Abstract
Objectives:
To investigate the diagnostic performance of fetal cardiovascular magnetic resonance imaging (MRI) using Doppler ultrasound (DUS) gating for the evaluation of the standardized five axial views in comparison with fetal echocardiography.
Methods:
In this prospective study 29 pregnant women (median: 34.4 weeks of gestation) underwent fetal cardiovascular MRI using DUS gating at 3 Tesla. The standardized five axial views in prenatal screening (fetal abdomen, four-chamber view, left ventricular outflow tract, right ventricular outflow tract, and three-vessel view) were independently assessed and analysed by both fetal MRI and fetal echocardiography on the same day. Image analysis included qualitative assessment and quantitative measurements of cardiovascular structures. MR image quality was assessed using a 4-point scale (from 1 = low to 4 = excellent). Postnatal echocardiography was performed for validation.
Results:
17/28 fetuses (60.7%) had pathological findings [16 congenital heart defect (CHD), one diaphragmatic hernia] in prenatal echocardiography. One fetus was excluded due to severe motion. Overall sensitivity and specificity in detecting fetal cardiac abnormalities was 88% and 100%, respectively, for fetal MRI and 100% and 100% for fetal echocardiography. MR image quality for evaluation of cardiac structures was high with a mean score of 2.8 (±0.8) (score 4: 15.9%, score 3: 53.8%, score 2: 19.3%, score 1: 11%). Quantitative measurements did not differ between fetal cardiovascular MRI and fetal echocardiography (all p > 0.05).
Conclusion:
Diagnostic performance of fetal cardiovascular MRI using DUS gating was comparable to fetal echocardiography. Fetal cardiovascular MRI using DUS gating might be a valuable diagnostic adjunct for the prenatal evaluation of CHD.
Introduction
Congenital heart defects (CHD) account for 1% of all live births (1, 2). Prenatal diagnosis of CHD optimizes perinatal management and improves neonatal outcome for several CHD (3). However, critical heart defects still have a mortality of 18% (4) and require prompt surgery or intervention (5), highlighting the importance of prenatal diagnosis.
Fetal echocardiography is the reference standard for prenatal detection of CHD. With the normal situs and the four-chamber view as the first established diagnostic axial planes (6) a detection rate of CHD of 40%–50% was achieved (7). Inclusion of additional axial views such as the left and right ventricular outflow tracts (LVOT, RVOT) plus the three-vessel (3V) view (8) established the current standard of the five-axial views. These five-axial views are recommended by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) guidelines for performing fetal echocardiography (9). Including these planes, the detection rate of CHD increased up to 60%–80% (10).
Oligohydramnios, dorsoanterior positions, late gestational age and obesity are restrictive conditions for fetal echocardiography and may lead to limited results (11–13). In these cases, a second cross-sectional imaging modality is desirable.
Fetal magnetic resonance imaging (MRI) is established as a second-tool approach especially in cases of fetal brain malformations (14, 15). However, its application for cardiovascular imaging is more challenging and just developing.
The technical challenge of fetal cardiovascular MRI is fetal cardiac gating, i.e., to synchronize the heart beat with MR image acquisition. One solution is to apply an external Doppler ultrasound (DUS) device for fetal cardiac gating (16, 17). Fetal cardiovascular MRI using DUS gating was successfully applied for morphological and functional imaging of the fetal cardiovascular system (18–23). However, a systematic approach for assessment of the five axial planes has not been investigated.
The aim of this study was to investigate the diagnostic performance of fetal cardiovascular MRI using DUS gating for the evaluation of the standardized five axial views in comparison with fetal echocardiography.
Methods
Study population
In this prospective study, 29 pregnant women underwent DUS gated fetal cardiovascular MRI and fetal echocardiography on the same day. The study group consisted of 17 fetuses with suspected CHD, anomalies of the central nervous system (CNS) (n = 5), and intestinal malformation (n = 2). Five voluntary pregnant women without evidence of fetal CHD were also enrolled. All pregnant women provided written informed consent prior to the imaging procedures to participate in this study. The local ethical committee granted ethical approval for this study.
Fetal echocardiography
Fetal echocardiography with color and pulse-wave Doppler ultrasound was performed using a convex curved-array transducer with a frequency of 2.0–6.0 MHz (Voluson E10, GE Healthcare, Solingen, Germany). The five axial views included the transverse view of the fetal abdomen, the four-chamber view, the left outflow tract (LVOT), the right outflow tract (RVOT) and the three-vessel (3V) view according to current guidelines (9).
Fetal cardiovascular MRI
DUS gated fetal cardiovascular MRI was performed at a 3T scanner (Ingenia Elition, Philips Medical Systems, Best, The Netherlands). Depending on individual preference, the pregnant women were placed in a supine or lateral position. A MR-compatible DUS sensor (Smart-Sync, northh Medical GmbH, Hamburg, Germany) was placed on the maternal abdomen for recording of the fetal heart beat and used for fetal cardiac gating (19).
T2-weighted turbo spin echo (TSE) sequences (repetition time TR = 2,436 ms, echo time TE = 80 ms, flip angle FA = 90°, field of view FOV = 350 × 300 mm, slice thickness = 4 mm, matrix size = 292 × 216, spatial resolution = 1.2 × 1.4 mm) of the fetal thorax were performed in 3 orthogonal planes for planning of the subsequent cardiac cine-sequences. DUS gated fetal cardiovascular MRI was performed using a multi-slice cine balanced steady-state free-precession (cine-bSSFP) sequence in transversal orientation to cover the fetal heart and great thoracic vessels (TR = 4.1 ms, TE = 2 ms, FA = 60°, FOV = 246 × 246 mm, slice thickness = 5 mm, slices = 12, spatial resolution = 1.5 × 1.5 mm, matrix size = 164 × 160, cardiac phases = 20, gap = −1 mm). In accordance to fetal echocardiography, imaging planes were angulated for 5°–10° in antero-posterior orientation (para-transversal). To avoid breathing artifacts, MR image acquisition was performed under maternal breath hold.
Postnatal echocardiography
Postnatal echocardiography was assessed within 1–3 days postpartum and served as the reference standard for diagnosis.
Qualitative analysis
Image quality
Image quality of fetal cardiovascular MRI was assessed for each of the five axial views using a 4-point scale. In addition, a detailed analysis of diagnostic quality was performed for every cardiac structure/morphology of each axial view using the same 4-point scale: 1 = low quality (anatomical structures not delineable with certainty, strong artifacts), 2 = moderate quality (anatomical structures recognizable with artifacts), 3 = high quality (reliable definition of cardiac structures, minor artifacts), and 4 = excellent quality (reliable definition of cardiac structures without artifacts). Image and diagnostic quality was rated by two readers in consensus (MDB and BS, with 2- and 13-years of experience in cardiovascular MRI).
