Abstract
Aims:
Few studies on early recurrence (ER) focused on patients with persistent atrial fibrillation (AF). We aimed to investigate the characteristics and clinical significance of ER in patients with persistent AF after catheter ablation (CA).
Methods:
A total of 348 consecutive patients who underwent first-time CA for persistent and long-standing persistent AF between January 2019 and May 2022 were investigated.
Results:
About 5/348 (1.44%) patients who failed to convert to sinus rhythm after CA were excluded. A total of 110/343 (32.1%) patients had ER, in which 98 (89.1%) were persistent and 50.9% occurred in the first 24 h after CA. Compared with the patients without ER, those with ER were more likely to have late recurrence (LR) (92.7% vs. 1.7%, P < 0.001) during a median follow-up of 13 (IQR 6–23) months. ER was the most significant independent predictor for LR (OR 120.5, 95% CI 41.5–349.8, P < 0.001). ER as atrial flutter (AFL) had a lower risk of LR when compared with ER as AF (P = 0.011) and both AF and AFL (P = 0.003). Early intervention of the patient with ER improved the short-term outcomes (P < 0.001), not long-term outcomes. Only 22/251 (8.76%) patients of LR appears among those who had no recurrence in the first month.
Conclusions:
Patients with persistent AF may not have a blanking period but rather have a risk period. Clinical significance of the blanking period should be given differential treatment between paroxysmal AF and persistent AF.
Introduction
Catheter ablation (CA) is used to treat symptomatic atrial fibrillation (AF), but recurrences are common after initially successful CA (1). Early recurrence (ER) occurs during the first 3 months after CA, the so-called “blanking period,” is estimated to be as high as 50% (2–4), and is not considered to be a failure of AF ablation (5, 6) also not suggesting reintervention.
However, patients with ER are at higher risk to develop late recurrence (LR) (3, 5, 7). ER is more common in the patients with persistent AF undergoing CA than in those with paroxysmal AF (5, 6). Previous studies on ER in AF mostly focused on patients with paroxysmal AF (8). The differences in ER between patients with persistent AF and those with paroxysmal AF have not been sufficiently identified. The current study sought to characterize ER in patients with persistent AF after CA during the blanking period and to determine its prognostic significance.
Methods
Study population
A total of 348 patients undergoing the first CA for drug-refractory persistent or long-standing persistent AF in the Yantai Yuhuangding Hospital from January 2019 to May 2022 were enrolled. The type of AF was defined according to generally accepted guidelines (1). All patients provided written informed consent for the ablation procedure and the use of their clinical data for this retrospective study. This study was approved by the Ethics Committee of our institution.
Ablation procedure
Details of the periprocedural management and catheter placement has been published previously (9). All procedures were guided by the CARTO 3 (Biosense Webster) electroanatomic mapping system, and ablation was performed using open irrigated catheters with contact force (CF) sensing (Thermocool Smart Touch, Biosense Webster). Ablation index (AI) was introduced as a novel algorithm of ablation lesion quality evaluation based on parameters that were formed by contact force-time power measured by the CF-sensing catheter. The AI could reliably predict the degree of necrosis in RF delivery. All patients underwent extensive encircling pulmonary vein isolation (PVI). Other additional substrate ablations [such as linear ablation, low-voltage zone ablation, or complex fractionated atrial electrogram (CFAE) ablation] (Figure 1A) depended on the operator's judgment. The endpoint of the linear ablation was a complete, bidirectional block across the linear lesion. If sinus rhythm (SR) was not transformed after ablation procedures, it was restored by Ibutilide. If it still failed, cardioversion was performed. All patients eventually returned to SR and were observed for 30 min without recurrence of arrhythmia. We selected previous studies about ER of AF as the control to research the difference in the characteristics and clinical significances of ER between persistent AF and paroxysmal AF.
Figure 1
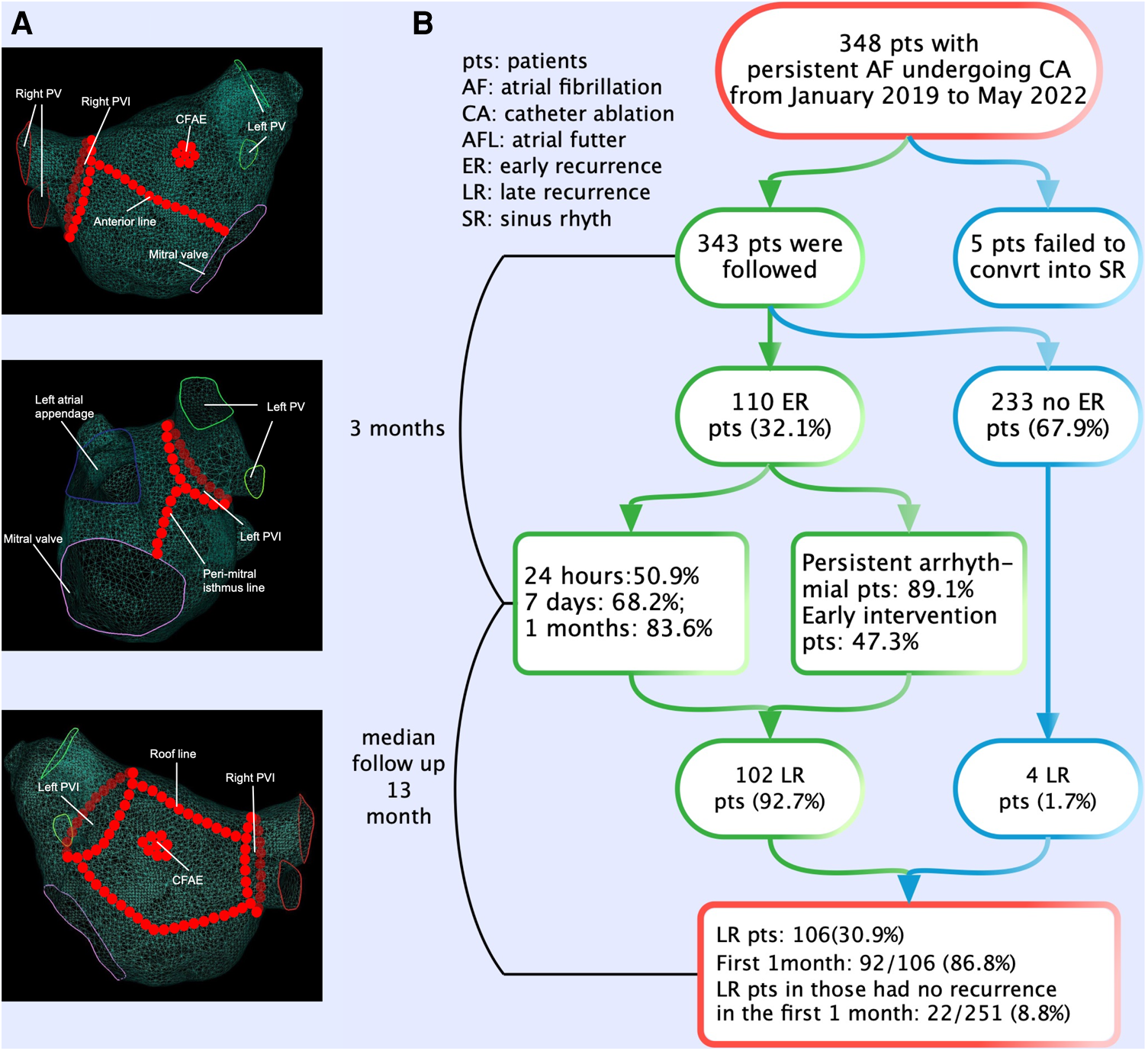
The ablation strategies and flowchart of the present study. (A) All patients underwent extensive encircling pulmonary vein isolation. Additional substrate ablations (such as linear ablation, low-voltage zone ablation, or complex fractionated atrial electrogram ablation) depended on the operator's judgment. (B) The flowchart of the study. A total of 348 patients with persistent AF underwent CA. Patients experiencing ER have a significantly higher risk (92.7%) of LR compared with those without ER. The majority (50.9%) of ER first occurred in the first 24 h after CA. ER was predominantly present as persistent. AF, atrial fibrillation; CA, catheter ablation; ER, early recurrence; LR, late recurrence.
Follow-up
Patients underwent continuous inpatient electrocardiogram (ECG) monitoring during the first 48 h after CA. After discharge, oral anticoagulation (OA) was continued for at least 3 months after CA. Further use of OA was determined according to the ESC guidelines (1). Regardless of the presence or absence of ER, antiarrhythmic drugs were discontinued 3 months after CA. Follow-up was scheduled 1, 2, 3, 6, and 12 months after CA in our outpatient clinic and comprised clinical assessment. At all follow-up visits, 12-lead ECG were obtained and 24-h Holter monitoring was performed at 3-month intervals during the follow-up period. Patients were strongly encouraged to obtain an ECG and advised to carry a portable event recorder if any arrhythmic symptom occurred.
ER and LR were defined as any atrial tachyarrhythmia recurrence occurring ≥30 s after CA ≤90 and >90 days after CA, respectively. Recurrence of arrhythmia consisted of both AF and atrial flutter (AFL). The manifestation of ER and LR can be divided into paroxysmal and persistent according to whether it can transform into SR within 7 days after recurrence. ER events were then categorized into the following time periods: (i) very early ER (first 24 h after CA), (ii) early ER (first 24 h–1 week after CA), (iii) intermediate ER (1 week–1 month after CA); and (iv) late ER (1–3 months after CA).
