- 1Graduate School, Clinical School of Thoracic, Tianjin Medical University, Tianjin, China
- 2Thoracic Clinical College, Tianjin Medical University, Tianjin, China
- 3Department of Cardiology, Tianjin Chest Hospital, Tianjin, China
- 4Cardiovascular Institute, Tianjin Chest Hospital, Tianjin, China
- 5Chest Hospital, Tianjin University, Tianjin, China
Background: The CLIMA study [Relationship between Optical Coherence Tomography (OCT) Coronary Plaque Morphology and Clinical Outcome; NCT02883088] introduced the concept of high-risk plaque (HRP) and demonstrated that HRP was associated with a high risk of major coronary events. HRP is defined by four simultaneous characteristics: minimum lumen area (MLA) <3.5 mm2, fibrous cap thickness (FCT) <75 μm, lipid arc circumferential extension >180°, and macrophage infiltration. Early prediction of HRP formation is critical for preventing and treating acute coronary syndrome (ACS), but no studies have been conducted on this topic.
Purpose: To identify the risk factors associated with OCT HRP in ACS and develop a risk prediction model for HRPs in ACS.
Methods: A prospective observational study was conducted on patients with ACS between September 2019 and August 2022. A total of 169 patients were divided into two groups: OCT HRP (n = 55) and OCT non-HRP (n = 114) groups. Clinical data, laboratory results, and OCT characteristics of the patients were collected. Least absolute shrinkage and selection operator (LASSO) regression was used to screen variables, while multivariate logistic regression was used to create a risk prediction model. A nomogram was created, and the receiver operating characteristic curve was used to assess the model's discrimination, as well as the bootstrap method to internally validate it.
Results: The most commonly observed HRP characteristic was lipid plague >180° (147 patients), followed by MLA < 3.5 mm2 (141 patients), macrophages (127 patients), and FCT < 75 μm (64 patients). The LASSO regression model was used to screen variables and develop an HRP risk factor model. The nomogram includes five predictors: age, BMI ≥ 25 kg/m2, triglycerides, low-density lipoprotein cholesterol, and Log N-terminal brain natriuretic peptide precursor. The model is highly differentiated (area under the curve 0.780, 95% confidence interval 0.705–855) and calibrated. The calibration curve and decision curve analysis demonstrated the model's clinical usefulness.
Conclusion: A simple and practical nomogram for predicting HRPs accurately in patients with ACS was developed and validated, and is expected to help clinicians diagnose and prevent plaque stability.
Introduction
Cardiovascular disease is the leading cause of death worldwide. As the unstable and progressive stage of coronary heart disease, acute coronary syndrome (ACS) is characterized by three serious and potentially fatal clinical manifestations: ST-segment elevation myocardial infarction (STEMI), non-STEMI, and unstable angina pectoris (1, 2).
In recent years, consensus has emerged that coronary atherosclerotic plaques with a propensity for thrombosis and a higher likelihood of rapid progression are commonly known as vulnerable or high-risk plaques (HRPs) (3, 4). The CLIMA study [Relationship between Optical Coherence Tomography (OCT) Coronary Plaque Morphology and Clinical Outcome; NCT02883088] introduced the concept of HRP and found that HRPs are associated with a higher risk of major coronary events (5). OCT, a high-resolution intravascular imaging technique, allows for precise identification of coronary plaque characteristics. HRPs are defined by four simultaneous characteristics: minimum lumen area (MLA) <3.5 mm2, fibrous cap thickness (FCT) <75 μm, lipid arc circumferential extension >180°, and macrophage infiltration (5). Previous research has found that HRPs are associated with an increased risk of cardiovascular events (6–8). Wang Ying et al. identified 274 patients with acute myocardial infarction using OCT-defined HRP plaques and followed them up for 2.2 years, finding that patients with HRP were 2.05 times more likely to have major adverse cardiovascular events than those without HRPs (7). Early prediction of HRPs formation and appropriate intervention are critical for the prevention and treatment of ACS, but no studies have been conducted on this topic.
Therefore, by analysing clinical data in conjunction with blood coherence indicators of circulation, relevant risk factors were identified, and a rapid early prediction model for HRPs was developed, providing novel insights into the prevention and treatment of ACS diseases.
Methods
Study population
This was a prospective observational study of ACS patients who underwent coronary angiography with OCT guidance between September 2019 and August 2022 at the Coronary Care Unit of Tianjin Chest Hospital, Tianjin, China. Patients aged 18 years or older with ACS who underwent coronary angiography (CAG) and pre-procedure OCT examination of the culprit lesion were enrolled (Figure 1). ACS patients were eligible if they had (1) angiographic evidence of ≥50% stenosis in ≥1 coronary vessel; (2) ischemic chest discomfort that increased or occurred at rest, and/or (3) electrocardiography (ECG) or cardiac biomarker criteria consistent with ACS. Participants with a history of chronic renal failure [glomerular filtration rate (eGFR) <60 ml/min/1.73 m2], sepsis, severe chronic liver disease, prior coronary stenting, or coronary artery bypass grafting were excluded from the study. The patients underwent a detailed history, full clinical examination, 12-lead ECG, echocardiography, and laboratory investigations such as the complete blood count, liver and kidney function, cardiac enzymes (troponin and creatine kinase isoenzyme MB), blood glucose, and serum lipid levels at the time of admission. All of the blood samples were assessed in the Department of Laboratory Medicine, Tianjin Chest Hospital. The concentrations of lipoprotein markers, such as low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TG), were determined using electro-chemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN). Other laboratory parameters were measured using standard test protocols.
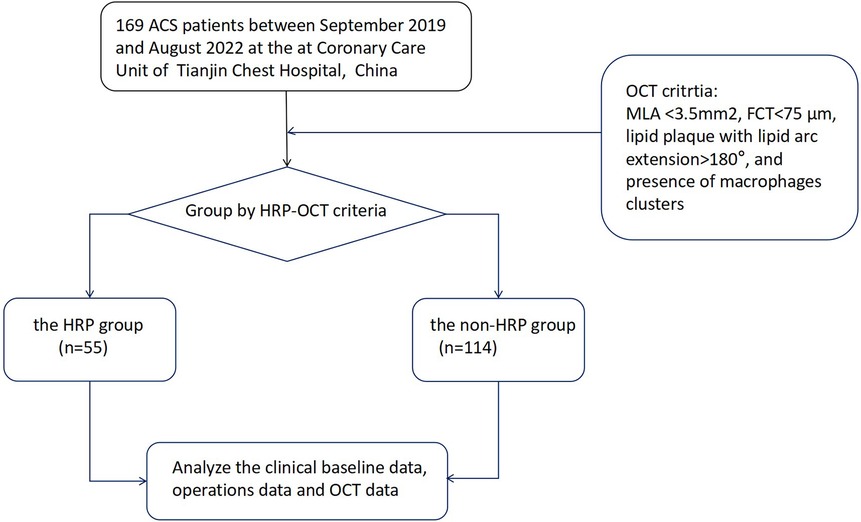
Figure 1. A flow diagram of the data selection process. ACS, acute coronary syndrome; OCT, optical coherence tomography; HRP, OCT-detected high-risk plaques.
