Abstract
Background:
Several studies have demonstrated an association between tricuspid regurgitation (TR) and organ dysfunction including hepatic and renal insufficiency. Improvement of liver function following transcatheter edge-to-edge repair (T-TEER) has already been linked to reduction of venous congestion due to TR reduction. This study analyzes whether TR-reduction using T-TEER is also associated with improved renal function.
Methods and results:
The TRIC-ULM registry includes 92 selected patients undergoing T-TEER between March 2017 and May 2023. Estimated glomerular filtration rate (eGFR) improvement was evident in 53 patients (57%) at 3-months follow-up (FU) and defined by FU eGFR > baseline eGFR. Median age was 80 [interquartile range 75–83] years, pre- and postinterventional TR grades were 4 [3–5] and 1 [1–2], baseline eGFR was 36 [30–53] ml/min and New Yeark Heart Association (NYHA) IV was evident in 15% of patients. Multiple logistic regression analysis revealed TR vena contracta reduction (Odds ratio (OR) 1.35 [95% CI: 1.12–1.64] per mm, p = 0.002) and reduced preinterventional tricuspid annular plane systolic excursion (TAPSE) [OR 0.89 (95% CI: 0.79–0.99) per mm, p = 0.033] to independently predict renal improvement at FU. An eGFR improvement threshold of >9 ml/min was associated with reduced 1-year heart failure hospitalization rates [adjusted hazard ratio 0.22 (95% CI: 0.07–0.62) p = 0.005].
Conclusion:
Effective tricuspid edge-to-edge repair is associated with improved renal function and reduced heart failure hospitalization. In patients without renal improvement at 3-months follow-up, residual tricuspid regurgitation should be reevaluated for reintervention.
Introduction
The TRILUMINATE trial demonstrated that symptomatic tricuspid regurgitation (TR) can successfully be treated using transcatheter edge-to-edge repair (T-TEER) (1). An association between atrioventricular valve regurgitation and hepatorenal function has already been reported and is assumed to be mediated by elevated venous congestion and reduced cardiac output (2–5).
The combination of reduced renal perfusion and elevated congestion is described as “renal tamponade” by Boorsma et al. in patients with heart failure and chronic kidney disease (6). Regarding mitral valve regurgitation (MR), large studies observed renal improvement in patients undergoing mitral valve edge-to-edge repair (M-TEER) (7). Regarding tricuspid valve regurgitation, Tanaka et al. found that an elevated model for end-stage liver disease excluding international normalized ratio (MELD-XI) score—reflecting hepatorenal function—predicts 1-year all-cause mortality and heart failure hospitalization (HFH) in patients who underwent T-TEER (8). Simultaneously, reducing TR to less than grade 3+ was independently associated with a reduction in MELD-XI score (8). Furthermore, postprocedural acute kidney injury has been linked to worse clinical outcomes following T-TEER (9). To further investigate the specific impact of T-TEER on renal function, our study aimed to investigate whether the effective reduction of tricuspid regurgitation is associated with improvement of renal function.
Methods
Study design and cohort
This retrospective single-center study included 92 consecutive patients undergoing T-TEER for symptomatic tricuspid regurgitation with 3 months available estimated glomerular filtration rate (eGFR) FU at our Ulm University Heart Center between March 2017 and May 2023. Patients undergoing reinterventions or patients on dialysis were excluded. Patients suffered from symptomatic heart failure equivalent to New York Heart Association (NYHA) ≥2 despite guideline-directed medical therapy. Baseline and postinterventional TR were defined according to the 2017 European Society of Cardiology (ESC) guidelines by intraprocedural transesophageal echocardiography (TEE) (10). eGFR was estimated using the Cockcroft-Gault-Formula. Each patient was evaluated by the interdisciplinary heart team according to current guidelines at the timepoint of procedure. All patients underwent T-TEER using the MitraClip (generation G1 to G3,) or TriClip (both Abbott Vascular) or PASCAL (PASCAL (P10) and PASCAL Ace (P5), Edwards Lifesciences). The objective of this study was to assess the impact of T-TEER on renal function. Therefore, eGFR improvement at 3 months following T-TEER was defined as primary endpoint. The closest eGFR value to 3 months follow-up was chosen within the time range 1 to 8 months after the T-TEER procedure. eGFR improvement was defined as follow-up eGFR > baseline eGFR considering that an eGFR slope of 0.75 ml/min/1.73 m2/year already impacts clinical outcomes as demonstrated by Odler et al. (11). One-year HFH was defined as secondary endpoint. Loop diuretic dosage was assessed in accordance with the established conversion standard: 20 mg torasemide = 40 mg furosemide oral administration. The investigation conforms with the principles outlined in the Declaration of Helsinki and was approved by the Ulm University ethics committee (435/16). Informed consent was obtained from all patients.
