Abstract
Background:
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited arrhythmia disorder characterized by ventricular arrhythmia triggered by adrenergic stimulation.
Case presentation:
A 9-year-old boy presented with convulsions following physical exertion. Bidirectional ventricular tachycardia (VT) during a treadmill test led to the diagnosis of catecholaminergic polymorphic ventricular tachycardia (CPVT). Genetic testing revealed a pathogenic variant of RYR2:c.720G>A (p.ArG2401His). Nadolol was initially started. However, he experienced aborted VT arrest three years later. Flecainide was thus added as dual therapy and he underwent left cardiac sympathetic denervation (LCSD). Subsequently, a transvenous implantable cardioverter-defibrillator (ICD) was implanted because he still had several episodes of bidirectional VT. Despite a good compliance to medication, the patient still had exercise induced VT episodes with new onset of atrial fibrillation. High dose nadolol was required and amiodarone was added. Despite maximizing the dosage of these three antiarrhythmics, the patient continued to experience multiple episodes of ventricular fibrillation with appropriate ICD shocks and persistent atrial arrhythmias. Right cardiac sympathetic denervation (RCSD) was performed as the last modality of treatment. Patient had a total elimination of VT post bilateral sympathectomy. He remained asymptomatic on follow up. A follow-up treadmill test showed no recurrence of exercise-induced PVCs and VT.
Conclusion:
We illustrated the challenges and the complex decision-making process encountered in managing refractory CPVT. In patients unresponsive to conventional therapies, RCSD in additional to LCSD is a safe and effective alternative treatment. A history of LCSD should not preclude physicians from considering RCSD in children with refractory CPVT.
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited channelopathy characterized by stress-induced bidirectional ventricular tachycardia leading to syncope and sudden cardiac death (1). To reduce the burden of ventricular arrhythmia in CPVT patients, various treatment options have been recommended in the latest European Society of Cardiology guideline in 2022. These include exercise restriction, pharmacological treatments such as β-blockers and flecainide, left cardiac sympathetic denervation (LCSD), and implantable cardioverter-defibrillator (ICD) (1). However, none of these treatments have shown complete efficacy, and in some cases, a combination of these treatments may be necessary to prevent cardiac death. We presented a teenager diagnosed with CPVT who was unresponsive to all of the aforementioned treatment. Finally, he underwent thoracoscopic right cardiac sympathetic denervation (RCSD), resulting in complete suppression of ventricular tachycardia (VT).
Case report
A 9-year-old Pakistani boy had been diagnosed with epilepsy and experienced repeated convulsions since the age of 4. Physical examination was unremarkable. However, subsequent convulsions occurring after physical exertion raised suspicion of arrhythmia syndrome. A stress exercise test revealed bidirectional ventricular tachycardia, leading to a diagnosis of CPVT (Figure 1). A genetic test confirmed the presence of a de novo heterozygous likely pathogenic RYR2 variant (RYR2:c.14861C>G; p.Ala4954Gly). He was started on non-selective beta blocker i.e., nadolol and experienced no syncope or convulsions thereafter. He remained symptom-free for 4 years. However, at the age of 13, he had an episode of aborted cardiac arrest, prompting the optimization of nadolol dosage to 40 mg daily (1.3 mg/kg/day) and the addition of flecainide 100 mg twice daily (6.6 mg/kg/day). LCSD was performed to enhance patient protection. The follow-up treadmill test was negative.
