Abstract
The gut microbiome refers to the collective genomes of the approximately 1,000–1,150 microbial species found in the human gut, called the gut microbiota. Changing the gut microbiota composition has been shown to affect cardiovascular health significantly. Numerous studies have demonstrated the part that gut microbiota and its metabolites play in the development and course of several illnesses, including colon cancer, heart failure, stroke, hypertension, and inflammatory bowel disease. With cardiovascular diseases responsible for more than 31% of all fatalities globally, conditions like hypertension, atherosclerosis, and heart failure are serious global health issues. Developing preventive measures to fight cardiovascular diseases requires understanding how the gut microbiota interacts with the cardiovascular system. Understanding the distinctive gut microbiota linked to cardiovascular diseases has been made possible by microbial sequencing analysis. The gut microbiota and cardiovascular diseases are closely related, and more profound knowledge of this association may result in treatment strategies and broad guidelines for enhancing cardiovascular health through gut microbiome modification. This review summarizes the role of gut microbiota in cardiovascular diseases, highlighting their influence on disease progression and potential therapeutic implications.
1 Introduction
It is estimated that between 1,000 and 1,150 different types of microorganisms reside in the human gut (1). These groups are referred to as “gut microbiota,” and “gut microbiomes” are the collective term for all of the genomes of microorganisms in the gut, including their DNA sequences and other genetic data (2). A small number of phyla, such as Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomicrobia, dominate the gut microbiota in healthy people, which maintains a very consistent composition (3). There is growing evidence that altering the composition of the gut microbiota impacts the cardiovascular phenotypes of the host (4–6).
Numerous research conducted in recent years have shown how the gut microbiota and its metabolites influence the development of both cardiovascular and non-cardiovascular diseases in the host, including inflammatory bowel disease, colon cancer, hypertension, heart failure, and stroke (7). One of the leading causes of death and morbidity globally is cardiovascular disease (CVD). It is the primary cause of death worldwide and includes heart failure, atherosclerosis, and hypertension (8). Atherosclerotic cardiovascular disease is now widely acknowledged as a serious global health hazard, accounting for almost 31% of all fatalities worldwide (9). Finding potential preventive measures is crucial to halting the onset and progression of CVD. Numerous details regarding the existence of distinctive gut microbiota linked to CVDs have been made available by microbial sequencing study (10–12). Gut microbiota has been linked to peripheral artery disease (PAD), especially in diabetic individuals, in addition to its well-established roles in hypertension and coronary artery disease. Atherosclerosis and vascular problems in PAD are accelerated by endothelial dysfunction, oxidative stress, and chronic inflammation, all of which are exacerbated by dysbiosis in the gut microbiota. Furthermore, metabolites originating from the gut, like trimethylamine-N-oxide (TMAO), have been connected to vascular dysfunction and enhanced platelet aggregation, which exacerbates ischemia conditions in PAD. The importance of microbiota in diabetic PAD was emphasized by Biscetti et al., who proposed that microbial manipulation might be a novel treatment approach to enhance vascular outcomes in these patients (13). A growing body of research has demonstrated a substantial correlation between CVDs and gut microbiota (14, 15). By better understanding the relationship between the gut microbiota and the cardiovascular system, we may be able to develop general guidelines and therapeutic strategies that support the gut microbiota's cardio-protective function. In this review, we summarize the role of gut microbiota in cardiovascular diseases.
2 Gut microbiota mechanisms of action in cardiovascular diseases
The gut microbiota might influence cardiovascular disorders through metabolites like short-chain fatty acids, trimethylamine-N-oxide, coprostanol, bile acid, indoxyl sulfate, phenylacetylglutamine, and vitamin K2 (Figure 1).
Figure 1
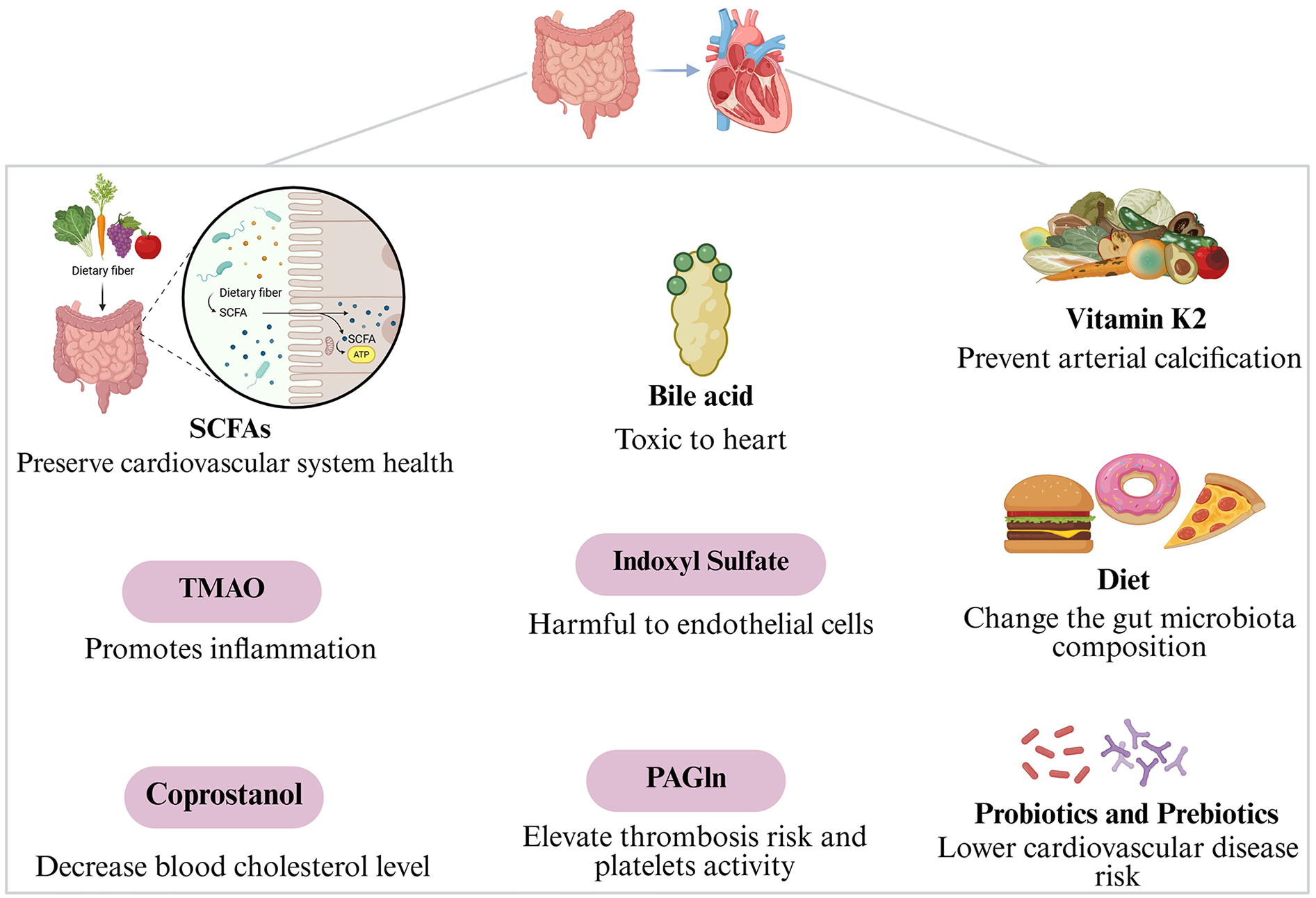
Gut microbiota mechanisms of action in cardiovascular diseases.
2.1 Short-chain fatty acid production
The metabolites acetate, butyrate, and propionate are categorized as short-chain fatty acids (SCFAs) and are produced when intestinal bacteria ferment the indigestible polysaccharides (16). SCFAs diffuse across the intestinal mucosa, enter the bloodstream through the portal system at low millimolar concentrations, and interact with G protein-coupled receptors (GPCRs) on the plasma membranes of various target cells throughout the mammalian body (17, 18). These fatty acids exhibit a positive correlation with Eubacterium rectale, Alistipes putredinis, Bacteroides spp., Roseburia, and Faecal prausnitzii (19). By improving intestinal barrier integrity through increased production of cellular junction proteins and acting as energy substrates for epithelial cells, SCFAs positively affect the gastrointestinal tract. Furthermore, they affect inflammation and metabolic processes. They can reduce oxidative stress, show anti-inflammatory and anti-tumorigenic properties, alter glycemic control and lipid metabolism, and alter the release of inflammatory cytokines and chemokines (20, 21).
The importance of SCFAs in preserving cardiovascular system health is suggested by their notable effects against various inflammatory illnesses, such as Coronary artery disease (CAD) and hypertension (22, 23). The amount of dietary fiber consumed and the composition of the gut flora's SCFA-producing bacteria control how much SCFA is produced. Accordingly, changes in the composition of the gut microbiota are a significant factor influencing the levels of SCFAs, thereby promoting the development of CVD linked to inflammation (24). There are two main ways that SCFAs might demonstrate their anti-inflammatory properties. The first is that SCFAs are known to inhibit histone deacetylase (HDAC). When HDAC inhibits the histone acetylation process, the chromatin structure decondenses, increasing gene expression and promoting the development of regulatory T cells to generate anti-inflammatory cytokines like interleukin 10 (IL-10) (22, 25). However, butyrate is a negative modulator of inflammation, and its anti-inflammatory action is achieved by inhibiting HDAC, which typically controls innate immunity pathways, regulating the differentiation of myeloid cells and the inflammatory response mediated by the expression of genes induced by toll-like receptors (TLR) and interferon (IFN) (26, 27). Thus, butyrate inhibits HDAC to limit the synthesis of pro-inflammatory cytokines like interferon-γ (IFN-γ), IL-12, and tumor necrosis factor-α (TNF-α). This increases monocytes' in vitro production of IL-10, which has anti-inflammatory effects (28). Second, it is found that SCFAs contribute to anti-inflammatory responses through the activation of specific G protein-coupled receptors (GPCRs). The intestinal epithelial cells and almost all immune cell types express GPCRs, such as GPCR41, GPCR43, and GPCR109A, which SCFAs bind to (29). SCFA binding to the GPCR can trigger several intracellular signaling cascades, including nuclear transcription, enzyme activation, and cell membrane ion transport (30). This binding protects the gut from inflammation by promoting regulatory T-cell differentiation to produce IL-10 and enhancing intestinal barrier integrity through the NLR family pyrin domain containing 3 (NLRP3) inflammasomes. These inflammasomes generate IL-19, which enhances epithelial integrity by improving the function of tight junction proteins (29, 31).
Butyrate regulates blood pressure by activating the GPCR41 and vasorelaxing the blood vessels (32). Butyrate specifically lowers diastolic blood pressure in humans. The effects of butyrate on 60 patients with type 2 diabetes mellitus were investigated in a randomized, double-blind trial. The results showed that the diastolic blood pressure of the treatment groups decreased statistically significantly (P < 0.05). Additionally, treated patients had higher levels of Akkermansia muciniphila, a bacteria with anti-inflammatory properties (33). Propionate activates GPCR41 to cause vasodilation, which lowers blood pressure slightly. The improvement of endothelial dysfunction may be the mechanism for the long-term decrease in blood pressure (34). By preventing the production of cholesterol and moving it to the liver, SCFAs can help reduce serum lipid levels (35). As a result, they have been proposed as a protective factor against the development of CAD. Additionally, there has been a decrease in SCFA-producing bacteria in some CAD cases and the gut dysbiosis of hypertension patients (36–39). Additionally, the intestinal metabolism of cholesterol is influenced by SCFAs. There is an inverse relationship between serum cholesterol levels and the conversion of cholesterol to coprostanol in patients with elevated fecal amounts of SCFAs; the precise mechanisms are still unclear, although this could be because the gut flora is different (40). Although there is growing evidence that short-chain fatty acids are involved in several cardiovascular disorders, more investigation is necessary to fully understand the underlying mechanism and the diverse effects of SCFA supplementation on the cardiovascular system.
