Abstract
Diabetic cardiomyopathy (DCM), a common diabetic complication independent of hypertension, coronary heart disease and heart valve disease is a major cause of cardiovascular mortality. The pathogenesis of DCM is complex. The Nrf2-related signaling pathway which regulates oxidative stress, energy metabolism and mitochondrial physiology may play important role in the pathogenesis of DCM. Current treatments for DCM focus on blood glucose and pressure control, cardiovascular protection, lipid lowering and blockade of the renin-angiotensin system. However, the adverse drug reactions are inevitable. Traditional Chinese medicine (TCM) as a multi-target, multi-pathway treatment approach is considered to be a promising therapy for DCM. We reviewed how the Nrf2 and related signaling pathway regulated pathophysiological processes such as oxidative stress, inflammation, myocardial fibrosis, apoptosis, ferroptosis, autophagy and mitochondrial dysfunction in the progression of DCM and explored the potential mechanism and clinical value of TCM in DCM treatment. Based on a literature review, we found that various herbal compounds and combinations could alleviate DCM via the Nrf2 signaling pathway. This review highlighted the role of the Nrf2 signaling pathway in DCM progression and put forward new therapeutic strategies for DCM.
1 Introduction
Diabetic cardiomyopathy (DCM) is a common complication of diabetes mellitus (DM) (1). It is a pathophysiological state characterized by metabolic disorders and microvascular lesions, which can lead to subclinical cardiac dysfunction, including left ventricular fibrosis, diastolic dysfunction and ultimately the heart failure (HF). In other words, cardiovascular disease is a major cause of death in diabetic patients. However, DCM as a unique disease is independent disease from traditional HF risks such as hypertension, coronary heart disease and valvular heart disease (2).
During the initial stage of DCM, metabolic disorders manifest as the impaired insulin metabolic signaling, the increased uptake of myocardial free fatty acids (FFAs) and mitochondrial dysfunction. These factors can worsen myocardial fibrosis, accelerate cardiac remodeling and ultimately reduce the ejection fraction (EF) in diabetic patients (3). In the later stages of DCM, there are more noticeable changes in cardiac structure, including myocardial cell necrosis, collagen accumulation, increased cross-linking of connective tissue, myocardial interstitial fibrosis and myocardial hypertrophy (4). With the increasing number of diabetic patients in China, the incidence of DCM has also risen rapidly (5). Currently, there is no specific therapy for DCM. Drug interventions mainly focus on blood glucose and pressure control, cardiovascular protection, lipid lowering and blockade of the renin-angiotensin system (2, 6). Unfortunately, the development and progression of cardiomyopathy in the patients with diabetes remains unrecoverable due to a poor prognosis (6). Additionally, the pathogenic factors of DCM can be proposed as the hyperlipidemia, inflammatory cytokines, oxidative stress, mitochondrial dysfunction and programmed cell death (7). However, the precise pathogenic mechanisms of these pathogenic factors in DCM still need to be clarified which is attractive for DCM therapy.
The Nrf2 signaling as one of the most critical intracellular signaling pathways and antioxidant defense systems controls essential cellular function including, but not limited to, cell proliferation, metabolism and extracellular matrix (ECM) remodeling (8, 9). Nrf2 belongs to the Cap “n”collar (CNC) transcription factor family and consists of multiple homologous domains, each one with a different functions (10). Under normal physiological conditions, Nrf2 binds to its inhibitor Keap1 in the cytoplasm, which facilitates the rapid ubiquitination and subsequently degradation of Nrf2 by the proteasome (11). However, when cells experience oxidative stress, electrophilic compounds, Nrf2 is unaffected by Keap1 and directly translocates to the nucleus. In the nucleus, it binds to antioxidant response elements (AREs) found in genes encoding antioxidant enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH), quinone oxidoreductase (NQO1), glutathione S-transferase (GST), heme oxygenase-1 (HO-1), and γ-glutamyl cysteine synthase (γ-GCS) to increase the expression of AREs which play the vital role of detoxification, antioxidant and anti-inflammatory effects (12, 13). Studies have confirmed that chronic hyperglycemia not only generates extra reactive oxygen species (ROS) but also impairs antioxidant capacity orchestrated by downregulation of Nrf2 in the heart (14). The molecule agonists targeting key kinase components of the Nrf2 signaling pathway have drawn extensive attention and have been developed and evaluated in preclinical models of DCM (15). Therefore, further understanding of characterization and regulatory mechanisms governing abnormal regulation of Nrf2 signaling in DCM from current literature researches will provide important insights into possible future directions for targeted therapeutic regimen and a new combinatory therapeutic approach for DCM.
Currently, the conventional treatment of DCM drugs include metformin, thiazolidinediones (TZDs), sulfonylureas, Glucagon-like peptide-1 receptor (GLP-1R) agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, SLGT-2 inhibitors and angiotensin-converting enzyme inhibitors (ACEI). Unfortunately, some contraindications and side effects from these drugs are inevitable on account of a long-time treatment (2, 16). Traditional Chinese medicine (TCM) has its own unique diagnosis and treatment system, which has been used in clinical treatment for more than 2000 years in Chinese history (17). Furthermore, TCM has been widely utilized in the treatment of DCM among clinicians in China (18). Increasing studies confirm that TCM ameliorate DCM through the synergistic benefits of its multiple components and multiple targets, which involves various signaling pathways (19). Compared to the conventional therapies, TCM is characterized by multiple targets, multiple pathways, fewer side effects and greater accessibility. It is considered to be a valuable and effective therapeutic way for chronic metabolic diseases such as DCM (20, 21). Beyond their direct protective effects on the heart, TCM can also assist in lowering blood glucose and lipid levels, thus indirectly reducing the metabolic burden on myocardium (22, 23). Besides, TCM emphasizes evidence-based and individual-based treatment, which can adjust the drug regimen according to the patient's physical situation in order to reduce unnecessary side effects (24, 25). What is noteworthy is that Nrf2 signaling is regarded as a key therapy targets based on TCM in preventing the progression of DCM (14).
In this review, we firstly summarized the characteristics of Nrf2 signaling participation in different pathogenic factors for DCM. Furthermore, we investigated up-to-date activators of the Nrf2 signaling pathway for DCM treatment, including compounds from TCM and TCM formulations as well as their characteristics on therapeutic mechanisms in order to provide valuable and effective direction for DCM therapy.
2 Correlation between the Nrf2 signaling pathway and diabetes cardiomyopathy
The pathogenesis of DCM is considered to involve complex interactions among multiple factors. Nrf2 as a key transcription factor plays a role in the progression of DCM by regulation of mitochondrial dysfunction, reactive oxygen species (ROS) production, apoptosis, inflammatory cytokines secretion, myocardial fibrosis, ferroptosis and autophagy.
2.1 The role of the Nrf2 signaling pathway in oxidative stress induced by DCM
Hyperglycemia stimulates the excessive production of ROS which impairs the endogenous antioxidant system and leads to oxidative stress in cardiomyocytes. As a results, inhibition of oxidative stress can improve heart function in patients with diabetes (26). Under normal physiological conditions, Nrf2 binds to Keap1 to form a stable complex. However, under oxidative stress, Nrf2 is released from Keap1 with phosphorylation and translocation into the nucleus. The phosphorylated Nrf2 then binds to the ARE and activates the transcription of antioxidant genes (27, 28) (Figure 1). PI3K/Akt pathway is also involved in Nrf2 activation and nuclear translocation. In doxorubicin (Dox)-induced H9c2 cardiomyocytes, activation of the PI3K/Akt signaling pathway upregulates the Nrf2 expression, which subsequently increases the protein expression of HO-1, NQO-1, and SOD and reduces oxidative stress (29). Studies have shown a significant downregulation of Nrf2 in both animal models of diabetes and diabetic patients' hearts which may be cause of angiogenic abnormalities, endothelial dysfunction and myocardial damage (14). While the upregulation of Nrf2 expression can protect cardiomyocytes from hyperglycemic injury (30). Priclincal experiments has confirmed that in the cardiomyocytes of streptozotocin (STZ)-induced diabetic rats and high glucose-induced H9c2 cells, the expression of Nrf2 is significantly downregulated. On the contrary, activation of Nrf2 can stimulate its downstream target genes expression such as SOD, HO-1, and NQO-1, thereby improving cardiomyocyte damage caused by oxidative stress, apoptosis and left ventricular dysfunction in DCM rats (31).
Figure 1
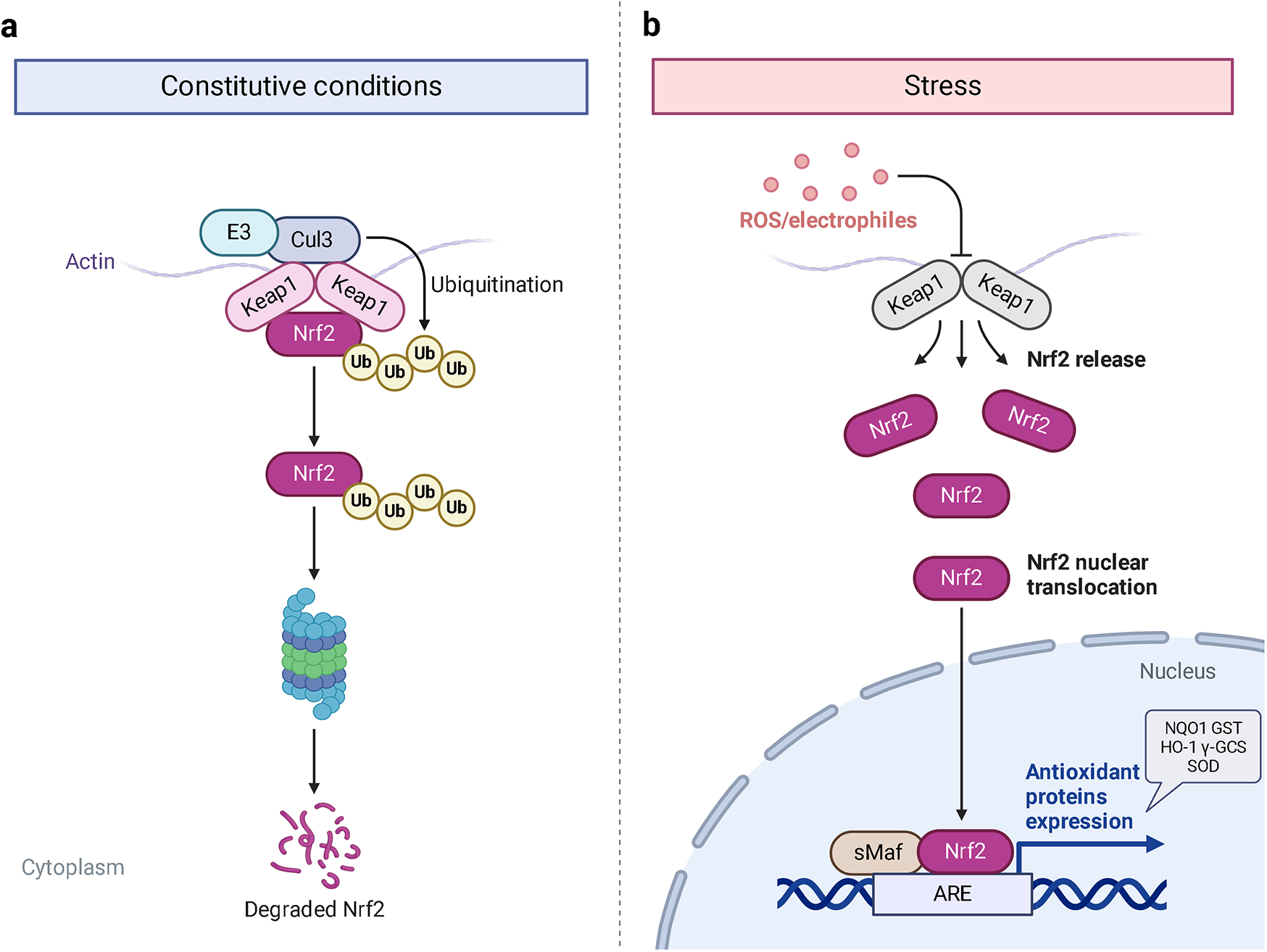
The process of Keap1 regulating Nrf2. E3 and Cul3 are ubiquitin ligases. (a) Normal physiological condition. (b) Oxidative stress condition.
2.2 Nrf2 signaling pathway participates in the inflammatory response caused by DCM
During the progress of inflammatory responses in DCM, cellular events are closely linked to redox balance (32). Metabolic disorders resulting from diabetes trigger the production of inflammatory factors such as IL-6, TNF-α and monocyte chemoattractant protein-1 (MCP-1). These factors, in turn, activate the NF-κB and Toll-like receptor-mediated inflammatory pathway and stimulate inflammatory cell infiltration (e.g., macrophages and neutrophils) which leads to myocardial inflammation. This process significantly damages cardiomyocytes and exacerbates the progression of DCM (33–35). Between the Nrf2 and NF-κB signaling pathways, there exists a complex and dynamic interplay. Both of the pathways modulate cellular redox homeostasis and mediate responses to stress and inflammation (36). The Nrf2 as an upstream inhibitor reduces intracellular ROS production, thereby inhibiting proinflammatory signaling. Researchers have shown that activation of Nrf2 signaling controls the redox balance that it exerts on inflammatory networks (37). Activation of the Nrf2/HO-1/NF-κB signaling pathway can reduce cardiomyocyte inflammation injury caused by ischemia-reperfusion (38).
