Abstract
Background:
Diastolic dysfunction (DD) represents an early indicator of cardiac impairment and is strongly linked to adverse cardiovascular outcomes. While obesity-related indices have been associated with DD, the role of Body Roundness Index (BRI)-a novel adiposity measure reflecting body shape-remains unclear. This study aimed to evaluate the association between BRI and diastolic function in a community-based cohort.
Methods:
This cross-sectional analysis included 1,466 participants from the Longitudinal Investigation of Osteoarthritis and Cardiovascular Health Status cohort. BRI was calculated using a validated formula, and DD was assessed via echocardiographic parameters. BRI's optimal cutoff was derived via 1000-iteration bootstrap ROC analysis. Multivariable regression models were used to evaluate associations, adjusting for age, sex, blood pressure, lipid profile, and cardiovascular comorbidities.
Results:
Higher BRI was significantly associated with increased odds of DD. In fully adjusted logistic regression, each 1-unit increase in BRI was associated with 14.4% higher odds of DD (OR = 1.144, 95% CI: 1.046–1.251, P = 0.003). High BRI (≥4.2) was linked to 41.7% higher odds of DD (OR = 1.417, 95% CI: 1.114–1.804, P = 0.004). Robust regression confirmed BRI was inversely associated with septal e’ (β = −0.164, P = 8.10 × 10−4) and lateral e' velocities (β=−0.167, P = 5.59 × 10−3), and marginally positively associated with E/e' ratio (β=0.121, P = 0.053). Restricted cubic spline models showed a nonlinear association between BRI and DD probability (P < 0.001 for overall association, P = 0.006 for linearity). Interaction analyses indicated BRI's effect on DD was not modulated by blood pressure and lipid profiles. Subgroup analyses indicated a consistent trend of association between BRI and DD.
Conclusions:
BRI is nonlinearly and independently associated with impaired diastolic function in middle-aged and older adults, with modest diagnostic performance. These findings provided evidence on the link between body shape metrics and DD.
Introduction
Cardiovascular diseases (CVDs) remain a leading cause of morbidity and mortality worldwide (1), with diastolic dysfunction (DD) emerging as a critical early marker of cardiac impairment. Diastolic dysfunction is associated with an increased risk of heart failure, arrhythmias, and cardiovascular events, underscoring the need for early identification of modifiable risk factors (2–7). While body mass index (BMI) has long been used to characterize adiposity, it fails to capture body shape and regional fat distribution, which may better reflect cardiometabolic risk (8–10). The Body Roundness Index (BRI), a novel adiposity metric derived from waist circumference and height, offers a more nuanced assessment of body shape independent of BMI (11, 12). Unlike BMI, BRI emphasizes central adiposity, a known contributor to cardiac remodeling and dysfunction. Preclinical and clinical studies have suggested that abdominal obesity is linked to impaired diastolic function, but the specific role of BRI in this context remains underexplored (13). Understanding this relationship is crucial for developing targeted screening strategies, as BRI may serve as a simple, non-invasive marker to identify individuals at risk for DD in primary care settings (14).
This cross-sectional study aims to fill this gap by investigating the association between BRI and diastolic function in a community-based cohort of middle-aged and older adults. We hypothesize that BRI is independently associated with DD, even after adjusting for traditional cardiovascular risk factors. By leveraging echocardiographic measures of diastolic function (e.g., E/e’ ratio, septal and lateral e’ velocities), we seek to characterize the nature of this association and explore potential effect modification by blood pressure, lipid profiles, and demographic factors. These findings may inform the integration of body shape metrics into routine cardiac health assessments, enhancing early detection and prevention of CVDs.
Methods
Study population
This cross-sectional analysis utilized baseline data from the Longitudinal Investigation of Osteoarthritis and Cardiovascular Health Status cohort, a prospective observational study (15) conducted in Luohe City and its surrounding areas in China between November 2023 and November 2024. The parent cohort aimed to examine the association between osteoarthritis and major adverse cardiovascular events (MACE), adhering to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (16).
Eligible participants were middle-aged and older adults (35–75 years) who had resided locally for ≥5 years, were willing to undergo cardiovascular and joint imaging (ultrasound and digital radiography), and could commit to ≥5 years of follow-up. Exclusion criteria included acute cardiovascular conditions (e.g., myocardial infarction, cardiogenic shock), malignancy, life-threatening trauma, severe psychiatric/cognitive impairment, or chronic kidney disease (estimated glomerular filtration rate <30 mL/min).
Of the 1,762 individuals enrolled in the parent cohort, 1,466 were included in this analysis after excluding 292 without sufficient echocardiographic data and 4 with missing waist circumference or height (required for BRI calculation).
Measurements
BRI was calculated using the formula: BRI = 364.2–365.5 * sqrt{1 – [WC/(2 * π * Height/2)]^2}, in which waist circumference was measured at the umbilical level, and height was recorded using a stadiometer.
Transthoracic echocardiography was performed by trained sonographers using a Philips Epic-7 system (Philips Healthcare, Amsterdam, Netherlands). Key diastolic function parameters were measured and defined in accordance with the 2025 American Society of Echocardiography (ASE) guidelines (17): Mitral annular e’ velocity was measured via tissue Doppler imaging (TDI) at the septal and lateral mitral annulus in the apical four-chamber view, with average e’ calculated as the mean of septal and lateral e’ velocities, and age-dependent cutoffs for impaired left ventricular (LV) relaxation applied-specifically, average e’ < 9 cm/s for 20–39 years, average e’ < 7 cm/s for 40–65 years, and average e’ < 6.5 cm/s for those >65 years; the E/e’ ratio was calculated as the ratio of transmitral early diastolic velocity (E, measured via pulsed-wave Doppler at the mitral leaflet tips) to average e’ velocity, with an average E/e’ > 14 defined as elevated LV filling pressure. Diastolic dysfunction was determined via two conditions: (1) reduced e' velocity; (2) additional markers of diastolic impairment were considered: mitral E/A ratio ≤ 0.8 or ≥ 2, mitral E/e' ratio > 14, and left atrial volume index (LAVI) > 34 mL/m2. Finally, diastolic dysfunction was diagnosed if either of the following criteria were satisfied: (1) reduced e' velocity plus at least 1 abnormal additional marker, or (2) preserved e' velocity plus at least 2 abnormal additional markers; all other cases were classified as normal diastolic function (17, 18). All the measurements of cardiac ultrasound followed clinical quality control standards.
