Abstract
Purpose of Review:
Pulmonary arterial hypertension (PAH) is a progressive clinical syndrome characterized by pulmonary vascular remodeling and elevated pulmonary artery pressure, associated with high morbidity and mortality. While targeted therapies have improved patient prognosis, restoring normal hemodynamics and reversing vascular pathology remain unmet challenges. Heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1), an RNA-binding protein integral to mRNA processing and post-transcriptional regulation, governs critical processes including cell proliferation, apoptosis, angiogenesis, and endothelial homeostasis. However, its role in PAH pathogenesis remains poorly defined. This review synthesizes current evidence on HNRNPA2B1 in PAH, evaluates its potential mechanistic contributions, and discusses therapeutic implications. Given the fact that much of the connections between PAH and HNRNPA2B1 are speculative, rigorous mechanistic studies are imperative to clarify its pathobiological relevance.
Recent Findings:
Emerging preclinical evidence suggests that HNRNPA2B1 silencing attenuates monocrotaline (MCT)-induced pulmonary hypertension (PH) in rat models. Mechanistically, HNRNPA2B1 modulates vascular smooth muscle cell (VSMC) proliferation via cross-talk between multiple signaling cascades and macrophage polarization dynamics, both central to pulmonary vascular remodeling. Nevertheless, clinical translatability remains uncertain, as no studies have yet conclusively validated HNRNPA2B1 as a druggable target in human PAH.
Summary:
Recent evidence suggests HNRNPA2B1 has emerged as a potential therapeutic target for PAH. However, further studies are essential to elucidate its role in modulating the pathogenic mechanisms underlying PAH.
1 Introduction
Pulmonary hypertension (PH) is a progressive disorder characterized by elevated pulmonary arterial pressure and vascular remodeling, culminating in right heart failure and premature mortality. Globally, PH imposes a significant economic burden, with an estimated prevalence affecting approximately 1% of the population (1).
PH encompasses five distinct clinical subtypes: group 1 pulmonary arterial hypertension (PAH), group 2 PH associated with left heart disease (PH-LHD), group 3 PH associated with lung diseases and/or hypoxia, group 4 PH associated with pulmonary artery obstruction, and group 5 PH with unclear and/or multifactorial mechanisms. The pathogenesis of PAH is intricately multifaceted, involving a spectrum of mechanisms, such as endothelial cell dysfunction, aberrant smooth muscle cell proliferation, plexiform lesions, inflammation, immune responses, cytokine activity, chronic thrombosis, small vessel occlusion, anti-apoptotic processes, as well as metabolic and hormonal influences (2). Currently, the most commonly studied animal models primarily replicate Group 1 and Group 3 PH. This review primarily focuses on Group 1 PH. Patients with PAH are hemodynamically defined by pre-capillary PH, except in cases of other underlying causes of pre-capillary PH (1). Although targeted therapies have significantly improved the prognosis of PAH patients, it remains a challenge to reduce pulmonary arterial pressure to normal levels and reverse pulmonary vascular remodeling. Targeted therapies for PAH primarily target three pathways: endothelin receptors on pulmonary arterial smooth muscle cells (PASMCs), which promote vasoconstriction and cell proliferation; the nitric oxide-induced activation of soluble guanylate cyclase (sGC); and the prostacyclin metabolic pathway. Therefore, calcium channel blockers are used to treat PAH patients who show a positive response to acute vasoreactivity testing (1). There are also new drugs under clinical investigation that target other pathways and have been reported to improve patient symptoms and hemodynamics (2). With these therapies, 5-year survival improved by more than 60% in 2015 (3). However, these drugs cannot reverse vascular remodeling, although they function by inducing vasodilation to alleviate clinical symptoms. Therefore, it is crucial to explore the pathophysiological mechanisms of PAH in order to provide early interventions and improve prognosis.
Heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) is an RNA-binding protein (RBP) that regulates the mRNA expression of several genes and exhibits diverse biological effects, making it a potential therapeutic target for multiple diseases.
The role of HNRNPA2B1 has primarily been studied in the field of cancer. The mechanisms driving PAH development partially overlap with those of carcinogenesis. Cell proliferation and anti-apoptotic phenotypes are also key features of PAH. HNRNPA2B1 can promote cell proliferation and prevent apoptosis through various pathways, and it can also promote tumor cell proliferation through mechanisms such as epithelial-mesenchymal transition (EMT) and angiogenesis (4, 5). However, its role in PAH was not elucidated until 2022. Silencing of HNRNPA2B1 can mitigate monocrotaline (MCT)-induced PAH in rats (6). Moreover, HNRNPA2B1 is upregulated and localized in the nucleus of patients with idiopathic pulmonary hypertension (IPAH), where it participates in the development of PAH by regulating the cell cycle (6). However, the detailed mechanisms remain under investigation. HNRNPA2B1 has been reported to promote proliferation and prevent apoptosis by activating the STAT3 and Erk1/2 signaling pathways (7). Importantly, these two signaling pathways are also crucial for promoting proliferation and preventing apoptosis in PASMCs (8, 9). Furthermore, HNRNPA2B1 regulates the maturation of mRNA precursors by recognizing the N6-methyladenosine (m6A) post-transcriptional modification (10). It is also associated with the exosomal secretion of miRNAs in endothelial cells (ECs) (11). Currently, miR-424/503 has been identified as an important component of pulmonary vascular EC homeostasis, and miRNAs may serve as potential targets for treating PAH (12–15). HNRNPA2B1 may also contribute to the occurrence of PAH partly through these pathways. Although HNRNPA2B1 expression in pulmonary arterial endothelial cells (PAECs) is not significantly different from that in normal control cells (6), it may still exert its effects in PAH ECs by sorting and exporting specific miRNAs. In conclusion, HNRNPA2B1 shows great potential as a therapeutic target for PAH. We have comprehensively reviewed and presented the potential mechanisms through which HNRNPA2B1 drives the development of PAH.
2 HNRNPA2B1
HNRNPA2B1 is a member of the heterogeneous nuclear ribonucleoprotein (HNRNP) family. Structurally, HNRNPA2B1 consists of two RNA recognition motifs (RRMs) located at its N-terminus, referred to as RRM1 and RRM2, and a C-terminal low-complexity region rich in glycine residues, which includes an RGG box, a core PrLD domain, and an M9 nuclear localization signal (M9-NLS) (16). The RRMs and the RGG box are crucial for RNA binding (16, 17). HNRNPA2B1 can bind to specific m6A-modified regions on mRNA through its RRM domains, thereby mediating the translation and degradation of downstream mRNA (18). Although the RGG box significantly affects binding strength, its impact on specific RNA binding is relatively small (19). HNRNPA2B1 plays a role in mRNA splicing, mRNA modification, promotion of pre-mRNA synthesis, transport of mature mRNA, and maintenance of mRNA stability; therefore, its role spans almost the entire process, from mRNA synthesis to maturation (10, 20, 21). As an m6A reader, HNRNPA2B1 can recognize the m6A-specific motifs RGAC and interact with the microprocessor complex protein DGCR8, regulating selective splicing and processing of target mRNAs (10). HNRNPA2B1 may regulate the splicing and processing of target mRNAs by recognizing m6A, thereby activating or inhibiting downstream mechanisms involved in various pathophysiological processes.
HNRNPA2B1 was initially discovered as a tumor-associated antigen in non-small cell lung cancer (22, 23). Patients with non-small cell lung cancer who are positive for HNRNPA2B1 have a worse prognosis (24). HNRNPA2B1 is involved in various pathological and physiological processes, including inflammation (25), immunity (26), oxidative stress, cell proliferation, apoptosis (27), EMT (28), and metabolism (29). It is implicated in the development of various diseases such as cancers, rheumatic immune system diseases, neurological disorders, and viral infections (30). However, few studies have elucidated the relationship between PAH and HNRNPA2B1. Research suggests that HNRNPA2B1 may play a potential role in the underlying mechanisms of PAH. Pulmonary vascular remodeling is a basic pathological feature in all groups of PH. In PAH, the affected vessels are small arteries, whereas in conditions like pulmonary venous obstructive disease, pulmonary capillary hemangiomatosis, or PH-LHD, the medium-sized veins and capillaries are primarily involved. It is characterized by the accumulation of different vascular cells (PASMCs, PAECs, fibroblasts, myofibroblasts, and pericytes) in the pulmonary artery wall, as well as the disappearance of precapillary arterioles and excessive infiltration of inflammatory cells around the vessels, such as B and T lymphocytes, mast cells, dendritic cells, and macrophages (31). The underlying mechanisms include abnormal proliferation and migration of PASMCs, dysfunction of ECs, infiltration of inflammatory cells, activation of fibroblasts in the vascular adventitia, and accumulation of extracellular matrix, all contributing to structural changes in the vessel wall. As an RNA-binding protein, HNRNPA2B1 is involved in pathological processes such as cell proliferation, migration, angiogenesis, EMT, cell metabolism, and inflammation through mechanisms including the regulation of gene transcription, m6A modification, miRNA sorting, and exosome secretion. HNRNPA2B1 has been implicated in the pathological mechanisms of various cell types, including smooth muscle cells (6, 32, 33), endothelial cells (11, 34), fibroblasts (35), and macrophages (36). Therefore, HNRNPA2B1 may contribute to pulmonary vascular remodeling by modulating the pathological processes in these cells.
3 HNRNPA2B1 as a key factor in PAH
3.1 The role of HNRNPA2B1 in smooth muscle cells
3.1.1 HNRNPA2B1 promotes PASMCs proliferation and phenotypic transformation
Microscopic remodeling of the pulmonary arteries is the hallmark pathological change in the progression of PAH. Excessive proliferation of PASMCs leads to thickening of the medial vascular layer. PASMC abnormalities are believed to be crucial in the early development of PAH. Normal PASMCs are highly differentiated cells with a contractile phenotype. However, in PAH, PASMCs undergo excessive proliferation, migration, and invasion, accompanied by increased extracellular matrix secretion, leading to a synthetic phenotype. This transition is known as phenotypic switching in PASMCs (38). In experimental PAH, including both MCT-induced and hypoxia-induced models, PASMCs undergo phenotype switching. Targeting phenotypic switching can improve preclinical pulmonary hypertension (39–41). Various signaling pathways, transcription factors, cytokines, and inflammatory mediators can trigger phenotypic switching of PASMCs. These details have been comprehensively discussed in previous reviews (42). The phenotypic switching of PASMCs ultimately results in pulmonary vascular thickening, loss of elasticity, distal vessel muscularization, and eventually vascular remodeling, particularly in the early stages of PAH (43). The dedifferentiation and proliferation of PASMCs can be regulated by signaling pathways such as STAT/ERK (7), PI3K/AKT/mTOR (44), AKT/STAT3 (27), ILF3/AKT (45), ERK/MAPK (46), Wnt-β/catenin (47), as well as epigenetic mechanisms like histone modification and DNA methylation, which control the phenotypic transformation of PASMCs (42). The treatment goal of PAH could also be to reverse or inhibit the abnormal proliferation and dedifferentiation of PASMCs.