Intervention for ER
For patients with paroxysmal ER, intervention is not carried out, and for patients with persistent ER, early intervention (cardioversion and/or in combination with anti-arrhythmic drugs (AADs)) was performed voluntarily during the blanking period. If there is failure intervention or recurrence of persistent arrhythmia, intervention is given again, but no more than three times in all.
Statistical analysis
Continuous variables are expressed as median and interquartile range (25th and 75th percentile; in case of non-normal distribution) and compared by Mann–Whitney tests. Categorical variables are presented as frequencies or percentages and compared by χ2 or Fisher’s exact tests. Time to first LR was plotted using the Kaplan–Meier survival analysis and compared by the log-rank test. Factors with a P-value <0.05 on univariate analysis were considered in the multivariate model. Cox-regression analysis was performed to analyze the correlation between timing of first ER and risk of LR. Statistical tests and confidence intervals (CIs) with two-sided P < 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS version 25.0 (IBM Inc., Armonk, NY, United States).
Results
Characteristics of patients
The flowchart of the present study is shown in Figure 1B. Out of 348 patients undergoing first ablation for persistent and long-standing persistent AF, 5 patients who failed to convert to SR were excluded after CA. A total of 343 patients (mean age 62.8 ± 9.0 years, male 63.3%) were included and followed up finally for a median of 13 months (inter-quartile range (IQR) 6–23 months) after CA. Long-standing persistent AF was noted in 237 (69.1%) patients. During the blanking period, 110 (32.1%) patients experienced ER with 78 (71.0%) and 16 (14.5%) patients as AF and AFL, respectively, and 16 (14.5%) patients as both AF and AFL. According to the occurrence of LR in patients with and without ER, differences in baseline characteristics are summarized in Table 1.
Table 1
| ER (+) | ER (−) | |||||
|---|---|---|---|---|---|---|
| LR (+) (102) | LR (−) (8) | P | LR (+) (4) | LR (−) (229) | P | |
| Age (year) | 64 (58–69) | 58 (48–65) | 0.509 | 57 (62–71) | 64 (57–70) | 0.770 |
| Male sex, n (%) | 59 (60.8) | 10 (76.9) | 0.260 | 3 (60.3) | 145 (63.6) | 0.869 |
| AF duration (months) | 36 (16–111) | 12 (11–54) | 0.058 | 32 (24–55) | 12 (2–24) | 0.042 |
| History of AFL, n (%) | 6 (6.2) | 1 (7.7) | 0.839 | 1 (20.0) | 19 (8.3) | 0.357 |
| CHA2DS2-VASc | 2 (1–3) | 2 (1–3) | 0.821 | 1.5 (0.25–2) | 2 (1–3) | 0.277 |
| Hypertension, n (%) | 52 (53.6) | 10 (76.9) | 0.111 | 3 (60.0) | 126 (55.3) | 0.833 |
| Diabetes mellitus, n (%) | 22 (22.7) | 5 (38.5) | 0.214 | 0 (0.0) | 33 (14.5) | 0.359 |
| Coronary artery disease, n (%) | 25 (25.8) | 4 (30.8) | 0.741 | 2 (40.0) | 48 (21.1) | 0.307 |
| Heart failure, n (%) | 25 (25.8) | 1 (7.7) | 0.150 | 0 (0.0) | 52 (22.8) | 0.226 |
| Chronic obstructive pulmonary disease, n (%) | 4 (4.1) | 0 (0.0) | 0.456 | 0 (0.0) | 7 (3.1) | 0.579 |
| History of ischemic stroke, n (%) | 15 (15.5) | 1 (7.7) | 0.688 | 0 (0.0) | 23 (10.1) | 0.454 |
| BMI (kg/m2) | 20.7 (19.8–21.4) | 21.5 (20.5–21.9) | 0.195 | 20.5 (20.1–20.9) | 20.7 (20.0–21.6) | 0.519 |
| LAA flow velocity (cm/s) | 32 (25–38) | 31 (24–47) | 0.575 | 34.5 (26.3–57) | 35 (26–47) | 0.976 |
| LA diameter (mm) | 48 (44–50) | 43 (39–45) | 0.014 | 47 (45–49) | 43 (40–46) | 0.064 |
| LV diameter (mm) | 49 (44–52) | 49 (47.5–52) | 0.756 | 50 (40–52) | 46 (43–50) | 0.655 |
| LV ejection fraction (%) | 60 (55.5–64) | 60 (57–63.5) | 0.802 | 59 (51–68) | 62 (56–66) | 0.727 |
| Albumin (g/L) | 40.5 (38.4–42.17) | 40.3 (39.0–41.7) | 0.886 | 42.56 (38.52–45.36) | 40 (38–43) | 0.201 |
| AST (µ/L) | 22 (18.5–26) | 20 (16–23) | 0.041 | 28 (17–31) | 23 (19–30) | 0.335 |
| ALT (µ/L) | 20 (16–30) | 25 (13–34.5) | 0.985 | 28 (21–42) | 22 (16–32) | 0.149 |
| Fasting blood Glucose (mmol/L) | 5.53 (4.84–6.62) | 6.16 (5.36–6.76) | 0.357 | 5.44 (4.54–6.60) | 5.4 (4.9–6.1) | 0.997 |
| Blood urea nitrogen (mmol/L) | 6.12 (4.95–7.39) | 5.85 (5.33–7.15) | 0.708 | 5.47 (4.29–6.67) | 6.0 (5.1–7.1) | 0.673 |
| Creatinine (mg/dl) | 67 (58.5–78.5) | 63 (61–84) | 0.653 | 72 (61–77) | 67 (59–75) | 0.835 |
| Uric acid (mmol/L) | 373 (319.5–430.5) | 362.0 (309.5–387.5) | 0.287 | 360 (253–414) | 377 (314–429) | 0.446 |
| TC (mmol/L) | 4.45 (3.79–5.22) | 4.22 (3.22–5.22) | 0.993 | 4.44 (3.47–5.77) | 4.5 (3.7–5.4) | 0.794 |
| Triglyceride (mmol/L) | 1.02 (0.83–1.5) | 1.19 (0.83–1.43) | 0.637 | 1.37 (0.80–2.52) | 1.1 (0.8–1.5) | 0.409 |
| HDL-c (mmol/L) | 1.2 (1.03–1.42) | 1.13 (0.94–1.23) | 0.125 | 1.33 (1.11–1.63) | 1.3 (1.1–1.5) | 0.308 |
| LDL-c (mmol/L) | 2.87 (2.34–3.49) | 2.73 (1.95–3.60) | 0.663 | 2.90 (1.97–3.64) | 2.7 (2.2–3.4) | 0.844 |
| HCY (mmol/L) | 12.8 (11.0–15.4) | 14.5 (10.7–16.1) | 0.277 | 12 (10.9–17.8) | 13.0 (10.8–15.2) | 0.720 |
| BNP (pg/ml) | 156.9 (88.2–27 7.8) | 161.1 (64.1–293.1) | 0.594 | 175.7 (159.1–345.2) | 162 (97–329) | 0.423 |
| D-Dimer (mg/L) | 0.58 (0.46–0.82) | 0.78 (0.53–0.93) | 0.402 | 0.57 (0.50–0.69) | 0.6 (0.5–0.8) | 0.899 |
| Hemoglobin (g/L) | 148 (136.5–158) | 152 (141–159.5) | 0.362 | 144 (135.5–155.5) | 149 (140–160) | 0.427 |
| Platelet (×109/L) | 206 (179–249.5) | 246 (185–274.5) | 0.313 | 194 (173.5–239.5) | 209 (176–246) | 0.551 |
| WBC (×109/L) | 6.57 (5.19–7.87) | 5.93 (4.73–7.35) | 0.372 | 5.07 (4.57–5.50) | 6.3 (5.3–7.4) | 0.031 |
| Substrate modification, n (%) | 80 (82.5) | 12 (92.3) | 0.368 | 4 (80.0) | 132 (57.9) | 0.321 |
| Anterior line ablation, n (%) | 6 (6.2) | 0 (0.0) | 0.356 | 0 (0.0) | 7 (3.1) | 0.691 |
| Roof line ablation, n (%) | 57 (58.8) | 6 (46.2) | 0.388 | 1 (20.0) | 78 (34.2) | 0.507 |
| Peri-mitral isthmus line ablation, n (%) | 11 (11.3) | 2 (15.4) | 0.650 | 0 (0.0) | 11 (4.8) | 0.484 |
| Ethanol infusion for Marshall bundle, n (%) | 0 (0.0) | 1 (7.7) | 0.118 | 0 (0.0) | 0 (0.0) | 0.651 |
| Peri-mitral isthmus line ablation, n (%) | 17 (17.5) | 4 (30.8) | 0.268 | 1 (20.0) | 68 (28.9) | 0.662 |
| CFAE-guided ablation, n (%) | 37 (38.1) | 3 (23.1) | 0.368 | 2 (40.0) | 45 (19.7) | 0.265 |
| Termination to SR by CA, n (%) | 4 (4.1) | 1 (7.7) | 0.473 | 0 (0.0) | 23 (10.1) | 0.454 |
| Termination to AFL by CA, n (%) | 7 (7.2) | 2 (15.4) | 0.288 | 1 (20.0) | 30 (13.2) | 0.656 |
| Termination to SR by Ibutilide, n (%) | 23 (23.7) | 5 (38.5) | 0.252 | 3 (60.0) | 113 (49.6) | 0.644 |
| Termination to SR by Ibutilide combined cardioversion, n (%) | 21 (21.6) | 1 (7.7) | 0.459 | 0 (0.0) | 24 (10.5) | 0.444 |
Baseline characteristics of the study population according to the occurrence of LR in patients with and without ER.
