Abstract
Cardiorespiratory function influences exercise capacity and is an important determinant of high-altitude adaptation. Some studies have investigated the characteristics of changes in cardiorespiratory fitness during high-altitude acclimatization. However, studies on changes in cardiorespiratory fitness during high-altitude de-acclimatization are still lacking and have not yet been elucidated. Furthermore, few drugs have been studied to improve cardiorespiratory function during both processes. The Shigatse CARdiorespiratory Fitness (SCARF) study is a single-center, randomized, double-blind, placebo-control clinical trial to explore the effects of ubiquinol on cardiorespiratory fitness during high-altitude acclimatization and de-acclimatization in healthy adults. Participants will be randomly assigned 1:1 to ubiquinol 200 mg daily or a placebo for 14 days before departure until the end of data collection after return in 7 days. Cardiorespiratory fitness is the primary outcome, while acute mountain sickness and high-altitude de-acclimatization symptoms are secondary endpoints. In addition, laboratory measurements, including routine blood tests and serological measurements, will be performed. To the best of our knowledge, the SCARF study will be the first to reveal the changes in the cardiorespiratory fitness characteristics during high-altitude acclimatization and de-acclimatization. Furthermore, the results of this study will contribute to exploring whether ubiquinol supplementation could be beneficial for endurance exercise capacity at different altitudes and help improve adaptation to acute hypoxia and de-acclimatization.
Clinical Trial Registration: This study has been registered in the Chinese Clinical Trial Register (www.chictr.org.cn) as ChiCTR2200059900 and ChiCTR2200066328.
1 Introduction
It is known that hypobaric hypoxia is the main characteristic of a high-altitude environment. Acute mountain sickness (AMS) is an environmental illness that can occur in people who arrive at high altitudes above about 2,500 m for the first time. The manifestations of AMS are a series of symptoms involving the brain, heart, gastrointestinal tract, and muscles (1). After returning to a low altitude, reoxygenation may also cause a series of symptoms, named high-altitude de-acclimatization symptoms (HADAS), which are adaptive reactions of the body when facing a normoxic environment (2). Both these symptoms stem from a maladaptation of the cardiovascular and respiratory systems when facing acute hypoxia and reoxygenation. Treatments targeting the cardiovascular and respiratory systems may alleviate these symptoms and help to restore cardiorespiratory fitness back to previous levels.
Reduced arterial oxygenation and increased pulmonary vascular resistance during acclimatization are the main causes of decreasing cardiorespiratory fitness at high altitude (3–6). Arterial oxygenation reduction is attributed to decreased partial pressure inspiratory oxygen and altered coupling of convectional and diffusional oxygen transport systems (7). Meanwhile, the elevated pulmonary vascular resistance is explained through hypoxia-induced pulmonary vasoconstriction and increased cardiac afterload in the right ventricle (8, 9). Although multiple reports have illustrated that the impairment of cardiorespiratory fitness is the main course of decreased physical performance capacity (10–12), there is far less information regarding the characteristics of cardiorespiratory fitness in the process of high-altitude acclimatization and de-acclimatization. Several prior attempts aimed to improve cardiorespiratory fitness after acute high-altitude exposure indicated that selective endothelin A receptor blockade, a PDE-5 inhibitor, and carbonic anhydrase inhibitor are beneficial in reducing cardiac afterload and restoring physical capacity (4, 13, 14). However, the severe side effects of sitaxsentan and acetazolamide limited their clinical applications, especially in elderly and severely ill patients (15, 16). Moreover, few studies have explored the potential medications for the prophylaxis and treatment of high-altitude de-acclimatization-associated symptoms and cardiorespiratory fitness impairment (17). Thus, exploring a new strategy to restore impaired cardiorespiratory fitness and exercise capacity during acclimatization and/or de-acclimatization is necessary.
Coenzyme Q10 (CoQ10) is a fat-soluble antioxidant occurring naturally in the inner membrane of the cell's mitochondria. It plays an essential role as a diffusible electron carrier in the electron transport chain (18, 19). CoQ10 has two forms: the oxidized form and the reduced, active form. This reduced form is widely used in the adjunctive treatment of cardiovascular diseases, nutritional health products, and food additives (20). Notably, CoQ10 plays a key role in myocardial hypoxia, significantly boosting heart health. In patients with myocardial ischemia and hypoxia, ubiquinol, the reduced-form supplement (19, 21, 22), increases aerobic metabolism efficiency and raises the anaerobic threshold. This signifies a lesser need to resort to anaerobic energy production at the same workload (19, 21, 22). Ubiquinol is necessary for the body to produce energy; however, its content in the organism decreases with age and may lead to diseases (23). The lack of ubiquinol causes less efficiency, quick fatigue, and weakened cellular protection ability against oxidative stress (19). These clinical trials implied that ubiquinol could reverse the loss of physical performance ability both at high altitudes and at return to sea level.
We designed this double-blinded, placebo-controlled clinical trial to investigate the hypothesis that ubiquinol supplementation could improve cardiorespiratory fitness, which may be impaired during high-altitude acclimatization and de-acclimatization. This novel Shigatse CARdiorespiratory Fitness (SCARF) study has the potential to identify whether ubiquinol supplementation may rescue the loss of exercise capacity and impaired cardiorespiratory fitness during acute hypoxia and the reoxygenation process. We believe this is the first study that prospectively and accurately evaluates cardiorespiratory fitness during high-altitude acclimatization and de-acclimatization. We also explore the effect of ubiquinol on blood metabolic substrate and automatic nervous function to illustrate the possible beneficial mechanisms further.
2. Materials and methods
2.1. Setting
This is a single-center, randomized, double-blind, placebo-control clinical trial conducted at Chongqing Xinqiao Hospital and Shigatse Branch Hospital [The Shigatse CARdiorespiratory Fitness (SCARF) Study] in 2022 and registered at www.chictr.org.cn (ChiCTR2200059900 and ChiCTR2200066328). The primary hypothesis of this study is that ubiquinol supplementation may improve cardiorespiratory fitness both at high-altitude acclimatization and de-acclimatization, while AMS and HADAS are secondary endpoints. The study complies with the SPIRIT 2013 recommendations (Standard Protocol Items: Recommendations for International Trials) (24) (Supplementary Material S1). Table 1 depicts the overall schedule and time commitment for trial participants.
