Abstract
Introduction:
Pediatric cardiac surgery with cardiopulmonary bypass (CPB) carries substantial risks of postoperative organ dysfunction and mortality, making the identification of biomarkers for postoperative organ dysfunction crucial for enhancing patient outcomes. As neutrophils play a major role in the perioperative setting and act as double-edge swords to the host, we examined neutrophil transcriptomic profiles in pediatric patients undergoing cardiac surgery with CPB.
Methods:
We enrolled into this study from May 31, 2022, to February 22, 2023.
Results:
32% developed postoperative complications, mainly in the respiratory and cardiovascular systems. Patients in the complication group showed higher PELOD-2 scores on postoperative day 2. These patients experienced longer duration of mechanical ventilation and extended ICU and hospital stays. RNA sequencing of neutrophils revealed significant changes in gene expression after CPB, with the myo5c gene emerging as a key downregulated transcript. Its expression was inversely correlated with PELOD-2 score, IL-6 levels, and markers of neutrophil and platelet activation. Furthermore, myo5c-knockout HL60 cells exhibited enhanced neutrophil extracellular traps (NETs) formation upon stimulation, supporting a potential regulatory role for myo5c in neutrophil activation and systemic inflammation.
Discussion:
While myo5c was not an independent predictor of complications, its expression was consistently associated with clinical severity, suggesting it may serve as a useful biomarker for early risk stratification of postoperative complications in this vulnerable pediatric population.
Introduction
Cardiac surgery for patients with congenital heart diseases (CHDs) is notably at high-risk of organ injury and mortality. Particularly neonates and infants undergoing cardiac surgery remain associated with the highest morbidity and mortality due to organ injury/failure (in-hospital mortality of 6.9%) (1, 2). In our previous investigation, neonates/infants had the highest rate (17.1%) of thrombosis and multiple organ dysfunction with the mortality rate of 4.3% (3). Identifying predictive markers for postoperative outcomes and developing intervention strategies is crucial for improving patient care.
Neutrophils, as first-line immune responders, are responsible for both microbial clearance and facilitating tissue repair (4). Thus, they are highly activated in the perioperative setting. While this activation is essential for immune defense, it can also paradoxically contribute to organ injury (5). Neutrophil extracellular traps (NETs), one of the strategies for neutrophils to eradicate pathogens (6), can contribute to microvascular thrombosis and organ injury (7, 8), complicating the postoperative recovery process.
This study focused on neutrophil transcriptomic profiles in pediatric patients undergoing cardiac surgery with cardiopulmonary bypass (CPB). The aim was to determine specific genetic markers that could serve as indicators of postoperative organ injury. The ultimate goal was to enhance postoperative management in pediatric cardiac surgery, mitigate the risk of organ injury, and improve overall patient outcomes.
Method
Study design
This prospective cohort study was conducted at a quaternary academic pediatric medical center. The study was approved by the Institutional Review Board of Boston Children's Hospital (Protocol number IRB-P00033314, approval date 02/18/2020). Written informed consent for participation was obtained from a parent or legal guardian.
Patient selection and perioperative course
All patients scheduled for CHD with CPB at our center were considered for inclusion. We excluded patients who had active infections, were on chronic steroid therapy, had immunodeficiencies, HIV, or a history of malignancy. The study period spanned from May 31, 2022, to February 22, 2023. Included patients received general anesthesia with endotracheal intubation and had arterial and central venous lines placed. Following surgical dissection, they were heparinized and cannulated for CPB. The CPB circuit was primed with red blood cells (RBC) and fresh frozen plasma (FFP) to maintain a hematocrit level above 30%, following our institutional protocol. RBC priming was selectively applied to patients with anticipated hemodilution risk, particularly those under 10 kg. All transfused RBC units were pre-storage leukocyte-depleted and used within 7 days of collection to minimize storage-related inflammatory activation. Circulatory arrest, regional perfusion, temperature management, and modified ultrafiltration (MUF) were used as needed. Platelet and cryoprecipitate were administered for non-surgical microvascular bleeding. Typically, neonates and infants did not receive FFP post-CPB in our institution. At the conclusion of surgery, patients were either extubated or remained intubated based on the anesthesiologist's decision and transferred to the ICU for further care.
Data collection
For the analysis of clinical data, we extracted a comprehensive range of data from electronic medical records. This included demographic details, comorbidities, diagnoses, surgical procedures, laboratory results, specifics of CPB, and details of postoperative complications. Additionally, data on mechanical ventilatory support, vital signs, and the duration of ICU and hospital stays were collected. It has been increasingly recognized that morbidity and mortality in the ICU are due to multiple organ failure (9). Frequently used methods to assess pediatric severity of illness in the pediatric ICUs include the Pediatric Logistic Organ Dysfunction score (PELOD), the Pediatric Risk of Mortality score (PRISM III), and the Pediatric Index of Mortality score (PIM2) (10–13). The comparison of the three scoring systems for mortality prediction demonstrated that the receiver operating characteristic curve (ROC) was 0.98 for PELOD (12), 0.900 for PIM-2 (11) and 0.82 for PRISM3 (9). In addition, PELOD has been used widely in pediatric CHD population (14). Accordingly, we used PELOD in this study to score organ function. PELOD-2 score was manually calculated as previously described (15). We also utilized the Society of Thoracic Surgeons—European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Categories (STAT Mortality Categories) to examine the risk of morbidity associated with surgical procedures. Postoperative complication was defined as organ dysfunction/injury or thrombosis. We adopted the criteria for organ dysfunction as previously described in pediatric critical illness (16) along with PELOD-2 as described above. Thrombosis was defined as the presence of any vascular thrombosis detected using ultrasound diagnostic imaging.
Blood sample collection
Blood samples were drawn from patients via existing central venous catheters at two distinct time points: (1) immediately after the induction of anesthesia, serving as the baseline, and (2) upon admission to the ICU. The volume of blood collected at each time point adhered to the guidelines specified in the “Protection of Human Subjects” document, totaling 1.8 ml for both collections (900 µl each). After sample collection, blood was immediately transported to the laboratory for assays. For each time point, 100 µl was used for flow cytometry analysis, and 800 µl was subjected to plasma and neutrophil purification. Flow cytometry analysis and RNA sequencing experiment methods are included in Supplementary document.
Inflammatory cytokine measurement
Plasma cytokine levels, including TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, and IL-13, were measured using V-plex proinflammatory panel 1 human kit (Meso Scale Discovery; Gaithersburg, Maryland), in accordance with the manufacturer's protocol.
Statistical analysis
For the analysis of clinical data, we utilized IBM SPSS Statistics, Version 30.0 (IBM Corp., NY, USA). Categorical variables were presented as numbers and percentages, while continuous variables were summarized as means and standard deviations for normally distributed variables, or medians and interquartile ranges for skewed distributions. The normality of variables was assessed using the Shapiro–Wilk test. Categorical variables were compared using chi-square test, Fisher exact test, or Kruskal–Wallis test as appropriate. Continuous variables were analyzed using Student's t test or Mann–Whitney U test, depending on their distribution. Logistic regression models were constructed to assess whether the association between myo5c expression (at baseline or ICU admission) and postoperative complications was confounded by clinical variables. Variables with a p-value <0.05 in univariable analysis were included in the models as potential confounders. For correlation analysis, Pearson or Spearman correlation coefficient was determined based on the normality of the data. Laboratory data were analyzed as outlined in the corresponding figure legends. Statistical significance was set at p value < 0.05. All statistical analyses were performed using Prism 10 software (GraphPad Software, La Jolla, CA, USA).
