- 1Department of Cardiology, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Beijing Key Laboratory of Metabolic Disorders Related Cardiovascular Disease, Beijing, China
- 3Laboratory for Clinical Medicine, Capital Medical University, Beijing, China
Background: Stress hyperglycemia ratio (SHR) is a commonly used predictor of acute hyperglycemia. The present study aimed to evaluate the prognostic significance of SHR in acute myocardial infarction (AMI) patients who underwent percutaneous coronary intervention (PCI).
Methods: A total of 3,212 consecutive AMI patients who underwent PCI were recruited and assigned to three groups, according to SHR tertiles. Then, the total number of major adverse cardiovascular and cerebrovascular events (MACCEs) and various cardiovascular events were recorded. The SHR was determined, as follows: admission blood glucose (mmol/L)/[1.59 × hemoglobin A1c (%) −2.59].
Results: The incidence of MACCEs was positively correlated to SHR during the median follow-up of 36 months. The multivariate COX regression analysis identified SHR as an independent predictor of composite MACCEs [hazard ratio: 2.279, 95% confidence interval (CI): 1.569–3.311, p < 0.001] and target vessel revascularization (hazard ratio: 1.998, 95% CI: 1.299–3.074, p = 0.002). In terms of gender, age, type of AMI, body mass index, left ventricular ejection fraction, and diabetes mellitus, SHR >1.45 was significantly associated to MACCEs across all subgroups (all, p < 0.001), except for patients with ejection fraction <50%. Furthermore, the area under the receiver operating characteristic curve for SHR in predicting MACCEs was 0.636 (95% CI: 0.613–0.659, p < 0.05), with a cut-off value of 1.317.
Conclusions: Stress hyperglycemia, as indicated by SHR, is significantly correlated to MACCEs, and independently predicts the prognosis of AMI patients undergoing PCI. These findings highlight the potential of SHR as an effective prognostic marker for AMI patients undergoing PCI.
1 Introduction
Acute myocardial infarction (AMI) remains as the leading cause of death globally, despite the significant advancements in primary percutaneous coronary intervention (PCI) and pharmacotherapy over the past decade (1). Thus, identifying risk factors for the poor prognosis of AMI patients following PCI is of great importance. Stress hyperglycemia refers to the metabolic disturbance that occurs during stress conditions, such as severe infection, trauma, excessive bleeding, or acute poisoning. Furthermore, this is characterized by disruptions in glucose metabolism. As a severe stressor on the body, AMI can trigger stress hyperglycemia (2). Epidemiological evidence has indicated that approximately 25%–50% of patients with acute coronary syndrome (ACS) may experience stress hyperglycemia (3). This acute physiological response to stress may destabilize and rupture atherosclerotic plaques, accelerate myocardial ischemia (4, 5), and become a strong predictor of mortality in critically ill patients (6, 7).
For patients with ACS, particularly for patients with AMI, stress hyperglycemia is independently associated to poor long-term and short-term outcomes (8, 9). However, there is no universally accepted definition of stress hyperglycemia for patients with AMI, and its predictive value remains controversial. Discrepancies in the study results may be attributed to the use of admission glucose levels, as a sole measure of stress hyperglycemia. Since absolute admission glucose concentrations can be affected by both chronic elevated baseline glucose and acute physiological stress, these may not always accurately reflect the true level of stress hyperglycemia (10). Thus, the stress hyperglycemia ratio (SHR), which is defined as the ratio of admission blood glucose (ABG) to the estimated average glucose, was introduced to address this issue (11). SHR has been shown to provide superior predictive value for cases with AMI, when compared to ABG alone (12, 13).
Most investigations on the relationship between stress hyperglycemia and AMI have primarily focused on ST-segment elevation myocardial infarction (STEMI). For instance, SHR was found to be independently associated to cardiac function and microvascular obstruction in patients who underwent primary PCI for acute STEMI (14). In addition, a previous study identified SHR as an independent predictor for in-hospital major adverse cardiovascular and cerebrovascular events (MACCEs) in acute STEMI patients, especially for patients without diabetes (15). Furthermore, SHR can independently predict in-hospital heart failure in patients with anterior STEMI (16). However, the predictive value of stress hyperglycemia across different subgroups of patients remain controversial. A multi-center nationwide registry study revealed that SHR was significantly positively correlated to increased risk of both all-cause and cardiovascular mortality in patients with coronary artery disease (CAD) and chronic kidney disease, while no such association was observed in CAD patients without chronic kidney disease (17). Furthermore, SHR was an independent predictor for hospitalization risk in patients with ischemia and non-obstructive coronary arteries, regardless of the diabetes status (18).
Therefore, the present study aimed to determine the correlation between SHR and adverse outcomes in AMI patients who underwent PCI, and determine whether these correlations differ among various subgroups.
2 Methods
2.1 Study participants
For the present retrospective, observational study, participants were recruited from the Cardiovascular Center of Beijing Friendship Hospital Database Bank. The enrollment process is presented in Figure 1. A total of 5,063 consecutive patients, who were diagnosed with AMI from January 2013 to October 2020, were screened. Inclusion criteria: (1) age ≥18 years old; (2) patients diagnosed with AMI; (3) patients who met the criteria for PCI, and underwent emergency or elective PCI during hospitalization based on the clinical condition. Exclusion criteria: (1) severe structural heart disease, severe valvulopathy, or cardiomyopathy; (2) severe hepatic dysfunction, kidney transplantation, eGFR <30 ml/min/1.73 m2, or chronic dialysis; (3) hemoglobin <60 g/L, malignant tumor, hematological disease, or serious infections with a life expectancy of <12 months; (4) missing important laboratory data, such as admission blood glucose and glycated hemoglobin (HbA1c) levels, or lacking follow-up data. Next, these patients were grouped according to tertiles of SHR: SHR < 1.14 group, 1.14 ≤ SHR ≤ 1.45 group, and SHR > 1.45 group. Coronary angiography and related procedures were performed in strict accordance to present guideline recommendations. Antiplatelet and perioperative anticoagulation therapies were administered following standardized protocols. After discharge, all patients received guideline-directed secondary prevention medications for CAD.
