Abstract
Objective:
To simulate plasma pharmacokinetics (PK) parameters of combining potassium citrate granules and potassium chloride extended-release tablets using real-world data, guiding optimal dosing and timing for cardiovascular emergency patients.
Methods:
A retrospective study analyzed PK characteristics in patients receiving both drugs at Fuwai Yunnan Hospital from January 1 to March 31, 2024, using non-compartmental analysis (NCA).
Results:
Among 330 patients, the Tmax values for 1-dose, 2-dose, and 3-dose administrations were 4.65 h, 6.00 h, and 6.11 h. The half-life (t1/2) was 1.33 h, 13.53 h, and 6.11 h, respectively. Each dose elevated plasma potassium by 0.31 ± 0.05 mmol/L. The limited areas under the plasma curve (AUClimit) were 30.37 mmol-h/L, 106.89 mmol-h/L, 141.74 mmol-h/L. Co-administered dose and AUClimit were linearly related (R2 = 0.9554).
Conclusion:
PK data fit a 1-compartment model, supporting safe, rational potassium supplementation in cardiovascular emergencies.
1 Introduction
Potassium dysregulation or abnormal potassium metabolism is common in patients with cardiovascular disease in the emergency setting due to the widespread use of diuretics (1). Persistent abnormalities in plasma potassium may lead to severe or life-threatening arrhythmias and death. It has been shown that the ideal plasma potassium level for patients with cardiovascular disease is 4.0–5.0 mmol/L (health: 3.5–5.0 mmol/L), with an optimal potassium value of 4.2 mmol/L (2). In clinical practice, a dual-strategy approach is sometimes adopted for rapid and sustained potassium replenishment. This involves co-administering a fast-absorbing preparation (e.g., potassium citrate granules)[1.46 g, Weight Equivalent (WEq):0.5589 g]) to promptly address the potassium deficit, alongside a slow-absorbing extended-release preparation (e.g., potassium chloride extended-release tablets)(1.0 g, WEq:0.5245 g) to maintain stable plasma levels and minimize gastrointestinal irritation (3). However, direct evidence supporting the pharmacokinetic (PK) profile, safety, and dosing rationale of this specific combination regimen in cardiovascular emergency patients is lacking. Previous PK studies have primarily focused on single formulations in healthy volunteers or used urinary excretion endpoints per FDA guidance for bioequivalence assessment (4, 5). Therefore, this study aims to characterize the plasma PK of this combined regimen in a real-world cohort of cardiovascular emergency patients, providing evidence to guide its rational use.
The pharmacokinetics of potassium in humans is Unusual (3). Plasma potassium levels are regulated by homeostasis in vivo and their absorption was hard assessed by conventional plasma pharmacokinetics (PK), the US FDA recommends the use of urinary PK parameters (e.g., maximal excretion rate, Rmax, and cumulative excretion over 24 h, Ae0-24 h) as the criteria for bioequivalence (BE) evaluation. Only three studies of plasma potassium have been reported. (1) In healthy people, after oral administration of 64 mEq of potassium chloride solution, the time to peak (Tmax) was 1.0 h and the increase in peak concentration (Cmax) was 1.7 meq/L (4). (2) the Tmax was 1.0 h and the increase in Cmax was 0.8 meq/L after potassium supplementation of 50 mEq (5). (3) In intensive care unit (ICU) patients, plasma potassium could be increased by 0.245 mmol/L by per 20 mmol administered via the intravenous and enteral routes (6). For clinical practice, the plasma potassium concentration is more responsive to whether potassium supplementation achieves the target effect than the excretion in the urine. Fluctuations in plasma potassium have not been reported in disease states, especially in patients in cardiovascular emergencies. Non-atrial model (NCA) is a conventional method to analyze drug pharmacokinetics, providing key parameters such as Tmax and Cmax. In order to further explore the rationality and safety of potassium supplementation with multi-group combination drugs, this study attempted to use NCA to analyze the plasma potassium pharmacokinetic parameters of patients with cardiovascular emergencies.
