- 1Department of Cardiology, Dazhou Second People’s Hospital, Dazhou, Sichuan, China
- 2Department of Otorhinolaryngology Head and Neck Surgery, Dazhou Second People’s Hospital, Dazhou, Sichuan, China
Background: Inflammatory markers are increasingly recognized as key contributors to the pathogenesis and progression of atrial fibrillation (AF). This meta-analysis aims to systematically assess the prognostic significance of various lymphocyte-based inflammation indices, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) with clinical outcomes in AF.
Methods: A comprehensive search was conducted in multiple databases until March 24, 2024. The included studies evaluated lymphocyte-based indices in relation to AF prognosis using a random-effects model. Weighted Mean Differences, Hazard ratios, and Odds Ratios with 95% Confidence Intervals were calculated. Subgroup and sensitivity analyses were performed, and evidence quality was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework.
Results: Twenty-one studies involving 63,687 patients with AF were included. Higher NLR was associated with increased risks of all-cause mortality (HR: 1.50, 95% CI: 1.16–1.92; I² = 74%), stroke (HR: 1.42, 95% CI: 1.26−1.61; I² = 0%), AF recurrence (OR: 1.47, 95% CI: 1.17−1.86; I² = 93%), and left atrial thrombosis (OR: 2.12, 95% CI: 1.41−3.19; I² = 82%). Sensitivity analyses yielded similar estimates. Evidence for PLR and SII was limited to two studies each for left atrial thrombosis, with inconsistent results and high heterogeneity; therefore, no firm conclusions could be drawn. Exploratory subgroup analyses suggested lower heterogeneity in larger studies, but tests for subgroup differences were underpowered. Overall certainty of evidence ranged from low to very low by GRADE.
Conclusion: Higher NLR shows an observational association with adverse outcomes in AF, but the certainty of evidence is low. Evidence for PLR and SII is extremely limited and inconsistent, precluding meaningful conclusions. Further large, well-designed prospective studies with standardized measurements are required.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024540368, identifier CRD42024540368.
1 Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia globally, affects approximately 50 million individuals worldwide. As the global population ages, the prevalence of AF is expected to increase (1, 2). AF significantly elevates the risk of cardiovascular diseases and is closely associated with all-cause mortality, strokes, and severe cardiovascular complications (3). Despite advances in management, predicting clinical outcomes in AF remains a challenge, necessitating reliable biomarkers to guide individualized treatment strategies. Emerging evidence suggests that AF is not merely an electrical disorder but also involves structural and functional remodeling of the atria, often referred to as atrial cardiomyopathy (4). Inflammation is a key contributor to this process, promoting atrial fibrosis, endothelial dysfunction, and thrombogenicity, all of which increase the risk of AF-related adverse events (5). Therefore, inflammatory biomarkers may provide valuable prognostic insights into AF progression and associated complications.
Inflammation plays a crucial role in the development and recurrence of cardiovascular diseases (5, 6). Lymphocyte-based inflammatory index, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), monocyte-to-lymphocyte ratio (MLR), and systemic inflammatory response index (SIRI), are readily available, cost-effective, and straightforward measures of inflammation. Research has shown that lymphocyte-based inflammatory indices correlate with poor outcomes in conditions such as acute heart failure (7, 8), acute coronary syndrome (9, 10), other coronary diseases (11, 12), and postoperative AF after cardiac surgery (13, 14). However, research on the relationship between these markers and adverse clinical outcomes like all-cause mortality, stroke, and AF recurrence following catheter ablation, as well as left atrial thrombosis among AF patients, remains limited. Therefore, investigating the predictive capabilities of lymphocyte-based inflammatory index for clinical outcomes in AF is essential.
This meta-analysis assessed the predictive value of lymphocyte-based inflammatory index for clinical outcomes in AF patients. By examining studies on all-cause mortality, AF recurrence post-catheter ablation, stroke, and left atrial thrombosis, this analysis provides a robust evidence base for evaluating the effectiveness of these indices as potential biomarkers in managing AF. Our research assesses the efficacy of these indices as prognostic biomarkers, which could be crucial for determining prognostic risks in AF patients and guiding targeted preventive treatments.
2 Methods
2.1 Protocol registration
This meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (Supplementary Table S1) and was previously registered with PROSPERO (CRD42024540368).
2.2 Search strategy
The Cochrane Library, Embase, Scopus, Web of Science, and PubMed were searched from their respective inceptions until March 24, 2024, using the following strategy: “lymphocytes AND ratio AND (atrial fibrillation OR AF)”. The detailed search protocol is provided in the Supplementary Table S2.
The search strategy was developed by the authors in accordance with the PRISMA 2020 guidelines. Although a medical librarian was not directly consulted, the strategy was refined through multiple pilot runs and cross-checks with previously published meta-analyses in related fields to ensure comprehensiveness and accuracy. Reference lists of included studies and relevant reviews were also manually screened to identify additional eligible articles.
2.3 Inclusion and exclusion criteria
Studies that met the following criteria were included: 1) Examined the predictive value of lymphocyte-related inflammatory indices in AF patients; 2) Evaluated the correlation between lymphocyte-based inflammatory indices (NLR, PLR, MLR, SII, SIRI) and prognostic outcomes (all-cause mortality, stroke, post-catheter ablation AF recurrence, left atrial thrombosis); 3) Provided extractable data; for inclusion in the meta-analysis, at least two studies must have evaluated the same inflammatory index in relation to the same outcome measure to ensure data pooling feasibility; 4) Prioritized studies with the largest sample size and most recent data when multiple studies used the same patient cohort; 5) Published in English or Chinese, or translated into these languages.
The exclusion criteria were as follows: 1) reviews, meta-analysis, letters, case reports, and comments; 2) non-clinical studies; 3) studies that did not investigate the association between lymphocyte-related inflammatory indices (NLR, PLR, MLR, SII, SIRI) and clinical outcomes in atrial fibrillation.
2.4 Literature screening, data extraction
Two investigators (XMC, XGZ) independently screened articles. The two authors (XMC, XGZ) independently extracted general study information (authors, publication year, country) and baseline demographic and clinical characteristics (age, gender, NLR, PLR, SII, MLR, SIRI, all-cause mortality, stroke, AF recurrence, left atrial thrombus). The third author (SHF) verified the extracted data. All the disagreements were resolved through discussions among the third author (SHF).