Table 1
| Timepoint | Study period | |||||
|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-allocation | Close-out | |||
| −14 days | 0 | 11 days | 17 days | 28 days | 6 months | |
| Enrolment | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Medical and travel history | X | |||||
| Vital signs | X | |||||
| Allocation/randomization | X | |||||
| Interventions | ||||||
| Ubiquinol group |

|
|||||
| Placebo group |

|
|||||
| Assessments | ||||||
| Baseline variables: age, sex, height, weight | X | |||||
| Outcome variables | ||||||
| CPET | X | X | X | X | ||
| Blood routine examination | X | X | X | |||
| Serum marker examination | X | X | X | |||
| The Lake Louise 2018 score assessment | X | |||||
| High altitude de-acclimatization syndrome assessment | X | X | ||||
Study schedule and time commitment.
2.2. Recruitment and study population
Enrolment occurred in Chongqing (low altitude area, 300 m) from June 1st, 2022, and ended on June 15th, 2022. Demographic and medical data of all participants were recorded through interviews. A potential participant must meet all the inclusion criteria and do not meet any exclusion criteria. The inclusion and exclusion criteria are detailed in Table 2. According to previous studies, the risks associated with CoQ10 are minor, even at doses as high as 1,800 mg (25). Moreover, pharmacokinetic studies suggest that exogenous CoQ10 does not influence the biosynthesis of endogenous CoQ10 and is less likely to accumulate in plasma or tissues after the cessation of supplementation (26).
Table 2
| Inclusion criteria | Age >18 years |
| Chinese Han people | |
| Have no recent (previous 6 months) experience in resistance exercise (including additional cardiopulmonary exercise tests, long-distance running, riding, swimming and skiing and other endurance exercise) | |
| Are able to perform leg-based resistance exercise | |
| Are free from musculoskeletal injury (past 6 months) | |
| Lived at low altitudes (<500 m) for at least 10 years | |
| Had no recent exposure to high altitude (>2,500 m) (in the last 6 months) | |
| Willing and capable of providing written informed consent and complying with the study protocol | |
| Exclusion criteria | Incapable of giving informed consent |
| Respiratory and cardiovascular diseases | |
| Liver and kidney dysfunctions | |
| Malignant tumors | |
| Coagulation disorders | |
| A history of AMS | |
| History of angioedema | |
| A history of syncope (hypoglycemia excepted) | |
| Psychiatric disorders or neuroses | |
| Ubiquinol supplementation with any side-effect in the first 14 days before departure | |
| Pregnant or nursing women | |
| A recent history (previous 1-month period) of drug use with acetaminophen, aspirin, nonsteroidal anti-inflammatory drugs | |
| A recent history (previous 1-month period) of drug use of acetazolamide, diuretics, or other drugs for the prevention or treatment of AMS | |
| A recent history (previous 1-month period) use of consuming ergogenic aids (e.g., creatine monohydrate), protein-based supplements, or nutritional supplements that may alleviate muscle damage (e.g., antioxidants, polyphenols) | |
| Other diseases that prevent the patient from going to high altitudes | |
| Involuntary participant status |
Inclusion and exclusion criteria.
The study protocol complying with the Declaration of Helsinki was approved by the Medical Ethics Committee of Xinqiao Hospital of Army Medical University (No. 2022-060-01). All participants had to provide written informed consent after the study details, procedures, benefits, and risks were explained to them verbally and literally. Failure to do so resulted in their exclusion from this study.
2.3. Sample size
The G-power tool (version 3.1.9.2, Germany, 2017) was used to calculate the sample size by prior power analysis and to estimate all statistically significant results. The α error probability was set at 0.05, and the power (1 − β) was set at 0.9. With a correlation among repeated measures of 0.5, a sample size of 39 was determined to be necessary to assess the effects of ubiquinol. A sample size of 36 within two groups (ubiquinol group and placebo group) and within-between interactions were calculated with a nonsphericity correction (ε) of 1. Considering a 5% dropout rate, based on a sample size of 39, 41 participants would be required finally.
2.4. Intervention
2.4.1. Randomization, blinding, study treatment, and follow-up of participants
Eligible subjects are randomized 1:1 into the ubiquinol group or placebo group by two physicians through a random code generator software (www.randomization.com) to receive either ubiquinol or placebo orally. These two physicians will solely operate randomization and drug distribution, and the randomization information will not be exposed to any participants or researchers, including laboratory staff, data gatherers, and statistical analysts throughout the study. Treatment allocation will be concealed in an opaque envelope opened on the day of treatment. Allocation will not be exposed until the end of the statistical data analysis or when any adverse event, such as severe diarrhea, occurs. A daily reminder email will be set up and sent to participants, and the rest of the medication will be sent back to us to assist in improving compliance. In addition, three serological tests of ubiquinol blood concentration can help monitor whether the participant is consistently taking the medication. The blinding will be maintained throughout the study. Clinical follow-up will be scheduled on days 11, 17, 28, and 6 months after the study began by performing cardiorespiratory tests or filling out self-administered questionnaires for symptoms. The primary outcome is cardiorespiratory fitness, while AMS and HADAS are the secondary endpoints. Safety endpoints include any reported adverse events (Table 3), exercise-limiting symptoms and pre-specified termination criteria (Table 4), and abnormal blood analysis results. The participants will have direct access to receive first aid from their treating team. Any adverse clinical event will be reported to the ethics committee.
Table 3
| 1. Adverse drug reaction in the digestive system |
| Upset stomach |
| Loss of appetite |
| Nausea |
| Diarrhea |
| 2. Adverse drug reaction in the cardiovascular system |
| Palpitation |
| Hypertension |
| Hypotension |
| Tachycardia |
| 3. Adverse drug reaction in the immune system |
| Allergic reactions (rashes, pruritus and hyperhidrosis) |
| Anaphylactic shock |
| 4. Adverse drug reaction in the nervous system |
| Activation insomnia |
| Dizziness |
Reported adverse events of ubiquinol (27).
