Abstract
Background:
Treating heavily calcified vessels is a challenging task in patients with an impaired left ventricular ejection fraction. Percutaneous mechanical circulatory support (pMCS) is increasingly used in patients in high-risk percutaneous coronary intervention (HRPCI).
Methods:
In this retrospective registry, we investigated 25 patients undergoing a protected HRPCI receiving either intravascular lithotripsy (IVL + pMCS; n = 11) or rotational atherectomy (RA + pMCS; n = 14). The primary endpoint was defined as peri-interventional hemodynamic stability. The secondary endpoint was defined as major adverse cardiac events (MACE).
Results:
Patients in the IVL + pMCS group had a significantly higher mean arterial pressure (MAP) at the end of the procedure (p = 0.04). However, the Δ-change in MAP was not significant [−12 mmHg (±20.3) vs. −16.1 mmHg (±23.9), p = 0.709]. The proportion of patients requiring post-interventional catecholamines was significantly lower in the IVL + pMCS group (p = 0.02). The Δ-change in Syntax Score was not significant between groups (IVL + pMCS −22 (±5.8) vs. RA + pMCS −21.2 (±7.6), p = 0.783). MACE did occur less in the group of IVL + pMCS (0% vs. 20%, p = 0.046). Patients with pMCS insertion as a bailout strategy had a higher probability for in-hospital death (p < 0.001) and the occurrence of the slow-reflow phenomenon was associated with long-term mortality (p = 0.021) in the cox regression analysis.
Conclusions:
In our cohort patients in the IVL + pMCS group were hemodynamically more stable which led to a lower rate of catecholamine usage. pMCS as a bailout strategy was associated with in-hospital death and the occurrence of the slow reflow phenomenon with all-cause mortality during follow-up.
1 Introduction
The use of percutaneous mechanical circulatory support (pMCS) systems in the treatment of patients with complex coronary artery disease who are unsuitable for coronary artery bypass surgery due to their clinical presentation, their comorbidities and/or an impaired left ventricular ejection fraction (LVEF) is on the uprise (1). Nonetheless, this population is potentially understudied and 1-year mortality varies from 1% to 11% (2, 3). Improvement in quality of life and a reduction of adverse events can be achieved through coronary intervention in these patients (4–7). With the use of pMCS the operator can perform complex interventions and reduce the risk of a peri-interventional hemodynamical deterioration. This has already been demonstrated in the PROTECT III trial where a significantly lower rate of hemodynamical deterioration, defined as procedural hypotension, cardiopulmonary resuscitation and ventricular arrhythmias occurred (8). However, a universal definition of these high-risk percutaneous coronary interventions (HRPCI) is still lacking (9). Considerations are mainly patient-related factors like criteria of the coronary anatomy and the hemodynamical situation. Also, the patients’ age and past medical history are of interest. Regarding the coronary anatomy, the presence of a coronary multi-vessel disease, last remaining vessel, chronic total occluded coronary arteries and heavily calcified vessels must be considered. Detection of these factors in combination with clinical or hemodynamic signs of cardiac decompensation or an expected long time of myocardial ischemia meets the criteria of a HRPCI (10). Further, clear recommendations of the usage of pMCS in elective cases undergoing HRPCI and especially in patients with severe coronary artery calcification are also lacking. In the presence of calcification, wiring and adequate lesion preparation may be a challenging task and may lead to longer procedure times (11–13). A more intensive lesion preparation carries the risk of complications such as the occurrence of the slow reflow or no reflow phenomenon or coronary artery perforation.
When conventional measures fail in the treatment of severely calcified coronary stenoses, there are alternative methods like intravascular lithotripsy (IVL) or rotational atherectomy (RA) (14). To optimize stent expansion and vessel compliance, IVL, a relatively new therapeutic method for lesion preparation, has been introduced. In this procedure calcium fracturation is achieved by sound wave emitters embedded in a balloon system. In addition, the use of low dilatation pressures (4 atm) reduces barotrauma, which is significantly higher with conventional high-pressure balloon catheters (up to 40 atm). The Disrupt CAD I-IV studies and registries (14–16) demonstrated the benefits and applicability of IVL, as well as the low complication and mortality rates (17). These results and IVL’s safe applicability have been confirmed in further studies (14, 18, 19). However, patients with a severely depressed LVEF were excluded from the Disrupt CAD I-IV studies. The theoretical advantage of RA over IVL is that the calcium is not fractured but ablated by the drill head. The ROTAXUS trial compared RA and stenting with standard therapy and stenting. The patient population had an average LVEF of 55% for the RA group and this trial also did not include patients with pMCS (20). Due to the lack of data in the context of HRPCI with pMCS in patients with severely calcified coronary stenoses, the use of coronary lesion preparation using RA vs. IVL is of interest. Here, with the support of pMCS, the operator can carry out longer procedures with complex interventions with a certain degree of safety, without having to worry about further hemodynamic deterioration of the patient. In this context this is the first study comparing IVL to RA in patients with pMCS.