2.3 Activation of the Nrf2 signaling pathway inhibits myocardial fibrosis caused by DCM
Myocardial fibrosis and collagen deposition represent the critical structural modifications observed in DCM (39). The transition from cardiac fibroblasts (CFs) to myofibroblasts is a crucial cellular event in myocardial fibrosis (40, 41). TGF-β1 is a well-known inducer involved in the differentiation of CFs into myofibroblasts (42). It has been demonstrated that hyperglycemia can upregulate the expression of TGF-β1 in CFs (43), thereby promoting myocardial fibrosis and impairing the compliance of cardiac tissue in diabetic patients (26). Studies have confirmed that the activation of Nrf2 signaling pathway plays a beneficial role in the development of myocardial fibrosis under hyperglycemic conditions involves inhibition of various redox signaling elements such as TGF-β1, profibrogenic genes, cardiac remodeling-associated lncRNAs (44, 45). Preclinical experiment indicates that activation of the Nrf2/HO-1 signaling axis inhibits the TGF-β1/Smad2/3 signaling pathway, effectively suppressing myocardial fibrosis in the DCM mice model (46, 47). The primary crosstalk mechanisms between Nrf2 and TGF-β1 are as follows. Firstly, Nrf2 reduces MMP-9 expression, which in turn decreases the levels of TGF-β1. Secondly, Nrf2-mediated Smads inhibition is associated with increased Smad7 levels, negatively modulating factor of the TGF-β1 signaling pathway (48).
2.4 Nrf2 signaling pathway inhibits apoptosis caused by DCM
It has been confirmed that metabolic disorders in DCM can induce maladaptive cardiomyocyte apoptosis. This situation can be caused by hyperglycemia, mitochondrial damage and dysfunction, energy metabolic disturbance, excessive ROS, endoplasmic reticulum stress (ERS), advanced glycation end products (AGEs) and inflammation (49, 50). Long-term hyperglycemia in diabetic patients can lead to cardiac contractile dysfunction and remodeling, attributed to cardiomyocyte apoptosis (51). Under hyperglycemic conditions, oxidative stress caused by ROS is heightened and may contribute to cardiomyocyte apoptosis (52, 53). Studies have shown that Nrf2 as an antioxidant response element plays a vital role in preventing ROS-induced cell apoptosis in both vasculature and heart tissue (54). In STZ-induced diabetic rat model, cardiomyocyte apoptosis was observed along with decreased protein expression of Nrf2 as well as its downstream antioxidant enzymes HO-1 and γ-GCS. Blocking the protein expression of Akt, Nrf2, HO-1, γ-GCS, and caspase3 by PI3K-specific siRNA and a PI3K inhibitor of LY294002 exacerbates high glucose-induced oxidative stress and cardiomyocyte apoptosis. These results suggest that the PI3K/Akt/Nrf2/HO-1 signaling pathway may play a significant role in the antioxidative effect of DCM (55). Additionally, overexpression of miR-155 in H9c2 cells induced by high glucose leads to decreasing expression of endonuclear Nrf2 and HO-1 which accompanied by the cell apoptosis (56). Furthermore, study demonstrates that activating the Nrf2-related signaling pathway alleviates cardiomyocyte apoptosis in DCM mice model (57).
2.5 Nrf2 signaling pathway inhibits ferroptosis caused by DCM
Ferroptosis is a form of cell death characterized by the accumulation of lipids and lipid peroxidation (58). Under conditions of metabolic disorder, cardiomyocytes predominantly derive energy from fatty acid oxidation, which induces excessive fatty acid oxidation and the accumulation of peroxides and inflammatory factors, ultimately resulting in ferroptosis and irreversible damage to cardiomyocytes (59). However, the activation of Nrf2 stimulates the expression of numerous antioxidant factors, such as HO-1 and glutathione peroxidase 4 (GPX4), thereby inhibiting the progression of ferroptosis (60). Research has shown that activation of the Nrf2/GPX4/glutathione (GSH) pathway leads to increased SOD levels and downregulation of both MDA and free ferrous iron (Fe2+) which effectively mitigates oxidative stress and ferroptosis in high glucose-induced H9c2 cells (61, 62). Besides, the cystine/glutamate antiporter SLC7A11 as one of the key regulators of ferroptosis is a downstream target of Nrf2. In cardiomyocytes of DCM mice model, activation of the AMPK/Nrf2 pathway can reverse the decrease expression of SLC7A11 and GSH levels and consequently balance iron metabolism (63).
2.6 The role of the Nrf2 signaling pathway in autophagy caused by DCM
Autophagy as an adaptive response can help cells to cope with various stresses, including hyperglycemia, hypoxia, oxidative stress, and exogenous stress (64). Autophagy occurs in almost all types of cardiovascular cells (65). Prolonged hyperglycemia can disrupt cardiomyocyte autophagy (66), exacerbating the progression of DCM. Nrf2, a gene with potential antioxidant functions, is capable of regulating autophagy through positive effect. Studies have shown that activation of the Nrf2 signaling pathway increases autophagy and alleviates high glucose-induced hypertrophy of H9c2 cells (67). Activation of the PP2A/Nrf2 signaling pathway can promote cardiomyocyte autophagy under high glucose condition (68). However, prolonged activation of Nrf2 may suppress autophagy via a non-canonical mechanism (69).
2.7 The role of the Nrf2 signaling pathway in mitochondrial dysfunction caused by DCM
Mitochondria are crucial organelles in cardiomyocytes responsible for energy supply. Studies have demonstrated that the production of adenosine 5′-triphosphate (ATP) by mitochondria is accompanied by the generation of ROS. Under normal physiological conditions, cardiomyocytes activate antioxidant defense mechanisms to counteract ROS damage. However, mitochondrial dysfunction in metabolic disorders can disrupt the respiratory chain, leading to excessive ROS production in the heart. The accumulation of ROS not only impairs mitochondrial structure and function but also promotes lipid buildup. These effects may contribute to myocardial fibrosis and cardiac diastolic dysfunction in the progression of DCM (70–72). Therefore, mitochondrial dysfunction is considered a key factor of oxidative stress. Sustained hyperglycemia acts as the primary driver of mitochondrial impairment in cardiomyocytes (73, 74). According to research findings, impairment of mitochondrial respiratory function, mitochondrial membrane potential and mitochondrial biogenesis in the hearts of diabetic rats contributes to atrial structure remodeling and alterations in electrical activity, thereby facilitating the onset of atrial fibrillation (75). Knockout of Nrf2 gene significantly impairs mitochondrial respiratory function and reduces mitochondrial membrane potential and decreases ATP production in cardiomyocytes of db/db mice (76). Conversely, activation of the Nrf2/HO-1 signaling pathway improves mitochondrial dysfunction in cardiomyocytes (77). In diabetic rats, activation of the SIRT1/Nrf2/HO-1/Nox-2 pathway effectively alleviates mitochondrial dysfunction and oxidative stress in cardiomyocytes. This is achieved by reducing ROS production, increasing ATP levels and enhancing the activity of mitochondrial enzymes in myocardial tissues. Ultimately, these improvements contribute to the restoration of cardiac function in diabetic rats (78). Therefore, the activation of Nrf2 may represent a promising therapeutic target for the treatment of diabetic cardiomyopathy by restoring mitochondrial function (Figure 2).
Figure 2
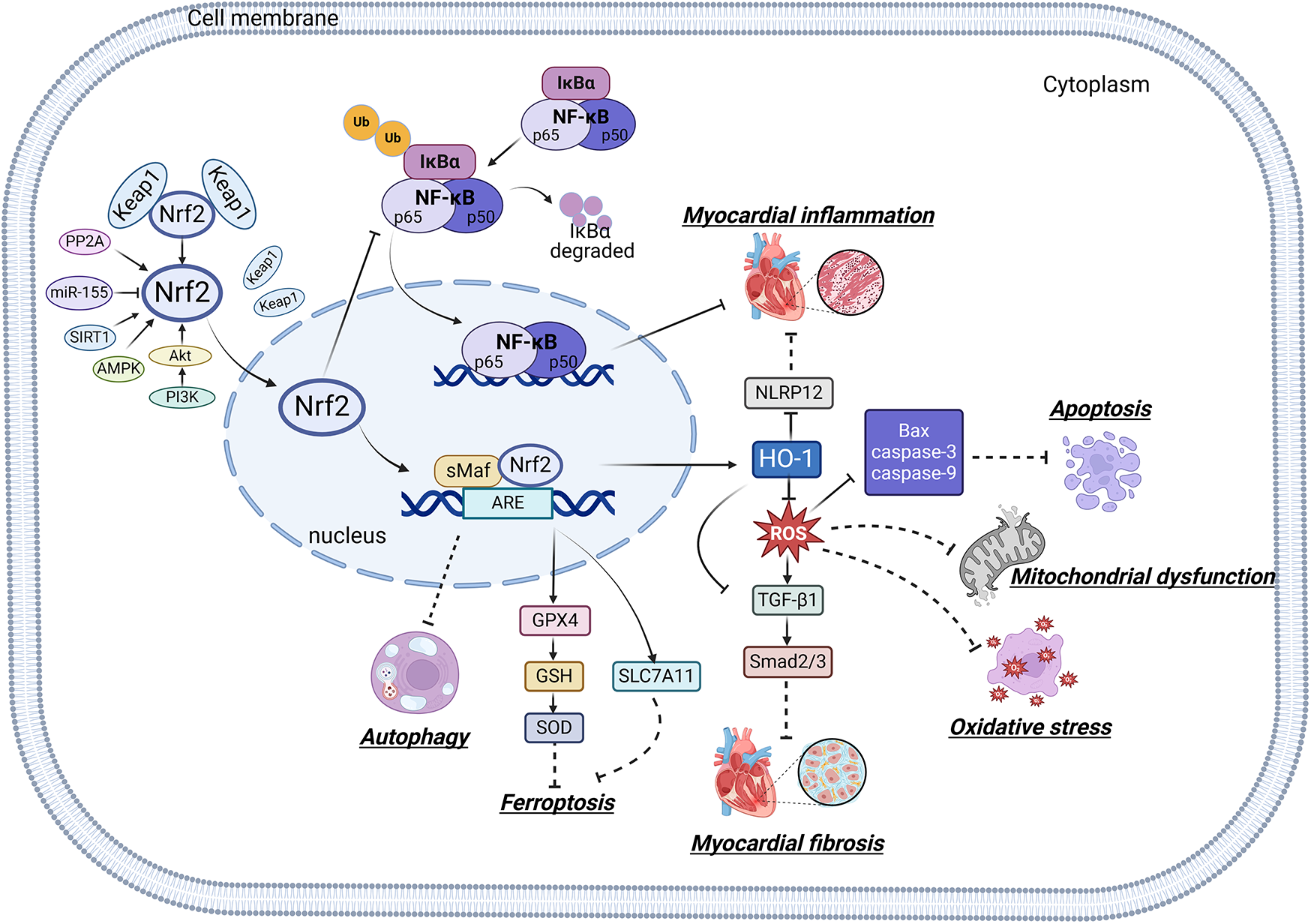
Relationship between Nrf2 signaling pathway and oxidative stress, inflammation, myocardial fibrosis, apoptosis, ferroptosis, autophagy and mitochondrial dysfunction.
3 Traditional Chinese medicine for the treatment of DCM through the activation of Nrf2 signaling pathway
Pharmacological NRF2 activators have shown significant protective effects across a range of disease models and have yielded promising results in human intervention trials, thereby solidifying NRF2 as a highly promising drug target (79). Existing evidences have confirmed that some specific TCMs and their bioactive components which have the potential efficacy of DCM are associated with the activation of Nrf2 signaling pathway. In this section, we presented a comprehensive overview of the effects and mechanisms of these TCMs and their bioactive ingredients in the context of DCM.
3.1 Bioactive compounds of Nrf2 agonists from TCM
3.1.1 Resveratrol
The natural polyphenol compound of resveratrol (RES) was originally derived from Veratrum grandiflorum (Maxim. ex Baker) Loes., Polygonum cuspidatum Sieb. et Zucc., Cassia obtusifolia L. and mulberry. In the past 20 years, nearly 200 clinical studies have evaluated RES for 24 indications, including cancer, menopause symptoms, diabetes, metabolic syndrome, and cardiovascular disease (80). Clinical trials confirm that RSV significantly improves left ventricular function and reduces premature atrial and ventricular contractions with decreasing the serum of aspartate aminotransferase, glucose, LDL cholesterol, alanine aminotransferase, total cholesterol and insulin resistance index (81, 82). It is suggested that RES, as a form of Nrf2 agonists has been shown the promising evidence of efficacy on DCM therapy both in clinical trials and lab experiments. Furthermore, numerous studies have demonstrated that RES exerts its antioxidant effects and anti-inflammatory and cardiovascular protective effects through the Nrf2 signaling pathway. It has been shown that RES protects the hyperglycemia-induced cardiomyocytes by promoting the Nrf2 protein expression and its downstream of antioxidant genes (83). Besides, intraperitoneal administration of RES for type 2 diabetic rats reduces myocardial ischemia/reperfusion injury (MIRI) by activating the AMPK/p38/Nrf2 signaling pathway (84, 85).
