Abstract
Background:
Left ventricular dysfunction and cardiomyopathy are well documented adverse effects associated with chemotherapy agents. Limited information exists regarding the impact of chemotherapeutic agents on the integrity and function of the right ventricle (RV).
Objectives:
The current metanalysis compared pre- chemotherapy versus post- chemotherapy RV parameters measured on 2D echocardiography in patients receiving anthracycline and/or trastuzumab across all breast cancer patients.
Methods:
A systematic search across PubMed, EMBASE and Cochrane databases were performed from inception of the databases until November 2021 for relevant studies. We used the inverse variance method with a random effect model and DerSimonian and Laird method of Tau2 generation to calculate mean difference [MD] with 95% confidence interval [CI]. The analysis was carried out using RevMan Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results:
Fifteen studies, constituting total of 644 patients, met the inclusion criteria, with most studies having a follow up period of less than 12 months from initiation of chemotherapy. Anthracycline and/or Trastuzumab chemotherapy resulted in a statistically significant reduction in right ventricular ejection fraction (RVEF) at follow-up [MD: 2.70, 95% CI: 0.27 to 5.13, P-value- 0.03, I2- 71%, χ2P-value < 0.05]. Treatment with Anthracycline and/or Trastuzumab chemotherapy resulted in a significant reduction in RV fractional area change (RVFAC) at follow-up [MD: 3.74, 95% CI: 1.33 to 6.15, P-value < 0.01, I2- 68%, χ2P-value < 0.05]. RV free wall longitudinal strain (RVFWLS) was lower at baseline, while LVEF was significantly reduced at follow-up [MD: -1.00, 95% CI: -1.86 to -0.15, P-value < 0.05, I2- 0%, χ2P-value-0.40], [MD: 4.04, 95% CI: 2.08 to 6.01, P-value < 0.01, I2- 91%, χ2P-value < 0.05], respectively. However, treatment with Anthracycline and/or Trastuzumab chemotherapy had no statistically significant effect on Tricuspid annular plane systolic excursion (TAPSE) at follow-up [MD: 0.53, 95% CI: -0.11 to 1.17, P-value-0.11, I2- 98%, χ2P-value < 0.05].
Conclusions:
Chemotherapy with anthracyclines and trastuzumab negatively affects right ventricular function leading to decline in RVEF, RVFAC, RVFWLS and LVEF.
Introduction
Cancer specific mortality rates have substantially declined over the past few decades owing to the diagnostic and therapeutic advances within the field. However, the adverse effects of cardiotoxicity of specific chemotherapeutic drugs, namely anthracyclines and trastuzumab continue to contribute to significant morbidity and mortality in this patient population. Deducing the adverse effects of these chemotherapeutic drugs on the cardiovascular system is pivotal due the presence of heart failure from chemotherapy-induced cardiomyopathy and premature coronary artery disease among a considerable number of patients (1). The first such effect was recognized in the 1960s with the advent of Daunorubicin, an anthracycline, which gave rise to the niche sub-specialty known as “Cardio-Oncology” (2–4). The increasing awareness and poor prognosis associated with chemotherapy-induced cardiomyopathy has ushered the development of protocols for screening, surveillance as well as interventions to reduce cardiac risk (5–8). The field continues to evolve to better identify, define predictive risk models and biomarkers of cancer therapy related cardiac dysfunction (CTRCD).
Left ventricular dysfunction and remodelling has been well defined in chemotherapy induced cardiotoxicity. The effect of chemotherapeutic agents on the right ventricle (RV) is not well researched. Several initial reports on CTRCD have demonstrated right ventricle involvement following chemotherapy, as seen on right ventricular biopsies (9). Nevertheless, there is a paucity of data with respect to the frequency of RV involvement and its prognostic value in CTRCD. Echocardiographic evaluation of the RV should include RV basal diameter, right atrial size (area), tricuspid annular plane systolic excursion via M-mode, tricuspid annular systolic peak velocity (TAPSE) using pulsed doppler tissue imaging (DTI), RV fractional area shortening (FAC) and provide estimates of the RV systolic pressure. Individual studies assessing the effects of cancer chemotherapeutics on the RV exist, but they are limited by a small patient cohort. In the current systematic review and meta-analysis we report the pooled differences in RV echo parameters pre and post anthracycline and/or trastuzumab across all breast cancer patients.
Methods
Data sources and search strategies
A systematic search across PubMed, EMBASE and Cochrane databases were performed from inception of the databases until November 2021 for relevant studies. Supplementary file provides the search strategy and MeSH terms used in each database (Supplementary Table S1). In addition to database search, we also reviewed the reference lists of included articles to identify additional studies.
The present analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and American Heart Association guidelines (10, 11). Since, only studies with prior ethical committee clearance were included in the present analysis, no separate ethical clearance was required.
Study selection
After checking for duplicates, the searched articles were screened for relevant studies. The inclusion criteria were as follows: (a) observational studies, reporting right ventricular echocardiography outcomes at baseline and post Anthracycline and/or Trastuzumab chemotherapy among breast cancer patients; (b) no exclusion based on sample size; (c) reporting one of the following right ventricular echocardiography outcomes; right ventricle ejection fraction (RVEF), right ventricular fractional area change (RVFAC), right ventricular global longitudinal strain (RVGLS), right ventricular free wall longitudinal strain (RVFWLS), tricuspid annular plane systolic excursion (TAPSE); (d) exclusion of studies conducted exclusively in hematologic malignancies and pediatric cancer survivors and (e) finally, studies published in English language. The screening for inclusion was performed independently by two reviewers (A.J., M.S.) at two separate levels. At level one, title and abstracts of searched citations were screened for relevance. At the second level, articles identified from level one screening were subjected to complete manuscript review and considered when they met the inclusion criteria.
Screening and data extraction
Data extraction from included studies was performed independently by two reviewers (A.J., M.S.), and later examined for similarity. The following information was extracted from original primary publications; author, year of study, sample size, diagnosis, cancer chemotherapy agent used, percentage of patients treated with Anthracyclines, follow-up period, baseline and follow-up values of RVEF, RVFAC, RVGLS, RVFWLS, TAPSE, and left ventricular ejection fraction (LVEF). The data extraction was performed as per a pre-designed data extraction form. Any disparity was resolved by mutual consensus and after consultation with other authors. Studies included observational studies without any interventions, therefore bias tools such as the ROBINS-I tool were not employed. The National Institute of Health (NIH) Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group was utilized for risk of bias assessment in the current analysis.
Quality assessment
We used the National Institutes of Health (NIH) Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group. It included the following assessments; pre-specified entry criteria, sample size, blinded assessors of data and clear descriptors of outcome measurements and others. Two authors independently judged each domain, with a third author resolving conflicts.