HNRNPA2B1 plays a role in vascular SMCs, including coronary artery, SMCs and PASMCs (48, 49). HNRNPA2B1 has the potential to regulate the phenotype of SMCs. HNRNPA2B1 can transcriptionally regulate the expression of SMCs genes by directly binding to the promoters of the Smαa and Sm22α genes, its knockdown leads to the downregulation of specific smooth muscle markers and transcription factors, indicating its crucial role in SMC differentiation (50). Besides, HNRNPA2B1 can promote cell proliferation in various pathways, these mechanisms can also promote PASMCs proliferation (see Table 1). HNRNPA2B1 regulates the biological processes of multiple mRNAs and exerts biological effects, while being itself regulated by various factors. All these interactions results in a tight regulation of cellular metabolism. HNRNPA2B1 can modulate the cell cycle by regulating the expression of cell cycle-related proteins such as cyclin-dependent kinases and cyclin-dependent kinase inhibitors, thereby influencing cell metabolism (32). Additionally, HNRNPA2B1 can promote cell proliferation and invasion through mechanisms involving exosome sorting and regulating of various microRNAs (miRNAs) (47). Furthermore, it is involved in regulating transcription factors such as ZEB (36), as well as lipid synthesis (51), oxidative stress, and serine metabolism (52). In conclusion, HNRNPA2B1 may play a role in the phenotypic switching of PASMCs through multiple mechanisms. The mechanisms by which HNRNPA2B1 may affect the proliferation and phenotypic transformation of PASMCs were illustrated in Figure 1 and Table 1.
Table 1
| Disease | Cells/Tissues | Upstream regulation | Role of gene overexpression | Role of gene knockout/knockdown | Mechanisms | Literatures | Literatures related to PH |
|---|---|---|---|---|---|---|---|
| PAH | PASMCs | - | - | ↓Cell proliferation; ↑Apoptosis | ↓Target mRNAs containing CUAGACUAGA, UAA[CG]UUAU, and GCC[GC]AAG[GA][AG][GA]CC motifs | (6) | - |
| Atherosclerosis | VSMCs | Down-regulated by lncRNA AC105942.1 | - | Reversal VSMCs proliferation induced by Ang II | ↓CDK4; ↑P27 | (32) | (53) |
| Breast cancer | Breast cancer cell | - | - | ↓Cell proliferation; ↑Apoptosis; ↑ S phase of the cell cycle | ↓STAT3/ERK1/2 | (7) | (54, 55) |
| Ovarian cancer | Ovarian cancer cell | - | - | ↓NUF2; ↓Cell proliferation; ↑Apoptosis | ↓PI3K/AKT/mTOR | (44) | (56) |
| Glioma | Glioma cell | - | - | ↓Cell proliferation; ↑Apoptosis | ↓AKT/STAT3 | (27) | (55, 57) |
| Multiple myeloma | Multiple myeloma cells | - | ↑Cell proliferation;↑ILF3/AKT | ↓Cell proliferation; ↑Apoptosis; ↓ILF3/AKT | ↑↓ILF3/AKT | (45) | (57, 58) |
| Colorectal cancer | Colorectal cancer cells | Interaction with circMYH9 | - | ↓Cell proliferation | ↓P53; ↓Serine metabolism and ROS | (52) | (59) |
| Colorectal cancer | Colorectal cancer cells | - | - | ↓Cell proliferation; ↑Apoptosis; ↑Cell cycle arrest | ↓ERK/MAPK | (46) | (63) |
| Hepatocellular carcinoma | Hepatocellular carcinoma cells | Decreased by knockdown of PCAT6 mediated by miR-326 | - | ↓Cell proliferation and invasion | - | (60) | (64) |
| Adenocarcinoma of lung | Adenocarcinoma of lung cells | Up-regulated by INC00963 | - | ↓Cell proliferation, invasion and EMT | ↓ZEB1 | (36) | (65) |
| Nasopharynx cancer | Nasopharynx cancer cells | Decreased by knockdown of SOX2-OT mediated by miR-146b-5 | ↓Cell proliferation, ↑Cell proliferation | - | - | (61) | (66) |
| Colorectal cancer | Colorectal cancer cells | Stabilized by CRNDE mediated by inhibiting ubiquitination through TRIM21 | ↑Cell proliferation and migration; ↑KRAS/MAPK | ↓Cell proliferation and migration; ↓KRAS/MAPK | ↑↓KRAS/MAPK | (62) | (63) |
| Adenocarcinoma of the lung | Lung adenocarcinoma cells; embryonic kidney cells | - | - | ↓Cell stemness, proliferation, migration and tumor growth | ↓miR-106b-5p; ↑SFRP2; ↓Wnt-β/catenin | (47) | (72) |
| Non-small cell lung cancer | Non-small cell lung cancer cells | - | - | ↓Cell proliferation and migration; ↓ MEG3 m6a; ↑MEG3 mRNA | ↓miR-21-5p; ↑PTEN; ↓PI3K/AKT | (67) | (73) |
| Pancreatic cancer | Pancreatic cancer cells | Decreased by knockdown of Fyn | ↓Bcl-x(s); ↓apoptosis | ↑Bcl-x(s) and Bcl-x(s)/Bcl-x(L); ↑apoptosis | ↓↑Bcl-x(s) | (68) | (74) |
| Esophagus cancer | Esophagus cancer cells | - | - | ↓Adipogenesis; ↓Cell proliferation, migration and invasion | ↓ACLY and ACC1 | (51) | (75) |
| Esophagus cancer | Esophagus cancer cells | - | - | ↓Cell proliferation | ↓miR-17, miR-18a, miR-20a, miR-93 and miR-106b | (69) | (76–78) |
| Esophagus cancer | Esophagus cancer cells | Interaction with p53-G245S | - | ↑Secretion of exosomes; ↑Cell proliferation | ↓AGAP1 | (70) | (59) |
| - | skeletal muscle cells | Up-regulated by miR-206 and lncRNA-lncA2B1 | - | ↓Cell proliferation | ↓miR-206 and MyHC | (71) | (79) |
| Non-small cell lung cancer | Non-small cell lung cancer cells | - | - | ↓Cell proliferation; ↑Apoptosis | ↓ERK/p53/HDM2 and CKD2; ↑P21 and P27 | (80) | (59, 63) |
| ovarian cancer | ovarian cancer cells | Decreased by knockdown of miR-30c-5p | - | ↓Cell proliferation, migration and invasion | ↓CDK19 | (81) | (81) |
| Non-small cell lung cancer | Non-small cell lung cancer cells | - | - | ↓Cell proliferation; ↑Apoptosis | c-Myc–LINC01234–HNRNPA2B1–miR-106b-5p–CRY2–c-Myc | (82) | (83) |
Mechanisms by which HNRNPA2B1 may regulate PASMC metabolism.
Figure 1
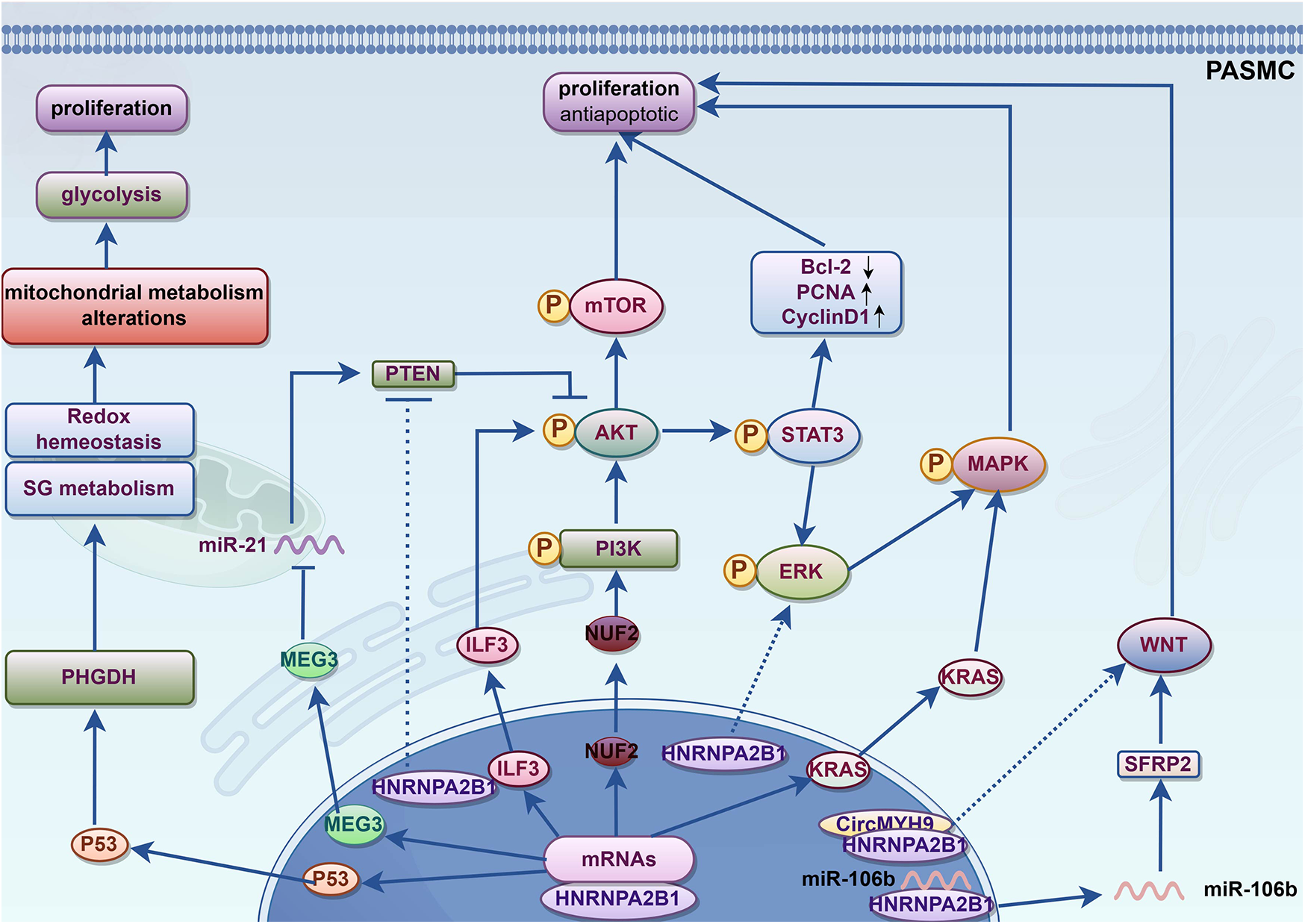
Potential mechanism of HNRNPA2B1 promotes phenotypic switching of PASMCs. KRAS, kirsten rat sarcoma viral oncogene homolog; MAPK, mitogen-activated protein kinase; ERK, extracellular regulated protein kinases; STAT3, signal transducer and activator of transcription; NUF2, Ndc80 kinetochore complex component; PI3K, Phosphoinositide 3-kinase; AKT, also known as protein kinase B or PKB; mTOR, Mammalian target of rapamycin; CyclinD1, G1/S specific cyclin D1; PCNA, Proliferating cell nuclear antigen; Bcl-2, B-cell lymphoma-2; ILF3, Interleukin enhancer binding factor 3; PTEN, Phosphatase and tensin homolog; MEG3, Maternally Expressed 3; PHGDH, Phosphoglycerate dehydrogenase; SG metabolism, serine/glycine metabolism; SFRP2, secreted frizzled related protein 2; WNT, Wingless/Integrated.