Diagnostic quality
Diagnostic quality was analyzed according to the current ISUOG guidelines (9). The 4-chamber view was evaluated by assessing the heart size, the thorax ratio, the cardiac axis, ventricle and atrium sizes, the foramen ovale, the attachment of leaflet valves, the cardiac crux, Moderator band and the ventricular septum. For the LVOT, the continuity between the ventricular septum and aorta, the origin of the aorta and the aortic valve with their morphology, opening, and movement were analyzed. Likewise, the origin and valves were studied for the evaluation of the RVOT. In the 3V view including the pulmonary artery, aorta and superior vena cava, the sequence of vessels, the size and relationship to each other, and the alignment were reviewed.
All structures from the five axial views were analyzed and defined as either “normal” or “pathological” to calculate sensitivities and specificities for each structure and for each of the five axial views. Two operators, who were blinded to each other's findings, evaluated fetal cardiovascular MRI (MDB, 2 years experience in cardiovascular MRI) and fetal echocardiography (BH, 6 years experience in fetal echocardiography).
Quantitative analysis
Quantitative measurements were also performed independently by the same two operators for fetal echocardiography and fetal cardiovascular MRI. Ventricular length and width were measured in the end-diastolic four-chamber view. The diameter of the LVOT and RVOT were compared by measuring below each semilunar valve. In the 3V view, the diameters of the pulmonary artery, aorta, and superior vena cava were measured following established recommendations (24).
Statistics
For statistical analysis, McNemar's-test was used for qualitative analysis. Measurements in fetal cardiovascular MRI and fetal echocardiography were compared and analyzed using the Student's t-test. P < 0.05 was defined as statistically significant. SPSS Statistics 27 software was used for the statistical analysis.
Results
One fetal MRI examination was excluded from further analysis due to severe fetal motion resulting in artifacts. In 8 of the remaining 28 cases (28.6%), the DUS sensor had to be repositioned on the maternal abdomen during MR examination due to fetal movement and intermittent loss of cardiac gating signal. Mean gestational age was 34.4 weeks (range: 29–38 weeks). Pregnant women had a mean age of 30.9 years (range: 19–39) and BMI of 28.8 kg/m2 (range: 21–38).
Qualitative analysis
Image quality score of fetal cardiovascular MRI, assessed for each of the five axial views per fetus, was high with a mean score of 2.8 ± 0.8 (score 4 in 15.9%, score 3 in 53.8%, score 2 in 19.3%, score 1 in 11%) (Figure 1).
Figure 1
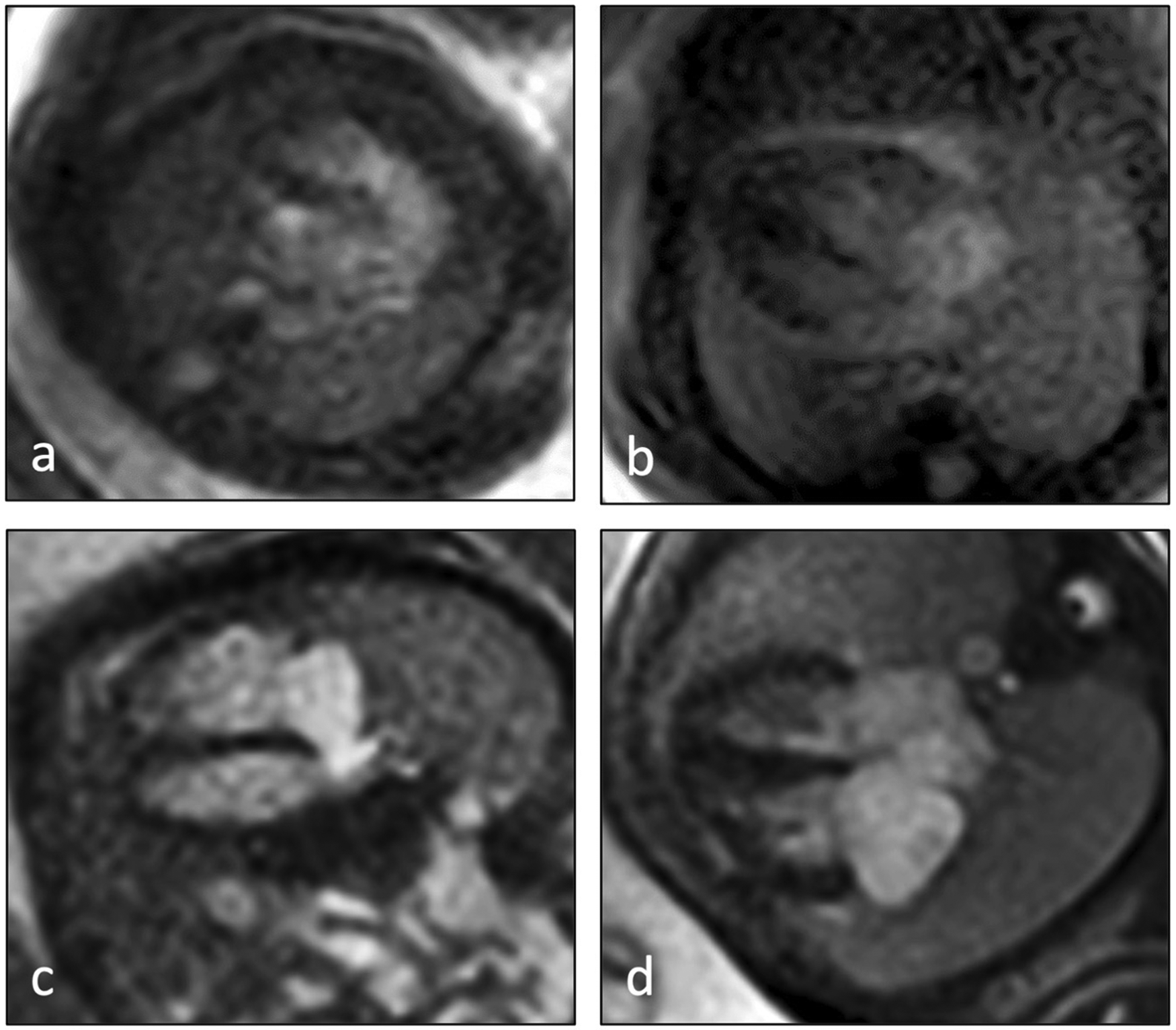
Examples of image quality scores of fetal cardiovascular MRI 4-chamber views according to the applied 4-point scale: (a) image score 1 = low quality (anatomical structures not delineable with certainty, strong artifacts), (b) image score 2 = moderate quality (anatomical structures recognizable with artifacts), (c) image score 3 = high quality (reliable definition of cardiac structures, minor artifacts), (d) image score 4 = excellent quality (reliable definition of cardiac structures without artifacts).