2 Materials and methods
This retrospective analysis was performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version. Ethical approval of the study was granted by the responsible local ethics committee of the Rhineland-Palatinate chamber of physicians and is listed under the file number 2023-17384. Patient confidentiality has been maintained by anonymizing patient data to remove any identifying information. Thus, patient consent was not required. A local database was generated using standard data collected during hospitalization and during treatment in the catheterization laboratory in the period from September 2019 to July 2023. The data is collected retrospectively from the clinic's internal IT database and is filtered according to the OPS coding for RA (8-837.50), IVL (8-83d.6), and pMCS (8-839.4). From this, patients are identified who received a combination of IVL (Shockwave® Medical Inc. Corporate Headquarters, 5403 Betsy Ross Drive, Santa Clara, CA 95054, USA) and pMCS or RA (Boston Scientific® World Headquarters, 300 Boston Scientific Way Marlborough, MA, USA) and pMCS (Impella® CP SmartAssist®, Abiomed Europe GmbH, Aachen, Deutschland). The use of either IVL or RA was left to the decision of the treating interventional cardiologist. We included all patients with severe coronary artery disease due to complex coronary anatomy, calcified stenoses and corresponding previous illnesses who are not suitable for surgical care. As a standardized approach we based our screening criteria on the recently published criteria suggested for identifying patients suitable for HRPCI (10) (Figure 1). We also included all patients who had an impaired LVEF or one is to be expected to be at risk of hemodynamic compromise during the intervention, based on the characteristics of the lesion. Exclusion criteria consisted of patients age below 18 years, contraindication for pMCS, patients suitable for cardiac surgical treatment, patients receiving Rota-Shock (RA + IVL), and patients in cardiac arrest. Analysis of the lesion characteristics (e.g., length of calcified portion, total length, eccentric, or concentric) was made afterward through a review of the coronary angiography by an operator blinded to the procedure groups. Measurements have been performed retrospectively using offline quantitative coronary angiography (QCA). The primary endpoint was the hemodynamic status after the intervention represented by mean arterial pressure (MAP). The MAP is recorded in our catheterization laboratory as standard before the start and at the end of the procedure. Data were available for all patients. The secondary endpoint was a composite of MACE [cardiac death, stroke, peri-interventional myocardial infarction according to the fourth universal definition of MI (21)] during hospital stay and during the follow-up, which was evaluated by telephone interview.
Figure 1
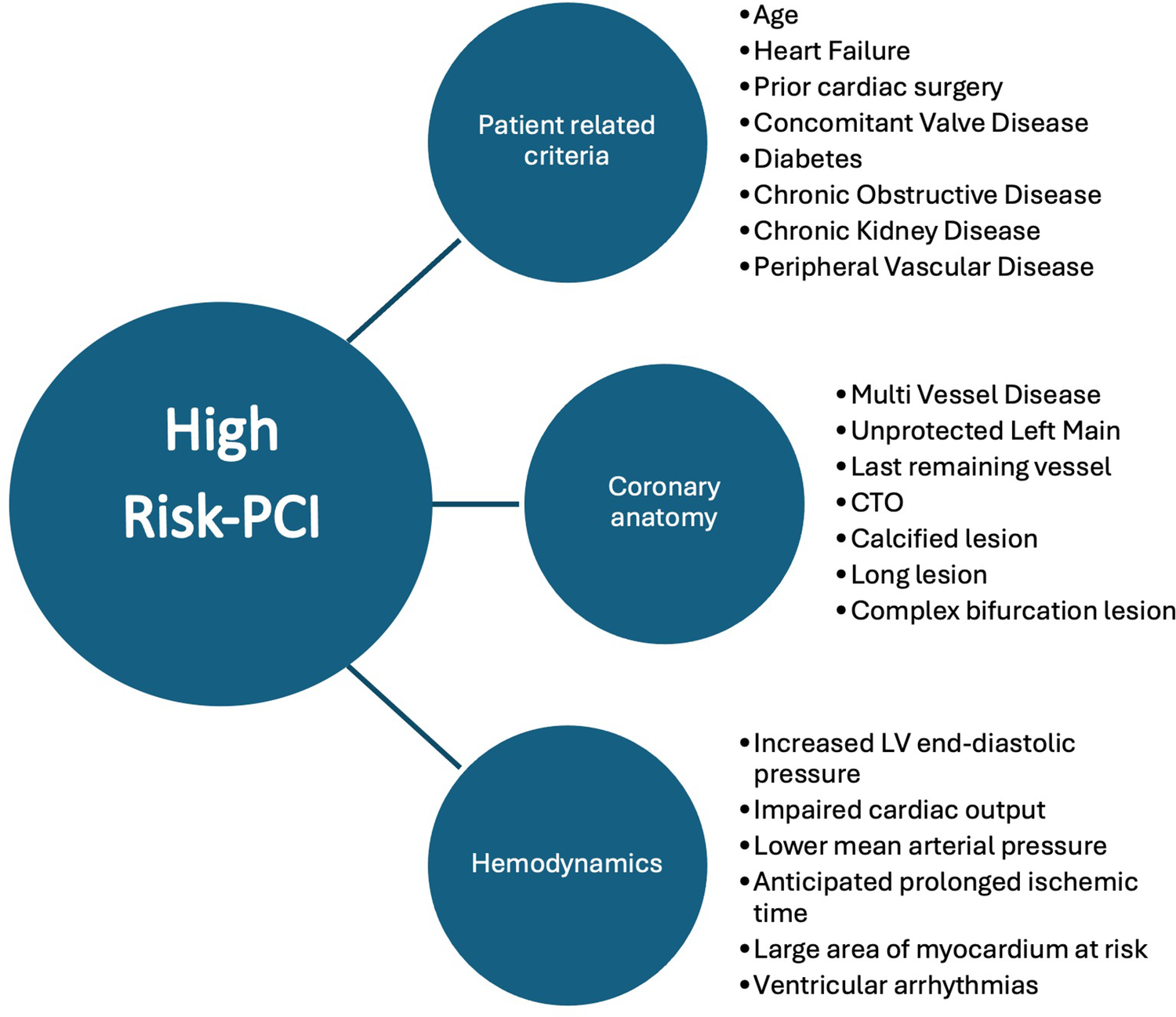
Suggested criteria for defining HRPCI (10).
