Abstract
Background:
Sodium-glucose cotransporter-2 inhibitors (SGLT2-i) are standard therapy for heart failure (HF). We performed a holistic evaluation of dapagliflozin, including its effects on exercise performance, left ventricle (LV) reverse remodeling, cardiac biomarkers, fluid retention, and renal and pulmonary function.
Methods:
We enrolled HF reduced ejection fraction (LVEF) outpatients (EF <40%) eligible for SGLT2-i and performed cardiopulmonary exercise tests (CPET), pulmonary function tests, bioelectrical impedance vector analysis, and laboratory and echocardiographic assessments at baseline (T = 0), after 2–4 weeks (T1), and after 6 months of treatment (T2).
Results:
None of the patients interrupted SGLT2-i for adverse events albeit follow-up was completed by 67 of 75 enrolled patients. At T2, mean LVEF increased (from 34.6 ± 7.8 to 37.5 ± 9.2%; p < 0.001) while end-diastolic (EDV) and end-systolic (ESV) volumes decreased [EDV: 186 (145–232) vs. 177 (129–225) mL, ESV: 113 (87–163) vs. 110 (76–145) mL; p < 0.001]. Peak oxygen intake was unchanged [peakVO2: 16.2 (13.4–18.7) vs. 16.0 (13.3–18.9) mL/kg/min; p = 0.297], while exercise ventilatory efficiency (VE/VCO2 slope) improved [from 34.2 (31.1–39.2) to 33.7 (30.2–37.6); p = 0.006]. Mean hemoglobin increased (from 13.8 ± 1.5 to 14.6 ± 1.7 g/dL; p < 0.001), while renal function did not change after a transient worsening at T1. NT-proBNP, ST-2, and hs-TNI did not change as overall body fluids and quality of life assessed by KCCQ. NYHA class improved (p=0.002), paralleled by a decrease of MECKI (Metabolic Exercise test data combined with Cardiac and Kidney Indexes) score, from 3.3% (1.9–8.0) to 2.8% (1.2–5.7), suggestive of a positive impact on 2 years prognosis (p < 0.001).
Conclusions:
Dapagliflozin induced positive LV remodeling, improvement of exercise ventilatory efficiency, and NYHA class but without peakVO2 fluid status and cardiac biomarkers changes.
1 Introduction
Dapagliflozin is a molecule belonging to the class of sodium-glucose cotransporter-2 inhibitors (SGLT2-i). This type of drug, initially used in the treatment of diabetes mellitus, has demonstrated significant clinical and prognostic benefit over the past several years in patients with reduced ejection fraction heart failure (HFrEF) even in the absence of diabetes mellitus (1, 2). HF guidelines have taken up this evidence suggesting the use of SGLT2-i therapy in patients with HFrEF (3, 4). More recently, this drug class has also demonstrated significant prognostic improvement in patients with HF with preserved and mildly reduced systolic function (5, 6) and in chronic kidney disease (7). Considering the recent introduction of the drug into clinical practice, direct field evaluation is very important to refine clinical management of patients treated with SGLT2-i and to understand the mechanism behind the clinical benefits. Indeed, in some small preliminary studies performed in patients with HFrEF, SGLT2-i, together with an otherwise optimized medical therapy, have shown to be effective in improving left ventricle ejection fraction (LVEF) and other echocardiographic parameters of ventricular remodeling (8, 9). However, a holistic evaluation of the potential effects of SGLT2-i therapy on body function, including exercise capacity assessed by the gold standard cardiopulmonary exercise test (CPET), pulmonary function, body fluid homeostasis, and biomarkers have not yet been reported in patients with HFrEF.
The present study was designed to evaluate changes in CPET-derived parameters, pulmonary function, echocardiographic parameters of LV systolic function, cardiac biomarkers, fluid homeostasis, and quality of life (QoL) in a single-center cohort of patients with HFrEF (NYHA functional class II–III) treated with dapagliflozin.
2 Methods
At the HF Unit of the Centro Cardiologico Monzino, IRCCS in Milan, we enrolled a cohort of stable HFrEF outpatients who were eligible for treatment with SGLT2-i. The inclusion criteria were as follows: age >18 years; stable clinical condition defined as absence of heart failure exacerbations in the past 3 months, i.e., no hospitalizations for heart failure requiring intravenous diuretic administration; LVEF ≤40% (echocardiography); and diagnosis of HFrEF (
3). In addition, they had to be able to undergo CPET and provide signed informed consent to participate in the study. The exclusion criteria included contraindications to SGLT2-i, moderate-to-severe chronic obstructive pulmonary disease (COPD), or an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m
2according to Modified Diet in Renal Disease (MDRD) criteria (
10). Patients who met the study’s inclusion and exclusion criteria underwent an initial evaluation (T0) that included:
- •
A clinical examination
- •
Kansas City Cardiomyopathy Questionnaire (KCCQ-12) to assess quality of life (QoL)
- •
Blood sample: complete blood count, creatinine, urea nitrogen, sodium, potassium, glycated hemoglobin (Hb), N-terminal BNP (NT-proBNP), suppression of tumorigenicity 2 (ST-2), high-sensitivity C-reactive protein (hsCRP), and high-sensitivity troponin I (hs-TNI)
- •
Standard spirometry
- •
Maximal ramp-protocol CPET on cycle ergometer
- •
Transthoracic echocardiogram
- •
Bioelectrical impedance vector analysis (BIVA)
Subsequently, patients were prescribed dapagliflozin at a dose of 10 mg/day. Between 2 and 4 weeks after starting the therapy, a safety evaluation was performed, including a clinical evaluation and blood tests. All parameters evaluated at T0 were reassessed 6 months after the start of treatment (T2). The study synopsis is shown in Figure 1.
