Abstract
Background:
The use of oral contraceptives (OCs) is associated with an increased risk of cardiovascular events such as arterial and venous thrombosis (VTE). Cardiovascular diseases (CVDs) are the leading cause of death worldwide, with low- and middle-income nations accounting for over three-quarter of CVD deaths. The aim of this systematic review is to provide a comprehensive synthesis of the available evidence on the link between OC use and CVD risk in premenopausal women and to further assess the role of geographic disparities in the reported prevalence of CVD risk in women on OCs.
Methods:
A comprehensive search of databases such as MEDLINE, Academic Search Complete, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Health Source: Nursing/Academic Edition was conducted, right from the inception to the present, by using the EBSCOhost search engine. The Cochrane Central Register of Clinical trials (CENTRAL) was also searched to augment relevant sources of information. OpenGrey, which is a repository of information providing open access to bibliographical references, was searched and the reference list of the selected studies was also scanned. The potential risk of bias of the included studies was assessed using the modified Downs and Black checklist. Data analysis was performed using the Review Manager (RevMan) version 5.3.
Results:
We included 25 studies that comprised 3,245 participants, of which 1,605 (49.5%) are OC users, while 1,640 (50.5%) are non-OC users. A total of 15 studies were included for meta-analysis, and the overall pooled estimates suggested a significant increase in the traditional cardiovascular risk variables [standardized mean difference (SMD) = 0.73, (0.46, 0.99) (Z = 5.41, p < 0.001)] and little to no difference in endothelial activation among OC users when compared with non-OC users [SMD = −0.11, (−0.81, 0.60) (Z = 0.30, p = 0.76)]. Europe [SMD = 0.03, (−0.21, 0.27), (Z = 0.25 p = 0.88)] had the least effect size, while North America had the highest effect size [SMD = 1.86, (−0.31, 4.04), (Z = 1.68 p = 0.09)] for CVD risk in OC users when compared with non-OC users.
Conclusion:
The use of OCs suggests a significant increase in the prevalence of traditional cardiovascular risk variables with little to no difference in the risk of endothelial dysfunction when compared with non-OC users, and the magnitude of CVD risks varies across different geographical regions.
Registration and protocol:
This systematic review was registered in the international prospective register of systematic reviews (PROSPERO) under the registration number: CRD42020216169.
1. Introduction
Hormonal contraceptives, primarily oral contraceptive pills (OCPs), are one of the most commonly-prescribed modern methods of birth control for premenopausal women aged 15–49 (1) because of its high efficacy and safety profile (2–5). There are an estimated 151 million women using OCPs worldwide (6) and developed countries account for over 30% of such women (6, 7). Despite the known health benefits of OCPs that include preventing pregnancy and treating reproductive disorders among others (8–10), their physiological impact on women's health, combined with the risk of cardiovascular events (2, 11) such as arterial and venous thrombosis (ATE and VTE), ischemic and hemorrhagic stroke, and myocardial infarction (12–15) at various phases of life, remains a major concern (1). Nonetheless, a previous report showed that the incidence of cardiovascular events is rare in young female adults (1–2 per 10,000 per year) but the rate increases to ∼1% per year in the elderly (16, 17), indicating age as a strong predisposing risk factor of cardiovascular disease (CVD) among women, especially in developed countries (18, 19).
Since the introduction of the first-generation combined oral contraceptives (COCs), efforts to reduce their adverse cardiovascular side effects has led to the development of subsequent second-generation and third-generation medications (levonorgestrel; LNG and desogestrel; DSG or norgestimate, respectively) with lower estrogen dose and a varying progestin component called “gonanes,” including the recent fourth-generation medication (drospirenone; DRSP) (1). However, emerging evidence shows conflicting differences regarding the individual impact of COCs on several cardiovascular risk variables such as metabolic, hemodynamic, and hemostatic parameters (1, 10, 13, 20–23), and their impact is attributed to the dose of estrogen and progestin type (24, 25) and the duration of use (26).
Notably, evidence from previous studies showed an association between third-generation COCs (desogestrel; DSG and gestodene; GSD) and elevated risk of thrombosis when compared with the second-generation COC (levonorgestrel) (27–29). More so, the reported incidence of thrombotic events associated with third-generation COCs, when compared with second- and fourth-generation COCs, remains high at 6.6 per 10,000-woman (27). However, the incidence rates for ATE events is lower in women on DRSP-containing OCs compared to other COCs (30, 31). More so, the relative risk of ATE for COCs containing 30–35 µg ethinylestradiol and gestodene, desogestrel, cyproterone acetate, or DRSP was similar, and approximately 50%–80% higher than, the second-generation LNG (32, 33).
In contrast, a previous study showed that the use of COCs are not associated with the occurrence of acute myocardial infarction in young women because no excess risk was reported among users of desogestrel and gestodene when compared with LNG (14). In fact, the study further reported a high amelioration of CVD-risk among smokers using the third-generation COC when compared with the second-generation LNG (14), which contradicts with the finding of another multicenter, case-control study that reported a 3-fold increased risk of ischemic stroke among COC users (34, 35). However, the incidence and risk of ischemic stroke attributable to OC use in the study was reportedly low in women of reproductive age who are non-smokers with no hypertension (34, 35).
Furthermore, a recent study showed an increased number of adverse events relating to CVD in fourth-generation COC (DRSP) users when compared with second/third-generation COC users, and the number of reported events was the highest in the 20-year age group, followed by the 30-year age group, and finally in those over 40 years (36). Meanwhile, available data on the risk of cardiovascular events among different formulations of COC remain inconclusive and further research is needed to identify the causality between COCs and CVDs (36). Therefore, the aim of this systematic review and meta-analysis was to provide a comprehensive synthesis of the available evidence on the link between COC use and CVD risk in premenopausal women and to further assess the role of geographic disparities in the reported prevalence of CVD risk in women on COCs.
2. Methods
This systematic review and meta-analysis was prepared according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (
37) and the protocol was published (
38). A comprehensive and systematic search of published studies was conducted to address the following research questions:
- 1.
Do COCs impact cellular and vascular markers of endothelial activation?
- 2.
What is the role of COCs in traditional cardiovascular risk variables?
2.1. Eligibility criteria
We included cross-sectional, cohort, and case control studies and randomized control trials. Studies reporting on the effect of OC use as a method of contraception on the risk of CVDs in healthy premenopausal women were also included. There were no language restrictions.
2.2. Exclusion criteria
Reviews, books, letters to editors including gray literature were excluded, the bibliographies that were searched for relevant citations.
2.3. Search strategy and information sources
The search strategy was developed using medical subheadings (MeSHs) and keywords related to oral contraceptives, cardiovascular disease, and premenopausal women (Supplementary File S1). The keywords and MeSH terms used included oral contraceptive pills, premenopausal women, cardiovascular disease, or coronary heart disease. A comprehensive search of databases such as MEDLINE, Academic Search Complete, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Health Source: Nursing/Academic Edition, APA PsycInfo, and MasterFILE Premier was conducted from inception to the present by using the EBSCOhost search engine. Furthermore, the Cochrane Central Register of Clinical trials (CENTRAL) was searched including OpenGrey (System Information on Grey Literature in Europe) (www.open.eu) to obtain relevant sources of information. In addition, the reference list of the selected studies was scanned, and forward citation tracking was done using Google scholar to identify the relevant literature. In instances of disagreements, a third reviewer (BBN) was consulted to conduct arbitration proceedings.
2.4. Study selection
The screening of studies was performed by two independent reviewers (OAF and PVD) to avoid inconsistencies with regard to the eligibility of the studies. The abstracts were screened, and the full texts of eligible studies were retrieved. In instances of discrepancies, BBN was consulted for arbitration.
2.5. Outcomes
The primary outcomes of this systematic review and meta-analysis were endothelial activation measured by nitric oxide (NO) and endothelin 1 (ET-1) level, flow-mediated dilation (FMD), and common carotid artery intima–media thickness (CCA-IMT). The secondary outcomes was cardiovascular risk evaluated by changes in blood pressure, lipid profile, and blood glucose levels.
2.6. Data items and collection process
A data extraction sheet was used to extract data items that included the name of the author, year of publication, country, population (sample size), study design, types of OC, dosage, and main findings of the study. Mendeley desktop reference manager software (version 1.19.4) was used to examine the retrieved citations and to remove study duplicates.
2.7. Quality assessment and risk of bias
The potential risk of bias of the included studies was assessed using the modified Downs and Black checklist (39). The tool assesses four domains, namely, reporting bias, external validity, internal validity, and selection bias. Each study was graded and scored as either “excellent” (24–28 points), “good” (19–23 points), “fair” (14–18 points), or “poor” (<14 points).
2.8. Certainty of evidence
The quality of evidence was evaluated using the grading of recommendations assessment, development, and evaluation (GRADE) tool (40). The findings are summarized and presented in the summary of findings table (Table 4).
2.9. Data synthesis and statistical analysis
Higgin's I2 statistic was used to assess statistical heterogeneity. In instances of substantial heterogeneity (I2 > 50%), a random-effects model was used to generate pooled effect estimates (41). Outcomes with same-effect estimates were reported as the mean difference (MD), while different-effect estimates were reported as the standardized mean difference (SMD) and a 95% confidence interval (CI). To explore potential sources of statistical heterogeneity, we conducted a subgroup analysis on the basis of the study design. Data analysis was performed using the software Review Manager (RevMan) version 5.3. The levels of inter-rater agreement were assessed using Cohen's kappa (39), in which a score of values 0.01–0.20 indicate none to slight agreement, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 an almost perfect agreement (42). A p-value of ≤0.05 was considered statistically significant.
2.10. Sensitivity analysis and publication bias
Sensitivity analysis was performed to test the robustness of our reported effect estimates by following a stepwise removal of studies. We performed repeated meta-analysis by taking into account participants’ characteristics and study design, and thereafter, sensitivity analysis was conducted on the basis of geographical location. Furthermore, the method of visual inspection of funnel plots was used to assess publication bias.
3. Results
A total of 165 studies were identified and retrieved using the search strategy and screened for eligibility. A total of 25 studies met the inclusion criteria, while total of 140 studies were excluded. Among the excluded studies, 17 were reviews, and 123 were not relevant to the topic of interest (Figure 1). In all, only 15 studies were shortlisted for quantitative and meta-analyses.
Figure 1
PRISMA flow diagram illustrating the study selection procedure.
