Abstract
The primary pharmacological action of sodium-glucose co-transporter 2 (SGLT2) inhibitors is to inhibit the reabsorption of glucose and sodium ions from the proximal tubules of the kidney and to promote urinary glucose excretion. Notably, several clinical trials have recently demonstrated potent protective effects of SGLT2 inhibitors in patients with heart failure (HF) or chronic kidney disease (CKD), regardless of the presence or absence of diabetes. However, the impact of SGLT2 inhibitors on sudden cardiac death (SCD) or fatal ventricular arrhythmias (VAs), the pathophysiology of which is partly similar to that of HF and CKD, remains undetermined. The cardiorenal protective effects of SGLT2 inhibitors have been reported to include hemodynamic improvement, reverse remodeling of the failing heart, amelioration of sympathetic hyperactivity, correction of anemia and impaired iron metabolism, antioxidative effects, correction of serum electrolyte abnormalities, and antifibrotic effects, which may lead to prevent SCD and/or VAs. Recently, as possible direct cardiac effects of SGLT2 inhibitors, not only inhibition of Na+/H+ exchanger (NHE) activity, but also suppression of late Na+ current have been focused on. In addition to the indirect cardioprotective mechanisms of SGLT2 inhibitors, suppression of aberrantly increased late Na+ current may contribute to preventing SCD and/or VAs via restoration of the prolonged repolarization phase in the failing heart. This review summarizes the results of previous clinical trials of SGLT2 inhibitors for prevention of SCD, their impact on the indices of electrocardiogram, and the possible molecular mechanisms of their anti-arrhythmic effects.
Introduction
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are increasingly gaining attention for their cardiorenal protective effects and their clinical significance (1). However, compared to the robust favorable effects of SGLT2 inhibitors on diabetes mellitus, heart failure, and chronic kidney disease, results of studies on their clinical impact on sudden cardiac death (SCD) have been inconsistent. Therefore, it is worth evaluating whether SGLT2 inhibitors can prevent the incidence of SCD in the presence or absence of diabetes mellitus, heart failure, or chronic kidney disease. This mini-review focuses on the results of recent clinical trials of SGLT2 inhibitors for prevention of SCD, the effects of SGLT2 inhibitors on ventricular indices of an electrocardiogram, and the possible molecular mechanisms of the anti-arrhythmic effects of SGLT2 inhibitors.
General background on SGLT2 inhibitors and their potential impact on SCD
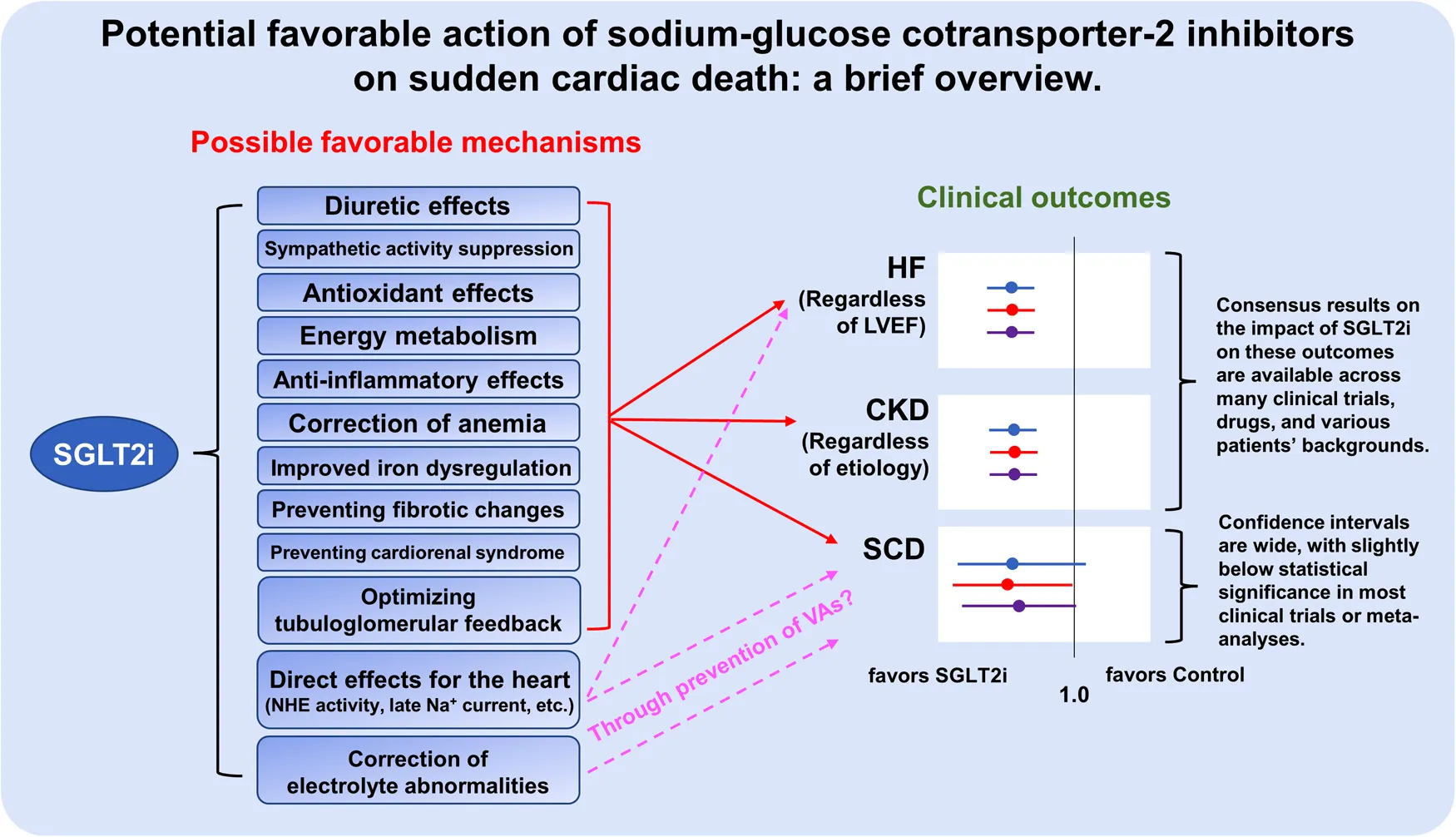
A major pharmacological action of SGLT2 inhibitors is to inhibit the reabsorption of glucose and sodium ions and to promote urinary glucose excretion by inhibiting SGLT2, which is abundant at the brush border membrane in the early S1 segment of the proximal tubules of the kidney (2). Thus, SGLT2 inhibitors were initially developed and introduced to the market as oral hypoglycemic agents with insulin-independent hypoglycemic effects. However, a number of large clinical trials in recent years have demonstrated the potent cardiorenal protective effects of SGLT2 inhibitors, and interestingly, their protective effects have been observed in both patients with and those without diabetes mellitus (3–8). Several SGLT2 inhibitors are now also recommended for the treatment of chronic heart failure or chronic kidney disease as well as diabetes mellitus (9–11). Compared to SGLT1, which has been reported to be expressed in multiple organs and cells (12), SGLT2 is expressed almost exclusively in the proximal tubules (13), and it is reasonable to assume that the cardiorenal protective effect of SGLT2 inhibitors is exerted via the pathway through the proximal tubule in the kidney. The cardiorenal protective mechanisms of SGLT2 inhibitors are thought to be complex, including diuretic effects that less activate the renin-angiotensin-aldosterone system with little change in intravascular volume (14, 15), suppression of sympathetic hyperactivity (16), antioxidant effects (17, 18), correction of impaired energy metabolism (19, 20), anti-inflammatory effects (21), improvement of anemia via increased erythropoietin production (22), improved iron metabolism (23), preventing fibrotic changes (24, 25), and preventing effects for the development of cardiorenal syndromes (26). Nevertheless, direct effects of SGLT2 inhibitors on cardiomyocytes, including inhibition of Na+/H+ exchanger (NHE) activity (27, 28) and suppression of late Na+ current (29–31), have also recently been reported, although it has been reported that SGLT2 inhibitors do not have an inhibitory effect on NHE activity in intact cardiomyocytes (32), and the precise mechanisms how SGLT2 inhibitors directly bind to cardiomyocytes that lack SGLT2 remain undetermined.
Although the absolute event rate of SCD is not as high as that of heart failure or chronic kidney disease (33), the impact of SCD is devasting because the people left behind have to deal with the sudden loss of a dear one without any preparation caused by a SCD event. It has been noted that fatal ventricular arrhythmias (VAs), regardless of the presence or absence of ischemic heart disease or heart failure, are involved in the majority of causes of SCD, although age and gender differences in the causes of SCD have been reported (34, 35). In addition, it has been known that the incidence of SCD via VAs is higher in patients with pre-existing heart diseases, including ischemic heart disease and cardiomyopathy (36). Indeed, an implantable cardioverter defibrillator (ICD) is recommended for the prevention of SCD in patients with documented VAs and in patients with heart disease (37). Even in individuals without cardiac disease, a recent genome-wide association study (GWAS) has proposed that risk factors for cardiac disease such as diabetes, obesity, hypertension, and dyslipidemia are risks for SCD in addition to QT interval prolongation (38), which is a well-established index of an electrocardiogram (ECG) for a risk of SCD. Interestingly, many of the pathophysiological mechanisms involved in the cardioprotective effects of SGLT2 inhibitors appear to overlap with those involved in the pathogenesis of SCD (39). However, clinical evidence on the favorable effects of SGLT2 inhibitors in preventing SCD or inhibiting VAs has been limited and remains controversial (Figure 1).
Figure 1
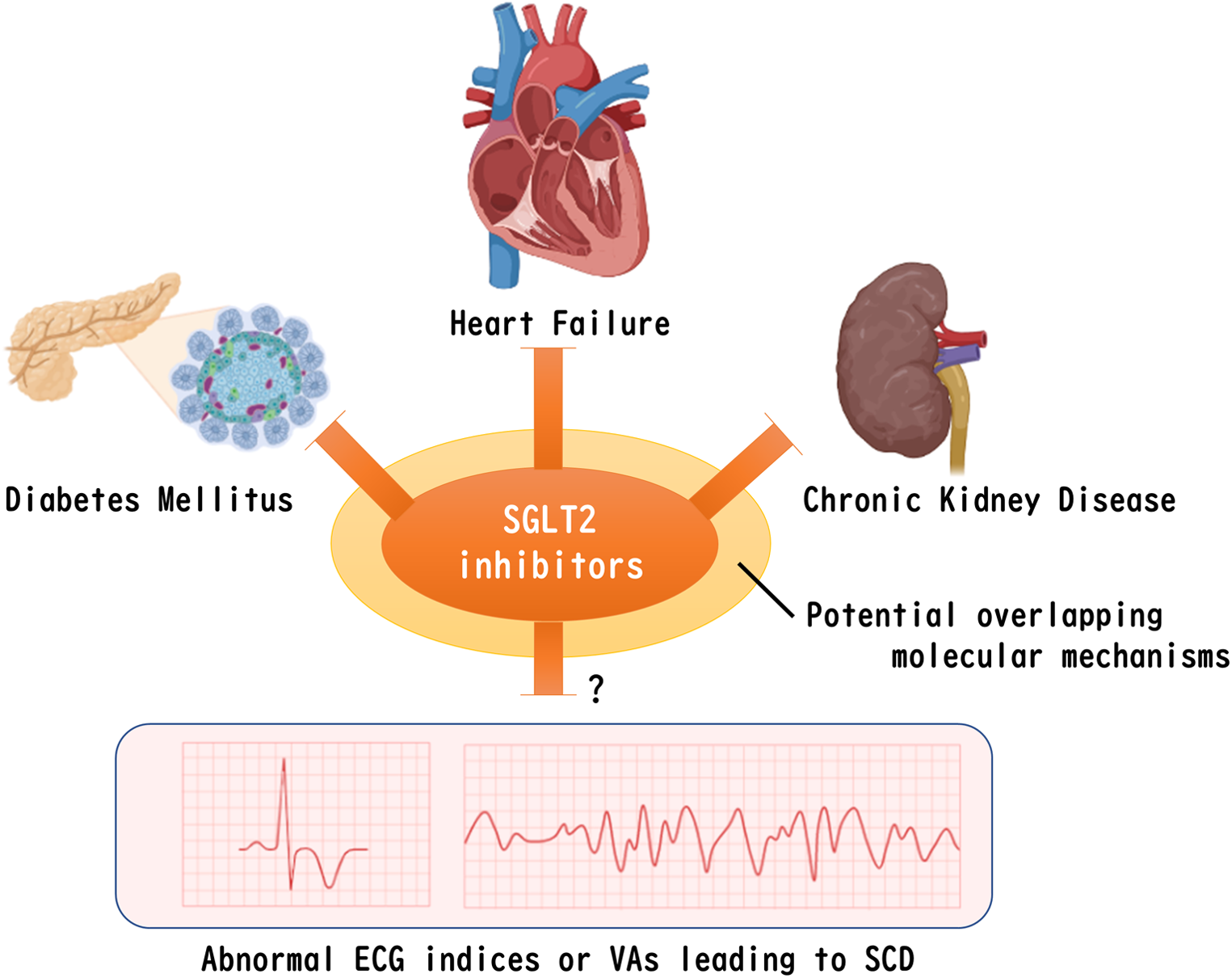
Remaining question of whether SGLT2 inhibitors improve abnormal ventricular ECG indices or VAs that leads to SCD. Despite the possible existence of a common molecular mechanism for SGLT2 inhibitors to ameliorate diabetes, heart failure, and chronic kidney disease, clinical evidence for a positive effect on VAs that leads to SCD has not yet been determined. The figure was created by using licensed BioRender. SGLT2, sodium-glucose co-transporter 2, ECG, electrocardiogram, VAs, fatal ventricular arrhythmias, SCD, sudden cardiac death.