3.1.2 HNRNPA2B1 regulates cell metabolic reprogramming
Metabolic reprogramming refers to the alteration of cellular metabolism in response to various stress conditions. Under normal circumstances, the main energy source for cells is oxidative phosphorylation through the tricarboxylic acid cycle (TCA cycle). However, in PAH, PASMCs shift their energy production toward glycolysis, a phenomenon known as the “Warburg effect”. During the Warburg effect, cells exhibit increased cytoplasmic glycolysis and glutaminolysis, while mitochondrial biogenesis and fatty acid oxidation are inhibited. Consequently, PASMCs in PAH primarily rely on glycolysis for energy production to support their growth. The promotion of glycolytic metabolism through metabolic reprogramming enhances PASMC survival and proliferation.
Abnormal PASMC metabolism may serve as a potential therapeutic target for PAH, with preliminary success observed in clinical trials. TEPP-46, an activator of pyruvate kinase M2 (PKM2), has been shown to normalize glycolysis and mitochondrial abnormalities in fibroblasts from PAH patients (84). Notably, PKM2 inhibitors reduce pulmonary artery pressure in Group 2 PH and reverse pulmonary vascular remodeling (85). Furthermore, PKM2 is highly expressed in PASMCs in Group 1 and Group 4 PH and accelerates their abnormal proliferation (86, 87). Interestingly, as a member of the HNRNP family, HNRNPA1 inhibition can downregulate PKM2 expression (87). Additionally, HNRNPA2B1 regulates PKM2 splicing, upregulates PKM2 expression, and subsequently modulates cellular metabolic reprogramming (29, 88). In an acute myocardial infarction model, shikonin inhibited HNRNPA2B1 activity and decreased PKM2 expression, thereby improving post-infarction inflammation, apoptosis, and fibrosis (89). These studies suggest that HNRNPA2B1, as an RBP, may regulate PASMC metabolic reprogramming by modulating PKM2 mRNA expression.
3.2 HNRNPA2B1 regulates EC function through exosome sorting
HNRNPA2B1 promotes cell proliferation and inhibits apoptosis. While tumor cells have been the main focus of studies on HNRNPA2B1, fewer studies have focused on its effects on SMCs, and even less evidence exists regarding its role in ECs. No significant difference in HNRNPA2B1 expression has been observed in PAECs from PAH mouse models (6), however, it is speculated to regulate miRNA sorting into exosomes, thereby modulating EC function through intercellular signaling. Notably, exosome therapy has shown promising results in experimental PAH (90). SMCs can promote the release of specific miRNA-loaded exosomes under hypoxia or TGFβ1 stimulation, which regulate EC metabolism through intercellular crosstalk (83, 91). Macrophage immune regulation plays an important role in PAH, and exosome therapy using mesenchymal stem cells has been shown to be effective in PAH animal models (90). miR-503 plays a critical role in PAEC proliferation and metabolism, not only inhibiting their proliferation but also exerting paracrine effects on PASMCs to suppress their proliferation and migration (12). In human umbilical vein endothelial cells (HUVECs), HNRNPA2B1 negatively regulates miR-503 exosomal sorting by inhibiting its secretion. However, in the presence of beraprost sodium, HNRNPA2B1 translocates to the nucleus and promotes miR-503 exosomal secretion, thereby exerting anti-tumor effects (11). In PAH, miR-503 overexpression inhibits ERK1/2 phosphorylation via targeting FGF2 and FGFR1, subsequently suppressing cell proliferation (12). Platelet-derived growth factor (PDGF) induces miR-185 expression in SMCs, and HNRNPA2B1 binds to the “GGAG” exosomal motif within miR-185. Through exosome transfer to ECs, this complex targets CXC motif chemokine ligand 12 (CXCL12), inducing angiogenesis. Importantly, HNRNPA2B1 inhibition significantly reduces neovascularization (49). These findings suggest that exosomes carrying functional miRNAs mediate crosstalk between ECs and SMCs.
HNRNPA2B1 can also promote the exosomal sorting of miR-122-5p (92, 93), miR-934, and lncRNA H19 (94). miR-122-5p is upregulated in hypoxia-induced pulmonary microvascular endothelial cells (PMECs), as well as in the lung tissues of SuHx rats, MCT rats, and IPAH patients. It may contribute to the pathogenesis of IPAH through the regulation of dihydrolipoamide S-acetyltransferase (DLAT) and regulating synaptic membrane exocytosis 1 (RIMS1), although the exact mechanisms require further investigation (95). Additionally, miR-122-5p regulates fatty acid utilization through 1-acylglycerol-3-phosphate O-acyltransferase 1 (AGPAT1) and promotes vascular development in ECs (96). miR-934 is transferred from tumor cells to macrophages via exosomal sorting, where it promotes macrophage polarization through the PTEN/PI3K/AKT signaling pathway. LncRNA H19 promotes the proliferation and migration of colorectal cancer cells through the Raf/ERK signaling pathway (97). Notably, H19 expression is significantly elevated in the pulmonary artery endothelium, and its deficiency improves pulmonary vascular remodeling. These effects are linked to the inhibition of EndMT via the TGF-β signaling pathway (98). Previous studies have established the role of these signaling pathways in EndMT.
In summary, exosomes released by SMCs, mesenchymal stem cells, and other cell types under pathological conditions can act on ECs through intercellular communication, with HNRNPA2B1 functioning as a key regulator of miRNA-loaded exosome sorting. Therefore, HNRNPA2B1 may regulate EC function by modulating the secretion of specific miRNAs via exosomal sorting mechanisms. The mechanisms through which HNRNPA2B1 regulates endothelial cell function via exosome-mediated signaling were illustrated in Figure 2.
Figure 2
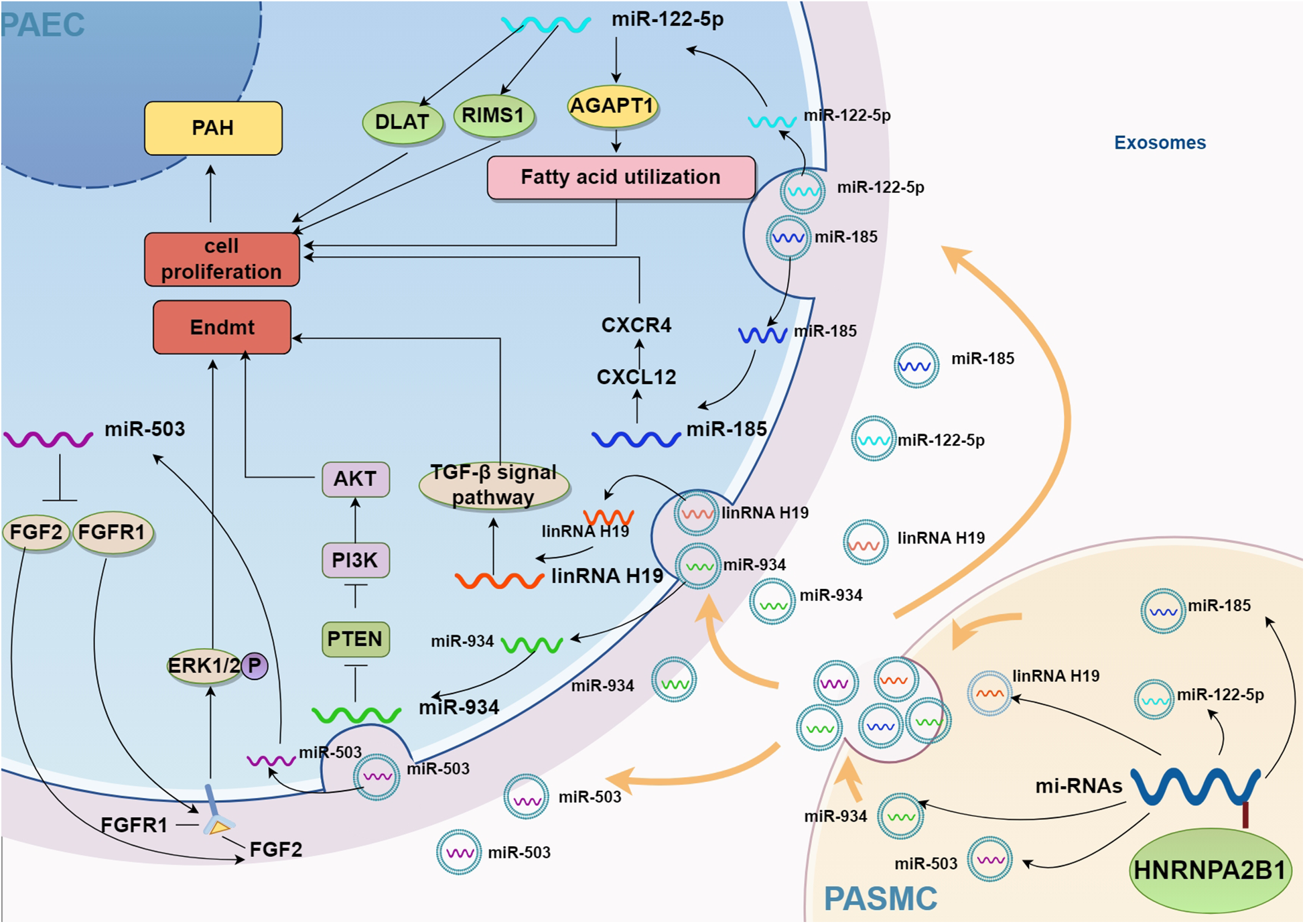
Potential mechanism of HNRNPA2B1 regulating exosome secretion. AGPAT1, 1-acylglycerol-3-phosphate O-acyltransferase 1; CXCL12, CXC motif ligand 12; CXCR4, CXC motif chemokine receptor 4; PTEN, phosphatase and tensin homolog; AKT, also known as protein kinase B or PKB; PI3K, phosphoinositide 3-kinase; ERK1/2, Extracellular regulated protein kinases 1/2; DLAT, dihydrolipoamide S-Acetyltransferase; RIMS1, regulating synaptic membrane exocytosis 1; PAH, pulmonary artery hypertension; Endmt, endothelial to mesenchymal transition; FGF2, fibroblast growth factor 2; FGFR1, fibroblast growth factor receptor 1; PAEC, pulmonary artery endothelial cell; PASMC, pulmonary arterial smooth muscle cell.