MR diagnostic quality of each anatomical structure/morphology assessed from the five axial views revealed highest scores for cardiac topography and situs and lowest scores for cardiac valves (Figures 2, 3).
Figure 2
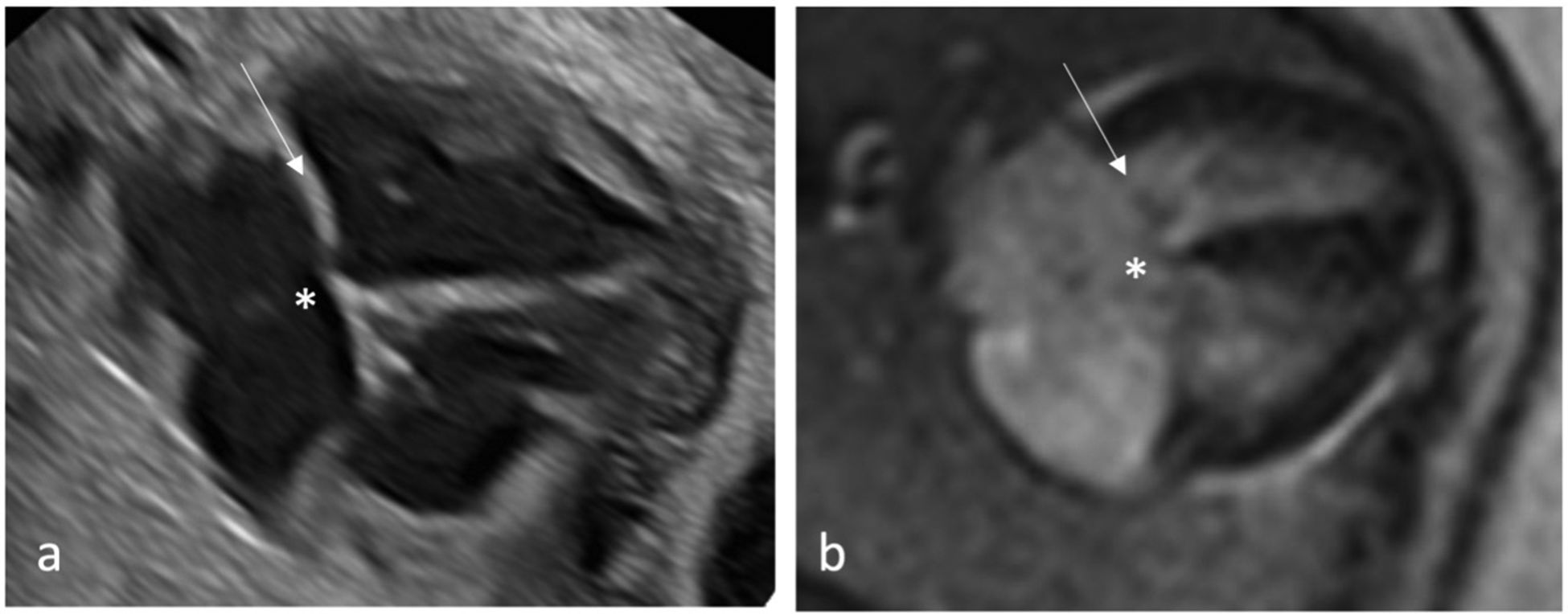
Qualitative assessment of fetal cardiac 4CV on fetal echocardiography (a) and DUS gated fetal cardiac MRI (b) with intermediate type AVSD detected by both methods. An AVSD, as shown here, is an atrial septal primum defect and an inlet ventricular septal defect (asterisk) with a combination of an abnormal common atrioventricular valve with a linear insertion (arrow).
Figure 3
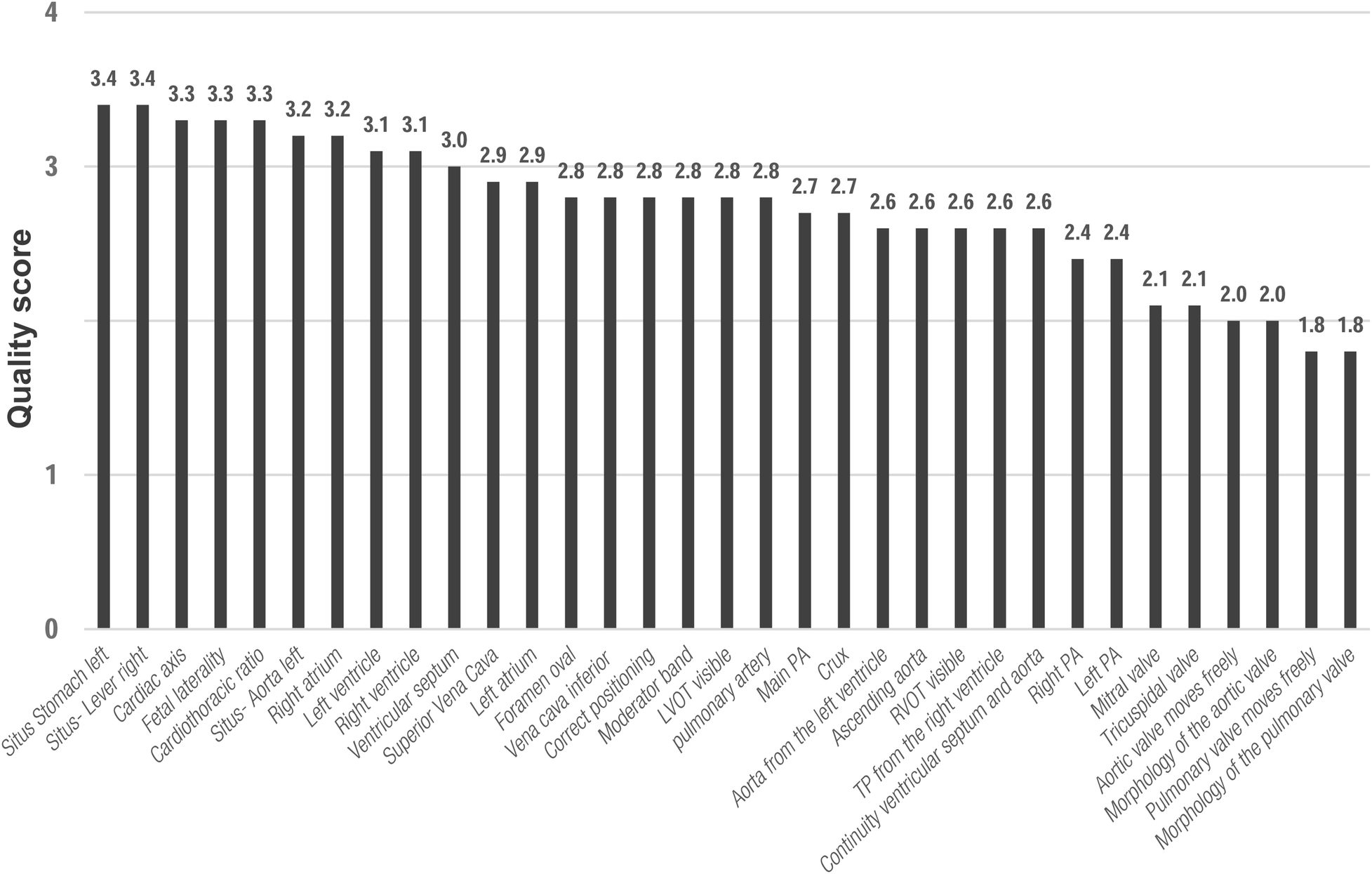
Mean scores for diagnostic quality of fetal cardiovascular MRI for cardiac structures according to the applied 4-point scale. 4 CV, four-chamber view; LVOT, left outflow tract; RVOT, right outflow tract; 3V, three-vessel view; PA, pulmonary artery; VCS, Vena cava superior.