Figure 1
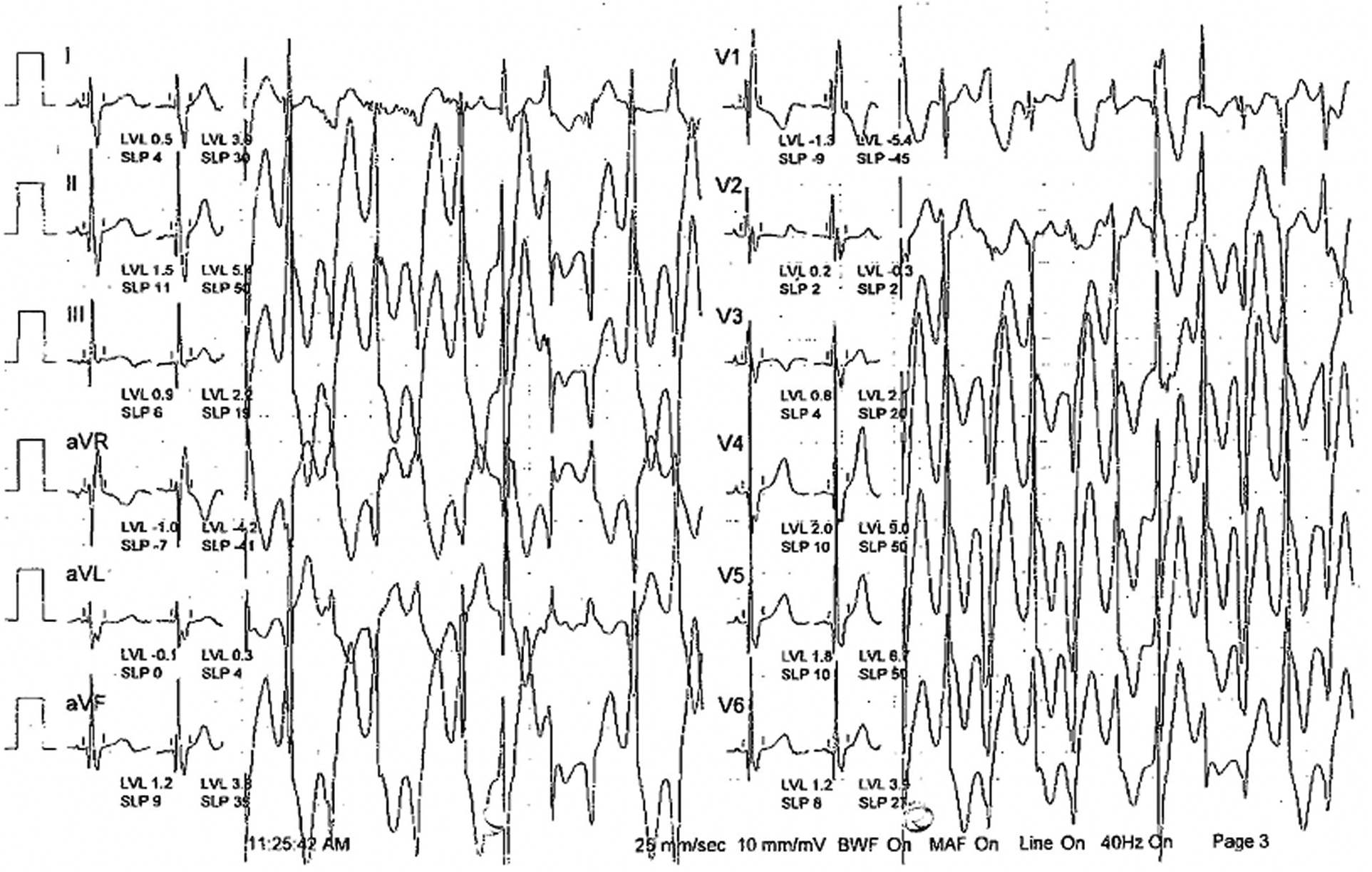
Stress exercise test revealed bidirectional ventricular tachycardia.
Two years following LSCD, the patient experienced syncope while playing boxing, and electrocardiogram (ECG) on ambulance revealed bidirectional ventricular tachycardia (VT), leading to the implantation of a transvenous dual-chamber implantable cardioverter-defibrillator (ICD) as secondary prevention. The ICD programming was set as follows: VT-1 zone was set at 200–230 beat per min (bpm), with a detection interval of 100 s. Six antitachycardia pacing (ATP) therapies were programmed before shock therapy. VT-2 zone was set at 230–260 bpm with the same detection interval. Three ATP therapies would been given before a shock is delivered. Ventricular fibrillation (VF) zone was set at heart rate exceeding 260 bpm, in which an ATP therapy was administered during charging. The rationale behind the VT-1 and VT-2 detection settings and an extended detection period, was to address clinical VT while minimizing the necessity for shocks that could trigger a VT storm. Despite this, patient still received multiple appropriate ICD shocks. ICD interrogation showed multiple episodes of polymorphic VT although the patient remained asymptomatic (Figure 2). ATP and ICD shocks were all ineffective in terminating these VT but they all reverted back to sinus rhythm spontaneously. Defibrillation threshold test was normal. The ICD was reprogrammed as follows to enhance our detection of the slow clinical VT: VT-1 monitoring was set at 176 bpm. VT-2 zone was set at 230–260 bpm, in which three ATP therapies would been given before a shock is delivered. Ventricular fibrillation (VF) zone was set at heart rate exceeding 260 bpm, in which one ATP therapy would be administered before shock delivery. Two years later, during ICD interrogation, the patient was found to have atrial fibrillation with rapid conduction, and the dosage of nadolol and flecainide were increased to 120 mg twice daily (4.5 mg/kg/day) and 150 mg twice daily (5.6 mg/kg/day) respectively. However, atrial fibrillation persisted. Amiodarone 200 mg daily (3.8 mg/kg/day) was added for rhythm control and subsequent treadmill test showed no exercise induced ventricular arrhythmia.