3.1.2 Quercetin
QUR, a natural flavonoid possessing antioxidant, anti-inflammatory, anti-atherosclerotic, anti-thrombotic and cardioprotective activities is widely distributed in Chinese herbal medicines, fruits, leaves, vegetables, seeds and plant roots (86, 87). Quercetin supplementation demonstrates moderate-to high-quality evidence for reducing cardiovascular disease (CVD) risk factors. Multiple randomized controlled trials examine the effects of quercetin on patients with coronary artery disease and demonstrate a significant reduction in chronic systemic inflammation (88). Priclincal studies have shown that QUR protects the myocardium from MIRI by inhibiting the inflammatory cascade and apoptosis via the PI3K/Akt signaling pathway (88). QUR also alleviates oxidative stress by enhancing the level of SOD, CAT and GPx, while mitigating inflammation and apoptosis through down-regulating the expression levels of IL-6 and Bax in cardiomyocytes of STZ-nicotinamide-induced diabetic rats. The Nrf2 signaling pathway may be a target for DCM therapy (89). In diabetic rats model, QUR reduces the accumulation of ROS in cardiomyocytes and delays the development of myocardial fibrosis by facilitating the nuclear translocation of Nrf2, which subsequently increases the expression levels of its downstream target genes including HO-1, SOD and glutamate-cysteine ligase catalytic (GCLC) (90). QUR has also been found to ameliorate hyperglycemia-induced myocardial bioenergetic damage and maintain intracellular energy homeostasis in diabetic rats by upregulating the expression levels of Nrf2, HO-1, SOD and proliferator-activated receptor gamma coactivator-1α (PGC-1α) (91, 92).
3.1.3 Curcumin
Curcumin (CUR), a naturally occurring polyphenolic compound derived from medicinal plants such as Curcuma wenyujin Y.H., Chen et C. Ling, Curcuma longa L., and Curcuma phaeocaulis Vai. (family Zingiberaceae), exhibits the antioxidant, anti-inflammatory, anticancer and antiapoptotic activities (93, 94). A randomized clinical trial suggests that curcumin significantly enhances both insulin sensitivity and the homeostatic model assessment of insulin resistance (HOMA-IR) (95). Preclincal study has confirmed that CUR has protective effects on DCM via the Nrf2-related signaling pathway (96). In high glucose-induced H9c2 cells and cardiomyocytes from type 2 diabetic rats, CUR increases cardiomyocyte viability and antioxidant enzyme activity, reduces ROS formation and cardiomyocyte apoptosis through activation of the Nrf2/HO-1 signaling pathway (97). Additionally, researchers have shown that CUR exerts protective effects against oxidative stress and ferroptosis-induced injury in the cardiomyocytes of diabetic rats by promoting Nrf2 nuclear translocation and upregulating the expression of its downstream genes (59, 98). CUR also can reduce the accumulation of superoxide and inhibit pyroptosis in the cardiomyocytes of diabetic rats through the activation of the AKT/Nrf2/ARE pathway (99).
3.1.4 Sulforaphane
Sulforaphane (SFN), a natural compound from the herb extracts belonging to the Cruciferae family (100) is one of the first identified and most potent naturally occurring Nrf2 activators (101). The overall outcomes of the clinical trials with sulforaphane-rich preparations have reinforced the preclinical evidence that sulforaphane has the potential to ameliorate a variety of diseases related to chronic metabolic and inflammatory stress (102, 103). A diversity preclinical experiments also shown improvement effect of sulforaphane on DCM. Studies have confirmed that SFN prevents cardiomyocyte oxidative damage, inflammation, and fibrosis in diabetic mouse models through the activation of the AMPK/AKT/GSK3β/NRF2 signaling pathway (104, 105). Furthermore, SFN alleviates cardiomyocyte hypertrophy and fibrosis in DCM mice through upregulating the expression and transcriptional activity of Nrf2. Silencing the Nrf2 gene in high glucose-induced H9c2 cells abolishes the protective effect of SFN on cardiomyocyte fibrosis (106). Moreover, SFN can prevent the cardiomyocyte ferroptosis in DCM mice model through activation of the AMPK/Nrf2 signaling pathway (63). These results suggest that Nrf2 and its related signaling pathway may be the key targets of SFN for treating DCM.
3.1.5 Luteolin
Luteolin (LUT) as a natural antioxidant is widely found in fruits, vegetables, flowers and herbs with excellent radical scavenging and cytoprotective properties. LUT is emerging as one of the most promising candidates in the biomedical and pharmaceutical fields (107). Recent studies have reported that LUT exhibits cardioprotective effects both in vitro and vivo (108). Researchers have confirmed that LUT has a protective effect on DCM via the Nrf2 signaling pathway. It has been proven that LUT ameliorates cardiac function and myocardial viability in diabetic rats with ischemia/reperfusion injury through the Nrf2-regulated antioxidative signaling pathway (109, 110). Evidence has demonstrated that LUT suppresses cardiomyocyte inflammation and oxidative stress, thereby preventing myocardial fibrosis and hypertrophy in DCM mice model via the upregulation of Nrf2, HO-1, and NQO1 (111). Although there were limited clinical trials correlated with the therapeutic effect of LUT on DCM, the exploration of its therapeutic effects on human DCM and related mechanisms targeting Nrf2 will become a hot topic among researchers.
3.1.6 Kaempferol
Kaempferol (KMP) as a plant-derived flavonoid has various pharmacological activities such as antioxidant, anti-inflammatory, anticancer and cardioprotective effects (112). KMP-containing plants are used worldwide in traditional systems to treat various conditions for centuries (113). Research has revealed that KMP as one of the main compounds of Eucommiae Folium exerted the cardioprotective effect on DCM mice (114). What's worth noting that KMP attenuates oxidative, inflammatory and fibrotic damages of the left ventricle (LV) in STZ-induced diabetic rats by upregulating the SIRT1/Nrf2 signaling pathway (115). KMP also protects against isoproterenol (ISO) -induced heart failure in diabetic rats by inhibiting cardiomyocyte apoptosis and activating the PI3K/Akt/GSK-3β/Nrf2 signaling pathway (116). Additionally, KMP as the Nrf2 activator demonstrates the beneficial effects on cardiac structure and function and its prominent anti-cardiac remodeling properties by inhibiting inflammatory responses and oxidative stress expression both in vitro and vivo DCM model (117). However, clinical studies are needed to confirm the protective effects of kaempferol observed in laboratory settings.
3.1.7 Notoginsenoside R1
Panax notoginseng (PN) root serves as a widely recognized nutritional supplement, health food ingredient, and traditional medicine. It plays a crucial role in maintaining homeostasis within the human microcirculatory system. Notoginsenoside R1 (NGR1), an active compound derived from Panax notoginseng (PN) root, has been reported to exhibit a range of pharmacological activities, including anti-inflammatory, antioxidant, anticancer, antimicrobial and angiogenic effects (118, 119). Clinical studies have demonstrated the efficacy of incorporating NGR1, the primary bioactive component of the XueShuanTong formula, into conventional treatments for ischemic diseases (120). Preclincal study has indicated that NGR1 prevents vascular smooth muscle cell (VSMC) proliferation, migration and neointimal proliferation by inhibiting activation of the PI3K/Akt signaling pathway (121). Recent experiment has confirmed that NGR1 inhibits cell apoptosis and hypertrophy by upregulating the AMPK/Nrf2 signaling pathway and HO-1 expression. The levels of cardiac hypertrophy markers, including auricular natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are significantly decreased (122).
3.1.8 Rg1 ginsenoside
Ginsenoside Rg1 (GRg1) is a primary active component of Panax ginseng C.A. Mey. It has been shown to have a protective effect on various cardiovascular diseases by regulating multiple cellular signaling pathways (123, 124). GRg1 has been proven to protect against DCM. Studies have demonstrated that GRg1 ameliorates cardiomyocyte oxidative stress and inflammation in DCM rats through activation of the AMPK/Nrf2/HO-1 signaling pathway (125). However, the precise role of GRg1 in regulating the Nrf2 signaling pathway within the treatment of DCM deserves further investigation.
3.1.9 Myricetin (杨梅素) and myricitrin (杨梅苷)
Myricetin, a flavonoid compound derived from various fruit, vegetables, tea, berries and red wine (126). Myricetin displays multiple preclinical biological effects including antioxidant, anti-inflammatory, anticancer, antidiabetic, antiviral, antibacterial and cardiovascular protective effects (127). Besides, clinical trials from various studies highlight the importance of myricetin as a chemo preventive reagent and its significant positive impact on key risk factors for coronary heart disease (128). In both lipopolysaccharide (LPS) -induced H9c2 cells and a C57BL/6J diabetic mouse model, myricetin mitigates cardiomyocyte oxidative stress and inflammation injuries (129). Studies have shown that myricetin alleviates pressure overload-induced cardiac hypertrophy in Nrf2 knockdown (Nrf2-KD) mice and phenylephrine (PE)-induced neonatal rat cardiomyocytes (NRCMs) via activation of the Nrf2 signaling pathway (130). Additionally, myricetin attenuates oxidative stress, inflammation and apoptosis in cardiomyocytes and improves myocardial diastolic dysfunction in STZ-induced diabetic mice through upregulation of the Nrf2/HO-1 signaling pathway (131, 132).
3.1.10 Naringenin (柚皮素) and naringin (柚皮苷)
Naringin, a flavanone glycoside exists in two forms: the glycosidic form of naringin and the aglycone form of naringenin (133). As flavonoids, naringenin and naringin possess a diverse range of pharmacological activities including antioxidant, anti-inflammatory, antidiabetic, anticancer and cardiovascular disease prevention effects (134). Naringenin exhibites beneficial effects on the lipid profile and reduces the percentages of non-alcoholic fatty liver disease (NAFLD) grades, serving as an indicator of the severity of hepatic steatosis for NAFLD patients. Clinical trials demonstrate that naringenin has a beneficial effect particularly related to cardiovascular diseases and diabetes (135). Preclinical study indicates that naringenin alleviates pathological damage, inflammation, lipid peroxidation and cellular ferroptosis by modulating the Nrf2/System Xc-/GPX4 axis in the myocardial tissue of MIRI-induced rats (136). Additionally, research has revealed that the protective effect of naringin on cardiomyocytes in diabetic mice which may be associated with reducing intracellular Ca2+ overload, limiting the increase in ROS levels and suppressing the expression level of TNF-α, IL-6, and NF-κB (137). Studies have also demonstrated that both naringenin and naringin ameliorate cardiomyocyte oxidative stress, inflammation and apoptosis in STZ-induced diabetic mice through activation of the Nrf2 signaling pathway (138, 139). Consequently, the preclinical studies indicate that Nrf2 may serve as a potential target for naringenin and naringin in the prevention of DCM (Figure 3).
Figure 3
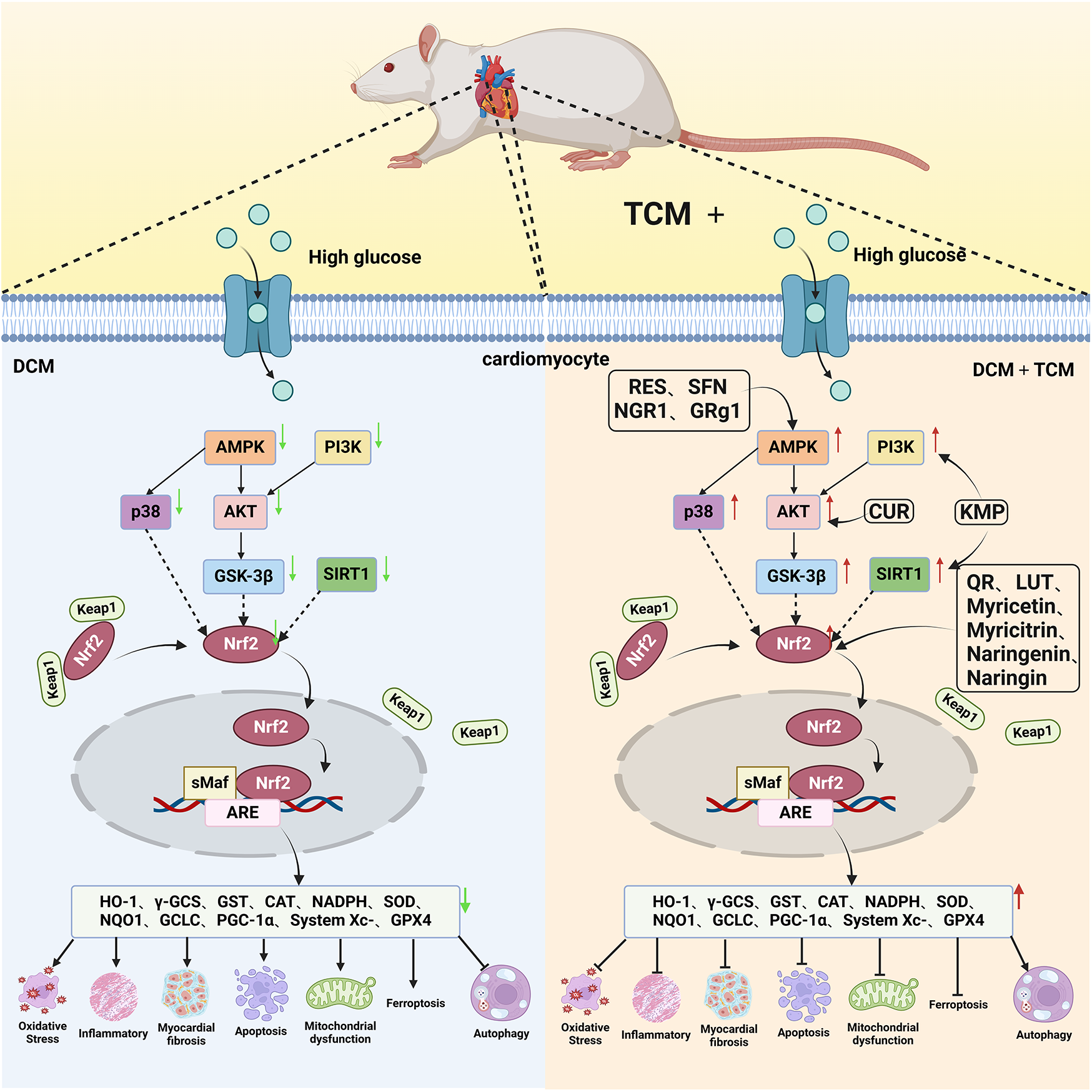
The cardioprotective effect of TCM on DCM rats via Nrf2 signaling pathway. RES, resveratrol SFN, sulforaphane; NGR1, Notoginsenoside R1; GRg1, Ginsenoside Rg1; CUR, curcumin; KMP, kaempferol; QR, quercetin; LUT, luteolin.