Follow up
Follow-up was performed by routine clinical visit, by telephone or hospitalization at our Heart Center.
Reference cohort
To demonstrate homogeneity, key baseline and procedure characteristics of the study cohort were compared to the excluded patients without follow-up. Male sex (57.6% in the included vs. 38.7% in the non-included group; p = 0.004) and higher MR grade (p < 0.001) were significantly more often and baseline creatinine (Median 140 [interquartile range 103–173] vs. 121 [97–154] µmol/L; p = 0.037) higher in patients with follow-up (FU). Left ventricular ejection fraction (LVEF) was lower (48 [38–57] vs. 50 [44–58]; p = 0.026) and postprocedural TR grade higher (p = 0.007) in the included cohort; Supplementary Table S1.
Statistical analysis
The study cohort was grouped according to 3-month eGFR improvement and non-improvement. Data are displayed as mean ± standard deviation, median with 25th–75th percentiles, proportions (%) or Kaplan–Meier estimates (%). The distribution of continuous variables was tested with the Kolmogorov–Smirnov test. Normally distributed variables were analyzed using the student's t-test and non-normally distributed variables using the unpaired U-test. Categorial variables were compared by the chi-square test. All variables at baseline and the procedure results were dichotomized according to eGFR improvement. All variables were further analyzed by univariate logistic regression analysis (Supplementary Table S2). Pearson's and Spearman Rho correlation functions were used to identify relevant correlation (r > 0.4) between variables. Multiple logistic regression analysis (full model) to predict eGFR improvement included all significantly tested pre- and periprocedural variables of univariate logistic regression analysis. The strength of the association with eGFR improvement was estimated by the adjusted odds ratio (OR) and the 95% confidence interval. Multiple Cox regression analysis was calculated using the backwards likelihood ratio and included the following significant variables from univariate Cox regression analysis: age, invasive sPAP (mmHg), NYHA grade, TR grade reduction and renal improvement >9 (ml/min) and was estimated by the adjusted Hazard ratio (HR) and the 95%-confidence interval (Supplementary Table S3). A receiver operating characteristic (ROC) analysis estimating sensitivity, specificity and area under the curve (AUC) was performed and the optimal cut-off was identified by Youden's Index. Statistical analysis was performed using IBM® SPSS® Statistics Version 28.
Results
Study cohort
92 patients undergoing T-TEER with 3-month [93 (65–117) days] eGFR follow-up were included. Three-month eGFR improvement was evident in 53 (57.6%) patients.
Baseline characteristics such as age (79 [75–83] in the improved vs. 80 [74–83] years) in the non-improved cohort; p = 0.74), male sex (p = 0.84) and MR severity (p = 0.28) did not significantly differ between patients. Beside betablocker intake (91% vs. 74%; p = 0.038), heart failure medication and diuretic intake at discharge were comparable. Baseline eGFR was significantly lower in the improved cohort (34 [29–43] vs. 41 [31–63] ml/min; p = 0.025). eGFR at follow-up was found to be 45 [34–55] ml/min at median. Hemodynamic parameters were comparable. Pre- and postinterventional tricuspid regurgitation grades did not show a significant difference (p = 0.16 and p = 0.65), whereas TR vena contract reduction was higher in the improved cohort [8 (5–10)] vs. 4 [3–6] mm; p < 0.001) (Table 1).
Table 1
