Abstract
Objectives:
Complications of intracranial hemorrhage (ICH) after percutaneous coronary intervention (PCI), although rare, have a poor prognosis with high mortality rates. This study aims to provide information on the clinical characteristics and outcomes of hospitalized patients with ICH after PCI.
Materials and methods:
This retrospective study included 24 patients enrolled from February 2014 to September 2023, which occurred ICH during post-PCI hospitalization. We mainly analyzed general, procedural, ICH features and subsequent outcomes. In addition, the predictive ability of the CRUSADE, ARC-HBR, and ACUITY scores was assessed with the receiver operating characteristics area under the curve (AUC).
Results:
Among the 24 patients, the mean age was 62.21 ± 10.01 years, and 66.7% (n = 16) were men. The mortality of ICH patients after PCI was very high (n = 13, 54.2%). In addition, the most common initial manifestation of ICH patients was the disturbance of consciousness (n = 14, 58.3%). Over half of the cases (58.3%) occurred ICH within the first 12 h following PCI. 13 patients (54.2%) had an ICH volume ≥30 cm3, and of these patients, a total of 11(84.6%) died. ICH volume ≥30 cm3 (p = 0.038), and the use of mechanical ventilators (p = 0.011) were significantly higher in patients who died. The AUC of CRUSADE, ARC-HBR, and ACUITY scores were 0.500, 0.619, and 0.545, respectively.
Conclusions:
In our study, the mortality of ICH after PCI was high. The high volume of ICH indicates a high risk of death.
1 Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide (1). Percutaneous coronary intervention (PCI) is the cornerstone of treatment for patients with acute coronary syndromes (ACS). In recent years, PCI has also been widely used in patients with chronic coronary syndromes (CCS) (2). In addition, antithrombotic therapy plays a crucial role in improving outcomes in patients who have undergone PCI (3). Over the past 30 years, using antiplatelet agents has significantly reduced thrombotic events and remains the standard of care following PCI in CAD patients (4). Although dual antiplatelet therapy (DAPT) reduced the risk of ischemic events, it also increased the rate of bleeding, which created a therapeutic dilemma for the clinician (5, 6). Therefore, identifying high-risk features associated with bleeding complications and implementing appropriate risk reduction approaches are essential.
Intracranial hemorrhage (ICH) is a rare but potentially life-threatening complication that may occur in patients undergoing PCI, which is the most severe bleeding complication and is often overlooked. For example, studies have shown that ICH can occur as a rare but severe complication in patients undergoing PCI, particularly those on dual antiplatelet therapy (DAPT), and may not always be adequately captured in routine clinical practice (7). Furthermore, ICH is internationally associated with significant morbidity and mortality (8, 9). Patients usually receive DAPT after PCI, and prior antiplatelet therapy is associated with higher mortality in patients with ICH (10, 11). Moreover, as there are few effective treatments for ICH, early identification of those at risk and effective preventive measures are essential. One study found that age >80 years, ICH volume >30 mm3, hematoma origin, intraventricular hemorrhage presence, and Glasgow Coma Scale score were associated with 30-day mortality after ICH (12).
Despite the severity of intracranial hemorrhage (ICH) following percutaneous coronary intervention (PCI), there is a paucity of detailed information regarding its clinical characteristics. Therefore, this study was conducted to provide a comprehensive description of the clinical features and outcomes of hospitalized patients who experienced ICH after PCI, thereby contributing to the existing body of knowledge in this area.
2 Patients and methods
2.1 Study population
Our study was a single-center, retrospective study of patients with ICH that occurred after PCI in the First Affiliated Hospital of Zhengzhou University from February 2014 to September 2023. A total number of 24 patients were finally enrolled after excluding individuals without PCI or ICH, patients with PCI after ICH, or those with missing data. In addition, patients were divided into a survival group (11 cases) and a death group (13 cases). Besides, the study received approval from the Human Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University and the informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
2.2 Data collection
Clinical data were sourced from the Medical Information Recording System at the First Affiliated Hospital of Zhengzhou University. We retrospectively collected data concerning patients’ demographic information, days of hospitalization, medical history, diagnosis, admission features, examinations, clinical characteristics, PCI procedural and ICH-related characteristics, as well as medication usage (prior-, during-, or post-procedure).
2.3 Definitions
The ICH volume was measured using ABC/2 according to the head CT, in which A represented the largest diameter of the hematoma on axial images, B represented the largest diameter perpendicular to A on the same image slice, and C represented the number of slices in which the hematoma is seen, multiplied by the slice thickness (13). All lengths were registered in centimeters (cm) and volumes in cubic centimeters (cm3) (14). For intracerebral hemorrhages with an intraventricular extension, only the parenchymal component was measured by the ABC/2 (15). ICH was classified into two categories small (<30 cm3) or large (≥30 cm3) (12). Besides, the ventricular extension means the CT head confirmed that ICH had extended into the ventricles. The mortality rate mentioned in our study refers to the overall mortality within the study population during the observation period of our research. It includes both in-hospital and post-discharge mortality up to the end of the follow-up period.
