- 1Department of Cardiology, Affiliated Zhongshan Hospital of Dalian University, Dalian, China
- 2Department of Geriatrics, No.903 Hospital of PLA Joint Logistics Support Force, Hangzhou, China
- 3Zhongshan Clinical College, Dalian University, Dalian, China
- 4Graduate School of Dalian Medical University, Dalian, China
- 5Department of Echocardiogram, Affiliated Zhongshan Hospital of Dalian University, Dalian, China
Objective: This study aims to evaluate the effects of angiotensin receptor–neprilysin inhibitor (ARNI) on myocardial energy metabolism and prognosis in patients with acute myocardial infarction (AMI) complicated by heart failure (HF).
Methods: A retrospective analysis was conducted on data from 244 inpatients admitted to our center, who were diagnosed with AMI complicated by HF. Among these patients, 210 completed a 1-year follow-up. According to the use of angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB)/ARNI, the 210 patients were divided into the ARNI group (107 cases, 51.0%) and the non-ARNI (ACEI/ARB) group (103 cases, 49.0%). The main outcome measures were the changes in myocardial energy expenditure (MEE) and prognostic indicators after 1-year follow-up.
Results: ARNI significantly reduced MEE after 1 year compared with the ACEI/ARB [(129.61 ± 40.81) kcal/min vs. (154.49 ± 47.58) kcal/min, P < 0.01]. The MEE level in the HFrEF group was significantly higher than that in the HFmrEF group (P < 0.05). The ARNI group showed significantly lower rates of heart failure (23.0% vs. 43.4%, P = 0.001), recurrent myocardial infarction (9.8% vs. 22.1%, P = 0.009), and renal function deterioration (5.7% vs. 13.1%, P = 0.049) than those in the non-ARNI group. ROC analysis identified an MEE (kcal/min) cutoff value of 178, with 85% sensitivity and 64% specificity for the prediction of cardiac death (AUC = 0.74, P = 0.007). During the 1-year follow-up, patients with MEE over 178 kcal/min were associated with increased risk of all-cause death compared with those with MEE below 178 kcal/min.
Conclusion: ARNI significantly reduced MEE compared with ACEI/ARB. MEE was significantly associated with the severity of left ventricular systolic dysfunction and long-term prognosis. An MEE value over 178 kcal/min was a powerful predictor of cardiac death and linked with increased risk of 1-year all-cause mortality in patients with AMI complicated by HF.
1 Introduction
Heart failure (HF) is a severe and end-stage condition of various cardiovascular diseases, with high rates of disability and mortality (1). Acute myocardial infarction (AMI) is currently one of the most common and significant etiologies of HF globally (2). The occurrence of HF following MI significantly increases the short-term and long-term risks of adverse events and mortality for patients (3). The heart is a highly energy-demanding organ with limited reserves. Therefore, the balanced state of myocardial energy metabolism (MEM) is crucial for sustaining cardiac function, and disturbances in MEM are one of the pathophysiological reasons for the occurrence and development of HF (4). Acute ischemia and hypoxia during AMI lead to MEM disorder which promotes the occurrence and development of HF. Therefore, MEM holds promise as a new target for the treatment of AMI complicated by HF (4–7). Studies have shown that angiotensin receptor–neprilysin inhibitor (ARNI) could reduce the infarct size of the left ventricle after AMI, enhance cardiac function, significantly improve the quality of life of patients, and lower the incidence of major adverse cardiovascular events (MACE) through multiple pathways to improve blood perfusion, thereby significantly improving the short-term and long-term prognosis of AMI patients (8, 9). However, there is still a lack of relevant research on the impact of ARNIs on MEM in patients with AMI complicated by HF. The purpose of this study is to evaluate the impact of ARNI on MEM and prognosis in patients with AMI complicated by HFrEF and HFmrEF, and this research used the angiotensin-converting enzyme inhibitor (ACEI)/ARB treatment group, the standard pharmacological treatment for AMI complicated by heart failure, as the control group. In addition, transthoracic echocardiography was used to measure myocardial energy expenditure (MEE).
2 Methods
2.1 Study subjects and grouping
A retrospective analysis was conducted on data from 244 inpatients admitted to the Department of Cardiology, Affiliated Zhongshan Hospital of Dalian University from February 2018 to February 2022. All patients were diagnosed with AMI complicated by HFrEF or HFmrEF, and 210 of them completed a 1-year follow-up. The study protocol was approved and carried out in accordance with the recommendations of the Ethics Committee of the Affiliated Zhongshan Hospital of Dalian University and adhered to the international ethical guidelines of the Declaration of Helsinki. All medical data information, images, and other relevant materials from enrolled patients were anonymized by the Medical Information Management Center of the hospital. Based on the medication status of ARNI/ACEI/ARB, the patients were divided into two groups: the ARNI group (107 cases, including 57 cases for HFrEF and 50 cases for HFmrEF) and the non-ARNI group (103 cases, including 50 cases for ACEI and 53 cases for ARB, 37 cases for HFrEF, and 66 cases for HFmrEF). In this study, ACEI/ARB/ARNI therapy was generally initiated within 24–48 h after hemodynamic stabilization following AMI. For patients who had started ACEI in the acute phase, ARNI was introduced after a ≥36 h washout period. The primary outcome measures were the changes in MEE and cardiac function before and after treatment, while the secondary outcome measures were the incidence of cardiovascular death, all-cause death, recurrent myocardial infarction, and readmission due to heart failure after a 1-year follow-up. Drug safety indicators included symptomatic hypotension, deterioration of renal function, hyperkalemia, angioedema, and dry cough.
2.2 Inclusion criteria
(1) Age > 18 years old; (2) history of AMI (diagnosed according to globally unified criteria) (≥30 days); (3) AMI complicated by HF meeting the diagnostic criteria for HFrEF and HFmrEF outlined in the “2020 Expert Consensus on Prevention and Treatment of Post-Myocardial Infarction Heart Failure” (3). (4) hemodynamically stable before initiating ARNI or non-ARNI treatment, with a systolic blood pressure (BP) not less than 90 mmHg within 6 h prior to ARNI initiation; (5) Exclusion of heart failure caused by other etiologies.
2.3 Exclusion criteria
(1) Intolerance to ACEI/ARB/ARNI; (2) presence of left ventricular aneurysm, pulmonary heart disease, severe hepatic or renal dysfunction, severe infections, malignancy, hematological or autoimmune diseases, or abnormal thyroid function; (3) use of medications that affect MEM (such as trimetazidine, coenzyme Q10, levocarnitine, and phosphocreatine); (4) lost to follow-up. Discontinuation criteria during treatment: severe renal dysfunction (serum creatinine ≥265 umol/L), hyperkalemia (serum potassium ≥5.5 mmol/L), symptomatic hypotension with systolic blood pressure <90 mmHg.
2.4 General data collection
Patient clinical data of selected individuals were collected from the Dragon and Jiahe Electronic Medical Record (EMR) systems. The data primarily included patient age, gender, BMI, New York Heart Association (NYHA) functional classification of heart failure, blood pressure (BP), heart rate (HR), comorbidities, echocardiographic findings, and biochemical indexes.
