Abstract
Objective:
To investigate the correlation between remnant cholesterol (RC) and premature coronary artery disease (PCAD) and the severity of coronary artery lesions in men.
Methods:
A total of 630 male subjects who underwent coronary angiography were included in the study. The general data, laboratory tests, and coronary angiography data of each group were statistically analyzed, and RC levels were calculated. According to the Gensini score, PCAD was divided into mild and severe lesion groups. The relationship between RC and PCAD and coronary artery lesions was analyzed using multivariate logistic regression and spearman correlation analysis, and the predictive value of RC for coronary artery lesions was evaluated using receiver operating characteristic (ROC) curves.
Results:
The RC levels in the PCAD group were significantly higher than those in the non-PCAD group (p < 0.05), and RC was an independent risk factor for PCAD (p < 0.05).The RC levels in the severe lesion group were higher than those in the mild lesion group (p < 0.05), and RC levels were positively correlated with the Gensini score (r = 0.335, p < 0.001).Multivariate logistic regression analysis showed that RC was an independent risk factor for severe coronary artery lesions (p < 0.05).The ROC curve calculated the value of RC in predicting severe coronary artery lesions, with an area under the curve of 0.693, a cutoff value of 0.485 mmol/L, a sensitivity of 64.7%, and a specificity of 66.2%.
Conclusion:
RC is an independent risk factor for PCAD and the severity of coronary artery lesions in adult men. RC levels are positively correlated with the severity of coronary artery lesions and can be used as an auxiliary indicator for clinical assessment of PCAD.
1 Introduction
In recent years, economic development and changes in lifestyle and dietary habits have made coronary artery disease (CAD) a leading cause of cardiovascular-related deaths. Notably, CAD is increasingly affecting younger populations. In patients with CAD, 4%–10% of patients with acute myocardial infarction (AMI) occur before the age of 45 (1), and among individuals aged 35 to 44, 62% of causes for sudden cardiac death can be attributed to CAD (2). PCAD is defined as CAD occurring in males under 55 years and females under 65 years (3). Although most studies indicate a low incidence rate for PCAD, the potential number of affected individuals may be significantly higher than currently estimated due to the rise in cardiovascular risk factors among younger populations. Statistics show that over half of PCAD patients experience significant coronary atherosclerosis progression within 10 years, with approximately 20% dying prematurely (4). Despite current preventive measures, PCAD still exhibits a high recurrence rate and mortality, intensifying the medical burden on younger populations and society (5). Therefore, it is crucial to identify high-risk PCAD populations early, provide interventions, and offer health guidance to reduce the incidence of PCAD.
Several major risk factors for CAD have been identified, including age, gender, smoking history, hypertension, diabetes, hyperuricemia, hyperlipidemia, obesity, and anxiety (6, 7). Among these, dyslipidemia is a significant independent risk factor in the occurrence and development of CAD. Traditionally, lipid-lowering therapy has focused on reducing low-density lipoprotein cholesterol (LDL-C). Research has shown that lowering LDL-C levels effectively improves CAD prognosis. However, many patients continue to experience major adverse cardiovascular events (MACEs), despite achieving target LDL-C levels and controlling other risk factors. Consequently, RC has emerged as a key marker of residual cardiovascular risk. RC is the cholesterol content in triglyceride-rich lipoproteins, including very low-density lipoprotein and intermediate-density lipoprotein in fasting states, and chylomicron remnants in non-fasting states (8). RC is now recognized as a residual cardiovascular risk factor even after achieving lipid targets, with substantial evidence linking it to CAD occurrence and progression (9).
Significant gender differences exist in CAD incidence, with men developing the disease at younger ages compared to women. Despite this, few studies have examined the correlation between RC and PCAD or the severity of coronary artery lesions in adult men. This study uses the Gensini score as the standard for evaluating the severity of coronary artery lesions to explore the correlation between RC and PCAD, and the severity of coronary artery lesions in adult men. The goal is to provide new insights into the prevention and treatment of PCAD in men.
2 Materials and methods
2.1 Study design and participants
From January 1, 2023, to December 31, 2023, a total of 9,276 patients suspected of having CAD and admitted to Weifang People's Hospital for coronary angiography were selected. The exclusion criteria were as follows: female patients; patients younger than 18 or older than 55; patients with a history of coronary revascularization; patients with organic heart diseases such as valvular heart disease, rheumatic heart disease, or dilated cardiomyopathy; patients with malignant tumors, infectious diseases, severe liver or kidney dysfunction, or autoimmune diseases; patients who recently used lipid-lowering drugs; patients with incomplete clinical data. Ultimately, 630 patients were included in the study, comprising 463 patients with PCAD and 167 patients without PCAD (Figure 1).