2.1 Statistical analysis
Statistical analysis was performed after collecting all data. Categorical variables are presented as n (%). Continuous variables are summarized as mean ± SD or as the median with interquartile range (IQR). For analyzing prevalence χ2 test and Fisher's test was calculated. The observations on the achievement of the secondary endpoints were evaluated by survival analyses with determination of univariate and multivariate Cox proportional hazard ratio (HR) and Kaplan–Meier curve. The following variables were included: group, slow reflow, cause of death (cardiac death vs. non-cardiac death) and death overall. For further clarification of predictors of mortality, we investigated the statistical relationship between using pMCS as a bailout procedure and the occurrence of death. Further, we performed multivariate testing for the event of slow reflow in both groups. Lastly, multivariate testing was done for the variable death in combination with the overall event of slow reflow. The analysis was performed with R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline and lesion characteristics
In total 25 patients have been enrolled during the screening episode of which 11 patients received IVL and pMCS and 14 patients received RA and pMCS. Use of either calcium modification technique (IVL or RA) for lesion preparation was at the discretion of the interventional cardiologist. Since this was an observational all-comer registry, we especially did not see any difference in patients presenting with acute coronary syndrome and in patients treated in an elective setting in both groups (p = 0.999). Further, we observed no differences in baseline characteristics (Table 1), and notably the Syntax Score did not differ between the groups [28.8 (±5.5) vs. 31.9 (±7.7), p = 0.255] (Table 2). Lesion characteristics are shown in Table 3. Regarding the group of RA + pMCS, the LAD was the predominantly targeted vessel (n = 11, p = 0.049). A significantly higher portion of calcification could be found in the RA + pMCS group [13.8 mm (±17.2) vs. 57.1 mm (±18.3), p < 0.0001]. This group also had a significantly higher portion of eccentric calcification (n = 14 vs. n = 7, p = 0.026).
Table 1
| Baseline characteristics | IVL + pMCS (n = 11) | RA + pMCS (n = 14) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 8 (32%) | 11 (44%) | 0.999 |
| Female | 3 (12%) | 3 (12%) | 0.999 |
| Age | |||
| Mean | 74.4 (7.9) | 76.6 (8.4) | 0.493 |
| BMI (kg/m2) | |||
| Mean | 27.7 (4.8) | 27.8 (3.7) | 0.94 |
| LVEF (%) | |||
| Mean | 42.7 (15.9) | 39.8 (16.1) | 0.652 |
| Euro score | 6.3 (4) | 9 (9.5) | 0.893 |
| CAD | |||
| CAD 1 | 0 | 0 | |
| CAD 2 | 3 (12%) | 2 (8%) | 0.623 |
| CAD 3 | 8 (32%) | 12 (48%) | 0.623 |
| Cardiovascular risk factors | |||
| Hypertension | 10 (40%) | 14 (56%) | 0.44 |
| Hypercholesterolemia | 9 (36%) | 10 (40%) | 0.661 |
| Diabetes mellitus | 5 (20%) | 6 (24%) | 0.999 |
| Smoking | 2 (8%) | 1 (4%) | 0.565 |
| Positive family history for CAD | 2 (8%) | 2 (8%) | 0.999 |
| NYHA class | |||
| NYHA I | 1 (4%) | 0 | 0.44 |
| NYHA II | 3 (12%) | 3 (12%) | 0.999 |
| NYHA III | 5 (20%) | 7 (28%) | 0.999 |
| NYHA IV | 1 (4%) | 5 (20%) | 0.18 |
| CCS class | |||
| CCS I | 0 | 1 (4%) | 0.999 |
| CCS II | 1 (4%) | 2 (8%) | 0.999 |
| CCS III | 0 | 4 (16%) | 0.105 |
| CCS IV | 7 (28%) | 6 (24%) | 0.428 |
| Acute coronary syndrome | 6 (24%) | 8 (32%) | 0.999 |
| Chronic kidney disease | 4 (16%) | 5 (20%) | 0.999 |
| Impaired renal function | 5 (20%) | 6 (24%) | 0.999 |
| Peripheral artery disease > Fontaine IIb | 1 (4%) | 3 (12%) | 0.604 |
Patient baseline characteristics.
Absolute prevalence (relative prevalence) for categorical variables. Mean (standard deviation) for metric variables.
Table 2
| Hemodynamics and procedural data |
IVL + pMCS (n = 11) | RA + pMCS (n = 14) | p-value |
|---|---|---|---|
| Mean arterial blood pressure (mmHg) | |||
| Pre PCI | 99.5 (18) | 86.8 (17.4) | 0.104 |
| Post PCI | 86 (10.5) | 73.8 (12.1) | 0 .04 |
| Δ Differences of arterial pressure | |||
| Systolic | −19.2 (36.3) | −21.9 (39.1) | 0.795 |
| Diastolic | −6.1 (17.1) | −9.4 (15.7) | 0.759 |
| MAP | −12 (20.3) | −16.1 (23.9) | 0.709 |
| Heart rate (bpm) | |||
| Pre PCI | 80.3 (13.8) | 72.2 (15.4) | 0.207 |
| During PCI | 79.9 (17.7) | 80.8 (16.5) | 0.935 |
| Post PCI | 75.2 (11) | 82.9 (12.6) | 0.218 |
| pMCS as bailout | 1 (4%) | 5 (20%) | 0.18 |
| Catecholamines post PCI | 0 | 6 (24%) | 0 .02 |
| Syntax Score | |||
| Syntax Score I pre PCI | 28.8 (5.5) | 31.9 (7.7) | 0.255 |
| Syntax Score I post PCI | 6.8 (3.4) | 10.6 (5.5) | 0.043 |
| Δ Syntax Score | |||
| Syntax Score I | −22 (5.8) | −21.2 (7.6) | 0.783 |
| Drug eluting stent length (mm) | |||
| Culprit | 31.8 (15.8) | 69.6 (22.2) | <0 .001 |
| Total | 103.8 (36.6) | 108.9 (34.5) | 0.73 |
| Drug eluting stent diameter (mm) | |||
| Culprit | 3.5 (0.6) | 3.6 (0.4) | 0.436 |
| Minimum | 2.7 (0.4) | 2.6 (0.2) | 0.379 |
| Maximum | 3.7 (0.5) | 3.8 (0.3) | 0.524 |
| Number of stents implanted | 4.3 (1.6) | 4.4 (1.7) | 0.901 |
| Slow reflow | 2 (8%) | 7 (28%) | 0.208 |
| Contrast agent used (ml) | 307.9 (102.9) | 283.8 (129.2) | 0.615 |
| Radiation time (min) | 41.7 (10.8) | 56.5 (19.4) | 0 .032 |
| Procedure time (min) | 136.1 (26.6) | 182.3 (46.8) | 0 .001 |
Hemodynamics and procedural data.