Figure 2
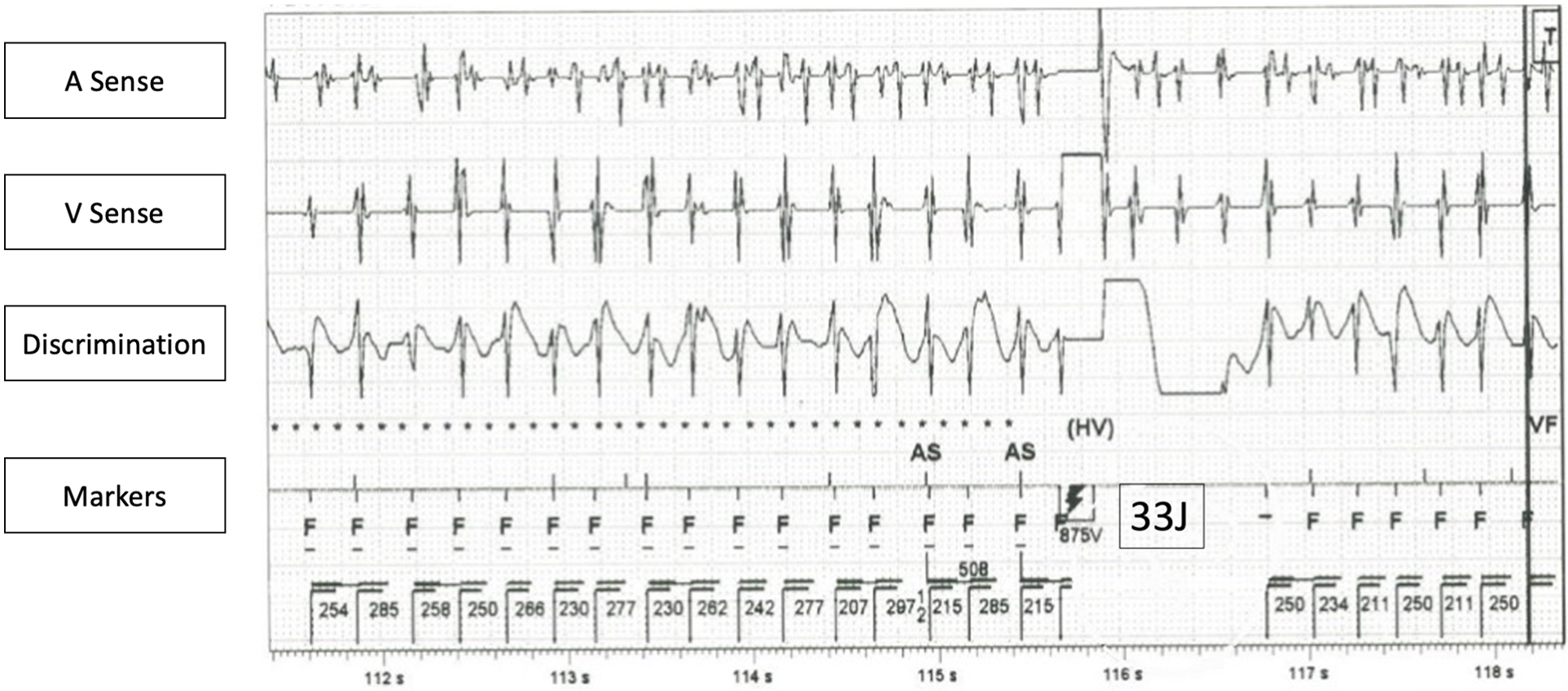
ICD interrogation of a VT episode, which failed to be terminated by ICD shock. (Remarks: Concurrent atrial fibrillation also noted in atrial channel, also failed to terminate by ICD shock).