The next section provided a review of the protective effects of representative natural compounds on DCM and their potential mechanisms related to the Nrf2 signaling pathway, as detailed in Table 1.
Table 1
| NO. | Chinese Medicine Monomer | Chemical Structural Formula | Model | Regulation of Nrf2 Signaling Pathway | Main Purposed Effects | Ref(s) |
|---|---|---|---|---|---|---|
| 1 | Resveratrol |
|
FVB mice | ↑Nrf2, HO-1, SOD, NADPH | Anti-oxidative stress | (83–85) |
| Anti-myocardial fibrosis | ||||||
| SD rats | Anti-myocardial hypertrophy | |||||
| 2 | Quercetin |
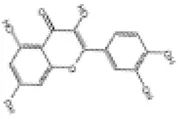
|
SD rats | ↑Nrf2, HO-1, SOD, GSH | Anti-oxidative stress | (90, 91) |
| H9c2 cells | ||||||
| Anti-myocardial fibrosis | ||||||
| Wistar rats | ||||||
| 3 | Curcumin |
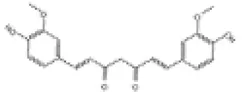
|
SD rats | ↑Nrf2, HO-1, GSH | Anti-oxidative stress | (59, 97–99) |
| H9c2 cells | Anti-cardiomyocyte apoptosis | |||||
| New Zealand rabbits | Anti-cardiomyocyte ferroptosis | |||||
| Wistar rats | Anti-inflammation | |||||
| 4 | Sulforaphane |

|
FVB mice | ↑Nrf2, HO-1, SOD, MT, CAT | Anti-oxidative stress | (63, 104–106) |
| Anti-myocardial fibrosis | ||||||
| Anti-inflammation | ||||||
| C57BL/6J mice | Anti-myocardial fibrosis | |||||
| Anti-myocardial hypertrophy | ||||||
| 5 | Luteolin |
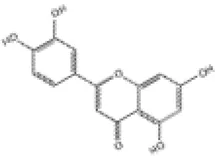
|
SD rats | ↑Nrf2, HO-1, SOD, GPx | Anti-oxidative stress | (109–111) |
| H9c2 cells | Anti-inflammation | |||||
| C57BL/6J mice | Anti-myocardial fibrosis | |||||
| Anti-myocardial hypertrophy | ||||||
| Anti-mitochondrial damage | ||||||
| 6 | Kaempferol |
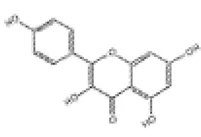
|
Wistar rats | ↑Nrf2, HO-1, γ-GCS | Anti-oxidative stress | (115–117) |
| C57BL/6J mice | Anti-inflammation | |||||
| Anti-myocardial fibrosis | ||||||
| H9c2 cells | ||||||
| Anti-cardiomyocyte apoptosis | ||||||
| 7 | Notoginsenoside R1 |
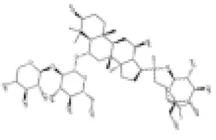
|
H9c2 cells | ↑Nrf2, HO-1 | Anti-oxidative stress | (122) |
| Anti-cardiomyocyte apoptosis | ||||||
| 8 | Ginsenoside Rg1 |
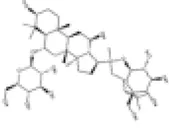
|
Wistar rats | ↑Nrf2, HO-1, SOD, CAT | Anti-oxidative stress | (125) |
| Anti-inflammation | ||||||
| 9 | Myricetin |
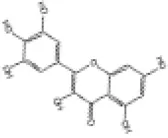
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD, NQO1 | Anti-oxidative stress | (131) |
| Anti-inflammation | ||||||
| Anti-cardiomyocyte apoptosis | ||||||
| 10 | Myricitrin |
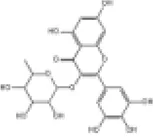
|
BALB/c mice | ↑Nrf2, HO-1, NQO-1 | Anti-oxidative stress | (132) |
| Anti-inflammation | ||||||
| H9c2 cells | Anti-cardiomyocyte apoptosis | |||||
| Anti-myocardial fibrosis | ||||||
| 11 | Naringenin |
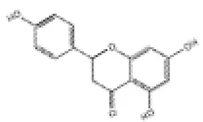
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD, NQO-1 | Anti-oxidative stress | (138) |
| Anti-inflammation | ||||||
| H9c2 cells | Anti-cardiomyocyte apoptosis | |||||
| 12 | Naringin |
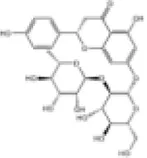
|
SD rats | ↑Nrf2 | Anti-oxidative stress | (139) |
| 13 | Thymoquinone |
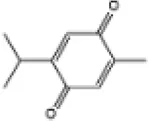
|
Wistar rats | ↑Nrf2, HO-1, SOD | Anti-oxidative stress | (140) |
| Anti-inflammation | ||||||
| 14 | Bakuchiol |
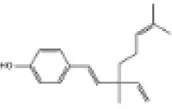
|
C57BL/6J mice | ↑Nrf2, SOD, GPx | Anti-oxidative stress | (141) |
| Anti-myocardial fibrosis | ||||||
| H9c2 cells | Anti-myocardial hypertrophy | |||||
| Anti-cardiomyocyte apoptosis | ||||||
| 15 | Andrographolide |
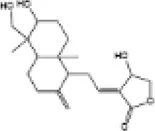
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD | Anti-oxidative stress | (142) |
| H9c2 cells | Anti-inflammation | |||||
| Anti-cardiomyocyte apoptosis | ||||||
| 16 | Dimethyl fumarate |
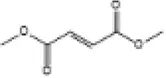
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD, CAT | Anti-oxidative stress | (143) |
| Anti-inflammation | ||||||
| Anti-myocardial fibrosis | ||||||
| 17 | Glycyrrhizin |
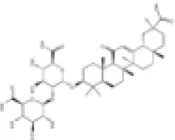
|
Zucker Diabetic Fatty rats | ↑Nrf2 | Anti-oxidative stress | (144) |
| Anti-inflammation | ||||||
| Anti-myocardial fibrosis | ||||||
| AC16 human cardiomyocyte | ||||||
| 18 | Scutellarin |
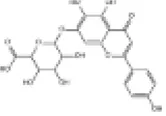
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD, CAT | Anti-oxidative stress | (145) |
| Anti-inflammation | ||||||
| Anti-myocardial fibrosis | ||||||
| 19 | Bail calin |
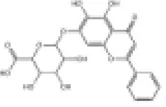
|
C57BL/6J mice | ↑Nrf2, HO-1, NQO1 | Anti-inflammation | (146) |
| Anti-myocardial fibrosis | ||||||
| Anti-myocardial hypertrophy | ||||||
| Anti-cardiomyocyte apoptosis | ||||||
| 20 | Honokiol |
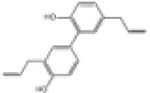
|
SD rats | ↑Nrf2, HO-1, NQO1 | Anti-oxidative stress | (147) |
| H9c2 cells | Anti-cardiomyocyte apoptosis | |||||
| 21 | Cyclovirobuxine D |
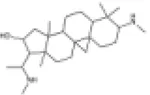
|
SD rats | ↑Nrf2, NQO1 | Anti-oxidative stress | (148) |
| The primary neonatalrat cardiomyocyte | ||||||
| 22 | Sinapic acid |
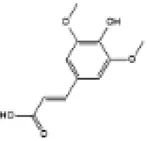
|
Wistar rats | ↑Nrf2, HO-1 | Anti-oxidative stress | (149) |
| Anti-inflammation | ||||||
| Anti-cardiomyocyte apoptosis | ||||||
| 23 | Oleanolic acid |
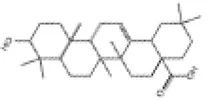
|
SD rats | ↑Nrf2, HO-1 | Anti-oxidative stress | (150) |
| Anti-cardiomyocyte apoptosis | ||||||
| 24 | Fucoxanthin |
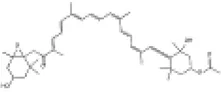
|
SD rats | ↑Nrf2, HO-1, SOD | Anti-oxidative stress | (151) |
| Anti-myocardial fibrosis | ||||||
| Anti-myocardial hypertrophy | ||||||
| 25 | 6-Gingerol |
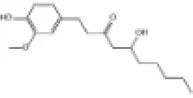
|
C57BL/6J mice | ↑Nrf2, HO-1 | Anti-oxidative stress | (152) |
| Anti-inflammation | ||||||
| H9c2 cells | Anti-cardiomyocyte ferroptosis | |||||
| Anti-cardiomyocyte apoptosis | ||||||
| 26 | Piceatannol |
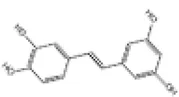
|
SD rats | ↑Nrf2, HO-1 | Anti-oxidative stress | (153) |
| Anti-inflammation | ||||||
| H9c2 cells | Anti-cardiomyocyte ferroptosis | |||||
| Anti-cardiomyocyte apoptosis | ||||||
| 27 | Fortunellin |
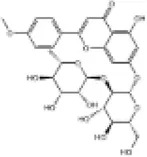
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD, CAT | Anti-oxidative stress | (154) |
| H9c2 cells | Anti-inflammation | |||||
| 28 | Costunolide |
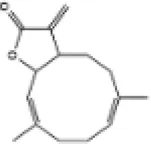
|
C57BL/6J mice | ↑Nrf2, HO-1 | Anti-oxidative stress | (155) |
| Anti-inflammation | ||||||
| H9c2 cells | Anti-cardiomyocyte ferroptosis | |||||
| Anti-myocardial hypertrophy | ||||||
| 29 | Geniposide |
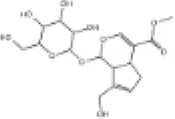
|
SD rats | ↑Nrf2, HO-1 | Anti-oxidative stress | (156) |
| 30 | Butin |
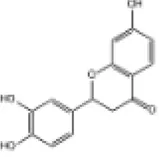
|
C57BL/6J mice | ↑Nrf2, HO-1 | Anti-oxidative stress | (157) |
| H9c2 cells | ||||||
| 31 | Kolaviron |
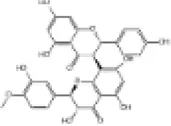
|
SD rats | ↑Nrf2, SOD | Anti-oxidative stress | (158) |
| Anti-inflammation | ||||||
| 32 | Diallyl trisulfide |

|
SD rats | ↑Nrf2, HO-1 | Anti-oxidative stress | (159) |
| 33 | Phloretin |
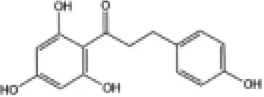
|
C57BL/6J mice | ↑Nrf2, HO-1, SOD, NQO1 | Anti-oxidative stress | (160) |
| Anti-myocardial fibrosis | ||||||
| Anti-myocardial hypertrophy | ||||||
| 34 | Gastrodin |
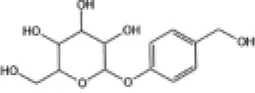
|
H9c2 cells | ↑Nrf2, GSH, SOD, CAT | Anti-oxidative stress | (161) |
| 35 | Esculeoside A |
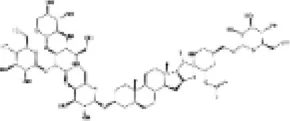
|
H9c2 cells | ↑Nrf2, OH-1, GSH, SOD | Anti-oxidative stress | (162) |
| Anti-inflammation | ||||||
| Anti-cardiomyocyte apoptosis | ||||||
| 36 | Ginsenoside Rb1 |
|
Wistar rats | ↑Nrf2, HO-1, SOD, CAT | Anti-oxidative stress | (163) |
| Anti-myocardial fibrosis | ||||||
| 37 | Asiaticoside |
|
db/db mice | ↑Nrf2, HO-1 | Anti-oxidative stress | (164) |
| 38 | Pterostilbene |
|
SD rats | ↑Nrf2, HO-1 | Anti-oxidative stress | (165) |
| Anti-inflammation |
Effects of Chinese medicine compounds on Nrf2 signaling pathway and their roles in DCM.