Absolute prevalence (relative prevalence) for categorical variables. Mean (standard deviation) for metric variables.
Bold italic values indicate statistical significance with a p-value <0.05.
Table 3
| Lesion characteristics |
IVL + pMCS (n = 11) | RA + pMCS (n = 14) | p-value |
|---|---|---|---|
| Target vessel | |||
| LM | 3 (12%) | 9 (36%) | 0.111 |
| LAD | 4 (16%) | 11 (44%) | 0 .049 |
| LCX | 3 (12%) | 1 (4%) | 0.288 |
| RCA | 2 (8%) | 1 (4%) | 0.565 |
| Lesion length (mm) | |||
| <10 | 0 | 0 | |
| 10–20 | 3 (12%) | 1 (4%) | 0.288 |
| >20 | 8 (32%) | 13 (52%) | 0.288 |
| Lesion morphology in treated vessels | |||
| Eccentric | 7 (28%) | 14 (56%) | 0.026 |
| Concentric | 5 (20%) | 6 (24%) | 0.999 |
| Ostial lesion | 6 (24%) | 9 (36%) | 0.934 |
| De novo lesion | 10 (40%) | 14 (56%) | 0.44 |
| Calcified portion (mm) | |||
| Mean | 13.8 (17.2) | 57.1 (18.3) | <0.001 |
| Bifurcation | 6 (24%) | 7 (28%) | 0.999 |
Lesion characteristics.
LM, left main coronary artery; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery.
Absolute prevalence (relative prevalence) for categorical variables. Mean (standard deviation) for metric variables.
Bold italic values indicate statistical significance with a p-value <0.05.
3.2 Procedural data and primary endpoint analysis
The baseline MAP was slightly higher in the IVL + pMCS group, but without statistical significance (p = 0.104). Primary endpoint analysis showed significant higher post-PCI MAP values in the IVL + pMCS group [99.5 mmHg (±18) vs. 86.8 mmHg (±17.4), p = 0.04]. However, the ΔMAP change was without statistical significance between the groups [−12 mmHg (±20.3) vs. −16.1 mmHg (±23.9), p = 0.709]. The need of catecholamines after PCI was significantly lower in the IVL + pMCS group [n = 0 (0%) vs. n = 6 (24%), p = 0.02].
The calculated baseline Syntax Score and the Δ-difference at the end of procedure did not differ between the groups [−22 (±5.8) vs. −21.2 (±7.6), p = 0.783] (Table 2). The length of the drug eluting stent used in the treated culprit lesion was significantly shorter in the group of IVL + pMCS (p < 0.001). Procedure and radiation time was significantly lower in the IVL + pMCS group (p < 0.001 and p < 0.032, respectively).
Detailed information about hemodynamics and procedural data can be found in Table 2.
3.3 Secondary endpoint analysis
We did not find any statistically significant difference for the occurrence of in-hospital death (all-cause mortality) between the groups [n = 1 (4%) vs. n = 6 (24%), p = 0.09] in general. We did find a statistically significant difference for the occurrence of cardiac death (overall) between the groups (n = 0 (0%) vs. n = 5 (20%), p = 0.046). Multivariate regression analysis could exclude an effect of the intervention group on the variable in-hospital death (HR 6.179, 95% CI: 0.741–51.561, p = 0.092). However, patients with pMCS insertion as a bailout strategy had a higher probability for in-hospital death in the univariate regression analysis (HR: 51.807, 95% CI: 5.874–456.906, p < 0.001). MACE did occur significantly less frequently in the group of IVL + pMCS (0% vs. 20%, p = 0.046) and in both group there were no access site bleedings or peripheral leg ischemia reported. Notably, the LVEF at hospital discharge was significantly higher in the group of IVL + pMCS [41.5% (±16.6) vs. 21.7% (±22.4), p = 0.035]. During the long-term follow-up [time to survival IVL + pMCS 504.6 days (±377.5) vs. RA + pMCS 278.9 days (±418); p = 0.028], we did find a statistically significant lower rate of cardiac death (0% vs. 20%, p = 0.046) in the group of IVL + pMCS. Further, all-cause mortality was lower in the IVL + pMCS group (12% vs. 40%, p = 0.047). Patients with the slow reflow phenomenon had a higher probability of long-term mortality (HR 3.989, 95% CI: 1.234–12.889, p = 0.021). The data on the individual causes of death are shown in Supplementary Table S1. In-hospital and long-term follow-up data are reported in detail in Table 4. The Kaplan–Meier plots for in-hospital death, slow reflow and all-cause mortality can be found in Figure 2.
Table 4
| Post-procedural data | IVL + pMCS (n = 11) |
RA + pMCS (n = 14) |
p-value |
|---|---|---|---|
| MACE | 0 | 5 (20%) | 0 .046 |
| In-hospital death | 1 (4%) | 6 (24%) | 0.09 |
| All-cause mortality overall | 3 (12%) | 10 (40%) | 0 .047 |
| Cardiac death overall | 0 (0%) | 5 (20%) | 0 .046 |
| Time of survival (days) | 504.6 (377.5) | 278.9 (418) | 0 .028 |
| Stroke | 0 | 0 | NA |
| Acute myocardial infarction | 0 | 1 (4%) | 0.999 |
| Access site complications | |||
| Access site bleeding | 0 | 0 | NA |
| Peripheral leg ischemia | 0 | 0 | NA |
| Other access site complications | 1 (4%) | 4 (16%) | 0.105 |
| Packed red blood cells | 0 | 4 (16%) | 0.105 |
| LVEF at discharge | 41.5 (16.6) | 21.7 (22.4) | 0 .035 |
| Days to hospital discharge | 11.7 (7) | 9.6 (2.9) | 0.641 |
In-hospital and long-term follow-up.
Absolute prevalence (relative prevalence) for categorical variables. Mean (standard deviation) for metric variables.
Bold italic values indicate statistical significance with a p-value <0.05.