Statistical analysis
We used the inverse variance method with a random effect model and DerSimonian and Laird method of Tau2 generation to calculate mean difference [MD] with 95% confidence interval [CI]. We used the method provided by Hozo et al., to calculate mean and standard deviation from studies reporting median and inter quartile range (12). To obtain a conservative result with a wide confidence interval, we used a correction (r = 0) between the pre-treatment and post treatment mean while calculating the standard deviation of the pooled estimate. Statistical heterogeneity was assessed using Higgins's I2 or chi-square P-value (13). The pooled estimate was deemed statistically heterogeneous if Higgins's I2 was >50% or chi-square P-value was < 0.05. Meta-analysis results were reported graphically using forest plots: the measure of effect (MD) was represented by a square, with the area being proportional to study weight. A p value < 0.05 was considered significant. The analysis was carried out using RevMan Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Study selection
The database search identified a total of 1,449 articles after checking for duplicates. Figure 1 represents the PRISMA flow chart for the inclusion of studies. 1,426 studies were excluded at the first level of screening and 15 studies were excluded at the second level of screening. Seven studies were included after manual search of reference list of the included studies. Finally, fifteen studies met the inclusion criteria and were included in the final analysis (14, 15, 24–26, 16–23). The fifteen studies constituted a total of 644 patients. All studies included breast cancer patients who were treated with Anthracycline and/or Trastuzumab chemotherapy, other than study by Cottin et al., 1996 which included women with breast cancer and lymphoma. Follow-up period of all included studies was less than 12 months. Baseline characteristics of patients from the included studies are presented in Table 1. We included studies which assessed right ventricular parameters by 2D echo parameters including RVEF, RVFAC, RVGLS, RVFWLS, TAPSE, as defined by the American Society of Echocardiography definitions and reference ranges for each parameter in Table 2. Summaries of individual studies included in the present systematic review and meta-analysis are presented in Table 3.
Figure 1
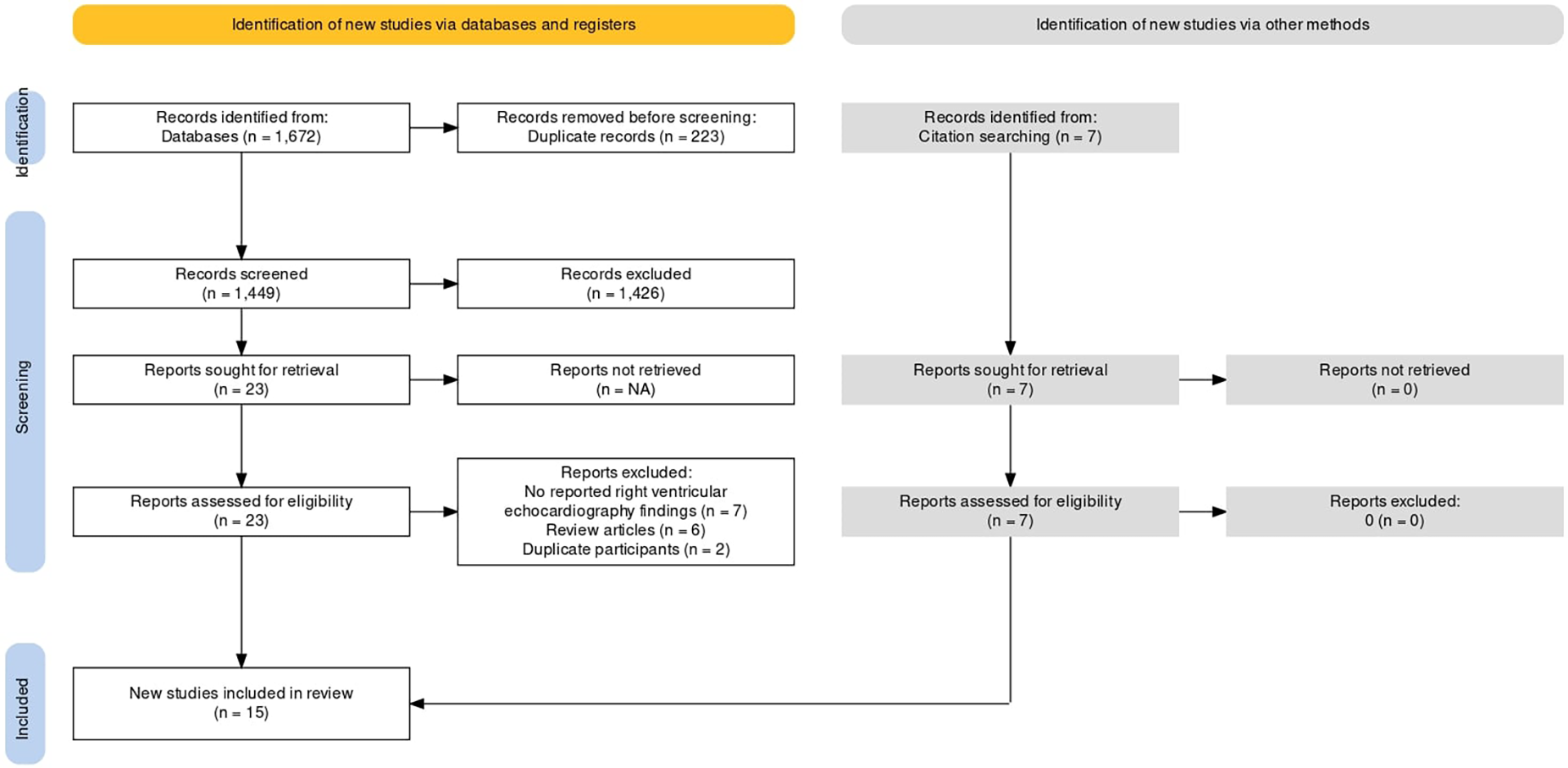
PRISMA flow chart.