| No renal improvement (n = 39) | Renal improvement (n = 53) | P-value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 80 [74–83] (39/39) | 79 [75–83] (53/53) | 0.742 |
| Height, cm | 168 ± 10 (39/39) | 168 ± 10 (53/53) | 0.793 |
| Weight, kg | 78 [63–93] (39/39) | 75 [67–82] (53/53) | 0.279 |
| Male | 22 (56.4%) (39/39) | 31 (58.5%) (53/53) | 0.842 |
| Arterial hypertension | 33 (84.6%) (39/39) | 46 (86.8%) (53/53) | 0.767 |
| Hyperlipoproteinemia | 27 (69.2%) (39/39) | 37 (69.8%) (53/53) | 0.952 |
| Diabetes | 14 (35.9%) (39/39) | 16 (30.2%) (53/53) | 0.546 |
| Smoker | 8 (20.5%) (39/39) | 10 (18.9%) (53/53) | 0.844 |
| Creatinine, µmol/L | 124 [81–170] (39/39) | 147 [122–176] (53/53) | 0.041 |
| eGFR, ml/min | 41 [31–63] (39/39) | 34 [29–43] (53/53) | 0.025 |
| Baseline NYHA grade | 0.226 | ||
| II | 8 (20.5%) | 9 (17.0%) | |
| III | 28 (71.8%) | 33 (62.3%) | |
| IV | 3 (7.7%) (39/39) | 11 (20.8%) (53/53) | |
| Coronary artery disease | 0.469 | ||
| None | 16 (41.0%) | 25 (47.2%) | |
| 1-vessel | 9 (23.1%) | 8 (15.1%) | |
| 2-vessel | 4 (10.3%) | 10 (18.9%) | |
| 3-vessel | 10 (25.6%) (39/39) | 10 (18.9%) (53/53) | |
| Dilatative cardiomyopathy | 4 (10.3%) (39/39) | 10 (17.0%) (53/53) | 0.360 |
| Pulmonary hypertension | 19 (48.7%) (39/39) | 31 (58.5%) (53/53) | 0.352 |
| Atrial fibrillation | 34 (87.2%) (39/39) | 47 (88.7%) (53/53) | 0.827 |
| COPD | 3 (7.7%) (39/39) | 4 (7.5%) (53/53) | 0.979 |
| EuroSCORE II | 5.5 [2.9–8.9] (39/39) | 6.5 [4.6–11.2] (53/53) | 0.120 |
| Prior mitral valve intervention | 19 (48.7%) (39/39) | 25 (47.2%) (53/53) | 0.883 |
| ACE-inhibitor | 14 (35.9%) (39/39) | 17 (32.1%) (53/53) | 0.702 |
| ARB | 10 (25.6%) (39/39) | 16 (30.2%) (53/53) | 0.632 |
| Betablocker | 29 (74.4%) (39/39) | 48 (90.6%) (53/53) | 0.038 |
| SGLT2-inhibitor | 7 (17.9%) (39/39) | 16 (30.2%) (53/53) | 0.180 |
| Aldosterone antagonist | 20 (51.3%) (39/39) | 32 (60.4%) (53/53) | 0.384 |
| Loop diuretics | 37 (94.9%) (39/39) | 49 (92.5%) (53/53) | 0.642 |
| Baseline loop diuretic dosage, mg | 20 [10–40] | 20 [10–40] | 0.266 |
| Thiazid diuretics | 7 (17.9%) (39/39) | 9 (17.0%) (53/53) | 0.904 |
| Right atrial pressure, mmHg | 18 ± 5 (32/39) | 17 ± 6 (43/53) | 0.334 |
| mPAP, mmHg | 33 [25–39] (32/39) | 31 [25–39] (47/53) | 0.893 |
| Invasive sPAP, mmHg | 51 [38–58] (34/39) | 50 [39–59] (47/53) | 0.985 |
| PCWP, mmHg | 22 [17–28] (29/39) | 20 [15–28] (44/53) | 0.502 |
| TR Etiology | 0.209 | ||
| Functional | 32 (82.1%) | 49 (92.5%) | |
| Degenerative | 2 (5.1%) | 0 | |
| Mixed | 4 (10.3%) | 4 (7.5%) | |
| PM-induced | 1 (2.6%) (39/39) | 0 (53/53) | |
| TR grade | 0.166 | ||
| 3 | 15 (38.5%) | 14 (26.9%) | |
| 4 | 17 (43.6%) | 19 (36.5%) | |
| 5 | 7 (17.9%) (39/39) | 21 (36.5%) (52/53) | |
| TR vena contracta, mm | 11 [8–12] (38/39) | 13 [10–16] (53/53) | 0.005 |
| LVEF, % | 46.1 ± 12.8% (36/39) | 46.9 ± 14.2% (50/53) | 0.752 |
| TAPSE, mm | 19 [15–23] (34/39) | 18 [13–20] (48/53) | 0.034 |
| MR severity | 0.283 | ||
| Mild | 27 (69.2%) | 29 (54.7%) | |
| Moderate | 8 (20.5%) | 16 (30.2%) | |
| Severe | 0 (39/39) | 3 (5.7%) (53/53) | |
| Vena cava inferior, mm | |||
| Preinterventional | 23 [21–28] (37/39) | 23 [20–30] (48/53) | 0.127 |
| 3-months FU | 22 [18–26] (33/39) | 20 [17–27] (48/53) | 0.893 |
| Echocardiographic sPAP, mmHg | 50 [35–65] (38/39) | 50 [39–62] (51/53) | 0.836 |
| Procedural data and in-hospital outcomes | |||
| Procedure duration, minutes | 111 [77–143] (39/39) | 100 [74–141] (52/53) | 0.639 |
| Fluoroscopy time, minutes | 33 [21–44] (31/39) | 31 [18–43] (45/53) | 0.428 |
| Device number | 0.799 | ||
| 1 | 14 (35.9%) | 20 (37.7%) | |
| 2 | 22 (56.4%) | 27 (50.9%) | |
