ORmax) with 95% credible interval (CI) in each cell is the maximum effect of each statin on the adverse event compared with non-statin controls (that is, the dose of the statin is 0), which is the natural exponential form of the estimated parameter, Emax, in each model.
We did not detect significant publication bias in each outcome (Supplementary Figure 3). In sensitivity analyses, the results were not influenced after excluding non-double-blind studies, Asian populations, or studies and individuals that did not reach a threefold elevation for transaminase (Supplementary Table 7). The results were also unchanged using the random-effects model, except for gastrointestinal discomfort [OR 1.07 (95% CI 0.73–1.57), P = 0.724].
Results from network meta-analyses
Thirty-five studies were included in the networks of treatment comparisons, but due to the inconsistency between direct and indirect treatment comparisons in analysis for muscle conditions, only direct comparisons were performed for this outcome. The evidence network plots are shown in Supplementary Figure 4. We found no significant inconsistencies between direct and indirect treatment comparisons in other safety outcomes analyses (Supplementary Table 8).
The results indicated that compared to atorvastatin, simvastatin was associated with a lower risk of muscle problems [OR 0.70 (95% CI 0.55 to 0.90)], while rosuvastatin showed a higher risk [OR 1.75 (95% CI 1.17 to 2.61)] (Supplementary Table 9). Compared to the non-statin control, atorvastatin was associated with an increased risk of transaminase elevations [OR 4.0 (95% CI 2.2 to 7.6)] (Figure 4). Atorvastatin showed a higher risk of transaminase elevations than pravastatin [OR 3.49 (95% CI 1.77 to 6.92)] and simvastatin [OR 2.77 (95% CI 1.31 to 5.09)]. Atorvastatin showed a lower risk, and pitavastatin showed a higher risk of cancer than other statins and controls with wide 95% CIs, which indicated poor precision, because each of the two statins was reported in only one study (Figure 4 and Supplementary Table 10); this result should be treated with caution.
FIGURE 4
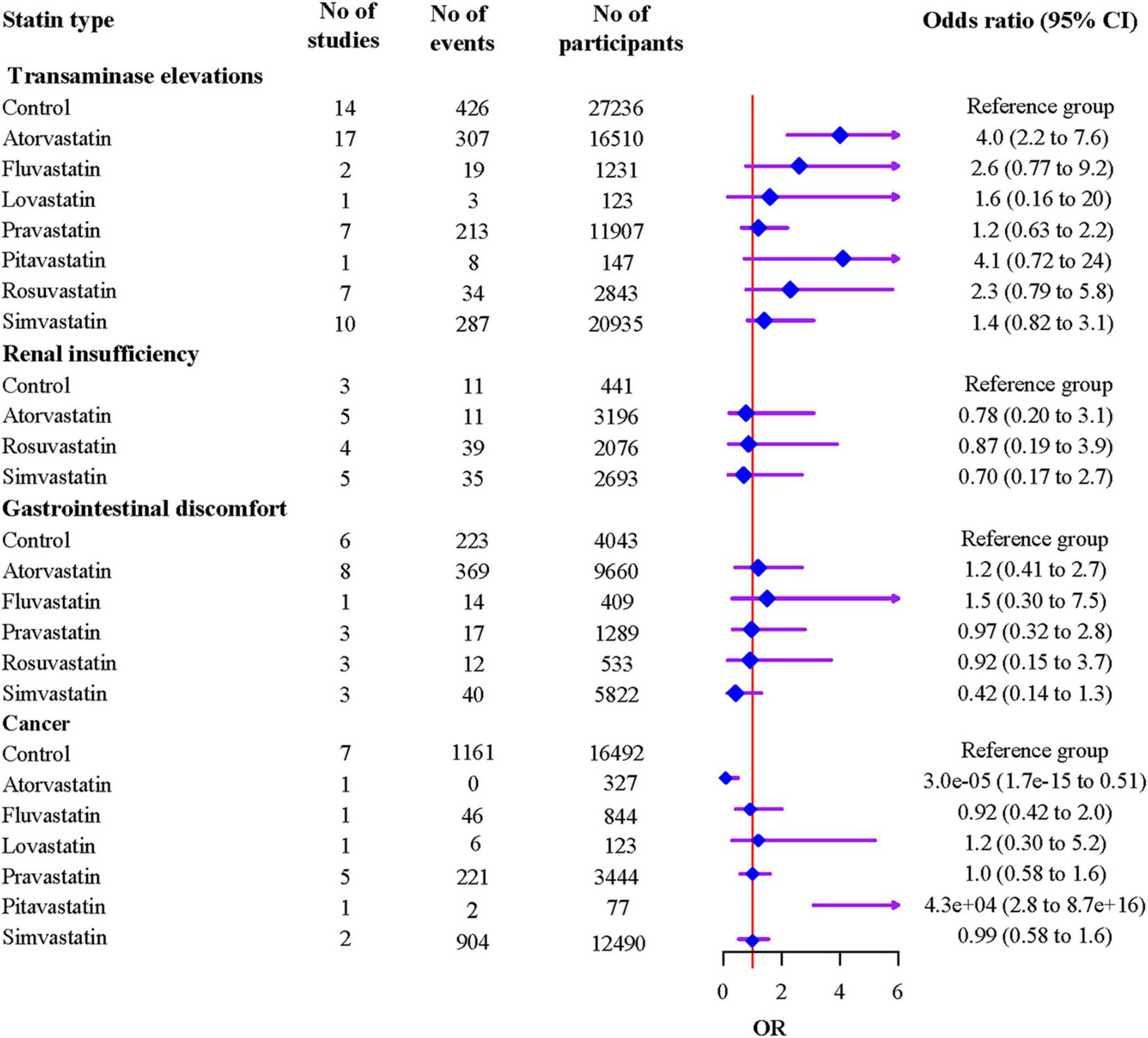
Associations of individual statins with adverse events from network meta-analyses.
