Abstract
Tetralogy of Fallot (ToF) is a chronic hypoxic condition that increases the risk of inflammatory responses during surgery. However, many inflammatory biomarkers used to predict surgical outcomes are costly and not widely accessible. This single-center, retrospective observational study aimed to compare the prognostic value of neutrophil-lymphocyte ratio (NLR), absolute lymphocyte count (ALC), and thrombocyte-lymphocyte ratio (TLR) in predicting outcomes of ToF primary repair. Between January 2020 and December 2022, 501 patients underwent ToF primary repair. Our findings revealed low postoperative reoperation (6.5%) and 30-day mortality (4.7%) rates, but prolonged LOS (92.2%) and a high complication rate (84%), with grade IVa (27.9%) and grade I (26.4%) complications being the most common. Elevated NLR (r = 0.132, p = 0.014), female gender (r = 0.117, p = 0.027), associated diagnoses (r = 0.104, p = 0.047), and prolonged surgical duration (r = 0.176, p = 0.001) were linked to higher complication burdens, though the correlations were weak. Among the three indexes, preoperative NLR demonstrated the strongest predictive value for complications, despite its low sensitivity and specificity. Given its accessibility and cost-effectiveness, NLR may serve as a useful tool for identifying high-risk patients and improving postoperative monitoring. Future multicenter prospective studies are necessary to validate the prognostic value of preoperative NLR under standardized conditions, across a broader patient population, and with more comprehensive confounding variables adjustments, ultimately improving ToF surgical outcomes (Clinical Trial NCT05976204).
1 Introduction
All surgical procedures, including congenital heart disease (CHD) surgeries, may induce inflammation as a result of a physiological response to trauma (1–3). In addition, most CHD operations are conducted at an early age and most of the organs are not fully developed, thus leading to poor prognosis and high risk of mortality (2). The use of cardiopulmonary bypass (CPB) may exacerbate this response and cause pathological complications as well as detrimental effects in various organs thus increasing morbidity and mortality in the postoperative period (2, 4, 5).
In the case of tetralogy of Fallot (ToF), chronic hypoxemia creates an imbalance between pro-inflammatory and anti-inflammatory responses in the preoperative period. This imbalance causes lethal injury to the myocardium which is then exacerbated by surgical trauma, CPB, and other factors resulting in prolonged mechanical ventilation time, duration of internsive care unit (ICU) stay, and total duration of hospitalization (2, 3, 6–9). Therefore, clinical indicators are required to help predict the morbidity and mortality risk of ToF surgery.
Various studies have recently shown some inflammatory biomarkers which may be closely related to increased morbidity and mortality in hypoxemic patients compared to those in acyanotic patients, but they are not readily available and expensive (4, 7, 10–12). Complete blood count with differential count was an inexpensive, repeatable, and widely available test for children undergoing ToF primary repair. Neutrophils are the first responders to inflammatory signals which are released from the damaged cells and tissues (3, 6, 9). On the contrary, lymphocytes decrease in response to inflammation as shown by its close relation with general health status and chronic inflammation state (13). Thrombocytes are not only involved in hemostasis, thrombosis, and wound healing, but also have important roles in immune regulation and inflammatory response (14).
Previous studies have shown the role of preoperative neutrophil-lymphocyte ratio (NLR) (15–21) and absolute lymphocyte count (ALC) (22–24) in predicting the outcome of congenital heart surgery. However, there were very limited studies on these inflammatory biomarkers as predictive indexes of ToF surgery outcomes (13, 19, 21). Despite its established association with coronary artery disease (25–29) and potential roles in pediatric cardiac surgery (30, 31), there was no study on preoperative thrombocyte-lymphocyte ratio (TLR) and its predictive value of ToF surgery. Furthermore, no study had compared these three preoperative indexes for their roles in predicting outcomes of ToF surgery. Therefore, this study aims to compare the prognostic value of these inflammatory biomarkers in predicting ToF surgery outcomes.
2 Materials and methods
2.1 Study population
This was a retrospective observational study on ToF primary repair in a single tertiary national cardiovascular center (National Cardiovascular Center Harapan Kita) between January 2020 and December 2022. The inclusion criteria were all ToF patients with any other associated cardiac anomalies, who underwent ToF primary repair and had a complete blood cell count with differential count available preoperatively. The exclusion criteria were as follows: surgery other than ToF primary repair, association with other procedures (except patent ductus arteriosus/PDA ligation, patent foramen ovale/PFO or atrial septal defect/ASD closure, or pulmonary arteries enlargement), preoperative hemodynamic instability, suspected or confirmed infection with prior antibiotic administration during the same hospital admission, and absence of complete blood count with differential count.
2.2 Preoperative and intraoperative data
All the data were obtained from the data registry in our center. The preoperative demographic data included were patients' gender, age, weight, oxygen saturation, and associated diagnoses other than ToF. The preoperative data of complete blood count and differential count must be tested from the most recent peripheral blood samples taken no later than 7 days before the surgery. The data obtained were the number of days of the most recent blood test including the leukocyte count, percentage neutrophil, percentage lymphocyte, and thrombocyte count, as well as the derived variables such as NLR, ALC, and TLR. The intraoperative data included were CPB time, aortic cross-clamp (AOX) time, and the total duration of surgery.
2.3 Evaluation and follow-up
Patients were evaluated and followed up for any complications and postoperative mortality during the same hospital stay until discharge. Patients were then followed up for any cause of mortality within 30 days postoperative. The primary endpoints were reoperation within the same hospital admission, mortality within 30 days postoperative, and complications. The complications were graded in severity using the modified Clavien-Dindo Complications Classification (CDCC) from least to most severe (grade I–V) (32). The Comprehensive Complication Index (CCI) was then used to convert these complications into cumulative severity scores using the formula (33). The secondary endpoints were hospital length of stay and postoperative length of stay.
2.4 Definition of variables
NLR was defined as the ratio of the absolute count of neutrophils to lymphocytes.
TLR was defined as the ratio of the absolute count of thrombocytes to lymphocytes.
Reoperation was defined as additional or corrective surgery after the initial primary ToF repair within the same hospital admission.
Hospital length of stay (HLOS) was defined as the duration of in-hospital stay from the initial admission to discharge.
Longer postoperative LOS was institutionally defined as in-hospital stay more than 5 days from perioperative to discharge.
Complication was defined as any adverse events that arose perioperative or postoperative to discharge.
2.5 Statistical analysis
Categorical data were expressed as counts and percentages. Kolmogorov–Smirnov test was used as a normality test for continuous variables expressed as means ± standard deviations in normally distributed data or median and interquartile range (IQR), in not normally distributed data. Student T test (normal distribution) or Mann–Whitney test (not normal distribution) was used to compare two groups with numerical data. Anova test (normal distribution) or Kruskal–Wallis test (not normal distribution) was used to compare more than two groups with numerical data. Chi-square test and Fischer's exact test (if did not meet the criteria for Chi-square test) were used for categorical data due to the small sample size. Pearson test (normal distribution) and Spearman test (not normal distribution) were used for correlation tests. In general, p-value <0.05 was considered statistically significant. Univariate and multivariate analyses were performed to evaluate the association between preoperative indexes with significant outcomes, based on the univariate analysis. Receiving Operating Characteristic (ROC) curve analysis was used to determine the optimal cutoff levels of the preoperative NLR, ALC, and TLR as well as comparing these markers to predict the significant outcomes. The statistical analyses were performed using SPSS 26.0.