| 3 | 3 (7.7%) (39/39) | 6 (11.3%) (53/53) | |
| Postinterventional TR grade | 0.655 | ||
| 1 | 19 (48.7%) | 27 (51.9%) | |
| 2 | 10 (25.6%) | 15 (28.8%) | |
| 3 | 10 (25.6%) | 9 (17.3%) | |
| 4 | 0 (39/39) | 1 (1.9%) (52/53) | |
| TR grade reduction | 0.096 | ||
| 0 | 1 (2.6%) | 1 (2.0%) | |
| 1 | 6 (15.4%) | 3 (5.9%) | |
| 2 | 26 (66.7%) | 26 (51.0%) | |
| 3 | 5 (12.8%) | 17 (33.3%) | |
| 4 | 1 (2.6%) (39/39) | 4 (7.8%) (51/53) | |
| Postinterventional TV mean gradient, mmHg | 2 [1–3] (27/39) | 2 [1–3] (45/53) | 0.363 |
| Time in-hospital, days | 7 [6–10] (39/39) | 7 [6–12] (53/53) | 0.194 |
| Postprocedural acute kidney injury | 9 (23.1%) (39/39) | 9 (17.0%) (53/53) | 0.466 |
| Postprocedural prolonged respiratoric weaning | 1 (2.6%) (39/39) | 4 (7.5%) (53/53) | 0.297 |
| Access site complication | 1 (2.6%) (39/39) | 2 (3.8%) (53/53) | 0.747 |
| Relevant pericardial effusion | 0 | 0 | – |
| Postprocedural need for catecholamines | 2 (5.1%) (39/39) | 6 (11.3%) (53/53) | 0.298 |
| Infection | 2 (5.1%) | 6 (11.3%) | 0.298 |
| Creatinine at discharge, µmol/L | 117 [85–159] (39/39) | 127 [97–152] (53/53) | 0.978 |
| eGFR at discharge, ml/min | 45 [27–60] (39/39) | 44 [31–57] (53/53) | 0.959 |
| TR vena contracta, mm | |||
| Postinterventional | 6 [4–8] (38/39) | 5 [3–7] (52/53) | 0.072 |
| Vena contracta reduction | 4 [3–6] (38/39) | 8 [5–10] (52/53) | <0.001 |
| 3-month follow-up | |||
| TR grade 3-month follow-up | 0.829 | ||
| 0 | 1 (2.7%) | 1 (2.3%) | |
| 1 | 19 (51.4%) | 22 (50.0%) | |
| 2 | 9 (24.3%) | 8 (18.2%) | |
| 3 | 8 (21.6%) | 12 (27.3%) | |
| 4 | 0 (37/39) | 1 (2.3%) (44/53) | |
| Creatinine at 3-month follow-up, µmol/L | 152 [100–242] (39/39) | 119 [101–145] (53/53) | 0.013 |
| eGFR at 3-month follow-up, ml/min | 31 [20–50] (39/39) | 45 [34–55] (53/53) | <0.001 |
| Difference eGFR follow-up and baseline | −8 [−13 to (−5)] (39/39) | 9 [4–15] (53/53) | <0.001 |
| Vena cava inferior, mm | |||
| 3-month FU | 22 [18–26] (33/39) | 20 [17–27] (48/53) | 0.893 |
| Difference to baseline | −3 [(−8) to 4] (31/39) | −2 [(−5) to 1] (45/53) | 0.217 |
| NYHA grade at 3-month follow-up | 0.127 | ||
| 1 | 5 (17.9%) | 11 (28.9%) | |
| 2 | 13 (46.4%) | 16 (42.1%) | |
| 3 | 10 (35.7%) | 7 (18.4%) | |
| 4 | 0 (28/39) | 4 (10.5%) (38/53) | |
| One-year outcomes | |||
| 1-year heart failure hospitalization | 18 (46.2%) (39/39) | 23 (43.4%) (53/53) | 0.793 |
| 1-year death | 6 (15.4%) (39/39) | 5 (9.4%) (53/53) | 0.385 |
Baseline and procedural characteristics and outcome in patients undergoing T-TEER stratified by improvement of renal function during mid-term follow up.
ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; FU, follow-up; PCWP, pulmonary capillary wedge pressure; LVEF, left ventricular ejection fraction; s-/mPAP, systolic-/mean pulmonary artery pressure; SGLT2, sodium glucose transport protein 2; NYHA, New York Heart Association; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; T-TEER, transcatheter edge-to-edge-repair.
P-values <0.05 are displayed bold and variable availability is displayed in brackets.
Predictors of renal improvement at 3-month follow-up
Multiple logistic regression analysis revealed TR vena contracta reduction (VC) [HR 1.35 (95% CI: 1.12–1.64); p = 0.002] and tricuspid annular plane systolic excursion (TAPSE) [OR 0.89 (0.79–0.99); p = 0.033] to independently impact renal improvement at 3-month follow-up (Table 2). ROC analysis identified a threshold of 6.5 mm TR VC reduction to predict eGFR improvement at follow-up with a sensitivity of 64% and 82% specificity (AUC 0.77, 95% CI: 0.67–0.87; p < 0.001, Figure 1).