Calculation of the CRUSADE score was based on the clinical data obtained at admission (heart rate, systolic blood pressure, hematocrit, creatinine clearance, gender, signs of chronic heart failure at presentation, history of vascular disease, and history of diabetes mellitus) (16). The CRUSADE score is categorized into five risk levels based on the patient's score: very low risk (≤20 points), low risk (21–30 points), moderate risk (31–40 points), high risk (41–50 points), and very high risk (>50 points). The ARC-HBR score consists of 14 primary criteria and 6 secondary criteria. If a patient meets at least 1 primary criterion or 2 secondary criteria, they may be considered to have a high risk of bleeding (17). The ACUITY score was based on the clinical data obtained at admission [age, gender, serum creatinine, white blood cell count, anemia, type of ACS (unstable angina, non-ST-elevation or ST-elevation acute myocardial infarction), use of bivalirudin] (18). The ACUITY score is categorized into four risk levels based on the patient's score: low risk (<10 points), moderate risk (10–14 points), high risk (15–19 points), and very high risk (≥20 points). The higher the scores, the higher the patient's risk of bleeding. In our study, all the scores of the patients were classified into bleeding risk strata by considering the very high risk and high risk as unique categories (high risk) and very low risk and low risk as low risk categories (low risk).
2.4 Statistical analysis
Categorical variables were shown as frequencies and percentages, whereas continuous variables were presented as mean ± standard deviations (SD). Continuous variables were compared using the one-way ANOVA analysis, whereas categorical variables were compared using Fisher's exact test. In addition, in our study, the ROC curve analysis was conducted to evaluate the predictive effectiveness of the CRUSADE, ARC-HBR, and ACUITY scores specifically for in-hospital bleeding events among patients with intracranial hemorrhage after percutaneous coronary intervention (PCI). The AUC values were calculated to assess the ability of these scores to predict the occurrence of bleeding complications during hospitalization.Statistical analyses were performed using SPSS 25.0 software. A two-sided P-value < 0.05 was considered statistically significant.
3 Results
3.1 General characteristics
A total of 24 patients experienced ICH after undergoing PCI between October 2015 and October 2023. Baseline characteristics are presented in Table 1. Of the 24 patients, the mean age was 62.21 ± 10.01 years, 66.7% (n = 16) were men, 11(45.8%) patients experienced myocardial infarction (MI), and 13 (54.2%) experienced unstable angina (UA). In addition, 15 patients (62.5%) had hypertension, and 5 patients (20.8%) had diabetes mellitus. The median follow-up time for our cohort was 11.00 days (IQR: 6.00–28.25 days). During this period, we observed an in-hospital mortality rate of 54.2% (n = 13).
Table 1
| Clinical data | Total (n = 24) | Survival (n = 11) | Death (n = 13) | P-value |
|---|---|---|---|---|
| Demographic variables | ||||
| Male, n (%) | 16 (66.7) | 8 (72.7) | 8 (61.5) | 0.679 |
| Age, years | 62.21 ± 10.01 | 61.64 ± 6.83 | 62.69 ± 12.35 | 0.803 |
| hospital stay, days | 15.33 ± 11.62 | 25.82 ± 8.27 | 6.46 ± 3.99 | <0.001 |
| Medical history, n (%) | ||||
| Hypertension | 15 (62.5) | 7 (63.6) | 8 (61.5) | 1.000 |
| Diabetes mellitus | 5 (20.8) | 2 (18.2) | 3 (23.1) | 1.000 |
| Peripheral vascular disease | 14 (58.3) | 4 (36.4) | 10 (76.9) | 0.095 |
| Heart failure | 8 (33.3) | 3 (27.3) | 5 (38.5) | 0.679 |
| Prior ischemia stroke/TIA | 6 (25.0) | 2 (18.2) | 4 (30.8) | 0.649 |
| Dyslipidemia | 4 (16.7) | 2 (18.2) | 2 (15.4) | 1.000 |
| Anemia | 8 (33.3) | 5 (45.5) | 3 (23.1) | 0.390 |
| Prior PCI | 4 (16.7) | 3 (27.3) | 1 (7.7) | 0.300 |
| Renal insufficiency | 13 (54.2) | 5 (45.5) | 8 (61.5) | 0.682 |
| Drinking | 8 (33.3) | 3 (27.3) | 5 (38.5) | 0.679 |
| Smoking | 10 (41.7) | 6 (54.5) | 4 (30.8) | 0.408 |
| Diagnosis, n (%) | 0.444 | |||
| Unstable angina | 13 (54.2) | 7 (63.6) | 6 (46.2) | |
| MI | 11 (45.8) | 4 (36.4) | 7 (53.8) | |
| Admission features | ||||
| SBP, mmHg | 119.50 ± 23.88 | 114.09 ± 23.74 | 124.08 ± 23.96 | 0.318 |
| DBP, mmHg | 72.54 ± 13.11 | 67.36 ± 12.52 | 76.92 ± 12.38 | 0.074 |
| LVEF, % | 54.46 ± 8.45 | 54.73 ± 9.23 | 54.23 ± 8.11 | 0.890 |
| LV, mm | 47.75 ± 4.50 | 48.18 ± 4.96 | 47.38 ± 4.23 | 0.675 |
