Abstract
Background:
Chronic kidney disease (CKD) is a significant cardiovascular (CV) risk factor, with dialysis-dependent CKD (DD-CKD) patients facing high mortality rates. Hypercholesterolemia is another crucial CV risk factor, typically managed with lipid-lowering therapy, though its efficacy in DD-CKD remains uncertain. Evidence shows mixed results regarding the benefits of statins in these patients. Citrate-based dialysates are known to reduce inflammatory biomarkers compared to acetate-based ones, potentially impacting lipid profiles and immune responses. This study aimed to determine the effects of citrate vs. acetate dialysate on lipid profiles and immunophenotypes in DD-CKD patients.
Methods:
This unicentric, cross-over, prospective study included 21 hemodialysis patients (10 males, 11 females, average age 62.25 years). Each patient underwent 24 dialysis sessions (12 with each dialysate) and acted as their own control. Lipid profiles, immunological parameters, and nutritional and inflammatory markers were measured before the last session with each dialysate.
Results:
After twelve dialysis sessions with citrate dialysate (CD), compared to acetate dialysate (AD), there was a statistically significant decline in TG and remnant cholesterol, with a decrease in HDL and an increase in LDL. Regarding immunology, C3 complement levels were higher, while CD3+ CD8+ and CD16+ 56+ lymphocytes were lower. Finally, total lymphocytes were lower with AD than with CD. We found no difference in predialysis nutritional nor inflammatory parameters except for ESR, which was higher when subjects used CD than AD.
Conclusion:
There are significant differences in lipid and immunophenotypic profiles with CD in comparison to AD. Interestingly, there could be an advantageous profile given the reduced amount of remnant cholesterol and TG. However, further studies are needed to understand if the observed changes lead to beneficial hard clinical outcomes in DD-CKD patients.
1 Introduction
Chronic kidney disease (CKD) is a substantial cardiovascular (CV) risk factor, being considered equivalent to having suffered a previous CV event (1–4). Patients with dialysis-dependent CKD (DD-CKD) have a 5-year mortality rate of approximately 50%, according to some authors (5–7), mainly due to CV causes. The dialysis fluid (i.e., dialysate) constitutes one of the main components of hemodialysis. Its components determine the direction and final concentration of the diffusive exchange of solutes through the dialyzer, aiming at the reduction of uremic toxins, as well as the correction of electrolyte and acid-base imbalances. The composition of the dialysate is as important as the dialyzer, the treatment time and the flows of both blood and dialysis fluid used during treatment. The most used dialysate is a bicarbonate based one that uses acetate as a weak acid to avoid the precipitation of calcium and magnesium carbonate. However, given the inflammatory and hemodynamic effects of acetate, there is a growing interest in the implementation of an alternative. The most used and studied alternative to acetate is citrate, however there is scant evidence and experience on how these different dialysates affect systemic physiological processes.
Hypercholesterolemia constitutes an important CV risk factor and is also a therapeutic objective to control in the general population (8–10). In this sense, low-density lipoprotein (LDL) levels are targeted to a certain threshold according to each patient's calculated risk, given the evidence on lipid control as a preventive measure both for primary and secondary CV events in those with high risk, including CKD patients (11–13). The association with mortality and cardiovascular disease between other relevant lipid biomarkers and DD-CKD has been studied in triglycerides (TG) (14, 15), high-density (HDL) (16–18) and very low-density (VLDL) lipoprotein cholesterol (19, 20), lipoprotein (a) [Lp(a)) (21–23), remnant cholesterol (24), as well as the calculation of the TG/HDL ratio (25). Unlike other high-cardiovascular-risk populations, the efficacy obtained from lipid-lowering therapy remains uncertain in DD-CKD patients. Two clinical trials and a systematic review failed to prove significant benefits from statins in these patients (26–28); nonetheless, there is data on the subgroup of dialysis patients from a randomized controlled trial and a real-life retrospective study that show a reduction in major cardiovascular events (29, 30). The discrepancy between findings is probably given to the exclusion in clinical trials of patients with very high LDL levels, their population's heterogeneity, accompanying cardiovascular morbidities, and the higher mortality risk explained in part by chronic inflammation (31–33). Interestingly, some studies in patients with DD-CKD have reported that high cholesterol is not associated with decreased mortality but with increased survival (34, 35). These findings suggest that LDL blood levels may indicate malnutrition, inflammation, or a sarcopenic state (34).
It is known that citrate-based dialysates reduce inflammatory biomarkers compared to acetate-based ones, and some authors state that this could be due to reduced oxidative stress and interfere with the immunological inflammation process. Acetate-based dialysates have also been associated with accumulating uremic toxins, membrane biocompatibility, and inflammation (36–38). There is evidence that inflammatory and immunological factors are relevant in establishing patients' CV risk. For instance, studies of lymphocyte subpopulations in non-CKD patients indicate that patients with a CD4/CD8 ratio higher than 1.5 (39) and an increased proportion of natural killer (NK) lymphocytes (40) are at a greater risk of CV problems than their counterparts. Also, the complement system, particularly C3 and the C3/C4 ratio, has been associated with CV disease and metabolic disorders (41–43). However, there is scarce data on the effect of different dialysates on the immune system.
This study aims to examine potential differences in the basic lipid profile and immunophenotype of patients on hemodialysis (HD).
2 Methods
2.1 Study design and population
This is a unicentric, cross-over, prospective study. Patients over 18 years old undergoing post-dilution online hemodiafiltration, who have been on dialysis for at least three months, receiving treatment three times a week for at least four hours each session, and maintaining a stable clinical condition during this period, were eligible for inclusion in the study. Each subject underwent 24 dialysis sessions, 12 with each dialysate acidifier, and acted as their own controls. Blood samples were retrieved predialysis on the last session with each acidifier.
All parameters of the dialysis session (calcium, sodium, and bicarbonate prescriptions; blood and dialysate flows; and dialysis duration), along with medical treatments, were kept constant throughout the study, except for the dialysate acidifier. The details of the dialysate characteristics can be found in Table 1. This study utilized Fresenius 6,008 CAREsystem™ dialysis monitors and FX CorDiax™ 60 dialyzers (Fresenius Medical Care, Bad Homburg v.d.H., Germany).
