Abstract
Emerging evidence from preclinical and observational studies indicates an association between gut flora dysbiosis and atrial fibrillation (AF). The gut flora constitutes a highly complex and dynamic community capable of producing diverse metabolites that influence host pathophysiology. External factors, such as diet and medications, further modulate its composition. This review synthesizes current knowledge on the relationship between gut flora and AF, emphasizing the role of microbial-derived metabolites in the underlying mechanisms of AF. We also explore the involvement of gut flora in AF-related conditions, including hypertension and diabetes. Additionally, we evaluate potential interventional strategies targeting the gut flora through dietary or pharmacological approaches for their antiarrhythmic effects. Finally, we address limitations in the current evidence and propose future research directions to clarify the causal relationship and therapeutic potential of targeting the gut flora in AF. While this review synthesizes the current evidence linking gut flora to AF, we also critically examine the limitations of the existing literature, including reliance on preclinical models, confounding factors in human studies, and inconsistencies in interventional outcomes, to provide a balanced perspective on the field's current state and future directions.
Graphical Abstract
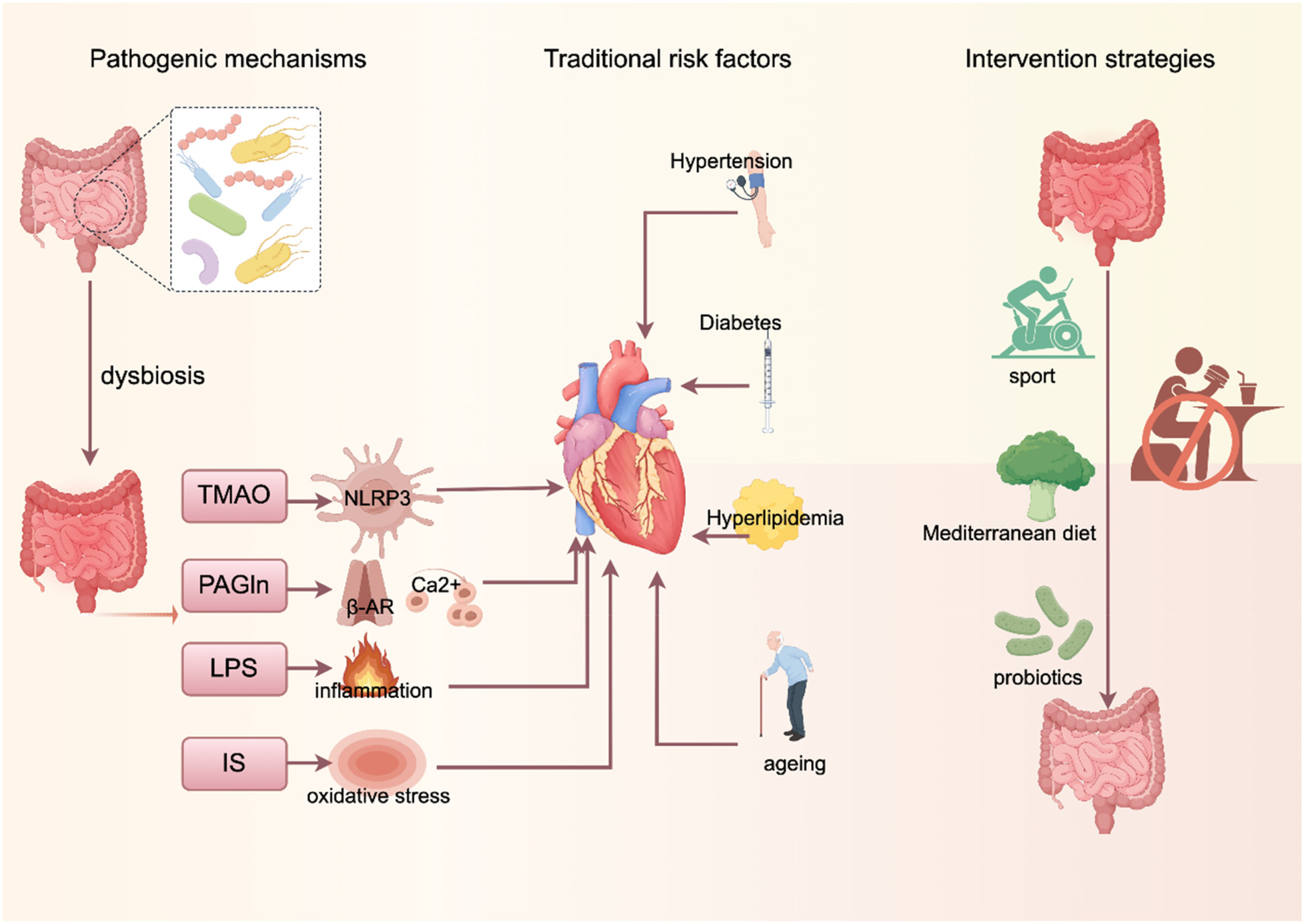
Created using Figdraw (https://www.figdraw.com/).
1 Introduction
Atrial fibrillation (AF) is an atrial disorder characterized by rapid and irregular beating (1), which results in the loss of effective atrial pumping function and imposes a significant health burden on patients and society. Although the prevention and treatment of AF and its associated risk factors have improved considerably in recent years, the incidence of AF continues to rise, affecting more than 37 million people worldwide (2).
Both clinical and preclinical studies have shown that the onset and progression of AF are significantly influenced by a variety of modifiable cardiovascular risk factors, including hypertension (3), diabetes mellitus (4) and obesity (5). It is worth noting that most of these risk factors are closely related to dietary structure and lifestyle (6). After food is digested and enters the gut, it interacts with the gut flora, a large and complex symbiotic ecosystem. In recent years, abnormal alterations in gut microbial composition and function have been found to be potentially closely associated with a variety of cardiovascular-metabolic diseases (7). Dysbiosis can affect intestinal barrier function (8), promote inflammatory responses, and participate in host physiological and pathological regulation through its metabolites. However, whether and how the gut flora and its metabolites are directly involved in the development of AF remains unclear. The aim of this paper is to review the current evidence linking gut microbial profiles to AF, with a focus on the potential mechanisms of microbiota-derived metabolites in the pathogenesis of AF and their influence on cardiovascular disease-related risk factors. We also discuss the potential value of pharmacological and dietary interventions targeting the gut flora for the treatment of AF, and finally, identify knowledge gaps in the field and suggest directions for future research.
Finally, it is crucial to note that the current evidence predominantly stems from animal and observational studies. The establishment of a definitive causal relationship and the translation of these findings into effective therapies require validation through more robust clinical trials.
2 Dysregulation of metabolites of intestinal flora on the pathogenesis of AF
2.1 Trimethylamine oxide (TMAO)
TMAO, an important metabolite of gut flora, has been shown to be strongly associated with the development of AF (9). A study on diabetes-associated AF showed that (10) diabetic cardiomyopathy (DCM) model rats not only exhibited dysbiosis of the intestinal flora and elevated levels of systemic TMAO, but also had a significantly higher rate of AF induction than control subjects. Mechanistically, current research indicates that TMAO may promote the onset and progression of AF through several potential mechanisms, including inflammatory activation, promotion of thrombosis, and pro-atrial fibrosis. Krishna M. Boini et al. demonstrated in mice that TMAO activates the NOD-like receptor protein 3 (NLRP3) inflammasome (11). This activation, in turn, impairs tight junction proteins and endothelial cell permeability, leading to a cascade of events including reactive oxygen species (ROS) activation, lysosomal rupture, and potassium ion efflux, thereby increasing AF risk (11). But addition of a specific TMAO inhibitor to drinking water significantly reduces plasma TMAO levels (10) and attenuates inflammatory cell infiltration in atrial tissue, ameliorating atrial inflammation and connexin remodelling.
TMAO can also increase the risk of thrombosis as it accelerates thrombus formation by inducing platelet aggregation, enhancing platelet reactivity, promoting tissue factor expression, and inducing endothelial cell injury (12, 13). The findings of Luo et al. that elevated plasma trimethylamine N-oxide enhances M1 macrophage infiltration in the atria, thereby inducing structural remodelling of the atria and increasing susceptibility to AF, provide further evidence for the mechanism by which TMAO induces AF (14). However, the translational relevance of these mechanistic pathways is challenged by clinical interventions targeting TMAO production. Specifically, randomized controlled trials demonstrate that supplementation with lactobacilli, a common probiotic, fails to significantly reduce circulating TMAO levels in humans (15, 16). Therefore, TMAO levels represent a promising candidate biomarker for predicting AF risk. However, its clinical utility requires further validation in diverse human cohorts, independent of renal function and other cardiovascular comorbidities (Figure 1).
Figure 1
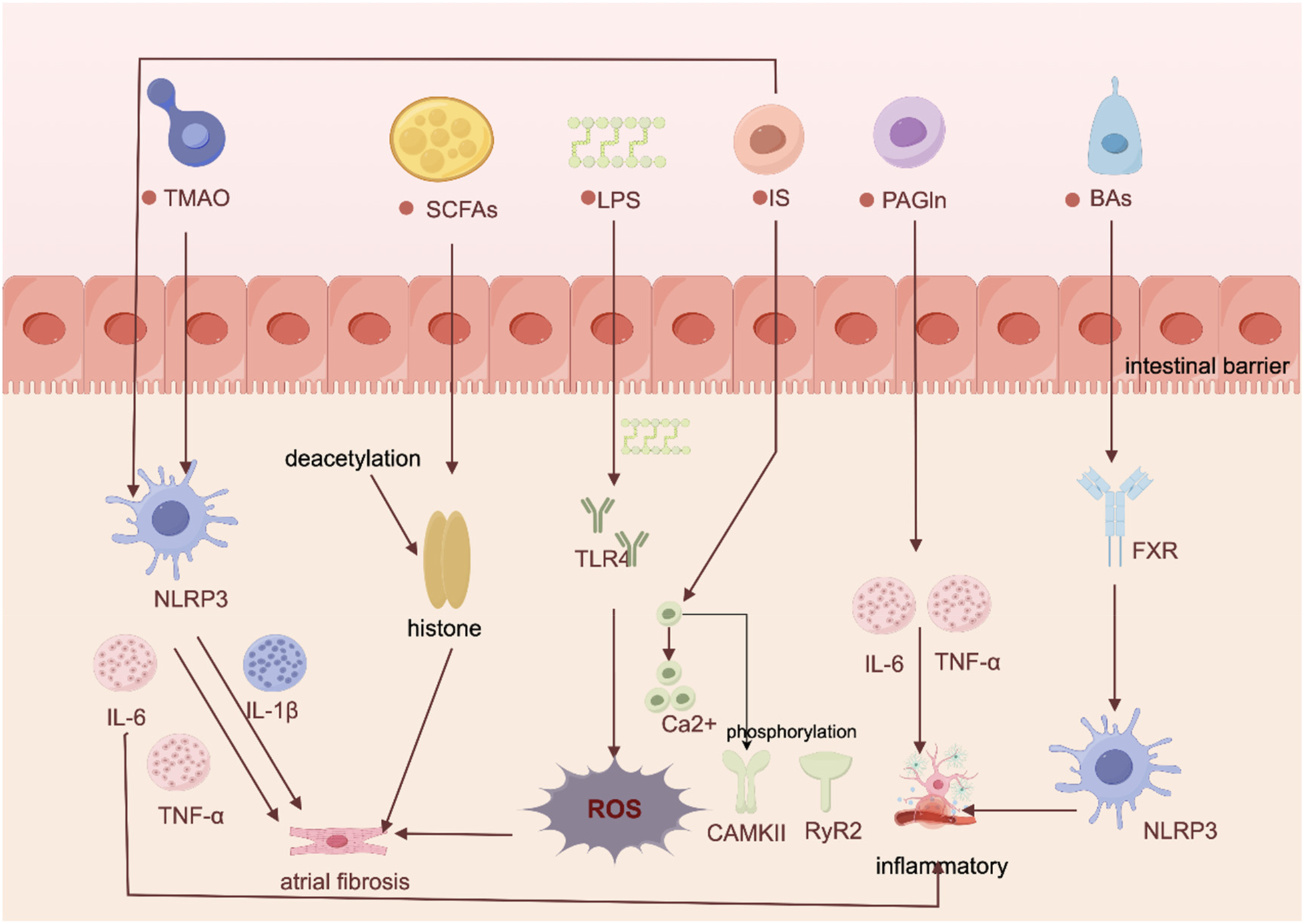
Proposed pathways and mechanisms linking gut flora-derived metabolites to AF development. Created using Figdraw (https://www.figdraw.com/).