| HR, beats/min | 77.25 ± 14.39 | 78.81 ± 12.00 | 75.08 ± 16.31 | 0.434 |
| Examinations | ||||
| WBC, 109/L | 13.65 ± 6.13 | 12.29 ± 5.00 | 14.79 ± 6.94 | 0.330 |
| RBC, 109/L | 3.60 ± 0.83 | 3.51 ± 0.91 | 3.68 ± 0.79 | 0.620 |
| Hemoglobin, g/dl | 109.84 ± 25.38 | 105.84 ± 27.40 | 113.23 ± 24.13 | 0.489 |
| Platelet, 109/L | 163.61 ± 77.97 | 160.18 ± 82.86 | 166.51 ± 76.86 | 0.848 |
| Cr, µmol/L | 93.06 ± 54.58 | 95.46 ± 55.20 | 91.03 ± 56.22 | 0.848 |
| UA, µmol/L | 325.71 ± 200.08 | 322.64 ± 221.33 | 328.31 ± 189.44 | 0.947 |
| eGFR, ml/min/1.73 m2 | 71.69 ± 24.34 | 71.87 ± 26.10 | 71.53 ± 23.92 | 0.974 |
| Albumin, g/L | 36.60 ± 5.27 | 35.94 ± 5.38 | 37.15 ± 5.33 | 0.584 |
| HbA1C | 6.04 ± 0.72 | 6.14 ± 0.91 | 5.96 ± 0.53 | 0.544 |
| PT, s | 12.18 ± 1.85 | 12.62 ± 1.93 | 11.81 ± 1.76 | 0.294 |
| APTT, s | 40.80 ± 32.90 | 32.29 ± 5.24 | 47.98 ± 43.93 | 0.253 |
| D-dimer, mg/L | 4.75 ± 5.14 | 3.71 ± 4.34 | 5.63 ± 5.76 | 0.374 |
| TC, mmol/L | 3.81 ± 1.19 | 4.14 ± 1.27 | 3.54 ± 1.10 | 0.228 |
| TG, mmol/L | 1.50 ± 0.98 | 1.49 ± 1.12 | 1.51 ± 0.88 | 0.969 |
| HDL, mmol/L | 1.01 ± 0.36 | 1.03 ± 0.40 | 0.99 ± 0.33 | 0.759 |
| LDL-C, mmol/L | 2.34 ± 1.12 | 2.67 ± 1.42 | 2.06 ± 0.74 | 0.191 |
| Tn I, µg/L | 4.34 ± 4.68 | 3.55 ± 4.06 | 5.01 ± 5.22 | 0.457 |
| Clinical characteristics, n (%) | ||||
| ECMO | 3 (12.5) | 1 (9.1) | 2 (15.4) | 1.000 |
| Mechanical ventilator | 19(79.2) | 6(54.5) | 13(100.0) | 0.011 |
Baseline characteristics.
CHD, coronary heart disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; MI, myocardial infarction; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; LV, left ventricular; HR, heart rate; WBC, white blood cell; RBC, red blood cell count; Cr, creatinine; UA: uric acid; eGFR, estimated glomerular filtration rate; HbAlC, hemoglobin A1C; PT, prothrombin time; APTT, activated partial thromboplastin time; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; Tn I, troponin I; ECMO, extracorporeal membrane oxygenation.
In our study, we found the proportion of patients requiring mechanical ventilation was higher in the death group [13(100.0%) vs. 6(54.5%), p = 0.011]. The remaining parameters were described in Table 1.
3.2 Procedural characteristics
Regarding procedure information, 17 patients (70.8%) underwent elective PCI, and 19 patients (79.2%) had multivessel disease. (Elective PCI was performed for patients who had stabilized after the initial acute event and required further intervention to address underlying coronary artery disease. This approach is consistent with clinical practice where elective PCI may be appropriate for post-myocardial infarction patients who have recurrent or inducible angina before hospital discharge, and for patients who have angina and remain symptomatic despite medical treatment.) Of all the patients with an indication to receive DAPT at baseline (prior to ICH onset), the majority (54.2%) received DAPT with aspirin plus ticagrelor. Overall, 20 patients (83.3%) received unfractionated heparin during PCI, and the remaining 4 patients (16.7%) received bivalirudin. Besides, one patient received tirofiban, and four received bivalirudin after PCI (Table 2).
Table 2
| Variables | Total number of cases (n = 24) | Survival group (n = 11) | Death group (n = 13) | P-value |
|---|---|---|---|---|
| Duration of procedure, mins | 76.00 ± 30.40 | 87.91 ± 26.15 | 65.92 ± 31.00 | 0.077 |
| Multivessel disease, n (%) | 19 (79.2) | 9 (81.8) | 10 (76.9) | 1.000 |
| Target vessel (%) | 0.718 | |||
| Anterior descending artery | 10 (41.7) | 4 (36.4) | 6 (46.2) | |
| Circumflex | 3 (12.5) | 2 (18.2) | 1 (7.7) | |
| Right coronary artery | 11 (45.8) | 5 (45.5) | 6 (46.2) | |
| Timing of PCI procedure, n (%) | 0.386 | |||
| Selective | 17 (70.8) | 9 (81.8) | 8 (61.5) | |
| Emergency | 7 (29.2) | 2 (18.2) | 5 (38.5) | |
| Total number of stents | 1.88 ± 1.12 | 1.55 ± 0.820 | 2.15 ± 1.28 | 0.189 |
| Antithrombotic therapy, n (%) | ||||
| Pre-procedure | 0.353 | |||
| Aspirin plus clopidogrel | 11 (45.8) | 6 (54.5) | 5 (38.5) | |
| Aspirin plus ticagrelor | 13 (54.2) | 5 (45.5) | 8 (61.5) | |
| During procedure | 0.300 | |||
| Unfractionated heparin | 20 (83.3) | 8 (72.7) | 12 (92.3) | |
| Bivalirudin | 4 (16.7) | 3 (27.3) | 1 (7.7) | |
| Post-procedure | 0.518 | |||
| Tirofiban | 1 (4.2) | 1 (9.1) | 0 (0.0) | |
| Bivalirudin | 4(16.7) | 2(18.2) | 2(15.4) | |
Procedural characteristics.
PCI, percutaneous coronary intervention.