A detailed analysis of each cardiovascular structure/morphology for fetal cardiovascular MRI and fetal echocardiography is presented in Table 1. In two cases with prenatal echocardiographic finding of VSD, no VSD was depicted by MRI and postnatal echocardiography. It was not always possible to evaluate all structures in each axial view of the 28 fetuses owing to insufficient image quality (score 1) in MRI or insufficient acoustic window in fetal echocardiography.
Table 1
| Echo | MRI | ||||
|---|---|---|---|---|---|
| Normal (specificity) | Pathologic (sensitivity) | Normal (specificity) | Pathologic (sensitivity) | ||
| Situs | Stomach left | 27/27 (100%) | 1/1 (100%) | 27/27 (100%) | 1/1 (100%) |
| Liver right | 28/28 (100%) | – | 28/28 (100%) | – | |
| Aorta left | 28/28 (100%) | – | 28/28 (100%) | – | |
| VCI | 28/28 (100%) | – | 28/28 (100%) | – | |
| Overall | 1/1 (100%) | 1/1 (100%) | |||
| 4CV | Fetal laterality | 27/27 (100%) | 1/1 (100%) | 27/27 (100%) | 1/1 (100%) |
| Heart occupies a 1/3 of thoracic area | 27/27 (100%) | 1/1 (100%) | 27/27 (100%) | 1/1 (100%) | |
| Cardiac axis | 24/24 (100%) | 4/4 (100%) | 22/24 (92%) | 4/4 (100%) | |
| Left ventricle | 21/21 (100%) | 7/7 (100%) | 21/21 (100%) | 4/7 (57%) | |
| Right ventricle | 19/19 (100%) | 9/9 (100%) | 19/19 (100%) | 7/9 (78%) | |
| Left atrium | 26/26 (100%) | 2/2 (100%) | 26/26 (100%) | 1/2 (50%) | |
| Right atrium | 26/26 (100%) | 2/2 (100%) | 26/26 (100%) | 2/2 (100%) | |
| Moderator band | 28/28 (100%) | – | 27/27 (100%) | – | |
| Crux | 26/26 (100%) | 2/2 (100%) | 25/25 (100%) | 1/2 (50%) | |
| Foramen oval | 28/28 (100%) | – | 26/26 (100%) | – | |
| Ventricular septum | 23/23 (100%) | 4/5 (80%) | 23/23 (100%) | 2/5 (40%) | |
| Mitral valve | 27/27 (100%) | 1/1 (100%) | 27/27 (100%) | 0/1 (0%) | |
| Tricuspidal valve | 27/27 (100%) | 1/1 (100%) | 27/27 (100%) | 1/1 (100%) | |
| Overall | 34/35 (97.1%) | 24/35 (68.6%) | |||
| LVOT | LVOT visible | 26/26 (100%) | 2/2 (100%) | 22/22 (100%) | 2/2 (100%) |
| Aorta originates from the left ventricle | 27/27 (100%) | 1/1 (100%) | 25/25 (100%) | 1/1 (100%) | |
| Continuity between the ventricular septum and the anterior wall of the aorta | 26/26 (100%) | 2/2 (100%) | 25/25 (100%) | 2/2 (100%) | |
| Aortic valve moves freely | 26/26 (100%) | 2/2 (100%) | 25/25 (100%) | 0/2 (0%) | |
| Morphology of the aortic valve | 26/26 (100%) | 1/2 (50%) | 26/26 (100%) | 0/2 (0%) | |
| Overall | 8/9 (88.9%) | 5/9 (55.6%) | |||
| RVOT | RVOT visible | 28/28 (100%) | – | 26/26 (100%) | – |
| TP originates from the right ventricle | 26/26 (100%) | – | 27/27 (100%) | – | |
| Pulmonary valve moves freely | 25/25 (100%) | 1/1 (100%) | 23/23 (100%) | – | |
| Morphology of the pulmonary valve | 24/24 (100%) | 2/2 (100%) | 22/22 (100%) | 0/1 (0%) | |
| Main PA | 23/23 (100%) | 2/3 (67%) | 25/25 (100%) | 2/3 (67%) | |
| Right PA | 23/23 (100%) | 2/2 (100%) | 21/21 (100%) | 1/2 (50%) | |
| Left PA | 22/22 (100%) | 2/2 (100%) | 21/21 (100%) | 1/2 (50%) | |
| Overall | 9/10 (90%) | 4/8 (50%) | |||
| 3V view | Pulmonary artery | 21/21 (100%) | 7/7 (100%) | 21/21 (100%) | 4/6 (67%) |
| Ascending aorta | 19/19 (100%) | 9/9 (100%) | 20/20 (100%) | 4/6 (67%) | |
| Superior vena cava | 28/28 (100%) | – | 26/26 (100%) | – | |
| Correct positioning | 26/26 (100%) | 1/2 (50%) | 26/26 (100%) | 0/1 (0%) | |
| Relationship | 19/19 (100%) | 9/9 (100%) | 18/18 (100%) | 4/8 (50%) | |
| Overall | 26/27 (96%) | 12/21 (57%) | |||
Detailed evaluation of cardiovascular structures and morphology for fetal cardiovascular MRI and fetal echocardiography.
4 CV, four-chamber view; LVOT, left outflow tract; RVOT, right outflow tract; 3V, three-vessel view; PA, pulmonary artery; VCS, Vena cava superior. Number of measurements varies from total number of fetuses (n = 28) when not all planes could be evaluated for this structure, e.g., due to insufficient image quality (score 1) in MRI or insufficient acoustic window in fetal echocardiography.
The Table 1 above shows an overall sensitivity for the single structures. However, we have also performed an analysis in which the detection of one pathology is sufficient to classify the level as pathological. Then the sensitivity for cardiovascular MRI and fetal echocardiography was 100% and 100% for the situs view, 81% and 94% for the 4-chamber view, 83% and 100% for the LVOT view, 100% and 67% for RVOT view, and 75% and 100% for the 3V view, respectively.