AF, atrial fibrillation; AFL, atrial flutter; CFAE, complex fractionated atrial electrogram; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, vascular disease, female sex; ER, early recurrence; LA, left atrium; LAA, left atrial appendage; LV, left ventricular; SR, sinus rhythm; BNP, B-type natriuretic peptide; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; ALT, alanine transaminase; AST, aspartate transaminase; WBC, white blood cell count; LR, late recurrence; CA, catheter ablation; BMI, body mass index; HCY, homocysteine.
Values are mean ± SD or %.
Characteristics of ER of persistent AF
We compared our results with previous studies on ER and found the following different characteristics of persistent AF (Figure 2). (1) First ER episode occurred in 50.9% of patients within 24 h and in 68.2% of patients within 7 days (Figure 3A). (2) ER is usually in the form of persistent atrial arrhythmia (89.1%). (3) LR was not related to timing of first ER manifestation events (P = 0.195) (Figure 3B).
Figure 2
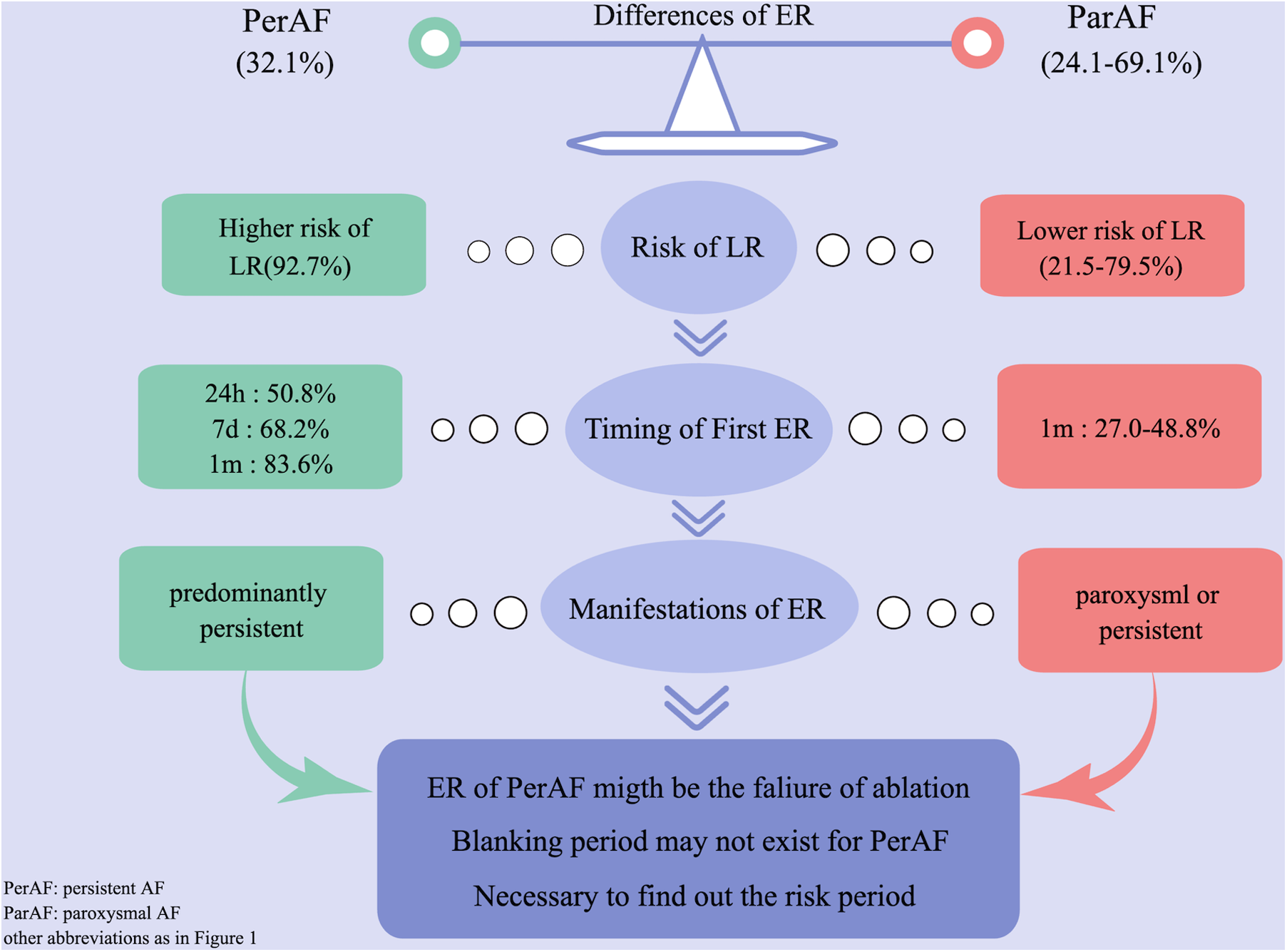
Difference of ER between persistent and paroxysmal AF by comparing with previous studies. AF, atrial fibrillation; ER, early recurrence.
Figure 3
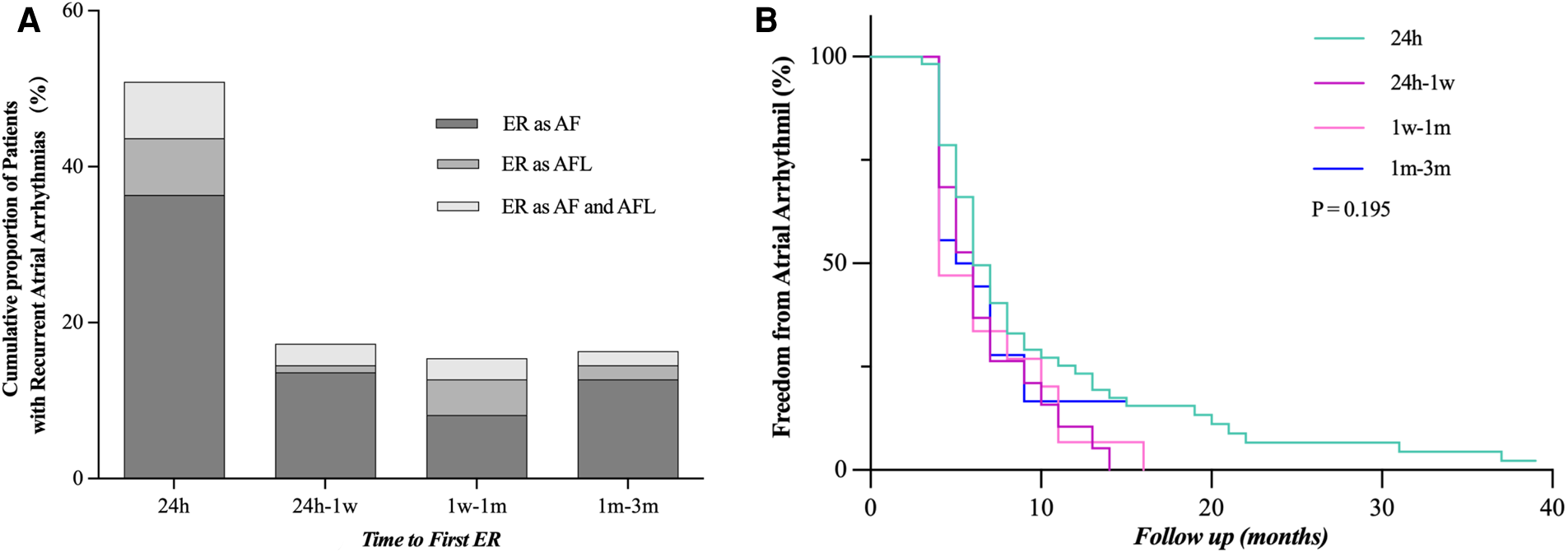
Time to the first episode of ER after catheter ablation. (A) Proportion of patients who developed ER after catheter ablation and time to recurrence are shown. (B) LR rate was not statistically different when compared between the groups distributed according to the timing of the first episode of ER (24 h, 24 h–1 week, 1 week–1 month, and 1–3 months, P = 0.195). ER, early recurrence, AF, atrial fibrillation, AFL, atrial flutter; LR, late recurrence.
Risk factors for ER
Baseline characteristics of patients with and without ER are described in Table 2. Patients with ER had a longer mean AF duration [36 (IQR 12–108) vs. 12 (IQR 2–24) months, P < 0.001], more diabetes mellitus (24.5% vs. 14.2%, P = 0.018), a larger left atrial (LA) size [47.0 mm (IQR 43.0–50.0) vs. 43.2 mm (IQR 40.0–46.5), P < 0.001], a larger left ventricular (LV) diameter [49.0 mm (IQR 44.0–52.0) vs. 46.0 mm (IQR 43.0–50.0), P = 0.005], a lower left ventricular ejection fraction (LVEF) [60% (IQR 56–64) vs. 62% (IQR 56–66), P = 0.029], a lower left atrial appendage (LAA) flow velocity [32 cm/s (IQR 25–40) vs. 35 cm/s (IQR 27–46), P = 0.033], a lower HDL-c [1.18 mmol/L (IQR 1.03–1.36) vs. 1.25 mmol/L (IQR 1.07, 1.47), P = 0.032], more additional substrate modification (83.6% vs. 58.4%, P < 0.001), roof line ablation (57.3% vs. 33.9%, P < 0.001), peri-mitral isthmus line ablation (11.8% vs. 4.7%, P < 0.001), CFAE ablation (36.4% vs. 20.2%, P < 0.001), termination to SR by Ibutilide (25.5% vs. 49.8%, P < 0.001), and termination to SR by Ibutilide combined cardioversion (20.0% vs. 10.3%, P = 0.014).
