Abstract
Objective:
Prolonged mechanical ventilation (PMV) is a significant postoperative complication in cardiac surgery, associated with increased mortality and healthcare costs. This study aims to develop and validate a novel scoring model to predict the risk of PMV in cardiac surgery patients.
Methods:
A retrospective analysis was conducted using data from 14 comprehensive hospitals in Jiangsu Province, including adult patients who underwent coronary artery bypass grafting (CABG), valve surgery, and aortic surgery from January 2021 to December 2022. Predictive variables were selected based on clinical expertise and prior literature, and a nomogram was developed using LASSO regression and multiple logistic regression. Model performance was evaluated using the C-index, calibration plots, and decision curve analysis (DCA).
Results:
A total of 5,206 patients were included in the final analysis. The incidence rate of PMV were 11.83% in the training set, 8.65% in the internal validation set, and 15.4% in the external validation set. The nomogram identified 9 significant predictors, including age, gender, preoperative conditions, and surgical factors. The model demonstrated robust performance with C-index values of 0.79 in the training and internal validation sets and 0.75 in the external validation set, indicating good predictive capability. Calibration curves confirmed the accuracy of predicted probabilities, and DCA indicated substantial net benefits for clinical decision-making.
Conclusions:
This study presents a validated scoring model for predicting PMV in cardiac surgery patients, integrating a comprehensive range of clinical variables. The model facilitates early identification of high-risk patients, enabling tailored perioperative strategies and potentially improving patient outcomes and resource utilization in cardiac surgery.
Introduction
Despite advancements in the perioperative management of cardiac surgery patients, prolonged mechanical ventilation (PMV) remains a significant postoperative challenge, with reported incidence rate reaching as high as one in five patients (1–3). PMV is associated with increased mortality, reduced quality of life, and substantial economic burden (4–6). Several recent studies and the Society of Thoracic Surgeons (STS) identify PMV lasting more than 24 h as a major morbidity endpoint in cardiac surgery, which aligns with our study's definition of PMV as a duration exceeding 24 h on the ventilator (1, 7, 8).
PMV following cardiac surgery places a considerable burden on both patients and healthcare systems, underscoring the need for a reliable predictive model to facilitate early detection and management. While previous studies have identified predictive factors for PMV after cardiac surgery, these efforts have often been limited by small sample sizes, population homogeneity, and the absence of independent external validation cohorts (2, 9, 10). To address these limitations, we conducted a retrospective analysis using data from 14 hospitals to develop a predictive model for the risk of PMV after cardiac surgery, which we subsequently validated in an independent cohort.
Methods
Patient selection
This study was a retrospective, observational research project that included inpatient records of adult patients who underwent CABG/valve surgery and aortic surgery, including combined procedures, at 14 level-three grade A comprehensive hospitals in Jiangsu Province from January 2021 to December 2022. Patients aged under 18 years, those who required preoperative intubation, had preoperative circulatory instability, were critically ill, underwent cardiac transplantation, received left ventricular assist devices, or underwent other non-cardiac open surgeries were excluded from the study. PMV was defined as the requirement for mechanical ventilation for more than 24 h following cardiac surgery. The research was approved by the Ethical Committee of Nanjing First Hospital (KY20170811-03), and patient informed consent was waived due to the retrospective nature of the study.
Variables selection
The selection of predictive variables in this study was based on clinical expertise and prior findings reported in the existing literature. Initially, demographic data including age, gender, height, weight, and smoking history were collected. Additionally, preoperative status and certain biomarkers were included in the dataset. These included diabetes, insulin use, preoperative hypertension, preoperative hyperlipidemia, preoperative dialysis, peripheral artery disease, chronic lung disease, history of cerebrovascular accidents, atrial fibrillation, history of percutaneous coronary intervention (PCI), preoperative serum creatinine, preoperative total bilirubin, preoperative hemoglobin, preoperative left ventricular ejection fraction (LVEF), and whether there was significant left main coronary artery disease. Moreover, surgical-related variables were collected, including whether the surgery was an emergency, the use of cardiopulmonary bypass, the duration of cardiopulmonary bypass, intraoperative red blood cell transfusion, intraoperative plasma transfusion, intraoperative cryoprecipitate transfusion, coronary artery bypass grafting, valve surgery, and aortic surgery.
Statistical analysis
Patients from Nanjing First Hospital were randomly divided into a training set and an internal validation set in a 7:3 ratio, while patients from other cardiac centers served as an external validation set. Continuous variables were presented as mean ± standard deviation. Student's t-test was employed to compare differences between groups. Categorical variables were reported as frequencies and percentages, and the Rao-Scott chi-square test was used for comparisons.
The variables in the training set underwent a filtering process via LASSO regression. After obtaining the predictors through LASSO regression, we constructed prediction models based on the multivariate logistic regression. Variables with non-zero coefficients in the LASSO regression model were selected to develop the nomogram prediction model. The accuracy of the risk prediction model was evaluated using several metrics, including the receiver operating characteristic curve (ROC) curve, calibration plot, and decision curve analysis (DCA) curve. These evaluations were performed for the training set, internal validation set, and external validation set. An area under the curve (AUC) value closer to 1 indicates better predictive capability of the model. Moreover, an AUC value greater than 0.7 signifies that the model has good predictive capacity. The calibration plot displays a scatter plot of the observed vs. predicted incidence; if the curves lie along the diagonal of the coordinate system, it indicates greater accuracy in the model's predictive ability. The DCA curve circumvents the issues associated with selecting cut-off values for the ROC curve, sensitivity, and specificity, directly calculating the net benefit in clinical settings. A DCA curve that remains above the two extremes suggests good clinical applicability of the model. In all the analyses mentioned above, a two-tailed p-value less than 0.05 was considered statistically significant.
Results
Characteristics of the cohorts
During the period spanning from January 2021 to December 2022, a comprehensive analysis was conducted involving a total of 5,351 participants. We excluded 13 individuals under the age of 18, 48 individuals who were intubated preoperatively, 14 individuals with unstable circulation or who underwent cardiopulmonary resuscitation before surgery, 21 individuals who had heart transplants, 11 individuals who underwent left ventricular assist device (LVAD) surgery, and 18 individuals who had non-cardiac valve surgeries, resulting in a final study cohort of 5,206 individuals (Figure 1).