2.2 Trimethylamine-N-oxide production
TMAO, a risk factor for the development of CAD, is produced by dietary choline, betaine, phosphatidylcholine, lecithin, and L-carnitine (41–44). Choline, phosphatidylcholine, and carnitine-trimethylamine are frequently found in meat, egg yolks, and high-fat dairy products. These substances undergo a two-step modification process: (A) undergo conversion by the gut bacteria into TMA, which is then taken up and transported to the liver via the portal circulation. 36 species with 102 genomes have been reported to create TMA. Firmicutes, Proteobacteria, and Actinobacteria are among the TMA producers; Bacteroidetes do not produce TMA. TMA production has been linked to firmicutes, such as Anaerococcus, Clostridium, Desulfitobacterium, Enterococcus, Streptococcus, and Proteobacteria, such as Pseudomonas, Enterobacter, Proteus, Escherichia, Dseulfovibrio, Actinobacter, Citrobacter, and Klebsiella (45, 46). (B) The flavin-containing monooxygenase (FMO) enzyme, which is expressed by the FMO gene in the liver, kidney, and other tissues, converts the microbiome-derived TMA molecule into TMAO once it enters the host's circulation and reaches hepatocytes (43, 47, 48). Microbiota, age, medicines, gender, and lifestyle all interact to affect TMAO levels (49). The volume of dietary precursor ingestion and the composition of each person's gut microbial flora determine how much TMAO each individual produces (50). Animal products with high concentrations of TMA precursors are a feature of Western diets, altering the gut flora and raising TMAO levels (51).
In contrast to SCFAs, TMAO is a bacterial metabolite that promotes inflammation and has also been linked to the pathophysiology of cardiovascular disease. To cause thrombus development, it works by triggering the immunological and inflammatory responses, upregulating the expression of inflammatory cytokines, and preventing the synthesis of bile acids (52). A higher risk of cardiovascular events and mortality has been associated with elevated TMAO concentrations by 23% and 62%, respectively. A meta-analysis of more than 25,000 participants found that all-cause mortality increased by 7.6% with every 10 μmol/L accumulation of TMAO (53, 54). TMAO may contribute to the pathogenic process of atherosclerosis development by stimulating macrophage migration and its conversion into foam cells. Foam cells have a proatherogenic action from the first lesion generation to the plaque rupture. The biological signature and initial stage of atherogenesis is the buildup of foam cells in artery intima. Most foam cells come from macrophages, which can control the metabolism of lipoproteins (41, 42). TMAO influences cholesterol metabolism and induces the production of foam cells in several ways. High-density lipoprotein (HDL) is a key component of the reverse cholesterol transport (RCT) pathway, which keeps cholesterol metabolism in equilibrium. It stimulates macrophages to release free cholesterol (55). Research revealed that HDL was considerably lower in the plasma of CVD patients with elevated TMAO levels, which prevented the RCT pathway and macrophage accumulation of cholesterol (41). Elevated TMAO levels are associated with increased cardiovascular risks and accelerated atherosclerosis. Additionally, TMAO increases endothelial dysfunction, changes lipid metabolism, and causes platelet hyperactivity by stimulating calcium release from the rough endoplasmic reticulum. Platelet hyperactivity and the consequent development of plaque are caused by TMAO, which also increases cholesterol influx, decreases cholesterol circulation, and blocks the bile acid route (56–58). Choline supplementation raises TMAO levels and enhances platelet aggregation and reactivity in healthy human volunteers (59). Non-culprit plaques show signs of vulnerability in CAD patients with increased TMAO. These include enhanced microvascularization, a higher incidence of thin-cap fibroatheroma, and decreased fibrous cap thickness (10, 60). C-reactive protein (CRP) and endothelial dysfunction with elevated gut permeability are also linked to TMAO (45, 58).
The relationship between elevated TMAO plasma levels and CVD, as well as its association with major adverse cardiac events (MACEs) like myocardial infarction, stroke, heart failure, and cardiovascular death, has been assessed by recent metabolomics investigations (42, 61, 62). The rate of MACEs was strongly correlated with plasma TMAO concentrations over a 3-year follow-up period in individuals who had elective coronary angiography. Even after controlling for conventional risk variables, patients in the highest quartile of circulating TMAO levels had a 2.5-fold increased risk of MACEs compared to those in the lowest (63). Even though numerous investigations have verified the link between TMAO levels and different CAD occurrences, some studies do not find a correlation between TMAO and CAD. One study, for instance, found no correlation between TMAO levels and atherosclerosis in the Framingham Heart Study Offspring group (1,215 participants) or supporting animal research (64, 65). Studies have demonstrated that the use of broad-spectrum antibiotics changed the composition of the gut microbiota and decreased TMAO levels, indicating the significance of the gut microbiota in the metabolism of TMAO, which is consistent with the mandatory role that the gut microbiota performed in TMAO formation (41). Elevated TMAO levels are linked to a greater risk of adverse cardiovascular disease, so further large-scale prospective cohorts are expected to characterize the association, especially the causality in the general population.
2.3 Coprostanol production
In addition to TMAO, there are various microbial pathways whose alteration or suppression could impact CVD risk. The gut bacteria's ability to convert cholesterol to coprostanol was initially documented in the 1930s (66). Humans begin producing coporstanol in the second half of their first year of life (67), as well as being sex-dependent, with young women having higher conversion rates than young men (68, 69). The gut converts cholesterol to coprostanol, a non-absorbable sterol removed with feces. Because it is linked to decreased blood cholesterol levels, converting cholesterol to coprostanol has a clinically significant effect (70). One potential method is the conversion of cholesterol to sterol coprostanol by the microbial cholesterol dehydrogenase enzyme (ismA gene) (71, 72). The number of bacteria that metabolize cholesterol was shown to be closely associated with factors that affect cholesterol conversion (73, 74). Intestinal bacteria that can convert cholesterol to coprostanol include Eubacterium coprostanoligenes, Bacteroides species, Lactobacillus species, and Bifidobacterium species. IsmA has a role in the oxidation of coprostanol to coprostanone and cholesterol to 4-cholesten-3-one. Interestingly, Escherichia colioverexpresses this enzyme, and other microbial species that have IsmA genes have been linked to lower blood cholesterol levels (75). Using animal models, giving hypercholesterolemic rabbits the cholesterol-lowering bacterium Eubacterium coprostanoligenes dramatically lowered their plasma cholesterol levels. The intestinal contents of rabbits-fed microorganisms showed higher than average coprostanol/cholesterol ratios (76). Many studies on the gut's metabolism of cholesterol have been conducted using human models (67, 77–80), and it has been proposed that the coprostanol/cholesterol ratio in human feces and human serum cholesterol have an inverse relationship (79, 81, 82). Coprostanoligenic strain research may be useful in the clinic to modify the microbiota and lower cardiovascular risk (83, 84). Further research is necessary because the precise mechanisms are still unclear.
2.4 Bile acid modulation
The main amphipathic, water-soluble byproduct of the breakdown of cholesterol is bile acids (BAs) (85). They have a role in inflammatory bowel disease, gastrointestinal cancer, metabolic disorders, and cardiovascular diseases (86). Since the 1960s, it has been shown that BAs are toxic to the heart. They can induce cardiac remodeling and electrophysiological changes, which can lead to deadly arrhythmias (87, 88). Because the ileum reabsorbs around 95% of the bile acids and the enterohepatic cycle returns them to the liver, bile acids can act as endocrine-like signaling molecules that alter host metabolism and energy homeostasis (89). The primary metabolites of cholesterol in the liver that aid in the absorption of lipids, nutrients, and lipophilic vitamins are bile acids, which are produced by the rate-limiting enzyme cholesterol 7-alpha-hydroxylase (CYP7A1) (90, 91), and also the energy metabolism, regulation of lipids and glucose (92, 93).
Bile salts are created when primary bile acids are coupled to the amino acids taurine or glycine. These salts are then secreted into bile and kept in the gallbladder until released into the small intestine, emulsifying fats and creating micelles that enterocytes can absorb (91). Primary bile acids like cholic acid (CA) and chenodeoxycholic acid (CDCA) are broken down in the gut by bile salt hydrolase (BSH) and the gut microbiota to produce secondary bile acids, including deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) (91, 94). Except for UDCA and LCA, primarily eliminated in feces, all conjugated and unconjugated bile acids in the lumen can be reabsorbed (95%) and returned to the liver (90). The nuclear receptor farnesoid X receptor (FXR) and the membrane G protein-coupled bile acid receptor Gpbar-1 can be activated by signaling molecules like bile acids in the gut (91, 95). Bile acids can suppress bile acid synthesis through this method (94), which may raise cholesterol levels (96). In the intestine, a wide range of aerobic and anaerobic bacteria (including Gram-negative Bacteroides, Lactobacillus, Clostridium, and Enterococcus, as well as Gram-positive Bifidobacterium) catalyze the deconjugation of the glycine and taurine moieties of PBA (97). Then, PBA (CA and CDCA) are changed into secondary bile acids (SBA): LCA and DCA, respectively, by bacterial hydroxysteroid dehydrogenase (HSDH) enzymes, which eliminate a hydroxyl group at the 7α position (98). Secondary bile acids (SBAs) have been linked to cardiovascular health and are ligands of the nuclear farnesoid X receptor (FXR). Diet can alter the composition of the gut microbiota and the metabolism of bile acids (99). The gut microbiota can impact how the liver regulates cholesterol metabolism (28, 81) and contribute to the modification of bile acids, which can affect systemic cholesterol levels (100). Bile acids may be a helpful biomarker to predict the severity of CVD, according to cohort studies conducted in various populations (101, 102). Elevated plasma levels of LCA were linked to an increased risk of CHD in a case-control study (103). Patients with CAD had higher fasting serum total BA than those without CAD, according to a survey of 7,438 participants (104). Additional research is necessary to validate and deepen our comprehension of BA's function in modulating the association between gut microbiota and the risk of CVD.
2.5 Indoxyl sulfate
Tryptophan, a necessary amino acid and a precursor to various important mediators such as tryptamine, serotonin, melatonin, kynurenines, and nicotinic acid, is converted in the intestines into the molecule indole by gut bacteria (105–107). Escherichia coli and other gut bacteria include tryptophanase, which converts a portion of tryptophan obtained from proteins into indole (108). The liver produces the indole metabolite indoxyl sulfate, which enters the bloodstream as a serum molecule coupled to albumin. Usually, indoxyl sulfate is eliminated by the urine. Because indoxyl sulfate is not adequately eliminated in patients with poor renal function, it increases in the blood and negatively impacts the endothelium by causing arterial stiffness and calcification. It is well-recognized that indoxyl sulfate damages various cell types, including vascular endothelial cells (109–112). In endothelial cells, elevated indoxyl levels cause oxidative stress, a pro-inflammatory response, and increased adhesion molecule expression. The pathophysiology of cardiovascular disorders in people with chronic kidney disease (CKD) is influenced by the harmful effects of indoxyl on endothelial cells. How indoxyl affects the pathophysiology of CAD in individuals who do not have renal impairment is still unknown (113). Among dialysis patients with normal renal function, indoxyl sulfate was associated with cardiovascular disease (114).