Figure 1
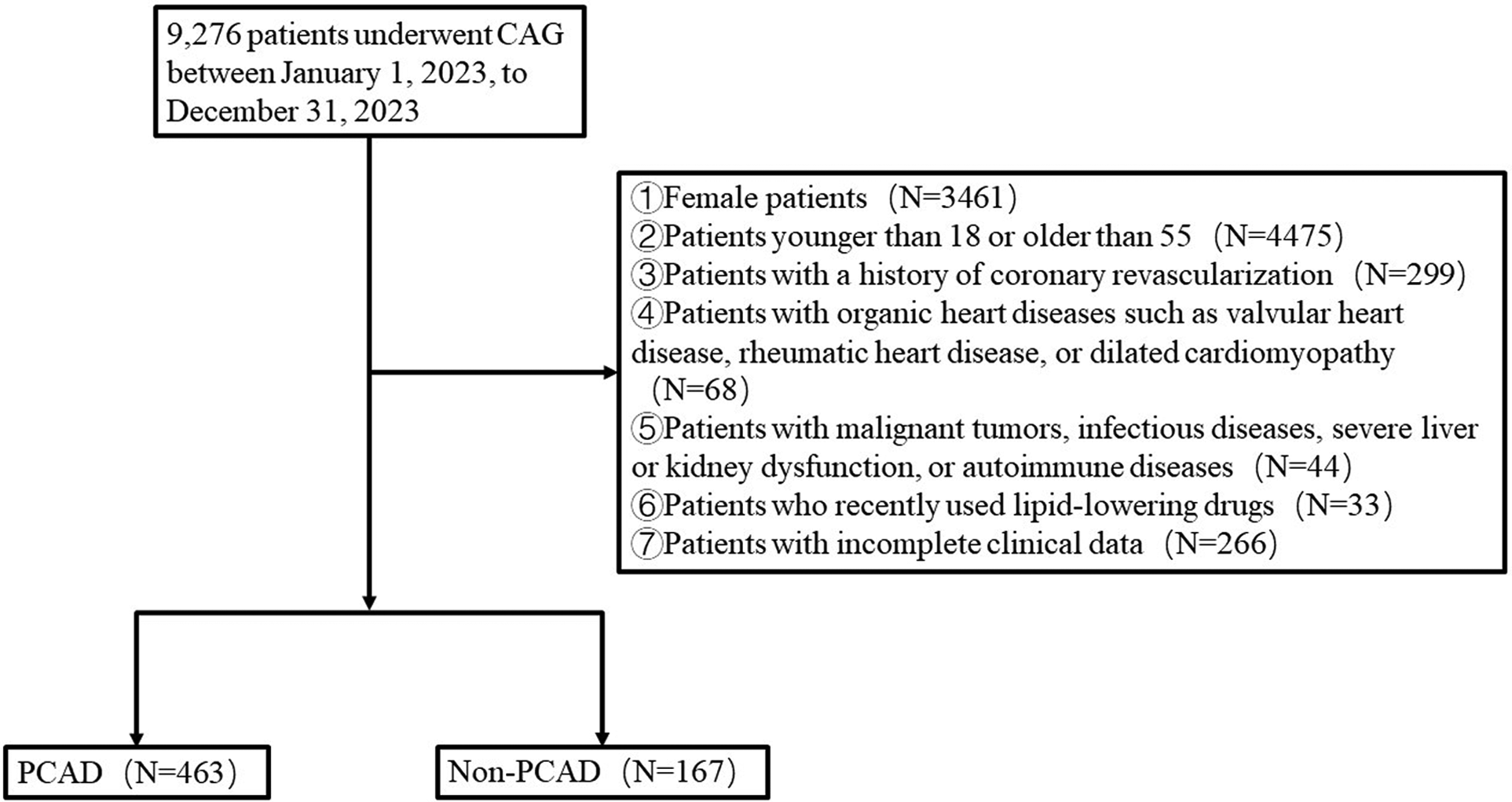
Flow chart.
2.2 Data collection
Clinical data were collected from the Hospital Information System, including demographic characteristics, clinical history, laboratory results, and coronary angiography (CAG) findings. The demographic characteristics included age, height, weight, smoking status, drinking habits. Clinical history included the history of hypertension, diabetes and family history of CAD.
All blood samples were fasting venous samples collected within 24 h of admission. The laboratory indicators primarily included total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, alanine aminotransferase (ALT), albumin (ALB), fasting blood glucose (FBG), uric acid (UA), and creatinine (CREA).
Invasive CAG was performed via percutaneous radial or femoral artery angiography. The angiographic equipment used was capable of accurately diagnosing all manifestations of coronary artery conditions.
2.3 Definitions
PCAD: (1) Age <55 years. (2) Obstructive stenosis ≥50% in the lumen diameter of any major coronary artery (including the left main coronary artery, left anterior descending artery, left circumflex artery, and right coronary artery) or their main branches.
BMI = Weight (kg)/Height (m)2
RC = TC (mmol/L)—HDL-C (mmol/L)—LDL-C (mmol/L) (10)
Gensini Score: The total score is calculated based on the Gensini scoring guidelines, which assess the severity of PCAD (11). The scoring is performed by experienced cardiologists. According to the median Gensini score, a score of <44 is defined as mild PCAD, and a score of ≥44 is defined as severe PCAD.