The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Chest Hospital (No. 2018KY-010-01). Informed consent was provided by all participants at our institution.
Angiographic procedure
Coronary angiography was performed using a transradial or transfemoral approach with a 6F or &7F sheath. Intravascular infusion of 30–50 IU/kg unfractionated heparin was administered prior to CAG. The culprit's vessel was identified through an analysis of angiography, echocardiography, and electrocardiographic changes (ischemic ST-segment changes, T-wave inversions, and/or pathological Q wave).
OCT imaging acquisition and definition
OCT images of the culprit coronary were obtained using the frequency-domain OPTIS imaging system (Abbott, St. Paul, Minnesota, USA). Following intracoronary administration of 0.2 mg nitroglycerin, an OCT imaging catheter was advanced distally to the lesion, and automated pullback began at a rate of 20 mm/s after manually flushing the guiding catheter with contrast media to create a nearly blood-free environment. The total length of the OCT pullback was 75 mm. Thrombus aspiration and/or gentle pre-dilation with a small balloon were performed for acute total occluded or severe stenosis lesions as needed to ensure that the OCT catheter passed through smoothly. The acquisition and analysis of OCT images has been described in detail (9–11). All OCT images were analyzed and scrutinized on an OCT workstation by two independent physicians who were blinded to the angiographic and clinical data. Inter-observer and intra-observer agreement for OCT-based HRP assessment was conducted, confirming excellent consistency and ensuring the reliability of image interpretation. The definition of image characteristics in OCT was primarily based on previous consensus (12). Culprit plaques were defined as fibrous plaques [homogeneous, highly backscattering region (Figure 2a)], or lipid-rich plaques [low signal region with a diffuse border (Figure 2b)]. Calcification within plaques was defined as the presence of well-defined, heterogeneous regions with low backscattering (Figure 2c). Thin-cap fibroatheroma (TCFA) is defined as a lipid-rich plaque with a maximum lipid arc greater than two quadrants and a thinnest FCT of <65 μm (Figure 2d). Plaque rupture was identified by its discontinuous fibrous cap and clear cavity formation (Figure 2e). Plaque erosion was defined as the presence of an attached thrombus over an intact and visible plaque (Figure 2f). The calcified nodule was identified as a nodular calcification that protruded into the lumen, resulting in thrombus formation (Figure 2g). A thrombus was defined as an irregular mass that adhered to the luminal surface, which could be white, red, or mixed (Figures 2h,i). Macrophage infiltration was identified as signal-rich, highly reflective, punctate strip regions with backward shadowing, typically found at the boundary between the fibrous cap and inner lipid core (Figure 2j). Cholesterol crystals were identified as linear, highly backscattering structures within plaques (Figure 2k). Micro-vessels were identified as black holes within a plaque that appeared in at least three consecutive frames (Figure 2l).
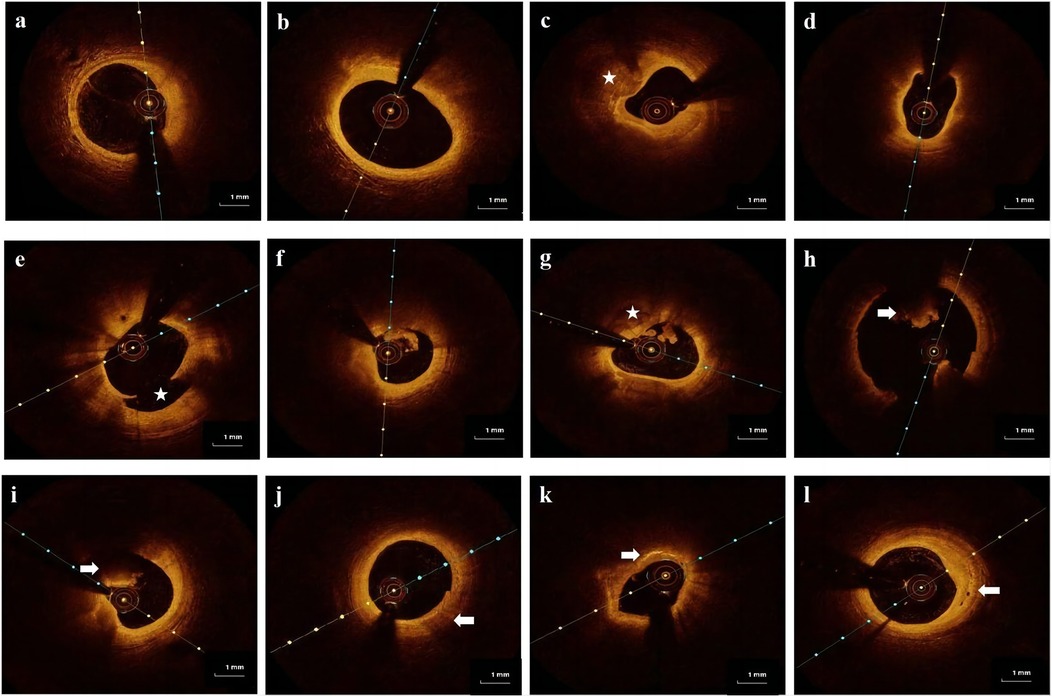
Figure 2. Representative cross-sectional optical coherence tomography images of the culprit's vessels: (a) fibrous plaque was identified as a homogeneous region with high backscatter. (b) Lipid-rich plaque was identified as a low signal region with a diffused border. (c) Calcification was detected as sharply defined, low backscattering heterogeneous regions (star). (d) Thin-cap fibroatheroma (TCFA) is a lipid-rich plaque with a fibrous cap thinner than 65 μm. (e) Plaque rupture is defined as a disruption of the fibrous cap with obvious cavity formation (star). (f) Plaque erosion is defined as the presence of an attached thrombus overlying an intact, visible plaque. (g) Calcified nodule identified as a nodular calcification protruding into the lumen and forming a thrombus (star). (h,i) Thrombus is defined as an irregular mass that adheres to the luminal surface, which can be a white thrombus, red thrombus (arrow), or mixed thrombus (arrow). (j) Macrophages are defined as signal-rich, distinct, or confluent punctuate regions with variable backward shadows (arrow). (k) Cholesterol crystals are linear, highly backscattering structures within plaques (arrow). (l) Micro-vessels are defined as black holes within a plaque that appear in at least three consecutive frames (arrow).
Quantitative OCT measurements contained the following information: the lipid arc was measured at 1-mm intervals throughout the lesion, and the largest arc was recorded; FCT was measured three times at the thinnest part of fibrous cap, and the average value was recorded; and MLA was assessed along the length of the target lesion. The calcification score is calculated by measuring the maximum Angle, thickness, and length of the calcification and scoring it.
Previous research identified four criteria for HRP: MLA < 3.5 mm2, FCT < 75 μm, lipid plaque with arc extension >180°, and macrophage clusters. OCT-defined HRP is defined as the simultaneous presence of all four criteria (5).