Besides, each participant provided a comprehensive set of clinical data including age, gender, smoking status (classified as current smoker or not), alcohol use (classified as yes or no), self-reported medical history data on the presence of hypertension, diabetes, coronary heart disease (CHD) and stroke which were confirmed by medical records when possible. Blood pressure measured in the sitting position with a calibrated sphygmomanometer where the average of two consecutive measurements was recorded, morning blood samples collected after at least 8 h of fasting to measure lipid profiles including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides and blood glucose levels. BFR was calculated using a validated anthropometric formula: BFR = 1.20 × BMI + 0.23 × Age − 16.2 (5.4 for female).
Statistical analysis
For baseline characteristics, continuous variables underwent normality testing using the Shapiro–Wilk test. Normally distributed variables were presented as mean ± standard deviation and compared via independent t-tests. Non-normally distributed variables were reported as median [interquartile range (IQR)] and compared using the Mann–Whitney U test. Categorical variables were expressed as frequencies (%) and analyzed via chi-square tests. To evaluate BRI's discriminatory ability for DD, a receiver operating characteristic (ROC) curve was constructed. A 1000-iteration bootstrap was performed to correct for optimism bias, generating the bootstrap-corrected area under the curve (AUC) and 95% confidence intervals (CIs). The optimal BRI cutoff was determined using the Youden index (sensitivity + specificity − 1), distinguishing participants into low BRI and high BRI groups. Additionally, the comparative discriminative performance of BRI vs. conventional adiposity indices was evaluated by constructing unadjusted ROC curves for waist circumference, body mass index (BMI), and body fat ratio (BFR), followed by comparison of their areas under the curve (AUCs). Logistic regression was used to assess the association between Body Roundness Index (BRI)-both as a continuous variable (per 1-unit increase) and a categorical variable (dichotomized at the optimal cutoff, rounded to 4.2 for clinical interpretability)-and DD, with three models constructed: Model 1 (unadjusted), Model 2 (adjusted for age and sex), and Model 3 [fully adjusted for age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), TC, LDL-C, HDL-C, triglycerides, current smoking, alcohol use, hypertension, diabetes, CHD, and stroke], and odds ratios (ORs) with 95% confidence intervals (CIs) are reported. We also used regression models to evaluate the association between BRI and continuous diastolic function metrics (septal e’, lateral e’, E/e’ ratio). Prior to analysis, residual normality was assessed. If residuals were non-normal, robust regression with Huber-White standard errors was used to mitigate the impact of outliers; otherwise, the linear regression model was adopted. Models were adjusted similarly to logistic regression, and beta coefficients (β) with standard errors (SEs) were reported. A restricted cubic spline (RCS) model with 3 knots was used to assess the linearity of the association between BRI and DD probability, and P-values for overall association and nonlinearity are reported. Interaction effects between BRI and cardiovascular risk factors (blood pressure, lipid profiles) on DD were evaluated using multiplicative terms in regression models, with statistically significant interactions visualized. Subgroup analyses involved stratified analyses conducted by age (<50, 50–60, >60 years), sex, current smoking, alcohol use, hypertension, diabetes, CHD, and stroke, and forest plots were used to visualize ORs and 95% CIs for each subgroup. All analyses were performed using R software (version 4.4.2).
Results
Baseline characteristics by BRI category
Using the optimal cutoff of 4.18 for BRI (derived from bootstrap ROC analysis, Figure 1A), participants were stratified into low BRI (<4.2, n = 662) and high BRI (≥4.2, n = 804) groups for clinical interpretability. Baseline characteristics differed significantly between groups (Table 1). High BRI participants had a higher median age (60 vs. 59 years, P = 0.040) and a higher prevalence of hypertension (45% vs. 33%, P < 0.001) and diabetes (10% vs. 6.8%, P = 0.022). Biochemical markers showed unfavorable profiles in the high BRI group, including lower high-density lipoprotein cholesterol (HDL-C: 1.35 vs. 1.43 mmol/L, P < 0.001) and higher triglycerides (1.80 vs. 1.39 mmol/L, P < 0.001), alongside higher systolic (152 vs. 150 mmHg, P < 0.001) and diastolic blood pressure (92 vs. 89 mmHg, P = 0.001). Anthropometrically, high BRI individuals had significantly larger waist circumference (94 vs. 80 cm, P < 0.001), higher body mass index (27.7 vs. 23.6 kg/m2, P < 0.001), and greater body fat ratio (0.38 vs. 0.27, P < 0.001). Echocardiographic parameters reflecting diastolic function also differed between groups. High BRI participants had lower septal e' velocity (6.70 vs. 7.22 cm/s, P < 0.001), lower lateral e’ velocity (9.2 vs. 9.8 cm/s, P < 0.001), and higher E/e' ratio (8.37 vs. 7.88, P = 0.009). The prevalence of DD, redefined per the 2025 American Society of Echocardiography (ASE) guidelines (either age-adjusted reduced average e' or elevated average E/e'), was significantly higher in the high BRI group (39% vs. 28%, P < 0.001). In contrast, left ventricular hypertrophy (6.5% vs. 5.9%, P = 0.735) and left atrial enlargement (21% vs. 19%, P = 0.501) showed no significant between-group differences.