2.4 Statistical analysis
Statistical analysis was performed using SPSS 25.0. The normality of continuous variables was tested using the Kolmogorov-Smirnov (K-S) test. Non-parametric tests were used for comparisons if the variables were not normally distributed. Categorical variables were expressed as rates and compared using the chi-square test. The correlation between RC and the Gensini score was analyzed using Spearman's correlation. Continuous variables were converted to binary variables for multivariate logistic regression analysis to compare the relationship between laboratory indicators, risk factors, and the severity of coronary artery lesions. ROC curves were plotted, and the optimal cutoff value of RC for predicting severe coronary artery lesions was determined based on the Youden index. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Clinical and demographic characteristics of PCAD and non-PCAD groups
The clinical data analysis involved 630 subjects, including 463 newly diagnosed PCAD patients and 167 non-PCAD individuals (Table 1). The results showed that compared with the non-PCAD group, the PCAD group had a higher proportion of smokers and higher rates of hypertension, diabetes. ALT, FBG, TC, TG, LDL-C, and RC levels were significantly higher in the PCAD group than in the non-PCAD group, with differences being statistically significant (all p < 0.05). HDL-C was significantly lower in the PCAD group compared to the non-PCAD group, with a statistically significant difference (p < 0.05). There were no statistically significant differences between the two groups in terms of age, alcoholism, family history of CAD, BMI, ALB, UA,and CERA (all p > 0.05).
Table 1
| Variables | PCAD (n = 463) | Non-PCAD (n = 167) | Z/χ2 | p-value |
|---|---|---|---|---|
| Age (years) | 49 (44, 52) | 49 (45, 52) | −0.368 | 0.713 |
| Hypertension, n (%) | 218 (47.1%) | 54 (32.3%) | 10.882 | 0.001 |
| Diabetes, n (%) | 114 (24.6%) | 15 (9%) | 18.437 | <0.001 |
| Smoking, n (%) | 275 (59.4%) | 70 (41.9%) | 15.136 | <0.001 |
| Alcoholism, n (%) | 93 (20.1%) | 26 (15.6%) | 1.635 | 0.201 |
| Family history of CAD, n (%) | 62 (13.4%) | 13 (7.8%) | 3.678 | 0.055 |
| BMI (Kg/m2) | 26.99 (24.61, 28.73) | 26.23 (23.88, 28.70) | −1.617 | 0.106 |
| ALT (U/L) | 28 (20, 38) | 23 (16, 33) | −3.677 | <0.001 |
| ALB (g/L) | 44 (41.3, 46.4) | 44.3 (42.4, 46.9) | −1.499 | 0.134 |
| FBG (mmol/L) | 5.6 (5, 6.8) | 5.1 (4.8, 5.7) | −5.948 | <0.001 |
| UA (umol/L) | 359 (296, 424) | 357 (303, 420) | −0.33 | 0.973 |
| CERA (umol/L) | 65 (56, 74) | 66 (60, 72) | −1.063 | 0.288 |
| TC (mmol/L) | 4.7 (4.1, 5.5) | 4.5 (3.8, 5.1) | −3.955 | <0.001 |
| TG (mmol/L) | 1.74 (1.16, 2.66) | 1.45 (1.05, 1.99) | −3.422 | <0.001 |
| HDL-C (mmol/L) | 1 (0.88, 1.2) | 1.17 (1.02, 1.33) | −6.759 | <0.001 |
| LDL-C (mmol/L) | 3.19 (2.6, 3.83) | 2.98 (2.36, 3.44) | −3.478 | 0.001 |
| RC (mmol/L) | 0.48 (0.31, 0.67) | 0.28 (0.18, 0.44) | −9.372 | <0.001 |
Clinical and demographic characteristics of PCAD and non-PCAD groups.
BMI, the body mass index; ALT, alanine aminotransferase; ALB, albumin; FBG, fasting blood glucose; UA, uric acid; CREA, creatinine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RC, remnant cholesterol.
3.2 Relationship between RC and PCAD
Using the presence of PCAD as the dependent variable, a multivariate logistic regression analysis was conducted. The variables with p < 0.1 in univariate analysis were adjusted for. The results indicated that hypertension, smoking, and family history of CAD, ALT, FBG, LDL-C, and RC were independent risk factors for PCAD (all p < 0.05), while HDL-C was an independent protective factor against PCAD (p < 0.05) (Table 2).
Table 2
| Variables | B | Wald | OR | 95%CI | p-value |
|---|---|---|---|---|---|
| Hypertension, n (%) | 0.451 | 4.053 | 1.571 | 1.012–2.437 | 0.044 |
| Diabetes, n (%) | 0.410 | 1.327 | 1.507 | 0.750–3.030 | 0.249 |
| Smoking, n (%) | 0.627 | 8.478 | 1.872 | 1.227–2.854 | 0.004 |
| Family history of CAD, n (%) | 0.891 | 5.892 | 2.439 | 1.187–5.009 | 0.015 |
| ALT (U/L) | 0.017 | 4.331 | 1.017 | 1.111–1.033 | 0.037 |
| FBG (mmol/L) | 0.380 | 11.528 | 1.462 | 1.174–1.820 | 0.001 |
| HDL-C (mmol/L) | −1.937 | 20.361 | 0.144 | 0.062–0.334 | <0.001 |
| LDL-C (mmol/L) | 0.403 | 10.181 | 1.497 | 1.168–1.971 | 0.001 |
| RC (mmol/L) | 3.772 | 39.352 | 43.453 | 13.373–141.187 | <0.001 |
Multivariate logistic regression analysis of PCAD risk factors.