Figure 1
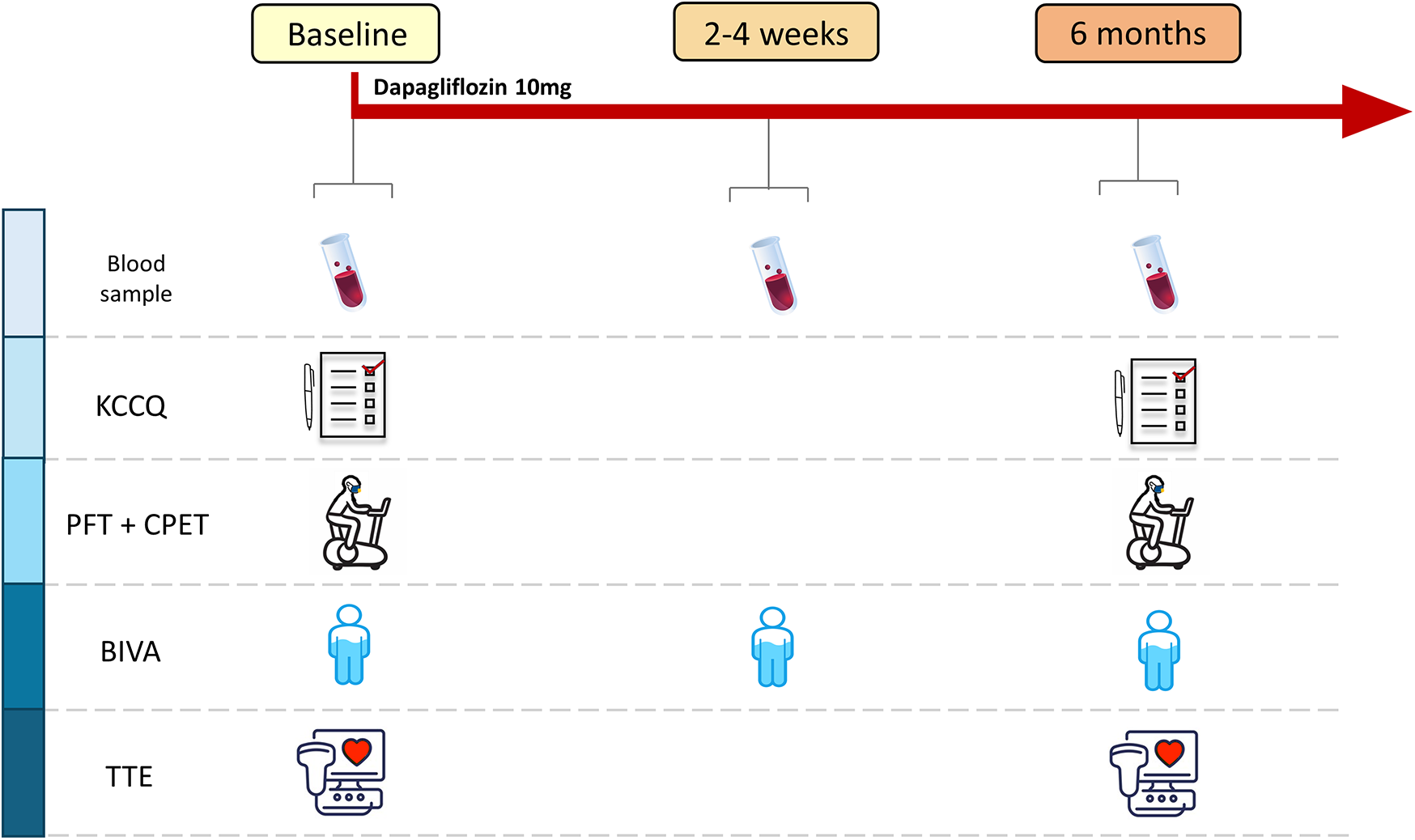
Study design. Scheduled activities at each study point. KCCQ, Kansas City Cardiomyopathy Questionnaire; PFT, pulmonary function test; CPET, cardiopulmonary exercise test; BIVA, bioelectrical impedance vector analysis; TTE, transthoracic echocardiography.
2.1 Kansas City Cardiomyopathy Questionnaire analysis
QoL was evaluated using the KCCQ-12 at baseline and at 6 months, administrated before any other assessment. The KCCQ-12 was analyzed combining the reported Physical Limitation, Symptom Frequency, QoL, and Social Limitation scales into the Summary Score, calculated as the average of the four scores. To calculate the summary score, at least one of the four scale scores must be present (11, 12). Only scales available at both T0 and T2 were considered.
2.2 Cardiopulmonary exercise testing
CPET was performed on an electronically braked cycle ergometer using a personalized ramp protocol set, to reach peak exercise in 10 ± 2 min (13) at T0 and applied unchanged at T2. CPET was performed and analyzed as standard (14). Specifically, in the absence of clinical events, tests were self-interrupted by patients when they reported the maximal effort. Patients wore a mask to measure ventilation (VE) and respiratory gases breath by breath. During the test, heart rate and a 12-lead ECG were continuously monitored, Hb O2 saturation was recorded by an oximeter, and blood pressure was monitored with a cuff sphygmomanometer at rest and every 2 min. PeakVO2 was calculated as the 30 s average of the highest VO2 recorded, while the VE/VCO2 slope was calculated based on the linear relationship between VE and VCO2, starting from 1 min after the initiation of loaded exercise until the end of the isocapnic buffering period. This value was also expressed as a percentage of the predicted value (15). Predicted peakVO2 was calculated using the Hansen and Wasserman equation as (height–age) × 20 for men and (height–age) × 14 for women (16). The anaerobic threshold (AT) was measured using a V-slope analysis of VO2 and VCO2 (17). The VO2/work relationship was measured through the entire exercise protocol. Other data are reported as the 20 s average.
The MECKI score, including six relevant prognostic parameters (Hb, LVEF, MDRD, Na, PeakVO2, and VE/VCO2 slope), was calculated as previously described (18).
2.3 Echocardiography
Transthoracic echocardiography (TTE) examinations were conducted using an Epiq CVx ultrasound machine (Philips Medical Systems, Andover, MA, USA) equipped with an X5-1 probe. A comprehensive standard 2D TTE analysis was performed, with left chamber volumes and LVEF measured from four-chamber and two-chamber views using the biplane Simpson’s method (19). All echocardiograms were conducted by highly trained operators. Pulmonary artery systolic pressure (PAP) was calculated by quantifying the peak velocity of tricuspid regurgitation and then adding the estimated pressure in the right atrium (20).
2.4 Bioelectrical impedance vector analysis
Bioimpedance measurements were conducted using an impedance plethysmograph (BIA 101 BIVA; AKERN SRL, Pisa, Italy) with a 250 µA RMS 50 kHz sinusoidal output signal. The device was calibrated using the standard control circuit with a known impedance [resistance = 383 ohms; reactance (Xc) = 45 ohms].
Measurements were taken with participants in a supine position, with their arms and legs by their sides. Values were recorded after a minimum rest of 5 min. Before measurement, the skin was cleaned with an alcohol solution and four contact electrodes (BIATRODES; AKERN SRL, Pisa, Italy) were placed on the dorsal surface of the right hand and foot as per the manufacturer's instructions.
KCCQ-12, CPET, spirometry, BIVA, and cardiac ultrasound were obtained and analyzed by medical personnel who were blinded to the study timeline, meaning they were unaware of whether the assessments were performed at T0, T1, or T2, while patients and referring physicians were unblinded.
The present research protocol complies with the World Medical Association’s Declaration of Helsinki and was approved by the Centro Cardiologico Monzino Ethical Committee (R 11637-22 CCM 1756). Each individual provided written informed consent to participate in the study. This study was registered on Clinicaltrials.gov (reference ID NCT05770167).
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Centro Cardiologico Monzino IRCCS (21, 22). REDCap is a secure, web-based software platform.
2.5 Statistical analysis
Continuous variables are described as mean ± standard deviation (SD) in case of normal distribution, and as median and interquartile range (IQR) in case of non-normal distribution. Categorical variables are expressed as numbers (percentages).
For continuous variables, differences between T0 and T6 were assessed with a paired t-test or a non-parametric test as appropriate. For categorical variables, McNemar’s test was used.
When variables were measured at all three protocol-specified time points (T0, T1, and T2), a statistical analysis was conducted using repeated measures tests for normally distributed variables or the Friedman test for non-normally distributed variables.
The correlation between the variables was evaluated using Pearson's correlation coefficient or Spearman’s non-parametric coefficient. A p-value <0.05 was considered statistically significant.