As shown in Figure 1, the PHILIPS EPIQ7 ultrasound system was used for cardiac ultrasound examination, with frequency set to 2–4 MHz. The heart was measured under a standard parasternal long-axis section. The patient took the left lying position to measure the left ventricular ejection fraction (LVEF), peak blood flow (E) in early mitral valve diastole, early mitral annulus velocity (e′), stroke volume (SV), tricuspid regurgitation velocity (TRvelocity), and E/e′. Additional measurements included ventricular septal thickness (IVSD), left ventricular posterior wall systolic thickness (PWTs), left ventricular end-systolic diameter (LVIDs), and end-diastolic diameter (LVIDd). Aortic valve ejection time (ET) was measured by Doppler blood flow spectrum. Each index was continuously measured for three cardiac cycles, and the average value was taken. Left ventricular end-systolic peripheral wall stress (cESS) was calculated, and MEE was calculated using the following formula (10, 11):
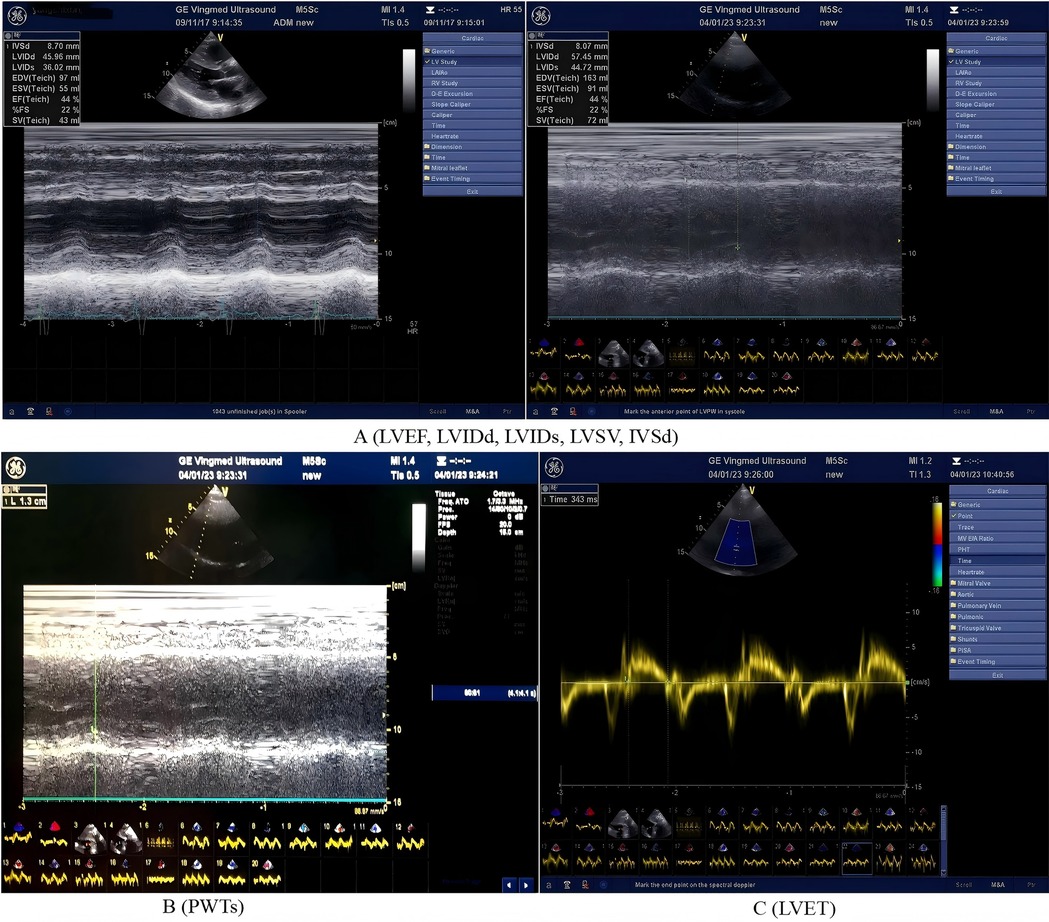
Figure 1. Echocardiographic parameters (including left ventricular structural, functional, volumetric and ejection time) and measurement methods. The panel A (LVEF, LVIDd, LVIDs, LVSV, and IVSd): LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal dimension in systole; LVSV, left ventricular stroke volume; IVSd, interventricular septal thickness in diastole. The panel B (PWTs): left ventricular posterior wall systolic thickness. The panel C (LVET): left ventricular ejection time.
2.5 Follow-up methods
All enrolled patients underwent on-site follow-up at 6 and 12 months. The observed indicators are as follows:
1. Baseline (data collection at the baseline was performed before the ARNI/ACEI/ARB drug treatment but after percutaneous coronary intervention (PCI) at the time of admission and diagnosis of AMI complicated by HFrEF/HFmrEF): cardiac ultrasound, electrocardiogram, and biochemical markers [including complete blood count, NT-proBNP, cTnI, liver function tests, kidney function tests, electrolytes, fasting blood glucose (FBG), glycated hemoglobin, lipid profile].
2. Six-month follow-up: cardiac ultrasound, electrocardiogram, and biochemical markers, including complete blood count, NT-proBNP, cTnI, liver function tests, kidney function tests, and electrolytes.
3. Twelve-month follow-up: the same assessments were repeated as in the 6-month follow-up.
Major cardiovascular events such as all-cause death, cardiac death, recurrent myocardial infarction, and HF-related rehospitalization were recorded. Drug safety indicators were also monitored, including symptomatic hypotension, renal function deterioration, hyperkalemia, vasovagal edema, and dry cough.
2.6 Statistical analysis
Data processing was conducted using SPSS version 26.0 statistical software. Normally distributed continuous variables are expressed as mean ± standard deviation and compared using the t-test. Non-normally distributed continuous variables are presented as median and quartiles [M (p25, p75)] and compared using the Mann–Whitney test. The paired Wilcoxon test was used for before-and-after comparisons. Categorical data were expressed as rates or proportions, and the chi-square test or Fisher's exact test was used to compare the two groups. Pearson's analysis method was used for correlation analysis of normal distribution continuous data, Spearman correlation analysis for correlation analysis of non-normal distribution continuous data, and multiple regression analysis to examine the relationship between MEE and various factors. The diagnostic value of indicators was evaluated using ROC curves, with the area under the curve (AUC) indicating diagnostic accuracy. The maximum Youden index was used to determine the optimal diagnostic threshold. Kaplan–Meier curve analysis was used for event-free survival analysis between the two groups. Statistical significance was defined as P < 0.05.
3 Results
3.1 Baseline characteristics
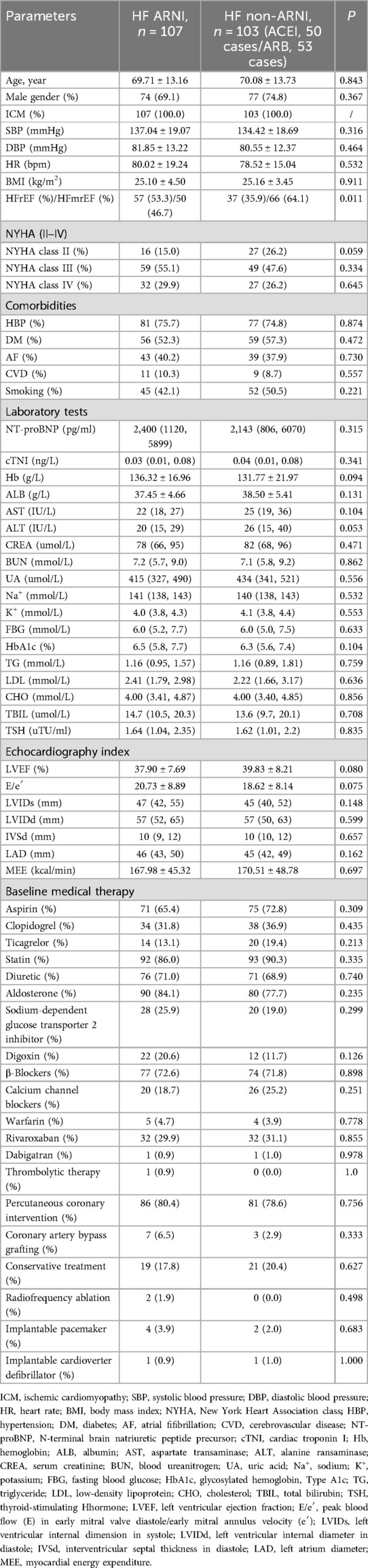
Refer to Table 1 for a comparison of baseline characteristics between the two patient groups, including age, gender, BP, HR, NYHA classification, comorbidities, coronary artery disease risk factors, NT-proBNP, cTNI, Hb, liver and kidney function, electrolytes, lipid profile, FBG, HbA1c, cardiac ultrasound, clinical background medications, and non-pharmacological treatments. There were no statistically significant differences observed (P > 0.05).
3.2 The impact of ARNI and non-ARNI on MEE, cardiac function, cardiac remodeling, and myocardial injury in patients with AMI complicated by HF
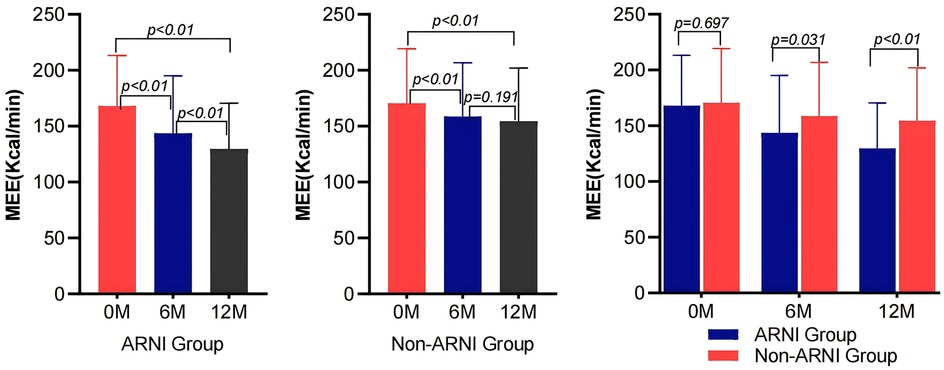
As shown in Table 2 and Figure 2, MEE in patients with AMI complicated by HF significantly decreased compared with baseline after treatment in both the ARNI and non-ARNI groups (P < 0.05). At the end of the follow-up, MEE in the ARNI group was significantly lower than that in the non-ARNI group (129.61 ± 40.81) kcal/min vs. (154.49 ± 47.58) kcal/min (P < 0.01). In both groups, LVEF was significantly increased after treatment compared with baseline in both groups (P < 0.05). As indicated in Table 2 and Figure 3, E/e′ in the ARNI group was significantly lower than that in the Non-ARNI group after 12 months of treatment (15.62 ± 7.62 vs. 18.20 ± 9.83, P = 0.035).