Inflammation plays a crucial role in the onset and progression of cardiovascular diseases (99, 100), including PAH (101). In lung biopsies of PAH patients, multiple inflammatory cell types—macrophages, mast cells, T lymphocytes, B lymphocytes, dendritic cells, and neutrophils—have been detected around the remodeled pulmonary vascular system (37, 102). Macrophages are pivotal in the inflammatory processes underlying pulmonary hypertension. Consequently, targeting inflammation and immunity has become a major therapeutic strategy for PAH management. However, the exact role and mechanism of anti-inflammatory therapy in PAH remain unclear. Reports indicate that during inflammation and hypoxia, circulating monocytes are recruited to the lungs, replace resident stromal macrophages, and contribute to pulmonary vascular remodeling (103). These recruited cells differentiate in response to microenvironmental changes during lung injury or hypoxic exposure. Early in hypoxia, macrophages accumulate around pulmonary vasculature, exhibit a hypoxic response, and release pro-inflammatory cytokines. Subsequently, perivascular macrophage accumulation decreases, and the cells adopt a tissue-repair and anti-inflammatory phenotype (104). Polarized macrophages drive PAEC dysfunction, PASMC proliferation, and activation of pro-inflammatory fibroblast phenotypes by coordinating pro- and anti-inflammatory mediators (105). Studies demonstrate that HNRNPA2B1 promotes macrophage polarization through multiple mechanisms. Under IL-4/IL-13 stimulation, lincRNA-MIR99AHG is upregulated in macrophages, translocates to the nucleus, and binds to HNRNPA2B1, thereby promoting polarization (106). In models of intestinal inflammation and obesity, HNRNPA2B1 exacerbates inflammation by enhancing mRNA stability of pro-inflammatory genes (e.g., TNF-α, IL-6, IL-1β), positioning it as a therapeutic target (25). Beyond direct macrophage regulation, HNRNPA2B1 modulates macrophage function by enhancing exosome secretion from other cell types. For example, in breast cancer, HNRNPA2B1 mediates exosomal sorting of miR-184-3p; upon macrophage uptake, this miRNA targets EGR1 to inhibit JNK signaling and induce polarization (107). In glioma, HNRNPA2B1 packages circNEIL3 into exosomes, which are delivered to tumor-associated macrophages to promote progression (108). Furthermore, neddylation-mediated degradation of HNRNPA2B1 downregulates mitochondrial trifunctional enzyme subunit α (MTPα), inhibiting NF-κB activation and inflammatory pathways (109).
In summary, the role of HNRNPA2B1 in inflammation remains unclear; however, as an RNA-binding protein (RBP), it regulates the expression of multiple RNAs and participates in exosome-mediated sorting processes. Given that inflammation is a complex process, a delicate balance between pro- and anti-inflammatory mechanisms exists under normal physiological conditions. However, when this balance is disrupted, pathological changes in the pulmonary vasculature occur. Therefore, the mechanisms of HNRNPA2B1 in inflammation may involve its regulation of both pro- and anti-inflammatory signaling pathways. In conclusion, HNRNPA2B1 may regulate the initiation and progression of pulmonary hypertension through inflammatory signaling pathways. Figure 3 illustrates how HNRNPA2B1 contributes to pulmonary hypertension via macrophage-mediated regulation of inflammation.
Figure 3
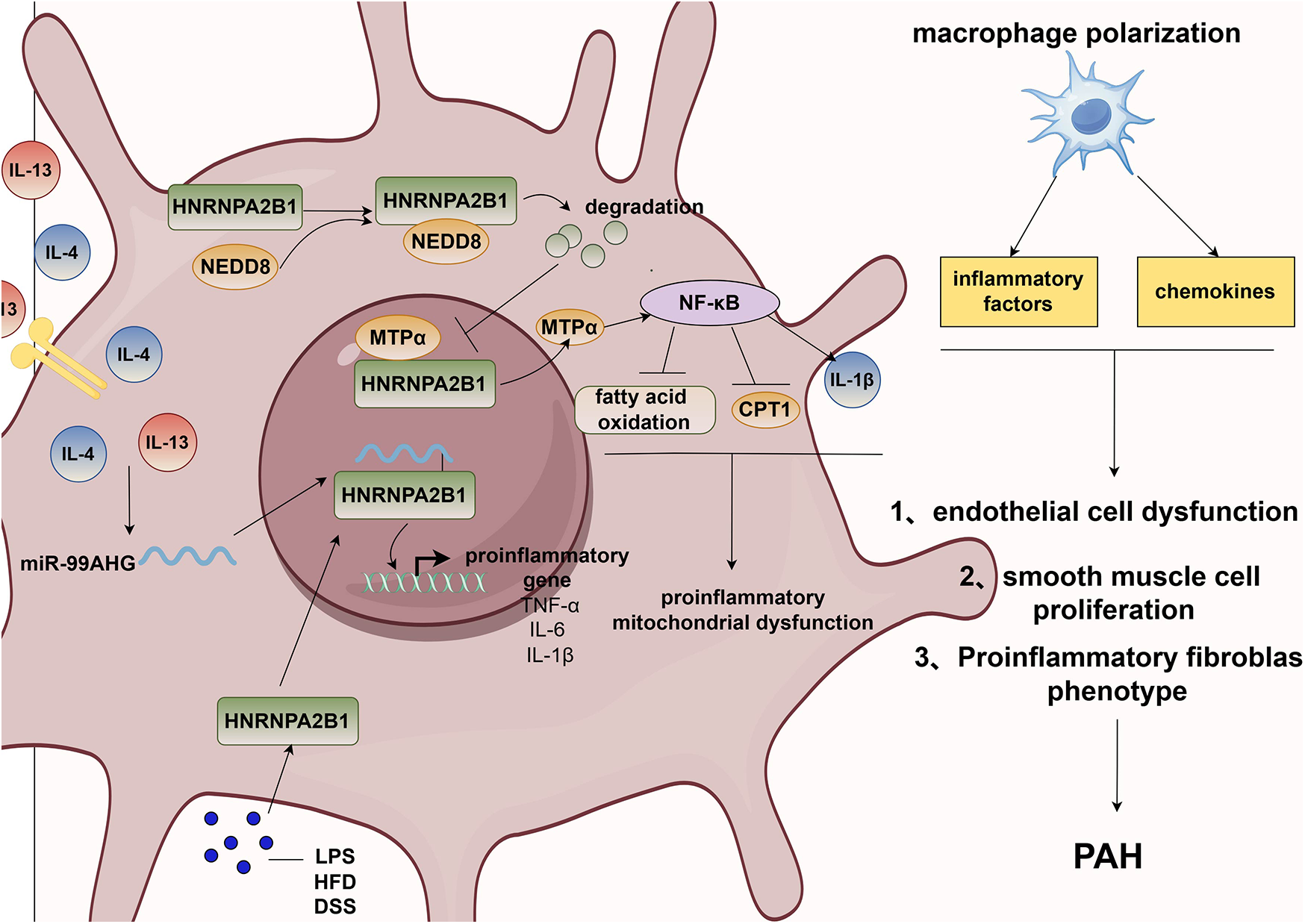
HNRNPA2B1 promotes pulmonary hypertension via macrophage polarization. PAH, pulmonary artery hypertension; IL-13, interleukin 13; IL-4, interleukin 4; NEDD8, neural precursor cell expressed developmentally down- regulated 8; MTPα, mitochondrial trifunctional protein α; CPT1, carnitine palmitoyltransferase 1A; NF-κB, nuclear factor kappa B subunit; TNF-α, tumor necrosis factor-α; IL-6, interleukin 6; IL-1β, interleukin 1β; LPS, lipopolysaccharides; HFD, high-fat-diet; DSS, dextran sodium sulfate.
3.3 Potential role of HNRNPA2B1 in orther types of cells
In addition to the aforementioned cell types, fibroblasts and platelets also play roles in the onset and progression of PAH. Upon activation, fibroblasts exhibit excessive proliferation and reduced apoptosis, accompanied by upregulated inflammatory cascades and metabolic reprogramming. These processes may drive the migration of myofibroblasts to the tunica media or intima, contributing to vascular wall thickening (110). Fibroblasts further disrupt the balance between extracellular matrix (ECM) protein production and degradation, thereby promoting ECM remodeling. Activated platelets, on the other hand, form white thrombi at sites of vascular intimal injury, exacerbating thrombosis and PAH progression (111). Additionally, they secrete vasoconstrictors, growth factors, and inflammatory mediators to further promote PAH development (112). However, the relationship between HNRNPA2B1 and fibroblasts or platelets remains poorly understood. Bioinformatics analyses have shown no significant difference in HNRNPA2B1 expression between PAH tissues and controls (113). Thus, further studies are required to investigate whether HNRNPA2B1 contributes to PAH pathogenesis by activating fibroblasts and platelets.
3.4 Therapeutic prospects of targeting HNRNPA2B1 in PAH
HNRNPA2B1 can serve as a potential therapeutic target for various diseases due to its biological functions. Several compounds that target HNRNPA2B1 exert therapeutic effects through pathways implicated in the development of PH. Table 2 shows the therapeutic prospect of Targeting HNRNPA2B1 in diseases. The broad-spectrum antiviral drug PAC5, for example, binds to a pocket near the Asp49 RNA recognition motif (RRM1) of HNRNPA2B1, inducing its translocation into the cytoplasm and activating the TBK1-IRF3 pathway to exert antiviral effects (114). In the tumor microenvironment, the natural compound Sanggenol-O and its synthetic derivative MO-460 inhibit hypoxia-inducible factor 1 alpha (HIF-1α) expression via HNRNPA2B1, thereby triggering apoptosis (115), highlighting their potential as anticancer agents.
