- 1Key Laboratory of Ultra-Weak Magnetic Field Measurement Technology, Ministry of Education, School of Instrumentation and Optoelectronic Engineering, Beihang University, Beijing, China
- 2Department of Cardiology, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3State Key Laboratory of Traditional Chinese Medicine Syndrome / National Institute of Extremely-Weak Magnetic Field Infrastructure, Hangzhou, China
- 4Hefei National Laboratory, Hefei, China
Magnetocardiography (MCG) is a highly sensitive, non-invasive, and functional imaging technique that records and examines magnetic fields generated by the electrical activity of the heart to reflect cardiac electrophysiological changes, including the first superconducting quantum interference device and optically pumped magnetometers-MCG. The 60-year research process yields new understanding in the areas of signal extraction, processing, and clinical application for the detection and treatment of cardiac diseases. Especially, the significant advancements in magnetic sensor technology, preprocessing methods and denoising methods have promoted the development of MCG. This article systematically reviews 83 studies to provide the latest and general overview of MCG in acute chest pain (6 studies), acute coronary syndrome (10 studies), ischemic heart disease (13 studies), non-ischemic cardiomyopathies (3 studies), arrhythmia (9 studies), and fetal congenital arrhythmia (11 studies). We highlight its incremental value in the triage of acute chest pain, diagnosis and prognosis prediction of chronic and acute coronary syndromes. We also discuss the limitations of this field and directions of future development.
1 Introduction
Local currents are produced by depolarization, repolarization, and ion transmembrane mobility in cells. The electrophysiological activity of cells will produce weak magnetic signals, which are strongest in excitable cells, including myocardial and nerve cells. Magnetocardiography (MCG) can detect alterations in the magnetic fields induced by abnormal electrical activity in the heart through magnetically shielded or unshielded superconducting quantum interference device (SQUID) systems, optically pumped magnetometers (OPM), or portable miniaturized induction coils (1). Compared to electrocardiogram (ECG), which obtains electrical signals and current conventional imaging detection methods, MCG provides additional information for disease diagnosis through changes in magnetic signals and has unique advantages. First, MCG is not affected by the thickness of the pericardium or chest wall, providing more accurate information through its multi-channel array (2). In addition, MCG has a greater spatiotemporal resolution and is sensitive to the magnetic field produced by tangential current, which can simultaneously collect tangential and eddy currents in the subepicardial and deep myocardium (3–5).
Recent decades have seen numerous studies on signal processing and clinical applications of MCG. Several studies have concluded that MCG is more sensitive to triage of acute chest pain and diagnosis of ischemic heart disease (IHD) than widely used screening tools such as ECG, echocardiography (ECHO), and stress tests (6–8). Additionally, increasing research has linked MCG to fetal heart disease, myocardial inflammation, and arrhythmia. As an exceptionally sensitive, non-contact, non-invasive, and radiation-free examination method, MCG has potential clinical value. Therefore, we aimed to comprehensively summarize the literature on the clinical application of MCG and provide updated data.
2 Methods
In this review, we conducted an extensive electronic search of all English-language studies published before Oct 31, 2024, from the PubMed, Web of Science, EMBASE, and Cochrane Library databases. We used the following search terms: “magnetocardiography” AND (“heart disease” OR “cardiac disease” OR “fetal disease” OR “clinical” OR “chest pain” OR “coronary syndrome” OR “arrhythmia” OR “cardiomyopathy”). After removing books, editorials, case reports, letters and duplicates, we included studies that reported MCG in cardiac diseases. Furthermore, references cited in the included publications were examined to identify additional relevant articles. Finally, 83 studies published between January 1971 and October 2024 were included in this review.
3 Results
3.1 Magnetocardiography
The MCG technology has been developed for nearly 60 years. In 1970, MCG based on a SQUID was created (9). This magnetic detector offered high sensitivity, a wide measurement range, and a broad frequency band, significantly enhancing the spatial accuracy and signal-to-noise ratio (9). In the past 60 years, the development of multichannel sensors, unshielded SQUID systems, and the emergence of OPM have contributed to the advancement of MCG. While SQUID have been the primary tool for clinical research, OPM are being explored for their potential in making MCG more accessible. In 1991, Fenici et al. developed non-magnetic catheter technology based on 10 years of practical experience in MCG and successfully achieved arrhythmia localization through a single-channel system, laying early evidence for the clinical research and application of MCG (10).
At present, there is still no standardized MCG consensus or database to define the parameters of one-dimensional butterfly diagram (BFD), two-dimensional magnetic field map and current density map in MCG. Several scholars have recorded commonly utilized parameters of one-dimensional and multi-dimensional MCG, including interval duration, waveform, dipole phenomenon, and vector parameters, and have identified significant sex-based and age-related differences in amplitude and repolarization angle parameters (11, 12).
3.2 Rapid triage and mortality prediction of acute chest pain
Acute chest pain (ACP) continues a prevalent complaint in emergency and internal medicine, with over 7 million annual visits, of which 20%–40% are non-cardiac (13, 14). As a heterogeneous group of diseases, accurate and expeditious recognition of acute coronary syndrome (ACS), acute pulmonary embolism, aortic dissection, and other high-risk ACP conditions represents the primary focus and challenge in the emergency management of ACP. There is a lack of sensitive, convenient and rapid stratification methods to reduce the risk of misdiagnosis. Same as avoiding unnecessary invasive tests and minimizing patient loss.