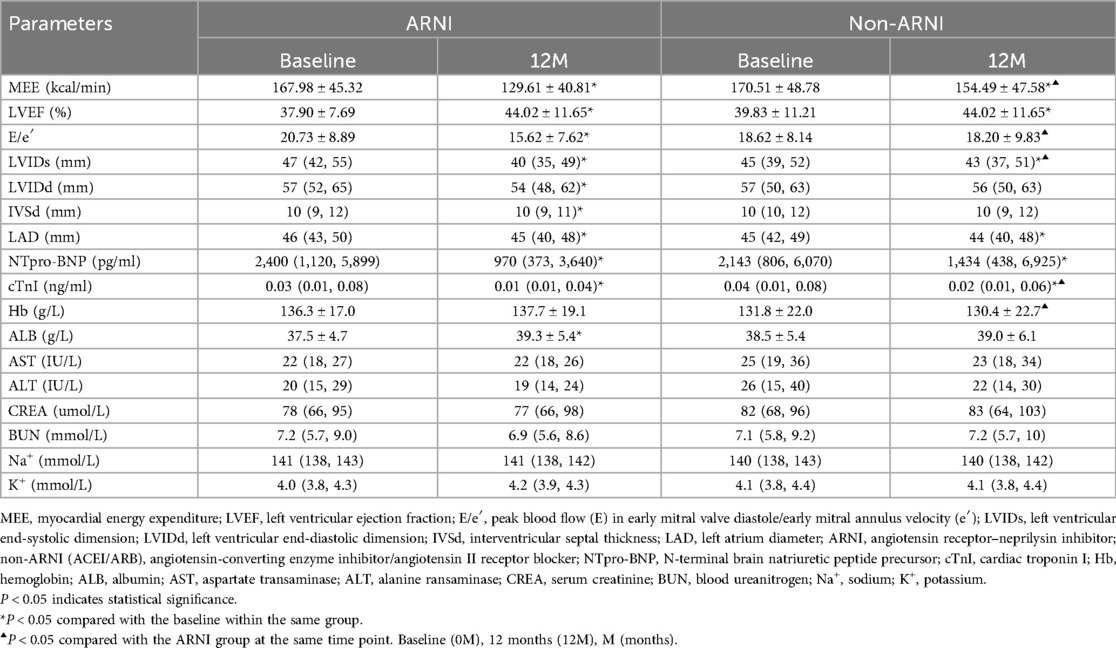
Table 2. Comparison of MEE, cardiac function, myocardial remodeling, myocardial injury markers, and laboratory biochemical parameters between the two groups before and after treatment.
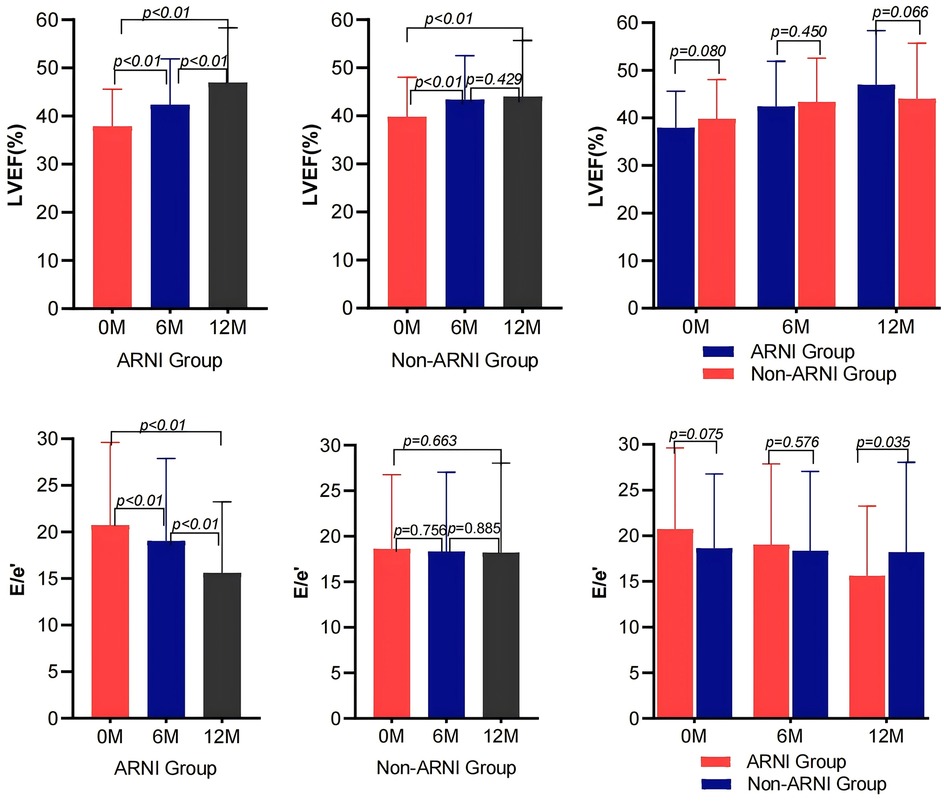
Figure 3. Comparison of LVEF and E/e′ before and after treatment between the ARNI and non-ARNI groups.
As shown in Table 2 and Figure 4, AMI complicated by HF patients showed significant reductions in LVIDd, LVIDs, left atrium diameter (LAD), and IVSd in the ARNI group compared with baseline after treatment (P < 0.05). In the non-ARNI group, LVIDs and LAD showed a significant decrease compared with baseline after treatment (P < 0.05). Although there was a decreasing trend in LVIDd and IVSd after treatment, the differences were not statistically significant (P > 0.05). At the end of the follow-up, LVIDs in the ARNI group was significantly lower than that in the non-ARNI group [40 (35, 49) mm vs. 43 (37, 51) mm, P = 0.039].
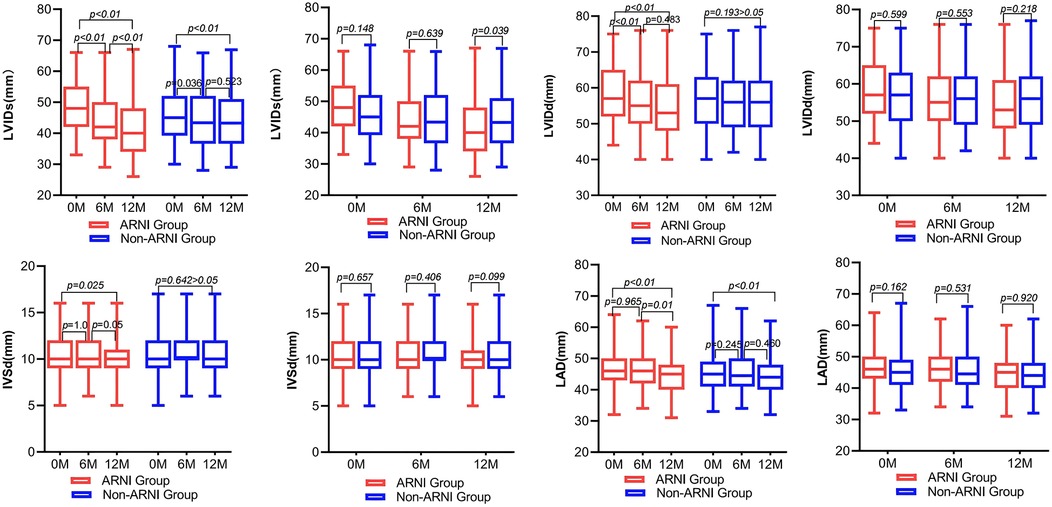
Figure 4. Comparison of left ventricular and left atrial structure between ARNI and non-ARNI before and after treatment.
Table 2 showed that in patients with AMI complicated by HF, NT-ProBNP and cTNI significantly decreased compared with baseline after treatment in both groups (P < 0.05). At the end of the follow-up, the cTNI levels in the ARNI group were significantly lower than those in the non-ARNI group [0.01 (0.01, 0.04) ng/ml vs. 0.02 (0.01, 0.06) ng/ml, P = 0.022], but Hb was higher than that in the non-ARNI group [136.3 ± 17.0 g/L vs. 130.4 ± 22.7 g/L, P = 0.012]. Additionally, ALB levels exhibited an increasing trend in the ARNI group and were notably higher than at baseline after treatment in the ARNI group [37.5 ± 4.7 g/L vs. 39.3 ± 5.4 g/L, P < 0.01]. There were no significant differences in AST, ALT, CREA, BUN, Na+, and K+ levels when comparing baseline and posttreatment values or when compared within the same time period between the two groups (P > 0.05).
3.3 Subgroup analysis on the effects of ARNI and non-ARNI on MEE, cardiac function, cardiac remodeling, and myocardial injury in the HFrEF and HFmrEF groups and exploring the impact of cardiac implantable devices on MEE
Tables 3 shows that there were no statistically significant differences in baseline characteristics between the two groups (P > 0.05).