2.6 Ethical approval
This study has been reviewed and approved by the Research Ethics Committee of National Cardiovascular Center Harapan Kita. No informed consent was needed from the patients as this study used secondary data from the database.
3 Results
3.1 Baseline characteristics and outcomes
Based on the inclusion and exclusion criteria, a total of 387 patients who underwent ToF primary repair between January 2020 and December 2022 were retrospectively enrolled. Most of the patients were male (56.6%), had associated diagnoses other than ToF (63.8%), and weighed ≥10 kg (64.6%). Other preoperative data of the patients are summarized in Table 1. The most common associated diagnosis was PDA (28.7%), followed by ASD (27.9%) with ASD secundum as the most common subtype (15.8%), see Table 2.
Table 1
| Variable | Groups | N (%)/Median (IQR)/Mean (SD) | 95% CI (%) |
|---|---|---|---|
| Age (months) | 44.8 (21.8–99.8) | 69.1–86.1 | |
| Gender | Female | 168 (43.4) | |
| Male | 219 (56.6) | ||
| Weight (kg) | 12 (8.5–18) | 15.8–18.6 | |
| <10 kg | 137 (35.4) | ||
| ≥10 kg | 250 (64.6) | ||
| SpO2 (%) | 77 (69–86) | 75–77.7 | |
| Associated Diagnosis | No | 140 (36.2) | |
| Yes | 247 (63.8) | ||
| Preoperative Days Blood Obtained | 3 (2–5) | 3.2–3.6 | |
| Leukocyte Count | 8,570 (7,190–10,150) | 8,719.7–9,239.7 | |
| Neutrophil Percentage | 42.1 (14.7) | 40.6–43.5 | |
| Lymphocyte Percentage | 46.5 (14.3) | 45–47.9 | |
| Thrombocyte Count | 2,99,685.7 (1,14,658) | 2,88,226.3–3,11,145.1 | |
| NLR | 0.88 (0.56–1.43) | 1.06–1.4 | |
| ALC | 3,854 (2,806–5,299) | 3,994.6–4,374.7 | |
| TLR | 72.3 (54.89–98.9) | 79.09–88.49 |
Preoperative data of the included patients.
ALC, absolute lymphocyte count; CI, confidence interval; IQR, interquartile range; NLR, neutrophil lymphocyte ratio; SpO2, blood oxygen saturation measured by pulse oximetry; TLR, thrombocyte lymphocyte ratio; kg, kilogram.
Table 2
| Associated Diagnosis | N | % | |
|---|---|---|---|
| Down Syndrome | 7 | 2 | |
| Persistent Left SVC | 10 | 2.6 | |
| Persistent Left IVC | 1 | 0.3 | |
| ASD | 108 | 27.9 | |
| Sinus Venosus | 1 | 0.3 | |
| Secundum | 61 | 15.8 | |
| PFO | 46 | 11.9 | |
| VSD Muscular | 1 | 0.3 | |
| VSD outlet | 5 | 1.3 | |
| AVSD incomplete | 2 | 0.5 | |
| PDA | 111 | 28.7 | |
| MAPCAs | 45 | 11.6 | |
| PA Stenosis | 15 | 3.9 | |
| PA Hypoplasia | 6 | 1.6 | |
| PV Stenosis | 2 | 0.5 | |
| PV Atresia | 32 | 8.3 | |
| PV Absent | 5 | 1.3 | |
| Others | 3 | 0.8 | |
| PA from PDA | 1 | 0.3 | |
| Coronary AV fistula | 1 | 0.3 | |
| Dextrocardia | 1 | 0.3 | |
Associated diagnosis of the patients.
ASD, atrial septal defect; AV, arteriovenous; AVSD, atrioventricular septal defect; IVC, inferior vena cava; MAPCAs, major aortopulmonary collateral arteries; PA, pulmonary artery; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PV, pulmonary valve; SVC, superior vena cava; VSD, ventricular septal defect.
The median of CPB time, AOX time, and surgery duration were 103 (82–128) min, 53 (42–67) min, and 241 (210–300) min respectively, see Table 3 for more details. There were 25 patients (6.5%) who needed reoperation due to residual ventricular septal defect and residual pulmonary stenosis as well as 18 deaths (4.7%) due to complications. The median hospital length of stay (HLOS) was 9 (6–14) days and the postoperative LOS was 7 (5–10) days. Most of the patients had complications (84%) with majority of grade IVa complications (27.9%), followed by grade I complications (26.4%). The median CCI was 25.7 (8.7–44.3), see Table 4. The recorded complications were cardiac complication (arrhythmia, pericardial effusion or tamponade, low cardiac output syndrome or right heart failure, ventricular dysfunction, cardiac arrest), pulmonary complication (pneumonia, pleural effusion, hemothorax, pneumothorax, atelectasis), kidney complication (acute kidney injury), other complications (anemia, thrombocytopenia, metabolic acidosis, electrolyte imbalance), including death.
Table 3
| Variable | Groups | N (%)/Median (IQR) | 95% CI (%) |
|---|---|---|---|
| CPB time (min) | 103 (82–128) | 106.6–123.4 | |
| <100 min | 186 (48.1) | ||
| ≥100 min | 201 (51.9) | ||
| AOX time (min) | 53 (42–67) | 55.6–61.1 | |
| Surgery duration (min) | 241 (210–300) | 252.1–271 |
Intraoperative data of the patients.
AOX, aortic cross clamp; CI, confidence interval; CPB, cardiopulmonary bypass; IQR, interquartile range.
Table 4
| Variable | Groups | N (%) | 95% CI (%) |
|---|---|---|---|
| Reoperation | No | 362 (93.5) | |
| Yes | 25 (6.5) | ||
| Mortality | No | 369 (95.3) | |
| Yes | 18 (4.7) | ||
| HLOS (days) | 9 (6–14) | 10.9–12.6 | |
| Postoperative LOS (days) | 7 (5–10) | 8.7–10.1 | |
| <5 days | 30 (7.8) | ||
| ≥5 days | 357 (92.2) | ||
| Complications | No | 62 (16) | |
| Yes | 325 (84) | ||
| Grade of Complication | No | 62 (16) | |
| I | 102 (26.4) | ||
| II | 31 (8) | ||
| III | 54 (14) | ||
| IIIa | 24 (6.2) | ||
| IIIb | 30 (7.8) | ||
| IV | 120 (31) | ||
| IVa | 108 (27.9) | ||
| IVb | 12 (3.1) | ||
| V | 18 (4.7) | ||
| CCI | 25.7 (8.7–44.3) | 28.1–33.4 |
Outcomes of the patients.
CCI, comprehensive complication index; CI, confidence interval; CDCC, Clavien-Dindo complications classification; HLOS, hospital length of stay; LOS, length of stay.
3.2 Univariate analysis
Univariate analyses between preoperative NLR, ALC, and TLR with the patients' outcomes were summarized in Tables 5, 6.