2.5 Definitions and outcomes
The outcome indicators for AF included all-cause mortality, stroke, AF recurrence, and left atrial thrombosis. AF recurrence was categorized into early and late recurrence. Early recurrence of AF was defined as any atrial tachyarrhythmia (including AF, atrial flutter, and atrial tachycardia) continuously recorded for at least 30 s within a blanking period of three months. Late recurrence of AF was defined as the continuous recording of any 30-seconds atrial tachyarrhythmia (including AF, atrial flutter, and atrial tachycardia) occurring after the three-month blanking period.
2.6 Quality assessment
The quality of the included studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS), with scores ranging from 0 to 9 (15). Studies with a NOS score of ≥7 were considered high-quality. Discrepancies arising during the evaluation process were resolved through consensus.
2.7 Data synthesis and analysis
Analyses were conducted in RevMan 5.4 and Stata 17.0 using inverse-variance weighting. For time-to-event outcomes we preferentially extracted hazard ratios (HRs); when HRs were unavailable, odds ratios (ORs) were used but not pooled together with HRs (separate syntheses or sensitivity analyses). Dichotomous outcomes were pooled as ORs with 95% CIs; continuous outcomes as (weighted) mean differences with 95% CIs. All meta-analyses used a random-effects model (DerSimonian–Laird).
Only contrasts with ≥2 independent studies and compatible effect measures were quantitatively pooled; contrasts not meeting these criteria were not synthesized or interpreted, and, if applicable, individual study estimates were tabulated in the Supplement for transparency. To avoid unit-of-analysis errors, when a study reported multiple cut-offs/categories for the same biomarker–outcome contrast, one effect per cohort was included according to a prespecified hierarchy [primary prespecified cut-off → ROC/Youden-derived cut-off → extreme-category contrast (highest vs. lowest)]; in all cases the most fully adjusted estimate was used. Alternative thresholds were examined in sensitivity analyses.
Heterogeneity was assessed with Cochran's Q and I² (substantial heterogeneity defined as I2 ≥ 50% or P ≤ 0.10). Prespecified exploratory subgroup analyses were performed by study design, region, sample size (e.g., ≥ 300 vs.<300), cut-off, measurement timing (pre- vs. post-procedure), and AF-recurrence timing (early vs. late); between-subgroup differences were tested using Q_between without multiplicity adjustment. Small-study effects were evaluated using funnel plots and Egger's regression only when k ≥ 5; formal tests were not performed when k < 5. Sensitivity analysis was conducted to evaluate result stability when the number of included studies was five or more. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to evaluate the quality of evidence (16).
3 Results
3.1 Literature search and study characteristics
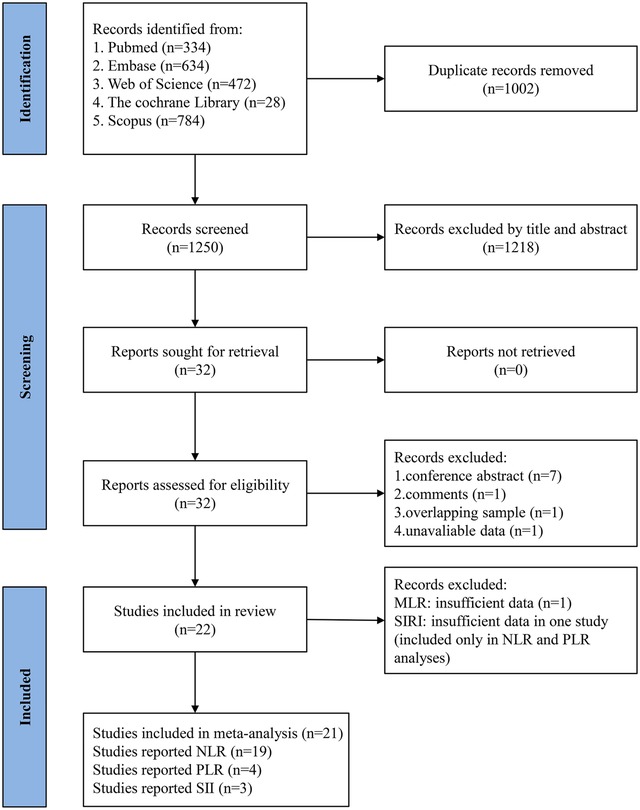
Using the described search strategies, 2,252 articles were initially retrieved. Of these, 1,002 were excluded due to duplication. After screening titles and abstracts, 1,218 studies not meeting the specified inclusion and exclusion criteria were removed. Subsequently, the full texts of 32 studies were reviewed. During this phase, 7 conference abstracts, 1 commentary article, 1 study with overlapping populations, and 1 article lacking critical data were excluded. Additionally, two studies on MLR (17, 18) reported different outcomes, precluding meta-analysis, and only one study on SIRI (18) was available, which was insufficient for data pooling. Ultimately, 21 studies were included in the final analysis (18–38). Figure 1 presents the flow chart depicting the study selection process.