Statistical analysis
Continuous data is presented as mean ± standard deviation or median (interquartile ranges). Student's t-test or non-parametric test was employed for statistical comparisons. Categorical variables were reported as numbers (percentages), and group comparisons were made using the chi-square test or Fisher's exact test. An upset plot was created to show the prevalence and intersections of HRP characteristics (13). Least absolute shrinkage and selection operator (LASSO) reduces regression coefficients of some unimportant variables to zero by including a penalty term λ in model estimation. This achieves variable screening. It reduces the impact of multicollinearity, prevents model overfitting, and improves model generalizability. Using LASSO regression, according to ten-fold cross-validation, the candidate predictive variables were tested. The variables identified by LASSO that were clinically significant were incorporated into a multivariate logistic stepwise regression analysis to create a nomogram to predict the risk of HRP in patients with ACS. Draw the receiver operating characteristic (ROC) curve, calculate the area under the curve (AUC) as the evaluation metric of discriminant ability, and use the bootstrap method to validate the model internally. The calibration curve was used to assess the calibration force of the final model, and a decision curve analysis (DCA) was performed to ensure the model's clinical feasibility.
Analyses were performed using IBM SPSS Statistics version 25.0 (IBM SPSS Statistics, IBM Corporation, Armonk, New York) and R 4.3.1 (http://www.rproject.org/) statistical packages. A bilateral P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
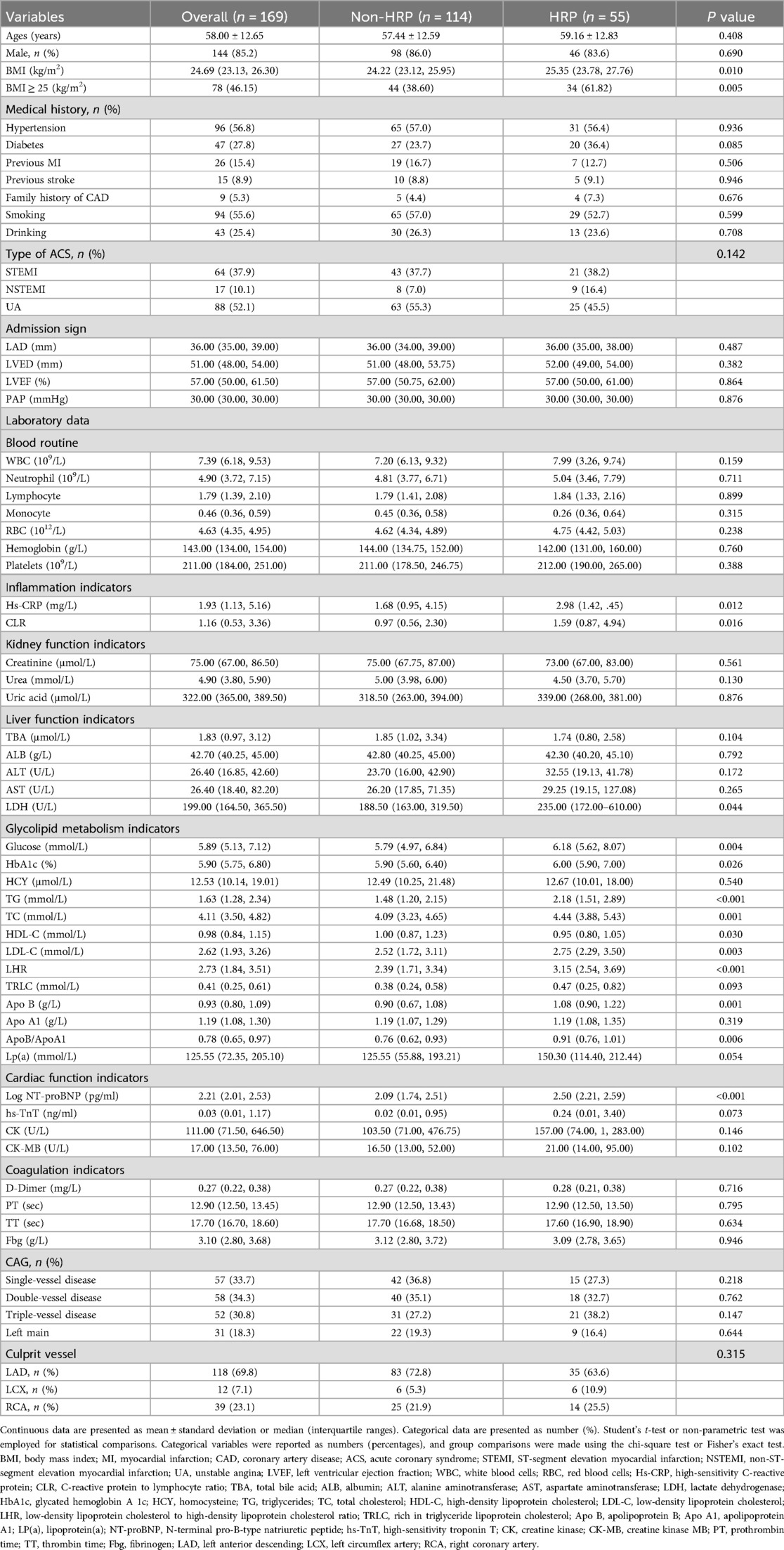
Table 1 shows the baseline and angiographic characteristics. Among the 169 enrolled patients, the average age was 58 ± 12.65 years. 85.2% were males, and 52.1% had unstable angina pectoris. Patients with HRP had a higher Body Mass Index (BMI) (25.35 [23.78, 27.76] vs. 24.22 [23.12, 25.95], P = 0.010) than the non-HRP group. Furthermore, there was no obvious difference in the distribution of culprit vessels. The angiographic findings for the culprit vessels were also presented.
There were significant variations in TG (P < 0.001), HDL (P = 0.030), LDL-C (P = 0.003), apolipoprotein b (P = 0.001), high-sensitivity C-reactive protein (Hs-CRP) (P = 0.012), and Log N-terminal brain natriuretic peptide precursor (Log NT-proBNP) (P < 0.001) between the two groups. There were no significant differences between the two groups in other circulating blood-related parameters (all P ≥ 0.05).