3 Results
In total, 75 patients have been enrolled between January 2022 and July 2023. Eight patients (10.7%) were excluded from the final analysis because they interrupted the drug or the study for personal reasons (specifically: two participants interrupted the study treatment for personal decision; six individuals continued the study treatment but did not perform the follow-up evaluation), while none of the enrolled patients stopped the treatment for clinical reasons or drug-related complains. All the remaining 67 HF patients (median age 66 years; age range 56–73 years) completed the evaluation at 6 months (T2), while 5 of 67 did not perform the safety evaluation at 2–4 weeks (T1). Table 1 reports the main parameters collected for the study population. At enrollment, all patients were on HF-optimized medical treatment, with 100% of patients were taking ACEi (n = 6, 9%), ARBs (n = 7, 10.4%), or Sacubitril/Valsartan (n = 54, 80.6%), 64 (95.5%) patients taking a β-blocker, 56 (83.6%) taking an MRA, and 35 (52.2%) taking a loop diuretic. With regard to comorbidities, at enrollment there were 7 (10.4%) patients with diabetes, 40 (59.7%) with dyslipidemia, 33 (49.3%) with hypertension, and 1 (1.5%) with moderate COPD. In total, 12 (18%) patients were current smokers while 26 (39%) were former smokers.
Table 1
| Variable | n | T0 | n | T1 | n | T2 | p T0 vs. T2 | p Repeated meas. | Bonferroni post-hoc test | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 vs. T0 | T1 vs. T0 | T1 vs. T2 | |||||||||
| Age (years) | 67 | 66 [56–73] | 67 | 66 [56–73] | 66 | 66 [57–73] | 0.604 | ||||
| Weight (kg) | 67 | 79.0 ± 14.3 | 61 | 79.1 ± 14.0 | 67 | 78.6 ± 14.2 | 0.016 | – | 0.020 | – | |
| Height (cm) | 67 | 172 ± 8 | 67 | 172 ± 8 | 67 | 172 ± 8 | 1 | ||||
| BMI (kg/m2) | 67 | 26.5 ± 3.5 | 61 | 26.5 ± 3.4 | 67 | 26.4 ± 3.4 | 0.014 | – | 0.019 | – | |
| LVEF (mL) | 67 | 34.6 ± 7.8 | 67 | 37.5 ± 9.2 | <0.001 | ||||||
| EDV (mL) | 67 | 186 [145–232] | 67 | 177 [129–225] | <0.001 | ||||||
| ESV (mL) | 67 | 113 [87–163] | 67 | 110 [76–145] | <0.001 | ||||||
| PAPs (mmHg) | 62 | 27.0 [23.7–29.0] | 58 | 25.0 [23.0–28.0] | 0.046 | ||||||
| SBP (mmHg) | 67 | 118.6 ± 16.1 | 61 | 109.3 ± 13.4 | <0.001 | ||||||
| DBP (mmHg) | 67 | 72.5 ± 9.5 | 60 | 68.4 ± 7.9 | 0.003 | ||||||
| Heart rate (bpm) | 63 | 63.4 ± 11.3 | 67 | 63.3 ± 12.0 | 0.796 | ||||||
| Vital capacity (L) | 65 | 3.74 ± 0.95 | 60 | 3.84 ± 0.89 | 61 | 3.79 ± 0.90 | 0.672 | ||||
| FEV1 (L) | 67 | 2.71 ± 0.74 | 61 | 2.74 ± 0.74 | 62 | 2.69 ± 0.69 | 0.226 | ||||
| FEV1 (%) | 67 | 87.34 ± 17.04 | 61 | 88.18 ± 16.22 | 62 | 87.20 ± 15.19 | 0.358 | ||||
| FVC (L) | 67 | 3.38 ± 0.91 | 61 | 3.45 ± 0.93 | 62 | 3.43 ± 0.87 | 0.476 | ||||
| FVC (%) | 67 | 84.36 ± 16.11 | 61 | 84.58 ± 18.08 | 62 | 85.51 ± 14.82 | 0.772 | ||||
| FEV1/FVC | 67 | 0.80 ± 0.06 | 61 | 0.80 ± 0.07 | 62 | 0.79 ± 0.07 | 0.366 | ||||
| Total body water (L) | 67 | 44.7 [39.8–51] | 60 | 44.9 [40.23–9.75] | 67 | 44.9 [39.5–50] | 0.145 | ||||
| Extracellular water (L) | 67 | 21.2 [18.2–23.1] | 60 | 21.0 [18.82–2.95] | 67 | 20.5 [18.4–22.9] | 0.145 | ||||
| Hydration index (%) | 67 | 73.6 [73.2–73.8] | 60 | 73.5 [73.3–73.8] | 67 | 73.5 [73.3–73.8] | 0.552 | ||||
Main variables at baseline (T0), 2–4 weeks (T1), and 6 months (T2).
BMI, body mass index; LVEF, left ventricular ejection fraction; EDV, left ventricle end-diastolic volume; ESV, left ventricle end-systolic volume; PAPs, systolic pulmonary artery pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
After 6 months of treatment with dapagliflozin, both systolic and diastolic blood pressure were lower than at T0, while heart rate was unchanged. Moreover, at the T2 visit, LVEF showed an 8% increase with a parallel reduction in LV volumes and a slight but significant decrease of PAPs (Table 1).
Medical treatment did not change during the course of the study, including the median dose of loop diuretics [25 mg/die (range 25–50) to 25 mg/die (range 25–25); p = 1.000]. None of the patients who were not on loop diuretics at baseline were prescribed them during the study, while one patient had loop diuretic therapy discontinued. Moreover, we detected a progressive increase of Hb (13.8 ± 1.5, 13.9 ± 1.5, and 14.6 ± 1.7 g/dL at T0, T1, and T2, respectively; p < 0.001) (Figure 2), and red cell distribution width (RDW) [13.7 (13.1–14.6), T1 13.8 (13.3–14.6), T2 13.8 (13.3–14.6); p < 0.019]. On the other hand, Na+ (140.3 ± 2.0, 140.1 ± 2.0, and 140.6 ± 2.2 at T0, T1, and T2, respectively; p = 0.192), NT-proBNP [from 852.0 pg/mL (455.3–1,845.3) at T0 to 916.5 pg/mL (301.7–1,831.0) at T2; p = 0.081], interleukin ST-2 [from 26.35 ng/mL (23.08–30.23) to 27.80 ng/mL (22.80–32.10); p = 0.829], hsCRP [from 1.07 mg/L (0.40–3.07) to 1.04 mg/L (0.46–2.82); p = 0.114], and hs-TNI [from 12.22 ng/L (7.31–25.53) to 9.49 ng/L (6.74–22.88); p = 0.201] did not significantly change during the study (Figure 2). We observed a short-term worsening of creatinine (T1) with a complete recovery of values at T2 (p = 0.009 T0 vs. T1) (Figure 2). The same temporal trend was confirmed if renal function was analyzed as MDRD (73.0 ± 22.8, 68.8 ± 21.3, and 70.2 ± 20.9 at T0, T1, and T2, respectively; p < 0.01).