Despite maximizing medical therapy with three anti-arrhythmic medications, he experienced multiple episodes of VF with appropriate ICD shocks and ongoing atrial arrhythmias. The episodes of VF were triggered either by emotions, dumbbell exercise, or playing computer games. The patient expressed dissatisfaction with adhering to exercise restrictions and taking numerous medications. Therefore, thoracoscopic exploration of left cardiac sympathetic stellate ganglion and right cardiac sympathetic denervation (RCSD) was performed. Intraoperatively, fibrous tissue was found over the left paraspinal region at the T1–T4 level, and no residual neural tissue was seen (Figure 3). The parietal pleura along the left second rib was cauterized. In view of satisfactory left-side denervation, we proceeded to the right-sided operation. Inferior stellate ganglionectomy, T2–T4 sympathectomy, and Kuntz's nerve ablation were performed (Supplementary video). Histopathological examination confirmed the presence of nerve bundles containing ganglion cells on the right side. The procedure was uneventful, and he was discharged on the day after the operation. Patient remained asymptomatic on follow up. He did not receive any ICD shocks and there was no more atrial and ventricular tachycardia detected. A subsequent treadmill test conducted three months after the sympathectomy showed no exercise induced PVCs and VT. The heart rate response was blunted, with a maximal heart rate of 76 beats per min achieved (Table 1). He remained asymptomatic and his antiarrhythmic medications were gradually tapered down. He was on nadolol 80 mg daily and flecainide 100 mg twice daily on 6-months follow-up.
Figure 3
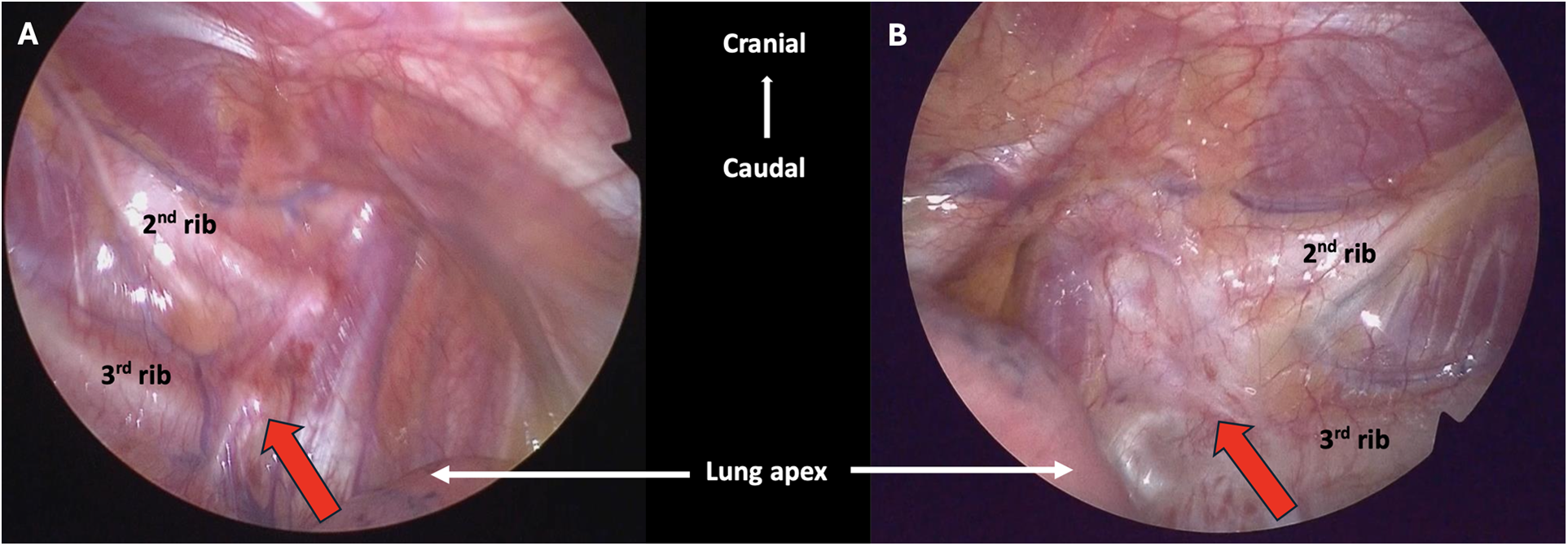
Intraoperative findings during bilateral cardiac sympathectomy. (A) The red arrow pointing to the right sympathetic trunk. (B) The red arrow pointing to the fibrotic left sympathetic trunk (status post LCSD).