Table 1
| Author | Year | N | Primary Site | Chemotherapy And/or Monoclonal Antibody | Cumulative anthracycline dose used (median) mg/m2 | Percentage of patients treated with anthracyclines | RV parameter evaluated/Modality | Follow up period |
|---|---|---|---|---|---|---|---|---|
| Havsteen et al. | 1989 | 14 | Breast | Anthracycline (Epirubicin) | 827 | 100% | RVEF | 2-3 weeks after the last chemotherapy |
| Cottin et al. | 1996 | 33 | Breast + Lymphoma | Anthracycline | The doses ranged from 100 to 550 mg/m2 of body surface area (mean: 260_+92 mg/m2). | 100% | RVEF PER TPER PFR TPFR |
1 month and 12 months |
| Grover et al. | 2013 | 46 | Breast | Anthracycline + Traztuzumab | Epirubicin 100 mg/m2 (3-6 cycles) Doxorubicin 50 m/m2 (3–6 cycles) |
67% | RVEF RVEDVI RVESVI |
4 months and 12 months |
| Calleja et al. # | 2015 | 30 | Breast | Anthracycline +/−Trastuzumab | Epirubicin = 302.4 Doxorubicin = 231.2 |
73% | RVSP RV FAC RV FWLS RV GLS |
Time to cardiotoxicity 5.0 months (A+) 7.5 months (A-) Follow up period 23 months |
| Nakano et al. | 2016 | 9 | Breast | Trastuzumab +/−Anthracycline | – | 66.7% | RV FWPSCS RV LS RVEF |
3, 6, 12 months |
| Barthur et al. | 2017 | 41 | Breast | Trastuzumab +/−Anthracycline | – | 56.1% | RVEF RVEDV RVESV |
6,12, 18 months |
| Tanindi et al. | 2011 | 37 | Breast | Anthracycline | 60 mg/m2 × 6 cycles = 360 | – | RVFAC TAPSE |
Echocardiography was performed before the onset of the chemotheurapeutic regimen (T1), on the day after the completion of the first cure (T2), and after the completion of two cures of the regimen (T3). |
| Moustafa et. al | 2015 | 50 | Breast | Trastuzumab +/−Anthracyclines | <240 mg/m2 | 28% | RVFAC TAPSE RVSP RVGLS |
12–15 months |
| Boczar et al. | 2016 | 49 | Breast | Anthracycline | –(N = 15)doxorubicin was 232 –(N = 34)fluorouracil/epirubicin/cyclophosphamide every 3weeks × 3 ± docetaxel was 294.12 |
100% | RV FAC RV FWLS |
Mean: 125 days; 95% CI: 107–142days |
| Esfahani et al. | 2016 | 49 | Breast | Anthracycline | 450–550 | 100% | RVFAC RVEDD |
6 months |
| Chang et al. | 2016 | 35 | Breast | Epirubicin | 354.19 ± 336.08 | 100% | RVFAC RV FWLS |
21, 42 and 63 days |
| Arciniegas et al. | 2018 | 66 | Breast | Trastuzumab + Anthracycline (doxorubicin and epirubicin) | anthracycline dose of 252 (±45) | 100% | RVLS RVGCS RVGLS RVGCSRs |
Till second echo after chemo initiation. Median 5.4 months |
| Keramida et al. | 2019 | 101 | Breast | Trastuzumab (Also Anthracycline And Taxanes) | Mean dose 483 (±145.1) mg/kg | 61.4% | RVGLS RV FWLS |
12 months |
| Lange et al. | 2012 | 42 | Breast | Trastuzumab +/−Anthracyclines | – | 73.8% | RVSP RV diameter |
3 and 6 months |
| Kilicasian et al. | 2015 | 42 | Breast | Trastuzumab and Anthracyclines | – | 33% | RV MPI TAPSE |
6 months |
Baseline characteristics of included studies.
30 cases and age and cardiac risk factor balanced women with a diagnosis of HER2 + breast cancer without any prior cardiac history and who had an echocardiogram prior to initiation of any cancer therapy were included as controls, since the cases didn't have a baseline echocardiogram. RV, Right ventricle; PER, peak ejection rate; PFR, peak filling rate; TPER, time to peak ejection rate; TPFR, time to peak filling rate; TAPSE, tricuspid annular plane systolic excursion; RVFWPSCS, right ventricular (RV) free wall peak systolic circumferential strains; RVLS, right ventricular longitudinal strain; RVFAC, right ventricular fractional area change; RVEDVI, right ventricular end-diastolic volume index; RVESVI, right ventricular end-systolic volume index; RV FWLS, right ventricular free wall longitudinal strain; RV GLS, right ventricular global longitudinal strain; RVGCS, right ventricular global circumferential strain; RVGCSRs, right ventricular global circumferential systolic strain rate; RVEDD, right ventricle end-diastolic diameter; RVSP, Right ventricular Systolic pressure; RV MPI, right ventricular myocardial performance index.
Table 2
| RV Parameter | Definition | Reference Range | What does it assess? |
|---|---|---|---|
| RVEF | End diastolic volume- end systolic volume/end diastolic volume | 45% | RV systolic function |
| RVFAC | End diastolic area-end systolic area/end diastolic area × 100 | 35% and above | Measure of RV systolic function Measures both longitudinal and radial components of RV |
| Strain: Strain is defined as percentage change in myocardial deformation | |||
| RVFWLS | Change in length in RV free wall over baseline length | <−20% | Assesses regional RV systolic function |
| RVGLS | Global change in length over baseline length | <−20% | Assesses global RV systolic function |
| TAPSE | A method used to measure the distance of systolic excursion of the RV annular segment along its longitudinal plane, from a standard apical 4-chamber window. | >16 mm | RV longitudinal function |
Description of common parameters used to assess right ventricular function as defined by the American society of echocardiography.