Table 1
| Components | Fresenius ACF 3A5 | Fresenius smartbag CA 211.5 |
|---|---|---|
| Sodium (mmol/L) | 140 | 138 |
| Potassium (mmol/L) | 2 | 2 |
| Calcium (mmol/ml) | 1.5 | 1.5 |
| Magnesium (mmol/ml) | 0.5 | 0.5 |
| Chloride (mmol/ml) | 106 | 109 |
| Acetate (mmol/L) | 4 | – |
| Citrate (mmol/L) | – | 1 |
| Glucose (g/L) | 1 | 1 |
Acetate and citrate dialysate components.
2.2 Variables
2.2.1 Lipid parameters
We registered the lipidic profile by assessing the plasma levels of total cholesterol, LDL, TG, HDL and VLDL lipoprotein cholesterol, and Lp(a), as well as the calculation of the TG/HDL ratio, comparing the use of citrate (CD) vs. acetate (AD) as dialysate acidifier, after twelve sessions with each one.
Total cholesterol, HDL and TG were measured using enzymatic assays while Lp(a) levels were determined using immunoassays. LDL cholesterol was calculated using the Friedewald formula: LDL = total cholesterol—(HDL + TG/5), provided TG levels were below 400 mg/dl; otherwise, LDL was measured directly. VLDL was estimated as TG/5 (44, 45). Remnant cholesterol was defined as total cholesterol minus LDL-C minus HDL-cholesterol (46). All measurements were performed using automated analyzers to ensure accuracy and consistency across samples.
2.2.2 Immunological parameters
The studied leucocyte populations were neutrophils, lymphocytes, neutrophil-to-lymphocyte ratio (NLR), natural killers (NK) cells, CD3 positive, CD4 positive, CD8 positive, and CD19 positive count. Complement levels (C3 and C4 levels) and the C3/C4 ratio.
The leucocyte analysis was performed using flow cytometry using whole blood samples on the Sysmex XR analyzer (Sysmex Corporation, Kobe, Japan) with software version SW 2.02. Complement levels and the C3/C4 ratio were determined using immunoturbidimetry, a technique in which antigen-antibody complexes induce changes in sample turbidity, allowing for precise quantification of complement components.
2.2.3 Other parameters
Blood levels for uric acid, glucose, folic acid, vitamin B12, magnesium, prealbumin, creatine kinase (CK), total proteins, albumin, transferrin, interleukin-6 (IL-6), high-sensitive C-reactive protein (hs-CRP), D-dimer, and erythrocyte sedimentation rate (ESR) were measured using standard laboratory techniques.
Uric acid, creatin kinase, and glucose levels were determined by enzymatic methods, with colorimetric detection of reaction products. Folic acid and vitamin B12 concentrations were quantified using chemiluminescence. Magnesium was measured using colorimetric assays, while prealbumin, transferrin, and total proteins were quantified via immunoturbidimetry. Albumin levels were determined using colorimetric binding assays. IL-6 and hs-CRP levels were quantified using high-sensitivity immunoassays. D-dimer was measured via immunoassays specific to fibrin degradation products. Finally, the ESR was determined using the Westergren method (47, 48).
2.3 Statistical analysis
Quantitative variables are reported with mean and standard deviation when normally distributed or median and 25th and 75th percentiles if skewed. Normal distribution was assessed with the Shapiro–Wilk test, and the comparisons were made with the Student's paired T-test or Wilcoxon's signed-rank test, accordingly. A two-sided p-value ≤0.05 was considered statistically significant.
3 Results
Two-hundred and fifty-two sessions were performed with CD and AD in total. Twenty-one participants, consisting of 10 (47.6%) males and 11 (52.4%) females, with an average age of 62.3 ± 13.8 years (ranging from 33.1 to 82.3 years), were included in the study. Dialysis access included arteriovenous fistula for 12 patients and tunneled catheter for 9 patients. Various underlying renal conditions were observed, including diabetic kidney disease (6 patients), autosomal dominant polycystic kidney disease (2 patients), HIV nephropathy (2 patients), hypertensive nephropathy (2 patients), chronic glomerulonephritis (1 patient), chronic pyelonephritis (1 patient), renal cell carcinoma (1 patient), other cystic diseases (1 patient), and undetermined causes (5 patients). Noteworthy, six patients had a past cardiovascular history where three had unresolved valvulopathy, two had coronary artery disease and one had symptomatic heart failure with preserved ejection fraction due to hypertensive cardiomyopathy.
3.1 Lipidic profile results
There were significantly lower LDL cholesterol levels with AD compared to CD (AD: 67.05 mg/dl ± 30.53 vs. CD: 75.71 mg/dl ± 31.01, p = 0.042), higher remnant cholesterol [AD: 20 mg/dl (95% CI: 17.5–23.5) vs. CD: 17 mg/dl (95% CI: 17–25) p = 0.036], higher TG [AD: 101 mg/dl (95% CI: 88.5–117) vs. CD: 83 mg/dl (95% CI: 73.5–126) p = 0.046], higher HDL cholesterol (AD: 51.19 mg/dl ± 11.25 vs. CD: 47.14 mg/dl ± 11.99, p = 0.013). However, there were no significant differences between predialysis total cholesterol (AD: 140.33 mg/dl ± 30.56 vs. CD: 142.33 mg/dl ± 33.4, p = 0.624), VLDL (AD: 22.14 mg/dl ± 7.36 vs. CD: 19.67 mg/dl ± 5.97, p = 0.057), TG/HDL ratio (AD: 2.35 ± 1.16 and CD: 2.29 ± 1.07, p = 0.876), and Lp(a) values (AD: 26.21 mg/dl ± 30.45 and CD: 27.04 mg/dl ± 34.21, p = 0.617). See Figure 1 for further details.