Clinical studies of SGLT2 inhibitors focusing on SCD
Investigations to determine whether SGLT2 inhibitors reduce the incidence of SCD were examined in the EMPA-REG OUTCOME trial, which demonstrated for the first time the potential of SGLT2 inhibitors to reduce cardiovascular events in patients with type 2 diabetes mellitus who have cardiovascular disease (40). In that trial, SCD was clearly defined as the death that occurred unexpectedly in a previously stable patient and included the following conditions: (1) witnessed and instantaneous without new or worsening symptoms, (2) witnessed within 60 min of the onset of new or worsening cardiac symptoms, (3) witnessed and attributed to an identified arrhythmia, (4) individuals unsuccessfully resuscitated from cardiac arrest or successfully resuscitated from cardiac arrest but who died within 24 h without identification of a non-cardiac etiology, and (5) unwitnessed death with no conclusive evidence of another non-cardiovascular death. In a sub-analysis of this study, the pooled empagliflozin group had a 31% lower risk of SCD compared to the placebo group, but did not reach statistical superiority (event rate: 1.1% in the empagliflozin group vs. 1.6% in the placebo group; hazard ratio: 0.69; 95% confidence interval: 0.45–1.04) (41). In a similar study with other SGLT2 inhibitors, the DECLARE-TIMI 58 trial (42), treatment with dapagliflozin significantly reduced by 17% the primary endpoint of heart failure plus cardiovascular death in patients with type 2 diabetes mellitus who had high risks for cardiovascular events, including those with existing cardiovascular diseases, although such cardioprotective effects were limited to the onset of heart failure. Interestingly, the results of the DECLARE-TIMI 58 trial revealed that SCD accounted for 58% of all deaths, raising the concern that SCD in this study may include deaths from multiple causes that are considered unassessable (43). Thus, that study also highlights the difficulty of etiological interpretation using SCD as an endpoint in such large randomized controlled clinical trials.
Nevertheless, a meta-analysis of multiple clinical trials may be useful for evaluating whether SGLT2 inhibitors reduce the incidence of SCD, which is not expected to be as common an event as the development of heart failure or chronic kidney disease. Interestingly, however, the results of two recent meta-analyses were different. Fernandes et al. performed a meta-analysis of a total of 34 randomized control trials including 63,166 patients with type 2 diabetes mellitus or heart failure for evaluating the effect of SGLT2 inhibitors on SCD (44). They showed that treatment with SGLT2 inhibitors was associated with a significant reduction in the risk of SCD (odds ratio: 0.72; 95% confidence interval: 0.54–0.97) compared with that in the control group. On the other hand, Sfairopoulos et al. conducted a meta-analysis of a total 19 randomized control trials that enrolled 55,590 patients with type 2 diabetes mellitus, heart failure, or chronic kidney disease for evaluating the effect of SGLT2 inhibitors on SCD (45). They concluded that there was no significant association between therapy with SGLT2 inhibitors and SCD (risk ratio: 0.74; 95% confidence interval: 0.50–1.08) and that treatment with SGLT inhibitors was not associated with a lower risk of VAs (risk ratio: 0.84; 95% confidence interval: 0.66–1.06). In a recent cohort study other than those included in the meta-analysis, Eroglu et al. analyzed data for 152,591 patients with type 2 diabetes mellitus to examine whether the use of SGLT2 inhibitors is more closely associated than the use of other anti-diabetic agents with SCD (46). They showed that the use of SGLT2 inhibitors was associated with a trend of lower event rates for SCD compared to other antidiabetic drugs after adjustment for common risk factors of SCD, but that this association did not reach statistical significance (hazard ratio: 0.62; 95% confidence interval: 0.38–1.01), while the hazard ratio for all-cause mortality for the use of SGLT2 inhibitors vs. other anti-diabetic drugs was significantly low (hazard ratio: 0.43; 95% confidence interval: 0.39–0.48). Of note, these different results may be related not only to the kind of patients, but also to a lower event rate of SCD because there were large confidence intervals for SCD events among those studies.
On the other hand, regarding the preventive effect of SGLT2 inhibitors on SCD in a heart failure population with expected higher SCD event rates, a recent combined analysis of DAPA-HF trial [in which the left ventricular ejection fraction (LVEF) of the participants was ≤40%] and DELIVER trial (LVEF >40%) demonstrated that treatment with the SGLT2 inhibitor dapagliflozin resulted in lower rates of SCD in patients with heart failure independent of the degree of LVEF (hazard ratio: 0.84; 95% confidence interval: 0.70–1.01), although the effect marginally failed to reach the nominal threshold for statistical significance (47). Indeed, a very recent meta-analysis exclusively for patients with heart failure, including 11 randomized control trials (10,796 patients received SGLT2 inhibitors and 10,796 patients received placebo), demonstrated that treatment with SGLT2 inhibitor was associated with a significant reduction in the risk of SCD (risk ratio: 0.68; 95% confidence interval: 0.48–0.95) (48). It has also been reported that patients with both type 2 diabetes mellitus and post-myocardial infarction have a higher incidence of SCD, even in the absence of residual ischemia (49). Interestingly, a recent observational study reported that patients with type 2 diabetes mellitus who were using SGLT2 inhibitors prior to the onset of myocardial infarction had a significantly lower rate of in-hospital VAs after myocardial infarction than those who were not using SGLT2 inhibitors (50). Thus, the preventive effect of SGLT2 inhibitors on SCD, if any, may be more prominent in high-risk populations such as patients with heart failure, ischemic heart disease, and cardiomyopathy.