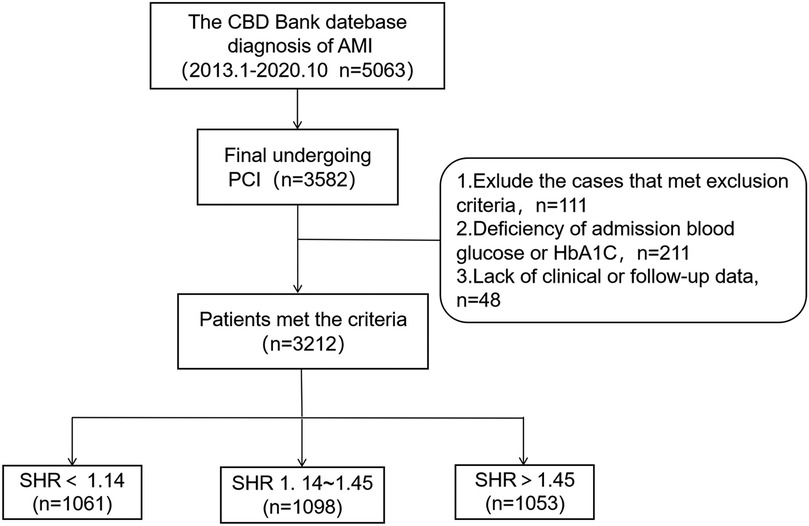
Figure 1. Flowchart for the enrollment of subjects. CBD, Cardiovascular Center of Beijing Friendship Hospital Database; PCI, percutaneous coronary intervention; SHR, stress hyperglycemia ratio.
2.2 Data collection and definitions
Demographic information, medical history, laboratory test results, medical treatment, and angiographic and echocardiographic evaluation results were obtained from all patients. Then, the MACCEs were documented and recorded during the clinical follow-up visits. Each patient was followed up at 1, 3, 6, and 12 months through outpatient clinic visits or telephone questionnaires. Subsequently, annual follow-ups were conducted.
The SHR was calculated, as follows: admission blood glucose (mmol/L)/[1.59 × HbA1c (%) - 2.59]. AMI included both non-STEMI and STEMI, which was defined as chest pain with newly detected ST-segment alterations, and elevation in myocardial necrosis markers to more than twice the upper limit of the normal range. The primary endpoint was the MACCE at follow-up, which included all-cause death, recurrent myocardial infarction (MI), cardiovascular death (CV death), target vessel revascularization, readmission for heart failure, and stroke. All-cause death was defined as mortality due to any cause, which comprised of both cardiac and non-cardiac origins. CV death refers to death caused by any heart disease. Target vessel revascularization refers to the revascularization of the target coronary artery through PCI (including both emergency and elective procedures) or coronary artery bypass grafting (CABG). Stroke was characterized as having a history of transient ischemic attacks, ischemic stroke, or cerebral hemorrhage.
2.3 Statistical analysis
The statistical analysis was conducted using IBM SPSS 23.0. Continuous data are expressed in mean ± standard deviation (SD) or median [interquartile range (IQR)]. Comparisons between the three groups were made using the Kruskal–Wallis test or one-way ANOVA. Categorical data were presented in numbers and percentages, and compared using Fisher's exact test or Pearson's Chi-square test. Kaplan–Meier survival curves were used to analyze the cumulative incidence of MACCEs, with group comparisons performed by log-rank test. The baseline and clinical characteristics that were significantly correlated to MACCEs in the univariate analysis were included in the multivariate model. Multivariate Cox regression analysis was performed to determine whether SHR can independently predict MACCEs, and identify other potential predictors. Receiver operating characteristic (ROC) curves were used to determine the optimal cut-off value for SHR in predicting MACCEs. A p-value of <0.05 indicated statistical significance, with 95% confidence interval (CI).
3 Results
3.1 Baseline characteristics
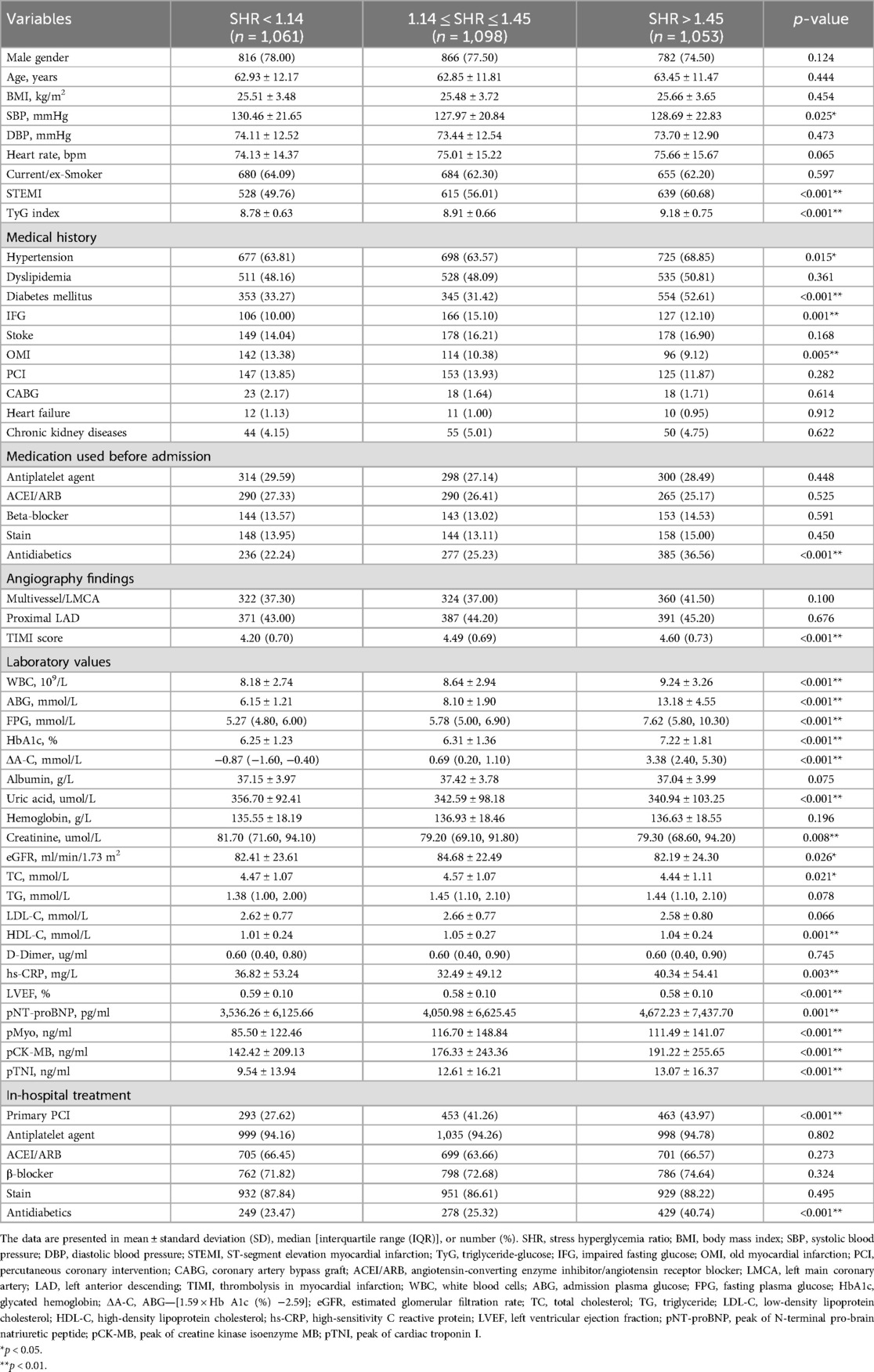