Figure 1
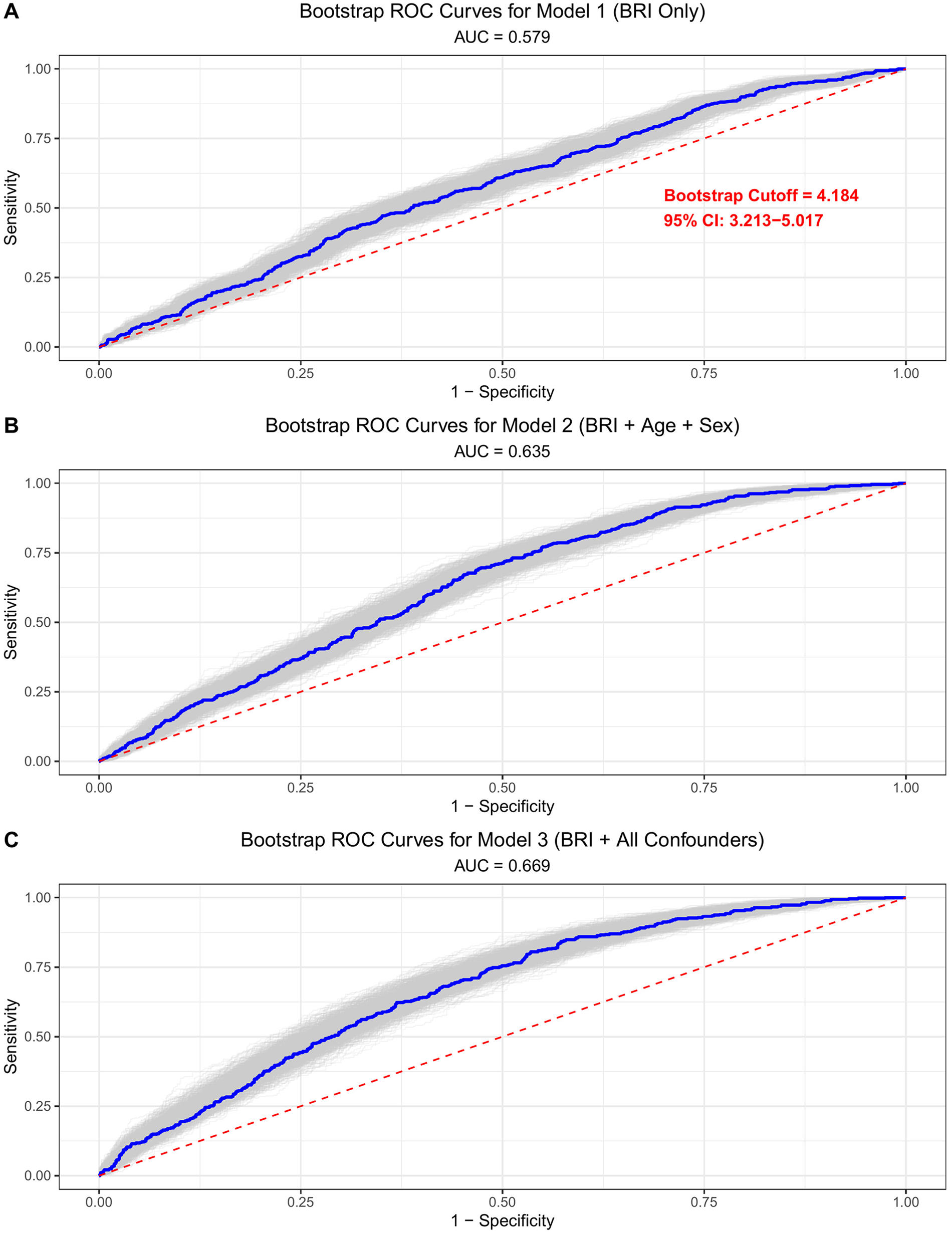
ROC curves illustrating the discriminative ability of BRI for identifying DD. Analyses were performed using 1000 bootstrap resamplings to assess stability; the optimal cutoff was determined by maximizing the Youden index (sensitivity + specificity − 1). (A) Model 1 is unadjusted. (B) Model 2 is adjusted for age and sex. (C) Model 3 is adjusted for all covariates (age, sex, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, current smoking, alcohol use, hypertension, diabetes, coronary heart disease, and stroke).
Table 1
| Variable | Overall N = 1,466a | Low BRI N = 662a | High BRI N = 804a | P-valueb |
|---|---|---|---|---|
| Age (years) | 59 (53, 67) | 59 (52, 67) | 60 (54, 68) | 0.040 |
| Male | 600 (41%) | 277 (42%) | 323 (40%) | 0.553 |
| Current Smoking | 321 (22%) | 156 (24%) | 165 (21%) | 0.181 |
| Alcohol use | 135 (9.2%) | 51 (8.2%) | 84 (10%) | 0.086 |
| Hypertension | 579 (39%) | 220 (33%) | 359 (45%) | <0.001 |
| Diabetes | 128 (8.7%) | 45 (6.8%) | 83 (10%) | 0.022 |
| Coronary Heart disease | 96 (6.5%) | 41 (6.2%) | 55 (6.8%) | 0.695 |
| Stroke | 164 (11%) | 69 (10%) | 95 (12%) | 0.448 |
| total cholesterol (mmol/L) | 5.21 (4.22, 6.43) [n = 3] | 5.13 (4.15, 6.46) [n = 1] | 5.27 (4.28, 6.40) [n = 2] | 0.377 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.97 (2.19, 4.16) [n = 46] | 2.95 (2.16, 4.21) [n = 25] | 2.97 (2.23, 4.10) [n = 21] | 0.869 |
| High-density lipoprotein cholesterol (mmol/L) | 1.39 (1.20, 1.61) [n = 4] | 1.43 (1.23, 1.69) [n = 4] | 1.35 (1.18, 1.57) | <0.001 |
| Triglycerides (mmol/L) | 1.64 (1.16, 2.30) [n = 1] | 1.39 (1.05, 2.13) [n = 1] | 1.80 (1.31, 2.44) | <0.001 |
| Glucose (mmol/L) | 5.60 (5.30, 6.30) | 5.50 (5.20, 6.10) | 5.70 (5.30, 6.50) | <0.001 |
| Systolic blood pressure (mmHg) | 151 (137, 163) | 150 (134, 162) | 152 (142, 163) | <0.001 |
| Diastolic blood pressure (mmHg) | 91 (81, 101) | 89 (80, 100) | 92 (82, 101) | 0.001 |
| Waist Circumference (cm) | 88 (81, 95) | 80 (76, 85) | 94 (90, 99) | <0.001 |
| Body mass index (kg/m2) | 25.7 (23.6, 28.1) | 23.6 (21.9, 25.0) | 27.7 (26.0, 29.5) | <0.001 |
| Body fat ratio | 0.32 (0.24, 0.40) | 0.27 (0.20, 0.33) | 0.38 (0.28, 0.43) | <0.001 |
| Left ventricular mass index (g/m2) | 77 (67, 89) [n = 1] | 77 (66, 89) [n = 1] | 79 (67, 90) | 0.034 |
| Left ventricular ejection fraction (%) | 68 (63, 72) [n = 4] | 68 (64, 72) [n = 4] | 68 (63, 71) | 0.033 |
| Left atrial volume index (mL/m2) | 27 (21, 34) [n = 2] | 27 (21, 34) [n = 1] | 28 (22, 34) [n = 1] | 0.002 |
| Relative wall thickness | 0.38 (0.34, 0.42) [n = 1] | 0.37 (0.33, 0.41) [n = 1] | 0.38 (0.34, 0.42) | <0.001 |
| Mitral valve E/e’ (ratio) | 8.12 (6.32, 10.16) | 7.88 (6.24, 9.82) | 8.37 (6.46, 10.43) | 0.009 |
| Mitral valve septal e’ velocity (cm/s) | 6.94 (5.60, 8.89) | 7.22 (5.83, 9.12) | 6.70 (5.36, 8.62) | <0.001 |
| Mitral valve lateral e’ velocity (cm/s) | 9.5 (7.5, 11.6) | 9.8 (7.9, 12.2) | 9.2 (7.4, 11.2) | <0.001 |
| Diastolic dysfunction | 495 (34%) | 185 (28%) | 310 (39%) | <0.001 |
| Left ventricular hypertrophy | 91 (6.2%) [n = 1] | 39 (5.9%) [n = 1] | 52 (6.5%) | 0.735 |
| Left atrial enlargement | 296 (20%) [n = 2] | 128 (19%) [n = 1] | 168 (21%) [n = 1] | 0.501 |
Baseline characteristics by BRI category (cutoff =4.2).