Figure 1
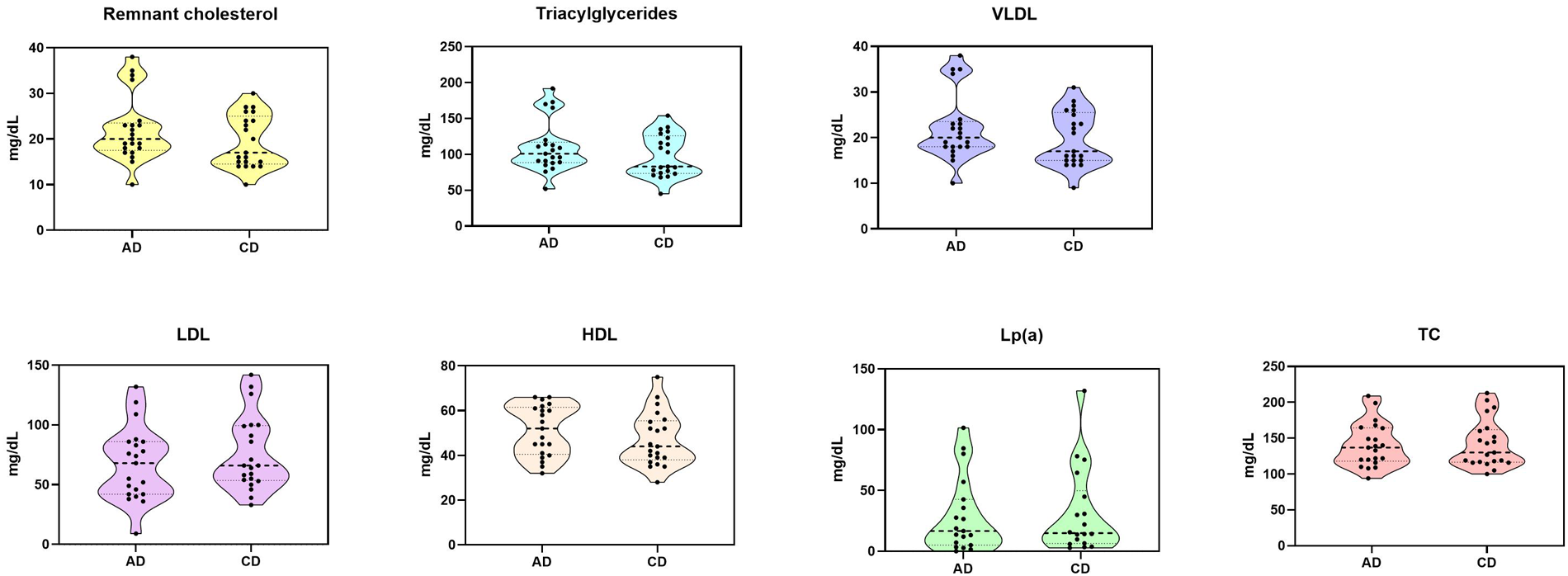
Measured lipid plasma levels after twelve dialysis sessions with each dialysate. AD, acetate dialysate; CD, citrate dialysate; HDL, high density lipoprotein; LDL, low density lipoprotein; Lp(a), lipoprotein (a); TC, total cholesterol; VLDL, very low-density lipoprotein.
3.2 Complement levels
We found a statistically significant difference in predialysis C3 levels between both dialysates. They were higher when patients were dialyzed with CD than with AD. However, there were no significant differences in C4 levels nor in the C3/C4 ratio (AD: 4.43 ± 0.98 vs. CD: 4.5 ± 0.92, p = 0.482). More details are in Table 2.
Table 2
| Variable | Acetate | Citrate | p-value |
|---|---|---|---|
| Total Leucocytes × 103/mm3, mean ± SD | 7.1 ± 3.9 | 6.3 ± 2.2 | 0.03 |
| Neutrophils × 103/mm3, mean ± SD | 4.72 ± 3.64 | 4.09 ± 1.75 | 0.412 |
| NLR, mean ± SD | 3.69 ± 3.01 | 3.85 ± 1.93 | 0.776 |
| Lymphocytes × 103/mm3, mean ± SD | 1.37 ± 0.46 | 1.21 ± 0.47 | 0.037 |
| CD19%, mean ± SD | 121.48 ± 103.26 | 133.95 ± 88.31 | 0.423 |
| CD3+ %, mean ± SD | 72.9 ± 12.69 | 68.48 ± 11.86 | 0.005 |
| CD8+ %, mean ± SD | 33.43 ± 14.56 | 30.67 ± 12.85 | 0.05 |
| CD4+ %, mean ± SD | 517.67 ± 244.83 | 479.05 ± 193.74 | 0.272 |
| CD4/CD8, mean ± SD | 1.53 ± 1.05 | 1.57 ± 1.08 | 0.393 |
| CD56+ CD16+ NK cells %, mean ± SD | 16.95 ± 7.85 | 19.24 ± 8.84 | 0.035 |
| C3 × 103/mm3, mean ± SD | 107.81 ± 19.71 | 115.14 ± 21.30 | 0.009 |
| C4 × 103/mm3, mean ± SD | 25.48 ± 7.05 | 26.57 ± 6.78 | 0.097 |
Leucocyte and complement subclasses' concentrations with acetate and citrate dialysates.
CD, cluster of differentiation; NLR, neutrophil to lymphocyte ratio; NK, natural killer; SD, standard deviation.
3.3 Leucocyte count and subpopulations
There was a higher number of total leucocytes (AD: 7.1 × 103/mm3 ± 3.9 vs. CD: 6.3 ± 2.2, p = 0.03) and lymphocytes (AD: 1.37 × 103/mm3 ± 0.46 vs. CD: 1.21 ± 0.47, p = 0.037) with AD than with CD. We also found a higher percentage of CD3+ (AD: 72.9 ± 12.69 vs. CD: 68.48 ± 11.86, p = 0.0049) and CD8+ T cells (AD: 33.43 ± 14.56 vs. CD: 30.67 ± 12.85, p = 0.05), and higher CD56+ CD16+ NK cells (AD: 16.95 ± 7.85 vs. CD: 19.24 ± 8.84, p = 0.035). There were no statistically significant differences in mean CD4/CD8 ratios nor the proportion of CD4/CD8 > 1.5 with each dialysate (AD: 42.1% vs. CD: 57.9%, p = 0.352). There were also no differences observed in predialysis CD19+ B-cells, and CD4+ T-cells, total leucocyte count, neutrophils, NLR, monocytes, nor eosinophils between dialysates. See Table 2 for further information.
3.4 Inflammatory and nutritional parameters
There were no statistically significant differences in predialysis IL-6 and hs-CRP between dialysates. However, the ESR was higher when patients used CD than AD (AD: 41.19 ± 22.92 vs. CD: 53.62 ± 35.24, p = 0.02). Regarding nutritional parameters, we did not find any significant differences between dialysates in predialysis albumin, prealbumin, total proteins, iron, transferrin, magnesium, iron, folic acid, or vitamin B12 levels. More detailed information is available in Table 3.