Table 5
| Variables | Groups | NLR | p-value | ALC | p-value | TLR | p-value |
|---|---|---|---|---|---|---|---|
| Mortality | No | 0.87 (0.56–1.39) | 0.530 | 3,864 (2,802.5–5,282.5) | 0.925 | 72.19 (54.82–98.84) | 0.471 |
| Yes | 0.9 (0.57–2.03) | 3,632 (2,800.25–5,449.5) | 81.82 (54.43–123.82) | ||||
| Reoperation | No | 0.88 (0.57–1.37) | 0.922 | 3,853.5 (2,807.5–5,258.5) | 0.761 | 72.25 (55.21–98.55) | 0.992 |
| Yes | 0.88 (0.4–2.35) | 4,218 (2,290.5–5,556.5) | 73.69 (49.68–116.86) | ||||
| Complications | No | 0.74 (0.47–1.26) | 0.077 | 3,802 (2,640–5,497) | 0.832 | 64.89 (53.42–89.15) | 0.126 |
| Yes | 0.89 (0.59–1.43) | 3,879 (2,855–5,261) | 76.37 (55.33–100.33) | ||||
| Grade of Complication | No | 0.74 (0.47–1.26) | 0.100 | 3,802 (2,640–5,497) | 0.019* | 64.89 (53.42–89.15) | 0.274 |
| I | 0.85 (0.55–1.32) | 4,192.5 (3,348.8–5,752.5) | 71.93 (54.43–96.14) | ||||
| II | 0.89 (0.66–1.69) | 3,942 (2,808–5,266) | 65.53 (42.14–97.6) | ||||
| IIIa | 1.11 (0.63–1.66) | 3,616 (2,501.5–4,351.5) | 87.32 (64.57–100.34) | ||||
| IIIb | 1.23 (0.71–1.86) | 3,063 (2,172.5–4,128.3) | 77.91 (61.96–116.83) | ||||
| IVa | 0.9 (0.55–1.35) | 3,704.5 (2,577.5–5,160.8) | 78.56 (53.99–104.82) | ||||
| IVb | 0.83 (0.53–0.98) | 4,462.6 (3,270–5,186.3) | 61.14 (37.26–115.29) | ||||
| V | 0.9 (0.57–2.03) | 3,632 (2,800.3–5,449.5) | 81.82 (54.43–123.82) | ||||
| Postoperative LOS | <5 days | 0.92 (0.57–1.43) | 0.822 | 3,732.5 (3,004–4,960.8) | 0.746 | 87.95 (67.64–115.5) | 0.026* |
| ≥5 days | 0.87 (0.56–1.42) | 3,882 (2,795–5,360.5) | 71 (54.68–98.46) |
Univariate categorical analysis of ToF surgery outcomes.
Significant p-value (<0.05).
ALC, absolute lymphocyte count; NLR, neutrophil lymphocyte ratio; TLR, thrombocyte lymphocyte ratio.
Table 6
| Variables | Correlation coefficient (NLR) | p-value | Correlation coefficient (ALC) | p-value | Correlation coefficient (TLR) | p-value |
|---|---|---|---|---|---|---|
| HLOS | −0.008 | 0.879 | −0.002 | 0.961 | −0.161 | 0.001* |
| Postoperative LOS | −0.01 | 0.844 | 0.026 | 0.603 | −0.157 | 0.002* |
| CCI | 0.87 | 0.086 | −0.078 | 0.126 | 0.095 | 0.061 |
Univariate numerical analysis of ToF surgery outcomes.
Significant p-value (<0.05).
ALC, absolute lymphocyte count; HLOS, hospital length of stay; LOS, length of stay; NLR, neutrophil lymphocyte ratio; TLR, thrombocyte lymphocyte ratio.
3.2.1 Neutrophil-Lymphocyte ratio
Higher preoperative NLR was observed in patients with mortality and complications, though the association lacked statistical significance. Preoperative NLR showed a strong positive correlation with CCI, though not statistically significant. Conversely, higher preoperative NLR was observed in patients with shorter HLOS and postoperative LOS, but without statistical significance.
3.2.2 Absolute lymphocyte count
Preoperative ALC was significantly associated with complication grade, though inconsistently. Median preoperative ALC was lower in non-survivors but higher in patients undergoing reoperation or experiencing complications. Weak, non-significant correlations were observed between preoperative ALC and HLOS, postoperative LOS, and CCI.
3.2.3 Thrombocyte-Lymphocyte ratio
Higher preoperative TLR was linked to poorer outcomes (mortality, reoperation, complication), though not statistically significant. Preoperative TLR showed a weak positive correlation with CCI and weak negative correlations with HLOS and postoperative LOS, all without statistical significance. Conversely, higher preoperative TLR was associated significantly with postoperative LOS < 5 days.
3.3 Univariate and multivariate logistic regression analysis of complications
We conducted univariate analyses, then a multivariate logistic regression to determine the association between NLR, TLR, and ALC with complication of ToF surgery which was categorized into two groups (without complication and with complication). Based on the univariate analysis, variables included in the multivariate analysis were weight, associated diagnosis, NLR, and TLR. Even though lacking statistical significance, there was a higher risk of complication in patients with higher NLR and no associated diagnosis (Table 7).
Table 7
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Gender (Female) | 1.16 (0.67–2.02) | 0.592 | ||
| Age (months) | 1 (1–1) | 0.316 | ||
| Weight (kg) | 1.01 (0.99–1.03) | 0.117 | 1 (0.98–1.03) | 0.840 |
| Weight (<10 kg) | 0.72 (0.41–1.25) | 0.240 | ||
| SpO2 (%) | 0.99 (0.97–1.02) | 0.724 | ||
| Associated Diagnosis (Yes) | 1.69 (0.98–2.93) | 0.058 | 1.71 (0.98–2.96) | 0.058 |
| NLR | 1.21 (0.87–1.68) | 0.077 | 1.22 (0.79–1.86) | 0.371 |
| ALC | 1 (1–1) | 0.832 | ||
| TLR | 1 (1–1.01) | 0.126 | 1 (0.99–1.01) | 0.879 |
| Surgery Duration (minutes) | 1 (1–1) | 0.637 | ||
| CPB time (minutes) | 1 (1–1.01) | 0.654 | ||
| CPB (<100 min) | 1.18 (0.69–2.04) | 0.541 | ||
| AOX time (minutes) | 1 (0.99–1.01) | 0.532 | ||
Univariate and multivariate logistic regression analysis of categorized complications.
ALC, absolute lymphocyte count; CI, confidence interval; IQR, interquartile range; NLR, neutrophil lymphocyte ratio; SpO2, blood oxygen saturation measured by pulse oxymetry; TLR, thrombocyte lymphocyte ratio; kg, kilogram AOX, aortic cross clamp; CI, confidence interval; CPB, cardiopulmonary bypass; IQR, interquartile range.
3.4 ROC curve
First, both preoperative NLR, ALC, and TLR were compared using ROC curves for the value in predicting complication in ToF primary repair (Figure 1). The areas under the curve (AUC) were the highest in preoperative NLR (0.571, 95% CI: 0.490–0.652; p = 0.077), followed by TLR (0.561, 95% CI: 0.486–0.637), and ALC (0.492, 95% CI: 0.409–0.575). Based on the ROC curve analysis, the cutoff values for preoperative NLR, TLR, and ALC as a predictor of moderate and severe complications were >0.61 (sensitivity: 75%, specificity: 57%), >76 (sensitivity: 50%, specificity: 29%), and >4346 (sensitivity: 40%, specificity: 38%), respectively. Using the model from multivariate logistic regression analysis, we compared the ROC curves using 2 models: one model which included NLR and another model which excluded NLR. In the model with preoperative NLR, the AUC was 0.591 (95% CI: 0.512–0.671, p = 0.022). Another model without preoperative NLR showed similar AUC of 0.594 (95% CI: 0.516–0.673, p = 0.019), see Figure 2.
