Abstract
Background:
Several studies have shown that serum copper levels are related to coronary heart disease, diabetes, and cancer. However, the association of serum copper levels with all-cause, cause-specific [including cardiovascular disease (CVD) and cancer] mortality remains unclear.
Objectives:
This study aimed to prospectively examine the association of copper exposure with all-cause, CVD, and cancer mortality among US adults.
Methods:
The data for this analysis was obtained from the National Health and Nutrition Examination Survey (NHANES) between 2011 and 2014. Mortality from all-causes, CVD, and cancer mortality was linked to US National Death Index mortality data. Cox regression models were used to estimate the association between serum copper levels and all-cause, CVD, and cancer mortality.
Results:
A total of 2,863 adults were included in the main study. During the mean follow-up time of 81.2 months, 236 deaths were documented, including 68 deaths from cardiovascular disease and 57 deaths from cancer. The weighted mean overall serum copper levels was 117.2 ug/L. After adjusting for all of the covariates, compared with participants with low (1st tertile, <103 μg/L)/medium (2st tertile, 103–124 μg/L) serum copper levels, participants with high serum copper levels (3rd tertile, ≥124 μg/L) had a 1.75-fold (95% CI, 1.05–2.92)/1.78-fold (1.19,2.69) increase in all-cause mortality, a 2.35-fold (95% CI, 1.04–5.31)/3.84-fold (2.09,7.05) increase in CVD mortality and a 0.97-fold (95% CI, 0.28–3.29)/0.86-fold (0.34,2.13) increase in cancer mortality. In addition, there was a linear dose-response association between serum copper concentration with all-cause and CVD mortality (P for nonlinear > 0.05).
Conclusions:
This prospective study found that serum copper concentrations were linearly associated with all-cause and CVD mortality in US adults. High serum copper levels is a risk factor for all-cause and CVD mortality.
1 Introduction
Cardiovascular disease (CVD) mortality is one of the leading causes of death worldwide (1). The economic burden of CVD and related mortality in the United States is particularly troubling (2). CVD mortality is usually attributed to physical inactivity, obesity, atherosclerosis, hypertension, and diabetes (3, 4). The imbalance of certain metallic elements in the body also contributes (5–7). Metal elements are exposed more and more frequently in our daily lives and can be ingested in a variety of ways, including air inhalation, skin contact, and ingestion of metal-contaminated food (8–10). Recent study evidence suggests that metal deficiency or excess in the body may adversely affect human health (11–16).
Copper, an essential trace element, has played a vital role in biochemical processes and physiological regulation in all forms of life (17, 18). Copper can participate in physiological functions such as oxidative phosphorylation, angiogenesis, blood coagulation, and anti-oxidation (17). As a cofactor for various enzymes, copper also plays an in dispensable in biochemical processes such as erythropoiesis, cellular respiration, cholesterol, and glucose metabolism, pigment formation, and hormone synthesis (18, 19). Serum copper homeostasis is very important to human health and is tightly regulated. Both serum copper deficiency (<63.7 ug/L) and excess (>140.12 ug/L) can have harmful effects that can lead to serious illness (11). Monk's disease and Wilson's disease are caused by serum copper deficiency (20). A retrospective cohort study involving 183 patients with cirrhosis or portal hypertension found that serum copper deficiency was an independent risk factor for death in patients with advanced liver disease (21). Serum copper levels were significantly lower in patients who died after COVID-19 infection than in the healthy population. Serum copper excess can generate reactive oxygen species that cause oxidative damage to lipids, proteins, and other molecules, increasing the risk of atrial fibrillation and heart failure (22, 23). Many epidemiological studies have confirmed the association between excessive serum copper exposure and impairments of renal function, lung function, and neurological function (24–26). High serum copper levels were found to increase the risk of CVD mortality in men in a previous small sample study (27). In patients with lung cancer, serum copper levels were positively associated with all-cause mortality (28). However, in the Turkish study, serum copper levels were not associated with all-cause mortality risk in patients with sepsis and systemic inflammatory response syndrome (29). Although these studies in small samples or subgroups of the population have suggested that lack of or excessive serum copper exposure may have some association with mortality, the correlation between serum copper levels and all-cause, CVD, and cancer mortality in a nationally representative general population remains unclear.
Notably, current research data on the association of serum copper levels with all-cause, CVD, and cancer mortality is limited. Therefore, the purpose of this study was to assess the association between serum copper levels and all-cause and cause-specific mortality in US adults using data from the National Health and Nutrition Examination Survey (NHANES).
2 Methods
2.1 Study population
Data for the current study has been abstracted from the NHANES database, which is aimed at investigating the fitness and nutrient composition of the US population. The survey is conducted through the National Center for health information. All the participants or their proxies provided written informed consent. The survey was approved by the Institutional Review Board of the United States Centers for Disease Control and Prevention (30). NHANES is a publicly available database. In this study, a total of 11,539 participants aged ≥18 years were included from NHANES 2011–2014. Excluding serum copper deficiency (n = 7,931) and failure to follow up (n = 4), 3,604 participants were included in the sensitivity analysis. After removing 741 participants with missing covariates, 2,863 participants were included in the final model analysis (Supplementary Figures S1, S2).
2.2 Assessment of serum copper
Blood samples were drawn in a fasted state and transported to the laboratory for analysis under suitable freezing (–20°C conditions). Serum copper was measured using inductively coupled plasma–dynamic reaction cell–mass spectrometry in a trace element clean laboratory. The lower limit of detection (LLOD) was 2.5 ug/dl for NHANES (2011–2014) samples. For serum copper concentrations below the LLOD, the value was replaced with a value equal to the limit of detection divided by the square root of 2. More details about the laboratory analysis strategy can be found on the NHANES official website (31).
2.3 Mortality ascertainment
The outcomes of this study included all-cause mortality, and cause-specific (CVD and cancer) mortality, which were ascertained by linkage to the National Death Index through December 31, 2019. The document linked NHANES to the national dying index through a rigorous opportunity matching and death certificate overview procedure. All-cause mortality was defined as death from any cause. Cause-specific mortality was defined using the International Classification of Diseases (ICD), 10th Revision (ICD-10) codes for potential causes of death for cardiovascular disease (ICD-10 codes 053-075) and cancer (ICD-10 codes 019-043) (32). Follow-up time was calculated as the interval between the date of the serum copper examination and the date of demise, or the end of follow-up, whichever befell earlier.