Table 1
| Before LCSD | After LCSD | Before RCSD | After RCSD | |
|---|---|---|---|---|
| Treadmill test results | ||||
| Rest HR (bpm) | Sinus rhythm | Sinus rhythm | Sinus rhythm | Atrial paced rhythm |
| 56 bpm | 60 bpm | 73 bpm | 60 bpm | |
| Peak HR (bpm) | VT | Sinus rhythm | Sinus rhythm | Sinus rhythm |
| 179 bpm | 139 bpm | 96 bpm | 76 bpm | |
| Induced arrhythmia | Bidirectional VT | PVCs only, no VT | Occasional PVC at rest, No VT | No PVCs |
| Threshold at onset | 120 bpm | 2 monomorphic PVCs noted at recovery | None during exercise | No PVC VT |
| Medications | Nadolol 40 mg daily | Nadolol 40 mg daily | Nadolol 120 mg twice daily | Nadolol 120 mg twice daily |
| Flecainide 100 mg twice daily | Flecainide 200 mg/150 mg twice daily Amiodarone 200 mg daily | Flecainide 100 mg twice daily Amiodarone 100 mg daily | ||
| Dual chamber ICD programming and interrogation results | ||||
| ICD program | N/A | VT-1 zone: 200–230 bpm, ATP × 6 → shock | VT-1 monitoring: >176 bpm | VT-1 monitoring > 176 bpm |
| VT-2 zone: 230–260 bpm, ATP × 3 → Shock | VT-2 zone: 230–260 bpm, ATP × 3 → Shock | VT-2: 230–260 bpm, ATP × 3 → Shock | ||
| VF zone: >260 bpm, ATP during charging, followed by shock | VF: >260 bpm, ATP × 1 → Shock | VF zone: >260 bpm, ATP × 1 → Shock | ||
| Percentages of atrial and ventricular pacing | Atrial pacing 60% | Atrial pacing 81% | Atrial pacing 70% | |
| Ventricular pacing <1% | Ventricular pacing <1% | Ventricular pacing <1% | ||
Summary of treadmill test results before and after LCSD and RCSD.
ATP, anti-tachycardia pacing; bpm, beat per minute; ICD, implantable cardioverter-defibrillator; LSCD, Left Cardiac Sympathetic Denervation; RSCD, Right Cardiac Sympathetic Denervation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Discussion
CPVT is a rare inherited arrhythmia characterized by the occurrence of bidirectional polymorphic ventricular tachycardia (VT) triggered by the presence of adrenergic stress. The primary approach in managing arrhythmic events in CPVT has traditionally involved the use of β-blockers, which target the underlying catecholaminergic mechanism of the arrhythmia (1). Among the β-blockers, non-selective β-blockers like nadolol and propranolol have been associated with a significant risk reduction of arrhythmic events in children compared to β1-selective β-blockers (2). Regarding the nadolol dosage for CPVT, the recommended daily dose was 2 mg/kg/day, with a median dosage of 1.1 (0.8–1.6) mg/kg/day reported in an international cohort (2). In our case, we attempted to administer nadolol at a notably high dosage of up to 5 mg/kg/day, inspired by its previous use in infants with supraventricular tachycardia (3). Despite this high dosage in our patient, there was no additional benefit observed, and as far as our knowledge extends, there is no supportive evidence for the efficacy of nadolol doses exceeding 2 mg/kg/day in CPVT patients.
In recent studies, flecainide has also demonstrated efficacy in reducing arrhythmic events (4). The mechanism of action of flecainide, whether through modulation of sodium channel-mediated intracellular calcium dynamics or inhibition of the cardiac ryanodine receptor (RYR2), remains a subject of ongoing debate (5). Unfortunately, although these medications show promising effects, none of them offer complete protection, as 9% of patients on flecainide and 13% of patients on β-blockers still experience arrhythmic events (2, 4, 6). In patients refractory to medical treatment, ICD was recommended despite its variable efficacy, which depend on the mechanism of ventricular arrhythmia, tachycardia cycle length and the presence of supraventricular arrhythmia (1, 7). It is important to note that the use of ICD is intended for “secondary prevention” of ventricular arrhythmia only, although it's essential to consider that repetitive shocks could precipitate an arrhythmic storm. For this young gentleman, albeit optimizing all the aforementioned treatments, he still experienced frequent breakthrough VT. This prompted us to consider alternative options, ultimately leading to the decision to perform cardiac sympathectomy.