Figure 1
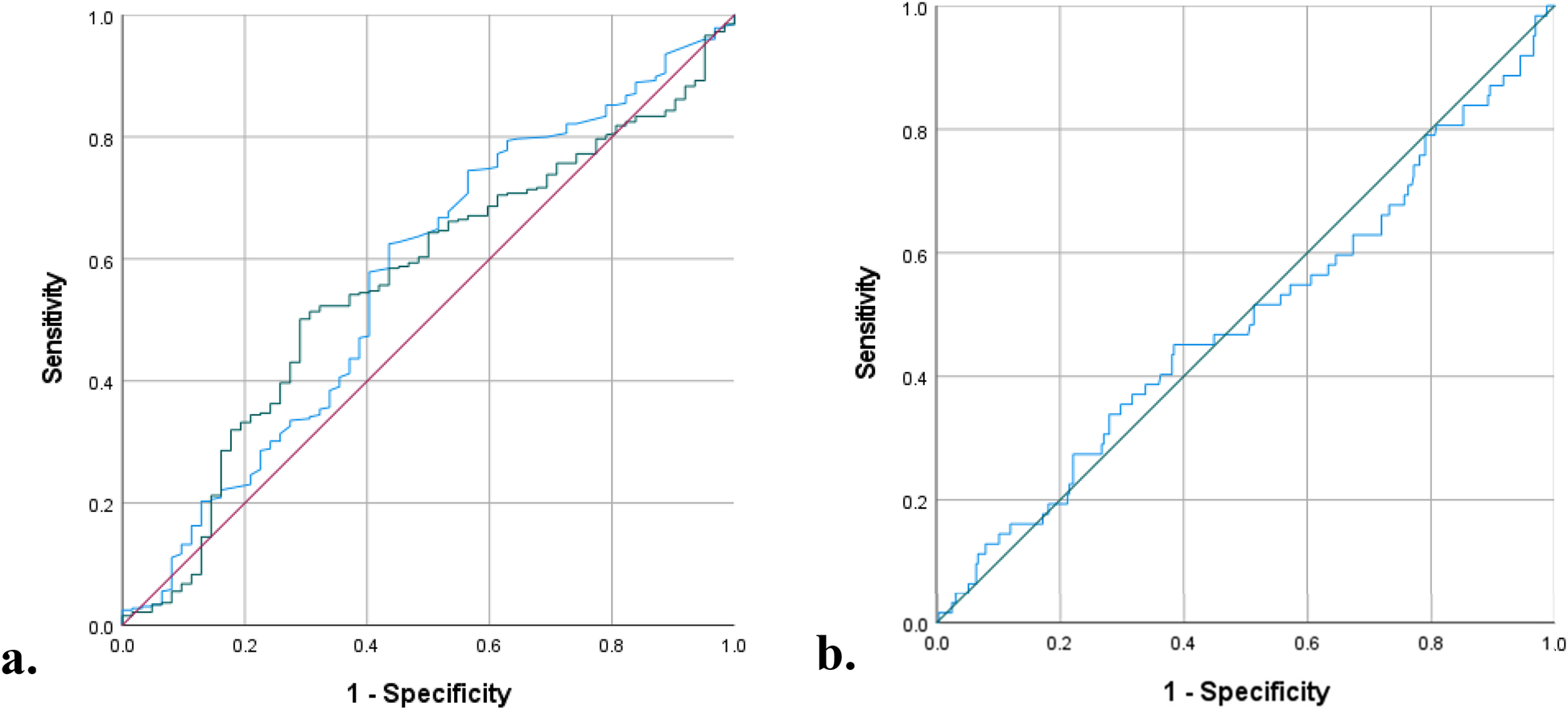
ROC curves: (a) preoperative NLR (blue curve) vs. TLR (green curve) (b) Preoperative ALC (blue curve), for prediction of complication.
Figure 2
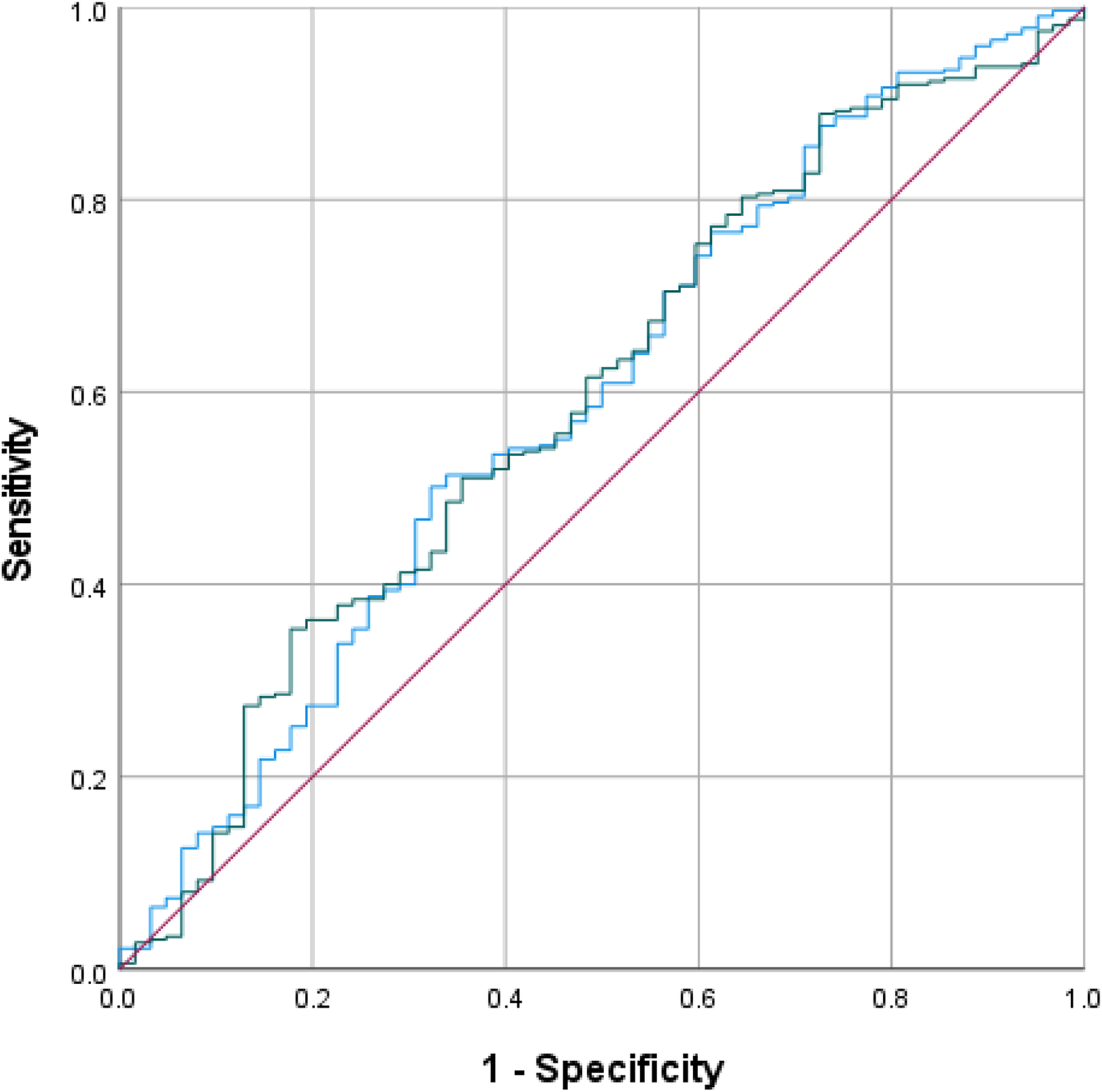
ROC curves for the model with (blue curve) and without (green curve) preoperative NLR.