Overall, it appears that SGLT2 inhibitors at least tend to decrease, rather than increase, events of SCD, but further de novo studies that take into account factors of a small event rate of SCD compared with that of heart failure or kidney disease, clear definition of SCD, and patients' backgrounds, are needed to conclude whether SGLT2 inhibitors can prevent SCD and/or VAs.
Effect of SGLT2 inhibitors on ventricular indices of electrocardiograms and underlying mechanisms for their potential favorable action in the heart
To assess the safety of a newly developed drug, it must be confirmed that the drug does not affect ECG indices, especially the QT interval. Consistent with this criterion, it has been reported that SGLT2 inhibitors do not affect ECG indices, including the QT interval, in healthy individuals (51, 52). On the other hand, patients with cardiomyopathy or heart failure, regardless of their etiology, often represent an abnormal ECG that reflects impaired excitation-contraction coupling and disturbance of conduction. Interestingly, there have even been attempts to detect certain cardiac diseases through ECG diagnosis using artificial intelligence in recent years (53). QT dispersion, a classic index of ventricular repolarization heterogeneity, has been reported to be increased in individuals with cardiomyopathy, ischemic heart disease, prolonged QT syndrome, and left ventricular hypertrophy of various etiologies (54), and an increase in its index is associated with overall mortality (55). Furthermore, Tpeak-Tend, defined as the interval from the peak of the T wave to the end of the T wave in an ECG at a chest lead, has also been reported to reflect ventricular repolarization heterogeneity and to be useful for predicting VAs and SCD (56, 57). In patients with type 2 diabetes mellitus, prolonged QT dispersion or Tpeak-Tend interval have been reported by the authors and other groups to be improved by SGLT2 inhibitors (58–60). It should be noted that there is a report showing that SGLT2 inhibitors had no effects on ECG indices including QT interval in patients with type 2 diabetes in a cohort study, although indices of ventricle repolarization heterogeneity were not assessed in that study (61). Since conventional hypoglycemic agents have no effect on these indices of ventricular repolarization heterogeneity (62), the improvement of ventricular repolarization heterogeneity must be independent of its glycemic control. Indeed, the degree of improvement in ventricular repolarization heterogeneity was shown to be associated with the degree of reduction of elevated blood pressure (58) or weight loss (59). Furthermore, an abnormal QRS-T angle, another index of ventricular repolarization heterogeneity and a predictor for SCD (63), has been reported to be improved by SGLT2 inhibitors in patients with type 2 diabetes mellitus with known cardiovascular diseases (64). Taken together, the results of studies indicate that SGLT2 inhibitors may improve impaired indices of ventricular repolarization heterogeneity, but whether similar results are observed in patients with heart failure or chronic kidney disease in the absence of diabetes mellitus requires further investigation.
In case that SGLT2 inhibitors do in fact ameliorate the abnormal heterogeneity of ventricular repolarization, its underlying molecular mechanisms are of interest. As a novel molecular mechanism of SGLT2 inhibitors on isolated cardiomyocytes, several studies using rodent animals have shown that SGLT2 inhibitors suppress late Na+ current, which is increased by oxidative stress, in a calmodulin-dependent protein kinase II (CAMKII)-dependent manner (30, 31). Late Na+ current can contribute to prolonged action potential duration by increasing an inward current during the repolarization phase (65). If the finding is also observed in the human myocardium, modification of increased late Na+ current by SGLT2 inhibitors may be a mechanism for improving abnormally prolonged ventricular repolarization, resulting in amelioration of impaired ECG indices of ventricular repolarization heterogeneity. In addition to the direct effects of SGLT2 inhibitors, it has been reported that systemic administration of SGLT2 inhibitors exerts antioxidant effects via increased ketone bodies in the blood (66) and improved metabolic flexibility with restored mitochondrial function in the heart (67). These indirect effects of SGLT2 inhibitors may also be involved in the suppression of cardiac late Na+ current via reduced oxidative stress. Another possibility of impaired repolarization in the failing cardiomyocytes is that voltage-gated K+ currents, which contribute to regulating repolarization heterogeneity (68), have been reported to be reduced in rodent models of diabetes mellitus and/or heart failure (69, 70). Indeed, there are reports that SGLT2 inhibitors can promote K+ currents that enhance repolarization in different species (71–74). Besides acting on ion channels themselves, SGLT2 inhibitors may also improve ECG abnormalities via improved serum electrolytes. In fact, SGLT2 inhibitors have been suggested to improve abnormal serum levels of potassium and/or magnesium (75–77), leading to improved ECG abnormalities independently of the direct effect of ion channels. In addition, increased myocardial stretch is known to promote arrhythmogenesis via modification of calcium handling (78), activation of stretch-induced angiotensin II type 1 receptor signaling (79), and activation of stretch-induced ion currents (80). Since it has been reported that SGLT2 inhibitors have a potential to reduce cardiac volumes (81), improved myocardial stretch by SGLT2 inhibitors in patients with heart failure, at least partially, could explain the anti-arrhythmic effect of SGLT2 inhibitors. Furthermore, the arrhythmogenic potential is also promoted not only by single myocyte abnormalities, but also by aberrant conduction via myocardial injury-induced scar (82). Indeed, a recent study demonstrated that administration of SGLT2 inhibitors ameliorates ischemia/reperfusion injury via reduction of microvascular obstruction and increases myocardial salvage (83), suggesting that the cardioprotective effect of SGLT2 inhibitors on myocardial injury may be indirectly related to another benefit of SGLT2 inhibitors on VAs. These reported findings suggest that SGLT2 inhibitors have potential protective effects on SCD and/or VAs via electrophysiologic mechanisms, and further studies in this area are warranted.