OCT findings and HRP characteristics
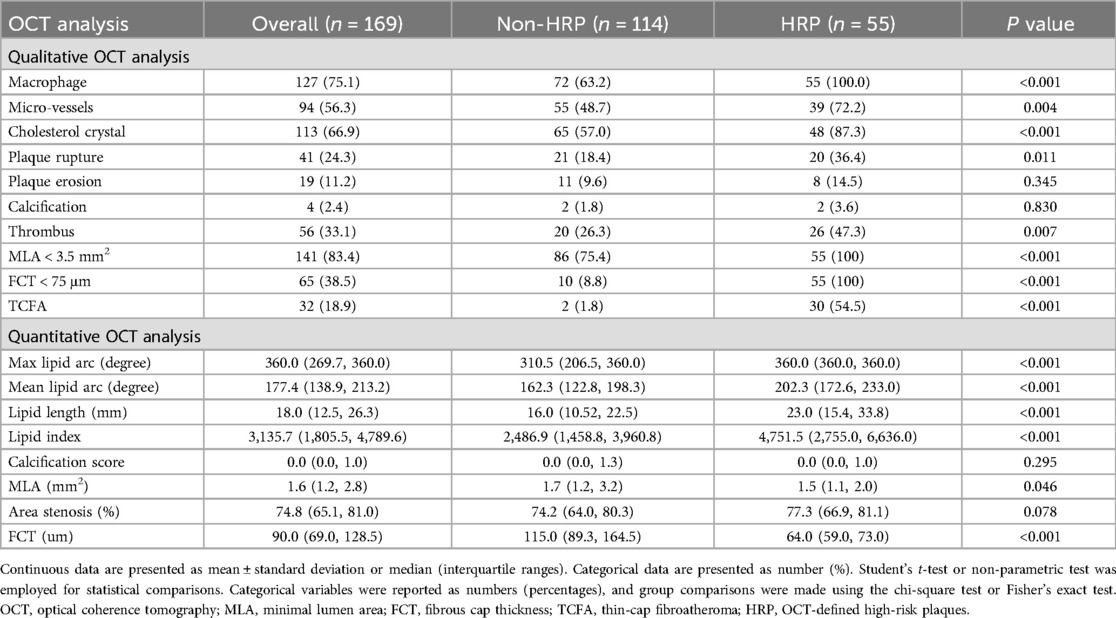
Table 2 displays the OCT characteristics. In addition to the OCT-defined HRP characteristics, patients with HRP had significantly higher rates of plaque rupture (36.4 vs. 18.4, P = 0.011), micro-vessels (72.2 vs. 48.7, P = 0.004), thrombus (47.3 vs. 26.3, P = 0.007), cholesterol crystal (87.3 vs. 57.0, P < 0.001), and TCFA (54.5 vs. 1.8, P < .001) compared to the non-HRP group. However, the difference in the prevalence of calcification between the HRP and non-HRP groups was not statistically significant.
Figure 3 depicts the prevalence of individual and HRP characteristics and their intersections. The most common HRP characteristic was MLA < 3.5 mm2 (83.4% of enrolled patients), followed by macrophage infiltration (75.1%), FCT < 75 μm (38.5%), and TCFA (18.9%).
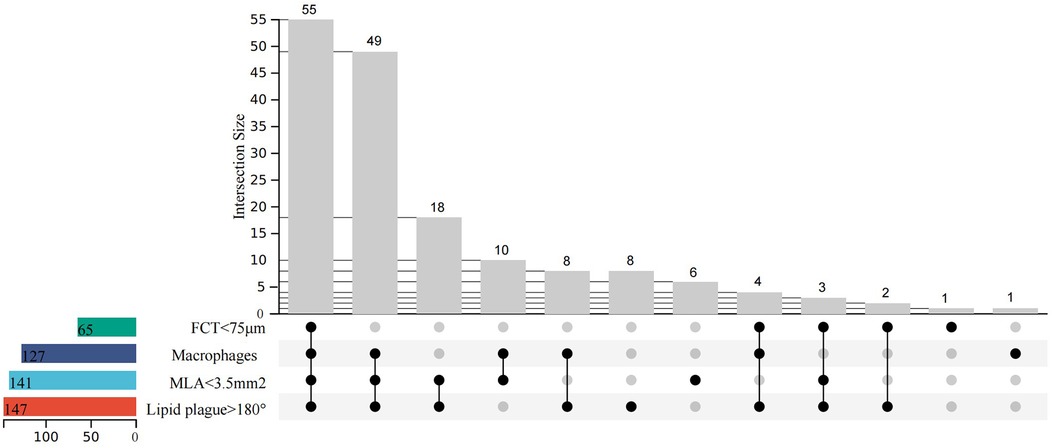
Figure 3. Upset plot of high-risk characteristics and combinations (minimal lumen area <3.5 mm2, fibrous cap thickness <75 μm, lipid arc circumferential extension >180°, and presence of macrophages). N = 169.
Identification of predictive factors and construction of nomograms
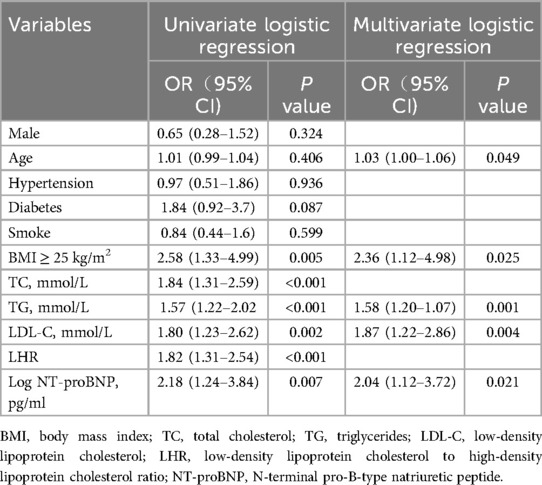
LASSO regression was used (Figures 4A,B) to identify five variables that affect plaque stability: BMI ≥ 25 kg/m2, LHR, TC, TG, and Log NT-proBNP. Combined with the variables screened by LASSO regression and clinically meaningful variables, the above 11 variables were included in a multivariate logistic stepwise regression model to create the HRP risk factor model (Table 3). The final model includes five variables: BMI ≥ 25 kg/m2, age, TG, LDL-C, and Log NT-proBNP. The nomogram is constructed (Figure 5). Each predictor is represented by a scale on the left, with the corresponding points derived from the regression coefficients. The total points, calculated by summing the individual points for each predictor, are mapped to the predicted probability of the event occurring on the rightmost scale. The nomogram uses odds ratios for each variable (shown as line markers), indicating how changes in each predictor affect the odds of the outcome. The colored density plot beneath the scales represents the distribution of data for each variable. Logistic regression was used to derive the model, with statistical significance indicated for variables with P-values less than 0.05. Confidence intervals for the predicted probabilities are also shown for clarity. A sum score was calculated as the total of the scores for related predictors with the risk of HRP on the basal axis. For example, in a patient with BMI < 25 kg/m2, age 74, TG 1.83 mmol/L, LDL-C 4.08 mmol/L and Log NT-proBNP 3.19, the total points was 150, and the 0dds was 1.84.