The ranking probabilities and cumulative probabilities plots of different statin types were shown in Supplementary Table 11 and Supplementary Figure 5, respectively. The ranking results based on SUCRA values showed that control had the highest rank for transaminase elevations (87.2%), and followed by pravastatin (76.3%), simvastatin (62.0%), lovastatin (56.2%), rosuvastatin (43.0%), fluvastatin (37.2%), pitavastatin (22.8%), and atorvastatin (15.3%); Simvastatin had the highest rank for renal insufficiency (65.9%), and followed by atorvastatin (54.8%), rosuvastatin (43.3%), and control (36.1%); Simvastatin had the highest rank for gastrointestinal discomfort (91.4%), and followed by rosuvastatin (52.7%), pravastatin (48.7%), control (48.2%), atorvastatin (32.8%), and fluvastatin (26.1%). Atorvastatin had the highest rank for cancer (99.0%), and followed by fluvastatin (58.7%), simvastatin (52.3%), control (51.6%), pravastatin (45.9%), lovastatin (41.7%), pitavastatin (0.7%).
Dose-response relationships in adverse effects of statins
Forty-three studies were included in the dose-response meta-analyses. A significant Emax dose-response relationship was detected for the effect of atorvastatin and fluvastatin on transaminase elevations, with a maximum effect that increased the risk with non-statin controls [maximum OR (ORmax) = 19.72, 95% CI (5.54 to 164.95); ORmax = 3.70, 95% CI (1.15 to 669.11), respectively] (Table 2). We detected no significant dose-response relationships for other statins on adverse effects.
The ranking probabilities in the dose-response meta-analysis showed similar results with network meta-analysis (Supplementary Table 12 and Supplementary Figure 6). The predicted dose-response curves, which had low precision with wide 95% CIs, were available for few doses of some statins, and only atorvastatin showed a precise shape (Supplementary Figure 7).
Discussion
The salient findings of the meta-analyses of 47 RCTs, including 107,752 patients, can be summarized as follows. First, pairwise meta-analyses show that statins were associated with a higher incidence of transaminase elevations, but not with muscle condition, gastrointestinal discomfort, renal insufficiency and cancer. As expected, the benefit-to-harm balance of statins for secondary prevention of CVD is still favorable. Second, network meta-analyses indicated that atorvastatin could increase the risk of transaminase elevations compared to control, pravastatin and simvastatin. Compared to atorvastatin, simvastatin was associated with a lower risk of muscle problems, and rosuvastatin showed a higher risk. Third, a significant dose-response relationship was identified for the effect of atorvastatin on transaminase elevations. The dose-response relationships for the other statins and adverse effects were inconclusive.
Although statin-associated muscle symptoms (SAMS) have been reported, occurring in 7–29% of statin-treated patients and covering a wide range of severities (9), the mechanism remains unclear and whether SAMS is caused by statin use remained controversial. Our study did not find a significant association between statin and muscle condition and consistent with our findings. Many previous systematic reviews examining statins did not find an association between statins and muscle problems in the primary and secondary prevention population (4, 72–74). In contrast, some reviews showed associations between statins and increased risk of muscle symptoms and muscle diseases in the primary and general populations (75, 76). Nevertheless, we can find that the increased risk is slight in these reviews. These conflicting results may be attributed to the different populations and a wide range of conditions with varying types and severities of muscle problems. Some research indicated that most muscle symptoms reported by users of statins were due to “nocebo” effects rather than statins (75, 77). A recent meta-analysis of 176 studies with 4,143,517 patients showed that the prevalence of complete statin intolerance, mainly including SAMS, might often be overestimated (78). Previous reviews were underpowered for clinically confirmed muscle disorders to detect the associations between statins and myopathy or rhabdomyolysis because of the low incidences (75, 79, 80). Based on the current evidence, statins tend to have little effect on muscle problems in primary and secondary prevention.
The serum AST and ALT are commonly used tests to assess liver diseases. Our findings that statins use was associated with elevated transaminase were consistent with previous reviews, especially atorvastatin which was associated with a four times higher risk of transaminase elevation than non-statin controls. Furthermore, a significant Emax dose-response relationship was detected for the effect of atorvastatin on transaminase elevation. This adverse effect was similar in primary and secondary prevention (74, 80). A recent meta-analysis indicated that atorvastatin had the highest and dose-dependent risk of elevated transaminase (81). Another meta-analysis stated that compared to non-statin controls, patients treated with high dose atorvastatin (80 mg/day) had a higher risk of transaminase elevation, specifically in patients with CHD (82). Some clinical trials showed acceptable safety profiles of atorvastatin (83, 84), probably due to the small population scales of the studies and rare incidence rate of transaminase elevation. Atorvastatin is one of the most commonly prescribed drugs in the United States, with more than 50 million prescriptions per year (85). Atorvastatin is significantly longer-acting than other statins and is primarily metabolized in liver, which may explain the higher risk of liver dysfunction (86, 87). Dujovne (88) hypothesized that atorvastatin had more pronounced activity in reducing serum low-density lipoprotein, which may affect cell membrane structure, resulting in greater leakage of cellular enzymes and increased incidence of liver dysfunction. According to the current evidence, clinicians should avoid using atorvastatin when transaminase elevation occurs, especially high-dose atorvastatin, and pravastatin may be a better choice.