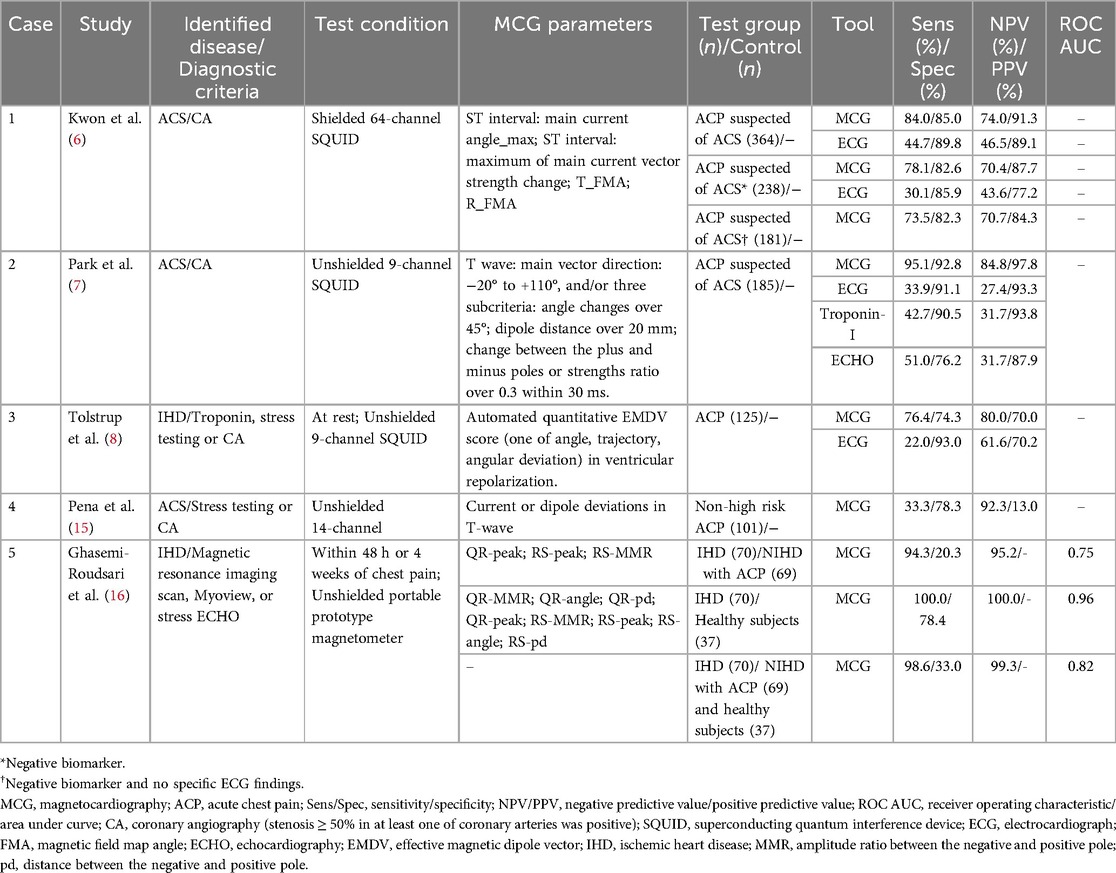
Previous studies have investigated the utility of MCG in triaging patients with ACP (Table 1). Compared to conventional diagnostic tools (ECG, troponin I, and ECHO) at admission, MCG exhibited superior performance in identifying ACS and IHD through multiple MCG parameters including the angle, intensity, shape, and additional characteristics of the current or magnetic dipole vector during repolarization (6–8). Pena et al. developed a novel imaging and analysis system to transform MCG data into dynamic 90-second images to evaluate non-high-risk ACP patients in the emergency observation unit, its specificity (78.3%) and negative predictive value (NPV, 92.3%) suggested potential utility in safely ruling out critical ischemia and guiding discharge decisions (15). Ghasemi-Roudsari et al. further explored a portable MCG and built a diagnostic prediction model for ACP patients by logistic regression analysis of 10 key parameters derived from QR and RS segments in magnetic field map (MFM) and current density map (CDM), but the model showed limited accuracy in differentiating IHD among ACP patients (16). Beyond diagnosis, MCG parameters showed strong prognostic significance. Two MCG parameters of QTc prolongation and low repolarization reserve and one clinical parameter of elevated serum creatinine were indicators of long-term mortality in ACP patients (P < 0.05), achieving 90.9% sensitivity, 85.6% specificity, and 99.4% NPV for cardiac death (17). Patients with abnormal MCG findings faced a nine-fold increased risk of cardiac death (17).
3.3 Acute coronary syndrome
ACS constitutes a continuous spectrum of life-threatening conditions initiated by coronary atherosclerotic plaque rupture and subsequent thrombotic occlusion, encompassing ST-elevation myocardial infarction (STEMI) and non-ST-elevation ACS (NSTE-ACS) (18). Characterized by high prevalence, sudden onset, and significant mortality, ACS requires urgent diagnosis to minimize the duration of emergency stay, as acute ischemia leads to irreversible myocardial cell necrosis. Currently, NSTE-ACS often lacks specific changes in ECG and myocardial damage indicators, requiring continuous testing for diagnosis. There is a pressing need for more efficient methods to facilitate early detection and risk stratification.
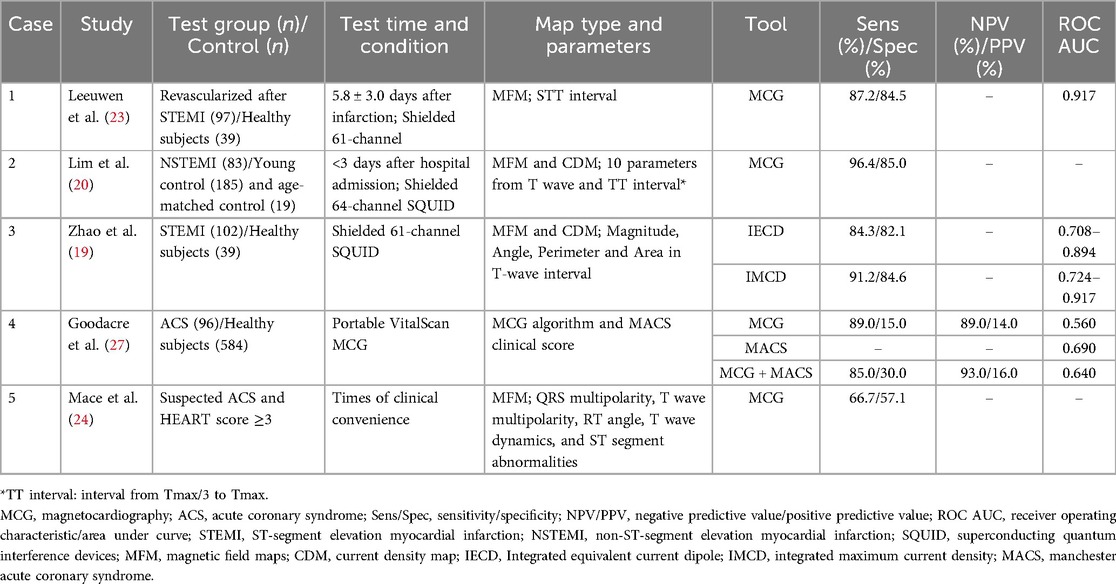
We compiled a comprehensive list of ACS articles pertaining to MCG (Table 2). In ACS, four qualitative and quantitative parameters including magnitude, angle, perimeter and area of MFM, as well as the T-wave CDM, exhibited marked abnormalities; T-peak maximum current angle and magnetic field angle had the highest sensitivity (96.4%), correlating positively with the severity of myocardial infarction (19–22). Among them, the integrated maximum CDM provided spatiotemporal information of ventricular repolarization process, with higher sensitivity (91.2%) and specificity (84.6%) for identifying STEMI than the integrated equivalent current dipole method (sensitivity, 84.3%; specificity, 82.1%) (19). ACS consistently exhibits non-dipole phenomena in MFM, with coherence impacted particularly during repolarization, the relationship of depolarization parameters and ACS was weak (23, 24). In 70% of unstable angina (UA) and 92.5% of non-ST-elevation myocardial infarction (NSTEMI), NSTE-ACS manifested as abnormal dipole position and shape, and was positively correlated with the severity of coronary artery stenosis (20).
MCG also demonstrated predictive capacity for clinical outcomes. Bang et al. illustrated that non-dipole phenomenon of T-peak independently predicted major adverse cardiac events, including all-cause death, reinfarction, and percutaneous coronary intervention (hazard ratio = 2.89, 95% confidence interval: 1.20–6.97, P = 0.02) (25). A three-year follow-up study of NSTEMI patients revealed that MCG outperformed troponin I, ECHO, and ECG in mortality prediction (relative risk: 4.58 vs. 2.48 vs. 1.58 vs. 1.69), with abnormal MCG independently portending poor prognosis (7, 26).
It can be seen that MFM and CDM during the repolarization phase have application prospects for both diagnosis and prognostic prediction in ACS. Future research requires further quantification of MFM and CDM parameters to establish a unified standard. Additionally, MCG has only been exclusively detected after the treatment of acute myocardial infarction, and point-of-care device reliability has not yet been fully validated (27). Further investigations are warranted to examine the feasibility of early detection in the post-event period.
3.4 Detection and classification of ischemic heart disease
IHD arises from a disparity between coronary blood supply and myocardial oxygen demand, caused by coronary spasm, stenosis, or obstruction, which ultimately leads to myocardial hypoxia or necrosis. IHD manifests as angina, myocardial infarction or even sudden cardiac death clinically. As a major global health concern, IHD affects 18.2 million cases in the United States alone and remains the leading cause of death worldwide, with 9.24 million global deaths attributed to IHD in 2022, representing 80% of sudden cardiac fatalities and 34% of cardiovascular disease-related deaths under before aged 70 (28–30). The heterogeneous clinical presentations and complex differential diagnosis of IHD pose significant challenges in developing rapid and accurate methods for case identification and risk stratification.