2.2 Endotoxins (lipopolysaccharide, LPS)
LPS is a major component of the cell membrane of gram-negative bacteria, and when the intestinal barrier function is impaired, LPS can enter the circulation and promote chronic systemic inflammation by activating a strong immune response (17). It has been suggested that chronic high-fat diets create favorable conditions for the proliferation of gram-negative bacteria (18), and is one of the causes of impaired intestinal barrier function (14). In terms of mechanisms (14), the high-fat diet tends to impair the intestinal barrier, leading to elevated circulating levels of LPS and the development of a pro-inflammatory state, for reasons related to the elevation of macrophage markers of inflammation signalled by LPS via Toll-like receptor 4 (TLR4) and CD14, but all of the above are reversible after antibiotic treatment. In addition, Zhang et al. demonstrated that (19) aging-associated gut flora dysbiosis enhances atrial NLRP3 inflammasome activity by increasing circulating LPS and glucose levels. This process promotes atrial fibrosis and ultimately contributes to AF development, providing compelling evidence for LPS's direct role in AF pathogenesis. Furthermore, the NLRP3 inflammatory signaling pathway was significantly enhanced in cardiomyocytes from patients with paroxysmal, persistent and postoperative AF (20). Upregulation of the NLRP3 system not only leads to atrial fibrosis but may also trigger arrhythmias by shortening the atrial action potential and increasing the frequency of Ca²⁺ release from the spontaneous diastolic sarcoplasmic reticulum, inducing delayed afterdepolarization and ectopic activity (21).
2.3 Short-chain fatty acids (SCFAs)
SCFAs are the main metabolites of fermentation of dietary fibre and glucose by colonic microbiota, and mainly include acetate, propionate and butyrate (22). SCFAs enhance intestinal barrier function, inhibit histone deacetylases (23), modulate immune responses, and regulate metabolic and inflammatory responses through G protein-coupled receptors (GPCRs). They are also involved in cardiac repair and arterial compliance regulation after ischemia/reperfusion injuries and myocardial infarction (24). However, there are differences in the role of different SCFAs in cardiovascular disease. Butyrate exerts beneficial metabolic effects through its anti-inflammatory action and increased insulin sensitivity (25). Propionate, on the other hand, reduces the risk of obesity by stimulating the release of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) and affects blood pressure by modulating renin release (26). Whereas acetate may promote insulin and growth hormone-releasing peptide secretion through activation of the parasympathetic nervous system, leading to dyslipidaemia and obesity (27).
However, that the evidence linking specific probiotic bacteria, which are putative enhancers of SCFA production, to these benefits is not always robust. For instance, meta-analyses on Bifidobacterium abundance indicate that the overall significance often hinges on a few studies, as the pooled result loses statistical significance upon the exclusion of specific trials (28–30). This highlights the fragility and inconsistency of the current evidence base connecting probiotic interventions to the SCFAs-related mechanisms discussed above (Table 1).
Table 1
| Metabolite class | Representative molecules | Key pathophysiological mechanisms | Impact on AF |
|---|---|---|---|
| TMAO | Trimethylamine N-oxide | 1. Activation of NLRP3 inflammatory vesicles promotes atrial inflammation. | Increased susceptibility and persistent risk of atrial fibrillation; biomarkers for predicting the occurrence of atrial fibrillation. |
| 2. Enhances platelet reactivity and promotes thrombosis. | |||
| 3. Induces M1 macrophage infiltration and promotes structural remodelling of atrial fibrosis. | |||
| LPS | Lipopolysaccharide | 1. Systemic chronic inflammation via the TLR4/CD14 pathway. | Disrupts electrophysiological stability, promotes atrial fibrosis and increases the risk of atrial fibrillation. |
| 2. Enhancement of atrial NLRP3 inflammatory vesicle activity. | |||
| 3. Leads to oxidative stress and abnormal calcium handling. | |||
| PAGln | Phenylacetylglutamine | 1. Agonises adrenergic β2 receptors (β2-AR), enhancing platelet activity and calcium cycling. | Is an independent predictor of atrial fibrillation in patients with heart failure and renal disease. |
| 2. Increased ROS production and leading to calcium leakage and posterior depolarisation. | |||
| IS | Indoxyl sulfate | 1. Induces oxidative stress and Ca²⁺ overload in cardiomyocytes. | It is strongly associated with the risk of atrial fibrillation associated primarily with chronic kidney disease. |
| 2. Promote the expression of inflammatory factors (TNF-α, IL-6, IL-1β). | |||
| 3. It has a pro-fibrotic and pro-hypertrophic effect. | |||
| SCFAs | Butyrate, acetate | A two-way street: | Butyrate may be protective; other SCFAs have complex roles and may indirectly influence AF risk factors. |
| Beneficial (Butyrate based): Strengthens intestinal barrier, anti-inflammatory, | |||
| Harmful effects (acetate based): May promote obesity and dyslipidaemia via vagal stimulation. | |||
| Bas | CDCA, UDCA | A two-way street: | CDCA promotes atrial remodelling; UDCA may have protective potential. |
| Harmful (CDCA): activates FXR and NLRP3, promotes apoptosis and fibrosis; affects ion channels. | |||
| Beneficial (UDCA): Stabilises cell membrane potential and may be antiarrhythmic. |
Dysregulated Gut Flora-derived metabolites and their pathogenic roles in atrial fibrillation.
NLRP3, NOD-like receptor thermoprotein structural domain-associated protein 3; TLR4, Toll-like receptor 4; ROS, activated oxygen; CaMKII, Calcium/calmodulin-dependent protein kinase II; RyR2, Ranibine receptor 2; FXR, Farnesol X receptor.
2.4 Phenylacetylglutamine (PAGln)
PAGln (31) is one of the metabolites of bacterial phenylalanine in humans, and is derived from the combination of phenylacetic acid and glutamine. A prospective clinical study confirms that elevated plasma PAGln promotes the expression of Interleukin-1β (IL-1β) and Interleukin-6 (IL-6) to increase the risk of developing AF (32). Moreover, PAGln is significantly elevated in end-stage renal disease, coronary artery disease (CAD) with stent stenosis (7), and has been used as an independent predictor of secondary AF in these diseases. Some animal studies have found that PAGln significantly increased the left atrial inner diameter (LAD) (33) and exacerbated the increase in plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration as well as promoted the expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) mRNA in atrial tissue of heart failure (HF) mice. Mechanistically, by acting as an agonist of the adrenergic β receptor 2 (β2-AR) (7), PAGln can potentiate platelet reactivity/thrombosis in arterial injury models and enhance spontaneous calcium release linked to AF (34). Thus, β2 AR has been identified as a potential key mediator of PAGln's action on AF, though the in vivo significance in humans remains to be fully elucidated. In addition, PAGln induces arrhythmias by increasing the accumulation of ROS, a product of oxidative stress, to promote activation of calmodulin kinase II (CaMKII) and ryanodine receptor 2 (RyR2), and by increasing Ca2+ release and calcium overload (35) as a means of promoting excitability and afterdepolarization of the cell membrane.
2.5 Indolephenol sulphate (IS)
IS is a uremic toxin, produced by gut flora through dietary protein metabolism (36, 37).Currently confirmed (37), increased levels of IS in the body contribute to the development of AF by increasing oxidative stress to exacerbate inflammation and cardiac fibrosis. Owing to its high protein-binding capacity and poor dialyzability, serum levels of IS remain elevated even after hemodialysis (38). Consequently, serum IS is considered a biomarker for predicting cardiovascular events in patients with advanced chronic kidney disease (38). In mechanisms (39), IS can alter electrical activity and induce Ca2 + overload in cardiomyocytes through oxidative stress, which interrupts regulatory proteins of calcium channels and increases intracellular Ca2+ overload and Ca2+ release. They also demonstrated in cellular experiments that IS has a pro-inflammatory effect, leading to significant increases in the inflammatory cytokines tumour necrosis factor- α, IL-6 and IL-1β. Thus, IS may have pro-fibrotic, pro-hypertrophic and pro-inflammatory effects on cardiomyocytes.
2.6 Bile acids (BAs)
BAs are the end products of cholesterol metabolism, mainly including free bile acids, primary bound bile acids and secondary free bile acids (40). Among them, secondary free bile acids can act as hormone-like molecules and regulate signalling pathways such as metabolism, inflammation and energy expenditure (41). However, excessive free bile acids may be cardiotoxic. For example, chenodeoxycholic acid (CDCA) may promote AF by binding to amino acids to form bile salts, further stimulating cardiac sodium-calcium exchange, inducing changes in membrane potential, and activating muscarinic M2 receptor/acetylcholine-regulated potassium currents in cardiomyocytes (42). In addition, experimental data suggest that CDCA can promote cardiac injury and fibrosis via farnesol X receptor (FXR) activation in model systems and exacerbates the inflammatory response by activating NLRP3 inflammasome (43). Animal experiments further validated this mechanism, Wang (44) found that CDCA levels in patients with AFwere positively correlated with left atrial low-voltage areas and that CDCA showed a dose-dependent relationship with atrial myocyte apoptosis in a mouse model, suggesting an important role in structural remodelling in AF. On the other hand, certain bile acids such as ursodeoxycholic acid (UDCA) are cardioprotective, and UDCA, as a hydrophilic bile acid, may play an active role in the prevention of cardiac arrhythmias by stabilising the potential of cell membranes (45).
3 Interaction of gut flora with traditional cardiovascular risk factors
3.1 There is a bidirectional relationship between gut flora and hypertension
Hypertension is a major risk factors for many cardiovascular diseases (46), and its pathogenesis is closely linked to intestinal microbiota disorders. Studies suggest a bidirectional regulatory relationship exists between hypertension and gut flora (47, 48). Animal experiments have confirmed that significant intestinal pathological alterations occur in hypertensive rat models. These alterations, which include impaired intestinal barrier integrity, dysregulated immune responses, and hemodynamic changes, may contribute to the development and maintenance of hypertension (48). The main manifestations were increased plasma fluorescein isothiocyanate (FITC)-dextran concentration, decreased tight junction proteins, increased fibrotic area of the intestinal wall, thickening of the muscularis propria, decreased number of cuprocytes and shortening of intestinal cilia (49).