Table 4
| 1. Symptoms or signs of poor breathing and circulation |
| Pale face, clammy skin |
| Cyanosis |
| Dizziness |
| Chest pain |
| Nausea |
| Severe difficulty breathing |
| Precardiac pain with ischemic ST changes greater than 2 mm |
| Severe hypertension (240/140 mmHg) |
| Systolic blood pressure drop >10 mmHg |
| 2. Occurrence of severe fatigue or severe leg pain, preventing the participants from pedaling |
| 3. Failure of instrument |
| More than three electrocardiogram patches fall off |
| An unexpected power failure |
| Bluetooth device disconnected |
| 4. Participant requests for reasons other than above |
Termination criteria of CPET.
2.5. Outcomes
The primary outcome will be the cardiorespiratory fitness characteristics both at high-altitude acclimatization and de-acclimatization after ubiquinol supplementation. Specifically, the parameters including peak oxygen consumption (VO2), % predicted peak VO2, VO2 at the anaerobic threshold (AT), peak O2 pulse, metabolic equivalents, % maximum predicted heart rate, rate pressure product, VE/VCO2 slope, oxygen uptake efficiency slope, VO2/work rate slope, cardiac output, exercise ventilatory power, circulatory power, and dead space ventilation / tidal volume at these four visits will be collected for further analysis. A brief definition of these indicators is as follows: peak VO2, peak oxygen consumption during an incremental exercise protocol with or without a plateau; % predicted peak VO2, peak VO2 divided by predicted peak VO2; VO2 at AT, the value of VO2 at AT time; peak O2 pulse, the peak oxygen extracted with each beat of the heart; metabolic equivalents, the ratio of exercise metabolic rate to resting metabolic rate calculated as VO2/3.5 ml/min/kg; % maximum predicted heart rate, heart rate divided by maximum predicted heart rate; rate pressure product, the product of heart rate and systolic blood pressure; VE/VCO2 slope, from exercise onset to peak exercise by the slope of linear regression of VE and VCO2; oxygen uptake efficiency slope, slope of the regression line of VO2 vs. log10 VE; VO2/work rate slope, ΔVO2/Δwork rate during incremental exercise; cardiac output, the amount of blood the heart pumps per minute; exercise ventilatory power, the ratio between peak SBP and the VE/VCO2 slope; circulatory power, the product of CO and mean arterial BP; dead space ventilation / tidal volume, an estimate of the ventilation-perfusion ratio. In addition, the detailed definitions and calculation methods of these indicators are shown in Table 5.
Table 5
| CPET fitness measure | Physiological significance | Measurement methodology |
|---|---|---|
| Peak oxygen consumption (VO2) | “Gold standard” assessment of cardiorespiratory fitness. | The VO2 value of plateau attained when there is no further (or relatively small) increases in VO2 despite further increases in work rate is determined as VO2max (28). Consequently, peak VO2 is often used as an estimate for VO2max. For practical purposes, VO2max and peak VO2 are used interchangeably. Symptom limitation during an incremental exercise protocol could be taken to reflect the maximal VO2 value even though a plateau in VO2 fails to appear (29). |
| % predicted peak VO2 | Comparison of peak VO2 achieved with predicted value for age, sex, and body size. | Using the Wasserman and Hansen formula (30). |
| VO2 at the anaerobic threshold (AT) | Anaerobic threshold refers to the critical point at which muscle metabolism transitions from aerobic to anaerobic metabolism in an incremental exercise test. | Measurement of VO2 and VCO2, with AT estimated as the breakpoint in the VCO2-VO2 relationship using the V-slope method (31). |
| Peak O2 pulse | Reflects stroke volume and peripheral O2 extraction. Indicates the peak capacity for oxygen extraction on each heart beat. | Peak VO2/peak heart rate. |
| Metabolic equivalents (METS) | The ratio of exercise metabolic rate to resting metabolic rate. Expresses the relative energy metabolism level of various activities based on energy consumption in quiet and sitting positions (32). 1 Met is defined as the consumption of 3.5 ml of oxygen per kilogram of body weight per minute (33). | VO2/3.5 ml/min/kg. |
| % maximum predicted heart rate | Heart rate response to exercise. | Peak heart rate as a percentage of the predicted peak heart rate calculated using the Tanaka formula (peak heart rate = 208–0.7*age) (34). |
| Rate pressure product (RPP) | Represents the relative level of myocardial oxygen consumption. | The product of heart rate and systolic blood pressure. |
| VE/VCO2 slope | Determined by the dead space fraction of the breath (VD/VT) and arterial PCO2. Typically taken to reflect the degree of match between alveolar ventilation and pulmonary perfusion. | Slope of the regression line of VE vs. VCO2 (35). |
| Oxygen uptake efficiency slope (OUES) | Reflects VO2 kinetics. | Slope of the regression line of VO2 vs. log10 VE (35). |
| VO2/work rate slope | Reflects the metabolic cost of performing external work. | Slope of the regression line of VO2 vs. work rate. |
| Cardiac output (CO) | Measures the strength of cardiac ejection | Using the equilibrium CO2 re-breathing technique of Collier (36). |
| Exercise ventilatory power (EVP) | An integrated index to predict risk representing synergistically linked assessment of ventilatory efficiency and hemodynamics, peak SBP reflecting systemic hemodynamics (37), and VE/VCO2 slope reflecting the complex interplay of peripheral and pulmonary abnormalities. | The ratio between peak SBP and the VE/VCO2 slope (38). |
| Circulatory Power (CP) | An index of cardiac systolic function (39). | The product of CO and mean arterial BP (40). |
| Dead space volume/tidal volume (VD/VT) | An estimate of the ventilation-perfusion ratio representing the efficiency of alveolar ventilation and blood flow matching. The physiological dead space is normally one-third of the tidal volume at rest (36). | [(PaCO2 − PECO2)/PaCO2] − (VDapp/tidal volume) where PaCO2 is the arterial CO2 pressure, PECO2 is the partial pressure of CO2 in mixed expired air, and VDapp is the apparatus dead space. PaCO2 can be estimated noninvasively using PETCO2 (41). |
The measurement approach for each CPET variable.