Table 2
| Medicine | siRNA | Target | Mechanisms | Diseases | Literatures | Literature related to PAH |
|---|---|---|---|---|---|---|
| PAC5 | HNRNPA2B1 | Transfer it into the cytoplasm, ↑TBK1-IRF3 | HBV, COVID-19 | (114) | - | |
| Sanggenol-O,MO-460 | - | HNRNPA2B1 | ↓HIF-1α, ↑apoptosis. | Cancer | (115) | (122) |
| Tripterygium wilfordii | - | HNRNPA2B1 | ↓PI3K-AKT, ↓cell proliferation | Cancer | (118) | (119) |
| Apigenin | - | HNRNPA2B1 | ↓proliferation of PASMCs, ↑apoptosis | PAH | (121) | (120) |
| - | HNRNPA2B1 | PTEN\AKT\mTOR | ↓proliferation, ↑apoptosis | CAD | (48) | - |
| - | HNRNPA2B1 | - | ↓cell proliferation, ↑apoptosis | PAH | (6) | - |
Therapeutic prospects of targeting HNRNPA2B1 in diseases.
Animal experiments demonstrate that HNRNPA2B1 interference reverses pulmonary hypertension in MCT-induced rat models (6). For instance, overexpression of the anti-aging enzyme SIRT6 inhibits hypoxia-induced proliferation of human pulmonary artery smooth muscle cells (HPASMCs) by activating the HIF-1α/PDK4 signaling pathway (116). SIRT6 also forms complexes with HNRNPA2B1, DGCR8, and Drosha to regulate macrophage pyroptosis and suppress inflammation under high glucose conditions (117). Additionally, Tripterygium wilfordii has emerged as a potential therapeutic agent for PAH (118). Studies indicate that it inhibits tumor cell proliferation by destabilizing HNRNPA2B1 mRNA and suppressing the PI3K-AKT pathway (119). The natural flavonoid apigenin, which inhibits PASMC proliferation and induces apoptosis, shows promise as a preventive or therapeutic option for PAH (120). Apigenin binds to the C-terminal of HNRNPA2B1, preventing its dimerization and potentially modulating its role in apoptosis (121). These findings collectively suggest HNRNPA2B1 as a promising therapeutic target for PAH, though further experimental validation is required.
4 Summary and prospect
This review article highlights the potential mechanisms of HNRNPA2B1 in the pathogenesis of PAH. Figure 4 illustrates how HNRNPA2B1 may influence pulmonary hypertension by regulating PASMCs, PAECs, and macrophages. To date, only one study has demonstrated the role of HNRNPA2B1 in MCT-induced PH, though the specific mechanisms remain unexplored. Numerous studies suggest that HNRNPA2B1 is involved in cell proliferation, apoptosis, and PASMC metabolism (Figure 1); however, its exact mechanism of action in PASMCs is still unclear. The pathophysiology of PAH is complex, and the MCT-induced PH model fails to fully replicate the clinical features observed in PAH patients. Whether the proposed mechanisms can be generalized to other experimental models remains uncertain. Currently, no drugs targeting HNRNPA2B1 for PAH treatment exist. While gene knockdown models show promise in animals, their translational relevance to humans has yet to be established, underscoring the need for further research.
Figure 4
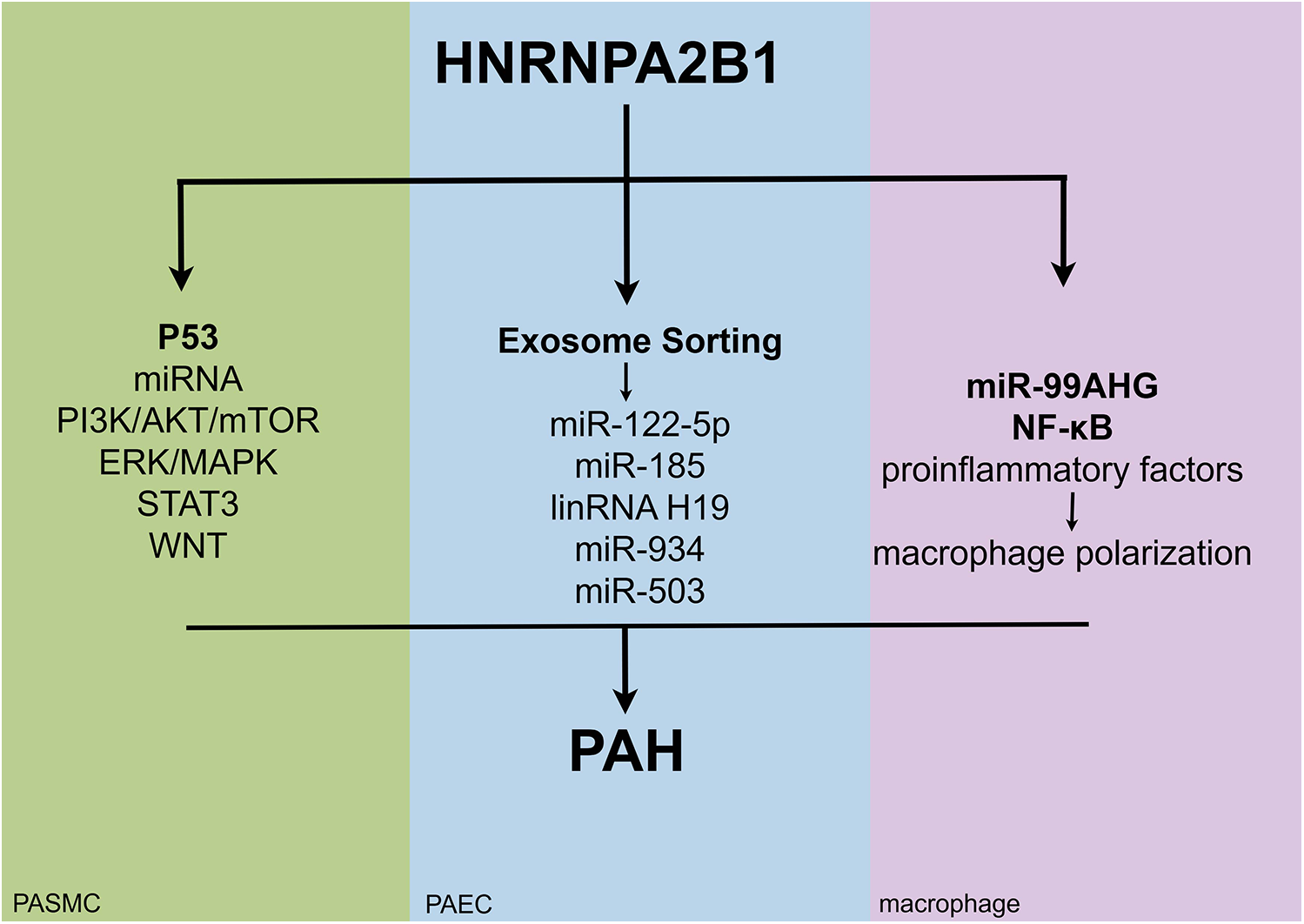
The potential mechanisms of action of HNRNPA2B1 in different cell types of pulmonary hypertension.
Additionally, the regulatory role of HNRNPA2B1 in EC function is poorly characterized. Although studies report no significant difference in HNRNPA2B1 expression in PAECs from PAH patients, it may still modulate exosome-mediated miRNA sorting mechanisms in ECs (Figure 2). Research on HNRNPA2B1-PAEC interactions in PAH remains in its early stages.
Structurally, the A2 and B1 isoforms of HNRNPA2B1 differ by only a 12-amino acid sequence, yet they exhibit distinct RNA-binding preferences. The ratio of these isoforms may vary across tissues and pathological stages, necessitating deeper functional characterization of this molecule (Figure 3).
Finally, HNRNPA2B1 regulates the Warburg effect and macrophage polarization—processes implicated in PAH progression. Nevertheless, the discussion of the relationship between HNRNPA2B1 and PAH is speculative, and there is currently no direct evidence to establish a connection between them. Therefore, further investigations are required to delineate its role in PAH mechanisms.
In summary, HNRNPA2B1 acts as an RBP through m6A-dependent mechanisms and regulates cell proliferation, apoptosis, metabolism, the immune microenvironment, and angiogenesis, all of which are important for the development of PAH. It is involved in the entire process, from mRNA generation to maturation, as well as in miRNA exosome sorting. However, the specific mechanisms need further confirmation. Targeted therapy involving HNRNPA2B1 is promising, but still in its early stages. Efforts to design and optimize treatment strategies targeting HNRNPA2B1 should continue.
Statements
Author contributions
YW: Writing – original draft, Writing – review & editing. DW: Writing – review & editing. ND: Writing – original draft. FX: Visualization, Writing – original draft. SL: Visualization, Writing – review & editing. XF: Visualization, Writing – original draft. HG: Visualization, Writing – original draft. JC: Writing – review & editing. WL: Supervision, Validation, Writing – review & editing. XS: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [grant numbers: 82260058 and 82160086]; the Guizhou Science and Technology(Qian Science and Technology Cooration Support) [grant numbers: (2020)4Y231 and (2020)1Y294] and Foundation ZK[2024] Key Project 040; and the Science and Technology Fund from Guizhou Provincial Health Commission [grant numbers: gzwkj2023-130 and gzwkj2023-304].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Humbert M Kovacs G Hoeper MM Badagliacca R Berger RMF Brida M et al 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. G Ital Cardiol (Rome). (2023) 24:e1–e116. 10.1714/4014.39906
2.
Prins KW Thenappan T Weir EK Kalra R Pritzker M Archer SL . Repurposing medications for treatment of pulmonary arterial hypertension: what’s old is new again. J Am Heart Assoc. (2019) 8:e011343. 10.1161/JAHA.118.011343
3.
Ruopp NF Cockrill BA . Diagnosis and treatment of pulmonary arterial hypertension: a review. JAMA. (2022) 327:1379–91. 10.1001/jama.2022.4402
4.
Gao LB Zhu XL Shi JX Yang L Xu ZQ Shi SL . HnRNPA2B1 promotes the proliferation of breast cancer MCF-7 cells via the STAT3 pathway. J Cell Biochem. (2021) 122:472–84. 10.1002/jcb.29875
5.
Wu Y Li A Chen C Fang Z Chen L Zheng X . Biological function and research progress of N(6)-methyladenosine binding protein heterogeneous nuclear ribonucleoprotein A2B1 in human cancers. Front Oncol. (2023) 13:1229168. 10.3389/fonc.2023.1229168
6.