Studies about the effect of statins on renal function are contradictory. Some reviews, which included studies in secondary prevention, showed that statins reduce the progression of kidney function decline and proteinuria (89, 90). The latest The Kidney Disease: Improving Global Outcomes (KDIGO) guideline and 2016 ESC/EAS guidelines both recommend the use of statins in all non-dialysis dependent chronic kidney diseases patients ≥ 50 years with an eGFR below 60 mL/min/1.73 m2 or at least 30 mg/g albuminuria (91, 92). In contrast, some reviews showed associations between statins and renal insufficiency in primary prevention (75, 89). We found in the present study no significant associations between any statins and renal insufficiency. Nevertheless, the current data that can be analyzed is limited, and there is no convincing indication that any statin at any currently marketed dose causes renal disease.
There is no consensus on whether statins have a causal relationship with common gastrointestinal conditions. Studies indicated that statins tended to increase the risk of gastrointestinal discomfort or hemorrhage than other chronic medication users (93–95). Conversely, some studies did not show significant associations (96). The inconsistencies may result from varieties symptoms and severities of gastrointestinal discomfort.
The current evidence on the link between statins and cancer is conflicting. Consistent with our findings of pairwise meta-analyses, numerous systematic reviews showed no association between statin use and cancer incidence (97–99). Conversely, some studies showed that statins could affect the risk or development of cancers. On one hand, some evidence shows that statin therapy may increase the risk of some cancer types (100, 101). The PROSPER study (102) found a 1.25 increased risk for cancer incidence for the statin-treated patients compared to the placebo group; however, the authors have extended their follow-up period by 10 years and found no increased risk of cancer incidence for participants treated with statins compared to placebo. On the other hand, various in vitro and in vivo studies have revealed the efficacy of statins against cancers (103, 104). The inconsistency in published studies regarding statin use and cancer prevalence or mortality may be due to marked differences in follow-up duration, as well as other inherent biases in different study designs. Scholars reviewed the current contradictory evidence and believed that there was no increased risk of incident cancer with statin treatment (105).
Trial data on diabetes, cognitive impairments and eye conditions are currently limited, and no significant result was found in our systematic review. The rare records also reflect a low incidence rate from the side. Though a few studies suggested possible relationships between statin use and these adverse events (106, 107), the results were contradictory as some studies showed no associations (75, 107). More research data are needed to draw convincing conclusions.
The present study has several strengths. First, to our best knowledge, this is the first study to comprehensively explore the association between different types and doses of statins and adverse events in secondary prevention. Second, we include only RCTs which are more likely to provide unbiased information. Third, we performed network meta-analyses to establish multiple treatments comparison and synthesize data with not only direct evidence but also indirect evidence. Fourth, we also used Emax model, a newly developed and reliable method to examine the dose-response relationship of adverse reactions of statins. Compared with other models, this model reflects the basic Emax pharmacodynamics of common inhibitors with clinically interpretable parameters (108). Our study also provides the ranking probabilities of interventions that may provide some references for clinicians to make optimal decisions. Finally, we evaluated the absolute risk difference in the number of events per 10,000 patients treated for a year, which indicated that the benefit-to-harm balance of statins was favorable. Though intolerance to statins was considered as one of the main causes of insufficient LDL-C response to statin treatment (109), our findings add strong evidence to the current view that the cardiovascular benefits of statins far outweigh non-cardiovascular harms in patients with cardiovascular risk (110).
While this study does provide helpful information for clinicians, several limitations should be noted. The first point is the inconsistent definition of outcome measures. As mentioned earlier, the muscle condition, renal insufficiency and gastrointestinal discomfort are not specific and involve various symptoms. We have emphasized this limitation in the GRADE profile for authors to evaluate the quality of evidence. Second, although atorvastatin and pitavastatin both show differences from other statins and controls for the cancer incidence, this result is affected by the rare incidence of adverse events and limited sample sizes. Therefore, the result should be treated with caution. Also, due to the insufficient data, a few analyses were underpowered to detect differences between groups, and estimates of Emax from the models in this study made it difficult to draw more specific conclusions about the dose-response relationships. Third, the node-splitting analysis showed inconsistency in network meta-analysis of muscle condition. Though we excluded the studies that caused inconsistency, the results still need to be interpreted with caution. Fourth, a few studies were open-labeled and may induce bias, although sensitivity analyses showed that excluding these trials does not influence the overall results. Finally, due to data limitations, we could not further analyze the association of statins with the severity of adverse effects. Nevertheless, none of these limitations affects the main conclusion of our analysis.
Conclusion
Statins were not associated with muscle condition, gastrointestinal discomfort, renal insufficiency and cancer but with increased risk of transaminases elevations in secondary prevention of ACSVD. Our study provides the ranking probabilities of statins that can help clinicians make optimal decisions when there is not enough literature to refer to. Future studies should systematically and detailly report adverse events of statins.