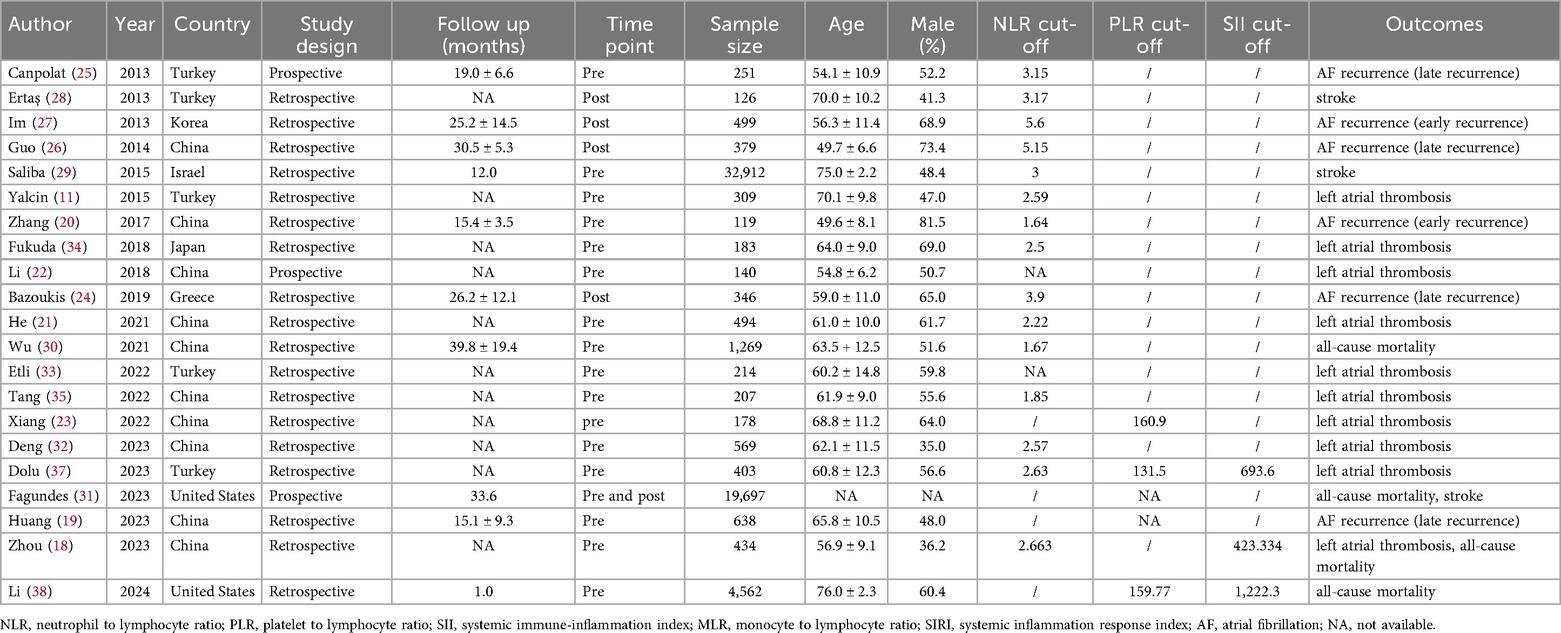
Table 1 presents the characteristics of the 21 included studies. Sample sizes ranged from 119 to 32,912, with a total of 63,687 participants. The reported age of participants ranged from 54.12 to 76.98 years. The studies comprised 18 retrospective and 3 prospective investigations. Regarding the inflammatory biomarkers, 19 studies focused exclusively on NLR, 4 on PLR, and 3 on SII. The research was conducted across multiple countries, including the United States, China, Japan, Korea, Ireland, Greece, and Turkey, with China as the primary research location. Details of the multivariable adjustment models and the covariates included in each study are summarized in Supplementary Table S3. According to the NOS, the studies were considered of high quality, with scores ranging from 7 to 8. Supplementary Table S4 provides details on NOS scores.
3.2 Predictive value of NLR
3.2.1 All-cause mortality
Three studies assessed the association between categorized NLR and all-cause mortality in AF patients. A higher NLR was associated with an increased risk of all-cause mortality (HR: 1.50, 95% CI: 1.16–1.92, P = 0.002, I2 = 74%) (Figure 2A).

Figure 2. Forest plot of HRs and ORs showing associations between lymphocyte-based inflammatory markers and clinical outcomes in patients with AF: (A) NLR and All-cause mortality; (B) NLR and stroke; (C) NLR and AF recurrence; (D) NLR and left atrial thrombosis.
3.2.2 Stroke
Three studies assessed the association between categorized NLR and stroke in AF. A higher NLR was associated with an increased stroke risk (HR: 1.42, 95% CI: 1.26–1.61, P < 0.00001, I2 = 0%) (Figure 2B).
3.2.3 AF recurrence
Five studies assessed the association between categorized NLR and AF recurrence. A higher NLR was associated with an increased AF recurrence risk (OR: 1.47, 95% CI: 1.17–1.86, P = 0.001, I2 = 93%) (Figure 2C).
3.2.4 Left atrial thrombosis
Six studies assessed the association between categorized NLR and left atrial thrombosis. A higher NLR was associated with an increased left atrial thrombosis risk (OR: 2.12, 95% CI: 1.41–3.19, P = 0.0003, I2 = 82%) (Figure 2D).
3.3 Sensitivity analysis and publication bias assessment
Leave-one-out sensitivity analyses were performed for AF recurrence and left atrial thrombosis by sequentially omitting each study to assess its influence on the pooled ORs. The pooled estimates remained stable; no single study materially altered the magnitude or direction of the effect (Supplementary Figure S1).
Visual inspection of funnel plots suggested slight asymmetry (Supplementary Supplementary Figure S2). Consistent with our prespecified rule (k ≥ 5), Egger's regression did not indicate small-study effects for the categorized NLR analyses (AF recurrence: P = 0.146; left atrial thrombosis: P = 0.440). Given the small numbers of studies, these tests were underpowered, and results should be interpreted cautiously.
3.4 Subgroup analysis
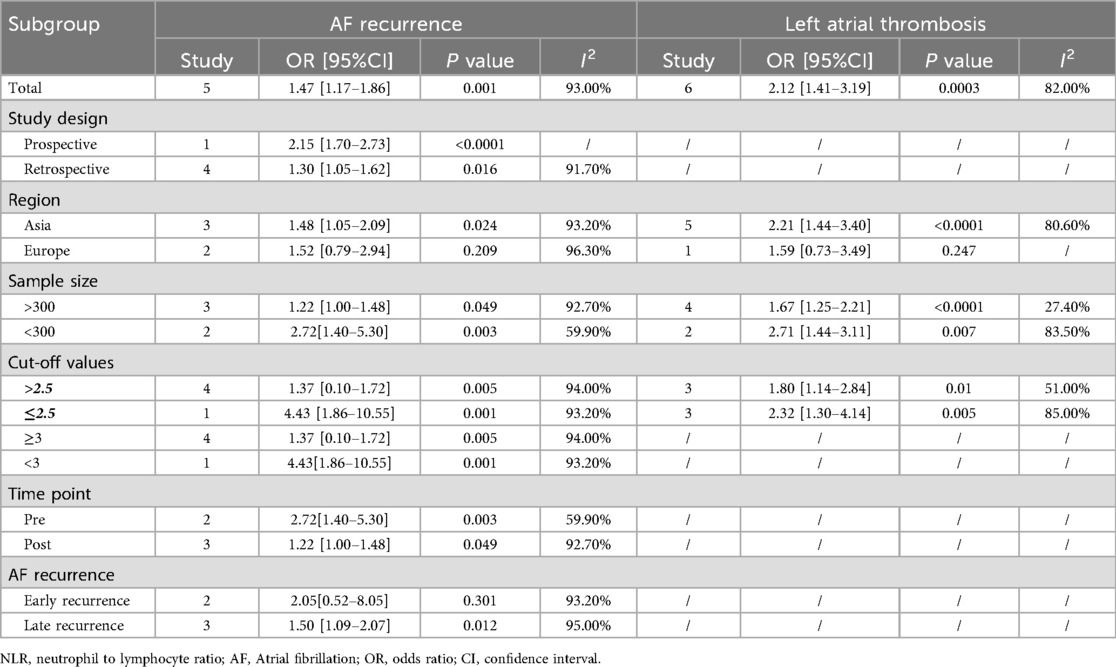
Given substantial between-study heterogeneity in several contrasts, we performed prespecified exploratory subgroup analyses by study design, region, sample size (≥300 vs. <300), NLR cut-off (≥2.5 vs. <2.5), measurement timing (pre- vs. post-procedure), and AF-recurrence timing (early vs. late). Overall, higher NLR was associated with greater odds of left atrial thrombosis across subgroups; within-subgroup heterogeneity tended to be lower in larger studies. For AF recurrence, the magnitude of association appeared to differ by region and recurrence timing; however, several strata included ≤3 studies (some single-study), so formal tests for subgroup differences (Q_between) were underpowered, and findings should be interpreted cautiously. Detailed pooled effects (k, ORs with 95% CIs, and I2) are shown in Table 2.