Table 3
| Variable | Acetate | Citrate | p-value |
|---|---|---|---|
| Glucose (mg/d), mean ± SD | 119.15 ± 35.70 | 126.7 ± 59.52 | 0.439 |
| Uric acid (mg/d), mean ± SD | 5.36 ± 1.43 | 5.38 ± 1.29 | 0.943 |
| Amylase (mg/d), mean ± SD | 134.05 ± 85.72 | 125.10 ± 88.86 | 0.457 |
| CK (mg/d), mean ± SD | 82.24 ± 95.51 | 135.67 ± 204.27 | 0.129 |
| Total Protein (mg/d), mean ± SD | 6.79 ± 0.75 | 6.77 ± 0.80 | 0.805 |
| Albumin (mg/d), mean ± SD | 4.01 ± 0.40 | 3.92 ± 0.46 | 0.119 |
| TSAT (mg/d), mean ± SD | 24.90 ± 9.47 | 29.14 ± 17.19 | 0.132 |
| Transferrin (mg/d), mean ± SD | 177.29 ± 29.93 | 169.62 ± 32.03 | 0.066 |
| Magnesium (mg/d), mean ± SD | 2.11 ± 0.19 | 2.05 ± 0.26 | 0.192 |
| Haptoglobin (mg/d), mean ± SD | 154.67 ± 67.57 | 153.64 ± 75.90 | 0.912 |
| Vitamin B12 (mg/d), mean ± SD | 745 ± 478.39 | 753.62 ± 435.85 | 0.865 |
| Folic acid (mg/d), mean ± SD | 16.26 ± 8.23 | 15.90 ± 7.26 | 0.844 |
| ESR (mg/d), mean ± SD | 41.19 ± 22.92 | 53.62 ± 35.24 | 0.03 |
| D-dimer (mg/d), mean ± SD | 1,497.5 ± 1,614.27 | 1,490.56 ± 1,344.63 | 0.978 |
| hs-CRP (mg/d), mean ± SD | 19.58 ± 59.26 | 16.46 ± 29.75 | 0.832 |
| Ferritin (mg/d), mean ± SD | 334.67 ± 231.94 | 398.71 ± 246.55 | 0.108 |
| Prealbumin (mg/d), mean ± SD | 26.21 ± 7.59 | 24.89 ± 8.089 | 0.096 |
Inflammatory and nutritional parameters with each dialysate.
CK, creatin kinase; ESR, erythrocyte sedimentation rate; hsCRP, high-sensitivity C-reactive protein; SD, standard deviation; TSAT, transferrin saturation.
4 Discussion
After twelve dialysis sessions with CD, compared to AD, there was a statistically significant decline in TG, remnant cholesterol and HDL, with an increase in LDL, and a tendency towards lower VLDL. Regarding immunology, C3 complement levels were higher, while CD3+ CD8+ and CD16+ 56+ lymphocytes were lower. Finally, the total lymphocyte-count was lower with AD than with CD. We found no difference in predialysis nutritional nor inflammatory parameters except for ESR, which was higher when subjects used CD than AD.
Few studies have measured the effect of the dialysates' weak acidifier in the lipidic profile, and when done -always as secondary variables only an increase in LDL has been described with the use of CD (49). Previous studies from the 1980s did not find clinically significant changes in the lipid profile when bicarbonate solutions with reduced concentrations of acetate became commonplace over only acetate solutions (50).
Real-life findings in HD patients seem to associate lipid values with the opposite of what is expected to be beneficial in the general population. For instance, high TG levels (i.e., >193 mg/dl) correlate with a lower mortality risk (15), while high HDL levels (i.e., >60 mg/dl) correlate with an increased mortality risk (18). In our cohort, the median TG was 101 mg/dl and the highest value registered was 192 mg/dl, not reaching the apparently beneficial value previously published (15), though, it is worth noting that TG levels were significantly higher with AD than with CD. Also, unlike us, a recent study by de Sequera, et al., showed no differences in TG between AD and CD (51). With respect to remnant cholesterol, we found lower levels with CD than with AD. The median levels when patients were on AD was 20 mg/dl, whereas it was 17 mg/dl when they used CD. Levels above 39 mg/dl which have been associated with a two-fold cardiovascular mortality risk recently in a large Danish cohort (52, 53) and above 15.4 mg/dl in patients on peritoneal dialysis (24). Remnant cholesterol provides an improved correlation due to a more linear association in comparison to the U-shaped one provided by LDL, where both lower and higher blood levels are related to an increased mortality risk (54). Regarding HDL, in our cohort we found lower levels with CD than with AD, with 38% of patients having values over 60 mg/dl when using AD while only 14% crossed this threshold while on CD. With regards to LDL, even though its blood levels were higher with CD, we do not actually know the long-term clinical impact of this finding. Previous data suggest a U-shaped association between LDL. In a study performed on peritoneal dialysis patients, LDL levels above 100 mg/dl and below 85 mg/dl were associated with all-cause and cardiovascular mortality. Mean LDL levels in that population were 98 mg/dl (55) whereas our population had a much lower mean LDL of 72 mg/dl. However, as stated earlier, no clear association has been established with mortality in HD patients. As to VLDL, we found lower blood levels when patients used CD instead of AD, though not significant. There is no data on VLDL cholesterol in hemodialysis patients, however, there is data associating high VLDL with mortality in both non-dialysis dependent CKD (56) and PD patients (20), making it a potential target for treatment though more studies are required in the HD population.
Further studies must elucidate if the short-term changes induced in the lipidic profile of DD-CKD patients by the dialysate's weak acid translate into clinical implications.
Regarding immunological parameters, there is recent evidence that the use of CD for three months did not affect leucocyte nor total B or T lymphocyte count in comparison to AD in patients on HD (57). Notably, the same authors measured NLR, which has been associated with worse survival in HD patients (58), and, like us, found no differences between dialysates.
Regarding lymphocytic subpopulations, previous studies have shown that there is a U-shaped relationship between the CD4/CD8T cell- ratio and atherosclerosis progression, being associated with lower ratios in HIV patients (mean ratio of around 0.5) (59–62) and more than 1.5 in Chinese elderly population (mean ratio of around 1.33) (36). It is reported that early atherosclerotic plaques have ratios below 1, while late atherosclerotic plaques, particularly in late fibroatheroma, are around 1.5 (63). We found no differences in the proportion of low and high CD4/CD8 ratios between dialysates. However, our population's mean CD4/CD8 ratio was higher than that from the Chinese study despite their subjects being on average older than ours. This higher ratio could potentially translate into a higher CV risk, though more research is needed in dialysis patients.