2 Manuscript formatting
2.1 Materials and methods
2.1.1 Patients' characteristics
The retrospective study analyzed PK characteristics in patients receiving both drugs. From January 1, 2024, to March 31, 2024, the patients who were prescribed both potassium chloride sustained-release tablets and potassium citrate granules were enrolled in Fuwai Yunnan Hospital, Kunming, China from Hospital Information System. The inclusion criteria, according to the enrollment criteria: (1) adult patients (>18 years old); (2) emergency patients with cardiovascular diseases (e.g., angina, arrhythmia, and heart failure); (3) patients who were co-administered potassium chloride sustained-release tablets and potassium citrate granules. The exclusion criteria were patients: (1) on diuretics in the emergency setting; (2) with communication problems or other problems such as visual or hearing impairment. The potassium citrate granules was purchased from Changchun Beihua Pharmaceutical Co., Ltd. (Changchun, Jilin, China)(0.5 g, Approval Number: H22024249). The potassium chloride extended-release tablets was purchased from Shanghai haihong Group Chaohu C-Dragon Pharmaceutical Co., Ltd. (Shanghai, China)(2 g, Approval Number: H34021120). Physicians prescribed three dosing regimens: 1-dose (potassium chloride extended-release tablets 0.5245 g + potassium citrate granules 0.5589 g), 2-dose (1.049 g + 1.1178 g), and 3-dose (1.5735 g + 1.6767 g). Each dose was administered at 0.5-hour intervals, with a maximum of three doses. The study protocol was approved by the Ethics Committee of Fuwai Yunnan Cardiovascular Hospital, Kunming, China (No.2024-046-01). The human samples used in this study were acquired from a by- product of routine care.
2.1.2 Statistical analysis
Plasma potassium parameters were calculated using the WinNonlin 7.0 Software (Pharsight Corporation, Mountain View, CA, USA) via non-compartmental modeling. Linear regression methods were employed to analyze the relationship between the limited areas under the plasma curve (AUClimit) and co-administered dose. The experimental data were expressed as mean ± standard deviation. Statistical analyses were using the GraphPad Prism 6 software (San Diego, CA, USA).
2.2 Result
2.2.1 Patients' characteristics
According to the criteria, 330 emergency patients who were prescribed both potassium chloride sustained-release tablets and potassium citrate granules were enrolled. The characteristics of the 330 patients are presented in Table 1. Due to subtle physiological adjustments in muscle mass, hormones, and kidney function, women's serum potassium levels are on average approximately 0.1–0.2 mmol/L lower than men's. Table 1 presents gender-specific subgroup data, though no statistically significant differences were observed in pre-co-administered values. Post-co-administered values is statistically significant differences(P < 0.05).
Table 1
| Characteristic | Male group (n = 182) |
Female group (n = 148) |
|
|---|---|---|---|
| Age, year | 56.72 ± 21.80 | 60.36 ± 15.46 | |
| Pre-co-administered values | plasma potassium concentration, mmol/L | 3.57 ± 0.31 | 3.55 ± 0.25 |
| Post-co-administered values | plasma potassium concentration, mmol/L | 3.95 ± 0.42 | 4.20 ± 0.45 |
| potassium chloride sustained-release tablets and potassium citrate granules | 1-dose administration group | 54 (29.67%) | 38 (25.68%) |
| 2-dose administration group | 92 (50.55%) | 37 (25%) | |
| 3-dose administration group | 36 (19.78%) | 73 (49%) | |
Clinical characteristics of 330 emergency patients before and after co-administered potassium chloride sustained-release tablets and potassium citrate granules.