A total of 3,582 patients underwent PCI. Among these patients, 370 patients were excluded based on the exclusion criteria, resulting in 3,212 patients who met the selection criteria for analysis. All patients were followed up until September 31, 2021, with a median follow-up duration of 36 months (IQR: 13–60 months). Then, these patients were classified into three groups based on the SHR: SHR < 1.14 group (n = 1,061), 1.14 ≤ SHR ≤ 1.45 group (n = 1,098), and SHR > 1.45 group (n = 1,053). Patients with SHR >1.45 exhibited higher rates of STEMI, diabetes mellitus (DM), and the prehospital use of antidiabetics, and higher TyG index levels, when compared to the other two groups (p < 0.001). Furthermore, these patients had higher rates of hypertension (p = 0.015). Moreover, the white cell count, admission plasma glucose (ABG), fasting plasma glucose (FPG), HbA1c, ΔA-C {which was calculated, as follows: ABG - [1.59 × HbA1c (%) - 2.59]}, high-sensitivity C-reactive protein, peak of N-terminal pro-brain natriuretic peptide (pNT-proBNP), peak of cardiac troponin I, and peak of creatine kinase isoenzyme MB were the highest in the SHR > 1.45 group, and the lowest in the SHR < 1.14 group (p < 0.01, Table 1).
The SHR > 1.45 group had the highest, while the SHR < 1.14 group had the lowest proportion of emergency PCI (p < 0.001). However, the SHR < 1.14 group had the highest rates of systolic blood pressure and old MI (p < 0.05). Furthermore, these patients presented with significantly higher creatinine levels and ejection fraction (EF) values measured by echocardiography, when compared to the other two groups (p < 0.01). In addition, the proportion of impaired fasting glucose (IFG) was significantly higher in the 1.14 ≤ SHR ≤ 1.45 group, when compared to the SHR < 1.14 group (p < 0.001). Moreover, the use of antidiabetics during hospitalization was higher in the SHR > 1.45 group, when compared to the other two groups (p < 0.001), while the use of other medications did not significantly differ among the three groups (Table 1).
3.2 Kaplan–Meier survival analysis for cardiovascular outcomes
The median follow-up duration was 36 months. During this period, a total of 655 (20.4%) patients experienced MACCEs, which included 290 (9.00%) cases of all-cause death, 146 (4.50%) cases of CV death, 216 (6.70%) cases of recurrent MI, 318 (9.90%) cases of target vessel revascularization, 109 (3.40%) cases of readmission for heart failure, and 56 (1.70%) cases of stroke. Composite MACCEs occurred in 121 (11.40%) patients in the SHR < 1.14 group, 208 (18.94%) patients in the 1.14 ≤ SHR ≤ 1.45 group, and 326 (30.96%) patients in the SHR > 1.45 group. The cumulative incidence curve for MACCEs in patients with different SHR levels is presented in Figure 2. The incidence of MACCEs was significantly higher in the SHR > 1.45 group, when compared to the SHR < 1.14 and 1.14 ≤ SHR ≤ 1.45 groups (p < 0.001).
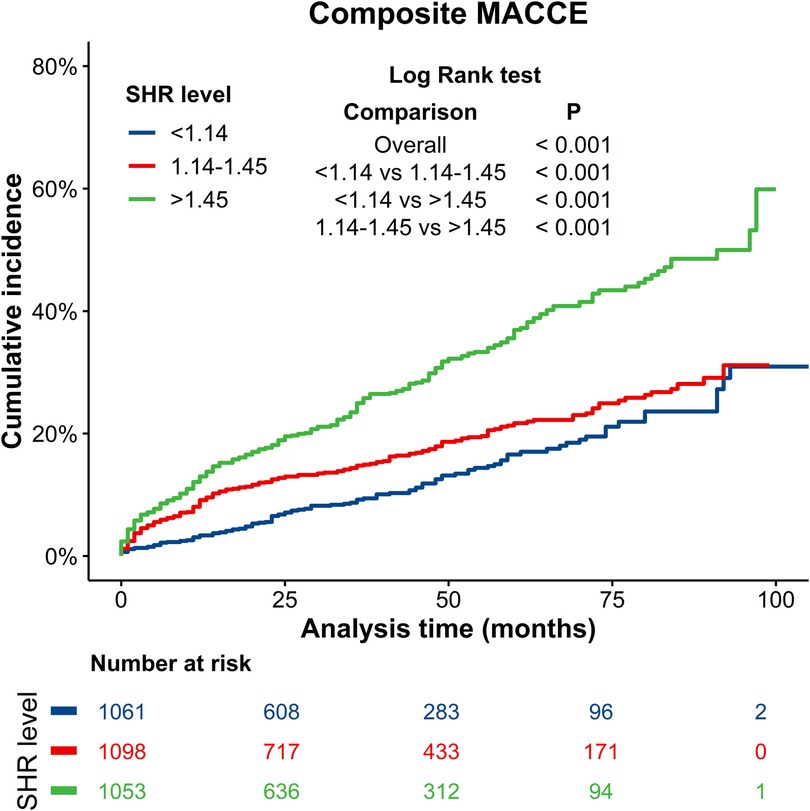
Figure 2. Kaplan–Meier curves for composite MACCEs. Blue line: SHR < 1.14 group; Red line: 1.14 ≤ SHR ≤ 1.45 group; Green line: SHR > 1.45 group. MACCEs, major adverse cardiac and cerebrovascular events.