Median (IQR) [n = missing];
Mann–Whitney U test; Pearson's Chi-squared test. [n = X] indicates the number of individuals lacking data of the measurement.
BRI and diastolic dysfunction: logistic regression results
Bootstrap ROC analysis (1000 iterations) demonstrated BRI's discriminatory ability for DD, with a corrected area under the curve (AUC) of 0.67 (95% CI: 0.65–0.70, Figure 1). In head-to-head comparison using unadjusted ROC curves, BRI showed marginally higher AUC than waist circumference (0.579 vs. 0.570), BMI (0.555), and BFR (0.551) (Supplementary Figure S1). Multivariable logistic regression results for the association between BRI and DD are presented in Table 2. In the unadjusted model (Model 1), each 1-unit increase in BRI was associated with 23.2% higher odds of DD (OR = 1.249, 95% CI: 1.147–1.360, P = 5.6 × 10−7). After adjusting for age and sex (Model 2), the association remained significant (OR = 1.205, 95% CI: 1.108–1.31, P = 1.3 × 10−5). In the fully adjusted model (Model 3), which included cardiovascular risk factors (blood pressure, lipid profile, smoking, alcohol use) and comorbidities (hypertension, diabetes, coronary heart disease, stroke), the association was attenuated but remained statistically significant: each 1-unit BRI increase was associated with a 14.4% higher odds of DD (OR = 1.144, 95% CI: 1.046–1.251, P = 0.003). When comparing BRI categories, high BRI (≥4.2) was associated with 41.7% higher odds of DD in the fully adjusted model (OR = 1.417, 95% CI: 1.114–1.804, P = 0.004).
Table 2
| BRI parameters | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Each 1 unit increment | 1.249 (1.147,1.360) | 5.6E−07 | 1.205 (1.108,1.31) | 1.3E−05 | 1.144 (1.046,1.251) | 0.003 |
| Low BRI (<4.2) vs. High BRI (≥4.2) | 1.618 (1.297,2.019) | 2.0E-05 | 1.573 (1.254,1.974) | 9.1E-05 | 1.417 (1.114,1.804) | 0.004 |
Logistic regression assessing the association between BRI and DD.
Model 1 is unadjusted. Model 2 is adjusted for age and sex. Model 3 is adjusted for all covariates (age, sex, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, current smoking, alcohol use, hypertension, diabetes, coronary heart disease, and stroke).
BRI and diastolic function parameters: robust regression results
Robust regression analyses revealed inverse associations between BRI and diastolic function indices (Table 3). In Model 3, each 1-unit BRI increase was associated with a 0.164 cm/s decrease in septal e’ velocity (β = −0.164, SE = 0.049, P = 8.10 × 10−4) and a 0.167 cm/s decrease in lateral e’ velocity (β = −0.167, SE = 0.060, P = 5.59 × 10−3). The E/e’ ratio showed a positive association with BRI (β = 0.121, SE = 0.062, P = 0.053). These findings indicate that higher BRI is linked to reduced myocardial relaxation and increased filling pressure.
Table 3
| Diastolic parameters | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (SE) | P-value | β (SE) | P-value | β (SE) | P-value | |
| Septal e' | −0.282 (0.048) | 5.21E-09 | -0.265 (0.048) | 3.02E-08 | −0.164 (0.049) | 8.10E-04 |
| Lateral e' | -0.319 (0.063) | 4.95E-07 | -0.283 (0.060) | 2.40E-06 | -0.167 (0.060) | 5.59E-03 |
| E/e' | 0.253 (0.059) | 1.91E-05 | 0.214 (0.060) | 3.31E-04 | 0.121 (0.062) | 0.053 |
Robust regression assessing the association between BRI and diastolic parameters.