3.5 Predictive value of PLR
3.5.1 Left atrial thrombosis
Two studies reported on continuous PLR data related to left atrial thrombosis. The PLR was significantly higher in the left atrial thrombus group compared to the non-thrombus group (WMD: 33.02, 95% CI: 24.76–41.29, P < 0.00001, I2 = 80%) (Figure 3A).

Figure 3. Forest plot of WMDs showing the association between left atrial thrombosis risk and PLR/SII: (A) PLR and left atrial thrombosis; (B) SII and left atrial thrombosis.
3.6 Predictive value of SII
3.6.1 Left atrial thrombosis
Two studies reported on continuous SII data related to left atrial thrombosis. The SII showed no statistically significant difference between the left atrial thrombus group and the non-thrombus group (WMD: 241.34, 95% CI: −32.48–515.17, P = 0.08, I2 = 96%) (Figure 3B).
3.7 GRADE classification
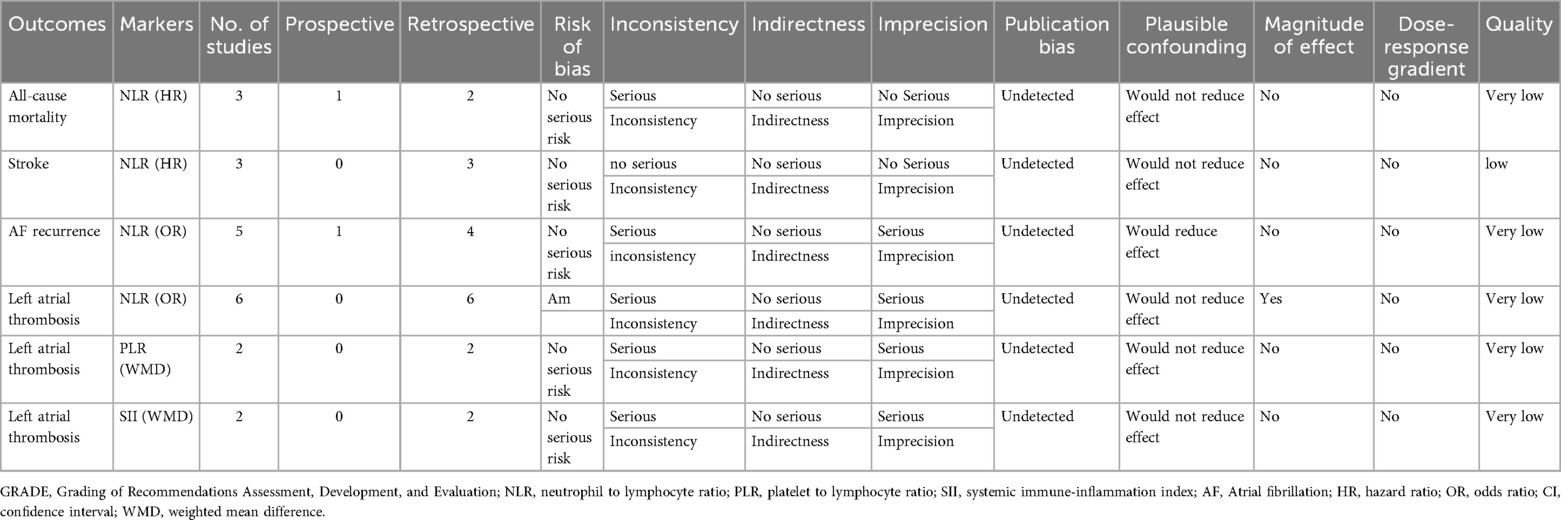
The GRADE framework was employed to assess the quality of evidence and strength of recommendations. Within this framework, the stroke outcome indicator related to NLR was rated as low quality. Despite the absence of significant downgrading factors in the analyzed domains, the inherent low level of evidence in observational study designs, specifically cohort and case-control studies, maintained this classification. For other outcome measures, the evidence quality was further rated as very low due to significant risk of bias identified in at least one critical area, such as study design, consistency of results, or measurement precision. Table 3 provides detailed scores for these assessments.
4 Discussion
4.1 Key findings
This meta-analysis of 21 studies (n = 63,687) shows that higher NLR is associated with increased risks of all-cause mortality, stroke, AF recurrence, and left atrial thrombosis, with pooled estimates generally ranging from 1.4 to 2.1. Substantial heterogeneity was observed across several contrasts, although sensitivity analyses supported the stability of the pooled results and no conclusive small-study effects were detected. Exploratory subgroup analyses indicated that heterogeneity was partly related to sample size and regional differences, but statistical power within subgroups was limited. Evidence for PLR and SII was very limited, with only two studies available for each and marked heterogeneity; these analyses are therefore exploratory and not suitable for firm prognostic inference. The certainty of evidence for the NLR–outcome associations was rated as low to very low according to GRADE.
4.2 Potential mechanisms linking inflammation to AF outcomes
AF is increasingly recognized as an inflammatory-driven arrhythmia, where systemic inflammation contributes to its initiation, progression, and thromboembolic complications. In this context, lymphocyte-based inflammatory markers such as NLR, PLR, and SII may serve as valuable indicators reflecting underlying pathophysiological mechanisms.