3.1. Characteristics of the included studies
The included studies were published between 1998 and 2019, and the study characteristics are given in Table 1. The included studies comprised 3,245 participants, of which 1,605 (49.5%) were on OCs, while 1,640 (50.5%) were non-OC users. Furthermore, 11 studies were cross-sectional studies (43–45, 47–50, 55, 56, 63, 64), seven were randomized control trials (51, 52, 59–61, 65, 66), three were cohort studies (54, 57, 62), two were clinical trials (53, 67), and one each was a prospective longitudinal study (46), and a case control study (58). In addition, the geographical distribution of the included studies comprised Europe (n = 6) (46, 51, 52, 58, 65, 66), North America (n = 6) (43–45, 47, 54, 57), South America (n = 6) (49, 53, 56, 59, 61, 67), Asia (n = 4) (50, 55, 62, 64), Africa (n = 2) (48, 63), and Australia (n = 1) (60).
Table 1
| Author | Year | Country | Main finding | Aim of study | Study design | Type of contraceptive | Dosage | Duration of use | Sample size | Age (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Friedman et al. (43) | 2011 | United States | OCPs significantly decreased serum iron and transferrin saturation and significantly increased FMD in the brachial artery. | To characterize the link between OCP use, iron stores, and cardiovascular risk in premenopausal women | Cross-sectional | COC | Estranes/gonanes and DRSP; 20–25 μg, >25 μg | >1 year | 23 (OC: 23; Non-user: NR) | OC (24.4 ± 2.3) Non-user (NR) |
| Blackmore et al. (44) | 2011 | Canada | OC use significantly lowered IGF-1 levels among younger women (18–21) when compared with non-users and older women (31–40). | Effect of past OC use and timing on circulating IGF-1 | Cross-sectional | COC | NR | >1 year | 329 (OC: 137; Non-user:192) | OC (27.5 ± 2.1) Non-user (27.5 ± 2.1) |
| Meendering et al. (45) | 2008 | United States | MPA treatment antagonized estradiol administration by decreasing endothelium-dependent vasodilation and increasing resting plasma ET-1 in healthy young women. | To investigate whether medroxyprogesterone acetate (MPA) antagonizes the favorable effects of exogenous estradiol on vascular function and biomarkers of cardiovascular risk in young women. | Cross-sectional | Progestin only | 5 mg MPA | 10 days | 14 (OC: 14; Non-user: NR) | OC (23 ± 5.7) Non-user (NR) |
| Merki-Feld et al. (46) | 2002 | Switzerland | No significant changes in the plasma levels of nitric oxide, ET-1, blood pressure (BP), and BMI. However, there was a significant negative correlation between nitric oxide and endothelin-1, and nitric oxide and cholesterol, and a positive correlation between endothelin-1 and cholesterol. | To examine the influence of LNG and GSD on plasma levels of the vasodilator NO, the vasoconstrictor endothelin 1, and the plasma lipids, cholesterol and HDL | Prospective Longitudinal | COC | 30 µg EE/150 µg LNG; 30 µg EE/75 µg GSD. | 6 months | 12 (OC: 12; Non-user: NR) | OC (21.7 ± 4.3) Non-user (NR) |
| Odutayo et al. (47) | 2015 | Canada | Baseline levels of angiotensinogen, angiotensin II, aldosterone, and plasma renin activity were significantly higher in OCP subjects compared with normotensive control and contraceptive patch subjects, while the mean arterial pressure and BMI were non-significant. | Effects of the contraceptive patch and OCP on circulating renin–angiotensin–aldosterone system (RAAS) mediators and systemic blood pressure. | Cross-sectional study | COC, Transdermal patch | 30 µg EE/150 µg LNG, 20 µg EE/150 µg norelgestromin | 3 months | 25 (OC: 10; Non-user: 15) | OC (28 ± 1) Non-user (24 ± 1) |
| Asare et al. (48) | 2014 | Ghana | Diastolic blood pressure, TC, LDL, Castelli risk indices I (TC/HDL) and II (LDL/HDL), and BMI were significantly higher in the HC group than in the control group. | Effect of hormonal contraceptives on lipid profile and the risk indices for CVD in a Ghanaian community | Cross-sectional | COC, Progestin only | 0.03 mg EE/0.15 mg LNG 0.035 mg norethindrone | >1 year | 43 (OC: 19; Non-user: 24) | OC (33.1 ± 6.3) Non-user (29.3 ± 8.1) |
| Lizarelli et al. (49) | 2009 | Brazil | The OC group had significantly lower FMD and HDL when compared with the control group, while the impact on CCA-IMT was not significantly different. | Both a combined oral contraceptive and depot medroxyprogesterone acetate impair endothelial function in young women. | Cross-sectional | COC, Progestin only | EE30 µg/LNG 150 µg DMPA (150 mg) |
6 months | 75 (OC: 25; Non-user: 50) | OC (23.7 ± 3.2) Non-user (23.4 ± 3.6) |
| Heidarzadeh et al. (50) | 2014 | Iran | COC use significantly reduced FMD% in comparison with the control group, while the mean CCA-IMT was significantly but slightly higher when compared with the age-matched control group | The effect of low-dose combined oral contraceptive pills on brachial artery endothelial function and CCA-IMT thickness | Cross-sectional | COC | EE 30 μg/LNG150 μg | >1 year | 60(OC: 30; Non-users: 30) | OC (33.3 ± 4.6) Non-users (33.9 ± 5) |
| Yildizhan et al. (51) | 2009 | Turkey | OCs resulted in significant reductions in systolic and diastolic BP and LDL levels and a significant increase in TG and HDL levels, resulting in increasing the HDL/LDL ratio. | Effects of two combined oral contraceptives containing ethinyl estradiol 30 μg combined with either gestodene or drospirenone on hemostatic parameters, lipid profiles, and BP | RCT | COC | EE 0.03 mg/GSD 0.075 mg, EE 0.03 mg/DRSP 3 mg |
1 year | 160 (OC: 160; Non-user: NR) | OC (27.5 ± 10.6) Non-user (NR) |
| Wiegratz et al. (52) | 2004 | Germany | OCs caused significant changes in the hemostatic parameters by increasing the levels of fibrinogen, prothrombin fragment 1 + 2, factor VII, protein C, plasminogen, Plasmin-Alpha-2-Antiplasmin (PAP) complexes, and D-dimer, while total and free protein S, t-PA, and PAI levels were significantly reduced. | Effect of four oral contraceptives on hemostatic parameters | RCT | COC | 30 µg EE/2 mg DNG, 20 µg EE/2 mg DNG, 10 µg EE/2 mg EV/2 mg DNG, 20 µg EE/100 µg LNG | 6 months | 100 (OC: 100; Non-user: NR) | OC (26.5 ± 12) Non-user (NR) |
| Giribela et al. (53) | 2012 | Brazil | The contraceptive formulation did not cause any significant changes in BP, heart rate (HR), cardiac output (CO), total peripheral resistance (TPR), and arterial endothelial function. | Effect of combined oral contraceptives containing drospirenone on endothelial function and hemodynamic parameters in healthy young women | Clinical trial | COC | 20 µg EE/3 mg DRSP | 6 months | 71 (OC:43; Non-user: 28) | OC (29.2 ± 6.8) Non-user (30.6 ± 6.8) |
| Kharbanda et al. (54) | 2014 | United States | COCs were not associated with clinically meaningful changes in weight or blood pressure. | Initiation of oral contraceptives and changes in blood pressure and BMI in healthy adolescents | Observational cohort | COC | 30/35 µg EE | >1 year | 1,422 (OC: 510; Non-user: 912) | OC (16.4 ± 1) Non-user (16.4 ± 1) |
| Fallah et al. (55) | 2012 | Iran | OCs significantly elevated the levels of homocysteine (HCY) and reduced the levels of NO concentration in the plasma. | Influence of oral contraceptive pills on homocysteine and nitric oxide levels | Cross-sectional | COC | 30 µg EE/I50 µg LNG | >1 year | 100 (OC: 50; Non-user: 50) | OC (27.5 ± 10.6) Non-user (27.5 ± 10.6) |
| Dos Santos et al. (56) | 2018 | Brazil | There was a significant increase in the levels of TG, HDL-cholesterol, CRP, and systolic blood pressure values in COCs. There was also a significant increase in plasma-oxidized LDL values when compared with the control group. | Elevation of oxidized lipoprotein of low density in users of combined oral contraceptives | Cross-sectional | COC | 150 µg LNG/30 µg EE | >1 year | 42 (OC: 21; Non-user: 21) | OC (23 ± 3.1) Non-user (23 ± 3.4) |