Table 3
| Author | Year | Mean Age (years) | Region | Drug | Radiation | Inclusion criteria | Conclusion |
|---|---|---|---|---|---|---|---|
| Havsteen et al. | 1989 | 53 (34–66) | Denmark | Epirubicin | – | Female breast cancer patients epirubicin compared to female breast cancer patients treated with cyclophosphamide, methotrexate and 5-fluorouracil | Treatment with epirubicin resulted in a significant decrease in LVEF at rest and during exercise compared to the CMF treatment in patients with advanced breast cancer. RVEF was unaffected. 1 patient developed fatal CHF |
| Cottin et al. | 1996 | 51+/–13 | France | Anthracycline | – | Women treated with Anthracycline chemotherapy | The study found a significant decrease in LVEF, and LV diastolic function after chemotherapy. There were no significant changes in RV parameters. |
| Grover et al. | 2013 | 55+/–10 | Australia | Anthracycline And/or traztuzumab | – | Women with breast cancer treated with anthracycline (3–6 cycles) and/or traztuzumab | The study found significant decreases in both the LVEF and RVEF following chemotherapy. |
| Calleja et al. | 2015 | 54+/−12 | Canada | Anthracyclines +/−Traztuzumab | – | Women > 18 years With HER-2 + breastcancer at any stage treated with Traztuzumab + −anthracyclines. Who developed cardiotoxicity during treatment |
The study showed Reduced RV-GLS and RV-FWLS in breast cancer patients with cardiotoxicity. These outcomes were compared with age matched breast cancer patients without cardiotoxicity |
| Nakano et al. | 2016 | 62.3+/−12.6 | Japan | Traztuzumab +/−Anthracylcines | 55.6% | Female patients newly diagnosed with HER-2 + breast cancer. | The study found a a decrease in LVEF. RV-FWLS and ejection fraction remained unchanged. |
| Barthur et al. | 2017 | 52+/−11 | Canada | Traztuzumab +/−Anthracylcines | 12% | Female patients > 18 years diagnosed with HER-2 + breast cancer with an LVEF > = 50% at baseline | Significant decreases in both the LVEF and RVEF following chemotherapy in breast cancer patients. In addition to significant increase in RV- end diastolic volume and RV-end systolic volume |
| Tanindi et al. | 2011 | 41.9+/–5.2 | Turkey | Anthracyclines | – | Female patients <50years, diagnosed with breast cancer treated with anthracylines that had no evidence of CAD or CAD risk factors | The study showed a decrease in RV FAC and RV TAPSE. LV systolic was not significantly affected |
| Moustafa et. al | 2015 | 60+/−13 | USA | Anthracylines +/−traztuzumab | 62% | Female patients diagnosed with HER-2 + breast cancer treated with traztumab+/- anthracyclines | The study showed no significant change in LVEF and RVEF. However there was significant decrease in RVGLS |
| Boczar et al. | 2016 | 53.4+/–3.3 | Canada | Anthracyclines | – | Female patients with early stage HER-2 - breast cancer | The study showed a decrease in LVEF and a significant worsening in RV-FAC and RV-FWLS |
| Esfahani et al. | 2016 | 40.75+/–7.14 | Iran | Anthracyclines | – | Female patients <50Yrs with breast cancer, no prior CAD and LVEF >50% | The study showed a decrease in LVEF in addition to RV end-diastolic diameter increase, RV-FAC decrease and Decrease in TAPSE |
| Chang et al. | 2016 | 45.33+/–8.48 | Taiwan | Traztuzumab | 10% | Female patients with breast cancer, LVEF >50%. With age and gender matched control populations | The study found that RV-FWLS significantly declined in addition to decline in TAPSE. No decline in LVEF. |
| Arciniegas et al. | 2018 | 52+/–9 | USA | Anthracylines +/−traztuzumab | 76% | Female patients with newly diagnosed breast cancer. LVEF >50% | The study showed a decrease in LVEF. In addition to a decrease in RV GLS was seen, which had predictive value in determining cardiotoxicity |
| Keramida et al. | 2019 | 54.3+/–11.4 | USA | Anthracyclines | 55% | Female patients with breast cancer, HER-2+ | The study showed a decrease in LVEF, LVGLS and RVGLS |
| Lange et al. | 2012 | 56 (38–75) | Germany | trastuzumab+/− Anthracyclines |
– | Female patients | The study showed a worsening diastolic LV filling, increased E Vmax, altered tissue Doppler velocities, and decreased systolic function. All Right heart parameters showed no significant change |
| Kilicasian et al. | 2015 | 50.4+/–11.6 | Turkey | trastuzumab+/− Anthracyclines |
31% | Female patients with HER 2 + breast cancer and LVEF >60% | The study showed a decrease in LVEF and TAPSE |
Summaries of individual studies included in the current systematic review and meta-analysis.
Per the NIH Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group, all the included studies had sample size not sufficiently large to provide confidence in the findings, and people assessing the outcomes were not blinded to the participants’ exposures/interventions, hence potentially leading to bias in the reported results.
Our study reported significant findings. Anthracycline and/or Trastuzumab chemotherapy led to a statistically significant decrease in Right Ventricular Ejection Fraction (RVEF), with six studies involving 168 patients providing data on RVEF [MD: 2.70, 95% CI: 0.27 to 5.13, P = 0.03, I2 = 71%, χ2 P < 0.05] (Figure 2A). Additionally, Right Ventricular Fractional Area of Change (RVFAC) decreased significantly following the chemotherapy treatment with Anthracycline and/or Trastuzumab, as depicted in five studies [MD: 3.74, 95% CI: 1.33 to 6.15, P < 0.01, I2 = 68%, χ2 P < 0.05] (Figure 2B). Chemotherapy with Anthracycline and/or Trastuzumab had no effect on Right Ventricular global longitudinal strain (RVGLS) [MD: -1.97, 95% CI: -4.16 to 0.23, P = 0.08, I2 = 51%, χ2 P = 0.15] (Figure 2C). Right Ventricular free wall longitudinal strain (RVFWLS) declined at follow-up, [MD: -1.00, 95% CI: -1.86 to -0.15, P < 0.05, I2 = 0%, χ2 P = 0.40] (Figure 3D). Furthermore, Left Ventricular Ejection Fraction (LVEF) was also reduced at follow-up [MD: 4.04, 95% CI: 2.08 to 6.01, P < 0.01, I2 = 91%, χ2 P < 0.05] (Figure 3F). Tricuspic annular plane systolic excursion (TAPSE) was not affected by the treatment [MD: 0.53, 95% CI: -0.11 to 1.17, P = 0.11, I2 = 98%, χ2 P < 0.05] (Figure 3E). However, most of the pooled estimates were associated with significant statistical heterogeneity, mostly because of the heterogeneity in the magnitude of effect measures rather than variation in effect of chemotherapy with Anthracycline and/or Trastuzumab on echocardiography parameters.