Ruffenach G Medzikovic L Aryan L Li M Eghbali M . HNRNPA2B1: RNA-binding protein that orchestrates smooth muscle cell phenotype in pulmonary arterial hypertension. Circulation. (2022) 146:1243–58. 10.1161/CIRCULATIONAHA.122.059591
7.
Hu Y Sun Z Deng J Hu B Yan W Wei H et al Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumour Biol. (2017) 39:1010428317694318. 10.1177/1010428317694318
8.
Jiang J Wang S Wang Z Ma J Liu S Li W et al The role of ERK1/2 in 15-HETE-inhibited apoptosis in pulmonary arterial smooth muscle cells. J Recept Signal Transduct Res. (2011) 31:45–52. 10.3109/10799893.2010.512013
9.
Paulin R Meloche J Jacob MH Bisserier M Courboulin A Bonnet S . Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. (2011) 301:H1798–809. 10.1152/ajpheart.00654.2011
10.
Alarcon CR Goodarzi H Lee H Liu X Tavazoie S Tavazoie SF . HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. (2015) 162:1299–308. 10.1016/j.cell.2015.08.011
11.
Perez-Boza J Boeckx A Lion M Dequiedt F Struman I . hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cell Mol Life Sci. (2020) 77:4413–28. 10.1007/s00018-019-03425-6
12.
Kim J Kang Y Kojima Y Lighthouse JK Hu X Aldred MA et al An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. (2013) 19:74–82. 10.1038/nm.3040
13.
Courboulin A Ranchoux B Cohen-Kaminsky S Perros F Bonnet S . MicroRNA networks in pulmonary arterial hypertension: share mechanisms with cancer?Curr Opin Oncol. (2016) 28:72–82. 10.1097/CCO.0000000000000253
14.
Chun HJ Bonnet S Chan SY . Translational advances in the field of pulmonary hypertension. Translating MicroRNA biology in pulmonary hypertension. It will take more than “miR” words. Am J Respir Crit Care Med. (2017) 195:167–78. 10.1164/rccm.201604-0886PP
15.
Wolowiec L Medlewska M Osiak J Wolowiec A Grzesk E Jasniak A et al MicroRNA and lncRNA as the future of pulmonary arterial hypertension treatment. Int J Mol Sci. (2023) 24:9735. 10.3390/ijms24119735
16.
Wu B Su S Patil DP Liu H Gan J Jaffrey SR et al Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun. (2018) 9:420. 10.1038/s41467-017-02770-z
17.
Dreyfuss G Matunis MJ Pinol-Roma S Burd CG . hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. (1993) 62:289–321. 10.1146/annurev.bi.62.070193.001445
18.
Liu Y Shi SL . The roles of hnRNP A2/B1 in RNA biology and disease. Wiley Interdiscip Rev RNA. (2021) 12:e1612. 10.1002/wrna.1612
19.
Kiledjian M Dreyfuss G . Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. (1992) 11:2655–64. 10.1002/j.1460-2075.1992.tb05331.x
20.
Villarroya-Beltri C Gutierrez-Vazquez C Sanchez-Cabo F Perez-Hernandez D Vazquez J Martin-Cofreces N et al Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. (2013) 4:2980. 10.1038/ncomms3980
21.
Singh R Gupta SC Peng WX Zhou N Pochampally R Atfi A et al Regulation of alternative splicing of Bcl-x by BC200 contributes to breast cancer pathogenesis. Cell Death Dis. (2016) 7:e2262. 10.1038/cddis.2016.168
22.
Mulshine JL Cuttitta F Bibro M Fedorko J Fargion S Little C et al Monoclonal antibodies that distinguish non-small cell from small cell lung cancer. J Immunol. (1983) 131:497–502. 10.4049/jimmunol.131.1.497
23.
Fielding P Turnbull L Prime W Walshaw M Field JK . Heterogeneous nuclear ribonucleoprotein A2/B1 up-regulation in bronchial lavage specimens: a clinical marker of early lung cancer detection. Clin Cancer Res. (1999) 5:4048–52.
24.
Qu XH Liu JL Zhong XW Li XI Zhang QG . Insights into the roles of hnRNP A2/B1 and AXL in non-small cell lung cancer. Oncol Lett. (2015) 10:1677–85. 10.3892/ol.2015.3457
25.
Meng M Cao Y Zhang Y Liu S Zhong Y Wang D et al HnRNPA2B1 aggravates inflammation by promoting M1 macrophage polarization. Nutrients. (2023) 15:1555. 10.3390/nu15071555
26.
Wang L Wen M Cao X . Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. (2019) 365:eaav0758. 10.1126/science.aav0758
27.
Yin D Kong C Chen M . Effect of hnRNPA2/B1 on the proliferation and apoptosis of glioma U251 cells via the regulation of AKT and STAT3 pathways. Biosci Rep. (2020) 40:BSR20190318. 10.1042/BSR20190318
28.
Dai S Zhang J Huang S Lou B Fang B Ye T et al HNRNPA2B1 Regulates the epithelial-mesenchymal transition in pancreatic cancer cells through the ERK/snail signalling pathway. Cancer Cell Int. (2017) 17:12. 10.1186/s12935-016-0368-4
29.
Liu Y Yang H Li L Chen S Zuo F Chen L . A novel VHLalpha isoform inhibits Warburg effect via modulation of PKM splicing. Tumour Biol. (2016) 37:13649–57. 10.1007/s13277-016-5191-y
30.
Fabbiano F Corsi J Gurrieri E Trevisan C Notarangelo M D'Agostino VG . RNA Packaging into extracellular vesicles: an orchestra of RNA-binding proteins?J Extracell Vesicles. (2020) 10:e12043. 10.1002/jev2.12043
31.
Humbert M Guignabert C Bonnet S Dorfmuller P Klinger JR Nicolls MR et al Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. (2019) 53:1801887. 10.1183/13993003.01887-2018
32.
Zhang RY Wu CM Hu XM Lin XM Hua YN Chen JJ et al LncRNA AC105942.1 downregulates hnRNPA2/B1 to attenuate vascular smooth muscle cells proliferation. DNA Cell Biol. (2021) 40:652–61. 10.1089/dna.2020.6451
33.
Wei R Chen L Li P Lin C Zeng Q . IL-13 alleviates idiopathic pulmonary hypertension by inhibiting the proliferation of pulmonary artery smooth muscle cells and regulating macrophage infiltration. Am J Transl Res. (2022) 14:4573–90.
34.
Chen Y Tang D Zhu L Yuan T Jiao Y Yan H et al hnRNPA2/B1 ameliorates LPS-induced endothelial injury through NF-kappaB pathway and VE-cadherin/beta-catenin signaling modulation in vitro. Mediators Inflamm. (2020) 2020:6458791. 10.1155/2020/6458791
35.
Hu HF Han L Fu JY He X Tan JF Chen QP et al LINC00982-encoded Protein PRDM16-DT regulates CHEK2 splicing to suppress colorectal cancer metastasis and chemoresistance. Theranostics. (2024) 14:3317–38. 10.7150/thno.95485
36.
Hu R Xu B Ma J Li L Zhang L Wang L et al LINC00963 Promotes the malignancy and metastasis of lung adenocarcinoma by stabilizing Zeb1 and exosomes-induced M2 macrophage polarization. Mol Med. (2023) 29:1. 10.1186/s10020-022-00598-y
37.
Stacher E. Graham B.B. Hunt J.M. Gandjeva A. Groshong S.D. McLaughlin V.V. Jessup M. Grizzle W.E. Aldred M.A. Cool C.D. , and TuderR.M., Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med186 (2012) 261–72.10.1164/rccm.201201-0164OC
38.
Chakraborty R Chatterjee P Dave JM Ostriker AC Greif DM Rzucidlo EM et al Targeting smooth muscle cell phenotypic switching in vascular disease. JVS Vasc Sci. (2021) 2:79–94. 10.1016/j.jvssci.2021.04.001
39.
Mao J Ma L . Research progress on the mechanism of phenotypic transformation of pulmonary artery smooth muscle cells induced by hypoxia. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2022) 51:750–7. 10.3724/zdxbyxb-2022-0282
40.
Li J Meng ZY Wen H Lu CH Qin Y Xie YM et al beta-sitosterol alleviates pulmonary arterial hypertension by altering smooth muscle cell phenotype and DNA damage/cGAS/STING signaling. Phytomedicine. (2024) 135:156030. 10.1016/j.phymed.2024.156030
41.
Fan Y Gu X Zhang J Sinn K Klepetko W Wu N et al TWIST1 drives smooth muscle cell proliferation in pulmonary hypertension via loss of GATA-6 and BMPR2. Am J Respir Crit Care Med. (2020) 202:1283–96. 10.1164/rccm.201909-1884OC
42.
Ma B Cao Y Qin J Chen Z Hu G Li Q . Pulmonary artery smooth muscle cell phenotypic switching: a key event in the early stage of pulmonary artery hypertension. Drug Discov Today. (2023) 28:103559. 10.1016/j.drudis.2023.103559
43.
Lechartier B Berrebeh N Huertas A Humbert M Guignabert C Tu L . Phenotypic diversity of vascular smooth muscle cells in pulmonary arterial hypertension: implications for therapy. Chest. (2022) 161:219–31. 10.1016/j.chest.2021.08.040
44.
Ren M Zhao H Gao Y Chen Q Zhao X Yue W . NUF2 Promotes tumorigenesis by interacting with HNRNPA2B1 via PI3K/AKT/mTOR pathway in ovarian cancer. J Ovarian Res. (2023) 16:17. 10.1186/s13048-023-01101-9
45.
Jiang F Tang X Tang C Hua Z Ke M Wang C et al HNRNPA2B1 promotes multiple myeloma progression by increasing AKT3 expression via m6A-dependent stabilization of ILF3 mRNA. J Hematol Oncol. (2021) 14:54. 10.1186/s13045-021-01066-6
46.
Tang J Chen Z Wang Q Hao W Gao WQ Xu H . hnRNPA2B1 promotes colon cancer progression via the MAPK pathway. Front Genet. (2021) 12:666451. 10.3389/fgene.2021.666451
47.
Rong L Xu Y Zhang K Jin L Liu X . HNRNPA2B1 Inhibited SFRP2 and activated Wnt-beta/catenin via m6A-mediated miR-106b-5p processing to aggravate stemness in lung adenocarcinoma. Pathol Res Pract. (2022) 233:153794. 10.1016/j.prp.2022.153794
48.
Fu Y Jia Q Ren M Bie H Zhang X Zhang Q et al Circular RNA ZBTB46 depletion alleviates the progression of atherosclerosis by regulating the ubiquitination and degradation of hnRNPA2B1 via the AKT/mTOR pathway. Immun Ageing. (2023) 20:66. 10.1186/s12979-023-00386-0
49.