| Harvey et al. (57) | 2015 | United States | Blood pressure was significantly higher in OC users than in OC non-users. Muscle sympathetic nerve activity (MSNA) at rest, as well as CO and TPR, is similar between the two study groups. | Effect of oral contraceptive use on MSNA, and systemic hemodynamics in young women | Retrospective cohort study | COC | 20–30 µg EE/3 mg DRSP, 150 µg LNG/30 µg EE | NR | 127 (OC: 53; Non-user: 74) | OC (25 ± 1) Non-user (25 ± 1) |
| John et al. (58) | 2000 | Germany | At the basal level, NO production and release was enhanced by oral contraceptive use, while upon stimulation, NO bioavailability remained unchanged in the participants’ group. | Effects of oral contraceptives on the vascular endothelium in premenopausal women | Case control | COC | 0.035 mg EE/0.125 mg LNG; 0.03 mg EE/0.15 mg DG; 0.03 mg EE/0.075 mg GSD; 0.03 mg EE/0.05 mg LNG; 0.02 mg EE/0.15 mg DSG. | 1 year | 16 (OC: 8; Non-user: 8) | OC (27 ± 2) Non-user (27 ± 2) |
| Nadai et al. (59) | 2015 | Brazil | There was no significant difference in BP associated with the use of combined oral contraceptives containing DRSP irrespective of the EE dose used | Effects of two contraceptives containing drospirenone on blood pressure in normotensive women: a randomized-controlled trial | RCT | COC | 30 μg EE/3 mg DRSP, 20 μg EE/3 mg DRSP | 6 months | 44 (OC: 44; Non-user: NR) | OC (24.7 ± 4.5) Non-user (NR) |
| Straznicky et al. (60) | 1998 | Australia | OCs significantly increased 24 h systolic and diastolic blood pressure levels, triglyceride levels, and insulin area under the curve in users when compared with non-users. | Effects of oral contraceptive use and dietary fat intake on blood pressure, cardiovascular reactivity, and glucose tolerance in normotensive women | RCT | COC | 0.03 mgEE/0.15 mg LNG,0.05 mg EE/0.125 mg LNG,0.03 mg EE/0.05 mg LNG, 0.04 mg EE/0.075 mg LNG, 0.03 mg EE/0.125 mg LNG | >1 year | 32 (OC: 16; Non-user: 16) | OC (29.8 ± 7.8) Non-user (31.3 ± 7.7) |
| Franceschini et al. (61) | 2013 | Brazil | A COC containing LNG is associated with a more pronounced reduction in FMD and increased IMT of healthy women than a COC containing CMA and non-hormonal contraception. | Effects of combined oral contraceptives containing levonorgestrel or chlormadinone on the endothelium | RCT | COC | EE 30 μg/CMA 2 mg, EE 30 μg/LNG 150 μg | 6 months | 64 (OC: 43; Non-user: 21) | OC (24.2 ± 6.1) Non-user (28.3 ± 3.7) |
| Zahra et al. (62) | 2019 | Iran | OCs significantly increase the plasma levels of homocysteine (HCY), TG, cholesterol (TC), and LDL-c among users when compared with non-users. | Effects of low-dose contraceptive pills on the risk factors of cardiovascular diseases among 15-35-year-old women | A retrospective cohort study | COC | 30 µg EE/150 µg LNG. | >1 year | 100 (OC: 50; Non-user: 50) | OC (30.1 ± 3.7) Non-user (30.1 ± 4.1) |
| El-Haggar and Mostafa (63) | 2015 | Egypt | The uptake of combined oral contraceptive pills (COCPs) significantly lowered adiponectin concentration and significantly increased leptin and resisting levels and the atherogenic index when compared with other studied groups. | To evaluate the associated cardiovascular risk in Egyptian healthy consumers of different types of COCPs. | Cross-sectional study | COC | 30 μg EE/150 μg LNG, 0.03 mg EE/0.075 mg GSD, 30 μg EE/3 mg DRSP | 6 months | 120 (OC: 90; Non-user: 30) | OC (31.2 ± 2.7) Non-user (31.7 ± 1.8) |
| Fallah et al. (64) | 2011 | Iran | Low-dose COC uptake results in a significant decrease in serum adiponectin concentration and an increased atherogenic lipid profile by significantly increasing LDL levels. | Adiponectin, leptin, and lipid profile evaluation in oral contraceptive pill consumers | Cross-sectional study | COC | 30 μg EE/150 μg LNG | >1 year | 100 (OC: 50; Nonuser: 50) | OC (31.7 ± 7.9) Nonuser (33.9 ± 6.3) |
| Winkler et al. (65) | 2009 | Germany | OC treatments significantly increased triglyceride and Apo AI levels and HDL levels, while LDL levels were reduced in OC users when compared with non-users. | The effects of two monophasic oral contraceptives containing 30 μμg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipids, hormones, and metabolic parameters | RCT | COC | EE 30 μg/CMA 2 mg, EE 30 μg/DSG 0.15 mg | 6 months | 43 (OC: 43; Non-user: NR) | OC (27.2 ± 5) Non-user (NR) |
| Piltonen et al. (66) | 2012 | Finland | The use of oral, transdermal, and vaginal combined contraceptives (CCs) decreases androgenicity, worsens insulin sensitivity, and increases the level of markers of chronic inflammation at the same rates. | Effect of alternative administration routes of CCs on androgen secretion, chronic inflammation, glucose tolerance, and lipid profile | RCT | COC, Patch, vaginal ring. | EE 20 μg/DSG 0.15 mg, EE 20 μg/norelgestromin 150 μg, EE 15 μg/120 μg etonogestrel | 2 months | 54 (OC: 18; Non-user: NR) | OC (23.5 ± 3.1) Non-user (NR) |
| Marcelo et al. (67) | 2014 | Brazil | There were no significant alterations in blood pressure, heart rate variability, and baroreflex sensitivity of healthy women during a 6-month period of use of a COC containing EE and DRSP. | To evaluate the effect of a contraceptive containing 20 μg of ethinyl estradiol and 3 mg of drospirenone on the heart rate variability, baroreflex sensitivity, and blood pressure of healthy women. | Prospective clinical trial | COC | 20 μg EE/3 mg DRSP | 6 months | 69 (OC: 36; Non-user: 33) | OC (28.8 ± 1.1) Non-user (30.3 ± 1) |
Characteristics of the included studies (n = 25).
NR, not reported; COC, combined oral contraceptives; EE, ethinylestradiol; DSG, desogestrel; DNG, dienogest; DRSP, drospirenone; CMA, chlormadinone acetate; LNG, levonorgestrel; MPA, medroxyprogesterone acetate; GSD, gestodene; IGF-1, insulin-like growth factor 1; RCT, randomized control trial.
3.2. Quality assessment and risk of bias of the included studies
The risk of bias was independently assessed by two reviewers (OAF and PVD) using the modified Downs and Black checklist (39). Overall, the included studies were rated as fair, with an average score of 18 out of a possible 26. Overall, the studies were scored as excellent for reporting the bias domain (with a score of nine out of a possible 10), poor for external validity (with a score of one out of a possible three), moderate for the internal validity domain (scoring three out of a possible seven), and moderate for selection bias (with a score three out of a possible six). The inter-rater reliability per domain was scored as k = 0.86 (CI = 0.8, 0.93) for reporting bias (perfect agreement), k = 0.54 (CI = 0.41, 0.68) for external validity (moderate agreement); k = 0.68 (CI = 0.53, 0.83) for internal validity (substantial agreement), and k = 0.63 (CI = 0.49, 0.77) for selection bias (substantial agreement) (Supplementary additional file S1, Figure 2).
Figure 2
Quality assessment of the included studies.
3.3. The impact of OC use on reported markers of endothelial activation in premenopausal women
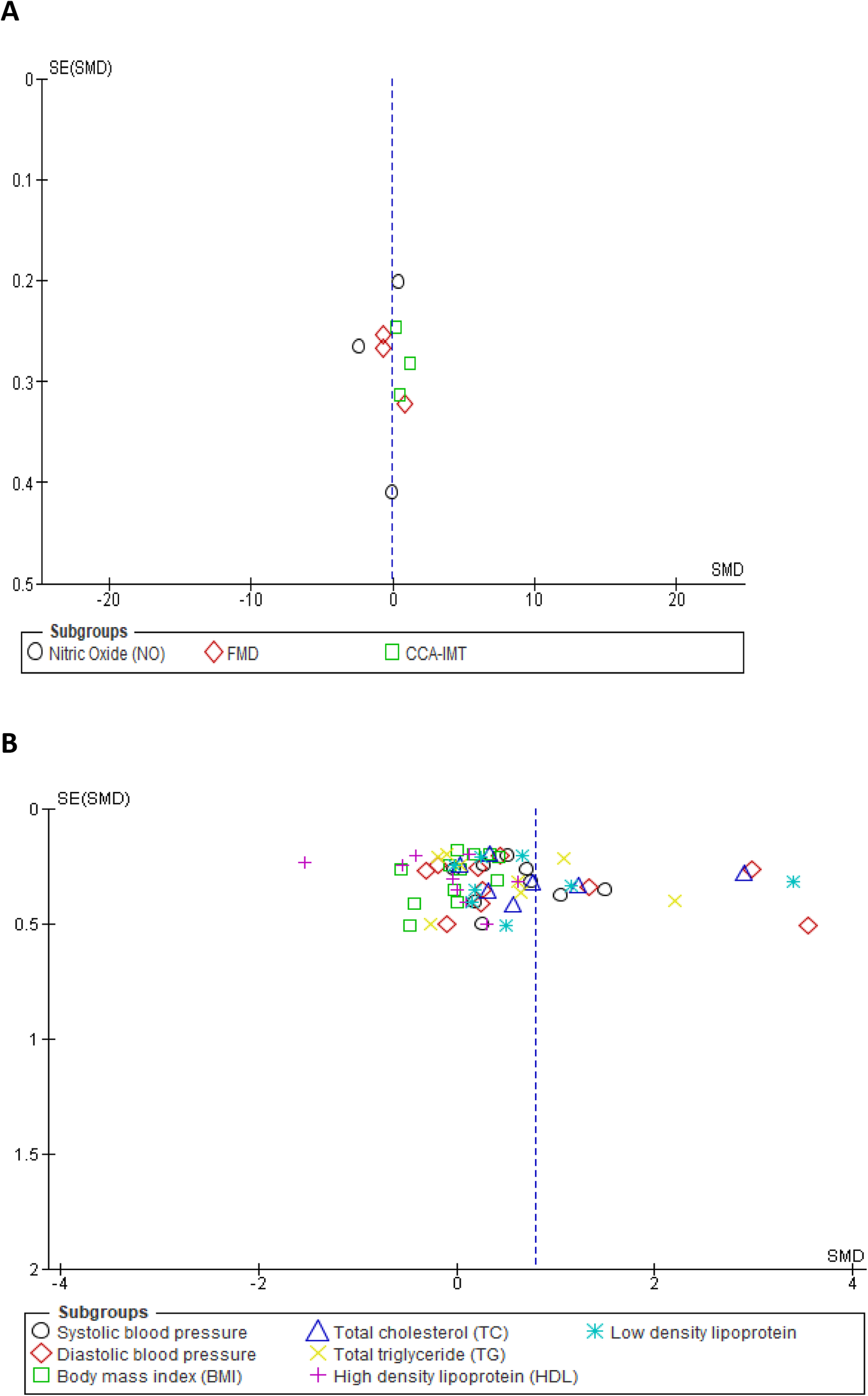
Overall, the results of our meta-analysis showed little to no difference in the pooled estimate for endothelial activation among participants on OCs when compared with non-users [SMD = −0.11, 95% CI (−0.81, 0.60), Z = 0.30, p = 0.76, low certainty evidence]. However, these results showed a substantial level of statistical heterogeneity (I2 = 94%, p < 0.00001) (Figure 3) and subgroup analyses based on study design, following which the reported measure of effect size of endothelial activation was estimated (Figure 3 and Table 2).