Results
Pediatric patients undergoing cardiac surgery demonstrated a high incidence of postoperative complications
We enrolled 50 patients undergoing congenital cardiac surgery with CPB between May 31, 2022–February 22, 2023. Table 1 presents the demographic data and baseline characteristics of the patient cohort. Median age was 5.8 months with 42% being male.
Table 1
| Covariates | All patients |
|---|---|
| (n = 50) | |
| Gender: male | 21 (42.0) |
| Age (mo) | 5.8 (1.3,24.2) |
| Body weight (kg) | 6.9 (3.9,12.1) |
| ASA classification | |
| 3 | 10 (20.0) |
| 4 | 40 (80.0) |
| Diagnosis | |
| Septal defect | 4 (8.0) |
| IAA/aortic arch hypoplasia | 8 (16.) |
| TOF | 2 (4.0) |
| PA/PS | 2 (4.0) |
| Aortic valvular disease | 4 (8.0) |
| Complete AVSD | 3 (6.0) |
| DORV | 3 (6.0) |
| HLHS | 5 (10.0) |
| Single ventricle, other | 3 (6.0) |
| Mitral valvular disease | 2 (4.0) |
| PVS | 3 (6.0) |
| TGA | 3 (6.0) |
| Conduit failure | 2 (4.0) |
| Coarctation of aorta | 1 (2.0) |
| CcTGA | 2 (4.0) |
| Other | 3 (6.0) |
| Associated disease | |
| Down syndrome | 3 (6.0) |
| Cerebrovascular disease | 4 (8.0) |
| Tracheo/broncho/laryngomalacia | 3 (6.0) |
| Lung hypoplasia | 2 (4.0) |
| DiGeorge syndrome | 1 (2.0) |
| Heterotaxy syndrome | 2 (4.0) |
| Other | 2 (4.0) |
| STAT mortality category | |
| 1 | 13 (26.0) |
| 2 | 18 (36.0) |
| 3 | 8 (16.0) |
| 4 | 8 (16.0) |
| 5 | 3 (6.0) |
Demographic and clinical characteristics of all patients.
In the table, data is presented as median (interquartile range), or number (percentage). The abbreviations in the Table are as follow.
AVSD, atrioventricular septal defect; ccTGA, congenitally corrected transposition of the great arteries; CPB, cardiopulmonary bypass; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; MUF, modified ultrafiltration; PA, pulmonary atresia; PS, pulmonary stenosis; PVS, pulmonary venous stenosis; RBC, red blood cells; STAT, society of thoracic surgeons-European association for cardio-thoracic surgery congenital heart surgery mortality categories; TGA, transposition of the great arteries; TOF, tetralogy of fallot.
Among these patients, 16 individuals (32%) developed postoperative complications (Table 2). These complications were predominantly respiratory (n = 10, 20%) and cardiovascular in nature (n = 9, 18%). Notably, eight patients (16%) exhibited multi-system involvement, indicating the complexity of these complications. A significantly higher proportion of patients with complications were in STAT mortality category ≥3 (62.5% vs. 26.5%, p = 0.027). RBC transfusion during CPB were administered to 37.5% of patients in the complication group (6 of 16) and 29.4% in the non-complication group (10 of 34) (p = 0.57). Postoperative lactate levels tended to be higher in patients with complications (4.1 vs. 2.8), with the difference approaching statistical significance (p = 0.055) (Table 2). Additionally, patients with complications had remarkably longer periods of mechanical ventilation (71.0 h vs. 28.0 h, p = 0.013), extended ICU stays (166.0 h vs. 72.0 h, p < 0.0001), and prolonged hospital stays (14.9 days vs. 8.9 days, p = 0.01). These patients exhibited higher PEdiatric Logistic Organ Dysfunction-2 (PELOD-2) scores on postoperative day 2 (4.5 vs. 2.0, p < 0.001), indicating a greater overall severity of organ dysfunction (Table 3).
Table 2
| Covariates | All patients | Complication | No complication | P value |
|---|---|---|---|---|
| (n = 50) | (n = 16) | (n = 34) | ||
| Age (mo) | 5.8 (1.3, 24.2) | 4.0 (1.4, 16.4) | 5.8 (0.8, 28.4) | 0.64 |
| STAT mortality category ≥3 | 19 (38.0) | 10 (62.5) | 9 (26.5) | 0.027* |
| ASA classification | ||||
| 3 | 10 (20.0) | 2 (12.5) | 8 (23.5) | 0.47 |
| 4 | 40 (80.0) | 14 (87.5) | 26 (76.5) | |
| Preoperative mechanical ventilation | 13 (26.0) | 3 (18.8) | 10 (29.4) | 0.51 |
| Operative time (min) | 356.0 (309.5, 461.0) | 386.0 (320.5, 531.0) | 353.0 (311.3, 453.8) | 0.16 |
| CPB time (min) | 170.0 (134.0, 252.5) | 235.0 (143.0, 292.0) | 170.5 (143.5, 232.3) | 0.18 |
| Aortic cross-clamp time (min) | 117.0 (82.5, 161.5) | 119.0 (72.5, 185.5) | 116.5 (85.5, 156.3) | 0.84 |
| Circulatory arrest use | 11 (22.0) | 6 (37.5) | 5 (14.7) | 0.052 |
| Regional perfusion | 12 (24.0) | 4 (25.0) | 8 (23.5) | 0.33 |
| MUF | 36 (72.0) | 24 (70.6) | 12 (75.0) | 0.29 |
| RBC transfusion during CPB (no. of patient) | 16 (32.0) | 6 (37.5) | 10 (29.4) | 0.57 |
| RBC transfusion during CPB (ml/kg) | 0 (0, 8.2) | 0 (0, 21.8) | 0 (0, 8.2) | 0.62 |
| Postoperative RBC transfusion (no. of patient) | 18 (36.0) | 7 (43.8) | 11 (32.4) | 0.43 |
| Postoperative RBC transfusion (ml/kg) | 0 (0, 120) | 0 (0, 15.4) | 0 (0, 14.3) | 0.55 |
| Postoperative lactate | 2.4 (1.8, 4.1) | 4.1 (3.0, 6.3) | 2.8 (2.3, 3.6) | 0.055 |
The comparison of demographic and perioperative characteristics between patients with and without complications.
In the table, data is presented as median (interquartile range) for continuous variable, or number (percentage) for categorical variable. For continuous variable, statistical analysis was performed using Mann–Whitney U test. For categorical variable, chi-square test was used.
The abbreviations in the table are as follow.
CPB, cardiopulmonary bypass; MUF, modified ultrafiltration; RBC, red blood cells; STAT, society of thoracic surgeons-European association for cardio-thoracic surgery congenital heart surgery mortality categories.
P < 0.05 was considered significant.
Table 3
| Covariates | All patients | Complication | No complication | P value |
|---|---|---|---|---|
| (n = 50) | (n = 16) | (n = 34) | ||
| Duration of mechanical ventilation (h) | 44.4 (20.7, 74.8) | 71.0 (27.5, 187.3) | 28.0 (20.4, 68.7) | 0.013 |
| ICU stay (h) | 93.5 (48.0, 162.3) | 166.0 (92.0, 323.0) | 72.0 (44.8, 127.5) | <0.0001* |
| Hospital stay (d) | 9.9 (8.0, 19.0) | 14.9 (11.9, 32.8) | 8.9 (6.0, 12.0) | 0.001* |
| Number of complication | ||||
| ≥2 | 8 (16.0) | - | - | - |
| 1 | 8 (16.0) | |||
| 0 | 34 (68.0) | |||
| Type of complication | ||||
| Respiratory dysfunction | 10 (20.0) | - | - | - |
| Cardiovascular dysfunction | 9 (18.0) | |||
| Neurologic dysfunction | 3 (6.0) | |||
| Coagulopathy | 3 (6.0) | |||
| Renal dysfunction | 0 (0) | |||
| Hepatic dysfunction | 0 (0) | |||
| Thrombosis | 2 (4.0) | |||
| PELOD-2 score | ||||
| Postoperative day 1 | 5 (5, 5) | 3.5 (3.0, 5.0) | 5.0 (5.0, 5.0) | 0.001* |
| Postoperative day 2 | 2 (2, 3) | 4.5 (3.0, 5.8) | 2.0 (2.0, 2.0) | <0001* |
| In-hospital death | 1 (2.0) | 1 (6.3) | 0 (0) | - |
The profile of postoperative outcomes after pediatric cardiac surgery.