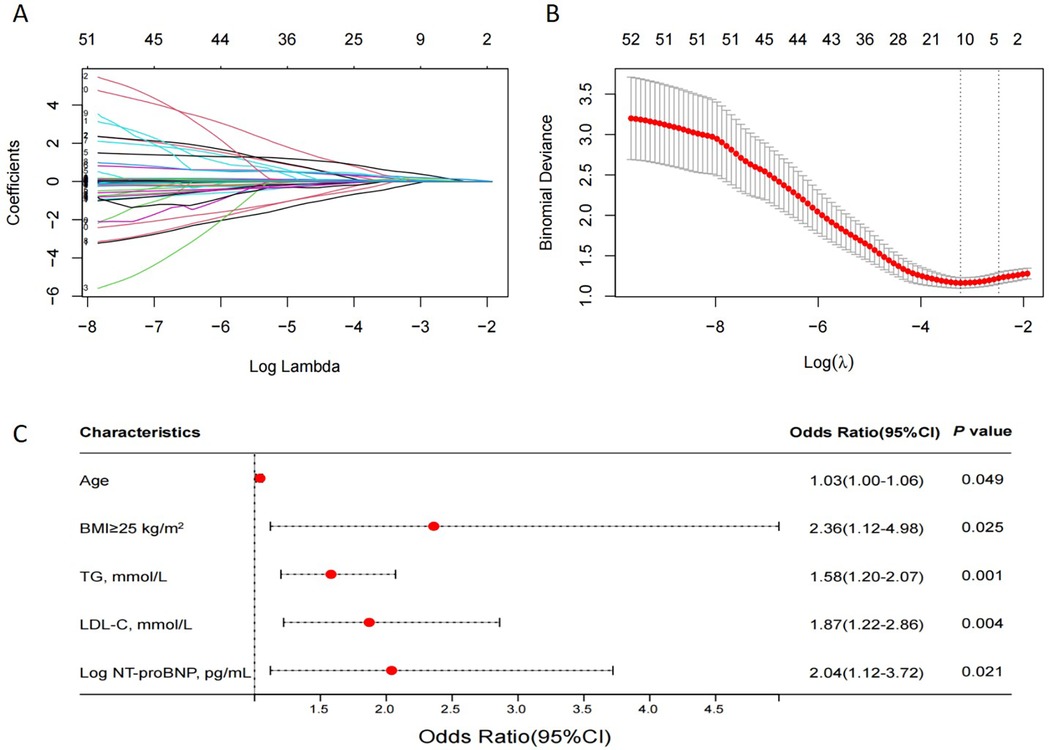
Figure 4. The LASSO regression model tested the factors affecting HRPs. (A) LASSO regression coefficient path diagram. (B) Cross-validation curve of LASSO regression, filtering out five predictor variables with non-zero coefficients using optimal lambda. (C) Multivariate logistic analysis forest plot of predictors. LASSO, least absolute shrinkage and selection operator; HRP, OCT-defined high-risk plaques; N = 169.
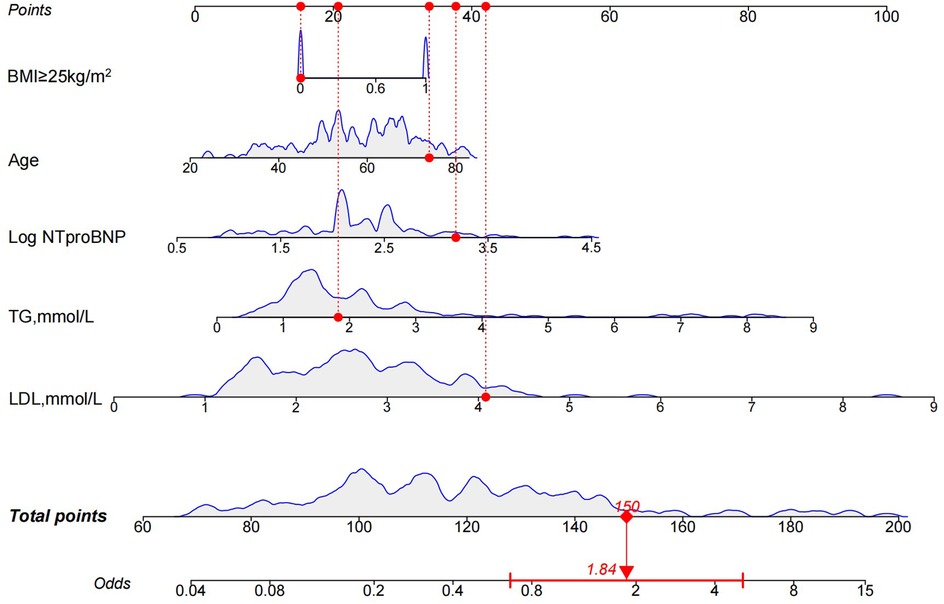
Figure 5. Nomogram for the HRP risk factor model. The nomogram is constructed based on age, BMI ≥ 25 kg/m2, TG, LDL-C, and Log NT-proBNP to assign the probability of high-risk plaques. HRP, high-risk plaque; BMI, body mass index; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; Log NT-proBNP, Log N-terminal brain natriuretic peptide precursor.
The results of the multivariate logistic analysis revealed that the variables listed above are independent risk factors for high risk of HRP (Figure 4C).
The discriminatory power of the nomogram was assessed using the area under the ROC curve. The ROC curve analysis revealed that the model had a high predictive capability for HRP, with an AUC of 0.780 [95% confidence interval (CI): 0.705–0.855] (Figure 6A). Internal validation is carried out using bootstrap resampling with a sample size of 1,000, and the calibration curve is plotted. The calibration curves of the model show that the predicted probabilities closely match the actual probabilities (Figure 6B). The DCA was used to assess clinical practicability. The DCA of the model is higher than the reference line, indicating that its clinical utility is generally superior (Figure 6C).
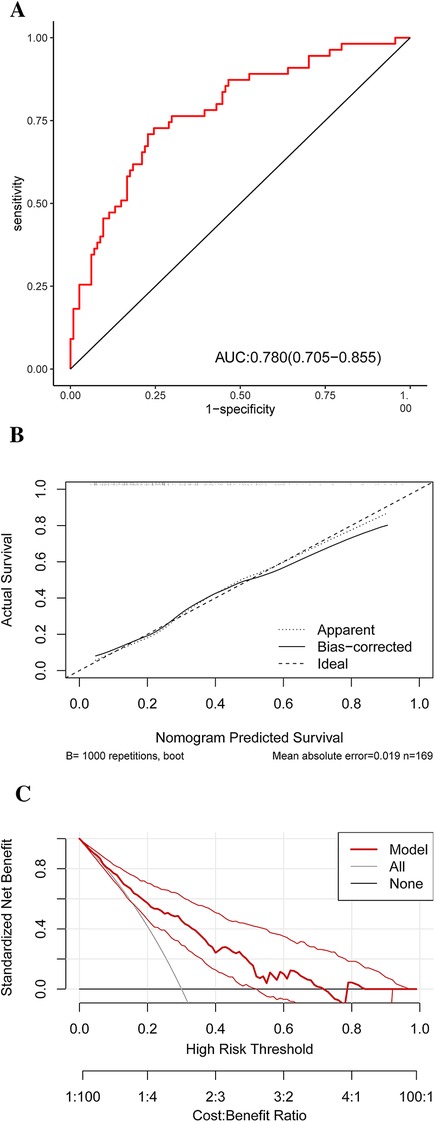
Figure 6. ROC (A) result for the diagnostic performances of the HRP risk factor model. A calibration curve (B) of the nomogram for probability prediction. The x-axis represents the nomogram-predicted probability, while the y-axis represents the actual probability. DCA (C) demonstrates the net benefit of the HRP risk factor model compared with the strategies of “treating all” or “treating none” for different decision thresholds. ROC, receiver operating characteristic curve analysis; HRP, high-risk plaque; DCA, decision curve analysis.