ALT, alanine aminotransferase; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RC, remnant cholesterol.
3.3 Clinical and demographic characteristics of different coronary artery lesion groups
The severity of coronary artery lesions was assessed using the Gensini score, with scores <44 defined as mild PCAD and scores ≥44 defined as severe PCAD. The results showed that, compared to the mild PCAD group, the severe PCAD group had a higher proportion of patients with diabetes. FBG, TG, and RC levels were significantly higher in the severe PCAD group than in the mild PCAD group, with statistically significant differences (all p < 0.05) (Table 3). HDL-C levels were significantly lower in the severe PCAD group compared to the mild PCAD group (p < 0.05). There were no statistically significant differences between the two groups in terms of age, hypertension, smoking, alcoholism, family history of CAD, BMI, ALT, ALB, UA, UREA, TC, and LDL-C (all p > 0.05).
Table 3
| Variables | Mild PCAD group (n = 231) | Severe PCAD group (n = 232) | Z/χ2 | p-value |
|---|---|---|---|---|
| Age (years) | 50 (45, 52) | 49 (44, 52) | −1.741 | 0.082 |
| Hypertension, n (%) | 107 (46.3%) | 111(47.8%) | 0.108 | 0.742 |
| Diabetes, n (%) | 41 (17.7%) | 73 (31.5%) | 11.734 | 0.001 |
| Smoking, n (%) | 130 (56.3%) | 145 (62.5%) | 1.859 | 0.173 |
| Alcoholism, n (%) | 50 (21.6%) | 43 (18.5%) | 0.698 | 0.404 |
| Family history of CAD, n (%) | 33 (14.3%) | 29 (12.5%) | 0.318 | 0.573 |
| BMI (Kg/m2) | 26.75 (24.62, 28.41) | 27.04 (24.52, 29.24) | −0.637 | 0.524 |
| ALT(U/L) | 27 (19, 37) | 30 (21, 40.75) | −1.867 | 0.062 |
| ALB (g/L) | 43.9 (41.3, 46.1) | 44.2 (41.4, 46.7) | −0.630 | 0.529 |
| FBG (mmol/L) | 5.5 (4.8, 6.2) | 5.9 (5.2, 7.8) | −4.502 | <0.001 |
| UA (umol/L) | 358 (306, 424) | 360 (287, 424) | −0.916 | 0.360 |
| CERA (umol/L) | 66 (56, 75) | 64 (56, 73) | −0.827 | 0.408 |
| TC (mmol/L) | 4.7 (4.1, 5.4) | 4.8 (4.2, 5.6) | −0.986 | 0.324 |
| TG (mmol/L) | 1.60 (1.08, 2.29) | 1.88 (1.23, 3.03) | −2.635 | 0.008 |
| HDL-C (mmol/L) | 1.07 (0.93, 1.24) | 0.95 (0.82, 1.13) | −5.176 | <0.001 |
| LDL-C (mmol/L) | 3.20 (2.622, 3.80) | 3.18 (2.60, 3.90) | −0.157 | 0.876 |
| RC (mmol/L) | 0.38 (0.26, 0.53) | 0.56 (0.4, 0.76) | −7.190 | <0.001 |
Clinical and demographic characteristics of mild PCAD group and severe PCAD group.
BMI, the body mass index; ALT, alanine aminotransferase; ALB, albumin; FBG, fasting blood glucose; UA, uric acid; CREA, creatinine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RC, remnant cholesterol.
3.4 Correlation analysis between the severity of coronary artery lesions and RC
Spearman correlation analysis showed a significant correlation between RC and the Gensini score (r = 0.335, p < 0.001). This indicates that as RC levels increase, the severity of coronary artery lesions in PCAD patients progressively worsens.
3.5 RC was an independent risk factor for severe coronary artery lesions
Using the presence of severe coronary artery lesions as the dependent variable, a multivariate logistic regression analysis was conducted. The variables with p < 0.1 in univariate analysis were adjusted for. The results indicated that FBG and RC were independent risk factors for severe coronary artery lesions (p < 0.05), while HDL-C was an independent protective factor against severe coronary artery lesions (p < 0.05) (Table 4).