↑ signifies increase/activation.
3.2 Nrf2 agonists from Chinese herbal prescriptions
In China, various medicinal plants are combined as TCM prescriptions for the treatment of diseases. TCM prescriptions known for their multi-target, multi-component and multi-pathway characteristics, exerts synergistic effects on DCM therapy. In this section, we reviewed representative TCM prescriptions for DCM treatment that were associated with the Nrf2 signaling pathway.
3.2.1 Guan Xin Dan Shen formulation
The Guan Xin Dan Shen Formulation (GXDSF) consists of three herbs: Dalbergia odoriferae Lignum, Salviae miltiorrhizae Radix et Rhizoma, and Panax notoginseng Radix et Rhizoma. It has been widely used for the management of coronary heart disease in China by activating blood circulation, resolving blood stasis and relieving pain (166). GXDSF has been used clinically for the treatment of cardiovascular diseases (23). The main active ingredients of GXDSF are ginsenoside Rg1 (9.51%), ginsenoside Rb1 (8.63%), notoginsenoside R1 (2.34%), tanshinone IIA (1.71%), cryptotanshinone (0.84%), tanshinone I (0.55%) and salvianolic acid B (0.50%) (167). Salvianolic acid B and notoginsenoside R1 in GXDSF have been shown to significantly protect H9c2 cardiomyocytes from hypoxia and reoxygenation injuries by reducing the levels of the inflammatory factors of TNF-α and IL-1β (168). Preclinical studies indicate that treatment with GXDSF improves cardiac hypertrophy and dysfunction and significantly increases the left ventricular ejection fraction (LVEF) in diabetic mice model. Additionally, GXDSF attenuates cardiac dysfunction and inhibits cardiomyocyte apoptosis by activating the Akt/Nrf2 signaling pathway (169).
3.2.2 Mulberry granules
Mulberry granules a traditional Chinese medicine prescription is derived from the fruit of Morus alba L (170). It contains various beneficial constituents especially flavonoids and alkaloids (171, 172). In China, mulberry is commonly used to treat diabetes palpitations, insomnia and hyperglycemia for many years (173). Clinical studies have confirmed that mulberry twig alkaloids are effective and safe for the treatment of type 2 diabetes (174). Besides, including mulberry in the diet could positively influence various cardiometabolic risk factors (175). Preclinical experiment indicates that mulberry has been found to improve insulin sensitivity by activating the AMPK signaling pathway in diabetic db/db mice (176). Research has shown that mulberry ethanol extracts ameliorate abnormal lipid metabolism and enhance antioxidant activity in atherosclerosis (AS) rats (177). It can be speculated that mulberry could be benefit for DCM. Furthermore, mulberry attenuates oxidative stress induced by myocardial ischemia-reperfusion injury through upregulating the expression of GSH, SOD, CAT and glutathione reductase (GR) in myocardial tissue via the AMPK/Nrf2 signaling pathway (178). These preclinical studies suggest that mulberry granules may exert protective effects on DCM through the AMPK/Nrf2 signaling pathway. However, further clinical and animal studies are required to investigate the mechanism by which mulberry granules influences the progression of DCM.
3.2.3 Polyherbal formulation
The polyherbal formulation (PHF) comprises Piper nigrum (fruit), Terminalia paniculata (bark) and Bauhinia purpurea (bark), three of which are ayurvedic medicines and is used India's conventional medicinal system. Based on animal experiments, researchers shown that PHF reduces oxidative stress and inflammation in cardiac tissues of diabetic rats. This mechanism may be associated with the upregulation of the Nrf2/HO-1 signaling pathway. Furthermore, PHF increases the serum levels of SOD, CAT and GSH and decreases the serum levels IL-1β, IL-6 and TNF-α, which is closely associated with the activation of the NF-κB/Nrf2/HO-1 signaling pathway (179). These findings demonstrate that Nrf2 may be considered as the key targets of PHF for preclinical treatment of DCM.
3.2.4 Benefits and challenges of TCMs as Nrf2 activators for DCM therapy
Due to the oxidative stress as an essential pathogenic factor for DCM pathogenesis, the TCMs as Nrf2 activators exert protective effects for cardiovascular tissues in diabetes. Aforementioned evidences reveal that mechanism and potential of the TCMs against DCM are prominent in the PI3K/Akt/GSK-3β, NF-κB, AMPK/p38, SIRT1 and TGF-β/Smads signaling pathway which are crosstalk with the activation of Nrf2. These evidences also support the conclusion that the TCM alleviates DCM by modulating pathological processes, including myocardial fibrosis, inflammation, oxidative stress, metabolism disorder, cardiac hypertrophy, apoptosis and etc. Considering the damage to multiple organs caused by hyperglycemia as well as the intricate and prolonged development of DCM, TCMs as the Nrf2 activators demonstrate significant promise as a potential option for DCM due to its advantages of multi-target and multi-pathway effects with limited adverse reactions. However, numerous challenges remain in elucidating both the biological activity and potential toxicity of TCMs for their future clinical application in DCM. Additionally, the larger clinical trials of the Nrf2 activators from TCMs are urgently needed to further evaluation the reliability of their therapeutic effects.
4 Future prospects
The pathological mechanism of DCM involves in the interplay of various molecular signal transduction pathways. Nrf2 as a key transcription factor in the pathogenesis of DCM provides protection effects by reducing oxidative stress, inflammation, myocardial fibrosis, apoptosis, ferroptosis, autophagy and mitochondrial dysfunction. However, there are still unexplored pathways or processes linked to Nrf2 that need further explained its role in DCM. Copper (Cu) regulation in the pathogenesis of DCM has been drawn attention as a new research hotspot. Researcher speculates that in the progression of DCM, the high levels of Cu in plasma may damage mitochondria of vascular endothelial cells through cuproptosis or oxidative stress pathway and then affects the diastolic and contractile function of cardiomyocytes (180). While Cu deficiency in myocardial cells may result in impairment of energy metabolism (181). Animal study confirms that Cu deficiency can inhibit the Nrf2 pathway, consequently induces oxidative damage in the liver (182). However, the exact mechanism by which Cu affects antioxidant related to regulation of Nrf2 deserves as another interesting research.
This review systematically analyses the role of the Nrf2 signaling pathway in the pathogenesis and treatment of DCM using TCM. Based on the literature, it can be concluded that Nrf2 as a classical transcription factor associated with anti-oxidative stress is a promising target for DCM treatment. However, the role of Nrf2 in DCM also represents complex and controversial aspect. One recent study has found that chronic Nrf2 activation in the context of autophagy deficiency can exacerbate DCM, suggesting that Nrf2 is not universally beneficial (183). Additionally, although TCM formulations have shown efficacy in ameliorating and treating DCM potentially by targeting the Nrf2 signaling pathway in preclinical experiments, further exploration of clinical researches is still required to validate the safety and efficacy of TCM formulations for DCM treatment. Furthermore, the majority studies on compounds from TCM have primarily focused on animal and cell experiments. How to translate these findings into clinical practice for DCM faces several potential limitations and challenges. Firstly, limitations of study methodology. Many studies focus on in vitro experiments, which may differ from the in vivo conditions. Although animal experiments are crucial for understanding TCM's mechanisms, it is different from human beings in terms of anatomy, physiology and metabolism. Secondly, the complexity of TCM components propose the challenges for revealing the precise molecular mechanism for treatment of DCM, which hinders their global recognition. TCM's complex composition makes it difficult to identify the major active ingredients. Thirdly, the clinical detection of Nrf2 presents another significant challenge. Although Nrf2 has been generally accepted as one of key anti-oxidative transcription factor in the progression of DCM, it has yet to be established as a reliable biomarker for both the diagnosis and treatment of DCM in clinical practice. Other possible diagnostic markers that indirectly reflect the Nrf2 activity needs to be explored. Nrf2 signaling, a key driver of antioxidation, is commonly down-regulated in DCM. Current TCM targeting the Nrf2 signaling pathway has been shown promise in preclinical trials. Further improvement understanding of the regulatory mechanisms of Nrf2 signaling and extensive clinical trial studies of natural Nrf2 activators will provide new approaches for the treatment of DCM patients in the near future.
Statements
Author contributions
HG: Writing – original draft. JL: Writing – review & editing. JJ: Writing – review & editing. HM: Writing – review & editing. SW: Writing – review & editing. LS: Funding acquisition, Writing – review & editing. LY: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Open Project of the Key Laboratory of Xinjiang Uygur Autonomous Region (No. 2023D04052) and the Major national science and technology projects (No. 2024ZD0528302).
Acknowledgments
The author expresses gratitude to the College of Traditional Chinese Medicine of Xinjiang Medical University, Xinjiang Uygur Autonomous Region Hospital of Traditional Chinese Medicine and Xinjiang Key Laboratory of Famous Prescription and Science of Formulas for their generous support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Dillmann WH . Diabetic cardiomyopathy. Circ Res. (2019) 124:1160–2. 10.1161/CIRCRESAHA.118.314665
2.
Murtaza G Virk HUH Khalid M Lavie CJ Ventura H Mukherjee D et al Diabetic cardiomyopathy—a comprehensive updated review. Prog Cardiovasc Dis. (2019) 62:315–26. 10.1016/j.pcad.2019.03.003
3.
Fuentes-Antrás J Picatoste B Gómez-Hernández A Egido J Tuñón J Lorenzo Ó . Updating experimental models of diabetic cardiomyopathy. J Diabetes Res. (2015) 2015:656795. 10.1155/2015/656795
4.
Jia G Whaley-Connell A Sowers JR . Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. (2018) 61:315–26. 10.1007/s00125-017-4390-4
5.
Lorenzo-Almorós A Cepeda-Rodrigo JM Lorenzo Ó . Diabetic cardiomyopathy. Revista Clínica Española. (2022) 222(2):100–11. 10.1016/j.rceng.2019.10.012
6.
Zhao X Liu S Wang X Chen Y Pang P Yang Q et al Diabetic cardiomyopathy: clinical phenotype and practice. Front Endocrinol. (2022) 13:1032268. 10.3389/fendo.2022.1032268
7.
Radzioch E Dąbek B Balcerczyk-Lis M Frąk W Fularski P Młynarska E et al Diabetic cardiomyopathy-from basics through diagnosis to treatment. Biomedicines. (2024) 12:765. 10.3390/biomedicines12040765
8.
Culletta G Buttari B Arese M Brogi S Almerico AM Saso L et al Natural products as non-covalent and covalent modulators of the KEAP1/NRF2 pathway exerting antioxidant effects. Eur J Med Chem. (2024) 270:116355. 10.1016/j.ejmech.2024.116355
9.
Yi M Toribio AJ Salem YM Alexander M Ferrey A Swentek L et al Nrf2 signaling pathway as a key to treatment for diabetic dyslipidemia and atherosclerosis. Int J Mol Sci. (2024) 25:5831. 10.3390/ijms25115831
10.
He F Ru X Wen T . NRF2, A transcription factor for stress response and beyond. Int J Mol Sci. (2020) 21:4777. 10.3390/ijms21134777
11.
Lee S Hu L . Nrf2 activation through the inhibition of Keap1-Nrf2 protein-protein interaction. Med Chem Res Int J Rapid Commun Des Mech Action Biol Act Agents. (2020) 29:846–67. 10.1007/s00044-020-02539-y
12.
Li R Jia Z Zhu H . Regulation of Nrf2 signaling. React Oxyg Species. (2019) 8:312–22. Available online at:https://rosj.org/index.php/ros/article/view/252(Accessed March 20, 2023).
13.
Li B Liu S Miao L Cai L . Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res. (2012) 2012:216512. 10.1155/2012/216512
14.
Chen J Zhang Z Cai L . Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J. (2014) 38:337–45. 10.4093/dmj.2014.38.5.337
15.
Sathibabu Uddandrao VV Brahmanaidu P Nivedha PR Vadivukkarasi S Saravanan G . Beneficial role of some natural products to attenuate the diabetic cardiomyopathy through Nrf2 pathway in cell culture and animal models. Cardiovasc Toxicol. (2018) 18:199–205. 10.1007/s12012-017-9430-2
16.
Gopal K Chahade JJ Kim R Ussher JR . The impact of antidiabetic therapies on diastolic dysfunction and diabetic cardiomyopathy. Front Physiol. (2020) 11:603247. 10.3389/fphys.2020.603247
17.
Li W Liu X Liu Z Xing Q Liu R Wu Q et al The signaling pathways of selected traditional Chinese medicine prescriptions and their metabolites in the treatment of diabetic cardiomyopathy: a review. Front Pharmacol. (2024) 15:1416403. 10.3389/fphar.2024.1416403
18.
Tian J Zhao Y Liu Y Liu Y Chen K Lyu S . Roles and mechanisms of herbal medicine for diabetic cardiomyopathy: current status and perspective. Oxid Med Cell Longev. (2017) 2017:8214541. 10.1155/2017/8214541
19.
Yan B Ren J Zhang Q Gao R Zhao F Wu J et al Antioxidative effects of natural products on diabetic cardiomyopathy. J Diabetes Res. (2017) 2017:2070178. 10.1155/2017/2070178
20.