Discussion
This study successfully developed and validated a nomogram for accurately predicting HRPs in patients with ACS. The nomogram included age, BMI ≥ 25 kg/m2, TG, LDL-C, and Log NT-proBNP levels. It demonstrated good discrimination, calibration, and clinical validity, making it a useful and clinically relevant tool for identifying HRPs defined by OCT and ACS.
ACS is typically caused by the rupture of vulnerable plaques, leading to thrombosis (14–16). Previous research has shown that vulnerable plaques have the following characteristics: TCFA, rich in macrophages, and a large central necrotic core (17, 18). The CLIMA study introduced the concept of HRP and found that HRPs are associated with a higher risk of major coronary events (5). Previous research has also found that HRPs defined by OCT are associated with an increased risk of cardiovascular events (6–8). Recent large-scale studies further support these findings: Matsumura et al. showed that OCT-defined TCFA and a minimal lumen area <3.5 mm2 are independent predictors of major adverse cardiovascular events (MACE) in patients with acute myocardial infarction (8), while van Veelen et al. reported that HRPs in nonculprit lesions, even when fractional flow reserve (FFR) negative, are linked to a higher incidence of MACE over 2 years (19). These accumulating data reinforce the clinical importance of early HRP detection and timely intervention. However, current imaging modalities, including OCT and intravascular ultrasonography, are invasive, require specialized equipment, and have limitations in the early detection of plaque stability. In low-middle-income countries and areas, OCT is not feasible due to economic and workforce constraints. Circulating biomarkers can be used as additional tools for predicting the risk of vulnerable plaques, and developing a risk prediction model based on circulating biomarkers has greater utility and potential for widespread use in clinical practice. Our proposed biomarker-based model is intended not to replace OCT but to provide a practical, non-invasive tool for early risk stratification and guiding the need for further invasive assessment.
Due to the crucial role of lipid accumulation and inflammation in atherosclerotic plaque formation, previous research on markers of vulnerable plaque circulation has primarily focused on these processes (20). Mechanistically, elevated levels of specific lipids or inflammatory factors indicate their involvement in initiating harmful events that lead to plaque destabilization, increasing the risk of unstable plaque formation. Conversely, certain molecules released by unstable plaque lesions into the bloodstream are easily detectable and may serve as predictors of vulnerable plaques. Hence, the use of circulating biomarkers has a high potential for detecting plaque vulnerability in patients. Nonetheless, no single reliable biological marker has demonstrated adequate sensitivity and specificity. This emphasizes the importance of identifying a panel of circulating markers for predicting the risk of AS plaque vulnerability, as well as developing a risk factor analysis model with greater utility in clinical practice. The current study analyzed relevant circulating blood indicators in patients, revealing statistically significant differences (P < 0.05) in BMI, Hs-CRP, Hemoglobin A1c, TG, TC, HDL-C, LDL-C, ApoB/ApoA1 ratio, and NT-proBNP levels between high-risk and non-HRP groups. These commonly used laboratory indicators may act as risk factors for HRPs.
In this study, general clinical data and laboratory examination indicators of patients were incorporated into the HRP risk prediction system. The aforementioned variables were used as influencing factors to identify factors affecting plaque stability using the LASSO regression model, which resulted in the creation of a model. Variables included “age”, “BMI ≥ 25 kg/m2”, “TG”, “LDL-C,” and “Log NT-proBNP”. Given the large number of indicators used in this study, there is a high risk of encountering issues such as variable collinearity when selecting variables using the least squares method. This can lead to important variables being overlooked, resulting in inaccuracies in the prediction model. The LASSO regression model addresses these concerns by efficiently selecting variables by compressing regression coefficients to zero (21). In the model development, we used the LASSO–Cox method to estimate the relationship between predictors and HRPs. LASSO regularization is a method for managing overfitting and variable selection that has been widely used in a variety of machine learning algorithms (22). When the LASSO method is applied to the Cox model, the estimation variance is reduced, and a subset of predictors is chosen, resulting in an interpretable Cox model (23). To ensure that the model was accurate, we used a nomogram to simplify the parameters in the model presentation. Furthermore, the nomogram model created using identified HRP risk factors is a simple and intuitive tool. Clinicians can calculate the cumulative scores of risk factors using the nomogram, allowing for quick and easy risk stratification for plaque vulnerability and HRP possibility. Thus, the risk factors evaluated in this study can be easily obtained through patient history collection and routine laboratory examination, allowing for early detection of HRPs. The risk prevention model's AUC of 0.780 (95% CI: 0.705–0.855) developed in this study indicates that assessing HRPs has a high predictive capability.
The results from screening variables in the LASSO regression model found that age, BMI ≥ 25 kg/m2, TG, LDL-C, and Log NT-proBNP levels are reliable indicators for predicting HRPs, implying that HRPs can be clinically identified using these indicators alone. Age is well-known as a traditional risk factor for cardiovascular events. Many studies have considered age to be an independent predictor of ACS (24), and in studies focusing on other risk factors, age is typically adjusted (25, 26). Furthermore, BMI ≥ 25 kg/m2 was associated with HRP. BMI > 25 kg/m2 was recently found to be associated with a significantly increased long-term risk of cardiovascular disease morbidity and mortality (27), and another study linked this epidemiologic evidence to HRP formation (28). Elevated triglyceride levels and triglyceride-rich lipoproteins (TRLs) have been increasingly recognized as important contributors to atherosclerotic cardiovascular disease beyond LDL-C (29). Recent evidence suggests that TRLs may promote the formation of lipid-rich necrotic cores, inflammation, and endothelial dysfunction—features commonly associated with high-risk plaques (HRPs) (30). For instance, the accumulation of TRLs in the arterial wall has been shown to induce macrophage activation and foam cell formation, both of which are implicated in plaque vulnerability. These pathophysiological mechanisms highlight the potential role of elevated TG in the development of OCT-defined HRPs and support its inclusion as a relevant biomarker in risk stratification models. LDL-C has long been recognized as an important risk factor for ASCVD, and numerous studies have consistently shown that LDL-C lowering interventions can effectively reduce plaque vulnerability, regardless of the imaging modality used to assess plaque characteristics (31–33). A recent study on intracoronary imaging using OCT found that high levels of small dense LDL-C are linked to the presence of vulnerable plaques (34). NT-proBNP concentration is regarded as a marker of cardiac function in heart disease, and myocardial ischemia can cause a reversible increase in regional wall stress, potentially leading to increased natriuretic peptide release (35). A previous study found significant associations between NT-proBNP and coronary atherosclerotic plaque parameters, which were consistent with the high-sensitivity cardiac Troponin T results (36).