We found higher total leucocytes and lymphocytes with AD than with CD, predominantly by a higher count of CD3+ and CD8+ lymphocytes, whereas NK cells were higher with CD than with AD. The interpretation of lymphocyte subpopulations needs to be clarified. There is evidence that both CD4 and CD8 decline post-hemodialysis, while their predialysis values remain unchanged compared to healthy populations (64). This is believed to be in response to poor biocompatibility with the dialyzer membrane or even with the dialysis solution, as postulated by data that has associated acetate with an increased number of activated CD3+ CD4+ CD69+ T cells than with citrate (57).
LDL receptors play a role in CD8+ T cell activation. Studies in ldlr −/− mice and individuals with familial hypercholesterolemia who carry a mutation in LDL receptor show decreased levels of CD8+ cytokine production and cell proliferation (60). Whether citrate alters LDL receptor function, increasing LDL levels and thus decreasing the number of CD8+ T remains unconfirmed but could partly explain the observed results compared to acetate.
Our cohort found that patients had higher circulating NK cells when exposed to CD than to AD. Activated natural killer (NK) cells utilize pyruvate, which is metabolized through the citrate-malate shuttle—a process that occurs across the mitochondrial membrane. This connection suggests that fluctuations in citrate levels could influence the metabolic reprogramming of NK cells, impacting their activation, cytotoxicity, and overall function. Citrate has been associated with promoting oxidative phosphorylation, a process essential for meeting the energy demands of NK cells during target cell elimination. This enhancement in citrate levels could potentially improve the ability of NK cells to carry out their cytotoxic functions effectively (65). NK cells constitute another subset of lymphocytes, which may also be necessary in vascular disease (66). These cells have been isolated from atherosclerotic plaques, particularly expanding the necrotic cores (37). Regarding circulating levels, a higher percentage of NK cells has been reported in patients with severe atherosclerosis awaiting revascularization (67), elderly patients with coronary disease (68), and has been associated with an increased number of CV and neurological complications after an endarterectomy (69).
Additionally, we found higher TG levels and decreased C3 levels when patients used AD than with CD. These findings may be related, given that data from experimental studies have shown that C3 stimulates glucose uptake and inhibits hormone-sensitive lipase in several cell types (40). C3 promotes lipophagy, which stimulates VLDL secretion in hepatocytes and balances TG levels in the liver (70). C3-deficient mice present with glucose intolerance delayed TG clearance and decreased TG storage (40, 71, 72). Therefore, C3 has been associated with metabolic disorders and is now recognized as a cardiometabolic risk factor (38). We also measured the C3/C4 ratio given recent data associating increased serum C3/C4 ratio as a novel marker for recurrent cardiovascular events in acute coronary syndrome (39). However, we found no differences in this parameter in our cohort.
Multiple sources state lower inflammatory parameters with CD than with AD (41–45, 73), but there are some discrepancies in the current literature (56, 74). We found no differences in IL-6 or hs-CRP, though we did find a statistically significant difference in ESR in favor of AD. It is worth noting that most of the previous evidence in this regard comes from patients on HD rather than HDF.
This study has several limitations that should be considered when interpreting the results. The single-center design, small sample size, and short follow-up limit the generalizability and long-term applicability of our findings. Although the crossover design minimizes interindividual variations, the influence of uncontrolled confounding factors, such as changes in diet, prior lipid lowering therapy, or physical activity, during the study, cannot be entirely excluded. Additionally, uncontrolled confounding factors and the absence of a non-intervention control group may have influenced the results. Larger multicenter studies are needed to validate these observations.
Based on our findings, we can conclude that using citrate dialysate in patients with DD-CKD results in significant changes to lipid profiles and immune parameters when compared to acetate dialysate. Specifically, citrate dialysate decreases TG and remnant cholesterol, lowers HDL levels and alters various lymphocyte subpopulations. These observations suggest that citrate dialysate may have distinct metabolic and immunological effects that could influence cardiovascular risk and inflammation in DD-CKD patients.
However, it remains unclear whether these changes lead to a favorable profile. While citrate dialysate reduces remnant cholesterol and has been associated with increased mortality independently of LDL-C and HDL-C levels (53), previous studies comparing mortality rates between citrate and acetate dialysate in HD patients have either shown no differences or suggested potential benefits for specific subpopulations using citrate (75). Additionally, there seems to be conflicting evidence on the role of citrate in preventing vascular calcification and magnesium supplementation and thus reducing CV morbidity and mortality (75–77). It still needs to be determined how our findings relate to those of other study groups and how they might explain certain clinical results.
To expand on our findings, future research should focus on long-term cardiovascular outcomes and immune responses. Longitudinal, multicenter trials with larger cohorts are needed to determine whether the short-term metabolic and immunological changes observed with citrate in this study result in clinically significant outcomes, such as cardiovascular event rates and mortality. Additionally, mechanistic experiments that explore the interactions among lipid metabolism, immune cell populations, and dialysate composition would provide deeper insights. Further studies that include lipidomics and immunophenotyping could help clarify the complex pathways involved, potentially guiding personalized dialysis strategies aimed at reducing cardiovascular risk and improving patient outcomes.
Statements
Data availability statement
The datasets presented in this study can be found here: https://github.com/Broseta/Citrate-dialysate.git. Further enquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of Hospital Clínic of Barcelona (HCB/2024/0173, 25/09/2023). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DR: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. EC-P: Investigation, Project administration, Writing – original draft. LM: Investigation, Writing – original draft. MG: Data curation, Investigation, Methodology, Writing – review & editing. FM: Conceptualization, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing. JB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
FM has received expenditures on travel and hospitality and conference fees from Fresenius Medical Care. JB has received expenditures on travel and hospitality from Fresenius Medical Care.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction Note
A correction has been made to this article. Details can be found at: 10.3389/fcvm.2025.1744975.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Levey AS Coresh J Balk E Kausz AT Levin A Steffes MW et al National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. (2003) 139(2). 10.7326/0003-4819-139-2-200307150-00013
2.
Tonelli M Muntner P Lloyd A Manns BJ Klarenbach S Pannu N et al Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. (2012) 380(9844):807–14. 10.1016/S0140-6736(12)60572-8
3.
Sarnak MJ Levey AS Schoolwerth AC Coresh J Culleton B Hamm LL et al Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. (2003) 108(17):2154–69. 10.1161/01.CIR.0000095676.90936.80
4.