Atrial cardiomyopathy plays a central role in AF onset, maintenance, and thromboembolic complications. It is characterized by structural abnormalities (atrial fibrosis, dilation), electrical remodeling (conduction disturbances), and autonomic dysregulation (39, 40). Inflammation is a key driver of atrial cardiomyopathy, where chronic inflammatory states—mediated by cytokines such as IL-6, TNF-α, and CRP—induce atrial fibrosis, alter intercellular connexins, and promote AF persistence (41–43). Additionally, endothelial dysfunction and platelet activation, both influenced by systemic inflammation, may contribute to the heightened stroke risk observed in AF patients (44). NLR has been proposed as a surrogate for inflammatory burden in AF, with elevated levels correlating with the extent of atrial fibrosis and serving as a predictor of AF recurrence and left atrial thrombus formation (45). These findings underscore the intricate link between inflammation and atrial remodeling, suggesting that inflammatory markers may provide insights into AF pathophysiology and risk stratification.
Catheter ablation, including radiofrequency catheter ablation (RFCA) and pulsed field ablation (PFA), is an effective treatment for symptomatic AF. RFCA utilizes thermal energy to disrupt abnormal conduction pathways, whereas PFA selectively ablates myocardial tissue using electroporation while sparing adjacent structures such as the esophagus and phrenic nerve. However, inflammation plays a crucial role in post-ablation AF recurrence. RFCA has been associated with a significant elevation in inflammatory mediators (IL-6, TNF-α, CRP) post-procedure, which may contribute to early AF recurrence (ERAF) and potentially impact long-term outcomes (46–49). In contrast, PFA appears to elicit a milder inflammatory response, with lower postoperative NLR and CRP levels, which may be linked to a reduced risk of AF recurrence (50). Recent evidence further supports that higher postoperative NLR is associated with increased rates of ERAF, suggesting a role for inflammation in post-ablation arrhythmogenesis (51). Similarly, elevated PLR and SII have been correlated with higher AF recurrence rates and left atrial thrombus formation following ablation (46). These findings highlight the importance of inflammation in procedural outcomes, reinforcing the need to consider inflammatory markers in post-ablation risk stratification. These modality-specific differences in inflammatory activation may partly explain the variation in early AF recurrence observed following RFCA vs. PFA. Moreover, the association between higher postoperative NLR and increased ERAF risk suggests that NLR may capture procedure-related inflammatory injury, linking biomarker dynamics to ablation-specific prognostic differences.
Together, these mechanistic insights suggest that inflammation is a crucial modulator of AF pathogenesis, influencing both disease progression and treatment response. Future studies should explore targeted anti-inflammatory strategies to mitigate AF burden and improve procedural outcomes.
In addition to these biological mechanisms, several important clinical and methodological confounders may also have contributed to the variability of the observed associations. First, AF duration and AF type (paroxysmal vs. persistent), both of which strongly influence baseline inflammatory burden and AF prognosis, were inconsistently reported and rarely adjusted across included studies. Second, comorbidities such as heart failure, hypertension, diabetes, and renal dysfunction can independently elevate inflammatory markers and modify AF outcomes, yet the degree of adjustment varied substantially among studies. Third, anticoagulation status and antiarrhythmic drug use, which profoundly influence stroke and thrombus risk, were not uniformly accounted for. Treatment modality represents another key confounder, particularly in analyses involving catheter ablation: radiofrequency ablation and pulsed field ablation elicit markedly different inflammatory responses, potentially influencing postoperative AF recurrence. Together, these discrepancies in baseline characteristics, treatment strategies, and adjustment models likely contributed to the substantial heterogeneity observed, beyond statistical explanations alone. Future studies should adopt standardized covariate adjustment frameworks to better isolate the independent prognostic value of inflammatory indices.
4.3 Clinical implications and incremental predictive value
The clinical implications of these findings should be interpreted cautiously. Whether NLR provides prognostic value beyond established risk scores such as CHA₂DS₂-VASc is unknown, as few included studies adjusted for key clinical predictors and none evaluated incremental metrics (e.g., NRI, IDI). Evidence supporting combined use of NLR with traditional scores in AF is therefore lacking. No existing studies have rigorously examined combined NLR–risk score models in AF populations, underscoring a current evidence gap. Differences in inflammatory response between RFCA and PFA also suggest potential modality-specific trajectories, but this remains unproven. Overall, NLR should be regarded as a research biomarker until studies establish standardized measurement, validated cutoffs, and demonstrable incremental predictive value.
4.4 Comparison with previous studies
Compared with previous studies, our meta-analysis provides a more comprehensive evaluation of multiple inflammatory markers beyond NLR, encompassing PLR, and SII in relation to various clinical outcomes in AF. The findings align with and expand upon those of Lu et al. (52), who demonstrated a significant association between NLR and stroke risk in AF patients, but our study incorporates a broader scope of inflammatory markers and additional prognostic outcomes, including all-cause mortality, AF recurrence, and left atrial thrombosis. Similarly, Lekkala et al. (53) reported the relationship between NLR and AF recurrence in patients undergoing catheter ablation, but their analysis lacked assessments of other inflammatory markers and did not apply subgroup analysis or the GRADE framework. In contrast, our study addresses these gaps by systematically evaluating the quality of evidence and identifying potential sources of heterogeneity. Furthermore, prior meta-analyses by Dentali et al. (54) and Vakhshoori et al. (55) have established the prognostic role of NLR in other cardiovascular conditions, including acute coronary syndrome and heart failure, findings that our study corroborates in the context of AF. The prognostic utility of SII, which Zhang et al. (56) linked to major adverse cardiac events in post-PCI patients, was also reinforced by our findings, highlighting SII as an important inflammatory marker associated with AF-related complications. By integrating these insights, our study provides an updated and more expansive perspective on the role of inflammation in AF, suggesting that lymphocyte-based inflammatory markers may serve as valuable prognostic indicators across a spectrum of cardiovascular diseases.
4.5 Limitations
This study has several limitations. First, most included studies were retrospective, and variations in NLR, PLR, and SII cutoff values introduced heterogeneity, affecting interpretability. Despite our standardized approach to extracting HR/OR, differences in NLR stratification may have influenced pooled estimates. Future studies should establish uniform classification criteria or report continuous values for better comparability.