Serum indoxyl sulfate levels may be a predictive mechanistic biomarker of the severity of coronary artery disease because they have a positive correlation with coronary atherosclerosis scores (115). Indoxyl sulfate causes thrombosis via increasing platelet activity and the body's reaction to collagen and thrombin (116). There is evidence linking an increase of the nephrotoxin indoxyl sulfate in the serum of uremic patients—which is caused by poor renal secretion—to gut microbiota dysbiosis toward a greater abundance of aerobic indole-producing bacteria (such as Escherichia coli) (117, 118). Studies by Takayama et al. demonstrated that by altering the composition of the gut microbiota, hemodialysis patients receiving oral treatment with non-indole-producing bacteria (such as Bifidobacterium) had a significant decrease in indoxyl sulfate serum levels (118). Another study demonstrated that giving indoxyl sulfate to rats with CKD accelerated the disease's progression and raised the expression of the genes for pro-α1(I) collagen, tissue inhibitor of metalloproteinase (TIMP)-1, and transforming growth factor (TGF)-β1 (119). Additional research has demonstrated that indoxyl sulfate exacerbates atrial fibrillation, cardiomyocyte hypertrophy, and cardiac fibrosis (120, 121). These investigations collectively show that indoxyl sulfate is biologically connected to CVD at both the molecular and cellular levels.
2.6 Phenylacetylglutamine
The gut flora of heart failure patients and healthy people varies in terms of both composition and function, according to a number of studies conducted in recent years. Glutamate, which lowers ammonia through the urea cycle bypass pathway, and phenylacetate/phenylbutyrate (phenylacetate precursor) were originally believed to be the only sources of phenylacetylglutamine (PAGln), one of the main toxins associated with chronic kidney illness (122). Recent research, however, has shown that PAGln, which is also produced by intestinal microbes that metabolize the essential amino acid phenylalanine, is regarded as an independent predictor of MACE risk and has high plasma concentrations in patients who have experienced MACE (104, 123). A metabolite called PAGln is produced when the gut bacteria conjugates glutamine and phenylacetate. PAGln has been linked to elevated thrombosis risk and platelet activity (103). Klebsiella pneumoniae, Acinetobacter baumannii, Proteus mirabilis, Lachnospiraceae, Christensenellaceae, and Ruminococcaceae. Among the main microbes that produce phenylacetylglutamine are Bacteroidetes, Firmicutes, Proteobacteria, and Staphylococcus aureus (124–127).
High levels of PAGln are independently linked to a higher risk of coronary in-stent restenosis, which is linked to a poorer prognosis for CAD patients (128). Hazen et al. were the first to show a positive association between the gut-derived metabolite PAGln and platelet and thrombosis functions in a nontargeted metabolomics investigation (123). Additionally, elevated PAGln plasma levels are a strong independent predictor of carotid plaque burden, indicating a potential link to atherosclerosis (129). According to a study by Fang et al, increased plasma PAGln levels and improved microbiota-derived PAGln synthesis-related functions were linked to in-stent stenosis and hyperplasia in CAD patients. To prevent stent stenosis in patients with CAD, an intervention that targets gut bacteria may be a promising approach (130).
2.7 Vitamin K2
There are two main types of vitamin K: phylloquinones (vitamin K1, VK1) and menaquinones (vitamin K2, VK2). Four isoprenoid residue side chains make up the single chemical VK1. However, the side chains of VK2 range in length from four to thirteen isoprene residues (131). Plants are the exclusive source of VK1, but gram-positive bacteria in the human gastrointestinal system create a number of congeners (menaquinone-5–menaquinone-13, MK5–MK13) that make up VK2 (131, 132). Vitamin K2 is an isoform of vitamin K and a cofactor that helps carboxylate several proteins, including matrix Gla-protein (MGP) (133). Fibroblasts, endothelial cells, vascular smooth muscle cells, and chondrocytes all express the MGP (134). This protein plays a role in preserving the vascular wall's structural and functional integrity (135). It prevents arterial calcifications by binding calcium crystals and blocking bone morphogenetic protein-2 (BMP-2), a pro-mineralizing factor (136). Additionally, MGP preserves the extracellular matrix's composition and inhibits the osteoblastic development of vascular smooth muscle cells (134).
Changes in vitamin K2 metabolism are linked to quantitative changes in the constitute of the gut microbiota, such as in small intestinal bacterial overgrowth (SIBO). Specifically, regardless of daily vitamin K2 intake, individuals with SIBO had considerably greater serum levels of dephosphorylated-uncarboxylated matrix Gla-protein, the inactive form of MGP, than controls. Higher levels of inactive MGP in patients were associated with an increase in arterial stiffness as determined by pulse-wave velocity, which was one of the early indicators of vascular dysfunction (137). One of the most reliable indicators of poor vitamin K2 status is generally thought to be elevated levels of the inactive form of MGP (138). Furthermore, it has been demonstrated that increases in MGP inactive form are linked to early vascular disease symptoms as well as cardiovascular morbidity and mortality (139–143).
3 New strategies in CVD prevention and treatment
Researchers have focused on gut microbiota and associated metabolites to prevent and treat CVD. Therefore, as a new regulator of CVD, the gut microbiota has emerged as a possible therapeutic target.
3.1 Diet
The composition and function of the gut microbiota can be altered by diet in several ways (144). These changes take time to manifest, and they have more significant impact if they are sustained over time (145). Short-term dietary changes can rapidly and temporarily change the diversity of human microbiota. Long-term dietary patterns influence the development of each person's stable microbiota profile (145, 146). The idea that a regulated diet may result in positive changes in the composition of the gut microbiota in the prevention of CVD is supported by an observational study by Wang et al. (147).
High-fiber diets inhibit the growth of known opportunistic pathogens and promote the growth of helpful commensal bacteria (148, 149). A high-fiber diet has been shown to reduce blood pressure, lessen heart hypertrophy and fibrosis, and boost the microbiota that produces acetate (150). According to Xiao et al., dietary interventions using whole grains and foods from traditional Chinese medicine can enhance intestine beneficial bacteria like Bifidobacterium and decrease Enterobacteriaceae harmful bacteria (151). Furthermore, a diet rich in fiber can boost the microbiota that produces acetic acid, which reduces blood pressure (150). The quantity of microbiomes that are members of the Bacteroidetes phylum with genera Prevotella and Bacteroides decreased in CAD patients, and it has been shown that those who eat a high-fiber diet create SCFAs (152). More SCFAs and phosphatidylcholine are produced when the gut bacterial community is modulated by a diet high in plant products, enabling the growth of species that can ferment fibers. A high-fat diet, on the other hand, causes adverse alterations in the fecal metabolomic composition, systemic inflammation, and gut flora (153). Fecal metabolomic patterns are a reflection of changes in the gut bacterial ecology. In fact, a high-fat meal causes circulating pro-inflammatory factors (such as plasminogen activator inhibitor-1, IL-1, and TNF-α mRNA) to rise while SCAs decrease and arachidonic acid and LPS production increases (154). Another study demonstrated the anti-inflammatory effects of a Mediterranean diet, showing a negative relationship between the production of SCFAs and the expression of inflammatory cytokines, including VEGF, MCP-1, IFN-γ-induced protein 10 (IP-10), IL-17, and IL-12. Additionally, Enterorhabdus, Lachnoclostridium, and Parabacteroides were more prevalent in the Mediterranean diet (155). Mediterranean diets high in fruits and vegetables decreased the incidence of heart failure by 70% in randomized controlled studies (156). Lower TMAO levels in both males and females were also associated with consuming a Mediterranean diet (157). Additionally, 200 g vs. 500 g of unprocessed lean red meat per week differed between two diet groups in a 5-week randomized experiment. The TMAO levels were lower in the group that consumed 200 g of red meat per week than in the group that consumed 500 g (158). When Organ et al. performed transverse aortic constriction surgery on C57BL6/J mice to cause HF, they discovered that the animals receiving TMAO or choline-supplemented diets showed worse HF symptoms and biomarkers than the mice receiving a control diet (159). According to another study, a lower ratio of Firmicutes, including Bacteroidetes and Streptococcus, and a higher ratio of Catenibacterium, Bifidobacterium, and fecal SCFAs were linked to a closer adherence to the Mediterranean dietary pattern and increased consumption of plant-based nutrients, such as vegetable proteins and polysaccharides (160). In contrast to a diet heavy in saturated fat, which can raise LDL cholesterol, a westernized diet high in unsaturated fat can boost Bacteroidetes and decrease Firmicutes and Bilophila wadsworthia (sulfite-reducing microorganisms) (161).
Sodium chloride, or NaCl, is another name for salt, an essential component of human nutrition. The words “salt” and “sodium” are commonly used in ways that imply they have the same meaning (162). A daily consumption of less than 5 g of salt is advised by the World Health Organization's (WHO) Healthy Diet Fact Sheet (163). Several recent studies have examined the effects of a high salt intake on gut microbiology and disease (164), including inflammatory bowel disease (162) and cardiovascular disease (165), given the well-established relationship between diet and gut microbiology and its implications for the development of disease. Recent research has shown that consuming too much salt, especially sodium, might raise blood pressure by altering the gut flora (164, 166). One example of this is the advice to limit salt consumption because some people and model organisms are sensitive to a diet high in salt (38, 167). A high-salt diet mechanistically increases intestinal permeability and pro-inflammatory cytokines in human and animal research, promoting local and systemic tissue inflammation (164, 168, 169). Certain bacteria, such as Bacteroides fragilis, may be responsible for some of the effects of dietary salt. They activate the mineralocorticoid receptor and raise blood pressure through intermediate metabolic actions (170). Ferguson JF and colleagues found that a high-salt diet decreased the number of lactate-producing bacteria, such as those belonging to the Bacilli class, the Lactobacillales order, the Leuconostocaceae family, and the Leuconostoc genus, in both people and mice. Usually, these bacteria prevent hypertension and T-cell activation by salt (164, 171). According to a different study by Wilck et al., eating a lot of salt impacts the gut microbiota, mainly by decreasing Lactobacillus murinus and raising Th17 cells and blood pressure. L. murinus supplementation reduced hypertension and inhibited Th17 activation (164).
These studies demonstrate how a diet high in salt can negatively impact gut microbiota, which can then affect gut health and contribute to the development and progression of cardiovascular disease. Given the intricate relationships between food, gut microbiota, and the metabolites that follow, diet is a significant risk factor for CVD and cardiovascular health. To maintain physical health, patients with CVD must optimise their food composition and make suitable dietary adjustments.