Figure 2
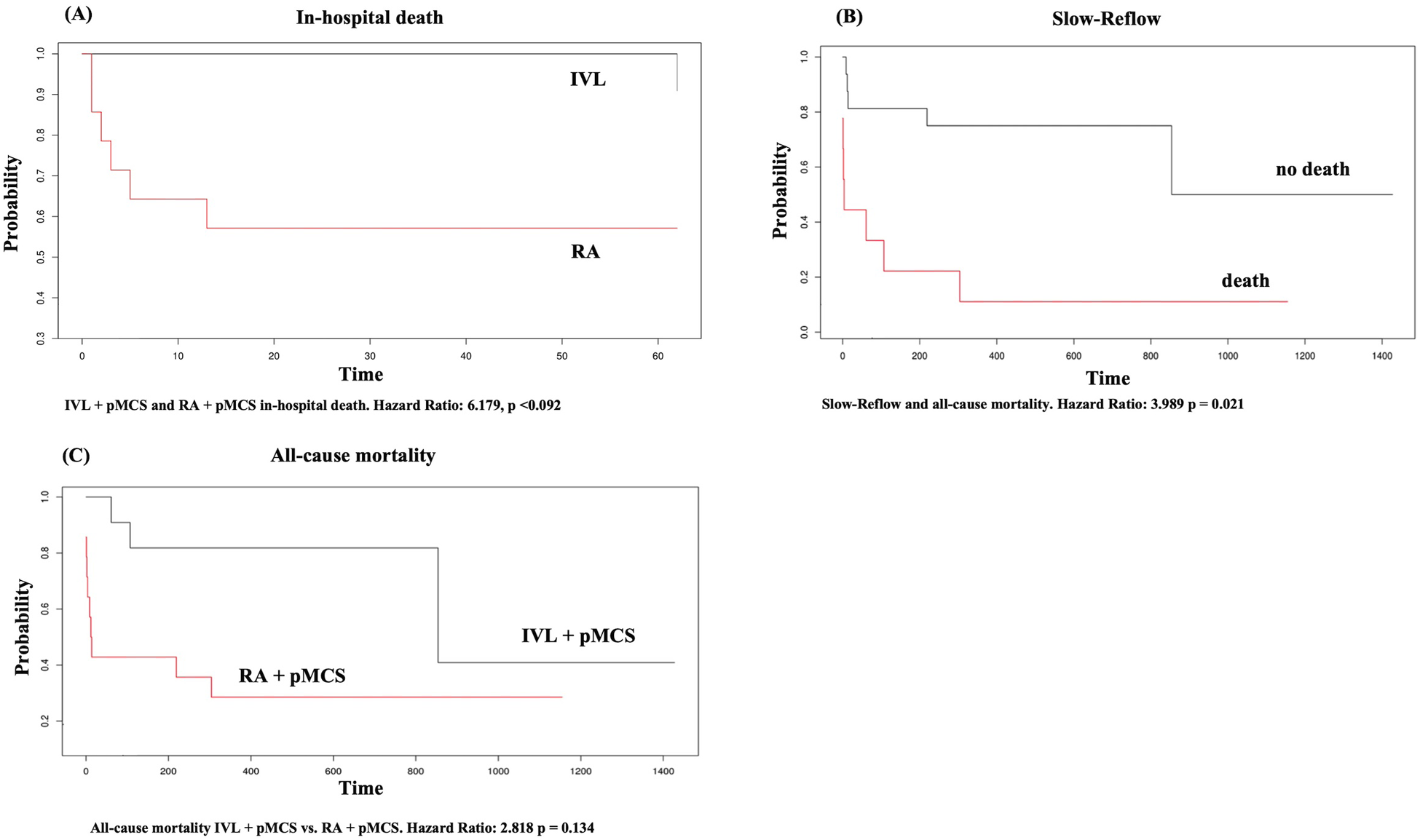
Kaplan Meier plot for (A) in-hospital death, (B) slow-reflow, (C) all-cause mortality. X-axis in (A–C) represents time given in days.
4 Discussion
HRPCI procedures are still a challenge, especially in patients with severe calcified coronary arteries. To our best knowledge there are no studies focusing on the comparison of patients receiving protected HRPCI with either IVL or RA so far. Our results can be summarized as follows: first we observed a significantly higher post-PCI MAP in the IVL + pMCS group. However, the ΔMAP change between the groups was not significant. There was no need for catecholamine therapy in the IVL + pMCS group, whereas patients in the RA + pMCS group required catecholamines significantly more often. Second, we observed a significantly lower rate of MACE in the IVL + pMCS group. Third, pMCS as a bailout strategy was associated with in-hospital death and the occurrence of the slow reflow phenomenon was associated with all-cause mortality during the follow-up in the univariate regression analysis.