The cumulative incidence curves for all-cause mortality, CV death, recurrent MI, revascularization, heart failure, and stroke in patients with different SHR levels are presented in Figure 3. There were no significant differences in follow-up stroke incidence among the three groups of SHR patients (p > 0.05). However, the SHR > 1.45 group had significantly higher incidences of all-cause death, CV death, recurrent MI and revascularization events, when compared to the SHR < 1.14 and 1.14 ≤ SHR ≤ 1.45 groups (p < 0.05), while no significant differences were observed between the 1.14 ≤ SHR ≤ 1.45 and SHR < 1.14 groups (p > 0.05). Compared to the SHR < 1.14 group, the SHR > 1.45 group had a significantly higher risk of heart failure events (p = 0.004), with no signifcant difference observed between the SHR > 1.45 and 1.14 ≤ SHR ≤ 1.45 groups (p > 0.05).
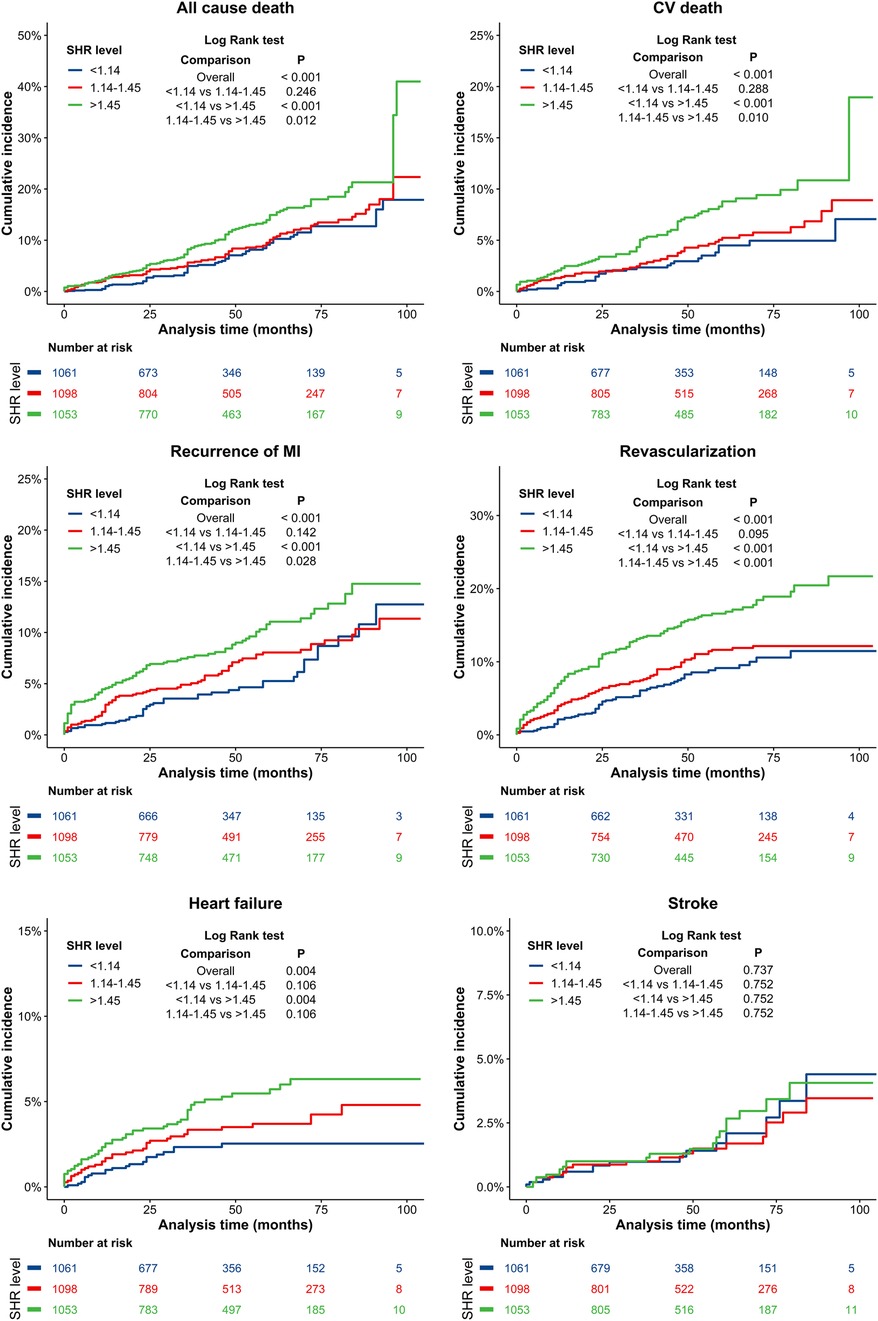
Figure 3. Kaplan–Meier curves for all-cause death, CV death, recurrence MI, revascularization, heart failure, and stroke. Blue line: SHR < 1.14 group; Red line: 1.14 ≤ SHR ≤ 1.45 group; Green line: SHR > 1.45 group. CV, cardiovascular; MI, myocardial infarction.