3.2 Probiotics and prebiotics
A growing body of research is investigating how probiotics can lower cardiovascular risk, particularly in light of recent findings linking the gut microbiota to the etiology of CVD. According to the Greek definition, probiotics are “live microorganisms which, when administered in adequate amounts, confer health benefits on the host” (Pro: promotion, Biotic: life) (172). Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus, and Enterococcus are common probiotics (173). Across many cultures, people eat a wide variety of fermented foods that contain probiotic strains, including yoghurt, kefir, sauerkraut, tempeh, and kimchi (174). Probiotics may work in a variety of ways, including adjusting pH, producing antibiotic compounds, and competing with pathogens (175). Prebiotics are defined as “non-digestible food components allowing the specificity of microbial changes in the intestinal tract, thereby exhibiting beneficial effects on host's health,” as was initially set there by Gibson and Roberfroid in 1995 and then revised in 2004 (176, 177). The International Scientific Association for Probiotics and Prebiotics has since maintained the most widely recognized definition of this term as a substrate that host bacteria preferentially use to provide health benefits (178). Studies conducted both in vitro and in vivo have shown that oligosaccharides, particularly fructans [fructooligosaccharides (FOS) and inulin] and galactans [galactooligosaccharides (GOS)], are the most well-known prebiotics (179). The majority of prebiotics are carbohydrates found in foods such as cereals, fruits, and vegetables (173). Probiotics and prebiotics' ability to alter gut microbiota has emerged as a new avenue for CVD prevention and treatment. Numerous studies have demonstrated the positive effects of prebiotics and probiotics on lipid regulation, which has indirect advantages for CVD (180–183). Furthermore, there is strong evidence that functional foods that contain prebiotics and probiotics may help prevent several cardio-metabolic conditions, such as type I diabetes, obesity, and hypertension (184–186). Another explanation for the protective benefits of probiotic and prebiotic treatments on CVD is host immune system regulation. Changes in epithelial cells, dendritic cells, effector lymphocytes, natural killer T cells, T regulatory cells, and B cells are among the immunological processes that probiotics and prebiotics support (187).
Some studies examined the positive benefits of inulin or inulin-containing prebiotics on a range of CVDs in both human and animal models, including coronary artery disease, chronic renal disease, atherosclerosis, hypercholesterolemia, and CHD or diabetes linked to CHD (188–192). Furthermore, it was discovered that the probiotics Lactobacillus fermentum and Bifidobacterium breve can lower blood pressure by preventing endothelial dysfunction and re-establishing the balance of the gut microbiota (193). In patients with CKD, the treatment of Lactobacillus sp. was linked to a considerable decrease in small intestinal toxins such as dimethylamine and nitrosodimethylamine, and in patients with carotid atherosclerosis, it was related to alterations in colon levels of certain SCFAs (194, 195). A 6-week daily supplementation with Lactobacillus plantarum 299v (Lp299v) has a positive effect on CVD by causing changes in circulating metabolites originating from the gut microbiome, according to a pilot trial including 21 men with stable coronary artery disease. Lp299v supplementation can decrease systemic inflammation and enhance endothelium-dependent brachial artery vasodilation by increasing nitric oxide bioavailability (196). Additionally, Lam et al. discovered that Lactobacillus plantarum could decrease the size of myocardial infarctions and enhance ventricular function (197). One gut microbiota component that has positive impacts on the pathophysiology of cardiovascular disease and arterial hypertension is Akkermansia muciniphila (198). Patients with heart failure can benefit from Saccharomyces boulardii's ability to lower serum creatinine and inflammatory marker levels (199). Although probiotics and prebiotics can be added to the diet of patients with CVD based on their circumstances to improve gut dysbiosis and regulate the gut microbiota, there are still unanswered questions regarding the precise immunological and physiological effects they may have on health and illness, necessitating more research.
3.3 Fecal microbiota transplantation
Transplanting a fecal solution from a healthy donor into the recipient's digestive tract is known as fecal microbiota transplantation (FMT), and it has been used to treat ulcerative colitis and Clostridium difficile infections (200, 201). Stools from healthy donors or recipients (self-FMT) are collected as part of this procedure before being administered to patients with illnesses or associated dysbioses. These complicated investigations about CVD endpoints have not yet been thoroughly examined. Although the clinical usefulness of this strategy for CVD is debatable, FMT has been shown to have a therapeutic effect against small intestine permeability and insulin resistance (202–204). The use of FMT via fecal enema as an adjuvant in treating pseudomembranous colitis was initially reported by Eisman et al. (205). FMT is much more effective than standard treatment procedures, including vancomycin antibiotics, and it has been instrumental in treating recurring Clostridium difficile infections (206, 207). Additionally, FMT is promising as a treatment for additional conditions linked to the microbiota, such as Crohn's disease, ulcerative colitis, obesity, type 2 diabetes, and cardiovascular disease.
FMT has also recently been investigated as a potential treatment for cardiometabolic diseases (208, 209). Fecal donation improved myocarditis in animal models (210). To maintain a balanced gut flora, it is thought to support beneficial microorganisms, compete with pathogens, and restore a healthy gut microbiota. Restoring the gut's health could aid in treating CVD because dysbiosis and gut microbiome are linked to the disease. In fact, in experimental mouse models of autoimmune myocarditis, FMT was shown to alleviate myocarditis (210). Increasing the Firmicutes/Bacteroidetes ratio and decreasing inflammatory infiltration are two proposed ways. Through the anti-inflammatory mechanism, the study validates the impact of gut flora on CVD and the significant function of Bacteroidetes in microbiota composition (211). More preclinical research expands our understanding of FMT and its potential by transferring the feces of healthy people and hypertension patients into germ-free mice. As expected, mice that received patient microbiomes had more tremendous blood pressure than mice that received healthy microbiomes (212). Furthermore, the impact of FMT on atherosclerosis was also investigated (213).
In a randomized, double-blind, controlled study with 20 patients with metabolic syndrome, it was discovered that vegetarians' post-single transplant fecal flora might alter the intestinal flora composition of specific individuals but not the vasculitis-related measures (214). Recently, Fan et al. reported their clinical trial design of FMT effects on primary hypertension patients. The expected outcomes include microbiota profile, blood pressure, blood glucose and lipids, ankle-branchial index, and the causes of events. This investigation may be among the earliest clinical trials to examine the effects of FMT on hypertension, offering crucial data for additional research. Patients with hypertension were found to benefit from a modified form of FMT known as the “washed microbiota transplantation effect.” (215).
However, due to the hazards involved, such as the potential for endotoxins or infectious organisms to spread and produce new gastrointestinal issues, the use of FMT is currently restricted (216, 217).
Without preclinical trials of FMT on CVD, including CAD, PAD, and heart failure, clinical trials may not have sufficient data and support to be carried out. Therefore, research on the FMT approach to CVD therapy and prevention is still needed.
3.4 Small-molecule antimicrobial enzyme therapeutics
As discussed earlier, gut microbiota produces a metabolite called TMA through the breakdown of dietary phosphatidylcholine/choline, L-carnitine, or betaine in consumed red meat or animal flesh (41, 42, 63, 218). Certain choline TMA lyase inhibitors can help lower plasma TMAO levels and improve atherosclerosis and thrombosis. It has been demonstrated that the natural substance 3,3-dimethyl-1-butanol (DMB), which is present in some red wines, balsamic vinegar, cold-pressed extra virgin olive oils, and grape seed oils, lowers plasma TMAO in mice given a chow diet supplemented with carnitine or choline (218). Furthermore, DMB reduces the in vivo rate of thrombus formation and platelet reactivity, which a choline diet improves (219).
By creating a small-molecule tool medication to limit microbial choline TMA lyase activity, this technique recently demonstrated proof of concept (218). Although there is currently little information available, new choline TMA-lyase inhibitors have recently been created, such as iodomethylcholine (IMC) and fluoromethylcholine (FMC) (220). Without prolonging the bleeding period, the IMC and FMC molecules can lower the host's TMA and TMAO levels, which will limit platelet aggregation and the in vivo rate of thrombus formation (219). FMO3, another regulator in the production pathway for TMAO, quickly transforms TMA into TMAO. Berberine, 3, 3C-diindolylmethane (DIM), and indole-3-carbinol are examples of phytochemicals that have demonstrated promise in lowering the generation of TMAO and suppressing FMO3 activity (221, 222). Research on the antisense oligonucleotide-based regulation of FMO3 in animal models revealed a decrease in TMAO serum levels concurrent with a reduction in diet-enhanced atherosclerosis (223). Thus, the possibility of developing microbial enzyme inhibitors to treat people's cardiometabolic abnormalities is quite exciting.
4 Conclusion
Despite mounting evidence linking gut microbiota to cardiovascular diseases (CVDs), significant information gaps still exist. The absence of clear evidence connecting particular changes in the microbiome to cardiovascular disease is one of the main drawbacks of current research. Since the majority of research uses observational and correlational data, it is challenging to determine whether gut dysbiosis is a direct cause of CVD development or merely an effect of the illness state. These linkages may become clearer in the future with the use of advanced microbiome sequencing, metabolomics, and causal inference techniques like Mendelian randomization.
Inter-individual variation in gut microbiota composition, which is impacted by environmental exposures, genetics, nutrition, and drugs, is a further major challenge. The development of standardized microbiome-based treatments for CVD is complicated by this diversity. Our review highlights the potential for patient-specific microbial profile-based specific gut microbiome therapies, an area that needs more investigation. Furthermore, although a number of metabolites originating from the gut (such as bile acids, SCFAs, and TMAO) have been linked to cardiovascular function, the exact molecular mechanisms behind the gut-heart axis are still not fully known. Finding new microbial metabolites and understanding how they specifically affect inflammation, atherosclerosis, and endothelial function may lead to the discovery of new treatment targets. Finally, there is still difficulty in implementing microbiome-targeted treatments in clinical settings. Although fecal microbiota transplantation (FMT), probiotics, prebiotics, and microbial enzyme inhibitors have demonstrated promise, their long-term safety and effectiveness in preventing and treating CVD are yet unknown. This review addresses new approaches that may help close this gap, such as targeted enzyme inhibitors and precision medicine based on the microbiome. Appropriate treatment approaches that target the gut microbiota promise improved CVD management and prevention in the future.
Statements
Author contributions
YZ: Data curation, Investigation, Methodology, Writing – original draft. HW: Investigation, Methodology, Writing – original draft. MJ: Data curation, Investigation, Methodology, Writing – original draft. GF: Data curation, Investigation, Methodology, Resources, Writing – original draft. SW: Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82160157 and No. 81970290), the Joint Funds of the National Natural Science Foundation of China (No. U20A2018), and the Natural Science Foundation of Beijing (No. 7242046 and No. 7222044).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Novakovic M Rout A Kingsley T Kirchoff R Singh A Verma V et al Role of gut microbiota in cardiovascular diseases. World J Cardiol. (2020) 12(4):110. 10.4330/wjc.v12.i4.110
2.
Tang WW Bäckhed F Landmesser U Hazen SL . Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73(16):2089–105. 10.1016/j.jacc.2019.03.024
3.
Gomaa EZ . Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. (2020) 113(12):2019–40. 10.1007/s10482-020-01474-7
4.
Sirisinha S . The potential impact of gut microbiota on your health: current status and future challenges. Asian Pac J Allergy Immunol. (2016) 34(4):249–64. 10.12932/AP0803
5.
Kootte R Vrieze A Holleman F Dallinga-Thie GM Zoetendal EG de Vos WM et al The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. (2012) 14(2):112–20. 10.1111/j.1463-1326.2011.01483.x
6.
Salehi M Kamali MJ Rajabzadeh A Minoo S Mosharafi H Saeedi F et al tRNA-derived fragments: key determinants of cancer metastasis with emerging therapeutic and diagnostic potentials. Arch Biochem Biophys. (2024) 753:109930. 10.1016/j.abb.2024.109930
7.