Table 4
| Variables | B | Wald | OR | 95%CI | p-value |
|---|---|---|---|---|---|
| Hypertension, n (%) | −0.034 | 0.028 | 0.967 | 0.652–1.433 | 0.866 |
| Diabetes, n (%) | 0.404 | 2.141 | 1.498 | 0.872–2.576 | 0.143 |
| Smoking, n (%) | 0.244 | 1.468 | 1.276 | 0.860–1.893 | 0.226 |
| ALT (U/L) | 0.011 | 2.668 | 1.011 | 0.998–1.025 | 0.102 |
| FBG (mmol/L) | 0.105 | 4.166 | 1.111 | 1.004–1.230 | 0.041 |
| HDL-C (mmol/L) | −1.681 | 16.235 | 0.186 | 0.082–0.422 | <0.001 |
| RC (mmol/L) | 0.768 | 8.375 | 2.154 | 1.281–3.623 | 0.004 |
Multivariate logistic regression analysis of coronary artery lesions risk factors.
ALT, alanine aminotransferase; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; RC, remnant cholesterol.
3.6 Predictive value of RC for severe coronary artery lesions
The value of RC in predicting severe coronary artery lesions was assessed using ROC curve analysis (Figure 2). The results showed that the area under curve (AUC) was 0.693 (95% CI 0.645–0.741, p < 0.001). The optimal cutoff value for RC was determined to be 0.485 mmol/L, with a sensitivity of 64.7% and a specificity of 66.2%.
Figure 2
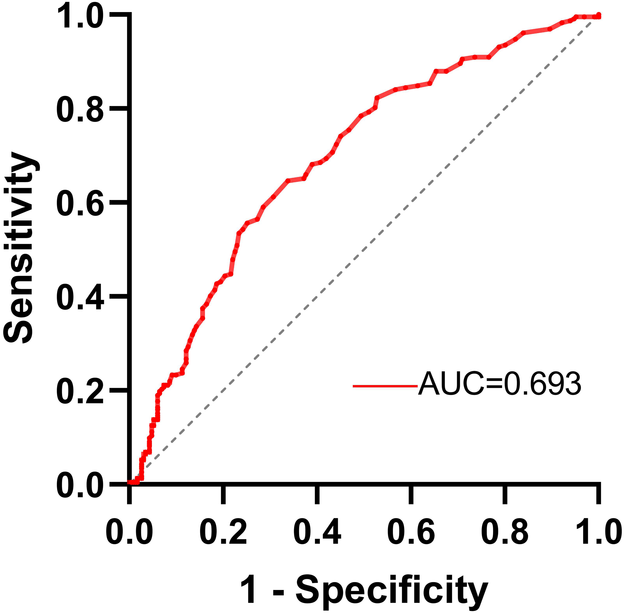
ROC curve analysis of RC for severe coronary artery lesions prediction.
4 Discussion
PCAD is a special type of CAD characterized by early onset and rapid progression, leading to considerable psychological and physiological harm to patients. Hence, early identification of clinical risk factors, along with timely diagnosis and treatment, is crucial (12). In this retrospective study, we compared the clinical data of populations with PCAD and non-PCAD, and examined the correlation between RC and the severity of coronary artery lesions. Our results indicated that the RC levels in the PCAD group were significantly higher than those in the non-PCAD group. Notably, RC did not correlate with traditional cardiovascular risk factors. However, after adjusting for other variables, RC showed a positive correlation with the severity of coronary artery lesions. Therefore, RC demonstrates significant predictive value for the severity of CAD.
Lipid metabolism disorders are significant pathogenic factors in the progression of atherosclerosis, making active lipid control a crucial strategy in preventing and treating CAD. Our study identified lipid metabolism abnormalities in PCAD patients, consistent with the findings of previous research (13). Research indicates that a 1 mmol/L reduction in LDL-C lowers cardiovascular risk by 22%, and maintaining TG levels below 100 mg/dl reduces the likelihood of future MACEs by 50% (14, 15). However, many patients continue to experience recurrent cardiovascular events despite appropriate LDL-C and TG control. Recent genetic and epidemiological studies have increasingly confirmed the association between high RC levels and increased cardiovascular disease risk (16, 17). Fujihara et al. (18) conducted a 38-month follow-up of 247 patients with stable CAD who maintained LDL-C levels below 70 mg/dl, finding that 33 patients experienced cardiovascular events related to RC levels, and RC was identified as a significant predictor of major endpoints (hazard ratio 1.62, 95% confidence interval 1.26–2.07, p < 0.01). A study evaluating 1,716 patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI) found that elevated RC levels (>0.79 mmol/L) were significantly associated with cardiovascular MACEs (19). Quispe et al. (20) analyzed 17,532 individuals without known CAD and found that elevated RC levels were associated with CAD, independent of traditional risk factors such as LDL-C and apolipoprotein B (apo B) levels. Luo et al. (21) discovered that fasting RC is an independent risk factor for in-stent restenosis (ISR) following PCI and a reliable predictor of ISR. In a prospective cohort study, elevated cumulative remnant cholesterol was significantly associated with the risk of developing ischemic heart disease (IHD), indicating consistent monitoring and modulation of RC may be instrumental in the prevention of IHD (22). These findings underscore the need for more comprehensive lipid management strategies that consider RC levels alongside traditional lipid parameters.
