Abstract
Background:
Mycophenolate mofetil (MMF) is a prodrug of mycophenolic acid (MPA) and a key immunosuppressant for improving graft survival in patients with heart transplantation (HTx). However, dose reduction or interruption is occasionally needed due to gastrointestinal (GI) side effects. Enteric-coated mycophenolate sodium (EC-MPS) is an alternative form of MPA delivery to improve GI tolerability. In the present study, the efficacy of EC-MPS compared with MMF in HTx patients was investigated.
Methods:
In this retrospective study, the Korean Organ Transplant Registry (KOTRY) data were used to analyze the efficacy and rejection rate of MMF and EC-MPS. A total of 611 patients was enrolled from 2014 to February of 2021. Patients were divided based on the use of MMF or EC-MPS at 6 months post-HTx. Patients who were not prescribed MMF or EC-MPS were excluded. Graft survival, all-cause mortality, and treated rejection were compared between the two groups. All statistical analyses were performed using SPSS; characteristics were compared using Pearson chi-square test and survival rate with Kaplan-Meier plot and log-rank test.
Results:
A total of 510 HTx patients was analyzed (mean age: 51.74 ± 13.16 years, males: 68.2%). At 6 months after HTx, 78 patients were taking EC-MPA (12.8%) and 432 patients were taking MMF (70.7%). The median follow-up was 42.0 months (IQR: 21.7–61.0 months). Post-HTx outcomes including overall survival, all cause mortality, acute cell mediated rejection (ACR), acute antibody mediated rejection (AMR), treated rejection, and cardiac allograft vasculopathy (CAV) were comparable between the two groups during follow-up.
Conclusion:
Notable differences were not observed in overall survival, all cause mortality, ACR, AMR, treated rejection, and CAV between MMF and EC-MPS groups. Efficacy of EC-MPS was similar to that of MMF in HTx patients during mid-term follow up after HTx.
Introduction
Heart transplantation (HTx) is the standard treatment for end-stage heart failure (HF). Survival and prognosis of HTx have improved over the last two decades with introduction of effective immunosuppression therapy (1, 2). Generally, for maintenance immunosuppression, HTx patients receive a combination of two or three classes of medication, calcineurin inhibitors (CNIs), anti-metabolites, and proliferation signal inhibitors (3).
Mycophenolate mofetil (MMF, CellCept®, Roche Laboratories, Nutley, NJ, USA) is a potent anti-proliferative drug that recently replaced azathioprine as the drug of choice due to improved survival and reduced rejection rates compared with azathioprine (4). MMF is a prodrug of mycophenolic acid (MPA) and inhibits inosine-5′-monophosphate dehydrogenase to block proliferation of T and B cells, leading to repression of both cell- and humoral-mediated immunity (5). However, gastrointestinal (GI) intolerance is a common dose-limiting side effect often leading to interruptions in therapy, which increases risk of rejection (6). Enteric-coated mycophenolate sodium (EC-MPS, Myfortic®, Novartis Pharmaceuticals, East Hanover, NJ, USA) was developed to reduce GI effects of MMF. Clinical trials in kidney transplant recipients demonstrated that EC-MPS is therapeutically equivalent to MMF (7, 8).
Unlike kidney transplantations, data regarding long-term HTx outcome with use of EC-MPS are limited. In the present study, using a nationwide organ transplant registry in Korea, post-HTx outcome was evaluated between patients taking MMF or EC-MPS in combination with CNIs and corticosteroids.
Methods
Study population
The nationwide multi-center HTx data submitted to the Korean Heart Transplant Registry (KOTRY), the first nationwide organ transplantation registry in Korea, was used in the present study (9). From 2014 to 2019, a total of 611 patients underwent HTx. With the exclusion of follow-up losses, a final number of 510 patients were included in this study. The study was reviewed and approved by the institutional review board of each transplantation center. The KOTRY registry includes baseline and follow-up data of transplanted patients. After HTx, follow-up visits were recorded at 1, 6, and 12 months and annually thereafter. Patients were classified into MMF or EC-MPS groups based on immunosuppressive regimen at 6 months after HTx.
