New wearable technologies for cardiac rhythm monitoring are gaining more importance in clinical routine in the field of cardiology and electrophysiology - by physicians as well as patients. These include, but are by far not restricted to, smartphone-based electrocardiogram (ECG) or photoplethysmograpy (PPG), finger-ECG, smartwatches, smart garments and more. This opens new horizons for mobile (m) Health-based patient care, mHealth-enhanced teleconsultations, but also mass screening for heart rhythm disorders.
The current Research Topic includes new research on these technologies covering methodological aspects on wearable single- and multiple-lead ECG or PPG devices as well as clinical implementation of digital devices (Figure 1).
Figure 1
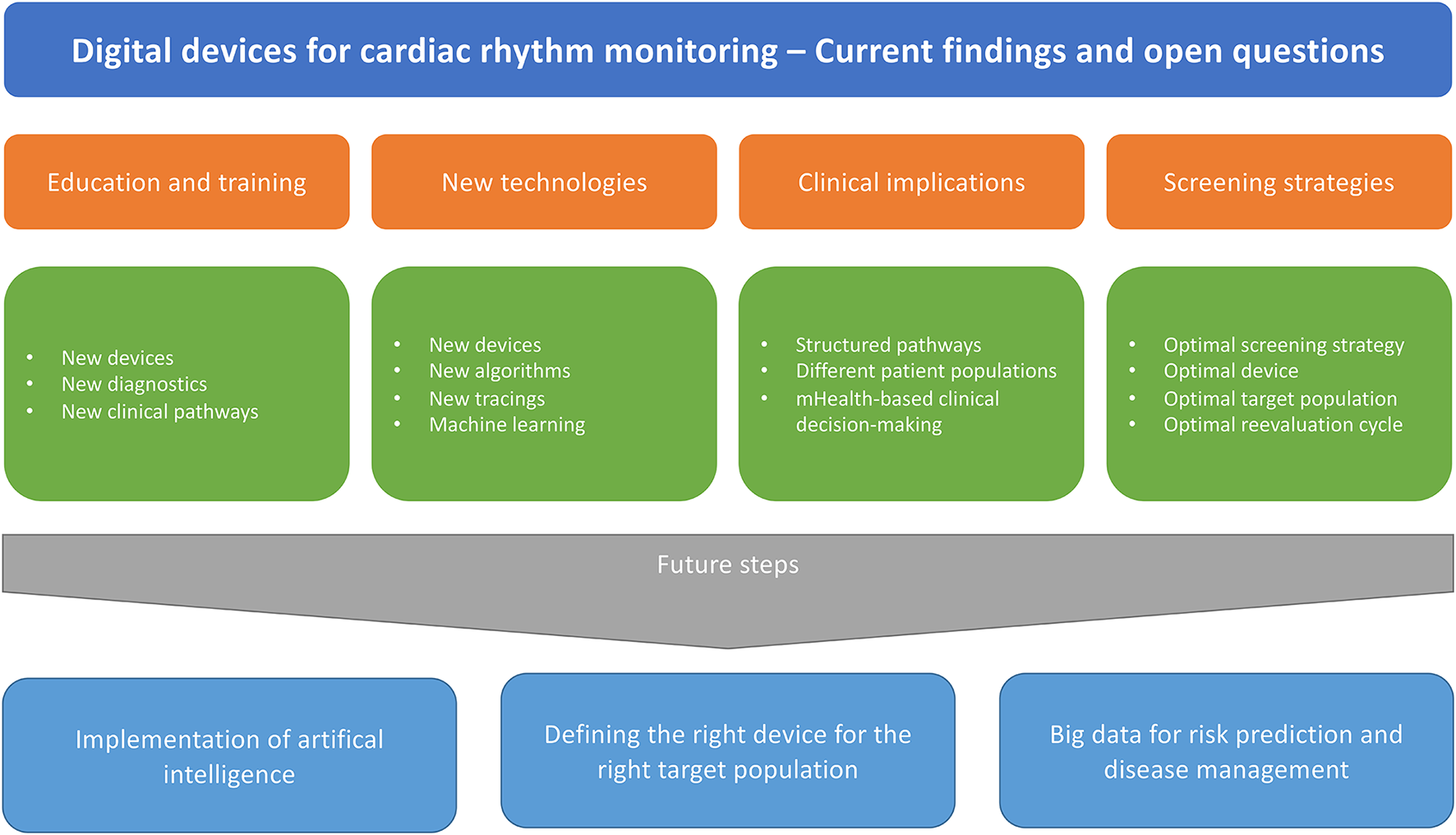
Clinical implementation of digital devices.