The secondary outcomes will be the questionnaire results of AMS and HADAS. The symptoms of these two diseases are shown in a later section. The effects of altitude and ubiquinol on them will be analyzed in detail in this study. Participants with severe disease symptoms, especially hypoxia-related symptoms, will receive additional treatment.
2.6. Study design
Figure 1 shows the flow chart of this study. Participants will take ubiquinol orally with a dose of 200 mg daily or a placebo for 14 days before departure. Physical examination, symptoms related to high-altitude acclimatization and de-acclimatization, cardiorespiratory exercise test, laboratory test, and any possible adverse reaction of ubiquinol and exercise will be collected at baseline detection and each visit. During the 14 days of the medication, only participants who did not experience these adverse effects would go to the high altitude as scheduled. It would help us identify related symptoms at high altitudes due to hypoxia rather than adverse events of ubiquinol. The laboratory and exercise tests will be conducted separately on the last three days of the 14 days. Subsequently, they will board a flight to Shigatse (high altitude area, 3,900 m) in 3 h. After arriving at Shigatse, the AMS symptom self-administered questionnaires and adverse reactions of ubiquinol will be followed up daily, and the other prior programs will be carried out on the third day. The subjects will then be flown back to a low altitude after staying for 7 days at a high altitude and scored for HADAS, followed by other prior programs at a low altitude on the 7th day after return. The last visit will be set in the sixth month of this study, which has not yet been conducted. Laboratory tests include a blood routine test of 19 parameters commonly used in the clinic and a serological test of 19 circulating indicators, including glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, lactate, non-esterified fatty acid, norepinephrine, epinephrine, plasma renin activity, angiotensin Ⅱ, angiotensin-converting enzyme 2, neuropeptide Y, atrial natriuretic peptide, lactate dehydrogenase, insulin, glucocorticoid, adiponectin, and leptin.
Figure 1
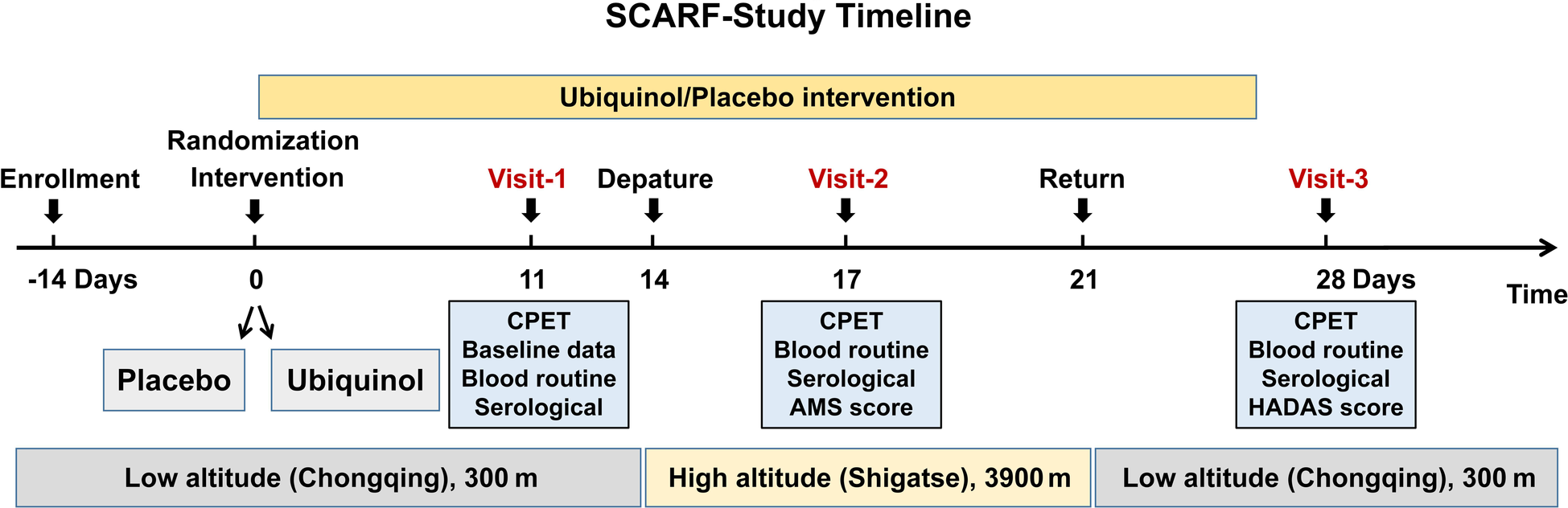
The flow chart of this study.
2.7. Laboratory tests
Participants will be requested to avoid eating or drinking anything apart from water for up to 12 h. Participants' blood samples will be collected between 8:00 and 8:15 am the day before the exercise tests at different altitudes using the same procedure. After sitting quietly for 15 min, approximately 5 ml of intravenous blood of participants will be collected from the vein at the cubital fossa in the elbow with a tourniquet and mixed immediately with 1 ml of the anticoagulant, dipotassium ethylenediaminetetraacetic acid. Blood samples will be divided into 1 ml and 4 ml parts. The first 1 ml will be analyzed using a BC-3000 plus automated hematology corpuscle analyzer (Shenzhen, China), and the 4 ml will be subjected to high sensitivity enzyme-linked immunosorbent assay kit (Jiangsu Jingmei Biological Technology Co., Ltd., China) for the 19 kinds of circulating parameters. Blood collected at high altitude will be frozen in a −20°C transfer tank and flown to Xinqiao Hospital within 3 h. All blood samples will be measured for biochemical parameters at the Clinical Laboratory of Cardiology Science of Xinqiao Hospital.
2.8. The Lake Louise consensus scoring system and high-altitude de-acclimatization syndrome score
The Lake Louise consensus scoring system 2018 version will be used to assess the presence of AMS at high altitudes according to the four main symptoms: headache, gastrointestinal symptoms, fatigue/weakness, and dizziness/vertigo. Scores of 0, 1, 2, and 3 correspond to none, mild, moderate, and severe degrees for each symptom, respectively. AMS is diagnosed as a total score of ≥3, combined with headache (1). We will collect this information every day through self-administered questionnaires at high altitudes.