Immunosuppression
CNI-based triple immunosuppressive therapy (tacrolimus, mycophenolate mofetil, and prednisone) was initially administered as maintenance therapy to most patients. Cyclosporine was administered if patients developed severe side effects from tacrolimus, such as seizures or encephalopathy. A regimen using a mammalian target of rapamycin (mTOR) inhibitor, either sirolimus or everolimus, in place of a CNI-free regimen was prescribed to eligible patients, including those with renal insufficiency or malignancy. An mTOR inhibitor was administered in conjunction with a CNI in patients who developed rejection with graft dysfunction, cytomegalovirus infection, or cardiac allograft vasculopathy (CAV). In case of intolerance to an mTOR inhibitor, a conventional CNI-based regimen was maintained. Patients at low risk of rejection were tapered off steroids 6 months after HTx according to transplantation clinic protocol. All HTx recipients underwent a protocol-based regular evaluation at their transplantation clinic (10). Post-HTx clinical outcome included overall survival, freedom from angiographic CAV (11), and any treated rejection. Rejection was diagnosed through endomyocardial biopsy and included both acute cellular rejection (ACR) and antibody-mediated rejection (AMR). Rejections were defined according to the revised International Society for Heart and Lung Transplantation (ISHLT) classification (12). Treated rejection was defined as events that require either intravenous steroids for acute cellular rejections or rituximab injections for antibody-mediated rejections.
Statistical analysis
Continuous variables are recorded as mean ± standard deviation, and categorical variables are reported as frequency and percentages. Baseline recipient/donor characteristics and clinical outcomes of HTx were compared between MMF and EC-MPS groups. Post-HTx outcomes included treated rejection and all cause mortality. The two groups were compared using chi-square test, and continuous variables using Student's t-test. The cumulative incidence of events and outcome analysis was assessed using the Kaplan-Meier method, and statistical significance was calculated using the log-rank test. Due to the number difference between the two groups, 1 to 1 individual matching within caliper by propensity score matching was performed. Analysis by chi-square test was used for group comparison, and two sample t-test was used for continuous variables. All data were analyzed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and R-version 4.2.0 (13).
Results
Baseline characteristics
Among the 510 HTx patients, 432 were taking MMF (70.7%) and 78 were taking EC-MPS (12.8%) post-HTx. Patients in EC-MPS group were younger and had longer warm ischemic time, aortic-cross clamp, and cardiopulmonary bypass time (Table 1). Significantly more patients in the EC-MPS group received extracorporeal membrane oxygenation before HTx (31.5 vs. 19.2%, p = 0.031). In addition, significantly more patients (65.3 vs. 91.0%, p < 0.001) in the EC-MPS group remained on steroid treatment at 6 months post-HTx compared with subjects in the MMF group. An average dose of 1,500 mg (1,000–2,000 mg) of MMF and 1,080 mg (720–1,440 mg) of EC-MPS was used in each group. This amount is an equivalent dose of the active form, MPA.