Plasma potassium was elevated in emergency patients using co-administration potassium supplements (potassium chloride extended-release tablets and potassium citrate granules). The mean concentration–time curves of potassium concentration in the plasma of emergency patients were determined, as shown in Figure 1A. The patient's plasma potassium concentration before potassium supplementation (1-dose administration group, 2-dose administration group, and 3-dose administration group) was 3.70 ± 0.24 mmol/L, 3.54 ± 0.29 mmol/L, and 3.36 ± 0.26 mmol/L. The 12-hour time point showed the largest difference in the increase in plasma potassium concentration, which is 0.005 ± 0.56 mmol/L, 0.45 ± 0.46 mmol/L, and 0.86 ± 0.51 mmol/L, respectively (Figure 1B) (P < 0.0001).
Figure 1
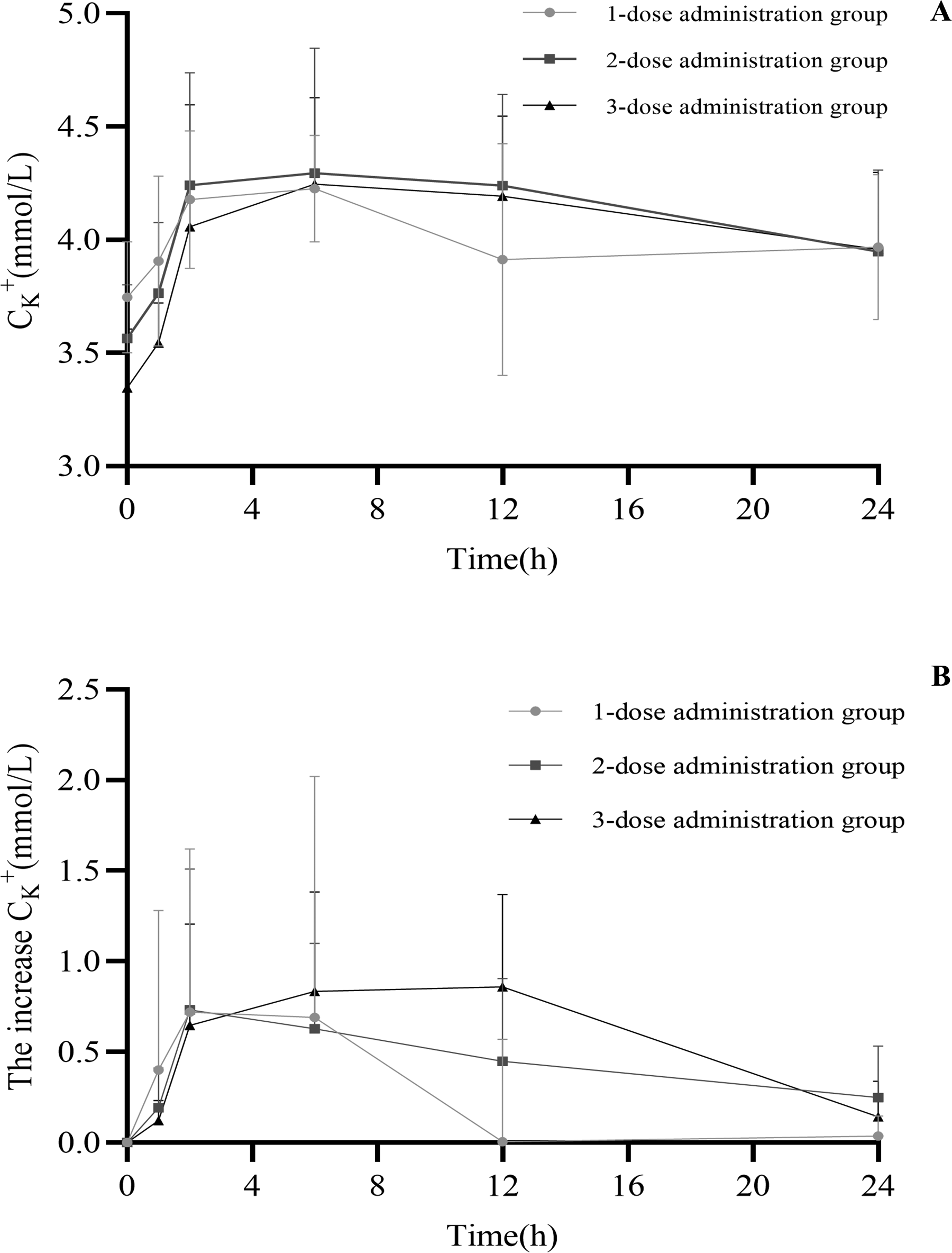
Mean concentration–time curves of the plasma potassium concentration (A) and the increase in plasma potassium concentration (B) in the emergency patients after co-administration.