Table 2
| ER (+) (110) | ER (−) (233) | P-value | |
|---|---|---|---|
| Age (years) | 64 (57.68) | 63 (57–70) | 0.619 |
| Male sex (%) | 69 (62.7) | 148 (63.5) | 0.490 |
| AF duration (months) | 36 (12–108) | 12 (2–24) | <0.001 |
| History of AFL, n (%) | 7 (6.4) | 20 (8.6) | 0.316 |
| CHA2DS2-VASc | 2 (1–3) | 2 (1–3) | 0.222 |
| Hypertension, n (%) | 62 (56.4) | 129 (55.4) | 0.478 |
| Diabetes mellitus, n (%) | 27 (24.5) | 33 (14.2) | 0.018 |
| Coronary artery disease, n (%) | 29 (26.4) | 50 (21.5) | 0.192 |
| Heart failure, n (%) | 26 (23.6) | 52 (22.3) | 0.786 |
| Chronic obstructive pulmonary disease, n (%) | 4 (3.6) | 7 (3.0) | 0.492 |
| History of ischemic stroke, n (%) | 16 (14.5) | 23 (9.9) | 0.138 |
| BMI (kg/m2) | 20.8 (19.8–21.5) | 20.8 (20.0–21.7) | 0.626 |
| LAA flow velocity (cm/s) | 32 (25–40) | 35 (27–46) | 0.033 |
| LA diameter (mm) | 47 (43–50) | 43.2 (40–46.5) | <0.001 |
| LV diameter (mm) | 49 (44–52) | 46 (43–50) | 0.005 |
| LV ejection fraction (%) | 60 (56–64) | 62 (56–66) | 0.029 |
| Blood tests | |||
| Albumin (g/L) | 40.45 (38.46–42.13) | 40.3 (38.0–42.7) | 0.921 |
| AST (µ/L) | 21.5 (18–26) | 23 (19–30) | 0.052 |
| ALT (µ/L) | 20 (16–30.25) | 22 (16–22) | 0.323 |
| Fasting blood glucose (mmol/L) | 5.60 (4.91–6.67) | 5.42 (4.91–6.12) | 0.163 |
| Blood urea nitrogen (mmol/L) | 6.09 (5.10–7.33) | 6.04 (5.08–7.02) | 0.738 |
| Creatinine (mg/dl) | 67 (59–79) | 67 (59–75) | 0.767 |
| Uric acid (mmol/L) | 370.5 (322.3–421.8) | 376 (314–428) | 0.993 |
| TC (mmol/L0 | 4.45 (3.79–5.27) | 4.52 (3.75–5.39) | 0.954 |
| Triglyceride (mmol/L) | 1.05 (5.83–1.49) | 1.08 (0.79–1.49) | 0.788 |
| HDL-c (mmol/L) | 1.18 (1.03–1.36) | 1.25 (1.07–1.47) | 0.032 |
| LDL-c (mmol/L) | 2.80 (2.22–3.48) | 2.75 (2.19–3.40) | 0.772 |
| HCY | 13 (10.98–15.43) | 13.0 (10.80–15.15) | 0.817 |
| BNP (pg/ml) | 160.13 (82.1–274.5) | 165.21 (98.85–329.21) | 0.161 |
| D-Dimer (mg/L) | 0.59 (0.47–0.85) | 0.57 (0.46–0.79) | 0.570 |
| Hemoglobin (g/L) | 148 (138–158) | 149 (140–160) | 0.372 |
| Platelet (×109/L) | 210 (180–252) | 207.0 (176.5–246.0) | 0.485 |
| WBC (×109/L) | 6.52 (5.11–7.73) | 6.28 (5.24–4.72) | 0.529 |
| Substrate modification, n (%) | 92 (83.6) | 136 (58.4) | <0.001 |
| Anterior line ablation, n (%) | 6 (5.5) | 7 (3.0) | 0.207 |
| Roof line ablation, n (%) | 63 (57.3) | 79 (33.9) | <0.001 |
| Peri-mitral isthmus line ablation, n (%) | 13 (11.8) | 11 (4.7) | 0.016 |
| Ethanol infusion for Marshall bundle, n (%) | 1 (0.9) | 0 (0.0) | 0.145 |
| Peri-tricuspid isthmus line ablation, n (%) | 21 (19.1) | 67 (28.8) | 0.056 |
| CFAE-guided ablation, n (%) | 40 (36.4) | 47 (20.2) | 0.001 |
| Termination to SR by CA, n (%) | 5 (4.5) | 23 (9.9) | 0.093 |
| Termination to AFL by CA, n (%) | 9 (8.2) | 31 (13.3) | 0.168 |
| Termination to SR by Ibutilide, n (%) | 28 (25.5) | 116 (49.8) | <0.001 |
| Termination to SR by Ibutilide combined cardioversion, n (%) | 73 (66.4) | 78 (33.5) | <0.001 |
Baseline characteristics and procedural data of patients with and without early recurrence.
AF, atrial fibrillation; AFL, atrial flutter; CFAE, complex fractionated atrial electrogram; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, vascular disease, female sex; ER, early recurrence; LA, left atrium; LAA, left atrial appendage; LV, left ventricular; SR, sinus rhythm; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; BNP, B-type natriuretic peptide; ALT, alanine transaminase; AST, aspartate transaminase; WBC, white blood cell count; CA, catheter ablation.
Values are mean ± SD or %.
Multivariate analysis revealed that AF duration (OR 1.006, 95% CI 1.004–1.008, P < 0.001), LA size (OR 1.063, 95% CI 1.018–1.110, P = 0.006), substrate modification (OR 2.34, 95% CI 1.237–4.427, P = 0.009), and termination to SR by Ibutilide combined cardioversion (OR 3.945, 95% CI 1.875–8.3, P < 0.001) were independent risk factors for ER (Table 3).
Table 3
| OR | 95% CI for OR | P-value | |
|---|---|---|---|
| AF duration | 1.006 | 1.004–1.008 | <0.001 |
| Diabetes mellitus | 2.083 | 1.305–3.325 | 0.102 |
| LAA flow velocity | 0.990 | 0.973–1.007 | 0.232 |
| LA diameter | 1.063 | 1.018–1.110 | 0.006 |
| LV diameter | 0.990 | 0.953–1.029 | 0.619 |
| LV ejection fraction | 0.978 | 0.955–1.002 | 0.069 |
| HDL-c | 0.59 | 0.278–1.254 | 0.170 |
| Substrate modification | 2.340 | 1.237–4.427 | 0.009 |
| Roof line ablation | 0.955 | 0.602–1.516 | 0.845 |
| Peri-mitral isthmus line ablation | 1.150 | 0.600–2.201 | 0.674 |
| CFAE-guided ablation | 0.970 | 0.604–1.557 | 0.899 |
| Termination to SR by Ibutilide, | 1.393 | 0.636–3.051 | 0.407 |
| Termination to SR by Ibutilide combined cardioversion | 3.945 | 1.875–8.300 | <0.001 |
Risk factors for early recurrence.
CI, confidence interval; AF, atrial fibrillation; LA, left atrium; LAA, left atrial appendage; LV, left ventricular; CFAE, complex fractionated atrial electrogram; HDL-c, high-density lipoprotein cholesterol; SR, sinus rhythm.
Hazard ratios are calculated by multivariate Cox-regression analysis.
Risk factors for LR
During the follow-up, LR occurred in 106 (30.9%) patients. On univariate analysis, patients with LR had a longer mean AF duration [36 months (IQR 19–108) vs. 12 months (IQR 2–24); P < 0.001], larger LA size [47 mm (IQR 44–50) vs. 43 (IQR 40–46); P < 0.001], a lower LVEF [60% (IQR 56–64) vs. 62% (IQR 57–66); P = 0.024], a larger left ventricular diameter [49 mm (IQR 44–52) vs. 46 mm (IQR 43–50); P = 0.005], a lower LAA flow velocity [32 cm/s (IQR 25–38) vs. 35 cm/s (IQR 26–47); P = 0.016], more substrate modification (83% vs. 59.1%, P < 0.001), roof line ablation (55.7% vs. 35.0%, P < 0.001), peri-mitral isthmus line ablation (12.3% vs. 4.6%, P = 0.012), peri-tricuspid isthmus line ablation (17.9% vs.29.1%, P = 0.018), CFAE-guided ablation (38.7% vs. 19.4%, P < 0.001), termination to SR by Ibutilide (29.1% vs. 17.9%, P = 0.018), and termination to SR by Ibutilide combined cardioversion (38.7% vs. 19.4%, P < 0.001) compared to patients without LR (Table 4).