3.3 Risk factors for MACCEs
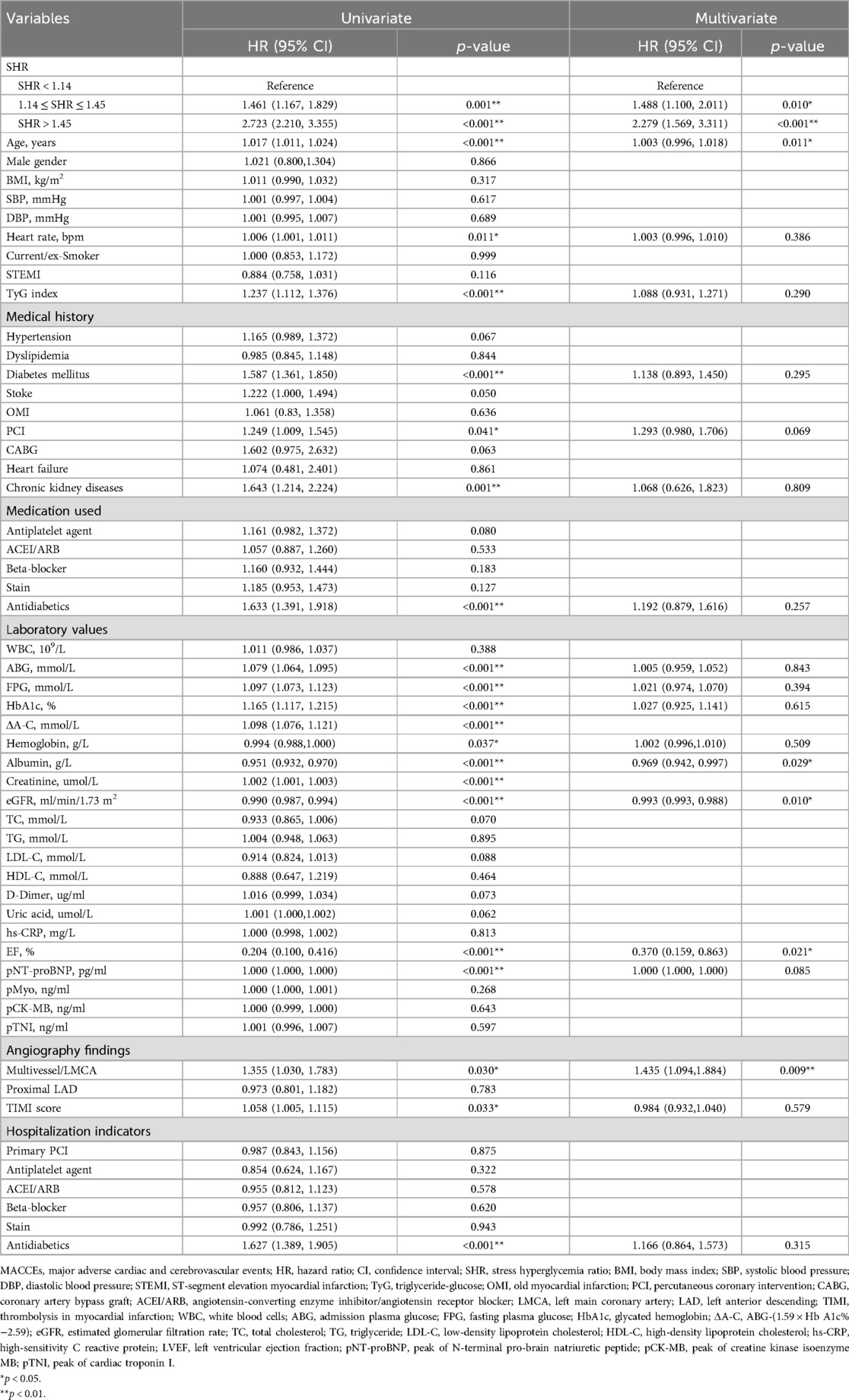
The predictors of composite MACCEs were detected by univariate and multivariate regression analysis (Table 2). Using the demographic characteristics, laboratory test results, and hospitalization data as independent variables, and MACCEs as the dependent variable, the univariate analysis identified the following as significantly associated to the occurrence of MACCEs in AMI patients who underwent PCI: SHR, age, heart rate, TyG index, DM, previous PCI, chronic kidney disease, use of antidiabetics, TIMI score, ABG, FPG, HbA1c, ΔA-C, hemoglobin, albumin, creatinine, eGFR, EF, pNT-proBNP, and multivessel/left main coronary artery (LMCA). Due to the significant correlation between eGFR and creatinine, creatinine was excluded from the multivariate model. After adjusting for potential confounding factors, such as age, the multivariate analysis identified the following as independent predictors of MACCEs in AMI patients who underwent PCI: SHR, age, albumin, eGFR, EF, and multivessel/LMCA (all, p < 0.05).
The unadjusted competing risk modeling revealed that the cumulative incidence of all-cause mortality, CV death, recurrent MI, target vessel revascularization, and readmission for heart failure significantly increased in the SHR > 1.45 group (all, p < 0.001). After adjusting for potential confounders, such as age and TyG, the multivariate-adjusted hazard ratio (HR) for target vessel revascularization remained significantly higher in patients with SHR >1.45 (p < 0.05, Table 3).
3.4 Independent associations of SHR with MACCEs in various subgroups
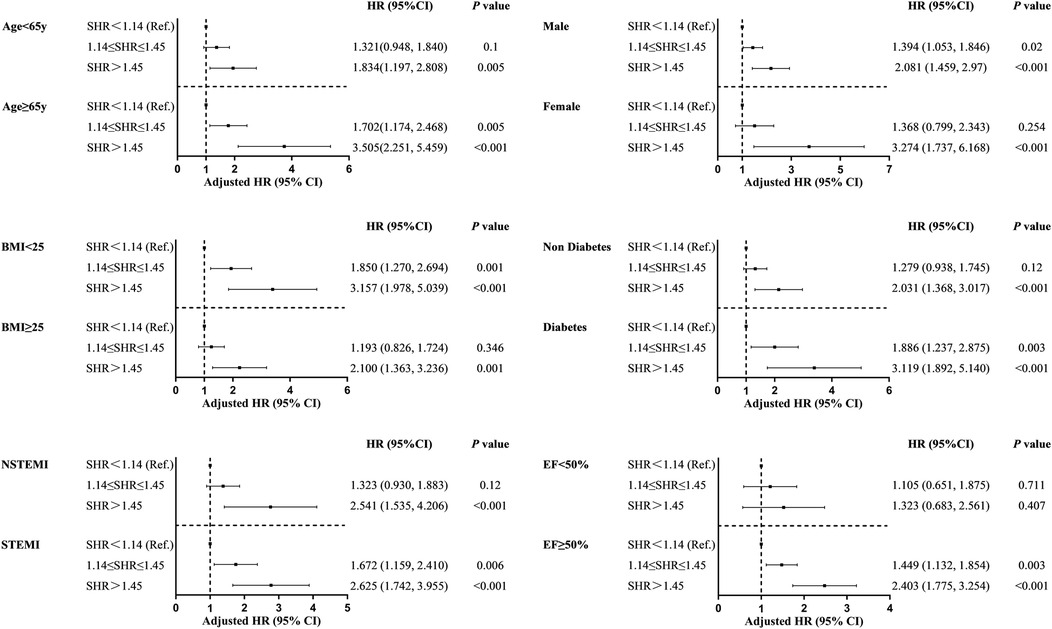
A subgroup analysis was conducted based on age, gender, DM, LVEF, BMI, and type of AMI (Figure 4). The results were generally consistent with the overall analysis results, except for the subgroup with EF <0.5. For all other groups, SHR predicted the occurrence of MACCEs during the follow-up period (p < 0.05). This suggests that the prognostic value of SHR was not significantly affected by the key factors correlated to AMI.