2.2.2 Non-compartmental model
The pharmacokinetic parameters for plasma potassium concentrations are presented in Table 2. The following parameters were calculated by WinNonlin Software. Each dose elevated plasma potassium by 0.31 ± 0.05 mmol/L. The AUClimit were: 30.37 mmol·h/L, 106.89 mmol·h/L, and 141.74 mmol·h/L. Linear regression of co-administered dose and AUC were showed as follows:
Table 2
| Group |
C
0
(mmol/L) |
T
max
(h) |
C
max
(mmol/L) |
ΔC
(mmol/L) |
K
a
(per hour) |
t
1/2
(h) |
AUClimit (mmol·h/L) |
|---|---|---|---|---|---|---|---|
| 1-dose administration group (extended-release tablets 0.5245 g and granules 0.5589 g) |
3.75 ± 0.24 | 4.65 | 4.11 ± 0.35 | 0.36 | 0.36 | 1.33 | 30.37 |
| 2-dose administration group (extended-release tablets 1.049 g and granules 1.1178 g) |
3.56 ± 0.29 | 6.00 | 4.14 ± 0.49 | 0.58 | 0.37 | 13.53 | 106.89 |
| 3-dose administration group (extended-release tablets 1.5735 g and granules 1.6767 g) |
3.34 ± 0.26 | 6.11 | 4.15 ± 0.55 | 0.81 | 0.32 | 6.55 | 141.74 |
| Literature 1 (granules 64 mEq) |
4.6 ± 0.2 | 1.0 | 5.8 ± 0.5 | 1.7 | - | - | - |
| Literature 2 (granules 50 mEq) |
4.1 ± 0.1 | 1.0 | 4.9 ± 0.2 | 0.8 | - | - | - |
PK parameters of the plasma potassium concentration in the emergency patients after co-administration (mean ± SD).
K a, absorption rate constant; t1/2, half-life.
AUC=55.69 × times of dose-18.37 (R2 = 0.9554).
where times of dose including 1, 2, or 3 represent 1-dose administration group(8.7583 mol), 2-dose administration group(17.5166 mol), or 3-dose administration group(26.2749 mol).
2.3 Discussion
2.3.1 The need for dietary potassium in patients with cardiovascular disease
Due to the widespread use of diuretics, hypokalemia often occurs in acute patients with cardiovascular disease. We suggest that patients with cardiovascular disease who use diuretics, especially potassium-excreting diuretics, could use plus supplements on a daily basis. Studies have shown that a low-potassium diet activates the potassium-sensing signaling pathway in the renal distal convoluted tubule, increasing the activity of the sodium-chloride cotransporter and sodium retention, thereby elevating blood pressure and enhancing salt sensitivity (7, 8). In contrast, increasing potassium intake could lower blood pressure by enhancing AngII-stimulated aldosterone secretion (without affecting cardiovascular responses), primarily through a diuretic mechanism (9). Hypertensive patients exhibit greater sensitivity to increased potassium intake compared to normotensive individuals, with particularly pronounced benefits for those on a high-salt diet (10). A high-potassium diet effectively reduces blood pressure in individuals with prehypertension and hypertensionand reverses endothelial dysfunction induced by high sodium intake, mitigating vascular damage (11). Additionally, dietary potassium not only improves blood pressure (12, 13). but also enhances glucose tolerance and insulin resistance (14). These studies confirm the multifaceted cardiovascular and metabolic benefits of dietary potassium. This might reduce the risk of developing serious cardiovascular disease.