Nesci A Carnuccio C Ruggieri V D’Alessandro A Di Giorgio A Santoro L et al Gut microbiota and cardiovascular disease: evidence on the metabolic and inflammatory background of a complex relationship. Int J Mol Sci. (2023) 24(10):9087. 10.3390/ijms24109087
8.
Murray CJ Barber RM Foreman KJ Ozgoren AA Abd-Allah F Abera SF et al Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. (2015) 386(10009):2145–91. 10.1016/S0140-6736(15)61340-X
9.
Benjamin EJ Virani SS Callaway CW Chamberlain AM Chang AR Cheng S et al Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. (2018) 137(12):e67–e492. 10.1161/CIR.0000000000000558
10.
Lindskog Jonsson A Caesar R Akrami R Reinhardt C Fåk Hållenius F Borén J et al Impact of gut microbiota and diet on the development of atherosclerosis in Apoe−/− mice. Arterioscler, Thromb, Vasc Biol. (2018) 38(10):2318–26. 10.1161/ATVBAHA.118.311233
11.
Jäckel S Kiouptsi K Lillich M Hendrikx T Khandagale A Kollar B et al Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via toll-like receptor-2. Blood. (2017) 130(4):542–53. 10.1182/blood-2016-11-754416
12.
Kiouptsi K Reinhardt C . Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis. Br J Pharmacol. (2018) 175(24):4439–49. 10.1111/bph.14483
13.
Biscetti F Nardella E Cecchini AL Landolfi R Flex A . The role of the microbiota in the diabetic peripheral artery disease. Mediat Inflamm. (2019) 2019(1):4128682. 10.1155/2019/4128682
14.
Witkowski M Weeks TL Hazen SL . Gut microbiota and cardiovascular disease. Circ Res. (2020) 127(4):553–70. 10.1161/CIRCRESAHA.120.316242
15.
Shojaeianforoud F Lahooti M . Cerebrospinal fluid flow dynamics in the aqueduct of Sylvius for rigid and deformable brain models. Comput Biol Med. (2025) 190:110047. 10.1016/j.compbiomed.2025.110047
16.
Louis P Flint HJ . Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. (2017) 19(1):29–41. 10.1111/1462-2920.13589
17.
Pluznick JL Zou D-J Zhang X Yan Q Rodriguez-Gil DJ Eisner C et al Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA. (2009) 106(6):2059–64. 10.1073/pnas.0812859106
18.
Gilissen J Jouret F Pirotte B Hanson J . Insight into SUCNR1 (GPR91) structure and function. Pharmacol Ther. (2016) 159:56–65. 10.1016/j.pharmthera.2016.01.008
19.
David LA Maurice CF Carmody RN Gootenberg DB Button JE Wolfe BE et al Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505(7484):559–63. 10.1038/nature12820
20.
Zhao P Zhao S Tian J Liu X . Significance of gut microbiota and short-chain fatty acids in heart failure. Nutrients. (2022) 14(18):3758. 10.3390/nu14183758
21.
Saeedi F Salehi M Kamali MJ Mir MA Kazemi S Shirafkan F et al Evaluation of the cytotoxic activities of the essential oil from Pistacia Atlantica Desf. Oleoresin on human gastric cancer cell lines. Med Oncol. (2024) 41(6):148. 10.1007/s12032-024-02339-z
22.
Zhang Z Zhang H Chen T Shi L Wang D Tang D . Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun Signal. (2022) 20(1):64. 10.1186/s12964-022-00869-5
23.
Shahed Shoarishoar S KaboodMehri R Fakor F Sorouri ZR Mansour-Ghanaei M Darkhaneh RF et al Assessment of decreased ovarian reserve and systemic inflammatory markers. Cell Mol Biol. (2024) 70(11):144–9. 10.14715/cmb/2024.70.11.21
24.
Tremaroli V Bäckhed F . Functional interactions between the gut microbiota and host metabolism. Nature. (2012) 489(7415):242–9. 10.1038/nature11552
25.
Hutchins AP Diez D Miranda-Saavedra D . The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. (2013) 12(6):489–98. 10.1093/bfgp/elt028
26.
Chang PV Hao L Offermanns S Medzhitov R . The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. (2014) 111(6):2247–52. 10.1073/pnas.1322269111
27.
Shakespear MR Halili MA Irvine KM Fairlie DP Sweet MJ . Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. (2011) 32(7):335–43. 10.1016/j.it.2011.04.001
28.
Säemann MD Böhmig GA Österreicher CH Burtscher H Parolini O Diakos C et al Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. (2000) 14(15):2380–2. 10.1096/fj.00-0359fje
29.
Sun M Wu W Liu Z Cong Y . Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. (2017) 52:1–8. 10.1007/s00535-016-1242-9
30.
Peti-Peterdi J Kishore BK Pluznick JL . Regulation of vascular and renal function by metabolite receptors. Annu Rev Physiol. (2016) 78(1):391–414. 10.1146/annurev-physiol-021115-105403
31.
Kamali MJ Salehi M Fath MK . Advancing personalized immunotherapy for melanoma: integrating immunoinformatics in multi-epitope vaccine development, neoantigen identification via NGS, and immune simulation evaluation. Comput Biol Med. (2025) 188:109885. 10.1016/j.compbiomed.2025.109885
32.
Xu J Moore BN Pluznick JL . Short-chain fatty acid receptors and blood pressure regulation: council on hypertension mid-career award for research excellence 2021. Hypertension. (2022) 79(10):2127–37. 10.1161/HYPERTENSIONAHA.122.18558
33.
Roshanravan N Mahdavi R Alizadeh E Ghavami A Saadat YR Alamdari NM et al The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J Cardiovasc Thorac Res. (2017) 9(4):183. 10.15171/jcvtr.2017.32
34.
Bartolomaeus H Balogh A Yakoub M Homann S Markó L Höges S et al Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. (2019) 139(11):1407–21. 10.1161/CIRCULATIONAHA.118.036652
35.
De Preter V Coopmans T Rutgeerts P Verbeke K . Influence of long-term administration of lactulose and Saccharomyces boulardii on the colonic generation of phenolic compounds in healthy human subjects. J Am Coll Nutr. (2006) 25(6):541–9. 10.1080/07315724.2006.10719570
36.
Karlsson FH Fåk F Nookaew I Tremaroli V Fagerberg B Petranovic D et al Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. (2012) 3(1):1245. 10.1038/ncomms2266
37.
Turnbaugh PJ Ley RE Mahowald MA Magrini V Mardis ER Gordon JI . An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444(7122):1027–31. 10.1038/nature05414
38.
Mell B Jala VR Mathew AV Byun J Waghulde H Zhang Y et al Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics. (2015) 47(6):187–97. 10.1152/physiolgenomics.00136.2014
39.
Pluznick J . A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. (2014) 5(2):202–7. 10.4161/gmic.27492
40.
Matysik S Krautbauer S Liebisch G Schött HF Kjølbæk L Astrup A et al Short-chain fatty acids and bile acids in human faeces are associated with the intestinal cholesterol conversion status. Br J Pharmacol. (2021) 178(16):3342–53. 10.1111/bph.15440
41.
Koeth RA Wang Z Levison BS Buffa JA Org E Sheehy BT et al Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19(5):576–85. 10.1038/nm.3145
42.
Wang Z Klipfell E Bennett BJ Koeth R Levison BS DuGar B et al Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. (2011) 472(7341):57–63. 10.1038/nature09922
43.
Zeisel SH Warrier M . Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. (2017) 37(1):157–81. 10.1146/annurev-nutr-071816-064732
44.
Tang WW Hazen SL . The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. (2014) 124(10):4204–11. 10.1172/JCI72331
45.
Al-Obaide MA Singh R Datta P Rewers-Felkins KA Salguero MV Al-Obaidi I et al Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. J Clin Med. (2017) 6(9):86. 10.3390/jcm6090086
46.
Falony G Vieira-Silva S Raes J . Microbiology meets big data: the case of gut microbiota–derived trimethylamine. Annu Rev Microbiol. (2015) 69(1):305–21. 10.1146/annurev-micro-091014-104422
47.
Xie Z Liu X Huang X Liu Q Yang M Huang D et al Remodelling of gut microbiota by Berberine attenuates trimethylamine N-oxide-induced platelet hyperreaction and thrombus formation. Eur J Pharmacol. (2021) 911:174526. 10.1016/j.ejphar.2021.174526
48.
Huang Y Zhang H Fan X Wang J Yin Y Zhang Y et al The role of gut microbiota and trimethylamine N-oxide in cardiovascular diseases. J Cardiovasc Transl Res. (2023) 16(3):581–9. 10.1007/s12265-022-10330-0
49.
Thomas MS Fernandez ML . Trimethylamine N-oxide (TMAO), diet and cardiovascular disease. Curr Atheroscler Rep. (2021) 23:1–7. 10.1007/s11883-021-00910-x
50.
Wang Z Xu K Zhou H . Characteristics of gut virome and microbiome in patients with stroke. Nan Fang Yi Ke Da Xue Xue Bao. (2021) 41(6):862–9. 10.12122/j.issn.1673-4254.2021.06.08
51.
Yoo W Zieba JK Foegeding NJ Torres TP Shelton CD Shealy NG et al High-fat diet–induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science. (2021) 373(6556):813–8. 10.1126/science.aba3683
52.
Coutinho-Wolino KS de F Cardozo LF de Oliveira Leal V Mafra D Stockler-Pinto MB . Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far?Eur J Nutr. (2021) 9:1–18. 10.1007/s00394-021-02491-6
53.
Heianza Y Ma W Manson JE Rexrode KM Qi L . Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. (2017) 6(7):e004947. 10.1161/JAHA.116.004947
54.
Schiattarella GG Sannino A Toscano E Giugliano G Gargiulo G Franzone A et al Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. (2017) 38(39):2948–56. 10.1093/eurheartj/ehx342
55.
Lee-Rueckert M Escola-Gil JC Kovanen PT . HDL Functionality in reverse cholesterol transport—challenges in translating data emerging from mouse models to human disease. Biochim Biophys Acta. (2016) 1861(7):566–83. 10.1016/j.bbalip.2016.03.004
56.
Zhen J Zhou Z He M Han H-X Lv E-H Wen P-B et al The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol (Lausanne). (2023) 14:1085041. 10.3389/fendo.2023.1085041
57.
Roncal C Martínez-Aguilar E Orbe J Ravassa S Fernandez-Montero A Saenz-Pipaon G et al Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci Rep. (2019) 9(1):15580. 10.1038/s41598-019-52082-z
58.
Zhu W Gregory JC Org E Buffa JA Gupta N Wang Z et al Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. (2016) 165(1):111–24. 10.1016/j.cell.2016.02.011
59.
Zhu W Wang Z Tang WW Hazen SL . Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation. (2017) 135(17):1671–3. 10.1161/CIRCULATIONAHA.116.025338
60.
Liu X Xie Z Sun M Wang X Li J Cui J et al Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int J Cardiol. (2018) 265:18–23. 10.1016/j.ijcard.2018.04.126
61.
Yang S Li X Yang F Zhao R Pan X Liang J et al Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. (2019) 10:1360. 10.3389/fphar.2019.01360
62.
Moore KJ Freeman MW . Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler, Thromb, Vasc Biol. (2006) 26(8):1702–11. 10.1161/01.ATV.0000229218.97976.43
63.
Tang WW Wang Z Levison BS Koeth RA Britt EB Fu X et al Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368(17):1575–84. 10.1056/NEJMoa1109400
64.