Second, follow-up durations varied, particularly in all-cause mortality analysis. One study had a 30-day follow-up, while others exceeded 12 months, complicating risk estimation. Inflammatory markers may reflect transient stress acutely but indicate chronic remodeling long-term. Standardized follow-up periods are needed to clarify these temporal dynamics.
Third, substantial heterogeneity was observed, likely due to differences in population characteristics, biomarker assessments, and study designs. Despite subgroup and sensitivity analyses, residual bias cannot be excluded. A key source of heterogeneity was the lack of standardized NLR cutoffs, which were derived using diverse statistical approaches and applied to different blood sampling time points across studies. Moreover, true harmonization of NLR values was not feasible because individual patient–level continuous data were unavailable, making recalculation or reclassification impossible within a study-level meta-analytic framework. This methodological variability not only constrained comparability across studies but also limits the practical clinical applicability of NLR, as no validated or universally accepted threshold currently exists. Future research should establish consensus definitions, standardized measurement protocols, and clinically validated cutoffs—ideally through large, prospective cohorts—before NLR can be reliably incorporated into routine risk stratification algorithms. Additionally, variations in adjusted covariates contributed to heterogeneity. Although we prioritized HRs/ORs from adjusted models, the included variables differed (e.g., cardiovascular risk factors vs. inflammatory markers), affecting comparability. To enhance the robustness of future meta-analyses, standardized adjustment strategies should be implemented. Furthermore, several clinically important determinants of both inflammatory marker levels and AF prognosis—such as AF type (paroxysmal vs. persistent), AF duration, major comorbidities (e.g., heart failure, hypertension, diabetes, renal dysfunction), and the use of anticoagulants or antiarrhythmic drugs—were inconsistently reported and rarely adjusted for across studies. In analyses involving catheter ablation, differences in procedural modality (radiofrequency vs. pulsed field ablation), which are known to elicit distinct inflammatory responses, may also have contributed to between-study variability. The inability to account adequately for these clinical and treatment-related confounders further limits interpretation of the pooled associations and likely explains part of the residual heterogeneity.
Finally, the limited number of studies restricted a comprehensive synthesis of individual inflammatory markers. While we removed biomarkers reported in only one study (MLR and SIRI), we retained those with two studies, including PLR and SII, as they met the minimum threshold for meta-analysis. However, results from these markers should be interpreted with caution due to the small sample size, substantial heterogeneity, and potential publication bias. The limited number of studies increases the risk of overestimated effect sizes, and variability in biomarker cutoff values further complicates comparability. Given these limitations, the PLR and SII findings should be regarded as exploratory only, as the very small evidence base and inconsistent effect directions preclude any reliable prognostic inference. Future large-scale prospective studies with standardized methodologies and predefined biomarker thresholds are needed to validate their clinical relevance and refine risk stratification.
5 Conclusion
This meta-analysis indicates a possible association between higher NLR and adverse outcomes in atrial fibrillation, including all-cause mortality, stroke, AF recurrence, and left atrial thrombosis; however, these findings are limited by substantial heterogeneity and an overall low certainty of evidence. Evidence for PLR and SII was extremely limited and heterogeneous, and current data do not support any reliable prognostic or clinically interpretable conclusions for these markers. Given these limitations, NLR should be interpreted cautiously as a potential signal rather than a validated risk stratification tool. Well-designed prospective studies with standardized measurement, uniform cut-offs, and comprehensive adjustment are required to clarify the independent prognostic value of lymphocyte-based inflammatory indices.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
XC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XF: Resources, Supervision, Validation, Writing – review & editing. SF: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1504163/full#supplementary-material
References
1. Ohlrogge AH, Brederecke J, Schnabel RB. Global burden of atrial fibrillation and flutter by national income: results from the global burden of disease 2019 database. J Am Heart Assoc. (2023) 12(17):e030438. doi: 10.1161/JAHA.123.030438
2. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2024) 83(1):109–279. doi: 10.1016/j.jacc.2023.08.017
3. Al-Khatib SM. Atrial fibrillation. Ann Intern Med. (2023) 176(7):Itc97–itc112. doi: 10.7326/AITC202307180
4. Schotten U, Goette A, Verheule S. Translation of pathophysiological mechanisms of atrial fibrosis into new diagnostic and therapeutic approaches. Nat Rev Cardiol. (2024) 22(4):225–40. doi: 10.1038/s41569-024-01088-w
5. Zhang H, Dhalla NS. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int J Mol Sci. (2024) 25(2):1082. doi: 10.3390/ijms25021082
6. Floege J, Lüscher B, Müller-Newen G. Cytokines and inflammation. Eur J Cell Biol. (2012) 91(6-7):427. doi: 10.1016/j.ejcb.2012.01.003
7. Cho JH, Cho HJ, Lee HY, Ki YJ, Jeon ES, Hwang KK, et al. Neutrophil-Lymphocyte ratio in patients with acute heart failure predicts in-hospital and long-term mortality. J Clin Med. (2020) 9(2):557. doi: 10.3390/jcm9020557
8. Ang SP, Chia JE, Jaiswal V, Hanif M, Iglesias J. Prognostic value of neutrophil-to-lymphocyte ratio in patients with acute decompensated heart failure: a meta-analysis. J Clin Med. (2024) 13(5):1212. doi: 10.3390/jcm13051212
9. Park JJ, Jang HJ, Oh IY, Yoon CH, Suh JW, Cho YS, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. (2013) 111(5):636–42. doi: 10.1016/j.amjcard.2012.11.012
10. Pruc M, Kubica J, Banach M, Swieczkowski D, Rafique Z, Peacock WF, et al. Diagnostic and prognostic performance of the neutrophil-to-lymphocyte ratio in acute coronary syndromes: a meta-analysis of 90 studies including 45 990 patients. Kardiol Pol. (2024) 82(3):276–84. doi: 10.33963/v.phj.99554
11. Yalcin AA, Topuz M, Akturk IF, Celik O, Erturk M, Uzun F, et al. Is there a correlation between coronary artery ectasia and neutrophil-lymphocyte ratio? Clin Appl Thromb Hemost. (2015) 21(3):229–34. doi: 10.1177/1076029613520488
12. Taşoğlu I, Turak O, Nazli Y, Ozcan F, Colak N, Sahin S, et al. Preoperative neutrophil-lymphocyte ratio and saphenous vein graft patency after coronary artery bypass grafting. Clin Appl Thromb Hemost. (2014) 20(8):819–24. doi: 10.1177/1076029613484086
13. Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. (2010) 105(2):186–91. doi: 10.1016/j.amjcard.2009.09.007
14. Chen YC, Liu CC, Hsu HC, Hung KC, Chang YJ, Ho CN, et al. Systemic immune-inflammation index for predicting postoperative atrial fibrillation following cardiac surgery: a meta-analysis. Front Cardiovasc Med. (2024) 11:1290610. doi: 10.3389/fcvm.2024.1290610
15. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa: The Ottawa Hospital (2016). Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed November 24, 2025).
16. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE Guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015
17. Yu Y, Wang S, Wang P, Xu Q, Zhang Y, Xiao J, et al. Predictive value of lymphocyte-to-monocyte ratio in critically ill patients with atrial fibrillation: a propensity score matching analysis. J Clin Lab Anal. (2022) 36(2):e24217. doi: 10.1002/jcla.24217
18. Zhou Y, Song X, Ma J, Wang X, Fu H. Association of inflammation indices with left atrial thrombus in patients with valvular atrial fibrillation. BMC Cardiovasc Disord. (2023) 23(1):9. doi: 10.1186/s12872-023-03036-x
19. Huang W, Sun H, Tang Y, Luo Y, Liu H. Platelet-to-Lymphocyte ratio improves the predictive ability of the risk score for atrial fibrillation recurrence after radiofrequency ablation. J Inflamm Res. (2023) 16:6023–38. doi: 10.2147/JIR.S440722
20. Zhang Z, Gao L, Zhang S, Yin X, Chang D, Xia Y, et al. Predictive value of NLR on lone atrial fibrillation recurrencepost radiofrequency catheter ablation. J Clin Cardiol. (2017) 33(3):246–50.
21. He C, Zhang Y, Ma J, Liu Q, Yang J, Hou Q, et al. Relationship between neutrophil/lymphocyte ratio and left atrial appendage thrombogenic milieu in patients with nonvalvular atrial fibrillation. Chin Circ J. (2021) 36(9):891–6.
22. Li X, Hu Y, Liu L, Wu P, Li X, Liu H. Significance of serum inflammatory markers and their predictive value for occurrence of left atrial appendage thrombosis in patients with atrial fibrillation. Chin J Integr Tradit West Med Intensive Crit Care. (2018) 25(3):278–82.
23. Xiang W, Kong L, Li X, Chen L, She F, He R, et al. Relationship between platelet/lymphocyte ratio and left atrial appendage thrombogenic milieu in patients with nonvalvular atrial fibrillation. Chin J Ultrason Med. (2022) 38(11):1229–33.
24. Bazoukis G, Letsas KP, Vlachos K, Saplaouras A, Asvestas D, Tyrovolas K, et al. Simple hematological predictors of AF recurrence in patients undergoing atrial fibrillation ablation. J Geriatr Cardiol. (2019) 16(9):671–5. doi: 10.11909/j.issn.1671-5411.2019.09.008
25. Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Kabakçı G, et al. Role of preablation neutrophil/lymphocyte ratio on outcomes of cryoballoon-based atrial fibrillation ablation. Am J Cardiol. (2013) 112(4):513–9. doi: 10.1016/j.amjcard.2013.04.015
26. Guo XY, Zhang S, Yan XL, Chen YW, Yu RH, Long DY, et al. Postablation neutrophil/lymphocyte ratio correlates with arrhythmia recurrence after catheter ablation of lone atrial fibrillation. Chin Med J. (2014) 127(6):1033–8. doi: 10.3760/cma.j.issn.0366-6999.20133001
27. Im SI, Shin SY, Na JO, Kim YH, Choi CU, Kim SH, et al. Usefulness of neutrophil/lymphocyte ratio in predicting early recurrence after radiofrequency catheter ablation in patients with atrial fibrillation. Int J Cardiol. (2013) 168(4):4398–400. doi: 10.1016/j.ijcard.2013.05.042
28. Ertaş G, Sönmez O, Turfan M, Kul S, Erdoğan E, Tasal A, et al. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. (2013) 324(1-2):49–52. doi: 10.1016/j.jns.2012.09.032
29. Saliba W, Barnett-Griness O, Elias M, Rennert G. Neutrophil to lymphocyte ratio and risk of a first episode of stroke in patients with atrial fibrillation: a cohort study. J Thromb Haemostasis. (2015) 13(11):1971–9. doi: 10.1111/jth.13006
30. Wu S, Yang Y-m, Zhu J, Ren J-m, Wang J, Zhang H, et al. Impact of baseline neutrophil-to-lymphocyte ratio on long-term prognosis in patients with atrial fibrillation. Angiology. (2021) 72(9):819–28. doi: 10.1177/00033197211000495
31. Fagundes A, Ruff CT, Morrow DA, Murphy SA, Palazzolo MG, Chen CZ, et al. Neutrophil-lymphocyte ratio and clinical outcomes in 19,697 patients with atrial fibrillation: analyses from ENGAGE AF- TIMI 48 trial. Int J Cardiol. (2023) 386:118–24. doi: 10.1016/j.ijcard.2023.05.031
32. Deng Y, Zhou F, Li Q, Guo J, Cai B, Li G, et al. Associations between neutrophil-lymphocyte ratio and monocyte to high-density lipoprotein ratio with left atrial spontaneous echo contrast or thrombus in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. (2023) 23(1):234. doi: 10.1186/s12872-023-03270-3
33. Etli M. Investigation of routine blood parameters for predicting embolic risk in patients with nonvalvular atrial fibrillation. Indian J Vascu Endovasc Surg. (2022) 9(1):36–9. doi: 10.4103/ijves.ijves_77_21
34. Fukuda Y, Okamoto M, Tomomori S, Matsumura H, Tokuyama T, Nakano Y, et al. In paroxysmal atrial fibrillation patients, the neutrophil-to-lymphocyte ratio is related to thrombogenesis and more closely associated with left atrial appendage contraction than with the left atrial body function. Intern Med. (2018) 57(5):633–40. doi: 10.2169/internalmedicine.9243-17
35. Tang L, Xia Y, Fang L. Correlation between left atrial thrombosis and neutrophil-to-lymphocyte ratio upon non-valvular atrial fibrillation. Clin Lab. (2022) 68(2):326–33. doi: 10.7754/Clin.Lab.2021.210409
36. Yalcin M, Aparci M, Uz O, Isilak Z, Balta S, Dogan M, et al. Neutrophil-lymphocyte ratio may predict left atrial thrombus in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. (2015) 21(2):166–71. doi: 10.1177/1076029613503398
37. Dolu AK, Akçay FA, Atalay M, Karaca M. Systemic immune-inflammation Index as a predictor of left atrial thrombosis in nonvalvular atrial fibrillation. J Tehran Uni Heart Cent. (2023) 18(2):87–93. doi: 10.11909/j.issn.1671-5411.2019.09.008