Figure 2
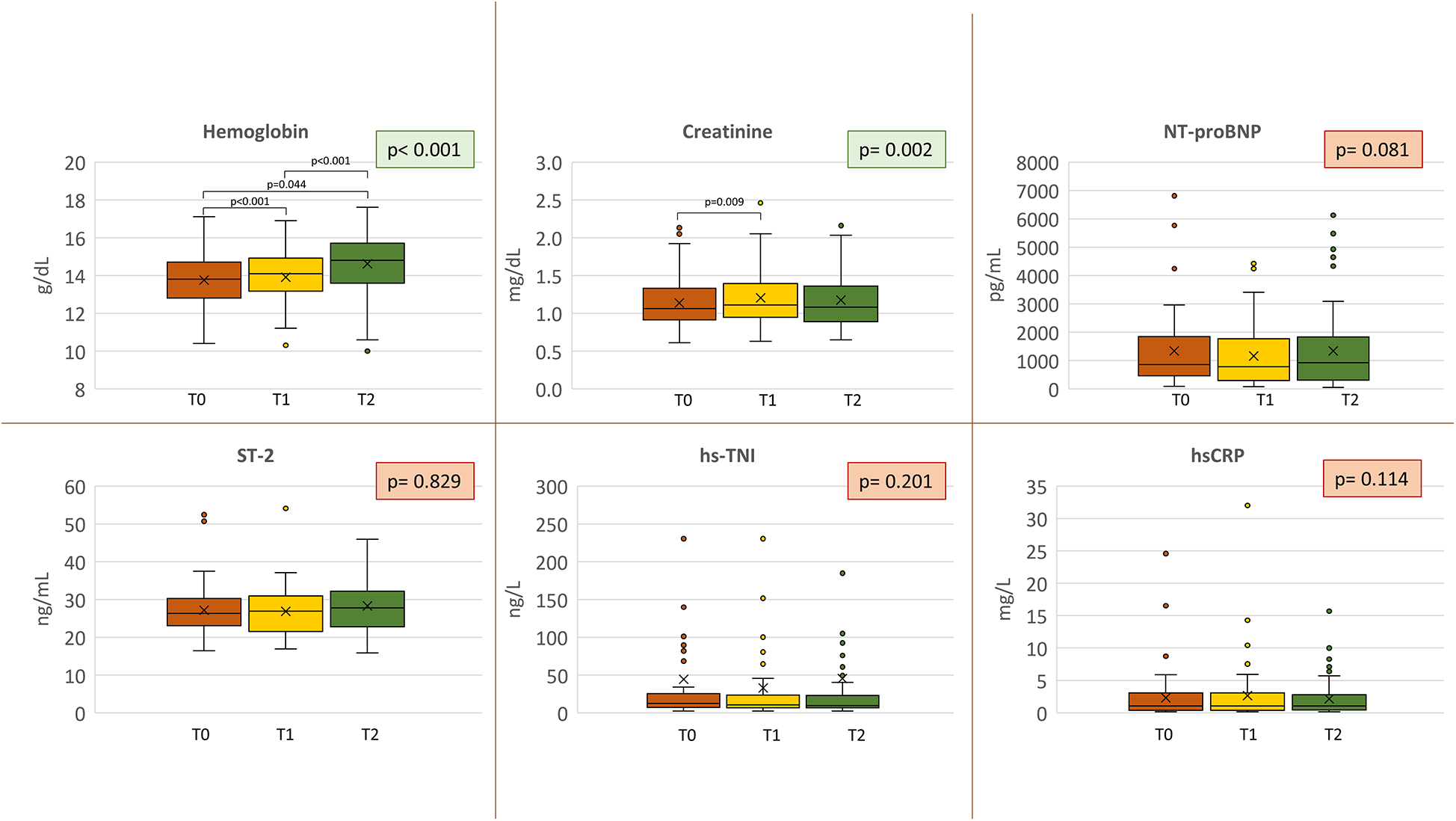
Biomarkers analysis. Biomarker variations at each study point. Orange = baseline; yellow = 2–4 weeks (T1); green = 6 months (T2). Nt-proBNP, N-terminal pro B-type natriuretic peptide; ST-2, suppression of tumorigenicity 2; hs-TNI, high-sensitivity troponin I; hsCRP, high-sensitivity C-reactive protein.
Regarding CPET evaluation, no significant changes in terms of peakVO2 and other VO2-derived CPET parameters were detected at 6 months, while the VE/VCO2 slope reduced both as absolute value and as percent of predicted (Table 2, Figure 3). Notably, two patients did not repeat the cardiopulmonary assessment at 6 months due to limitations not related to HF; therefore, they were excluded from the present analysis.
Table 2
| Variable | n | T0 | n | T2 | p |
|---|---|---|---|---|---|
| NYHA I | 67 | 5 (7.5%) | 67 | 18 (26.9%) | <0.001 |
| NYHA II-III | 67 | 62 (92.5%) | 67 | 49 (73.1%) | |
| VO2-AT (mL) | 60 | 885.2 ± 268.9 | 61 | 874.9 ± 265.6 | 0.386 |
| VO2/kg-AT (mL/kg) | 60 | 11.3 ± 3.1 | 61 | 11.1 ± 2.6 | 0.345 |
| Heart rate-AT (bpm) | 60 | 87.33 ± 16.26 | 61 | 86.15 ± 15.41 | 0.529 |
| PeakVO2 (mL/min) | 65 | 1,212 [996–1,593] | 65 | 1,246 [993–1,583] | 0.190 |
| PeakVO2 (mL/min/kg) | 65 | 16.21 [13.43–18.67] | 65 | 15.98 [13.26–18.85] | 0.297 |
| PeakVO2 (% pred) | 65 | 64.3 ± 17.1 | 65 | 63.1 ± 16.5 | 0.375 |
| Peak heart rate (bpm) | 65 | 114 ± 25 | 65 | 115 ± 25 | 0.586 |
| Peak workload (watt) | 65 | 112.0 [84.5–128.5] | 65 | 109.0 [78–141.5] | 0.297 |
| Peak pulse (mL/beat) | 65 | 11.71 ± 3.25 | 65 | 11.43 ± 3.35 | 0.251 |
| Peak Systolic blood pressure (mmHg) | 65 | 150 [130–170] | 65 | 140 [127–160] | 0.264 |
| VE/VCO2 slope | 65 | 34.2 [31.1–39.2] | 65 | 33.7 [30.2–37.6] | 0.006 |
| VE/VCO2 slope (% pred) | 65 | 130 [117–147] | 65 | 126 [115–140] | 0.003 |
| VO2/work slope (mL/min/watt) | 65 | 9.21 [7.86–9.78] | 65 | 9.08 [8.30–9.74] | 0.799 |
| Peak respiratory rate (L/min) | 65 | 35.09 ± 7.25 | 65 | 34.54 ± 6.88 | 0.422 |
| Peak ventilation (L/min) | 65 | 62.37 ± 19.17 | 64 | 60.76 ± 20.31 | 0.527 |
| Rest PetCO2 (mmHg) | 65 | 28.0 ± 3.3 | 65 | 27.9 ± 3.8 | 0.750 |
| PetCO2-AT (mmHg) | 60 | 34.8 ± 4.7 | 61 | 35.0 ± 4.7 | 0.628 |
| PetCO2-RCP (mmHg) | 50 | 33.9 ± 5.0 | 44 | 34.2 ± 5.4 | 0.953 |
| Peak PetCO2 (mmHg) | 65 | 30.3 ± 4.8 | 65 | 30.7 ± 4.9 | 0.225 |
| Peak respiratory exchange ratio | 65 | 1.12 ± 0.12 | 65 | 1.13 ± 0.14 | 0.568 |
| KCCQ-Physical limitation | 64 | 4.20 ± 0.9 | 63 | 4.29 ± 0.85 | 0.404 |
| KCCQ-Symptoms | 64 | 5.19 ± 0.96 | 63 | 5.30 ± 0.91 | 0.438 |
| KCCQ-QoL | 64 | 3.66 ± 1.08 | 63 | 3.70 ± 1.12 | 0.847 |
| KCCQ-Social limitation | 64 | 4.17 ± 1.09 | 63 | 4.22 ± 1.11 | 0.789 |
| KCCQ-Mean | 64 | 4.31 ± 0.88 | 63 | 4.38 ± 0.87 | 0.529 |
| KCCQ-Total | 64 | 53.1 ± 10.4 | 63 | 53.8 ± 10.3 | 0.509 |
Functional and QoL evaluation of study population at baseline (T0) and after 6 months (T2).