Cardiac sympathectomy, recognized as a last resort for medically refractory CPVT, involves removal of the lower third to half of the stellate ganglion (T1), together with the thoracic ganglia from T2 to T4 (1, 8). Initially, Moss and McDonald described the use of LCSD in long QT syndrome (LQTS), based on the belief that increased sympathetic activity on the left side contributes to LQTS (9). This sparked interest in the potential antiarrhythmic benefits of LCSD, leading to its application in other life-threatening arrhythmias, including CPVT (8). In recent decades, our understanding of the role of cardiac sympathetic denervation in ventricular arrhythmia has advanced and found that the molecular mechanisms in the final anti-arrhythmic effect was boarder than expected. Specifically, research has revealed the significant involvement of the stellate ganglion in the conduction system and heart contraction, predominantly through ß1-adrenergic receptors and noradrenaline activity. In addition, the stellate ganglion also releases neuropeptide Y (NPY) to modulate sympathetic activity not only by inhibiting acetylcholine release from parasympathetic neurons, but also through NPY receptors present on cardiomyocytes and coronary arteries (10). Consequently, the removal of the stellate ganglion during sympathectomy not only mitigates the decrease in norepinephrine release and counteracts the effects of neuropeptide Y, but also the reduction in sympathetic stimulation. This reduction leads to a decrease in the overall dispersion of repolarization, thereby exerting its antiarrhythmic effects (11, 12). While LSCD has shown effectiveness in reducing arrhythmia burden in CPVT, its recurrence is possible. In the most extensive study on LSCD in CPVT conducted by Ferrari et al., although the findings showed promising outcomes with a reduction in significant cardiac events, 32% of patients still encountered major cardiac events following LSCD (13). Another case series by Hofferbecrth et al., only four out of nine CPVT patients remained free of ICD shocks after LSCD, and similar ineffectiveness was observed in our patient two years post-procedure, leading to the need for ICD implantation (14). Despite being aware of the potential for LSCD failure, upon reviewing the 2017 American Heart Association/American College of Cardiology (ACC)/Heart Rhythm Society and 2022 European Society of Cardiology guidelines on management of ventricular arrhythmias, it is evident that both guidelines endorse LCSD as a last-resort option but do not offer recommendations on handling cases where LCSD has proven ineffective (1, 15). Our patients underwent thorough medical and surgical interventions as per the recommendations in these guidelines; however, he continues to experience breakthrough arrhythmias. Our experience highlights the potential effectiveness of RSCD for patients with resistant CPVT after LCSD attempts.
The inefficacy of LCSD has been theorized to stem from the hypertrophy of the right cardiac sympathetic chain, which can reinnervate areas previously serviced by the surgically excised left ganglia (16). Additionally, it has been described in patients suffering from heart failure and structural heart disease may experience a restructuring of postganglionic sympathetic neurons in the bilateral stellate ganglia due to pronounced abnormal afferent signaling, potentially resulting in severe arrhythmias (17, 18). Considering these factors, bilateral cardiac sympathectomy emerges as a reasonable alternative for patients with medically refractory ventricular arrhythmia. This approach has been supported by a study conducted by Vaseghi et al., which demonstrated a notable decrease in ICD shocks in adults with medically resistant ventricular arrhythmia who underwent bilateral cardiac sympathectomy compared to those who had LSCD. Given that most patients in this research had underlying cardiomyopathy, there was a compelling argument to initiate treatment directly with bilateral cardiac sympathetic denervation rather than LCSD (19). However, this does not diminish in any way the additional value of right cardiac sympathetic denervation in very selected cases of CPVT patients with LCSD refractory arrhythmias, albeit with limited supporting data from a handful of case reports that highlight its safety and efficacy in adult populations (20, 21). In a study conducted by Ertugrul et al., positive outcomes were observed in a group of fourteen children aged 8–19 who underwent bilateral cardiac sympathectomy. Among these children, six patients had CPVT. The study findings indicated that there were no instances of ventricular arrhythmia recurrence in the children with CPVT during the follow-up period (22).