Figure 1
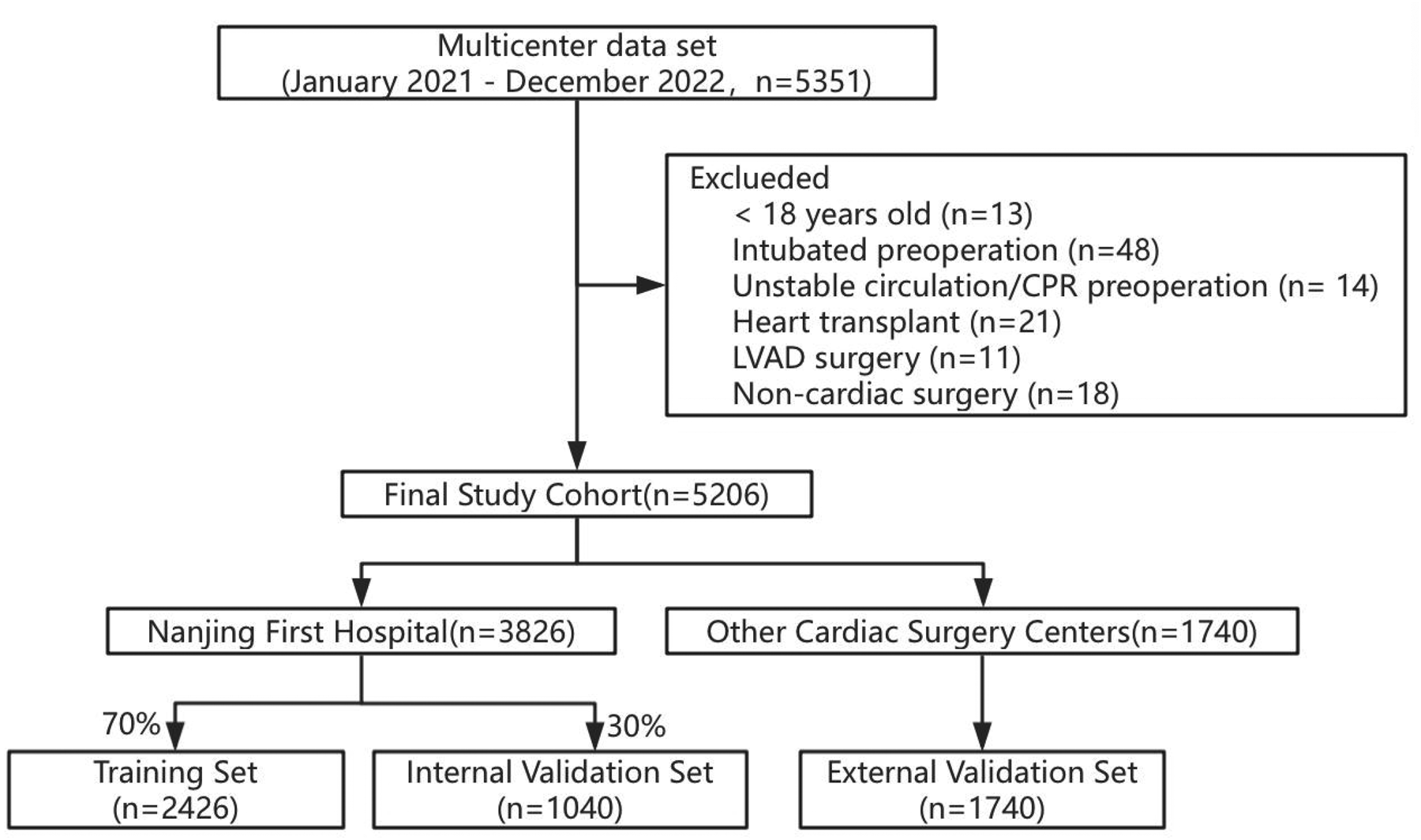
Participant selection and dataset division.
Among them, 3,826 individuals were from the patient cohort of Nanjing First Hospital, and the remaining 1,740 individuals were from 13 other cardiac centers. Using the random seed method, the patient queue of Nanjing First Hospital was divided into a training set (N = 2,426) and an internal validation set (n = 1,040) in a ratio of 7:3. The patient queues of the other 13 cardiac centers were used as an external validation set (n = 1,740) (Figure 1).
Baseline characteristics differed significantly across the 3 groups. The incidence rate of PMV in the training set, internal validation set, and external validation set were 11.83%, 8.65%, and 15.4% respectively (Table 1). In the training set, patient characteristics of the PMV and non-PMV groups were compared (Table 2).