In the table, data is presented as median (interquartile range) for continuous variable, or number (percentage) for categorical variable. For continuous variable, statistical analysis was performed using Mann–Whitney U test. For categorical variable, chi-square test was used.
The abbreviations used in the table are as follow.
ICU, intensive care unit; PELOD-2, pediatric logistic organ dysfunction-2.
P < 0.05 was considered significant.
RNA sequencing analysis demonstrated inflammatory responses and cellular stress in neutrophils in the early postoperative period following congenital cardiac surgery with CPB
Neutrophils are the first responders to inflammation/ stress during the perioperative phase, exhibiting an increase in numbers and activation in response to surgical triggers and associated inflammatory stimuli. Given this physiological response, we posited that variations in neutrophil RNA transcription might distinguish patients with postoperative complications from those without. To test this hypothesis, we performed RNA sequencing (RNA-seq) analysis of neutrophils, both at baseline and upon admission to the ICU.
In our RNA-seq analysis, we utilized the Uniform Manifold Approximation and Projection (UMAP) technique to visualize the complex gene expression data in high dimensions. The resulting UMAP plot (Figure 1A) distinctly delineated clusters between the baseline and ICU admission time points, indicating significant alterations in RNA expression patterns attributed to the cardiac surgery and CPB procedure.
Figure 1
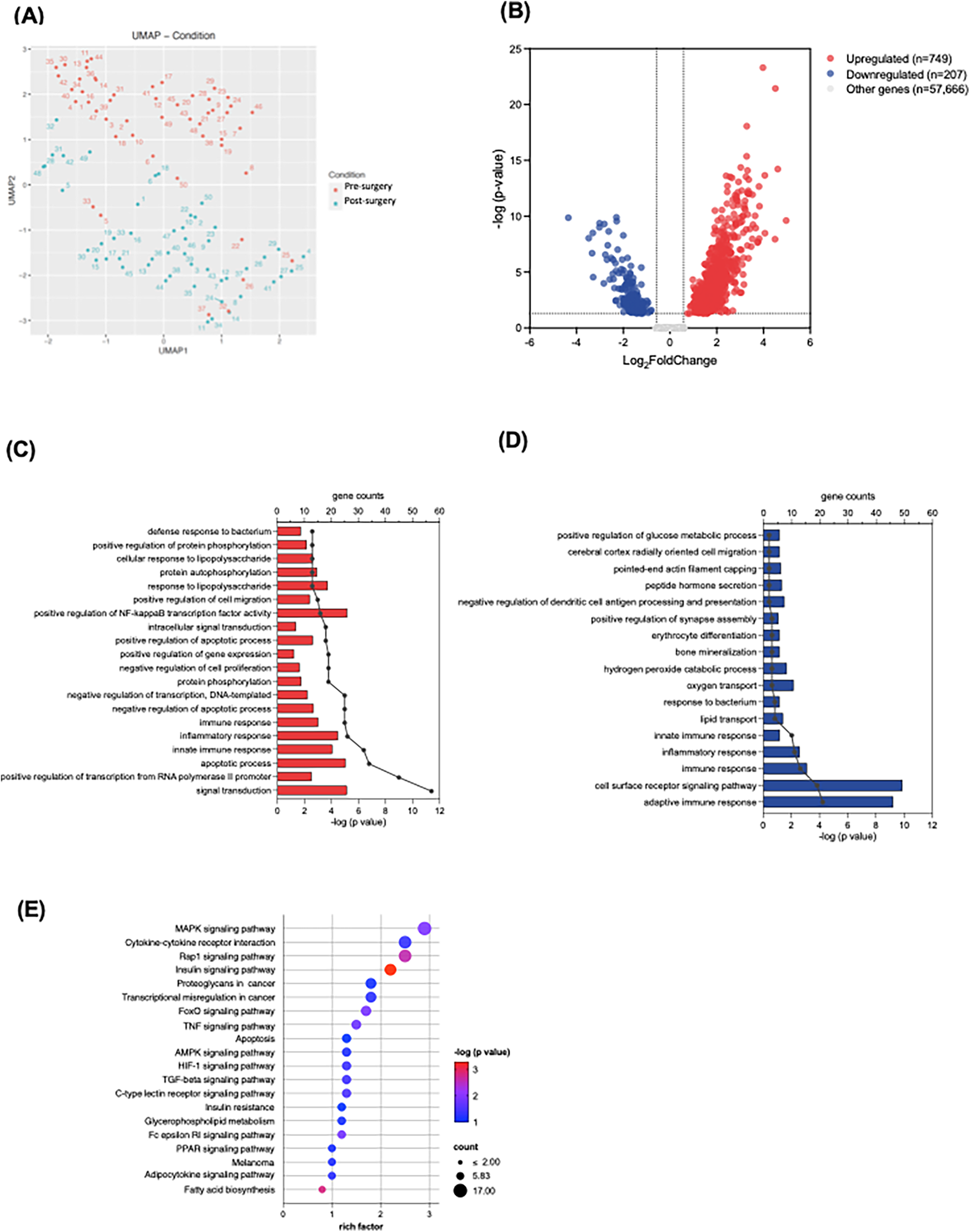
RNA sequencing analysis of neutrophils at baseline and upon ICU admission. (A) UMAP plot demonstrates clusters between the baseline (blue) and ICU admission (red) time points. (B) The volcano plot shows upregulated and downregulated genes; the dotted line represents the threshold of differential gene screening criteria. (C,D) Gene ontology (GO) of biological process enrichment analysis of top 20 significantly upregulated (C) and downregulated (D) differentially expressed genes (DEGs). Bar plots represent –log (adjusted p value) calculated using the Benjamini-Hochberg correction. Line and dotted plots represent gene counts for the corresponding GO terms. (E) KEGG pathway enrichment analysis of upregulated DEGs. Rich factor refers to the ratio of the number of DEGs to the number of total annotated genes in the pathway. The point color represents –log (adjusted p value) using the Benjamini-Hochberg correction, and the point size represents the number of DEG mapped to the reference pathway.
The bulk RNA-seq analysis identified a total of 58,622 transcripts. We used criteria of an absolute log2 fold change >0.58 and a statistically significant adjusted p-value < 0.05 to determine DEGs at ICU admission compared to baseline. The volcano plot illustrated the 749 upregulated and 207 downregulated DEGs (Figure 1B). Further exploration on these DEGs included GO enrichment analysis, primarily focusing on GO Biological Processes (GO-BP). Within the top 20 enriched GO-BP terms associated with upregulated DEGs, we observed notable enrichment in various inflammatory processes, such as apoptotic processes, innate immune responses, and the regulation of nuclear factor-kappa B (NFκB) transcription factor activity (Figure 1C). Conversely, the adaptive immune response appeared to be downregulated in the early postoperative period (Figure 1D). Our KEGG pathway analysis further demonstrated the upregulated DEGs involved several inflammatory signaling pathways and apoptosis, encompassing pathways such as mitogen-activated protein kinase (MAPK), Ras-associated protein-1 (Rap 1), hypoxia-inducible factor (HIF)-1 and tumor necrosis factor (TNF) signaling pathways (Figure 1F). These findings substantiate the presence of robust inflammatory responses in the neutrophils during the early postoperative period following congenital cardiac surgery with CPB.