NYHA, New York Heart Association; VO2, oxygen intake; %pred, percentage of predicted value; AT, anaerobic threshold; VE/VCO2, minute ventilation/carbon dioxide production relationship; PetCO2, end tidal pressure of CO2; RCP, respiratory compensation point; KCCQ, Kansas City Cardiomyopathy Questionnaire; QoL, quality of life.
Figure 3
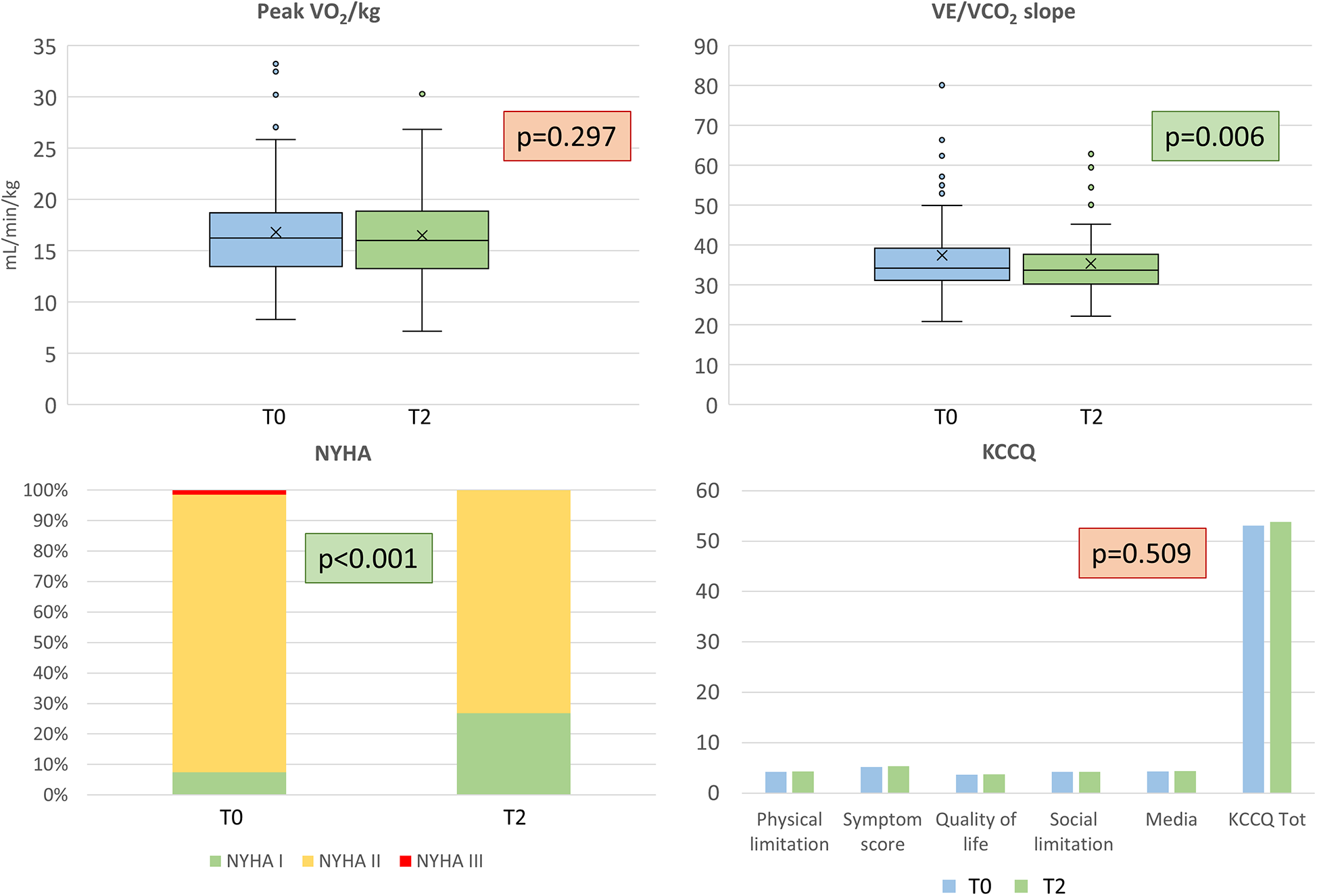
Functional and quality of life evaluation. Top: peak oxygen uptake (VO2/kg) on the left, and ventilatory efficiency (VE/VCO2 slope), on the right. Bottom: New York Heart Association (NYHA) class and Kansas City Cardiomyopathy Questionnaire results are reported on the left and right respectively. T0 = baseline, T2 = 6 months.
KCCQ did not reveal an improvement in the subjective perception of QoL. No significant differences with respect to the T0 evaluation were found in either the total score or in the analyzed domains (Table 2, Figure 3). VE/VCO2 slope and Hb improvements were not correlated (R2 for ΔVE/VCO2 vs. ΔHb 0.029). NYHA class improved in 13 patients (from NYHA II or III at baseline to NYHA I at T2) (p <0.001). Only one patient worsened from NYHA I to NYHA II. Regarding BIVA, we did not find any significant difference (Table 1). We observed a significant improvement in median MECKI score (18) from 3.3% (range 1.9–8.0) to 2.8% (range 1.2–5.7), suggestive of a positive impact on prognosis at 2 years (p <0.001).
4 Discussion
The main finding of this study is that in our cohort of HFrEF patients, dapagliflozin had no impact on exercise capacity, as assessed by peakVO2 and workload. Similarly, other relevant CPET parameters, including VO2 at AT, VO2/work, peakO2-pulse, and peak heart and respiratory rates, remained unchanged, with patients achieving maximal or near maximal effort (Table 2). However, dapagliflozin improved exercise ventilatory efficiency, as shown by a modest but significant VE/VCO2 slope reduction (34.2 vs. 33.7; p < 0.001).