Si Y Liu F Wang D Fang C Tang X Guo B et al Exosomal transfer of miR-185 is controlled by hnRNPA2B1 and impairs Re-endothelialization after vascular injury. Front Cell Dev Biol. (2021) 9:619444. 10.3389/fcell.2021.619444
50.
Wang G Xiao Q Luo Z Ye S Xu Q . Functional impact of heterogeneous nuclear ribonucleoprotein A2/B1 in smooth muscle differentiation from stem cells and embryonic arteriogenesis. J Biol Chem. (2012) 287:2896–906. 10.1074/jbc.M111.297028
51.
Guo H Wang B Xu K Nie L Fu Y Wang Z et al M(6)A reader HNRNPA2B1 promotes esophageal cancer progression via up-regulation of ACLY and ACC1. Front Oncol. (2020) 10:553045. 10.3389/fonc.2020.553045
52.
Liu X Liu Y Liu Z Lin C Meng F Xu L et al CircMYH9 drives colorectal cancer growth by regulating serine metabolism and redox homeostasis in a p53-dependent manner. Mol Cancer. (2021) 20:114. 10.1186/s12943-021-01412-9
53.
Xu Y Bei Y Shen S Zhang J Lu Y Xiao J et al MicroRNA-222 promotes the proliferation of pulmonary arterial smooth muscle cells by targeting P27 and TIMP3. Cell Physiol Biochem. (2017) 43:282–92. 10.1159/000480371
54.
Wei C Li HZ Wang YH Peng X Shao HJ Li HX et al Exogenous spermine inhibits the proliferation of human pulmonary artery smooth muscle cells caused by chemically-induced hypoxia via the suppression of the ERK1/2- and PI3K/AKT-associated pathways. Int J Mol Med. (2016) 37:39–46. 10.3892/ijmm.2015.2408
55.
Bisserier M Katz MG Bueno-Beti C Brojakowska A Zhang S Gubara S et al Combination therapy with STAT3 inhibitor enhances SERCA2a-induced BMPR2 expression and inhibits pulmonary arterial hypertension. Int J Mol Sci. (2021) 22:9105. 10.3390/ijms22179105
56.
Liu G Zhang S Yang S Shen C Shi C Diao W . Circdiaph3 influences PASMC apoptosis by regulating PI3K/AKT/mTOR pathway through IGF1R. 3 Biotech. (2023) 13:342. 10.1007/s13205-023-03739-0
57.
Dai J Zhou Q Chen J Rexius-Hall ML Rehman J Zhou G . Alpha-enolase regulates the malignant phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt pathway. Nat Commun. (2018) 9:3850. 10.1038/s41467-018-06376-x
58.
Liu Y Zhang H Li Y Yan L Du W Wang S et al Long noncoding RNA Rps4l mediates the proliferation of hypoxic pulmonary artery smooth muscle cells. Hypertension. (2020) 76:1124–33. 10.1161/HYPERTENSIONAHA.120.14644
59.
Zheng Q Lu W Yan H Duan X Chen Y Zhang C et al Established pulmonary hypertension in rats was reversed by a combination of a HIF-2alpha antagonist and a p53 agonist. Br J Pharmacol. (2022) 179:1065–81. 10.1111/bph.15696
60.
Luo J Zheng J Hao W Zeng H Zhang Z Shao G . lncRNA PCAT6 facilitates cell proliferation and invasion via regulating the miR-326/hnRNPA2B1 axis in liver cancer. Oncol Lett. (2021) 21:471. 10.3892/ol.2021.12732
61.
Zhang E Li X . LncRNA SOX2-OT regulates proliferation and metastasis of nasopharyngeal carcinoma cells through miR-146b-5p/HNRNPA2B1 pathway. J Cell Biochem. (2019) 120:16575–88. 10.1002/jcb.28917
62.
Lu Y Zou R Gu Q Wang X Zhang J Ma R et al CRNDE Mediated hnRNPA2B1 stability facilitates nuclear export and translation of KRAS in colorectal cancer. Cell Death Dis. (2023) 14:611. 10.1038/s41419-023-06137-9
63.
Silva GF da Silva JS de Alencar AKN de Moraes Carvalho da Silva M Montagnoli TL de Souza Rocha B et al Novel p38 mitogen-activated protein kinase inhibitor reverses hypoxia-induced pulmonary arterial hypertension in rats. Pharmaceuticals (Basel). (2022) 15:900. 10.3390/ph15070900
64.
Luo X Hang C Zhang Z Le K Ying Y Lv Y et al PVECs-Derived exosomal microRNAs regulate PASMCs via FoxM1 signaling in IUGR-induced pulmonary hypertension. J Am Heart Assoc. (2022) 11:e027177. 10.1161/JAHA.122.027177
65.
Yuan C Xu M Rong R Mei Y Cai W Li L et al miR-200c regulates endothelin-1 induced PASMCs abnormal proliferation and apoptosis. IUBMB Life. (2017) 69:877–86. 10.1002/iub.1686
66.
Jiang Y Hei B Hao W Lin S Wang Y Liu X et al Clinical value of lncRNA SOX2-OT in pulmonary arterial hypertension and its role in pulmonary artery smooth muscle cell proliferation, migration, apoptosis, and inflammatory. Heart Lung. (2022) 55:16–23. 10.1016/j.hrtlng.2022.04.002
67.
Li K Gong Q Xiang XD Guo G Liu J Zhao L et al HNRNPA2B1-mediated M(6)A modification of lncRNA MEG3 facilitates tumorigenesis and metastasis of non-small cell lung cancer by regulating miR-21–5p/PTEN axis. J Transl Med. (2023) 21:382. 10.1186/s12967-023-04190-8
68.
Chen ZY Cai L Zhu J Chen M Chen J Li ZH et al Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis. (2011) 32:1419–26. 10.1093/carcin/bgr088
69.
Li K Chen J Lou X Li Y Qian B Xu D et al HNRNPA2B1 Affects the prognosis of esophageal cancer by regulating the miR-17–92 cluster. Front Cell Dev Biol. (2021) 9:658642. 10.3389/fcell.2021.658642
70.
Feng R Yin Y Wei Y Li Y Li L Zhu R et al Mutant p53 activates hnRNPA2B1-AGAP1-mediated exosome formation to promote esophageal squamous cell carcinoma progression. Cancer Lett. (2023) 562:216154. 10.1016/j.canlet.2023.216154
71.
Zhang J Sheng H Zhang L Li X Guo Y Wang Y et al Bta-miR-206 and a novel lncRNA-lncA2B1 promote myogenesis of skeletal muscle satellite cells via common binding protein HNRNPA2B1. Cells. (2023) 12:1028. 10.3390/cells12071028
72.
Zhou JJ Li H Qian YL Quan RL Chen XX Li L et al Nestin represents a potential marker of pulmonary vascular remodeling in pulmonary arterial hypertension associated with congenital heart disease. J Mol Cell Cardiol. (2020) 149:41–53. 10.1016/j.yjmcc.2020.09.005
73.
Sun Z Nie X Sun S Dong S Yuan C Li Y et al Long non-coding RNA MEG3 downregulation triggers human pulmonary artery smooth muscle cell proliferation and migration via the p53 signaling pathway. Cell Physiol Biochem. (2017) 42:2569–81. 10.1159/000480218
74.
Kurahara LH Hiraishi K Yamamura A Zhang Y Abe K Yahiro E et al Eicosapentaenoic acid ameliorates pulmonary hypertension via inhibition of tyrosine kinase fyn. J Mol Cell Cardiol. (2020) 148:50–62. 10.1016/j.yjmcc.2020.08.013
75.
Jiang L Goncharov DA Shen Y Lin D Chang B Pena A et al Akt-Dependent glycolysis-driven lipogenesis supports proliferation and survival of human pulmonary arterial smooth muscle cells in pulmonary hypertension. Front Med (Lausanne). (2022) 9:886868. 10.3389/fmed.2022.886868
76.
Bienertova-Vasku J Novak J Vasku A . MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens. (2015) 9:221–34. 10.1016/j.jash.2014.12.011
77.
Lu Z Li S Zhao S Fa X . Upregulated miR-17 regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation and apoptosis by targeting mitofusin 2. Med Sci Monit. (2016) 22:3301–8. 10.12659/msm.900487
78.
Zhou Y Fang XL Zhang Y Feng YN Wang SS . miR-20a-5p promotes pulmonary artery smooth muscle cell proliferation and migration by targeting ABCA1. J Biochem Mol Toxicol. (2020) 34:e22589. 10.1002/jbt.22589
79.
Jalali S Ramanathan GK Parthasarathy PT Aljubran S Galam L Yunus A et al Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS One. (2012) 7:e46808. 10.1371/journal.pone.0046808
80.
Kim MK Choi MJ Lee HM Choi HS Park YK Ryu CJ . Heterogeneous nuclear ribonucleoprotein A2/B1 regulates the ERK and p53/HDM2 signaling pathways to promote the survival, proliferation and migration of non-small cell lung cancer cells. Oncol Rep. (2021) 46:153. 10.3892/or.2021.8104
81.
Wu Q Li G Gong L Cai J Chen L Xu X et al Identification of miR-30c-5p as a tumor suppressor by targeting the m(6) A reader HNRNPA2B1 in ovarian cancer. Cancer Med. (2023) 12:5055–70. 10.1002/cam4.5246
82.
Chen Z Chen X Lei T Gu Y Gu J Huang J et al Integrative analysis of NSCLC identifies LINC01234 as an oncogenic lncRNA that interacts with HNRNPA2B1 and regulates miR-106b biogenesis. Mol Ther. (2020) 28:1479–93. 10.1016/j.ymthe.2020.03.010
83.
Ailifeire M-M Gao J Yu Z Ma Y . Up-regulated miR-106b-5p in exosomes derived from pulmonary artery smooth muscle cells enhances Warburg effect of pulmonary artery endothelial cells and promotes arterial pulmonary hypertensio. J Pract Med. (2023) 39:2190–5. 10.3969/j.issn.1006-5725.2023.17.007
84.
Zhang H D'Alessandro A Li M Reisz JA Riddle S Muralidhar A et al Histone deacetylase inhibitors synergize with sildenafil to suppress purine metabolism and proliferation in pulmonary hypertension. Vascul Pharmacol. (2023) 149:107157. 10.1016/j.vph.2023.107157
85.
Xiong PY Motamed M Chen KH Dasgupta A Potus F Tian L et al Inhibiting pyruvate kinase muscle isoform 2 regresses group 2 pulmonary hypertension induced by supra-coronary aortic banding. Acta Physiol (Oxf). (2022) 234:e13764. 10.1111/apha.13764
86.