It is important to mention that bilateral cardiac sympathectomy was rarely performed on younger children due to clinician concerns about potential complications like Horner's syndrome, harlequin facial flushing, dry skin, significant bradycardia, or neuropathic pain. Generally, sympathectomy has shown to be a safe procedure in children and pain are usually short-lasting (22). Specifically, clinicians might be apprehensive about the possibility that bilateral cardiac sympathectomy could reduce chronotropic competence and escalate the necessity for atrial pacing (23). On top of that, prior literature has highlighted that the primary innervation of the sinoatrial node originates from cardiac postganglionic sympathetic nerve terminals in the right stellate ganglion (17). Therefore, additional limitation to heart rate increase provided by RCSD on top of LCSD, could explain the effectiveness of RCSD or bilateral cardiac sympathectomy in the very few cases with severe and recurring malignant arrhythmias after LCSD.
Our case study further exemplified the potential benefits of RCSD in CPVT patients underwent LCSD, as it showcased how this procedure can help avoid complications associated with implantable cardioverter-defibrillators (ICDs) and mitigate the side effects of high-dose antiarrhythmic drugs. More importantly, our case also demonstrated that RSCD is a safe procedure, even for children with a history of LSCD, as it does not pose a higher risk in such cases.
Conclusion
For refractory CPVT patients with treatment failure using conventional therapies, RCSD can be considered as a safe and effective alternative treatment. A history of previous LCSD should not deter clinicians from considering RCSD as a potential therapeutic approach for these patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
HL: Software, Writing – original draft, Writing – review & editing, Visualization. SK: Supervision, Writing – original draft, Writing – review & editing. ML: Writing – original draft, Writing – review & editing. KL: Supervision, Writing – original draft, Writing – review & editing. ST: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1477359/full#supplementary-material
References
1.
Zeppenfeld K Tfelt-Hansen J de Riva M Winkel BG Behr ER Blom NA et al 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. (2022) 43(40):3997–4126. 10.1093/eurheartj/ehac262
2.
Peltenburg PJ Kallas D Bos JM Lieve KVV Franciosi S Roston TM et al An international multicenter cohort study on β-blockers for the treatment of symptomatic children with catecholaminergic polymorphic ventricular tachycardia. Circulation. (2022) 145(5):333–44. 10.1161/CIRCULATIONAHA.121.056018
3.
Capponi G Belli G Giovannini M Remaschi G Brambilla A Vannuccini F et al Supraventricular tachycardias in the first year of life: what is the best pharmacological treatment? 24 years of experience in a single centre. BMC Cardiovasc Disord. (2021) 21(1):137. 10.1186/s12872-020-01843-0
4.
Bergeman AT Lieve KVV Kallas D Bos JM Rosés I Noguer F Denjoy I et al Flecainide is associated with a lower incidence of arrhythmic events in a large cohort of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. (2023) 148(25):2029–37. 10.1161/CIRCULATIONAHA.123.064786
5.
Bannister ML Thomas NL Sikkel MB Mukherjee S Maxwell C MacLeod KT et al The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circ Res. (2015) 116(8):1324–35. 10.1161/CIRCRESAHA.116.305347
6.
Mazzanti A Kukavica D Trancuccio A Memmi M Bloise R Gambelli P et al Outcomes of patients with catecholaminergic polymorphic ventricular tachycardia treated with β-blockers. JAMA Cardiol. (2022) 7(5):504. 10.1001/jamacardio.2022.0219
7.
Miyake CY Webster G Czosek RJ Kantoch MJ Dubin AM Avasarala K et al Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ Arrhythm Electrophysiol. (2013) 6(3):579–87. 10.1161/CIRCEP.113.000170
8.
Odero A Bozzani A De Ferrari GM Schwartz PJ . Left cardiac sympathetic denervation for the prevention of life-threatening arrhythmias: the surgical supraclavicular approach to cervicothoracic sympathectomy. Heart Rhythm. (2010) 7(8):1161–5. 10.1016/j.hrthm.2010.03.046
9.
Moss AJ McDonald J . Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. N Engl J Med. (1971) 285(16):903–4. 10.1056/NEJM197110142851607
10.
Tan CMJ Green P Tapoulal N Lewandowski AJ Leeson P Herring N . The role of neuropeptide Y in cardiovascular health and disease. Front Physiol. (2018) 9:1281. 10.3389/fphys.2018.01281
11.