Tang WW Wang Z Li XS Fan Y Li DS Wu Y et al Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. (2017) 63(1):297–306. 10.1373/clinchem.2016.263640
65.
Luk S Doma H Reimark R Stephen JS . Plasma levels of TMAO can be increased with ‘healthy’and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability.
66.
Schoenheimer R . New contributions in sterol metabolism. Science. (1931) 74(1928):579–84. 10.1126/science.74.1928.579
67.
Midtvedt A-C Midtvedt T . Conversion of cholesterol to coprostanol by the intestinal microflora during the first two years of human life. J Pediatr Gastroenterol Nutr. (1993) 17(2):161–8. 10.1097/00005176-199308000-00006
68.
Benno P Midtvedt K Alam M Collinder E Norin E Midtvedt T . Examination of intestinal conversion of cholesterol to coprostanol in 633 healthy subjects reveals an age-and sex-dependent pattern. Microb Ecol Health Dis. (2005) 17(4):200–4. 10.1080/08910600500519854
69.
Salehi M Kamali MJ Ashuori Z Ghadimi F Shafiee M Babaei S et al Functional significance of tRNA-derived fragments in sustained proliferation of tumor cells. Gene Rep. (2024) 35:101901. 10.1016/j.genrep.2024.101901
70.
Kazemian N Mahmoudi M Halperin F Wu JC Pakpour S . Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. (2020) 8:1–17. 10.1186/s40168-020-00821-0
71.
Kriaa A Bourgin M Mkaouar H Jablaoui A Akermi N Soussou S et al Microbial reduction of cholesterol to coprostanol: an old concept and new insights. Catalysts. (2019) 9(2):167. 10.3390/catal9020167
72.
Shojaeianforoud F Marin L Anderl WJ Marino M Coats B Monson KL . Repeated loading and damage progression in cerebral arteries. Acta Biomater. (2025) 73:5742–9. 10.1016/j.actbio.2025.03.027
73.
Brinkley AW Gottesman AR Mott GE . Growth of cholesterol-reducing Eubacterium on cholesterol-brain agar. Appl Environ Microbiol. (1980) 40(6):1130–2. 10.1128/aem.40.6.1130-1132.1980
74.
Snog-Kjaer A Prange I Dam H . Conversion of cholesterol into coprosterol by bacteria in vitro. Microbiology. (1956) 14(2):256–60. 10.1099/00221287-14-2-256
75.
Kenny DJ Plichta DR Shungin D Koppel N Hall AB Fu B et al Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe. (2020) 28(2):245–57.e6. 10.1016/j.chom.2020.05.013
76.
Li L Buhman K Hartman P Beitz D . Hypocholesterolemic effect of Eubacterium coprostanoligenes ATCC 51222 in rabbits. Lett Appl Microbiol. (1995) 20(3):137–40. 10.1111/j.1472-765X.1995.tb00410.x
77.
Rosenfeld R Fukushima DK Hellman L Gallagher T . The transformation of cholesterol to coprostanol. J Biol Chem. (1954) 211(1):301–11. 10.1016/S0021-9258(18)71221-0
78.
Midtvedt T Lingaas E Carlstedt-Duke B Höverstad T Midtvedt A Saxerholt H et al Intestinal microbial conversion of cholesterol to coprostanol in man: influence of antibiotics. APMIS. (1990) 98(7–12):839–44. 10.1111/j.1699-0463.1990.tb05004.x
79.
Gérard P Lepercq P Leclerc M Gavini F Raibaud P Juste C . Bacteroides sp. strain D8, the first cholesterol-reducing bacterium isolated from human feces. Appl Environ Microbiol. (2007) 73(18):5742–9. 10.1128/AEM.02806-06
80.
Antharam VC McEwen DC Garrett TJ Dossey AT Li EC Kozlov AN et al An integrated metabolomic and microbiome analysis identified specific gut microbiota associated with fecal cholesterol and coprostanol in Clostridium difficile infection. PLoS One. (2016) 11(2):e0148824. 10.1371/journal.pone.0148824
81.
Lye H-S Rusul G Liong M-T . Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J Dairy Sci. (2010) 93(4):1383–92. 10.3168/jds.2009-2574
82.
Tahri K Grill JP Schneider F . Involvement of trihydroxyconjugated bile salts in cholesterol assimilation by bifidobacteria. Curr Microbiol. (1997) 34:79–84. 10.1007/s002849900148
83.
Kriaa A Bourgin M Potiron A Mkaouar H Jablaoui A Gérard P et al Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. (2019) 60(2):323–32. 10.1194/jlr.R088989
84.
Shoarishoar SS Milani F Adineh S Sorouri ZR Attari SM . Comparison of pregnancy outcomes in amniocentesis recipients with normal and abnormal maternal serum analytes. Cell Mol Biol. (2024) 70(11):109–14. 10.14715/cmb/2024.70.11.16
85.
Hofmann AF . The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. (1999) 159(22):2647–58. 10.1001/archinte.159.22.2647
86.
Zhang S Zhou J Wu W Zhu Y Liu X . The role of bile acids in cardiovascular diseases: from mechanisms to clinical implications. Aging Dis. (2023) 14(2):261. 10.14336/AD.2022.0817
87.
Lefebvre P Cariou B Lien F Kuipers F Staels B . Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. (2009) 89(1):147–91. 10.1152/physrev.00010.2008
88.
Li C Li Y Gai Z . Bile acids and farnesoid X receptor: novel target for the treatment of diabetic cardiomyopathy. Curr Protein Pept Sci. (2019) 20(10):976–83. 10.2174/1389203720666190726152847
89.
Wahlström A Sayin SI Marschall H-U Bäckhed F . Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24(1):41–50. 10.1016/j.cmet.2016.05.005
90.
Chiang JY . Bile acids: regulation of synthesis: thematic review series: bile acids. J Lipid Res. (2009) 50(10):1955–66. 10.1194/jlr.R900010-JLR200
91.
Ferrell JM Boehme S Li F Chiang JY . Cholesterol 7α-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders [S]. J Lipid Res. (2016) 57(7):1144–54. 10.1194/jlr.M064709
92.
Li T Francl JM Boehme S Ochoa A Zhang Y Klaassen CD et al Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem. (2012) 287(3):1861–73. 10.1074/jbc.M111.305789
93.
Broeders EP Nascimento EB Havekes B Brans B Roumans KH Tailleux A et al The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. (2015) 22(3):418–26. 10.1016/j.cmet.2015.07.002
94.
Kumar PS Mason MR Brooker MR O'Brien K . Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol. (2012) 39(5):425–33. 10.1111/j.1600-051X.2012.01856.x
95.
Khaje N Salehan F Shakiba D Shabestari AM Shoarishoar SS . In vitro assessment of paclitaxel-loaded niosome nanoparticles and their cytotoxic effects on the ovarian cancer cell line A2780CP. Asian Pac J Cancer Biol. (2024) 9(4):479–86. 10.31557/apjcb.2024.9.4.479-486
96.
de Aguiar Vallim TQ Tarling EJ Edwards PA . Pleiotropic roles of bile acids in metabolism. Cell Metab. (2013) 17(5):657–69. 10.1016/j.cmet.2013.03.013
97.
Busnelli M Manzini S Chiesa G . The gut microbiota affects host pathophysiology as an endocrine organ: a focus on cardiovascular disease. Nutrients. (2019) 12(1):79. 10.3390/nu12010079
98.
Chen ML Takeda K Sundrud MS . Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. (2019) 12(4):851–61. 10.1038/s41385-019-0162-4
99.
Lu Q Chen J Jiang L Geng T Tian S Liao Y et al Gut microbiota-derived secondary bile acids, bile acids receptor polymorphisms, and risk of cardiovascular disease in individuals with newly diagnosed type 2 diabetes: a cohort study. Am J Clin Nutr. (2024) 119(2):324–32. 10.1016/j.ajcnut.2023.08.023
100.
Jones BV Begley M Hill C Gahan CG Marchesi JR . Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. (2008) 105(36):13580–5. 10.1073/pnas.0804437105
101.
Li W Shu S Cheng L Hao X Wang L Wu Y et al Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. (2020) 292:193–200. 10.1016/j.atherosclerosis.2019.11.026
102.
Jovanovich A Isakova T Block G Stubbs J Smits G Chonchol M et al Deoxycholic acid, a metabolite of circulating bile acids, and coronary artery vascular calcification in CKD. Am J Kidney Dis. (2018) 71(1):27–34. 10.1053/j.ajkd.2017.06.017
103.
Li Y Zhang D He Y Chen C Song C Zhao Y et al Investigation of novel metabolites potentially involved in the pathogenesis of coronary heart disease using a UHPLC-QTOF/MS-based metabolomics approach. Sci Rep. (2017) 7(1):15357. 10.1038/s41598-017-15737-3
104.
Ottosson F Brunkwall L Smith E Orho-Melander M Nilsson PM Fernandez C et al The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J Hypertens. (2020) 38(12):2427–34. 10.1097/HJH.0000000000002569
105.
Vaziri ND Zhao Y-Y Pahl MV . Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. (2016) 31(5):737–46. 10.1093/ndt/gfv095
106.
Michael AF Drummond KN Doeden D Anderson JA Good RA . Tryptophan metabolism in man. J Clin Invest. (1964) 43(9):1730–46. 10.1172/JCI105048
107.
Rose D . Aspects of tryptophan metabolism in health and disease: a review. J Clin Pathol. (1972) 25(1):17. 10.1136/jcp.25.1.17
108.
Niwa T . Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci. (2010) 72(1–2):1–11.
109.
Hung S-C Kuo K-L Huang H-L Lin C-C Tsai T-H Wang C-H et al Indoxyl sulfate suppresses endothelial progenitor cell–mediated neovascularization. Kidney Int. (2016) 89(3):574–85. 10.1016/j.kint.2015.11.020
110.
Niwa T Takeda N Maeda K Shibata M Tatematsu A . Accumulation of furancarboxylic acids in uremic serum as inhibitors of drug binding. Clin Chim Acta. (1988) 173(2):127–38. 10.1016/0009-8981(88)90250-1
111.
Dou L Jourde-Chiche N Faure V Cerini C Berland Y Dignat-George F et al The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemostasis. (2007) 5(6):1302–8. 10.1111/j.1538-7836.2007.02540.x
112.
Dou L Bertrand E Cerini C Faure V Sampol J Vanholder R et al The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. (2004) 65(2):442–51. 10.1111/j.1523-1755.2004.00399.x
113.
Fujii H Goto S Fukagawa M . Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins (Basel). (2018) 10(5):202. 10.3390/toxins10050202
114.
Rossi M Campbell K Johnson D Stanton T Pascoe E Hawley C et al Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: an observational study. Nutr Metab Cardiovasc Dis. (2014) 24(9):1035–42. 10.1016/j.numecd.2014.04.006
115.
Hsu C-C Lu Y-C Chiu C-A Yu T-H Hung W-C Wang C-P et al Levels of indoxyl sulfate are associated with severity of coronary atherosclerosis. Clin Investig Med. (2013) 36(1):E42–E9. 10.25011/cim.v36i1.19404
116.
Yang K Du C Wang X Li F Xu Y Wang S et al Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease–associated thrombosis in mice. Blood. (2017) 129(19):2667–79. 10.1182/blood-2016-10-744060
117.
Deguchi T Ohtsuki S Otagiri M Takanaga H Asaba H Mori S et al Major role of organic anion transporter 3 in the transport of indoxyl sulfate in the kidney. Kidney Int. (2002) 61(5):1760–8. 10.1046/j.1523-1755.2002.00318.x
118.