Although HbA1c and diabetes are recognized cardiovascular risk factors, neither was retained in the final model. HbA1c was excluded during the LASSO regression due to its limited independent predictive value after penalization. Diabetes was initially selected but subsequently removed in the multivariate logistic regression, likely due to collinearity with other glycemic markers and limited statistical significance (P = 0.08 in univariate analysis). In contrast, variables such as age, overweight BMI, TG, LDL-C, and NT-proBNP showed stronger and more consistent associations with high-risk plaques and were prioritized in the final model. This reflects the relative predictive contributions of different variables within our cohort. Importantly, we acknowledge that the exclusion of glycemic variables may also reflect the limited sample size of our study, which could reduce the statistical power to detect their independent associations. Future large-scale, multicenter investigations are warranted to validate these findings and comprehensively clarify the prognostic value of glycemic factors in cardiovascular risk prediction.
The predictive value of circulating biomarkers for high-risk coronary plaque features has been increasingly supported by evidence from non-invasive imaging studies. Russo et al. demonstrated that low HDL-C and elevated levels of leptin and interleukin-6 were independently associated with high-risk coronary anatomy as assessed by coronary CT angiography (CCTA) in patients with stable chest pain (37). Similarly, Nidorf et al. reported that high-sensitivity C-reactive protein (hsCRP) levels were significantly associated with vulnerable plaque features, including low attenuation and positive remodeling, identified by CCTA (38). These findings underscore the role of systemic inflammation and lipid metabolism in plaque vulnerability and support the integration of serological biomarkers into non-invasive risk prediction models. Our current study aligns with this direction, aiming to develop a practical, blood-based tool for identifying high-risk plaques defined by OCT, especially in settings where intracoronary imaging may not be routinely feasible.
It is important to note that the model's strong predictive performance does not imply that other indicators have a weak predictive effect on HRPs. Previous research has shown that individual indicators are closely related to the onset and progression of HRPs, as exemplified by the European Society of Cardiology/European Atherosclerosis Blood Lipid Management Guidelines, which emphasize the causal relationship between LDL-C and all apolipoprotein B lipoproteins in arteriosclerotic cardiovascular disease. It is suggested that the role of apolipoprotein B and lipoprotein in cardiovascular risk stratification should be investigated further (25). More research is needed to understand the underlying mechanisms of plaque instability, and progression and to develop more reliable biomarkers for the early detection of HRPs in ACS.
Therefore, we believe that our model will help patients better understand the disease and doctors make clinical decisions. Particularly for patients with a high risk of ACS, doctors can use this model to determine whether patients would benefit from treatment.
There are some limitations to our study. First, the single-center design and limited sample size reduced the statistical power, restricted the robustness of subgroup analyses, and lacked external validation, which may affect the generalizability of the results. Second, for safety considerations regarding the use of iodinated contrast, patients with an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 were excluded; Additionally, in a subset of patients (n = 26) with severely stenotic or occluded lesions, low-pressure (4–6 atm) balloon pre-dilatation was performed to facilitate OCT catheter passage. These procedures are consistent with standard clinical practice and their impact on plaque parameters (such as MLA and FCT) is minimal or negligible. All images were interpreted by blinded observers, and sensitivity analyses were conducted to minimize potential bias.
Despite these limitations, OCT technology enabled accurate classification of high-risk plaque populations, and rigorous statistical methods were applied for risk prediction, thereby providing the proposed prediction model with high accuracy and reliability.
Conclusion
In this study, an OCT examination was used to accurately identify HRPs associated with risk factors in the ACS cohort. The nomogram risk prediction model developed in response to these findings has high predictive efficacy and clinical applicability, making it critical for identifying, preventing, and treating HRP vulnerability. However, due to the single-center observational cohort design and limited sample size, future research will focus on validating the nomogram model's clinical utility in multi-center studies with larger samples.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy concerns and institutional regulations. Additionally, the data are part of an ongoing research project and have not been fully released. However, they are available from the corresponding author upon reasonable request and with appropriate institutional approval.
Ethics statement
The studies involving humans were approved by the Bioethics Committee of Tianjin Chest Hospital (No. 2018KY-010-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
M-NB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. Ji-XW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. X-WL: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – review & editing. Jin-XW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing. Y-HW: Data curation, Investigation, Methodology, Resources, Software, Writing – review & editing. YL: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. JG: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key disciplines of the Tianjin Health Science and Technology Project (No. TJWJ2022XK032), the Key Projects of the Tianjin Natural Science Foundation (No. 22JCZDJC00130), the Key Project of the Scientific and Technological Support Plan of Tianjin in 2020 (No. 20YFZCSY00820).
Acknowledgments
We express our gratitude to every participant for donating their data and test results, and we are grateful for all of the staff's outstanding work during the specimen collection and clinical data acquisition. We also thank the Chest Hospital Biospecimen Bank for collecting, processing, and storing the blood samples for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
3. Tomaniak M, Katagiri Y, Modolo R, de Silva R, Khamis RY, Bourantas CV, et al. Vulnerable plaques and patients: state-of-the-art. Eur Heart J. (2020) 41(31):2997–3004. doi: 10.1093/eurheartj/ehaa227
4. Kurihara O, Russo M, Kim HO, Araki M, Shinohara H, Lee H, et al. Clinical significance of healed plaque detected by optical coherence tomography: a 2-year follow-up study. J Thromb Thrombolysis. (2020) 50(4):895–902. doi: 10.1007/s11239-020-02076-w
5. Prati F, Romagnoli E, Gatto L, La Manna A, Burzotta F, Ozaki Y, et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J. (2020) 41(3):383–91. doi: 10.1093/eurheartj/ehz520
6. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. doi: 10.1038/s41586-021-03392-8