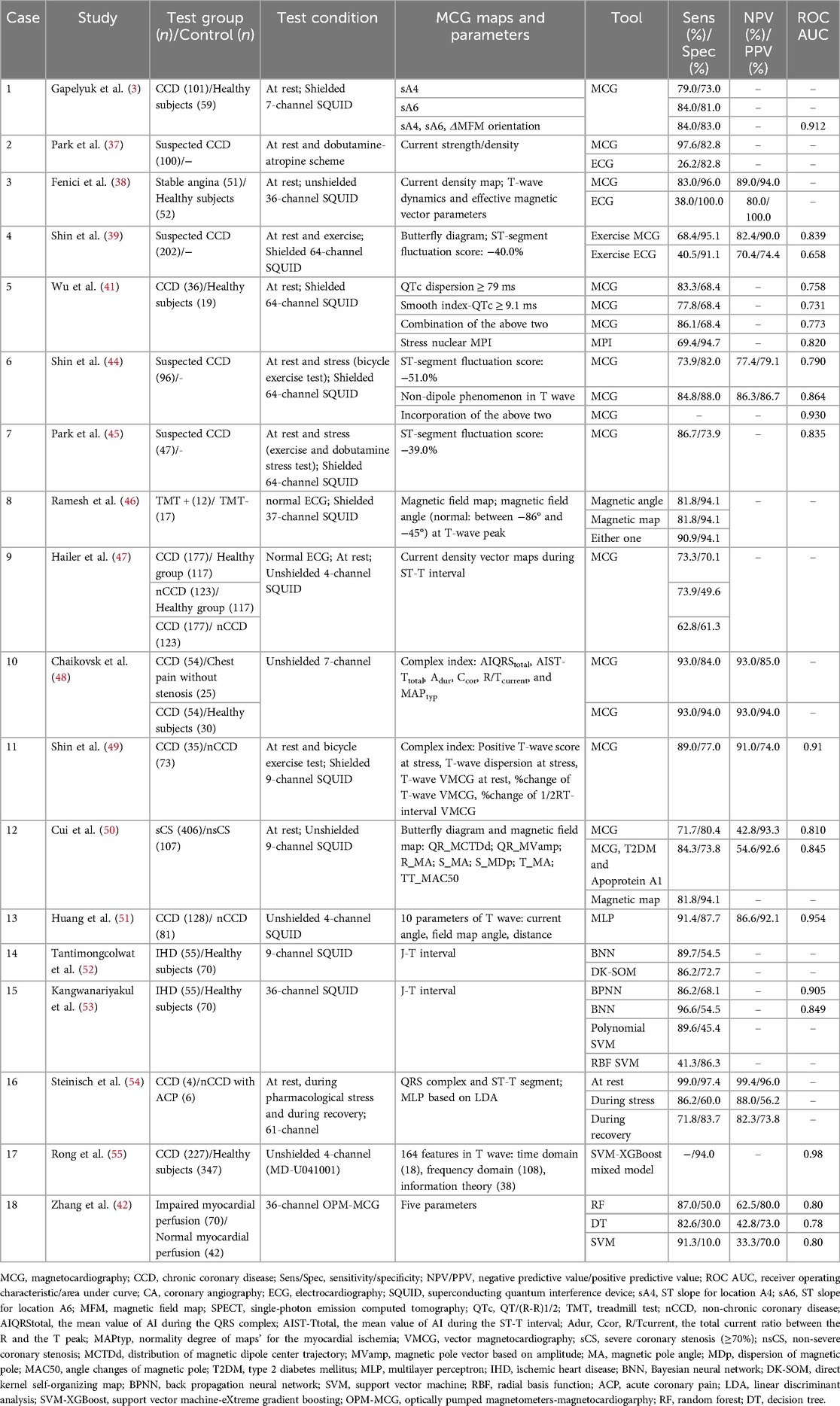
ECG and ECHO serve as first-line screening tools for IHD detection. A meta-analysis of 147 studies involving 24,074 patients reported a sensitivity of 68% and specificity of 77% for ECG stress tests (31). Coronary computed tomography angiography (CCTA) offers a high diagnostic accuracy in assessing anatomic coronary stenosis, with a sensitivity of 94%, specificity of 83%, and NPV of 99% (32, 33). However, its poor positive predictive value (PPV) (48%–64%) and inability to reliably evaluate hemodynamic changes or screen large-scale population necessitate additional supplementary testing to determine ischemia severity (33, 34). Existing non-invasive functional tests use perfusion imaging with radioactive tracers to evaluate myocardial viability, though these methods carry inherent risks of radiation exposure and potential adverse effects. In contrast, MCG demonstrates promising diagnostic performance. Three studies have shown that abnormal ST-segment and T-wave in MCG during myocardial ischemia can be correctly classified in 80% of cases using only one parameter (sA4), irrespective of the number or location of affected vessels, when combining all three parameters achieved 84% sensitivity and 83% specificity (area under the curve, AUC: 0.912) (3, 35). Comparative analyses revealed that MCG exhibited greater mean differences in QRS complex, T-wave, and ST-segment abnormalities than ECG (37% vs. 26%) (36).
We visually listed the literature on the diagnostic value of MCG for coronary stenosis in Table 3. Whether at rest or under stress testing, MCG demonstrated superior efficacy than ECG in identifying chronic coronary disease (CCD), regardless of whether recordings were obtained in magnetically shielded or unshielded environments (37–40). Additionally, QTc parameters of MCG successfully screened CCD patients at rest, providing a viable alternative for individuals unable to tolerate stress testing (41). Machine learning-based MCG diagnostic models have achieved high sensitivity (82.6%–91.3%) in detecting myocardial ischemia, though specificity remains modest (10.0%–50.0%) (42). For borderline coronary lesions (40%–90% stenosis), MCG yielded an AUC of 0.864 (95% CI: 0.803–0.925), suggesting its potential to reduce unnecessary invasive procedures (43).
Different MCG parameters and analytical approaches have been explored to optimize coronary stenosis detection in suspected CCD patients. The combination of ST-segment fluctuation score and qualitative non-dipole parameter achieved the highest diagnostic accuracy (AUC: 0.93) (39, 44, 45). In chronic chest pain patients with normal ECG, magnetic field map and angles distinguished coronary stenosis with 90.9% sensitivity and 94.1% specificity (46). CDM during ST-T interval effectively differentiated healthy individuals from CCD patients, though their ability to distinguish non-CCD from CCD cases was inferior to MFM (47). Integrating one-dimensional BFD with MFM enhanced diagnostic performance and localized stenosis with 69%–77% accuracy (48–50). To expedite data processing and minimize manual errors, automated machine learning approaches are applied for parameter extraction and analysis. Bayesian neural networks and multilayer perceptron demonstrated high sensitivity, making them suitable for high-risk population screening (51–54). A hybrid classifier Support Vector Machine-eXtreme Gradient Boosting, incorporating 164T-wave features (time domain, frequency domain, and information theory characteristics), achieved exceptional performance (AUC, 0.98; specificity, 94.0%) while effectively reducing false positives (55). In summary, MCG showed particular strengths in identifying ischemia in resting patients and those with borderline lesions. Individual parameters in MFM and CDM as well as the complex index in BFD are all strong predictors, AI-enhanced approaches further improved diagnostic accuracy. Notably, MCG also showed acceptable potential for detecting non-obstructive coronary microvascular dysfunction (sensitivity: 68%, specificity: 65%) (56).