2.4 Covariates
Self-reported demographic information (age, sex, education, race); health behaviors (alcohol intake, smoking); and diagnoses of diabetes, coronary heart disease, stroke, cancer, hypertension, chronic obstructive pulmonary disease, and hyperlipidemia were collected. Body weight and height were measured to calculate Body mass index (BMI). Self-reported race or ethnicity using fixed categories was collected to characterize the population. The race was classified as non-Hispanic, white, and other. Education levels were categorized as less than high school or high school and over. The smoking status was subdivided into nonsmokers, former smokers, and current smokers, including daily and often. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or taking antihypertensive agents. Diabetes was defined as a fasting blood glucose level ≥126 mg/dl, non-fasting blood glucose level ≥200 mg/dl, self-reported history of diabetes, or taking glucose-lowering medications. BMI was calculated as weight in kilograms divided by height in meters squared. The estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology equation (2021). Total cholesterol and high-density lipoprotein (HDL) cholesterol were assessed under a standardized process and protocol (33, 34).
3 Statistical analysis
All analyses were performed using SAS version 9.4 (SAS Institute). Two-sided P < 0.05 was considered statistically significant. The overall sampling design and sampling weights were adjusted to account for non-response bias and sampling design using the PROC SURVEY (SVY) commands in SAS (35).
The weighted continuous and categorical variables were expressed using means (95%CI) and frequencies (%). Continuous variables were analyzed using ANOVA (36), while those with categorical variables were analyzed using the Chi-square test. Participants' serum copper levels were divided into three equal groups, low (<103 ug/L), medium (103–123 ug/L), and high (≥124 ug/L). Kaplan-Meier curves have been used to estimate the survival probability of serum copper level tertiles. The Cox proportional-hazard regression model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between serum copper exposure and all-cause, cardiovascular disease, and cancer mortality. We constructed two models. Model 1 was adjusted for covariates, which included age, sex, race, and education levels; model 2 was adjusted for model 1 plus BMI, smoking status, hypertension, diabetes, eGFR, HDL cholesterol, and total cholesterol. The linear trend was tested by a median value for each tertile as a continuous variable. We investigated the nonlinear association between serum copper and mortality by including restricted cubic spline curves with nodes at the 10th, 50th, and 90th quartiles in the fully adjusted model. We also examined the association between serum copper levels and all-cause and cardiovascular disease mortality by performing analyses stratified by age (<60 and ≥60 years), sex, and race (non-Hispanic white or other). The interplay between non-stop serum copper and stratification becomes tested. Considering the exclusion of 741 participants with missing covariates, we used multiple interpolations for further analysis of 3,604 participants to assess the robustness of our results. Multiple interpolations were performed using Surveyimpute and MIANALYZE methods (37).
4 Results
A total of 2,863 participants were enrolled in the main analysis and during the mean follow-up time of 81.2 months, 236 deaths were documented, including 68 deaths from cardiovascular disease and 57 deaths from cancer. The weighted mean overall serum copper concentration was 117.2 ug/L. The numbers of Serum copper deficiency (<63.7 ug/L), normal ranges (63.7–140.12 ug/L), and copper excesses (>140.12 ug/L) were observed in 25, 2,861, and 718, respectively (Supplementary Table S1). According to the tertiles of serum copper levels, participants were divided into low, medium, and high serum copper levels groups (respectively, serum copper concentration < 103 ug/L, 103 ug/L ≤ serum copper concentration < 124 ug/L and serum copper concentration ≥ 124 ug/L). Compared with participants with low serum copper levels, participants with higher serum copper levels were more likely to be female, older, of other races, have a high annual family income, former smokers, have antihypertensive medications, hypertension, and have a higher BMI, as well as elevated total cholesterol and HDL cholesterol (Table 1).
Table 1
| Characteristic | No. (%) | ||||
|---|---|---|---|---|---|
| All | Serum copper levels (μg/L) | ||||
| Low (<103) | Middle (103–123) | High (≥124) | P value | ||
| Number of participantsa | N = 2,863 | N = 898 | N = 934 | N = 1,031 | |
| Serum copper, mean (95% CI) | 117.2 (114.75,119.7) | 90.3 (89.4,91.19) | 112.7 (112.24,113.24) | 148.6 (145.92,151.38) | <.0001 |
| Age, years, mean (95% CI) | 47.8 (46.73,48.82) | 45.1 (43.63,46.47) | 49.4 (47.79,50.97) | 48.8 (47.13,50.53) | <.0001 |
| Sex | <.0001 | ||||
| Male | 1,455 (49.9) | 688 (77.2) | 518 (52.2) | 249 (20.5) | |
| Female | 1,408 (50.1) | 210 (22.8) | 416 (47.8) | 782 (79.5) | |
| Races/ethnicity | <.0001 | ||||
| Non-Hispanic White | 1,230 (68.6) | 403 (71.3) | 429 (71.4) | 398 (63.2) | |
| Non-Hispanic Black | 628 (10.4) | 124 (5.7) | 191 (9.1) | 313 (16.3) | |
| Mexican American | 325 (8.1) | 91 (7.9) | 107 (8.1) | 127 (8.3) | |
| Other Race | 680 (12.9) | 280 (15.1) | 207 (11.4) | 193 (12.2) | |
| Education levels | 0.0778 | ||||
| Less than high school | 605 (14.5) | 152 (12.2) | 211 (15.4) | 242 (15.9) | |
| More high school than | 2,258 (85.5) | 746 (87.8) | 723 (84.6) | 789 (84.1) | |
| Annual family income | <.0001 | ||||
| Under $20,000 | 701 (17.4) | 180 (13.9) | 212 (14.6) | 309 (23.7) | |
| Over $20,000 | 2,162 (82.6) | 718 (86.1) | 722 (85.4) | 722 (76.3) | |