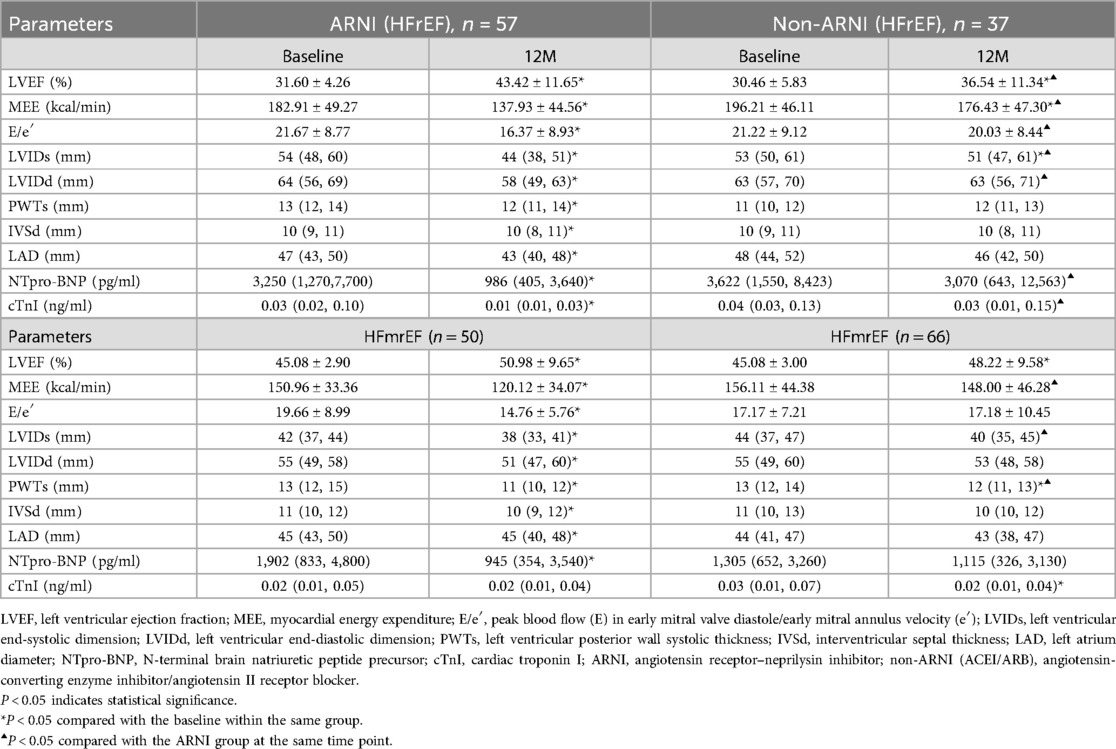
Table 3. Effects of ARNI and non-ARNI on the change of MEE, cardiac function, cardiac remodeling, and myocardial injury in the HFrEF and HFmrEF groups.
In both the ARNI and non-ARNI groups, LVEF in patients with AMI complicated by HFrEF significantly increased compared with baseline after treatment (P < 0.05), and LVEF in the ARNI group was significantly higher than that in the non-ARNI group (43.42 ± 11.65% vs. 36.54 ± 11.34%, P = 0.006). E/e′ in the ARNI group significantly decreased compared with baseline after treatment (P < 0.01) and was also significantly lower than that in the non-ARNI group (16.37 ± 8.93 vs. 20.03 ± 8.44, P = 0.012). In both groups, MEE in patients with AMI complicated by HFrEF significantly decreased compared with baseline after treatment (P < 0.05), and MEE in the ARNI group was significantly lower than that in the non-ARNI group (137.93 ± 44.56 kcal/min vs. 176.43 ± 47.30 kcal/min, P < 0.01). In the ARNI group, LVIDd, LVIDs, PWTs, LAD, and IVSd showed significant reductions compared with baseline after treatment (P < 0.05). In the non-ARNI group, LVIDs showed a significant decrease compared with baseline after treatment (P < 0.05). Although there was a decreasing trend in LVIDd, LAD, and IVSd after treatment, the differences were not statistically significant (P > 0.05). At the end of the follow-up, LVIDs [44 (38, 51) mm vs. 51 (47, 61) mm, P = 0.004] and LVIDd [58 (49, 63) mm vs. 63 (56, 71) mm, P = 0.014] in the ARNI group were significantly lower than those in the non-ARNI group. In the ARNI group, NT-ProBNP and cTNI significantly decreased compared with baseline after treatment (P < 0.05), and NT-ProBNP [986 (405, 3,640) pg/ml vs. 3,070 (643, 12,563) pg/ml, P < 0.01] and cTNI [0.01 (0.01, 0.03) ng/ml vs. 0.03 (0.01, 0.15) ng/ml, P < 0.01] in the ARNI group were significantly lower than those in the non-ARNI group.
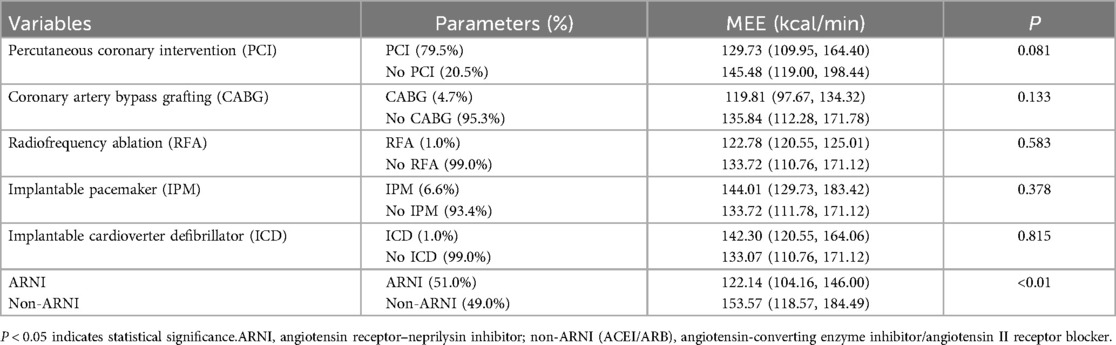
In both groups, LVEF in patients with AMI complicated by HFmrEF increased compared with baseline after treatment (P < 0.05). E/e′ in the ARNI group significantly decreased compared with baseline after treatment (P < 0.01). In the ARNI group, MEE in patients with AMI complicated by HFmrEF significantly decreased compared with baseline after treatment (P < 0.01), and MEE in the ARNI group was significantly lower than in the non-ARNI group (120.12 ± 34.07 kcal/min vs. 148.00 ± 46.28 kcal/min, P < 0.01). In the ARNI group, LVIDd, LVIDs, PWTs, LAD, and IVSd showed significant reductions compared with baseline after treatment (P < 0.05). In the non-ARNI group, PWTs showed a significant decrease compared with baseline after treatment (P < 0.01). Although there was a decreasing trend in LVIDd, LVIDs, LAD, and IVSd after treatment, the differences were not statistically significant (P > 0.05). At the end of the follow-up, LVIDs [38 (33, 41) mm vs. 40 (35, 45) mm, P = 0.036] and PWTs [11 (10, 12) mm vs. 12 (11, 13) mm, P < 0.01] in the ARNI group were significantly lower than those in the non-ARNI group. In the ARNI group, NT-ProBNP significantly decreased compared with baseline after treatment (P = 0.04), and cTNI significantly decreased in the non-ARNI group compared with baseline after treatment (P = 0.03). A comparison of MEE after 1-year follow-up revealed no significant differences among cardiac implantable devices groups (P > 0.05) (Table 4).
3.4 Comparison of clinical endpoints and safety between the ARNI and non-ARNI groups
At the end of the 12-month follow-up, there were 15 cases of all-cause mortality, with 9 in the ARNI group and 6 in the non-ARNI group. Of these, 13 cases were due to cardiovascular death, with 7 in the ARNI group and 6 in the non-ARNI group. The difference in the all-cause mortality rate (7.4% vs. 4.9%, P = 0.424) and cardiovascular mortality rate (5.7% vs. 4.9%, P = 0.776) was similar between the two groups. The ARNI group had significantly lower rates of hospitalization for heart failure (23.0% vs. 43.4%, P = 0.001), recurrent myocardial infarction (9.8% vs. 22.1%, P = 0.009), and renal function deterioration (5.7% vs. 13.1%, P = 0.049) compared with the non-ARNI group. There were no statistically significant differences in the rates of symptomatic hypotension, hyperkalemia, vascular angioedema, and dry cough between the two groups (P > 0.05).