Table 1
| Characteristics | Training set (N = 2,426) | Internal validation set (N = 1,040) | External validation set (N = 1,740) | P value |
|---|---|---|---|---|
| Age | 61.47 ± 11.6 | 61.44 ± 11.54 | 62.86 ± 10.42 | <0.001 |
| Sex | <0.001 | |||
| Male | 1,495 (61.62) | 639 (61.44) | 1,318 (75.75) | |
| Female | 931 (38.38) | 401 (38.56) | 422 (24.25) | |
| BMI | 24.25 ± 3.44 | 24.21 ± 3.31 | 24.54 ± 3.29 | 0.010 |
| Medical history | ||||
| Smoking | 853 (35.16) | 364 (35) | 703 (40.4) | <0.001 |
| Diabetes | 598 (24.65) | 241 (23.17) | 553 (31.78) | <0.001 |
| Insulin-treated diabetes | 252 (10.39) | 88 (8.46) | 209 (12.01) | 0.012 |
| Hypertension | 1,371 (56.51) | 552 (53.08) | 1,203 (69.14) | <0.001 |
| Hyperlipidemia | 261 (10.76) | 105 (10.1) | 359 (20.63) | <0.001 |
| Dialysis | 19 (0.78) | 7 (0.67) | 18 (1.03) | 0.543 |
| Peripheral arterial disease | 50 (2.06) | 21 (2.02) | 32 (1.84) | 0.874 |
| Chronic lung disease | 60 (2.47) | 34 (3.27) | 44 (2.53) | 0.379 |
| Carotid artery stenosis | <0.001 | |||
| None | 2,312 (95.3) | 996 (95.77) | 1,460 (83.91) | |
| Unilateral | 69 (2.84) | 27 (2.6) | 83 (4.77) | |
| Bilateral | 45 (1.85) | 17 (1.63) | 197 (11.32) | |
| Previous cerebrovascular accident | 224 (9.23) | 84 (8.08) | 164 (9.43) | 0.452 |
| Atrial fibrillation | 470 (19.37) | 205 (19.71) | 42 (2.41) | <0.001 |
| Previous PCI | 155 (6.39) | 56 (5.38) | 128 (7.36) | 0.118 |
| LVEF | 62 (57, 64) | 62 (58, 64) | 61 (54, 65) | 0.253 |
| Left main disease | 275 (11.34) | 114 (10.96) | 531 (30.52) | <0.001 |
| Laboratory test | ||||
| Creatinine | 73.95 (62, 88.84) | 72 (61.58, 86.78) | 76 (63, 91) | <0.001 |
| Bilirubin | 10.7 (7.7, 15.32) | 10.8 (7.8, 15.5) | 11.3 (8.3, 16.1) | 0.003 |
| Hemoglobin | 132 (120, 143) | 132 (121, 143) | 129 (118, 141) | <0.001 |
| Surgical information | ||||
| Emergency | 170 (7.01) | 79 (7.6) | 176 (10.3) | <0.001 |
| CPB | 2,287 (94.27) | 982 (94.42) | 1,599 (91.9) | 0.004 |
| CPB time | 112 (85, 144) | 114 (84, 144.25) | 115 (81, 145) | 0.906 |
| Intraoperative RBC transfusion | 246 (10.14) | 125 (12.02) | 679 (39.02) | <0.001 |
| Intraoperative plasma transfusion | 138 (5.69) | 70 (6.73) | 530 (30.46) | <0.001 |
| Intraoperative cryoprecipitate transfusion | 439 (18.1) | 183 (17.6) | 337 (19.37) | 0.432 |
| CABG | 1,108 (45.67) | 448 (43.08) | 1,445 (83.05) | <0.001 |
| Valve surgery | 1,396 (57.54) | 621 (59.71) | 266 (15.29) | <0.001 |
| Aorta surgery | 403 (16.61) | 184 (17.69) | 284 (16.32) | 0.630 |
| In-hospital outcomes | ||||
| Postoperative reintubation | 64 (2.64) | 31 (2.98) | 40 (2.3) | 0.539 |
| In-hospital death | 64 (2.64) | 27 (2.6) | 35 (2.01) | 0.396 |
| PMV | 287 (11.83) | 90 (8.65) | 268 (15.4) | <0.001 |
Clinical characteristics in training set, internal and external validation set.
Values are presented as mean ± SD, median (quartiles) or n (%).
BMI, body mass index; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection function; CPB, cardiopulmonary bypass; RBC, red blood cell; CABG, coronary artery bypass grafting; PMV, prolonged mechanical ventilation.
Table 2
| Characteristics | Non-PMV (N = 2,139) | PMV (N = 287) | P value |
|---|---|---|---|
| Age | 61.28 ± 11.60 | 62.92 ± 11.54 | 0.024 |
| Sex | 0.760 | ||
| Male | 1,321 (61.76) | 174 (60.63) | |
| Female | 818 (38.24) | 113 (39.37) | |
| BMI | 24.22 ± 3.37 | 24.50 ± 3.90 | 0.239 |
| Medical history | |||
| Smoking | 749 (35.02) | 104 (36.24) | 0.733 |
| Diabetes | 531 (24.82) | 67 (23.34) | 0.636 |
| Insulin-treated diabetes | 225 (10.52) | 27 (9.41) | 0.634 |
| Hypertension | 1,178 (55.07) | 193 (67.25) | <0.001 |
| Hyperlipidemia | 236 (11.03) | 25 (8.71) | 0.275 |
| Dialysis | 16 (0.75) | 3 (1.05) | 0.485 |
| Peripheral arterial disease | 39 (1.82) | 11 (3.83) | 0.042 |
| Chronic lung disease | 45 (2.1) | 15 (5.23) | 0.003 |
| Carotid artery stenosis | 0.109 | ||
| None | 2,041 (95.42) | 271 (94.43) | |
| Unilateral | 56 (2.62) | 13 (4.53) | |
| Bilateral | 42 (1.96) | 3 (1.05) | |
| Previous cerebrovascular accident | 196 (9.16) | 28 (9.76) | 0.828 |
| Atrial fibrillation | 404 (18.89) | 66 (23) | 0.115 |
| Previous PCI | 134 (6.26) | 21 (7.32) | 0.578 |
| LVEF | 62 (57, 64) | 61 (49, 64) | <0.001 |
| Left main disease | 247 (11.55) | 28 (9.76) | 0.424 |
| Laboratory test | |||
| Creatinine | 73 (62, 87.75) | 79.8 (64, 105.5) | <0.001 |
| Bilirubin | 10.6 (7.6, 14.9) | 12.1 (8.25, 18.2) | <0.001 |
| Hemoglobin | 132 (120, 143) | 129 (118, 141) | 0.027 |
| Surgical information | |||
| Emergency | 90 (4.21) | 80 (27.87) | <0.001 |
| CPB | 2,005 (93.74) | 282 (98.26) | 0.003 |
| CPB time | 108 (83, 137) | 147 (111.5, 177.5) | <0.001 |
| Intraoperative RBC transfusion | 189 (8.84) | 57 (19.86) | <0.001 |
| Intraoperative plasma transfusion | 87 (4.07) | 51 (17.77) | <0.001 |
| Intraoperative cryoprecipitate transfusion | 311 (14.54) | 128 (44.60) | <0.001 |
| CABG | 992 (46.38) | 116 (40.42) | 0.066 |
| Valve surgery | 1,220 (57.04) | 176 (61.32) | 0.188 |
| Aorta surgery | 308 (14.40) | 95 (33.10) | <0.001 |
| In-hospital outcomes | |||
| Postoperative reintubation | 15 (0.7) | 49 (17.07) | <0.001 |
| In-hospital death | 26 (1.22) | 38 (13.24) | <0.001 |
Characteristics of patients with PMV and non-PMV in training set.