Model 1 is unadjusted. Model 2 is adjusted for age and sex. Model 3 is adjusted for all covariates (age, sex, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, current smoking, alcohol use, hypertension, diabetes, coronary heart disease, and stroke).
Nonlinearity and interaction effects
Restricted cubic spline modeling (Figure 2) showed a nonlinear relationship between BRI and DD probability, with P < 0.001 for overall association and P = 0.006 for linearity. Interaction analyses (Figure 3) revealed that BRI's effect on DD was not modified by blood pressure and lipid profiles.
Figure 2
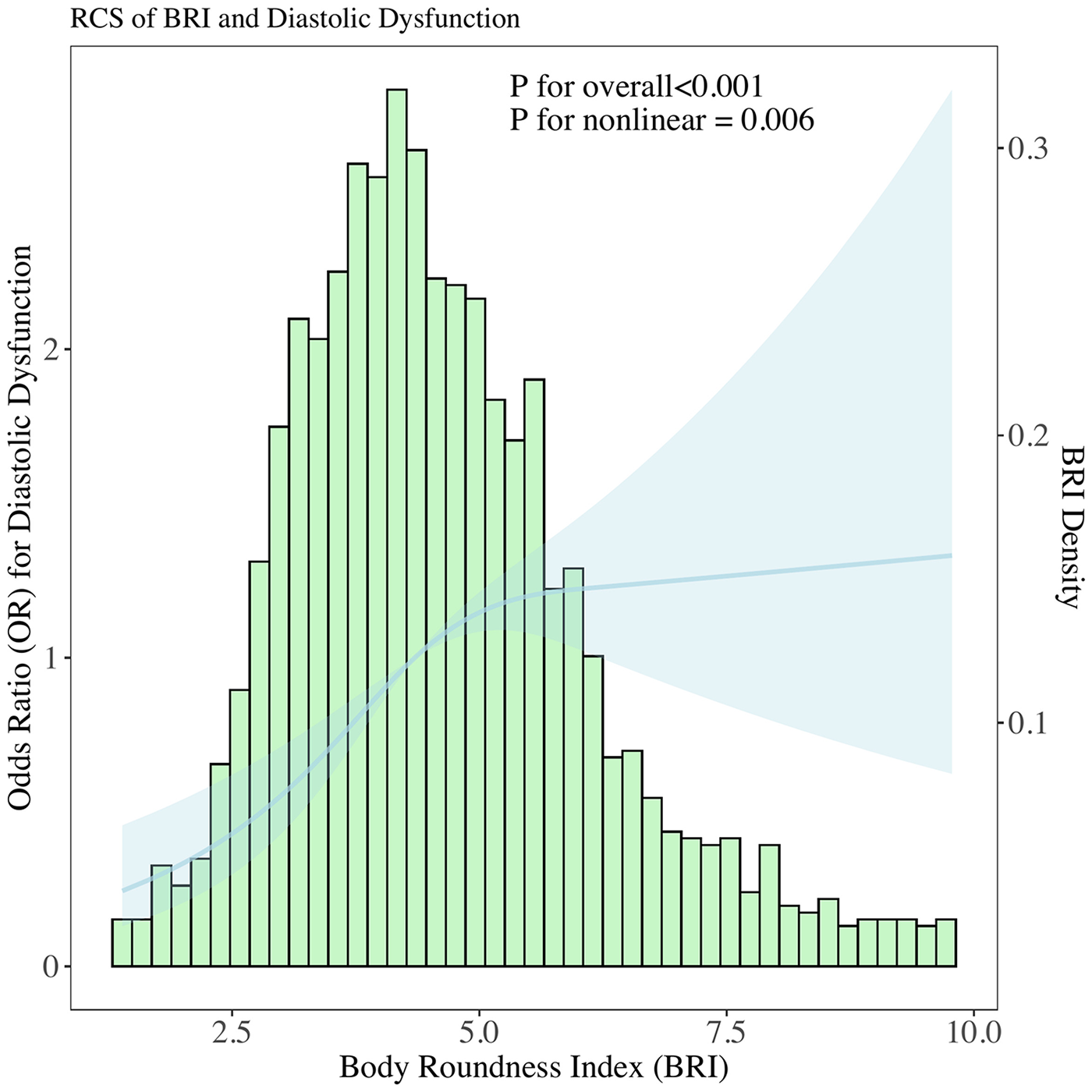
Histogram of BRI distribution overlaid with a restricted cubic spline model, examining the functional form of the association between BRI and the probability of DD. Spline included 3 knots to flexibly capture potential non-linear relationships.