3.5 ROC curve analysis of SHR
The predictive efficacy of SHR for MACCEs during the follow-up period was determined by ROC curve analysis. The area under the ROC curve was 0.636 (95% CI: 0.613–0.659, p < 0.05), indicating that SHR has significant predictive value for follow-up MACCEs. The optimal cut-off value for SHR in predicting MACCEs was 1.317, with a Youden's index of 0.221, a sensitivity of 0.631, and a specificity of 0.591 (Figure 5).
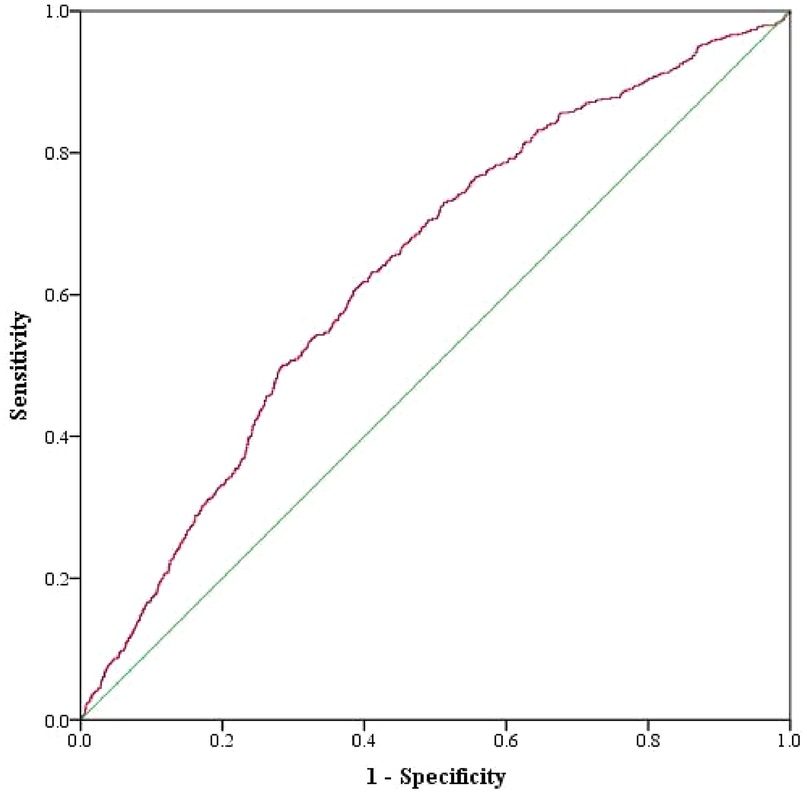
Figure 5. ROC curves for SHR, as a predictor of composite MACCEs, in AMI patients who underwent PCI.
4 Discussion
The present study investigated the prognostic value of SHR for cardiovascular events in AMI patients who underwent PCI, including both STEMI and non-STEMI. The present main findings were, as follows: (1) the incidence of MACCEs was significantly correlated to the increase in SHR; (2) SHR was an independent predictor of MACCEs in AMI patients who underwent PCI, which included all-cause mortality, CV death, recurrent MI, revascularization, heart failure, and composite MACCEs; (3) in terms of gender, age, type of AMI, BMI, LVEF, and DM, SHR >1.45 was significantly correlated to MACCEs in all subgroups, except for EF <50%; (4) the area under the ROC curve for SHR in predicting MACCEs was 0.636, with a cut-off value of 1.317; (5) age, albumin, eGFR, EF, and multivessel/LMCA were the independent predictors of MACCEs in AMI patients who underwent PCI.
The activation of sympathetic nerves after AMI induces the release of glucagon, growth hormones, glucocorticoids and catecholamine, leading to the increase in blood glucose through the promotion of gluconeogenesis, accelerating the liver glycogen breakdown, and reducing the insulin uptake of glucose. Stress hyperglycemia has been identified as a key risk factor for cardiovascular diseases and adverse clinical outcomes (9, 19). The harm of stress hyperglycemia on AMI patients is mainly correlated to metabolic disorders, inflammation, hypercoagulability, oxidative stress, and endothelial dysfunction. MI patients with hyperglycemia exhibited an increase in left ventricular dysfunction, and a broader range of myocardial necrosis, as evidenced by the wider extent of microvascular obstruction and late gadolinium enhancement observed on the cardiac magnetic imaging, indicating a positive correlation between admission hyperglycemia and MI (20). The adverse effect of stress hyperglycemia on patient prognosis is independent of the DM itself. Elevated SHR is associated to increased risk of heart failure progression in patients with significant secondary mitral regurgitation after PCI, particularly in patients with normoglycemia (21). For MI patients without diabetes, the stress hyperglycemia on admission was associated to the increase in in-hospital death and late-follow-up MACCEs (i.e., stroke, recurrent MI, and all-cause mortality). In addition, stress-induced glycemic increase, older age, and low baseline LVEF predicted the MACCEs during the follow-up period (22). Furthermore, elevated SHR independently predicted the poor short- and long-term outcomes in patients with acute heart failure (23). Cui et al. reported that high ABG is positively correlated to 2-year mortality in patients with AMI complicated by DM, prediabetes, and normal glucose regulation. Stress hyperglycemia can be a useful marker for risk stratification, in both diabetic patients and subjects with normal glucose regulation. However, the ABG threshold needs to be adjusted according to different glucose metabolism statuses (24). Compared to a single blood glucose measurement, SHR provides better adjustment for the effects of the last meal and basal blood glucose, reflects the relative increase in blood glucose, and helps to identify patients with true stress hyperglycemia. Therefore, SHR can be used to identify stress hyperglycemia in clinical practice.