Table 1
| MMF group (n = 432) | EC-MPS group (n = 78) | p-value | |
|---|---|---|---|
| Age, years | |||
| Recipient | 52.4 ± 12.6 | 48 ± 15.5 | 0.001 |
| Donor | 39.8 ± 11.4 | 40.4 ± 10.8 | 0.184 |
| Recipient | |||
| BMI (kg/m2) | 22.6 ± 3.7 | 22.4 ± 3.5 | 0.898 |
| Sex (male) | |||
| Recipient | 290 (66.9%) | 59 (75.6%) | 0.146 |
| Donor | 301 (69.7%) | 53 (67.9%) | 0.790 |
| Male recipient/female donor | 64 (14.8%) | 24 (16.7%) | 0.731 |
| Female recipient/male donor | 76 (17.6%) | 7 (9.0%) | 0.066 |
| Recipient | |||
| Hypertension | 109 (25.2%) | 40 (51.3%) | 0.035 |
| Diabetes mellitus | 117 (27.1%) | 35 (44.9%) | 0.277 |
| Chronic kidney disease | 62 (14.4%) | 13 (16.7%) | 0.055 |
| Previous malignancy | 33 (7.6%) | 6 (7.7%) | 1.000 |
| Cold ischemia time (min) | 113.5 ± 59.2 | 103.6 ± 64.1 | 0.323 |
| Warm ischemia time (min) | 56.9 ± 25.6 | 75.5 ± 44.7 | 0.001 |
| ACC time (min) | 113.1 ± 49.1 | 140.9 ± 52.5 | <0.001 |
| CPB time (min) | 152.3 ± 65.1 | 181.3 ± 66.5 | <0.001 |
| Most recent PRA > 10% | |||
| Overall | 171 (39.6%) | 33 (42.3%) | 0.707 |
| Class I | 124 (29.0%) | 21 (27.3%) | 0.891 |
| Class II | 116 (26.9%) | 24 (30.8%) | 0.492 |
| LVEF at HTx | 26.8 ± 15.1 | 27.0 ± 12.8 | 0.698 |
| Cr at time of HTx (mg/dL) | 1.21 ± 0.87 | 1.43 ± 1.59 | 0.070 |
| Diagnosis | |||
| Dilated cardiomyopathy | 224 (51.9%) | 39 (50.0%) | 0.806 |
| Ischemia | 89 (20.6%) | 13 (16.7%) | 0.538 |
| Retransplant | 15 (3.5%) | 3 (3.8%) | 0.750 |
| Pre-HTx support | |||
| Mechanical ventilator | 11 (14.1%) | 102 (23.6%) | 0.075 |
| ECMO | 136 (31.5%) | 15 (19.2%) | 0.031 |
| LVAD | 21 (4.9%) | 4 (5.1%) | 1.000 |
| Induction therapy | 372 (86.1%) | 71 (91.0%) | 0.278 |
| Immunosuppression at 6 months post-HTx | |||
| Tacrolimus | 368 (86.2%) | 66 (84.6%) | 0.863 |
| Cyclosporine | 11 (2.5%) | 6 (7.7%) | 0.032 |
| Everolimus | 111 (25.7%) | 23 (29.5%) | 0.487 |
| Steroid | 282 (65.3%) | 71 (91.0%) | <0.001 |
Comparison of donor/recipient baseline characteristics between the MMF and EC-MPS groups.
Data are shown as mean ± standard deviation or number.
ACC, aortic cross-clamp; BMI, body mass index; CPB, cardiopulmonary bypass; Cr, creatinine; ECMO, extracorporeal membrane oxygenation; HLA, human leukocyte antigen; IABP, intraaortic balloon pump; LVAD, left ventricular assisting device; PRA, panel reactive antibodies; RVSP, right ventricular systolic pressure; HTx, heart transplantation; MMF, mycophenolate mofetil; EC-MPS, enteric-coated mycophenolate sodium.
Post-HTx clinical outcomes
Significant differences in all-cause mortality and freedom from treated rejection were not observed between the two groups (Figures 1A,B) during the mean follow-up period (40 ± 23 months). Post-Hx overall survival, all cause mortality, freedom from ACR, AMR, and CAV were similar between the two groups (Table 2). Although significantly more patients in the EC-MPS group (4.4 vs. 10.3%, p = 0.049) experienced treated rejection during the first year compared with the MMF group, graft survival and overall survival were comparable between two groups. During follow-up, incidences of an infection requiring hospitalization were similar between the two groups.