Statements
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
XW, HX, and KC: conceptualization. XW and JL: data curation and writing—original draft. XW, TW, and ZZ: formal analysis. QL and DM: methodology. HX and KC: project administration and supervision. ZC, JJ, and HX: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Major Achievements in Chinese Medicine Science and Technology Guiding Project of China Academy of Chinese Medical Sciences (ZZ13-ZD-03) and CACMS Innovation Fund (CI2021A00917).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.929020/full#supplementary-material
References
1.
Mach F Baigent C Catapano AL Koskinas KC Casula M Badimon L et al 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. 10.15829/1560-4071-2020-3826
2.
Macedo AF Taylor FC Casas JP Adler A Prieto-Merino D Ebrahim S . Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis.BMC Med. (2014) 12:51. 10.1186/1741-7015-12-51
3.
Chaipichit N Krska J Pratipanawatr T Jarernsiripornkul N . Statin adverse effects: patients’ experiences and laboratory monitoring of muscle and liver injuries.Int J Clin Pharm. (2015) 37:355–64. 10.1007/s11096-015-0068-5
4.
Yebyo HG Aschmann HE Kaufmann M Puhan MA . Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: a systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants.Am Heart J. (2019) 210:18–28. 10.1016/j.ahj.2018.12.007
5.
Stone NJ Robinson JG Lichtenstein AH Bairey Merz CN Blum CB Eckel RH et al 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2889–934. 10.1161/01.cir.0000437738.63853.7a
6.
Silva M Matthews ML Jarvis C Nolan NM Belliveau P Malloy M et al Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther. (2007) 29:253–60. 10.1016/j.clinthera.2007.02.008
7.
Dale KM White CM Henyan NN Kluger J Coleman CI . Impact of statin dosing intensity on transaminase and creatine kinase.Am J Med. (2007) 120:706–12. 10.1016/j.amjmed.2006.07.033
8.
Moher D Liberati A Tetzlaff J Altman DG Group P . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097
9.
Knuuti J Ballo H Juarez-Orozco LE Saraste A Kolh P Rutjes AWS et al The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. (2018) 39:3322–30. 10.1093/eurheartj/ehy267
10.
Stroes ES Thompson PD Corsini A Vladutiu GD Raal FJ Ray KK et al Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. (2015) 36:1012–22. 10.1093/eurheartj/ehv043
11.
Higgins JP Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928
12.
Schünemann H Brozek J Guyatt G Oxman A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. (2013). Available online at: https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2
13.
Puhan MA Schünemann HJ Murad MH Li T Brignardello-Petersen R Singh JA et al GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. (2014) 349:g5630. 10.1136/bmj.g5630
14.
Higgins JP Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses.BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557
15.
Higgins J Thomas J Chandler J Cumpston M Li T Mj P. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. London: The Cochrane Collaboration (2020). 10.1002/9781119536604
16.
Viechtbauer W Cheung MW . Outlier and influence diagnostics for meta-analysis.Res Synth Methods. (2010) 1:112–25. 10.1002/jrsm.11
17.
Lee E Ryan S Birmingham B Zalikowski J March R Ambrose H et al Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. (2005) 78:330–41. 10.1016/j.clpt.2005.06.013
18.
Jansen JP Crawford B Bergman G Stam W . Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons.Value Health. (2008) 11:956–64. 10.1111/j.1524-4733.2008.00347.x
19.
Laird NM Mosteller F . Some statistical methods for combining experimental results.Int J Technol Assess Health Care. (1990) 6:5–30. 10.1017/S0266462300008916
20.
Dias S Welton NJ Caldwell DM Ades AE . Checking consistency in mixed treatment comparison meta-analysis.Stat Med. (2010) 29:932–44. 10.1002/sim.3767
21.
Salanti G Ades AE Ioannidis JP . Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial.J Clin Epidemiol. (2011) 64:163–71. 10.1016/j.jclinepi.2010.03.016
22.
Mawdsley D Bennetts M Dias S Boucher M Welton NJ . Model-based network meta-analysis: a framework for evidence synthesis of clinical trial data.CPT Pharmacometrics Syst Pharmacol. (2016) 5:393–401. 10.1002/psp4.12091
23.
Langford O Aronson JK van Valkenhoef G Stevens RJ . Methods for meta-analysis of pharmacodynamic dose-response data with application to multi-arm studies of alogliptin.Stat Methods Med Res. (2018) 27:564–78. 10.1177/0962280216637093
24.
Kirby S Brain P Jones B . Fitting E(max) models to clinical trial dose-response data.Pharm Stat. (2011) 10:143–9. 10.1002/pst.432
25.
Blankenhorn DH Azen SP Kramsch DM Mack WJ Cashin-Hemphill L Hodis HN et al Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann Intern Med. (1993) 119:969–76. 10.7326/0003-4819-119-10-199311150-00002
26.
Keech A Collins R MacMahon S Armitage J Lawson A Wallendszus K et al Three-year follow-up of the Oxford cholesterol study: assessment of the efficacy and safety of simvastatin in preparation for a large mortality study. Eur Heart J. (1994) 15:255–69. 10.1093/oxfordjournals.eurheartj.a060485
27.
Pedersen TR Kjekshus J Berg K Haghfelt T Faergeman O Thorgeirsson G et al Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the scandinavian simvastatin survival study (4S). Lancet. (1994) 344:1383–9. 10.1016/S0140-6736(94)90566-5
28.