| Smoking status | 0.0004 | ||||
| Never | 1,618 (56) | 546 (61) | 480 (49.8) | 592 (57.5) | |
| Former | 686 (24.7) | 208 (23.5) | 268 (29.3) | 210 (21.1) | |
| Now | 559 (19.3) | 144 (15.5) | 186 (20.8) | 229 (21.3) | |
| Alcohol consumption | 0.1565 | ||||
| Never | 428 (11.3) | 114 (10) | 134 (10.2) | 180 (13.6) | |
| Former | 462 (13.8) | 136 (12.9) | 161 (14.4) | 165 (14) | |
| Now | 1,973 (74.9) | 648 (77.1) | 639 (75.4) | 686 (72.4) | |
| Medications use | |||||
| Lipid-lowering medicationsd | 595 (20.1) | 176 (20) | 214 (21.6) | 205 (18.7) | 0.5305 |
| Hypoglycemic medicationsc | 321 (9) | 84 (8.2) | 97 (8.3) | 140 (10.6) | 0.3411 |
| Antihypertensive medicationsb | 197 (6.2) | 34 (3.3) | 72 (7.3) | 91 (7.8) | 0.0002 |
| Baseline comorbidities | |||||
| Coronary heart disease | 105 (3.3) | 31 (3.2) | 40 (3.6) | 34 (3.1) | 0.8711 |
| Stroke | 97 (2.4) | 24 (1.9) | 23 (1.9) | 50 (3.4) | 0.1142 |
| COPD | 126 (5) | 21 (3.1) | 46 (6.3) | 59 (5.6) | 0.0118 |
| Hypertension | 1,215 (38.8) | 312 (33.3) | 414 (40.8) | 489 (42.2) | 0.0105 |
| Diabetes | 527 (14.4) | 137 (12.4) | 149 (13.3) | 241 (17.6) | 0.0211 |
| Hyperlipidemia | 1,993 (68.9) | 560 (62.4) | 668 (71.2) | 765 (73) | 0.0043 |
| Cancer | 297 (9.8) | 79 (9.3) | 119 (11.8) | 99 (8.4) | 0.1387 |
| BMI, kg/m2, mean (95% CI) | 28.9 (28.47,29.24) | 27 (26.55,27.48) | 28.8 (28.33,29.33) | 30.7 (30.01,31.43) | <.0001 |
| Biochemical tests, mean (95% CI) | |||||
| EGFR, ml/min per 1.73 m2 | 93.7 (92.58,94.8) | 93.7 (91.73,95.72) | 93.3 (91.52,95.05) | 94.1 (92.33,95.8) | 0.5684 |
| Total cholesterol, mmol/L | 5 (4.93,5.03) | 4.8 (4.68,4.92) | 5 (4.93,5.1) | 5.1 (5.05,5.23) | <.0001 |
| HDL cholesterol, mmol/L | 1.4 (1.35,1.4) | 1.3 (1.26,1.33) | 1.4 (1.36,1.43) | 1.4 (1.4,1.48) | <.0001 |
| Follow-up time, months, mean (95% CI) | 81.2 (79.19,83.16) | 81.7 (79.42,84.05) | 81.8 (79.58,84.09) | 79.9 (77.52,82.36) | 0.0011 |
| Cause of death | |||||
| All-cause mortality | 236 (7.2) | 60 (5.3) | 70 (6.6) | 106(9.7) | 0.0135 |
| Cardiovascular mortality | 68(1.8) | 19(1.3) | 15(1) | 34(3.2) | 0.0062 |
| Cancer mortality | 57(2) | 13(1.6) | 19(2.5) | 25(2) | 0.5279 |
Baseline characteristics of participants by serum copper levels among 2,863 participants in NHANES (2011–2014).
NHANES, national health, and nutrition examination survey; COPD, chronic obstructive pulmonary disease; BMI, body mass index; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; CI, confidence interval.
Percentages and means (95% CI) were estimated using US population weights.
P values were computed separately for each covariate and indicate statistically significant differences between step groups if P < 0.05.
Race/ethnicity was determined using preferred terminology from the National Center for Health Statistics as non-Hispanic white, non-Hispanic black, Mexican American, and Other Race.
Numbers in the table were unweighted.
Antihypertensive medications referred to self-reported taking antihypertensive drugs in NHANES.
Hypoglycemic medications included oral hypoglycemic agents and treatment with insulin (participant self-report).
Lipid-lowering medications included atorvastatin, simvastatin, pravastatin, rosuvastatin, lovastatin, and pitavastatin. fluvastatin, niacin, and ezetimibe.
The association between serum copper exposure and all-cause, CVD, and cancer mortality was demonstrated (Table 2 and Supplementary Table S2). In Model 1, HRs for all-cause mortality, CVD mortality, and cancer mortality in participants with medium/high serum copper levels compared with those with low serum copper concentrations were 1.88 (95% CI, 1.17–3.03)/2.06 (1.37,3.08), 2.81 (95% CI, 1.16–6.79)/4.72 (2.66,8.4), and 1.00 (95% CI, 0.33–3.05)/0.95 (0.39,2.3), respectively. The full-adjusted HRs (Model 2) for all-cause, CVD, and cancer mortality were 1.75 (95% CI, 1.05–2.92)/1.78(1.19,2.69), 2.35 (95% CI, 1.04–5.31)/3.84(2.09,7.05), and 0.97 (95% CI, 0.28–3.29)/0.86(0.34,2.13) for participants with high serum copper level compared with those with low/medium serum copper concentrations, respectively. In addition, for per SD (30 ug/L), increase in serum copper concentration, all-cause and CVD mortality risk increased 1.53 times (95% CI, 1.02–2.30), and 1.32 times (95% CI, 1.04–1.69), respectively, after the adjustment for all covariates. However, the serum copper was not associated with cancer mortality [HR: 1.01 (95% CI–0.53,1.9)].
Table 2
| Serum copper levels | Patients, No.a | Events, No.a | Mortality rate per 1,000 person-years | Model 1 | Model 2 |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Low | 898 | 60 | 8.1 | 1 (ref) | 1 (ref) |
| Middle | 934 | 70 | 9.9 | 0.92 (0.60,1.40) | 0.98 (0.63,1.54) |
| High | 1,031 | 106 | 14.8 | 1.88 (1.17,3.03) | 1.75 (1.05,2.92) |
| P for trend | 0.0049 | 0.0179 | |||
| Serum copper, per 30 μg/L | 2,863 | 236 | 10.7 | 1.38 (1.1,1.72) | 1.32 (1.04,1.69) |
| Cardiovascular disease mortality | |||||
| Low | 898 | 19 | 2 | 1 (ref) | 1 (ref) |
| Middle | 934 | 15 | 1.6 | 0.60 (0.28,1.29) | 0.61 (0.29,1.28) |
| High | 1,031 | 34 | 5.3 | 2.81 (1.16,6.79) | 2.35 (1.04,5.31) |
| P for trend | 0.0086 | 0.0151 | |||
| Serum copper, per 30 μg/L | 2,863 | 68 | 2.8 | 1.57 (1.10,2.23) | 1.53 (1.02,2.30) |
| Cancer mortality | |||||
| Low | 898 | 13 | 2.6 | 1 (ref) | 1 (ref) |
| Middle | 934 | 19 | 3.8 | 1.06 (0.39,2.82) | 1.13 (0.40,3.16) |
| High | 1,031 | 25 | 3.1 | 1.00 (0.33,3.05) | 0.97 (0.28,3.29) |
| P for trend | 0.9823 | 0.912 | |||
| Serum copper, per 30 μg/L | 2,863 | 57 | 3 | 0.97 (0.56,1.7) | 1.01(0.53,1.9) |
Hazard ratios (95% CIs) of all-cause, cardiovascular disease and cancer cortality according to serum copper levels among 2,863 participants in NHANES (2011–2014).
Model 1 was adjusted for age, sex, race, and education levels, annual family income, smoking status, alcohol consumption.