However, overall diagnostic performance, referring to a diagnosis as pathological if at least one axial view revealed a pathological finding, did not differ between fetal cardiovascular MRI und fetal echocardiography. Pathological cases were correctly diagnosed in 15/17 and 17/17 cases by fetal cardiovascular MRI and fetal echocardiography, resulting in a sensitivity of 88% and 100%, respectively. Normal findings were correctly assessed in all fetuses without cardiac abnormalities by both, fetal cardiovascular MRI and fetal echocardiography resulting in a specificity of 100%.
A summary of all cases with prenatal and postnatal findings and neonatal outcome is provided in Table 2.
Table 2
| Fetus | GA (weeks) | Situs | 4CV | LVOT | RVOT | 3V view | Postnatal Echo | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Echo | MRI | Echo | MRI | Echo | MRI | Echo | MRI | Echo | MRI | ||||
| 1 | 38 + 3 | N | N | P | P | N | N | N | N | P | P | Narrow aortic isthmus | Conservative |
| 2 | 32 + 5 | N | N | N | N | P | N | N | N | P | P | Bicuspid aortic valve + aortic stenosis | Intervention |
| 3 | 33 + 4 | N | N | P | P | P | P | N | N | P | P | Hypoplastic aortic arch | Conservative |
| 4 | 35 + 6 | N | N | N | P | P | P | P | P | P | P | Tetralogy of Fallot with high-grade infundibular and valvular pulmonary stenosis. Hypoplastic proximal right pulmonary artery | Surgery |
| 5 | 33 + 0 | N | N | P | P | P | P | N | not evaluable | P | N | Right aortic arch | Conservative |
| 6 | 35 + 4 | N | N | P | P | N | N | N | not evaluable | not evaluable | not evaluable | Ebstein's anomaly Carpentier type C, pulmonary atresia with constricted confluence, ASD of the secondary type | Surgery |
| 7 | 37 + 1 | N | N | P | P | N | N | N | N | N | N | Intermediate type AVSD, or partial AVSD,ASD I,ASD II | Surgery |
| 8 | 36 + 3 | N | N | P | N | N | N | N | N | N | N | Trisomy 13, Microphtalmia Extremely narrow palpebral fissures Left/right shunt over atrial septum, persistent ductus arteriosus with left/right shunt, impaired left ventricular function |
No cardial intervention |
| 9 | 29 + 2 | N | N | P | P | N | N | N | N | P | P | CoA,VDS,AVSD, partial Trisomy 8, partial Monosomy 18 | Surgery |
| 10 | 38 + 2 | N | N | P | P | N | N | N | N | P | P | Coarctation aortae | Surgery |
| 11 | 35 + 1 | N | N | P | N | P | P | N | N | P | N | Mid-muscular VSD, narrow aortic arch | Conservative |
| 12 | 34 + 3 | N | N | P | P | N | N | N | N | P | N | Interrupted aortic arch type A Multiple collaterals to the descending aorta Atrial septal defect of secondary type |
Surgery |
| 13 | 33 + 0 | N | N | P | P | N | N | P | P | P | P | Absent pulmonary valve syndroym, Fallot-Type | Surgery |
| 14 | 36 + 3 | N | N | P | N | N | N | N | N | N | N | Prenatal VSD | Control |
| 15 | 37 + 6 | N | N | P | P | P | P | N | N | P | P | Severe valvular aortic valve stenosis, bicuspid aortic valve Mitral valve dysplasia,left ventricular dilatation with endocardial fibroelastosis |
Normal |
| 16 | 38 + 5 | N | N | P | P | N | N | N | N | P | P | Limited hypolplastic aortic arch | Surgery |
| 17 | 34 + 5 | P | P | P | P | N | N | N | N | N | N | Diaphragmatic hernia | Surgery |
Summary of all fetuses with prenatal and postnatal pathologic findings and neonatal outcome.
GA, gestational age; P, pathological; N, normal; 4 CV, four-chamber view; LVOT, left outflow tract; RVOT, right outflow tract; 3V, three-vessel view. ASD, atrial septal defect; AVSD, atrioventricular septal defect; CoA, Coarctation of the aorta; VSD, ventricular septal defect.
In relation to the postnatal cardiac diagnoses, this means for each axial view the following:
The 4CV by fetal cardiovascular MRI revealed 13 anomalies and had one false positive anomalie (case 4) and could not detect the three VSD (case 8,11,15).
The LVOT by fetal cardiovascular MRI revealed 5 anomalies (5/6, 83.3%) and missed an aortic stenosis with a bicuspid aortic valve (case 2).
Prenatal pathological findings of the RVOT in both examination techniques were present. In case 6, pulmonary stenosis was not seen by echocardiography. This plane and a second one was not evaluable on cardiovascular MRI.
The fetal cardiovascular MRI revealed 9 anomalies (9/12, 75%) in the 3V view and could not detected a right aortic arch, an interrupted aortic arch and narrowed aortic arch (case 5,11,12). One fetus could not be evaluated in both modalities.
Quantitative analysis
Quantitative evaluation of diameter measurements in the five axial views showed no differences between fetal cardiovascular MRI and fetal echocardiography (Table 3). Examples of measurements are provided in Figure 4.
Table 3
| n | Echo | MRI | P-value | |
|---|---|---|---|---|
| Longitudinal cardiac diameter (mm) | 24 | 44.8 ± 5.6 | 45.1 ± 5.7 | 0.71 |
| Transverse cardiac diameter (mm) | 25 | 37.1 ± 5.9 | 38.4 ± 7.2 | 0.17 |
| LVOT | 23 | 5.8 ± 0.9 | 5.4 ± 0.8 | 0.10 |
| RVOT | 22 | 8.4 ± 1.8 | 8.6 ± 2.9 | 0.75 |
| PA | 22 | 8.6 ± 1.9 | 8.6 ± 1.6 | 0.70 |
| Aorta | 23 | 5.5 ± 1.4 | 5.8 ± 1.1 | 0.08 |
| VCS | 21 | 4.7 ± 0.9 | 4.7 ± 1.0 | 0.8 |
Comparison of quantitative measurements for cardiovascular MRI and fetal echocardiography.
Comparison of measurements were only included in cases where images were available for both methods. LVOT, left outflow tract; RVOT, right outflow tract; PA, pulmonary artery; VCS, Vena cava superior.