Pitt B Mancini GB Ellis SG Rosman HS Park JS McGovern ME . Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC I): reduction in atherosclerosis progression and clinical events. PLAC I investigation.J Am Coll Cardiol. (1995) 26:1133–9. 10.1016/0735-1097(95)00301-0
29.
Sacks FM Pfeffer MA Moye LA Rouleau JL Rutherford JD Cole TG et al The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. (1996) 335:1001–9. 10.1056/NEJM199610033351401
30.
Long-Term Intervention with Pravastatin in Ischaemic Disease Study Group [LIPID]. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. (1998) 339:1349–57. 10.1056/NEJM199811053391902
31.
März W Wollschläger H Klein G Neiss A Wehling M . Safety of low-density lipoprotein cholestrol reduction with atorvastatin versus simvastatin in a coronary heart disease population (the TARGET TANGIBLE trial).Am J Cardiol. (1999) 84:7–13. 10.1016/S0002-9149(99)00183-6
32.
Serruys PW Foley DP Jackson G Bonnier H Macaya C Vrolix M et al A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. (1999) 20:58–69. 10.1053/euhj.1998.1150
33.
Schwartz GG Olsson AG Ezekowitz MD Ganz P Oliver MF Waters D et al Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. (2001) 285:1711–8. 10.1001/jama.285.13.1711
34.
Karalis DG Ross AM Vacari RM Zarren H Scott R . Comparison of efficacy and safety of atorvastatin and simvastatin in patients with dyslipidemia with and without coronary heart disease.Am J Cardiol. (2002) 89:667–71. 10.1016/S0002-9149(01)02337-2
35.
Serruys PW de Feyter P Macaya C Kokott N Puel J Vrolix M et al Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. (2002) 287:3215–22. 10.1001/jama.287.24.3215
36.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. (2002) 360:7–22. 10.1016/S0140-6736(02)09327-3
37.
Olsson AG Eriksson M Johnson O Kjellström T Lanke J Larsen ML et al A 52-week, multicenter, randomized, parallel-group, double-blind, double-dummy study to assess the efficacy of atorvastatin and simvastatin in reaching low-density lipoprotein cholesterol and triglyceride targets: the treat-to-target (3T) study. Clin Ther. (2003) 25:119–38. 10.1016/S0149-2918(03)90015-4
38.
Nissen SE Tuzcu EM Schoenhagen P Brown BG Ganz P Vogel RA et al Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. (2004) 291:1071–80. 10.1001/jama.291.9.1071
39.
Schwartz GG Bolognese MA Tremblay BP Caplan R Hutchinson H Raza A et al Efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: a randomized, controlled trial. Am Heart J. (2004) 148:e4. 10.1016/j.ahj.2004.01.020
40.
Cannon CP Braunwald E McCabe CH Rader DJ Rouleau JL Belder R et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. (2004) 350:1495–504. 10.1056/NEJMoa040583
41.
Mizuno K Nakamura H Ohashi Y Kaburagi T Kitabatake A Tochihara T . A randomized, open-label, comparative study of simvastatin plus diet versus diet alone on angiographic retardation of coronary atherosclerosis in adult Japanese patients: Japanese utilization of simvastatin therapy (JUST) study.Clin Ther. (2004) 26:878–88. 10.1016/S0149-2918(04)90131-2
42.
Pedersen TR Faergeman O Kastelein JJ Olsson AG Tikkanen MJ Holme I et al High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. (2005) 294:2437–45. 10.1001/jama.294.19.2437
43.
LaRosa JC Grundy SM Waters DD Shear C Barter P Fruchart JC et al Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. (2005) 352:1425–35. 10.1056/NEJMoa050461
44.
Yokoi H Nobuyoshi M Mitsudo K Kawaguchi A Yamamoto A . Three-year follow-up results of angiographic intervention trial using an HMG-CoA reductase inhibitor to evaluate retardation of obstructive multiple atheroma (ATHEROMA) study.Circ J. (2005) 69:875–83. 10.1253/circj.69.875
45.
Amarenco P Bogousslavsky J Callahan A III Goldstein LB Hennerici M Rudolph AE et al High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. (2006) 355:549–59. 10.1056/NEJMoa061894
46.
Insull W Jr. Ghali JK Hassman DR Y As JW Gandhi SK Miller E et al Achieving low-density lipoprotein cholesterol goals in high-risk patients in managed care: comparison of rosuvastatin, atorvastatin, and simvastatin in the SOLAR trial. Mayo Clin Proc. (2007) 82:543–50. 10.4065/82.5.543
47.
Danchin N Chadarevian R Gayet JL Licour M Valensi P . [Compared with atorvastatin at the dose of 10 mg per day rosuvastatin was more effective to reach an LDL goal of < 1.00 g/l in high cardiovascular risk patients (ARIANE study)].Ann Cardiol Angeiol. (2007) 56:82–7. 10.1016/j.ancard.2007.01.003
48.
Yun KH Park HY Choi JH Song MJ Park EM Kim YK et al Comparison of efficacy and safety after administering high potency statin to high risk patients: rosuvastatin 10 mg versus atorvastatin 20 mg. Korean Circ J. (2007) 37:154–60. 10.4070/kcj.2007.37.4.154
49.
Yu CM Zhang Q Lam L Lin H Kong SL Chan W et al Comparison of intensive and low-dose atorvastatin therapy in the reduction of carotid intimal-medial thickness in patients with coronary heart disease. Heart. (2007) 93:933–9. 10.1136/hrt.2006.102848
50.