Limitation
The recent COVID-19 pandemic, at least partially, has affected several recent clinical trials, although it has been shown that SGLT2 inhibitors failed to improve outcomes in patients with COVID-19 (84). The possibility that some events of SCD may have been caused by COVID-19 rather than VAs or cardiac events cannot be ruled out. Therefore, caution is needed in interpreting the results of recent clinical trials and the meta-analysis including the studies that were affected by COVID-19. To address questions of whether SGLT2 inhibitors decrease events of SCD, it is necessary to obtain direct evidence of whether SGLT2 inhibitors reduce VAs or not in various patients. For example, results of ongoing clinical trials, including those evaluating the incidence of arrhythmic events before and after SGLT2 inhibitor treatment in patients with type 2 diabetes mellitus with ICD (85) and in patients with heart failure with ICD (86), may provide a major clue for revealing the association between the use of SGLT2 inhibitors and the incidence of SCD as well as VAs. Finally, in terms of arrhythmias, this review did not address the effect of SGLT2 inhibitors on atrial fibrillation, but SGLT2 inhibitors are likely to be effective in preventing the onset of atrial fibrillation (87, 88).
Conclusion and perspective
SGLT2 inhibitors have the potential to improve abnormal electrophysiological remodeling in the failing myocardium, possibly leading to a reduction in the incidence of SCD and VAs. Clinical studies using SGLT2 inhibitors with a clear definition of SCD and VAs may help to answer the remaining question of whether SGLT2 inhibitors can reduce SCD and/or VAs. Resolving these questions also has the potential to advance current treatment strategies for the prevention of SCD.
Statements
Author contributions
TS wrote the original draft. HK, TY, IS, MF, and NT provided significant suggestions and revisions from their respective professional standpoints. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by the educational research grant of Sapporo Medical University and JSPS KAKENHI (22K08210).
Acknowledgments
We acknowledge S.E.S. Translation and Proofreading Services for editing and proofreading this manuscript.
Conflict of interest
TS, HK, and TY have been engaged in a research project on SGLT2 inhibitors funded by Kowa Co., Ltd. TS has research grants from Kowa Co. Ltd. and Taisho Pharmaceutical Co., Ltd. HK has a research grant from Eli Lilly Japan K.K. IS received research grants from AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd. TS, TY, and MF received honoraria from Eli Lilly Japan K.K. TS, HK, TY, and MF received honoraria from Nippon Boehringer lngelheim Co., Ltd. TS, HK, TY, and MF received honoraria from AstraZeneca K.K. TS, HK, TY, and MF received honoraria from Ono Pharmaceutical Co., Ltd. TS, TY, and MF received honoraria from Mitsubishi Tanabe Pharma Corporation. TS, TY, and MF received honoraria from Daiichi Sankyo Co., Ltd. TS and MF received honoraria from Taisho Pharmaceutical Co., Ltd. TS, HK, TY, IS, and MF received honoraria from Kowa Co., Ltd. TS, TY, and MF received honoraria from Astellas Pharma Inc.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor IM declared a past co-authorship with the author IS.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Kubota Y Shimizu W . Clinical benefits of sodium-glucose cotransporter 2 inhibitors and the mechanisms underlying their cardiovascular effects. JACC Asia. (2022) 2(3):287–93. 10.1016/j.jacasi.2022.03.009
2.
Abdul-Ghani MA Norton L DeFronzo RA . Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. (2015) 309(11):F889–900. 10.1152/ajprenal.00267.2015
3.
McMurray JJV Solomon SD Inzucchi SE Køber L Kosiborod MN Martinez FA et al Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. 10.1056/NEJMoa1911303
4.
Packer M Anker SD Butler J Filippatos G Pocock SJ Carson P et al Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383(15):1413–24. 10.1056/NEJMoa2022190
5.
Heerspink HJL Stefánsson BV Correa-Rotter R Chertow GM Greene T Hou F et al Dapagliflozin in patients with chronic kidney disease. N Engl J Med. (2020) 383(15):1436–46. 10.1056/NEJMoa2024816
6.
Anker SD Butler J Filippatos G Ferreira JP Bocchi E Böhm M et al Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. 10.1056/NEJMoa2107038
7.
Solomon SD McMurray JJV Claggett B Boer RA DeMets D Hernandez AF et al Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. 10.1056/NEJMoa2206286
8.
Herrington WG Staplin N Wanner C Green JB Hauske SJ Emberson JR et al Empagliflozin in patients with chronic kidney disease. N Engl J Med. (2023) 388(2):117–27. 10.1056/NEJMoa2204233
9.
Heidenreich PA Bozkurt B Aguilar D Allen LA Byun JJ Colvin MM et al 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(18):e895–1032. 10.1161/CIR.0000000000001063
10.
Rossing P Caramori ML Chan JCN Heerspink HJL Hurst C Khunti K . Executive summary of the KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. (2022) 102(5):990–9. 10.1016/j.kint.2022.06.013
11.
ElSayed NA Aleppo G Aroda VR Bannuru RR Brown FM Bruemmer D et al 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. (2023) 46(Suppl 1):S97–110. 10.2337/dc23-S006
12.
Song P Onishi A Koepsell H Vallon V . Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets. (2016) 20(9):1109–25. 10.1517/14728222.2016.1168808
13.
Nair S Wilding JPH . Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. (2010) 95(1):34–42. 10.1210/jc.2009-0473
14.
Hallow KM Helmlinger G Greasley PJ McMurray JJV Boulton DW . Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. (2018) 20(3):479–87. 10.1111/dom.13126
15.
Nakagaito M Joho S Ushijima R Nakamura M Kinugawa K . Comparison of canagliflozin, dapagliflozin and empagliflozin added to heart failure treatment in decompensated heart failure patients with type 2 diabetes Mellitus. Circ Rep. (2019) 1(10):405–13. 10.1253/circrep.CR-19-0070
16.
Herat LY Magno AL Rudnicka C Hricova J Carnagarin R Ward NC et al SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci. (2020) 5(2):169–79. 10.1016/j.jacbts.2019.11.007
17.
Oshima H Miki T Kuno A Mizuno M Sato T Tanno M et al Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. (2019) 368(3):524–34. 10.1124/jpet.118.253666
18.