Our study found a positive correlation between the severity of coronary artery lesions and RC in PCAD patients, independent of LDL-C levels. This suggests that RC may influence the progression of cardiovascular disease through distinct pathways. Researchers have noted that elevated RC levels are associated with an increased risk of early atherosclerosis and contribute to the pathogenesis of CAD (23–25). Wu et al. (26) discovered that higher concentrations of RC are significantly correlated with the progression of atherosclerosis, and that screening for RC can help assess the extent of coronary atherosclerosis. Unlike LDL-C, RC can penetrate the vessel wall and is directly degraded by scavenger receptors in macrophages without undergoing oxidative modification (27). This leads to the formation of foam cells and promotes the development and progression of atherosclerotic plaques. Additionally, RC also participates in the formation and progression of atherosclerosis by activating monocyte-macrophages and upregulating pro-inflammatory cytokines (28, 29). In a study involving 60,608 patients with IHD, elevated non-fasting RC levels were associated with low-grade inflammation and IHD (30). Liao et al. (31) studied 5,778 CAD patients treated with statins and undergoing PCI, discovering that high residual inflammation and cholesterol risk, assessed by high-sensitivity C-reactive protein and RC were closely related to an increased risk of ischemic events, cardiovascular death, and all-cause mortality. Wu et al. (32) found that higher concentrations of RC are associated with an increased risk of hypertension and type 2 diabetes, potentially mediated by inflammatory responses involving leukocytes, lymphocytes, monocytes, and neutrophils. Given RC's unique role in atherogenesis and inflammation, it represents a potential target for more comprehensive lipid management strategies.
Our study also confirmed the impact of traditional risk factors such as hypertension, diabetes, smoking, and LDL-C on the development of PCAD, while HDL-C may be an independent protective factor against PCAD. In multivariate logistic regression analysis, blood glucose was closely related to the occurrence of PCAD and was an independent risk factor for the severity of coronary artery lesions, which may be associated with different states of glucose metabolism (33). The CAD group with concurrent diabetes represents a special cohort whose residual risk should receive more attention. Wang et al. (34) found that an elevated triglyceride-glucose (TyG) index, which reflects insulin resistance, was associated with an increased risk of multi-vessel CAD, and TyG may be a valuable predictor of CAD severity, particularly in individuals with prediabetes. Another follow-up study involving 4,095 diabetic patients found that RC levels were associated with all-cause and cardiovascular mortality, suggesting that maintaining appropriate RC levels may help reduce mortality risk in diabetic patients (35). In high cardiovascular risk diabetic patients, RC was closely associated with MACEs, independent of traditional risk factors and LDL-C, and its visit-to-visit variability was an independent predictor of adverse cardiovascular events (36). These findings highlight the need for a more comprehensive approach to managing lipid profiles in patients with CAD, particularly those with concurrent diabetes or prediabetes. In clinical practice, in addition to early detection and appropriate management of blood glucose and LDL-C, RC levels should also be actively monitored. Overall, RC can be widely used in clinical settings to identify high-risk individuals and may serve as a potential target for future therapeutic interventions.
The advantage of this study is that it focuses on adult males, a group with a high incidence of CAD, and explores the predictive value of RC for adult male PCAD. However, several limitations should be considered. Firstly, this study is a single-center retrospective study, which may introduce selection bias in the included population. Secondly, the sample size is relatively small, and larger sample sizes are needed to validate the results. Thirdly, we used estimated RC levels, which might not be as accurate as direct measurements, but this approach avoids additional costs in the study. Fourthly, this study did not include left ventricular ejection fraction (LVEF), and the interactive relationship between RC and LVEF with PCAD requires further investigation. Finally, this study did not include dynamic monitoring of RC levels in PCAD patients or follow-up of outpatient endpoint events.
5 Conclusions
RC is an independent risk factor for PCAD in adult men and is closely associated with the severity of coronary artery stenosis, demonstrating good predictive capability. Clinically, RC can be used as one of the parameters for dyslipidemia to optimize lipid-lowering regimens, facilitate early identification of high-risk individuals for CAD, and reduce the residual risk in CAD patients. This provides more accurate and effective monitoring and new prevention strategies for the clinical management of CAD.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Weifang Peoples Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XD: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Visualization. KC: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology, Visualization. XL: Writing – original draft, Writing – review & editing, Formal Analysis, Validation, Visualization. YT: Writing – original draft, Writing – review & editing, Formal Analysis, Visualization. RZ: Conceptualization, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Natural Science Foundation of Shandong Province [ZR2020QH005, ZR2021QH262, ZR2022QH142, ZR2022QH225]; the National Natural Science Foundation of China [82202427, 82202376]; the Science and Technology Development Program of Shandong Second Medical University (2023FYQ007); the Science and Technology Development Program of Weifang [2024YX002, 2024YX003]; and Weifang Yuandu Scholar Young Expert Program of Shandong Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PH declared a past co-authorship with the author(s) RZ to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Rallidis LS Xenogiannis I Brilakis ES Bhatt DL . Causes, angiographic characteristics, and management of premature myocardial infarction: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79(24):2431–49. 10.1016/j.jacc.2022.04.015
2.