Li L Chen X Su C Wang Q Li R Jiao W et al Si-Miao-Yong-An decoction preserves cardiac function and regulates GLC/AMPK/NF-κB and GLC/PPARα/PGC-1α pathways in diabetic mice. Biomed Pharm Biomed Pharm. (2020) 132:110817. 10.1016/j.biopha.2020.110817
21.
Yan M Liu S Zeng W Guo Q Mei Y Shao X et al The Chinese herbal medicine Fufang Zhenzhu Tiaozhi ameliorates diabetic cardiomyopathy by regulating cardiac abnormal lipid metabolism and mitochondrial dynamics in diabetic mice. Biomed Pharm Biomed Pharm. (2023) 164:114919. 10.1016/j.biopha.2023.114919
22.
Lv S Wang Q Zhang X Ning F Liu W Cui M et al Mechanisms of multi-omics and network pharmacology to explain traditional Chinese medicine for vascular cognitive impairment: a narrative review. Phytomed Int J Phytother Phytopharm. (2024) 123:155231. 10.1016/j.phymed.2023.155231
23.
Hao P Jiang F Cheng J Ma L Zhang Y Zhao Y . Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. (2017) 69:2952–66. 10.1016/j.jacc.2017.04.041
24.
Shao Y Wang K Jiang Z Yu X An W Han Y et al Comparative efficacy and acceptability of traditional Chinese medicine for adult major depression: a protocol for network meta-analysis. Medicine (Baltimore). (2020) 99:e23199. 10.1097/MD.0000000000023199
25.
Matos LC Machado JP Monteiro FJ Greten HJ . Understanding traditional Chinese medicine therapeutics: an overview of the basics and clinical applications. Healthc Basel Switz. (2021) 9:257. 10.3390/healthcare9030257
26.
Ritchie RH Abel ED . Basic mechanisms of diabetic heart disease. Circ Res. (2020) 126:1501–25. 10.1161/CIRCRESAHA.120.315913
27.
Peng ML Fu Y Wu CW Zhang Y Ren H Zhou SS . Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front Endocrinol. (2022) 13:907757. 10.3389/fendo.2022.907757
28.
Zhou S Jin J Bai T Sachleben LR Cai L Zheng Y . Potential drugs which activate nuclear factor E2-related factor 2 signaling to prevent diabetic cardiovascular complications: a focus on fumaric acid esters. Life Sci. (2015) 134:56–62. 10.1016/j.lfs.2015.05.015
29.
Hsieh P-L Chu P-M Cheng H-C Huang Y-T Chou W-C Tsai K-L et al Dapagliflozin mitigates doxorubicin-caused myocardium damage by regulating AKT-mediated oxidative stress, cardiac remodeling, and inflammation. Int J Mol Sci. (2022) 23:10146. 10.3390/ijms231710146
30.
Wu X Huang L Liu J . Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (review). Exp Ther Med. (2021) 22:678. 10.3892/etm.2021.10110
31.
Li H Yao W Irwin MG Wang T Wang S Zhang L et al Adiponectin ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction by concomitantly activating Nrf2 and Brg1. Free Radic Biol Med. (2015) 84:311–21. 10.1016/j.freeradbiomed.2015.03.007
32.
Dyson A Bryan NS Fernandez BO Garcia-Saura M-F Saijo F Mongardon N et al An integrated approach to assessing nitroso-redox balance in systemic inflammation. Free Radic Biol Med. (2011) 51:1137–45. 10.1016/j.freeradbiomed.2011.06.012
33.
Bellezza I Giambanco I Minelli A Donato R . Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta BBA—Mol Cell Res. (2018) 1865:721–33. 10.1016/j.bbamcr.2018.02.010
34.
Frati G Schirone L Chimenti I Yee D Biondi-Zoccai G Volpe M et al An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. (2017) 113:378–88. 10.1093/cvr/cvx011
35.
He F Antonucci L Karin M . NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. (2020) 41:405–16. 10.1093/carcin/bgaa039
36.
Gao Y Liu W Su X Li X Yu F Zhang N . The beneficial effects of Chinese herbal monomers on ameliorating diabetic cardiomyopathy via Nrf2 signaling. Oxid Med Cell Longev. (2022) 2022:3959390. 10.1155/2022/3959390
37.
Ryter SW . Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells. (2021) 10:515. 10.3390/cells10030515
38.
Wang W Shen Q . Tranilast reduces cardiomyocyte injury induced by ischemia-reperfusion via Nrf2/HO-1/NF-κB signaling. Exp Ther Med. (2023) 25:160. 10.3892/etm.2023.11859
39.
Brunner F Brás-Silva C Cerdeira AS Leite-Moreira AF . Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. (2006) 111:508–31. 10.1016/j.pharmthera.2005.11.001
40.
Jiang W Xiong Y Li X Yang Y . Cardiac fibrosis: cellular effectors, molecular pathways, and exosomal roles. Front Cardiovasc Med. (2021) 8:715258. 10.3389/fcvm.2021.715258
41.
Li L Zhao Q Kong W . Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol J Int Soc Matrix Biol. (2018) 68–69:490–506. 10.1016/j.matbio.2018.01.013
42.
Turner NA . Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol. (2016) 94:189–200. 10.1016/j.yjmcc.2015.11.002
43.
Yue Y Meng K Pu Y Zhang X . Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract. (2017) 133:124–30. 10.1016/j.diabres.2017.08.018
44.
Vashi R Patel BM . NRF2 In cardiovascular diseases: a ray of hope!. J Cardiovasc Transl Res. (2021) 14:573–86. 10.1007/s12265-020-10083-8
45.
Goh KY He L Song J Jinno M Rogers AJ Sethu P et al Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol. (2019) 21:101100. 10.1016/j.redox.2019.101100
46.
Syed AM Kundu S Ram C Kulhari U Kumar A Mugale MN et al Up-regulation of Nrf2/HO-1 and inhibition of TGF-β1/Smad2/3 signaling axis by daphnetin alleviates transverse aortic constriction-induced cardiac remodeling in mice. Free Radic Biol Med. (2022) 186:17–30. 10.1016/j.freeradbiomed.2022.04.019
47.
Chen R-R Fan X-H Chen G Zeng G-W Xue Y-G Liu X-T et al Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/ TGFβ1/Smad2/3 signaling axis. Chem Biol Interact. (2019) 302:11–21. 10.1016/j.cbi.2019.01.031
48.
Ryoo I-G Ha H Kwak M-K . Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: a modulatory effect on SMAD signaling. PLoS One. (2014) 9:e93265. 10.1371/journal.pone.0093265
49.
Nishida K Yamaguchi O Otsu K . Crosstalk between autophagy and apoptosis in heart disease. Circ Res. (2008) 103:343–51. 10.1161/CIRCRESAHA.108.175448
50.
Lee Y Gustafsson AB . Role of apoptosis in cardiovascular disease. Apoptosis Int J Program Cell Death. (2009) 14:536–48. 10.1007/s10495-008-0302-x
51.
Joubert M Manrique A Cariou B Prieur X . Diabetes-related cardiomyopathy: the sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab. (2019) 45:238–47. 10.1016/j.diabet.2018.07.003
52.
Fiordaliso F Bianchi R Staszewsky L Cuccovillo I Doni M Laragione T et al Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. (2004) 37:959–68. 10.1016/j.yjmcc.2004.07.008
53.
Giacco F Brownlee M . Oxidative stress and diabetic complications. Circ Res. (2010) 107:1058–70. 10.1161/CIRCRESAHA.110.223545
54.
Ji L Liu Y Zhang Y Chang W Gong J Wei S et al The antioxidant edaravone prevents cardiac dysfunction by suppressing oxidative stress in type 1 diabetic rats and in high-glucose-induced injured H9c2 cardiomyoblasts. Can J Physiol Pharmacol. (2016) 94:996–1006. 10.1139/cjpp-2015-0587
55.
Tsai C-Y Wang C-C Lai T-Y Tsu H-N Wang C-H Liang H-Y et al Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int J Cardiol. (2013) 168:1286–97. 10.1016/j.ijcard.2012.12.004
56.
Li Y Duan J-Z He Q Wang C-Q . Mir-155 modulates high glucose-induced cardiac fibrosis via the Nrf2/HO-1 signaling pathway. Mol Med Rep. (2020) 22:4003–16. 10.3892/mmr.2020.11495
57.
Gao L Liu Y Guo S Xiao L Wu L Wang Z et al LAZ3 protects cardiac remodeling in diabetic cardiomyopathy via regulating miR-21/PPARa signaling. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:3322–38. 10.1016/j.bbadis.2018.07.019
58.
Song Z Wang J Zhang L . Ferroptosis: a new mechanism in diabetic cardiomyopathy. Int J Med Sci. (2024) 21:612–22. 10.7150/ijms.88476
59.
Wei Z Shaohuan Q Pinfang K Chao S . Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovasc Ther. (2022) 2022:3159717. 10.1155/2022/3159717
60.
Cui C Yang F Li Q . Post-Translational modification of GPX4 is a promising target for treating ferroptosis-related diseases. Front Mol Biosci. (2022) 9:901565. 10.3389/fmolb.2022.901565
61.
Li F Hu Z Huang Y Zhan H . Dexmedetomidine ameliorates diabetic cardiomyopathy by inhibiting ferroptosis through the Nrf2/GPX4 pathway. J Cardiothorac Surg. (2023) 18:223. 10.1186/s13019-023-02300-7
62.
Wang M Tang J Zhang S Pang K Zhao Y Liu N et al Exogenous H2S initiating Nrf2/GPx4/GSH pathway through promoting Syvn1-Keap1 interaction in diabetic hearts. Cell Death Discov. (2023) 9:394. 10.1038/s41420-023-01690-w
63.
Wang X Chen X Zhou W Men H Bao T Sun Y et al Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. (2022) 12:708–22. 10.1016/j.apsb.2021.10.005
64.
Chen Y Hua Y Li X Arslan IM Zhang W Meng G . Distinct types of cell death and the implication in diabetic cardiomyopathy. Front Pharmacol. (2020) 11:42. 10.3389/fphar.2020.00042
65.
Lavandero S Chiong M Rothermel BA Hill JA . Autophagy in cardiovascular biology. J Clin Invest. (2015) 125:55–64. 10.1172/JCI73943
66.
Lusha E Jiang H . Simvastatin protects high glucose-induced H9c2 cells from injury by inducing autophagy. Pharm Biol. (2020) 58:1077–84. 10.1080/13880209.2020.1839512
67.
Fang Q Liu X Ding J Zhang Z Chen G Du T et al Soluble epoxide hydrolase inhibition protected against diabetic cardiomyopathy through inducing autophagy and reducing apoptosis relying on Nrf2 upregulation and transcription activation. Oxid Med Cell Longev. (2022) 2022:3773415. 10.1155/2022/3773415
68.
Guan Y Zhou L Zhang Y Tian H Li A Han X . Effects of PP2A/Nrf2 on experimental diabetes mellitus-related cardiomyopathy by regulation of autophagy and apoptosis through ROS dependent pathway. Cell Signal. (2019) 62:109339. 10.1016/j.cellsig.2019.06.004
69.
Dewanjee S Vallamkondu J Kalra RS John A Reddy PH Kandimalla R . Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. (2021) 68:101338. 10.1016/j.arr.2021.101338
70.
Yu T Jhun BS Yoon Y . High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal. (2011) 14:425–37. 10.1089/ars.2010.3284
71.
Cai C Wu F He J Zhang Y Shi N Peng X et al Mitochondrial quality control in diabetic cardiomyopathy: from molecular mechanisms to therapeutic strategies. Int J Biol Sci. (2022) 18:5276–90. 10.7150/ijbs.75402
72.
Khan S Ahmad SS Kamal MA . Diabetic cardiomyopathy: from mechanism to management in a nutshell. Endocr Metab Immune Disord—Drug Targets. (2021) 21:268–81. 10.2174/1871530320666200731174724
73.
Yan W Zhang H Liu P Wang H Liu J Gao C et al Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol. (2013) 108:329. 10.1007/s00395-013-0329-1
74.
Maharjan S Oku M Tsuda M Hoseki J Sakai Y . Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci Rep. (2014) 4:5896. 10.1038/srep05896
75.
Shao Q Meng L Lee S Tse G Gong M Zhang Z et al Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. (2019) 18:165. 10.1186/s12933-019-0964-4
76.
Holmström KM Baird L Zhang Y Hargreaves I Chalasani A Land JM et al Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. (2013) 2:761–70. 10.1242/bio.20134853
77.
Cao G Yang C Jin Z Wei H Xin C Zheng C et al FNDC5/irisin Reduces ferroptosis and improves mitochondrial dysfunction in hypoxic cardiomyocytes by Nrf2/HO-1 axis. Cell Biol Int. (2022) 46:723–36. 10.1002/cbin.11763
78.
Zhang J Cai X Zhang Q Li X Li S Ma J et al Hydrogen sulfide restores sevoflurane postconditioning mediated cardioprotection in diabetic rats: role of SIRT1/Nrf2 signaling-modulated mitochondrial dysfunction and oxidative stress. J Cell Physiol. (2021) 236:5052–68. 10.1002/jcp.30214
79.