Table 4
| LR (+) (106) | LR (−) (237) | P-value | |
|---|---|---|---|
| ER (%) | 102 (96.2) | 8 (3.4) | <0.001 |
| Age (years) | 64 (58–68) | 64 (57–69) | 0.990 |
| Male sex, n (%) | 64 (60.4) | 153 (64.6) | 0.267 |
| AF duration (months) | 36 (19–108) | 12 (2–24) | <0.001 |
| History of AFL, n (%) | 7 (6.6) | 20 (8.4) | 0.365 |
| CHA2DS2-VASc | 2 (1–3) | 2 (1–3) | 0.286 |
| Hypertension, n (%) | 58 (54.7) | 133 (56.1) | 0.450 |
| Diabetes mellitus, n (%) | 25 (23.6) | 35 (14.8) | 0.035 |
| Coronary artery disease, n (%) | 31 (29.2) | 48 (20.3) | 0.047 |
| Heart failure, n (%) | 26 (24.5) | 51 (21.5) | 0.314 |
| Chronic obstructive pulmonary disease, n (%) | 4 (3.8) | 7 (3.0) | 0.457 |
| History of ischemic stroke (%) | 15 (14.2) | 24 (10.1) | 0.183 |
| BMI (kg/m2) | 20.8 (19.8–21.6) | 20.7 (20.0–21.7) | 0.626 |
| LAA flow velocity (cm/s) | 32 (25–38) | 35 (26–47) | 0.016 |
| LA diameter (mm) | 47 (44–50) | 43 (40–46) | <0.001 |
| LV diameter (mm) | 49 (44–52) | 46 (43–50) | 0.005 |
| LV ejection fraction (%) | 60 (56–64) | 62 (57–66) | 0.024 |
| Albumin (g/L) | 40.5 (38.4–42.2) | 40.2 (38.0–42.7) | 0.979 |
| AST (µ/L) | 22 (18–26) | 23 (19–30) | 0.056 |
| ALT (µ/L) | 20 (16.30) | 22 (16–33) | 0.204 |
| Fasting blood glucose (mmol/L) | 5.53 (4.81–6.68) | 5.42 (4.92–6.17) | 0.379 |
| Blood urea nitrogen (mmol/L) | 6.13 (4.91–7.33) | 6.03 (5.10–7.02) | 0.792 |
| Creatinine (mg/dl) | 67 (59–77) | 67 (59–75) | 0.894 |
| Uric acid (mmol/L) | 368 (313–422) | 374 (316–428) | 0.764 |
| TC (mmol/L) | 4.45 (3.81–5–23) | 4.52 (3.74–5.40) | 0.883 |
| Triglyceride (mmol/L) | 1.05 (0.84–1.53) | 1.07 (0.79–1.48) | 0.936 |
| HDL-c (mmol/L) | 1.19 (1.03–1.41) | 1.24 (1.03–1.46) | 0.484 |
| LDL-c (mmol/L) | 2.81 (2.24–3.84) | 2.75 (2.18–3.41) | 0.652 |
| HCY (mmol/L) | 12.9 (11.0–15.3) | 13.0 (10.8–15.3) | 0.925 |
| BNP (pg/ml) | 160.1 (85.8–266.1) | 165.2 (97.4–329.7) | 0.147 |
| D-Dimer (mg/L) | 0.58 (0.47–0.83) | 0.57 (0.46–0.79) | 0.210 |
| Hemoglobin (g/L) | 148 (139–158) | 149 (140–160) | 0.188 |
| Platelet (×109/L) | 205.5 (180.0–215.5) | 210.0 (176.5–247.5) | 0.792 |
| WBC (×109/L) | 6.41 (5.12–7.71) | 6.34 (5.29–7.48) | 0.971 |
| Substrate modification, n (%) | 88 (83.0) | 140 (59.1) | <0.001 |
| Anterior line ablation, n (%) | 6 (5.7) | 7 (3.0) | 0.180 |
| Roof line ablation, n (%) | 59 (55.7) | 83 (35.0) | <0.001 |
| Peri-mitral isthmus line ablation, n (%) | 13 (12.3) | 11 (4.6) | 0.012 |
| Ethanol infusion for Marshall bundle, n (%) | 0 (0) | 1 (0.4) | 0.515 |
| Peri-tricuspid isthmus line ablation, n (%) | 69 (29.1) | 19 (17.9) | 0.018 |
| CFAE-guided ablation, n (%) | 41 (38.7) | 46 (19.4) | <0.001 |
| Termination to SR by CA, n (%) | 5 (4.7) | 23 (9.7) | 0.085 |
| Termination to AFL by CA, n (%) | 8 (7.5) | 32 (13.5) | 0.077 |
| Termination to SR by Ibutilide, n (%) | 26 (24.5) | 118 (49.8) | <0.001 |
| Termination to SR by Ibutilide combined cardioversion, n (%) | 72 (67.9) | 79 (33.3) | <0.001 |
Baseline characteristics and procedural data of patients with and without late recurrence.
ER, early recurrence; AF, atrial fibrillation; AFL, atrial flutter; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, vascular disease, female sex; LA, left atrium; LAA, left atrial appendage; LV, left ventricular; ALT, alanine transaminase; AST, aspartate transaminase; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; BNP, B-type natriuretic peptide; WBC, white blood cell count; CFAE, complex fractionated atrial electrogram; SR, sinus rhythm; CA, catheter ablation.
Values are mean ± SD or %.
In multivariate analysis, ER (OR 120.505, 95% CI 41.517–349.77, P < 0.001), LA size (OR 1.063, 95% CI 1.015–1.112, P = 0.009), and termination to SR by Ibutilide combined cardioversion (OR 2.347, 95% CI 1.042–5.288, P = 0.039) were the independent predictors of LR (Table 5). ER was observed at a significantly higher rate in patients with LR (96.2% vs. 3.4%, P < 0.001) than in patients without LR (Table 5).
Table 5
| OR | 95% CI for OR | P-value | |
|---|---|---|---|
| ER | 120.505 | 41.517–349.77 | <0.001 |
| AF duration | 1.001 | 0.998–1.003 | 0.596 |
| Coronary artery disease | 1.287 | 0.814–2.035 | 0.280 |
| LAA flow velocity | 0.999 | 0.979–1.018 | 0.886 |
| LA diameter | 1.063 | 1.015–1.112 | 0.009 |
| LV diameter | 0.979 | 0.936–1.025 | 0.375 |
| LV ejection fraction | 0.996 | 0.971–1.022 | 0.747 |
| Substrate modification | 0.925 | 0.459–1.864 | 0.827 |
| Roof line ablation | 0.672 | 0.411–1.097 | 0.112 |
| Peri-mitral isthmus line ablation | 1.403 | 0.748–2.633 | 0.291 |
| Peri-tricuspid isthmus line ablation | 0.668 | 0.353–1.264 | 0.215 |
| CFAE-guided ablation | 1.031 | 0.633–1.678 | 0.904 |
| Termination to SR by Ibutilide | 1.513 | 0.629–3.637 | 0.355 |
| Termination to SR by Ibutilide combined cardioversion | 2.347 | 1.042–5.288 | 0.039 |
Risk factors for late recurrence.
CI, confidence interval; ER, early recurrence; AF, atrial fibrillation; LA, left atrium; LAA, left atrial appendage; LV, left ventricular; CFAE, complex fractionated atrial electrogram; SR, sinus rhythm.
Hazard ratios are calculated by multivariate Cox-regression analysis.
Relationship between ER and LR
During long-term follow-up, the LR rate was significantly higher in patients with ER (92.7% vs. 1.7%, P < 0.001) than those without ER (Figure 4A). ER as AFL had a lower risk of LR compared with ER as AF and both AF and AFL (P = 0.011; P = 0.003) (Figures 4B,D). Compared with ER as AF (Figure 4C), ER as both AF and AFL had a similar risk of LR (P = 0.622). The relationship between the types of ER and LR can be further clarified by expanding the sample size.