Xintarakou et al. present an elaborated review about smart wearables for monitoring and management of cardiac arrhythmias. The sensitivity and specificity of PPG-based devices in detecting AF is very good. Interpreting the PPG waveforms and tracings, however, requires some training (1). The INTERPRET-AF study by Gruwez et al. show that the accuracy of physicians interpreting PPGs is quite high and that using all available information from the PPG signal, the tachogram, the Poincaré plot and an automated algorithm increases the diagnostic accuracy and is comparable to a single lead ECG or 12-lead ECG. However, a call for training and education of PPG tracings and validity and limitations of interpretation should be made as this is rare in cardiological curricula, except in the recently updated curricula by the German Cardiac Society (2).
New Devices
Comparative studies on different devices are rare. Abu-Alrub et al. compared recording quality of three single-lead smartwatches in 100 patients with atrial fibrillation vs. 100 patients in sinus rhythm. Diagnosing AF is possible using various ECG smartwatch models, but differences in diagnostic accuracy of the related automated algorithms were noted.
An electronic-textile-based ECG monitoring was evaluated by Teferra et al. showing an effective option for continuous cardiac monitoring implemented into textiles.
Combining digital devices with machine learning algorithms represents a unique opportunity for individualized approaches or early identification of patients at risk. Luongo et al. evaluated a machine learning algorithm using a single-lead Holter ECG to identify patients with AF-induced cardiomyopathy. In clinical routine this could be a time and cost-efficient discriminator for general practitioners performing Holter ECG to identify patients requiring referral to a cardiologist.
Clinical Implications
Still, these new technologies require validation in clinical settings and a substantiated choice of the appropriate method using the appropriate device for the patient or user (3, 4). The DoubleCheck-AF validation study from Bacevicius et al. prospectively evaluated a wrist-worn device providing both continuous PPG-based rhythm monitoring and simultaneous 6-lead ECG. The study confirms a high specificity of the underlying algorithm to detect atrial fibrillation and to differentiate atrial fibrillation from other differential diagnoses, like frequent premature contractions.
The optimal screening strategy remains to be found (5). Following important screening trials like the STROKESTOP study (6), current consensus documents extended their recommendations on target populations and settings for screening for atrial fibrillation (3). Furthermore, the implementation of systematic screening for AF to achieve long-term reduction in a combined outcome of mortality, stroke, and severe bleeding is supported by current evidence (7), but will require establishment of clear diagnostic patient pathways.
The DoubleCheck-AF study opens the door for new screening strategies using PPG-based technology as the initial screening device and extending with ECG-based devices in case of irregular pulse notifications Bacevicius et al.. Fabritz et al. present the study design of the investigator-initiated multicenter Smart in OAC – AFNET 9 study which will include 1,000 unselected individuals of 65 years or older on wearable-based screening for PPG-detected atrial arrhythmias.
In post stroke patients, searching for AF is of utmost importance and strongly recommended (3, 8). Wouters, Gruwez, Vranken, Ernon, et al. present a nice case report of a patient simultaneously monitored by an implantable loop recorder and a PPG device.
In preliminary results from the REMOTE trial, Wouters, Gruwez, Vranken, Vanhaen, et al. present their initial results from 39 patients monitored with an implantable loop recorder and a PPG-based device. Interestingly, using the implantable loop recorder as the gold standard compared to a PPG-based monitoring, they identified limitations of the mHealth technology, but also registered false-positive recordings by the implantable loop record requiring revision by a physician.