3.3 ICH characteristics
The clinical and imaging characteristics of 24 patients with ICH are represented in Table 3. The most common initial manifestation of ICH patients was the disturbance of consciousness (n = 14, 58.3%), followed by focal neurological signs (n = 10, 41.7%). More than half of the cases (58.3%) occurred ICH within the first 12 h following PCI. All 24 patients received brain CT scans. The mean ICH volume was 40.41 ± 32.28 cm3, and 10 of the 13(76.9%) patients who died in the hospital had ICH volumes on CT exceeding 30 cm3, whereas 7 of 11(63.6%) surviving patients were discharged with cerebral hemorrhage volumes below 30 cm3. Out of the 9 patients with ICH who suffered ventricular extension, 6 (66.7%) died. In addition, the most common treatment of ICH patients was conservative medicine (n = 21, 87.5%), followed by invasive surgery (n = 3, 12.5%) (Table 3).
Table 3
| Variables | Total number of cases (n = 24) | Survival group (n = 11) | Death group (n = 13) | P-value |
|---|---|---|---|---|
| Initial symptoms, n (%) | 0.408 | |||
| Focal neurological signs | 10 (41.7) | 6 (54.5) | 4 (30.8) | |
| Disturbance of consciousness | 14 (58.3) | 5 (45.5) | 9 (69.2) | |
| Time to symptoms after procedure | ||||
| Median time, hours | 26.48 ± 41.91 | 35.82 ± 45.10 | 18.58 ± 39.03 | 0.326 |
| Within 12 h, n (%) | 14 (58.3) | 5 (45.5) | 9 (69.2) | 0.408 |
| More than 12 h, n (%) | 10 (41.7) | 6 (54.5) | 4 (30.8) | 0.408 |
| ICH volume, cm3 | 40.41 ± 32.28 | 21.08 ± 16.20 | 56.77 ± 33.85 | 0.004 |
| Small (<30) | 11 (45.8) | 8 (72.7) | 3 (23.1) | 0.038 |
| Large (≥30) | 13 (54.2) | 3 (27.3) | 10 (76.9) | 0.038 |
| Treatment, n (%) | 0.576 | |||
| Conservative medicine | 21 (87.5) | 9 (81.8) | 12 (92.3) | |
| Minimally invasive surgery | 3 (12.5) | 2(18.2) | 1(7.7) | |
ICH-related characteristics (n = 24).
ICH, intracranial hemorrhage.
Among the 24 patients, 8 patients (33.3%) were classified as high or very high risk of bleeding on admission according to the CRUSADE score, 14 patients (58.3%) were classified as high risk according to the ARC-HBR score, and13 patients (54.2%) were classified as high or very high risk according to the ACUITY score. Furthermore, according to the ARC-HBR score, the mortality rate among patients with high bleeding risk was higher at 64.3% as compared to non-high bleeding risk patients, whose mortality rate was 40.0% (Table 4). Besides, ROC curve analysis was conducted to determine the AUC to judge the predictive effectiveness of CRUSADE, ARC-HBR, and ACUITY scores, with their AUC were 0.500, 0.619, and 0.545, respectively (Figure 1; Table 5).
Table 4
| ID | Death | Age | Gender | Dual antiplatelet therapy, aspirin plus | During procedural anti-coagulants | Time since PCI, hours | Onset symptoms | CT Manifestations | Score | Mechanical ventilator | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bleeding location | Volume, cm3 | CRUSADE score | ARC-HBR score | ACUITY score | |||||||||
| 1 | YES | 55 | Female | Clopidogre | UFH | 12.5 | FNS, V | CH | 20.5 | Medium | Low | Medium | YES |
| 2 | YES | 78 | Male | Clopidogre | UFH | 144 | DC, FNS | FL, TL, V | 50 | Low | High | High | YES |
| 3 | YES | 56 | Male | Ticagrelor | UFH | 8 | DC, V | BS, V | 38.2 | Low | High | Medium | YES |
| 4 | NO | 55 | Male | Clopidogrel | UFH | 65 | HA | V | 82 | Very low | Low | High | NO |
| 5 | NO | 58 | Male | Ticagrelor | UFH | 15 | HA | TL | 1 | Medium | High | Very high | YES |
| 6 | YES | 55 | Male | Ticagrelor | Bivalirudin | 2 | DC, V | PL, OL, SS, CH, V | 82.25 | Medium | Low | Medium | YES |
| 7 | NO | 55 | Female | Ticagrelor | UFH | 5.5 | DC, HA, V | FL | 41.4 | High | Low | Very high | YES |
| 8 | NO | 66 | Female | Clopidogre | UFH | 15 | DC, FNS | BG, V | 26 | Very high | Low | Very high | YES |
| 9 | YES | 55 | Male | Clopidogre | UFH | 29 | DC, FNS | TL, SS | 58.5 | Medium | Low | Low | YES |
| 10 | NO | 69 | Female | Clopidogre | UFH | 144 | FNS | CE, V | 46 | Medium | Low | Medium | N0 |
| 11 | YES | 66 | Male | Ticagrelor | UFH | 30 | DC, HA | PL, TL, OL, SS | 46.25 | Medium | Low | Medium | YES |
| 12 | NO | 62 | Male | Clopidogrel | UFH | 9 | V | CE, SS | 8.1 | Low | High | Low | N0 |
| 13 | NO | 69 | Male | Clopidogrel | Bivalirudin | 24 | FNS | TL, SS | 40 | Low | Low | Low | NO |
| 14 | NO | 50 | Male | Clopidogre | UFH | 90 | HA, FNS | OL | 3.2 | Very low | High | Low | NOS |
| 15 | NO | 66 | Male | Ticagrelor | UFH | 11.5 | DC | TL, SS, V | 15.2 | Very high | High | Very high | YES |
| 16 | YES | 72 | Female | Ticagrelor | UFH | 0.5 | PL, TL, OL, SS | 50 | Medium | High | Very high | YES | |
| 17 | YES | 66 | Female | Ticagrelor | UFH | 0.5 | DC | SS, CH, V | 121.5 | Very high | High | Very high | YES |
| 18 | YES | 32 | Male | Ticagrelor | UFH | 5 | DC | SS | 3.8 | Very high | High | Very high | YES |
| 19 | NO | 58 | Male | Clopidogrel | UFH | 10 | DC | TL | 14 | Medium | Low | Low | YES |
| 20 | YES | 73 | Female | Clopidogrel | UFH | 2 | DC | PL | 55 | Low | High | High | YES |
| 21 | YES | 64 | Female | Ticagrelor | UFH | 1 | DC | TL, SS, V | 108 | Very high | High | High | YES |
| 22 | NO | 70 | Male | Ticagrelor | UFH | 5 | DC | OL | 29 | Very high | High | Very high | YES |
| 23 | YES | 77 | Male | Ticagrelor | UFH | 1 | FNS | TL, BG, V | 80 | Low | High | Medium | YES |
| 24 | YES | 66 | Male | Clopidogrel | UFH | 6 | V | TL, OL | 24 | Very high | High | Very high | YES |
Detailed information of the 24 post-PCI patients who suffered ICH.