Deedwania P Stone PH Bairey Merz CN Cosin-Aguilar J Koylan N Luo D et al Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation. (2007) 115:700–7. 10.1161/CIRCULATIONAHA.106.654756
51.
Bonnet J McPherson R Tedgui A Simoneau D Nozza A Martineau P et al Comparative effects of 10-mg versus 80-mg Atorvastatin on high-sensitivity C-reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clin Ther. (2008) 30:2298–313. 10.1016/j.clinthera.2008.12.023
52.
Hiro T Kimura T Morimoto T Miyauchi K Nakagawa Y Yamagishi M et al Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. (2009) 54:293–302. 10.1161/circ.118.suppl_18.S_657-c
53.
Zhao Z Geng J Ge ZM Wang W Zhang Y Kang WQ . Efficacy and safety of atorvastatin during early hospitalization in elderly patients with unstable angina.Clin Exp Pharmacol Physiol. (2009) 36:554–8. 10.1111/j.1440-1681.2008.05110.x
54.
Hall AS Jackson BM Farrin AJ Efthymiou M Barth JH Copeland J et al A randomized, controlled trial of simvastatin versus rosuvastatin in patients with acute myocardial infarction: the Secondary Prevention of Acute Coronary Events–Reduction of Cholesterol to Key European Targets Trial. Eur J Cardiovasc Prev Rehabil. (2009) 16:712–21. 10.1097/HJR.0b013e3283316ce8
55.
Mok VC Lam WW Chen XY Wong A Ng PW Tsoi TH et al Statins for asymptomatic middle cerebral artery stenosis: the regression of cerebral artery stenosis study. Cerebrovasc Dis. (2009) 28:18–25. 10.1159/000215939
56.
Lablanche JM Leone A Merkely B Morais J Alonso J Santini M et al Comparison of the efficacy of rosuvastatin versus atorvastatin in reducing apolipoprotein B/apolipoprotein A-1 ratio in patients with acute coronary syndrome: results of the CENTAURUS study. Arch Cardiovasc Dis. (2010) 103:160–9. 10.1016/j.acvd.2010.01.005
57.
Ostadal P Alan D Vejvoda J Kukacka J Macek M Hajek P et al Fluvastatin in the first-line therapy of acute coronary syndrome: results of the multicenter, randomized, double-blind, placebo-controlled trial (the FACS-trial). Trials. (2010) 11:61. 10.1186/1745-6215-11-61
58.
Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine Collaborative Group [SEARCH], Armitage J Bowman L Wallendszus K Bulbulia R Rahimi K et al Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. (2010) 376:1658–69. 10.1016/S0140-6736(10)60310-8
59.
Pitt B Loscalzo J Monyak J Miller E Raichlen J . Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study).Am J Cardiol. (2012) 109:1239–46. 10.1016/j.amjcard.2011.12.015
60.
Nozue T Yamamoto S Tohyama S Umezawa S Kunishima T Sato A et al Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. (2012) 163:191–9.e1. 10.1016/j.ahj.2011.11.004
61.
Kaul U Varma J Kahali D Hiremath MS Dani S Dalal J et al Post-marketing study of clinical experience of atorvastatin 80 mg vs 40 mg in Indian patients with acute coronary syndrome- a randomized, multi-centre study (CURE-ACS). J Assoc Physicians India. (2013) 61:97–101.
62.
Thompson PL Meredith I Amerena J Campbell TJ Sloman JG Harris PJ . Effect of pravastatin compared with placebo initiated within 24 hours of onset of acute myocardial infarction or unstable angina: the pravastatin in acute coronary treatment (PACT) trial.Am Heart J. (2004) 148:e2. 10.1016/j.ahj.2003.10.052
63.
Zhou P Lu Z Gao P Wang P Cao Z Zhang G et al Efficacy and safety of intensive statin therapy in Chinese patients with atherosclerotic intracranial arterial stenosis: a single-center, randomized, single-blind, parallel-group study with one-year follow-up. Clin Neurol Neurosurg. (2014) 120:6–13. 10.1016/j.clineuro.2014.02.001
64.
Khurana S Gupta S Bhalla H Nandwani S Gupta V . Comparison of anti-inflammatory effect of atorvastatin with rosuvastatin in patients of acute coronary syndrome.J Pharmacol Pharmacother. (2015) 6:130–5. 10.4103/0976-500X.162011
65.
Hosomi N Nagai Y Kohriyama T Ohtsuki T Aoki S Nezu T et al The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. (2015) 2:1071–8. 10.1016/j.ebiom.2015.08.006
66.
Liu Z Xu Y Hao H Yin C Xu J Li J et al Efficacy of high intensity atorvastatin versus moderate intensity atorvastatin for acute coronary syndrome patients with diabetes mellitus. Int J Cardiol. (2016) 222:22–6. 10.1016/j.ijcard.2016.07.140
67.
Priti K Agrawal A Ranwa BL . High versus low dose statin therapy in Indian patients with acute ST-segment elevation myocardial infarction undergoing thrombolysis.Indian Heart J. (2017) 69:453–7. 10.1016/j.ihj.2017.05.026
68.
Kulik A Abreu AM Boronat V Ruel M . Intensive versus moderate statin therapy and early graft occlusion after coronary bypass surgery: the aggressive cholesterol therapy to inhibit vein graft events randomized clinical trial.J Thorac Cardiovasc Surg. (2019) 157:151–61.e1. 10.1016/j.jtcvs.2018.05.123
69.
