- 1Houston Methodist-DeBakey Heart and Vascular Center, Houston, TX, United States
- 2Aurora St. Luke’s Medical Center, Milwaukee, WI, United States
- 3Medtronic, Minneapolis, MN, United States
A Commentary on
By Senguttuvan NB, Bhatt H, Balakrishnan VK, Krishnamoorthy P, Goel S, Reddy PMK, Subramanian V, Claessen BE, Kumar A, Majmundar M, Ro R, Lerakis S, Jayaraj R, Kalra A, Flather M, Dangas G, Tang GHL (2023). Front. Cardiovasc. Med. 10: doi: 10.3389/fcvm.2023.1130354
We read with interest the meta-analysis of Senguttuvan and colleagues (1) that concludes that balloon-expandable (BE) transcatheter aortic valve replacement (TAVR) is associated with reduced all-cause mortality and cardiovascular mortality at 30 days compared to self-expanding (SE) TAVR in high surgical risk patients. Their conclusions underscore the inherent limitations of meta-analyses, such as mixing historical randomized controlled trials (RCTs) and registries that are nearly a decade old, use of early term (30-day) rather than long term (up to 5 years) outcomes, and incomplete propensity matching of very different risk populations. Most importantly, the authors grouped all SE bioprostheses together, when, in fact, there may be important differences amongst SE devices. For example, a network analysis of BE Sapien TAVR (Edwards Lifesciences, USA) and SE CoreValve/Evolut TAVR (Medtronic, USA) RCTs in high risk patients would use surgery as the comparator group for both devices. The BE Sapien PARTNER 1A RCT in high surgical risk patients showed similar rates of 30-day (TAVR, 3.4%; surgery, 6.5%; P = 0.07) and 1-year (TAVR, 24.2%; surgery, 26.8%; P = 0.44) all-cause mortality in the two groups (Figure 1A) (2). The SE CoreValve high surgical risk RCT found similar rates of 30-day mortality (TAVR, 3.3%; surgery, 4.5%; P = 0.43), but a statistically 1-year lower mortality with TAVR compared with surgery (TAVR, 14.2%; surgery, 19.1%; P = 0.04) (Figure 1B) (3).
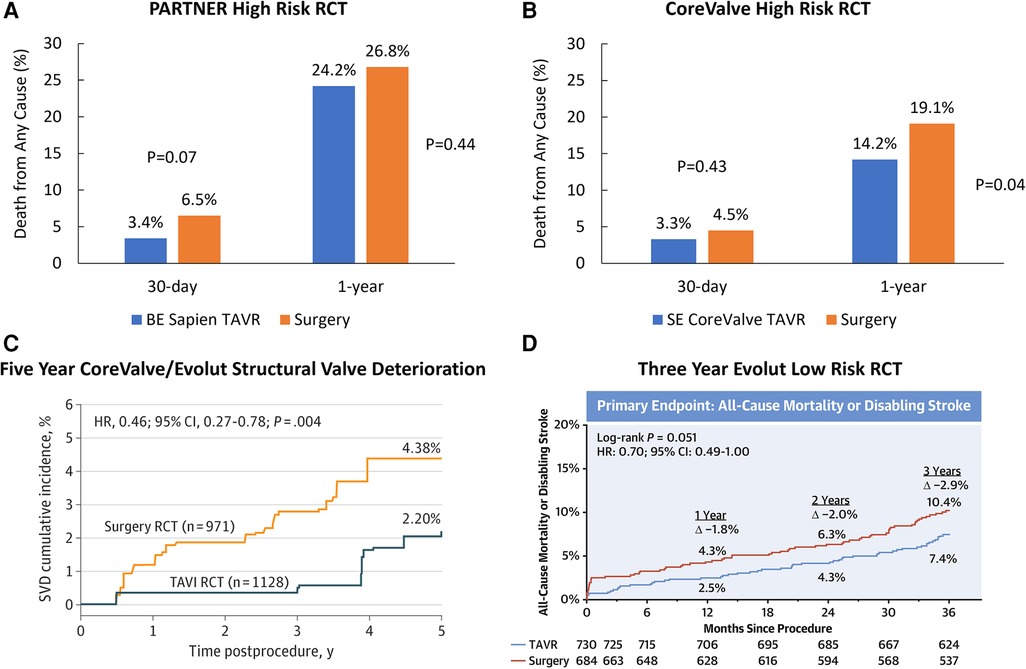
Figure 1. Randomized controlled trials comparing TAVR with surgery. (A) Primary endpoint of the PARTNER 1 A randomized controlled trial in high surgical risk patients. (B) Primary endpoint of the CoreValve high risk randomized controlled trial in high surgical risk patients. (C) Rates of structural valve deterioration in patients treated with CoreValve/Evolut TAVR or surgery. Reproduced with permission from O'Hair D, Yakubov SJ, Grubb KJ, Oh JK, Ito S, Deeb GM, et al. Structural Valve Deterioration After Self-Expanding Transcatheter or Surgical Aortic Valve Implantation in Patients at Intermediate or High Risk. JAMA Cardiol. 2022. Copyright© 2022 American Medical Association. All rights reserved. (D) Three Year primary endpoint in the Evolut low risk randomized controlled trial. Reprinted from Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J Am Coll Cardiol. 2023;81(17):1663–74, with permission from Elsevier.