UFH, unfractionated heparin; HA, headache; V, vomiting; DC, disturbance of consciousness; FNS, focal neurological signs; CH, cerebral; FL, frontal lobe; PL, parietal lobe; TL, temporal lobe; OL, occipital lobe; SS, subarachnoid space; BG, basal ganglia; CE, cerebellum; V, ventricle.
Figure 1
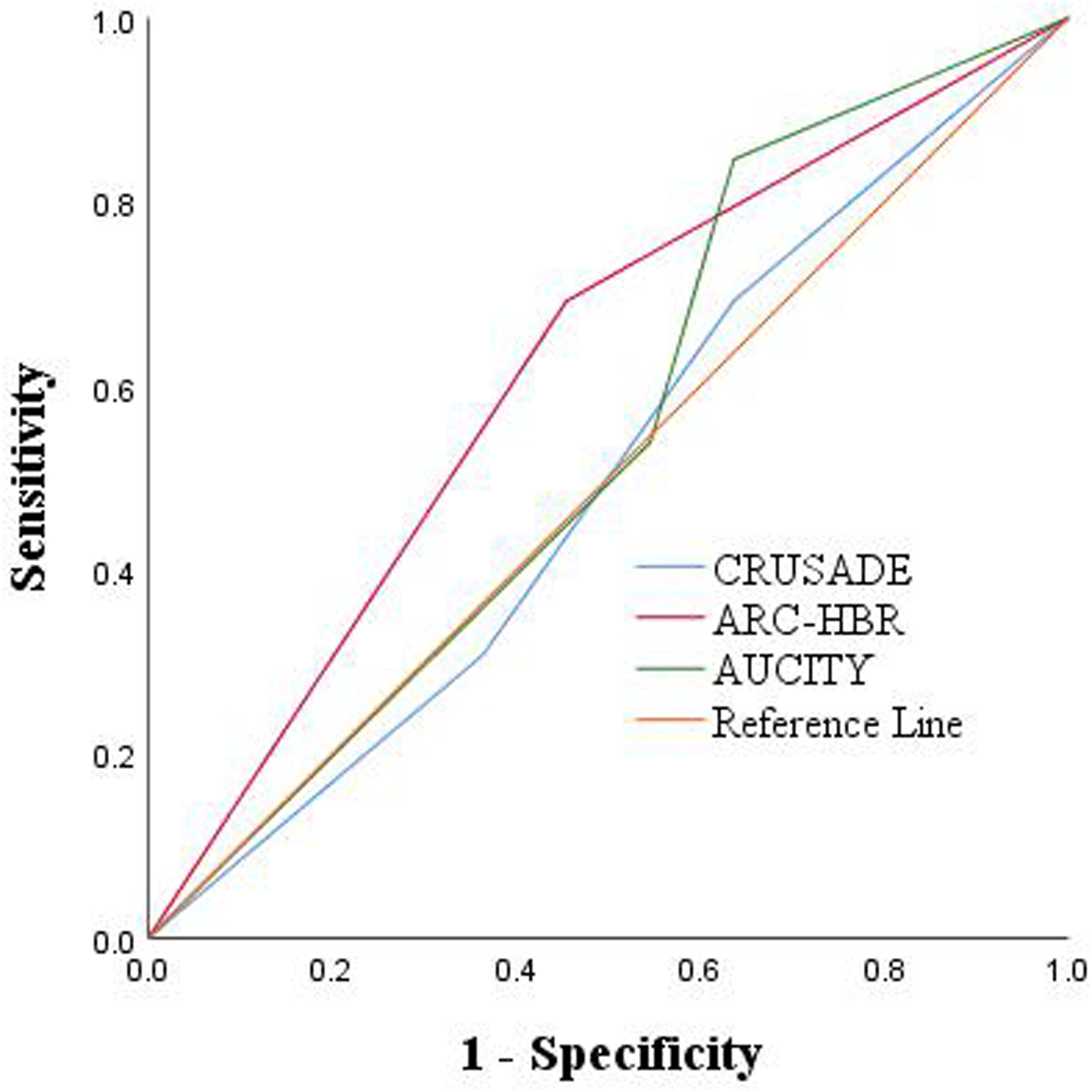
ROC: predictive outcomes with the 3 risk scores.
Table 5
| Variables | AUC (95% CI) | P-value | Sensitivity | Specificity |
|---|---|---|---|---|
| CRUSADE score | 0.500 (0.261–0.739) | 1.000 | 0.692 | 0.364 |
| ARC-HBR score | 0.619 (0.388–0.849) | 0.325 | 0.692 | 0.545 |
| AUCITY score | 0.545 (0.305–0.786) | 0.706 | 0.846 | 0.364 |
ROC: predictive outcomes with the 3 risk scores.