Neutrophil myo5c gene was downregulated in patients exhibiting high PELOD-2 scores on postoperative day 2
To delineate the association between neutrophil mRNA expression and postoperative complications, we employed the PELOD-2 score as a surrogate marker for postoperative complications, capitalizing on its well-established correlation with pediatric organ dysfunction and its ability to provide a broader range for a more nuanced understanding of complications (15). Among several candidate genes identified from the RNA sequencing dataset, myo5c was the only transcript that consistently correlated with indicators of postoperative organ dysfunction, including PELOD-2 scores. Although the scores were assessed for both postoperative days 1 and 2, the association with myo5c expression was more pronounced and statistically significant on day 2, which was therefore prioritized in our analysis. Our analysis showed a notable reduction in myo5c expression at a PELOD-2 score on postoperative day 2 of 6, with levels also lower at a score of 7 (Figure 2A). Additionally, we demonstrated a strong negative correlation between myo5c expressions upon ICU admission and PELOD-2 scores on postoperative day 2 (r = −0.6393, p < 0.0001) (Figure 2B). Myo5c expression also negatively correlated with the ICU length of stay (r = −0.3030, p = 0.0325) (Figure 2C). The receiver operating characteristic (ROC) curve showed an acceptable discriminatory ability of myo5c expression upon ICU admission in relation to PELOD-2 score on postoperative day 2 (AUROC = 0.6754, 95% CI 0.56–0.79, p = 0.0025) (Supplementary Figure S2).
Figure 2
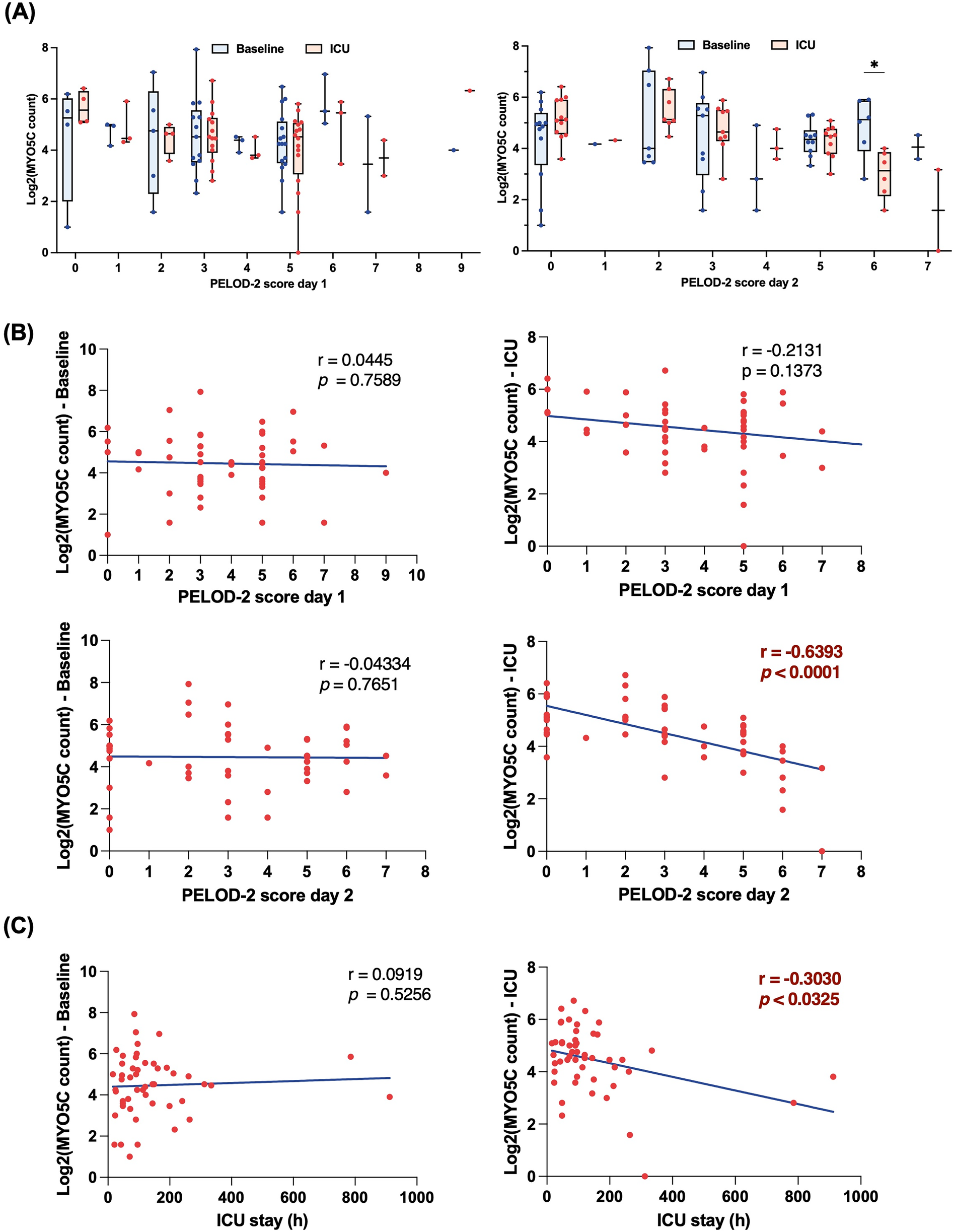
Downregulation of myo5c gene expression in neutrophil is associated with high PELOD-2 score on postoperative day 2. (A) Comparison of myo5c gene expression in neutrophil between baseline (blue) and upon ICU admission (red) at different PELOD-2 score on postoperative day 1 (left panel) and day 2 (right panel). Each dot indicates raw data. Data are presented as median (IQR). Statistical analysis was performed using mixed-effect model. The Bonferroni correction was applied to adjust p-values for multiple comparisons. (B) Correlation analyses between myo5c gene expression in neutrophil and PELOD-2 score at different time point. Statistical analysis was performed using Spearman correlation analysis between myo5c expression levels and PELOD-2 score. Spearman correlation analysis was used, and correlation coefficients (r) and corresponding p-values are shown. (C) Correlation analyses between myo5c gene expression in neutrophil and ICU stay (h) Statistical analysis was performed using Spearman correlation analysis between myo5c expression levels and ICU stay (h) Spearman correlation analysis was used, and correlation coefficients (r) and corresponding p-values are shown.
We further assessed whether myo5c expression declined after surgery by comparing baseline and ICU admission levels within each group. No significant within-group differences were found in either the complication or non-complication group (Supplementary Figure S3). Additionally, logistic regression models were constructed to assess whether the association between myo5c expression and postoperative complications was confounded by other clinical variables. In all models, only STAT category ≥3 emerged as a significant independent predictor of complications, while myo5c expression was not statistically significant (Supplementary Tables S1, S2).
In addition, we performed subgroup analyses to determine whether myo5c expression was influenced by lesion type or surgical complexity. Patients were stratified by cyanotic vs. acyanotic CHD, as well as by STAT mortality category. No significant differences in myo5c expression were observed between cyanotic and acyanotic groups at either baseline or ICU admission (Supplementary Figure S4). Similarly, no consistent trend was identified across the full range of STAT categories (1–5) (Supplementary Figure S5). In contrast, when patients were grouped by STAT mortality category <3 vs. ≥3, myo5c expression at ICU admission showed a downward trend in the STAT ≥3 group, though this did not reach statistical significance (Supplementary Figure S6).