Values are presented as mean ± SD, median(quartiles) or n (%).
PMV, prolonged mechanical ventilation; BMI, body mass index; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection function; CPB, cardiopulmonary bypass; RBC, red blood cell; CABG, coronary artery bypass grafting.
Feature selection and nomogram
When 29 variables are included in the LASSO regression model for variable selection, the regression coefficients of all variables progressively diminish towards zero with increasing penalty, ultimately converging to zero (Figure 2A). The significant variables were determined through ten-fold cross-validation, illustrated in Figure 2B. Ultimately, 14 variables were identified, encompassing gender, age, preoperative condition, hypertension, peripheral arterial disease, chronic lung disease, preoperative atrial fibrillation, blood creatinine level, preoperative bilirubin level, preoperative hemoglobin level, LVEF value, emergency surgery status, cardiopulmonary bypass duration, intraoperative plasma transfusion, and intraoperative cryoprecipitate transfusion.
Figure 2
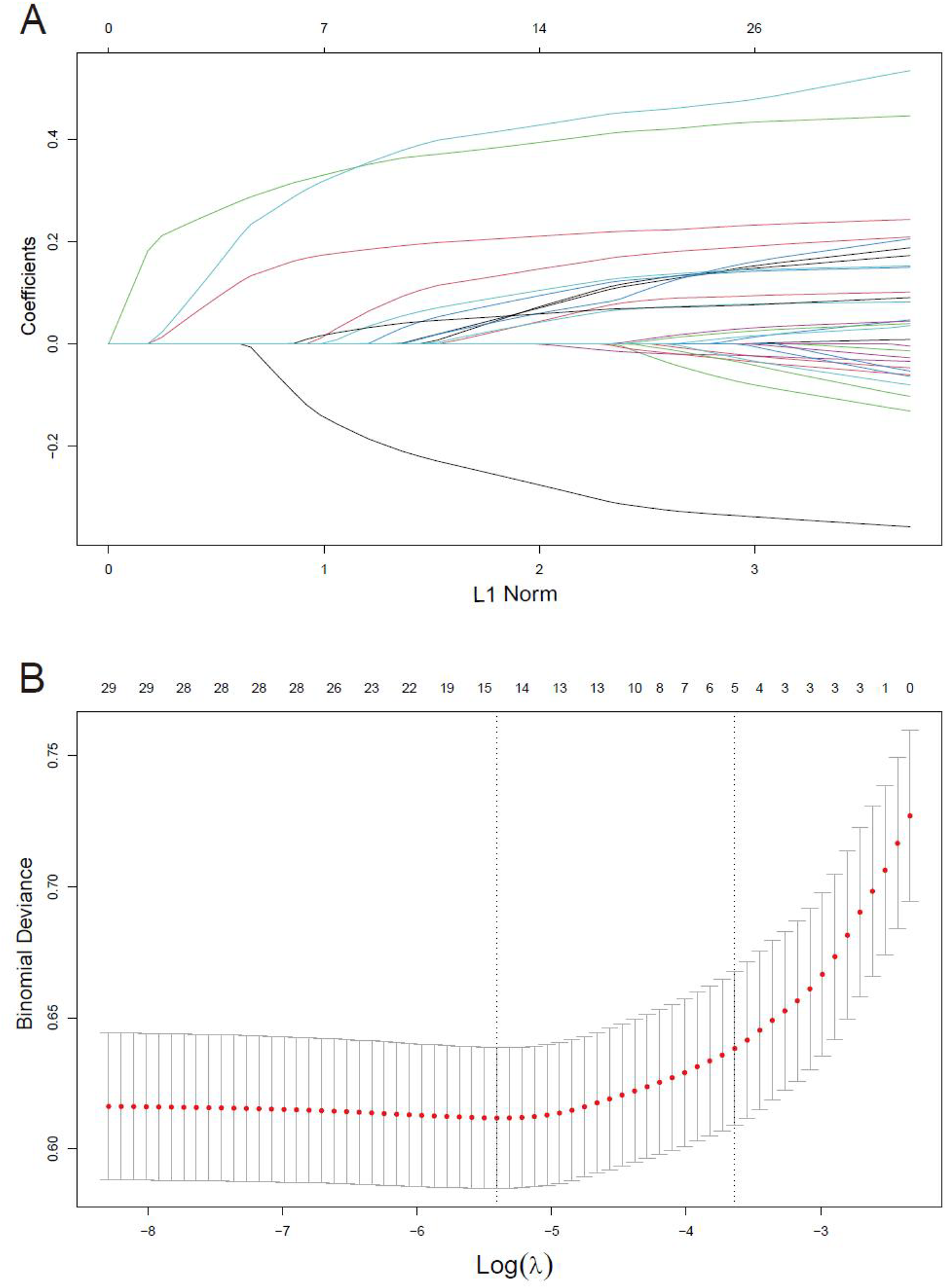
Variables selection using the LASSO regression models. (A) LASSO coefficient profiles of the 29 variables. Variables are included in the LASSO regression model for selection, the regression coefficients progressively diminish towards zero with increasing penalty. (B) Optimal parameter (lambda) selection in the LASSO model used 10-fold cross-validation via minimum criteria.
Subsequently, these 14 variables were incorporated into a multiple logistic regression model (Table 3). The analysis unveiled that gender, age, peripheral arterial disease, chronic lung disease, preoperative creatinine level, LVEF value, emergency surgery status, cardiopulmonary bypass duration, and intraoperative cryoprecipitate transfusion emerged as independent risk factors for postoperative PMV in cardiac surgery patients. A nomogram model was developed utilizing these 9 variables (Figure 3).