Model 2 was adjusted for the variables in model 1 plus lipid-lowering medications, hypoglycemic medications, antihypertensive medications, stroke, COPD, hypertension, hyperlipidemia, diabetes, BMI, eGFR, total cholesterol, and HDL cholesterol.
NHANES, national health, and nutrition examination survey; CI, confidence interval; COPD, chronic obstructive pulmonary disease; BMI, body mass index; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
Numbers in the table were unweighted.
Kaplan–Meier curves of all-cause and risk for the serum copper tertiles were presented in Figure 1. Participants with high serum copper concentration had higher all-cause and CVD mortality risk than those with low serum copper concentration. After multivariate adjustment, there was a linear dose-response association between serum copper concentration with CVD mortality and all-cause mortality (p for nonlinear > 0.05) (Figure 2).
Figure 1
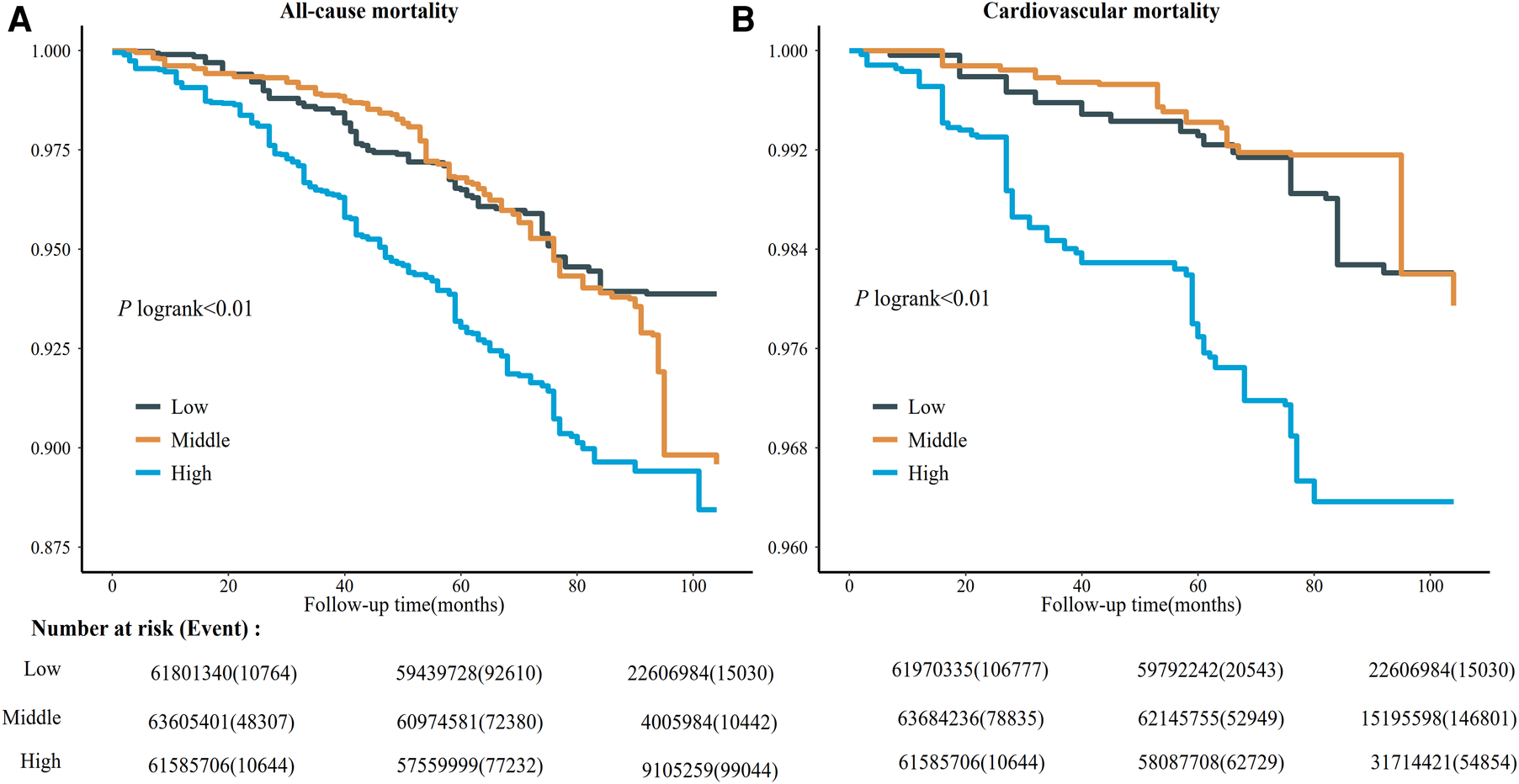
Kaplan-meier survival curve for all-cause (A) and cardiovascular disease mortality (B) among different levels of serum copper concentration. The survival rate was evaluated after adjusting for the variables for age, sex, race, education levels, annual family income, smoking status, alcohol consumption, lipid-lowering medications, hypoglycemic medications, antihypertensive medications, stroke, COPD, hypertension, hyperlipidemia, diabetes, BMI, eGFR, total cholesterol, and HDL cholesterol. The number at risk (event) was estimated using US population weights. NHANES, national health, and nutrition examination survey; CI, confidence interval; COPD, chronic obstructive pulmonary disease; BMI, body mass index; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate. Participants were subdivided into three subgroups according to serum copper levels: low, serum copper <103 μg/L; middle, 103 μg/L ≤ serum copper <124 μg/L; high, serum copper ≥124 μg/L.