However, given the limited sample size in higher STAT categories, these results are considered exploratory and require validation in larger, stratified cohort.
Myo5C gene expression was negatively correlated with IL-6 release, neutrophil and platelet activations
To further elucidate the association between myo5c expression and the inflammatory response, we conducted an analysis examining the correlation of myo5c expression levels with various parameters, including cytokine release, neutrophil and platelet activation, as well as neutrophil and platelet counts.
Our analysis revealed a statistically significant negative correlation between myo5c expression and organ injury marker, IL-6 levels upon ICU admission (r = −0.4993, p = 0.0094) (Figure 3). Additionally, elevated myo5c expression was significantly associated with less activation of both neutrophils (at T2, m24 vs. myo5c, r = −0.4284, p + 0.0024) and platelets (at T2, PAC1 vs. myo5c, r = −0.4190, p = 0.0027) (Figure 4A). However, neutrophil and platelet counts did not exhibit a statistically significant correlation (Figure 4B). These findings supported the hypothesis that myo5c potentially play a substantial role in the regulation of systemic inflammatory response following CPB.
Figure 3
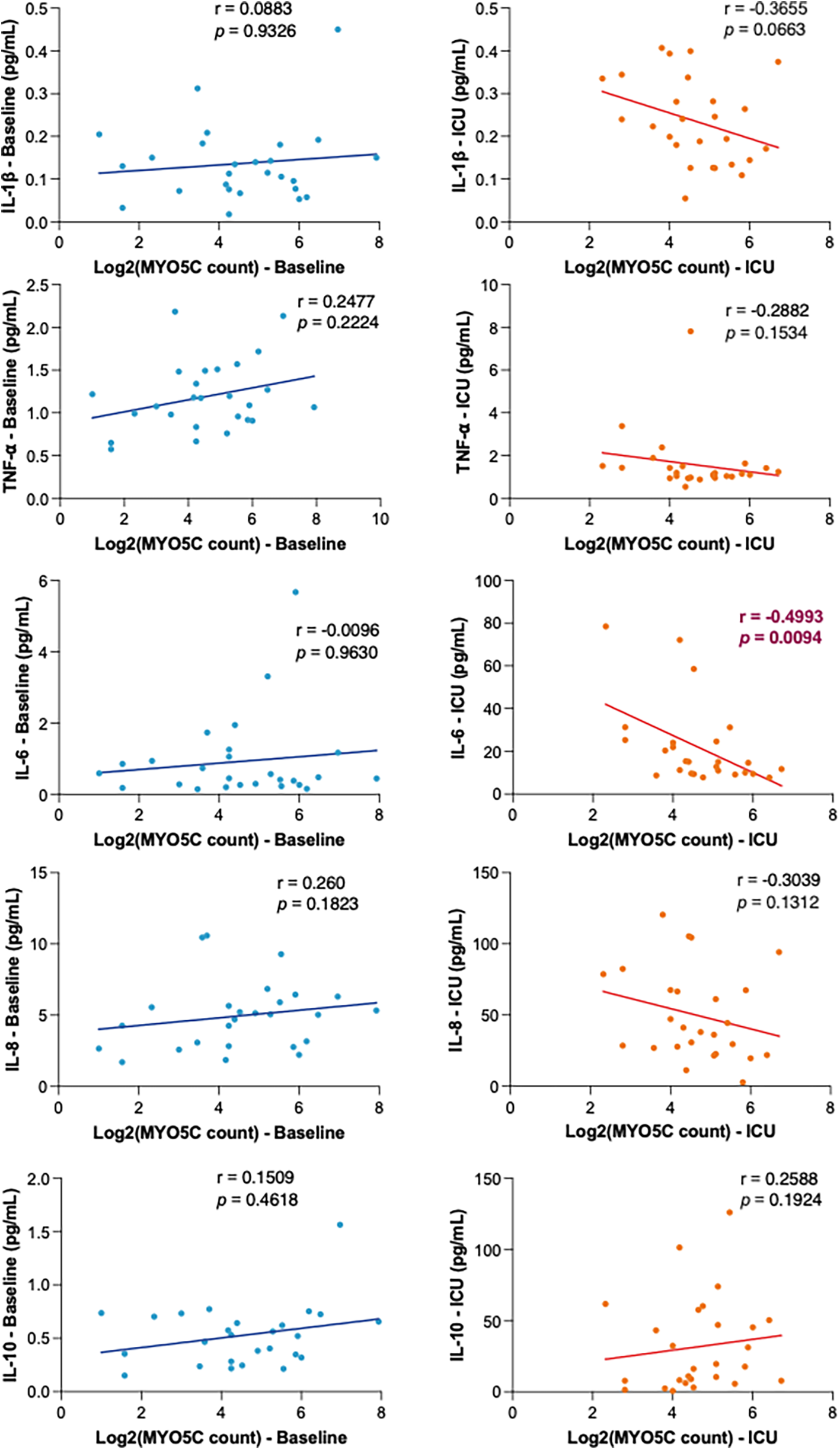
Correlation analyses of myo5c gene expression in inflammatory response. Correlation of myo5c gene expression and cytokine levels (IL-1β, TNF-α, IL-6, IL-8, and IL-10 top to bottom) at baseline (blue) and upon ICU admission (red) was determined using linear regression analysis. Spearman correlation coefficients (r) and corresponding p-values are shown for each marker.