3.5 Clinical correlation between LV dysfunction and MEE
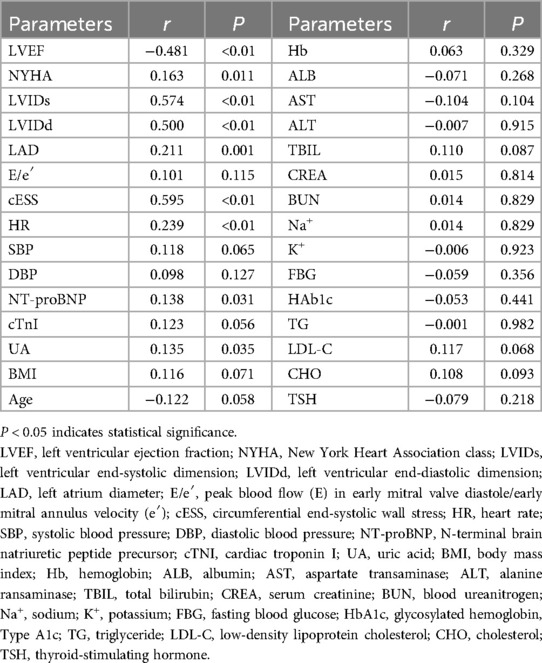
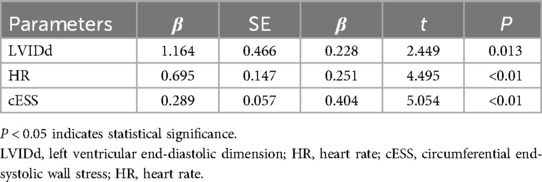
The correlation analysis of baseline MEE and other variables in AMI complicated by HF patients revealed that there was a negative correlation between MEE and LVEF (r = −0.481, P < 0.01), and MEE showed positive correlations with NYHA classification (r = 0.163, P = 0.011), LVIDs (r = 0.574, P < 0.01), LVIDd (r = 0.500, P < 0.01), LAD (r = 0.211, P = 0.001), cESS (r = 0.595, P < 0.01), HR (r = 0.239, P < 0.01), NT-proBNP (r = 0.138, P = 0.031), and UA (r = 0.135, P = 0.035). However, there were no significant correlations between MEE and cTNI, Hb, ALB, AST, ALT, CREA, BUN, Na, K, TG, CHO, LDL-C, TBIL, TSH, FBG, and HbA1c (Table 5). Using MEE as the dependent variable and LVEF, NYHA, LVIDs, LVIDd, LAD, cESS, HR, NT-proBNP, and UA as independent variables, a stepwise multiple linear regression analysis was performed. Table 6 shows that MEE was independently associated with LVIDd (β = 0.228, P = 0.013), HR (β = 0.251, P < 0.01), and cESS (β = 0.404, P < 0.01). Furthermore, as LVIDd, HR, cESS, and MEE increased.
3.6 Predictive ability of MEE for cardiovascular mortality
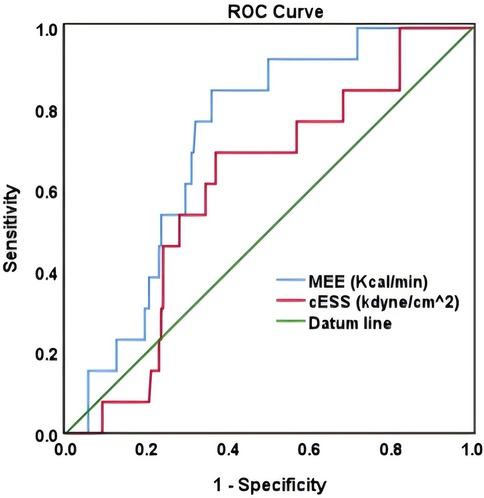
In ROC analysis, MEE (kcal/min) at a cutoff value of 178 had 85% sensitivity and 64% specificity for prediction of cardiac death (AUC = 0.74, P = 0.007) (Table 7; Figure 5). All the patients were divided into Groups 1 and 2 again based on the MEE cutoff value of 178 (Group 1, MEE >178 kcal/min, 97 patients; Group 2, MEE ≤178 kcal/min, 147 patients). Kaplan–Meier analysis according to the long-term event-free survival revealed that the occurrence of events was higher in Group 1 compared with Group 2 (P = 0.005) (Figure 6).
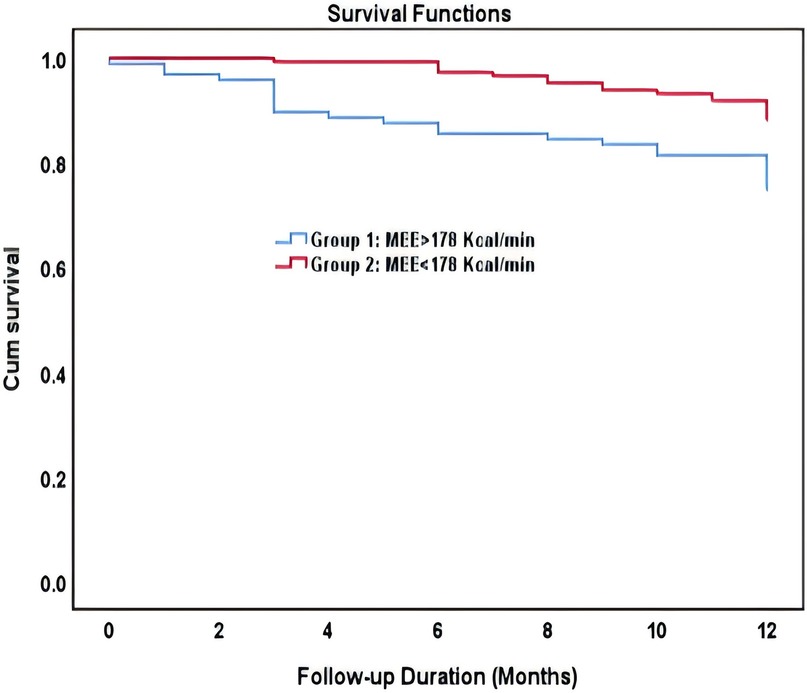
Figure 6. Kaplan–Meier survival curve of participants demonstrating long-term all-cause mortality among patient groups specified based on the myocardial energy expenditure cutoff value of 178 kcal/min.
4 Discussion
The present study showed that in patients with HFmrEF and HFrEF after myocardial infarction, MEE reduction, cardiac functional improvement, reverse remodeling, and mitigation of myocardial injury are more significant in the ARNI group compared with the ACEI/ARB group. The ARNI group has a significantly lower rate of hospitalization for heart failure, recurrent myocardial infarction, and renal function deterioration compared with the ACEI/ARB group. Moreover, MEE (kcal/min) at a cutoff value of 178 could be used as a biomarker to predict cardiac death with 85% sensitivity and 64% specificity (AUC = 0.74), and the occurrence of events was significantly higher in patients with MEE >178kcal/min.
4.1 Effects of ARNI on MEE in patients with AMI complicated by HF
Previous studies have demonstrated the benefits of ARNI in cardiac function, ventricular remodeling, myocardial injury, anti-arrhythmias, prognosis, and quality of life of patients with AMI complicated by HF. However, there is limited research regarding the effects of ARNI on myocardial energy metabolism. As part of the GDMT in AMI complicated by HF, the ACEI/ARB treatment group was used as the control group. There are four primary methods available to estimate MEE. Based on safety, simplicity, accuracy, feasibility, cost-effectiveness, stability, and repeatability, transthoracic echocardiography was selected to evaluate MEE in the present study.
This study found that ARNI significantly reduced MEE in patients with AMI complicated by HFrEF and HFmrEF. The known mechanisms underlying the improvement in MEE by ARNI involve its dual blockade of the RASS and neprilysin. It inhibits neprilysin to increase the activity of natriuretic peptides, adrenomedullin, glucagon-like peptide 1 (GLP-1), and skeletal muscle cyclic guanosine monophosphate (cGMP), while simultaneously reducing the activity of dipeptidyl peptidase 4, thus increasing insulin sensitivity (12). Furthermore, ARNI can improve insulin resistance and pancreatic β-cell function and promote glucose metabolism (13). By downregulating NADPH oxidase-1, NADPH oxidase-2, oxidized proteins, γ-H2A histone family member X, and hypoxia-inducible factor 1 alpha and by upregulating Sirtuin-1, Sirtuin-3, superoxide dismutase, catalase, glutathione peroxidase, it alleviates mitochondrial damage induced by oxidative stress and inflammatory response, simultaneously maintain the stability of mitochondrial membrane potential, restore mitochondrial activity, promote autophagy to clear damaged mitochondria, reduce energy consumption, and thus maintain myocardial energy metabolism homeostasis (14–17). Additionally, sacubitril/valsartan dilates blood vessels, reduces both preload and afterload on the heart, decreases myocardial energy consumption, increases blood flow perfusion in ischemic regions, and facilitates the delivery of metabolic substrates (glucose, oxygen), thereby improving myocardial energy metabolism. The further mechanisms and metabolic signaling pathways by which ARNI reduces MEE and improves myocardial energy metabolism still need to be explored and validated through fundamental experiments and clinical research. In this study, ARNI demonstrated a significantly greater improvement in MEE compared with ACEI/ARB, and subgroup analyses revealed that PCI, coronary artery bypass grafting (CABG), radiofrequency ablation (RFA), implantable pacemaker (IPM), and implantable cardioverter defibrillator (ICD) did not have a substantial impact on MEE in patients with AMI complicated by HFrEF and HFmrEF. However, ACEI/ARB also displayed a reduction in MEE during treatment. The reduction in MEE is more pronounced in the first 6 months of medication initiation and gradually diminishes in effectiveness from 6 to 12 months of treatment. The mechanisms underlying this reduction involve the enhanced activity of RASS associated with HF, which can lead to alterations in the insulin and insulin-like growth factor 1 signaling pathway and the generation of reactive oxygen species, resulting in endothelial dysfunction and insulin resistance (18). ACEI can increase fatty acid uptake in HF patients, improve myocardial energy metabolism, and cause inactivation of adrenomedullin, which has a favorable effect on glucose absorption, oxidation, and glycolysis (19). Studies on obese and insulin-resistant animals have shown that ACEI can improve the heart's response to insulin (20).