Figure 2
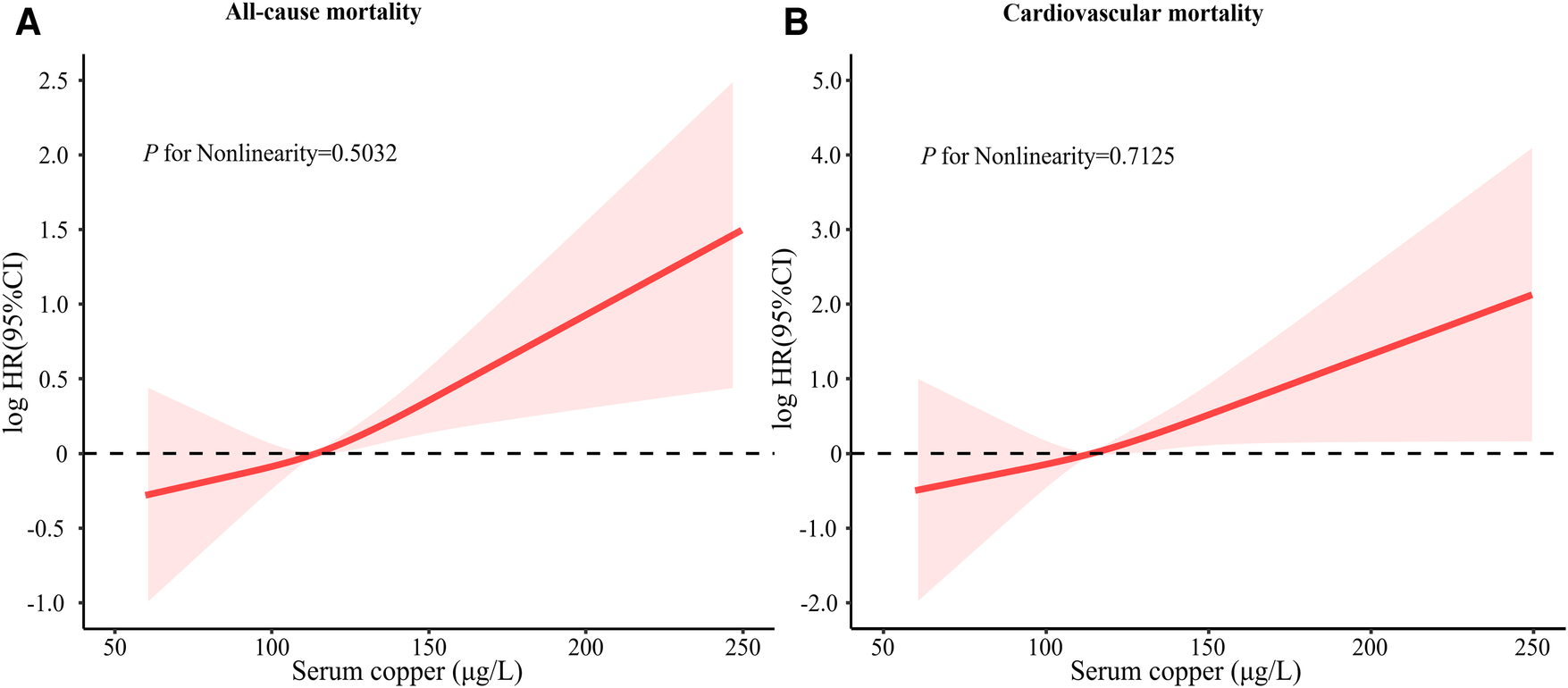
Restricted cubic spline of log HR of serum copper concentration with CVD mortality and all-cause mortality. In a fully adjusted model, restricted cubic spline curves with sections at the 10th, 50th, and 90th quartiles were added to examine the nonlinear relationship between serum copper concentration with CVD mortality and all-cause mortality. The model was adjusted for age, sex, race, education levels, annual family income, smoking status, alcohol consumption, lipid-lowering medications, hypoglycemic medications, antihypertensive medications, stroke, COPD, hypertension, hyperlipidemia, diabetes, BMI, eGFR, total cholesterol, and HDL cholesterol. NHANES, national health, and nutrition examination survey; CI, confidence interval; COPD, chronic obstructive pulmonary disease; BMI, body mass index; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
The stratified analysis according to age (<60 years, 60 years≤), sex, race (Non-Hispanic White, Other Race), or BMI (<30 kg/m2, 30 kg/m2≤) were given in Table 3. There was no evidence that the association between serum copper levels and all-cause and CVD mortality differed by race, age, or sex.
Table 3
| Serum copper levels | ||||
|---|---|---|---|---|
| Characteristic | Low | Middle | High | P- interaction |
| All-cause mortality | ||||
| Age, year | 0.3564 | |||
| <60 | 1 (ref) | 0.79 (0.26,2.42) | 1.92 (0.62,5.91) | |
| 60≤ | 1 (ref) | 1.03 (0.63,1.7) | 1.63 (0.87,3.06) | |
| Sex | 0.0702 | |||
| Male | 1 (ref) | 1.14 (0.68,1.91) | 2.25 (1.22,4.14) | |
| Female | 1 (ref) | 0.52 (0.22,1.23) | 0.96 (0.43,2.14) | |
| Races/ethnicity | 0.1524 | |||
| Non-Hispanic White | 1 (ref) | 1.02 (0.59,1.76) | 1.97 (1.06,3.67) | |
| Other Race | 1 (ref) | 0.74 (0.34,1.63) | 1.3 (0.71,2.4) | |
| BMI, kg/m2 | 0.8777 | |||
| <30 | 1 (ref) | 0.87 (0.49,1.53) | 1.6 (0.92,2.79) | |
| 30≤ | 1 (ref) | 1.18 (0.52,2.66) | 2.26 (0.82,6.2) | |
| Cardiovascular disease mortality | ||||
| Age, year | 0.1939 | |||
| <60 | 1 (ref) | 0.26 (0,33.73) | 2.15 (0.06,78.6) | |
| 60≤ | 1 (ref) | 0.6 (0.3,1.19) | 1.75 (0.77,3.98) | |
| Sex | 0.1428 | |||
| Male | 1 (ref) | 0.43 (0.12,1.5) | 3.13 (1.34,7.29) | |
| Female | 1 (ref) | 0.46 (0.09,2.28) | 1.16 (0.3,4.5) | |
| Races/ethnicity | 0.4752 | |||
| Non-Hispanic White | 1 (ref) | 0.41 (0.15,1.12) | 2.47 (1.05,5.8) | |
| Other Race | 1 (ref) | 2.17 (0.63,7.48) | 3.03 (0.96,9.59) | |
| BMI, kg/m2 | 0.413 | |||
| <30 | 1 (ref) | 0.36 (0.1,1.24) | 1.46 (0.45,4.79) | |
| 30≤ | 1 (ref) | 1.68 (0.52,5.44) | 6.36(1.21,33.32) | |
Stratified analyses of the associations between serum copper levels and all-cause mortality or cardiovascular disease mortality among 2,863 participants in NHANES (2011–2014).
COX proportional hazards models were used to estimate HRs (95% CI) for all-cause and cardiovascular disease mortality based on serum copper levels. Results were adjusted for age, sex, race, educations levels, annual family income, smoking status, alcohol consumption, lipid-lowering medications, hypoglycemic medications, antihypertensive medications, stroke, COPD, hypertension, hyperlipidemia, diabetes, BMI, eGFR, total cholesterol, HDL cholesterol, except for the sub-group variable.
NHANES, national health, and nutrition examination survey; HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; BMI, body mass index; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
To assess potential bias from missing values, we used multiple imputations to impute the missing values of the covariates (n = 741) and then repeated the analysis. The analysis was consistent with the results of the main analysis (Supplementary Table S3). After adjusting for all of the covariates, compared with the low serum copper group, participants with high serum copper levels had a 1.72-fold (95% CI, 1.13–2.62) increase in all-cause mortality, a 2.36-fold (95% CI, 1.09–5.11) increase in CVD mortality, respectively. HRs for cancer mortality were 1.07 (0.43,2.66)/0.98 (0.37,2.6) for participants with medium/high serum copper levels compared to individuals with low serum copper concentrations, respectively.