Table 3
| Variables | OR | 95%CI | P-value |
|---|---|---|---|
| Sex | 0.026 | ||
| Male | Ref | – | |
| Female | 1.42 | (1.04, 1.93) | |
| Age | 1.02 | (1.00, 1.03) | 0.012 |
| Hypertension | 1.38 | (1.03, 1.87) | 0.033 |
| Peripheral arterial disease | 3.00 | (1.39, 6.50) | 0.005 |
| Chronic lung disease | 2.74 | (1.41, 5.32) | 0.003 |
| Atrial fibrillation | 1.37 | (0.97, 1.94) | 0.066* |
| Preoperative creatinine | 1.00 | (1.00, 1.00) | 0.046 |
| Preoperative bilirubin | 1.01 | (1.00, 1.02) | 0.092* |
| Preoperative hemoglobin | 1.01 | (0.99, 1.01) | 0.656* |
| Preoperative LVEF | 0.96 | (0.95, 0.98) | <0.001 |
| Emergency surgery | 5.61 | (3.59, 8.76) | <0.001 |
| Cardiopulmonary bypass time | 1.01 | (1.01, 1.01) | <0.001 |
| Intraoperative plasma transfusion | 1.43 | (0.89, 2.30) | 0.145* |
| Intraoperative cryoprecipitate transfusion | 1.83 | (1.29, 2.59) | <0.001 |
Multivariate logistic regression of predictors associated with prolonged mechanical ventilation in the training set.
OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection function.
P > 0.05.
Figure 3
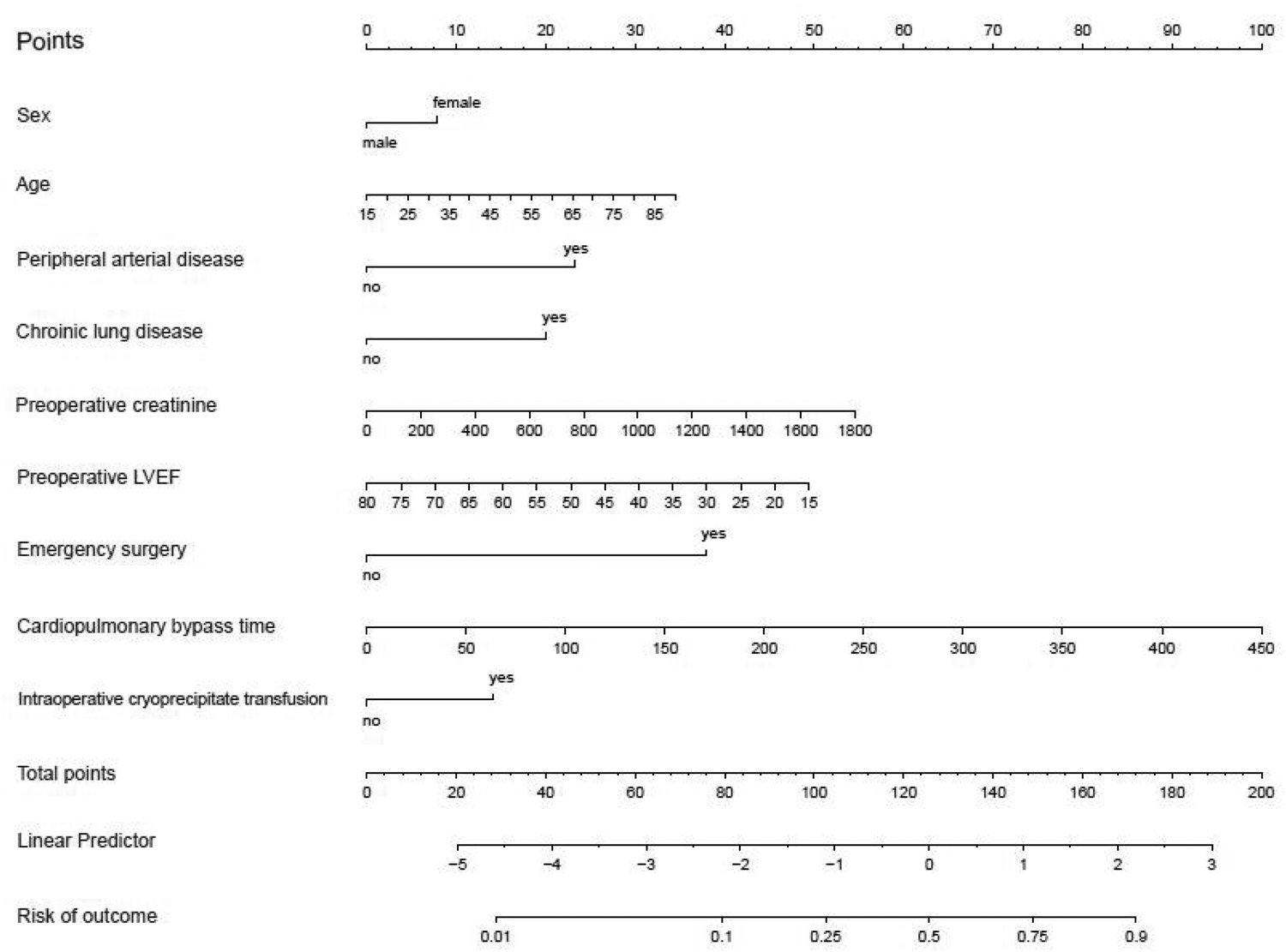
Nomogram derived from training set for predicting prolonged mechanical ventilation.
Model performance
To assess the robustness of the clinical prediction model established, we conducted testing on the training set, internal validation set, and external validation dataset. Initially, we computed the model's C-index. In the training set, the nomogram model's C-index was 0.79 (95% confidence interval, 0.76–0.81; p < 0.001), while in the internal and external validation sets, the C-index was 0.79 (95% confidence interval, 0.73–0.84; p < 0.001) and 0.75 (95% confidence interval, 0.73–0.78; p < 0.001), respectively. Calibration curves indicated that the fitted actual occurrence rate of PMV (Y-axis) and the predicted occurrence rate (X-axis) in the nomogram model were distributed around a line with a slope of approximately 45°. The decision curve, within the horizontal coordinate range of 0.1–0.4, positioned above the lines representing None and All, suggesting that the model exhibits good predictive capability within this range (Figure 4).