In contrast, the antihypertensive drug chlorosartan (50) restores the expression of colonic closure proteins and mucins by promoting α-defensin production and inhibiting sympathetic excitation (51), a finding that confirms the bi-directional regulatory relationship between hypertension and intestinal barrier function. Notably, studies have found significantly lower levels of butyrate in hypertensive patients (52, 53). Butyrate is highly active in regulating blood pressure, dilating blood vessels and reducing inflammatory responses (54). In terms of mechanisms (55), butyrate acts on the receptor GPR109A to control the development of hypertension by modulating the immune response so that macrophages exhibit a higher capacity to induce differentiation of juvenile cells to regulatory T cells. In addition, the acetate and propionate salts in SCFAs can also lower blood pressure by relaxing blood vessels (56, 57). Animal experiments confirmed that SCFAs significantly dilated mesenteric and thin femoral arteries in phenylephrine preconstricted rats (58). However, the regulation of blood pressure by SCFAs is bidirectional: when they bind to the vascular OLFR78 receptor they promote renin release, leading to an increase in blood pressure, whereas when they interact with the G-protein-coupled receptor GPR43 they exert a vasodilatory effect and reduce blood pressure (59). This receptor-dependent dual regulatory action reveals a complex mechanism of SCFAs in blood pressure regulation. Gut flora is also involved in the development of hypertension through immunomodulatory pathways. A high-salt diet can, in turn, induce the formation of a pro-inflammatory environment in the body, which in turn promotes the development of hypertension. Specific mechanisms involve T-cell activation/inflammatory cytokines/gut flora pathways, etc. (60). Notably, propionic acid in SCFAs has significant anti-inflammatory properties, inhibits the progression of cardiovascular disease in animal models, and exhibits antihypertensive effects (58), its cardioprotective effects are mainly dependent on regulatory T cells (Treg), which reduce cardiac hypertrophy, fibrosis, arrhythmia susceptibility and atherosclerotic lesions by regulating T helper cell homeostasis. Supplementation of SCFAs restores endotoxaemia and regulates Th17/Treg cell balance in mesenteric lymph nodes in hypertensive rats, thus exerting an antihypertensive effect (61).
In conclusion, intestinal microecology is closely related to the pathogenesis of hypertension. A dysregulated intestinal microenvironment is involved in traditional mechanisms of hypertension, such as the autonomic nervous system, renin-angiotensin-aldosterone system and the immune system, by altering the composition and distribution of the bacterial flora, influencing the function of the intestinal barrier, regulating the metabolites of the flora and mediating immune-inflammatory responses (62). These traditional mechanisms, in turn, interact with the gut flora and work together to promote the progression of hypertension (63). These findings provide an important theoretical basis for an in-depth understanding of the pathogenesis of hypertension and the development of new therapeutic strategies (Table 2).
Table 2
| Risk factor | Nature of interaction with Gut flora | Core mechanisms | Key microbial/metabolite changes | Key Insights and therapeutic implications |
|---|---|---|---|---|
| Hypertension | Bidirectional Regulation | 1. Destruction of the intestinal barrier: Leads to endotoxemia and systemic inflammation. | Deleterious: Increased LPS, decreased butyrate | The gut flora is a central regulator of hypertension. Supplementing SCFAs, using specific probiotics, or drugs can lower blood pressure by repairing the intestinal barrier and modulating immunity. |
| 2. Immunomodulation: SCFAs regulate Treg cells via receptors, influencing blood pressure. | Beneficial: Propionate, acetate | |||
| 3. Neurological & RAAS regulation: SCFAs bidirectionally regulate renin release via Olfactory Receptor 78 (OLFR78) and GPR43. | ||||
| 4. High-salt diet: Induces pro-inflammatory Th17 cell response and disrupts microbial balance. | ||||
| Insulin Resistance & Diabetes | Driver | 1. Inflammation: Microbiota-derived LPS triggers chronic low-grade inflammation via the TLR4 pathway, interfering with insulin signaling. | Deleterious: Increased LPS, BCAAs, succinate Beneficial: Decreased SCFAs, indole derivatives | The gut flora is central to the “chronic inflammation” of T2DM. Targeting the microbiota can reduce LPS translocation and increase beneficial metabolites, offering new strategies for preventing diabetes and associated AF. |
| 2. Metabolite regulation: SCFAs, indole derivatives exert protective effects by stimulating GLP-1 secretion and improving insulin sensitivity; BCAAs and succinate promote resistance. | ||||
| 3. Iron overload: Gut flora-associated iron dysregulation reduces insulin secretion and promotes resistance. | ||||
| Hyperlipidemia | Main Regulator | 1. Lipid metabolism regulation: Microbiota modulates lipid balance by affecting epithelial integrity, cholesterol metabolism, and lipid oxidation/storage. | Deleterious: Increased Firmicutes, TMAO | Blood lipid levels are closely tied to microbiota composition. Reducing TMAO levels and supplementing SCFAs are novel approaches to modulate lipids and reduce cardiovascular risk. |
| 2. TMAO pathway: High-fat/high-choline diet → promotes gut microbial TMA production → hepatic FMO3 oxidation to TMAO → promotes atherosclerosis and thrombosis. | Beneficial: Butyrate, propionate | |||
| 3. SCFAs effects: Butyrate and propionate lower cholesterol and triglycerides by activating receptors | ||||
| Aging | Accelerator | 1. Microbial evolution: With age, glycolytic bacteria decrease, while proteolytic bacteria increase. | Protective decrease: Butyrate-producing bacteria | Age-related dysbiosis is an independent risk factor for AF. Transplanting aged microbiota increases AF susceptibility. Modulating the microbiota during aging (e.g., increasing dietary fiber) may delay cardiovascular aging and prevent age-related AF. |
| 2. Inflammation & aging: Increased entry of harmful metabolites into circulation activates the NLRP3 inflammasome, promoting atrial fibrosis. | Deleterious increase: Proteolytic bacteria, PAGln, TMAO | |||
| 3. Molecular aging: PAGln induces mitochondrial dysfunction and DNA damage via the ADR-AMPK pathway; telomere dysfunction exacerbates gut leakiness and inflammation |
Summary of gut flora interactions with traditional cardiovascular risk factors.
SCFAs, short-chain fatty acids; TMAO, trimethylamine N-oxide; LPS, lipopolysaccharide; Treg, regulatory T cells; RAAS, renin-angiotensin-aldosterone system; PAGln, phenylacetylglutamine; BCAAs, branched-chain amino acids.
3.2 Dysbiosis of gut flora is associated with insulin resistance
Diabetes mellitus (DM) is an established independent risk factor for AF. And the gut flora has been suggested as a therapeutic target for pre-diabetes and early diagnosis (64),that can lead to diabetes by affecting intestinal permeability, inflammation, the immune system and energy metabolism.
One study (65) revealed the central role of the inflammatory response in the development of type 2 diabetes mellitus (T2DM) (66). T2DM is now redefined as a chronic inflammatory disease (10). Insulin resistance (IR) is strongly associated with a chronic low-grade inflammatory state (67), this chronic inflammation interferes with insulin signalling and reduces insulin bioavailability through direct or indirect mechanisms. Notably, endotoxaemia induced by LPS produced by gut flora may be a key trigger of inflammation and insulin resistance. After bacterial lysis, LPS enters the circulation and triggers systemic chronic low-grade inflammation (67) through activation of Toll-like receptor 4 (TLR4), which in turn induces serine phosphorylation of insulin receptor substrate-1, ultimately leading to the development of insulin resistance.
Mechanistically, SCFAs are produced in the intestinal tract by the phylum Serratia marcescens and Serratia marcescens to stimulate the release of glucagon-like peptide-1 (GLP-1) and activate the G protein-coupled receptor (gpcr) expressed in enteroendocrine cells, inducing insulin secretion from the pancreas (65). In addition, indole and its derivatives have been shown to bind and activate the aromatic hydrocarbon receptor (AhR), which is associated with improved insulin secretion and insulin sensitivity. N-acylamides have also been found to enhance glucose metabolism in humans by binding to G protein-coupled receptor 119 (GPR119). However, branched chain amino acids (BCAAs) and succinate from bacteria can accelerate the development of insulin resistance (68). In addition gut flora-associated iron overload increases susceptibility to type 2 diabetes by reducing insulin secretion, promoting insulin resistance and facilitating hepatic gluconeogenesis (69).
DM is strongly associated with elevated levels of systemic inflammatory mediators (70), study shows significant activation of multiple pro-inflammatory pathways in myocardial tissue of diabetes model animals (71),suggests chronic cardiac inflammation may be an important pathophysiological bridge between diabetes and arrhythmias (66). Specifically, a persistent low-grade inflammatory state may lead to altered myocardial electrophysiological properties, increased fibrosis, and autonomic dysfunction, which may increase the risk of arrhythmias such as AF. This mechanism provides a new perspective for understanding the pathogenesis of diabetes-associated AF, as well as a theoretical basis for the development of therapeutic approaches based on anti-inflammatory strategies.
3.3 Intestinal flora and hyperlipidaemia
Significant correlation between blood lipid levels and cardiovascular disease incidence (72). The relative abundance of Firmicutes was significantly higher in patients with hyperlipidemia, a finding that suggests a strong association between the composition of the intestinal flora and blood lipid levels (73). Gut flora are involved in the regulation of lipid metabolism by altering the integrity of intestinal epithelial cells, regulating cholesterol metabolism, promoting lipid oxidation in muscle tissue, and modulating lipid storage in adipose tissue (74), these mechanisms work together to maintain the body's lipid metabolic balance. Recent studies have revealed the key role of gut flora metabolites in lipid metabolism. Butyrate in SCFAs exerts a lipid-regulating effect by activating the intestinal GPR109A receptor, which in turn activates macrophages, reduces total lipase activity and inhibits triglyceride synthesis and accumulation (26). In addition, in vitro and in vivo studies have demonstrated that propionic acid reduces cholesterol levels by activating free fatty acid receptors GPR41 and GPR43, which promote the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) (75). It is worth noting that TMAO is strongly associated with high-fat diets. These diets are rich in choline, a key substrate for TMAO production. Choline is first broken down in the gut to trimethylamine (TMA), which is subsequently oxidized by hepatic flavin monooxygenase 3 (FMO3) to produce TMAO (76). It was further found that a high-fat diet alters intestinal epithelial physiology by impairing colonic epithelial mitochondrial bioenergetics and eliminating epithelial hypoxia, promoting intestinal TMA production and increasing circulating TMAO levels (77). A randomised crossover design study confirms that a diet high in saturated fat increases systemic TMAO levels by increasing dietary precursors and reducing renal TMAO excretion (17),this finding consistent with clinical observations of elevated plasma TMAO levels.