Regarding the primary endpoint, different factors must be considered. As far as we know there are no randomized studies addressing specifically hemodynamics during IVL in combination with pMCS. Rather, the literature predominantly contains case reports that focus primarily on RA in combination with extracorporeal membrane oxygenation (ECMO) (22, 23). Marchese et al. describe two cases of patients with severe aortic valve stenosis and accompanying severely calcified coronary stenoses that were successfully treated with ECMO and IVL in the left main (24). Dallan et al. were able to describe a successful intervention of RA in the left main using Impella (25). Buono et al. published a case report of a heavily calcified left circumflex in a patient with an impaired LVEF of 25% treated by IVL and Impella (26). A larger group of patients receiving RA + pMCS can be found in the PROTECT III trial (8). It is of note that these were only elective patients and a carefully selected study collective. Furthermore, in the PROTECT III trial there was no subgroup analysis investigating RA with other plaque-modifying procedures. In none of the PROTECT III cases a hemodynamic instability was reported. In our study we observed a significantly higher rate of post-PCI usage of vasopressor in the RA + pMCS group. Although a significantly higher MAP was seen in the IVL + pMCS group during the procedure, the Δ-difference of the MAP in the two groups did not differ in statistically significant terms. We therefore conclude that patients in the RA + pMCS group in our cohort were in a significantly higher need of vasopressors to maintain stable hemodynamics for the same degree of revascularization reflected by the statistically non-significant post-procedural Syntax Score delta change. Notably, although the Syntax Score did not differ before the intervention the above-mentioned findings might indicate that the patient group of RA + pMCS is more complex than the group of IVL + pMCS patients. In addition, IVL has been reported in the ROTA.shock trial as non-inferior to RA regarding minimal stent area (27). However, this complexity seems not to be reflected by the Syntax Score appropriately. One must keep in mind that although not statistically significant the higher amount of slow reflow in the RA + pMCS group and the higher rate of pMCS as a bailout strategy might contribute to the higher rate of catecholamine usage and the higher rate of MACE. Furthermore, for pMCS as a bailout strategy we did find a statistical significance in the probability of in-hospital death in the univariate testing. Although there is no difference in the event of in-hospital death between the groups there is a higher risk of fatal outcome if pMCS is used as a bailout strategy during PCI in cases of hemodynamic instability. This is in line with the findings of Basir et al. who demonstrated an early use of pMCS improves hemodynamics and survival rates in patients with acute myocardial infarction (28). The same was demonstrated in the DanGer Shock study (29). However, these studies treat patients in cardiogenic shock and the data are therefore not comparable with our data. We therefore emphasize the necessity of a standardized patient selection, strategy planning beforehand as well as an early usage of pMCS in the context of HRPCI, also in elective cases. A combination of different characteristics consisting of patient-related criteria (e.g., age, heart failure), the coronary anatomy (e.g., multi-vessel disease, last remaining vessel) and hemodynamics (e.g., increased left ventricular end-diastolic pressure, impaired cardiac output) have been published recently to identify patients at risk (10) (Figure 1).
Addressing the secondary endpoint, a significantly higher MACE rate in the RA + pMCS group was observed. Patients in the RA + pMCS group had longer calcified lesions treated which led to a more intensive lesion preparation, as well as longer procedural duration which might resulted in a numerical but statistical not significant higher rate of slow reflow phenomenon in this group. In addition, during and after the usage of RA thermal injury and platelet activation are reported (30), which might be associated with the increased MACE rate in the RA + pMCS group. However, multivariate testing did not show any difference in the probability of slow reflow between the groups, but when slow reflow occurred, a statistically significant increase in the probability of all-cause mortality was observed. Further, a recent study reports a significant higher 2-year mortality rate in patients with an impaired LVEF (<35%) receiving RA (31). In our cohort, the LVEF change in the RA + pMCS group must be considered as multifactorial and a combination of slow reflow, longer length of calcification and a non-statistically significant higher percentage of pMCS as a bailout strategy in the RA group. The significantly lower LVEF at discharge in the RA + pMCS group might also contribute to the fact that a higher rate of cardiac death (0% vs. 20%, p = 0.046) and all-cause mortality (12% vs. 40%, p = 0.047) in the long-term follow-up could be seen. Observational studies have demonstrated a nearly two times higher rate of death and electric instability in patients with coronary artery disease and reduced LVEF receiving incomplete revascularization (32, 33). In this context the combined data of the Protect II study and the cVAD registry shows that a more complete revascularization is independently associated with a better LVEF (34).
5 Limitations
Several limitations of our study have to be considered. This single center experience study with a retrospective approach and a small number of patients limits the generalizability of the statistical findings. However, the operator driven choice of either using IVL or RA reflects a real-world population but inherits a potential selection bias. Excluding patients who received a combination of RA and IVL further limits the generalizability in especially difficult lesions. Also, only RA and IVL were used as calcium modification methods but other devices like orbital atherectomy were not included due to a lack of routine use in this single center study. Further, some patients were treated in an elective setting, others were admitted during acute coronary syndrome which might influence the overall outcome of the patients, although there was no significant difference in the number of acute coronary syndrome patients in both groups. Intravascular imaging was not standardly used in all cases (16%). Although this rate was even higher than the average rate of intravascular imaging of 6.6% as reported by Lemor et al. in 2020, it was not valid to be used for statistical analysis (35). The choice of calcium modification tool was mostly done angiographically. Use of either calcium modification technique (IVL or RA) for lesion preparation was at the discretion of the interventional cardiologist. As the selection of the calcium modification method was subject to the operator, a possible bias cannot be ruled out. Nevertheless, in future studies systematic intravascular imaging will give a better understanding of plaque morphology and will help identify the best plaque modification device to choose. Despite multivariate testing confounders like degree of calcification and procedural complexity were more prevalent in the RA group and this study was not adequately powered for its secondary endpoint. There was no routine follow-up angiography to identify long term procedural failure (e.g., in stent restenosis).
6 Conclusion
In our cohort patients in the IVL + pMCS group were hemodynamical more stable, which led to a lower rate of catecholamine usage. This might be related to our findings of a higher LVEF, a lower MACE rate, a lower overall cardiac death rate and a lower all-cause mortality in patients receiving IVL + pMCS. However, in the multivariate analysis, a correlation between the intervention group and the occurrence of in-hospital death could be ruled out. We did observe a statistical significance between the occurrence of slow reflow and all-cause mortality, whereas the slow reflow phenomenon did not differ statistically between the two groups. In addition, we did observe a significant correlation between the event of in-hospital death if pMCS is used as a bailout strategy in patients qualifying for HRPCI. This emphasizes the need of a common definition for HRPCI to identify patients at risk to be able to perform proper procedure planning and patient preparation. Even more, it substantially underlines the necessity of early pMCS usage if these patients are identified, as already reported by Basir et al. (28). When screening for HRPCI patients, the LVEF is one of the key factors that determines whether to use or not to use pMCS. This is in line with recent studies which included patients with a LVEF <45% and a planned HRPCI with or without pMCS (36, 37). Furthermore, the occurrence of slow reflow during HRPCI should indicate a closer observation of the treated patient as it may trigger a higher all-cause mortality and can be helpful in categorizing patients into HRPCI groups. In light of the limitations of this study, the findings can only be used for hypothesis generation. Larger patient cohorts, standardized patient selection and a standardized procedure protocol with routine follow-up investigations are needed.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Rhineland-Palatinate chamber of physicians. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
TK: Investigation, Writing – original draft, Writing – review & editing. SA: Writing – review & editing. AG: Writing – review & editing. ML: Writing – review & editing. LS: Writing – review & editing. MS: Writing – review & editing. RZ: Writing – review & editing. AH: Writing – review & editing. NW: Writing – review & editing. JL: Writing – original draft, Writing – review & editing, Conceptualization, Supervision, Data curation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge the contribution of Runge Statistik GmbH in consulting and performing statistical analysis.