Figure 3
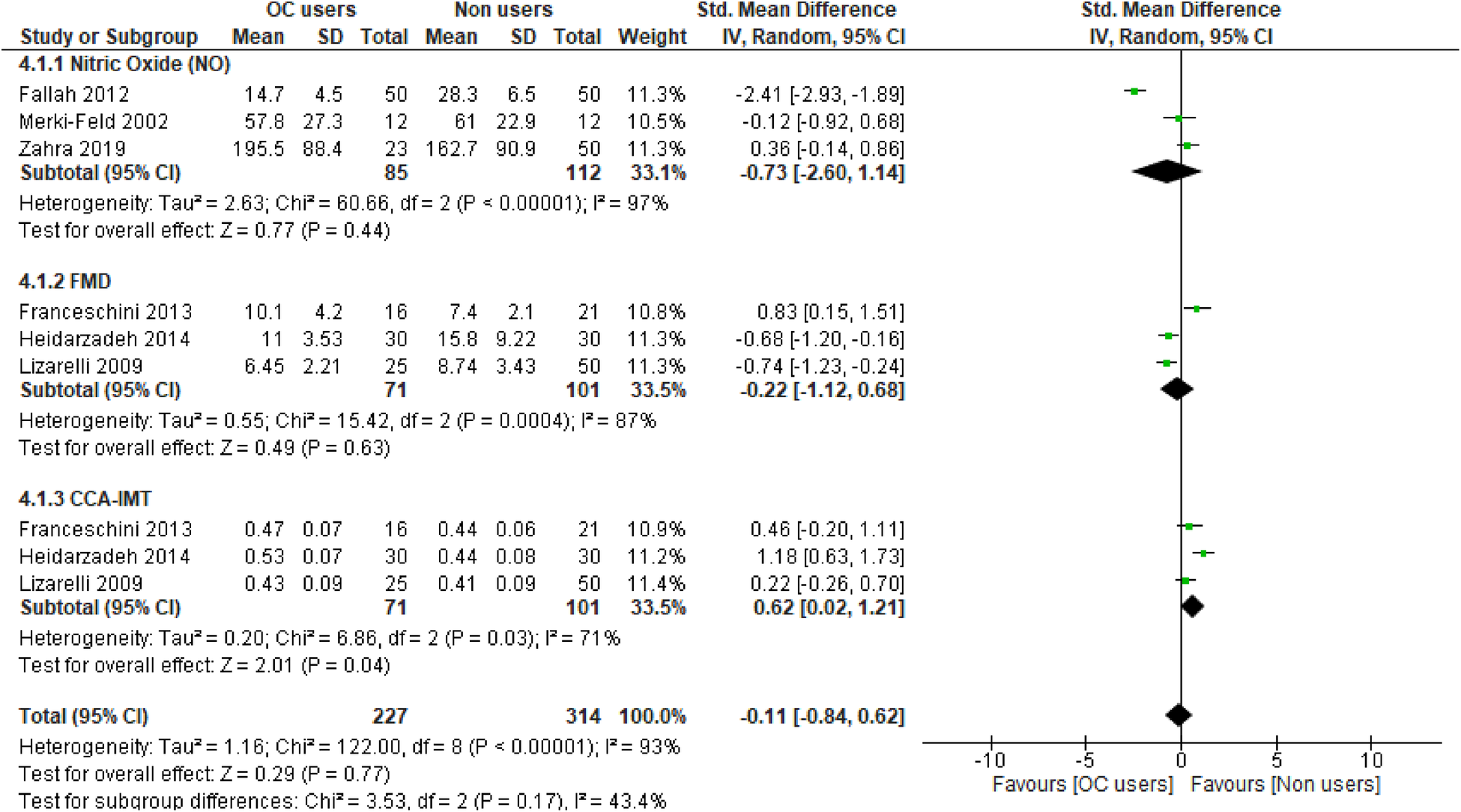
Forest plot of cellular and vascular markers of endothelial activation in premenopausal women on OCs vs. non-OC users.
Table 2
| Effect Measure | Number of Studies | Number of participants | Effect estimate | ||||
|---|---|---|---|---|---|---|---|
| Model | SMD | 95% CI | I 2, p-value | p-value | |||
| Blood pressure | |||||||
| SBP | 12, (46, 48, 62, 67, 49, 50, 55–58, 60, 61) | 752 | RE | 1.96 | 0.94–2.97 | 97%, p < 0.001 | 3.78, p = 0.002 |
| DBP | 12, (46, 48, 62, 67, 49, 50, 55–58, 60, 61) | 752 | RE | 1.74 | 0.71–2.78 | 97%, p < 0.001 | 3.3, p = 0.001 |
| BMI | 14, (46, 47, 61–63, 67, 48–50, 55–58, 60) | 897 | RE | 0.22 | −0.14–0.57 | 82%, p < 0.001 | 1.21, p = 0.23 |
| Lipid metabolism | |||||||
| Total cholesterol | 8, (46, 48, 55, 56, 60, 62, 63) | 536 | RE | 0.94 | 0.22–1.66 | 92%, p < 0.001 | 2.55, p = 0.01 |
| HDL cholesterol | 9, (46, 48, 49, 55, 56, 58, 60, 62, 63) | 552 | RE | −0.20 | −0.64–0.25 | 82%, p < 0.001 | 0.85, p = 0.39 |
| LDL cholesterol | 8, (48, 55, 56, 58, 60, 62, 63) | 509 | RE | 0.79 | −0.04–1.59 | 92%, p < 0.001 | 1.93, p = 0.05 |
| Triglycerides | 8, (48, 55, 56, 58, 60, 62, 63) | 528 | RE | 0.48 | −0.02–0.99 | 85%, p < 0.001 | 1.87, p = 0.06 |
| Glucose metabolism | |||||||
| Fasting blood glucose | 3, (55, 56, 60) | 87 | RE | 0.07 | −0.23–0.37 | 0%, p = 0.59 | 0.45, p = 0.65 |
| Total effect estimate | 14 | 4,320 | − | 0.74 | 0.47–1.01 | 94%, p < 0.001 | 5.41, p < 0.001 |
Traditional cardiovascular-risk variables of included participants.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; RE, random effects, MD, mean difference; SMD, standard mean difference.
3.3.1. NO level
The qualitative findings of our study, as reported in Table 1, showed that at the basal level, NO production and release was enhanced by OCs [second-generation (LNG) and third-generation gestodene and desogestrel (GSD, DSG) types], but upon stimulation with different dosages of acetylcholine, the plasma level of NO remained unchanged (58). Meanwhile, a study by Merki-Feld et al. showed that the use of second-generation (LNG) and third-generation (GSD) OC did not alter the plasma levels of nitric oxide (46). In contrast, the use of second-generation (LNG) OCs was associated with reduced plasma levels of NO when compared with the control group (55). However, the pooled estimate of our subgroup analysis suggests that OC use may result in little to no difference in theplasma level of NO when compared with non-OC users (SMD = −0.73, 95% CI (−2.60, 1.14), p = 0.44 (low certainty evidence) with a substantial level of heterogeneity (I2 = 97%, p < 0.00001) (Figure 3).
3.3.2. Flow-mediated dilation
The qualitative findings of our study, as reported in Table 1, showed that the use of fourth-generation drospirenone (DRSP) OC significantly increased flow-mediated dilation (FMD) in the brachial artery of the participants (43), which contrasted with the findings of other studies (49, 50, 61), where the use of second-generation levonorgestrel (LNG) and fourth-generation chlormadinone acetate (CMA) OC by the participants significantly lowered FMD when compared with non-users. However, the results of our meta-analysis suggest little to no difference in the pooled estimate for FMD in the participants on OCs when compared with non-OC users [SMD = −0.22, 95% CI (−1.12, 0.68), p = 0.63 (low certainty evidence) with a substantial level of heterogeneity (I2 = 87%, p = 0.0004)] (Figure 3).
3.3.3. Common carotid artery intima–media thickness
The qualitative findings of our study, as reported in Table 1, showed that the mean CCA-IMT was significantly higher in participants who used second- and third-generation OCs (50, 61), which contrasted with the findings of a study by Lizarelli et al. that reported no significant difference between users of the second-generation levonorgestrel (LNG) and non-users (49). However, the results of our meta-analysis showed a significant increase in the pooled estimate for CCA-IMT in participants not on OCs when compared with OC users [SMD = 0.62, 95% CI (0.02, 1.21), p = 0.04, low certainty evidence], although a substantial level of statistical heterogeneity was observed in these studies (I2 = 71%, p = 0.03) (Figure 3). Thus, our evidence suggests that OC use may result in a significant reduction in CCA-IMT among users.
3.4. Prevalence of traditional cardiovascular risk variables among OC users when compared with non-users
The overall pooled estimates of our meta-analysis suggest an increased CVD risk among OC users when compared with non-users [SMD = 0.73, 95% CI (0.46, 0.99), Z = 5.41, p < 0.001] (I2 = 94%, p < 0.001, low certainty evidence). However, due to a substantial level of heterogeneity, a subgroup analysis of the reported effect estimates was conducted (Table 2).
3.4.1. Blood pressure measurements
3.4.1.1. Systolic blood pressure
The qualitative findings of our study, as reported in Table 1, showed that systolic blood pressure increased significantly among users of second- (levonorgestrel; LNG) and third- (gestodene; GSD) generation COCs (56, 57, 60, 62, 64), which contrasted with those of a study by Franceschini et al. that reported a significant reduction among users of second (LNG)-generation COC when compared with non-users (61). However, several other studies reported a non-significant change in systolic blood pressure (SBP) among COC users despite the similarity in the duration of use (46, 48–50, 58, 67). Furthermore, the results of our subgroup analysis suggest a significant increase in the SBPof participants on OCs when compared with non-users [SMD = 1.96, 95% CI (0.94, 2.97), p = 0.002, low certainty evidence] and a substantial level of heterogeneity (I2 = 97%, p < 0.001) (Table 2).
3.4.1.2. Diastolic blood pressure
The qualitative findings of our study, as reported in Table 1, showed that diastolic blood pressure (DBP) increased significantly among users of second- (levonorgestrel; LNG) and third- (gestodene; GSD) generation COCs (48, 57, 64), which contrasted with those of a study by Franceschini et al. that reported a significant reduction among users of second- (LNG) and fourth- (CMA) generation COCs when compared with non-users (61). However, several other studies reported a non-significant change among COC users despite the similarity in the duration of use (46, 49, 56, 58, 60, 62, 67). In addition, evidence from our meta-analysis suggests a significant increase in DBP of participants on OCs when compared with non-users [SMD = 1.74, 95% CI (0.86, 3.03), p = 0.001, low certainty evidence], although there was a substantial level of heterogeneity (I2 = 97%, p < 0.001) (Table 2).
3.4.2. Body mass index
The qualitative findings of our study, as reported in Table 1, showed that the use of second- (levonorgestrel; LNG) and third- (gestodene; GSD, desogestrel; DSG) generation COCs does not significantly increase body mass index (BMI) (46, 47, 49, 50, 55–58, 60, 61–63, 67), which contrasted with those of a study by Asare et al. that reported a significant increase in BMI among users of the second- (LNG) generation COC despite the similarity in the duration of use (48). However, the pooled estimate of our subgroup analysis suggests that OC use may result in little to no difference in BMI when compared with non-users [SMD = 0.22, 95% CI (−0.14, 0.57), p = 0.23, low certainty evidence] and a substantial level of heterogeneity (I2 = 82%, p < 0.001) (Table 2).