Based on the available evidence, the intestinal flora in hyperlipidemic patients increases the risk of AF mainly by affecting lipid metabolism, altering intestinal epithelial integrity and permeability, and increasing the level of TMAO in vivo, these mechanisms may ultimately increase the risk of AF by promoting atrial remodelling, increasing inflammatory responses and oxidative stress. These findings provide new perspectives for understanding the pathogenesis of hyperlipidaemia-associated AF and a theoretical basis for developing intervention strategies based on the regulation of gut flora.
3.4 Correlation of ageing gut flora with age
The risk of developing AF was significantly and positively associated with age, a phenomenon that may be closely related to age-related changes in gut flora. Research suggests that the gut flora plays a key role in regulating the organism's aging process (78), and the dynamics of gut flora with age is an independent risk factor for the development of atrial fibrillation (79).
With age, the composition of the gut flora shows a decrease in the proportion of glycolytic bacteria and an increase in the proportion of proteolytic bacteria (80). Glycolytic bacteria provide cardiovascular protection by producing beneficial metabolites such as short-chain fatty acids (SCFA) (for example, butyrate),which can maintain the intestinal barrier function (81, 82) and reduces the entry of pro-inflammatory molecules such as LPS, TMAO and PAGln into the blood circulation by, thus regulating macrophage activity and inhibiting atherosclerotic plaque formation. On the other hand, proteolytic bacteria may produce potentially harmful metabolites such as trimethylamine, which is converted to trimethylamine oxide (TMAO) in the liver (83), which may accelerate the ageing process of endothelial cells and the vascular system by activating oxidative stress pathways.
Its animal experiments further confirmed this mechanism, as transplantation of faecal flora from aged rats (22–24 months old) to young rats (2–3 months old) significantly increased atrial NLRP3 inflammasome activity, exacerbated atrial fibrosis and increased AF susceptibility, whereas reverse transplantation showed a protective effect. Other evidence for this conclusion comes from Zhang et al's (19) research which reveals that gut dysbiosis associated with aging promotes AF through multiple mechanisms: including increased circulating LPS and glucose levels, enhanced atrial NLRP3 inflammasome activity, and ultimately atrial fibrosis.
At the microscopic level, telomere wear, epigenetic alterations, loss of protein homeostasis, mitochondrial dysfunction and altered intercellular communication have been recognised as major molecular and cellular hallmarks of ageing (78, 84). These biological aging, in turn, are directly related to the decline in the functioning of the flora. Yang et al. found that (85) increased production of PAGln in the gut of the elderly and validation of the PAGln -induced senescence phenotype in cellular and mouse models suggests a mechanism by which PAGln induces mitochondrial dysfunction and DNA damage via the adrenergic receptor (ADR)-amp activated protein kinase (AMPK) signalling pathway. And telomere dysfunction may also directly affect the integrity of intestinal tissue (86),this leads to increased leaky gut, systemic inflammation and ecological dysregulation, and further accelerates telomere shortening. For example, telomerase-deficient mice have shorter intestinal telomeres than wild-type, reduced expression of intestinal genes that tightly link the F11 receptor, and increased expression of inflammatory markers (87).
These findings not only confirm the strong association between age and gut flora, but also provide a theoretical basis for the development of microbiota intervention strategies for age-related diseases such as AF. Based on this evidence, modulation of the gut flora may be a new strategy for the prevention and treatment of cardiovascular disease in adults, which is expected to reduce the probability of cardiovascular events in the elderly population (Figure 2).
Figure 2
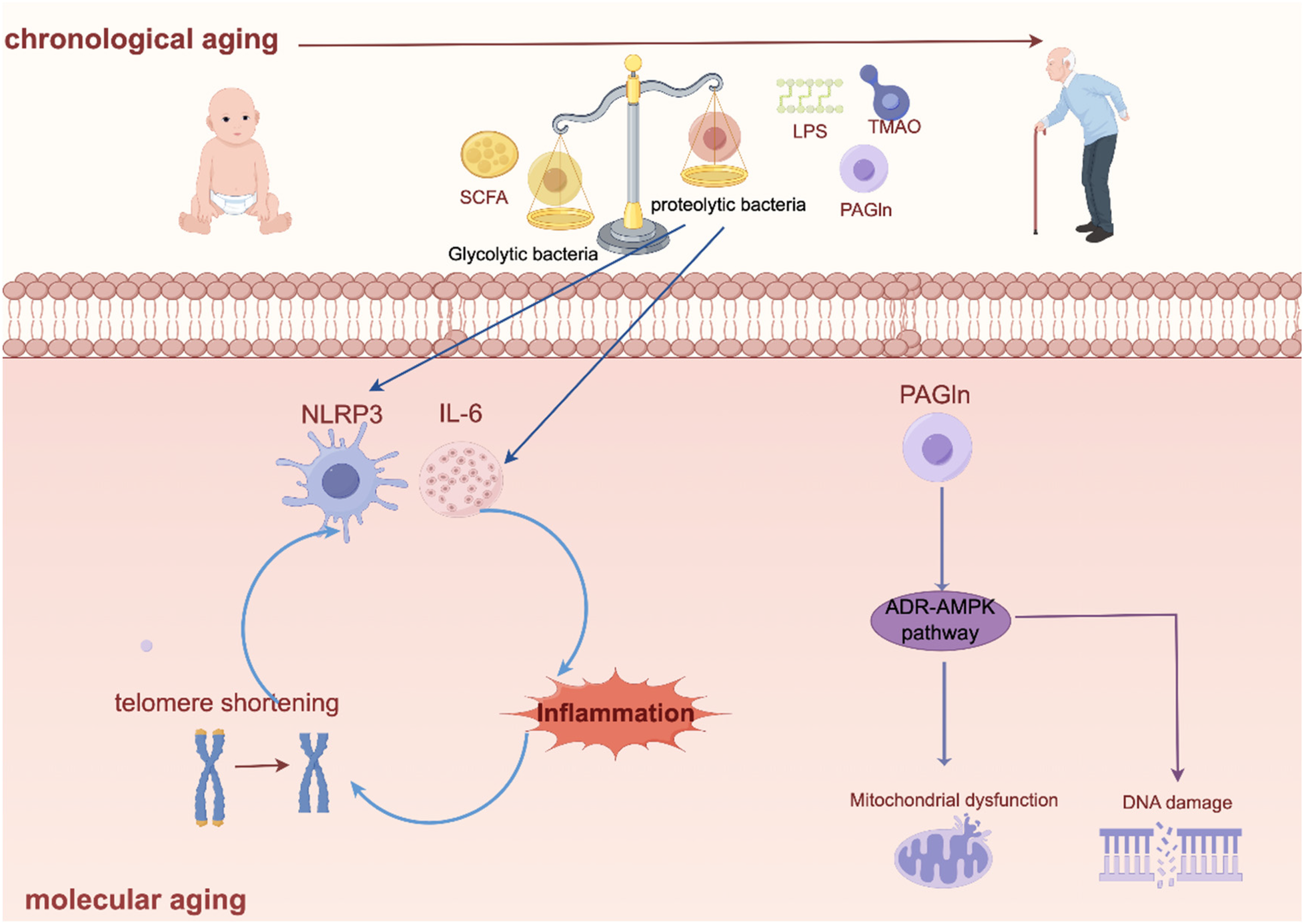
Intestinal flora leads to chronological ageing and molecular ageing. Created using Figdraw (https://www.figdraw.com/).
4 Intervention strategies targeting intestinal flora
4.1 Dietary regulation
Dietary structure is one of the key factors influencing the composition and function of intestinal flora (Table 3). Dietary patterns characterised by high fat and sugar have been shown to lead to a decrease in the abundance of beneficial flora such as bifidobacteria in the gut, which in turn triggers dysregulation of lipid metabolism (88) and local inflammatory responses (88). Whereas high dietary fibre intake in the Mediterranean diet (MD) significantly increased the proportion of thick-walled phyla (89), thick-walled phylum is able to produce butyric acid with anti-inflammatory effects, which plays an important role in maintaining microbial homeostasis and reducing LPS production (90). In addition adherence to MD significantly increased the level of SCFAs in faeces (91), however, inadequate adherence to MD can lead to elevated levels of TMAO in the blood, which can increase the risk of a number of chronic diseases, including cardiovascular disease (92). Dietary fibre also exerts its cardioprotective effects by modulating the renin-angiotensin-aldosterone (RAAS) system and blood pressure-related signalling pathways via SCFAs (89). Under the influence of a high-fibre diet, SCFAs and their metabolites interacted with the RAAS system in the host kidney and down-regulated RAAS in the kidneys of experimental rats, resulting in a substantial down-regulation of systolic and diastolic blood pressures (55). Animal studies confirm that a low dietary fibre diet leads to increased expression of Clostridium difficile, which in turn promotes the development of hypertension and cardiac remodelling (93). Treatment with SCFAs improves cardiovascular function through multiple mechanisms, including upregulation of intestinal tight junction protein mRNA expression levels, increased splenic Treg cell abundance, inhibition of pro-inflammatory factors (IL-17a, IL-6) and fibrosis markers (TNF-α, Col3a) expression, and mediation of G-protein-coupled receptor signal transduction (94). Collectively, these findings suggest that modifying dietary patterns may reduce cardiovascular risk through mechanisms such as promoting the production of SCFAs and attenuating the inflammatory response.
Table 3
| Dietary pattern | Key impacts on gut flora & metabolites | Potential impact on AF risk and mechanisms |
|---|---|---|
| Mediterranean Diet (MD) | 1. Significantly increases Firmicutes, particularly butyrate-producing bacteria. | Likely Reduces Risk Mechanisms:
|
| 2. Increases fecal SCFAs levels. | ||
| 3. High adherence can reduce blood TMAO levels. | ||
| High-Fiber Diet | 1. Promotes the proliferation of SCFA-producing bacteria (e.g., Roseburia). | Mechanisms: |
| 1. SCFAs improve cardiovascular function via GPCR signaling. | ||
| 2. Significantly increases production of SCFAs (especially butyrate, propionate). | 2. Upregulates intestinal tight junction proteins, enhancing the gut barrier and reducing endotoxemia. | |
| 3. Inhibits pro-inflammatory factorsand fibrosis markers | ||
| 3. Low-fiber diet increases abundance of harmful bacteria like C. difficile. | 4. Increases splenic Treg cell abundance, modulating immune response. | |
| 5. Low-fiber diet promotes hypertension and cardiac remodeling. | ||
| High-Fat/High-Sugar Diet (Western Pattern) | 1. Leads to a decrease in beneficial bacteria (e.g., Bifidobacteria). | Mechanisms: |
| 1. Triggers lipid metabolism disorders and local inflammatory responses. | ||
| 2. Creates favorable conditions for Gram-negative bacteria, increasing LPS (endotoxin) levels. | 2. Compromises gut barrier function, allowing LPS into circulation and causing systemic chronic inflammation. | |
| 3. Impairs colonic epithelial mitochondrial bioenergetics, promotes intestinal TMA production, and increases circulating TMAO levels. | 3. High TMAO levels directly promote AF progression through inflammation, thrombosis, and fibrosis mechanisms. |
Main evidence on dietary interventions targeting intestinal Flora.
SCFAs, short-chain fatty acids TMAO, trimethylamine N-oxide LPS, lipopolysaccharide RAAS, renin-angiotensin-aldosterone system; Treg, regulatory T cells.