Figure 4
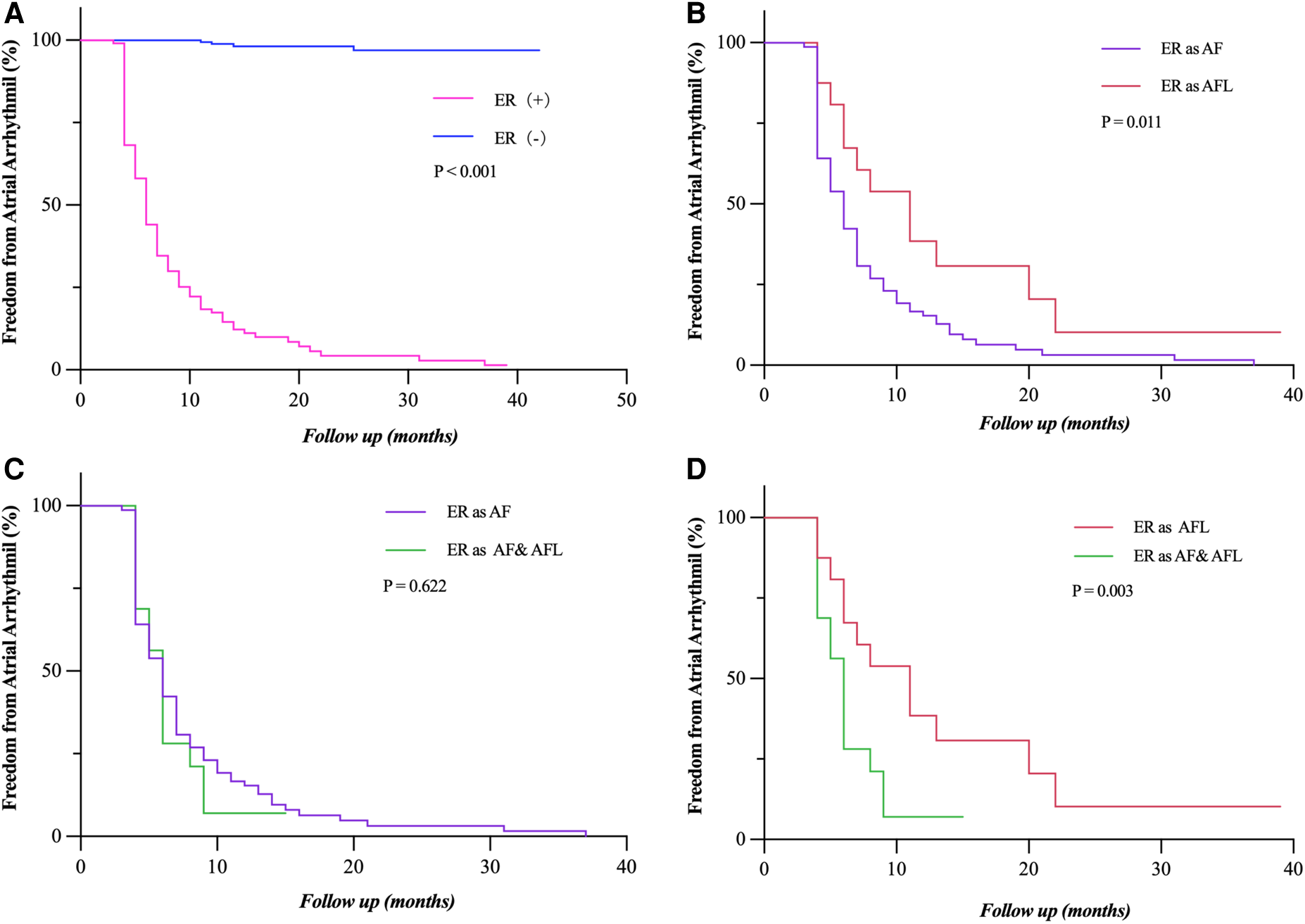
Freedom from atrial tachyarrhythmia recurrence. (A) Patients who experienced ER (as AF, AFL, and both AF and FL) had a significantly lower rate of freedom from LR (P < 0.001). (B,D) Patients who had ER as AF and both AF and AFL showed a significantly lower rate of freedom from LR compared to those with ER as AFL (P = 0.011; P = 0.003). (C) The rate of freedom from LR had no statistical difference between the patients who had ER as AF and as both AF and AFL (P = 0.622). Incidence curves were compared using the log-rank test. ER, early recurrence; LR, late recurrence; AF, atrial fibrillation; AFL, atrial flutter.
Relationship between early intervention and LR
Early intervention was performed in 48 (47.3%) patients with persistent ER during the blanking period. Patients who had early intervention after ER (as AF, AFL, and both AF and AFL) had a significantly lower rate of freedom from LR (P = 0.001) in the short term, but not in the long term, which is more obvious in patients who had ER as AF (P = 0.001) (Figures 5A,B). The rate of freedom from LR had no statistical difference between the patients who had early intervention after ER as AFL and as both AF and AFL (P = 0.914; P = 0.714) (Figures 5C,D). The sample size of patients with ER as AFL and both AF and AFL is too small that cannot be accurately analyzed.
Figure 5
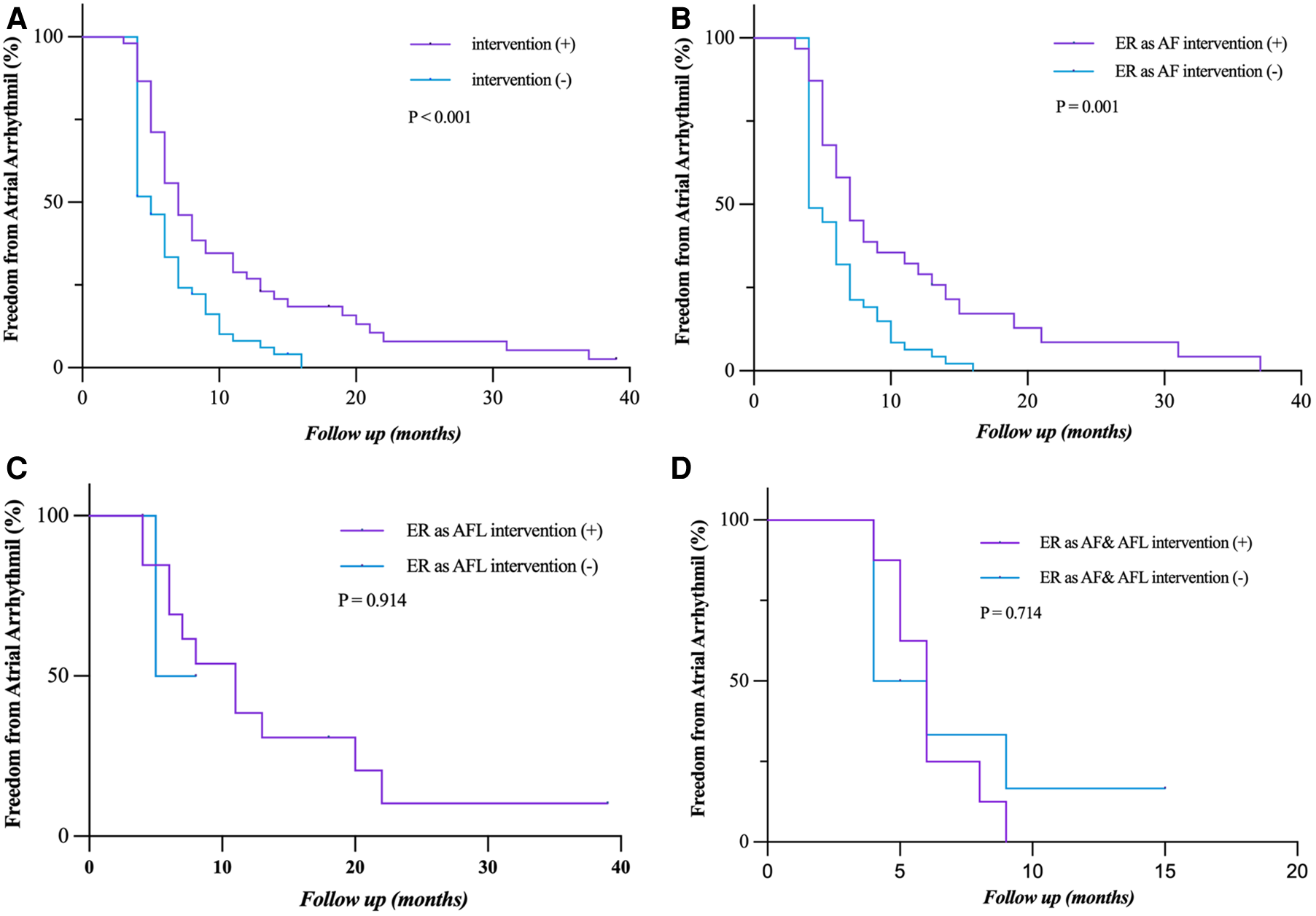
Freedom from atrial tachyarrhythmia recurrence after early intervention for ER. (A,B) Patients who had early intervention after ER (as AF, AFL, and both AF and AFL) had a significantly lower rate of freedom from LR (P = 0.001) in the short term, but not in the long term, which is more obvious in the patients who had ER as AF (P = 0.001). (C,D) The rate of freedom from LR had no statistical difference between the patients who had early intervention after ER as AFL and as both AF and AFL (P = 0.914, P = 0.714). Incidence curves were compared using the log-rank test. ER, early recurrence; AF, atrial fibrillation; AFL, atrial flutter; LR, late recurrence.
Risk period for LR
In a subgroup analysis (LR, n = 106), we assume that there is no blanking period after CA. Recurrence of arrhythmia occurred in 71/106 (70.0%) patients during the first week and 92/106 (86.8%) patients within the first month (Figure 6A). The occurrence rate of LR was 35/343 (14.45%) and 22/251 (8.76%) in patients who did not experience recurrence in the first week and first month, respectively (Figure 6B). LR occurred within the first month in 25.4% of all patients (Figure 6C).