2.3.2 Plasma PK of co-administration in emegency patients
The conventional opinion is that fluctuations in plasma potassium caused by oral potassium-supplementing preparations are difficult to detect, and that intravenous administration without an absorptive phase is easier for raising plasma potassium. Oral extended-release preparations could delay potassium release in order to minimize gastrointestinal irritation (3). In this study, we found a linear relationship between the administered dose and plasma AUC in patients with cardiovascular emergencies. This provides an evidence-based basis for potassium supplementation in these patients. To rapidly replenish a patient's plasma potassium, physicians use the strategy of fast-absorbing granules preparations (potassium citrate granules) and slow-absorbing extended-release preparations. This combination achieves rapid, prolonged potassium supplementation.
The Tmax of the combination was prolonged to 4–6 h compared to the 1 h Tmax of granules KCl in the literature (4, 5). Patient's plasma potassium rose rapidly over a 2 h period and Ka averaged 0.35. Drug absorption was at 2–6 h for 3-dose administration due to the extended-release dosage form. 3-dose administration approximated an equilibrium of absorption and elimination at 6–12 h, with Ka = Ke = 0.10. It can be hypothesized that the Ka of KCl extended-release tablets alone might be 0.10 with a t1/2 of 6.93 h. Based on this, if the goal is to maintain target plasma potassium levels, the recommended dosing interval should approximate the apparent half-life of the extended-release component multiplied by the number of doses administered. The number of administrations can be calculated based on the patient's pre-administration plasma potassium level. Therefore, (1) 1-dose administration: the need to maintain attained plasma potassium concentrations suggests that a second dose be given after 6.93 h. (2) 2-dose administration: it is recommended to be re-dosed after 13.86 h to maintain the standardized. (3) 3-dose administration: it is recommended to be re-dosed after 20.79 h to maintain the standardized. Further studies would be needed to confirm the Ka results for KCl extended-release tablets.
Among our 330 patients, 8 cases (2.4%) developed transient serum potassium elevation >5.0 mmol/L after administration, with the highest value being 5.3 mmol/L. All were asymptomatic, received no specific treatment, and returned to normal levels during subsequent monitoring. This relatively low incidence of hyperkalemia may be attributed to the following factors: (1) The extended-release formulation avoided rapid spikes in serum potassium; (2) The combination of liquid and solid formulations provided a relatively stable serum potassium profile; (3) Close monitoring of emergency patients.
There are some limitations of this study. Patients using diuretics were not selected during the study, reducing the interference of the elimination phase. Further studies are needed to investigate the effect of diuretics on potassium-supplementing medications in real-world.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fuwai Yunnan Cardiovascular Hospital, Kunming, China (No.2024-046-01). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XL: Writing – original draft, Data curation, Formal analysis. MQ: Data curation, Writing – original draft. HL: Writing – original draft, Data curation. TL: Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This research was funded by Kunming Medical University Alliance Special Project (No.202501AY070001-233), Yunnan Provincial Department of Education Scientific Research Fund (No.2024J0310), Talent cultivation Program of Fuwai Yunnan Hospital, Chinese Academy of Medical Sciences (No.2024RCTJ-QN-005, No.2025YFKT-PY-07, No.2025YFKT-ZL-10), the Yunnan Provincial Clinical Medicine Research Special Program (No.202405AJ310003).
Acknowledgments
We wish to thank the colleagues from Fuwai Yunnan Hospital/Chinese Academy of Medical Sciences for their support.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Cooper LB Benson L Mentz RJ Savarese G DeVore AD Carrero JJ et al Association between potassium level and outcomes in heart failure with reduced ejection fraction: a cohort study from the Swedish heart failure registry. Eur J Heart Fail. (2020) 22(8):1390–8. 10.1002/ejhf.1757
2.