Conflict of interest
JL and NW received speaker honorarium from Boston Scientific, Shockwave Medical, and Abiomed (Johnson & Johnson Med Tech).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1451229/full#supplementary-material
References
1.
Neumann FJ Sousa-Uva M Ahlsson A Alfonso F Banning AP Benedetto U et al 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165. 10.1093/eurheartj/ehy394
2.
Kirtane AJ Doshi D Leon MB Lasala JM Ohman EM O'Neill WW et al Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. (2016) 134(5):422–31. 10.1161/CIRCULATIONAHA.116.022061
3.
Brener SJ Cunn GJ Desai PH Faroqui M Ha LD Handa G et al A novel risk score to predict one-year mortality in patients undergoing complex high-risk indicated percutaneous coronary intervention (CHIP-PCI). J Invasive Cardiol. (2021) 33(4):E253–8. 10.25270/jic/20.00515
4.
Abdallah MS Wang K Magnuson EA Spertus JA Farkouh ME Fuster V et al Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. (2013) 310(15):1581–90. 10.1001/jama.2013.279208
5.
Cohen DJ Van Hout B Serruys PW Mohr FW Macaya C den Heijer P et al Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med. (2011) 364(11):1016–26. 10.1056/NEJMoa1001508
6.
Norris CM Ghali WA Galbraith PD Graham MM Jensen LA Knudtson ML et al Women with coronary artery disease report worse health-related quality of life outcomes compared to men. Health Qual Life Outcomes. (2004) 2:21. 10.1186/1477-7525-2-21
7.
Velazquez EJ Lee KL Jones RH Al-Khalidi HR Hill JA Panza JA et al Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. (2016) 374(16):1511–20. 10.1056/NEJMoa1602001
8.
O'Neill WW Anderson M Burkhoff D Grines CL Kapur NK Lansky AJ et al Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am Heart J. (2022) 248:139–49. 10.1016/j.ahj.2022.02.006
9.
Werner N Burzotta F Sinning JM . European Best practice: a step forward to optimize Impella-protected percutaneous coronary intervention to improve outcome after high-risk coronary interventions. Eur Heart J Suppl. (2022) 24(Suppl J):J1–3. 10.1093/eurheartjsupp/suac065
10.
Leick J Werner N Mangner N Panoulas V Aurigemma C . Optimized patient selection in high-risk protected percutaneous coronary intervention. Eur Heart J Suppl. (2022) 24(Suppl J):J4–10. 10.1093/eurheartjsupp/suac060
11.
Kini AS Vengrenyuk Y Pena J Motoyama S Feig JE Meelu OA et al Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. (2015) 86(6):1024–32. 10.1002/ccd.26000
12.
Tzafriri AR Garcia-Polite F Zani B Stanley J Muraj B Knutson J et al Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. (2017) 264:203–10. 10.1016/j.jconrel.2017.08.037
13.
Wiemer M Butz T Schmidt W Schmitz KP Horstkotte D Langer C . Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. (2010) 75(6):905–11. 10.1002/ccd.22347
14.
Aksoy A Salazar C Becher MU Tiyerili V Weber M Jansen F et al Intravascular lithotripsy in calcified coronary lesions: a prospective, observational, multicenter registry. Circ Cardiovasc Interv. (2019) 12(11):e008154. 10.1161/CIRCINTERVENTIONS.119.008154
15.
Leick J Rheude T Denne M Cassese S Kastrati A Hauptmann F et al Comparison of long-term outcome in patients with calcified stenosis treated with intravascular lithotripsy or with modified balloon angioplasty: a propensity score-adjusted study. Front Cardiovasc Med. (2023) 10:1185422. 10.3389/fcvm.2023.1185422
16.
Leick J Rheude T Denne M Tobias K Cassese S Kastrati A et al Comparison of long-term outcome in patients with in-stent restenosis treated with intravascular lithotripsy or with modified balloon angioplasty. Clin Res Cardiol. (2023) 113(7):1030–40. 10.1007/s00392-023-02357-3
17.
Kereiakes DJ Di Mario C Riley RF Fajadet J Shlofmitz RA Saito S et al Intravascular lithotripsy for treatment of calcified coronary lesions: patient-level pooled analysis of the disrupt CAD studies. JACC Cardiovasc Interv. (2021) 14(12):1337–48. 10.1016/j.jcin.2021.04.015
18.
Wong B El-Jack S Newcombe R Glenie T Armstrong G Khan A . Shockwave intravascular lithotripsy for calcified coronary lesions: first real-world experience. J Invasive Cardiol. (2019) 31(3):46–8. 10.1016/j.hlc.2019.05.022
19.
Ali ZA Brinton TJ Hill JM Maehara A Matsumura M Karimi Galougahi K et al Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovascular Imaging. (2017) 10(8):897–906. 10.1016/j.jcmg.2017.05.012
20.
Abdel-Wahab M Richardt G Joachim Buttner H Toelg R Geist V Meinertz T et al High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (rotational atherectomy prior to Taxus stent treatment for Complex native coronary artery disease) trial. JACC Cardiovasc Interv. (2013) 6(1):10–9. 10.1016/j.jcin.2012.07.017
21.
Domienik-Karlowicz J Kupczynska K Michalski B Kaplon-Cieslicka A Darocha S Dobrowolski P et al Fourth universal definition of myocardial infarction. Selected messages from the European Society of Cardiology document and lessons learned from the new guidelines on ST-segment elevation myocardial infarction and non-ST-segment elevation-acute coronary syndrome. Cardiol J. (2021) 28(2):195–201. 10.5603/CJ.a2021.0036
22.