Figure 4
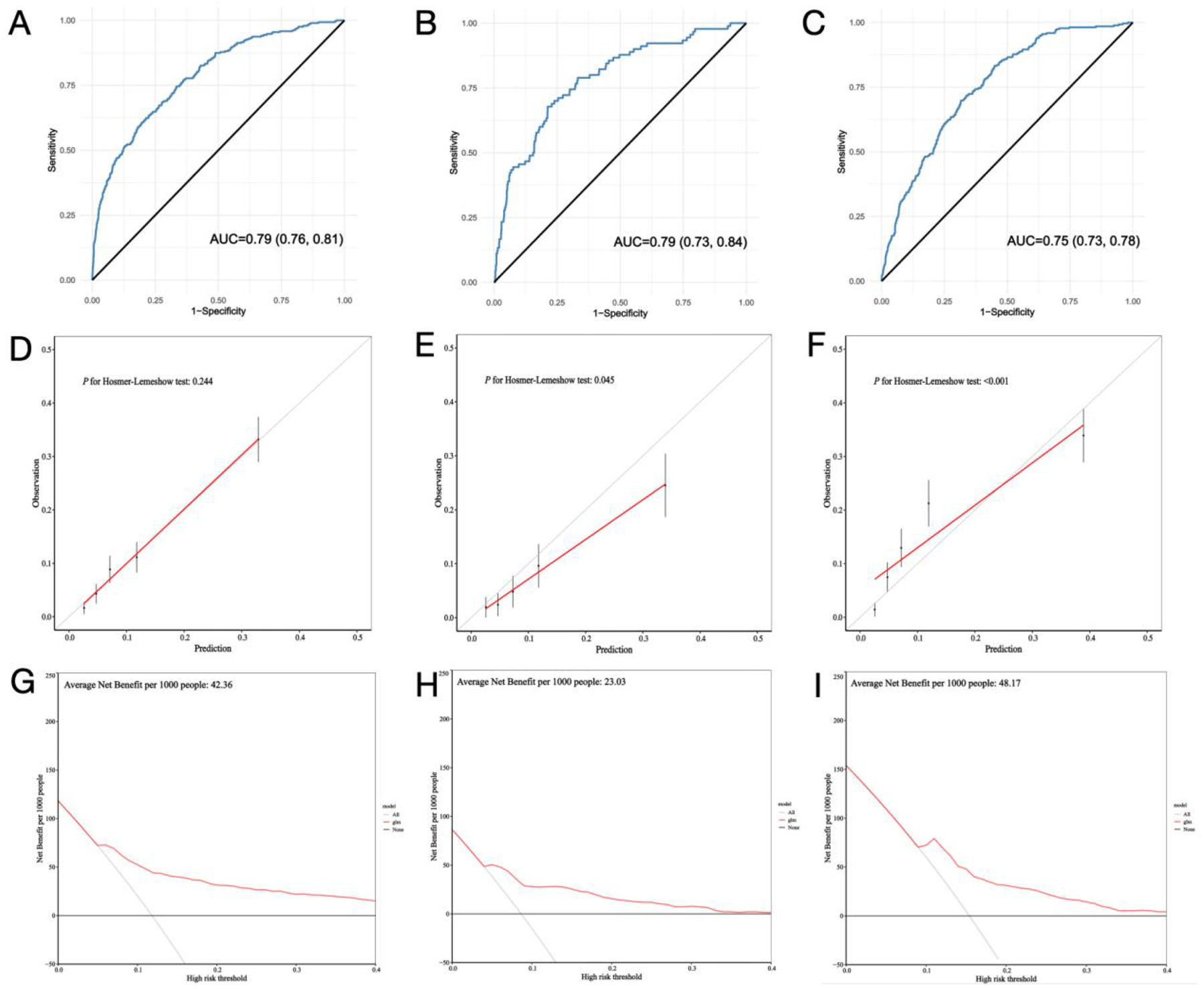
Model performance of the nomogram for predicting prolonged mechanical ventilation after cardiac surgery in train set (A,D,G), internal validation set (B,E,H) and external validation set (C,F,I).
Discussion
PMV is a significant postoperative complication in cardiac surgery, impacting patient outcomes and healthcare resources (11). Our study corroborates that inpatient mortality rate is higher in the PMV group compared to the non-PMV group. While most studies use a 24 or 48 h threshold for extubation time to determine PMV duration, our research aligns with previous findings by adopting a 24-h threshold, given its association with faster recovery post-surgery (1, 12, 13).
This study aimed to address the challenge of predicting PMV by developing and validating a novel scoring model. The nomogram was constructed based on a rigorous selection process that identified nine key predictors, chosen for their statistical significance and clinical relevance. This model incorporates a comprehensive range of factors, including patient demographics, comorbidities, and intraoperative variables, reflecting the multifactorial nature of PMV. Its primary strength lies in using multi-center data, which encompasses a large sample size and includes an independent external validation cohort.
Age and chronic lung disease are common risk factors for PMV after cardiac surgery (13–16). The female gender was found to be independently associated with failure of the spontaneous breathing trial and failure of prolonged weaning in a previous study (2). The main reason for this may be related to the changes in female hormone levels. Sharma et al. reveals that lower LVEF is a significant predictor of PMV following cardiac surgery (12). Huan et al. identified LVEF as a critical predictor influencing the occurrence of PMV after robot-assisted CABG (14). Patients with a low ejection fraction may experience greater fluctuations in circulation after cardiac surgery. Since stable circulation is a prerequisite for extubation, these patients are likely to require prolonged mechanical ventilation. Many studies have confirmed that cardiopulmonary bypass (CPB) time are independent risk factors for PMV (9, 12, 14, 17, 18). Prolonged CPB can trigger a systemic inflammatory response that activates various mediators, and disrupt normal physiological homeostasis, leading to increased capillary permeability in the pulmonary circulation (12).