Figure 2
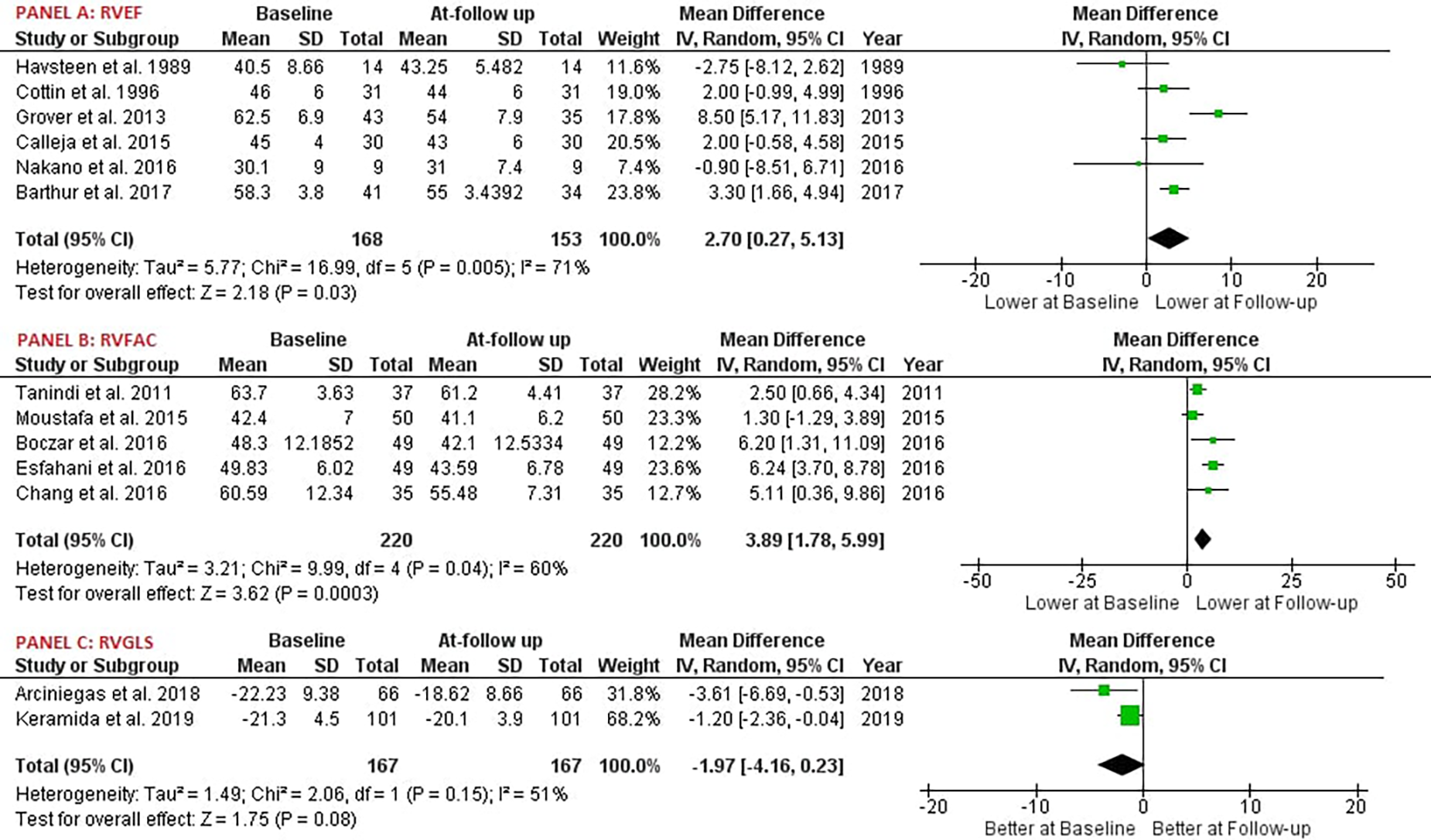
Forest plot; RVEF, right ventricular ejection fraction; RVFAC, right ventricular fractional area change; RVGLS, right ventricular global longitudinal strain.
Figure 3
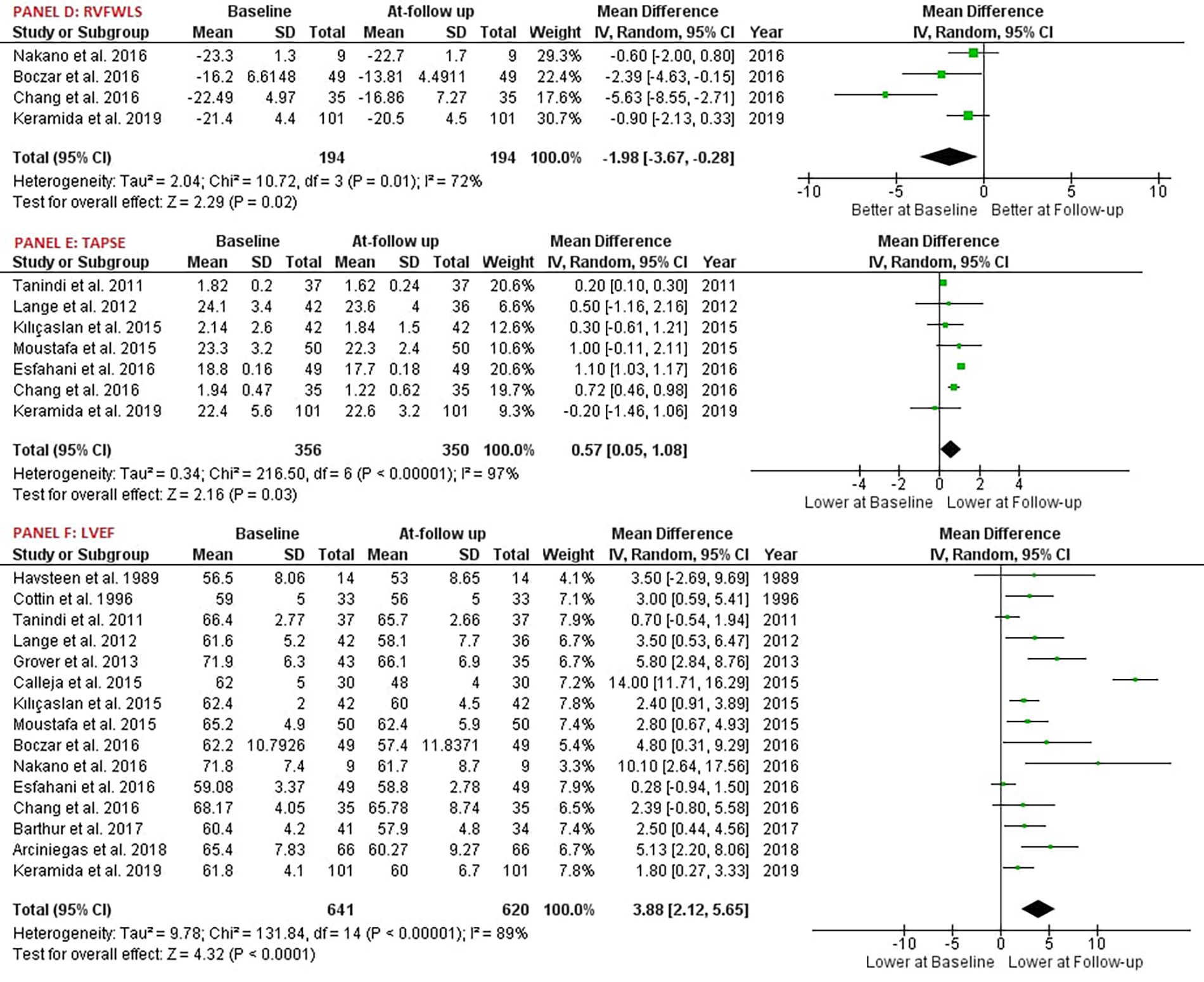
Forest plot; RVFWLS, right ventricular free wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion; LVEF, left ventricular ejection fraction.
Discussion
The present meta-analysis analysed the echocardiographic changes of RV following treatment with Anthracycline and/or Trastuzumab. This is one of the first studies with a large sample size of 644 patients to study the effects of chemotherapeutic agents on the RV, thereby, increasing its statistical power.