Figure 4
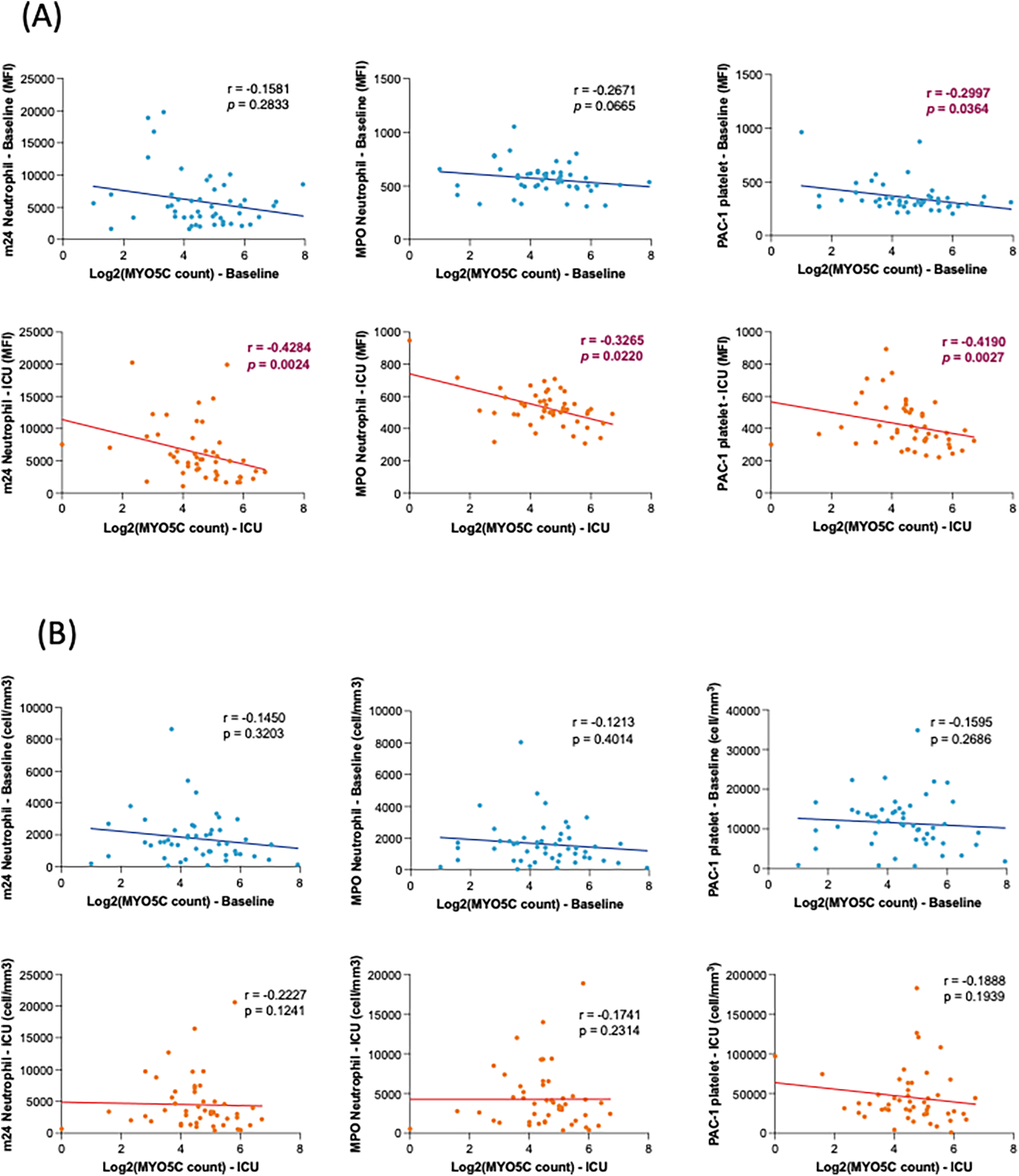
Correlation analyses of myo5c gene expression with neutrophil/platelet activation. (A) Correlation between myo5c gene expression and neutrophil and platelet activation at baseline (blue) and upon ICU admission (red). Neutrophil activation was assessed by m24 and MPO staining; platelet activation was measured by PAC-1 staining. Activation levels are represented as mean fluorescence intensity (MFI). (B) Correlation of myo5c gene expression and the number of activation marker-positive neutrophils (m24, MPO) and platelets (PAC-1) activation at baseline (blue) and upon ICU admission (red). Cell counts (cells/mm³) represent the number of marker-positive cells, measured by flow cytometry. (A,B) Spearman correlation analysis was used, and correlation coefficients (r) and corresponding p-values are shown.
Myo5C deficiency enhanced neutrophil extracellular trap (NET) formation
The observed downregulation of the neutrophil myo5c gene in patients with elevated PELOD-2 scores suggested its potential involvement in postoperative complications. To determine the functional role of myo5c, we performed its deletion in HL60 cells using CRISPR/Cas9. Our investigation into NET formation in HL60 cells with myo5c knockout phenotypes revealed enhanced NET formation in the absence of myo5cprotein following PMA stimulation (Figure 5), suggesting that myo5c would be a functionally important molecule. This experimental evidence supported our initial hypothesis, strengthening the association between myo5c, systemic inflammation, and NET formation.
Figure 5
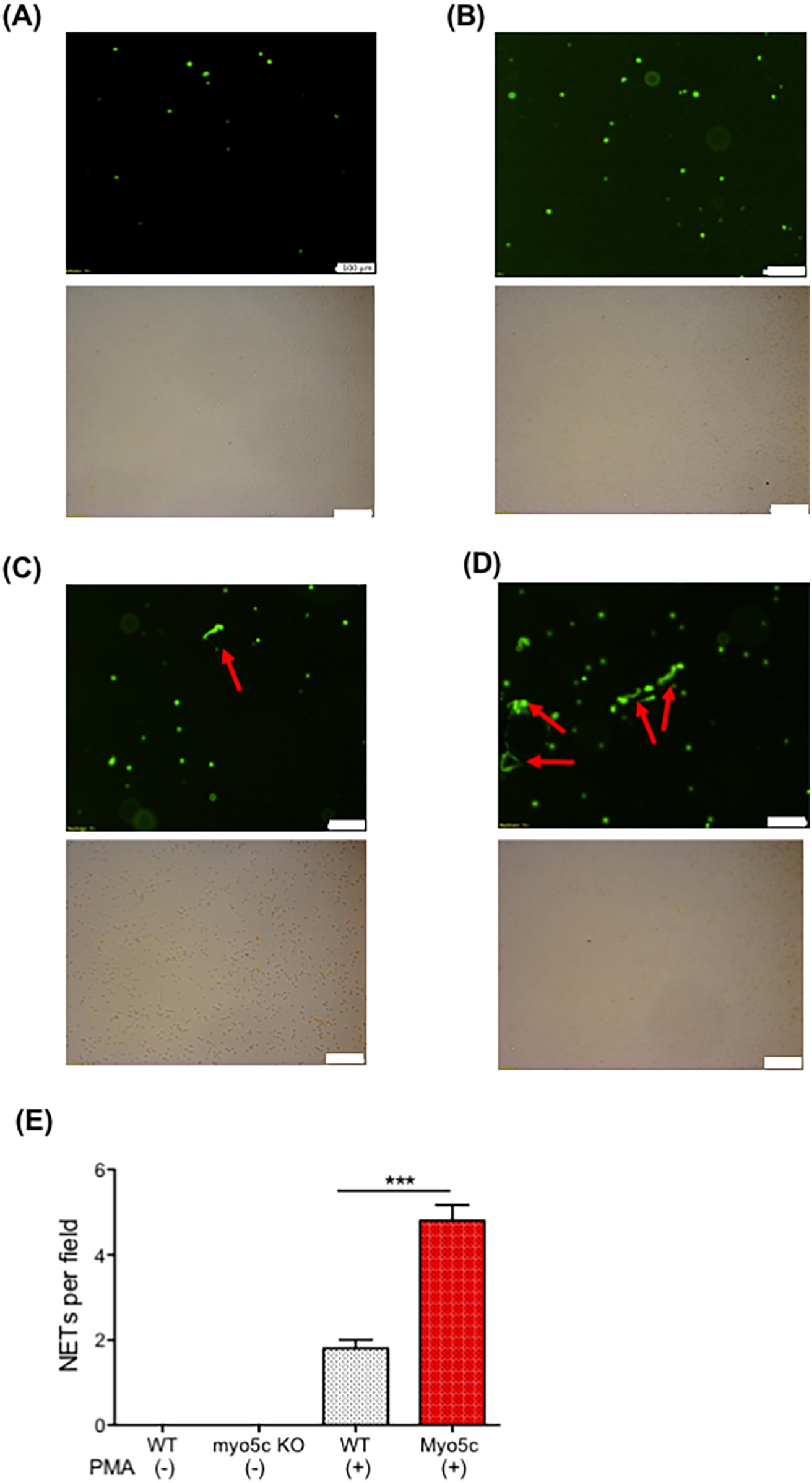
The role of myo5c in NETs formation. NETs formation was examined using neutrophil-differentiated HL-60 cells. Neutrophil-differentiated HL-60 cells were subjected to NETs induction by incubating with PMA for 4 h. (A) HL60 WT cells. (B) HL60 myo5c KO, (C) HL60 + PMA, (D) HL60 myo5c KO + PMA. Top image shows Sytox green image. The bottom shows bright-field image. The white bar denotes 100 µm. (E) The number of NETs formation per field in the five independent experiments was counted and shown. Data are representative of the two independent experiments. One-way ANOVA was performed. *** p < 0.001.