It is important to emphasize that all aforementioned studies were conducted on patients with CCD, indicating that MCG can detect lesions with coronary stenosis of ≥70%. However, for vascular stenosis that has not yet affected the structure or function of cells, it does not cause abnormal conduction or magnetic signals, MCG will be unable to detect it. In addition, the relationship between the degree of coronary stenosis and changes in magnetocardiographic vector has not yet been standardized, so the current evidence supporting magnetocardiographic evaluation in this context remains limited and can not contribute to clinical decision-making.
3.5 Non-ischemic cardiomyopathies
Beyond IHD, a spectrum of cardiac disorders arising from genetic predisposition, metabolic dysfunction, and structural myocardial changes are collectively termed non-ischemic cardiomyopathies (57). Integrating more dimensions to achieve precise classification of cardiomyopathies is a way forward for efficient diagnosis and treatment. Emerging research have investigated the promising role of MCG in the diagnosis, treatment monitoring, and recurrence prediction of non-ischemic cardiomyopathies.
Changes of magnetic vector (the value ≥ 0.051) discriminated cardiomyopathy from controls with 59% sensitivity, 95% specificity, 93% PPV, and 64% NPV, and reflected immune-suppressive therapeutic effect earlier than ECHO (7 vs. 30 days) (58). In dilated cardiomyopathy, MCG effectively predicted major adverse cardiac events (MACE) by detecting left intraventricular disorganized conduction (LIDC) with high spatiotemporal resolution to facilitate risk stratification, and the predictive value of LIDC was superior to that of traditional ECG indices such as fragmented QRS waves and late potential (59). By capturing early changes of myocardial electrical remodeling and quantifying the Kullback Leibler (KL) entropy of the cardiac magnetic field topology, MCG differentiated hypertrophic cardiomyopathy from healthy individuals or cardiac hypertrophy caused by other reasons, and the accuracy increased to 87.9% when combined with regional magnetic field strength parameters (sensitivity from 78.8% to 84.8% and specificity from 86.9% to 88.9%), and identified all patients with hypertrophic cardiomyopathy carrying genetic mutation in familial screening (60).
3.6 Diagnosis and localization of arrhythmia
Compared to ECG, MCG has a enhanced spatial resolution of cardiac current distribution and more comprehensive insights into ventricular depolarization and repolarization. As summarized in Table 4, growing evidence supports the utility of MCG in risk stratification, original localization, and management guidance.
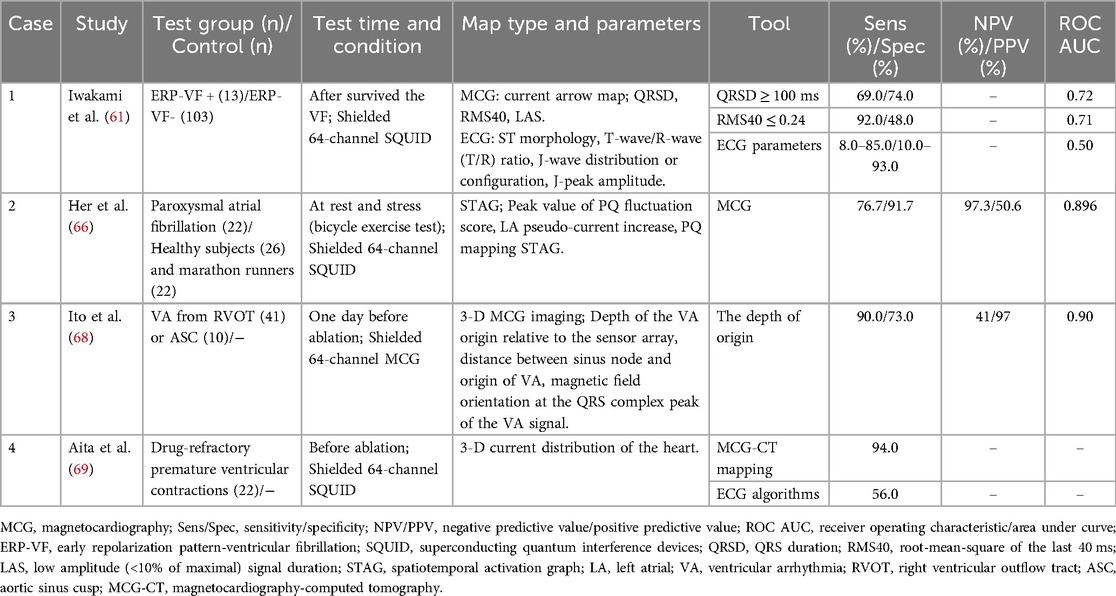
There was no statistical difference in ECG parameters between benign and malignant early repolarization patterns (ERP). Conversely, ERP-ventricular fibrillation (ERP-VF) patients in MCG had prolonged QRS complex (108 ± 24 vs. 91 ± 23 ms, P = 0.02) and diminished root-mean-square voltage of the last 40 ms (0.10 ± 0.08 vs. 0.25 ± 0.20, P = 0.01) (61). In addition, compared with invasive electrophysiological mapping, the accuracy of MCG in differentiating benign and malignant ventricular arrhythmia was 94.7% (62). Beyond non-invasive risk stratification for VF, the low-amplitude QRS complex also predicted arrhythmia risk after MI, with QRS duration > 121 ms as an independent predictor (63, 64). The P-wave duration, PR interval and P-wave depolarization of patients with atrial fibrillation were significantly longer than those of healthy controls, providing more sensitive atrial fibrillation susceptibility markers than ECG, the angle dynamics of P-wave depolarization was an independent predictor of AF recurrence (P = 0.037) (65). The alteration in left atrial pseudo-current conversion detected left atrial dysfunction in paroxysmal atrial fibrillation, and identify different atrial conduction pathways (such as Bachmann bundles, margin of fossa ovalis, and coronary sinus ostial region) with an accuracy of 93%, providing a new perspective for the mechanism study of atrial fibrillation (66, 67).
The multi-channel sensors of MCG provide insights into the temporal and spatial distribution of cardiac magnetic fields and enable the localization of arrhythmia origins through three-dimensional imaging. Ito et al. developed a new spatial filter to differentiate ventricular arrhythmia originating from the right ventricular outflow tract and the aortic sinus cusp, through three parameters, with depth being the most powerful predictor (68). By integrating cardiac computed tomography to reconstruct the 3-D current distribution, the origin of premature ventricular complexes throughout the ventricle was pinpointed with 94% accuracy (17/18) (69).
3.7 Fetal magnetocardiography
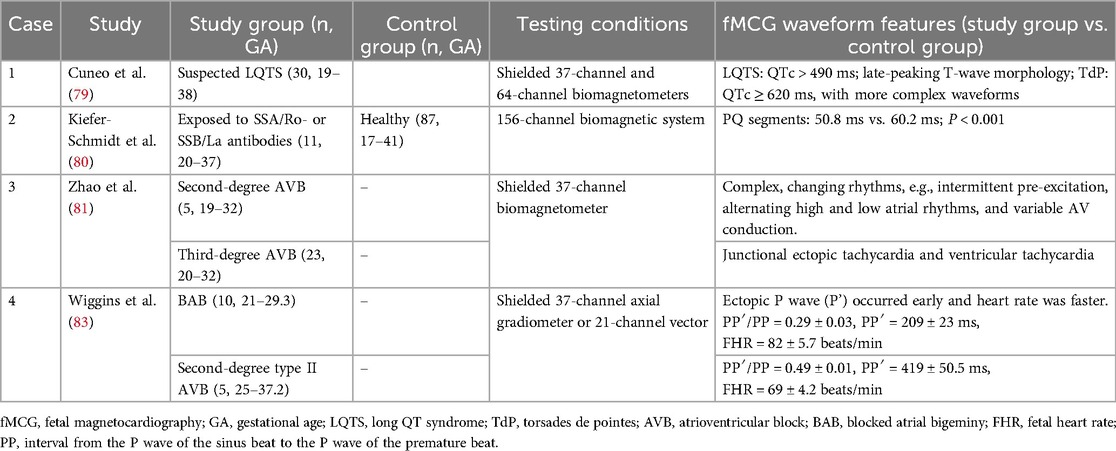
Current evaluation of fetal cardiac structure and function relies on cardiotocography and ECHO, but they lack electrophysiological data on the conduction system and cannot identify certain malignant arrhythmia such as torsade de pointes (70). Fetal MCG (fMCG) allows for precise assessment of cardiac time intervals, signal characteristics, and various rhythm patterns by extracting fetal cardiac magnetic signals after the 15 weeks of pregnancy (71–74). It provides vital information about fetal cardiac development and function, as shown in Table 5.