The two most commonly used chemotherapeutic agents associated with cardiotoxicity are Doxorubicin (an Anthracycline) and Trastuzumab (a recombinant DNA derived humanized monoclonal antibody targeting human epidermal growth receptor 2 (HER 2) (28). Doxorubicin associated cardiotoxicity is cumulative dose-dependent and has been attributed to an upsurge in inflammation, oxidative stress, dysregulation of autophagy, mitochondrial dysfunction and apoptosis (29). Trastuzumab causes asymptomatic decrease in LVEF and less frequently, overt congestive heart failure (30–32). These effects are not dose dependent and are reversible. Trastuzumab can potentiate the cardiac side-effects of Anthracyclines. The risk of cardiotoxicity with Trastuzumab monotherapy is 3%–7% as compared to 27% when combined with Anthracyclines (33).
Cancer Therapy-Related Cardiac Dysfunction (CTRCD) has been classified as Type I and Type II. Type I CTRCD encompasses Anthracycline-induced cardiotoxicity which begins at the time of drug initiation and progresses algorithmically with increase in cumulative dose (34). Once a threshold maximum is achieved, myofibrillar disarray and cardiac myocyte apoptosis ensues, manifesting as left ventricular dysfunction and irreversible cardiac injury. Type II CTRCD is associated with, but not unique to, Trastuzumab. There is loss of myocardial contractility, likely due to the phenomena of myocyte hibernation or stunning without cell death. This effect is reversible and less likely to be associated with overt congestive heart failure (35).
There are no existing evidence-based guidelines for the screening and surveillance of CTRCD, however, expert consensus has been published by several committees (36, 37). Baseline cardiac imaging, typically with 2D echocardiography, is obtained with the main aim of evaluating left ventricular function prior to initiation of Anthracyclines or HER-2 inhibitors. The modified biplane Simpson's technique is the method of choice for assessment of left ventricular function. If available, global longitudinal strain should also be employed. Although, multigated acquisition (MUGA) scan has consistently outperformed standard 2D echocardiography in quantifying LVEF measurements, it is not a good assessment tool for right ventricular function, valvular dysfunction, atrial enlargement or pericardial disease and is used as an adjunct to echocardiography (38, 39). Cardiac magnetic resonance imaging (CMR) is the reference standard for assessing ventricular volume and function. The ASE defined CTRCD as ⩾10% decline in LVEF to a final value less than 53% confirmed on subsequent imaging performed 2 to 3 weeks after the initial measurement and >15% relative decline in global longitudinal strain (GLS) compared with baseline strain (36, 40). In recognition of the limitation regarding evaluation of RV and to promote a more uniformed approach, the ASE published an updated guideline detailing the methodology (41).
Right ventricular parameters
Results from one of the earlier trials investigating the effect of Anthracyclines on RV systolic and diastolic function using radionuclide angiography showed no significant change in RV EF during the one-year follow-up (42, 43). This is non-congruent to our own findings, which demonstrate a statistically significant drop in RV EF at follow-up. A study by Grover et al. using CMR to assess RV and LV function, not only concurred with our findings, demonstrating a decline in RV EF at 12-month follow-up but also, demonstrated a steeper decline in RV EF as compared to reduction in LVEF (20). Our meta-analysis established a statistically significant reduction in RVFAC, the parameter for RV radial systolic function among patients treated with Anthracyclines and/or Trastuzumab. This is on par with prior reports (19, 23, 26). Evaluation of RV strain is an important component of quantitating RV dysfunction, however it is a measurement that is subject to several methodological complexities. Consistent with our findings, several studies have demonstrated a significant decline in RVFWLS. Chang et al. (27) reported that RVFWLS may be a critical parameter for evaluation of occult RV dysfunction, suggestive of the increased susceptibility for damage within the thinner RV wall. Overall, among the studies that evaluated RV parameters, evaluation of strain may provide the most utility in early prediction of the effect of chemotherapy on the right ventricle. The discrepancies among the studies are most probably due to echocardiographic imaging techniques, as image acquisition and interobserver variability which may compromise the overall assessment. Additionally, the study population consisted of varying cardiovascular comorbidities, follow up periods and small sample sizes.
Despite the heterogeneity among the study population and the various parameters studied, there appears to be substantial evidence to suggest chemotherapy induced RV dysfunction. Larger studies with longer follow-up is necessary to confirm and establish the clinical impact and long term effects of these findings.
There is a growing body of evidence emphasizing the predictive significance of right ventricular structure and pathophysiology in different cardiac conditions (5, 6, 44–49). RV function is adversely affected even in untreated cancer patients, in part due to the abnormal physiology of increased pro-inflammatory markers, neurohumoral changes and circulating reactive oxidative species (47–49). However, there exists less evidence and guidance regarding the evaluation of right ventricle in patients undergoing chemotherapy. The implications of right ventricular dysfunction in cancer patients treated with Anthracycline and/or Trastuzumab have been reported in literature. Oliviera et al. (50) reported significant differences in the need for right ventricular assist devices in patients with chemotherapy associated cardiomyopathy, the majority of whom were treated with Anthracycline compared to all other causes of cardiomyopathy (19% vs. 9.3%, p < 0.0001), suggesting that RV dysfunction likely occurs in parallel with the more defined left ventricular dysfunction in this patient population (50).
Milano et al. (51) demonstrated a decrease in RV and LV thickness among mice infused with Doxorubicin or a combination of Doxorubicin and Trastuzumab. Both groups expressed increased RV free wall fibrosis in contrast to placebo or mice treated with Trastuzumab alone (51). Thus, it is intuitive that RV structure and function might be compromised following chemotherapy in human beings.
Strengths and study limitations
The present meta-analysis has several limitations. First, this is a study level meta-analysis. Study level meta-analyses are limited in their ability to identify inter study heterogeneity. The dose of Anthracyclines and/or Trastuzumab used varied across studies. However, this has not been accounted for in the present analysis. All studies included in the present analysis had a follow-up period of less than 12 months. This was planned to avoid selection bias of survivors and because Anthracyclines and/or Trastuzumab induced cardiotoxicity is reversible. The study by Cottin et al., included both breath cancer and lymphoma patients. Finally, the present analysis did not assume any correlation between the pre and post treatment mean, so that a conservative pooled estimate with a wide confidence interval is obtained.