Briasoulis A Bakri GL . Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. (2013) 15(3):1534–3170. 10.1007/S11886-012-0340-4
5.
Ahmed M Alalawi F Alnour H Gulzar K Alhadari A . Five-Year mortality analysis in hemodialysis patients in a single-center in dubai. Saudi J Kidney Dis Transpl. (2020) 31(5):1062–8. 10.4103/1319-2442.301172
6.
de Arriba G Avila GG Guinea MT Alia IM Herruzo JA Ruiz BR et al Mortality of hemodialysis patients is associated with their clinical situation at the start of treatment. Nefrología. (2021) 41(4):461–6. 10.1016/j.nefroe.2021.10.006
7.
Saran R Robinson B Abbott KC Agodoa LYC Bragg-Gresham J Balkrishnan R et al US Renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. (2019) 73(3):A7–8. 10.1053/j.ajkd.2019.01.001
8.
Grundy SM Stone NJ Bailey AL Beam C Birtcher KK Blumenthal RS et al 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 139(25):E1082–143. 10.1016/j.jacc.2018.11.002
9.
Mach F Baigent C Catapano AL Koskina KC Casula M Badimon L et al 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. (2019) 290:140–205. 10.1016/j.atherosclerosis.2019.08.014
10.
Alloubani A Nimer R Samara R . Relationship between hyperlipidemia, cardiovascular disease and stroke: a systematic review. Curr Cardiol Rev. (2021) 17(6):051121189015. 10.2174/1573403X16999201210200342
11.
Wanner C Tonelli M ; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. (2014) 85(6):1303–9. 10.1038/ki.2014.31
12.
Sarnak MJ Bloom R Muntner P Rahman M Saland JM Wilson PWF et al KDOQI US commentary on the 2013 KDIGO clinical practice guideline for lipid management in CKD. Am J Kidney Dis. (2015) 65(3):354–66. 10.1053/j.ajkd.2014.10.005
13.
Ferro CJ Mark PB Kanbay M Sarafidis P Heine GH Rossignol P et al Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. (2018) 14(12):727–49. 10.1038/s41581-018-0072-9
14.
Soohoo M Moradi H Obi Y Kovesdy CP Kalantar-Zadeh K Streja E . Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million U.S. Veterans. J Clin Lipidol. (2019) 13(5):744–753.e15. 10.1016/j.jacl.2019.08.001
15.
Huang Y Zhong Q Chen J Qin X Yang Y He Y et al Relationship of serum total cholesterol and triglyceride with risk of mortality in maintenance hemodialysis patients: a multicenter prospective cohort study. Ren Fail. (2024) 46(1):2334912. 10.1080/0886022X.2024.2334912
16.
Kopecky C Ebtehaj S Genser B Drechsler C Krane V Antlanger M et al HDL Cholesterol efflux does not predict cardiovascular risk in hemodialysis patients. J Am Soc Nephrol. (2017) 28(3):769–75. 10.1681/ASN.2016030262
17.
Zewinger S Speer T Kleber ME Scharnagl H Woitas R Lepper PM et al HDL Cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol. (2014) 25(5):1073–82. 10.1681/ASN.2013050482
18.
Moradi H Streja E Kashyap ML Vaziri ND Fonarow GC Kalantar-Zadeh K . Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. (2014) 29(8):1554–62. 10.1093/ndt/gfu022
19.
Yu J Xia X Lin T Huang N Qiu Y Yang X et al Non-high-density lipoprotein cholesterol and mortality among peritoneal dialysis patients. J Clin Lipidol. (2021) 15(5):732–42. 10.1016/j.jacl.2021.06.005
20.
Xie X Zhang X Xiang S Yan X Huang H Tian Y et al Association of very low-density lipoprotein cholesterol with all-cause and cardiovascular mortality in peritoneal dialysis. Kidney Blood Press Res. (2017) 42(1):52–61. 10.1159/000469714
21.
Webb AT Reaveley DA O’Donnell M O’Connor B Seed M Brown EA . Lipoprotein (a) in patients on maintenance haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. (1993) 8(7):609–13.
22.
Goldwasser P Michel MA Collier J Mittman N Fein PA Gusik SA et al Prealbumin and lipoprotein(a) in hemodialysis: relationships with patient and vascular access survival. Am J Kidney Dis. (1993) 22(1):215–25. 10.1016/S0272-6386(12)70189-0
23.
Cressman MD Heyka RJ Paganini EP O’Neil J Skibinski CI Hoff HF . Lipoprotein(a) is an independent risk factor for cardiovascular disease in hemodialysis patients. Circulation. (1992) 86(2):475–82. 10.1161/01.CIR.86.2.475
24.
Deng J Tang R Chen J Zhou Q Zhan X Long H et al Remnant cholesterol as a risk factor for all-cause and cardiovascular mortality in incident peritoneal dialysis patients. Nutr Metab Cardiovasc Dis. (2023) 33(5):1049–56. 10.1016/j.numecd.2023.02.009
25.
Gonzáles-Rubianes DZ Figueroa-Osorio LK Benites-Zapata VA Pacheco-Mendoza J Herrera-Añazco P . Utility of TG/HDL-c ratio as a predictor of mortality and cardiovascular disease in patients with chronic kidney disease undergoing hemodialysis: a systematic review. Hemodial Int. (2022) 26(2):137–46. 10.1111/hdi.12981
26.
Wanner C Krane V März W Olschewski M Mann JFE Ruf G et al Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. (2005) 353(3):238–48. 10.1056/NEJMoa043545
27.
Fellström BC Jardine AG Schmieder RE Holdaas H Bannister K Beutler J et al Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. (2009) 360(14):1395–407. 10.1056/NEJMoa0810177
28.
Palmer SC Navaneethan SD Craig JC Johnson DW Perkovic V Nigwekar SU et al HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. (2013) 2013(9):CD004289. 10.1002/14651858.CD004289.PUB5
29.
Kim JE Park S Kim Ms Kang SJ Lee JW Kim KS et al Statin initiation and all-cause mortality in incident statin-naïve dialysis patients. Atherosclerosis. (2021) 337:59–65. 10.1016/j.atherosclerosis.2021.08.026
30.
Baigent C Landray MJ Reith C Emberson J Wheeler DC Tomson C et al The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet. (2011) 377(9784):2181–92. 10.1016/S0140-6736(11)60739-3
31.