Due to systemic atherosclerosis, patients with peripheral arterial disease may also experience indirect effects on their lungs, leading to reduced pulmonary function (19). After surgery, these patients may require prolonged mechanical ventilation to maintain adequate oxygenation levels. Patients with elevated preoperative creatinine levels often face more complex fluid management issues postoperatively after cardiac surgery (7, 20–22). If fluid management is not handled properly, it may lead to pulmonary edema or other respiratory-related complications, which can delay weaning from mechanical ventilation. Patients undergoing emergency surgery are often in a severe state, potentially experiencing shock, severe infections, or acute heart failure (9). These factors increase the risk of surgery and may result in delayed postoperative recovery of respiratory function, necessitating a longer duration of mechanical ventilation. The intraoperative administration of large amounts of blood products, such as cryoprecipitate, can trigger a systemic inflammatory response (23–26). This response may affect lung function, leading to delayed recovery of pulmonary function postoperatively, thereby prolonging the duration of mechanical ventilation.
To illustrate the model's application, consider a 50-year-old female patient with peripheral vascular disease and chronic pulmonary disease, a preoperative serum creatinine level of 200 µmol/L, and an LVEF of 50%. She underwent emergency cardiac surgery with a 100-min CPB time and received cryoprecipitate. The patient would receive 8 points for female, 16 points for age, 23 for peripheral vascular disease, 20 points for chronic pulmonary disease, 6 points for serum creatinine, 23 points for LVEF, 38 points for emergency surgery, 22 points for CPB and 14 points for cryoprecipitate for a score of 170, indicating a 90% risk of PMV.
Hypertension was initially included in model construction but was subsequently excluded due to its limited predictive value for PMV and adverse effects on model performance. The model demonstrated strong performance across various datasets, with consistent C-index values of 0.79 in both the training and internal validation sets and 0.75 in the external validation set, reflecting high accuracy in distinguishing between high- and low-risk patients for PMV. Heng Yang et al. developed a potential nomogram to predict the risk of PMV after valve surgery in a single-center retrospective study with a C-index of 0.782 (27), which indicate the robustness of our model. Calibration curves confirmed that predicted probabilities closely matched actual outcomes. The decision curve analysis revealed substantial net benefits in clinical decision-making within a probability threshold range of 0.1–0.4, emphasizing the model's practical value in guiding early interventions and resource allocation.
This predictive model has significant implications. It allows for early identification of high-risk patients, enabling more tailored perioperative strategies, such as increased surveillance, proactive respiratory support, and optimized comorbidity management. Additionally, the model can facilitate informed discussions with patients and families about expected postoperative courses and potential interventions. It also offers potential benefits in resource utilization, reducing ICU stays, ventilator dependency, and associated healthcare costs.
Despite promising results, this study has limitations. The retrospective nature of the data may introduce biases, and variability in clinical practices across multiple centers could affect the generalizability of the findings. For example, NT-ProBNP, NYHA class was excluded due to grossly incomplete data. Although the model performed well in the external validation cohort, further prospective studies are needed to validate its effectiveness in broader and more diverse patient populations. Future research should explore incorporating emerging biomarkers and integrating machine learning techniques to enhance predictive accuracy (28).
Conclusion
Our study introduces a validated scoring model for predicting PMV in cardiac surgery patients. By incorporating a range of clinical variables, the model provides a practical tool for improving perioperative planning and patient care. Its adoption could enhance outcomes through early identification and management of at-risk patients, advancing the field of cardiac surgery and postoperative care.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of Nanjing First Hospital (KY20170811-03). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
QL: Data curation, Formal analysis, Methodology, Resources, Software, Supervision, Validation, Writing – original draft. PC: Formal analysis, Methodology, Software, Writing – original draft. WW: Conceptualization, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal analysis, Writing – original draft. YX: Data curation, Methodology, Resources, Software, Writing – original draft. XC: Data curation, Methodology, Software, Writing – original draft. RF: Resources, Software, Supervision, Writing – original draft, Writing – review & editing. WC: Methodology, Supervision, Validation, Visualization, Writing – original draft. FH: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. XC: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Rahimi S Abdi A Salari N Shohaimi S Naghibeiranvand M . Factors associated with long-term mechanical ventilation in patients undergoing cardiovascular surgery. BMC Cardiovasc Disord. (2023) 23(1):276. 10.1186/s12872-023-03315-7
2.
Aksoy R Karakoc AZ Cevirme D Elibol A Yigit F Yilmaz Ü et al Predictive factors of prolonged ventilation following cardiac surgery with cardiopulmonary bypass. Braz J Cardiovasc Surg. (2021) 36(6):780–7. 10.21470/1678-9741-2020-0164
3.
Jin M Ma WG Liu S Zhu J Sun L Lu J et al Predictors of prolonged mechanical ventilation in adults after acute type-a aortic dissection repair. J Cardiothorac Vasc Anesth. (2017) 31(5):1580–7. 10.1053/j.jvca.2017.03.036
4.
Diaz-Castrillon CE Brown JA Navid F Serna-Gallegos D Yousef S Thoma F et al The impact of prolonged mechanical ventilation after acute type a aortic dissection repair. J Thorac Cardiovasc Surg. (2022) 167(5):1672–9.S0022522322007309.
5.
Mathis MR Duggal NM Likosky DS Haft JW Douville NJ Vaughn MT et al Intraoperative mechanical ventilation and postoperative pulmonary complications after cardiac surgery. Anesthesiology. (2019) 131(5):1046–62. 10.1097/ALN.0000000000002909
6.
Papathanasiou M Mincu R Lortz J Horacek M Koch A Pizanis N et al Prolonged mechanical ventilation after left ventricular assist device implantation: risk factors and clinical implications. ESC Heart Fail. (2019) 6(3):545–51. 10.1002/ehf2.12428
7.
Michaud L Dureau P Kerleroux B Charfeddine A Regan M Constantin JM et al Development and validation of a predictive score for prolonged mechanical ventilation after cardiac surgery. J Cardiothorac Vasc Anesth. (2022) 36(3):825–32. 10.1053/j.jvca.2021.07.016
8.
McCarthy C Fletcher N . Early extubation in enhanced recovery from cardiac surgery. Crit Care Clin. (2020) 36(4):663–74. 10.1016/j.ccc.2020.06.005
9.
Hessels L Coulson TG Seevanayagam S Young P Pilcher D Marhoon N et al Development and validation of a score to identify cardiac surgery patients at high risk of prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. (2019) 33(10):2709–16. 10.1053/j.jvca.2019.03.009
10.