Table 2
| Odds ratio | 95%-Confidence interval | P-value | |
|---|---|---|---|
| Univariate regression analysis | |||
| Preinterventional betablocker | 3.31 | 1.03–10.65 | 0.045 |
| Baseline eGFR, ml/min | 0.97 | 0.95–0.99 | 0.030 |
| Preinterventional TAPSE, mm | 0.89 | 0.82–0.98 | 0.018 |
| Vena contracta reduction, mm | 1.39 | 1.17–1.65 | <0.001 |
| Multiple regression analysis | |||
| TR vena contracta reduction, mm | 1.35 | 1.12–1.64 | 0.002 |
| Preinterventional TAPSE, mm | 0.89 | 0.79–0.99 | 0.033 |
| Baseline eGFR, ml/min | 1.00 | 0.97–1.03 | 0.890 |
| Preinterventional betablocker | 2.87 | 0.66–12.53 | 0.161 |
Logistic regression analysis to identify predictors for renal function improvement during mid-term follow-up.
eGFR, estimated glomerular filtration rate; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
P-values <0.05 are presented bold.
Figure 1
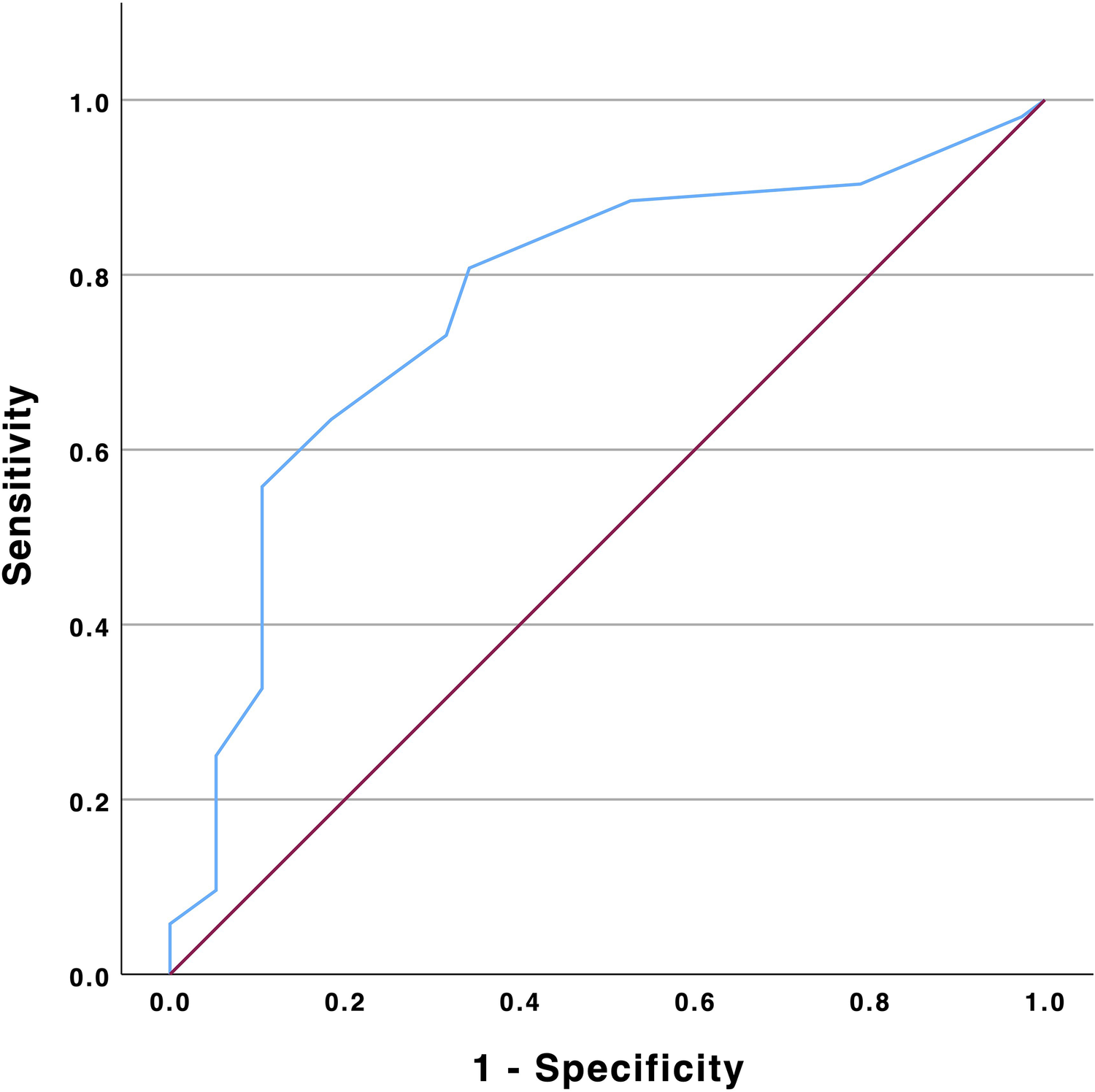
ROC analysis of TR vena contracta reduction and eGFR improvement. eGFR, estimated glomerular filtration rate; TR, tricuspid regurgitation.