Both the 2014 and 2024 American Heart Association scientific statements advocated fMCG to assess cardiac conduction and rhythm abnormalities in fetuses with suspected or confirmed congenital heart disease (Class 2a) (75, 76). Strand et al. used SQUID to characterize fMCG waveforms of 132 healthy fetuses at 15.7–39.9 weeks of gestation, P-wave, PR-interval, QRS complex, and RR interval increased with gestational age (P < 0.001), while QT-interval and QTc (QTc = QT/RR1/2) remained constant throughout gestation (73). In contrast, another study used new magnetograph dedicated to fetal recordings and concluded that P wave, QRS complex, ST-segment, QT-interval and QTc increased with gestational age, PQ segment and T-wave were independent of gestation (77). It is imperative to elucidate the waveform characteristics of fetuses at different developmental stages.
Wacker-Gussmann et al. reviewed 144 fetuses with tachycardia, bradycardia atrioventricular block (AVB), or familial Long QT syndrome (LQTS) (71). As a result, fMCG facilitated additional diagnoses in 81% (117/144) of cases, with 56% (81/144) exhibiting critical alterations, prompting a change in the treatment regimen for 35 patients (71). LQTS is a hereditary ion channel disorder that leads to potentially fatal heart rhythm abnormalities and accounts for 10% of sudden infant death and unexplained stillbirths (78). Early detection of abnormal heart rates and intrauterine treatment can reduce mortality. Research published in Circulation in 2013 showed that QTc > 490 ms, corroborated by genetic testing, achieved 89% sensitivity and specificity for LQTS between 19 and 38 weeks of gestation, with QTc ≥ 620 ms predicting a severe TdP phenotype, characterized by various uncommon rhythms such as second-degree AVB, T-wave alternation, and QRS alternation (79).
The waveform characteristics of AVB with different degrees of severity are much more complex than those of ECHO. Fetuses with positive maternal SSA/Ro SSB/La antibodies are at elevated risk of developing immune AVB. The prolonged PQ segment predicts early atrioventricular node involvement in fetuses (60.2 ms vs. 50.8 ms; P < 0.001) (80). Different waveforms classified AVB and accurately identified third-degree AVB with structural heart disease and poor prognosis (81, 82). ECHO struggled to distinguish blocked atrial bigeminy (BAB) from 2nd degree AVB, despite their divergent clinical management and prognosis (83). fMCG discerned differences between the two entities, with the ectopic P wave (P’) manifesting earlier and a higher heart rate observed in BAB cases, thus offering pivotal evidence for differential diagnosis (83).
4 Limitations
Although many studies have proved that MCG has excellent clinical diagnostic and prognostic predictive capabilities across various cardiac diseases and is a promising clinical tool in the future, certain limitations persist within this domain. First, there exists heterogeneity in study design, and parameters, with different methodologies, MCG systems, and analytical parameters employed across studies. The absence of standardized protocols in the acquisition of cardiomagnetic images and data, as well as in parameter definitions and diagnostic thresholds constrains the general applicability and clinical advancement of research findings. Establishing uniform guidelines for MCG interpretation is crucial for advancing the field. Secondly, most clinical studies of MCG are conducted in single-center with small cohorts and meticulously screened populations. This may introduce selection bias and lead to an overestimation of diagnostic accuracy. Consequently, larger, multi-center studies are necessary to validate the reproducibility of MCG across diverse populations, devoid of established clinical backgrounds and in varied environments. Finally, research evidence concerning non-ischemic cardiomyopathy and UA is limited, and there are few studies on MCG parameters and long-term prognosis verification, thereby limiting the robustness of MCG results. Therefore, future research must focus on optimizing the standardization of MCG data collection processes, establishing unified parameter reference ranges, and identifying specific parameters for various diseases.
5 Conclusion
Magnetocardiography provides a new perspective for the rapid and non-invasive identification of critical and severe heart diseases. The high spatial and temporal resolution and absence of interference from human tissue with the magnetic signal confer excellent functional imaging capabilities to MCG, demonstrating superior diagnostic performance for adult and fetal heart disease, including MI, adult and fetal arrhythmia, and rapid triage of ACP. However, its application is still limited to a few research centers, and the lack of uniform equipment standards and specifications makes it difficult to directly compare the results between different devices. Furthermore, there remains a paucity of large-scale clinical evidence and well-defined diagnostic criteria for MCG.
Future research should focus on validating parameters, establishing usage standards, and integrating MCG into clinical practice to develop high-performance devices suitable for routine environments, ensuring the rapid and accurate diagnosis of acute and severe heart diseases.
Author contributions
JL: Data curation, Writing – original draft, Writing – review & editing. YS: Writing – review & editing. CS: Writing – review & editing, Validation. XN: Supervision, Writing – review & editing. MX: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was partially supported by the National Key Research and Development Program of China (2022YFC2407000), and the National Natural Science Foundation of China (U23A20485).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MCG, magnetocardiography; fMCG, fetal magnetocardiography; IHD, ischemic heart disease; CCD, chronic cardiac disease; ACS, acute coronary syndrome; NICM, non-ischemic cardiomyopathy.