Le A Peng H Golinsky D Di Scipio M Lali R Paré G . What causes premature coronary artery disease?Curr Atheroscler Rep. (2024) 26(6):189–203. 10.1007/s11883-024-01200-y
3.
Michos ED Choi AD . Coronary artery disease in young adults: a hard lesson but a good teacher. J Am Coll Cardiol. (2019) 74(15):1879–82. 10.1016/j.jacc.2019.08.1023
4.
Zeitouni M Clare RM Chiswell K Abdulrahim J Shah N Pagidipati NP et al Risk factor burden and long-term prognosis of patients with premature coronary artery disease. J Am Heart Assoc. (2020) 9(24):e017712. 10.1161/JAHA.120.017712
5.
Collet JP Zeitouni M Procopi N Hulot JS Silvain J Kerneis M et al Long-term evolution of premature coronary artery disease. J Am Coll Cardiol. (2019) 74(15):1868–78. 10.1016/j.jacc.2019.08.1002
6.
Malakar AK Choudhury D Halder B Paul P Uddin A Chakraborty S . A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. (2019) 234(10):16812–23. 10.1002/jcp.28350
7.
Shao C Wang J Tian J Tang YD . Coronary artery disease: from mechanism to clinical practice. Adv Exp Med Biol. (2020) 1177:1–36. 10.1007/978-981-15-2517-9_1
8.
Heo JH Jo SH . Triglyceride-rich lipoproteins and remnant cholesterol in cardiovascular disease. J Korean Med Sci. (2023) 38(38):e295. 10.3346/jkms.2023.38.e295
9.
Kexin W Yaodong D Wen G Rui W Jiaxin Y Xiaoli L et al Association of increased remnant cholesterol and the risk of coronary artery disease: a retrospective study. Front Cardiovasc Med. (2021) 8:740596. 10.3389/fcvm.2021.740596
10.
Cordero A Alvarez-Alvarez B Escribano D García-Acuña JM Cid-Alvarez B Rodríguez-Mañero M et al Remnant cholesterol in patients admitted for acute coronary syndromes. Eur J Prev Cardiol. (2023) 30(4):340–8. 10.1093/eurjpc/zwac286
11.
Rampidis GP Benetos G Benz DC Giannopoulos AA Buechel RR . A guide for gensini score calculation. Atherosclerosis. (2019) 287:181–3. 10.1016/j.atherosclerosis.2019.05.012
12.
Wang J Xu Y Liu L Wu W Shen C Huang H et al Comparison of LASSO and random forest models for predicting the risk of premature coronary artery disease. BMC Med Inform Decis Mak. (2023) 23(1):297. 10.1186/s12911-023-02407-w
13.
Sharma SK Makkar JS Bana A Sharma K Kasliwal A Sidana SK et al Premature coronary artery disease, risk factors, clinical presentation, angiography and interventions: hospital based registry. Indian Heart J. (2022) 74(5):391–7. 10.1016/j.ihj.2022.08.003
14.
Weintraub WS Arbab-Zadeh A . Should we adjust low-density lipoprotein cholesterol management to the severity of coronary artery disease?JACC Cardiovasc Imaging. (2020) 13(9):1973–5. 10.1016/j.jcmg.2020.04.007
15.
Bazarbashi N Miller M . Triglycerides: how to manage patients with elevated triglycerides and when to refer?Med Clin North Am. (2022) 106(2):299–312. 10.1016/j.mcna.2021.11.006
16.
Navarese EP Vine D Proctor S Grzelakowska K Berti S Kubica J et al Independent causal effect of remnant cholesterol on atherosclerotic cardiovascular outcomes: a Mendelian randomization study. Arterioscler Thromb Vasc Biol. (2023) 43(9):e373–80. 10.1161/ATVBAHA.123.319297
17.
Wang K Ding Y Wang R Yang J Liu X Han H et al Remnant cholesterol and the risk of coronary artery disease in patients with type 2 diabetes. Angiology. (2023) 74(8):745–53. 10.1177/00033197221121008
18.
Fujihara Y Nakamura T Horikoshi T Obata JE Fujioka D Watanabe Y et al Remnant lipoproteins are residual risk factor for future cardiovascular events in patients with stable coronary artery disease and on-statin low-density lipoprotein cholesterol levels <70 mg/dl. Circ J. (2019) 83(6):1302–8. 10.1253/circj.CJ-19-0047
19.
Shao Q Yang Z Wang Y Li Q Han K Liang J et al Elevated remnant cholesterol is associated with adverse cardiovascular outcomes in patients with acute coronary syndrome. J Atheroscler Thromb. (2022) 29(12):1808–22. 10.5551/jat.63397
20.
Quispe R Martin SS Michos ED Lamba I Blumenthal RS Saeed A et al Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. (2021) 42(42):4324–32. 10.1093/eurheartj/ehab432
21.