Dusi V De Ferrari GM Pugliese L Schwartz PJ . Cardiac sympathetic denervation in channelopathies. Front Cardiovasc Med. (2019) 6:27. 10.3389/fcvm.2019.00027
12.
Dusi V De Ferrari GM Schwartz PJ . There are 100 ways by which the sympathetic nervous system can trigger life-threatening arrhythmias. Eur Heart J. (2020) 41(23):2180–2. 10.1093/eurheartj/ehz950
13.
De Ferrari GM Dusi V Spazzolini C Bos JM Abrams DJ Berul CI et al Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation. (2015) 131(25):2185–93. 10.1161/CIRCULATIONAHA.115.015731
14.
Hofferberth SC Cecchin F Loberman D Fynn-Thompson F . Left thoracoscopic sympathectomy for cardiac denervation in patients with life-threatening ventricular arrhythmias. J Thorac Cardiovasc Surg. (2014) 147(1):404–11. 10.1016/j.jtcvs.2013.07.064
15.
Al-Khatib SM Stevenson WG Ackerman MJ Bryant WJ Callans DJ Curtis AB et al 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. Circulation. (2018) 138(13):e226–7. 10.1161/CIR.0000000000000549
16.
Ajijola OA Vaseghi M Mahajan A Shivkumar K . Bilateral cardiac sympathetic denervation: why, who and when?Expert Rev Cardiovasc Ther. (2012) 10(8):947–9. 10.1586/erc.12.93
17.
Li YL . Stellate ganglia and cardiac sympathetic overactivation in heart failure. IJMS. (2022) 23(21):13311. 10.3390/ijms232113311
18.
Shivkumar K Ajijola OA Anand I Armour JA Chen PS Esler M et al Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol (Lond). (2016) 594(14):3911–54. 10.1113/JP271870
19.
Vaseghi M Gima J Kanaan C Ajijola OA Marmureanu A Mahajan A et al Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm. (2014) 11(3):360–6. 10.1016/j.hrthm.2013.11.028
20.
Scott PA Sandilands AJ Morris GE Morgan JM . Successful treatment of catecholaminergic polymorphic ventricular tachycardia with bilateral thoracoscopic sympathectomy. Heart Rhythm. (2008) 5(10):1461–3. 10.1016/j.hrthm.2008.07.007
21.
Okajima K Kiuchi K Yokoi K Teranishi J Aoki K Shimane A et al Efficacy of bilateral thoracoscopic sympathectomy in a patient with catecholaminergic polymorphic ventricular tachycardia. J Arrhythm. (2016) 32(1):62–6. 10.1016/j.joa.2015.07.002
22.
Ertugrul I Donmez YN Aydın A Aykan HH Sel K Uysal S et al Bilateral thoracoscopic sympathectomy for cardiac denervation in pediatric population: does kuntz nerve cauterization have an impact on success? J Card Surg. (2021) 36(8):2705–13. 10.1111/jocs.15652
23.
Dusi V Sorg JM Gornbein J Gima J Yanagawa J Lee JM et al Prognostic impact of atrial rhythm and dimension in patients with structural heart disease undergoing cardiac sympathetic denervation for ventricular arrhythmias. Heart Rhythm. (2020) 17(5):714–20. 10.1016/j.hrthm.2019.12.007
Summary
Keywords
catecholaminergic polymorphic ventricular tachycardia (CPVT), right cardiac sympathetic denervation, left cardiac sympathetic denervation, bilateral cardiac sympathectomy, paediatric, case report
Citation
Leung H-T, Kwok S-Y, Lau M, Lee LK-F and Tsao S (2024) Case Report: The unrelenting journey—successful resolution of catecholaminergic polymorphic ventricular tachycardia (CPVT) through right cardiac sympathetic denervation in a teenager after left cardiac sympathetic denervation. Front. Cardiovasc. Med. 11:1477359. doi: 10.3389/fcvm.2024.1477359
Received
07 August 2024
Accepted
25 November 2024
Published
13 December 2024
Volume
11 - 2024
Edited by
Inga Voges, University Medical Center Schleswig-Holstein, Germany
Reviewed by
Roman A. Gebauer, Leipzig University, Germany
Veronica Dusi, University of Turin, Italy
Updates
Copyright
© 2024 Leung, Kwok, Lau, Lee and Tsao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Sit-Yee Kwok sityeekwok@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.