Conclusive statements
In conclusion, our meta-analysis establishes that Anthracyclines and/or Trastuzumab significantly impact RV function as assessed by ecocardiography. While the impact on left ventricular function has long been recognised, our findings indicate that a comprehensive assessment should include right ventricular function. The implications of RV dysfunction in the context of CTRCD could be far-reaching, potentially affecting treatment decisions and patient prognosis. Future clinical guidelines and research should focus on this aspect, paving the way for improved cardiovascular care in patients undergoing chemotherapy. Our study underlines the need for larger studies with extended follow-ups to further confirm the clinical significance and long-term effects of RV dysfunction in this population.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
PTK conceived the idea and was responsible for the final edits and review AK performed the statistical analysis and contributed to manuscript writing AJ, AJ, MS, YC, SD performed the literature review, contributed to the manuscript writing and illustrated the tables and figures. AJ* performed the literature review, contributed to the manuscript writing and was responsible for the final edits and review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1103941/full#supplementary-material
References
1.
Carver JR Shapiro CL Ng A Jacobs L Schwartz C Virgo KS et al American Society of clinical oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. (2007) 25(25):3991–4008. 10.1200/JCO.2007.10.9777
2.
Tan C Tasaka H Yu KP Murphy ML Karnofsky DA . Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. (1967) 20(3):333–53. 10.1002/1097-0142(1967)20:3%3C333::aid-cncr2820200302%3E3.0.co;2-k
3.
Marmagkiolis K Finch W Tsitlakidou D Josephs T Iliescu C Best JF et al Radiation toxicity to the cardiovascular system. Curr Oncol Rep. (2016) 18(3):15. 10.1007/s11912-016-0502-4
4.
Chen-Scarabelli C Hundley WG Scarabelli TM . Cardio-oncology: an evolving hybrid subspecialty. Future Cardiol. (2018) 14(3):197–201. 10.2217/fca-2017-0080
5.
Plana JC Galderisi M Barac A Ewer MS Ky B Scherrer-Crosbie M et al Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2014) 27(9):911–39. 10.1016/j.echo.2014.07.012
6.
Zamorano JL Lancellotti P Rodriguez Munoz D Aboyans V Asteggiano R Galderisi M et al 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European society of cardiology (ESC). Eur Heart J. (2016) 37(36):2768–801. 10.1093/eurheartj/ehw211
7.
Coviello JS . Cardiovascular and cancer risk: the role of cardio-oncology. J Adv Pract Oncol. (2018) 9(2):160–76.
8.
Felker GM Thompson RE Hare JM Hruban RH Clemetson DE Howard DL et al Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. (2000) 342(15):1077–84. 10.1056/NEJM200004133421502
9.
Mason JW Bristow MR Billingham ME Daniels JR . Invasive and noninvasive methods of assessing Adriamycin cardiotoxic effects in man: superiority of histopathologic assessment using endomyocardial biopsy. Cancer Treat Rep. (1978) 62(6):857–64.
10.
Liberati A Altman DG Tetzlaff J Mulrow C Gotzsche PC Ioannidis J et al The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. (2009) 339:b2700. 10.1136/bmj.b2700
11.
Goutham R Francisco LJ Jack B Frank D Durant NH Hlatky MA et al Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American heart association. Circulation. (2017) 136(10):e172–94. 10.1161/CIR.0000000000000523
12.
Hozo SP Djulbegovic B Hozo I . Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5(1):13. 10.1186/1471-2288-5-13
13.
Higgins JPT Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. 10.1136/bmj.327.7414.557
14.
Havsteen H Brynjolf I Svahn T Dombernowsky P Godtfredsen J Munck O . Prospective evaluation of chronic cardiotoxicity due to high-dose epirubicin or combination chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil. Cancer Chemother Pharmacol. (1989) 23(2):101–4. 10.1007/BF00273525
15.
Cottin Y Touzery C Coudert B Richebourg S Cohen M Toubeau M et al Diastolic or systolic left and right ventricular impairment at moderate doses of anthracycline? A 1-year follow-up study of women. Eur J Nucl Med. (1996) 23(5):511–6. 10.1007/BF00833384
16.
Barthur A Brezden-Masley C Connelly KA Dhir V Chan KKW Haq R et al Longitudinal assessment of right ventricular structure and function by cardiovascular magnetic resonance in breast cancer patients treated with trastuzumab: a prospective observational study. J Cardiovasc Magn Reson. (2017) 19(1):44. 10.1186/s12968-017-0356-4
17.
Arciniegas Calle MC Sandhu NP Xia H Cha SS Pellikka PA Ye Z et al Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer. (2018) 18(1):1037. 10.1186/s12885-018-4935-z
18.
Keramida K Farmakis D Bingcang J Sulemane S Sutherland S Bingcang RA et al Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail. (2019) 21(4):529–35. 10.1002/ejhf.1385
19.
Tanindi A Demirci U Tacoy G Buyukberber S Alsancak Y Coskun U et al Assessment of right ventricular functions during cancer chemotherapy. Eur J Echocardiogr. (2011) 12(11):834–40. 10.1093/ejechocard/jer142
20.
Grover S Leong DP Chakrabarty A Joerg L Kotasek D Cheong K et al Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol. (2013) 168(6):5465–7. 10.1016/j.ijcard.2013.07.246
21.
Moustafa S Murphy K Nelluri BK Northfelt D Shah P Lee H et al Temporal trends of cardiac chambers function with trastuzumab in human epidermal growth factor receptor II–positive breast cancer patients. Echocardiography. (2016) 33(3):406–15. 10.1111/echo.13087
22.
Kılıçaslan B Özdoğan Ö Demir Pişkin G Kahya Eren N Dursun H . Echocardiographic signs of right ventricle changes after trastuzumab treatment in breast cancer patients with erb-2 overexpression. Anatol J Cardiol. (2015) 15(2):143–8. 10.5152/akd.2014.5220
23.
Calleja A Poulin F Khorolsky C Shariat M Bedard PL Amir E et al Right ventricular dysfunction in patients experiencing cardiotoxicity during breast cancer therapy. J Oncol. (2015) 2015. 10.1155/2015/609194
24.
Abdar Esfahani M Mokarian F Karimipanah M . Alterations in the echocardiographic variables of the right ventricle in asymptomatic patients with breast cancer during anthracycline chemotherapy. Postgrad Med J. (2017) 93(1099):271–4. 10.1136/postgradmedj-2016-134286
25.
Nakano S Takahashi M Kimura F Senoo T Saeki T Ueda S et al Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: a pilot study to evaluate the feasibility of the method. Cardiol J. (2016) 23:270–80. 10.5603/CJ.a2016.0023
26.
Boczar KE Aseyev O Sulpher J Johnson C Burwash IG Turek M et al Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo Res Pract. (2016) 3(3):79–84. 10.1530/ERP-16-0020
27.
Chang WT Shih JY Feng YH Chiang CY Kuo YH Chen WY et al The early predictive value of right ventricular strain in epirubicin-induced cardiotoxicity in patients with breast cancer. Acta Cardiologica Sinica. (2016) 32(5):550. 10.6515/acs20151023a
28.