3.4.3. Lipid profile
3.4.3.1. Total cholesterol
The qualitative findings of our study, as reported in Table 1, showed that second- (levonorgestrel; LNG), third- (gestodene; GSD), and fourth-generation (drospirenone; DRSP) COCs significantly increased the total cholesterol (TC) level among users when compared with non-users (48, 55, 62, 63). However, some studies reported no significant difference among users of second- and third-generation COCs when compared with non-users despite similarity in the duration of use (46, 49). Furthermore, evidence from our subgroup analysis suggests a significant increase in the total cholesterol level among OC users when compared with non-users [SMD = 0.94, 95% CI (0.22, 1.66), p = 0.01, low certainty evidence] and a substantial level of heterogeneity (I2 = 92%, p < 0.001) (Table 2).
3.4.3.2. High-density lipoprotein
The qualitative findings of our study, as reported in Table 1, showed a significant increase in the high-density lipoprotein (HDL) level among users of second- (levonorgestrel; LNG) and third-generation (gestodene; GSD) COCs when compared with non-users (46, 56). However, these findings contrasted with the results of other studies that reported a significant decrease in the HDL level among users of second- (LNG) generation COC when compared with non-users and among third- (GSD) and fourth- (drospirenone; DRSP) generation COC users (49, 63). Nonetheless, several other studies reported non-significant changes in the HDL level among COC users despite similarity in the duration of use (55, 58, 60, 62). Furthermore, our subgroup analysis suggests that OC use may result in little to no difference in HDL levels when compared with non-users [SMD = −0.20, 95% CI (−0.64, 0.25), p = 0.39, low certainty evidence] and a substantial level of heterogeneity (I2 = 82%, p < 0.001) (Table 2).
3.4.3.3. Low-density lipoprotein
The qualitative findings of our study, as reported in Table 1, showed an increased level of low-density lipoprotein (LDL) among users of second- (LNG) generation COC when compared with non-users and among third- (GSD) and fourth- (DRSP) generation COC users (48, 55, 62, 63). This contrasted with the findings of other studies that reported no significant differences among users of second- (LNG) and third- (GSD, DSG) generation COCs when compared with non-users despite similarity in the duration of use (55, 56, 58, 60). Nevertheless, the pooled estimate of our subgroup analysis suggests a significant increase in LDL levels among OC users when compared with non-users [SMD = 0.79, 95% CI (−0.04, 1.59), p = 0.05, low certainty evidence] and a substantial level of heterogeneity (I2 = 92%, p < 0.001) (Table 2).
3.4.3.4. Triglyceride
The qualitative findings of our study, as reported in Table 1, showed an increased level of triglyceride (TG) among second-generation (LNG) users when compared with non-users (56, 62). While several studies reported no significant differences among users of second- (levonorgestrel; LNG) and third- (gestodene; GSD, desogestrel; DSG) generation COC users (48, 55, 58, 60), a study by El-Haggar and Mostafa showed a significant reduction in the levels of TG among users of second-generation COC (LNG) when compared with non-users and among third- (GSD) and fourth- (drospirenone; DRSP) generation COC users (63) despite similarity in the duration of use. In addition, the pooled estimate of our subgroup analysis suggests that OC use may result in little to no difference in triglyceride levels when compared with non-users [SMD = 0.48, 95% CI (−0.02, 0.99), p = 0.06, low certainty evidence] and a substantial level of heterogeneity (I2 = 85%, p < 0.001) (Table 2).
3.5. Glucose metabolism
3.5.1. Fasting blood glucose
The qualitative findings of our study, as reported in Table 1, showed no significant change in fasting blood glucose (FBG) among users of second- (levonorgestrel; LNG) generation COC when compared with non-users (55, 56, 60). Moreover, the pooled estimate of our subgroup analysis also suggests that OC use may result in little to no difference in FBG levels [SMD = 0.07, 95% CI (−0.23, 0.37), p = 0.45, low certainty evidence] when compared with non-users (I2 = 0%, p = 0.59) and a low level of heterogeneity (Table 2).
3.6. Sensitivity analyses and publication bias
We assessed the robustness of our results and further explored the sources of heterogeneity in the reported outcomes by performing sensitivity and subgroup analyses. The meta-analysis was repeated by a stepwise omission of studies based on the geographical location of each reported outcome. The sensitivity analysis of the traditional cardiovascular risk variables showed that studies conducted in Europe [SMD = 0.03, 95% CI (−0.21, 0.27), (I2 = 0%, p = 0.88)] and Australia [SMD = 0.33, 95% CI (0.05, 0.61), (I2 = 7%, p = 0.38)] had low levels of heterogeneity when compared with other studies conducted in Africa, Asia, and North and South America; however, the effect size was quite small in South America when compared with that in Africa, Asia, and North America (Supplementary additional file S1 and Table 3). This suggested geographical location to be a potential source of statistical heterogeneity in the included studies. However, an assessment of the funnel plot suggests evidence of publication bias (Supplementary additional file S1 and Figure 4B).
Figure 4
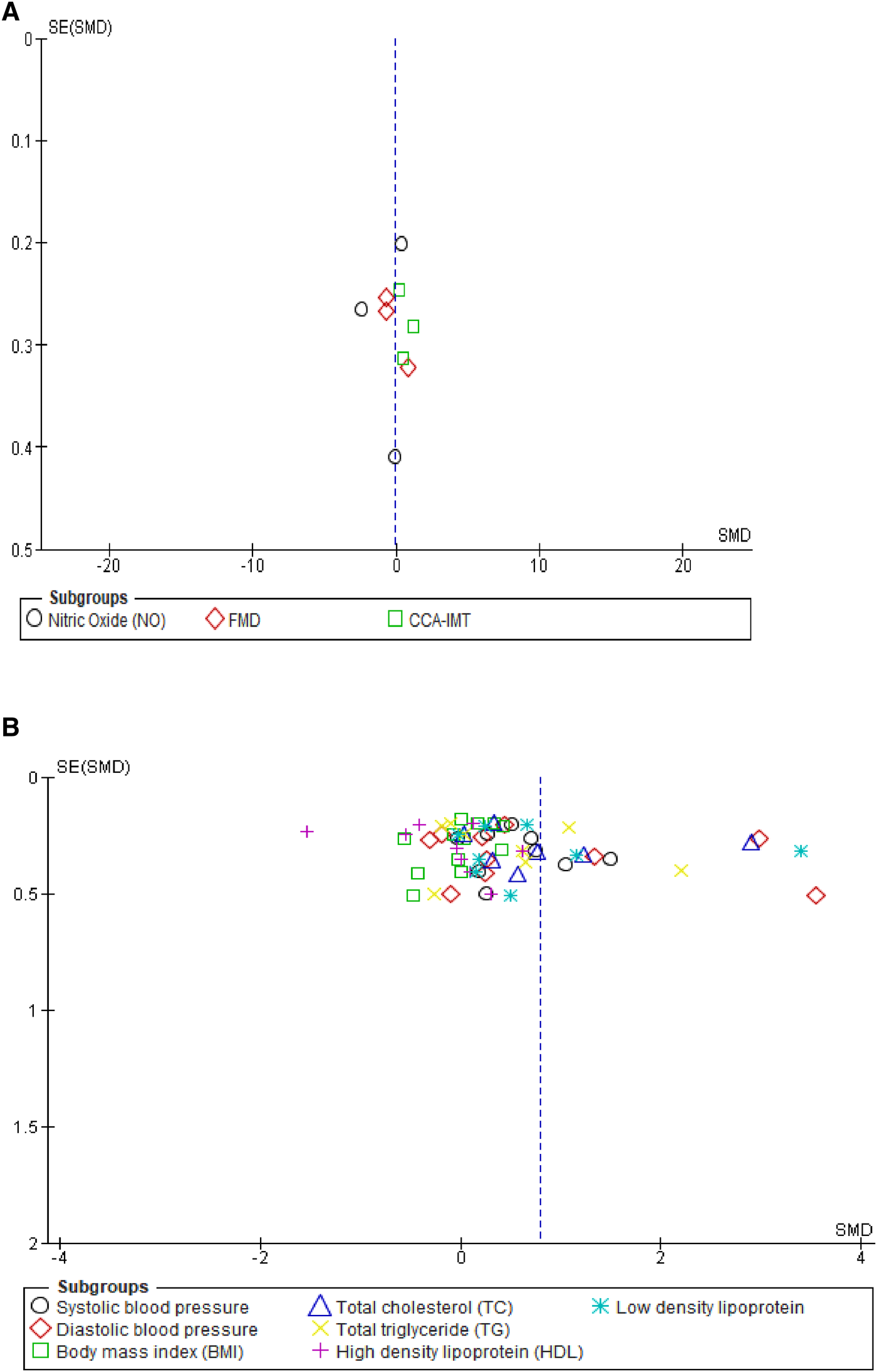
Funnel plot of vascular markers and cardiovascular risk factors showing a perfect symmetry. Hence, there was no publication bias in these studies. (A) Vascular markers, (B) Traditional cardiovascular risk variables.