Ajmi I Mahnkopf C Brachmann J Sinani M Oudeh M Schnupp S . Rotablation of heavily calcified left main stenosis and aortic valve valvuloplasty under ECMO cardiopulmonary support. JACC Case Rep. (2020) 2(15):2448–54. 10.1016/j.jaccas.2020.10.015
23.
Dardas P Mezilis N Ninios V Theofilogiannakos EK Tsikaderis D Tsotsolis N et al ECMO as a bridge to high-risk rotablation of heavily calcified coronary arteries. Herz. (2012) 37(2):225–30. 10.1007/s00059-011-3489-5
24.
Marchese A Tarantini G Tito A Margari V Resta F Dhojniku I et al Mechanical circulatory support and intravascular lithotripsy in high-risk patients undergoing percutaneous coronary intervention and transcatheter aortic valve replacement: a case series. Eur Heart J Case Rep. (2021) 5(12):ytab498. 10.1093/ehjcr/ytab498
25.
Dallan LAP Ribeiro MH Igwe C Tensol Pereira Rodrigues G Zago EI Zimin V et al Rotational atherectomy and mechanical support to treat left main. JACC Case Rep. (2019) 1(5):811–4. 10.1016/j.jaccas.2019.11.012
26.
Buono A Ielasi A De Blasio G Tespili M . “Shock-Pella”: combined management of an undilatable ostial left circumflex stenosis in a complex high-risk interventional procedure patient. Cardiol J. (2020) 27(4):427–8. 10.5603/CJ.2020.0112
27.
Blachutzik F Meier S Weissner M Schlattner S Gori T Ullrich H et al Coronary intravascular lithotripsy and rotational atherectomy for severely calcified stenosis: results from the ROTA.shock trial. Catheter Cardiovasc Interv. (2023) 102(5):823–33. 10.1002/ccd.30815
28.
Basir MB Lemor A Gorgis S Patel KC Kolski BC Bharadwaj AS et al Early utilization of mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the national cardiogenic shock initiative. J Am Heart Assoc. (2023) 12(23):e031401. 10.1161/JAHA.123.031401
29.
Moller JE Gerke O DanGer Shock I . Danish-German cardiogenic shock trial-DanGer shock: trial design update. Am Heart J. (2023) 255:90–3. 10.1016/j.ahj.2022.10.078
30.
MacIsaac AI Bass TA Buchbinder M Cowley MJ Leon MB Warth DC et al High speed rotational atherectomy: outcome in calcified and noncalcified coronary artery lesions. J Am Coll Cardiol. (1995) 26(3):731–6. 10.1016/0735-1097(95)00206-J
31.
Zhang HP Zhao Y Ai H Li H Tang GD Zheng NX et al Outcomes of coronary rotational atherectomy in patients with reduced left ventricular ejection fraction. J Int Med Res. (2020) 48(4):300060519895144. 10.1177/0300060519895144
32.
Sohn GH Yang JH Choi SH Song YB Hahn JY Choi JH et al Long-term outcomes of complete versus incomplete revascularization for patients with multivessel coronary artery disease and left ventricular systolic dysfunction in drug-eluting stent era. J Korean Med Sci. (2014) 29(11):1501–6. 10.3346/jkms.2014.29.11.1501
33.
Takano T Tanaka K Ozaki K Sato A Iijima K Yanagawa T et al Clinical predictors of recurrent ventricular arrhythmias in secondary prevention implantable cardioverter defibrillator recipients with coronary artery disease—lower left ventricular ejection fraction and incomplete revascularization. Circ J. (2018) 82(12):3037–43. 10.1253/circj.CJ-18-0646
34.
Russo JJ Prasad M Doshi D Karmpaliotis D Parikh MA Ali ZA et al Improvement in left ventricular function following higher-risk percutaneous coronary intervention in patients with ischemic cardiomyopathy. Catheter Cardiovasc Interv. (2020) 96(4):764–70. 10.1002/ccd.28557
35.
Lemor A Patel N Jain T Baber U Hernandez G Villablanca P et al Trends and outcomes of intravascular imaging-guided percutaneous coronary intervention in the United States. Crit Pathw Cardiol. (2020) 19(2):69–74. 10.1097/HPC.0000000000000194
36.
Baumann S Werner N Ibrahim K Westenfeld R Al-Rashid F Sinning JM et al Indication and short-term clinical outcomes of high-risk percutaneous coronary intervention with microaxial Impella(R) pump: results from the German Impella(R) registry. Clin Res Cardiol. (2018) 107(8):653–7. 10.1007/s00392-018-1230-6
37.
Ameloot K Bastos MB Daemen J Schreuder J Boersma E Zijlstra F et al New-generation mechanical circulatory support during high-risk PCI: a cross-sectional analysis. EuroIntervention. (2019) 15(5):427–33. 10.4244/EIJ-D-18-01126
Summary
Keywords
high-risk PCI, percutaneous mechanical circulatory support, rotational atherectomy, intravascular lithotripsy, slow reflow
Citation
Krause TT, Afzal SS, Gjata A, Lindner M, Saad L, Steinbach M, Zayat R, Haneya A, Werner N and Leick J (2024) Comparison of patients undergoing protected high risk percutaneous coronary intervention using either intravascular lithotripsy or rotational atherectomy. Front. Cardiovasc. Med. 11:1451229. doi: 10.3389/fcvm.2024.1451229
Received
18 June 2024
Accepted
24 October 2024
Published
29 November 2024
Volume
11 - 2024
Edited by
Istvan Szokodi, University of Pécs, Hungary
Reviewed by
Mehmet Cilingiroglu, University of California, Irvine, United States
Adem Aksoy, University Hospital Bonn, Germany
Attila Kónyi, University of Pécs, Hungary
Mariuca Nicotera, Klinikverbund Südwest, Germany
Updates
Copyright
© 2024 Krause, Afzal, Gjata, Lindner, Saad, Steinbach, Zayat, Haneya, Werner and Leick.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Juergen Leick j.leick@bbtgruppe.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.