Zhang A Yu F Yu W Ye P Liu P Gu Y et al Pyruvate kinase M2 activation protects against the proliferation and migration of pulmonary artery smooth muscle cells. Cell Tissue Res. (2020) 382:585–98. 10.1007/s00441-020-03245-2
87.
Liu L Pang W Liu J Xu S Zhang Z Hao R et al Inhibition of heterogeneous nuclear ribonucleoproteins A1 and oxidative stress reduces glycolysis via pyruvate kinase M2 in chronic thromboembolic pulmonary hypertension. J Transl Int Med. (2024) 12:437–51. 10.2478/jtim-2022-0051
88.
Zhou B Lu D Wang A Cui J Zhang L Li J et al Endoplasmic reticulum stress promotes sorafenib resistance via miR-188–5p/hnRNPA2B1-mediated upregulation of PKM2 in hepatocellular carcinoma. Mol Ther Nucleic Acids. (2021) 26:1051–65. 10.1016/j.omtn.2021.09.014
89.
Rihan M Sharma SS . Inhibition of pyruvate kinase M2 (PKM2) by shikonin attenuates isoproterenol-induced acute myocardial infarction via reduction in inflammation, hypoxia, apoptosis, and fibrosis. Naunyn Schmiedebergs Arch Pharmacol. (2024) 397:145–59. 10.1007/s00210-023-02593-4
90.
Willis GR Fernandez-Gonzalez A Reis M Mitsialis SA Kourembanas S . Macrophage immunomodulation: the gatekeeper for mesenchymal stem cell derived-exosomes in pulmonary arterial hypertension?Int J Mol Sci. (2018) 19:2534. 10.3390/ijms19092534
91.
de la Cuesta F Passalacqua I Rodor J Bhushan R Denby L Baker AH . Extracellular vesicle cross-talk between pulmonary artery smooth muscle cells and endothelium during excessive TGF-beta signalling: implications for PAH vascular remodelling. Cell Commun Signal. (2019) 17:143. 10.1186/s12964-019-0449-9
92.
Zhang M Wang Y Yu L Zhang Y Wang Y Shang Z et al Fusobacterium nucleatum promotes colorectal cancer metastasis by excretion of miR-122–5p from cells via exosomes. iScience. (2023) 26:107686. 10.1016/j.isci.2023.107686
93.
Li C Qin F Wang W Ni Y Gao M Guo M et al hnRNPA2B1-Mediated extracellular vesicles sorting of miR-122–5p potentially promotes lung cancer progression. Int J Mol Sci. (2021) 22:12866. 10.3390/ijms222312866
94.
Lei Y Guo W Chen B Chen L Gong J Li W . Tumor-released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non-small cell lung cancer. Oncol Rep. (2018) 40:3438–46. 10.3892/or.2018.6762
95.
Zhao H Duan R Wang Q Hu X Zhao Q Wu W et al MiR-122–5p as a potential regulator of pulmonary vascular wall cell in idiopathic pulmonary arterial hypertension. Heliyon. (2023) 9:e22922. 10.1016/j.heliyon.2023.e22922
96.
Lou J Wu J Feng M Dang X Wu G Yang H et al Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122–5p. J Sport Health Sci. (2022) 11:495–508. 10.1016/j.jshs.2021.09.009
97.
Zhang Y Huang W Yuan Y Li J Wu J Yu J et al Long non-coding RNA H19 promotes colorectal cancer metastasis via binding to hnRNPA2B1. J Exp Clin Cancer Res. (2020) 39:141. 10.1186/s13046-020-01619-6
98.
Yu X Huang J Liu X Li J Yu M Li M et al LncRNAH19 acts as a ceRNA of let-7 g to facilitate endothelial-to-mesenchymal transition in hypoxic pulmonary hypertension via regulating TGF-beta signalling pathway. Respir Res. (2024) 25:270. 10.1186/s12931-024-02895-y
99.
Sun K Li YY Jin J . A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther. (2021) 6:79. 10.1038/s41392-020-00455-6
100.
Zhang Y Tu J Li Y Wang Y Lu L Wu C et al Inflammation macrophages contribute to cardiac homeostasis. Cardiology. (2023) 8(1):6–17. 10.1097/CP9.0000000000000035
101.
Hu Y Chi L Kuebler WM Goldenberg NM . Perivascular inflammation in pulmonary arterial hypertension. Cells. (2020) 9. 10.3390/cells9112338
102.
Savai R Pullamsetti SS Kolbe J Bieniek E Voswinckel R Fink L et al Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. (2012) 186:897–908. 10.1164/rccm.201202-0335OC
103.
Florentin J Coppin E Vasamsetti SB Zhao J Tai YY Tang Y et al Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol. (2018) 200:3612–25. 10.4049/jimmunol.1701287
104.
Pugliese SC Kumar S Janssen WJ Graham BB Frid MG Riddle SR et al A time- and compartment-specific activation of lung macrophages in hypoxic pulmonary hypertension. J Immunol. (2017) 198:4802–12. 10.4049/jimmunol.1601692
105.
Zuo Y Li B Gao M Xiong R He R Li N et al Novel insights and new therapeutic potentials for macrophages in pulmonary hypertension. Respir Res. (2024) 25:147. 10.1186/s12931-024-02772-8
106.
Gcanga L Tamgue O Ozturk M Pillay S Jacobs R Chia JE et al Host-directed targeting of LincRNA-MIR99AHG suppresses intracellular growth of Mycobacterium tuberculosis. Nucleic Acid Ther. (2022) 32:421–37. 10.1089/nat.2022.0009
107.
Zhou X Hong Y Liu Y Wang L Liu X Li Y et al Intervening in hnRNPA2B1-mediated exosomal transfer of tumor-suppressive miR-184–3p for tumor microenvironment regulation and cancer therapy. J Nanobiotechnology. (2023) 21:422. 10.1186/s12951-023-02190-w
108.
Pan Z Zhao R Li B Qi Y Qiu W Guo Q et al EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. (2022) 21:16. 10.1186/s12943-021-01485-6
109.
Chen W Wang Y Xia W Zhang J Zhao Y . Neddylation-mediated degradation of hnRNPA2B1 contributes to hypertriglyceridemia pancreatitis. Cell Death Dis. (2022) 13:863. 10.1038/s41419-022-05310-w
110.
Li M Riddle S Zhang H D'Alessandro A Flockton A Serkova NJ et al Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation. (2016) 134:1105–21. 10.1161/CIRCULATIONAHA.116.023171
111.
Koupenova M Clancy L Corkrey HA Freedman JE . Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. (2018) 122:337–51. 10.1161/CIRCRESAHA.117.310795
112.
Kazimierczyk R Kaminski K . The role of platelets in the development and progression of pulmonary arterial hypertension. Adv Med Sci. (2018) 63:312–6. 10.1016/j.advms.2018.04.013
113.
Zheng H Hua J Li H He W Chen X Ji Y et al Comprehensive analysis of the expression of N6-methyladenosine RNA methylation regulators in pulmonary artery hypertension. Front Genet. (2022) 13:974740. 10.3389/fgene.2022.974740
114.
Zuo D Chen Y Cai JP Yuan HY Wu JQ Yin Y et al A hnRNPA2B1 agonist effectively inhibits HBV and SARS-CoV-2 omicron in vivo. Protein Cell. (2023) 14:37–50. 10.1093/procel/pwac027
115.
Han HJ Sivaraman A Kim M Min KH Song ME Choi Y et al HIF-1alpha inhibition by MO-2097, a novel chiral-free benzofuran targeting hnRNPA2B1. J Adv Res. (2024) 64:67–81. 10.1016/j.jare.2023.11.016
116.
Li M Ying M Gu S Zhou Z Zhao R . SIRT6 Inhibits hypoxia-induced pulmonary arterial smooth muscle cells proliferation via HIF-1alpha/PDK4 signaling. Life Sci. (2023) 312:121192. 10.1016/j.lfs.2022.121192
117.
Li B Xin Z Gao S Li Y Guo S Fu Y et al SIRT6-regulated macrophage efferocytosis epigenetically controls inflammation resolution of diabetic periodontitis. Theranostics. (2023) 13:231–49. 10.7150/thno.78878
118.
Wang S Liu Y Wang Q Xu X Huang T Dong P et al Utilizing network pharmacology and molecular docking integrated surface plasmon resonance technology to investigate the potential targets and mechanisms of Tripterygium wilfordii against pulmonary artery hypertension. Evid Based Complement Alternat Med. (2022) 2022: 9862733. 10.1155/2022/9862733
119.
Chen P Zhao P Hu M Wang L Lei T Liu B et al HnRNP A2/B1 as a potential anti-tumor target for triptolide based on a simplified thermal proteome profiling method using XGBoost. Phytomedicine. (2023) 117:154929. 10.1016/j.phymed.2023.154929
120.
He Y Fang X Shi J Li X Xie M Liu X . Apigenin attenuates pulmonary hypertension by inducing mitochondria-dependent apoptosis of PASMCs via inhibiting the hypoxia inducible factor 1alpha-KV1.5 channel pathway. Chem Biol Interact. (2020) 317:108942. 10.1016/j.cbi.2020.108942
121.
Arango D Morohashi K Yilmaz A Kuramochi K Parihar A Brahimaj B et al Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc Natl Acad Sci U S A. (2013) 110:E2153–62. 10.1073/pnas.1303726110
122.
Dunham-Snary KJ Wu D Sykes EA Thakrar A Parlow LRG Mewburn JD et al Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. (2017) 151:181–92. 10.1016/j.chest.2016.09.001
Summary
Keywords
pulmonary arterial hypertension, HNRNPA2B1, vascular remodeling, smooth muscle cells, endothelial cell
Citation
Wei Y, Wu D, Deng N, Xu F, Luo S, Fan X, Guo H, Chen J, Li W and Si X (2025) HNRNPA2B1: a novel target in pulmonary arterial hypertension. Front. Cardiovasc. Med. 12:1497938. doi: 10.3389/fcvm.2025.1497938
Received
21 September 2024
Accepted
10 June 2025
Published
09 July 2025
Volume
12 - 2025
Edited by
Ming-Lin Liu, University of Pennsylvania, United States
Reviewed by
Wei Zhang, Temple University, United States
Suzette Riddle, University of Colorado Anschutz Medical Campus, United States
Updates
Copyright
© 2025 Wei, Wu, Deng, Xu, Luo, Fan, Guo, Chen, Li and Si.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Wei Li liwei249188@sina.com Xiaoyun Si sixiaoyun1@qq.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.