7. Wang Y, Zhao X, Zhou P, Liu C, Liao Z, Yan S, et al. High-risk culprit plaque predicts cardiovascular outcomes independently of plaque rupture in ST-segment elevation myocardial infarction: insight from optical coherence tomography. Angiology. (2022) 73(10):946–55. doi: 10.1177/00033197221087778
8. Jiang S, Fang C, Xu X, Xing L, Sun S, Peng C, et al. Identification of high-risk coronary lesions by 3-vessel optical coherence tomography. J Am Coll Cardiol. (2023) 81(13):1217–30. doi: 10.1016/j.jacc.2023.01.030
9. Otake H, Kubo T, Shinke T, Hibi K, Tanaka S, Ishida M, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention in patients with acute coronary syndrome: study protocol for a randomized controlled trial. J Cardiol. (2020) 76(3):317–21. doi: 10.1016/j.jjcc.2020.03.010
10. Johnson TW, Räber L, di Mario C, Bourantas C, Jia H, Mattesini A, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. (2019) 40(31):2566–84. doi: 10.4244/EIJY19M06_02
11. Raber L, Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. (2018) 39(35):3281–300. doi: 10.1093/eurheartj/ehy285
12. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. (2012) 59(12):1058–72. doi: 10.1016/j.jacc.2011.09.079
13. Conway JR, Lex A, Gehlenborg N. Upsetr: an R package for the visualization of intersecting sets and their properties. Bioinformatics. (2017) 33(18):2938–40. doi: 10.1093/bioinformatics/btx364
14. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. (2017) 376(21):2053–64. doi: 10.1056/NEJMra1606915
15. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. (2017) 389(10065):197–210. doi: 10.1016/S0140-6736(16)30677-8
16. Dweck MR, Maurovich-Horvat P, Leiner T, Cosyns B, Fayad ZA, Gijsen FJH, et al. Contemporary rationale for non-invasive imaging of adverse coronary plaque features to identify the vulnerable patient: a position paper from the European society of cardiology working group on atherosclerosis and vascular biology and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2020) 21(11):1177–83. doi: 10.1093/ehjci/jeaa201
17. Beg F, Rehman H, Al-Mallah MH. The vulnerable plaque: recent advances in computed tomography imaging to identify the vulnerable patient. Curr Atheroscler Rep. (2020) 22(10):58. doi: 10.1007/s11883-020-00879-z
18. Kowara M, Cudnoch-Jedrzejewska A. Different approaches in therapy aiming to stabilize an unstable atherosclerotic plaque. Int J Mol Sci. (2021) 22(9):4354. doi: 10.3390/ijms22094354
19. Volleberg R, Mol J-Q, Belkacemi A, Hermanides RS, Meuwissen M, Protopopov AV, et al. High-risk plaques in non-culprit lesions and clinical outcome after NSTEMI vs. STEMI. Eur Heart J Cardiovasc Imaging. (2025) 26(2):197–206. doi: 10.1093/ehjci/jeae289
20. Kumric M, Borovac JA, Martinovic D, Ticinovic Kurir T, Bozic J. Circulating biomarkers reflecting destabilization mechanisms of coronary artery plaques: are we looking for the impossible? Biomolecules. (2021) 11(6):881. doi: 10.3390/biom11060881
21. Heinze G, Wallisch C, Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom J. (2018) 60(3):431–49. doi: 10.1002/bimj.201700067
22. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. (1996) 58:267–88. doi: 10.1111/j.2517-6161.1996.tb02080.x
23. Tibshirani R. The lasso method for variable selection in the cox model. Stat Med. (1997) 16:385–95. doi: 10.1002/(sici)1097-0258(19970228)16:4%3C385::aid-sim380%3E3.0.co;2-3
24. Lechner K, von Schacky C, McKenzie AL, Worm N, Nixdorff U, Kränkel N, et al. Lifestyle factors and high-risk atherosclerosis: pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol. (2020) 27(4):394–406. doi: 10.1177/2047487319869400
25. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41(1):111–88. doi: 10.1093/eurheartj/ehz455
26. Zhao Y, Guo Z, Liu Z, Yang X. Predictive value of a combination of the age, creatinine and ejection fraction (ACEF) score and fibrinogen level in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Innov Appl. (2023) 8(1):e990. doi: 10.15212/CVIA.2023.0027
27. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. (2018) 3(4):280–7. doi: 10.1001/jamacardio.2018.0022
28. Senoner T, Plank F, Langer C, Beyer C, Steinkohl F, Barbieri F, et al. Smoking and obesity predict high-risk plaque by coronary CTA in low coronary artery calcium score (CACS). J Cardiovasc Comput Tomogr. (2021) 15(6):499–505. doi: 10.1016/j.jcct.2021.04.003
29. Xu D, Xie L, Cheng C, Xue F, Sun C. Triglyceride-rich lipoproteins and cardiovascular diseases. Front Endocrinol. (2024) 15:1409653. doi: 10.3389/fendo.2024.1409653
30. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease. Circ Res. (2016) 118(4):547–63. doi: 10.1161/CIRCRESAHA.115.306249
31. Catalano O, Bendotti G, Aloi TL, Bardile AF, Memmi M, Gambelli P, et al. Evidence of carotid atherosclerosis vulnerability regression in real life from magnetic resonance imaging: results of the MAGNETIC prospective study. J Am Heart Assoc. (2023) 12(2):e026469. doi: 10.1161/JAHA.122.026469
32. Dong N, Xie Z, Dai J, Wang W, Sun R, Zhan Y, et al. Statin-induced improvements in vulnerable plaques are attenuated in poorly controlled diabetic patients with coronary atherosclerosis disease: a serial optical coherence tomography analysis. Acta Diabetol. (2016) 53(6):999–1008. doi: 10.1007/s00592-016-0902-9
33. Hattori K, Ozaki Y, Ismail TF, Okumura M, Naruse H, Kan S, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging. (2012) 5(2):169–77. doi: 10.1016/j.jcmg.2011.11.012
34. Sekimoto T, Koba S, Mori H, Arai T, Matsukawa N, Sakai R, et al. Impact of small dense low-density lipoprotein cholesterol on cholesterol crystals in patients with acute coronary syndrome: an optical coherence tomography study. J Clin Lipidol. (2022) 16(4):438–46. doi: 10.1016/j.jacl.2022.04.008
35. Goetze JP, Christoffersen C, Perko M, Arendrup H, Rehfeld JF, Rastrup J, et al. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. (2003) 17(9):1105–7. doi: 10.1096/fj.02-0796fje
36. Altintas S, Cardinaels EPM, Versteylen MO, Joosen IA, Seifert M, Wildberger JE, et al. Unstable coronary plaque characteristics are associated with high-sensitivity cardiac troponin T and N-terminal pro-brain natriuretic peptide. J Cardiovasc Comput Tomogr. (2016) 10(1):82–8. doi: 10.1016/j.jcct.2015.10.001
37. Caselli C, De Graaf MA, Lorenzoni V, Rovai D, Marinelli M, Del Ry S, et al. HDL cholesterol, leptin and interleukin-6 predict high risk coronary anatomy assessed by CT angiography in patients with stable chest pain. Atherosclerosis. (2015) 241(1):55–61. doi: 10.1016/j.atherosclerosis.2015.04.811
Keywords: acute coronary syndrome, nomogram, high-risk plaque, optical coherence tomography, LASSO regression algorithm
Citation: Bai M-N, Wang J-X, Li X-W, Wang J-X, Wang Y-H, Liu Y and Gao J (2025) Nomogram for predicting the severity of high-risk plaques in acute coronary syndrome. Front. Cardiovasc. Med. 12:1618038. doi: 10.3389/fcvm.2025.1618038
Received: 25 April 2025; Accepted: 13 June 2025;
Published: 25 June 2025.
Edited by:
Vasile Bogdan Halatiu, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Takanori Sato, Eastern Chiba Medical Center, JapanYinsu Zhu, Nanjing Medical University, China
Copyright: © 2025 Bai, Wang, Li, Wang, Wang, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Liu, bGl1eWluMjA4OEAxNjMuY29t; Jing Gao, Z2FvamluZzIwODlAMTYzLmNvbQ==; Z2FvamluZzIwODhAMTYzLmNvbQ==;, Z2FvamluZ0B0bXUuZWR1LmNu
 Miao-Na Bai1,2,3
Miao-Na Bai1,2,3 Yu-Hang Wang
Yu-Hang Wang Yin Liu
Yin Liu Jing Gao
Jing Gao