References
1. Wang Y, Zhao ZG, Chai Z, Fang JC, Chen M. Electromagnetic field and cardiovascular diseases: a state-of-the-art review of diagnostic, therapeutic, and predictive values. FASEB J. (2023) 37(10):e23142. doi: 10.1096/fj.202300201RR
2. Plonsey R. Capability and limitations of electrocardiography and magnetocardiography. IEEE Trans Biomed Eng. (1972) 19(3):239–44. doi: 10.1109/TBME.1972.324123
3. Gapelyuk A, Wessel N, Fischer R, Zacharzowsky U, Koch L, Selbig D, et al. Detection of patients with coronary artery disease using cardiac magnetic field mapping at rest. J Electrocardiol. (2007) 40(5):401–7. doi: 10.1016/j.jelectrocard.2007.03.013
4. Dutz S, Bellemann ME, Leder U, Haueisen J. Passive vortex currents in magneto- and electrocardiography: comparison of magnetic and electric signal strengths. Phys Med Biol. (2006) 51(1):145–51. doi: 10.1088/0031-9155/51/1/011
5. Smith FE, Langley P, van Leeuwen P, Hailer B, Trahms L, Steinhoff U, et al. Comparison of magnetocardiography and electrocardiography: a study of automatic measurement of dispersion of ventricular repolarization. Europace. (2006) 8(10):887–93. doi: 10.1093/europace/eul070
6. Kwon H, Kim K, Lee YH, Kim JM, Yu KK, Chung N, et al. Non-invasive magnetocardiography for the early diagnosis of coronary artery disease in patients presenting with acute chest pain. Circ J. (2010) 74(7):1424–30. doi: 10.1253/circj.CJ-09-0975
7. Park JW, Hill PM, Chung N, Hugenholtz PG, Jung F. Magnetocardiography predicts coronary artery disease in patients with acute chest pain. Ann Noninvasive Electrocardiol. (2005) 10(3):312–23. doi: 10.1111/j.1542-474X.2005.00634.x
8. Tolstrup K, Madsen BE, Ruiz JA, Greenwood SD, Camacho J, Siegel RJ, et al. Non-invasive resting magnetocardiographic imaging for the rapid detection of ischemia in subjects presenting with chest pain. Cardiology. (2006) 106(4):270–6. doi: 10.1159/000093490
9. Cohen D, Norman JC, Molokhia F, Hood W Jr. Magnetocardiography of direct currents: S-T segment and baseline shifts during experimental myocardial infarction. Science. (1971) 172(3990):1329–33. doi: 10.1126/science.172.3990.1329
10. Fenici RR, Melillo G, Masselli M. Clinical magnetocardiography. 10 years experience at the catholic university. Int J Card Imaging. (1991) 7(3-4):151–67. doi: 10.1007/BF01797748
11. Kandori A, Ogata K, Watanabe Y, Takuma N, Tanaka K, Murakami M, et al. Space-time database for standardization of adult magnetocardiogram-making standard MCG parameters. Pacing Clin Electrophysiol. (2008) 31(4):422–31. doi: 10.1111/j.1540-8159.2008.01011.x
12. Sorbo AR, Lombardi G, La Brocca L, Guida G, Fenici R, Brisinda D. Unshielded magnetocardiography: repeatability and reproducibility of automatically estimated ventricular repolarization parameters in 204 healthy subjects. Ann Noninvasive Electrocardiol. (2018) 23(3):e12526. doi: 10.1111/anec.12526
13. Kontos MC, de Lemos JA, Deitelzweig SB, Diercks DB, Gore MO, Hess EP, et al. 2022 ACC expert consensus decision pathway on the evaluation and disposition of acute chest pain in the emergency department: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. (2022) 80(20):1925–60. doi: 10.1016/j.jacc.2022.08.750
14. Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting–a population-based study. Aliment Pharmacol Ther. (2003) 17(9):1115–24. doi: 10.1046/j.1365-2036.2003.01557.x
15. Pena ME, Pearson CL, Goulet MP, Kazan VM, DeRita AL, Szpunar SM, et al. A 90-second magnetocardiogram using a novel analysis system to assess for coronary artery stenosis in emergency department observation unit chest pain patients. Int J Cardiol Heart Vasc. (2020) 26:100466. doi: 10.1016/j.ijcha.2019.100466
16. Ghasemi-Roudsari S, Al-Shimary A, Varcoe B, Byrom R, Kearney L, Kearney M. A portable prototype magnetometer to differentiate ischemic and non-ischemic heart disease in patients with chest pain. PLoS One. (2018) 13(1):e0191241. doi: 10.1371/journal.pone.0191241
17. Wessel N, Kim JS, Joung BY, Ko YG, Dischl D, Gapelyuk A, et al. Magnetocardiography at rest predicts cardiac death in patients with acute chest pain. Front Cardiovasc Med. (2023) 10:1258890. doi: 10.3389/fcvm.2023.1258890
18. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44(38):3720–826. doi: 10.1093/eurheartj/ehad191
19. Zhao C, Jiang S, Wu Y, Zhu J, Zhou D, Hailer B, et al. An integrated Maximum current density approach for noninvasive detection of myocardial infarction. IEEE J Biomed Health Inform. (2018) 22(2):495–502. doi: 10.1109/JBHI.2017.2649570
20. Lim HK, Kwon H, Chung N, Ko YG, Kim JM, Kim IS, et al. Usefulness of magnetocardiogram to detect unstable angina pectoris and non-ST elevation myocardial infarction. Am J Cardiol. (2009) 103(4):448–54. doi: 10.1016/j.amjcard.2008.10.013
21. Lim HK, Chung N, Kim K, Ko YG, Kwon H, Lee YH, et al. Can magnetocardiography detect patients with non-ST-segment elevation myocardial infarction? Ann Med. (2007) 39(8):617–27. doi: 10.1080/07853890701538040
22. Lim H K, Kim K, Lee YH, Chung N. Detection of non-ST-elevation myocardial infarction using magnetocardiogram: new information from spatiotemporal electrical activation map. Ann Med. (2009) 41(7):533–46. doi: 10.1080/07853890903107883
23. Van Leeuwen P, Hailer B, Beck A, Eiling G, Grönemeyer D. Changes in dipolar structure of cardiac magnetic field maps after ST elevation myocardial infarction. Ann Noninvasive Electrocardiol. (2011) 16(4):379–87. doi: 10.1111/j.1542-474X.2011.00466.x
24. Mace SE, Peacock WF, Stopyra J, Mahler SA, Pearson C, Pena M, et al. Accelerated magnetocardiography in the evaluation of patients with suspected cardiac ischemia: the MAGNETO trial. Am Heart J Plus. (2024) 40:100372. doi: 10.1016/j.ahjo.2024.100372
25. Bang WD, Kim K, Lee YH, Kwon H, Park Y, Pak HN, et al. Repolarization heterogeneity of magnetocardiography predicts long-term prognosis in patients with acute myocardial infarction. Yonsei Med J. (2016) 57(6):1339–46. doi: 10.3349/ymj.2016.57.6.1339
26. Park JW, Leithäuser B, Hill P, Jung F. Resting magnetocardiography predicts 3-year mortality in patients presenting with acute chest pain without ST segment elevation. Ann Noninvasive Electrocardiol. (2008) 13(2):171–9. doi: 10.1111/j.1542-474X.2008.00217.x
27. Goodacre S, Walters SJ, Qayyum H, Coffey F, Carlton E, Coats T, et al. Diagnostic accuracy of the magnetocardiograph for patients with suspected acute coronary syndrome. Emerg Med J. (2021) 38(1):47–52. doi: 10.1136/emermed-2020-210396
28. Mensah GA, Fuster V, Roth GA. A heart-healthy and stroke-free world: using data to inform global action. J Am Coll Cardiol. (2023) 82(25):2343–9. doi: 10.1016/j.jacc.2023.11.003
29. Mensah GA, Fuster V, Murray CJL, Roth GA. Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. (2023) 82(25):2350–473. doi: 10.1016/j.jacc.2023.11.007
30. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2100–32. doi: 10.1016/S0140-6736(24)00367-2