Tsai K Chen Y Chiou TT Chu T Li L Ng H et al Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants (Basel). (2021) 10(8):1166. 10.3390/antiox10081166
19.
Santos-Gallego CG Requena-Ibanez JA Antonio RS Ishikawa K Watanabe S Picatoste B et al Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. (2019) 73(15):1931–44. 10.1016/j.jacc.2019.01.056
20.
Gao Y Feng S Wen Y Tang T Wang B Liu B et al Cardiorenal protection of SGLT2 inhibitors-perspectives from metabolic reprogramming. EBioMedicine. (2022) 83:104215. 10.1016/j.ebiom.2022.104215
21.
Scisciola L Cataldo V Taktaz F Fontanella RA Pesapane A Ghosh P . Anti-inflammatory role of SGLT2 inhibitors as part of their anti-atherosclerotic activity: data from basic science and clinical trials. Front Cardiovasc Med. (2022) 9:1008922. 10.3389/fcvm.2022.1008922
22.
Sano M Goto S . Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. (2019) 139(17):1985–7. 10.1161/CIRCULATIONAHA.118.038881
23.
Packer M . Potential interactions when prescribing SGLT2 inhibitors and intravenous iron in combination in heart failure. JACC Heart Fail. (2023) 11(1):106–14. 10.1016/j.jchf.2022.10.004
24.
Requena-Ibáñez JA Santos-Gallego CG Rodriguez-Cordero A Vargas-Delgado AP Mancini D Sartori S et al Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. (2021) 9(8):578–89. 10.1016/j.jchf.2021.04.014
25.
Chen X Yang Q Bai W Yao W Liu L Xing Y et al Dapagliflozin attenuates myocardial fibrosis by inhibiting the TGF-β1/smad signaling pathway in a normoglycemic rabbit model of chronic heart failure. Front Pharmacol. (2022) 13:873108. 10.3389/fphar.2022.873108
26.
Salvatore T Galiero R Caturano A Rinaldi L Martino AD Albanese G . An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. (2022) 23(7):3651. 10.3390/ijms23073651
27.
Baartscheer A Schumacher CA Wüst RCI Fiolet JWT Stienen GJM Coronel R et al Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. (2017) 60(3):568–73. 10.1007/s00125-016-4134-x
28.
Uthman L Baartscheer A Bleijlevens B Schumacher CA Fiolet JWT Koeman A et al Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. (2018) 61(3):722–6. 10.1007/s00125-017-4509-7
29.
Philippaert K Kalyaanamoorthy S Fatehi M Long W Soni S Byrne NJ et al Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. (2021) 143(22):2188–204. 10.1161/CIRCULATIONAHA.121.053350
30.
Hegyi B Hernandez JM Shen EY Habibi NR Bossuyt J Bers DM . Empagliflozin reverses late Na+ current enhancement and cardiomyocyte proarrhythmia in a translational murine model of heart failure with preserved ejection fraction. Circulation. (2022) 145(13):1029–31. 10.1161/CIRCULATIONAHA.121.057237
31.
Mustroph J Baier MJ Pabel S Stehle T Trum M Provaznik Z et al Empagliflozin inhibits cardiac late sodium current by ca/calmodulin-dependent kinase II. Circulation. (2022) 146(16):1259–61. 10.1161/CIRCULATIONAHA.122.057364
32.
Chung YJ Park KC Tokar S Eykyn TR Fuller W Pavlovic D et al Off-target effects of sodium-glucose co-transporter 2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart. Cardiovasc Res. (2021) 117(14):2794–806. 10.1093/cvr/cvaa323
33.
Adabag AS Luepker RV Roger VL Gersh BJ . Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. (2010) 7(4):216–25. 10.1038/nrcardio.2010.3
34.
Lynge TH Nielsen JL Risgaard B Werf C Winkel BG Tfelt-Hansen J . Causes of sudden cardiac death according to age and sex in persons aged 1-49 years. Heart Rhythm. (2023) 20(1):61–8. 10.1016/j.hrthm.2022.08.036
35.
Skjelbred T Rajan D Svane J Lynge TH Tfelt-Hansen J . Sex differences in sudden cardiac death in a nationwide study of 54 028 deaths. Heart. (2022) 108(13):1012–8. 10.1136/heartjnl-2021-320300
36.
Hayashi M Shimizu W Albert CM . The spectrum of epidemiology underlying sudden cardiac death. Circ Res. (2015) 116(12):1887–906. 10.1161/CIRCRESAHA.116.304521
37.
Zeppenfeld K Tfelt-Hansen J Riva M Winkel BG Behr ER Blom NA et al 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. (2022) 43(40):3997–4126. 10.1093/eurheartj/ehac262
38.
Ashar FN Mitchell RN Albert CM Newton-Cheh C Brody JA Müller-Nurasyid M . A comprehensive evaluation of the genetic architecture of sudden cardiac arrest. Eur Heart J. (2018) 39(44):3961–9. 10.1093/eurheartj/ehy474
39.
Deo R Albert CM . Epidemiology and genetics of sudden cardiac death. Circulation. (2012) 125(4):620–37. 10.1161/CIRCULATIONAHA.111.023838
40.
Zinman B Wanner C Lachin JM Fitchett D Bluhmki E Hantel S et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373(22):2117–28. 10.1056/NEJMoa1504720
41.
Inzucchi SE Zinman B McGinniss J Schnee J George J Fitchett D . Consistent effect of empagliflozin on composite outcomes related to heart failure: results from empa-reg outcome. J Am Coll Cardiol. (2017) 69(11 Suppl):1656. 10.1016/S0735-1097(17)35045-3
42.
Wiviott SD Raz I Bonaca MP Mosenzon O Kato ET Cahn A et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380(4):347–57. 10.1056/NEJMoa1812389
43.
Terpening TM . A call for more complete reporting of cardiovascular death. Circulation. (2019) 140(11):887–8. 10.1161/CIRCULATIONAHA.119.041607
44.
Fernandes GC Fernandes A Cardoso R Penalver J Knijnik L Mitrani RD et al Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: a meta-analysis of 34 randomized controlled trials. Heart Rhythm. (2021) 18(7):1098–105. 10.1016/j.hrthm.2021.03.028
45.