3.5 Univariate and multivariate linear regression analysis of CCI
Gender, associated diagnosis, NLR, ALC, TLR, surgery duration, CPB time, and AOX time were correlated significantly with CCI. However, multivariate linear regression revealed that only gender, associated diagnosis, NLR, and surgery duration were significantly correlated with CCI, though the correlations were weak (R 0.294, R2 8.6%, Adjusted R2 7.5%, Standard Error of the Estimate 25.3), see Table 8. Based on the regression (Figure 3), the following equation was produced: CCI = 7.901 + 6.186*Gender (Female = 1, Male = 0) + 5.754*Associated Diagnosis (Yes = 1, No = 0) + NLR*2.147 + Duration of Surgery*0.052.
Table 8
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Coefficient correlation | p-value | Coefficient correlation | p-value | |
| Gender (Female) | 0.101 | 0.046* | 0.117 | 0.027* |
| Age (months) | 0.007 | 0.898 | ||
| Weight (kg) | 0.028 | 0.585 | ||
| SpO2 (%) | −0.040 | 0.431 | ||
| Associated Diagnosis (Yes) | 0.141 | 0.006* | 0.104 | 0.047* |
| NLR | 0.087 | 0.086* | 0.132 | 0.014* |
| ALC | −0.078 | 0.126* | ||
| TLR | 0.095 | 0.061* | ||
| Surgery Duration (minutes) | 0.135 | 0.011* | 0.176 | 0.001* |
| CPB time (minutes) | 0.175 | <0.001* | ||
| AOX time (minutes) | 0.124 | 0.017* | ||
Univariate and multivariate linear regression analysis of comprehensive complication index.
Significant p-value <0.05.
ALC, absolute lymphocyte count; CI, confidence interval; IQR, interquartile range; NLR, neutrophil lymphocyte ratio; SpO2, blood oxygen saturation measured by pulse oxymetry; TLR, thrombocyte lymphocyte ratio; kg, kilogram AOX, aortic cross clamp; CI, confidence interval; CPB, cardiopulmonary bypass; IQR, interquartile range.
Figure 3
Regression linear plot of CCI.
4 Discussion
Tetralogy of Fallot (ToF) is a common cyanotic congenital heart diseases that leads to chronic hypoxia, disrupting long-term energy metabolism and causing myocardial and systemic organ injury (34, 35). This condition induces hypoxia-related inflammation due to an imbalance between pro-inflammatory and anti-inflammatory responses, increasing the expression of genes linked to apoptosis and remodeling while decreasing the expression of genes associated with myocardial contractility and function. Consequently, myocardial stress triggers the activation of various genes, including cytokines such as IL-6 and TNF-α, further contributing to inflammatory processes (8, 36, 37).
Since ToF often coexists with associated diagnoses, we did not exclude such cases to ensure our findings can also reflect these conditions. Additionally, we consider RVOT obstruction and shunting as the baseline physiology, with minimal impact from PDA, ASD, and similar anomalies (38). Our study found that most ToF primary repairs resulted in prolonged LOS (92.2%), likely due to preoperative inflammation increasing postoperative morbidity and mortality (2, 4, 5). Cyanotic CHD, including ToF, is an independent risk factor for exacerbated systemic inflammatory response syndrome (SIRS) after cardiopulmonary bypass (CPB) (3, 6, 7). Additional risks include young age, low weight, extended CPB and aortic cross-clamp times, preoperative leukocytosis, and postoperative liver dysfunction (3, 6). This inflammatory burden leads to prolonged ICU stays, mechanical ventilation, and hospitalization, posing challenges in ToF surgical management (2, 34).
Our study observed a low mortality rate of 4.7% following ToF primary repair, with only 6.5% requiring reoperation, consistent with previous findings of a 0%–2% surgical mortality rate, even in neonates (39–41). Despite its overall safety, ToF primary repair is frequently associated with ICU and postoperative morbidity, which can contribute to mortality (42). In our study, 84% of patients experienced complications either during or after surgery, with grade IVa complications (27.9%) being the most common, followed by grade I complications (26.4%). These findings highlight the importance of identifying patients at risk for postoperative morbidity and mortality based on their preoperative characteristics to improve outcomes.
There were many studies on potential inflammatory biomarkers for predicting increased morbidity and mortality in hypoxemic patients (4, 7, 10–12). However, these biomarkers are expensive and may not be readily available in some centers, especially those in developing countries. The three indexes NLR, ALC, and TLR which are derived from complete blood count with differential count are very attractive alternatives to these biomarkers as they may be obtained from a simple, conventional, inexpensive, repeatable, and widely available blood test for children undergoing ToF primary repair.
Our study found that patients with mortality and complications following ToF primary repair had higher preoperative NLR. Manuel et al. identified a link between preoperative NLR and prolonged ICU and hospital LOS and its potential role in predicting grade III acute kidney injury following ToF primary repair (19, 21). Conversely, higher preoperative NLR was linked to shorter HLOS and postoperative LOS, possibly due to the time gap between admission, blood specimen collection, and surgery. While our analysis showed a significant association between preoperative ALC and complication grade, the trend was inconsistent. This result was supported by finding by Wu et al. that low preoperative ALC may predict poor prognosis in children undergoing ToF repair (13). We observed weak negative correlations between preoperative TLR and both HLOS and postoperative LOS, though these appeared coincidental, with higher preoperative thrombocyte levels possibly offering protection against complications like bleeding.
In our study, preoperative NLR, ALC, and TLR were not significantly associated with complications in univariate and multivariate analysis. However, preoperative NLR emerged as the strongest predictor among the three with a cutoff of >0.61 (sensitivity: 75%, specificity: 57%, AUC = 0.571, p = 0.077). Previous studies by Manuel et al. reported higher cutoff values for predicting longer HLOS (>0.80) and grade III acute kidney injury (>0.93) (19, 21). Other cardiac surgeries have shown even greater preoperative NLR thresholds, such as >3.23 in general cardiac surgery, >3.4 in coronary artery bypass graft (CABG), and >8 in emergent surgery for acute type I aortic dissection to predict mortality (43–45). Despite its predictive potential, ROC curve analyses demonstrated that preoperative NLR was unreliable for predicting complications in ToF primary repair, possibly due to differences in study design and the types of complications analyzed. Nonetheless, previous studies have linked preoperative NLR with cardiovascular surgery complications including atrial fibrillation, acute kidney injury, and mortality (21, 44–46).
Conversely, preoperative NLR, ALC, and TLR were significantly correlated with complication severity based on comprehensive complication index (CCI). Multivariate analysis revealed that only preoperative NLR (p = 0.014) along with gender (p = 0.027), associated diagnosis (p = 0.047), and duration of surgery (p = 0.001) had significant correlations with CCI. Tasoglu et al. identified preoperative NLR as an independent predictor of saphenous vein graft patency in CABG patients. Their study categorized patients into three groups based on preoperative NLR levels (low, intermediate, and high), demonstrating that higher NLR was linked to an increased incidence of saphenous vein graft failure (47). This finding aligns with our results, where higher preoperative NLR correlated with higher CCI. However, the relatively weak correlation in our study may be attributed to methodoclical differences, particularly blood sample collection timing (up to 7 days in our study vs. 1–3 days in other studies) (21, 45, 47).