Luo Y Cui S Zhang C Huang R Zhao J Su K et al Prognostic role of fasting remnant cholesterol with in-stent restenosis after drug-eluting stent implantation. Int J Gen Med. (2022) 15:1733–42. 10.2147/IJGM.S348148
22.
Zhao X Wang Y Li W Gao H Wu H Yu J et al Cumulative remnant cholesterol as a causal risk factor for ischemic heart disease: a prospective cohort study. Curr Probl Cardiol. (2024) 49(2):102215. 10.1016/j.cpcardiol.2023.102215
23.
Twickler T Dallinga-Thie GM Chapman MJ Cohn JS . Remnant lipoproteins and atherosclerosis. Curr Atheroscler Rep. (2005) 7(2):140–7. 10.1007/s11883-005-0037-x
24.
Sandesara PB Virani SS Fazio S Shapiro MD . The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocrine Reviews. (2019) 40(2):537–57. 10.1210/er.2018-00184
25.
Wang K Wang R Yang J Liu X Shen H Sun Y et al Remnant cholesterol and atherosclerotic cardiovascular disease: metabolism, mechanism, evidence, and treatment. Front Cardiovasc Med. (2022) 9:913869. 10.3389/fcvm.2022.913869
26.
Wu S Su X Zuo Y Chen S Tian X Xu Q et al Discordance between remnant cholesterol and low-density lipoprotein cholesterol predicts arterial stiffness progression. Hellenic J Cardiol. (2023) 74:24–31. 10.1016/j.hjc.2023.05.008
27.
Baratta F Cocomello N Coronati M Ferro D Pastori D Angelico F et al Cholesterol remnants, triglyceride-rich lipoproteins and cardiovascular risk. Int J Mol Sci. (2023) 24(5):4268. 10.3390/ijms24054268
28.
Attiq A Afzal S Ahmad W Kandeel M . Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. (2024) 966:176338. 10.1016/j.ejphar.2024.176338
29.
Elías-López D Doi T Nordestgaard BG Kobylecki CJ . Remnant cholesterol and low-grade inflammation jointly in atherosclerotic cardiovascular disease: implications for clinical trials. Curr Opin Clin Nutr Metab Care. (2024) 27(2):125–35. 10.1097/MCO.0000000000000999
30.
Varbo A Benn M Tybjærg-Hansen A Nordestgaard BG . Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. (2013) 128(12):1298–309. 10.1161/CIRCULATIONAHA.113.003008
31.
Liao J Qiu M Su X Qi Z Xu Y Liu H et al The residual risk of inflammation and remnant cholesterol in acute coronary syndrome patients on statin treatment undergoing percutaneous coronary intervention. Lipids Health Dis. (2024) 23(1):172. 10.1186/s12944-024-02156-3
32.
Wu Y Wei Q Li H Yang H Wu Y Yu Y et al Association of remnant cholesterol with hypertension, type 2 diabetes, and their coexistence: the mediating role of inflammation-related indicators. Lipids Health Dis. (2023) 22(1):158. 10.1186/s12944-023-01915-y
33.
Li J Dong Z Wu H Liu Y Chen Y Li S et al The triglyceride-glucose index is associated with atherosclerosis in patients with symptomatic coronary artery disease, regardless of diabetes mellitus and hyperlipidaemia. Cardiovasc Diabetol. (2023) 22(1):224. 10.1186/s12933-023-01919-z
34.
Wang X Xu W Song Q Zhao Z Meng X Xia C et al Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. (2022) 21(1):168. 10.1186/s12933-022-01606-5
35.
Wang H Guo Y Zhang H Wang X Zheng X . The U-shaped association between remnant cholesterol and risk of all-cause and cardiovascular deaths in diabetic adults: findings from NHANES 1999–2018. Nutr Metab Cardiovasc Dis. (2024) 34(10):2282–8. 10.1016/j.numecd.2024.05.011
36.
Fu L Tai S Sun J Zhang N Zhou Y Xing Z et al Remnant cholesterol and its visit-to-visit variability predict cardiovascular outcomes in patients with type 2 diabetes: findings from the ACCORD cohort. Diabetes Care. (2022) 45(9):2136–43. 10.2337/dc21-2511
Summary
Keywords
premature coronary artery disease, remnant cholesterol, coronary artery lesions, Gensini score, retrospective study
Citation
Dong X, Chen K, Li X, Tang Y, Zhang R and Wang J (2024) Correlation between remnant cholesterol and premature coronary artery disease and the severity of coronary artery lesions in men: a retrospective study. Front. Cardiovasc. Med. 11:1462142. doi: 10.3389/fcvm.2024.1462142
Received
09 July 2024
Accepted
13 November 2024
Published
25 November 2024
Volume
11 - 2024
Edited by
Wilfried Le Goff, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Reviewed by
Panpan Hao, Shandong University, China
Ivica Bosnjak, Osijek Clinical Hospital Center, Croatia
Hongya Han, Capital Medical University, China
Updates
Copyright
© 2024 Dong, Chen, Li, Tang, Zhang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Rui Zhang zhang6969rui@163.com Jian Wang rmyywangjian@wfmc.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.