Table 3
| Outcome | Geographical location | Number of studies | Omitted studies | SMD (95% CI) | I 2 (%), p-value | Overall effect: Z, p-value |
|---|---|---|---|---|---|---|
| Vascular markers of endothelial dysfunction | All | 6, (1–6) | None | −0.11 (−0.81, 0.60) | 94%, p < 0.00001 | Z = 0.30, p = 0.76 |
| Europe | 1, (1) | 5, (3–7) | −0.12 (−0.92, 0.68) | N/A | Z = 0.30, p = 0.76 | |
| Asia | 3, (3, 5, 7) | 3, (1, 4, 6) | −0.39 (−1.83, 1.06) | 97%, p < 0.00001 | Z = 0.52, p = 0.60 | |
| South America | 2, (4, 6) | 4, (1–3, 5) | 0.17 (−0.49, 0.82) | 82%, p = 0.0007 | Z = 0.49, p = 0.62 | |
| Traditional cardiovascular risk variables | All | 14, (1–6, 8–15) | None | 0.74 (0.47, 1.01) | 94%, p < 0.00001 | Z = 5.34, p < 0.00001 |
| Europe | 2, (1, 10) | 12, (2, 3, 14, 15, 4–6, 8, 9, 11–13) | 0.03 (−0.21, 0.27) | 0%, p = 0.88 | Z = 0.25, p = 0.88 | |
| North America | 2, (9, 14) | 12, (1, 2, 13, 15, 3–6, 8, 10–12) | 1.86 (−0.31, 4.04) | 98%, p < 0.00001 | Z = 1.68, p = 0.09 | |
| South America | 4, (4, 6, 11, 13) | 10, (1–3, 5, 8–10, 12, 14, 15) | 0.24 (0.01, 0.47) | 72%, p < 0.00001 | Z = 2.03, p = 0.04 | |
| Africa | 2, (8, 12) | 12, (1, 2, 14, 15, 3–6, 9–11, 13) | 1.44 (0.51, 2.38) | 97%, p < 0.00001 | Z = 3.02, p = 0.003 | |
| Asia | 3, (1, 2, 12–15, 3–6, 8–11) | 11, (1, 2, 12–15, 3–6, 8–11) | 1.30 (0.69, 1.91) | 96%, p < 0.00001 | Z = 4.19, p < 0.001 | |
| Australia | 1, (15) | 13, (1, 2, 12–14, 3–6, 8–11) | 0.33 (0.05, 0.61) | 7%, p = 0.38 | Z = 2.34, p = 0.02 |
Sensitivity analysis of outcomes based on geographical location.
Table 4
| Oral contraceptive treatment compared with non-users (controls) | ||||||
|---|---|---|---|---|---|---|
| Patient or population: [premenopausal women] | ||||||
| Intervention: [oral contraceptive] | ||||||
| Comparison: [non-user] | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with [comparison] | Risk with [intervention] | – | 197 (3 observational studies) | ⨁⨁◯◯ Lowa,b | ||
| Cellular marker of endothelial activation—NO | – | SMD 0.73lower (2.6 lower to 1.14 higher) | ||||
| Vascular marker of endothelial activation—FMD | – | SMD 0.22 lower (1.12 lower to 0.68 higher) | – | 172 (2 observational studies, 1 RCT) | ⨁⨁◯◯ Lowa,b | |
| Vascular marker of endothelial activation—CCA-IMT | – | SMD 0.62 higher (0.02 higher to 1.21 higher) | – | 172 (2 observational studies, 1 RCT) | ⨁⨁◯◯ Lowa,b | |
| Traditional cardiovascular risk variables | – | SMD 0.74 higher (0.47 higher to 1.01 higher) | – | 4,320 (12 observational studies, 2 RCTs) | ⨁⨁◯◯ Lowa,b | |
Summary of findings: use of oral contraceptives in premenopausal women compared with non-users.
CI, confidence interval; SMD, standardized mean difference.
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
4. Discussion
The aim of this systematic review was to provide a comprehensive synthesis of the available evidence on the link between OC use and CVD risk in premenopausal women. Cumulative evidence summarized in this review highlights the impact of OC use on endothelial function and some traditional cardiovascular risk variables. The results of our study show that the use of progestin-only type of OC is associated with increased levels of plasma endothelin 1 (ET-1) in healthy young women (45). In contrast, the use of second-generation (levonorgestrel; LNG) and third-generation (gestodene; GSD) COCs does not significantly impact the plasma levels of ET-1 and NO (46). It is noteworthy that the imbalance in quotient between NO and ET-1 can impact the vascular tone. Meanwhile, a study by John et al. showed that the use of second-generation (LNG) OC significantly impacted the production and release of NO at the basal level and the levels of NO remained unchanged despite stimulating its release with acetylcholine and sodium nitroprusside (58). However, change in several hemodynamic, mechanical, and chemical factors, including blood pressure, vascular resistance, angiotensin II, as well as transforming growth factor-β, among others, can influence the activation and functions of endothelial cells leading to multiple inflammatory responses involving the innate and adaptive immune cells across the body system.
Furthermore, our study findings showed that fourth-generation (drospireneone; DRSP) OC significantly increased FMD (43). In contrast, the findings of other studies (49, 50, 61) involving the use of second-generation levonorgestrel (LNG) and another type of fourth-generation CMA OC showed lowered FMD. However, the reported pooled estimate of our meta-analysis showed no significant change in FMD in participants who used the second- (LNG) and third-generation (GSD, DSG) OCs (49, 50, 61).
More so, our study findings showed a significantly increased mean Common Carotid Artery Intima–Media thickness (CCA-IMT) in those who used second-generation (LNG) OC when compared with non-users and among fourth-generation (CMA) OC users (50, 61). However, the pooled estimate of our meta-analysis showed a significant decrease in CCA-IMT of participants on OCs when compared with non-users (49, 50, 61). In clinical settings, both FMD and IMT are strong predictors of endothelial dysfunction where FMD reflects early and predominant functional changes in the vascular wall, and IMT serves as a marker of more advanced structural changes (68). Nonetheless, understanding these changes may provide an insight into the power and effectiveness of the deep nerve stimulation to regulate systemic blood pressure (69).
Of note, endogenous estrogen is known to guard against vascular damage and atherosclerosis via the estrogen receptor (Eps), especially ERα and Erβ (70). However, the demonstrated changes in endothelial activation markers can be attributed to the type of progestin where a COC containing LNG was shown to result in 3–7.5-fold greater reduction in mean FMD among users when compared with non-users (61) and among users of fourth-generation (CMA) OC, which is derived from 17-hydroxyprogesterone, with high affinity for the progesterone receptor (PR) and moderate antiandrogenic activity (61). Furthermore, high androgenic properties associated with second-generation LNG progestin can antagonize the vasodilatory effects of estrogens and impact endothelial function (71, 72).
Furthermore, evidence emerging from our summary of findings showed that the OC use significantly increased systolic and diastolic blood pressure levels (60, 73, 74). Chronic use of COCs can induce increases in arterial pressure, primarily by activating the renin–angiotensin system (61) and via oxidative stress (75). However, some studies reported contradictory findings where the use of OCs did not significantly impact the blood pressure of the participants irrespective of the estrogen component (59, 67). Of note, endogenous female sex hormones are known to play a role in maintaining body fluid homeostasis (76) during the menstrual cycle. However, emerging evidence suggests that exogenous sex hormones may alter body fluid homeostasis in women of reproductive age (77, 78), which may depend on progestin type (76). While the progestin component may increase plasma volume through the combined mechanisms of increased osmolarity in the vascular space as well as overall expansion of ECF, the estrogen component may increase the plasma volume by reducing the operating point for osmoregulation of arginine vasopressin (AVP) and thirst, leading to a greater fluid retention in the vascular space (76).
AVP is a key hormone synthesized in the paraventricular and supraoptic nuclei of the hypothalamus (79, 80) they are released together with copeptin from the axonal terminals of the magnocellular neurons located in the posterior lobe of the pituitary gland (79). They are involved in the regulation of other body functions besides the control of the body's osmotic balance, respiratory and blood pressure regulation, sodium homeostasis, kidney functioning (80), fear conditioning, and love making (81–83). It is noteworthy that the synthetic progestins, apart from acting at the PR, can also influence the activity of other steroid receptors to induce androgenic, glucocorticoid, antiandrogenic, and antimineralocorticoid effects (84, 85).
Furthermore, findings from our data synthesis showed that the use of OCs is associated with dyslipidemia. Due to imbalance in the lipid profile, dyslipidemia may result in cardiovascular complications (86). The results showed that second- (LNG), third- (GSD), and fourth- generation (DRSP) COCs significantly increased the TC levels of OC users when compared with non-OC users (48, 55, 62, 63). In contrast, the findings from other studies showed that second- and third-generation COCs do not impact the TC level (46, 49). Furthermore, our study results showed that second- (LNG) generation COC increased the levels of LDL in users when compared with non-users, as also third- (GSD) and fourth- (DRSP) generation COCs (48, 55, 62, 63). This contrasted with the findings of other studies that showed that second- (LNG) and third- (GSD, DSG) generation COCs do not impact the LDL levels (55, 56, 58, 60). However, the pooled estimate of our meta-analysis showed that OC significantly increased the levels of TC and LDL in OC users when compared with non-users (62–67).
In addition, the results showed that second- (LNG) and third-generation (GSD) increased the HDL levels (46, 56). However, these findings contrasted with the results of other studies where second- (LNG) generation COC decreased the HDL levels when compared with third- (GSD) and fourth- (DRSP) generation COCs (49, 63). Nonetheless, the findings of several other studies showed that COCs do not impact the HDL levels (55, 58, 60, 62). More so, our study results showed that second- (LNG) generation COC increased the levels of TG (56, 62). On the other hand, second-generation COC (LNG) reduced the levels of TG when compared with the third- (GSD) and fourth- (DRSP) generation COCs (63). However, several other studies showed that COCs do not impact the TG levels (48, 55, 58, 60). Furthermore, the pooled estimate of our subgroup analysis showed an insignificant increase in the levels of TG and HDL among OC users.
Moreover, the results showed that COCs do not impact BMI (46, 47, 49, 50, 55–58, 60, 61–63, 67), although a study by Asare et al. showed that second- (LNG) generation COC increased BMI (48). However, the pooled estimate of our subgroup analysis showed that OCs do not impact BMI as well as FBG levels. Of note, emerging evidence showed the existence of regional disparities in cardiovascular disease incidence and mortality (87, 88). Moreover, three-quarter of the world's CVD deaths occur in low- and middle-income countries (89). Despite limited data on known risk factors to explain these regional variations in CVD among women of reproductive age, the results of our meta-analysis showed a high prevalence of traditional cardiovascular risk variables among OC users from North America when compared with Europe and other regions, which had the lowest prevalence.
There are several limitations in the evidence presented in this systematic review. These include substantial levels of statistical heterogeneity among included studies and unavailability of data on some prespecified effect measures. Therefore, caution should be exercised in interpreting and extrapolating these findings in different populations of various geographical locations.
5. Conclusion
The evidence presented in this review highlights the impact of second-generation (LNG) OC use on FMD, CCA-IMT, and NO levels in premenopausal women. In conclusion, evidence from our findings suggests that second-generation OC may result in little to no difference in endothelial activation. Although, among the variables assessed, our evidence suggests that the use of LNG may result in a significant reduction in CCA-IMT among users. Furthermore, our evidence suggests that the use of LNG may significantly increase other traditional cardiovascular risk variables. However, more independently conducted studies are needed to determine the long-term impact of individually available COCs on CVD risk.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
OAF and BBN conceptualized and designed the study, and OAF drafted the protocol. PVD helped draft the protocol. All authors wrote and approved the final manuscript. BBN is the guarantor of the review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Williams JS MacDonald MJ . Influence of hormonal contraceptives on peripheral vascular function and structure in premenopausal females: a review. Am J Physiol - Heart Circ Physiol. (2021) 320:H77–89. 10.1152/AJPHEART.00614.2020
2.