Extensive research has demonstrated the predictive value of SHR in AMI patients. A meta-analysis and systematic review of 32 studies suggested that elevated SHR is associated to increased all-cause mortality risk in hospitalized patients with AMI or acute ischemic stroke (25). In a retrospective study that analyzed 905 patients diagnosed with STEMI, the incidence of no-reflow increased with the increase in SHR levels in STEMI patients who underwent emergency PCI, regardless of the DM status (26). In a study conducted on coronary artery disease patients who underwent PCI with a median follow-up of 2.5 years, the highest SHR quartile group had significantly higher risk of MACCEs in the overall population (HR: 1.31, 95% CI: 1.05–1.64), and in non-diabetic patients (HR: 1.45, 95% CI: 1.02–2.06), when compared to the lower three quartiles. SHR can effectively predict the occurrence of MACCEs after PCI, particularly in STEMI patients without DM (27). Wei et al. reported that SHR is independently correlated to risk of mortality and major adverse cardiovascular events in patients with STEMI. Furthermore, incorporating SHR into the Thrombolysis in Myocardial Infarction risk score may improve its predictive accuracy for STEMI patients, especially for patients with DM (28). In the present study, it was found that SHR independently predicted the occurrence of MACCEs in AMI patients who underwent PCI, which is consistent with the aforementioned studies. In addition, a study conducted on 5,562 patients revealed that ACS patients who received drug-eluting stent implantation had the lowest incidence of MACCEs in the third quintile of SHR, according to the Kaplan–Meier survival analysis results. Furthermore, SHR exhibited a U-shaped relationship with the incidence rates of MACCEs and major adverse cardiovascular events after the 2-year follow-up period, and a J-shaped relationship with in-hospital CV death and MI incidence after the 2-year follow-up period (3). In the present study, there was no significant difference in cumulative incidence of cardiovascular events between the two groups with lower SHR tertiles, and no corresponding phenomenon was observed. However, it was suggested that the sample size should be expanded, and the groupings should be refined to further verify the findings.
The present study revealed that the cumulative incidence of other MACCEs significantly increased with the increase in SHR, except for stroke. However, SHR did not independently predict the occurrence of cardiovascular events, which may be correlated to the small number of follow-up events, and the limited follow-up time. The novel aspect of the present study was that the predictive value of SHR for MACCEs in various subgroups was analyzed, which included BMI, gender, age, presence or absence of DM, LVEF, and type of AMI. The results revealed that SHR effectively predicted the MACCEs in most subgroups, except for patients with EF <50%. In a meta-analysis conducted for a heart failure cohort, elevated SHR had an increased risk ratio for all-cause mortality, although the confidence interval was wide, and included the null [RR 1.34 (95% CI: 0.89–2.01), p = 0.17] (25).
In the present study, among patients with EF <50%, the rates of primary PCI (p < 0.001) and antidiabetic therapy (p = 0.002) were higher in patients in the SHR > 1.45 group, when compared to the other groups, with no differences in other treatments. This was consistent with the overall population. A study conducted on 2,596 patients with MI revealed that despite the advancements in the epidemiology and management of MI, the mortality rate for heart failure patients remained high at 70% during an average follow-up of 7.6 years (29). After adjusting for age and gender, heart failure was identified to have a strong association to mortality, as a time-dependent variable. Therefore, it can be reasonably considered that patients with heart failure have a significantly poor prognosis, and that a high SHR would not further exacerbate the poor prognosis.
The present study has several limitations. First, despite the large sample size, the present study had a single-center design. Thus, the findings need to be verified through multi-center and larger cohort studies. Second, the present study excluded patients with missing data, and patients who were lost to follow-up, which may have introduced selection bias. Third, a recent study has demonstrated for the first time that periprocedural myocardial injury, especially type 4a MI, is associated with a significant worse prognosis (30). However, in this study, reinfarction did not include periprocedural myocardial infarctions, which may have resulted in the loss of valuable prognostic information. Forth, due to the retrospective observational nature of the present study, further explorations with a prospective design on the effects of stress hyperglycemia control in reducing long-term MACCEs are needed. As an emerging non-invasive marker, SHR deserves greater clinical attention. Although no randomized trials have been conducted to date, large observational studies have highlighted its relevance in real-world cardiovascular disease management and stress hyperglycemia intervention. Future research should focus in clarifying the diagnostic utility of SHR in cardiovascular disease, optimizing its integration into cardiovascular risk assessment, and exploring its potential as a therapeutic target.
5 Conclusions
The present study confirms that stress hyperglycemia, as expressed by SHR, is a strong independent predictor of MACCEs in AMI patients undergoing PCI. These findings provide important guidance for clinicians in predicting follow-up clinical events in patients with AMI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by ethics committee of Beijing Friendship Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because retrospective, observational study.
Author contributions
WM: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. HQ: Conceptualization, Visualization, Writing – review & editing. WL: Visualization, Writing – review & editing. HL: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Laboratory for Clinical Medicine, Capital Medical University.
Acknowledgments
Special thanks to Dr. Ye Wu from the Laboratory for Clinical Medicine, Capital Medical University, for providing assistance in literature review and methodological guidance during this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. (2017) 389:197–210. doi: 10.1016/s0140-6736(16)30677-8
2. Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response!. Crit Care. (2013) 17:305. doi: 10.1186/cc12514
3. Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. (2022) 45:947–56. doi: 10.2337/dc21-1526
4. D'Onofrio N, Sardu C, Paolisso P, Minicucci F, Gragnano F, Ferraraccio F, et al. MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J Cell Physiol. (2020) 235:1438–52. doi: 10.1002/jcp.29064