4.2 The impact of ARNI on cardiac function, myocardial remodeling, and myocardial injury in patients with AMI complicated by HF
Sacubitril/valsartan, the first ARNI, exerts cardioprotective effects through dual inhibition of the AT1 receptor of angiotensin II and neprilysin. This dual mechanism attenuates RAAS-mediated vasoconstriction and remodeling while enhancing natriuretic peptide activity, promoting natriuresis, vasodilation, and possessing antifibrotic effects (21, 22).
In this study, the ARNI group showed a significantly stronger inhibition of cardiac remodeling, reduction in MEE and myocardial injury, and improvement in left ventricular systolic and filling compared with the ACEI/ARB group, indicating a more pronounced effect in improving cardiac function. In the PARADISE-MI echocardiographic substudy, at 8-month follow-up, ARNI exhibited a significant advantage over enalapril in inhibiting left ventricular remodeling and reducing left ventricular filling pressure (23). Moreover, in this study, E/e′ in the ARNI group significantly decreased compared with baseline after treatment, and E/e′ in the ARNI group was significantly lower than that in the ACEI/ARB group. Additionally, ARNI is superior to ACEI/ARB in inhibiting myocardial remodeling at the end of follow-up. A multicenter/prospective randomized controlled trial, aimed primarily at observing the improvement of myocardial remodeling in patients with AMI treated with PCI, found that early administration of ARNI effectively prevented cardiac remodeling in AMI patients (24). The SAVE-STEMI trial (25) and a study using an AMI rabbit model (8) both demonstrated that ARNI significantly outperforms enalapril and valsartan in delaying or even reversing left ventricular remodeling and improving systolic function post-MI. Additionally, the latter study found that ARNI was significantly superior to valsartan in reducing post-MI left ventricular infarct area. Another study investigating the therapeutic effects of ARNI in patients with STEMI complicated by HF after emergency PCI found that ARNI significantly delayed ventricular remodeling and improved cardiac function (9). The results of this study are consistent with the aforementioned studies, which demonstrated that ARNI is significantly superior to ACRI/ARB in inhibiting myocardial remodeling, improving cardiac function, and reducing myocardial injury. A study indicated that during the initial week post-high-risk MI, NT-proBNP correlates with the incidence of HF, mortality, and atherosclerotic incidents (26). The PIONEER-HF trial demonstrated that ARNI therapy was superior to ACEI therapy in terms of reducing NT-ProBNP levels among heart failure patients with a diminished ejection fraction during an acute phase (27). Similarly, within this research, NT-proBNP levels significantly decreased in the ARNI group and the ACEI/ARB group in posttreatment, and NT-ProBNP in the ARNI group was significantly lower than in the Non-ARNI group in patients with AMI complicated by HFrEF. A substudy of PARADIGM-HF indicated hemoglobin (Hb) decreased less and the incidence of new anemia was lower with ARNI (28). Correspondingly, at the end of the follow-up of this study, the levels of Hb were found to be higher in the ARNI group compared with the ACEI/ARB groups. The prevalence of liver function abnormalities is common in patients with HFrEF. A substudy of the PARADIGM-HF trial revealed that sacubitril/valsartan improved indicators of liver function compared with enalapril (29). Similarly, ALB levels exhibited a trend of increasing in the ARNI group, which were observed to be higher than at baseline after treatment in this study. The changes in Hb and ALB levels were likely attributed to the ARNI's improvement in cardiac function. Improved cardiac function can enhance systemic organ perfusion, reduce digestive system congestion, and enhance gastrointestinal absorption of proteins and iron supplements which are essential for hematopoiesis, thereby mitigating the decline in Hb. Additionally, the alleviation of hepatic congestion and subsequent reduction in hepatocyte damage thereby contributes to improved liver function and increased synthesis of ALB. In summary, ARNI may improve the overall physiological function in patients with heart failure after myocardial infarction through various indirect or direct mechanisms.
The subanalysis revealed that MEE in the HFrEF group was significantly higher than that in the HFmrEF group in patients with AMI complicated by HF. Several experts have highlighted that MEE shows a significant increase as LVEF decreases and cardiac function grade increases. Moreover, there is a strong correlation between MEE and NT-proBNP levels (30, 31). In this study, MEE showed a significantly negative correlation with LVEF, and MEE showed positive correlations with NYHA classification, NT-proBNP, LVIDs, LVIDd, LAD, cESS, and HR. Multiple regression analysis revealed that MEE was independently associated with LVIDd, HR, and cESS. In the calculation formula for MEE, MEE is directly proportional to HR and cESS. Moreover, reducing HR alleviates myocardial oxygen consumption and decreases MEE. cESS reflects the circumferential stress experienced by the ventricular wall at the end of systole, serving as one of the indices used in cardiac biomechanics to assess myocardial contractility and wall tension (32). Higher cESS indicates that the myocardium requires more energy expenditure and performs more work to overcome ventricular wall stress to maintain normal cardiac output. In addition, this study found that LVIDd had a significant impact on MEE. LVIDd is an indirect indicator for assessing left ventricular preload and myocardial remodeling and is also one of the important parameters for evaluating left ventricular function. Reducing ventricular preload can decrease myocardial work and thus reduce MEE. Reversing myocardial remodeling can improve cardiac function, leading to less energy expenditure under the same conditions of myocardial work. In summary, the results proved that MEE was significantly correlated with the cardiac function, especially LV systolic function, cardiac remodeling, degree of myocardial damage, ventricular wall tension, and ventricular rate. Additionally, the worse the LV systolic function in post-myocardial infarction heart failure patients, the greater the energy expenditure. Namely, the aggravated myocardial oxygen and energy expenditure coexist with enhanced neurohumoral activity, myocardial remodeling, myocardial injury, etc., which may further result in the diminution of myocardial energy storage and deterioration of cardiac function and form the vicious cycle of the above adverse factors in patients with AMI complicated by HF.
At 1-year follow-up, both HFrEF and HFmrEF patients overall had higher LVEF and lower MEE, E/e′, LVIDs, LVIDd, PWTs, IVSd, LAD, NT-proBNP, and cTNI than at baseline, which illustrated that for patients with AMI complicated by HF, cardiac function and MEE can be improved through timely revascularization of the occluded vessel to restore myocardial blood perfusion, long-term standard treatment, and relevant cardiac implantable electronic devices. Furthermore, in the HFrEF group, the change rates for LVEF, NT-proBNP, cTnI, MEE, and cESS were all greater than those in the HFmrEF group. This may reflect that certain treatment regimens have better efficacy in HFrEF than in HFmrEF, particularly ARNI (33). However, in a subset of patients, the LVEF decreased, while MEE, E/e′, LVIDs, LVIDd, PWTs, IVSd, LAD, NT-proBNP, and cTNI increased compared with their baseline, which indicated a persistent deterioration characteristic of LV systolic function in patients with AMI complicated by HFrEF and HFmrEF. Research has demonstrated that any activity resulting in an energy metabolism disorder can precipitate both systolic and diastolic dysfunction of the cardiac mechanics, subsequently triggering ventricular remodeling. The presence of aberrant cardiac energy metabolism is closely related to a decline in cardiac function (34). Reduced MEE might be one mechanism responsible for attenuating myocardial remodeling, relieving myocardial injury and improving cardiac function and prognosis in patients with acute myocardial infarction complicated by heart failure.
4.3 The impact of ARNI on the prognosis of patients with AMI complicated by HF
The results of the PARADIGM-HF study showed that ARNI was superior to enalapril in reducing the risk of death from cardiovascular causes or all-cause and hospitalization for heart failure. Additionally, it alleviates symptoms and enhances exercise tolerance in patients with HF (35). The PARADISE-MI study demonstrated that ARNI was not associated with a significantly lower incidence of mortality from cardiovascular causes or incident heart failure than ramipril in patients with AMI complicated by a reduced LVEF, pulmonary congestion, or both (36). However, in subsequent analyses of PARADISE-MI, ARNI was significantly more effective than enalapril in reducing any cause or cardiovascular death and hospitalization for HF. In this study, the ARNI group had a significantly lower rate of hospitalization for heart failure, but there was no significant difference in the rate of cardiovascular mortality and all-cause between the ARNI group and the ACEI/ARB group at the end of the 12-month follow-up, which may be relevant to the small sample size and short observation period of this study. The occurrence rates of adverse events were low in both groups, with no difference in vascular angioedema, hyperkalemia, renal insufficiency, or hepatic dysfunction, and the incidence of hypotension was higher in patients treated with ARNI, while the incidence of dry cough was higher in patients treated with enalapril in the PARADISE-MI study (36–40). In this research regarding the safety profile of ARNI compared with ACEI/ARB, there were no statistically significant differences between the two groups in the occurrence rates of symptomatic hypotension, hyperkalemia, vasomotor edema, or dry cough. Additionally, this study found that ARNI was significantly superior to ACEI/ARB in delaying the deterioration of renal function, which is closely related to its potent effect on improving cardiac function. Improved cardiac function can enhance systemic organ perfusion in heart failure patients, increase renal blood flow, and thus delay the deterioration of renal function. Another study aimed at comparing the cardiovascular outcomes of ARNI and ACEI/ARB in AMI patients found that ARNI was superior to ACEI/ARB in reducing long-term MACE (41). Within this clinical investigation, the ARNI group had a significantly lower rate of hospitalization for heart failure and recurrent myocardial infarction compared with the ACEI/ARB group. Moreover, studies found that 13%–32% of MI patients have left ventricular systolic dysfunction or pulmonary congestion, which is associated with a 2–3 times increased risk of subsequent death or heart failure hospitalization (42). A substudy in PARADISE-MI found that ARNI significantly reduced pulmonary congestion (43), thereby improving prognosis. In conclusion, for patients with AMI complicated by HFrEF and HFmrEF, both ARNI and ACEI/ARB have shown varying degrees of reduction in MEE levels, inhibition or even reversal of myocardial remodeling, improvement in cardiac function, attenuation of myocardial injury, and reduction in MACE, thereby improving prognosis. Moreover, ARNI exhibits superior efficacy over ACEI/ARB in all the aforementioned aspects observed from this study.