Figure 3
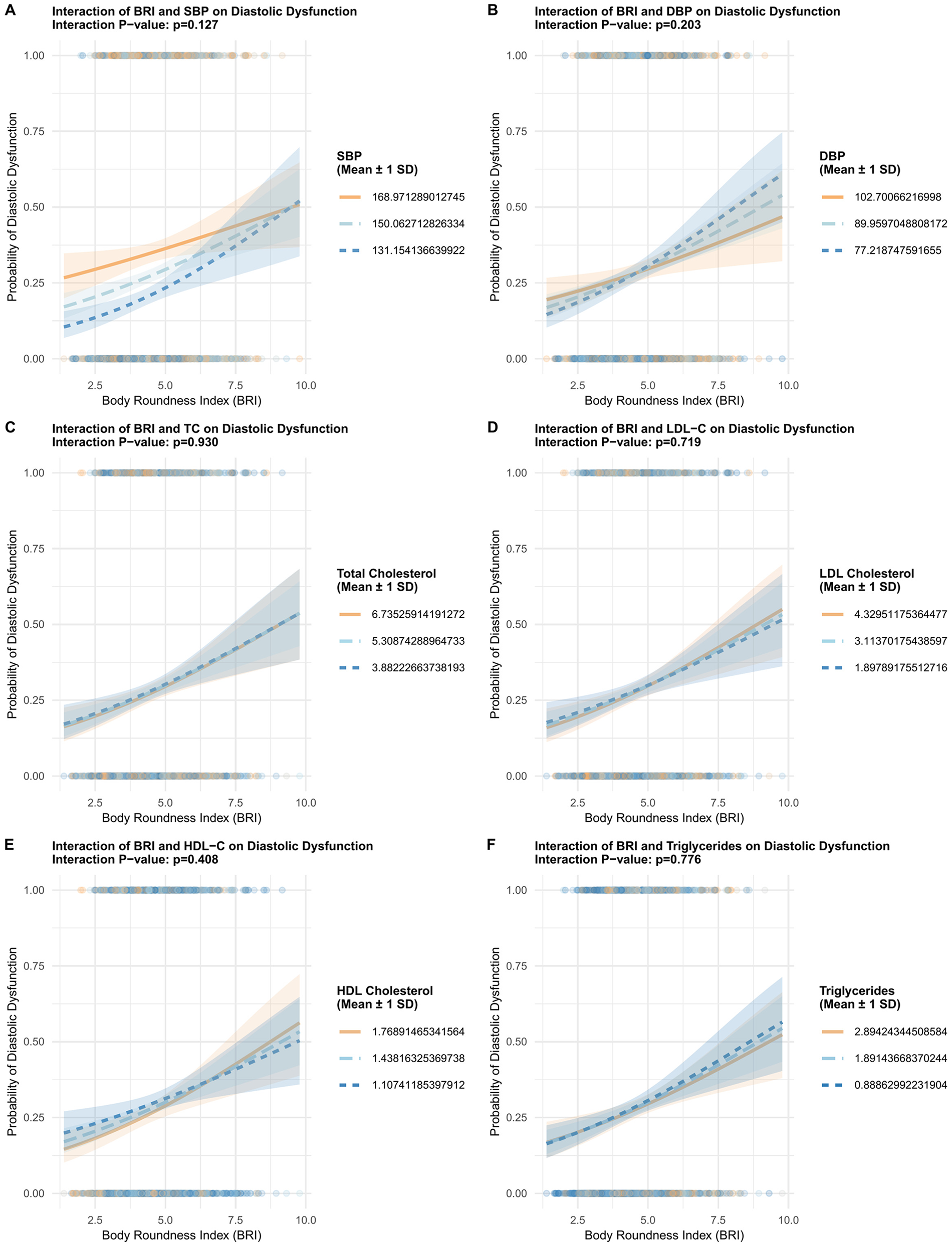
Interaction analyses investigating the modifying effects of blood pressure and lipid profiles on the association between BRI and DD. Panel A shows BRI and systolic blood pressure (SBP) with p = 0.127. Panel B displays BRI and diastolic blood pressure (DBP) with p = 0.203. Panel C depicts BRI and total cholesterol with p = 0.930. Panel D presents BRI and LDL cholesterol with p = 0.719. Panel E illustrates BRI and HDL cholesterol with p = 0.408. Panel F shows BRI and triglycerides with p = 0.776. Each graph features shaded areas representing mean plus or minus one standard deviation.
Subgroup analyses
Subgroup analyses by age, sex, current smoking, alcohol use, hypertension, diabetes, coronary heart disease, and stroke indicated a consistent trend of association between BRI and DD in almost all subgroups, either by each 1-unit BRI increase of by high and low levels of BRI (Figure 4).
Figure 4
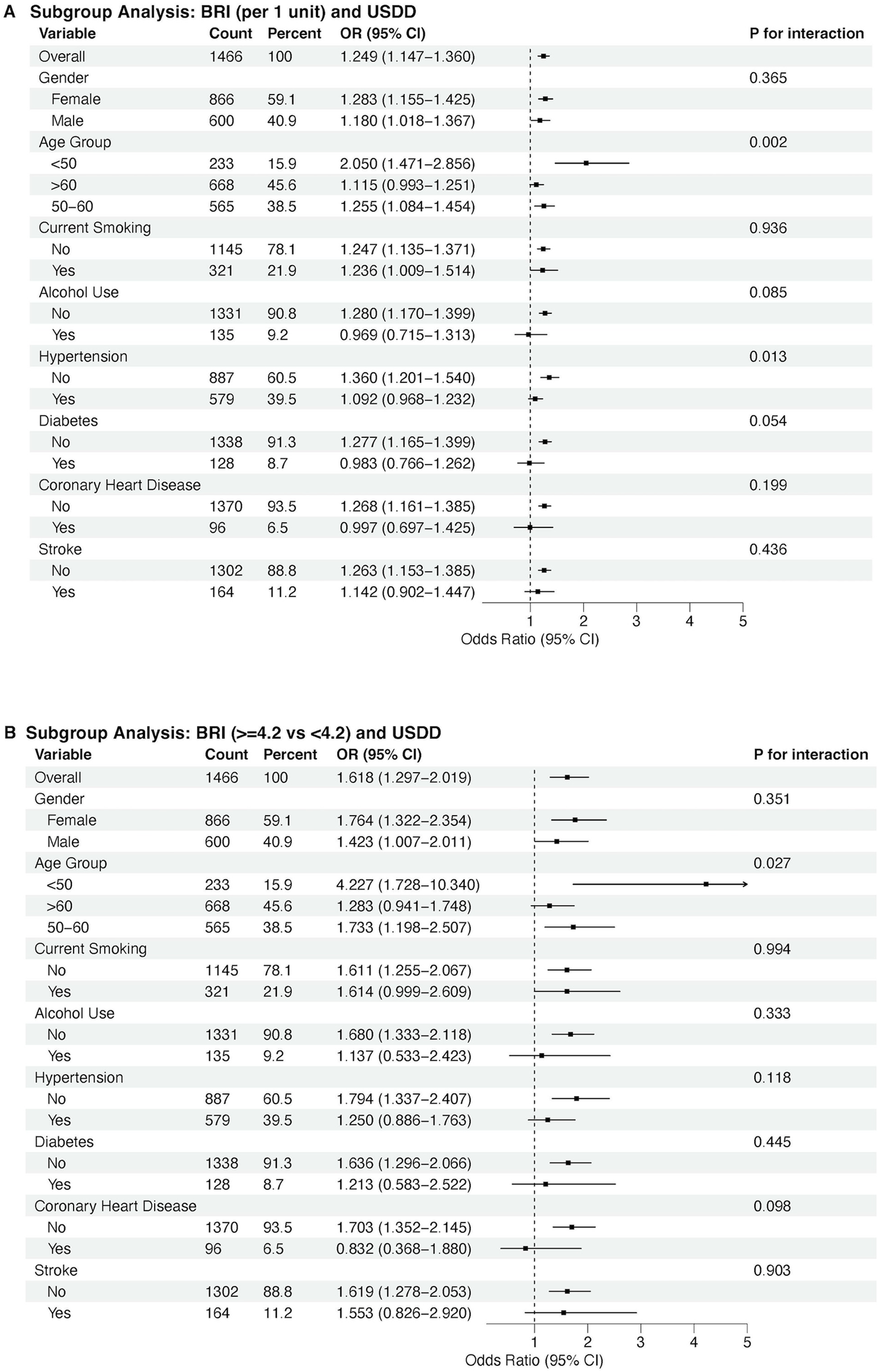
Subgroup analyses by age, sex, current smoking, alcohol use, hypertension, diabetes, coronary heart disease, and stroke. Panel A shows associations per 1-unit increase in BRI; Panel B compares low BRI (<4.2) and high BRI (≥4.2) groups.