Nowak KL Chonchol M . Does inflammation affect outcomes in dialysis patients?Semin Dial. (2018) 31(4):388. 10.1111/sdi.12686
32.
Yang Y Xu Y Liu S Lu P Zhou H Yang M . The systemic inflammation indexes predict all-cause mortality in peritoneal dialysis patients. Ren Fail. (2023) 45(1):2160348. 10.1080/0886022X.2022.2160348
33.
Panichi V Rizza GM Paoletti S Bigazzi R Aloisi M Barsotti G et al Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. (2008) 23(7):2337–43. 10.1093/ndt/gfm951
34.
Liu Y Coresh J Eustace JA Longenecker JC Jaar B Fink NE et al Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. (2004) 291(4):451–9. 10.1001/jama.291.4.451
35.
Iseki K Yamazato M Tozawa M Takishita S . Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. (2002) 61(5):1887–93. 10.1046/j.1523-1755.2002.00324.x
36.
de Sequera Ortiz P Pérez García R Molina Nuñez M Muñoz González RI Fernández GA Herrero EM et al Prospective randomised multicentre study to demonstrate the benefits of haemodialysis without acetate (with citrate): ABC-treat study. Acute effect of citrate. Estudio prospectivo aleatorizado multicéntrico para demostrar los beneficios de la hemodiálisis sin acetato (con citrato): estudio ABC-treat. Efecto agudo del citrato. Nefrologia. (2019) 39(4):424–33. 10.1016/j.nefro.2018.11.002
37.
Molina Nuñez M De Alarcón R Roca S Álvarez G Ros MS Jimeno C et al Citrate versus acetate-based dialysate in on-line haemodiafiltration. A prospective cross-over study. Blood Purif. (2015) 39(1–3):181–7. 10.1159/000371569
38.
Broseta JJ López-Romero LC Cerveró A Devesa-Such R Soldevila A Bea-Granell S et al Improvements in inflammation and calcium balance of citrate versus acetate as dialysate buffer in maintenance hemodialysis: a unicentric, cross-over, prospective study. Blood Purif. (2021) 50(6):914–20. 10.1159/000513419
39.
Gao P Rong HH Lu T Tang G Si LY Lederer JA et al The CD4/CD8 ratio is associated with coronary artery disease (CAD) in elderly Chinese patients. Int Immunopharmacol. (2017) 42:39–43. 10.1016/j.intimp.2016.11.007
40.
Selathurai A Deswaerte V Kanellakis P Tipping P Toh BH Bobik A et al Natural killer (NK) cells augment atherosclerosis by cytotoxic-dependent mechanisms. Cardiovasc Res. (2014) 102(1):128–37. 10.1093/cvr/cvu016
41.
Hertle E Van Greevenbroek MMJ Stehouwer CDA . Complement C3: an emerging risk factor in cardiometabolic disease. Diabetologia. (2012) 55(4):881. 10.1007/s00125-012-2462-z
42.
Palikhe A Sinisalo J Seppänen M Haario H Meri S Valtonen V et al Serum complement C3/C4 ratio, a novel marker for recurrent cardiovascular events. Am J Cardiol. (2007) 99(7):890–5. 10.1016/j.amjcard.2006.11.034
43.
Phieler J Garcia-Martin R Lambris JD Chavakis T . The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. (2013) 25(1):47–53. 10.1016/j.smim.2013.04.003
44.
Friedewald WT Levy RI Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. (1972) 18(6):499–502. 10.1093/clinchem/18.6.499
45.
Cao J Donato L El-Khoury JM Goldberg A Meeusen JW Remaley AT . ADLM Guidance document on the measurement and reporting of lipids and lipoproteins. J Appl Lab Med. (2024) 9(5):1040–56. 10.1093/jalm/jfae057
46.
Nordestgaard BG Varbo A . Triglycerides and cardiovascular disease. Lancet. (2014) 384:626–35. 10.1016/S0140-6736(14)61177-6
47.
Grzybowski A Sak J . Edmund biernacki (1866–1911): discoverer of the erythrocyte sedimentation rate. On the 100th anniversary of his death. Clin Dermatol. (2011) 29(6):697–703. 10.1016/j.clindermatol.2011.08.033
48.
Tishkowski K Gupta V . Erythrocyte sedimentation rate. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2025). Available at:https://www.ncbi.nlm.nih.gov/books/NBK557485/(Accessed July 12, 2024).
49.
Mahmood D Stegmayr BG . Haemodialysis with tinzaparin versus dialysate citrate as anticoagulation. Blood Purif. (2018) 46(3):257–63. 10.1159/000490409
50.
Morin RJ Srikantaiah Mv Woodley Z Davidson WD . Effect of hemodialysis with acetate vs bicarbonate on plasma lipid and lipoprotein levels in uremic patients. J Dial. (1980) 4(1):9–20. 10.3109/08860228009065323
51.
de Sequera P Pérez-García R Molina M Álvarez-Fernández G Muñoz-González RI Mérida E et al Advantages of the use of citrate over acetate as a stabilizer in hemodialysis fluid: a randomized ABC-treat study. Nefrologia. (2022) 42(3):327–37. 10.1016/j.nefroe.2021.12.003
52.
Wadström BN Pedersen KM Wulff AB Nordestgaard BG . Elevated remnant cholesterol, plasma triglycerides, and cardiovascular and non-cardiovascular mortality. Eur Heart J. (2023) 44(16):1432–45. 10.1093/eurheartj/ehac822
53.
Stürzebecher PE Katzmann JL Laufs U . What is ‘remnant cholesterol’?Eur Heart J. (2023) 44:1446–8. 10.1093/EURHEARTJ/EHAC783
54.
Johannesen C Langsted A Mortensen MB Nordestgaard BG . Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. Br Med J. (2020) 371:m4266. 10.1136/BMJ.M4266
55.
Wu X Zhou L Zhan X Wen Y Wang X Feng X et al Low-Density lipoprotein cholesterol and mortality in peritoneal dialysis. Front Nutr. (2022) 9:910348. 10.3389/FNUT.2022.910348/FULL
56.
Bajaj A Xie D Cedillo-Couvert E Charleston J Chen J Deo R et al Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am J Kidney Dis. (2019) 73:827. 10.1053/J.AJKD.2018.11.010
57.
Shen Y Schmaderer C Ossadnik A Hammitzsch A Carbajo-Lozoya J Bachmann Q et al Immunophenotypic characterization of citrate-containing A concentrates in maintenance hemodialysis: a Pre-post study. Int J Nephrol. (2023) 2023:7772677. 10.1155/2023/7772677
58.