Shufelt CL Bairey Merz CN . Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol. (2009) 53:221–31. 10.1016/j.jacc.2008.09.042
3.
Burkman R Bell C Serfaty D . The evolution of combined oral contraception: improving the risk-to-benefit ratio. Contraception. (2011) 84:19–34. 10.1016/j.contraception.2010.11.004
4.
Ahrendt HJ Nisand I Bastianelli C Gómez MA Gemzell-Danielsson K Urdl W et al Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 µg of ethinyl estradiol and 3 mg of drospirenone. Contraception. (2006) 74:451–7. 10.1016/j.contraception.2006.07.004
5.
Oddsson K Leifels-Fischer B De Melo NR Wiel-Masson D Benedetto C Verhoeven CHJ et al Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. (2005) 71:176–82. 10.1016/j.contraception.2004.09.001
6.
UN. Population Division. Contraceptive use by method 2019: data booklet. Contracept Use by Method 2019 (2019) 25.
7.
Christin-Maitrei S . History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. (2013) 27:3–12. 10.1016/j.beem.2012.11.004
8.
Schindler AE . Non-contraceptive benefits of oral hormonal contraceptives. Int J Endocrinol Metab. (2013) 11:41–7. 10.5812/ijem.4158
9.
Badawy A Elnashar A . Treatment options for polycystic ovary syndrome. Int J Womens Health. (2011) 3:25–35. 10.2147/IJWH.S11304
10.
Hee L Kettner LO Vejtorp M . Continuous use of oral contraceptives: an overview of effects and side-effects. Acta Obstet Gynecol Scand. (2013) 92:125–36. 10.1111/aogs.12036
11.
Burkman R . Cardiovascular issues with oral contraceptives: evidenced-based medicine. Int J Fertil Womens Med. (2000) 45:166–74. PMID: .
12.
Farley TMM Collins J Schlesselman JJ . Hormonal contraception and risk of cardiovascular disease: an international perspective. Contraception. (1998) 57:211–30. 10.1016/S0010-7824(98)00019-5
13.
Zakharova MY Meyer RM Brandy KR Datta YH Joseph MS Schreiner PJ et al Risk factors for heart attack, stroke, and venous thrombosis associated with hormonal contraceptive use. Clin Appl Thromb. (2011) 17:323–31. 10.1177/1076029610368670
14.
Lewis MA Heinemann LAJ Spitzer WO MacRae KD Bruppacher R . The use of oral contraceptives and the occurrence of acute myocardial infarction in young women: results from the transnational study on oral contraceptives and the health of young women. Contraception. (1997) 56:129–40. 10.1016/S0010-7824(97)00118-2
15.
Heinemann LAJ Lewis MA Thorogood M Spitter WO Guggenmoos-Holzmann I Bruppacher R . Case-control study of oral contraceptives and risk of thromboembolic stroke: results from international study on oral contraceptives and health of young women. Br Med J. (1997) 315:1502–4. 10.1136/bmj.315.7121.1502
16.
Lidegaard Ø Løkkegaard E Svendsen AL Agger C . Hormonal contraception and risk of venous thromboembolism: national follow-up study. Br Med J. (2009) 339:557–60. 10.1136/bmj.b2890
17.
Engbers MJ van Hylckama Vlieg A Rosendaal FR . Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost. (2010) 8:2105–12. 10.1111/j.1538-7836.2010.03986.x
18.
Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Centers Dis Control Prev (2020). Available at:https://www.cdc.gov/ncbddd/dvt/data.html(Accessed October 27, 2021).
19.
Heit JA . Epidemiology of venous thromboembolism. Nat Rev Cardiol. (2015) 12:464–74. 10.1038/nrcardio.2015.83
20.
Nawrot TS Den HE Fagard RH Hoppenbrouwers K Staessen JA . Blood pressure, serum total cholesterol and contraceptive pill use in 17-year-old girls. Eur J Prev Cardiol. (2003) 10:438–42. 10.1097/01.hjr.0000103463.31435.1e
21.
Du Y Rosner BM Knopf H Schwarz S Dören M Scheidt-Nave C . Hormonal contraceptive use among adolescent girls in Germany in relation to health behavior and biological cardiovascular risk factors. J Adolesc Heal. (2011) 48:331–7.. doi: 10.1016/J.JADOHEALTH.2011.01.004
22.
Paulus D Saint-Remy A Jeanjean M . Oral contraception and cardiovascular risk factors during adolescence. Contraception. (2000) 62:113–6. 10.1016/S0010-7824(00)00159-1
23.
Douxfils J Klipping C Duijkers I Kinet V Mawet M Maillard C et al Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. (2020) 102:396–402. 10.1016/j.contraception.2020.08.015
24.
Roach REJ Lijfering WM Helmerhorst FM Cannegieter SC Rosendaal FR van Hylckama Vlieg A . The risk of venous thrombosis in women over 50years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost. (2013) 11:124–31. 10.1111/jth.12060
25.
Khialani D Rosendaal F Vlieg AVH . Hormonal contraceptives and the risk of venous thrombosis. Semin Thromb Hemost. (2020) 46:865–71. 10.1055/s-0040-1715793
26.
Mayeda ER Torgal AH Westhoff CL . Weight and body composition changes during oral contraceptive use in obese and normal weight women. J Women’s Heal. (2014) 23:38–43. 10.1089/jwh.2012.4241
27.
Kemmeren JM Algra A Grobbee DE . Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. Br Med J. (2001) 323:131–4. 10.1136/bmj.323.7305.131
28.
World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. Lancet. (1995) 346:1582–8. 10.1016/S0140-6736(95)91927-9
29.
Stegeman BH De Bastos M Rosendaal FR Van Hylckama Vlieg A Helmerhorst FM Stijnen T et al Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. Br Med J. (2013) 347:1–12. 10.1136/bmj.f5298
30.
Dinger J Möhner S Heinemann K . Cardiovascular risks associated with the use of drospirenone-containing combined oral contraceptives. Contraception. (2016) 93:378–85. 10.1016/j.contraception.2016.01.012
31.
Dinger JC Heinemann LAJ Kühl-Habich D . The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance study on oral contraceptives based on 142,475 women-years of observation. Contraception. (2007) 75:344–54. 10.1016/j.contraception.2006.12.019
32.
de Bastos M Stegeman BH Rosendaal FR Van Hylckama Vlieg A Helmerhorst FM Stijnen T et al Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. (2014) 2014(3):1–51. 10.1002/14651858.CD010813.pub2
33.
Van Hylckama Vlieg A Helmerhorst FM Vandenbroucke JP Doggen CJM Rosendaal FR . The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. Br Med J. (2009) 339:561. 10.1136/bmj.b2921
34.
Poulter NR Chang CL Farley TMM Meirik O Marmot MG . Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. (1996) 348:498–505. 10.1016/S0140-6736(95)12393-8
35.
Poulter NR Meirik O . Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. (1996) 348:505–10. 10.1016/S0140-6736(95)12394-6
36.
Lee Y Choi A Noh Y Jeon HL Choe SA Shin JY . Signal detection of drospirenone-containing oral contraceptives: a disproportionality analysis using the Korea Adverse Event Reporting System Database, 2008–2017. BMJ Open. (2021) 11:e045948. 10.1136/bmjopen-2020-045948
37.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. 10.1136/bmj.n71
38.
Fabunmi OA Dludla PV Ngcobo SR Nkambule BB . Investigating the risks of cardiovascular disease among premenopausal women using oral contraceptive: a protocol for a systematic review and meta-analysis. BMJ Open (2023) 13:e071118. 10.1136/bmjopen-2022-071118
39.
Downs SH Black N . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. (1998) 52:377–84. 10.1136/jech.52.6.377
40.
Ryan R Hill S . Supporting implementation of Cochrane methods in complex communication reviews: resources developed and lessons learned for editorial practice and policy. Health Res Policy Syst. (2019) 17:1–11. 10.1186/s12961-019-0435-0
41.
Schroll JB Moustgaard R Gøtzsche PC . Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol. (2011) 11:1–8. 10.1186/1471-2288-11-22
42.
McHugh ML . Interrater reliability: the kappa statistic. Biochem Med. (2012) 22:276–82. 10.11613/bm.2012.031
43.
Friedman J Cremer M Jelani QUA Huang X Jian J Shah S et al Oral contraceptive use, iron stores and vascular endothelial function in healthy women. Contraception. (2011) 84:285–90. 10.1016/j.contraception.2011.01.012
44.
Blackmore KM Wong J Knight JA . A cross-sectional study of different patterns of oral contraceptive use among premenopausal women and circulating IGF-1: implications for disease risk. BMC Womens Health. (2011) 11:15. 10.1186/1472-6874-11-15
45.
Meendering JR Torgrimson BN Miller NP Kaplan PF Minson CT . Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol - Heart Circ Physiol. (2008) 294:1–8. 10.1152/ajpheart.01314.2007
46.
Merki-Feld GS Rosselli M Dubey RK Jäger AW Keller PJ . Long-term effects of combined oral contraceptives on markers of endothelial function and lipids in healthy premenopausal women. Contraception. (2002) 65:231–6. 10.1016/S0010-7824(01)00312-2
47.
Odutayo A Cherney D Miller J Ahmed SB Lai V Dunn S et al Transdermal contraception and the renin–angiotensin–aldosterone system in premenopausal women. Am J Physiol - Ren Physiol. (2015) 308:F535–40. 10.1152/ajprenal.00602.2014
48.
Asare GA Santa S Angala RA Asiedu B Afriyie D Amoah AG . Effect of hormonal contraceptives on lipid profile and the risk indices for cardiovascular disease in a Ghanaian community. Int J Womens Health. (2014) 6:597–603. 10.2147/IJWH.S59852
49.
Lizarelli PM Martins WP Vieira CS Soares GM Franceschini SA Ferriani RA et al Both a combined oral contraceptive and depot medroxyprogesterone acetate impair endothelial function in young women. Contraception. (2009) 79:35–40. 10.1016/j.contraception.2008.07.024
50.
Heidarzadeh Z Asadi B Saadatnia M Ghorbani A Fatehi F . The effect of low-dose combined oral contraceptive pills on brachial artery endothelial function and common carotid artery intima-media thickness. J Stroke Cerebrovasc Dis. (2014) 23:675–80. 10.1016/j.jstrokecerebrovasdis.2013.06.007
51.