Figure 1
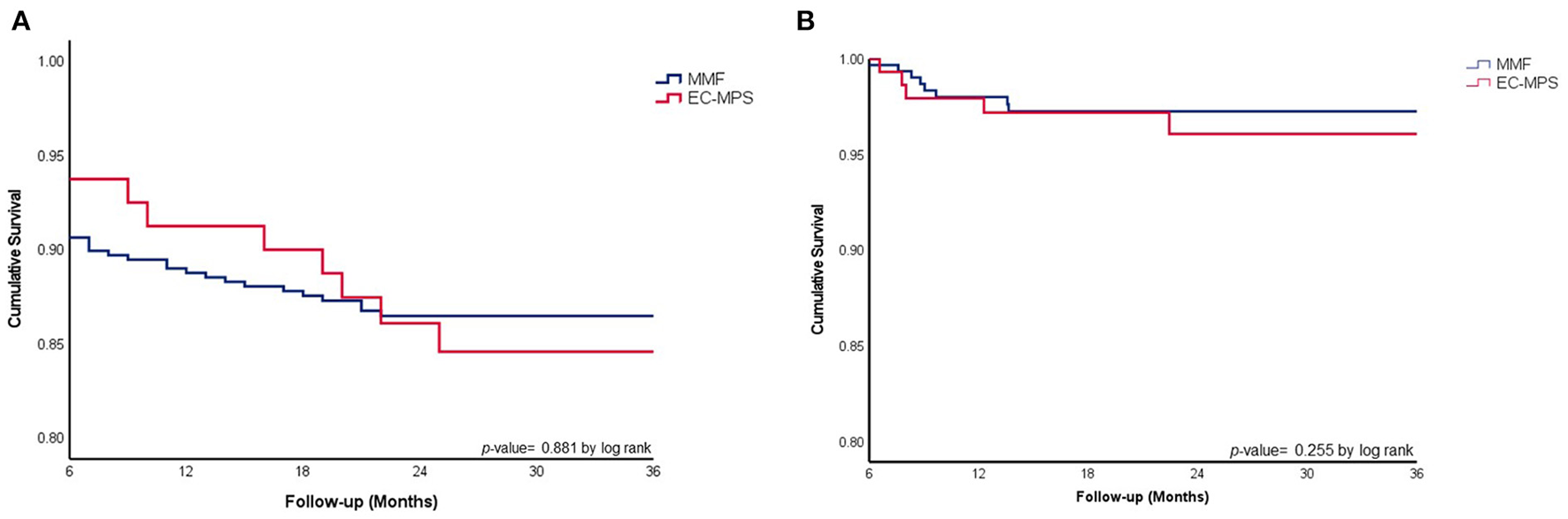
(A) Comparison of overall survival between HTx patients in EC-MPS and MMF groups. (B) Cumulative survival in freedom from treated rejection in heart transplantation patients using MMF vs. EC-MPS. Overall survival rates (A) and cumulative survival in freedom from treated rejection (B) were similar between HTx patients in the EC-MPS and MMF groups. HTx, heart transplantation; EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil.
Table 2
| MMF group (n = 432) | EC-MPS group (n = 78) | p-value | |
|---|---|---|---|
| All cause mortality | 61 (14.1%) | 12 (15.4%) | 0.855 |
| 1-year freedom from ACR | 195 (45.1%) | 29 (37.2%) | 0.325 |
| 3-year freedom from ACR | 180 (41.7%) | 24 (30.7%) | 0.567 |
| 1-year freedom from AMR | 416 (96.3%) | 72 (92.3%) | 0.942 |
| 3-year freedom from AMR | 415 (96.3%) | 72 (92.3%) | 0.567 |
| 1-year freedom from treated rejection | 414 (95.8%) | 71 (91.0%) | 0.047 |
| 3-year freedom from treated rejection | 408 (95.8%) | 68 (87.2%) | 0.658 |
| 1-year freedom from CAV | 418 (96.7%) | 72 (92.3%) | 0.078 |
| 3-year freedom from CAV | 390 (90.3%) | 67 (85.9%) | 0.490 |
| Infection requiring hospitalization | 15 (3.5%) | 3 (3.8) | 0.746 |
Comparison of clinical outcomes after HTx between the MMF and EC-MPS groups.
HTx, heart transplantation; MMF, mycophenolate mofetil; EC-MPS, enteric-coated mycophenolate sodium; ACR, Acute cell mediated rejection; AMR, Acute antibody mediated rejection; CAV, cardiac allograft vasculopathy.
We performed a subgroup analysis of patients treated with cyclosporine, because cyclosporine is known to influence MPA pharmacokinetics. Post-HTx clinical outcomes were comparable between MMF and EC-MPS group in subgroup of patients who were treated with cyclosporine (Supplementary Table 1).
Due to significant difference of baseline characteristics between MMF and EC-MPS groups, a propensity score matching analysis was conducted with adjustment of age, gender, pre-HTx ECMO and prolonged steroid use (Table 3). In this analysis, post-HTx clinical outcomes including all-cause mortality were comparable between two groups.