The neutral effect of the drug on peakVO2 is surprising, given previously reported data. The DAPA-VO2 study (23) documented a significant improvement in peakVO2 after 1 and 3 months of treatment (+Δ 1.09 mL/kg/min and +Δ 1.06 mL/kg/min, respectively). Compared to that trial, our cohort had less advanced HF, as indicated by the relatively higher baseline peakVO2 (16.2 mL/kg/min vs. 13.4 mL/kg/min), LVEF (34.6% vs. 33.7%), and lower NT-proBNP levels (774 vs. 1,085 pg/ml). Therefore, a smaller effect on peakVO2 in our population may have been expected. The lack of peakVO2 improvement with dapagliflozin remains unexpected, especially considering the increases in LVEF and Hb. O2 delivery is directly linked to cardiac output (CO) and Hb levels. In this study, the former was possibly unchanged, being the improvement of LVEF obtained in parallel with the reduction in LV volumes, while Hb was undoubtedly and significantly increased by dapagliflozin (from 13.8 to 14.6 g/dL; p < 0.001), consistent with previous studies (24, 25). We hypothesize two possible explanations for this. First, the reduction in LV volumes might have led to a relevant decrease in peak CO regardless of the improvement in LVEF, though this seems unlikely. Alternatively, dapagliflozin may have influenced exercise-induced blood flow redistribution. This phenomenon, which we recently reported, is rarely considered in clinical practice but may explain the blunted changes in peakVO2, despite significant hemodynamic improvement in HF patients receiving effective treatments (26). Indeed, as the severity of HF increases, blood flow distribution during exercise is, as a percentage of total blood flow, progressively directed toward the working muscles, leading to differences in increased arteriovenous O2 content (Δa-vCO2). However, as HF improves and CO increases, the percentage of blood flow to the muscle decreases, leading to a reduction in Δa-vCO2 and affecting peakVO2 measurements. This phenomenon may explain the discrepancy observed between peakVO2 and LVEF/Hb changes. Therefore, dapagliflozin’s improvement on exercise hemodynamics cannot be ruled out by the unchanged peakVO2 we observed.
The reduction of the VE/VCO2 slope, indicating improved VE efficiency during exercise, is another interesting observation. Indeed, the VE/VCO2 slope is an important parameter directly related to HF prognosis in HFrEF as well as in other cardiomyopathies (27, 28) with a prognostic significance comparable to peakVO2 (29). Therefore, it is included in heart transplant screening guidelines (30) and in HF prognostic scores involving exercise evaluation, such as the MECKI score (18). The VE/VCO2 slope depends on chemoreflex-mediated VE regulation and VE/perfusion mismatch in the lungs. While there are no available data on the direct effects of SGLT-i on chemo- or metaboreceptor activity, the absence of changes in PetCO2 at rest, during exercise, and specifically at AT, respiratory compensation point, and peak exercise, suggests a change in the effects of reflex on ventilation during exercise. An improvement in VE/perfusion mismatch at the lung level seems likely, as pulmonary pressure was significantly reduced in the cardiac ultrasound evaluation. This postulated hemodynamic improvement may be due to the well-known dapagliflozin-related diuretic effect (31), which helps reduce pulmonary pressures and interstitial edema. However, our data do not support a relevant diuretic effect, as patients' weight loss was negligible (−0.4 kg) and NT-proBNP and BIVA (i.e., overall hydration index and body water) remained unchanged (Table 1, Figure 2). Notably, BIVA does not assess thoracic fluids. Therefore, although the overall hemodynamic effect of dapagliflozin at rest is limited, an improvement during exercise may still be possible.
In addition to exercise, we demonstrated a favorable LV reverse remodeling with a statistically significant (though small) reduction in both EDV and ESV, along with an improvement in LVEF (Table 1). These changes are in line with previous data (9, 32, 33) and confirm the positive effects of SGLT2-i on LV geometry and HF progression. Prevention and reversal of adverse cardiac remodeling is one of the mechanisms through which SGLT2-i may exert cardiovascular benefits, involving molecular, cellular, and interstitial changes related to increased apoptosis and necrosis, decreased autophagy, impairments of myocardial oxygen supply and demand, and altered energy metabolism (32). However, in the present study, these positive changes in cardiac volumes and function were not accompanied by significant improvements in cardiac biomarkers. In fact, NT-proBNP, hs-TNI, and ST-2 levels did not change.
The results of the present trial should be considered in the context of existing data. Most of our patients were non-diabetic and NYHA class II at baseline. The KCCQ demonstrated a mild degree of QoL impairment; similarly, peakVO2 and NT-proBNP values indicate a non-severe spectrum of HF. Importantly, our population had a higher degree of HF therapeutic optimization with disease-modifying therapy compared to most studies. For example, in the DAPA-HF study (1), only 10% of the patients were taking sacubitril/valsartan, compared to 81% of our population. This, together with the other standard HF therapies, contributed to a baseline mild impairment in peakVO2 (16.21 mL/kg/min). Moreover, the same trial observed a significant reduction in NT-proBNP (−196 ± 2,387 pg/mL), but from slightly higher baseline values. In fact, other studies conducted in specific HF phenotypes (e.g., amyloidosis) have confirmed a positive effect of dapagliflozin on NT-proBNP (34). In other words, the drug’s impact on biomarkers related to LV stretch/overload seems to depend on the baseline value and/or on the length of the follow-up. Therefore, it is difficult to expect further clinical benefits in this parameter in a population with stable non-severe HF.
In a population with moderate HF and uptitrated treatment, dapagliflozin does not seem to have additional effects on top of sacubitril/valsartan in terms of peakVO2 and cardiac biomarkers, which are considered pivotal for assessing HF treatment efficacy. However, these mechanistic findings, which may initially seem disappointing, should not limit the use of SGLT2-i. Even in our well-treated, stable, low-severity population, we observed an additional favorable effect on the prognostic balance, as shown by a significant improvement in the MECKI score (Figure 4). This confirms that the evaluation of HF patients should not be limited to a single variable, even if it is prognostically important (e.g., peakVO2), but requires a holistic evaluation. Furthermore, dapagliflozin demonstrated excellent tolerability without significant side effects across the entire population. Finally, it is also important to remember that HF is a progressive and debilitating disease characterized by the worsening of parameters over time (including peakVO2 and biomarkers). Therefore, even “freezing” the situation, as we did in the present study, can be considered a success.