Takayama F Taki K Niwa T . Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. (2003) 41(3):S142–S5. 10.1053/ajkd.2003.50104
119.
Miyazaki T Ise M Seo H Niwa T . Indoxyl sulfate increases the gene expressions of TGF-β1 TIMP-1 and pro-α1 (1) collagen in uremic rat kidneys. Kidney Int Suppl. (1997) 62.
120.
Yisireyili M Shimizu H Saito S Enomoto A Nishijima F Niwa T . Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci. (2013) 92(24–26):1180–5. 10.1016/j.lfs.2013.05.008
121.
Aoki K Teshima Y Kondo H Saito S Fukui A Fukunaga N et al Role of indoxyl sulfate as a predisposing factor for atrial fibrillation in renal dysfunction. J Am Heart Assoc. (2015) 4(10):e002023. 10.1161/JAHA.115.002023
122.
Mokhtarani M Diaz G Rhead W Lichter-Konecki U Bartley J Feigenbaum A et al Urinary phenylacetylglutamine as dosing biomarker for patients with urea cycle disorders. Mol Genet Metab. (2012) 107(3):308–14. 10.1016/j.ymgme.2012.08.006
123.
Nemet I Saha PP Gupta N Zhu W Romano KA Skye SM et al A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. (2020) 180(5):862–77.e22. 10.1016/j.cell.2020.02.016
124.
Mayrand D . Identification of clinical isolates of selected species of Bacteroides: production of phenylacetic acid. Can J Microbiol. (1979) 25(8):927–8. 10.1139/m79-138
125.
Kaczmarek F Coykendall A . Production of phenylacetic acid by strains of Bacteroides asaccharolyticus and Bacteroides gingivalis (sp. nov.). J Clin Microbiol. (1980) 12(2):288–90. 10.1128/jcm.12.2.288-290.1980
126.
Dodd D Spitzer MH Van Treuren W Merrill BD Hryckowian AJ Higginbottom SK et al A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. (2017) 551(7682):648–52. 10.1038/nature24661
127.
Zhu Y Dwidar M Nemet I Buffa JA Sangwan N Li XS et al Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe. (2023) 31(1):18–32.e9. 10.1016/j.chom.2022.11.015
128.
Fu Y Yang Y Fang C Liu X Dong Y Xu L et al Prognostic value of plasma phenylalanine and gut microbiota-derived metabolite phenylacetylglutamine in coronary in-stent restenosis. Front Cardiovasc Med. (2022) 9:944155. 10.3389/fcvm.2022.944155
129.
Bogiatzi C Gloor G Allen-Vercoe E Reid G Wong RG Urquhart BL et al Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. (2018) 273:91–7. 10.1016/j.atherosclerosis.2018.04.015
130.
Fang C Zuo K Fu Y Li J Wang H Xu L et al Dysbiosis of gut microbiota and metabolite phenylacetylglutamine in coronary artery disease patients with stent stenosis. Front Cardiovasc Med. (2022) 9:832092. 10.3389/fcvm.2022.832092
131.
Beulens JW Booth SL van den Heuvel EG Stoecklin E Baka A Vermeer C . The role of menaquinones (vitamin K2) in human health. Br J Nutr. (2013) 110(8):1357–68. 10.1017/S0007114513001013
132.
Merli GJ Fink J . Vitamin K and thrombosis. Vitam Horm. (2008) 78:265–79. 10.1016/S0083-6729(07)00013-1
133.
Demir Y . Naphthoquinones, benzoquinones, and anthraquinones: Molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular diseases. Drug Dev Res. (2020) 81(5):628–36. 10.1002/ddr.21667
134.
Evrard S Delanaye P Kamel S Cristol J-P Cavalier E Arnaud J et al Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. (2015) 438:401–14. 10.1016/j.cca.2014.08.034
135.
Gröber U Reichrath J Holick M Kisters K . Vitamin K: an old vitamin in a new perspective. Dermatoendocrinol. (2014) 6(1):e968490. 10.4161/19381972.2014.968490
136.
Evrard S Delanaye P Kamel S Cristol J Cavalier E Arnaud J et al Vascular calcification: from pathophysiology to biomarkers Clin Chim Acta. (2015) 438:401–14. 10.1016/j.cca.2014.08.034
137.
Ponziani FR Pompili M Di Stasio E Zocco MA Gasbarrini A Flore R . Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J Gastroenterol. (2017) 23(7):1241. 10.3748/wjg.v23.i7.1241
138.
Schurgers LJ Cranenburg EC Vermeer C . Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemostasis. (2008) 100(10):593–603. 10.1160/TH08-02-0087
139.
Pivin E Ponte B Pruijm M Ackermann D Guessous I Ehret G et al Inactive matrix Gla-protein is associated with arterial stiffness in an adult population–based study. Hypertension. (2015) 66(1):85–92. 10.1161/HYPERTENSIONAHA.115.05177
140.
Hermans MM Vermeer C Kooman JP Brandenburg V Ketteler M Gladziwa U et al Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif. (2008) 25(5–6):395–401. 10.1159/000108629
141.
Mayer Jr O Seidlerová J Vaněk J Karnosová P Bruthans J Filipovský J et al The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int J Cardiol. (2016) 203:916–22. 10.1016/j.ijcard.2015.10.226
142.
Mayer Jr O Seidlerová J Bruthans J Filipovský J Timoracká K Vaněk J et al Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis. (2014) 235(1):162–8. 10.1016/j.atherosclerosis.2014.04.027
143.
Dalmeijer G Van Der Schouw Y Magdeleyns E Vermeer C Verschuren W Boer J et al Circulating desphospho-uncarboxylated matrix γ-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemostasis. (2014) 12(7):1028–34. 10.1111/jth.12609
144.
Illiano P Brambilla R Parolini C . The mutual interplay of gut microbiota, diet and human disease. FEBS J. (2020) 287(5):833–55. 10.1111/febs.15217
145.
Leeming ER Johnson AJ Spector TD Roy CIL . Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. (2019) 11(12):2862. 10.3390/nu11122862
146.
David LA Materna AC Friedman J Campos-Baptista MI Blackburn MC Perrotta A et al Host lifestyle affects human microbiota on daily timescales. Genome Biol. (2014) 15:1–15. 10.1186/gb-2014-15-7-r89
147.
Wang DD Nguyen LH Li Y Yan Y Ma W Rinott E et al The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. (2021) 27(2):333–43. 10.1038/s41591-020-01223-3
148.
Foye OT Huang I-F Chiou CC Walker WA Shi HN . Early administration of probiotic Lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and Smad 7 cell signaling. FEMS Immunol Med Microbiol. (2012) 65(3):467–80. 10.1111/j.1574-695X.2012.00978.x
149.
Alimi N Hamidi Madani Z Saranjam R Shoarishoar SS Massoudifar A Haghighi Z et al Investigating body dysmorphic disorder in pregnant mothers requesting cesarean section. J Obstet Gynecol Cancer Res. (2024) 9.
150.
Marques FZ Nelson E Chu P-Y Horlock D Fiedler A Ziemann M et al High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. (2017) 135(10):964–77. 10.1161/CIRCULATIONAHA.116.024545
151.
Xiao S Fei N Pang X Shen J Wang L Zhang B et al A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol Ecol. (2014) 87(2):357–67. 10.1111/1574-6941.12228
152.
Luu M Pautz S Kohl V Singh R Romero R Lucas S et al The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. (2019) 10(1):760. 10.1038/s41467-019-08711-2
153.
Palabıyık E Sulumer AN Uguz H Avcı B Askın S Askın H et al Assessment of hypolipidemic and anti-inflammatory properties of walnut (Juglans regia) seed coat extract and modulates some metabolic enzymes activity in triton WR-1339-induced hyperlipidemia in rat kidney, liver, and heart. J Mol Recognit. (2023) 36(3):e3004. 10.1002/jmr.3004
154.
Cani PD Bibiloni R Knauf C Waget A Neyrinck AM Delzenne NM et al Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. (2008) 57(6):1470–81. 10.2337/db07-1403
155.
Pagliai G Russo E Niccolai E Dinu M Di Pilato V Magrini A et al Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG study. Eur J Nutr. (2020) 59:2011–24. 10.1007/s00394-019-02050-0
156.
Liyanage T Ninomiya T Wang A Neal B Jun M Wong MG et al Effects of the Mediterranean diet on cardiovascular outcomes—a systematic review and meta-analysis. PLoS One. (2016) 11(8):e0159252. 10.1371/journal.pone.0159252
157.
Barrea L Annunziata G Muscogiuri G Laudisio D Di Somma C Maisto M et al Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex? Nutrition. (2019) 62:7–17. 10.1016/j.nut.2018.11.015
158.
Krishnan S O’Connor LE Wang Y Gertz ER Campbell WW Bennett BJ . Adopting a Mediterranean-style eating pattern with low, but not moderate, unprocessed, lean red meat intake reduces fasting serum trimethylamine N-oxide (TMAO) in adults who are overweight or obese. Br J Nutr. (2022) 128(9):1738–46. 10.1017/S0007114521004694
159.
Organ CL Otsuka H Bhushan S Wang Z Bradley J Trivedi R et al Choline diet and its gut microbe–derived metabolite, trimethylamine N-oxide, exacerbate pressure overload–induced heart failure. Circ Heart Fail. (2016) 9(1):e002314. 10.1161/CIRCHEARTFAILURE.115.002314
160.
Garcia-Mantrana I Selma-Royo M Alcantara C Collado MC . Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. (2018) 9:890. 10.3389/fmicb.2018.00890
161.
Berger S Raman G Vishwanathan R Jacques PF Johnson EJ . Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. (2015) 102(2):276–94. 10.3945/ajcn.114.100305
162.
Kuang R O’Keefe SJ del Aguila de Rivers CR Koutroumpakis F Binion DG . Is salt at fault? Dietary salt consumption and inflammatory bowel disease. Inflamm Bowel Dis. (2023) 29(1):140–50. 10.1093/ibd/izac058
163.
Herforth A Arimond M Álvarez-Sánchez C Coates J Christianson K Muehlhoff E . A global review of food-based dietary guidelines. Adv Nutr. (2019) 10(4):590–605. 10.1093/advances/nmy130
164.
Wilck N Matus MG Kearney SM Olesen SW Forslund K Bartolomaeus H et al Salt-responsive gut commensal modulates TH17 axis and disease. Nature. (2017) 551(7682):585–9. 10.1038/nature24628
165.
He FJ Tan M Ma Y MacGregor GA . Salt reduction to prevent hypertension and cardiovascular disease: jACC state-of-the-art review. J Am Coll Cardiol. (2020) 75(6):632–47. 10.1016/j.jacc.2019.11.055
166.
Robinson AT Edwards DG Farquhar WB . The influence of dietary salt beyond blood pressure. Curr Hypertens Rep. (2019) 21:1–11. 10.1007/s11906-019-0948-5
167.
Ha SK . Dietary salt intake and hypertension. Electrolyte Blood Press. (2014) 12(1):7. 10.5049/EBP.2014.12.1.7
168.
Kleinewietfeld M Manzel A Titze J Kvakan H Yosef N Linker RA et al Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. (2013) 496(7446):518–22. 10.1038/nature11868
169.
Yi B Titze J Rykova M Feuerecker M Vassilieva G Nichiporuk I et al Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. (2015) 166(1):103–10. 10.1016/j.trsl.2014.11.007
170.