Table 3
| MMF group | EC-MPS group | p-value | |
|---|---|---|---|
| Number | 75 | 75 | |
| Sex (male) | 55.0 (73.3%) | 58.0 (77.3%) | 0.41 |
| Age (years) | 46.39 ± 14.90 | 47.53 ± 15.33 | 0.341 |
| Pre-HTx support, ECMO | 19.0 ± 25.3 | 21.0 ± 28.0 | 0.62 |
| Steroid use at 6 months | 74.0 (98.7%) | 74.0 (98.7%) | 1 |
| All cause mortality | 11 (14.7%) | 12 (16%) | 0.7 |
| 1-year freedom from ACR | 27 (36.0%) | 31 (41.3%) | 0.346 |
| 3-year freedom from ACR | 25 (33.3%) | 26 (34.7%) | 0.168 |
| 1-year freedom from AMR | 72 (96.0%) | 72 (96.0%) | 0.914 |
| 3-year freedom from AMR | 71 (94.7%) | 72 (96.0%) | 0.168 |
| 1-year freedom from treated rejection | 27 (36.0%) | 23 (30.7%) | 0.488 |
| 3-year freedom from treated rejection | 21 (28.0%) | 20 (26.7%) | 0.855 |
| 1-year freedom from CAV | 70 (93.3%) | 71 (94.7%) | 0.754 |
| 3-year freedom from CAV | 68 (90.7%) | 68 (90.7%) | 1 |
1 to 1 individual matching between MMF vs. EC-MPS within caliper by propensity score.
HTx, heart transplantation; MMF, mycophenolate mofetil; EC-MPS, enteric-coated mycophenolate sodium; ACR, Acute cell mediated rejection; AMR, Acute antibody mediated rejection; CAV, cardiac allograft vasculopathy.
Discussion
To the best of our knowledge, this is the first mid-term study in which post-HTx outcomes from a multi-center registry were compared between patients receiving MMF or EC-MPS. During the mean follow-up of 40 months, post-HTx clinical outcomes were similar between MMF and EC-MPS groups, although significantly more patients with EC-MPS experienced treated rejection at 1-year follow-up.
MMF, due to its effectiveness in reducing acute rejection rates (4), is now the drug of choice for post-transplantation immunosuppression in multiple organs including the heart. However, GI side effects caused by MPA, the active form of MMF (14, 15), and leukopenia are dose-limiting side effects. Dose reduction due to MMF intolerance increases the risk of acute rejection (16). In previous kidney transplantation registries, the incidence of MMF intolerance leading to dose reductions reportedly ranged from 42 to 59% (15–17). In a single-center study, the recommended dose of 3 mg/day of MMF in adult HTx patients was poorly tolerated, and the median dose of MMF at 6 months post-HTx was 1,560 ± 984 mg/day (18), similar to median doses of MMF at 6 months post-HTx in the present study. In the study cohort, 12.8% were taking EC-MPS at 6 months after HTx because patients either experienced or were predisposed to GI disorder.
EC-MPS was developed to decelerate the release of MPA, contrary to MMF, which shows instant release of MPA into the GI tract. In pharmacokinetic studies, despite delayed delivery, administration of EC-MPS resulted in a similar maximal plasma concentration and MPA exposure (19, 20). In maintenance and de novo trials, similar safety and efficacy of EC-MPS compared with MMF were observed in kidney transplant patients with similar rejection, infection, and graft survival at 12 months post-kidney transplant (7, 8).
In a previous single-blinded multi-center trial including 154 HTx patients, similar rates of treatment failure and combination of treated acute rejection, graft loss, and death at 6 months (52.6 vs. 57.9%) and 12 months (57.7 vs. 60.5%) (21, 22) post-HTx were reported. A previous single center randomized study showed that EC-MPS treated HTx patients are less likely to require multiple dose reductions than those on MMF and was associated with a significant lower incidence of treated rejection (23). However, these studies are limited due to a short follow-up of 12 months with a small numbers of patients. In the present study, we analyzed real-world clinical outcomes after HTx with nation-wide multi-center data and longer follow-up duration. Results of the present study indicate similar long-term efficacy of EC-MPS to MMF in HTx patients compared to previous studies (20–22). In addition, our study showed that eventually the average of MPA dose in both groups did not significantly differ.