Collins AJ Pitt B Reaven N Funk S McGaughey K Wilson D et al Association of Serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. (2017) 46(3):213–21. 10.1159/000479802
3.
Le Thi TV Le Quan N Le H . Development and bioequivalence study of potassium chloride extended release tablets. Asian J Pharm Sci. (2018) 13(4):353–9. 10.1016/j.ajps.2017.05.010
4.
Hinderling PH . The pharmacokinetics of potassium in humans is unusual. J Clin Pharmacol. (2016) 56(10):1212–20. 10.1002/jcph.713
5.
Mittapalli RK Marroum P Qiu Y Apfelbaum K Xiong H . Development of in vitro-in vivo correlation for potassium chloride extended release tablet formulation using urinary pharmacokinetic data. Pharm Res. (2017) 34(7):1527–33. 10.1007/s11095-017-2179-2
6.
DeCarolis DD Kim GM Rector TS Ishani A . Comparative dose response using the intravenous versus enteral route of administration for potassium replenishment. Intensive Crit Care Nurs. (2016) 36:17–23. 10.1016/j.iccn.2015.08.005
7.
Gritter M Rotmans JI Hoorn EJ . Role of dietary K+ in natriuresis, blood pressure reduction, cardiovascular protection, and renoprotection. Hypertension. (2019) 73(1):15–23. 10.1161/HYPERTENSIONAHA.118.11209
8.
Dreier R Andersen UB Forman JL Sheykhzade M Egfjord M Jeppesen JL . Effect of increased potassium intake on adrenal cortical and cardiovascular responses to angiotensin II: a randomized crossover study. J Am Heart Assoc. (2021) 10(9):e018716. 10.1161/JAHA.120.018716
9.
He FJ MacGregor GA . Beneficial effects of potassium on human health. Physiol Plant. (2008) 133(4):725–35. 10.1111/j.1399-3054.2007.01033.x
10.
Welling PA Little R Al-Qusairi L Delpire E Ellison DH Fenton RA et al Potassium-Switch signaling pathway dictates acute blood pressure response to dietary potassium. Hypertension. (2024) 81(5):1044–54. 10.1161/HYPERTENSIONAHA.123.22546
11.
Sacks FM Svetkey LP Vollmer WM Appel LJ Bray GA Harsha D et al Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med. (2001) 344(1):3–10. 10.1056/NEJM200101043440101
12.
Aburto NJ Hanson S Gutierrez H Hooper L Elliott P Cappuccio FP . Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. Br Med J. (2013) 346:f1378. 10.1136/bmj.f1378
13.
Cook NR Obarzanek E Cutler JA Buring JE Rexrode KM Kumanyika SK et al Trials of hypertension prevention collaborative research group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the trials of hypertension prevention follow-up study. Arch Intern Med. (2009) 169(1):32–40. 10.1001/archinternmed.2008.523
14.
Smiljanec K Mbakwe A Ramos Gonzalez M Farquhar WB Lennon SL . Dietary potassium attenuates the effects of dietary sodium on vascular function in salt-resistant adults. Nutrients. (2020) 12(5):1206. 10.3390/nu12051206
Summary
Keywords
cardiovascular disease, emergency patient, non-compartmental model, plasma potassium parameters, potassium
Citation
Li X, Qu M, Li H and Li T (2026) Plasma pharmacokinetics of the combination of potassium chloride extended-release tablets and potassium citrate granules in patients with cardiovascular emergencies. Front. Cardiovasc. Med. 12:1636090. doi: 10.3389/fcvm.2025.1636090
Received
27 May 2025
Revised
29 November 2025
Accepted
15 December 2025
Published
06 January 2026
Volume
12 - 2025
Edited by
Xiaofeng Yang, Temple University, Philadelphia, United States
Reviewed by
Dotan Shaniv, Kaplan Medical Center, Israel
Chongzhuang Tang, XenoFinder Co LTD, China
Updates
Copyright
© 2026 Li, Qu, Li and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Te Li at1029@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.