Our findings indicate that female ToF patients experienced greater postoperative complications. Women are generally at greater risk of morbidity and mortality in cardiac surgery, often presenting at an older age with more comorbidities and atypical symptoms, making them more likely to require urgent than elective revascularization (48, 49), Similarly, Kochilas et al. reported higher mortality rates among girls undergoing high-risk cardiac surgeries, reinforcing our findings (50). Chromosomal and gonadal hormone factors have been shown to play a role in sex-specific responses to cardiac ischemic or hypoxic injury (51–53). However, some studies, including a meta-analysis, have found no significant gender differences in pediatric cardiac surgery outcomes (54, 55).
Patients with additional diagnoses beyond ToF experienced higher cumulative complications following ToF repair in our study. Previous researches have shown that individuals with genetic disorders or other cardiac anomalies, such as coronary abnormalities, tend to have poorer surgical outcomes (56, 57). These patients often present with compromised baseline health compared to those with isolated ToF, which may contribute to increased postoperative risks. Furthermore, the presence of complex cardiac anatomy can make can make surgical repair more challenging, further elevating the likelihood of morbidity and mortality.
Our study found that longer surgery duration, CPB time, and AOX time were associated with a higher complication burden, though regression analysis identified surgery duration as the only statistically significant factor. Prolonged operative time, including extended CPB and AOX durations, is known to exacerbate inflammatory responses in cardiac surgery (3, 6). A systematic review and meta-analysis have linked longer surgical procedures to an increased risk of complications (58). The prolonged CPB and AOX times likely contribute to heightened inflammation through ischemia-reperfusion injury, further impacting postoperative outcomes (3, 6, 59).
Previous studies have shown that patients with oxygen saturation <90% exhibit elevated preoperative or intramyocardial cytokine levels (7, 36). Additionally, researches have linked increased IL-6 with high neutrophils counts and low lymphocytes levels in chronically hypoxia patients, indicating a potential inflammatory imbalance (8, 9). Chronic hypoxia may also contribute to reperfusion injury caused by oxygen-derived free radicals, leading to myocardial and endothelial damage and remodeling, similar to patients undergoing CABG (60). CABG. However, the lack of significant findings in our study may be attributed to the presence of associated cardiac anomalies, such as PDA, ASD, PFO, AVSD, and MAPCAs, which could have influenced the results.
Low body weight has traditionally been considered a risk factor for exacerbated inflammatory responses in cardiac surgery (3, 6). However, our study found that patients weighing less than 10 kg had a lower complication risk, suggesting that advancements in surgical techniques may have contributed to improved outcomes. A study on very low birth weight infants (<2.2 kg) also reported relatively favorable cardiac surgery results, despite an increased risk of ICU morbidity and mortality (61). Additionally, higher body weight was associated with older age and prolonged exposure to chronic hypoxia-induced inflammation, which may contribute to a poorer prognosis.
5 Limitations
As a single-center, retrospective study, our study is susceptible to recall bias and missing data. However, we minimized this risk by utilizing a comprehensive, updated database. The study population was heterogeneous, with multiple potential confounding factors. While we attempted to address bias through multivariate analysis, the scope of included variables was constrained by data limitations. Another limitation was the use of blood tests conducted up to seven days before surgery due to institutional regulations, which may not fully reflect the patients' preoperative condition. Additionally, CRP was not included as a standard preoperative laboratory investigation, limiting the assessment of inflammatory profiles. Lastly, patient outcome evaluations were restricted to 30 days, as many participants traveled from across the country and preferred to return to their hometowns post-surgery.
6 Conclusion
Elevated preoperative NLR was associated with increased complication burdens in ToF primary repair, as well as factors such as female gender, associated diagnoses, and prolonged surgical duration. Although the correlation was weak, with low sensitivity and specificity, preoperative NLR proved to be the most reliable among the three indexes for predicting complications. Its simplicity, cost-effectiveness, and accessibility make it a promising tool for identifying high-risk patients and optimizing postoperative monitoring. Future multicenter prospective studies are necessary to validate the prognostic value of preoperative NLR under standardized conditions, across a broader patient population, and with more comprehensive confounding variables adjustments, ultimately improving ToF surgical outcomes.
Statements
Data availability statement
The dataset associated with this study is not readily available due to strict institutional policies that govern data sharing and confidentiality. These policies ensure compliance with regulatory and ethical guidelines, particularly concerning sensitive information and proprietary data. Requests to access the dataset should be directed to the corresponding author and we would further decide whether to share the dataset based on the purposes.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of National Cardiovascular Center Harapan Kita. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SS: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. CC: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank those who have supported us in the making of this article. We are especially grateful to the Department of Cardiology and Vascular Medicine, Faculty of Medicine Universitas Indonesia, for their guidance and assistance in teaching the authors about research methodology and for proofreading this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Seghaye MC Duchateau J Grabitz RG Nitsch G Marcus C Messmer BJ et al Complement, leukocytes, and leukocyte elastase in full-term neonates undergoing cardiac operation. J Thorac Cardiovasc Surg. (1994) 108(1):29–36. 10.1016/S0022-5223(94)70214-4
2.
Soares L Ribas D Spring R Silva J Miyague N . Perfil clínico da resposta inflamatória sistêmica após cirurgia cardíaca pediátrica com circulação extracorpórea. Arq Bras Cardiol. (2010) 94(1):119–24. 10.1590/S0066-782X2010000100019
3.
Güvener M Korun O Demirtürk OS . Risk factors for systemic inflammatory response after congenital cardiac surgery. J Card Surg. (2015) 30(1):92–6. 10.1111/jocs.12465
4.
Allan CK Newburger JW McGrath E Elder J Psoinos C Laussen PC et al The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. (2010) 111(5):1244–51. 10.1213/ANE.0b013e3181f333aa
5.
Levy JH Tanaka KA . Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. (2003) 75(2):S715–20. 10.1016/S0003-4975(02)04701-X
6.
Bhatia M Moochhala S . Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. (2004) 202(2):145–56. 10.1002/path.1491
7.
Qing M Schumacher K Heise R Wöltje M Vazquez-Jimenez JF Richter T et al Intramyocardial synthesis of pro- and anti-inflammatory cytokines in infants with congenital cardiac defects. J Am Coll Cardiol. (2003) 41(12):2266–74. 10.1016/S0735-1097(03)00477-7
8.
Eltzschig HK Carmeliet P . Hypoxia and inflammation. N Engl J Med. (2011) 364(7):656–65. 10.1056/NEJMra0910283
9.
Vinten-Johansen J . Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. (2004) 61(3):481–97. 10.1016/j.cardiores.2003.10.011
10.
Nagy O Baráth S Ujfalusi A . The role of microRNAs in congenital heart disease. Ejifcc. (2019) 30(2):165–78.
11.
Zloto K Tirosh-Wagner T Bolkier Y Bar-Yosef O Vardi A Mishali D et al MiRNA-208a as a sensitive early biomarker for the postoperative course following congenital heart defect surgery. Pediatr Cardiol. (2018) 39(8):1565–71. 10.1007/s00246-018-1931-7
12.