Figure 4
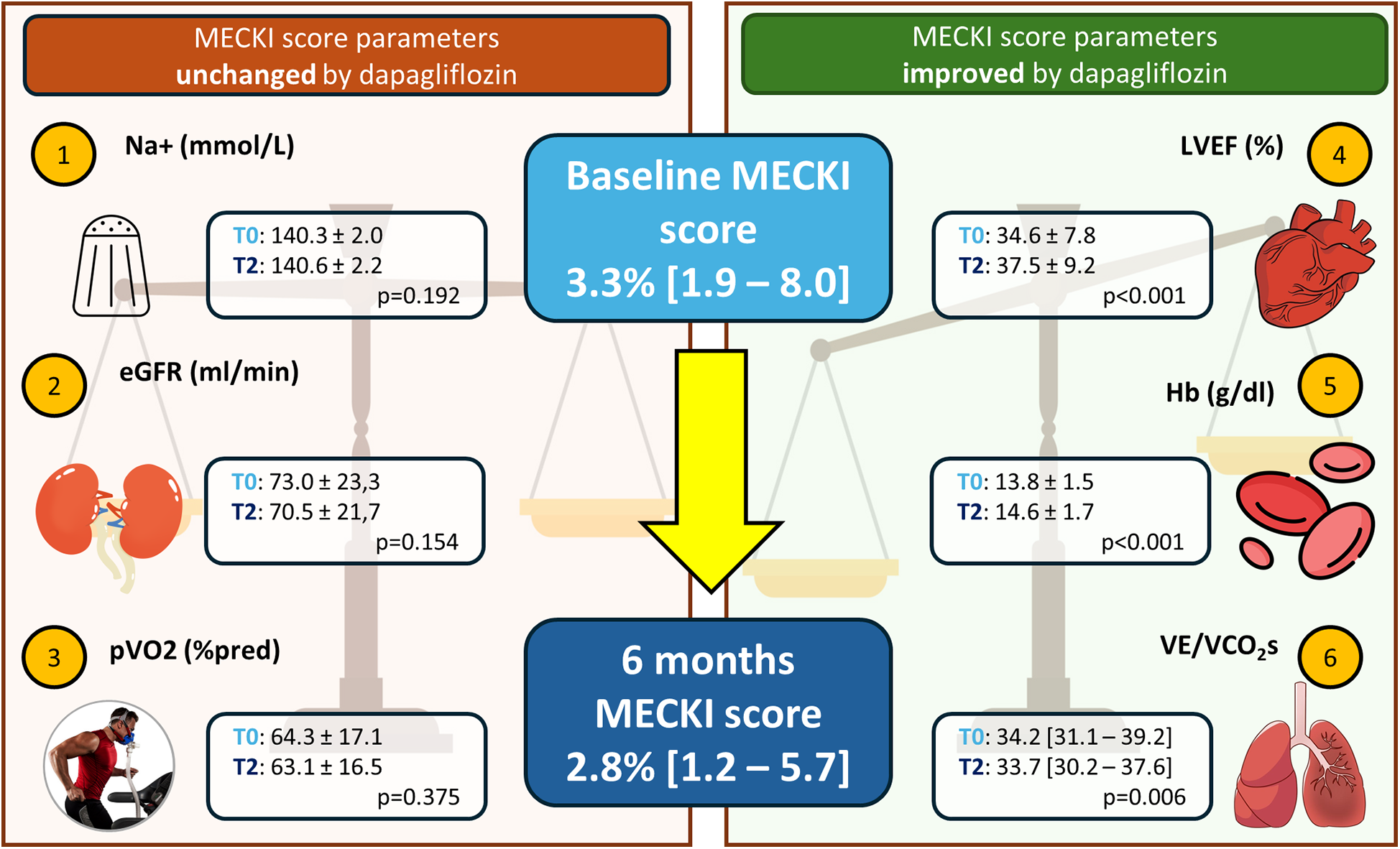
Effect of dapagliflozin on prognosis estimation by MECKI score. Schematic representation of the six variables constituting the MECKI score, which assesses prognosis. Left: variables that remained unchanged in our study; right: variables that significantly improved after 6 months with dapagliflozin. Na+, sodium; eGFR, estimated glomerular filtration rate calculated by modification of diet in renal disease (MDRD) formula; MECKI, Metabolic Exercise test data combined with Cardiac and Kidney Index; pVO2 (%pred), peak oxygen uptake expressed as% of predicted; LVEF, left ventricular ejection fraction; Hb, hemoglobin; VE/VCO2s, ventilatory efficiency.
4.1 Limitations
This study has some limitations. First, due to ethical reasons, it is randomized, meaning a direct comparison of interventions is not possible. Second, the monocentric nature of the study with a small sample size limits the automatic generalization of the results to other populations. Third, most of the patients were in NYHA class II, with relatively stable, non-advanced heart failure. Therefore, the effects of the drug in more severe HF populations deserve to be studied in dedicated trials. Fourth, the study was designed to detect changes during the first few months of treatment in patients with reduced LVEF. Further studies are needed to analyze the long-term effects and outcomes in other HF groups (with midrange or preserved LVEF). Finally, we only studied patients treated with dapagliflozin, not other SGLT2-i. Therefore, we do not know whether similar results can be obtained with different SGLT2-i.
In conclusion, our trial highlighted a beneficial impact of dapagliflozin on key HF parameters, such as VE/VCO2 slope, Hb, LV volumes, and ejection fraction, despite a neutral effect on peakVO2 and cardiac biomarkers. These findings help to understand the type of benefits to expect from this pillar of HF therapy, even in a well-treated population of clinically stable patients with moderate HFrEF.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IEO-Monzino Ethic committee, Centro Cardiologico Monzino, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MMa: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – original draft. IM: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft. ES: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft. NC: Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. VM: Investigation, Validation, Visualization, Writing – review & editing. AG: Data curation, Investigation, Validation, Visualization, Writing – review & editing. JC: Data curation, Validation, Visualization, Writing – review & editing. FR: Investigation, Validation, Visualization, Writing – review & editing. CP: Investigation, Validation, Visualization, Writing – review & editing. TC: Investigation, Validation, Visualization, Writing – review & editing. AN: Investigation, Validation, Visualization, Writing – review & editing. RC: Data curation, Validation, Visualization, Writing – review & editing. PG: Data curation, Validation, Visualization, Writing – review & editing. CV: Validation, Visualization, Writing – review & editing. BP: Investigation, Validation, Visualization, Writing – review & editing. FD: Investigation, Validation, Visualization, Writing – review & editing. GG: Investigation, Validation, Visualization, Writing – review & editing. MS: Validation, Visualization, Writing – review & editing. AB: Formal analysis, Validation, Visualization, Writing – review & editing. GS: Supervision, Validation, Visualization, Writing – review & editing. MMu: Investigation, Validation, Visualization, Writing – review & editing. PA: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Italian Ministry of Health, Ricerca Corrente (Project number: CUP B43C24000090001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer IJI declared a past coauthorship with the author MM to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
McMurray JJV Solomon SD Inzucchi SE Kober L Kosiborod MN Martinez FA et al Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. 10.1056/NEJMoa1911303
2.
Packer M Anker SD Butler J Filippatos G Pocock SJ Carson P et al Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383(15):1413–24. 10.1056/NEJMoa2022190
3.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Bohm M et al 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. 10.1093/eurheartj/ehab368
4.
McDonald M Virani S Chan M Ducharme A Ezekowitz JA Giannetti N et al CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. (2021) 37(4):531–46. 10.1016/j.cjca.2021.01.017
5.
Anker SD Butler J Filippatos G Ferreira JP Bocchi E Bohm M et al Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. 10.1056/NEJMoa2107038
6.
Solomon SD McMurray JJV Claggett B de Boer RA DeMets D Hernandez AF et al Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. 10.1056/NEJMoa2206286
7.
Heerspink HJL Stefansson BV Correa-Rotter R Chertow GM Greene T Hou FF et al Dapagliflozin in patients with chronic kidney disease. N Engl J Med. (2020) 383(15):1436–46. 10.1056/NEJMoa2024816
8.
Singh JSS Mordi IR Vickneson K Fathi A Donnan PT Mohan M et al Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. (2020) 43(6):1356–9. 10.2337/dc19-2187
9.