ROC, receiver operating characteristics.
4 Discussion
Although rare, complications of ICH after PCI in patients with coronary artery disease have a poor prognosis and high mortality. In this single-center retrospective study, 24 patients were finally included for analysis. Of these, our findings were as follows: (1) More than half of the patients (n = 13) with concomitant ICH after PCI had a poor prognosis, especially those with an ICH volume of more than 30 cm3 and those who were on a ventilator during their hospital stay. (2) Of the 9 patients with ICH who occurred ventricular extension, a total of 6 (66.7%) died. (4) The CRUSADE, ACUITY, and ARC-HBR scores can complement each other in assessing the risk of ICH occurring after PCI.
Studies on ICH after PCI are scarce so far, especially during hospitalization. Yang et al. studied 121,066 patients undergoing PCI between 2013 and 2022 in the Fu Wai Hospital; they found that the incidence of ICH was 0.015%, and the 90-day mortality was very high (72.2%) (19). Myint et al. analyzed 560,439 patients undergoing PCI between 2007 and 2012 in the British Cardiovascular Intervention Society (BCIS) database and found that the incidence of ICH after PCI during hospitalization was 0.02% (20). Our study ultimately included 24 patients with ICH after PCI, with more than half of them (54.2%) occurring in-hospital death. There were some studies of ICH complicating PCI during postoperative follow-up. In one study that included 11,136 patients, 30 (0.27%) patients developed ICH in the first year after PCI (21). Furthermore, a study using the Korean National Health Insurance Service database found that the cumulative incidence of ICH was 0.54% at 1 year after PCI and increased relatively steadily by 0.25–0.30% per year thereafter (6).
The incidence of ICH after PCI is relatively rare but may result in lifechanging disabilities or even death, so the prevention and treatment of ICH as well as the timely identification of high-risk groups are crucial, but the information of previous studies is limited, so more clinical studies are needed to provide support. Several studies have found advanced age, hypertension, and a history of stroke or transient ischemic attack to be independent predictors of ICH after PCI (6, 21). In our analysis, we found that more than half of ICH appeared within 12 h after PCI and early symptoms of impaired consciousness. Jeffrey et al. found that all hemorrhagic strokes occurred within 48 h of PCI in a study that included 5,372 patients with AMI treated with PCI, suggesting that the risk of early ICH after PCI is high (22). Awareness of complications of ICH is crucial in the early stages of patients undergoing PCI, especially in patients at high risk of bleeding. Therefore, clinicians should appropriately and comprehensively evaluate post-PCI patients for early identification of those at high risk of bleeding and provide aggressive symptomatic management.
One study found that clopidogrel and P2Y12 inhibitors were associated with a similar risk of ICH, which is consistent with our findings (23). The “Bleeding Academic Research Consortium” has put forward a standardized definition for post-PCI bleeding, with ICH being defined as a Type 3C bleed (24). The risk of ICH associated with DAPT is related to the individual and total potency of the drug. In the Stent Anticoagulation Restenosis Study, the risk of hemorrhagic complications in patients using aspirin, aspirin-ticlopidine, and aspirin-warfarin was 1.8%, 5.5%, and 6.2%, respectively (25). The above findings suggest that dual antiplatelet therapy in the perioperative period of PCI and the use of anticoagulant medications may be high-risk factors for the development of ICH, thus necessitating a thorough evaluation of antithrombotic strategies. In our study, aspirin combined with clopidogrel was used preoperatively in 45.8% of patients, and aspirin combined with ticagrelor in 54.2%.
As previously reported by Tuhrim et al., the 30-day mortality rate in patients with intracranial hemorrhage (ICH) was significantly higher when ventricular extension was present (26). Consistent with this, our data showed a high mortality rate among patients with ventricular extension. Specifically, among the 9 patients with ICH who experienced ventricular extension, a substantial proportion succumbed to their illness. Further investigation is warranted to explore the underlying mechanisms and potential interventions to improve outcomes in such high-risk cases. Therefore, it is necessary to review the head CT after the occurrence of ICH to detect any erroneous ventricular extension. Besides, we found that among the 24 patients included, 15(62.5%) patients had hypertension. A systematic review that enrolled 14 studies that examined the relationship between hypertension and ICH showed a positive correlation between hypertension and ICH (27). In addition, a multicenter, randomized phase III trial (ATACH II) demonstrated that aggressive control of blood pressure (target SBP level <140 mmHg) within 3 h of the onset of ICH reduced the risk of death or disability in the 3 months following ICH (28). Therefore, effective management of blood pressure might reduce the risk of bleeding after PCI. The American Heart Association guidelines on the management of ICH, recommend maintaining blood pressure levels below a mean arterial pressure of 130 mmHg (29).
In recent years, several risk scores have been utilized to evaluate bleeding in patients with coronary artery disease (CAD), including the CRUSADE score, ARC-HBR score, and ACUITY score. These scores have been widely applied to assess the risk of nosocomial bleeding in patients with CAD (30). For instance, Costa et al. found that in the overall patient population undergoing PCI, the CRUSADE score predicted major bleeding similarly to ACUITY (16). However, it should be noted that none of these scores have been specifically validated for predicting ICH after PCI. In our study, we explored the potential of these scores in assessing the risk of bleeding after PCI. Although we observed that the ARC-HBR score may have a slight advantage over CRUSADE and ACUITY in predicting poor ICH prognosis, this finding should be interpreted with caution due to the limited power of our study (30). Further large-scale, multi-center studies are needed to comprehensively evaluate the predictive ability of these scores for ICH after PCI.