The HADAS will be evaluated according to the scoring criteria for syndromes, which include complaining of dizziness, fatigue, weakness, headache, blurred vision, lethargy, insomnia, dreaminess, difficulty concentrating, memory loss, slow reaction, and physical signs, including chest tightness, cyanosis, palpitation, slow pulse, precordial pain, changes in blood pressure, decreased appetite, changes in body mass, abdominal distension, diarrhea, edema, constipation, cough, asthma, hair loss, sexual dysfunction, tooth loss, numbness of hands and feet, and skin hemorrhagic spots or ecchymosis (2). In addition, these symptoms do not improve under short-term rehabilitation after organic diseases of the heart, lungs, kidneys, and other organs are ruled out. Physicians who have been systematically trained will score the presence of three or more of the above symptoms or signs according to the following criteria: (1) have a slight reaction but not affect daily work for 0 points; (2) affect work but the symptoms improve soon after rest for score 1; (3) seriously affect daily work and symptoms improve significantly after drug treatment for score 2; (4) seriously affect daily work and symptoms do not improve significantly after drug treatment for score 3.
2.9. Respiratory function assessment
Participants will be notified in advance to avoid smoking and consuming caffeinated or heavy meals for at least 2 h before the tests. It will be ensured that the indoor environment is suitable for the entire measurement process [in a dedicated temperature-controlled (20–25°C) laboratory]. Lung function will be assessed using the spiroergometry system with Breath-by-Breath technology (Metalyzer 3B, Cortex, Germany, 2022). Standardized assessments of resting lung function will be performed before respiratory tests during exercises. The best attained values of three attempts of forced expiratory volume in one second (FEV1) and forced vital capacity will be recorded. Before each test, the volume sensor will be calibrated using a standard three-liter syringe, and the gas analyzer will be adjusted with two-point calibration using air and a 16.5% O2:CO2 mixture. Participants will be provided with a fitting face mask, and volumetric and gas-exchange data will be directly transferred to the metabolic cart software (MetaSoft® Studio, Cortex Biophysik GmbH, 2022), with real-time viewing capacity. Maximum voluntary ventilation (MVV) will be measured from a 12-s maneuver of deep and rapid breathing as the corresponding minute volume. Standards for the performance and interpretation of spirometry data will be obtained from the European Respiratory Society and the American Thoracic Society (42).
2.10. Cardiopulmonary exercise test
Before CPET, standardized assessments of resting lung function will be performed. Whole tests will be conducted in a dedicated temperature-controlled (20–25°C) laboratory by a physician and a skilled technician, who is blinded to other procedures and details of this study. The baseline physiological measures for all devices used in this study will be measured for 5 min in a resting state and subsequently in a standing position. CPET will be performed in an erect position through an electronically braked cycle ergometer (EC3000e, Customed, Germany) with Breath-by-Breath technology (Metalyzer 3B, Cortex, Germany, 2022). The cycle ergometry exercise protocol comprises four stages of exercise: 3 min of rest, 3 min of unloaded exercise, a continual increase in resistance of 25 W/min, and recovery with 3 min of unloaded cycling. All patients will continue CPET until the development of limiting symptoms such as angina, dyspnea, or muscular fatigue. CPET will be terminated at the discretion of the operating physician if pre-specified termination criteria develop, such as hemodynamic abnormalities, heart rhythm abnormalities, or neurological impairment.
The AT time is estimated from breath-by-breath data using the V-slope method and validated with other plots using the ventilatory-equivalent method and the respiratory exchange ratio method (31, 43). The parameters for AT will be obtained at this time point. Other peak values will be calculated at identical time points. The calculation method for each indicator detected using the metabolic cart software (MetaSoft® Studio, Cortex Biophysik GmbH, 2022) is described in detail in Table 5.
Standard 12-lead electrocardiogram, blood pressure, and SpO2 will be obtained throughout the procedure using a 12-lead connection (Custo-Cardio 3000BT-A, Cortex, Germany, 2022) in real-time, blood pressure cuffs (Suntech Tango M2, Cortex, Germany) in the upper arm and a portable finger clip oximeter (Nonin wristOx2 3150, Nonin, United States), respectively. All these parameters of CPET are the direct output from the cardiopulmonary exercise testing system.
2.11. Data collection, management, and analysis
2.11.1. Data collection and management
The data collection tools of this study include oral inquiry, questionnaire, laboratory examination report, and export of cardiopulmonary exercise test report. We have collected relevant information about the participants, including socio-demographic characteristics such as age, sex, alcohol use, tobacco use, medical history, nature and duration of illness, etc. The data, including all primary and secondary outcomes collected at Chongqing Xinqiao Hospital and Shigatse Branch Hospital, will be organized in an Excel spreadsheet in the proper format by a trained research assistant. All the data collected will be stored in Human Plateau Resources Biobank at Chongqing Xinqiao Hospital after being checked by the principal investigator and may only be consulted with the permission of the principal investigator. All personal data will be legitimately stored in encrypted folders and kept unavailable to any third party without the consent of the participants in advance. The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
2.11.2. Statistical considerations
Continuous variables will be expressed as means ± standard deviations (SD) or as median (interquartile spacing), and categorical variables as n (%) according to the Shapiro–Wilk test. Generalized estimating equations (GEE) with an exchangeable working correlation will be employed. Treatment, altitude, and the treatment multiplied by altitude interaction will be considered independent variables under mixed models. At each altitude, a comparative analysis will be carried out between two groups (ubiquinol group and placebo group), and the least significant difference method will be performed to adjust within-altitude differences for multiple comparisons. Correlation analyses will be performed using Pearson correlation for continuous variables, and Spearman Rank tests will be used between categorical variables. In addition, subgroups will be defined by age for analysis since it is known that ubiquinol levels decrease with age (23). Two-sided tests with p-values less than 0.05 will be considered statistically significant. IBM SPSS Statistics software for Windows (version 26, IBM, Armonk, NY, United States) will be used for statistical analyses.