5 Discussion
5.1 Major findings
In this large prospective cohort study, we investigated the association between serum copper exposure and all-cause, CVD, and cancer mortality in US adults. Our study found that higher serum copper concentrations in US adults were associated with increased all-cause and CVD mortality after adjusting for important potential confounders, but not cancer mortality. The study provides new evidence for the association of copper exposure with all-cause and CVD mortality in US adults. These results are noteworthy because serum copper concentrations have gotten little attention for many years.
5.2 Comparisons with previous studies
This study suggests that higher serum copper concentration is associated with higher CVD mortality in US adults. We also found higher serum copper levels in patients with hypertension, diabetes, and hyperlipidemia (Table 1), implying that higher serum copper levels are associated with mortality from systemic metabolic diseases/disorders. Several previous studies have supported our findings. Elevated serum copper levels have been reported to increase heart failure by affecting systolic and diastolic blood pressure functions (38). The oxidation of low-density lipoprotein cholesterol is exacerbated by copper, causing atherosclerosis (39) and increasing the risk of cardiovascular disease (40).In addition, high serum copper concentrations lead to insulin resistance and abnormal insulin secretion, which promotes diabetes (41, 42). In a study of 3,253 Germans undergoing coronary angiography, elevated serum copper concentrations were associated with an increased risk of death from cardiovascular causes (43). Also, after reviewing 37 studies, Chowdhury et al. found that higher circulating copper concentrations were associated with an increased risk of cardiovascular disease (44). On the other hand, copper binds to homocysteine to form a copper-homocysteine complex, which leads to vascular dysfunction and increases the risk of cardiovascular disease (45).
At the same time, our study also found that higher serum copper concentrations in US adults were associated with increased all-cause mortality. A longitudinal study of elderly Italians showed a positive association between serum copper concentrations and all-cause mortality (46). Similarly, a study of 167 lung cancer patients showed that higher serum copper levels were associated with increased all-cause mortality in lung cancer patients (28), and in a study of 498 elderly patients, Malavolta et al. (47) indicated that elevated plasma copper led to increased plasma copper/zinc ratio in patients with cardiovascular disease and that copper/zinc ratio was an important predictor of all-cause mortality in the elderly. The above studies are consistent with our results.
Interestingly, our study found no significant effect of high or low serum copper levels on the risk of cancer mortality in the general U.S. adult population. Studies have demonstrated that the risk of colorectal and breast cancers is not associated with serum copper (48, 49). However, high serum copper levels may increase the risk of lung cancer (50). Higher serum copper levels may be associated with decreased survival in patients with liver and lung cancers (28, 51). These findings indicate that serum copper has been differentially associated with different cancers, but in the general population, serum copper is not associated with the risk of cancer death. In conclusion, the link between copper and cancer is complicated and calls for more investigation into the underlying mechanisms.
5.3 Underlying mechanism
However, the underlying mechanism by which higher blood copper concentrations increase all-cause mortality remains unclear. Some studies suggest that copper may play a role in diabetes (52), dyslipidemia (53, 54), and cancer (55), exacerbating all-cause mortality. In a Japanese study, higher copper intake was strongly associated with an increased risk of type 2 diabetes, and the association was more pronounced in overweight and smokers (52). Galiardi et al. (53) found that high serum copper levels increase cholesterol levels, leading to dyslipidemia. A study in NHANES (2011–2014) revealed that high serum copper concentrations were associated with elevated total serum cholesterol concentrations and increased risk of dyslipidemia (54). In addition, a Chinese study demonstrated that high serum copper levels significantly increased the risk of oral cancer (55). Excessive serum copper increases the risk of cell death. A novel form of cell death, copper-dependent, has recently been reported to cause proteotoxic stress and cell death through copper binding to the thiooctylated components of the tricarboxylic acid cycle (56). Although serum copper is an essential micronutrient, excess copper can produce reactive oxygen radicals that lead to oxidative stress (57). When copper binds to superoxide dismutase 1, it increases the level of reactive oxygen species, which in turn causes oxidative stress (58, 59). In addition, copper may also be involved in inflammatory processes, with high levels of copper promoting reactive oxygen species formation and participating in inflammation (60).
5.4 Strengths and limitations
This study has many strengths. Strengths of the current study include a prospective study design, a relatively large sample size, and the use of a nationally representative sample of US adults, which help generalize our findings. In addition, with the detailed and high-quality data collection in the NHANES, this study was able to control potential confounding effects.
Our study has several limitations. First, the number of deaths from cardiovascular and cancer in this study was small, which may have limited the statistical power to detect a significant association. Second, in baseline data, the number of cancer patients was relatively small, so it was impossible to analyze the association between the mortality rate of cancer patients and blood copper level. In the end, we get death results from death certificates, which may contain inaccurate information—some causes of death will be miscoded and may not generalize to non-fatal events. Therefore, our study could only provide a limited view of the effects of copper on cardiovascular health.
6 Conclusions
In summary, this nationally representative cohort study revealed that serum copper concentrations were linearly associated with all-cause and CVD mortality. High serum copper levels is a risk factor for all-cause and CVD mortality, and not cancer mortality. Future studies of all-cause and CVD mortality should take serum copper concentrations into account.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES database: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by participants were reviewed and approved by the NCHS Institutional Review Board and all participants signed intormed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. LZ: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft. QZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. HZ: Project administration, Supervision, Validation, Writing – review & editing. JL: Data curation, Supervision, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the NHANES database for providing open access.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1340968/full#supplementary-material
References
1.
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1736–88. 10.1016/S0140-6736(18)32203-7
2.
Tsao CW Aday AW Almarzooq ZI Anderson CAM Arora P Avery CL et al Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. (2023) 147(8):e93–621. 10.1161/CIR.0000000000001123
3.
Ndumele CE Rangaswami J Chow SL Neeland IJ Tuttle KR Khan SS et al Cardiovascular-kidney-metabolic health: a presidential advisory from the American heart association. Circulation. (2023) 148(20):1606–35. 10.1161/CIR.0000000000001184
4.
Liang Y Liu F Yin H Shi X Chen Y Wang H et al Trends in unhealthy lifestyle factors in US NHANES respondents with cardiovascular disease for the period between 1999 and 2018. Front Cardiovasc Med. (2023) 10:1169036. 10.3389/fcvm.2023.1169036
5.
Wechselberger C Messner B Bernhard D . The role of trace elements in cardiovascular diseases. Toxics. (2023) 11(12):956. 10.3390/toxics11120956
6.