Heart failure hospitalization
HFH occurred in 41 (44.6%) patients. These patients suffered more often from NYHA class IV (24% in rehospitalized vs. 8% in non-rehospitalized patients; p = 0.028), had an elevated invasive sPAP (55 ± 15 vs. 46 ± 14 mmHg; p = 0.007) and a lower baseline eGFR (32 [27–42] vs. 39 [32–63] ml/min; p = 0.023) (Supplementary Table S4). In patients with improved eGFR, ROC analysis identified a threshold of eGFR increase by 9 ml/min to predict a reduced HFH risk with a sensitivity of 67% and 78% specificity (AUC 0.72, 95% CI: 0.58–0.86; p = 0.006; Supplementary Figure S1). Patients with eGFR improvement >9 ml/min had a 1-year rehospitalization probability of 26.6%, whereas no improvement <9 ml/min was associated with a risk of 61.8% (Log rank p = 0.013). Multiple Cox logistic regression analysis revealed NYHA IV [HR 12.24 (95% CI: 3.15–47.53); p < 0.001] and eGFR improvement >9 ml/min [0.22 (95% CI: 0.07–0.62), p = 0.005] to independently impact HFH (Table 3).
Table 3
| Hazard ratio | 95%-Confidence interval | P-value | |
|---|---|---|---|
| Univariate Cox regression analysis | |||
| Age, years | 1.07 | 1.01–1.13 | 0.019 |
| Invasive sPAP, mmHg | 1.02 | 1.00–1.05 | 0.031 |
| Baseline eGFR, ml/min | 0.97 | 0.95–0.99 | 0.004 |
| Preinterventional echocardiographic sPAP, mmHg | 1.02 | 1.00–1.03 | 0.016 |
| Baseline NYHA grade | |||
| II | |||
| III | 3.38 | 1.02–11.18 | 0.046 |
| IV | 12.47 | 3.36–46.33 | <0.001 |
| Pulmonary hypertension | 2.20 | 1.15–4.22 | 0.017 |
| TR grade reduction | |||
| 0 | |||
| 1 | 0.08 | 0.08–0.83 | 0.035 |
| 2 | 0.16 | 0.02–1.26 | 0.082 |
| 3 | 0.12 | 0.15–1.02 | 0.052 |
| 4 | 0.08 | 0.05–1.41 | 0.085 |
| eGFR improvement >9 ml/min | 0.27 | 0.09–0.76 | 0.013 |
| Multiple Cox regression analysis | |||
| Baseline NYHA grade | |||
| II | |||
| III | 2.49 | 0.75–8.37 | 0.138 |
| IV | 12.24 | 3.15–47.53 | <0.001 |
| eGFR improvement >9 ml/min | 0.22 | 0.07–0.62 | 0.005 |
Cox regression analysis to identify predictors for unplanned 1-year heart failure hospitalization.
eGFR, estimated glomerular filtration rate; sPAP, systolic pulmonary artery pressure; NYHA, New York Heart Association; TR, tricuspid regurgitation.
P-values <0.05 are displayed bold.
Discussion
To date, this is the largest study to investigate the impact of T-TEER on renal function. The main results can be summarized as follows:
- -
Renal improvement during mid-term follow-up was evident in the majority of patients (57%)
- -
Tricuspid regurgitation reduction indicated by TR vena contracta reduction and lower preinterventional TAPSE indicating right ventricular dysfunction predict renal improvement during follow-up
- -
Significant renal improvement is associated with reduced heart failure hospitalization (adjusted HR 0.22)
Several studies demonstrated an association between tricuspid regurgitation and impaired renal function (
4,
5). Reduced cardiac output and elevated venous congestion are both assumed to contribute to the negative effect of TR on renal function (
3–
5,
12). In patients with relevant TR undergoing T-TEER, renal function may improve by reducing tricuspid regurgitation. Our study confirms this assumption and demonstrates renal improvement in 57% of patients. Furthermore, TR predicts renal improvement emphasizing the procedures’ beneficial effect on renal function. Additionally, patients with reduced TAPSE had a higher chance of mid-term eGFR improvement. Thus, sicker patients seem to benefit even more from the effect of venous decongestion.