While these beneficial mechanistic changes are corroborated by clinical outcomes (95, 96), extensive research has established the Mediterranean Diet as an effective strategy for cardiovascular disease prevention, demonstrating significant reductions in major adverse cardiovascular events, including myocardial infarction, stroke, and cardiovascular mortality. However, this clinical profile is nuanced: notably, a significant reduction in all-cause mortality has not been consistently verified across all studies (97). Furthermore, the limited reporting of adverse events in most research challenges a comprehensive assessment of the risk-benefit balance associated with the MD.
4.2 Supplementation of specific strains
Dai et al. found that six bacterial taxa- Fusicatenibacter, Holdemania, Howardella, Intestinibacter, Ruminococcaceae UCG014 and Turicibacter- are associated with a reduced risk of AF (98). Zuo et al. also found that (99) psaf-rich Holosporaceae family and Holospora genus, Methylovulum genus and Methylovulum miyakonense species were positively correlated with psaf-rich serum choline, which is the main factor contributing to atrial enlargement in AF patients. Study shows (100) the MD diet significantly increased the abundance of Clostridium difficile groups XVIa and Clostridium pumilus, which are not only involved in butyric acid and secondary bile acid production, but also play an important role in the formation of T-regulatory cells (101). Among them, butyric acid, as a histone deacetylase inhibitor, can exert significant anti-inflammatory effects by inhibiting the expression of pro-inflammatory cytokines (102). Furthermore, Roseburia species (103) also as one of the main producers of butyrate, which is negatively associated with cardiovascular complications associated with chronic kidney disease (CKD) (104). In summary, targeted supplementation of specific beneficial flora may also be a new strategy for the prevention of cardiovascular disease.
4.3 Exercise may play a role in preventing AF through gut flora
Numerous studies have shown that regular exercise is effective in improving intestinal barrier function and positively affecting the composition of intestinal flora (63). However, there were significant differences in the effects of different types of exercise on the gut flora (105).
Endurance exercise has been found to significantly alter the gut metabolite profile, as evidenced by increased levels of organic acids and decreased levels of nucleic acids. Notably, the effect of exercise on the metabolism of short-chain fatty acids (SCFAs) was weight-dependent: in lean individuals, running exercise significantly increased faecal SCFAs concentrations, whereas similar changes were not observed in obese individuals. This difference may be related to exercise-induced changes in the expression of the butyrate-regulated gene BCoAT and the propionate-regulated gene mmDA. Skeletal muscle-dominated exercise can potentially influence the composition of the gut flora, and it has now been found that exercise exerts beneficial regulatory effects on the composition and metabolic function of the gut flora (68). For example (106), the abundance of microorganisms involved in processes such as carbohydrate and amino acid metabolism and the production of SCFAs increased significantly, while playing a regulatory role in iron metabolism. From the point of view of the intestinal flora, this may be related to the elevated intestinal permeability which promotes the entry of pathogenic bacteria, lipopolysaccharides and other toxic substances into the body circulation, thus disrupting the internal homeostasis of the organism.
These findings provide new mechanistic explanations for the prevention of cardiovascular disease by exercise, and also suggest that gut flora may be an important target for exercise intervention (Table 4).
Table 4
| Intervention category | Specific intervention/finding | Impact on gut flora & key mechanisms | Potential effect on AF risk |
|---|---|---|---|
| Specific bacterial strains | Fusicatenibacter, Holdemania, Howardella, Intestinibacter, Ruminococcaceae UCG014, Turicibacter | Identification of these six bacterial taxa as being associated with a reduced risk of AF in Mendelian randomization analysis. | Likely Protective Mechanism: Their presence in the gut microbiome is causally linked to a lower incidence of AF, suggesting potential therapeutic benefits. |
| Clostridium groups XVIa and Clostridium pumilus (enriched by MD diet) | 1. Involved in butyrate and secondary bile acid production. | Likely Protective | |
| 2. Play a crucial role in the formation of regulatory T cells (Tregs). | Mechanism: Butyrate acts as an HDAC inhibitor, exerting anti-inflammatory effects; Tregs modulate immune response and reduce inflammation. | ||
| Roseburia species | One of the main producers of butyrate. Its abundance is negatively associated with cardiovascular complications in chronic kidney disease (CKD). | Likely Protective | |
| Mechanism: Butyrate production enhances intestinal barrier function, reduces systemic inflammation, and improves metabolic health. | |||
| Holosporaceae family, Methylovulum genus | Positively correlated with serum choline, a major dietary precursor for the pro-arrhythmic metabolite TMAO. Associated with atrial enlargement in AF patients. | May Increase Risk | |
| Mechanism: These taxa may promote TMAO production, which facilitates AF through inflammation, fibrosis, and thrombosis. | |||
| Exercise | Endurance Exercise | 1. Alters the gut metabolite profile (increases organic acids, decreases nucleic acids). 2. Effect on SCFA production is weight-dependent: increases fecal SCFAs in lean individuals but not in obese individuals. 3. Increases abundance of microbes involved in carbohydrate/amino acid metabolism and SCFA production. | Likely Protective |
| Mechanism: Improves intestinal barrier function, reduces permeability, and decreases entry of pathogens/LPS. Modulates iron metabolism. Promotes an anti-inflammatory environment. | |||
| Skeletal Muscle-Dominated Exercise | Exerts beneficial regulatory effects on the composition and metabolic function of the gut flora. | Likely Protective Mechanism: The gut-muscle axis may influence host physiology, potentially reducing systemic inflammation and improving metabolic parameters linked to AF risk. |
Main evidence on supplementation with specific strains and exercise.
MD, mediterranean diet; TMAO, trimethylamine N-oxide; SCFAs: short-chain fatty acids; Tregs, regulatory T cells; HDAC, histone deacetylase; LPS, lipopolysaccharide.
5 Evidence quality, disputes and challenges
This review systematically presents the role of the gut flora and its metabolites in AF. However, before translating these promising findings into clinical practice, it is imperative to critically evaluate the level of current evidence, existing controversies, and potential confounding factors.
The current body of evidence supporting the gut flora-AF axis primarily consists of animal studies and related observational research. Although animal models, such as the various rat and mouse models cited in this paper, have provided key evidence for elucidating the molecular mechanisms of metabolites like TMAO, PAGln, and LPS, significant interspecies differences must be acknowledged. For instance, mice and humans differ in both macroscopic and microscopic intestinal structures (107), as well as in cellular composition and function (108, 109). Furthermore, approximately 85% of mouse gut bacterial species are not present in the human gut (110). These fundamental differences highlight the need for a cautious evaluation of the relative importance and translatability of the relevant pathways in humans. For instance, while TMAO promotes AF in rodent models through pathways such as NLRP3 inflammasome activation, its association with AF in human observational studies has been inconsistent across different cohorts. Moreover, this association is often strongly confounded by renal function (111). Specifically, patients with reduced glomerular filtration rates (112) and those with chronic kidney disease exhibiting progressive renal impairment often exhibit elevated plasma TMAO levels (113). In contrast, TMAO levels can normalize following kidney transplantation (113), suggesting that the association between TMAO and AF may be partly driven by renal function status. Similarly, the pathogenic mechanisms of PAGln are currently supported mainly by cellular and animal experimental evidence (7, 85, 114). Its predictive value in broader populations and its causal relationship with AF require further validation through larger-scale prospective cohort studies and genetic evidence.
A core challenge in elucidating the relationship between gut flora and AF lies in disentangling the pervasive confounding factors. AF patients often present with multiple comorbidities, such as heart failure (115), hypertension (116), diabetes (117), and chronic kidney disease (118), and are frequently on long-term medications, including antibiotics, proton pump inhibitors, and antiarrhythmic drugs. These comorbidities and medications themselves are known to significantly alter the structure and function of the gut flora (119, 120). Consequently, it remains difficult to definitively determine whether the “AF-associated microbial signatures” observed in studies are a cause, a consequence, or an epiphenomenon of AF. Furthermore, diet is the strongest environmental factor shaping the microbiota. Variations in geographical regions, cultural backgrounds, and individual dietary habits can lead to difficulties in replicating and generalizing study findings (121). Host genetic background may also simultaneously influence susceptibility to AF and the colonization of the gut flora (122), further adding to the complexity of the analysis.
Current intervention studies targeting the microbiota, such as probiotic supplementation and dietary modifications, not only show inconsistent results (123) but have also raised safety concerns. For instance, a meta-analysis of 8 randomized controlled trials (n = 270) concluded that probiotic supplementation, overall, had no significant effect on circulating TMAO levels compared to controls (124). This finding directly challenges the presumed pathway from probiotic-modulated microbiota to reduced production of pro-arrhythmic metabolites. While a subgroup analysis suggested a potential benefit in individuals under 50 years of age, the primary null result underscores the gap between theoretical mechanisms and clinical outcomes. Cases of Lactobacillus bacteremia and complications have been reported in immunocompromised patients following long-term probiotic use (125). Regarding probiotics, although certain specific strains have shown antiarrhythmic potential in animal models, their effects often become weak or inconsistent when translated to human clinical trials. While the MD and dietary fiber demonstrate robust cardiovascular protective effects in observational studies, randomized controlled trial evidence regarding their direct impact on AF incidence remains limited (123, 126). These “unsuccessful” intervention studies are nonetheless of significant value, as they reveal the complexity of microbiota-targeted interventions and suggest that their efficacy may highly depend on the host's baseline microbiota, as well as the timing, dosage, and duration of the intervention. Future efforts will require more targeted and personalized intervention strategies.
6 Summary and outlook
In recent years, the role of gut flora in the development of AF has been gradually revealed. Gut flora and their metabolites are involved in the onset and development of AF through a variety of mechanisms, including inflammatory response, lipid metabolism, oxidative stress, atrial fibrosis, and regulation of the autonomic nervous system. These findings offer novel insights into AF pathogenesis and provide a theoretical basis for the development of targeted therapeutic strategies based on gut flora.
First, gut flora directly or indirectly promotes atrial inflammation and fibrosis through metabolites such as TMAO and LPS, increasing the risk of AF. Second, metabolites such as bile acids and short-chain fatty acids play an important role in regulating cardiac electrophysiological properties, inflammatory responses, and lipid metabolism, further influencing the onset and progression of AF. In addition, the interaction between gut flora and traditional risk factors for AF, such as hypertension, diabetes and hyperlipidemia, further emphasises the centrality of gut flora in AF. Based on these findings, gut flora and its metabolites are expected to be new targets for AF prediction, diagnosis and treatment. Future studies should further explore the causal relationship between gut flora and AF, and clarify the specific mechanism of action of specific flora and their metabolites in the development of AF. In addition, the development of intervention strategies based on gut flora (e.g., probiotics, faecal transplantation, etc.) may provide new directions for the prevention and treatment of AF.