Yildizhan R Yildizhan B Adali E Yoruk P Birol F Suer N . Effects of two combined oral contraceptives containing ethinyl estradiol 30 µg combined with either gestodene or drospirenone on hemostatic parameters, lipid profiles and blood pressure. Arch Gynecol Obstet. (2009) 280:255–61. 10.1007/s00404-008-0907-x
52.
Wiegratz I Lee JH Kutschera E Winkler UH Kuhl H . Effect of four oral contraceptives on hemostatic parameters. Contraception. (2004) 70:97–106. 10.1016/j.contraception.2004.03.004
53.
Giribela CRG Melo NR Silva RCG Hong VM Guerra GM Baracat EC et al A combined oral contraceptive containing drospirenone changes neither endothelial function nor hemodynamic parameters in healthy young women: a prospective clinical trial. Contraception. (2012) 86:35–41. 10.1016/j.contraception.2011.08.017
54.
Kharbanda EO Parker ED Sinaiko A Daley MF Margolis K Becker M et al NIH public access. J Pediatr. (2015) 165:1029–33. 10.1016/j.jpeds.2014.07.048.Initiation
55.
Fallah S Nouroozi V Seifi M Samadikuchaksaraei A Aghdashi EM . Influence of oral contraceptive pills on homocysteine and nitric oxide levels: as risk factors for cardiovascular disease. J Clin Lab Anal. (2012) 26:120–3. 10.1002/jcla.21492
56.
Dos Santos ACN Petto J Diogo DP Seixas CR de Souza LH Araújo WS et al Elevation of oxidized lipoprotein of low density in users of combined oral contraceptives. Arq Bras Cardiol. (2018) 111:764–70. 10.5935/abc.20180194
57.
Harvey RE Hart EC Charkoudian N Curry TB Carter JR Fu Q et al Oral contraceptive use, muscle sympathetic nerve activity, and systemic hemodynamics in young women. Hypertension. (2015) 66:590–7. 10.1161/HYPERTENSIONAHA.115.05179
58.
John S Jacobi J Schlaich MP Delles C Schmieder RE . Effects of oral contraceptives on vascular endothelium in premenopausal women. Am J Obstet Gynecol. (2000) 183:28–33. 10.1067/mob.2000.105739
59.
De Nadai MN Nobre F Ferriani RA Vieira CS . Effects of two contraceptives containing drospirenone on blood pressure in normotensive women: a randomized-controlled trial. Blood Press Monit. (2015) 20:310–5. 10.1097/MBP.0000000000000139
60.
Straznicky NE Barrington VE Branley P Louis WJ . A study of the interactive effects of oral contraceptive use and dietary fat intake on blood pressure, cardiovascular reactivity and glucose tolerance in normotensive women. J Hypertens. (1998) 16:357–68. 10.1097/00004872-199816030-00013
61.
Franceschini SA Vieira CS Martins WP França JB Ferriani RA . Effects of combined oral contraceptives containing levonorgestrel or chlormadinone on the endothelium. Contraception. (2013) 87:766–72. 10.1016/j.contraception.2012.09.023
62.
Momeni Z Dehghani A Fallahzadeh H Koohgardi M Dafei M Mohammadi M . Effects of low-dose contraceptive pills on the risk factors of cardiovascular diseases among 15-35-year-old women: a retrospective cohort. Int J Reprod Biomed. (2019) 17:841–50. 10.18502/ijrm.v17i10.5496
63.
El-Haggar SM Mostafa TM . Cardiovascular risk in Egyptian healthy consumers of different types of combined oral contraceptives pills: a comparative study. Endocrine. (2015) 49:820–7. 10.1007/s12020-014-0507-4
64.
Fallah S Pour MS Chadegani AR Korani M . Adiponectin, leptin and lipid profiles evaluation in oral contraceptive pill consumers. Arch Gynecol Obstet. (2012) 285:1747–52. 10.1007/s00404-011-2192-3
65.
Winkler UH Sudik R . The effects of two monophasic oral contraceptives containing 30 mcg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipid, hormone and metabolic parameters. Contraception. (2009) 79:15–23. 10.1016/j.contraception.2008.08.011
66.
Piltonen T Puurunen J Hedberg P Ruokonen A Mutt SJ Herzig KH et al Oral, transdermal and vaginal combined contraceptives induce an increase in markers of chronic inflammation and impair insulin sensitivity in young healthy normal-weight women: a randomized study. Hum Reprod. (2012) 27:3046–56. 10.1093/humrep/des225
67.
Nisenbaum MG De Melo NR Giribela CRG De Morais TL Guerra GM De Angelis K et al Effects of a contraceptive containing drospirenone and ethinyl estradiol on blood pressure and autonomic tone: a prospective controlled clinical trial. Eur J Obstet Gynecol Reprod Biol. (2014) 175:62–6. 10.1016/j.ejogrb.2014.01.006
68.
Koivistoinen T Virtanen M Hutri-Kähönen N Lehtimäki T Jula A Juonala M et al Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. (2012) 220:387–93. 10.1016/J.ATHEROSCLEROSIS.2011.08.007
69.
Gonzalez-Gonzalez MA Romero K Beitter J Lloyd D Lam DV Hernandez-Reynoso AG et al Renal nerve activity and arterial depressor responses induced by neuromodulation of the deep peroneal nerve in spontaneously hypertensive rats. Front Neurosci. (2022) 16:511. 10.3389/fnins.2022.726467
70.
Dos Santos RL Da Silva FB Ribeiro RF Stefanon I . Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig. (2014) 18:89–103. 10.1515/hmbci-2013-0048
71.
Ganz P . Vasomotor and vascular effects of hormone replacement therapy. Am J Cardiol. (2002) 90:F11–6. 10.1016/S0002-9149(01)02218-4
72.
Kawano H Motoyama T Ohgushi M Kugiyama K Ogawa H Yasue H . Menstrual cyclic variation of myocardial ischemia in premenopausal women with variant angina. Ann Intern Med. (2001) 135:977–81. 10.7326/0003-4819-135-11-200112040-00009
73.
Cardoso F Polónia J Santos A Silva-Carvalho J Ferreira-De-Almeida J . Low-dose oral contraceptives and 24-hour ambulatory blood pressure. Int J Gynecol Obstet. (1997) 59:237–43. 10.1016/S0020-7292(97)00239-7
74.
The WHO multicentre trial of the vasopressor effects of combined oral contraceptives: 1. Comparisons with IUD. Task Force on Oral Contraceptives. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. (1989) 40:129–45. 10.1016/0010-7824(89)90001-2
75.
Chen JT Kotani K . Oral contraceptive therapy increases oxidative stress in pre-menopausal women. Int J Prev Med. (2012) 3:893–6. 10.4103/2008-7802.104862
76.
Stachenfeld NS . Hormonal changes during menopause and the impact on fluid regulation. Reprod Sci. (2014) 21:555. 10.1177/1933719113518992
77.
Cagnacci A Ferrari S Napolitano A Piacenti I Arangino S Volpe A . Combined oral contraceptive containing drospirenone does not modify 24-h ambulatory blood pressure but increases heart rate in healthy young women: prospective study. Contraception. (2013) 88:413–7. 10.1016/j.contraception.2012.12.002
78.
Burrows M Peters CE . The influence of oral contraceptives on athletic performance in female athletes. Sport Med. (2007) 37:557–74. 10.2165/00007256-200737070-00001
79.
Proczka M Przybylski J Cudnoch-Jędrzejewska A Szczepańska-Sadowska E Żera T . Vasopressin and breathing: review of evidence for respiratory effects of the antidiuretic hormone. Front Physiol. (2021) 12:1828. 10.3389/fphys.2021.744177
80.
Cuzzo B Padala SA Lappin SL . Physiology, vasopressin (antidiuretic hormone, ADH). StatPearls (2020).
81.
Carter CS . The oxytocin–vasopressin pathway in the context of love and fear. Front Endocrinol. (2017) 8:356. 10.3389/fendo.2017.00356
82.
Bosch OJ Neumann ID . Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. (2012) 61:293–303. 10.1016/j.yhbeh.2011.11.002
83.
Carter CS . Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. (2014) 65:17–39. 10.1146/annurev-psych-010213-115110
84.
Adeyanju OA Michael OS Soladoye AO Olatunji LA . Blockade of mineralocorticoid receptor ameliorates oral contraceptive-induced insulin resistance by suppressing elevated uric acid and glycogen synthase kinase-3 instead of circulating mineralocorticoid. Arch Physiol Biochem. (2018):1–10. 10.1080/13813455.2018.1509220
85.
Adeyanju OA Olatunji LA . Drospirenone-containing oral contraceptives do not affect glucose regulation and circulating corticosterone. J Basic Clin Physiol Pharmacol. (2019) 30:1–9. 10.1515/jbcpp-2018-0184
86.
Diniz ET Bandeira F . Dyslipidemia. Endocrinol Diabetes A Probl Approach. (2014) 489–502. 10.1007/978-1-4614-8684-8_40
87.
Kim LG Carson C Lawlor DA Ebrahim S . Geographical variation in cardiovascular incidence: results from the British Women’s Heart and Health Study. BMC Public Health. (2010) 10:1–10. 10.1186/1471-2458-10-696
88.
Parcha V Kalra R Suri SS Malla G Wang TJ Arora G et al Geographic variation in cardiovascular health among American adults. Mayo Clin Proc. (2021) 96:1770–81. 10.1016/j.mayocp.2020.12.034
89.
WHO. Cardiovascular diseases (CVDs) (2017). Available at:https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)(Accessed June 14, 2020).
Summary
Keywords
oral contraceptives, combined oral contraceptives, ethinylestradiol, progestins, cardiovascular disease
Citation
Fabunmi OA, Dludla PV and Nkambule BB (2023) Investigating cardiovascular risk in premenopausal women on oral contraceptives: Systematic review with meta-analysis. Front. Cardiovasc. Med. 10:1127104. doi: 10.3389/fcvm.2023.1127104
Received
19 December 2022
Accepted
09 March 2023
Published
25 April 2023
Volume
10 - 2023
Edited by
Gino Seravalle, University of Milano-Bicocca, Italy
Reviewed by
Rafael Sanchez-Borrego, Hospital Quirón Teknon, Spain Flavia Franconi, Istituto Nazionale Biostrutture e Biosistemi, Italy
Updates
Copyright
© 2023 Fabunmi, Dludla and Nkambule.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Bongani B. Nkambule nkambuleb@ukzn.ac.za
Specialty Section: This article was submitted to Hypertension, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.