Wang et al. demonstrated both MR reduction and more advanced chronic kidney disease to predict renal improvement following the M-TEER procedure (7). Moreover, CKD stage correlated directly with worsened prognosis in their analysis (7). In our study, impaired preinterventional renal function showed a trend towards higher risk of heart failure rehospitalization (p = 0.08). However, in patients with a relevant eGFR improvement >9 ml/min at mid-term follow-up, the likelihood of heart failure rehospitalization was significantly reduced by 68% (95% CI: 18%–87%).
Renal improvement was observed in the majority of patients (57%). Notably, patients who experienced renal improvement had a higher preinterventional TR VC, and the reduction of TR VC was more in these patients. Each millimeter reduction of VC reduction was associated with a 35% increased likelihood of renal improvement (OR 1.35), highlighting the direct link between venous renal decongestion and a patients’ individual absolute TR reduction.
Additionally, a lower TAPSE was associated with a higher likelihood of renal improvement (OR 0.89). This finding aligns with recent research by Vogelhuber et al., which showed that TAPSE improved following T-TEER in patients with reduced preinterventional TAPSE (13). This oberservation underscores the previously described critical role of right ventricular function in influencing renal function and demonstrates a potentially benefit of T-TEER in this context (14).
However, renal improvement was not observed in every subject of the whole cohort, which may be explained by the following: chronic kidney disease itself displays a risk-factor of valvular heart disease mediated by volume overload causing annulus dilatation and degeneration by calcification and therefore, renal improvement seems unlikely in these patients (15). On the other hand, patients without preinterventional renal impairment are not expected to show renal improvement during follow-up.
Consequently, renal improvement may be used as a surrogate of the T-TEER procedures’ efficacy indicating improved organ function and being associated with reduced HFH in selected patients.
The observed HFH rate of 45% is higher than in other studies, such as the randomized TRILUMINATE study reporting 14.9% (1). This can be explained by the fact that only patients with follow-up eGFR were included in our study. Consequently, patients without need for short-term blood control or not experiencing heart failure rehospitalization are not included in our study cohort due to lacking eGFR control. When comparing the included and the not included cohort, LVEF was lower and mitral regurgitation severity was higher in the included cohort being both associated with elevated rehospitalization rates (16, 17). Hence, the results of our study especially focus on patients with a high risk of heart failure rehospitalization and demonstrates that optimal TR reduction is crucial in this vulnerable group.
Limitations
The results of our study have to be interpreted with several confinements. The retrospective study design is associated with all the inherent limitations ascribed to this study type. Patients were dichotomized by renal improvement at mid-term follow-up irrespective of their CKD stage. Renal improvement was included to the Cox regression model for 1-year heart failure hospitalization, even though it was assessed at 3-month follow-up and not at baseline. However, it considers that complex hemodynamic changes have to apply and cannot be expected immediately after the procedure, underlining its value as an indicator of improved outcome.
Conclusion
In patients with effective TR reduction and impaired renal function tricuspid edge-to-edge repair is associated with improvement of renal function. Renal improvement itself is associated with reduced heart failure hospitalization. In patients without renal improvement at 3-months follow-up, residual TR should be reevaluated.
Statements
Data availability statement
The datasets presented in this article are not readily available due to privacy reasons, but the raw data supporting the conclusions of this article will be made available on reasonable request from the corresponding author.
Ethics statement
The studies involving humans were approved by Ulm University Ethics Committee (435/16). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DF: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft. JW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. MP: Methodology, Validation, Writing – review & editing. MG: Data curation, Investigation, Validation, Writing – review & editing. EW: Data curation, Writing – review & editing. SA: Data curation, Writing – review & editing. LS: Validation, Writing – review & editing. SM: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. WR: Conceptualization, Resources, Supervision, Validation, Writing – review & editing. MK: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Uta Dichristin and Viktor Riedl for study coordination and data management.
Conflict of interest
LS has received speaker honoraria from Edwards Lifesciences and Abbott. SM has received speaker honoraria from Abbott. WR has received speaker honoraria from Edwards Lifesciences and Abbott. MK has received speaker honoraria from Edwards Lifesciences and Abbott.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1452446/full#supplementary-material
Supplementary Table S1Baseline characteristics in patients undergoing T-TEER stratified by patients with available follow-up for eGFR.
Supplementary Table S2Univariate logistic regression analysis to identify predictors for renal function improvement during mid-term follow-up.
Supplementary Table S3Univariate Cox logistic regression analysis to identify predictors for unplanned 1-year heart failure hospitalization.
Supplementary Table S4Baseline and procedural characteristics in patients undergoing T-TEER stratified by 1-year heart failure hospitalization.
Supplementary Figure S1ROC analysis of eGFR improvement and heart failure hospitalization. eGFR, estimated glomerular filtration rate.
References
1.
Sorajja P Whisenant B Hamid N Naik H Makkar R Tadros P et al Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. (2023) 388(20):1833–42. 10.1056/NEJMoa2300525
2.