3. Discussion
To the best of our knowledge, this study is the first to evaluate cardiorespiratory fitness prospectively and accurately during the high-altitude acclimatization and de-acclimatization process. Moreover, it is a double-blinded, placebo-controlled trial to explore the effects of ubiquinol on cardiorespiratory fitness during acclimatization and de-acclimatization. The clinical trial design depicts cardiorespiratory fitness characteristics at high-altitude acclimatization and de-acclimatization and is designed to reveal cardiovascular and respiratory function restoration during de-acclimatization. In addition, we determine whether ubiquinol supplementation is beneficial for endurance exercise capacity at a high altitude and helps with better adaptation to acute hypoxia exposure. Furthermore, we explore the relationship between the circulating substances and physical performance capacity at high altitudes to be able to account for the possible mechanisms.
The exploration of medicines for high-altitude acclimatization has been underway for decades. A few drugs, such as acetazolamide and Rhodiola rosea, have been recommended to promote acclimatization (16, 44, 45). However, limited research focused on the process of de-acclimatization and related symptoms. Thus, in this study, we recruited healthy Han Chinese from Chongqing to assess the possible effect of ubiquinol on cardiorespiratory fitness, AMS, and HADAS symptoms. A previous trial has reported that CoQ10 supplementation with a dose of 90 mg for 6 weeks orally could improve VO2max, aerobic, and anaerobic thresholds, indicating the increase of cardiorespiratory fitness of endurance athletes (21). In addition, studies demonstrated that CoQ10 deficiency was positively associated with fatigue, whereas serum CoQ10 levels were inversely correlated with fatigue severity (46, 47). In addition, another meta-regression also confirmed that a daily dose of CoQ10 was associated with fatigue reduction (48). Therefore, we suppose that ubiquinol supplementation at high altitudes may alleviate AMS and HADAS-related symptoms and maintain cardiorespiratory fitness.
Cardiorespiratory fitness reflects the comprehensive ability of the body to transport and utilize oxygen and embodies the overall function of the respiratory and cardiovascular systems (49, 50). VO2max is the gold standard for assessing cardiorespiratory fitness and an independent predictor of cardiovascular events and mortality (51–53). Previous studies have indicated that VO2max decreases linearly by approximately 8% for every 1,000 m of additional elevation above 700 m, while it descends in an accelerated curve over 6,300 m, suggesting that cardiorespiratory function is impaired at high altitudes (54). However, there is limited information on cardiorespiratory fitness changes during de-acclimatization from a high altitude in healthy individuals, and the underlying mechanisms remain to be elucidated. Thus, in this study, we try to illuminate the possible mechanism by circulating markers of metabolism and the autonomic nervous system (55).
This study has some limitations. First, although this is not a small clinical trial in the CPET trial at a high altitude, this study is limited by a small sample size (n = 41) and short follow-up (on days 17 and 28, respectively). Therefore, it is troublesome to implement hierarchical analysis, and these preliminary results need to be confirmed in a large cohort. Second, long-term follow-up may be more meaningful in evaluating ubiquinol supplements on cardiorespiratory fitness.
4. Conclusions
This SCARF study will attempt to unravel the mystery of cardiorespiratory fitness during high-altitude acclimatization and de-acclimatization and reveal whether ubiquinol supplementation could be beneficial for physical performance capacity at different altitudes. These preliminary results will provide novel insight into cardiorespiratory fitness and a new pharmacological intervention strategy for a high-altitude exposure population of more than 100 million every year.
Statements
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Xinqiao Hospital of Army Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LH conceived and supervised this study. JY, XY, MS, SY, ZL, HL, and BW followed all subjects both at low and high altitudes, collected the exercise data, and recorded the physiological parameters. ZL, HL, and CZ guided subjects in performing the exercise tests both at low and high altitudes. XY, MS, JH, WG, and XW analyzed the data with guidance from YS and JY. The manuscript was written by JY, MS, and XY. LH reviewed and revised this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Research Project of PLA, National Natural Science Foundation of China (grant no. 81730054), and Chongqing Talents: Exceptional Young Talents Project, Program for Excellent Talents in Army Medical University (2019R007, 2022XKRC008).
Acknowledgments
The authors appreciate the participants and all the researchers for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1129144/full#supplementary-material
References
1.
Roach RC Hackett PH Oelz O Bärtsch P Luks AM MacInnis MJ et al The 2018 lake louise acute mountain sickness score. High Alt Med Biol. (2018) 19(1):4–6. 10.1089/ham.2017.0164
2.
He B Wang J Qian G Hu M Qu X Wei Z et al Analysis of high-altitude de-acclimatization syndrome after exposure to high altitudes: a cluster-randomized controlled trial. PLoS One. (2013) 8(5):e62072. 10.1371/journal.pone.0062072
3.
Mollard P Woorons X Letournel M Lamberto C Favret F Pichon A et al Determinants of maximal oxygen uptake in moderate acute hypoxia in endurance athletes. Eur J Appl Physiol. (2007) 100(6):663–73. 10.1007/s00421-007-0457-0
4.
Naeije R Huez S Lamotte M Retailleau K Neupane S Abramowicz D et al Pulmonary artery pressure limits exercise capacity at high altitude. Eur Respir J. (2010) 36(5):1049–55. 10.1183/09031936.00024410
5.
Karinen HM Peltonen JE Kähönen M Tikkanen HO . Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt Med Biol. (2010) 11(4):325–32. 10.1089/ham.2009.1060
6.
Seiler T Nakas CT Brill AK Hefti U Hilty MP Perret-Hoigné E et al Do cardiopulmonary exercise tests predict summit success and acute mountain sickness? A prospective observational field study at extreme altitude. Br J Sports Med. (2023):1–9. 10.1136/bjsports-2022-106211
7.
Naeije R . Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis. (2010) 52(6):456–66. 10.1016/j.pcad.2010.03.004
8.
Chen X Liu B Deng Y Yang F Wang W Lin X et al Cardiac adaptation to prolonged high altitude migration assessed by speckle tracking echocardiography. Front Cardiovasc Med. (2022) 9:856749. 10.3389/fcvm.2022.856749
9.
Bian SZ Zhang C Rao RS Ding XH Huang L . Systemic blood predictors of elevated pulmonary artery pressure assessed by non-invasive echocardiography after acute exposure to high altitude: a prospective cohort study. Front Cardiovasc Med. (2022) 9:866093. 10.3389/fcvm.2022.866093
10.
Brawner CA Ehrman JK Bole S Kerrigan DJ Parikh SS Lewis BK et al Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. (2021) 96(1):32–9. 10.1016/j.mayocp.2020.10.003
11.