In addition, we did not collect data on the proportion of patients with intracranial hemorrhage (ICH) and concomitant recent ischemic stroke after percutaneous coronary intervention (PCI), nor did we gather information on other potential causes of ICH. Future studies should consider collecting data on these aspects to provide a more comprehensive understanding of the clinical characteristics and outcomes of ICH after PCI. And the current study mainly focuses on the overall clinical characteristics and outcomes of hospitalized patients with intracranial hemorrhage (ICH) after percutaneous coronary intervention, without further subgroup division for survivors and non—survivors. Future studies may consider conducting subgroup analyses to further explore the differences and potential influencing factors in hospital stay days among patients with different characteristics.
In summary, because of the rapid deterioration, high mortality, and high healthcare costs once a patient undergoes ICH after PCI, it is critical to provide more clinical information to identify those at high risk of bleeding as early as possible and to help determine treatment strategies and clinical decisions. In addition, for the management of ICH after PCI, we recommend close neuromonitoring and early intervention to prevent sustained ICH extension while avoiding cardiovascular events during the temporary interruption of DAPT.
5 Limitations
Our study, as a single-center study, has several limitations that may affect the outcome and analysis. Firstly, this is a retrospective study and, therefore, suffers from the inherent limitations of observational databases. Secondly, out-of-hospital and asymptomatic ICH were not included in our study. Besides, our study did not routinely perform CT scans in patients after PCI; therefore, the incidence of subclinical ICH may have been underestimated. Thirdly, our study did not routinely explore the etiology of patients with ICH, and it is difficult to determine the cause of ICH because only an initial CT scan of the brain was performed without further imaging. Finally, there were some missing patient history data when collected in this study, which may affect the overall reliability of the analysis. Therefore, future large-scale, multicenter, and prospective studies are necessary to provide more evidence. Despite its limitations, it also has essential strengths worth considering. This study provides clinical practitioners with additional clinical evidence regarding the management of bleeding related to PCI, which can help to improve the prognosis of these patients.
6 Conclusions
The mortality of ICH after PCI was high and high volume of ICH indicates a high risk of death. Although ICH post-PCI is a rare, it remains a great challenge and dilemma regarding how to manage these patients. In our study, we provide clinical information on the clinical features, imaging manifestations, and mortality of ICH patients after PCI during hospitalization, providing assistance in developing optimal treatments to improve the prognosis of these patients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Human Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Investigation, Methodology, Writing – original draft, Writing – review & editing. XS: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – review & editing. PL: Investigation, Methodology, Writing – review & editing. YT: Investigation, Supervision, Writing – review & editing. DC: Investigation, Supervision, Writing – review & editing. HL: Investigation, Supervision, Writing – review & editing. HS: Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Li DL Kronenberg MW . Myocardial perfusion and viability imaging in coronary artery disease: clinical value in diagnosis, prognosis, and therapeutic guidance. Am J Med. (2021) 134(8):968–75. 10.1016/j.amjmed.2021.03.011
2.
Neumann FJ Sousa-Uva M Ahlsson A Alfonso F Banning AP Benedetto U et al 2018 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. (2019) 14(14):1435–534. 10.4244/EIJY19M01_01
3.
Cao D Chandiramani R Chiarito M Claessen BE Mehran R . Evolution of antithrombotic therapy in patients undergoing percutaneous coronary intervention: a 40-year journey. Eur Heart J. (2021) 42(4):339–51. 10.1093/eurheartj/ehaa824
4.
van der Sangen NMR Rozemeijer R Chan Pin Yin DRPP Valgimigli M Windecker S James SK et al Patient-tailored antithrombotic therapy following percutaneous coronary intervention. Eur Heart J. (2021) 42(10):1038–46. 10.1093/eurheartj/ehaa1097
5.
Simonsson M Wallentin L Alfredsson J Erlinge D Hellström Ängerud K Hofmann R et al Temporal trends in bleeding events in acute myocardial infarction: insights from the SWEDEHEART registry. Eur Heart J. (2020) 41(7):833–43. 10.1093/eurheartj/ehz593Erratum in: Eur Heart J. 2022 September 1;43(33):3184. doi: 10.1093/eurheartj/ehac310.
6.
Lee PH Park S Nam H Kang DY Kang SJ Lee SW et al Intracranial bleeding after percutaneous coronary intervention: time-dependent incidence, predictors, and impact on mortality. J Am Heart Assoc. (2021) 10(15):e019637. 10.1161/JAHA.120.019637
7.
Al-Shammari AS Ibrahim A Shalabi L Khan M Rafiqul Islam M Alsawadi RA et al Comparison between mono vs dual vs triple antiplatelet therapy in patients with ischemic heart disease undergoing PCI, a network meta-analysis. Curr Probl Cardiol. (2024) 49(11):102755. 10.1016/j.cpcardiol.2024.102755
8.
van Asch CJ Luitse MJ Rinkel GJ van der Tweel I Algra A Klijn CJ . Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9(2):167–76. 10.1016/S1474-4422(09)70340-0
9.
Broderick J Connolly S Feldmann E Hanley D Kase C Krieger D et al Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Circulation. (2007) 116(16):e391–413. 10.1161/CIRCULATIONAHA.107.183689
10.
Levine GN Bates ER Bittl JA Brindis RG Fihn SD Fleisher LA et al 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. (2016) 134(10):e123–55. 10.1161/CIR.0000000000000404Epub 2016 March 29. Erratum in: Circulation. 2016 September 6;134(10):e192-4. doi: 10.1161/CIR.0000000000000452.
11.
An SJ Kim TJ Yoon B-W . Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. (2017) 19(1):3–10. 10.5853/jos.2016.00864
12.