Figure 6
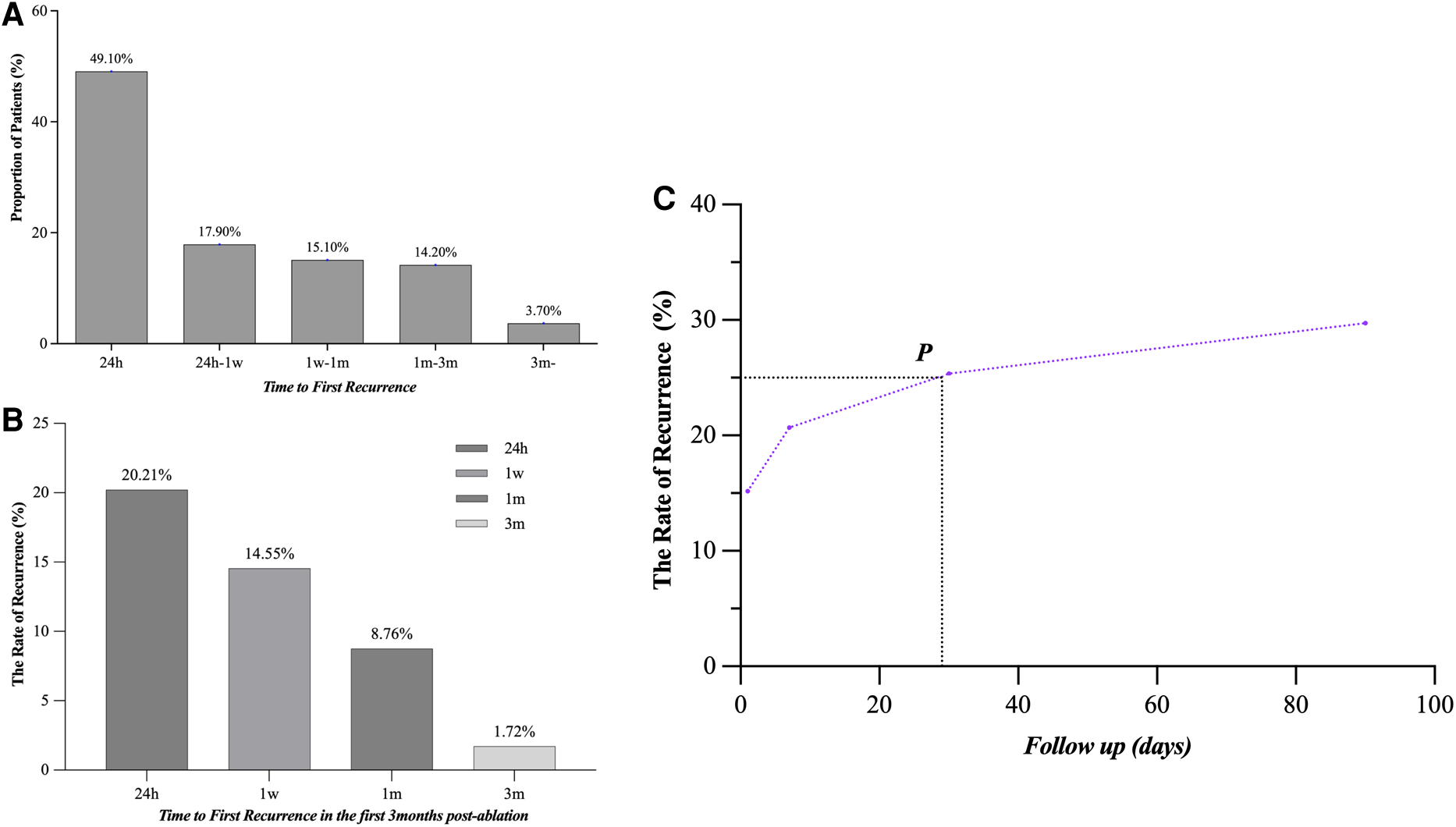
Time to the atrial tachyarrhythmia recurrence after CA. (A) The proportion was 49.1%, 17.9%, 15.1%, 14.2%, and 3.7%, respectively, in 24 h, 24 h–1 week, 1 week–1 month, 1–3 months, and after 3 months in AF patients with LR. (B) The LR probabilities of patients without recurrence in 24 h, 1 week, 1 month, and 3 months after CA were 20.21%, 14.55%, 8.76% and 1.72%, respectively. (C) The overall recurrence rate at 1 month after CA reached 25%, with 86.8% of these patients still recurring during the follow-up. LR, late recurrence; AF, atrial fibrillation; CA, catheter ablation.
Discussion
Main findings
In the present study, we first summarized the characteristics and significances of ER after CA in patients with persistent AF (Figure 2). (1) Patients experiencing ER have a significantly higher risk (92.7%) of LR compared with those without ER. (2) The majority (50.9%) of first ER occurred in the first 24 h after CA. (3) ER was predominantly present as persistent. (4) LR was not related to the timing of the first ER. (5) ER as AFL had a lower risk of LR compared with ER as AF and as both AF and AFL. (6) Early intervention during blanking period could improve the prognosis in the short term, but could not prevent more LR. (7) Only 8.76% of patients who did not have recurrence within the first 1 month after CA had LR; we called this period the “risk period” of recurrence.
Our findings are not in accordance with the current guideline recommendation that ER is a common phenomenon after CA for AF.
Characteristics of ER in patients with persistent AF
The majority of ER first occurred in the initial 24 h after CA
ER showed the highest incidence in the first week after CA. Nearly half of the patients had ER within the first 24 h, which was not consistent with the findings of previous studies (3, 10–13). The theoretical basis of the blanking period is related to transient inflammatory response, autonomic nervous system imbalance, and lesion maturation after CA. These studies do not explain the earlier recurrence in persistent AF, as is usually present after CA in paroxysmal AF. Another theory that ER is associated with pulmonary vein reconnection is also not supported in persistent AF (14). ER confined to the first 4 weeks after PVI is not associated with pulmonary vein reconnection. Patients with ER have a higher risk of LR after repeat CA, but pulmonary vein reconnection is less likely after repeat CA (15). Furthermore, AI-guided ablation may also reduce pulmonary vein reconnection. It is inferred that the presence of substrates may lead to earlier ER.
ER was predominantly persistent
ER was predominantly persistent, which is in striking contrast to previous reports of paroxysmal AF cohorts (10, 11, 16). In the early stage of AF, triggers from the pulmonary veins are the main mechanism. However, the more important mechanism in the persistence of AF is the change of underlying atrial substrate with remodeling in the later stage (17). We consider that the atrial substrate in those patients was too strong to be sufficiently modified by the ablation strategies applied in our study, and it may result in the recurrence of persistent atrial arrhythmias. Popa reported that 18% of patients of ER in persistent AF experienced spontaneous conversion to SR (18). This phenomenon was also confirmed in our study that it was observed in only 10.9% of patients with ER. Ibutilide combined cardioversion and larger LA were the independent predictors of LR, which indicated that the substrate of these patients may be stronger and may be a more important mechanism in the persistence of AF.
The timing of ER was not associated with the incidence of LR
We also found a different correlation between ER timing and LR than previously described for paroxysmal AF, where the risk of LR is known to gradually increase with later ER (3, 10, 19). In our cohort, the risk of LR was rather equally high regardless of the timing of ER.
The above characteristics confirm that the occurrence of ER may reflect the original AF substrate but is not a result of a solely transient mechanism. If so, this would support early re-ablation in these patients.
Value of ER and LR
We found that the patients with ER are inevitably subject to LR. This adds to the growing body of evidence that ER is highly predictive of LR in paroxysmal AF (3, 19, 16). The type of LR (68.0% AF and 17.0% AFL, 15% both AF and AFL) mainly corresponded to the ER type (71.0% AF, 14.5% AFL, and 14.5% both AF and AFL), as reported previously (20). These data support an almost equal relationship between ER and LR in patients with persistent AF. Thus, we guess that the occurrence of ER might be a surrogate marker of the severity of only AF itself.
In our study, AF often transforms into AFL during the ablation of atrial substrate rather than PVI, and the patients with ER as AFL had a significantly lower risk of LR. That may indicate that ER as AFL might suggest a reduction in AF substrate after CA. Thus, the clinical significance and underlying pathophysiology of ER as AFL should be explored further.
Among LR patients, 86.8% occurred within 1 month after CA, and among patients without ER within 1 month, only 8.76% had LR, suggesting that 1 month after CA may be the “risk period” for LR, which has never been reported in previous studies.
Intervention for ER
Early intervention is thought to promote reverse remodeling processes in patients with ER (21). However, previous studies have failed to reach a consistent conclusion as to whether intervention usage in patients with ER after CA was associated with the reduction of AF recurrence, and there are few studies on patients with persistent AF. Several studies with a majority of patients with persistent AF have found that early intervention after CA did not prevent more LR (22, 23). On the contrary, in other studies, intervention after CA could prevent LR in patients with paroxysmal AF (21, 24). Our results showed that intervention could not affect the outcome of patients with ER of persistent AF after CA, which is consistent with the former.
CF and AI-guided ablation could improve durability of line isolation
Our study also reports the ER of AF ablated by the CF catheter and AI-guided ablation for the first time. With the introduction of CF-sensing catheters and AI algorithms to improve lesion quality or more transmural energy sources, we enhanced PVI and linear block of the additional ablation. In previous studies, it has been demonstrated that CF-sensing catheters could increase efficiency in the ablation of persistent AF caused by the reduction of PV reconnection (25–27). Successful complex electrogram ablation and the achievement of linear block minimized the occurrence of new arrhythmias (28). In this case, there is further evidence that the unablated substrates may be a more important mechanism for the recurrence of persistent AF.
Outlook and clinical implications
In the present study, ER in persistent AF shows different characteristics and prognostic significance. We recommend that patients with persistent AF undergoing ER should be informed of their high risk for LR without waiting for the onset and end of ER. We initially proposed that there is no so-called “blanking period” of 3 months after CA for patients with persistent AF, but there is a “risk period” of 1 month after CA, which is in contrast with the guideline. For patients with ER of persistent AF after CA, early re-ablation may reduce LR.
Limitations
The results from this retrospective single-center analysis are not free from the inherent limitations of this design. First, ER was not determined by implantable event recorder devices or trans-telephonic monitoring, which have not provided a more accurate time of ER, and as a result, the exact time of what we call the risk period is not obtained. Second, we did not study further whether or not early re-ablation vs. late re-ablation following an unsuccessful CA for persistent AF would provide any additional benefit or value. Third, many patients received non-PVI ablation, but we have not explored further whether non-PVI ablation was associated with ER. Fourth, although we concluded the difference of ER between paroxysmal and persistent AF by collecting previous studies on ER of paroxysmal AF, we did not conduct a controlled study of patients with persistent and paroxysmal AF in this study. Finally, the study consisted entirely of East Asian patients, and whether these results apply to other ethnic groups needs further investigation.