Figure 4
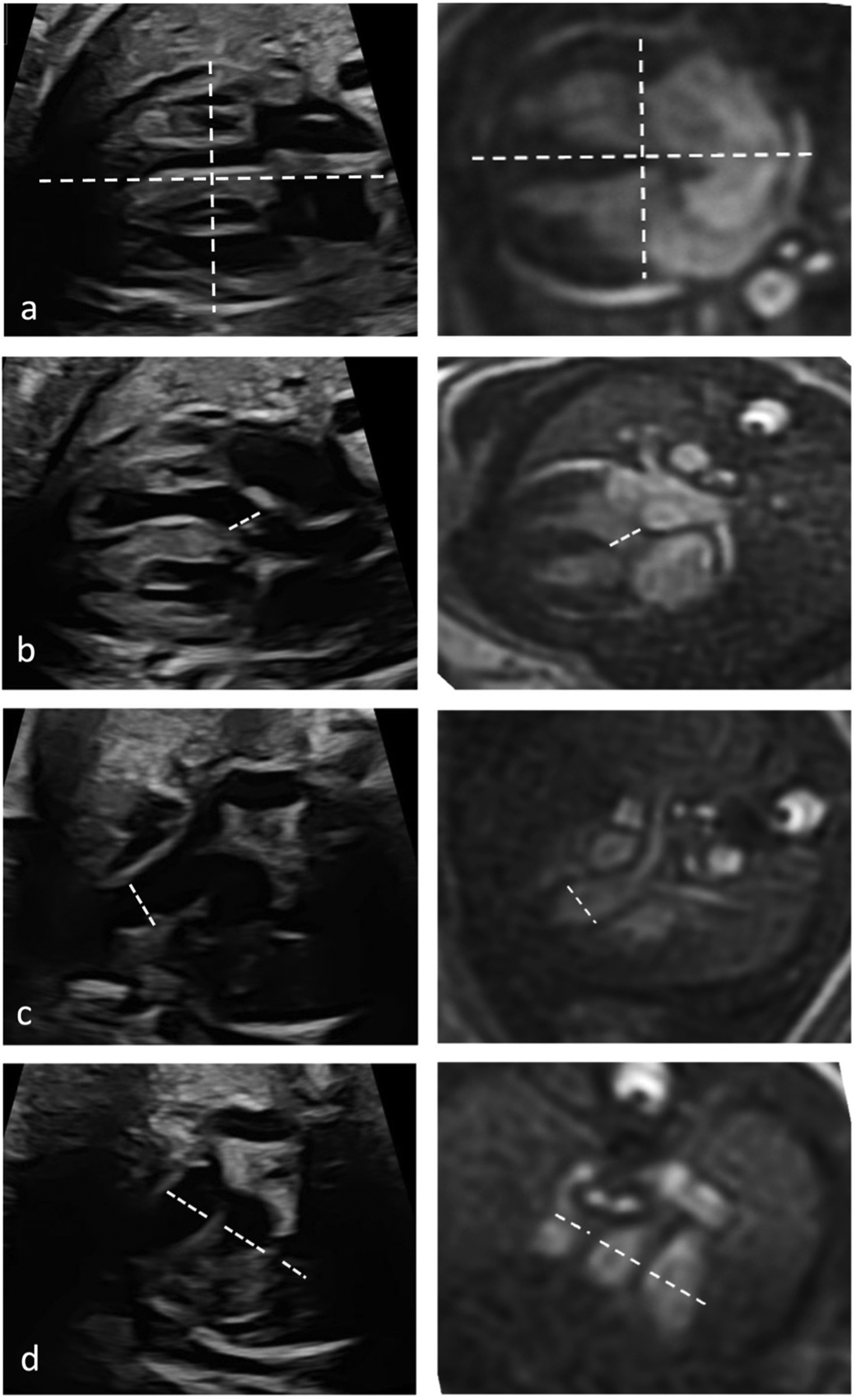
Quantitative assessment of cardiac diameters. Diameter measurements in fetal cardiac five axial views in a healthy Fetus (35 + 1 weeks gestation) in fetal echocardiography (left column) and cardiac MRI (right column). Longitudinal and transverse cardiac diameter from 4-chmaber view (a), diameter of the LVOT (b), the RVOT diameter (c) and diameters of pulmonary artery, aorta, and superior vena cava from the three-vessel view (d).
Discussion
Fetal cardiovascular MRI using DUS gating for the assessment of the five axial views revealed similar diagnostic performance in comparison to fetal echocardiography. Our study indicates that the five axial views established for fetal echocardiography can be transferred to fetal cardiovascular MRI for prenatal evaluation of CHD.
Considering the high fetal heart rates and small dimensions of the fetal heart and vessels, cardiac gating is necessary to allow dynamic imaging with high spatial and temporal resolution (19). The method of DUS gating provides a promising approach for anatomical and functional MRI of the fetal cardiovascular system (18–21). An overall high image quality was achieved in this study.
Diagnostic performance of fetal cardiovascular MRI was similar to fetal echocardiography assessing the five axial views. While specificity of MRI was 100% for all five axial views, sensitivities were slightly inferior to fetal echocardiography with 88% vs. 100%, respectively. In two cases a prenatally diagnosed VSD by fetal echocardiography was not depicted by MRI. However, these prenatally described VSDs were not visualized in postnatal echocardiography either. It remains unclear whether MRI revealed false negative, or fetal echocardiography false positive results in these two cases. To note, VSDs may close and disappear during pregnancy or after delivery (25, 26). It must be said that fetal echocardiography has still a better sensitivity at analyzing the single structures, but the MRI achieves high overall diagnostic performance because the detection of one pathology is sufficient to classify the level as pathological. With this increasing improvement in MR imaging, showing in this paper, it can be expected that complete diagnosis of congenital heart defects will be possible in the future.
Evaluation of the cardiac valves revealed to be difficult by cardiovascular MRI, indicated by lower diagnostic quality scores. However, it is also known from adult cardiovascular MRI that fetal echocardiography is superior to MRI in the evaluation of valve abnormalities (27). Difficulties in the detection of specific pathologies may be seen for both, fetal echocardiography and MRI, e.g., Ebstein's anomaly revealed limitations in the interpretability of both imaging modalities due to the enlarged right atrium. Detection of pathology in the 3V view, e.g., evaluation of the relationship of VCI, aorta and main pulmonary artery, with cardiovascular MRI also proved difficult (Table 1). One reason for the lower sensitivity of cardiovascular MRI in the detection of CHD in this study may be the limited experience of the analyzing radiologist with cardiac MRI in general and specifically with CHD. Therefore, detection rates could be higher with increasing routine and expertise in this complex field. However, quantitative analysis demonstrated that fetal cardiovascular MRI is comparable to fetal echocardiography as all diameters were similar for both methods.
This study provides insight into diagnostic performance of MRI in comparison to the reference standard of echocardiography in a fetal population with and without CHD including a detailed analysis of all cardiac and vascular structures. It demonstrated that the evaluation of the five axial views is possible for both imaging techniques including qualitative and quantitative assessment of particular structures. If CHD is suspected by fetal echocardiography after the second trimester scan, MRI may provide a valuable alternative or imaging adjunct because fetal echocardiographic imaging becomes difficult with increasing gestational age and increasing fetal ossification. However, this did not appear to be a limitation in our study.