Limitations
The present study had limitations due to the retrospective design and the different number of subjects in the MMF and EC-MPS groups. Data regarding MPA level were lacking, as this is not a routine practice in many centers. Time-dependent dose change of MMF and EC-MPS might not have been reported due to pre-specified follow-up intervals in the KOTRY registry and other immunosuppressive regimens determined based on the protocol of each transplantation clinic.
The main use of EC-MPS being an alternative for MMF intolerance due to its gastrointestinal side effects, and this study might been more profound if the actual incidence and severity of GI side effects was assessed after HTx. Unfortunately, due to the nature of our retrospective study, the severity and rate of intolerance were not consistently assessed with objective tools in this registry, therefore, difference in gastrointestinal complications between two groups could not be provided. However, the present study is valuable because our study described and compared real world post-HTx clinical outcomes between MMF and EC-MPS groups from a multi-center, nationwide registry.
Conclusion
In conclusion, HTx patients treated with EC-MPS and MMF have similar incidence of overall survival, ACR, AMR, treated rejection, CAV, and all cause mortality. Mid-term post-HTx clinical efficacy of EC-MPS was similar to that of MMF in HTx patients.
Funding
This research was supported by a fund (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00, 2017-ER6301-01, and 2017-ER6301-02) by Research of Korea Centers for Disease Control and Prevention Agency. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
Relevant data are available from the corresponding author on reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by KOTRY. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.957299/full#supplementary-material
References
1.
ChambersDCPerchMZuckermannACherikhWSHarhayMOHayesDJret al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-eighth adult lung transplantation report-2021; focus on recipient characteristics. J Heart Lung Transplant. (2021) 40:1060–72. 10.1016/j.healun.2021.07.021
2.
YangYAgbor-EnohSIlkerTHsuSRussellSFellerEet al. Cardiac allograft injury in patients of African ancestry: trends of donor-derived cell-free DNA based on genetic ancestry. J Heart Lung Transpl. (2021) 40:S254-S. 10.1016/j.healun.2021.01.725
3.
SoderlundCRadegranG. Immunosuppressive therapies after heart transplantation-the balance between under- and over-immunosuppression. Transplant Rev. (2015) 29:181–9. 10.1016/j.trre.2015.02.005
4.
KobashigawaJMillerLRenlundDMentzerRAldermanEBourgeRet al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenol Mofetil Investig Transplant. (1998) 66:507–15. 10.1097/00007890-199808270-00016
5.
StaatzCETettSE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. (2014) 88:1351–89. 10.1007/s00204-014-1247-1
6.
LindenfeldJMillerGGShakarSFZoltyRLowesBDWolfelEEet al. Drug therapy in the heart transplant recipient: Part II: Immunosuppressive drugs. Circulation. (2004) 110:3858–65. 10.1161/01.CIR.0000150332.42276.69
7.
BuddeKCurtisJKnollGChanLNeumayerHHSeifuYet al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant. (2004) 4:237–43. 10.1046/j.1600-6143.2003.00321.x
8.
SalvadoriMHolzerHde MattosASollingerHArnsWOppenheimerFet al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. (2004) 4:231–6. 10.1046/j.1600-6143.2003.00337.x
9.
KimDLeeGYChoiJOKimKKimSJJuESet al. Prognostic values of novel biomarkers in patients with Al amyloidosis. Sci Rep. (2019) 9:12200. 10.1038/s41598-019-48513-6
10.
ChangDHYounJ-CDiliberoDPatelJKKobashigawaJA. Heart transplant immunosuppression strategies at Cedars-Sinai Medical Center. Int J Heart Fail. (2021) 3:15–30. 10.36628/ijhf.2020.0034
11.
MehraMRCrespo-LeiroMGDipchandAEnsmingerSMHiemannNEKobashigawaJAet al. International society for heart and lung transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. (2010) 29:717–27. 10.1016/j.healun.2010.05.017
12.