In the early and intermediate phases of heart failure, myocardial energy metabolism is relatively normal, while in the terminal phase, the myocardial cells transition from fatty acid metabolism to glucose metabolism as the main source of energy supply (30). However, in the advanced stage, with increased myocardial oxygen and energy consumption, abnormal MEE may reflect the progressive cardiac function deterioration, and MEE may be associated with the prognosis of patients with AMI complicated by HF. Our study provides significant findings on prognostic implications of estimated MEE in relation to LV systolic dysfunction in middle-aged and elderly patients with HFrEF and HFmrEF after myocardial infarction. Increased MEE was correlated with severe LV systolic dysfunction. Furthermore, MEE was a powerful predictor of cardiac death independent of established prognostic factors, including reduced EF, NT-proBNP, cTnI, significant cardiovascular risk factors, comorbidities, and renal function. Stepwise higher rates of cardiac death occurred at MEE values above 178 kcal/min, and MEE over 178 kcal/min is linked with an increased risk of 1-year all-cause mortality in HFrEF and HFmrEF. Similar to the study by Palmieri et al. (10), the present study indicates that depressed EF was associated independently with higher MEE and elevated MEE as an independent predictor of cardiac death in a population-based sample of adults with depressed LV systolic function but without overt congestive heart failure. HF patients generally exhibit a state of cardiac overload. Research indicates that a higher myocardial energy requirement in overloaded hearts is expected based on experimental data on myocardial bioenergetics (44). In patients with AMI complicated with HF, especially those with HFrEF complicated with cardiac overload, increased myocardial energy consumption leads to worsened heart function, thus forming a vicious cycle. The disturbance of myocardial energy metabolism might also be an important factor responsible for the continuous progression of patients with heart failure after myocardial infarction. Therefore, in the treatment of patients with AMI complicated by HF, greater emphasis should be placed on the therapy of myocardial energy metabolism.
5 Conclusions
Reduced MEE might be one mechanism responsible for reversing myocardial remodeling, attenuating myocardial injury, elevating cardiac function, lowering MACE, and improving prognosis in patients with AMI complicated by HF. ARNI exhibits superior efficacy over ACEI/ARB in all the aforementioned aspects observed from this study at the end of the 12-month follow-up. MEE is significantly associated with the severity of left ventricular systolic dysfunction and long-term prognosis. MEE is a powerful predictor of cardiac death, and MEE over 178 kcal/min is linked with increased risk of 1-year all-cause mortality in HFrEF and HFmrEF.
5.1 Limitations and outlook of this study
Due to the limited study duration, the current analysis is based on the 1-year follow-up data from a single medical center. Further research is needed to initiate a multicenter study with an expanded sample size with follow-up at 1 year and 2 years to better assess the long-term effects of ARNI on MEE, cardiac function, prognosis, and adverse events in patients with post-myocardial infarction heart failure. The calculation formula for MEE used in this study is complex, involving the conversion of mechanical and energetic parameters. However, all measurements in this study were performed by the same experienced cardiac sonographer, minimizing potential confounding factors. In future research protocols, it is recommended to use a variety of methods that can be explored to investigate myocardial energy metabolism while ensuring accuracy. In addition, more studies are needed in the future to clarify the long-term effects of sacubitril/valsartan on myocardial metabolism in patients with post-myocardial infarction heart failure and to explore its long-term effects at the metabolomic level.
Highlights
The effects of sacubitril/valsartan on myocardial energy metabolism and prognosis in patients with acute myocardial infarction complicated by heart failure are as follows:
1. Previous studies have shown that angiotensin receptor–neprilysin inhibitor (ARNI) can improve and delay ventricular remodeling after AMI. Our research focuses on the impact of ARNI on myocardial energy metabolism in patients with AMI complicated by heart failure.
2. As a kind of non-invasive method to evaluate myocardial energy expenditure (MEE), compared with PET and MRI, echocardiography is simpler, safer, more cost-effective, stable, and repeatable.
3. Compared with GDMT medicine ACEI/ARB, ARNI could reduce MEE, improve myocardial remodeling, and relieve myocardial injury in patients with AMI complicated by heart failure, which improves cardiac function and prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Affiliated Zhongshan Hospital of Dalian University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CC: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. YN: Data curation, Investigation, Methodology, Writing – original draft. DC: Data curation, Investigation, Software, Writing – original draft. YY: Data curation, Investigation, Software, Writing – original draft. SL: Data curation, Formal analysis, Methodology, Software, Writing – original draft. QY: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number: 81770405).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships, and all authors declare no conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. (2014) 63(12):1123–33. doi: 10.1016/j.jacc.2013.11.053
2. Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. (2014) 385(9966):441–51. doi: 10.1016/S0140-6736(14)60921-1
3. Chinese Physician Association Cardiovascular Medicine Physician BranchChina Cardiovascular Health Alliance, Expert Consensus Working Group on Prevention and Treatment of Heart Failure after Myocardial Infarction. 2020 Expert consensus on the prevention and treatment of heart failure after myocardial infarction. Chin Circ J. (2020) 35(12):1166–80. doi: 10.3969/j.issn.1000-3614.2020.12.002
4. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. (2018) 15(8):457–70. doi: 10.1038/s41569-018-0044-6
5. Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic origins of heart failure. JACC Basic Transl Sci. (2017) 2(3):297–310. doi: 10.1016/j.jacbts.2016.11.009
6. Chinese Geriatrics Society Electrophysiology and Cardiac Function Branch, Chinese Physician Association Cardiovascular Medicine Branch, Expert Committee of the China Heart Failure Center Alliance. Chinese Expert consensus on clinical application of drugs to improve cardiac metabolism (2021). Chin J Geriatr. (2021) 40(9):1081–92. doi: 10.3760/cma.j.issn.0254-9026.2021.09.001
7. Bertero E, Maack C. Reply to ‘metabolic remodelling in heart failure revisited’. Nat Rev Cardiol. (2018) 15(12):780–1. doi: 10.1038/s41569-018-0116-7
8. Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, et al. Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. Jam Coll Cardiol. (2018) 72(19):2342–56. doi: 10.1016/j.jacc.2018.07.102
9. Tian JF, Song XT, He Y, Zhang MD, Zhang DF, Nan N, et al. Effect of sakubatril valsartan on heart function in patients with anterior old myocardial infarction. Chin J Evid-Bases Cardiovasc Med. (2021) 13(3):289–92. doi: 10.3969/j.issn.1674-4055.2021.03.07
10. Palmieri V, Roman MJ, Bella JN, Liu JE, Best LG, Lee ET, et al. Prognostic implications of relations of left ventricular systolic dysfunction with body composition and myocardial energy expenditure: the Strong Heart Study. J Am Soc Echocardiog. (2007) 21(1):66–71. doi: 10.1016/j.echo.2007.05.008
11. Shen AN, Du ZY, Wang P, Xie ZB, Xu DL. Value of Doppler echocardiography derived myocardial energy expenditure measurements in chronic heart failure patients. Chin J Cardiovasc Dis. (2010) 38(3):209–14. doi: 10.3760/cma.j.issn.0253-3758.2010.03.003
12. Jordan J, Stinkens R, Jax T, Engeli S, Blaak EE, May M, et al. Improved insulin sensitivity with angiotensin receptor neprilysin inhibition in individuals with obesity and hypertension. Clin Pharmacol Ther. (2016) 101(2):254–63. doi: 10.1002/cpt.455