Sugimoto M Kuwata S Kurishima C Kim JH Iwamoto Y Senzaki H . Cardiac biomarkers in children with congenital heart disease. World J Pediatr. (2015) 11(4):309–15. 10.1007/s12519-015-0039-x
13.
Wu X Luo Q Su Z Li Y Wang H Yuan S et al Prognostic value of preoperative absolute lymphocyte count in children with tetralogy of fallot. J Am Heart Assoc. (2021) 10(11):e019098. 10.1161/JAHA.120.019098
14.
Chen Y Zhong H Zhao Y Luo X Gao W . Role of platelet biomarkers in inflammatory response. Biomark Res. (2020) 8(1):28. 10.1186/s40364-020-00207-2
15.
Manuel V Miana LA Guerreiro GP Tenório DF Turquetto A Penha JG et al Prognostic value of the preoperative neutrophil-lymphocyte ratio in patients undergoing the bidirectional Glenn procedure. J Card Surg. (2020) 35(2):328–34. 10.1111/jocs.14381
16.
Savluk OF Guzelmeric F Yavuz Y Ukil F Yilmaz A Cevirme D et al Neutrophil-lymphocyte ratio as a mortality predictor for Norwood stage I operations. Gen Thorac Cardiovasc Surg. (2019) 67(8):669–76. 10.1007/s11748-019-01081-y
17.
Xu H Sun Y Zhang S . The relationship between neutrophil to lymphocyte ratio and clinical outcome in pediatric patients after cardiopulmonary bypass surgery: a retrospective study. Front Pediatr. (2019) 7:308. 10.3389/fped.2019.00308
18.
Iliopoulos I Alder MN Cooper DS Villarreal EG Loomba R Sahay RD et al Pre-operative neutrophil-lymphocyte ratio predicts low cardiac output in children after cardiac surgery. Cardiol Young. (2020) 30(4):521–5. 10.1017/S1047951120000487
19.
Manuel V Miana LA Guerreiro GP Turquetto A Santos RM Fernandes N et al Preoperative neutrophil-lymphocyte ratio can predict outcomes for patients undergoing tetralogy of fallot repair. Braz J Cardiovasc Surg. (2021) 36(5):607–13. 10.21470/1678-9741-2020-0408
20.
Yin X Xin M Ding S Gao F Wu F Wang J et al Predictive role of perioperative neutrophil to lymphocyte ratio in pediatric congenital heart disease associated with pulmonary arterial hypertension. BMC Surg. (2021) 21(1):3. 10.1186/s12893-020-01009-x
21.
Manuel V Miana LA Turquetto A Guerreiro GP Fernandes N Jatene MB . The role of the neutrophil-lymphocyte ratio for pre-operative risk stratification of acute kidney injury after tetralogy of Fallot repair. Cardiol Young. (2021) 31(6):1009–14. 10.1017/S1047951121001943
22.
Lomivorotov VV Efremov SM Boboshko VA Leyderman IN Lomivorotov VN Cheung AT et al Preoperative total lymphocyte count in peripheral blood as a predictor of poor outcome in adult cardiac surgery. J Cardiothorac Vasc Anesth. (2011) 25(6):975–80. 10.1053/j.jvca.2010.12.006
23.
Cabrera AG Dyamenahalli U Gossett J Prodhan P Morrow WR Imamura M et al Preoperative lymphopenia is a predictor of postoperative adverse outcomes in children with congenital heart disease. J Thorac Cardiovasc Surg. (2009) 138(5):1172–9. 10.1016/j.jtcvs.2009.06.016
24.
Jones SM McCracken C Alsoufi B Mahle WT Oster ME . Association of preoperative cell counts with outcomes after operation for congenital heart disease. Ann Thorac Surg. (2018) 106(4):1234–40. 10.1016/j.athoracsur.2018.04.022
25.
Ozcan Cetin EH Cetin MS Aras D Topaloglu S Temizhan A Kisacik HL et al Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology. (2016) 67(4):336–45. 10.1177/0003319715591751
26.
Azab B Shah N Akerman M McGinn JT Jr . Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. (2012) 34(3):326–34. 10.1007/s11239-012-0718-6
27.
Kurtul A Murat SN Yarlioglues M Duran M Ergun G Acikgoz SK et al Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. (2014) 114(7):972–8. 10.1016/j.amjcard.2014.07.005
28.
Akboga MK Canpolat U Yayla C Ozcan F Ozeke O Topaloglu S et al Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. (2016) 67(1):89–95. 10.1177/0003319715583186
29.
Li XT Fang H Li D Xu FQ Yang B Zhang R et al Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J. (2020) 133(4):415–23. 10.1097/CM9.0000000000000650
30.
Walian A Kohli JK Magoon R Kashav RC Shri I Dey S et al Retrospective evaluation of platelet-leukocyte indices and cardiac surgical outcomes in acyanotic heart disease patients with pulmonary hypertension (REPLICA-PH). Braz J Cardiovasc Surg. (2022) 37(6):866–74. 10.21470/1678-9741-2020-0648
31.
Arslanoğlu E Çine N Kara KA Tunçer E Ukil Işıldak F Yavuz Y et al Do platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) have a predictive value on pediatric extracorporeal membrane oxygenation (ECMO) results? Cardiol Young. (2021) 31(6):1003–8. 10.1017/S1047951121001918
32.
Hébert M Cartier R Dagenais F Langlois Y Coutu M Noiseux N et al Standardizing postoperative complications—validating the clavien-dindo complications classification in cardiac surgery. Semin Thorac Cardiovasc Surg. (2021) 33(2):443–51. 10.1053/j.semtcvs.2020.09.029
33.
Slankamenac K Graf R Barkun J Puhan MA Clavien P-A . The comprehensive complication Index: a novel continuous scale to measure surgical morbidity. Ann Surg. (2013) 258(1):1–7. 10.1097/SLA.0b013e318296c732
34.
Huang J Ding J Wu X Jia Y Liu Q Yuan S et al Chronic hypoxia prolongs postoperative mechanical ventilation and reduces the left atrial pressure threshold in children with tetralogy of Fallot. Front Pediatr. (2022) 10:965703. 10.3389/fped.2022.965703
35.
Liu L Huang L Yao L Zou F He J Zhao X et al Energy metabolism disorder dictates chronic hypoxia damage in heart defect with tetralogy of fallot. Front Cardiovasc Med. (2022) 9:1096664. 10.3389/fcvm.2022.1096664
36.
Sethi BS Kapoor PM Chauhan S Chowdhury UK Kiran U Choudhury M . Perioperative levels of tumor necrosis factor-α correlate with outcomes in children and adults with tetralogy of fallot undergoing corrective surgery. World J Pediatr Congenit Heart Surg. (2014) 5(1):38–46. 10.1177/2150135113507290
37.
Hövels-Gürich HH Schumacher K Vazquez-Jimenez JF Qing M Hüffmeier U Buding B et al Cytokine balance in infants undergoing cardiac operation. Ann Thorac Surg. (2002) 73(2):601–8. discussion 8–9. 10.1016/S0003-4975(01)03391-4
38.
Bailliard F Anderson RH . Tetralogy of Fallot. Orphanet J Rare Dis. (2009) 4:2. 10.1186/1750-1172-4-2
39.