StewartSWintersGLFishbeinMCTazelaarHDKobashigawaJAbramsJet al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. (2005) 24:1710–20. 10.1016/j.healun.2005.03.019
13.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2022).
14.
BehrendM. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf. (2001) 24:645–63. 10.2165/00002018-200124090-00002
15.
MouradMMalaiseJChaib EddourDDe MeyerMKönigJSchepersRet al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem. (2001) 47:88–94. 10.1093/clinchem/47.1.88
16.
KnollGAMacDonaldIKhanAVan WalravenC. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. (2003) 14:2381–6. 10.1097/01.ASN.0000079616.71891.F5
17.
SquiffletJPBackmanLClaessonKDietlKHEkbergHForsytheJLRet al. Dose optimization of mycophenolate mofetil when administered with a low dose of tacrolimus in cadaveric renal transplant recipients. Transplantation. (2001) 72:63–9. 10.1097/00007890-200107150-00014
18.
PassiNSneadJGoldsteinDJMurthySPatelSRSaeedOet al. Mycophenolate mofetil in adult orthotopic heart transplant recipients: how much is too much?J Heart Lung Transpl. (2016) 35:S422-S. 10.1016/j.healun.2016.01.1229
19.
BuddeKBauerSHambachPHahnURoblitzHMaiIet al. Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transplant. (2007) 7:888–98. 10.1111/j.1600-6143.2006.01693.x
20.
ArnsWBreuerSChoudhurySTaccardGLeeJBinderVet al. Enteric-coated mycophenolate sodium delivers bioequivalent mpa exposure compared with mycophenolate mofetil. Clin Transplant. (2005) 19:199–206. 10.1111/j.1399-0012.2004.00318.x
21.
KobashigawaJARenlundDGGerosaGAlmenarLEisenHJKeoghAMet al. Similar efficacy and safety of enteric-coated mycophenolate sodium (EC-MPS, Myfortic) compared with mycophenolate mofetil (Mmf) in de novo heart transplant recipients: results of a 12-month, single-blind, randomized, parallel-group, multicenter study. J Heart Lung Transplant. (2006) 25:935–41. 10.1016/j.healun.2006.04.005
22.
LehmkuhlHHummelMKobashigawaJLadenburgerSRothenburgerMSackFet al. Enteric-coated mycophenolate-sodium in heart transplantation: efficacy, safety, and pharmacokinetic compared with mycophenolate mofetil. Transplant Proc. (2008) 40:953–5. 10.1016/j.transproceed.2008.03.046
23.
SegoviaJGerosaGAlmenarLLiviUViganòMArizónJMet al. Impact of dose reductions on efficacy outcome in heart transplant patients receiving enteric-coated mycophenolate sodium or mycophenolate mofetil at 12 months post-transplantation. Clin Transplant. (2008) 22:809–14. 10.1111/j.1399-0012.2008.00887.x
Summary
Keywords
heart transplantation, prognosis, mycophenolate mofetil, mycophenolic acid, rejection
Citation
Jeon K, Kim D, Choi J-O, Cho YH, Sung K, Oh J, Cho HJ, Jung S-H, Lee H-Y, Park JJ, Choi D-J, Kang S-M, Kim J-J and Jeon E-S (2022) Comparison of mid-term clinical outcome in heart transplantation patients using mycophenolate mofetil vs. enteric-coated mycophenolate sodium. Front. Cardiovasc. Med. 9:957299. doi: 10.3389/fcvm.2022.957299
Received
30 May 2022
Accepted
01 August 2022
Published
23 August 2022
Volume
9 - 2022
Edited by
Matteo Cameli, University of Siena, Italy
Reviewed by
Kenichi Hongo, Jikei University School of Medicine, Japan; Joanna Sobiak, Poznan University of Medical Sciences, Poland; Norihide Fukushima, National Cerebral and Cardiovascular Center, Japan
Updates
Copyright
© 2022 Jeon, Kim, Choi, Cho, Sung, Oh, Cho, Jung, Lee, Park, Choi, Kang, Kim and Jeon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darae Kim daraekyrie@gmail.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.