Discussion
Our study identifies a potential role for myo5c in modulating neutrophil activation and inflammation in pediatric patients undergoing cardiac surgery with CPB. Reduced myo5c expression in circulating neutrophils was associated with greater postoperative organ dysfunction, as reflected by higher PELOD-2 scores, increased surgical complexity (STAT mortality category ≥3), and prolonged ICU and hospital stays. Although myo5c expression was not independently associated with the presence of complications, its strong inverse correlation with PELOD-2 scores suggests a closer relationship with the severity of physiological stress and evolving organ injury.
Functionally, myo5c-deficient HL60 cells exhibited enhanced NET formation upon PMA stimulation, supporting a role of Myosin Vc protein in regulating neutrophil activation. Transcriptomic downregulation of myo5c also correlated with elevated IL-6 levels, and markers of neutrophil and platelet activation, further linking reduced myo5c expression to a proinflammatory and potentially prothrombotic phenotype.
Class V myosin, key member of the myosin superfamily, serve as actin-based molecular motors essential for cellular motility and organelle transport (17–19). These myosin V proteins are ubiquitous and fundamental in establishing the molecular mechanisms of actin-mediated cellular activities (20). In vertebrates, the class V myosin family comprises three genes: myo5a, myo5b, and myo5c (21–23). While the roles of Myosin Va and Vb proteins have been extensively studied, Myosin Vc remains less investigated. This novel variant, prevalent in secretory and glandular tissues, is implicated in diverse cellular functions such as secretory granule trafficking (24), transferrin trafficking (22), melanosome biogenesis and secretion (25), and von Willebrand factor (vWF) externalization (26). Despite its presence in various immune cells, including neutrophils (27, 28), the specific roles of Myosin Vc in immune responses are not well-defined.
Our findings suggest that myo5c downregulation may be associated with postoperative organ dysfunction via enhanced neutrophil activation and NET formation, which in turn may promote vascular microthrombosis, impair tissue perfusion, and contribute to subsequent organ injury (29, 30). While direct evidence of Myosin Vc's role in NET formation is lacking, its established functions in actin cytoskeletal rearrangement may be important for chromatin decondensation and release of nuclear DNA during NET formation (31, 32). In our study, myo5c-knockout cells produced more NETs, suggesting a previously unrecognized role for Myosin Vc in neutrophil effector functions.
Following cardiac surgery with CPB, extensive tissue injury and cellular damage leads to the release of damage-associated molecular patterns (DAMPs), such as histones and high mobility group box 1 (HMGB1), which activate neutrophils (33) and trigger their degranulation and NET release (34, 35). Our previous work demonstrated elevated circulating DAMPs in patients with postoperative organ dysfunction and showed that DAMPs enhance NET formation in a mouse model (3). These findings underscore the relevance of NET-driven mechanisms in postoperative organ dysfunction and raise the possibility that Myosin Vc may modulate this response.
Taken together, these cellular processes may help explain the observed association between lower myo5c expression and higher PELOD-2 scores, linking transcriptomic alterations with postoperative organ dysfunction severity. Although our study did not include direct imaging of actin remodeling or vesicle transport, future mechanistic studies will be essential to define how Myosin Vc regulates neutrophil activation and NET formation. To avoid overinterpretation, we emphasize that this represents a hypothesis-generating observation based on indirect mechanistic parallels from correlative data and in vitro observations, and additional research is needed to validate the proposed pathway.
This study has several limitations. It was exploratory in nature, with no formal sample size calculation. The relatively small sample size, modest number of complication cases, and single-center design may reduce statistical power and limit generalizability. The ROC AUC for myo5c expression indicates moderate discriminatory ability and should be interpreted with caution. Additionally, heterogeneity in CHD diagnoses and surgical complexity may influence gene expression. Nevertheless, myo5c downregulation may have clinical value as part of a multi-marker panel for early risk stratification. Future studies with larger, more homogeneous cohorts are needed to validate the clinical utility of myo5c and elucidate its mechanistic role in inflammation.
In conclusion, this study highlights the association between myo5c gene expression, neutrophil activation, and postoperative organ dysfunction severity in pediatric cardiac surgery with CPB. Our findings support the potential role of myo5c in modulating NET formation and systemic inflammation. While myo5c was not identified as an independent predictor of clinical outcomes, its consistent association with markers of illness severity suggests it may serve as a valuable biomarker, particularly as part of a multi-marker panel, for early risk stratification. Further research is warranted to elucidate the regulatory mechanisms underlying myo5c expression and to explore the molecular regulation and functional relevance of Myosin Vc protein in neutrophil-mediated inflammation.
Statements
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE297377.
Ethics statement
The studies involving humans were approved by Boston children's Hospital IRB. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
WM: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SS: Formal analysis, Writing – review & editing. SK: Resources, Writing – review & editing. HV: Resources, Writing – review & editing. SK: Supervision, Writing – review & editing. JI: Writing – review & editing. KY: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by NICHD R21 HD109110.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1380606/full#supplementary-material
Supplementary Figure S1Neutrophils and platelets gating strategy. (A) Neutrophils gated by CD15(+) population. Activation antibody m24 was gated among the neutrophils. (B) Platelets gated by CD41 (+) population. Activation antibody PAC-1 was gated among the platelets.
Supplementary Figure S2Receiver Operating Characteristic (ROC) curve analysis comparing discrimination performance of myo5c expression upon ICU admission and PELOD-2 score on postoperative day 2.
Supplementary Figure S3Myo5c expression in neutrophils stratified by complication status. Log2-transformed myo5c counts at baseline (blue) and upon ICU admission (red) in patients with (A) and without (B) postoperative complications. ns = not significant.
Supplementary Figure S4Myo5c expression in neutrophils stratified by cyanosis status. Log2-transformed myo5c counts at baseline (blue) and upon ICU admission (red) in acyanotic vs. cyanotic patients. ns = not significant.
Supplementary Figure S5Myo5c expression across full STAT mortality categories (1–5). Box plots showing expression at baseline (blue) and upon ICU admission (red). ns = not significant.
Supplementary Figure S6Myo5c expression in STAT category <3 vs. ≥3. Box plots showing expression at baseline (blue) and upon ICU admission (red). ns = not significant.
Supplementary Table S1Logic regression analysis of baseline myo5c expression and clinical variables.
Supplementary Table S2Logic regression analysis of myo5c expression at ICU admission and clinical variables.
References
1.
Brown KL Ridout D Pagel C Wray J Anderson D Barron DJ et al Incidence and risk factors for important early morbidities associated with pediatric cardiac surgery in a UK population. J Thorac Cardiovasc Surg. (2019) 158(4):1185–96. 10.1016/j.jtcvs.2019.03.139
2.
Marelli A Gauvreau K Landzberg M Jenkins K . Sex differences in mortality in children undergoing congenital heart disease surgery: a United States population-based study. Circulation. (2010) 122(11 Suppl):S234–40. 10.1161/CIRCULATIONAHA.109.928325
3.
Maisat W Hou L Sandhu S Sin YC Kim S Van Pelt H et al Neutrophil extracellular traps formation is associated with postoperative complications in congenital cardiac surgery. Pediatr Res. (2024):1–14. 10.1038/s41390-024-03717-z
4.
Kraus RF Gruber MA . Neutrophils-from bone marrow to first-line defense of the innate immune system. Front Immunol. (2021) 12:767175. 10.3389/fimmu.2021.767175
5.
Esper SA Subramaniam K Tanaka KA . Pathophysiology of cardiopulmonary bypass: current strategies for the prevention and treatment of anemia, coagulopathy, and organ dysfunction. Semin Cardiothorac Vasc Anesth. (2014) 18(2):161–76. 10.1177/1089253214532375
6.
Ríos-López AL González GM Hernández-Bello R Sánchez-González A . Avoiding the trap: mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol Res. (2021) 243:126644. 10.1016/j.micres.2020.126644
7.