Although a substantial body of research has revealed associations between gut flora and AF, establishing causality and translating these findings into effective therapies remain formidable challenges. Analysis of ongoing clinical trials (Table 5) reveals critical gaps that future studies must address with greater precision. Key unresolved questions include whether gut flora contributes equally to various AF subtypes (e.g., paroxysmal vs. persistent), and how demographic factors (age, gender, ethnicity) modulate this risk. To move beyond correlation, future research should prioritize three actionable strategies: First, employ humanized animal models—transplanting microbiota from well-phenotyped AF patients into germ-free mice—to directly test the arrhythmogenic potential of human microbial communities. Second, transition from generic probiotic trials to biomarker-enriched interventions, by recruiting patients with elevated pro-arrhythmic metabolites (e.g., TMAO, PAGln) to test targeted microbial therapies. Third, leverage metagenomic sequencing to identify not just correlated taxa, but the specific bacterial genes [e.g., the choline-utilizing cutC/D cluster (127)] responsible for producing AF-associated metabolites. By adopting these focused approaches, the field can advance from documenting associations to defining causal mechanisms and developing personalized interventions.
Table 5
| Trial name | NCT or Identifier | Planned completion date | Study design & intervention | Primary outcome measures |
|---|---|---|---|---|
| Microbiota-Inflammation-Brain Axis in Heart Failure: New Functional Food for the Prevention of undeRnutrition in Older (AMBROSIA) | NCT07076329 | 30/08/2025 | Interventional: Patients receiving probiotics vs. placebo after catheter ablation. | help improve muscle mass and physical performance in older adults with heart conditions |
| Impact of a probiotic supplement on menopause | ISRCTN56831337 | 31/12/2025 | Interventional: All participants will receive a 12-week course of billions of live and active bacteria. | Questionnaire to measure quality of life |
| The survey of oral and intestinal microbiota in patients with diabetes and periodontitis and the effect of probiotic consumption | IRCT20250107064315N1 | — | Interventional: One probiotic capsule daily for 12 weeks | Quantitative real-time_ Polymerase Change Reaction. |
| effects of synbiotics on protein absorption for muscle mass improvement in recreational male bodybuilders | TCTR20250803008 | 31/12/2025 | Interventional: Placebo Comparator Dietary Supplement | Probiotic levels pre-test, post-test Colony forming units |
| comparison of zinc and probiotics on neonates with indirect hyperbilirubinemia undergoing phototherapy ZIPP | NCT07102836 | — | interventional: Drug1: Zinc sulfate Drug2: Probiotic lactobacillus rhamnosus GG | Rate of decline in Bilirubin levels during phototherapy with this adjuvant therapy |
| Effect of probiotic on chemotherapy-induced side effects | IRCT20130211012438N37 | — | Interventional: One probiotic capsule daily for 12 week | Chemotherapy induced side effects include gastrointestinal symptoms such as nausea |
| Adipose Tissue Gene Expression and Metabolomics Links to the Gut Microbiome-brain Axis POINSETTIA | NCT06869941 | — | Observational: Blood sugar and percentage of deep sleep | Possible links between lipid metabolism and gut flora |
| How a Single Workout Affects Gut Bugs in Women With Different Fitness Levels and Body Types FITGut-W | NCT06691100 | — | Interventional: aerobics | Gut Microbiome Composition: Before the exercise intervention and immediately after the intervention |
Ongoing clinical trials investigating the role of Gut Flora in risk factors associated with AF.
Statements
Author contributions
CW: Writing – original draft, Writing – review & editing. MS: Writing – review & editing, Writing – original draft. YZ: Writing – review & editing, Supervision. ZX: Resources, Writing – review & editing. YH: Writing – review & editing, Validation. HX: Writing – review & editing, Supervision, Funding acquisition.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Shandong Provincial Natural Science Foundation [grant numbers ZR2021LZY019]; the Shandong Provincial Key Discipline Construction Project of Traditional Chinese Medicine (Clinical Discipline of Integrated Traditional Chinese and Western Medicine); National Natural Science Foundation of China [grant numbers 82104723]; Key Project of Traditional Chinese Medicine Science and Technology Development in Shandong Province [grant numbers 2020Z14].
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MF declared a shared affiliation with the authors ZX and YH to the handling editor at the time of review.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Brundel BJJM Ai X Hills MT Kuipers MF Lip GYH de Groot NMS . Atrial fibrillation. Nat Rev Dis Primers. (2022) 8(1):21. 10.1038/s41572-022-00347-9
2.
Wang L Ze F Li J Mi L Han B Niu H et al Trends of global burden of atrial fibrillation/flutter from global burden of disease study 2017. Heart. (2021) 107(11):881–7. 10.1136/heartjnl-2020-317656
3.
Gawałko M Linz D . Atrial fibrillation detection and management in hypertension. Hypertension. (2023) 80(3):523–33. 10.1161/HYPERTENSIONAHA.122.19459
4.
Alijla F Buttia C Reichlin T Razvi S Minder B Wilhelm M et al Association of diabetes with atrial fibrillation types: a systematic review and meta-analysis. Cardiovasc Diabetol. (2021) 20(1):230. 10.1186/s12933-021-01423-2
5.
Memelink RG Pasman WJ Bongers A Tump A van Ginkel A Tromp W et al Effect of an enriched protein drink on muscle mass and glycemic control during combined lifestyle intervention in older adults with obesity and type 2 diabetes: a double-blind rct. Nutrients. (2020) 13(1):64. 10.3390/nu13010064
6.
Tosti V Bertozzi B Fontana L . Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. (2018) 73(3):318–26. 10.1093/gerona/glx227
7.
Nemet I Saha PP Gupta N Zhu W Romano KA Skye SM et al A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. (2020) 180(5):862–77.e22. 10.1016/j.cell.2020.02.016
8.
Patterson E Ryan PM Cryan JF Dinan TG Ross RP Fitzgerald GF et al Gut microbiota, obesity and diabetes. Postgrad Med J. (2016) 92(1087):286–300. 10.1136/postgradmedj-2015-133285
9.
Yu L Meng G Huang B Zhou X Stavrakis S Wang M et al A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. (2018) 255:92–8. 10.1016/j.ijcard.2017.11.071
10.
Jiang W-Y Huo J-Y Wang S-C Cheng Y-D Lyu Y-T Jiang Z-X et al Trimethylamine N-oxide facilitates the progression of atrial fibrillation in rats with type 2 diabetes by aggravating cardiac inflammation and connexin remodeling. J Physiol Biochem. (2022) 78(4):855–67. 10.1007/s13105-022-00908-2
11.
Boini KM Hussain T Li P-L Koka SS . Trimethylamine-N-Oxide instigates Nlrp3 inflammasome activation and endothelial dysfunction. Cell Physiol Biochem. (2017) 44(1):152–62. 10.1159/000484623
12.
Cheng X Qiu X Liu Y Yuan C Yang X . Trimethylamine N-oxide promotes tissue factor expression and activity in vascular endothelial cells: a new link between trimethylamine N-oxide and atherosclerotic thrombosis. Thromb Res. (2019) 177:110–6. 10.1016/j.thromres.2019.02.028
13.
Zhu W Gregory JC Org E Buffa JA Gupta N Wang Z et al Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. (2016) 165(1):111–24. 10.1016/j.cell.2016.02.011
14.
Pendyala S Walker JM Holt PR . A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. (2012) 142(5):1100–e2. 10.1053/j.gastro.2012.01.034
15.
Tripolt NJ Leber B Triebl A Köfeler H Stadlbauer V Sourij H . Effect of Lactobacillus casei shirota supplementation on trimethylamine-N-oxide levels in patients with metabolic syndrome: an open-label, randomized study. Atherosclerosis. (2015) 242(1):141–4. 10.1016/j.atherosclerosis.2015.05.005
16.
Boutagy NE Neilson AP Osterberg KL Smithson AT Englund TR Davy BM et al Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring). (2015) 23(12):2357–63. 10.1002/oby.21212
17.
Hsu C-N Hou C-Y Hsu W-H Tain Y-L . Cardiovascular diseases of developmental origins: preventive aspects of gut Microbiota-targeted therapy. Nutrients. (2021) 13(7):2290. 10.3390/nu13072290
18.
Kim K-A Gu W Lee I-A Joh E-H Kim D-H . High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the Tlr4 signaling pathway. PLoS One. (2012) 7(10):e47713. 10.1371/journal.pone.0047713
19.
Zhang Y Zhang S Li B Luo Y Gong Y Jin X et al Age-related changes in the gut microbiota promote atrial fibrillation (2020).
20.
Scott L Jr Fender AC Saljic A Li L Chen X Wang X et al Nlrp3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res. (2021) 117(7):1746–59. 10.1093/cvr/cvab024
21.
Liu H Zhao Y Xie A Kim T-Y Terentyeva R Liu M et al Interleukin-1β, oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. Jacc Basic Transl Sci. (2021) 6(1):42–52. 10.1016/j.jacbts.2020.11.002
22.
Carley AN Maurya SK Fasano M Wang Y Selzman CH Drakos SG et al Short-Chain fatty acids outpace ketone oxidation in the failing heart. Circulation. (2021) 143(18):1797–808. 10.1161/CIRCULATIONAHA.120.052671
23.
Yamashita T Yoshida N Emoto T Saito Y Hirata K-I . Two gut Microbiota-derived toxins are closely associated with cardiovascular diseases: a review. Toxins (Basel). (2021) 13(5):297. 10.3390/toxins13050297
24.
Gao X Lin S-H Ren F Li J-T Chen J-J Yao C-B et al Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun. (2016) 7:11960. 10.1038/ncomms11960
25.
Gao Z Yin J Zhang J Ward RE Martin RJ Lefevre M et al Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. (2009) 58(7):1509–17. 10.2337/db08-1637
26.
Chambers ES Viardot A Psichas A Morrison DJ Murphy KG Zac-Varghese SEK et al Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. (2015) 64(11):1744–54. 10.1136/gutjnl-2014-307913
27.
Pluznick JL Protzko RJ Gevorgyan H Peterlin Z Sipos A Han J et al Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. (2013) 110(11):4410–5. 10.1073/pnas.1215927110
28.
Salvesi C Silvi S Fiorini D Scortichini S Sagratini G Palermo FA et al Impact of a probiotic diet on well-being of healthy senior: the ProbioseniOR project. J Appl Microbiol. (2022) 133(5):2941–53. 10.1111/jam.15747
29.
Valentini L Pinto A Bourdel-Marchasson I Ostan R Brigidi P Turroni S et al Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota—the “ristomed project": randomized controlled trial in healthy older people. Clin Nutr. (2015) 34(4):593–602. 10.1016/j.clnu.2014.09.023
30.
Akatsu H Iwabuchi N Xiao J Matsuyama Z Kurihara R Okuda K et al Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. Jpen J Parenter Enteral Nutr. (2013) 37(5):631–40. 10.1177/0148607112467819
31.
Zhu Y Dwidar M Nemet I Buffa JA Sangwan N Li XS et al Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe. (2023) 31(1):18–32.e9. 10.1016/j.chom.2022.11.015
32.
Wei H Wu J Wang H Huang J Li C Zhang Y et al Increased circulating phenylacetylglutamine concentration elevates the predictive value of cardiovascular event risk in heart failure patients. J Intern Med. (2023) 294(4):515–30. 10.1111/joim.13653
33.
Fu H Li D Shuai W Kong B Wang X Tang Y et al Effects of phenylacetylglutamine on the susceptibility of atrial fibrillation in overpressure-induced HF mice. Mol Cell Biol. (2024) 44(4):149–63. 10.1080/10985549.2024.2345363
34.