Discussion
Main findings
This study demonstrates a significant association between BRI and DD in middle-aged and older adults, independent of traditional cardiovascular risk factors. Key findings include: 1) BRI exhibited a nonlinear association with DD probability (restricted cubic spline: P < 0.001 for overall association, P = 0.006 for linearity) and a modest discriminative ability for DD (AUC = 0.67), with an optimal cutoff of 4.2; 2) multivariable regression confirmed BRI's independent association with DD, even after adjusting for traditional cardiovascular risk factors. Each 1-unit BRI increase was associated with 14.4% higher odds of DD (P = 0.003) and high BRI (≥4.2) was associated with 41.7% higher odds of DD (P = 0.004), and each 1-unit BRI increase correlated with unfavorable changes in continuous diastolic parameters: reduced septal e’ (β = −0.164, P = 8.10 × 10−4) and lateral e’ velocities (β = −0.167, P = 5.59 × 10−3), and a marginally elevated E/e’ ratio (β = 0.121, P = 0.053). These findings link BRI-an index of body shape and central adiposity-to impaired myocardial relaxation and subclinical filling pressure elevation. These results highlight BRI as a novel adiposity marker for early DD detection.
Comparison with existing literature
Previous studies have predominantly focused on the association between obesity and cardiac structure, while research on the correlation between obesity and diastolic function remains scarce. This may be attributed to the fact that the assessment of diastolic function imposes relatively high requirements on time and equipment-early cardiac ultrasound examinations typically did not include evaluations of diastolic function, leading to a relative paucity of clinical epidemiological studies incorporating diastolic function indices. Our findings build on and extend this limited evidence: rather than confirming a linear link between adiposity and diastolic dysfunction (DD), we identified a nonlinear association between BRI and DD probability (restricted cubic spline: P < 0.001 for overall association, P = 0.006 for linearity). This overall association aligns with prior research highlighting central adiposity as a key driver of cardiac remodeling (19–24).
This nonlinearity manifests as a “decelerating risk trajectory”: as BRI increases, the probability of DD rises rapidly in the lower-to-moderate BRI range, but this upward trend slows markedly in the moderate-to-high BRI range. This contrasts with BMI, which often exhibits a J-shaped association with cardiac outcomes (25, 26). The interaction effects observed with blood pressure and lipids further underscore the complex interplay between body shape, hemodynamics, and metabolism in driving DD. For example, BRI's effect on DD risk is more pronounced in participants with lower diastolic blood pressure (DBP), which suggests that in the absence of elevated DBP-a known driver of myocardial stiffening, BRI-related central adiposity becomes a more dominant contributor to DD risk. A similar pattern was observed with triglycerides: lower triglycerides amplified the BRI-DD association, while higher HDL-C mitigated this effect. Collectively, these interactions highlight that BRI is a more impactful marker of DD risk in individuals with less pre-existing cardiometabolic stress (lower DBP, higher HDL-C and lower triglycerides)-reinforcing the value of BRI for early screening in these subgroups, where interventions to reduce central adiposity may most effectively prevent DD progression.
Clinical implications
BRI offers several advantages over traditional adiposity measures in primary care: 1) It is easily calculable from waist circumference and height, requiring no additional equipment; 2) It captures regional fat distribution, which is more strongly linked to cardiometabolic risk than BMI; and 3) Its linear association with DD allows for graduated risk stratification. Given its modest discriminatory performance (AUC ≈ 0.67), BRI should not be used as a standalone screening tool; however, it may serve as an adjunctive or risk enrichment marker to help prioritize individuals-particularly those with borderline risk profiles-for further echocardiographic evaluation when combined with clinical judgment and other risk factors.
The observed sex and age differences have important screening implications. Younger males with high BRI showed particularly strong associations with DD, highlighting this subgroup as a priority for early intervention. Lifestyle modifications (e.g., diet, exercise) to reduce central adiposity might mitigate DD progression, as supported by prior trials showing that weight loss improves diastolic function.
Limitations
Several limitations should be considered. First, the cross-sectional design precludes causal inference, and longitudinal studies are needed to establish temporality. Second, BRI was derived from waist circumference and height, potentially missing contributions from visceral fat or muscle mass. Future studies could incorporate dual-energy x-ray absorptiometry or MRI to validate BRI's relationship with body composition. Third, the study population was predominantly Chinese, limiting generalizability to other ethnic groups. Fourth, while bootstrap validation reduced optimism bias in ROC analysis and BRI showed slightly higher AUC than BMI, waist circumference, or BFR, the absolute differences were small and all indices exhibited limited discriminatory accuracy (AUCs < 0.58 in unadjusted models), reinforcing that BRI should be interpreted as a supplementary risk indicator rather than a diagnostic test. Finally, DD was defined using combined echocardiographic parameters, but regional variations in diagnostic criteria may affect reproducibility.