Fetal cardiovascular MRI has gained substantial interest in recent years, mainly due to technical developments (16). In 2009, Manganaro was one of the first to investigate the performance of fetal cardiac MRI for the detection of CHD, evaluating direct and indirect criteria such as malrotation of the ventricles or cardiomegaly (28). However, assessment of particular anatomical structures was not possible due to the lack of cardiac-gating, which is necessary to allow high spatio-temporal resolutions in fetal cardiovascular MRI (28). Furthermore, no comparison to the reference standard of fetal echocardiography was provided. Dong et al. were able to demonstrate the effective use of fetal cardiac MRI over a period of 5 years and 68 pregnant women with fetuses with a congenital heart defect (29). The detection rate in this study was 79% with MRI and 82% with fetal echocardiography, although no additional cardiac gating or analysis of the 5 axial planes was used (29). They emphasize the examiner's wealth of experience here and also highlight this in the Dong et al. paper from 2020, which could explain the high detection rate even in the early development phase of fetal cardiac MRI (30).
In 2012, Votino et al. examined fetuses with CHD assessing 4-chamber views without cardiac gating (31). The assessed 4-chamber view achieved a comparable sensitivity to our study of 88%. However, for the LVOT and RVOT sensitivities of only 63% and 59% were reported (31). The lacking opportunity to apply fetal cardiac gating at that time could explain the lower sensitivities for LVOT and RVOT in their study. Using DUS-gated cardiovascular MRI we could achieve higher detection rates for the LVOT and RVOT with 83% and 100%, respectively.
Vollbrecht et al. recently investigated 23 fetuses with CHD in a prospective setting and evaluated the diagnostic performance of fetal cardiovascular MRI using DUS-gating, similar to our study (22). Specific structures were analyzed and compared to postnatal findings. High specificity and sensitivity of 99.9% and 91.8% for CHD was found (29). Our results are comparable to Vollbrecht et al., indicating the diagnostic potential of fetal cardiovascular MRI for evaluation and detection of CHD. In addition to that former study we analyzed all cardiac structures referring to current ISUOG guidelines (9). Furthermore, we assessed quantitative measurements of cardiovascular structures, revealing high agreement for fetal echocardiography and MRI. Another noteworthy difference is that in contrast to Vollbrecht et al. the radiologist in our study was blinded to the referral diagnosis.
Nevertheless, a general limitation of fetal MRI is fetal movement causing motion artefacts with resulting low image quality in some of our examined fetuses or in single imaging planes and our study is the relatively small number of included fetuses. The preliminary data suggests that while fetal MRI provides detailed anatomical information, technical challenges such as fetal motion need to be addressed to minimize data loss and ensure reliable cardiac gating. Further studies are also warranted to identify potential subgroups of fetuses that may benefit from additional cardiovascular MRI regarding counseling and neonatal care.
Conclusion
In conclusion, our study indicates the diagnostic potential of dynamic fetal cardiovascular MRI in the evaluation of the five axial views for prenatal assessment of CHD. In certain conditions, when fetal echocardiography is inconclusive or limited by anatomical or maternal conditions, fetal cardiovascular MRI offers an alternative or adjunct to fetal echocardiography and may therefore improve prenatal assessment of CHD. Our study showed a higher sensitivity for the axial plane of the RVOT in fetal cardiovascular MRI and could be particularly helpful for this question in the diagnosis of CHD. Further studies are warranted to evaluate the clinical impact and influence on fetal outcome of this promising technique.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethik-Kommission der Ärztekammer Hamburg. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BH: Writing – original draft, Writing – review & editing. MT: Writing – original draft, Writing – review & editing. JH: Writing – review & editing. PB: Writing – review & editing. LH: Supervision, Writing – review & editing. SG: Writing – review & editing. KH: Writing – review & editing. GA: Writing – review & editing. MD: Writing – original draft, Writing – review & editing. BS: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant of the German Research Foundation (DFG) (SCHO 1564/2–1 and BA 5893/6–1)
Conflict of interest
MT and BS are Co-founder and stakeholder of northh medical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Lindinger A Schwedler G Hense HW . Prevalence of congenital heart defects in newborns in Germany: results of the first registration year of the PAN study (July 2006 to June 2007). Klin Padiatr. (2010) 222:321–6. 10.1055/s-0030-1254155
2.
Van der Linde D Konings E Slager M Witsenburg M Helbing W Takkenberg J et al Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. (2011) 58:2241–7. 10.1016/j.jacc.2011.08.025
3.
Strauss A Toth B Schwab B Fuchshuber S Schulze A Netz H et al Prenatal diagnosis of congenital heart disease and neonatal outcome–a six years experience. Eur J Med Res. (2001) 6(2):66–70.
4.
Eckersley L Sadler L Parry E Finucane K Gentles TL . Timing of diagnosis affects mortality in critical congenital heart disease. Arch Dis Child. (2016) 101(6):516–20. 10.1136/archdischild-2014-307691
5.
Khalil M Speer CP Gahr M Dötsch J . In: SpeerCPGahrMDötschJ, editors. Herz und Gefäße. Berlin, Heidelberg: Springer (2019). p. 453–83.
6.
Allan LD Tynan MJ Campbell S Wilkinson JL Anderson RH . Echocardiographic and anatomical correlates in the fetus. Br Heart J. (1980) 44:444–51. 10.1136/hrt.44.4.444
7.
Tegnander E Williams W Johansen OJ Blaas HG Eik-Nes S . Prenatal detection of heart defects in a non-selected population of 30149 fetuses- detection rates and outcome. Ultrasound Obstet Gynecol. (2006) 27:252–65. 10.1002/uog.2710
8.
Yoo S Lee Y Kim ES Ryu HM Kim MY Choi HK et al Three-vessel view of the fetal upper mediastinum: an easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetric screening. Ultrasound Obstet Gynecol. (1997) 9:173–82. 10.1046/j.1469-0705.1997.09030173.x
9.
Carvalho JS Axt-Fliedner R Chaoui R Copel JA Cuneo BF Goff D et al ISUOG practice guidelines (updated): fetal cardiac screening. UOG. (2023) 61(6):788–803. 10.1002/uog.26224
10.
Li Y Hua Y Fang J Wang C Qiao L Wan C et al Performance of different scan protocols of fetal echocardiography in the diagnosis of fetal congenital heart disease: a systematic review and meta-analysis. PLoS One. (2013) 8:e65484. 10.1371/journal.pone.0065484
11.
Hendler I Blackwell SC Bujold E Treadwell MC Wolfe HM Sokol RJ et al The impact of maternal obesity on midtrimester sonographic visualization of fetal cardiac and craniospinal structures. Int J Obes Relat Metab Disord. (2004) 28(12):1607–11. 10.1038/sj.ijo.0802759
12.