Raghuveer G Hartz J Lubans DR Takken T Wiltz JL Mietus-Snyder M et al Cardiorespiratory fitness in youth: an important marker of health: a scientific statement from the American heart association. Circulation. (2020) 142(7):e101–18. 10.1161/cir.0000000000000866
12.
Kokkinos P Myers J Franklin B Narayan P Lavie CJ Faselis C . Cardiorespiratory fitness and health outcomes: a call to standardize fitness categories. Mayo Clin Proc. (2018) 93(3):333–6. 10.1016/j.mayocp.2017.10.011
13.
Schwartz BG Levine LA Comstock G Stecher VJ Kloner RA . Cardiac uses of phosphodiesterase-5 inhibitors. J Am Coll Cardiol. (2012) 59(1):9–15. 10.1016/j.jacc.2011.07.051
14.
Collier DJ Wolff CB Hedges AM Nathan J Flower RJ Milledge JS et al Benzolamide improves oxygenation and reduces acute mountain sickness during a high-altitude trek and has fewer side effects than Acetazolamide at sea level. Pharmacol Res Perspect. (2016) 4(3):e00203. 10.1002/prp2.203
15.
Xu Y Liu Y Liu J Qian G . Meta-analysis of clinical efficacy of sildenafil, a phosphodiesterase type-5 inhibitor on high altitude hypoxia and its complications. High Alt Med Biol. (2014) 15(1):46–51. 10.1089/ham.2013.1110
16.
Ritchie ND Baggott AV Andrew Todd WT . Acetazolamide For the prevention of acute mountain sickness–a systematic review and meta-analysis. J Travel Med. (2012) 19(5):298–307. 10.1111/j.1708-8305.2012.00629.x
17.
He B Hu M Liang Z Ma Q Zi Y Dong Z et al Efficacy of shenqi pollen capsules for high-altitude deacclimatization syndrome via suppression of the reoxygenation injury and inflammatory response. J Immunol Res. (2019) 2019:4521231. 10.1155/2019/4521231
18.
Dai S Tian Z Zhao D Liang Y Liu M Liu Z et al Effects of coenzyme Q10 supplementation on biomarkers of oxidative stress in adults: a GRADE-assessed systematic review and updated meta-analysis of randomized controlled trials. Antioxidants (Basel). (2022) 11(7):1360. 10.3390/antiox11071360
19.
Sarmiento A Diaz-Castro J Pulido-Moran M Kajarabille N Guisado R Ochoa JJ . Coenzyme Q10 supplementation and exercise in healthy humans: a systematic review. Curr Drug Metab. (2016) 17(4):345–58. 10.2174/1389200216666151103115654
20.
Campisi L La Motta C . The use of the coenzyme Q(10) as a food supplement in the management of fibromyalgia: a critical review. Antioxidants (Basel). (2022) 11(10):1969. 10.3390/antiox11101969
21.
Ylikoski T Piirainen J Hanninen O Penttinen J . The effect of coenzyme Q10 on the exercise performance of cross-country skiers. Mol Aspects Med. (1997) 18(Suppl 1):S283–90. 10.1016/s0098-2997(97)00038-1
22.
Mizuno K Tanaka M Nozaki S Mizuma H Ataka S Tahara T et al Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. (2008) 24(4):293–9. 10.1016/j.nut.2007.12.007
23.
Marcheggiani F Kordes S Cirilli I Orlando P Silvestri S Vogelsang A et al Anti-ageing effects of ubiquinone and ubiquinol in a senescence model of human dermal fibroblasts. Free Radic Biol Med. (2021) 165:282–8. 10.1016/j.freeradbiomed.2021.01.032
24.
Chan AW Tetzlaff JM Gøtzsche PC Altman DG Mann H Berlin JA et al SPIRIT 2013 Explanation and elaboration: guidance for protocols of clinical trials. Br Med J. (2013) 346:e7586. 10.1136/bmj.e7586
25.
Yeung CK Billings FT Claessens AJ Roshanravan B Linke L Sundell MB et al Coenzyme Q10 dose-escalation study in hemodialysis patients: safety, tolerability, and effect on oxidative stress. BMC Nephrol. (2015) 16:183. 10.1186/s12882-015-0178-2
26.
Bhagavan HN Chopra RK . Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. (2007) 7(Suppl):S78–88. 10.1016/j.mito.2007.03.003
27.
Raizner AE Quiñones MA . Coenzyme Q(10) for patients with cardiovascular disease: jACC focus seminar. J Am Coll Cardiol. (2021) 77(5):609–19. 10.1016/j.jacc.2020.12.009
28.
American Thoracic Society. Board of directors, and American College of chest physicians (ACCP) health science policy Committee. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. (2003) 167(2):211–77. 10.1164/rccm.167.2.211
29.
Radtke T Crook S Kaltsakas G Louvaris Z Berton D Urquhart DS et al ERS Statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev. (2019) 28(154):180101. 10.1183/16000617.0101-2018
30.
Hansen JE Sue DY Wasserman K . Predicted values for clinical exercise testing. Am Rev Respir Dis. (1984) 129(2 Pt 2):S49–55. 10.1164/arrd.1984.129.2P2.S49
31.
Beaver WL Wasserman K Whipp BJ . A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. (1986) 60(6):2020–7. 10.1152/jappl.1986.60.6.2020
32.
Ainsworth BE Haskell WL Herrmann SD Meckes N Bassett DR Jr. Tudor-Locke C et al 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. (2011) 43(8):1575–81. 10.1249/MSS.0b013e31821ece12
33.
Fletcher GF Ades PA Kligfield P Arena R Balady GJ Bittner VA et al Exercise standards for testing and training: a scientific statement from the American heart association. Circulation. (2013) 128(8):873–934. 10.1161/CIR.0b013e31829b5b44
34.
Tanaka H Monahan KD Seals DR . Age-predicted maximal heart rate revisited. J Am Coll Cardiol. (2001) 37(1):153–6. 10.1016/s0735-1097(00)01054-8
35.