Sousa Uva M Lacour-Gayet F Komiya T Serraf A Bruniaux J Touchot A et al Surgery for tetralogy of Fallot at less than six months of age. J Thorac Cardiovasc Surg. (1994) 107(5):1291–300. 10.1016/S0022-5223(12)70161-7
40.
Kolcz J Pizarro C . Neonatal repair of tetralogy of Fallot results in improved pulmonary artery development without increased need for reintervention. Eur J Cardiothorac Surg. (2005) 28(3):394–9. 10.1016/j.ejcts.2005.05.014
41.
Touati GD Vouhé PR Amodeo A Pouard P Mauriat P Leca F et al Primary repair of tetralogy of Fallot in infancy. J Thorac Cardiovasc Surg. (1990) 99(3):396–402. discussion -3. 10.1016/S0022-5223(19)36968-5
42.
van Dongen EI Glansdorp AG Mildner RJ McCrindle BW Sakopoulos AG VanArsdell G et al The influence of perioperative factors on outcomes in children aged less than 18 months after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. (2003) 126(3):703–10. 10.1016/S0022-5223(03)00035-7
43.
Lafçi G Ciçek ÖF Uzun HA Yalçinkaya A Diken A Turak O et al Relationship of admission neutrophil-to-lymphocyte ratio with in-hospital mortality in patients with acute type I aortic dissection. Turk J Med Sci. (2014) 44(2):186–92. 10.3906/sag-1301-136
44.
Haran C Gimpel D Clark H McCormack DJ . Preoperative neutrophil and lymphocyte ratio as a predictor of mortality and morbidity after cardiac surgery. Heart Lung Circ. (2021) 30(3):414–8. 10.1016/j.hlc.2020.05.115
45.
Lim HA Kang JK Kim HW Song H Lim JY . The neutrophil-to-lymphocyte ratio as a predictor of postoperative outcomes in patients undergoing coronary artery bypass grafting. J Chest Surg. (2023) 56(2):99–107. 10.5090/jcs.22.082
46.
Weedle RC Da Costa M Veerasingam D Soo AWS . The use of neutrophil lymphocyte ratio to predict complications post cardiac surgery. Ann Transl Med. (2019) 7(23):778. 10.21037/atm.2019.11.17
47.
Taşoğlu I Turak O Nazli Y Ozcan F Colak N Sahin S et al Preoperative neutrophil-lymphocyte ratio and saphenous vein graft patency after coronary artery bypass grafting. Clin Appl Thromb Hemost. (2014) 20(8):819–24. 10.1177/1076029613484086
48.
Dixon LK Di Tommaso E Dimagli A Sinha S Sandhu M Benedetto U et al Impact of sex on outcomes after cardiac surgery: a systematic review and meta-analysis. Int J Cardiol. (2021) 343:27–34. 10.1016/j.ijcard.2021.09.011
49.
Dixon LK Dimagli A Di Tommaso E Sinha S Fudulu DP Sandhu M et al Females have an increased risk of short-term mortality after cardiac surgery compared to males: insights from a national database. J Card Surg. (2022) 37(11):3507–19. 10.1111/jocs.16928
50.
Kochilas LK Vinocur JM Menk JS . Age-dependent sex effects on outcomes after pediatric cardiac surgery. J Am Heart Assoc. (2014) 3(1):e000608. 10.1161/JAHA.113.000608
51.
Stone G Choi A Meritxell O Gorham J Heydarpour M Seidman CE et al Sex differences in gene expression in response to ischemia in the human left ventricular myocardium. Hum Mol Genet. (2019) 28(10):1682–93. 10.1093/hmg/ddz014
52.
Zampino M Yuzhakova M Hansen J McKinney RD Goldspink PH Geenen DL et al Sex-related dimorphic response of HIF-1 alpha expression in myocardial ischemia. Am J Physiol Heart Circ Physiol. (2006) 291(2):H957–64. 10.1152/ajpheart.00580.2005
53.
Ostadal B Ostadal P . Sex-based differences in cardiac ischaemic injury and protection: therapeutic implications. Br J Pharmacol. (2014) 171(3):541–54. 10.1111/bph.12270
54.
DiBardino DJ Pasquali SK Hirsch JC Benjamin DK Kleeman KC Salazar JD et al Effect of sex and race on outcome in patients undergoing congenital heart surgery: an analysis of the society of thoracic surgeons congenital heart surgery database. Ann Thorac Surg. (2012) 94(6):2054–9. discussion 9–60. 10.1016/j.athoracsur.2012.05.124
55.
Crain AK Lim ZN Sarfatis CJ Arias M Holder T Moreira AG et al Meta-analysis on sex differences in mortality and neurodevelopment in congenital heart defects. Sci Rep. (2025) 15(1):8152. 10.1038/s41598-025-92894-w
56.
Saygi M Ergul Y Tola HT Ozyilmaz I Ozturk E Onan IS et al Factors affecting perioperative mortality in tetralogy of Fallot. Pediatr Int. (2015) 57(5):832–9. 10.1111/ped.12627
57.
Smith CA McCracken C Thomas AS Spector LG St Louis JD Oster ME et al Long-term outcomes of tetralogy of fallot: a study from the pediatric cardiac care consortium. JAMA Cardiol. (2019) 4(1):34–41. 10.1001/jamacardio.2018.4255
58.
Cheng H Clymer JW Po-Han Chen B Sadeghirad B Ferko NC Cameron CG et al Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. (2018) 229:134–44. 10.1016/j.jss.2018.03.022
59.
Parmana IMA Boom CE Rachmadi L Hanafy DA Widyastuti Y Mansyur M et al Correlation between cardiac index, plasma troponin I, myocardial histopathology, CPB and AoX duration in glutamine versus No glutamine administered patients with low ejection fraction undergoing elective on-pump CABG surgery: secondary analysis of an RCT. Vasc Health Risk Manag. (2023) 19:93–101. 10.2147/VHRM.S399925
60.
Gibson PH Croal BL Cuthbertson BH Small GR Ifezulike AI Gibson G et al Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. (2007) 154(5):995–1002. 10.1016/j.ahj.2007.06.043
61.
Mehmood A Ismail SR Kabbani MS Abu-Sulaiman RM Najm HK . Outcome of low body weight (<2.2 kg) infants undergoing cardiac surgery. J Saudi Heart Assoc. (2014) 26(3):132–7. 10.1016/j.jsha.2014.03.002
Summary
Keywords
absolute lymphocyte count, complications, mortality, neutrophil-lymphocyte ratio, tetralogy of fallot, thrombocyte-lymphocyte ratio
Citation
Siagian SN and Christianto C (2025) Prognostic value of neutrophil-lymphocyte ratio, absolute lymphocyte count, and thrombocyte-lymphocyte ratio in predicting the outcomes of tetralogy of fallot primary repair. Front. Cardiovasc. Med. 12:1489242. doi: 10.3389/fcvm.2025.1489242
Received
31 August 2024
Accepted
22 May 2025
Published
05 June 2025
Volume
12 - 2025
Edited by
Daniel De Wolf, Ghent University Hospital, Belgium
Reviewed by
Ömer Faruk Çiçek, Selcuk University, Türkiye
Nikoletta Rachel Czobor, Royal Papworth Hospital NHS Foundation Trust, United Kingdom
Carla Tanamati, Heart Institute (InCor), Brazil
Updates
Copyright
© 2025 Siagian and Christianto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Sisca Natalia Siagian sisca.ped.car@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.