Nolla-Colomer C Casabella-Ramon S Jimenez-Sabado V Vallmitjana A Tarifa C Herraiz-Martínez A et al β2-adrenergic stimulation potentiates spontaneous calcium release by increasing signal mass and co-activation of ryanodine receptor clusters. Acta Physiol. (2021) 234(4):e13736. 10.1111/apha.13736
35.
Ren X Wang X Yuan M Tian C Li H Yang X et al Mechanisms and treatments of oxidative stress in atrial fibrillation. Curr Pharm Des. (2018) 24(26):3062–71. 10.2174/1381612824666180903144042
36.
Yamagami F Tajiri K Yumino D Ieda M . Uremic toxins and atrial fibrillation: mechanisms and therapeutic implications. Toxins (Basel). (2019) 11(10):597. 10.3390/toxins11100597
37.
Heo GY Park JT Kim HJ Kim KW Kwon YU Kim SH et al Adequacy of dialysis and incidence of atrial fibrillation in patients undergoing hemodialysis. Circ Cardiovasc Qual Outcomes. (2024) 17(8):e010595. 10.1161/CIRCOUTCOMES.123.010595
38.
Ferrari R Bertini M Blomstrom-Lundqvist C Dobrev D Kirchhof P Pappone C et al An update on atrial fibrillation in 2014: from pathophysiology to treatment. Int J Cardiol. (2016) 203:22–9. 10.1016/j.ijcard.2015.10.089
39.
Huang S-Y Chen Y-A Chen S-A Chen Y-J Lin Y-K . Uremic toxins– novel arrhythmogenic factor in chronic kidney disease– related atrial fibrillation. Acta Cardiol Sin. (2016) 32(3):259. 10.6515/acs20151116a
40.
Bogatyrev SR Rolando JC Ismagilov RF . Self-reinoculation with fecal flora changes microbiota density and composition leading to an altered bile-acid profile in the mouse small intestine. Microbiome. (2020) 8(1):19. 10.1186/s40168-020-0785-4
41.
Ridlon JM Harris SC Bhowmik S Kang D-J Hylemon PB . Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. (2016) 7(1):22–39. 10.1080/19490976.2015.1127483
42.
Rainer PP Primessnig U Harenkamp S Doleschal B Wallner M Fauler G et al Bile acids induce arrhythmias in human atrial myocardium–implications for altered serum bile acid composition in patients with atrial fibrillation. Heart. (2013) 99(22):1685–92. 10.1136/heartjnl-2013-304163
43.
Pu J Yuan A Shan P Gao E Wang X Wang Y et al Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. (2013) 34(24):1834–45. 10.1093/eurheartj/ehs011
44.
Wang X-H Li Z Zang M-H Yao T-B Mao J-L Pu J . Circulating primary bile acid is correlated with structural remodeling in atrial fibrillation. J Interv Card Electrophysiol. (2020) 57(3):371–7. 10.1007/s10840-019-00540-z
45.
Desai MS Penny DJ . Bile acids induce arrhythmias: old metabolite, new tricks. Heart. (2013) 99(22):1629–30. 10.1136/heartjnl-2013-304546
46.
van Oort S Beulens JW van Ballegooijen AJ Grobbee DE Larsson SC . Association of cardiovascular risk factors and lifestyle behaviors with hypertension A Mendelian randomization study. Hypertension. (2020) 76:1971–9. 10.1161/HYPERTENSIONAHA.120.15761
47.
Yang T Santisteban MM Rodriguez V Li E Ahmari N Carvajal JM et al Gut dysbiosis is linked to hypertension. Hypertension. (2015) 65(6):1331–40. 10.1161/HYPERTENSIONAHA.115.05315
48.
Richards EM Li J Stevens BR Pepine CJ Raizada MK . Gut microbiome and neuroinflammation in hypertension. Circ Res. (2022) 130(3):401–17. 10.1161/CIRCRESAHA.121.319816
49.
Kim S Goel R Kumar A Qi Y Lobaton G Hosaka K et al Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. (2018) 132(6):701–18. 10.1042/CS20180087
50.
Robles-Vera I Toral M de la Visitación N Sánchez M Gómez-Guzmán M Muñoz R et al Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br J Pharmacol. (2020) 177(9):2006–23. 10.1111/bph.14965
51.
Santisteban MM Qi Y Zubcevic J Kim S Yang T Shenoy V et al Hypertension-Linked pathophysiological alterations in the gut. Circ Res. (2017) 120(2):312–23. 10.1161/CIRCRESAHA.116.309006
52.
Li J Zhao F Wang Y Chen J Tao J Tian G et al Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. (2017) 5:14. 10.1186/s40168-016-0222-x
53.
Naqvi S Asar TO Kumar V Al-Abbasi FA Alhayyani S Kamal MA et al A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother. (2021) 134:111156. 10.1016/j.biopha.2020.111156
54.
Avery EG Bartolomaeus H Maifeld A Marko L Wiig H Wilck N et al The gut microbiome in hypertension: recent advances and future perspectives. Circ Res. (2022) 128:934–50. 10.1161/CIRCRESAHA.121.318065
55.
Wang X Chen W Jin R Xu X Wei J Huang H et al Engineered probiotics Clostridium butyricum-pmtl007-glp-1 improves blood pressure via producing glp-1 and modulating gut microbiota in spontaneous hypertension rat models. Microb Biotechnol. (2022) 16(4):799–812. 10.1111/1751-7915.14196
56.
Xu J Moore BN Pluznick JL . Short-Chain fatty acid receptors and blood pressure regulation: council on hypertension mid-career award for research excellence 2021. Hypertension. (2022) 79(10):2127–37. 10.1161/HYPERTENSIONAHA.122.18558
57.
Bartolomaeus H Balogh A Yakoub M Homann S Markó L Höges S et al Short-Chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. (2019) 139(11):1407–21. 10.1161/CIRCULATIONAHA.118.036652
58.
Robles-Vera I Toral M de la Visitación N Sánchez M Gómez-Guzmán M Romero M et al Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Mol Nutr Food Res. (2020) 64(6):e1900616. 10.1002/mnfr.201900616
59.
Peng J Xiao X Hu M Zhang X . Interaction between gut microbiome and cardiovascular disease. Life Sci. (2018) 214:153–7. 10.1016/j.lfs.2018.10.063
60.
O’Donnell JA Zheng T Meric G Marques FZ . The gut microbiome and hypertension. Nat Rev Nephrol. (2023) 19(3):153–67. 10.1038/s41581-022-00654-0
61.
Ferguson JF Aden LA Barbaro NR Van Beusecum JP Xiao L Simons AJ et al High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. Jci Insight. (2019) 5(13):e126241. 10.1172/jci.insight.126241
62.
Rahman MM Islam F -Or-Rashid MH Mamun AA Rahaman MS Islam MM et al The gut Microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front Cell Infect Microbiol. (2022) 12:903570. 10.3389/fcimb.2022.903570
63.
Kaye DM Shihata WA Jama HA Tsyganov K Ziemann M Kiriazis H et al Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. (2020) 141(17):1393–403. 10.1161/CIRCULATIONAHA.119.043081
64.
Palacios T Vitetta L Coulson S Madigan CD Lam YY Manuel R et al Targeting the intestinal Microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: a randomised controlled pilot study. Nutrients. (2020) 12(7):2041. 10.3390/nu12072041
65.
Aydin OC Aydın S Barun S . Role of natural products and intestinal flora on type 2 diabetes mellitus treatment. World J Clin Cases. (2023) 11(1):65–72. 10.12998/wjcc.v11.i1.65
66.
Phang RJ Ritchie RH Hausenloy DJ Lees JG Lim SY . Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy. Cardiovasc Res. (2023) 119(3):668–90. 10.1093/cvr/cvac049
67.
Ciesielska A Matyjek M Kwiatkowska K . Tlr4 and CD14 trafficking and its influence on lps-induced pro-inflammatory signaling. Cell Mol Life Sci. (2021) 78(4):1233–61. 10.1007/s00018-020-03656-y
68.
Yan Y Zhang W Wang Y Yi C Yu B Pang X et al Crosstalk between intestinal flora and human iron metabolism: the role in metabolic syndrome-related comorbidities and its potential clinical application. Microbiol Res. (2024) 282:127667. 10.1016/j.micres.2024.127667
69.
Harrison AV Lorenzo FR Mcclain DA . Iron and the pathophysiology of diabetes. Annu Rev Physiol. (2023) 85:339–62. 10.1146/annurev-physiol-022522-102832
70.
Fuentes-Antrás J Ioan AM Tuñón J Egido J Lorenzo Ó . Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol. (2014) 2014:847827. 10.1155/2014/847827
71.
Murtaza G Virk HUH Khalid M Lavie CJ Ventura H Mukherjee D et al Diabetic cardiomyopathy—a comprehensive updated review. Prog Cardiovasc Dis. (2019) 62(4):315–26. 10.1016/j.pcad.2019.03.003
72.
Adhyaru BB Jacobson TA . Safety and efficacy of statin therapy. Nat Rev Cardiol. (2018) 15(12):757–69. 10.1038/s41569-018-0098-5
73.
Li C Stražar M Mohamed AMT Pacheco JA Walker RL Lebar T et al Gut microbiome and metabolome profiling in framingham heart study reveals cholesterol-metabolizing bacteria. Cell. (2024) 187(8):1834–52.e19. 10.1016/j.cell.2024.03.014
74.
Fan Y Pedersen O . Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19(1):55–71. 10.1038/s41579-020-0433-9
75.
Gawałko M Agbaedeng TA Saljic A Müller DN Wilck N Schnabel R et al Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc Res. (2022) 118(11):2415–27. 10.1093/cvr/cvab292
76.
Yoo W Zieba JK Foegeding NJ Torres TP Shelton CD Shealy NG et al High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science. (2021) 373(6556):813–8. 10.1126/science.aba3683
77.
Wang Z Bergeron N Levison BS Li XS Chiu S Jia X et al Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. (2019) 40(7):583–94. 10.1093/eurheartj/ehy799
78.
Ling Z Liu X Cheng Y Yan X Wu S . Gut microbiota and aging. Crit Rev Food Sci Nutr. (2022) 62(13):3509–34. 10.1080/10408398.2020.1867054
79.
Jie Z Xia H Zhong S-L Feng Q Li S Liang S et al The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. (2017) 8(1):845. 10.1038/s41467-017-00900-1
80.
Milani C Duranti S Bottacini F Casey E Turroni F Mahony J et al The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut Microbiota. Microbiol Mol Biol Rev. (2017) 81(4):10–128. 10.1128/MMBR.00036-17
81.
Galkin F Mamoshina P Aliper A Putin E Moskalev V Gladyshev VN et al Human gut microbiome aging clock based on taxonomic profiling and deep learning. iScience. (2020) 23(6):101199. 10.1016/j.isci.2020.101199
82.
Dehoux P Marvaud JC Abouelleil A Earl AM Lambert T Dauga C . Comparative genomics of Clostridium bolteae and Clostridium clostridioforme reveals species-specific genomic properties and numerous putative antibiotic resistance determinants. Bmc Genomics. (2016) 17(1):819. 10.1186/s12864-016-3152-x
83.
Ohira H Fujioka Y Katagiri C Mamoto R Aoyama-Ishikawa M Amako K et al Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb. (2013) 20(5):425–42. 10.5551/jat.15065
84.