Dashe JS McIntire DD Twickler DM . Effect of maternal obesity on the ultrasound detection of anomalous fetuses. Obstet Gynecol. (2009) 113:1001–7. 10.1097/AOG.0b013e3181a1d2f5
13.
Kawabata I Takahashi Y Iwagaki S Tamaya T . MRI during pregnancy. J Perinat Med. (2003) 31(6):449–58. 10.1515/JPM.2003.070
14.
Manganaro L Bernardo S Antonelli A Vinci V Saldari M Catalano C . Fetal MRI of central nervous system: state-of-the-art. Eur J Radiol. (2017) 93:273–83. 10.1016/j.ejrad.2017.06.004
15.
Masselli G Vaccaro Notte MR Zacharzewska-Gondek A Laghi F Manganaro L Brunelli R . Fetal MRI of CNS abnormalities. Clin Radiol. (2020) 75(8):640.e1–e11. 10.1016/j.crad.2020.03.035
16.
Roy CW van Amerom JF Marini D Seed M Macgowan CK . Fetal cardiac MRI: a review of technical advancements. Top Magn Reson Imaging. (2019) 28(5):235–44. 10.1097/RMR.0000000000000218
17.
Kording F Yamamura J De Sousa MT Ruprecht C Hedström E Aletras AH et al Dynamic fetal cardivascular magnetic resonance imaging using Doppler ultrasound gating. J Cardiovasc Magn Reson. (2018) 20(1):17. 10.1186/s12968-018-0440-4
18.
Tavares de Sousa M Hecher K Yamamura J Kording F Ruprecht C Fehrs K et al Dynamic fetal cardiac magnetic resonance four chamber view imaging using Doppler ultrasound gating in the normal fetal heart and congenital heart disease: comparison to fetal echocadriography. Ultrasound Obstet Gynecol. (2019) 53:669–75. 10.1002/uog.20167
19.
Tavares de Sousa M Hecher K Kording F Yamamura J Lenz A Adam G et al Fetal dynamic magnetic resonance imaging using Doppler ultrasound gating for the assessment of the aortic isthmus: a feasibility study. Acta Obstet Gynecol Scand. (2021) 100(1):67–73. 10.1111/aogs.13957
20.
Knapp J Tavares de Sousa M Lenz A Herrmann J Zhang S Kording F et al Fetal 4D flow MRI of the great thoracic vessels at 3 tesla using Doppler-ultrasound gating: a feasibility study. Eur Radiol. (2022) 33(3):1698–706. 10.1007/s00330-022-09167-7
21.
Schoennagel BP Yamamura J Kording F Fischer R Bannas P Adam G et al Fetal dynamic phase-contrast MR angiography using ultrasound gating and comparison with Doppler ultrasound measurements. Eur Radiol. (2019) 29(8):4169–76. 10.1007/s00330-018-5940-y
22.
Vollbrecht TM Hart C Zhang S Katemann C Isaak A Pieper CC et al Fetal cardiac cine MRI with Doppler US gating in complex congenital heart disease. Radiol Cardiothorac Imaging. (2023) 5(1):e220129. 10.1148/ryct.220129
23.
Vollbrecht TM Hart C Luetkens JA . Fetal cardiac MRI of complex interrupted aortic arch. Radiology. (2023) 307(5):e223224. 10.1148/radiol.223224
24.
Moon-Grady AJ Donofrio MT Gelehrter S Hornberger L Kreeger J Lee W et al Guidelines and recommendations for performance of the fetal echocardiogram: an update from the American society of echocardiography. J Am Soc Echocardiogr. (2023) 36(7):679–723. 10.1016/j.echo.2023.04.014
25.
Raucher Sternfeld A Sheffy A Tamir A Mizrachi Y Assa S Shohat M et al Isolated ventricular septal defects demonstrated by fetal echocardiography:prenatal course and postnatal outcome. J Matern Fetal Neonatal Med. (2022) 35(1):129–33. 10.1080/14767058.2020.1712710
26.
Svirsky R Brabbing-Goldstein D Rozovski U Kapusta L Reches A et al The genetic and clinical outcome of isolated fetal muscular ventricular septal defect (VSD). J Matern Fetal Neonatal Med. (2019) 32(17):2837–41. 10.1080/14767058.2018.1449829
27.
Roshin CM Löffler AI Salerno M . Role of cardiac magnetic resonance imaging in valvular heart disease: diagnosis, assessment, and management. Curr Cardiol Rep. (2018) 20(11):119. 10.1007/s11886-018-1057-9
28.
Manganaro L Savelli S Di Maurizio M Perrone A Francioso A La Barbera L et al Assessment of congenital heart diseases (CHD): is there a role for fetal magnetic resonance imaging (MRI)? Eur J Radiol. (2009) 72(1):172–80. 10.1016/j.ejrad.2008.06.016
29.
Dong SZ Zhu M Li F . Preliminary experience with cardiovascular magnetic resonance in evaluation of fetal cardiovascular anomalies. J Cardiovasc Magn Reson. (2013) 15(1):40. 10.1186/1532-429X-15-40
30.
Dong SZ Zhu M Hui J Ren J-Y Liu K . Fetal cardiac MRI: a single center experience over 14-years on the potential utility as an adjunct to fetal technically inadequate echocardiography. Sci Rep. (2020) 10(1):12373. 10.1038/s41598-020-69375-3
31.
Vitino L Votino C Jani J Damry N Dessy H Kang X et al Magnetic resonance imaging in the normal fetal heart and in congenital heart diseases. Ultrasound Obstet Gynecol. (2012) 39(3):322–9. 10.1002/uog.10061
Summary
Keywords
fetal MRI, fetal echocardiography, five axial views, congenital heart disease, DUS gating MRI, fetal heart
Citation
Hergert B, Tavares de Sousa M, Herrmann J, Bannas P, Huber L, Götz S, Hecher K, Adam G, Dargahpour Barough M and Schoennagel BP (2024) A comparative study of fetal cardiovascular assessment: utilizing Doppler ultrasound gated MRI and echocardiography with detailed analysis using five axial views. Front. Cardiovasc. Med. 11:1408071. doi: 10.3389/fcvm.2024.1408071
Received
27 March 2024
Accepted
02 September 2024
Published
23 September 2024
Volume
11 - 2024
Edited by
Christopher W. Roy, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Reviewed by
Luis Goncalves, Phoenix Children’s Hospital, United States
Liqun Sun, University of Toronto, Canada
Updates
Copyright
© 2024 Hergert, Tavares de Sousa, Herrmann, Bannas, Huber, Götz, Hecher, Adam, Dargahpour Barough and Schoennagel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: B. Hergert b.hergert@uke.de M. Tavares de Sousa m.tavares-de-sousa@uke.de B. P. Schoennagel bschoenn@uke.de
†These authors share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.