Singal PK. Iliskovic N. Doxorubicin-Induced cardiomyopathy. N Engl J Med. (1998) 339(13):900–5. 10.1056/NEJM199809243391307
29.
Prathumsap N. Shinlapawittayatorn K. Chattipakorn SC. Chattipakorn N . Effects of doxorubicin on the heart: from molecular mechanisms to intervention strategies. Eur J Pharmacol. (2020) 866:172818. 10.1016/j.ejphar.2019.172818
30.
Keefe DL . Trastuzumab-associated cardiotoxicity. Cancer. (2002) 95(7):1592–600. 10.1002/cncr.10854
31.
Perez EA Rodeheffer R . Clinical cardiac tolerability of trastuzumab. J Clin Oncol. (2004) 22(2):322–9. 10.1200/JCO.2004.01.120
32.
Ewer MS Vooletich MT Durand JB Woods ML Davis JR Valero V et al Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. (2005) 23(31):7820–6. 10.1200/JCO.2005.13.300
33.
Fiúza M . Cardiotoxicity associated with trastuzumab treatment of HER2+breast cancer. Adv Ther. (2009) 26(1):9–17. 10.1007/s12325-009-0048-z
34.
Ewer MS Vooletich MT Benjamin RS . A mathematical model for doxorubicin cardiotoxicity: added evidence for the concept of sequential stress. J Clin Oncol. (2004) 22(14_suppl):2086. 10.1200/jco.2004.22.90140.2086
35.
Ewer MS Lippman SM . Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. (2005) 23(13):2900–2. 10.1200/JCO.2005.05.827
36.
Plana JC Galderisi M Barac A Ewer MS Ky B Scherrer-Crosbie M et al Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J–Cardiovasc Imag. (2014) 15(10):1063–93. 10.1093/ehjci/jeu192
37.
Dobson R Ghosh AK Ky B Marwick T Stout M Harkness A et al BSE And BCOS guideline for transthoracic echocardiographic assessment of adult cancer patients receiving anthracyclines and/or trastuzumab. Cardio Oncology. (2021) 3(1):1–16. 10.1016/j.jaccao.2021.01.011
38.
Naik MM. Diamond GA. Pai T. Soffer A. Siegel RJ . Correspondence of left ventricular ejection fraction determinations from two-dimensional echocardiography, radionuclide angiography and contrast cineangiography. J Am Coll Cardiol. (1995) 25(4):937–42. 10.1016/0735-1097(94)00506-L
39.
Bellenger NG Burgess MI Ray SG Lahiri A Coats AJS Cleland JGF et al Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J. (2000) 21(16):1387–96. 10.1053/euhj.2000.2011
40.
Perez IE Alam ST Hernandez GA Sancassani R . Cancer therapy-related cardiac dysfunction: an overview for the clinician. Clinical Medicine Insights: Cardiology. (2019) 13:1179546819866445. 10.1177/1179546819866445
41.
Rudski LG Lai WW Afilalo J Hua L Handschumacher MD Chandrasekaran K et al “Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography: endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography”. J Am Soc Echocardiogr. (2010) 23(7):685–713. 10.1016/j.echo.2010.05.010
42.
Parmentier S Melin JA Piret L Beckers C . Assessment of left ventricular diastolic function in patients receiving anthracycline therapy. Eur J Nucl Med. (1988) 13(11):563–7. 10.1007/bf02574768
43.
Lange SA Ebner B Wess A Kögel M Gajda M Hitschold T et al Echocardiography signs of early cardiac impairment in patients with breast cancer and trastuzumab therapy. Clin Res Cardiol. (2012) 101(6):415–26. 10.1007/s00392-011-0406-0
44.
Kawut SM Barr RG Lima JAC Praestgaard A Johnson WC Chahal H et al Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the multi-ethnic study of atherosclerosis (MESA)–right ventricle study. Circulation. (2012) 126(14):1681–8. 10.1161/CIRCULATIONAHA.112.095216
45.
Iacoviello M Citarelli G Antoncecchi V Romito R Monitillo F Leone M et al Right ventricular longitudinal strain measures independently predict chronic heart failure mortality. Echocardiography. (2016) 33(7):992–1000. 10.1111/echo.13199
46.
Roşca M Călin A Beladan CC Enache R Mateescu AD Gurzun MM et al Right ventricular remodeling, its correlates, and its clinical impact in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2015) 28(11):1329–38. 10.1016/j.echo.2015.07.015
47.
Essick EE Sam F . Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev. (2010) 3(3):168–77. 10.4161/oxim.3.3.12106
48.
Tadic M Cuspidi C Hering D Venneri L Danylenko O . The influence of chemotherapy on the right ventricle: did we forget something?Clin Cardiol. (2017) 40(7):437–43. 10.1002/clc.22672
49.
Haarmark C Haase C Jensen MM Zerahn B . Pre-chemotherapy values for left and right ventricular volumes and ejection fraction by gated tomographic radionuclide angiography using a cadmium-zinc-telluride detector gamma camera. J Nucl Cardiol. (2016) 23(1):87–97. 10.1007/s12350-015-0177-5
50.
Oliveira GH Dupont M Naftel D Myers SL Yuan Y Tang WHW et al “Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS registry (interagency registry for mechanically assisted circulatory support)”. J Am Coll Cardiol. (2014) 63(3):240–8. 10.1016/j.jacc.2013.09.040
51.
Milano G Raucci A Scopece A Daniele R Guerrini U Sironi L et al Doxorubicin and trastuzumab regimen induces biventricular failure in mice. J Am Soc Echocardiogr. (2014) 27(5):568–79. 10.1016/j.echo.2014.01.014
Summary
Keywords
right ventri cular dysfunction, anthracycline, trastuzumab, cardiomyopathy, chemotherapy, breast cancer, cardiooncology
Citation
Theetha Kariyanna P, Kumar A, Jayarangaiah A, Shetty M, Chowdhury Y, Das S and Jayarangaiah A (2023) Chemotherapy induced right ventricular cardiomyopathy; a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1103941. doi: 10.3389/fcvm.2023.1103941
Received
21 November 2022
Accepted
29 June 2023
Published
03 August 2023
Volume
10 - 2023
Edited by
Aaron L. Sverdlov, Hunter Medical Research Institute, The University of Newcastle, Australia
Reviewed by
Anja Karlstaedt, Cedars Sinai Medical Center, United States Christian Cadeddu Dessalvi, University of Cagliari, Italy
Updates
Copyright
© 2023 Theetha Kariyanna, Kumar, Jayarangaiah, Shetty, Chowdhury, Das and Jayarangaiah.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Apoorva Jayarangaiah apoorvajay@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.