5. Schmitz T, Freuer D, Harmel E, Heier M, Peters A, Linseisen J, et al. Prognostic value of stress hyperglycemia ratio on short- and long-term mortality after acute myocardial infarction. Acta Diabetol. (2022) 59:1019–29. doi: 10.1007/s00592-022-01893-0
6. Lee TF, Drake SM, Roberts GW, Bersten A, Stranks SN, Heilbronn LK, et al. Relative hyperglycemia is an independent determinant of in-hospital mortality in patients with critical illness. Crit Care Med. (2020) 48:e115–e22. doi: 10.1097/ccm.0000000000004133
7. Kim EJ, Jeong MH, Kim JH, Ahn TH, Seung KB, Oh DJ, et al. Clinical impact of admission hyperglycemia on in-hospital mortality in acute myocardial infarction patients. Int J Cardiol. (2017) 236:9–15. doi: 10.1016/j.ijcard.2017.01.095
8. Ferreira JA, Baptista RM, Monteiro SR, Gonçalves FM, Monteiro PF, Gonçalves LM. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: a tertiary center analysis. Intern Emerg Med. (2021) 16:2109–19. doi: 10.1007/s11739-021-02693-0
9. Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, et al. Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction. Am J Cardiol. (2020) 125:851–9. doi: 10.1016/j.amjcard.2019.12.034
10. Wexler DJ, Nathan DM, Grant RW, Regan S, Van Leuvan AL, Cagliero E. Prevalence of elevated hemoglobin A1c among patients admitted to the hospital without a diagnosis of diabetes. J Clin Endocrinol Metab. (2008) 93:4238–44. doi: 10.1210/jc.2008-1090
11. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
12. Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. (2018) 41:847–53. doi: 10.2337/dc17-1732
13. Sia CH, Chan MH, Zheng H, Ko J, Ho AF, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. (2021) 20:211. doi: 10.1186/s12933-021-01395-3
14. Bo K, Li W, Zhang H, Wang Y, Zhou Z, Gao Y, et al. Association of stress hyperglycemia ratio with left ventricular function and microvascular obstruction in patients with ST-segment elevation myocardial infarction: a 3.0 T cardiac magnetic resonance study. Cardiovasc Diabetol. (2024) 23:179. doi: 10.1186/s12933-024-02271-6
15. Guo W, Zhu J, Liu W. Stress hyperglycemia ratio: an independent predictor for in-hospital major adverse cardiovascular and cerebrovascular events in patients with st-segment elevation myocardial infarction. BMC Cardiovasc Disord. (2023) 23:195. doi: 10.1186/s12872-023-03219-6
16. Zia Ul S, Alqahtani SAM, Wani JI, Aziz S, Durrani HK, Patel AA, et al. Stress hyperglycaemia ratio is an independent predictor of in-hospital heart failure among patients with anterior ST-segment elevation myocardial infarction. BMC Cardiovasc Disord. (2024) 24:751. doi: 10.1186/s12872-024-04362-4
17. Wu J, Liu J, Yuan Z, Tang S, Zhang W, Xiang Y, et al. Effects of stress hyperglycemia ratio upon long-lasting prognosis in coronary artery disease patients with or lacking chronic renal impairment: findings from a Chinese multi-center observational study. Diabetol Metab Syndr. (2024) 16:316. doi: 10.1186/s13098-024-01521-7
18. Mone P, Lombardi A, Salemme L, Cioppa A, Popusoi G, Varzideh F, et al. Stress hyperglycemia drives the risk of hospitalization for chest pain in patients with ischemia and nonobstructive coronary arteries (INOCA). Diabetes Care. (2023) 46:450–4. doi: 10.2337/dc22-0783
19. Ye N, Yang L, Wang G, Bian W, Xu F, Ma C, et al. Admission fasting plasma glucose is associated with in-hospital outcomes in patients with acute coronary syndrome and diabetes: findings from the improving care for cardiovascular disease in China—acute coronary syndrome (CCC-ACS) project. BMC Cardiovasc Disord. (2020) 20:380. doi: 10.1186/s12872-020-01662-3
20. Pak S, Yatsynovich Y, Markovic JP. A meta-analysis on the correlation between admission hyperglycemia and myocardial infarct size on CMRI. Hellenic J Cardiol. (2018) 59:174–8. doi: 10.1016/j.hjc.2017.09.009
21. Huang H, Liu J, Li Q, Qiao L, Chen S, Kang Y, et al. Relationship between stress hyperglycemia and worsening heart failure in patients with significant secondary mitral regurgitation. Atherosclerosis. (2024) 394:117306. doi: 10.1016/j.atherosclerosis.2023.117306
22. Eskandari R, Matini P, Emami S, Rezaei Y. Association between admission blood glucose and prognosis in non-diabetic patients with first-ever acute myocardial infarction. Rom J Intern Med. (2022) 60:34–41. doi: 10.2478/rjim-2021-0033
23. Ge T, Hu J, Zhou Y. The association between stress hyperglycemia ratio with mortality in critically ill patients with acute heart failure. Front Cardiovasc Med. (2024) 11:1463861. doi: 10.3389/fcvm.2024.1463861
24. Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Admission blood glucose and 2-year mortality after acute myocardial infarction in patients with different glucose metabolism Status: a prospective, nationwide, and multicenter registry. Front Endocrinol (Lausanne). (2022) 13:898384. doi: 10.3389/fendo.2022.898384
25. Esdaile H, Khan S, Mayet J, Oliver N, Reddy M, Shah ASV. The association between the stress hyperglycaemia ratio and mortality in cardiovascular disease: a meta-analysis and systematic review. Cardiovasc Diabetol. (2024) 23:412. doi: 10.1186/s12933-024-02454-1
26. Şimşek B, Çınar T, Tanık VO, İnan D, Zeren G, Avcı İ, et al. The association of acute–to–chronic glycemic ratio with no-reflow in patients with ST–segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Kardiol Pol. (2021) 79:170–8. doi: 10.33963/kp.15736
27. Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. (2017) 241:57–63. doi: 10.1016/j.ijcard.2017.02.065
28. Xu W, Yang YM, Zhu J, Wu S, Wang J, Zhang H, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol. (2022) 21:48. doi: 10.1186/s12933-022-01479-8
29. Gerber Y, Weston SA, Enriquez-Sarano M, Berardi C, Chamberlain AM, Manemann SM, et al. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail. (2016) 9:e002460. doi: 10.1161/circheartfailure.115.002460
30. Armillotta M, Bergamaschi L, Paolisso P, Belmonte M, Angeli F, Sansonetti A, et al. Prognostic relevance of type 4a myocardial infarction and periprocedural myocardial injury in patients with non-ST-segment-elevation myocardial infarction. Circulation. (2025) 151:760–72. doi: 10.1161/circulationaha.124.070729
Keywords: acute myocardial infarction, major adverse cardiovascular and cerebrovascular events, percutaneous coronary intervention, stress hyperglycemia, stress hyperglycemia ratio
Citation: Meng W, Qiu H, Li W and Li H (2025) Correlation between stress hyperglycemia ratio and prognosis in acute myocardial infarction patients following percutaneous coronary intervention. Front. Cardiovasc. Med. 12:1493635. doi: 10.3389/fcvm.2025.1493635
Received: 9 September 2024; Accepted: 9 April 2025;
Published: 9 May 2025.
Edited by:
Sri Harsha Patlolla, Mayo Clinic, United StatesReviewed by:
Pasquale Mone, University of Molise, ItalyMatteo Armillotta, University of Bologna, Italy
Copyright: © 2025 Meng, Qiu, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Li, bGh3MTk2NTZAc2luYS5jb20=; c2VsaW5kYXh4QDE2My5jb20=; Weiping Li, eHVlZXIwOUAxNjMuY29t
 Wei Meng1
Wei Meng1 Hui Qiu
Hui Qiu Weiping Li
Weiping Li Hongwei Li
Hongwei Li