Ahbap E Sakaci T Kara E Sahutoglu T Koc Y Basturk T et al Neutrophil-to-lymphocyte ratio and platelet-to lymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin Nephrol. (2016) 85(4):199–208. 10.5414/CN108584
59.
Castilho JL Shepherd BE Koethe J Turner M Bebawy S Logan J et al CD4+/CD8+ ratio, Age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS. (2016) 30(6):899–907. 10.1097/QAD.0000000000001005
60.
Morell EB Cabeza JS Muñoz Á Marín I Masiá M Gutiérrez F et al The CD4/CD8 ratio is inversely associated with carotid intima-Media thickness progression in human immunodeficiency virus-infected patients on antiretroviral treatment. AIDS Res Hum Retroviruses. (2016) 32(7):648–53. 10.1089/aid.2015.0385
61.
Bernal E Serrano J Perez A Valero S Garcia E Marín I et al The CD4:cD8 ratio is associated with IMT progression in HIV-infected patients on antiretroviral treatment. J Int AIDS Soc. (2014) 17(4 Suppl 3):19723. 10.7448/IAS.17.4.19723
62.
Lo J Abbara S Shturman L Soni A Wei J Rocha-Filho JA et al Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. (2010) 24(2):243. 10.1097/QAD.0b013e328333ea9e
63.
van Dijk RA Duinisveld AJF Schaapherder AF Mulder-Stapel A Hamming JF Kuiper J et al A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc. (2015) 4(4):e001403. 10.1161/JAHA.114.001403
64.
Lisowska KA Dębska-Ślizień A Jasiulewicz A Heleniak Z Bryl E Witkowski JM . Hemodialysis affects phenotype and proliferation of CD4-positive T lymphocytes. J Clin Immunol. (2012) 32(1):189. 10.1007/s10875-011-9603-x
65.
Sohn H Cooper MA . Metabolic regulation of NK cell function: implications for immunotherapy. Immunometabolism (Cobham). (2023) 5(1):e00020. 10.1097/IN9.0000000000000020
66.
Hedrick CC . Lymphocytes in atherosclerosis. Arterioscler Thromb Vasc Biol. (2015) 35(2):253–7. 10.1161/ATVBAHA.114.305144
67.
Clerc G Roux PM . Lymphocyte subsets in severe atherosclerosis before revascularization. Ann Intern Med. (1997) 126(12):1004–5. 10.7326/0003-4819-126-12-199706150-00028
68.
Bruunsgaard H Pedersen AN Schroll M Skinhoj P Pedersen BK . Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol. (2001) 37(1):127–36. 10.1016/S0531-5565(01)00162-0
69.
Kotfis K Biernawska J Zegan-Barańska M Żukowski M . Peripheral blood lymphocyte subsets (CD4+, CD8+ T cells, NK cells) in patients with cardiovascular and neurological complications after carotid endarterectomy. Int J Mol Sci. (2015) 16(5):10077. 10.3390/ijms160510077
70.
Li Y Sha Y Wang H He L Li L Wen S et al Intracellular C3 prevents hepatic steatosis by promoting autophagy and very-low-density lipoprotein secretion. FASEB J. (2021) 35(12):e22037. 10.1096/FJ.202100856R
71.
Murray I Sniderman AD Havel PJ Cianflone K . Acylation stimulating protein (ASP) deficiency alters postprandial and adipose tissue metabolism in male mice. J Biol Chem. (1999) 274(51):36219–25. 10.1074/jbc.274.51.36219
72.
Paglialunga S Fisette A Yan Y Deshaies Y Brouillette JF Pekna M et al Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. (2008) 294(3):E521–9. 10.1152/AJPENDO.00590.2007
73.
Kossmann RJ Gonzales A Callan R Ahmad S . Increased efficiency of hemodialysis with citrate dialysate: a prospective controlled study. Clin J Am Soc Nephrol. (2009) 4(9):1459–64. 10.2215/CJN.02590409
74.
Grundström G Christensson A Alquist M Nilsson LG Segelmark M . Replacement of acetate with citrate in dialysis fluid: a randomized clinical trial of short term safety and fluid biocompatibility. BMC Nephrol. (2013) 14:216. 10.1186/1471-2369-14-216
75.
Pérez-García R Jaldo MT Puerta M Ortega M Corchete E de Sequera P et al Hypomagnesaemia in haemodialysis is associated with increased mortality risk: its relationship with dialysis fluid. La hipomagnesemia en hemodiálisis se asocia a mayor riesgo de mortalidad: su relación con el líquido de diálisis. Nefrologia. (2020) 40(5):552–62. 10.1016/j.nefro.2020.04.013
76.
Cejka D Thiem U Blinzler E Machacek J Voelkl J Smith ER et al Citrate-Buffered, magnesium-enriched dialysate on calcification propensity in hemodialysis patients—the CitMag study. Kidney Int Rep. (2024) 9(6):1765–73. 10.1016/j.ekir.2024.03.023
77.
Noris M Todeschini M Casiraghi F Roccatello D Martina G Minetti L et al Effect of acetate, bicarbonate dialysis, and acetate-free biofiltration on nitric oxide synthesis: implications for dialysis hypotension. Am J Kidney Dis. (1998) 32(1):115–24. 10.1053/ajkd.1998.v32.pm9669432
Summary
Keywords
lipids, dyslipidemia, acetate, acetate-free, citrate, hemodialysis, dialysate
Citation
Rodríguez-Espinosa D, Cuadrado-Payán E, Morantes L, Gomez M, Maduell F and Broseta JJ (2025) Lipid and immunophenotypic profiles in hemodialysis patients with citrate vs. acetate dialysates. Front. Cardiovasc. Med. 12:1497353. doi: 10.3389/fcvm.2025.1497353
Received
20 November 2024
Accepted
18 March 2025
Published
10 April 2025
Corrected
26 November 2025
Volume
12 - 2025
Edited by
Chu-Huang Chen, Texas Heart Institute, United States
Reviewed by
Amani Kallel, Ministry of Public Health, Tunisia
Eşref Araç, Dicle University, Türkiye
Updates
Copyright
© 2025 Rodríguez-Espinosa, Cuadrado-Payán, Morantes, Gomez, Maduell and Broseta.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: José Jesús Broseta jjbroseta@clinic.cat
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.