Sfairopoulos D Zhang N Wang Y Chen Z Letsas KP Tse G et al Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: a meta-analysis of randomized controlled trials. Europace. (2022) 24(1):20–30. 10.1093/europace/euab177
46.
Eroglu ET Coronel R Zuurbier CJ Blom M Boer A Souverein PK . Use of sodium-glucose cotransporter-2 inhibitors and the risk for sudden cardiac arrest and for all-cause death in patients with type 2 diabetes mellitus. Eur Heart J Cardiovasc Pharmacother. (2022) 9(1):18–25. 10.1093/ehjcvp/pvac043
47.
Desai AS Jhund PS Claggett BL Vaduganathan M Miao ZM Kondo T et al Effect of dapagliflozin on cause-specific mortality in patients with heart failure across the Spectrum of ejection fraction: a participant-level pooled analysis of DAPA-HF and DELIVER. JAMA Cardiol. (2022) 7(12):1227–34. 10.1001/jamacardio.2022.3736
48.
Oates CP Santos-Gallego CG Smith A Basyal B Moss N Kawamura I et al SGLT2 inhibitors reduce sudden cardiac death risk in heart failure: meta-analysis of randomized clinical trials. J Cardiovasc Electrophysiol. Mar. (2023) 23. 10.1111/jce.15894. In Press
49.
Yeung C Lam KS Li S Lam K Tse H Siu C . Sudden cardiac death after myocardial infarction in type 2 diabetic patients with no residual myocardial ischemia. Diabetes Care. (2012) 35(12):2564–9. 10.2337/dc12-0118
50.
Cesaro A Gragnano F Paolisso P Bergamaschi L Gallinoro E Sardu C et al In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: insights from the SGLT2-I AMI PROTECT study. Front Cardiovasc Med. (2022) 27(9):1012220. 10.3389/fcvm.2022.1012220
51.
Carlson GF Tou CKP Parikh S Birmingham BK Butler K . Evaluation of the effect of dapagliflozin on cardiac repolarization: a thorough QT/QTc study. Diabetes Ther. (2011) 2(3):123–32. 10.1007/s13300-011-0003-2
52.
Ring A Brand T Macha S Breithaupt-Groegler K Simons G Walter B et al The sodium glucose cotransporter 2 inhibitor empagliflozin does not prolong QT interval in a thorough QT (TQT) study. Cardiovasc Diabetol. (2013) 12:70. 10.1186/1475-2840-12-70
53.
Siontis KC Noseworthy PA Attia ZI Friedman PA . Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol. (2021) 18(7):465–78. 10.1038/s41569-020-00503-2
54.
Malik M Batchvarov VN . Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. (2000) 36(6):1749–66. 10.1016/s0735-1097(00)00962-1
55.
Okin PM Devereux RB Howard BV Fabsitz RR Lee ET Welty KT . Assessment of QT interval and QT dispersion for prediction of all-cause and cardiovascular mortality in American Indians: the strong heart study. Circulation. (2000) 101(1):61–6. 10.1161/01.cir.101.1.61
56.
Prenner SB Shah SJ Goldberger JJ Sauer AJ . Repolarization heterogeneity: beyond the QT interval. J Am Heart Assoc. (2016) 5(5):e003607. 10.1161/JAHA.116.003607
57.
Tse G Gong M Wong WT Georgopoulos S Letsas KP Vassiliou VS et al The tpeak—tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: a systematic review and meta-analysis. Heart Rhythm. (2017) 14(8):1131–7. 10.1016/j.hrthm.2017.05.031
58.
Sato T Miki T Ohnishi H Yamashita T Takada A Yano T et al Effect of sodium-glucose co-transporter-2 inhibitors on impaired ventricular repolarization in people with type 2 diabetes. Diabet Med. (2017) 34(10):1367–71. 10.1111/dme.13424
59.
Sato T Miki T Furukawa S Matsuura B Hiasa Y Ohnishi H et al Longitudinal impact of dapagliflozin treatment on ventricular repolarization heterogeneity in patients with type 2 diabetes. J Diabetes Investig. (2019) 10(6):1593–4. 10.1111/jdi.13063
60.
Duran M Ziyrek M Alsancak M . Effects of SGLT2 inhibitors as an add-on therapy to metformin on electrocardiographic indices of ventricular repolarization. Acta Cardiol Sin. (2020) 36(6):626–32. 10.6515/ACS.202011_36(6).20200511A
61.
Wu VC Chiu K Wang C Hsu C Tu H Huang Y et al Electrocardiographic changes associated with SGLT2 inhibitors and non-SGLT2 inhibitors: a multi-center retrospective study. Front Cardiovasc Med. (2022) 9:934193. 10.3389/fcvm.2022.934193
62.
Miki T Tobisawa T Sato T Tanno M Yano T Akasaka H et al Does glycemic control reverse dispersion of ventricular repolarization in type 2 diabetes? Cardiovasc Diabetol. (2014) 13:125. 10.1186/s12933-014-0125-8
63.
Aro AL Huikuri HV Tikkanen JT Junttila MJ Rissanen HA Reunanen A et al QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace. (2012) 14(6):872–6. 10.1093/europace/eur393
64.
Eyuboglu M Celik A . Empagliflozin has favourable effect on frontal plane QRS-T angle in diabetic patients with cardiovascular disease. J Clin Pharm Ther. (2022) 47(11):1783–8. 10.1111/jcpt.13734
65.
Horváth B Hézső T Kiss D Kistamás K Magyar J Nánási PP et al Late sodium current inhibitors as potential antiarrhythmic agents. Front Pharmacol. (2020) 11:413. 10.3389/fphar.2020.00413
66.
Ferrannini E Baldi S Frascerra S Astiarraga B Heise T Bizzotto R et al Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. (2016) 65(5):1190–5. 10.2337/db15-1356
67.
Santos-Gallego CG Mayr M Badimon J . SGLT2 inhibitors in heart failure: targeted metabolomics and energetic metabolism. Circulation. (2022) 146(11):819–21. 10.1161/CIRCULATIONAHA.122.060805
68.
Meo M Meste O Signore S Sorrentino A Cannata A Zhou Y et al Reduction in Kv current enhances the temporal dispersion of the action potential in diabetic myocytes: insights from a novel repolarization algorithm. J Am Heart Assoc. (2016) 5(2):e003078. 10.1161/JAHA.115.003078
69.