Fan Y Tao C Li Z Huang Y Yan W Zhao S et al Association of endocrine-disrupting chemicals with all-cause and cause-specific mortality in the U.S.: a prospective cohort study. Environ Sci Technol. (2023) 57(7):2877–86. 10.1021/acs.est.2c07611
7.
Rashidmayvan M Mansoori A Aghasizadeh M Dianati M Barati S Sahranavard T et al Prediction of cardiovascular disease risk by serum zinc and copper concentrations and anthropometric measurements. J Trace Elem Med Biol. (2024) 83:127385. 10.1016/j.jtemb.2024.127385
8.
Iwegbue CMA Bassey FI Tesi GO Onyeloni SO Obi G Martincigh BS . Safety evaluation of metal exposure from commonly used moisturizing and skin-lightening creams in Nigeria. Regul Toxicol Pharmacol. (2015) 71(3):484–90. 10.1016/j.yrtph.2015.01.015
9.
Chen XC Cao JJ Ward TJ Tian LW Ning Z Gali NK et al Characteristics and toxicological effects of commuter exposure to black carbon and metal components of fine particles (PM2.5) in Hong Kong. Sci Total Environ. (2020) 742:140501. 10.1016/j.scitotenv.2020.140501
10.
Zhang T Ruan J Zhang B Lu S Gao C Huang L et al Heavy metals in human urine, foods and drinking water from an e-waste dismantling area: identification of exposure sources and metal-induced health risk. Ecotoxicol Environ Saf. (2019) 169:707–13. 10.1016/j.ecoenv.2018.10.039
11.
Scheiber IF Mercer JFB Dringen R . Metabolism and functions of copper in brain. Prog Neurobiol. (2014) 116:33–57. 10.1016/j.pneurobio.2014.01.002
12.
Zhang Y Liu W Zhang W Cheng R Tan A Shen S et al Association between blood lead levels and hyperlipidemiais: results from the NHANES (1999–2018). Front Public Health. (2022) 10:981749. 10.3389/fpubh.2022.981749
13.
Dubey P Thakur V Chattopadhyay M . Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. (2020) 12(6):1864. 10.3390/nu12061864
14.
Gammoh NZ Rink L . Zinc in infection and inflammation. Nutrients. (2017) 9(6):624. 10.3390/nu9060624
15.
Kieliszek M . Selenium−fascinating microelement, properties and sources in food. Molecules. (2019) 24(7):1298. 10.3390/molecules24071298
16.
Xu J Zhu X Hui R Xing Y Wang J Shi S et al Associations of metal exposure with hyperuricemia and gout in general adults. Front Endocrinol. (2022) 13:1052784. 10.3389/fendo.2022.1052784
17.
Araya M Pizarro F Olivares M Arredondo M González M Méndez M . Understanding copper homeostasis in humans and copper effects on health. Biol Res. (2006) 39(1):183–7. 10.4067/S0716-97602006000100020
18.
Grubman A White AR . Copper as a key regulator of cell signalling pathways. Expert Rev Mol Med. (2014) 16:e11. 10.1017/erm.2014.11
19.
Lalioti V Muruais G Tsuchiya Y Pulido D Sandoval IV . Molecular mechanisms of copper homeostasis. Front Biosci (Landmark Ed). (2009) 14(13):4878–903. 10.2741/3575
20.
van den Berghe PVE Klomp LWJ . New developments in the regulation of intestinal copper absorption. Nutr Rev. (2009) 67(11):658–72. 10.1111/j.1753-4887.2009.00250.x
21.
Yu L Yousuf S Yousuf S Yeh J Biggins SW Morishima C et al Copper deficiency is an independent risk factor for mortality in patients with advanced liver disease. Hepatol Commun. (2023) 7(3):e0076. 10.1097/HC9.00000000000000076
22.
Nishito Y Kambe T . Absorption mechanisms of iron, copper, and zinc: an overview. J Nutr Sci Vitaminol. (2018) 64(1):1–7. 10.3177/jnsv.64.1
23.
Grandis DJ Nah G Whitman IR Vittinghoff E Dewland TA Olgin JE et al Wilson’s disease and cardiac myopathy. Am J Cardiol. (2017) 120(11):2056–60. 10.1016/j.amjcard.2017.08.025
24.
Lilis R Valciukas JA Weber JP Malkin J Selikoff IJ . Epidemiologic study of renal function in copper smelter workers. Environ Health Perspect. (1984) 54:181–92. 10.1289/ehp.8454181
25.
Lilis R Valciukas JA Weber JP Malkin J . Effects of low-level lead and arsenic exposure on copper smelter workers. Arch Environ Health. (1985) 40(1):38–47. 10.1080/00039896.1985.10545887
26.
Skoczyńska A Gruszczyński L Wojakowska A Ścieszka M Turczyn B Schmidt E . Association between the type of workplace and lung function in copper miners. Biomed Res Int. (2016) 2016:5928572. 10.1155/2016/5928572
27.
Reunanen A Knekt P Marniemi J Mäki J Maatela J Aromaa A . Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. (1996) 50(7):431–7.
28.
Zabłocka-Słowińska K Prescha A Płaczkowska S Porębska I Kosacka M Pawełczyk K . Serum and whole blood Cu and Zn status in predicting mortality in lung cancer patients. Nutrients. (2020) 13(1):60. 10.3390/nu13010060
29.
Ayoglu H Sezer U Akin M Okyay D Ayoglu F Can M et al Selenium, copper, zinc, iron levels and mortality in patients with sepsis and systemic inflammatory response syndrome in Western Black Sea Region, Turkey. J Pak Med Assoc. (2016) 66(4):447–52.
30.
NHANES Survey Methods and Analytic Guidelines. Available online at:https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx(cited January 3, 2024).
31.
NHANES 2013–2014: Copper, Selenium & Zinc—Serum Data Documentation, Codebook, and Frequencies. Available online at:https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CUSEZN_H.htm#LBDSCUSI(cited January 3, 2024).
32.
The Linkage of National Center for Health Statistics Survey Data to the National Death Index—2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. Available online at:https://www.cdc.gov/nchs/data/datalinkage/LMF2015_Methodology_Analytic_Considerations.pdf(cited November 27, 2023).
33.
NHANES 2013–2014: Cholesterol—HDL Data Documentation, Codebook, and Frequencies. Available online at:https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/HDL_H_MET_COBAS.pdf(cited January 3, 2024).
34.
NHANES 2011–2012: Cholesterol—Total Data Documentation, Codebook, and Frequencies. Available online at:https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/TCHOL_G.htm#LBDTCSI(cited January 3, 2024).
35.
NHANES Tutorials—Weighting Module. Available online at:https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx(cited October 18, 2023).