31. Detrano R, Gianrossi R, Froelicher V. The diagnostic accuracy of the exercise electrocardiogram: a meta-analysis of 22 years of research. Prog Cardiovasc Dis. (1989) 32(3):173–206. doi: 10.1016/0033-0620(89)90025-X
32. Sirajuddin A, Mirmomen SM, Kligerman SJ, Groves DW, Burke AP, Kureshi F, et al. Ischemic heart disease: noninvasive imaging techniques and findings. Radiographics. (2021) 41(4):990–1021. doi: 10.1148/rg.2021200125
33. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. (2008) 52(21):1724–32. doi: 10.1016/j.jacc.2008.07.031
34. Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. (2010) 55(25):2816–21. doi: 10.1016/j.jacc.2009.11.096
35. Hänninen H, Takala P, Korhonen P, Oikarinen L, Mäkijärvi M, Nenonen J, et al. Features of ST segment and T-wave in exercise-induced myocardial ischemia evaluated with multichannel magnetocardiography. Ann Med. (2002) 34(2):120–9. doi: 10.1080/07853890252953518
36. Alday EA, Ni H, Zhang C, Colman MA, Gan Z, Zhang H. Comparison of electric- and magnetic-cardiograms produced by myocardial ischemia in models of the human ventricle and torso. PLoS One. (2016) 11(8):e0160999. doi: 10.1371/journal.pone.0160999
37. Park JW, Leithäuser B, Vrsansky M, Jung F. Dobutamine stress magnetocardiography for the detection of significant coronary artery stenoses—a prospective study in comparison with simultaneous 12-lead electrocardiography. Clin Hemorheol Microcirc. (2008) 39(1–4):21–32. doi: 10.3233/CH-2008-1064
38. Fenici R, Brisinda D. Predictive value of rest magnetocardiography in patients with stable angina. Int Congr Ser. (2007) 1300:737–40. doi: 10.1016/j.ics.2007.02.022
39. Shin ES, Chung JH, Park SG, Saleh A, Lam YY, Bhak J, et al. Comparison of exercise electrocardiography and magnetocardiography for detection of coronary artery disease using ST-segment fluctuation score. Clin Hemorheol Microcirc. (2019) 73(2):283–91. doi: 10.3233/CH-180485
40. Huang X, Hua N, Tang F, Zhang S. Effectiveness of magnetocardiography to identify patients in need of coronary artery revascularization: a cross-sectional study. Cardiovasc Diagn Ther. (2020) 10(4):831–40. doi: 10.21037/cdt-20-121
41. Wu YW, Lin LC, Tseng WK, Liu YB, Kao HL, Lin MS, et al. QTc heterogeneity in rest magnetocardiography is sensitive to detect coronary artery disease: in comparison with stress myocardial perfusion imaging. Acta Cardiol Sin. (2014) 30(5):445–54.27122818
42. Zhang H, Ma Z, Mi H, Jiao J, Dong W, Yang S, et al. Diagnostic value of magnetocardiography to detect abnormal myocardial perfusion: a pilot study. Rev Cardiovasc Med. (2024) 25(10):379. doi: 10.31083/j.rcm2510379
43. Yang S, Feng L, Zhang M, Zhang M, Ma Z, Zhang H, et al. Development and validation of a clinical diagnostic model for myocardial ischaemia in borderline coronary lesions based on optical pumped magnetometer magnetocardiography: a prospective observational cohort study. BMJ Open. (2024) 14(10):e086433. doi: 10.1136/bmjopen-2024-086433
44. Shin ES, Lam YY, Her AY, Brachmann J, Jung F, Park JW. Incremental diagnostic value of combined quantitative and qualitative parameters of magnetocardiography to detect coronary artery disease. Int J Cardiol. (2017) 228:948–52. doi: 10.1016/j.ijcard.2016.11.165
45. Park JW, Shin ES, Ann SH, Godde M, Park LS, Brachmann J, et al. Validation of magnetocardiography versus fractional flow reserve for detection of coronary artery disease. Clin Hemorheol Microcirc. (2015) 59(3):267–81. doi: 10.3233/CH-141912
46. Ramesh R, Senthilnathan S, Satheesh S, Swain PP, Patel R, Ananthakrishna Pillai A, et al. Magnetocardiography for identification of coronary ischemia in patients with chest pain and normal resting 12-lead electrocardiogram. Ann Noninvasive Electrocardiol. (2020) 25(3):e12715. doi: 10.1111/anec.12715
47. Hailer B, Chaikovsky I, Auth-Eisernitz S, Schäfer H, Van Leeuwen P. The value of magnetocardiography in patients with and without relevant stenoses of the coronary arteries using an unshielded system. Pacing Clin Electrophysiol. (2005) 28(1):8–16. doi: 10.1111/j.1540-8159.2005.09318.x
48. Chaikovsky I, Hailer B, Sosnytskyy V, Lutay M, Mjasnikov G, Kazmirchuk A, et al. Predictive value of the complex magnetocardiographic index in patients with intermediate pretest probability of chronic coronary artery disease: results of a two-center study. Coron Artery Dis. (2014) 25(6):474–84. doi: 10.1097/MCA.0000000000000107
49. Shin ES, Park SG, Saleh A, Lam YY, Bhak J, Jung F, et al. Magnetocardiography scoring system to predict the presence of obstructive coronary artery disease. Clin Hemorheol Microcirc. (2018) 70(4):365–73. doi: 10.3233/CH-189301
50. Cui JG, Tian F, Miao YH, Jin QH, Shi YJ, Li L, et al. Accurate diagnosis of severe coronary stenosis based on resting magnetocardiography: a prospective, single-center, cross-sectional analysis. J Geriatr Cardiol. (2024) 21(4):407–20. doi: 10.26599/1671-5411.2024.04.006
51. Huang X, Chen P, Tang F, Hua N. Detection of coronary artery disease in patients with chest pain: a machine learning model based on magnetocardiography parameters. Clin Hemorheol Microcirc. (2021) 78(3):227–36. doi: 10.3233/CH-200905
52. Tantimongcolwat T, Naenna T, Isarankura-Na-Ayudhya C, Embrechts MJ, Prachayasittikul V. Identification of ischemic heart disease via machine learning analysis on magnetocardiograms. Comput Biol Med. (2008) 38(7):817–25. doi: 10.1016/j.compbiomed.2008.04.009
53. Kangwanariyakul Y, Nantasenamat C, Tantimongcolwat T, Naenna T. Data mining of magnetocardiograms for prediction of ischemic heart disease. EXCLI J. (2010) 9:82–95. doi: 10.17877/DE290R-15805
54. Steinisch M, Torke PR, Haueisen J, Hailer B, Gronemeyer D, Van Leeuwen P, et al. Early detection of coronary artery disease in patients studied with magnetocardiography: an automatic classification system based on signal entropy. Comput Biol Med. (2013) 43(2):144–53. doi: 10.1016/j.compbiomed.2012.11.014
55. Rong T, Shulin Z, Xiao H, Minfang T, Jian M, Shixin M, et al. Magnetocardiography-based ischemic heart disease detection and localization using machine learning methods. IEEE Trans Biomed Eng. (2019) 66(6):1658–67. doi: 10.1109/TBME.2018.2877649
56. Ashokprabhu N, Ziada K, Daher E, Cho L, Schmidt CW, Roca Y, et al. Evaluation of coronary microvascular dysfunction using magnetocardiography: a new application to an old technology. Am Heart J Plus. (2024) 44:100424. doi: 10.1016/j.ahjo.2024.100424
57. Wang Y, Jia H, Song J. Accurate classification of non-ischemic cardiomyopathy. Curr Cardiol Rep. (2023) 25(10):1299–317. doi: 10.1007/s11886-023-01944-0
58. Brala D, Thevathasan T, Grahl S, Barrow S, Violano M, Bergs H, et al. Application of magnetocardiography to screen for inflammatory cardiomyopathy and monitor treatment response. J Am Heart Assoc. (2023) 12(4):e027619. doi: 10.1161/JAHA.122.027619