Rangaswami J Bhalla V Blair JEA Chang TI Costa S Lentine KL et al Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. (2019) 139(16):E840–78. 10.1161/CIR.0000000000000664
3.
Mullens W Abrahams Z Francis GS Sokos G Taylor DO Starling RC et al Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. (2009) 53(7):589–96. 10.1016/J.JACC.2008.05.068
4.
Maeder MT Holst DP Kaye DM . Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J Card Fail. (2008) 14(10):824–30. 10.1016/J.CARDFAIL.2008.07.236
5.
Benfari G Antoine C Miller WL Thapa P Topilsky Y Rossi A et al Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. (2019) 140(3):196–206. 10.1161/CIRCULATIONAHA.118.038946
6.
Boorsma EM ter Maaten JM Voors AA van Veldhuisen DJ . Renal compression in heart failure. JACC Heart Fail. (2022) 10(3):175–83. 10.1016/j.jchf.2021.12.005
7.
Wang A Sangli C Lim S Ailawadi G Kar S Herrmann HC et al Evaluation of renal function before and after percutaneous mitral valve repair. Circ Cardiovasc Interv. (2015) 8(1):e001349. 10.1161/CIRCINTERVENTIONS.113.001349
8.
Tanaka T Kavsur R Sugiura A Vogelhuber J Öztürk C Weber M et al Prognostic impact of hepatorenal function in patients undergoing transcatheter tricuspid valve repair. Sci Rep. (2021) 11(1):14420. 10.1038/s41598-021-93952-9
9.
Tanaka T Kavsur R Sugiura A Haurand JM Galka N Öztürk C et al Acute kidney injury following tricuspid transcatheter edge-to-edge repair. JACC Cardiovasc Interv. (2022) 15(19):1936–45. 10.1016/j.jcin.2022.07.018
10.
Baumgartner H Falk V Bax JJ De Bonis M Hamm C Holm PJ et al 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. 10.1093/eurheartj/ehx391
11.
Odler B Fu EL . eGFR slope as a primary endpoint for clinical trials of CKD progression: one size fits all?Clin Kidney J. (2024) 17(1):1–2. 10.1093/ckj/sfae001
12.
Andersen MJ Nishimura RA Borlaug BA . The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. (2014) 7(6):911–7. 10.1161/CIRCHEARTFAILURE.114.001575
13.
Vogelhuber J Tanaka T Kavsur R Goto T Öztürk C Silaschi M et al Outcomes of transcatheter tricuspid edge-to-edge repair in patients with right ventricular dysfunction. Circ Cardiovasc Interv. (2024) 17(6):e013156. 10.1161/CIRCINTERVENTIONS.123.013156
14.
Dini FL Demmer RT Simioniuc A Morrone D Donati F Guarini G et al Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail. (2012) 14(3):287–94. 10.1093/eurjhf/hfr176
15.
Marwick TH Amann K Bangalore S Cavalcante JL Charytan DM Craig JC et al Chronic kidney disease and valvular heart disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2019) 96(4):836–49. 10.1016/J.KINT.2019.06.025
16.
De la Espriella R Santas E Miñana G Bodí V Valero E Payá R et al Functional mitral regurgitation predicts short-term adverse events in patients with acute heart failure and reduced left ventricular ejection fraction. Am J Cardiol. (2017) 120(8):1344–8. 10.1016/J.AMJCARD.2017.07.023
17.
Janwanishstaporn S Feng S Teerlink J Metra M Cotter G Davison BA et al Relationship between left ventricular ejection fraction and cardiovascular outcomes following hospitalization for heart failure: insights from the RELAX-AHF-2 trial. Eur J Heart Fail. (2020) 22(4):726–38. 10.1002/EJHF.1772
Summary
Keywords
tricuspid regurgitation, renal improvement, heart failure hospitalization, edge-to-edge repair, T-TEER
Citation
Felbel D, von Winkler J, Paukovitsch M, Gröger M, Walther E, Andreß S, Schneider L, Markovic S, Rottbauer W and Keßler M (2024) Effective tricuspid regurgitation reduction is associated with renal improvement and reduced heart failure hospitalization. Front. Cardiovasc. Med. 11:1452446. doi: 10.3389/fcvm.2024.1452446
Received
20 June 2024
Accepted
07 October 2024
Published
21 October 2024
Volume
11 - 2024
Edited by
Verena Veulemans, University Heart and Vascular Center Frankfurt, Germany
Reviewed by
Elric Zweck, University Hospital of Düsseldorf, Germany
Tetsu Tanaka, University Hospital Bonn, Germany
Updates
Copyright
© 2024 Felbel, von Winkler, Paukovitsch, Gröger, Walther, Andreß, Schneider, Markovic, Rottbauer and Keßler.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Mirjam Keßler mirjam.kessler@uniklinik-ulm.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.