Mereweather LJ Constantinescu-Bercu A Crawley JTB Salles C II . Platelet-neutrophil crosstalk in thrombosis. Int J Mol Sci. (2023) 24(2):1266. 10.3390/ijms24021266
8.
Kumar S Payal N Srivastava VK Kaushik S Saxena J Jyoti A . Neutrophil extracellular traps and organ dysfunction in sepsis. Clin Chim Acta. (2021) 523:152–62. 10.1016/j.cca.2021.09.012
9.
Jenkins KJ Gauvreau K Bergersen L Nathan M Thiagarajan R . Pediatric Risk Adjustment for Congenital Heart Disease. London: Springer-Verlag London (2014).
10.
Pollack MM Patel KM Ruttimann UE . PRISM III: an updated pediatric risk of mortality score. Crit Care Med. (1996) 24(5):743–52. 10.1097/00003246-199605000-00004
11.
Slater A Shann F Pearson G , Paediatric Index of Mortality Study G. PIM2: a revised version of the paediatric index of mortality. Intensive Care Med. (2003) 29(2):278–85. 10.1007/s00134-002-1601-2
12.
Leteurtre S Martinot A Duhamel A Proulx F Grandbastien B Cotting J et al Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. (2003) 362(9379):192–7. 10.1016/S0140-6736(03)13908-6
13.
Lacroix J Cotting J , Pediatric Acute Lung I, Sepsis Investigators N. Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med. (2005) 6(3 Suppl):S126–34. 10.1097/01.PCC.0000161287.61028.D4
14.
Russell RA Ghanayem NS Kuhn EM Jeffries HE Scanlon MC Rice TB . Relationship between risk-adjustment tools and the pediatric logistic organ dysfunction score. World J Pediatr Congenit Heart Surg. (2014) 5(1):16–21. 10.1177/2150135113510008
15.
Leteurtre S Duhamel A Salleron J Grandbastien B Lacroix J Leclerc F . PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. (2013) 41(7):1761–73. 10.1097/CCM.0b013e31828a2bbd
16.
Bembea MM Agus M Akcan-Arikan A Alexander P Basu R Bennett TD et al Pediatric organ dysfunction information update mandate (PODIUM) contemporary organ dysfunction criteria: executive summary. Pediatrics. (2022) 149(1 Suppl 1):S1–s12. 10.1542/peds.2021-052888B
17.
DePina AS Langford GM . Vesicle transport: the role of actin filaments and myosin motors. Microsc Res Tech. (1999) 47(2):93–106. 10.1002/(sici)1097-0029(19991015)47:2%3C93::Aid-jemt2%3E3.0.Co;2-p
18.
Eddy RJ Pierini LM Matsumura F Maxfield FR . Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. (2000) 113(Pt 7):1287–98. 10.1242/jcs.113.7.1287
19.
Tuxworth RI Titus MA . Unconventional myosins: anchors in the membrane traffic relay. Traffic. (2000) 1(1):11–8. 10.1034/j.1600-0854.2000.010103.x
20.
Berg JS Powell BC Cheney RE . A millennial myosin census. Mol Biol Cell. (2001) 12(4):780–94. 10.1091/mbc.12.4.780
21.
Cheney RE O’Shea MK Heuser JE Coelho MV Wolenski JS Espreafico EM et al Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. (1993) 75(1):13–23. 10.1016/S0092-8674(05)80080-7
22.
Rodriguez OC Cheney RE . Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J Cell Sci. (2002) 115(Pt 5):991–1004. 10.1242/jcs.115.5.991
23.
Zhao LP Koslovsky JS Reinhard J Bähler M Witt AE Provance DW Jr. et al Cloning and characterization of myr 6, an unconventional myosin of the dilute/myosin-V family. Proc Natl Acad Sci USA. (1996) 93(20):10826–31. 10.1073/pnas.93.20.10826
24.
Jacobs DT Weigert R Grode KD Donaldson JG Cheney RE . Myosin Vc is a molecular motor that functions in secretory granule trafficking. Mol Biol Cell. (2009) 20(21):4471–88. 10.1091/mbc.e08-08-0865
25.
Farquhar RE Rodrigues E Hamilton KL . The role of the cytoskeleton and myosin-Vc in the targeting of KCa3.1 to the basolateral membrane of polarized epithelial cells. Front Physiol. (2016) 7:639. 10.3389/fphys.2016.00639
26.
Holthenrich A Terglane J Naß J Mietkowska M Kerkhoff E Gerke V . Spire1 and myosin Vc promote Ca(2+)-evoked externalization of von Willebrand factor in endothelial cells. Cell Mol Life Sci. (2022) 79(2):96. 10.1007/s00018-021-04108-x
27.
Maravillas-Montero JL Santos-Argumedo L . The myosin family: unconventional roles of actin-dependent molecular motors in immune cells. J Leukoc Biol. (2012) 91(1):35–46. 10.1189/jlb.0711335
28.
Monaco G Lee B Xu W Mustafah S Hwang YY Carré C et al RNA-Seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. (2019) 26(6):1627–40.e7. 10.1016/j.celrep.2019.01.041
29.
Chen Z Zhang H Qu M Nan K Cao H Cata JP et al Review: the emerging role of neutrophil extracellular traps in sepsis and sepsis-associated thrombosis. Front Cell Infect Microbiol. (2021) 11:653228. 10.3389/fcimb.2021.653228
30.
Retter A Singer M Annane D . "The NET effect": neutrophil extracellular traps-a potential key component of the dysregulated host immune response in sepsis. Crit Care. (2025) 29(1):59. 10.1186/s13054-025-05283-0
31.
Sprenkeler EGG Tool ATJ Henriet SSV van Bruggen R Kuijpers TW . Formation of neutrophil extracellular traps requires actin cytoskeleton rearrangements. Blood. (2022) 139(21):3166–80. 10.1182/blood.2021013565
32.
Wang H Kim SJ Lei Y Wang S Wang H Huang H et al Neutrophil extracellular traps in homeostasis and disease. Signal Transduct Target Ther. (2024) 9(1):235. 10.1038/s41392-024-01933-x
33.
Huang H Tohme S Al-Khafaji AB Tai S Loughran P Chen L et al Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. (2015) 62(2):600–14. 10.1002/hep.27841
34.
Pircher J Engelmann B Massberg S Schulz C . Platelet-neutrophil crosstalk in atherothrombosis. Thromb Haemost. (2019) 119(8):1274–82. 10.1055/s-0039-1692983
35.
Mandel J Casari M Stepanyan M Martyanov A Deppermann C . Beyond hemostasis: platelet innate immune interactions and thromboinflammation. Int J Mol Sci. (2022) 23(7):3868. 10.3390/ijms23073868
Summary
Keywords
congenital heart disease, cardiopulmonary bypass, organ injury, myo5c , neutrophil extracellular traps
Citation
Maisat W, Sandhu S, Kim S, Van Pelt H, Kong SW, Ibla J and Yuki K (2025) Neutrophil Myo5c gene downregulation is associated with postoperative organ dysfunction following pediatric cardiac surgery with cardiopulmonary bypass. Front. Cardiovasc. Med. 12:1380606. doi: 10.3389/fcvm.2025.1380606
Received
01 February 2024
Accepted
09 May 2025
Published
27 May 2025
Volume
12 - 2025
Edited by
Lei Du, Sichuan University, China
Reviewed by
Albert Gyllencreutz Castellheim, University of Gothenburg, Sweden
Xin Li, Fudan University, China
Updates
Copyright
© 2025 Maisat, Sandhu, Kim, Van Pelt, Kong, Ibla and Yuki.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Koichi Yuki koichi.yuki@childrens.harvard.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.