Pascual-Figal DA Zamorano JL Domingo M Morillas H Nunez J Cobo Marcos M et al Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: the DAPA-MODA study. Eur J Heart Fail. (2023) 25(8):1352–60. 10.1002/ejhf.2884
10.
Levey AS Coresh J Greene T Marsh J Stevens LA Kusek JW et al Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. (2007) 53(4):766–72. 10.1373/clinchem.2006.077180
11.
Spertus JA Jones PG . Development and validation of a short version of the Kansas city cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. (2015) 8(5):469–76. 10.1161/CIRCOUTCOMES.115.001958
12.
Stubblefield WB Jenkins CA Liu D Storrow AB Spertus JA Pang PS et al Improvement in Kansas city cardiomyopathy questionnaire scores after a self-care intervention in patients with acute heart failure discharged from the emergency department. Circ Cardiovasc Qual Outcomes. (2021) 14(10):e007956. 10.1161/CIRCOUTCOMES.121.007956
13.
Agostoni P Bianchi M Moraschi A Palermo P Cattadori G La Gioia R et al Work-rate affects cardiopulmonary exercise test results in heart failure. Eur J Heart Fail. (2005) 7(4):498–504. 10.1016/j.ejheart.2004.06.007
14.
Agostoni P Dumitrescu D . How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int J Cardiol. (2019) 288:107–13. 10.1016/j.ijcard.2019.04.053
15.
Salvioni E Corra U Piepoli M Rovai S Correale M Paolillo S et al Gender and age normalization and ventilation efficiency during exercise in heart failure with reduced ejection fraction. ESC Heart Fail. (2020) 7(1):371–80. 10.1002/ehf2.12582
16.
Wasserman K Hansen JE Sue DY Stringer WW Whipp BJ . Clinical Exercise Testing, in Principles of Exercise Testing and Interpretation Including Pathophysiology and Clinical Applications. Philadelphia: Lippincott Williams & Wilkins (2005). p. 138–9.
17.
Beaver WL Wasserman K Whipp BJ . A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). (1986) 60(6):2020–7. 10.1152/jappl.1986.60.6.2020
18.
Agostoni P Corra U Cattadori G Veglia F La Gioia R Scardovi AB et al Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol. (2013) 167(6):2710–8. 10.1016/j.ijcard.2012.06.113
19.
Lang RM Badano LP Mor-Avi V Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. 10.1093/ehjci/jev014
20.
Pepi M Tamborini G Galli C Barbier P Doria E Berti M et al A new formula for echo-Doppler estimation of right ventricular systolic pressure. J Am Soc Echocardiogr. (1994) 7(1):20–6. 10.1016/s0894-7317(14)80414-8
21.
Harris PA Taylor R Thielke R Payne J Gonzalez N Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. 10.1016/j.jbi.2008.08.010
22.
Harris PA Taylor R Minor BL Elliott V Fernandez M O'Neal L et al The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. 10.1016/j.jbi.2019.103208
23.
Palau P Amiguet M Dominguez E Sastre C Mollar A Seller J et al Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction [DAPA-VO2]: a randomized clinical trial. Eur J Heart Fail. (2022) 24(10):1816–26. 10.1002/ejhf.2560
24.
Sano M Takei M Shiraishi Y Suzuki Y . Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. (2016) 8(12):844–7. 10.14740/jocmr2760w
25.
Heyman SN Khamaisi M Rosenberger C Szalat A Abassi Z . Increased hematocrit during sodium-glucose cotransporter-2 inhibitor therapy. J Clin Med Res. (2017) 9(2):176–7. 10.14740/jocmr2849w
26.
Agostoni P Cattadori G Vignati C Apostolo A Farina S Salvioni E et al Deceived by the Fick principle: blood flow distribution in heart failure. Eur J Prev Cardiol. (2024) 31(17):2001–10. 10.1093/eurjpc/zwae203
27.
Francis DP Shamim W Davies LC Piepoli MF Ponikowski P Anker SD et al Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J. (2000) 21(2):154–61. 10.1053/euhj.1999.1863
28.
Sinagra G Iorio A Merlo M Cannata A Stolfo D Zambon E et al Prognostic value of cardiopulmonary exercise testing in idiopathic dilated cardiomyopathy. Int J Cardiol. (2016) 223:596–603. 10.1016/j.ijcard.2016.07.232
29.
Arena R Myers J Guazzi M . The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. (2008) 13(2):245–69. 10.1007/s10741-007-9067-5
30.
Mehra MR Canter CE Hannan MM Semigran MJ Uber PA Baran DA et al The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. (2016) 35(1):1–23. 10.1016/j.healun.2015.10.023
31.
Lambers Heerspink HJ de Zeeuw D Wie L Leslie B List J . Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. (2013) 15(9):853–62. 10.1111/dom.12127
32.
Salah HM Verma S Santos-Gallego CG Bhatt AS Vaduganathan M Khan MS et al Sodium-glucose cotransporter 2 inhibitors and cardiac remodeling. J Cardiovasc Transl Res. (2022) 15(5):944–56. 10.1007/s12265-022-10220-5
33.
Xanthopoulos A Katsiadas N Skoularigkis S Magouliotis DE Skopeliti N Patsilinakos S et al Association between dapagliflozin, cardiac biomarkers and cardiac remodeling in patients with diabetes mellitus and heart failure. Life (Basel). (2023) 13(8):1778. 10.3390/life13081778
34.
Porcari A Cappelli F Nitsche C Tomasoni D Sinigiani G Longhi S et al SGLT2 Inhibitor therapy in patients with transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. (2024) 83(24):2411–22. 10.1016/j.jacc.2024.03.429
Summary
Keywords
dapagliflozin, SGLT2-i, heart failure, cardiopulmonary exercise testing (CPET), reverse remodeling, HFrEF
Citation
Mapelli M, Mattavelli I, Salvioni E, Capra N, Mantegazza V, Garlaschè A, Campodonico J, Rubbo FM, Paganin C, Capovilla TM, Nepitella AA, Caputo R, Gugliandolo P, Vignati C, Pezzuto B, De Martino F, Grilli G, Scatigna M, Bonomi A, Sinagra G, Muratori M and Agostoni P (2025) Dapagliflozin effects on exercise, cardiac remodeling, biomarkers, and renal and pulmonary function in heart failure patients: not as good as expected?. Front. Cardiovasc. Med. 12:1542870. doi: 10.3389/fcvm.2025.1542870
Received
16 December 2024
Accepted
26 February 2025
Published
17 March 2025
Volume
12 - 2025
Edited by
Federica Fogacci, University of Bologna, Italy
Reviewed by
Emilia D'Elia, Papa Giovanni XXIII Hospital, Italy
Inga Jona Ingimarsdottir, National University Hospital of Iceland, Iceland
Updates
Copyright
© 2025 Mapelli, Mattavelli, Salvioni, Capra, Mantegazza, Garlaschè, Campodonico, Rubbo, Paganin, Capovilla, Nepitella, Caputo, Gugliandolo, Vignati, Pezzuto, De Martino, Grilli, Scatigna, Bonomi, Sinagra, Muratori and Agostoni.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Massimo Mapelli massimo.mapelli@cardiologicomonzino.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.