Liu Q Wang Y Cheng X . The functional effect of atorvastatin dose-dependent via inflammation factors on acute ST segment elevation myocardial infarction after emergency percutaneous coronary intervention.J Cardiovasc Med. (2019) 20:215–9. 10.2459/JCM.0000000000000711
70.
Wang D Bai L Cui XR Yang XH Zhang JD . Effectiveness of atorvastatin in the treatment of asymptomatic heart failure after myocardial infarction: a clinical study.Adv Ther. (2020) 37:4649–59. 10.1007/s12325-020-01441-8
71.
Kim JB Song WH Park JS Youn TJ Park YH Kim SJ et al A randomized, open-label, parallel, multi-center Phase IV study to compare the efficacy and safety of atorvastatin 10 and 20 mg in high-risk Asian patients with hypercholesterolemia. PLoS One. (2021) 16:e0245481. 10.1371/journal.pone.0245481
72.
Iwere RB Hewitt J . Myopathy in older people receiving statin therapy: a systematic review and meta-analysis.Br J Clin Pharmacol. (2015) 80:363–71. 10.1111/bcp.12687
73.
Tramacere I Boncoraglio GB Banzi R Del Giovane C Kwag KH Squizzato A et al Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med. (2019) 17:67. 10.1186/s12916-019-1298-5
74.
Palmer SC Craig JC Navaneethan SD Tonelli M Pellegrini F Strippoli GF . Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis.Ann Intern Med. (2012) 157:263–75. 10.7326/0003-4819-157-4-201208210-00007
75.
Cai T Abel L Langford O Monaghan G Aronson JK Stevens RJ et al Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. (2021) 374:n1537. 10.1136/bmj.n1537
76.
Lu Y Cheng Z Zhao Y Chang X Chan C Bai Y et al Efficacy and safety of long-term treatment with statins for coronary heart disease: a bayesian network meta-analysis. Atherosclerosis. (2016) 254:215–27. 10.1016/j.atherosclerosis.2016.10.025
77.
Penson PE Mancini GBJ Toth PP Martin SS Watts GF Sahebkar A et al Introducing the ‘Drucebo’ effect in statin therapy: a systematic review of studies comparing reported rates of statin-associated muscle symptoms, under blinded and open-label conditions. J Cachexia Sarcopenia Muscle. (2018) 9:1023–33. 10.1002/jcsm.12344
78.
Bytyçi I Penson PE Mikhailidis DP Wong ND Hernandez AV Sahebkar A et al Prevalence of statin intolerance: a meta-analysis. Eur Heart J. (2022). [Epub ahead of print]. 10.1093/eurheartj/ehac015
79.
Law M Rudnicka AR . Statin safety: a systematic review.Am J Cardiol. (2006) 97:52C–60C. 10.1016/j.amjcard.2005.12.010
80.
Cholesterol Treatment Trialists’ Collaboration [CTT] Baigent C Blackwell L Emberson J Holland LE Reith C et al Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. (2010) 376:1670–81. 10.1016/S0140-6736(10)61350-5
81.
Villani R Navarese EP Cavallone F Kubica J Bellanti F Facciorusso A et al Risk of statin-induced hypertransaminasemia: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc Innov Qual Outcomes. (2019) 3:131–40. 10.1016/j.mayocpiqo.2019.01.003
82.
Li H Wang C Zhang S Sun S Li R Zou M et al Safety profile of atorvastatin 80 mg: a meta-analysis of 17 randomized controlled trials in 21,910 participants. Drug Saf. (2016) 39:409–19. 10.1007/s40264-016-0394-0
83.
Kalantari S Naghipour M . Statin therapy and hepatotoxicity: appraisal of the safety profile of atorvastatin in hyperlipidemic patients.Adv Biomed Res. (2014) 3:168. 10.4103/2277-9175.139133
84.
Wu CC Sy R Tanphaichitr V Hin AT Suyono S Lee YT . Comparing the efficacy and safety of atorvastatin and simvastatin in Asians with elevated low-density lipoprotein-cholesterol–a multinational, multicenter, double-blind study.J Formos Med Assoc. (2002) 101:478–87.
85.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] . Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
86.
Malinowski JM . Atorvastatin: a hydroxymethylglutaryl-coenzyme A reductase inhibitor.Am J Health Syst Pharm. (1998) 55:2253–67. 10.1093/ajhp/55.21.2253
87.
Ward NC Watts GF Eckel RH . Statin Toxicity.Circ Res. (2019) 124:328–50. 10.1161/CIRCRESAHA.118.312782
88.
Dujovne CA . Side effects of statins: hepatitis versus “transaminitis”-myositis versus “CPKitis”.Am J Cardiol. (2002) 89:1411–3. 10.1016/S0002-9149(02)02356-1
89.
Esmeijer K Dekkers OM de Fijter JW Dekker FW Hoogeveen EK . Effect of different types of statins on kidney function decline and proteinuria: a network meta-analysis.Sci Rep. (2019) 9:16632. 10.1038/s41598-019-53064-x
90.
Su X Zhang L Lv J Wang J Hou W Xie X et al Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis. (2016) 67:881–92. 10.1053/j.ajkd.2016.01.016
91.
Wanner C Tonelli M Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. (2014) 85:1303–9. 10.1038/ki.2014.31
92.