Shao D Straub J Matrka L . Obesity as a predictor of prolonged mechanical ventilation. Otolaryngol Head Neck Surg. (2020) 163(4):750–4. 10.1177/0194599820923601
11.
Suarez-Pierre A Fraser CD Zhou X Crawford TC Lui C Metkus TS et al Predictors of operative mortality among cardiac surgery patients with prolonged ventilation. J Card Surg. (2019) 34(9):759–66. 10.1111/jocs.14118
12.
Sharma V Rao V Manlhiot C Boruvka A Fremes S Wąsowicz M . A derived and validated score to predict prolonged mechanical ventilation in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2017) 153(1):108–15. 10.1016/j.jtcvs.2016.08.020
13.
Daza-Arana JE Lozada-Ramos H Ávila-Hernández DF Ordoñez-Mora LT Sánchez DP . Prolonged mechanical ventilation following coronary artery bypass graft in Santiago de Cali, Colombia. Vasc Health Risk Manag. (2022) 18:767–81. 10.2147/VHRM.S367108
14.
Hsu H Lai HC Liu TJ . Factors causing prolonged mechanical ventilation and peri-operative morbidity after robot-assisted coronary artery bypass graft surgery. Heart Vessels. (2019) 34(1):44–51. 10.1007/s00380-018-1221-6
15.
Xiao Y Xu J Zhao C Pan G . Risk factors of prolonged mechanical ventilation in patients undergoing redo valve surgery. Heart Surg Forum. (2022) 25(5):E683–8. 10.1532/hsf.4913
16.
Luo Q Su Z Jia Y Liu Y Wang H Zhang L et al Risk factors for prolonged mechanical ventilation after total cavopulmonary connection surgery: 8 years of experience at Fuwai hospital. J Cardiothorac Vasc Anesth. (2020) 34(4):940–8. 10.1053/j.jvca.2019.10.043
17.
Fernandez-Zamora MD Gordillo-Brenes A Banderas-Bravo E Arboleda-Sánchez JA Hinojosa-Pérez R Aguilar-Alonso E et al Prolonged mechanical ventilation as a predictor of mortality after cardiac surgery. Respir Care. (2018) 63(5):550–7. 10.4187/respcare.04915
18.
Chen P Chen M Chen L Ding R Chen Z Wang L . Risk factors for severe acute kidney injury post complication after total arch replacement combined with frozen elephant trunk, in acute type A aortic dissection. Cardiovasc Diagn Ther. (2022) 12(6):880–91. 10.21037/cdt-22-313
19.
Gilbert RF Cichowitz C Bibangambah P Kim JH Hemphill LC Yang IT et al Lung function and atherosclerosis: a cross-sectional study of multimorbidity in rural Uganda. BMC Pulm Med. (2022) 22(1):12. 10.1186/s12890-021-01792-0
20.
Lei G Wang G Liu Q Zhou H Fang Z Zhang C et al Single-stage hybrid aortic arch repair is associated with a lower incidence of postoperative acute kidney injury than conventional aortic surgery. J Cardiothorac Vasc Anesth. (2019) 33(12):3294–300. 10.1053/j.jvca.2019.05.024
21.
Gumus F Polat A Yektas A Totoz T Bagci M Erentug V et al Prolonged mechanical ventilation after CABG: risk factor analysis. J Cardiothorac Vasc Anesth. (2015) 29(1):52–8. 10.1053/j.jvca.2014.09.002
22.
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100(4S):S1–276. 10.1016/j.kint.2021.05.021
23.
Downey LA Andrews J Hedlin H Kamra K McKenzie ED Hanley FL et al Fibrinogen concentrate as an alternative to cryoprecipitate in a postcardiopulmonary transfusion algorithm in infants undergoing cardiac surgery: a prospective randomized controlled trial. Anesth Analg. (2019) 130(3):740–51. 10.1213/ANE.0000000000004384
24.
Hinton JV Xing Z Fletcher CM Perry LA Karamesinis A Shi J et al Cryoprecipitate transfusion after cardiac surgery. Heart Lung Circ. (2022) 32(3):414–23. 10.1016/j.hlc.2022.11.007
25.
Sankar A Rotstein AJ Teja B Carrier FM Belley-Côté EP Bolliger D et al Prolonged mechanical ventilation after cardiac surgery: substudy of the transfusion requirements in cardiac surgery III trial. Can J Anesth. (2022) 69(12):1493–506. 10.1007/s12630-022-02319-9
26.
Voelker MT Spieth P . Blood transfusion associated lung injury. J Thorac Dis. (2019) 11(8):3609–15. 10.21037/jtd.2019.06.61
27.
Yang H Kong L Lan W Yuan C Huang Q Tang Y . Risk factors and clinical prediction models for prolonged mechanical ventilation after heart valve surgery. BMC Cardiovasc Disord. (2024) 24(1):250. 10.1186/s12872-024-03923-x
28.
Wu Q Lin Q Xie L Qiu Z Chen L . High summation of preoperative and postoperative interleukin-6 levels predicts prolonged mechanical ventilation in patients with acute DeBakey type I aortic dissection: a single center retrospective study. Heliyon. (2023) 9(4):e15465. 10.1016/j.heliyon.2023.e15465
Summary
Keywords
prolonged mechanical ventilation, cardiac surgery, predicting model, multiple centers, retrospective study
Citation
Liu Q, Chen P, Wang W, Zhou Y, Xu Y, Cao X, Fan R, Chen W, Huang F and Chen X (2025) A novel scoring model for predicting prolonged mechanical ventilation in cardiac surgery patients: development and validation. Front. Cardiovasc. Med. 12:1573874. doi: 10.3389/fcvm.2025.1573874
Received
10 February 2025
Accepted
11 March 2025
Published
26 March 2025
Volume
12 - 2025
Edited by
Hongtao Tie, First Affiliated Hospital of Chongqing Medical University, China
Reviewed by
Lijun Wang, Second Hospital of Nanchang, China
Yuxiang Luo, Erasmus Medical Center, Netherlands
Updates
Copyright
© 2025 Liu, Chen, Wang, Zhou, Xu, Cao, Fan, Chen, Huang and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xin Chen stevecx@njmu.edu.cn Fuhua Huang huangfuhua@sina.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.