Yan X Jin J Su X Yin X Gao J Wang X et al Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res. 2020;126(7):839–53. 10.1161/CIRCRESAHA.119.316394
171.
Ferguson JF Aden LA Barbaro NR Van Beusecum JP Xiao L Simons AJ et al High dietary salt–induced DC activation underlies microbial dysbiosis-associated hypertension. JCI Insight. (2019) 4(13). 10.1172/jci.insight.126241
172.
Gerritsen J Smidt H Rijkers GT de Vos WM . Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. (2011) 6:209–40. 10.1007/s12263-011-0229-7
173.
Markowiak P Śliżewska K . Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. (2017) 9(9):1021. 10.3390/nu9091021
174.
Kothari D Patel S Kim S-K . Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother. (2019) 111:537–47. 10.1016/j.biopha.2018.12.104
175.
Ojetti V Lauritano EC Barbaro F Migneco A Ainora ME Fontana L et al Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol. (2009) 5(6):675–82. 10.1517/17425250902973695
176.
Gibson GR Probert HM Van Loo J Rastall RA Roberfroid MB . Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. (2004) 17(2):259–75. 10.1079/NRR200479
177.
Gibson GR Roberfroid MB . Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. (1995) 125(6):1401–12. 10.1093/jn/125.6.1401
178.
Hill C Guarner F Reid G Gibson GR Merenstein DJ Pot B et al Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:133–42. 10.1038/nrgastro.2014.66
179.
Ouwehand AC Derrien M de Vos W Tiihonen K Rautonen N . Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol. (2005) 16(2):212–7. 10.1016/j.copbio.2005.01.007
180.
Mohania D Kansal VK Shah D Nagpal R Kumar M Gautam SK et al Therapeutic effect of probiotic dahi on plasma, aortic, and hepatic lipid profile of hypercholesterolemic rats. J Cardiovasc Pharmacol Ther. (2013) 18(5):490–7. 10.1177/1074248413487431
181.
Yoo SR Kim YJ Park DY Jung UJ Jeon SM Ahn YT et al Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity. (2013) 21(12):2571–8. 10.1002/oby.20428
182.
Lew L-C Choi S-B Khoo B-Y Sreenivasan S Ong K-L Liong M-T . Lactobacillus plantarum DR7 reduces cholesterol via phosphorylation of AMPK that down-regulated the mRNA expression of HMG-CoA reductase. Korean J Food Sci Anim Resour. (2018) 38(2):350. 10.5851/kosfa.2018.38.2.350
183.
Korcz E Kerényi Z Varga L . Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: potential health benefits with special regard to cholesterol-lowering effects. Food Funct. (2018) 9(6):3057–68. 10.1039/C8FO00118A
184.
Cerdó T García-Santos JA Bermúdez MG Campoy C . The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. (2019) 11(3):635. 10.3390/nu11030635
185.
Mishra S Wang S Nagpal R Miller B Singh R Taraphder S et al Probiotics and prebiotics for the amelioration of type 1 diabetes: present and future perspectives. Microorganisms. (2019) 7(3):67. 10.3390/microorganisms7030067
186.
Upadrasta A Madempudi RS . Probiotics and blood pressure: current insights. Integr Blood Press Control. (2016) 123:33–42. 10.2147/IBPC.S73246
187.
Frei R Akdis M O’Mahony L . Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol. (2015) 31(2):153–8. 10.1097/MOG.0000000000000151
188.
Farrokhian A Raygan F Soltani A Tajabadi-Ebrahimi M Sharifi Esfahani M Karami AA et al The effects of synbiotic supplementation on carotid intima-media thickness, biomarkers of inflammation, and oxidative stress in people with overweight, diabetes, and coronary heart disease: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob Proteins. (2019) 11:133–42. 10.1007/s12602-017-9343-1
189.
Tajabadi-Ebrahimi M Sharifi N Farrokhian A Raygan F Karamali F Razzaghi R et al A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes. (2017) 125(01):21–7. 10.1055/s-0042-105441
190.
Moludi J Khedmatgozar H Nachvak SM Abdollahzad H Moradinazar M Sadeghpour Tabaei A . The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci. (2022) 25(8):1659–68. 10.1080/1028415X.2021.1889451
191.
Lai S Mazzaferro S Muscaritoli M Mastroluca D Testorio M Perrotta A et al Prebiotic therapy with inulin associated with low protein diet in chronic kidney disease patients: evaluation of nutritional, cardiovascular and psychocognitive parameters. Toxins (Basel). (2020) 12(6):381. 10.3390/toxins12060381
192.
Aliasgharzadeh A Khalili M Mirtaheri E Gargari BP Tavakoli F Farhangi MA et al A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: a randomized controlled clinical trial. Adv Pharm Bull. (2015) 5(4):507. 10.15171/apb.2015.069
193.
Chi C Li C Wu D Buys N Wang W Fan H et al Effects of probiotics on patients with hypertension: a systematic review and meta-analysis. Curr Hypertens Rep. (2020) 22:1–8. 10.1007/s11906-020-01041-5
194.
Simenhoff M Dunn S Zollner G Fitzpatrick M Emery S Sandine W et al Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab. (1996) 22(1–3):92–6.
195.
Karlsson C Ahrné S Molin G Berggren A Palmquist I Fredrikson GN et al Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis. (2010) 208(1):228–33. 10.1016/j.atherosclerosis.2009.06.019
196.
Malik M Suboc TM Tyagi S Salzman N Wang J Ying R et al Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. (2018) 123(9):1091–102. 10.1161/CIRCRESAHA.118.313565
197.
Lam V Su J Koprowski S Hsu A Tweddell JS Rafiee P et al Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. (2012) 26(4):1727. 10.1096/fj.11-197921
198.
Lakshmanan AP Murugesan S Al Khodor S Terranegra A . The potential impact of a probiotic: Akkermansia muciniphila in the regulation of blood pressure—the current facts and evidence. J Transl Med. (2022) 20(1):430. 10.1186/s12967-022-03631-0
199.
Costanza AC Moscavitch SD Neto HCF Mesquita ET . Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. (2015) 179:348–50. 10.1016/j.ijcard.2014.11.034
200.
Gupta S Allen-Vercoe E Petrof EO . Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. (2016) 9(2):229–39. 10.1177/1756283X15607414
201.
Moayyedi P Surette MG Kim PT Libertucci J Wolfe M Onischi C et al Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. (2015) 149(1):102–9.e6. 10.1053/j.gastro.2015.04.001
202.
Woodworth MH Carpentieri C Sitchenko KL Kraft CS . Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes. (2017) 8(3):225–37. 10.1080/19490976.2017.1286006
203.
de Groot P Scheithauer T Bakker GJ Prodan A Levin E Khan MT et al Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. (2020) 69(3):502–12. 10.1136/gutjnl-2019-318320
204.
Craven L Rahman A Parvathy SN Beaton M Silverman J Qumosani K et al Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. (2020) 115(7):1055–65. 10.14309/ajg.0000000000000661
205.
Eiseman B Silen W Bascom GS Kauvar AJ . Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. (1958) 44(5):854–9.
206.
Van Nood E Vrieze A Nieuwdorp M Fuentes S Zoetendal EG de Vos WM et al Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. (2013) 368(5):407–15. 10.1056/NEJMoa1205037
207.
Khan MY Dirweesh A Khurshid T Siddiqui WJ . Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. (2018) 30(11):1309–17. 10.1097/MEG.0000000000001243
208.
Vrieze A Van Nood E Holleman F Salojärvi J Kootte RS Bartelsman JF et al Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. (2012) 143(4):913–6.e7. 10.1053/j.gastro.2012.06.031
209.
Gregory JC Buffa JA Org E Wang Z Levison BS Zhu W et al Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. (2015) 290(9):5647–60. 10.1074/jbc.M114.618249
210.
Hu X-F Zhang W-Y Wen Q Chen W-J Wang Z-M Chen J et al Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. (2019) 139:412–21. 10.1016/j.phrs.2018.11.042
211.
Toral M Robles-Vera I De la Visitacion N Romero M Yang T Sánchez M et al Critical role of the interaction gut microbiota–sympathetic nervous system in the regulation of blood pressure. Front Physiol. (2019) 10:231. 10.3389/fphys.2019.00231
212.
Kim TT Parajuli N Sung MM Bairwa SC Levasseur J Soltys C-LM et al Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am J Physiol Endocrinol Metab. (2018) 315(4):E511–E9. 10.1152/ajpendo.00471.2017
213.
Kim ES Yoon BH Lee SM Choi M Kim EH Lee B-W et al Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp Mol Med. (2022) 54(2):103–14. 10.1038/s12276-022-00728-w
214.
Smits LP Kootte RS Levin E Prodan A Fuentes S Zoetendal EG et al Effect of vegan fecal microbiota transplantation on carnitine-and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. (2018) 7(7):e008342. 10.1161/JAHA.117.008342
215.
Fan L Ren J Chen Y Wang Y Guo Z Bu P et al Effect of fecal microbiota transplantation on primary hypertension and the underlying mechanism of gut microbiome restoration: protocol of a randomized, blinded, placebo-controlled study. Trials. (2022) 23(1):178. 10.1186/s13063-022-06086-2
216.
Brandt LJ . FMT: first step in a long journey. Am J Gastroenterol. (2013) 108(8):1367–8. 10.1038/ajg.2013.165
217.
De Leon LM Watson JB Kelly CR . Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. (2013) 11(8):1036–8. 10.1016/j.cgh.2013.04.045
218.
Wang Z Roberts AB Buffa JA Levison BS Zhu W Org E et al Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. (2015) 163(7):1585–95. 10.1016/j.cell.2015.11.055
219.
Roberts AB Gu X Buffa JA Hurd AG Wang Z Zhu W et al Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. (2018) 24(9):1407–17. 10.1038/s41591-018-0128-1
220.
Jing L Zhang H Xiang Q Shen L Guo X Zhai C et al Targeting trimethylamine N-oxide: a new therapeutic strategy for alleviating atherosclerosis. Front Cardiovasc Med. (2022) 9:864600. 10.3389/fcvm.2022.864600
221.
Shi Y Hu J Geng J Hu T Wang B Yan W et al Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/-mice. Biomed Pharmacother. (2018) 107:1556–63. 10.1016/j.biopha.2018.08.148
222.
Chen S Henderson A Petriello MC Romano KA Gearing M Miao J et al Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. (2019) 30(6):1141–51.e5. 10.1016/j.cmet.2019.08.021
223.
Miao J Ling AV Manthena PV Gearing ME Graham MJ Crooke RM et al Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. (2015) 6(1):6498. 10.1038/ncomms7498
Summary
Keywords
gut microbiota, cardiovascular diseases, heart failure, metabolites, microbiota composition
Citation
Zhang Y, Wu H, Jin M, Feng G and Wang S (2025) The gut-heart axis: unveiling the roles of gut microbiota in cardiovascular diseases. Front. Cardiovasc. Med. 12:1572948. doi: 10.3389/fcvm.2025.1572948
Received
13 February 2025
Accepted
05 May 2025
Published
26 May 2025
Volume
12 - 2025
Edited by
Chengxing Shen, Shanghai Jiao Tong University, China
Reviewed by
Maria Margherita Rando, Agostino Gemelli University Polyclinic (IRCCS), Italy
Ayoola Awosika, University of Illinois at Chicago, United States
Updates
Copyright
© 2025 Zhang, Wu, Jin, Feng and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Sheng Wang shengwang@mail.ccmu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.