For patients after cryptogenic stroke, the CANDLE-AF study will clarify the role of a single-lead patch ECG for the early detection of AF (Jung et al.).
In a novel outlook on use of wearables, patients after coronary bypass surgery used a digital device to study the relationship between heart rate variability and pulse rate variability (Chen et al.).
Digital devices not only measure the cardiac rhythm, but can also be used for further risk stratification and clinical decision-making. In a sub-study from the TeleCheck-AF project (9), Hermans et al. analyzed the patient responses to an app-based 10-item questionnaire on risk factors. They found that self-reported mHealth-based assessment of AF risk factors is feasible, but still bears the risk of over- or underreporting. This sets the stage for new approaches to mHealth-based clinical pathways.
Conclusions and Future Perspectives
Wearable devices for cardiac rhythm monitoring are common. For practical implementation it is key that health care professionals learn about the benefits and pitfalls of new devices, how to interpret the tracings, but also how to integrate this knowledge in practical patient pathways. Further studies are needed to identify the optimal target populations, the best screening settings, establish gold standards, and identify appropriate interventions.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
DD and ES drafted the work and revised it critically for important intellectual content. Both authors approved publication of the content and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
DD has received speaker honoraria and/or travel grants from Abbott, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, CVRx, Medtronic, Pfizer, and Zoll. ES has received lecture fees from Bayer, Bristol-Myers Squibb-Pfizer, Boehringer- Ingelheim, Merck Sharp & Dohme, and Sanofi.
References
1.
Velden RMJ van der Verhaert DVM Hermans ANL Duncker D Manninger M et al . The photoplethysmography dictionary: practical guidance on signal interpretation and clinical scenarios from TeleCheck-AF. Eur Heart J Digital Heal. (2021) 2:363–73. 10.1093/ehjdh/ztab050
2.
Werdan K Baldus St Bauersachs J Baumgartner H Bongarth CM Buerke M et al . Curriculum Kardiologie. Der Kardiologe. (2020) 14:1–28. 10.1007/s12181-020-00425-w
3.
Svennberg E Tjong F Goette A Akoum N Biaise LD Bordachar P et al . How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Ep Europace. (2022). 10.1093/europace/euac038. [Epub ahead of print].
4.
Hillmann HAK Soltani S Mueller-Leisse J Hohmann S Duncker D . Cardiac rhythm monitoring using wearables for clinical guidance before and after catheter ablation. J Clin Medicine. (2022) 11:2428. 10.3390/jcm11092428
5.
Duncker D Svennberg E . The catch in atrial fibrillation detection: don't forget to treat. Lancet Heal Longev. (2021) 2:e447–8. 10.1016/s2666-7568(21)00176-8
6.
Svennberg E Friberg L Frykman V Al-Khalili F Engdahl J Rosenqvist M . Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet. (2021) 398:1498–506. 10.1016/s0140-6736(21)01637-8
7.
Schnabel RB Marinelli EA Arbelo E Boriani G Boveda S Buckley C et al . Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. EP Europace. (2022). 10.1093/europace/euac062. [Epub ahead of print].
8.
Hindricks G Potpara T Dagres N Bax JJ Blomström-Lundqvist C Castella M et al . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehaa612
9.
Gawalko M Duncker D Manninger M Velden RM van der Hermans AN Verhaert DV et al . The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace. (2021) 23:1003–15. 10.1093/europace/euab050
Summary
Keywords
mobile health (mHealth), wearable device, atrial fibrillation, screening, rhythm monitoring
Citation
Duncker D and Svennberg E (2022) Editorial: Wearable Devices for Cardiac Rhythm Monitoring. Front. Cardiovasc. Med. 9:951769. doi: 10.3389/fcvm.2022.951769
Received
24 May 2022
Accepted
30 May 2022
Published
21 June 2022
Volume
9 - 2022
Edited and reviewed by
Matteo Anselmino, University of Turin, Italy
Updates
Copyright
© 2022 Duncker and Svennberg.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Duncker duncker.david@mh-hannover.de
This article was submitted to Cardiac Rhythmology, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.