Borghi-Silva A Labate V Arena R Bandera F Generati G Pellegrino M et al Exercise ventilatory power in heart failure patients: functional phenotypes definition by combining cardiopulmonary exercise testing with stress echocardiography. Int J Cardiol. (2014) 176(3):1348–9. 10.1016/j.ijcard.2014.07.268
36.
Balady GJ Arena R Sietsema K Myers J Coke L Fletcher GF et al Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. (2010) 122(2):191–225. 10.1161/CIR.0b013e3181e52e69
37.
Forman DE Guazzi M Myers J Chase P Bensimhon D Cahalin LP et al Ventilatory power: a novel index that enhances prognostic assessment of patients with heart failure. Circ Heart Fail. (2012) 5(5):621–6. 10.1161/circheartfailure.112.968529
38.
Guazzi M Arena R Halle M Piepoli MF Myers J Lavie CJ . 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. (2016) 133(24):e694–711. 10.1161/cir.0000000000000406
39.
Cohen-Solal A Tabet JY Logeart D Bourgoin P Tokmakova M Dahan M . A non-invasively determined surrogate of cardiac power (‘circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J. (2002) 23(10):806–14. 10.1053/euhj.2001.2966
40.
Williams SG Cooke GA Wright DJ Parsons WJ Riley RL Marshall P et al Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur Heart J. (2001) 22(16):1496–503. 10.1053/euhj.2000.2547
41.
Jones NL Robertson DG Kane JW . Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol Respir Environ Exerc Physiol. (1979) 47(5):954–60. 10.1152/jappl.1979.47.5.954
42.
Crapo RO Casaburi R Coates AL Enright PL Hankinson JL Irvin CG et al Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American thoracic society was adopted by the ATS board of directors, July 1999. Am J Respir Crit Care Med. (2000) 161(1):309–29. 10.1164/ajrccm.161.1.ats11-99
43.
Ganesananthan S Rajkumar CA Foley M Thompson D Nowbar AN Seligman H et al Cardiopulmonary exercise testing and efficacy of percutaneous coronary intervention: a substudy of the ORBITA trial. Eur Heart J. (2022) 43(33):3132–45. 10.1093/eurheartj/ehac260
44.
Imray C . Acetazolamide For the prophylaxis of acute mountain sickness. Br Med J. (2012) 345:e7077. 10.1136/bmj.e7077
45.
Molano Franco D Nieto Estrada VH Gonzalez Garay AG Martí-Carvajal AJ Arevalo-Rodriguez I . Interventions for preventing high altitude illness: part 3. Miscellaneous and non-pharmacological interventions. Cochrane Database Syst Rev. (2019) 4(4):Cd013315. 10.1002/14651858.Cd013315
46.
Maes M Mihaylova I Kubera M Uytterhoeven M Vrydags N Bosmans E . Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. (2009) 30(4):470–6.
47.
Maes M Mihaylova I Kubera M Uytterhoeven M Vrydags N Bosmans E . Lower plasma coenzyme Q10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuro Endocrinol Lett. (2009) 30(4):462–9.
48.
Tsai IC Hsu CW Chang CH Tseng PT Chang KV . Effectiveness of coenzyme Q10 supplementation for reducing fatigue: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. (2022) 13:883251. 10.3389/fphar.2022.883251
49.
Sovová M Sovová E Asswad AG Sova M . Is population’s cardiorespiratory fitness really declining?Cent Eur J Public Health. (2020) 28(2):120–3. 10.21101/cejph.a5912
50.
Shigdel R Stubbs B Sui X Ernstsen L . Cross-sectional and longitudinal association of non-exercise estimated cardiorespiratory fitness with depression and anxiety in the general population: the HUNT study. J Affect Disord. (2019) 252:122–9. 10.1016/j.jad.2019.04.016
51.
Khan H Jaffar N Rauramaa R Kurl S Savonen K Laukkanen JA . Cardiorespiratory fitness and nonfatalcardiovascular events: a population-based follow-up study. Am Heart J. (2017) 184:55–61. 10.1016/j.ahj.2016.10.019
52.
Davidson T Vainshelboim B Kokkinos P Myers J Ross R . Cardiorespiratory fitness versus physical activity as predictors of all-cause mortality in men. Am Heart J. (2018) 196:156–62. 10.1016/j.ahj.2017.08.022
53.
Nauman J Stensvold D Coombes JS Wisløff U . Cardiorespiratory fitness, sedentary time, and cardiovascular risk factor clustering. Med Sci Sports Exerc. (2016) 48(4):625–32. 10.1249/mss.0000000000000819
54.
Wehrlin JP Hallén J . Linear decrease in.VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol. (2006) 96(4):404–12. 10.1007/s00421-005-0081-9
55.
Hammett CJ Prapavessis H Baldi JC Varo N Schoenbeck U Ameratunga R et al Effects of exercise training on 5 inflammatory markers associated with cardiovascular risk. Am Heart J. (2006) 151(2):367.e7–16. 10.1016/j.ahj.2005.08.009
Summary
Keywords
cardiorespiratory fitness, study design, cardiopulmonary exercise test, clinical trial, ubiquinol, high altitude
Citation
Yang J, Ye X, Liu Z, Sun M, Yu S, Lv H, Wu B, Zhang C, Gu W, He J, Wang X and Huang L (2023) Effect of ubiquinol on cardiorespiratory fitness during high-altitude acclimatization and de-acclimatization in healthy adults: the Shigatse CARdiorespiratory fitness study design. Front. Cardiovasc. Med. 10:1129144. doi: 10.3389/fcvm.2023.1129144
Received
21 December 2022
Accepted
10 July 2023
Published
25 July 2023
Volume
10 - 2023
Edited by
Mieczyslaw Pokorski, Opole University, Poland
Reviewed by
Ricardo Jorge Gelpi, University of Buenos Aires, Argentina Susan Ward, Human Bio-energetics Research Centre, United Kingdom Volodymyr Portnichenko, National Academy of Sciences of Ukraine, Ukraine
Updates
Copyright
© 2023 Yang, Ye, Liu, Sun, Yu, Lv, Wu, Zhang, Gu, He, Wang and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Lan Huang huanglan260@126.com
†These authors have contributed equally to this work
ORCID Lan Huang orcid.org/0000-0001-6200-2309
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.