Conclusions
For persistent AF, ER is a strong independent predictor of LR, which challenges the fact that ER is merely a transient phenomenon. In patients with persistent AF, most of the LR occurred in the first month after CA. Based on the above two points, we hypothesized that there may not be a so-called 3-month “blanking period” for patients with persistent AF, but there is a “risk period” of 1 month after CA, which can be tested in future studies.
Statements
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Yantai Yuhuangding Hospital Affiliated to Qingdao University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XZ and KF designed the study. XZ, KF, HC, LZ, and CW performed the experiments. ZW analyzed the data. XZ, KF, RY, and WL prepared the manuscript. GW helped complete ECG and 24-h Holter monitoring. LW helped complete the preoperative transthoracic echocardiographic and transesophageal echocardiography. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Calkins H Hindricks G Cappato R Kim YH Saad EB Aguinaga L et al HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14(10):e275–444. 10.1016/j.hrthm.2017.05.012
2.
Oral H Knight BP Ozaydin M Tada H Chugh A Hassan S et al Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. (2002) 40(1):100–4. 10.1016/S0735-1097(02)01939-3
3.
Willems S Khairy P Andrade JG Hoffmann BA Levesque S Verma A et al Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation: insights from the ADVICE (adenosine following pulmonary vein isolation to target dormant conduction elimination) trial. Circ Arrhythm Electrophysiol. (2016) 9(8):e003909. 10.1161/CIRCEP.115.003909
4.
Yanagisawa S Inden Y Kato H Fujii A Mizutani Y Ito T et al Effect and significance of early reablation for the treatment of early recurrence of atrial fibrillation after catheter ablation. Am J Cardiol. (2016) 118(6):833–41. 10.1016/j.amjcard.2016.06.045
5.
Themistoclakis S Schweikert RA Saliba WI Bonso A Rossillo A Bader G et al Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. (2008) 5(5):679–85. 10.1016/j.hrthm.2008.01.031
6.
Richter B Gwechenberger M Socas A Marx M Gössinger HD . Frequency of recurrence of atrial fibrillation within 48 h after ablation and its impact on long-term outcome. Am J Cardiol. (2008) 101(6):843–7. 10.1016/j.amjcard.2007.11.021
7.
Liang JJ Elafros MA Chik WW Santangeli P Zado ES Frankel DS et al Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: timing and frequency of early recurrences predicts long-term ablation success. Heart Rhythm. (2015) 12(12):2461–8. 10.1016/j.hrthm.2015.07.015
8.
Loghin C Karimzadehnajar K Ekeruo IA Mukerji SS Memon NB Kantharia BK . Outcome of pulmonary vein isolation ablation for paroxysmal atrial fibrillation: predictive role of left atrial mechanical dyssynchrony by speckle tracking echocardiography. J Interv Card Electrophysiol. (2014) 39(1):7–15. 10.1007/s10840-013-9841-3
9.
Zhu X Chu H Li J Wang C Li W Wang Z et al New discovery of left atrial macroreentry tachycardia: originating from the spontaneous scarring of left atrial anterior wall. J Interv Cardiol. (2021) 2021:2829070. 10.1155/2021/2829070
10.
von Olshausen G Uijl A Jensen-Urstad M Schwieler J Drca N Bastani H et al Early recurrences of atrial tachyarrhythmias post pulmonary vein isolation. J Cardiovasc Electrophysiol. (2020) 31(3):674–81. 10.1111/jce.14368
11.
Kim YG Boo KY Choi JI Choi YY Choi HY Roh SY et al Early recurrence is reliable predictor of late recurrence after radiofrequency catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. (2021) 7(3):343–51. 10.1016/j.jacep.2020.09.029
12.
Alipour P Azizi Z Pirbaglou M Ritvo P Pantano A Verma A et al Defining blanking period post-pulmonary vein antrum isolation. JACC Clin Electrophysiol. (2017) 3(6):568–76. 10.1016/j.jacep.2017.01.006
13.
Takayama K Hachiya H Iesaka Y Hirao K Isobe M . Early recurrence after longstanding persistent atrial fibrillation ablation. Int Heart J. (2018) 59(2):321–7. 10.1536/ihj.16-652
14.
Das M Wynn GJ Morgan M Lodge B Waktare JE Todd DM et al Recurrence of atrial tachyarrhythmia during the second month of the blanking period is associated with more extensive pulmonary vein reconnection at repeat electrophysiology study. Circ Arrhythm Electrophysiol. (2015) 8(4):846–52. 10.1161/CIRCEP.115.003095
15.
Mujović N Marinković M Marković N Vučićević V Lip GYH Bunch TJ et al The relationship of early recurrence of atrial fibrillation and the 3-month integrity of the ablation lesion set. Sci Rep. (2018) 8(1):9875. 10.1038/s41598-018-28072-y
16.
Miyazaki S Taniguchi H Nakamura H Takagi T Iwasawa J Hachiya H et al Clinical significance of early recurrence after pulmonary vein antrum isolation in paroxysmal atrial fibrillation—insight into the mechanism. Circ J. (2015) 79(11):2353–9. 10.1253/circj.CJ-15-0475
17.
Pak HN Hwang C Lim HE Kim JW Lee HS Kim YH . Electroanatomic characteristics of atrial premature beats triggering atrial fibrillation in patients with persistent versus paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. (2006) 17(8):818–24. 10.1111/j.1540-8167.2006.00503.x
18.
Popa MA Kottmaier M Risse E Telishevska M Lengauer S Wimbauer K et al Early arrhythmia recurrence after catheter ablation for persistent atrial fibrillation: is it predictive for late recurrence? Clin Res Cardiol. (2022) 111(1):85–95. 10.1007/s00392-021-01934-8
19.
Uetake S Miyauchi Y Mitsuishi T Maruyama M Seino Y Shimizu W . Re-definition of blanking period in radiofrequency catheter ablation of atrial fibrillation in the contact force era. J Cardiovasc Electrophysiol. (2020) 31(9):2363–70. 10.1111/jce.14643
20.
Alipour P Azizi Z Pirbaglou M Ritvo P Pantano A Verma A et al Defining blanking period post-pulmonary vein antrum isolation. JACC Clin Electrophysiol. (2017) 3(6):568–76. 10.1016/j.jacep.2017.01.006
21.
Baman TS Gupta SK Billakanty SR Ilg KJ Good E Crawford T et al Time to cardioversion of recurrent atrial arrhythmias after catheter ablation of atrial fibrillation and long-term clinical outcome. J Cardiovasc Electrophysiol. (2009) 20(12):1321–5. 10.1111/j.1540-8167.2009.01553.x
22.
Chilukuri K Dukes J Dalal D Marine JE Henrikson CA Scherr D et al Outcomes in patients requiring cardioversion following catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. (2010) 21(1):27–32. 10.1111/j.1540-8167.2009.01593.x
23.
Ebert M Stegmann C Kosiuk J Dinov B Richter S Arya A et al Predictors, management, and outcome of cardioversion failure early after atrial fibrillation ablation. Europace. (2018) 20(9):1428–34. 10.1093/europace/eux327
24.
Malasana G Day JD Weiss JP Crandall BG Bair TL May HT et al A strategy of rapid cardioversion minimizes the significance of early recurrent atrial tachyarrhythmias after ablation for atrial fibrillation. J Cardiovasc Electrophysiol. (2011) 22(7):761–6. 10.1111/j.1540-8167.2010.02005.x
25.
Hussein A Das M Riva S Morgan M Ronayne C Sahni A et al Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol. (2018) 11(9):e006576. 10.1161/CIRCEP.118.006576
26.
Taghji P El Haddad M Phlips T Wolf M Knecht S Vandekerckhove Y et al Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol. (2018) 4(1):99–108. 10.1016/j.jacep.2017.06.023
27.
Kautzner J Neuzil P Lambert H Peichl P Petru J Cihak R et al EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. (2015) 17(8):1229–35. 10.1093/europace/euv057
28.
Sawhney N Anousheh R Chen W Feld GK . Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. (2010) 3(3):243–8. 10.1161/CIRCEP.109.924878
Summary
Keywords
persistent atrial fibrillation, blanking period, catheter ablation, early recurrence, late recurrence
Citation
Fu K, Zhu X, Chu H, Zhong L, Wang Z, Li W, Wang C, Li J, Gong L, Wang G, Yao R and Wang L (2023) Re-recognize early recurrence of persistent atrial fibrillation. Front. Cardiovasc. Med. 10:1145695. doi: 10.3389/fcvm.2023.1145695
Received
16 January 2023
Accepted
02 May 2023
Published
30 May 2023
Volume
10 - 2023
Edited by
Yongquan Wu, Capital Medical University, China
Reviewed by
Chen-Xi Jiang, Capital Medical University, China Mauricio Pimentel, Clinical Hospital of Porto Alegre, Brazil
Updates
Copyright
© 2023 Fu, Zhu, Chu, Zhong, Wang, Li, Wang, Li, Gong, Wang, Yao and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Hongxia Chu chuhx1972@163.com Lin Zhong yizun1971@126.com
†These authors have contributed equally to this work
Abbreviations AADs, anti-arrhythmic drugs; AF, atrial fibrillation; AFL, atrial flutter; CFAE, complex fractionated atrial electrogram; ER, early recurrence; LA, left atrial; LAA, left atrial appendage; LV, left ventricular; SR, sinus rhythm; CA, catheter ablation; LR, late recurrence; CF, contact force; AI, ablation index; PVI, pulmonary vein isolation; ECGs, electrocardiograms; OA, oral anticoagulation, IQR, inter-quartile range; BMI, body mass index; HCY, homocysteine.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.