38. Li Q, Nie J, Cao M, Luo C, Sun C. Association between inflammation markers and all-cause mortality in critical ill patients with atrial fibrillation: analysis of the multi-parameter intelligent monitoring in intensive care (MIMIC-IV) database. IJC Heart and Vasculature. (2024) 51:101372. doi: 10.1016/j.ijcha.2024.101372
39. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res. (2020) 127(1):51–72. doi: 10.1161/CIRCRESAHA.120.316363
40. Goette A, Lendeckel U. Atrial cardiomyopathy: pathophysiology and clinical consequences. Cells. (2021) 10(10):2605. doi: 10.3390/cells10102605
41. Hohmann C, Pfister R, Mollenhauer M, Adler C, Kozlowski J, Wodarz A, et al. Inflammatory cell infiltration in left atrial appendageal tissues of patients with atrial fibrillation and sinus rhythm. Sci Rep. (2020) 10(1):1685. doi: 10.1038/s41598-020-58797-8
42. Grune J, Yamazoe M, Nahrendorf M. Electroimmunology and cardiac arrhythmia. Nat Rev Cardiol. (2021) 18(8):547–64. doi: 10.1038/s41569-021-00520-9
43. Ihara K, Sasano T. Role of inflammation in the pathogenesis of atrial fibrillation. Front Physiol. (2022) 13:862164. doi: 10.3389/fphys.2022.862164
44. Ajoolabady A, Nattel S, Lip GYH, Ren J. Inflammasome signaling in atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79(23):2349–66. doi: 10.1016/j.jacc.2022.03.379
45. Ying Y, Yu F, Luo Y, Feng X, Liao D, Wei M, et al. Neutrophil-to-Lymphocyte ratio as a predictive biomarker for stroke severity and short-term prognosis in acute ischemic stroke with intracranial atherosclerotic stenosis. Front Neurol. (2021) 12:705949. doi: 10.3389/fneur.2021.705949
46. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12(4):230–43. doi: 10.1038/nrcardio.2015.2
47. Ozkan E, Elcik D, Barutcu S, Kelesoglu S, Alp ME, Ozan R, et al. Inflammatory markers as predictors of atrial fibrillation recurrence: exploring the C-reactive protein to albumin ratio in cryoablation patients. J Clin Med. (2023) 12(19):6313. doi: 10.3390/jcm12196313
48. Zhao Z, Jiang B, Zhang F, Ma R, Han X, Li C, et al. Association between the systemic immune-inflammation index and outcomes among atrial fibrillation patients with diabetes undergoing radiofrequency catheter ablation. Clin Cardiol. (2023) 46(11):1426–33. doi: 10.1002/clc.24116
49. Suehiro H, Kiuchi K, Fukuzawa K, Yoshida N, Takami M, Watanabe Y, et al. Circulating intermediate monocytes and atrial structural remodeling associated with atrial fibrillation recurrence after catheter ablation. J Cardiovasc Electrophysiol. (2021) 32(4):1035–43. doi: 10.1111/jce.14929
50. Liu D, Li Y, Zhao Q. Effects of inflammatory cell death caused by catheter ablation on atrial fibrillation. J Inflamm Res. (2023) 16:3491–508. doi: 10.2147/JIR.S422002
51. Yano M, Egami Y, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, et al. Atrial fibrillation type modulates the clinical predictive value of neutrophil-to-lymphocyte ratio for atrial fibrillation recurrence after catheter ablation. Int J Cardiol Heart Vasc. (2020) 31:100664. doi: 10.1016/j.ijcha.2020.100664
52. Lu M, Zhang Y, Liu R, He X, Hou B. Predictive value of neutrophil to lymphocyte ratio for ischemic stroke in patients with atrial fibrillation: a meta-analysis. Front Neurol. (2022) 13:1029010. doi: 10.3389/fneur.2022.1029010
53. Lekkala SP, Mellacheruvu SP, Gill KS, Khela PS, Singh G, Jitta SR, et al. Association between preablation and postablation neutrophil-lymphocyte ratio and atrial fibrillation recurrence: a meta-analysis. J Arrhythm. (2024) 40(2):214–21. doi: 10.1002/joa3.12996
54. Dentali F, Nigro O, Squizzato A, Gianni M, Zuretti F, Grandi AM, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: a systematic review and meta-analysis of the literature. Int J Cardiol. (2018) 266:31–7. doi: 10.1016/j.ijcard.2018.02.116
55. Vakhshoori M, Nemati S, Sabouhi S, Yavari B, Shakarami M, Bondariyan N, et al. Neutrophil to lymphocyte ratio (NLR) prognostic effects on heart failure; a systematic review and meta-analysis. BMC Cardiovasc Disord. (2023) 23(1):555. doi: 10.1186/s12872-023-03572-6
56. Zhang C, Li M, Liu L, Deng L, Yulei X, Zhong Y, et al. Systemic immune-inflammation index as a novel predictor of major adverse cardiovascular events in patients undergoing percutaneous coronary intervention: a meta-analysis of cohort studies. BMC Cardiovasc Disord. (2024) 24(1):189. doi: 10.1186/s12872-024-03849-4
Keywords: neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, atrial fibrillation, meta-analysis
Citation: Chen X, Zhang X, Fang X and Feng S (2025) Association of inflammatory markers with clinical outcomes in atrial fibrillation: a meta-analysis. Front. Cardiovasc. Med. 12:1504163. doi: 10.3389/fcvm.2025.1504163
Received: 30 September 2024; Revised: 24 November 2025;
Accepted: 1 December 2025;
Published: 17 December 2025.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Marco Valerio Mariani, Sapienza University of Rome, ItalyUğur Canpolat, Hacettepe University, Türkiye
Yin Huang, Sichuan University, China
Copyright: © 2025 Chen, Zhang, Fang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomei Chen, eG14bWNoZW54aWFvbWVpQDE2My5jb20=
†These authors have contributed equally to this work
 Xiaomei Chen
Xiaomei Chen Xuge Zhang2,†
Xuge Zhang2,† Shenghong Feng
Shenghong Feng