59. Kawakami S, Takaki H, Hashimoto S, Kimura Y, Nakashima T, Aiba T, et al. Utility of high-resolution magnetocardiography to predict later cardiac events in nonischemic cardiomyopathy patients with normal QRS duration. Circ J. (2016) 81(1):44–51. doi: 10.1253/circj.CJ-16-0683
60. Schirdewan A, Gapelyuk A, Fischer R, Koch L, Schütt H, Zacharzowsky U, et al. Cardiac magnetic field map topology quantified by Kullback-Leibler entropy identifies patients with hypertrophic cardiomyopathy. Chaos. (2007) 17(1):015118. doi: 10.1063/1.2432059
61. Iwakami N, Aiba T, Kamakura S, Takaki H, Furukawa TA, Sato T, et al. Identification of malignant early repolarization pattern by late QRS activity in high-resolution magnetocardiography. Ann Noninvasive Electrocardiol. (2020) 25(4):e12741. doi: 10.1111/anec.12741
62. Lombardi G, Sorbo AR, Guida G, La Brocca L, Fenici R, Brisinda D. Magnetocardiographic classification and non-invasive electro-anatomical imaging of outflow tract ventricular arrhythmias in recreational sport activity practitioners. J Electrocardiol. (2018) 51(3):433–9. doi: 10.1016/j.jelectrocard.2018.02.004
63. Korhonen P, Husa T, Tierala I, Väänänen H, Mäkijärvi M, Katila T, et al. QRS Duration in high-resolution methods and standard ECG in risk assessment after first and recurrent myocardial infarctions. Pacing Clin Electrophysiol. (2006) 29(8):830–6. doi: 10.1111/j.1540-8159.2006.00448.x
64. Korhonen P, Tierala I, Simelius K, Väänänen H, Mäkijärvi M, Nenonen J, et al. Late QRS activity in signal-averaged magnetocardiography, body surface potential mapping, and orthogonal ECG in postinfarction ventricular tachycardia patients. Ann Noninvasive Electrocardiol. (2002) 7(4):389–98. doi: 10.1111/j.1542-474X.2002.tb00190.x
65. Guida G, Sorbo AR, Fenici R, Brisinda D. Predictive value of unshielded magnetocardiographic mapping to differentiate atrial fibrillation patients from healthy subjects. Ann Noninvasive Electrocardiol. (2018) 23(6):e12569. doi: 10.1111/anec.12569
66. Her AY, Shin ES, Zhou Q, Wierzbinski J, Vidal-Lopez S, Saleh A, et al. Magnetocardiography detects left atrial dysfunction in paroxysmal atrial fibrillation. Clin Hemorheol Microcirc. (2019) 72(4):353–63. doi: 10.3233/CH-180528
67. Jurkko R, Mäntynen V, Tapanainen JM, Montonen J, Väänänen H, Parikka H, et al. Non-invasive detection of conduction pathways to left atrium using magnetocardiography: validation by intra-cardiac electroanatomic mapping. Europace. (2009) 11(2):169–77. doi: 10.1093/europace/eun335
68. Ito Y, Shiga K, Yoshida K, Ogata K, Kandori A, Inaba T, et al. Development of a magnetocardiography-based algorithm for discrimination between ventricular arrhythmias originating from the right ventricular outflow tract and those originating from the aortic sinus cusp: a pilot study. Heart Rhythm. (2014) 11(9):1605–12. doi: 10.1016/j.hrthm.2014.05.032
69. Aita S, Ogata K, Yoshida K, Inaba T, Kosuge H, Machino T, et al. Noninvasive mapping of premature ventricular contractions by merging magnetocardiography and computed tomography. JACC Clin Electrophysiol. (2019) 5(10):1144–57. doi: 10.1016/j.jacep.2019.06.010
70. Wacker-Gussmann A, Eckstein GK, Strasburger JF. Preventing and treating torsades de pointes in the mother, fetus and newborn in the highest risk pregnancies with inherited arrhythmia syndromes. J Clin Med. (2023) 12(10):3379. doi: 10.3390/jcm12103379
71. Wacker-Gussmann A, Strasburger JF, Wakai RT. Contribution of fetal magnetocardiography to diagnosis, risk assessment, and treatment of fetal arrhythmia. J Am Heart Assoc. (2022) 11(15):e025224. doi: 10.1161/JAHA.121.025224
72. Yuan SM. Fetal arrhythmias: surveillance and management. Hellenic J Cardiol. (2019) 60(2):72–81. doi: 10.1016/j.hjc.2018.12.003
73. Strand SA, Strasburger JF, Wakai RT. Fetal magnetocardiogram waveform characteristics. Physiol Meas. (2019) 40(3):035002. doi: 10.1088/1361-6579/ab0a2c
74. Stingl K, Paulsen H, Weiss M, Preissl H, Abele H, Goelz R, et al. Development and application of an automated extraction algorithm for fetal magnetocardiography—normal data and arrhythmia detection. J Perinat Med. (2013) 41(6):725–34. doi: 10.1515/jpm-2013-0031
75. Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. (2014) 129(21):2183–242. doi: 10.1161/01.cir.0000437597.44550.5d
76. Batra AS, Silka MJ, Borquez A, Cuneo B, Dechert B, Jaeggi E, et al. Pharmacological management of cardiac arrhythmias in the fetal and neonatal periods: a scientific statement from the American Heart Association: endorsed by the pediatric & congenital electrophysiology society (PACES). Circulation. (2024) 149(10):e937–e52. doi: 10.1161/CIR.0000000000001206
77. Kiefer-Schmidt I, Lim M, Wacker-Gussmann A, Ortiz E, Abele H, Kagan KO, et al. Fetal magnetocardiography (fMCG): moving forward in the establishment of clinical reference data by advanced biomagnetic instrumentation and analysis. J Perinat Med. (2012) 40(3):277–86. doi: 10.1515/jpm.2011.139
78. Van Norstrand DW, Ackerman MJ. Sudden infant death syndrome: do ion channels play a role? Heart Rhythm. (2009) 6(2):272–8. doi: 10.1016/j.hrthm.2008.07.028
79. Cuneo BF, Strasburger JF, Yu S, Horigome H, Hosono T, Kandori A, et al. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation. (2013) 128(20):2183–91. doi: 10.1161/CIRCULATIONAHA.113.004840
80. Kiefer-Schmidt I, Lim M, Preissl H, Draganova R, Weiss M, Abele H, et al. Fetal magnetocardiography (fMCG) to monitor cardiac time intervals in fetuses at risk for isoimmune AV block. Lupus. (2014) 23(9):919–25. doi: 10.1177/0961203314527364
81. Zhao H, Cuneo BF, Strasburger JF, Huhta JC, Gotteiner NL, Wakai RT. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. (2008) 51(1):77–84. doi: 10.1016/j.jacc.2007.06.060
82. Cuneo BF, Bitant S, Strasburger JF, Kaizer AM, Wakai RT. Assessment of atrioventricular conduction by echocardiography and magnetocardiography in normal and anti-Ro/SSA-antibody-positive pregnancies. Ultrasound Obstet Gynecol. (2019) 54(5):625–33. doi: 10.1002/uog.20245
Keywords: magnetocardiography, acute chest pain, ischemic heart disease, acute coronary syndrome, chronic cardiac disease, non-ischemic cardiomyopathy, arrhythmia, fetal magnetocardiography
Citation: Li J, Shen Y, Shen C, Ning X and Xiang M (2025) Advances of magnetocardiography in application of adult and fetal cardiac diseases. Front. Cardiovasc. Med. 12:1522467. doi: 10.3389/fcvm.2025.1522467
Received: 4 November 2024; Accepted: 30 June 2025;
Published: 16 July 2025.
Edited by:
Sebastian Kelle, German Heart Center Berlin, GermanyReviewed by:
Phillip Suwalski, Charité Universitätsmedizin Berlin, GermanySebastian Bannasch, Steinbeis Foundation, Germany
Copyright: © 2025 Li, Shen, Shen, Ning and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Ning, bmluZ3hpYW9saW5AYnVhYS5lZHUuY24=; Min Xiang, eGlhbmdfbWluQGJ1YWEuZWR1LmNu
 Junting Li
Junting Li Yaqian Shen
Yaqian Shen Chengxing Shen2
Chengxing Shen2 Xiaolin Ning
Xiaolin Ning Min Xiang
Min Xiang