Dinkova-Kostova AT Copple IM . Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol Sci. (2023) 44:137–49. 10.1016/j.tips.2022.12.003
80.
Brown K Theofanous D Britton RG Aburido G Pepper C Sri Undru S et al Resveratrol for the management of human health: how far have we come? A systematic review of resveratrol clinical trials to highlight gaps and opportunities. Int J Mol Sci. (2024) 25:747. 10.3390/ijms25020747
81.
Chekalina NI . Resveratrol has a positive effect on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiadomosci Lek Wars Pol. (2017) 70:286–91.
82.
Chen S Zhao X Ran L Wan J Wang X Qin Y et al Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. (2015) 47:226–32. 10.1016/j.dld.2014.11.015
83.
Wang G Song X Zhao L Li Z Liu B . Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. BioMed Res Int. (2018) 2018:2150218. 10.1155/2018/2150218
84.
Xu G Ma Y Jin J Wang X . Activation of AMPK/p38/Nrf2 is involved in resveratrol alleviating myocardial ischemia-reperfusion injury in diabetic rats as an endogenous antioxidant stress feedback. Ann Transl Med. (2022) 10:890. 10.21037/atm-22-3789
85.
Xu G Zhao X Fu J Wang X . Resveratrol increase myocardial Nrf2 expression in type 2 diabetic rats and alleviate myocardial ischemia/reperfusion injury (MIRI). Ann Palliat Med. (2019) 8:565–75. 10.21037/apm.2019.11.25
86.
Sun Z-G Li Z-N Zhang J-M Hou X-Y Yeh SM Ming X . Recent developments of flavonoids with various activities. Curr Top Med Chem. (2022) 22:305–29. 10.2174/1568026622666220117111858
87.
Ferenczyova K Kalocayova B Bartekova M . Potential implications of quercetin and its derivatives in cardioprotection. Int J Mol Sci. (2020) 21:1585. 10.3390/ijms21051585
88.
Papakyriakopoulou P Velidakis N Khattab E Valsami G Korakianitis I Kadoglou NP . Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals. (2022) 15:1019. 10.3390/ph15081019
89.
Roslan J Giribabu N Karim K Salleh N . Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharm Biomed Pharm. (2017) 86:570–82. 10.1016/j.biopha.2016.12.044
90.
Wei Z Jing Z Pinfang K Chao S Shaohuan Q . Quercetin inhibits pyroptosis in diabetic cardiomyopathy through the Nrf2 pathway. J Diabetes Res. (2022) 2022:9723632. 10.1155/2022/9723632
91.
Castillo RL Herrera EA Gonzalez-Candia A Reyes-Farias M de la Jara N Peña JP et al Quercetin prevents diastolic dysfunction induced by a high-cholesterol diet: role of oxidative stress and bioenergetics in hyperglycemic rats. Oxid Med Cell Longev. (2018) 2018:7239123. 10.1155/2018/7239123
92.
Cantó C Auwerx J . PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. (2009) 20:98–105. 10.1097/MOL.0b013e328328d0a4
93.
Peng Y Ao M Dong B Jiang Y Yu L Chen Z et al Anti-Inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug Des Devel Ther. (2021) 15:4503–25. 10.2147/DDDT.S327378
94.
Kotha RR Luthria DL . Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Mol Basel Switz. (2019) 24:2930. 10.3390/molecules24162930
95.
Sangouni AA Taghdir M Mirahmadi J Sepandi M Parastouei K . Effects of curcumin and/or coenzyme Q10 supplementation on metabolic control in subjects with metabolic syndrome: a randomized clinical trial. Nutr J. (2022) 21:62. 10.1186/s12937-022-00816-7
96.
Li H Sureda A Devkota HP Pittalà V Barreca D Silva AS et al Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. (2020) 38:107343. 10.1016/j.biotechadv.2019.01.010
97.
Wu X Zhou X Lai S Liu J Qi J . Curcumin activates Nrf2/ HO -1 signaling to relieve diabetic cardiomyopathy injury by reducing ROS in vitro and in vivo. FASEB J. (2022) 36:e22505. 10.1096/fj.202200543RRR
98.
Abdelsamia EM Khaleel SA Balah A Abdel Baky NA . Curcumin augments the cardioprotective effect of metformin in an experimental model of type I diabetes mellitus; impact of Nrf2/HO-1 and JAK/STAT pathways. Biomed Pharmacother Biomedecine Pharmacother. (2019) 109:2136–44. 10.1016/j.biopha.2018.11.064
99.
Wu X Zhou X Lai S Liu J Qi J . Curcumin improves diabetic cardiomyopathy by inhibiting pyroptosis through AKT/Nrf2/ARE pathway. FASEB J. (2022) 36(9):e22505. 10.1155/2023/3906043
100.
Elkashty OA Tran SD . Sulforaphane as a promising natural molecule for cancer prevention and treatment. Curr Med Sci. (2021) 41:250–69. 10.1007/s11596-021-2341-2
101.
Zhang Y Talalay P Cho CG Posner GH . A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. (1992) 89:2399–403. 10.1073/pnas.89.6.2399
102.
Yagishita Y Fahey JW Dinkova-Kostova AT Kensler TW . Broccoli or sulforaphane: is it the source or dose that matters?Mol Basel Switz. (2019) 24:3593. 10.3390/molecules24193593
103.
Yagishita Y Gatbonton-Schwager TN McCallum ML Kensler TW . Current landscape of NRF2 biomarkers in clinical trials. Antioxid Basel Switz. (2020) 9:716. 10.3390/antiox9080716
104.
Gu J Cheng Y Wu H Kong L Wang S Xu Z et al Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. (2017) 66:529–42. 10.2337/db15-1274
105.
Sun Y Zhou S Guo H Zhang J Ma T Zheng Y et al Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metab Clin Exp. (2020) 102:154002. 10.1016/j.metabol.2019.154002
106.
Bai Y Cui W Xin Y Miao X Barati MT Zhang C et al Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. (2013) 57:82–95. 10.1016/j.yjmcc.2013.01.008
107.
Zhu M Sun Y Su Y Guan W Wang Y Han J et al Luteolin: a promising multifunctional natural flavonoid for human diseases. Phytother Res PTR. (2024) 38:3417–43. 10.1002/ptr.8217
108.
Luo Y Shang P Li D . Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front Pharmacol. (2017) 8:692. 10.3389/fphar.2017.00692
109.
Xiao C Xia M-L Wang J Zhou X-R Lou Y-Y Tang L-H et al Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid Med Cell Longev. (2019) 2019:2719252. 10.1155/2019/2719252
110.
Zhou X-R Ru X-C Xiao C Pan J Lou Y-Y Tang L-H et al Sestrin2 is involved in the Nrf2-regulated antioxidative signaling pathway in luteolin-induced prevention of the diabetic rat heart from ischemia/reperfusion injury. Food Funct. (2021) 12:3562–71. 10.1039/d0fo02942d
111.
Li L Luo W Qian Y Zhu W Qian J Li J et al Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomed Int J Phytother Phytopharm. (2019) 59:152774. 10.1016/j.phymed.2018.11.034
112.
Dabeek WM Marra MV . Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. (2019) 11:2288. 10.3390/nu11102288
113.
Periferakis A Periferakis K Badarau IA Petran EM Popa DC Caruntu A et al Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int J Mol Sci. (2022) 23:15054. 10.3390/ijms232315054
114.
Zhang S-N Liu Q Li X-Z Yang W Zhou Y . Cardioprotection of eucommiae folium: the extract and pharmaceutically active compounds attenuate hyperglycemia, mitochondrial dysfunction, calcium dyshomeostasis, insulin resistance, and oxidative stress in diabetic cardiomyopathy db/db mice. J Pharm Pharmacol. (2023) 75:1509–20. 10.1093/jpp/rgad095
115.
Alshehri AS El-Kott AF Eleawa SM El-Gerbed MSA Khalifa HS El-Kenawy AE et al Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J Physiol Pharmacol Off J Pol Physiol Soc. (2021) 72:339–55. 10.26402/jpp.2021.3.04
116.
Zhang L Guo Z Wang Y Geng J Han S . The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev Res. (2019) 80:294–309. 10.1002/ddr.21495
117.
Chen X Qian J Wang L Li J Zhao Y Han J et al Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine. (2018) 60:83–94. 10.1007/s12020-018-1525-4
118.
Wen C Liao X Ye X Lai W . Pharmacokinetics and biological activities of notoginsenoside R1: a systematical review. Am J Chin Med. (2025) 53:205–49. 10.1142/S0192415X25500090
119.
Xie Y Wang C . Herb-drug interactions between panax notoginseng or its biologically active compounds and therapeutic drugs: a comprehensive pharmacodynamic and pharmacokinetic review. J Ethnopharmacol. (2023) 307:116156. 10.1016/j.jep.2023.116156
120.
Zhang H-Y Niu W Olaleye OE Du F-F Wang F-Q Huang Y-H et al Comparison of intramuscular and intravenous pharmacokinetics of ginsenosides in humans after dosing XueShuanTong, a lyophilized extract of Panax notoginseng roots. J Ethnopharmacol. (2020) 253:112658. 10.1016/j.jep.2020.112658
121.
Fang H Yang S Luo Y Zhang C Rao Y Liu R et al Notoginsenoside R1 inhibits vascular smooth muscle cell proliferation, migration and neointimal hyperplasia through PI3K/Akt signaling. Sci Rep. (2018) 8:7595. 10.1038/s41598-018-25874-y
122.
Du F Huang H Cao Y Ran Y Wu Q Chen B . Notoginsenoside R1 protects against high glucose-induced cell injury through AMPK/Nrf2 and downstream HO-1 signaling. Front Cell Dev Biol. (2021) 9:791643. 10.3389/fcell.2021.791643
123.
Fan W Huang Y Zheng H Li S Li Z Yuan L et al Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: pharmacology and mechanisms. Biomed Pharm Biomed Pharm. (2020) 132:110915. 10.1016/j.biopha.2020.110915
124.
Zhu G-X Zuo J-L Xu L Li S-Q . Ginsenosides in vascular remodeling: cellular and molecular mechanisms of their therapeutic action. Pharmacol Res. (2021) 169:105647. 10.1016/j.phrs.2021.105647
125.
Qin Q Lin N Huang H Zhang X Cao X Wang Y et al Ginsenoside Rg1 ameliorates cardiac oxidative stress and inflammation in streptozotocin-induced diabetic rats. Diabetes Metab Syndr Obes Targets Ther. (2019) 12:1091–103. 10.2147/DMSO.S208989
126.
Song X Tan L Wang M Ren C Guo C Yang B et al Myricetin: a review of the most recent research. Biomed Pharmacother. (2021) 134:111017. 10.1016/j.biopha.2020.111017
127.
Xu C Liu Y-L Gao Z-W Jiang H-M Xu C-J Li X . Pharmacological activities of myricetin and its glycosides. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J Chin Mater Medica. (2020) 45:3575–83. 10.19540/j.cnki.cjcmm.20200426.605
128.
Taheri Y Suleria HAR Martins N Sytar O Beyatli A Yeskaliyeva B et al Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complement Med Ther. (2020) 20:241. 10.1186/s12906-020-03033-z
129.
Zhang N Feng H Liao H-H Chen S Yang Z Deng W et al Myricetin attenuated LPS induced cardiac injury in vivo and in vitro. Phytother Res PTR. (2018) 32:459–70. 10.1002/ptr.5989
130.
Liao H-H Zhang N Meng Y-Y Feng H Yang J-J Li W-J et al Myricetin alleviates pathological cardiac hypertrophy via TRAF6/TAK1/MAPK and Nrf2 signaling pathway. Oxid Med Cell Longev. (2019) 2019:6304058. 10.1155/2019/6304058
131.
Liao H-H Zhu J-X Feng H Ni J Zhang N Chen S et al Myricetin possesses potential protective effects on diabetic cardiomyopathy through inhibiting IκBα/NFκB and enhancing Nrf2/HO-1. Oxid Med Cell Longev. (2017) 2017:8370593. 10.1155/2017/8370593
132.
Zhang B Shen Q Chen Y Pan R Kuang S Liu G et al Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep. (2017) 7:44239. 10.1038/srep44239
133.
Patel K Singh GK Patel DK . A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. (2018) 24:551–60. 10.1007/s11655-014-1960-x
134.
Heidary Moghaddam R Samimi Z Moradi SZ Little PJ Xu S Farzaei MH . Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur J Pharmacol. (2020) 887:173535. 10.1016/j.ejphar.2020.173535
135.
Salehi B Fokou PVT Sharifi-Rad M Zucca P Pezzani R Martins N et al The therapeutic potential of naringenin: a review of clinical trials. Pharm Basel Switz. (2019) 12:11. 10.3390/ph12010011
136.
Xu S Wu B Zhong B Lin L Ding Y Jin X et al Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/system xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered. (2021) 12:10924–34. 10.1080/21655979.2021.1995994
137.
Uryash A Mijares A Flores V Adams JA Lopez JR . Effects of naringin on cardiomyocytes from a rodent model of type 2 diabetes. Front Pharmacol. (2021) 12:719268. 10.3389/fphar.2021.719268
138.
He Y Wang S Sun H Li Y Feng J . Naringenin ameliorates myocardial injury in STZ-induced diabetic mice by reducing oxidative stress, inflammation and apoptosis via regulating the Nrf2 and NF-κB signaling pathways. Front Cardiovasc Med. (2022) 9:946766. 10.3389/fcvm.2022.946766
139.