Authors/Task Force Members Catapano AL Graham I De Backer G Wiklund O Chapman MJ . 2016 ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and European atherosclerosis society (EAS) Developed with the special contribution of the European assocciation for cardiovascular prevention & rehabilitation (EACPR).Atherosclerosis. (2016) 253:281–344.
93.
Urina-Jassir M Pacheco-Paez T Paez-Canro C Urina-Triana M . Statin associated adverse reactions in Latin America: a scoping review.BMJ Open. (2021) 11:e050675. 10.1136/bmjopen-2021-050675
94.
Kim H Kim N Lee DH Kim HS . Analysis of National Pharmacovigilance Data Associated with Statin Use in Korea.Basic Clin Pharmacol Toxicol. (2017) 121:409–13. 10.1111/bcpt.12808
95.
Martinez AI Freeman PR Moga DC . Statin use and gastrointestinal hemorrhage: a large retrospective cohort study.Am J Cardiovasc Drugs. (2019) 19:65–74. 10.1007/s40256-018-0301-4
96.
Pearlman M Covin Y Schmidt R Mortensen EM Mansi IA . Statins and lower gastrointestinal conditions: a retrospective cohort study.J Clin Pharmacol. (2017) 57:1053–63. 10.1002/jcph.895
97.
Cholesterol Treatment Trialists’ Collaborators [CTT] Mihaylova B Emberson J Blackwell L Keech A Simes J et al The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. (2012) 380:581–90. 10.1016/S0140-6736(12)60367-5
98.
Cholesterol Treatment Trialists’ Collaboration [CTT] Emberson JR Kearney PM Blackwell L Newman C Reith C et al Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. (2012) 7:e29849. 10.1371/journal.pone.0029849
99.
Haukka J Sankila R Klaukka T Lonnqvist J Niskanen L Tanskanen A et al Incidence of cancer and statin usage–record linkage study. Int J Cancer. (2010) 126:279–84. 10.1002/ijc.24536
100.
Liao J Farmer JA . Aggressive statin therapy and the risk of malignancy.Curr Atheroscler Rep. (2013) 15:316. 10.1007/s11883-013-0316-x
101.
Ahmadi Y Karimian R Panahi Y . Effects of statins on the chemoresistance-The antagonistic drug-drug interactions versus the anti-cancer effects.Biomed Pharmacother. (2018) 108:1856–65. 10.1016/j.biopha.2018.09.122
102.
Shepherd J Blauw GJ Murphy MB Bollen EL Buckley BM Cobbe SM et al PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. (2002) 360:1623–30. 10.1016/S0140-6736(02)11600-X
103.
Emami A Shojaei S da Silva Rosa SC Aghaei M Samiei E Vosoughi AR et al Mechanisms of simvastatin myotoxicity: the role of autophagy flux inhibition. Eur J Pharmacol. (2019) 862:172616. 10.1016/j.ejphar.2019.172616
104.
Khanzada UK Pardo OE Meier C Downward J Seckl MJ Arcaro A . Potent inhibition of small-cell lung cancer cell growth by simvastatin reveals selective functions of Ras isoforms in growth factor signalling.Oncogene. (2006) 25:877–87. 10.1038/sj.onc.1209117
105.
Jukema JW Cannon CP de Craen AJ Westendorp RG Trompet S . The controversies of statin therapy: weighing the evidence.J Am Coll Cardiol. (2012) 60:875–81. 10.1016/j.jacc.2012.07.007
106.
Ooi KG Khoo P Vaclavik V Watson SL . Statins in ophthalmology.Surv Ophthalmol. (2019) 64:401–32. 10.1016/j.survophthal.2019.01.013
107.
Altwairgi AK . Statins are potential anticancerous agents (review).Oncol Rep. (2015) 33:1019–39. 10.3892/or.2015.3741
108.
Warren JB . Translating the dose response into risk and benefit.Br J Clin Pharmacol. (2019) 85:2187–93. 10.1111/bcp.13949
109.
Šimiæ I Reiner Ž . Adverse effects of statins - myths and reality.Curr Pharm Des. (2015) 21:1220–6. 10.2174/1381612820666141013134447
110.
Reiner Z . Resistance and intolerance to statins.Nutr Metab Cardiovasc Dis. (2014) 24:1057–66. 10.1016/j.numecd.2014.05.009
Summary
Keywords
statin, prevention, adverse events, meta-analysis, randomized controlled trials
Citation
Wang X, Li J, Wang T, Zhang Z, Li Q, Ma D, Chen Z, Ju J, Xu H and Chen K (2022) Associations between statins and adverse events in secondary prevention of cardiovascular disease: Pairwise, network, and dose-response meta-analyses of 47 randomized controlled trials. Front. Cardiovasc. Med. 9:929020. doi: 10.3389/fcvm.2022.929020
Received
26 April 2022
Accepted
01 August 2022
Published
25 August 2022
Volume
9 - 2022
Edited by
Jinfeng Xu, The University of Hong Kong, Hong Kong SAR, China
Reviewed by
Bruria Raccah, Hebrew University of Jerusalem, Israel; Željko Reiner, University Hospital Center Zagreb, Croatia; Chun Chao Chen, Taipei Medical University, Taiwan
Updates
Copyright
© 2022 Wang, Li, Wang, Zhang, Li, Ma, Chen, Ju, Xu and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Xu, xuhaotcm@hotmail.comKeji Chen, kjchenvip@163.com
This article was submitted to Cardiovascular Therapeutics, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.