36.
NHANES Tutorials—Variance Estimation Module. Available online at:https://wwwn.cdc.gov/nchs/nhanes/tutorials/VarianceEstimation.aspx(cited October 18, 2023).
37.
Saint-Maurice PF Troiano RP Bassett DR Graubard BI Carlson SA Shiroma EJ et al Association of daily step count and step intensity with mortality among US adults. JAMA. (2020) 323(12):1151–60. 10.1001/jama.2020.1382
38.
Alexanian I Parissis J Farmakis D Athanaselis S Pappas L Gavrielatos G et al Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clin Res Cardiol. (2014) 103(11):938–49. 10.1007/s00392-014-0735-x
39.
Worthley SG Osende JI Helft G Badimon JJ Fuster V . Coronary artery disease: pathogenesis and acute coronary syndromes. Mt Sinai J Med. (2001) 68(3):167–81.
40.
Gaetke LM Chow CK . Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. (2003) 189(1–2):147–63. 10.1016/S0300-483X(03)00159-8
41.
Bjørklund G Dadar M Pivina L Doşa MD Semenova Y Aaseth J . The role of zinc and copper in insulin resistance and diabetes mellitus. Curr Med Chem. (2020) 27(39):6643–57. 10.2174/0929867326666190902122155
42.
Kant R Verma V Patel S Chandra R Chaudhary R Shuldiner AR et al Effect of serum zinc and copper levels on insulin secretion, insulin resistance and pancreatic β cell dysfunction in US adults: findings from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Diabetes Res Clin Pract. (2021) 172:108627. 10.1016/j.diabres.2020.108627
43.
Grammer TB Kleber ME Silbernagel G Pilz S Scharnagl H Lerchbaum E et al Copper, ceruloplasmin, and long-term cardiovascular and total mortality (the ludwigshafen risk and cardiovascular health study). Free Radic Res. (2014) 48(6):706–15. 10.3109/10715762.2014.901510
44.
Chowdhury R Ramond A O’Keeffe LM Shahzad S Kunutsor SK Muka T et al Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. Br Med J. (2018) 362:k3310. 10.1136/bmj.k3310
45.
Kang YJ . Copper and homocysteine in cardiovascular diseases. Pharmacol Ther. (2011) 129(3):321–31. 10.1016/j.pharmthera.2010.11.004
46.
Mocchegiani E Malavolta M Lattanzio F Piacenza F Basso A Abbatecola AM et al Cu to Zn ratio, physical function, disability, and mortality risk in older elderly (ilSIRENTE study). Age. (2012) 34(3):539–52. 10.1007/s11357-011-9252-2
47.
Malavolta M Giacconi R Piacenza F Santarelli L Cipriano C Costarelli L et al Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology. (2010) 11(3):309–19. 10.1007/s10522-009-9251-1
48.
Jouybari L Kiani F Islami F Sanagoo A Sayehmiri F Hosnedlova B et al Copper concentrations in breast cancer: a systematic review and meta-analysis. Curr Med Chem. (2020) 27(37):6373–83. 10.2174/0929867326666190918120209
49.
Zhang C Cheng R Ding J Li X Niu H Li X . Serum copper and zinc levels and colorectal cancer in adults: findings from the national health and nutrition examination 2011–2016. Biol Trace Elem Res. (2022) 200(5):2033–9. 10.1007/s12011-021-02826-8
50.
Zhang X Yang Q . Association between serum copper levels and lung cancer risk: a meta-analysis. J Int Med Res. (2018) 46(12):4863–73. 10.1177/0300060518798507
51.
Fang AP Chen PY Wang XY Liu ZY Zhang DM Luo Y et al Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int J Cancer. (2019) 144(11):2823–32. 10.1002/ijc.31991
52.
Eshak ES Iso H Maruyama K Muraki I Tamakoshi A . Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: a large population-based prospective cohort study. Clin Nutr. (2018) 37(2):667–74. 10.1016/j.clnu.2017.02.010
53.
Galhardi CM Diniz YS Faine LA Rodrigues HG Burneiko RCM Ribas BO et al Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol. (2004) 42(12):2053–60. 10.1016/j.fct.2004.07.020
54.
Song X Wang W Li Z Zhang D . Association between serum copper and serum lipids in adults. Ann Nutr Metab. (2018) 73(4):282–9. 10.1159/000494032
55.
Chen F Wang J Chen J Yan L Hu Z Wu J et al Serum copper and zinc levels and the risk of oral cancer: a new insight based on large-scale case-control study. Oral Dis. (2019) 25(1):80–6. 10.1111/odi.12957
56.
Tsvetkov P Coy S Petrova B Dreishpoon M Verma A Abdusamad M et al Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. (2022) 375(6586):1254–61. 10.1126/science.abf0529
57.
Jomova K Valko M . Advances in metal-induced oxidative stress and human disease. Toxicology. (2011) 283(2–3):65–87. 10.1016/j.tox.2011.03.001
58.
Gralla EB Valentine JS . Null mutants of Saccharomyces cerevisiae Cu,Zn superoxide dismutase: characterization and spontaneous mutation rates. J Bacteriol. (1991) 173(18):5918–20. 10.1128/jb.173.18.5918-5920.1991
59.
Bertinato J L’Abbé MR . Copper modulates the degradation of copper chaperone for Cu,Zn superoxide dismutase by the 26 S proteosome. J Biol Chem. (2003) 278(37):35071–8. 10.1074/jbc.M302242200
60.
Chen Z Zhu J Zhou H Jia Y Ruan H Diao Q et al The involvement of copper, circular RNAs, and inflammatory cytokines in chronic respiratory disease. Chemosphere. (2022) 303(Pt 2):135005. 10.1016/j.chemosphere.2022.135005
Summary
Keywords
serum copper, all-cause mortality, cancer mortality, cardiovascular disease mortality, national health and nutrition examination survey
Citation
Zeng X, Zhou L, Zeng Q, Zhu H and Luo J (2024) High serum copper as a risk factor of all-cause and cause-specific mortality among US adults, NHANES 2011–2014. Front. Cardiovasc. Med. 11:1340968. doi: 10.3389/fcvm.2024.1340968
Received
23 November 2023
Accepted
08 April 2024
Published
19 April 2024
Volume
11 - 2024
Edited by
Gen-Min Lin, Hualien Armed Forces General Hospital, Taiwan
Reviewed by
Muhammad Abdul Qayyum, University of Education Lahore, Pakistan
Rahul Mallick, University of Eastern Finland, Finland
Susan Darroudi, Mashhad University of Medical Sciences, Iran
Updates
Copyright
© 2024 Zeng, Zhou, Zeng, Zhu and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jianping Luo luojianping@mail.gzsrmyy.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.