13. Kashiwagi Y, Nagoshi T, Ogawa K, Kawai M, Yoshimura M. Heart failure treatments such as angiotensin receptor/neprilysin inhibitor improve heart failure Status and glucose metabolism. Cureus. (2022) 14(3):e22762. doi: 10.7759/cureus.22762
14. Dogan Z, Ergun DD, Durmus S, Sahin H, Senturk GE, Gelisgen R, et al. Empagliflozin and sacubitril/valsartan reverse methotrexate cardiotoxicity by repressing oxidative stress and hypoxia in heart embryonic H9c2 cardiomyocytes—the role of morphology of mitochondria observed on electron microscopy. EUR Rev Med Pharmaco. (2023) 27(9):3979–92. doi: 10.26355/eurrev_202305_32304
15. Raffa S, Forte M, Gallo G, Ranieri D, Marchitti S, Magrì D, et al. Atrial natriuretic peptide stimulates autophagy/mitophagy and improves mitochondrial function in chronic heart failure. Cell Mol Life Sci. (2023) 80(5):134. Published 2023 April 26. doi: 10.1007/s00018-023-04777-w
16. Ko SF, Sung PH, Yang CC, Chiang JY, Yip HK. Combined therapy with dapagliflozin and entresto offers an additional benefit on improving the heart function in rat after ischemia-reperfusion injury. Biomed J. (2023) 46(3):100546. doi: 10.1016/j.bj.2022.06.002
17. Vergaro G, Del Franco A, Carecci A, Ferrari Chen YF, Aimo A, Forini F, et al. Effects of sacubitril-valsartan on remodelling, fibrosis and mitochondria in a murine model of isoproterenol-induced left ventricular dysfunction. Int J Cardiol. (2023) 409:132203. doi: 10.1016/j.ijcard.2024.132203
18. Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol-Heart C. (2007) 293(4):H2009–23. doi: 10.1152/ajpheart.00522.2007
19. Mori J, Zhang L, Oudit GY, Lopaschuk GD. Impact of the renin-angiotensin system on cardiac energy metabolism in heart failure. J Mol Cell Cardiol. (2013) 63:98–106. doi: 10.1016/j.yjmcc.2013.07.010
20. Kadkhodayan A, Coggan AR, Peterson LR. A “PET” area of interest: myocardial metabolism in human systolic heart failure. Heart Fail Rev. (2013) 18(5):567–74. doi: 10.1007/s10741-012-9360-9
21. Docherty KF, McMurray JJV. Angiotensin receptor-neprilysin inhibitors: a new paradigm in heart failure with reduced ejection fraction. Int J Cardiol. (2018) 281:179–85. doi: 10.1016/j.ijcard.2018.05.124
22. Ishii M, Kaikita K, Sato K, Sueta D, Fujisue K, Arima Y, et al. Cardioprotective effects of LCZ696 (sacubitril/valsartan) after experimental acute myocardial infarction. JACC Basic Transl Sci. (2017) 2(6):655–68. doi: 10.1016/j.jacbts.2017.08.001
23. Shah AM, Claggett B, Prasad N, Li G, Volquez M, Jering K, et al. Impact of sacubitril/valsartan compared with ramipril on cardiac structure and function after acute myocardial infarction: the PARADISE-MI echocardiographic substudy. Circulation. (2022) 146(14):1067–81. doi: 10.1161/CIRCULATIONAHA.122.059210
24. Li Z, Fu G. Assessment of ultra-early administration of sacubitril valsartan to improve cardiac remodeling in patients with acute myocardial infarction following primary PCI: rational and design of a prospective, multicenter, randomized controlled trial. Front Physiol. (2022) 13:831212. doi: 10.3389/fphys.2022.831212
25. Rezq A, Saad M, El Nozahi M. Comparison of the efficacy and safety of sacubitril/valsartan versus ramipril in patients with ST-segment elevation myocardial infarction. Am J Cardiol. (2021) 143:7–13. doi: 10.1016/j.amjcard.2020.12.037
26. Jering KS, Claggett BL, Pfeffer MA, Granger CB, Køber L, Lewis EF, et al. Prognostic importance of NT-proBNP (N-terminal pro-B-type natriuretic peptide) following high-risk myocardial infarction in the PARADISE-MI trial. Circ-Heart Fail. (2023) 16(5):e010259. doi: 10.1161/CIRCHEARTFAILURE.122.010259
27. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. New Engl J Med. (2019) 380(6):539–48. doi: 10.1056/NEJMoa1812851
28. Curtain JP, Adamson C, Docherty KF, Jhund PS, Desai AS, Lefkowitz MP, et al. Prevalent and incident Anemia in PARADIGM-HF and the effect of sacubitril/valsartan. JACC-Heart Fail. (2023) 11(7):749–59. doi: 10.1016/j.jchf.2022.12.012
29. Suzuki K, Claggett B, Minamisawa M, Packer M, Zile MR, Rouleau J, et al. Liver function and prognosis, and influence of sacubitril/valsartan in patients with heart failure with reduced ejection fraction. Eur J Heart Fail. (2020) 22(9):1662–71. doi: 10.1002/ejhf.1853
30. Cetin MS, Ozcan Cetin EH, Canpolat U, Sasmaz H, Temizhan A, Aydogdu S. Prognostic significance of myocardial energy expenditure and myocardial efficiency in patients with heart failure with reduced ejection fraction. Int J Cardiovas Imag. (2017) 34(2):211–22. doi: 10.1007/s10554-017-1226-8
31. Chen P, Zhan Q, Bai Y, Huang X, Wang P, Pan Y, et al. Serum peroxisome proliferator-activated receptor gamma coactivator-1α related to myocardial energy expenditure in patients with chronic heart failure. Am J Med Sci. (2018) 357(3):205–12. doi: 10.1016/j.amjms.2018.12.002
32. Gui YX, Si XD. Correlation between biomechanical changes at the end of left ventricular systole and pathogenesis of diastolic heart failure. Med Biomech. (2020) 35(6):744–9. doi: 10.16156/j.1004-7220.2020.06.015
33. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. (2014) 16(7):817–25. doi: 10.1002/ejhf.115
34. Guzun R, Timohhina N, Tepp K, Gonzalez-Granillo M, Shevchuk I, Chekulayev V, et al. Systems bioenergetics of creatine kinase networks: physiological roles of creatine and phosphocreatine in regulation of cardiac cell function. Amino Acids. (2011) 40(5):1333–48. doi: 10.1007/s00726-011-0854-x
35. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. New Engl J Med. (2014) 371(11):993–1004. doi: 10.1056/NEJMoa1409077
36. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, et al. Angiotensin receptor-neprilysin inhibition in acute myocardial infarction. New Engl J Med. (2021) 385(20):1845–55. doi: 10.1056/NEJMoa2104508
37. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, et al. Impact of sacubitril/valsartan versus ramipril on total heart failure events in the PARADISE-MI trial. Circulation. (2021) 145(1):87–9. doi: 10.1161/CIRCULATIONAHA.121.057429
38. Gatto L. Does sacubitril/valsartan work in acute myocardial infarction? The PARADISE-AMI study. Eur Heart J Suppl. (2021) 23(Suppl E):E87–90. doi: 10.1093/eurheartj/suab098
39. Mehran R, Steg PG, Pfeffer MA, Jering K, Claggett B, Lewis EF, et al. The effects of angiotensin receptor-neprilysin inhibition on major coronary events in patients with acute myocardial infarction: insights from the PARADISE-MI trial. Circulation. (2022) 146(23):1749–57. doi: 10.1161/CIRCULATIONAHA.122.060841
40. Berwanger O, Pfeffer M, Claggett B, Jering KS, Maggioni AP, Steg PG, et al. Sacubitril/valsartan versus ramipril for patients with acute myocardial infarction: win-ratio analysis of the PARADISE-MI trial. Eur J Heart Fail. (2022) 24(10):1918–27. doi: 10.1002/ejhf.2663
41. She J, Lou B, Liu H, Zhou B, Jiang GT, Luo Y, et al. ARNI Versus ACEI/ARB in reducing cardiovascular outcomes after myocardial infarction. ESC Heart Fail. (2021) 8(6):4607–16. doi: 10.1002/ehf2.13644
42. Hamilton E, Desta L, Lundberg A, Alfredsson J, Christersson C, Erlinge D, et al. Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Fail. (2023) 10(2):1347–57. doi: 10.1002/ehf2.14301
43. Platz E, Claggett B, Jering KS, Kovacs A, Cikes M, Winzer EB, et al. Trajectory and correlates of pulmonary congestion by lung ultrasound in patients with acute myocardial infarction: insights from PARADISE-MI. Eur Heart J-Acute CA. (2023) 12(3):155–64. doi: 10.1093/ehjacc/zuad001
Keywords: acute myocardial infarction, heart failure, ARNI, MEE, energy metabolism
Citation: Cheng C, Nie Y, Chen D, Yang Y, Liang S and Yu Q (2025) Effects of angiotensin receptor–neprilysin inhibition on myocardial energy metabolism and prognosis in patients with acute myocardial infarction complicated by heart failure. Front. Cardiovasc. Med. 12:1550624. doi: 10.3389/fcvm.2025.1550624
Received: 23 December 2024; Accepted: 8 July 2025;
Published: 12 August 2025.
Edited by:
Dobrin Vassilev, University of Ruse, BulgariaReviewed by:
Yuanyuan Dai, Stanford University, United StatesSandeep Bhushan, Chengdu Second People's Hospital, China
Copyright: © 2025 Cheng, Nie, Chen, Yang, Liang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Yu, eXVxaW5AZGx1LmVkdS5jbg==
 Caiming Cheng
Caiming Cheng Yu Nie1,4
Yu Nie1,4 Qin Yu
Qin Yu