Sato T Kobayashi T Kuno A Miki T Tanno M Kouzu H et al Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol Heart Circ Physiol. (2014) 306(7):H1054–65. 10.1152/ajpheart.00414.2013
70.
Näbauer M Kääb S . Potassium channel down-regulation in heart failure. Cardiovasc Res. (1998) 37(2):324–34. 10.1016/s0008-6363(97)00274-5
71.
Durak A Olgar Y Degirmenci S Akkus E Tuncay E Turan B . A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc Diabetol. (2018) 17(1):144. 10.1186/s12933-018-0790-0
72.
Hasan A Hasan R . Empagliflozin relaxes resistance mesenteric arteries by stimulating multiple smooth muscle cell voltage-gated K+ (KV) channels. Int J Mol Sci. (2021) 22(19):10842. 10.3390/ijms221910842
73.
Karpushev AV Mikhailova VB Klimenko ES Kulikov AN Ivkin DY Kaschina E et al SGLT2 inhibitor empagliflozin modulates ion channels in adult zebrafish heart. Int J Mol Sci. (2022) 23(17):9559. 10.3390/ijms23179559
74.
Barış VÖ Dinçsoy B Gedikli E Erdemb A . Empagliflozin significantly attenuates sotalol-induced QTc prolongation in rats. Kardiol Pol. (2021) 79(1):53–7. 10.33963/KP.15666
75.
Filippatos TD Tsimihodimos V Liamis G Elisaf MS . SGLT2 inhibitors-induced electrolyte abnormalities: an analysis of the associated mechanisms. Diabetes Metab Syndr. (2018) 12(1):59–63. 10.1016/j.dsx.2017.08.003
76.
Zhang J Huan Y Leibensperger M Seo B Song Y . Comparative effects of sodium-glucose cotransporter 2 inhibitors on Serum electrolyte levels in patients with type 2 diabetes: a pairwise and network meta-analysis of randomized controlled trials. Kidney360. (2022) 3(3):477–87. 10.34067/KID.0006672021
77.
Neuen BL Oshima M Agarwal R Arnott C Cherney DZ Edwards R et al Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. (2022) 145(19):1460–70. 10.1161/CIRCULATIONAHA.121.057736
78.
Kaur S Shen X Power A Ward M . Stretch modulation of cardiac contractility: importance of myocyte calcium during the slow force response. Biophys Rev. (2020) 12(1):135–42. 10.1007/s12551-020-00615-6
79.
Seo K Parikh VN Ashley EA . Stretch-induced biased signaling in angiotensin II type 1 and apelin receptors for the mediation of cardiac contractility and hypertrophy. Front Physiol. (2020) 11:181. 10.3389/fphys.2020.00181
80.
Kamkin A Kiseleva I Isenberg G . Stretch-activated currents in ventricular myocytes: amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc Res. (2000) 48(3):409–20. 10.1016/s0008-6363(00)00208-x
81.
Santos-Gallego CG Vargas-Delgado AP Requena-Ibanez JA Garcia-Ropero A Mancini D Pinney S et al Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2021) 77(3):243–55. 10.1016/j.jacc.2020.11.008
82.
Amoni M Dries E Ingelaere S Vermoortele D Roderick HL Claus P . Ventricular arrhythmias in ischemic cardiomyopathy-new avenues for mechanism-guided treatment. Cells. (2021) 10(10):2629. 10.3390/cells10102629
83.
Santos-Gallego CG Requena-Ibáñez JA Picatoste B Fardman B Ishikawa K Mazurek R et al Cardioprotective effect of empagliflozin and circulating ketone bodies during acute myocardial infarction. Circ Cardiovasc Imaging. (2023) 16(4):e015298. 10.1161/CIRCIMAGING.123.015298
84.
Kosiborod MN Esterline R Furtado RMH Oscarsson J Gasparyan SB Koch GG et al Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. (2021) 9(9):586–94. 10.1016/S2213-8587(21)00180-7
85.
Fujiki S Iijima K Okabe M Niwano S Tsujita K Naito S et al Placebo-controlled, double-blind study of empagliflozin (EMPA) and implantable cardioverter-defibrillator (EMPA-ICD) in patients with type 2 diabetes (T2DM): rationale and design. Diabetes Ther. (2020) 11(11):2739–55. 10.1007/s13300-020-00924-9
86.
Lewinski D Tripolt NJ Sourij H Pferschy PN Oulhaj A Alber H et al Ertugliflozin to reduce arrhythmic burden in ICD/CRT patients (ERASe-trial)—a phase III study. Am Heart J. (2022) 246:152–60. 10.1016/j.ahj.2022.01.008
87.
Li W Chen X Xu L Li Y Luo B . SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. (2020) 19(1):130. 10.1186/s12933-020-01105-5
88.
Zhuo M D'Andrea E Paik JM Wexler DJ Everett BM Glynn RJ et al Association of sodium-glucose cotransporter-2 inhibitors with incident atrial fibrillation in older adults with type 2 diabetes. JAMA Netw Open. (2022) 5(10):e2235995. 10.1001/jamanetworkopen.2022.35995
Summary
Keywords
SGLT2 inhibitors, sudden cardiac death (SCD), ventricular arrhythmia (VAs), electrophysiology, mini review
Citation
Sato T, Kouzu H, Yano T, Sakuma I, Furuhashi M and Tohse N (2023) Potential favorable action of sodium-glucose cotransporter-2 inhibitors on sudden cardiac death: a brief overview. Front. Cardiovasc. Med. 10:1159953. doi: 10.3389/fcvm.2023.1159953
Received
06 February 2023
Accepted
21 April 2023
Published
12 May 2023
Volume
10 - 2023
Edited by
Ichiro Manabe, Chiba University, Japan
Reviewed by
Gwo-Ping Jong, Chung Shan Medical University Hospital, Taiwan Manuel Kraft, Heidelberg University Hospital, Germany Ting-Yung Chang, Taipei Veterans General Hospital, Taiwan Carlos Garcia Santos-Gallego, Mount Sinai Hospital, United States
Updates
Copyright
© 2023 Sato, Kouzu, Yano, Sakuma, Furuhashi and Tohse.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Tatsuya Sato sato.tatsuya@sapmed.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.