With respect to long-term valve durability, the 5-year outcomes of the PARTNER IIA RCT in patients at intermediate surgical risk found similar rates of structural valve deterioration (SVD) between BE Sapien 3 and surgery (TAVR, 3.9%; surgery 3.5%; P = 0.65), and a higher rate of SVD with BE Sapien XT (TAVR, 9.5%; surgery 3.5%; P < 0.001) (4). The 5-year durability post-hoc analysis performed in similar intermediate and high risk patients showed lower rates of SVD with SE CoreValve/Evolut TAVR compared to surgery (TAVR, 2.2%; surgery, 4.4%; P = 0.004) (5) (Figure 1C). In lower surgical risk patients, there were no differences in death or disabling stroke in patients treated with BE Sapien 3 vs. surgery at 2 years (TAVR 3.0%; surgery 3.8%; P = 0.47), although valve thrombosis at 2 years was higher after TAVR (TAVR 2.6%; surgery, 0.7%; P = 0.02) (6). The Evolut low risk RCT found a numerically lower rate of all-cause mortality or disabling stroke in patients treated with SE Evolut TAVR compared to surgery at 3 years (TAVR, 7.4%; surgery, 10.4%; P = 0.051). (Figure 1D) (7).
Nevertheless, these comparative RCT findings vs. surgery as the common denominator are not conclusive, underscoring the need for specific device-device RCTs. The SMART trial (ClinicalTrials.gov Identifier: NCT04464421) has completed randomization of 700 patients with a small (<430 mm2) aortic annulus treated with BE Sapien 3 or SE Evolut TAVR, and will compare valve performance at 1 and 5 years. More direct comparative analyses are needed before drawing any conclusions that one class of device is safer than another from these types of meta-analyses, particularly those that are not concordant with prospective RCTs. Meta-analysis as a statistical combination of the outcomes of different trials is limited by the quality of the studies included, the heterogeneity of the individual studies, as well as potential publication bias.
Author contributions
MR: Writing – original draft, Writing – review & editing. TB: Writing – original draft, Writing – review & editing. JP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Andrés Caballero, an employee of Medtronic, assisted in the drafting of the general commentary.
Conflict of interest
MR reports receiving personal consulting fees from Abbott, Boston Scientific, Gore Medical and Medtronic outside of the submitted work. TB serves as a proctor for Medtronic and reports receiving personal consulting fees from Medtronic outside the submitted work. JP is an employee and shareholder of Medtronic.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Senguttuvan NB, Bhatt H, Balakrishnan VK, Krishnamoorthy P, Goel S, Reddy PMK, et al. The safety and efficacy of balloon-expandable versus self-expanding trans-catheter aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis. Front Cardiovasc Med. (2023) 10:1130354. doi: 10.3389/fcvm.2023.1130354
2. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364(23):2187–98. doi: 10.1056/NEJMoa1103510
3. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. (2014) 370(19):1790–8. doi: 10.1056/NEJMoa1400590
4. Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM, et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. (2020) 76(16):1830–43. doi: 10.1016/j.jacc.2020.08.049
5. O'Hair D, Yakubov SJ, Grubb KJ, Oh JK, Ito S, Deeb GM, et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. (2022) 8(2):111–9. doi: 10.1001/jamacardio.2022.4627
6. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. (2021) 77(9):1149–61. doi: 10.1016/j.jacc.2020.12.052
Keywords: TAVR, TAVI, balloon-expandable, self-expanding, aortic stenosis, randomized controlled trial
Citation: Reardon MJ, Bajwa T and Popma JJ (2023) Commentary: The safety and efficacy of balloon-expandable vs. self-expanding trans-catheter aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis. Front. Cardiovasc. Med. 10:1295274. doi: 10.3389/fcvm.2023.1295274
Received: 15 September 2023; Accepted: 1 December 2023;
Published: 18 December 2023.
Edited by:
Luca Testa, IRCCS San Donato Polyclinic, ItalyReviewed by:
Andreas Schaefer, University Medical Center Hamburg-Eppendorf, Germany© 2023 Reardon, Bajwa and Popma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Reardon bXJlYXJkb25AaG91c3Rvbm1ldGhvZGlzdC5vcmc=
 Michael J. Reardon
Michael J. Reardon Tanvir Bajwa2
Tanvir Bajwa2