Hemphill JC 3rd Bonovich DC Besmertis L Manley GT Johnston SC . The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. (2001) 32(4):891–7. 10.1161/01.str.32.4.891
13.
Kwak R Kadoya S Suzuki T . Factors affecting the prognosis in thalamic hemorrhage. Stroke. (1983) 14(4):493–500. 10.1161/01.STR.14.4.493
14.
Hillal A Sultani G Ramgren B Norrving B Wassélius J Ullberg T . Accuracy of automated intracerebral hemorrhage volume measurement on non-contrast computed tomography: a Swedish stroke register cohort study. Neuroradiology. (2023) 65(3):479–88. 10.1007/s00234-022-03075-9
15.
Krishnan K Mukhtar SF Lingard J Houlton A Walker E Jones T et al Performance characteristics of methods for quantifying spontaneous intracerebral haemorrhage: data from the efficacy of nitric oxide in stroke (ENOS) trial. J Neurol Neurosurg Psychiatry. (2015) 86(11):1258–66. 10.1136/jnnp-2014-309845
16.
Zhang P Pang S Du L Li J Su X . Clinical characteristics and outcomes of patients with intracerebral hemorrhage after acute myocardial infarction. Int J Cardiol. (2023) 390:131218. 10.1016/j.ijcard.2023.131218
17.
Urban P Mehran R Colleran R Angiolillo DJ Byrne RA Capodanno D et al Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research consortium for high bleeding risk. Eur Heart J. (2019) 40(31):2632–53. 10.1093/eurheartj/ehz372
18.
Correia LC Ferreira F Kalil F Silva A Pereira L Carvalhal M et al Comparison of ACUITY and CRUSADE scores in predicting major bleeding during acute coronary syndrome. Arq Bras Cardiol. (2015) 105(1):20–7. 10.5935/abc.20150058
19.
Yang C Sui YG Wang BC Xu YL Wu NQ Wu YJ et al Intracranial hemorrhage in hospitalized patients following percutaneous coronary intervention: a large cohort analysis from a single center. Diagnostics (Basel). (2023) 13(14):2422. 10.3390/diagnostics13142422
20.
Myint PK Kwok CS Roffe C Kontopantelis E Zaman A Berry C et al Determinants and outcomes of stroke following percutaneous coronary intervention by indication. Stroke. (2016) 47(6):1500–7. 10.1161/STROKEAHA.116.012700
21.
Raposeiras-Roubín S Abu-Assi E Caneiro Queija B Cobas Paz R D'Ascenzo F Henriques JPS et al Incidence, predictors and prognostic impact of intracranial bleeding within the first year after an acute coronary syndrome in patients treated with percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care. (2020) 9(7):764–70. 10.1177/2048872619827471
22.
Guptill JT Mehta RH Armstrong PW Horton J Laskowitz D James S et al Stroke after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: timing, characteristics, and clinical outcomes. Circ Cardiovasc Interv. (2013) 6(2):176–83. 10.1161/CIRCINTERVENTIONS.112.000159
23.
Ng AK Ng PY Ip A Lau KK Siu CW . Risk of ischaemic and haemorrhagic stroke in Chinese undergoing percutaneous coronary intervention treated with potent P2Y12 inhibitor versus clopidogrel. Stroke Vasc Neurol. (2022) 7(4):310–8. 10.1136/svn-2021-001294
24.
Mehran R Rao SV Bhatt DL Gibson CM Caixeta A Eikelboom J et al Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123(23):2736–47. 10.1161/CIRCULATIONAHA.110.009449
25.
Leon MB Baim DS Popma JJ Gordon PC Cutlip DE Ho KK et al A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent anticoagulation restenosis study investigators. N Engl J Med. (1998) 339(23):1665–71. 10.1056/NEJM199812033392303
26.
Tuhrim S Horowitz DR Sacher M Godbold JH . Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. (1999) 27(3):617–21. 10.1097/00003246-199903000-00045
27.
Ariesen MJ Claus SP Rinkel GJ Algra A . Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. (2003) 34(8):2060–5. 10.1161/01.STR.0000080678.09344.8D
28.
Qureshi AI Palesch YY . Antihypertensive treatment of acute cerebral hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. (2011) 15(3):559–76. 10.1007/s12028-011-9538-3
29.
Broderick J Brott T Kothari R Miller R Khoury J Pancioli A et al The greater cincinnati/northern Kentucky stroke study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. (1998) 29(2):415–21. 10.1161/01.str.29.2.415
30.
Liu J He H Su H Hou J Luo Y Chen Q et al The predictive value of the ARC-HBR criteria for in-hospital bleeding risk following percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol Heart Vasc. (2024) 55:101527. 10.1016/j.ijcha.2024.101527
Summary
Keywords
intracranial hemorrhage, percutaneous coronary intervention, coronary artery disease, mortality, clinical characteristics
Citation
Zhou Y, Su X, Liu P, Tang Y, Cheng D, Li H and Sang H (2025) Clinical characteristics and outcomes of hospitalized patients with intracranial hemorrhage after percutaneous coronary intervention. Front. Cardiovasc. Med. 12:1424598. doi: 10.3389/fcvm.2025.1424598
Received
11 June 2024
Accepted
25 February 2025
Published
11 March 2025
Volume
12 - 2025
Edited by
Christian Cadeddu Dessalvi, University of Cagliari, Italy
Reviewed by
Mona Laible, Ulm University, Germany
Luca Fazzini, University of Cagliari, Italy
Updates
Copyright
© 2025 Zhou, Su, Liu, Tang, Cheng, Li and Sang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xin Su suxin901524@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.