López-Otín C Blasco MA Partridge L Serrano M Kroemer G . The hallmarks of aging. Cell. (2013) 153(6):1194–217. 10.1016/j.cell.2013.05.039
85.
Yang H Wang T Qian C Wang H Yu D Shi M et al Gut microbial-derived phenylacetylglutamine accelerates host cellular senescence. Nat Aging. (2025) 5(3):401–18. 10.1038/s43587-024-00795-w
86.
Pepke ML Hansen SB Limborg MT . Telomere dynamics as mediators of gut microbiota-host interactions. Trends Cell Biol. (2024) 34(10):805–8. 10.1016/j.tcb.2024.08.003
87.
Liu X Khalil AEMM Muthukumarasamy U Onogi Y Yan X Singh I et al Reduced intestinal lipid absorption improves glucose metabolism in aged G2-terc knockout mice. Bmc Biol. (2023) 21(1):150. 10.1186/s12915-023-01629-8
88.
Hold GL . Western lifestyle: a ‘master’ manipulator of the intestinal microbiota?Gut. (2014) 63(1):5–6. 10.1136/gutjnl-2013-304969
89.
Atarashi K Tanoue T Oshima K Suda W Nagano Y Nishikawa H et al Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. (2013) 500(7461):232–6. 10.1038/nature12331
90.
Pagliai G Russo E Niccolai E Dinu M Di Pilato V Magrini A et al Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and scfa: the cardiveg study. Eur J Nutr. (2020) 59(5):2011–24. 10.1007/s00394-019-02050-0
91.
Haro C Montes-Borrego M Rangel-Zúñiga OA Alcalá-Díaz JF Gómez-Delgado F Pérez-Martínez P et al Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J Clin Endocrinol Metab. (2016) 101(1):233–42. 10.1210/jc.2015-3351
92.
Raghuveer G Hartz J Lubans DR Takken T Wiltz JL Mietus-Snyder M et al Cardiorespiratory fitness in youth: an important marker of health: a scientific statement from the American Heart Association. Circulation. (2020) 142(7):e101–18. 10.1161/CIR.0000000000000866
93.
Campaniello D Corbo MR Sinigaglia M Speranza B Racioppo A Altieri C et al How diet and physical activity modulate gut Microbiota: evidence, and perspectives. Nutrients. (2022) 14(12):2456. 10.3390/nu14122456
94.
Verhaar BJH Prodan A Nieuwdorp M Muller M . Gut Microbiota in hypertension and atherosclerosis: a review. Nutrients. (2020) 12(10):2982. 10.3390/nu12102982
95.
Canudas S Becerra-Tomás N Hernández-Alonso P Galié S Leung C Crous-Bou M et al Mediterranean Diet and telomere length: a systematic review and meta-analysis. Adv Nutr. (2020) 11(6):1544–54. 10.1093/advances/nmaa079
96.
Khan MS Khan F Fonarow GC Sreenivasan J Greene SJ Khan SU et al Dietary interventions and nutritional supplements for heart failure: a systematic appraisal and evidence map. Eur J Heart Fail. (2021) 23(9):1468–76. 10.1002/ejhf.2278
97.
Sebastian SA Padda I Johal G . Long-term impact of Mediterranean diet on cardiovascular disease prevention: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. (2024) 49(5):102509. 10.1016/j.cpcardiol.2024.102509
98.
Dai X-C Yu Y Zhou S-Y Yu S Xiang M-X Ma H . Assessment of the causal relationship between gut microbiota and cardiovascular diseases: a bidirectional Mendelian randomization analysis. BioData Min. (2024) 17(1):6. 10.1186/s13040-024-00356-2
99.
Zuo K Yin X Li K Zhang J Wang P Jiao J et al Different types of atrial fibrillation share patterns of gut Microbiota dysbiosis. mSphere. (2020) 5(2):10–1128. 10.1128/mSphere.00071-20
100.
De Filippis F Pellegrini N Vannini L Jeffery IB La Storia A Laghi L et al High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. (2016) 65(11):1812–21. 10.1136/gutjnl-2015-309957
101.
Lombardo M Aulisa G Marcon D Rizzo G Tarsisano MG Di Renzo L et al Association of urinary and plasma levels of trimethylamine N-oxide (tmao) with foods. Nutrients. (2021) 13(5):1426. 10.3390/nu13051426
102.
Gutiérrez-Díaz I Fernández-Navarro T Salazar N Bartolomé B Moreno-Arribas MV de Andres-Galiana EJ et al Adherence to a Mediterranean diet influences the fecal metabolic profile of microbial-derived phenolics in a Spanish cohort of middle-age and older people. J Agric Food Chem. (2017) 65(3):586–95. 10.1021/acs.jafc.6b04408
103.
Flint HJ Scott KP Duncan SH Louis P Forano E . Microbial degradation of complex carbohydrates in the gut. Gut Microbes. (2012) 3(4):289–306. 10.4161/gmic.19897
104.
Nie K Ma K Luo W Shen Z Yang Z Xiao M et al Roseburia intestinalis: a beneficial gut organism from the discoveries in genus and Species. Front Cell Infect Microbiol. (2021) 11:757718. 10.3389/fcimb.2021.757718
105.
Yan Q Zhai W Yang C Li Z Mao L Zhao M et al The relationship among physical activity, intestinal Flora, and cardiovascular disease. Cardiovasc Ther. (2021) 2021:3364418. 10.1155/2021/3364418
106.
Ticinesi A Lauretani F Tana C Nouvenne A Ridolo E Meschi T . Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc Immunol Rev. (2019) 25:84–95. PMID: 30753131
107.
Casteleyn C Rekecki A Van Der Aa A Simoens P Van Den Broeck W . Surface area assessment of the murine intestinal tract as a prerequisite for oral dose translation from mouse to man. Lab Anim. (2010) 44(3):176–83. 10.1258/la.2009.009112
108.
Cunliffe RN Rose FR Keyte J Abberley L Chan WC Mahida YR . Human defensin 5 is stored in precursor form in normal paneth cells and is expressed by some villous epithelial cells and by metaplastic paneth cells in the colon in inflammatory bowel disease. Gut. (2001) 48(2):176–85. 10.1136/gut.48.2.176
109.
Ghosh D Porter E Shen B Lee SK Wilk D Drazba J et al Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. (2002) 3(6):583–90. 10.1038/ni797
110.
Ley RE Bäckhed F Turnbaugh P Lozupone CA Knight RD Gordon JI . Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. (2005) 102(31):11070–5. 10.1073/pnas.0504978102
111.
Zhang J Zhu P Li S Gao Y Xing Y . From heart failure and kidney dysfunction to cardiorenal syndrome: tmao may be a bridge. Front Pharmacol. (2023) 14:1291922. 10.3389/fphar.2023.1291922
112.
Pignanelli M Bogiatzi C Gloor G Allen-Vercoe E Reid G Urquhart BL et al Moderate renal impairment and toxic metabolites produced by the intestinal microbiome: dietary implications. J Ren Nutr. (2019) 29(1):55–64. 10.1053/j.jrn.2018.05.007
113.
Missailidis C Hällqvist J Qureshi AR Barany P Heimbürger O Lindholm B et al Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. (2016) 11(1):e0141738. 10.1371/journal.pone.0141738
114.
Li Z Gu M Zaparte A Fu X Mahen K Mrdjen M et al Alcohol-induced gut microbial reorganization and associated overproduction of phenylacetylglutamine promotes cardiovascular disease. Nat Commun. (2024) 15(1):10788. 10.1038/s41467-024-55084-2
115.
Reddy YNV Borlaug BA Gersh BJ . Management of atrial fibrillation across the Spectrum of heart failure with preserved and reduced ejection fraction. Circulation. (2022) 146(4):339–57. 10.1161/CIRCULATIONAHA.122.057444
116.
Verdecchia P Angeli F Reboldi G . Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res. (2018) 122(2):352–68. 10.1161/CIRCRESAHA.117.311402
117.
Granger CB Mahaffey KW . Preventing atrial fibrillation with treatments for diabetes Mellitus. Circulation. (2020) 141(15):1235–7. 10.1161/CIRCULATIONAHA.120.045864
118.
Song J Navarro-Garcia JA Wu J Saljic A Abu-Taha I Li L et al Chronic kidney disease promotes atrial fibrillation via inflammasome pathway activation. J Clin Invest. (2023) 133(19):e167517. 10.1172/JCI167517
119.
Chen AT Zhang J Zhang Y . Gut microbiota in heart failure and related interventions. Imeta. (2023) 2(3):e125. 10.1002/imt2.125
120.
Weersma RK Zhernakova A Fu J . Interaction between drugs and the gut microbiome. Gut. (2020) 69(8):1510–9. 10.1136/gutjnl-2019-320204
121.
Smith MI Yatsunenko T Manary MJ Trehan I Mkakosya R Cheng J et al Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. (2013) 339(6119):548–54. 10.1126/science.1229000
122.
Wang J Thingholm LB Skiecevičienė J Rausch P Kummen M Hov JR et al Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. (2016) 48(11):1396–406. 10.1038/ng.3695
123.
Díaz-López A Babio N Martínez-González MA Corella D Amor AJ Fitó M et al Erratum. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes care 2015;38:2134–2141. Diabetes Care. (2018) 41(10):2260–1. 10.2337/dc18-er10a
124.
Sohouli MH Ozovanu OD Fatahi S Hekmatdoost A . Impact of probiotic supplementation on trimethylamine N-oxide (tmao) in humans: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr Espen. (2022) 50:56–62. 10.1016/j.clnesp.2022.06.006
125.
Eze UJ Lal A Elkoush MI Halytska M Atif S . Recurrent Lactobacillus rhamnoses bacteremia and complications in an immunocompromised patient with history of probiotic use: a case report. Cureus. (2024) 16(2):e54879. 10.7759/cureus.54879
126.
Basterra-Gortari FJ Ruiz-Canela M Martínez-González MA Babio N Sorlí JV Fito M et al Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the predimed trial. Diabetes Care. (2019) 42(8):1390–7. 10.2337/dc18-2475
127.
Martínez-del Campo A Bodea S Hamer HA Marks JA Haiser HJ Turnbaugh PJ et al Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio. (2015) 6(2):10–1128. 10.1128/mBio.00042-15
Summary
Keywords
atrial fibrillation, autonomic nervous system, gut flora, inflammasome, microbial metabolites, myocardial fibrosis
Citation
Wang C, Shen M, Zhao Y, Xu Z, Huang Y and Xiang H (2025) Exploring the effect of gut flora on atrial fibrillation based on epidemiological history. Front. Cardiovasc. Med. 12:1655007. doi: 10.3389/fcvm.2025.1655007
Received
27 June 2025
Revised
05 December 2025
Accepted
05 December 2025
Published
19 December 2025
Volume
12 - 2025
Edited by
Rui Providencia, University College London, United Kingdom
Reviewed by
Nikolaos Fragakis, Aristotle University of Thessaloniki, Greece
Xintian Cai, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Manlu Fan, Shandong University of Traditional Chinese Medicine, China
Updates
Copyright
© 2025 Wang, Shen, Zhao, Xu, Huang and Xiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Hongjie Xiang xhjlyh@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.