Amini N Sarkaki A Dianat M Mard SA Ahangarpour A Badavi M . Protective effects of naringin and trimetazidine on remote effect of acute renal injury on oxidative stress and myocardial injury through Nrf-2 regulation. Pharmacol Rep PR. (2019) 71:1059–66. 10.1016/j.pharep.2019.06.007
140.
Atta MS El-Far AH Farrag FA Abdel-Daim MM Al Jaouni SK Mousa SA . Thymoquinone attenuates cardiomyopathy in streptozotocin-treated diabetic rats. Oxid Med Cell Longev. (2018) 2018:7845681. 10.1155/2018/7845681
141.
Ma W Guo W Shang F Li Y Li W Liu J et al Bakuchiol alleviates hyperglycemia-induced diabetic cardiomyopathy by reducing myocardial oxidative stress via activating the SIRT1/Nrf2 signaling pathway. Oxid Med Cell Longev. (2020) 2020:3732718. 10.1155/2020/3732718
142.
Liang E Liu X Du Z Yang R Zhao Y . Andrographolide ameliorates diabetic cardiomyopathy in mice by blockage of oxidative damage and NF-κB-mediated inflammation. Oxid Med Cell Longev. (2018) 2018:9086747. 10.1155/2018/9086747
143.
Hu X Rajesh M Zhang J Zhou S Wang S Sun J et al Protection by dimethyl fumarate against diabetic cardiomyopathy in type 1 diabetic mice likely via activation of nuclear factor erythroid-2 related factor 2. Toxicol Lett. (2018) 287:131–41. 10.1016/j.toxlet.2018.01.020
144.
Thakur V Alcoreza N Delgado M Joddar B Chattopadhyay M . Cardioprotective effect of glycyrrhizin on myocardial remodeling in diabetic rats. Biomolecules. (2021) 11:569. 10.3390/biom11040569
145.
Xu L Chen R Zhang X Zhu Y Ma X Sun G et al Scutellarin protects against diabetic cardiomyopathy via inhibiting oxidative stress and inflammatory response in mice. Ann Palliat Med. (2021) 10:2481–93. 10.21037/apm-19-516
146.
Li R Liu Y Shan Y-G Gao L Wang F Qiu C-G . Bailcalin protects against diabetic cardiomyopathy through Keap1/Nrf2/AMPK-mediated antioxidative and lipid-lowering effects. Oxid Med Cell Longev. (2019) 2019:3206542. 10.1155/2019/3206542
147.
Zhang B Zhai M Li B Liu Z Li K Jiang L et al Honokiol ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by reducing oxidative stress and apoptosis through activating the SIRT1-Nrf2 signaling pathway. Oxid Med Cell Longev. (2018) 2018:3159801. 10.1155/2018/3159801
148.
Jiang Z Fu L Xu Y Hu X Yang H Zhang Y et al Cyclovirobuxine D protects against diabetic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Sci Rep. (2020) 10:6427. 10.1038/s41598-020-63498-3
149.
Raish M Ahmad A Bin Jardan YA Shahid M Alkharfy KM Ahad A et al Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed Pharm Biomed Pharm. (2022) 145:112412. 10.1016/j.biopha.2021.112412
150.
Li W-F Wang P Li H Li T-Y Feng M Chen S-F . Oleanolic acid protects against diabetic cardiomyopathy via modulation of the nuclear factor erythroid 2 and insulin signaling pathways. Exp Ther Med. (2017) 14:848–54. 10.3892/etm.2017.4527
151.
Zheng D Chen L Wei Q Zhu Z Liu Z Jin L et al Fucoxanthin regulates Nrf2/Keap1 signaling to alleviate myocardial hypertrophy in diabetic rats. Nan Fang Yi Ke Da Xue Xue Bao. (2022) 42:752–9. 10.12122/j.issn.1673-4254.2022.05.18
152.
Wu S Zhu J Wu G Hu Z Ying P Bao Z et al 6-Gingerol Alleviates ferroptosis and inflammation of diabetic cardiomyopathy via the Nrf2/HO-1 pathway. Oxid Med Cell Longev. (2022) 2022:3027514. 10.1155/2022/3027514
153.
Li H Shi Y Wang X Li P Zhang S Wu T et al Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem Biol Interact. (2019) 310:108754. 10.1016/j.cbi.2019.108754
154.
Zhao C Zhang Y Liu H Li P Zhang H Cheng G . Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem Biophys Res Commun. (2017) 490:552–9. 10.1016/j.bbrc.2017.06.076
155.
Jin B Chen Y Wang J Chen Y Zhang M Huang J et al Costunolide alleviates hyperglycaemia-induced diabetic cardiomyopathy via inhibiting inflammatory responses and oxidative stress. J Cell Mol Med. (2023) 27:831–45. 10.1111/jcmm.17686
156.
Wang J De-Qiong X Hong D-Q Zhang Q-Q Zhang J . Attenuation of myocardial ischemia reperfusion injury by geniposide preconditioning in diabetic rats. Curr Res Transl Med. (2019) 67:35–40. 10.1016/j.retram.2019.03.002
157.
Duan J Guan Y Mu F Guo C Zhang E Yin Y et al Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci Rep. (2017) 7:41491. 10.1038/srep41491
158.
Adoga JO Channa ML Nadar A . Type-2 diabetic rat heart: the effect of kolaviron on mTOR-1, P70S60K, PKC-α, NF-kB, SOD-2, NRF-2, eNOS, AKT-1, ACE, and P38 MAPK gene expression profile. Biomed Pharm Biomed Pharm. (2022) 148:112736. 10.1016/j.biopha.2022.112736
159.
Yu L Li S Tang X Li Z Zhang J Xue X et al Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: role of SIRT1 activation. Apoptosis Int J Prog Cell Death. (2017) 22:942–54. 10.1007/s10495-017-1378-y
160.
Ying Y Jin J Ye L Sun P Wang H Wang X . Phloretin prevents diabetic cardiomyopathy by dissociating Keap1/Nrf2 Complex and inhibiting oxidative stress. Front Endocrinol. (2018) 9:774. 10.3389/fendo.2018.00774
161.
Dong Z Bian L Wang Y-L Sun L-M . Gastrodin protects against high glucose-induced cardiomyocyte toxicity via GSK-3β-mediated nuclear translocation of Nrf2. Hum Exp Toxicol. (2021) 40:1584–97. 10.1177/09603271211002885
162.
ALTamimi JZ AlFaris NA Alshammari GM Alagal RI Aljabryn DH Yahya MA . Esculeoside a decreases diabetic cardiomyopathy in streptozotocin-treated rats by attenuating oxidative stress, inflammation, fibrosis, and apoptosis: impressive role of Nrf2. Med Kaunas Lith. (2023) 59:1830. 10.3390/medicina59101830
163.
Bingbing L Jieru LI Jianchao SI Qi C Shengchang Y Ensheng JI . Ginsenoside Rb1 alleviates chronic intermittent hypoxia-induced diabetic cardiomyopathy in db/db mice by regulating the adenosine monophosphate-activated protein kinase/Nrf2/heme oxygenase-1 signaling pathway. J Tradit Chin Med Chung Tsa Chih Ying Wen Pan. (2023) 43:906–14. 10.19852/j.cnki.jtcm.20221206.004
164.
Xu C Xia L Xu D Liu Y Jin P Zhai M et al Cardioprotective effects of asiaticoside against diabetic cardiomyopathy: activation of the AMPK/Nrf2 pathway. J Cell Mol Med. (2024) 28:e18055. 10.1111/jcmm.18055
165.
Kosuru R Kandula V Rai U Prakash S Xia Z Singh S . Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc Drugs Ther. (2018) 32:147–63. 10.1007/s10557-018-6780-3
166.
Tang SM Xing XY Deng XH Zhao NN Li G Yang RC et al Research progress of Guanxin Danshen formula and its effective components in treating coronary artery heart disease. China J Chin Mater Medica. (2016) 41(20):3721–6. 10.4268/cjcmm20162004
167.
Deng X Xing X Sun G Xu X Wu H Li G et al Guanxin Danshen formulation protects against myocardial ischemia reperfusion injury-induced left ventricular remodeling by upregulating estrogen receptor β. Front Pharmacol. (2017) 8:777. 10.3389/fphar.2017.00777
168.
Li L Liu B Wang M Ye J Sun G . Protective effect of Guanxin Danshen formula on myocardial ischemiareperfusion injury in rats. Acta Cir Bras. (2023) 38:e380123. 10.1590/acb380123
169.
Zhang B Zhang C-Y Zhang X-L Sun G-B Sun X-B . Guan Xin Dan Shen formulation protects db/db mice against diabetic cardiomyopathy via activation of Nrf2 signaling. Mol Med Rep. (2021) 24:531. 10.3892/mmr.2021.12170
170.
Maqsood M Anam Saeed R Sahar A Khan MI . Mulberry plant as a source of functional food with therapeutic and nutritional applications: a review. J Food Biochem. (2022) 46:e14263. 10.1111/jfbc.14263
171.
Isabelle M Lee BL Ong CN Liu X Huang D . Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J Agric Food Chem. (2008) 56:9410–6. 10.1021/jf801527a
172.
Huang H-P Ou T-T Wang C-J . Mulberry (sang shèn zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J Tradit Complement Med. (2013) 3:7–15. 10.4103/2225-4110.106535
173.
Kimura T Nakagawa K Kubota H Kojima Y Goto Y Yamagishi K et al Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. (2007) 55:5869–74. 10.1021/jf062680g
174.
Qu L Liang X Tian G Zhang G Wu Q Huang X et al Efficacy and safety of mulberry twig alkaloids tablet for the treatment of type 2 diabetes: a multicenter, randomized, double-blind, double-dummy, and parallel controlled clinical trial. Diabetes Care. (2021) 44:1324–33. 10.2337/dc20-2109
175.
Chen X Sohouli MH Nateghi M Melekoglu E Fatahi S . Impact of mulberry consumption on cardiometabolic risk factors: a systematic review and meta-analysis of randomized-controlled trials. J Clin Pharm Ther. (2022) 47:1982–93. 10.1111/jcpt.13822
176.
Choi KH Lee HA Park MH Han J-S . Mulberry (Morus alba L.) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice. J Med Food. (2016) 19:737–45. 10.1089/jmf.2016.3665
177.
Liu Y-G Yan J-L Ji Y-Q Nie W-J Jiang Y . Black mulberry ethanol extract attenuates atherosclerosis-related inflammatory factors and downregulates PPARγ and CD36 genes in experimental atherosclerotic rats. Food Funct. (2020) 11:2997–3005. 10.1039/c9fo02736j
178.
Liu Y Zhao Y-B Wang S-W Zhou Y Tang Z-S Li F . Mulberry granules protect against diabetic cardiomyopathy through the AMPK/Nrf2 pathway. Int J Mol Med. (2017) 40:913–21. 10.3892/ijmm.2017.3050
179.
Uddandrao VVS Parim B Singaravel S Ponnusamy P Ponnusamy C Sasikumar V et al Polyherbal formulation ameliorates diabetic cardiomyopathy through attenuation of cardiac inflammation and oxidative stress via NF-κB/Nrf-2/HO-1 pathway in diabetic rats. J Cardiovasc Pharmacol. (2022) 79:e75–86. 10.1097/FJC.0000000000001167
180.
Zhang S Liu H Amarsingh GV Cheung CCH Wu D Narayanan U et al Restoration of myocellular copper-trafficking proteins and mitochondrial copper enzymes repairs cardiac function in rats with diabetes-evoked heart failure. Met Integr Biometal Sci. (2020) 12:259–72. 10.1039/c9mt00223e
181.
Saari JT . Copper deficiency and cardiovascular disease: role of peroxidation, glycation, and nitration. Can J Physiol Pharmacol. (2000) 78:848–55. 10.1139/cjpp-78-10-848
182.
Pan Z Deng C Shui L Yin H Liu B . Copper deficiency induces oxidative stress in liver of mice by blocking the Nrf2 pathway. Biol Trace Elem Res. (2024) 202:1603–11. 10.1007/s12011-023-03769-y
183.
Zang H Wu W Qi L Tan W Nagarkatti P Nagarkatti M et al Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. (2020) 69:2720–34. 10.2337/db19-1176
Summary
Keywords
Nrf2 signaling pathway, diabetic cardiomyopathy, pathogenesis, traditional Chinese medicine, therapeutic mechanism
Citation
Gu H, Liu J, Jiang J, Ma H, Wu S, Sun L and Yao L (2025) The role of Nrf2 signaling pathway in diabetic cardiomyopathy: from pathogenesis to traditional Chinese medicine interventions. Front. Cardiovasc. Med. 12:1492499. doi: 10.3389/fcvm.2025.1492499
Received
07 September 2024
Accepted
26 June 2025
Published
14 July 2025
Volume
12 - 2025
Edited by
Xiaofeng Yang, Temple University, United States
Reviewed by
Khaja Shameem Mohammed Abdul, Huntington Medical Research Institutes, United States
Priyanka Choudhury, Medical College of Wisconsin, United States
Updates
Copyright
© 2025 Gu, Liu, Jiang, Ma, Wu, Sun and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Longfei Sun longfeisundoc@163.com Lan Yao 56174475@qq.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.