Future directions
Prospective studies should evaluate whether BRI changes over time predict DD incidence and cardiovascular outcomes. Mechanistic research is needed to clarify how BRI influences myocardial structure and function, including inflammatory and neurohumoral pathways. Additionally, evaluating BRI's utility in conjunction with other markers (e.g., natriuretic peptides) could enhance risk prediction. Finally, randomized controlled trials are needed to test whether BRI-targeted interventions improve diastolic function.
Conclusion
This study identifies BRI as an independent correlate of diastolic dysfunction in middle-aged and older adults. Given its modest discriminatory performance, BRI is not recommended as a standalone screening tool; however, it may serve as a supplementary anthropometric indicator that reflects body shape related cardiometabolic risk. When considered alongside established clinical and echocardiographic data, BRI could contribute to more nuanced risk stratification in preventive cardiovascular assessment. Future research should focus on validating its incremental value in prospective cohorts and exploring its mechanistic links to myocardial structure and function.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board (IRB) of Luohe Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Writing – original draft. JW: Writing – original draft, Investigation. BH: Investigation, Writing – original draft, Data curation. JB: Data curation, Formal analysis, Methodology, Writing – original draft. NW: Data curation, Formal analysis, Investigation, Writing – original draft. DL: Data curation, Investigation, Project administration, Writing – original draft. QW: Data curation, Investigation, Writing – original draft. HW: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. QX: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The study is funded by the National Natural Science Foundation of China (81803318), Henan Provincial Science and Technology Research Project (232300420069, 232102310231, 232300420289), and Henan Ministry of Education (22B320003). The funding sources only provided financial support for this research. It had no role in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The authors alone are responsible for the study's design, conduct, and the content of this manuscript.
Acknowledgments
The authors gratefully acknowledge the valuable contributions of the staff and participants of the “Longitudinal Investigation of Osteoarthritis and Cardiovascular Health Status” cohort study.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was used in the creation of this manuscript. During the preparation of this work the authors used Doubao, an AI language model, in order to assist with English language polishing. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1659587/full#supplementary-material
Supplementary Figure S1ROC curves comparing the discriminative ability of waist circumference, BMI, BRI, and BFR for predicting DD.
References
1.
Vaduganathan M Mensah GA Turco JV Fuster V Roth GA . The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. (2022) 80:2361–71. 10.1016/j.jacc.2022.11.005
2.
Jeong E-M Dudley SC . Diastolic dysfunction. Circ J. (2015) 79:470–7. 10.1253/circj.CJ-15-0064
3.
Johansen ND Biering-Sørensen T Jensen JS Mogelvang R . Diastolic dysfunction revisited: a new, feasible, and unambiguous echocardiographic classification predicts major cardiovascular events. Am Heart J. (2017) 188:136–46. 10.1016/j.ahj.2017.03.013
4.
Kuznetsova T Cauwenberghs N Sabovčik F Kobayashi Y Haddad F . Evaluation of diastole by echocardiography for detecting early cardiac dysfunction: an outcome study. ESC Heart Fail. (2022) 9:1775–83. 10.1002/ehf2.13863
5.
Saba S Mulukutla S Thoma F Aronis KN Bhonsale A Kancharla K et al Impact of diastolic dysfunction on the risk of sudden cardiac arrest. Circ Arrhythm Electrophysiol. (2023) 16:475–7. 10.1161/CIRCEP.123.012089
6.
Rasmussen-Torvik LJ Colangelo LA Lima JAC Jacobs DR Rodriguez CJ Gidding SS et al Prevalence and predictors of diastolic dysfunction according to different classification criteria. Am J Epidemiol. (2017) 185:1221–7. 10.1093/aje/kww214
7.
Lee H-J . The evolution of diastolic function may be a marker of myocardial ischemia in coronary slow flow phenomenon. J Cardiovasc Imaging. (2021) 29:357–60. 10.4250/jcvi.2021.0057
8.
Suthahar N Bergman RN de Boer RA . Replacing body mass index with relative fat mass to accurately estimate adiposity. Nat Rev Endocrinol. (2025) 21:393–4. 10.1038/s41574-025-01120-0
9.
Lucas E Aronne LJ . Is it time to define obesity by body composition and not solely body mass Index?J Clin Endocrinol Metab. (2025) 110:e1278–9. 10.1210/clinem/dgae473
10.
Shah NR Braverman ER . Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. (2012) 7:e33308. 10.1371/journal.pone.0033308
11.
Rico-Martín S Calderón-García JF Sánchez-Rey P Franco-Antonio C Martínez Alvarez M Sánchez Muñoz-Torrero JF . Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev (2020) 21:e13023. 10.1111/obr.13023
12.
Thomas DM Bredlau C Bosy-Westphal A Mueller M Shen W Gallagher D et al Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring). (2013) 21:2264–71. 10.1002/oby.20408
13.
Cho D-H Kim M-N Joo HJ Shim WJ Lim D-S Park S-M . Visceral obesity, but not central obesity, is associated with cardiac remodeling in subjects with suspected metabolic syndrome. Nutr Metab Cardiovasc Dis. (2019) 29:360–6. 10.1016/j.numecd.2019.01.007
14.
Zhang X Ma N Lin Q Chen K Zheng F Wu J et al Body roundness Index and all-cause mortality among US adults. JAMA Netw Open. (2024) 7:e2415051. 10.1001/jamanetworkopen.2024.15051
15.
Wang H Wang Q He B Bai J Wang N Liu D et al Association between knee cartilage degradation and cardiac remodeling: a cross-sectional analysis in a community-based cohort. Cardiac Res. (2025) 1(1):38–49. 10.1097/re9.0000000000000004
16.
von Elm E Altman DG Egger M Pocock SJ Gøtzsche PC Vandenbroucke JP , STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. 10.1016/j.jclinepi.2007.11.008
17.
Nagueh SF Sanborn DY Oh JK Anderson B Billick K Derumeaux G et al Recommendations for the evaluation of left ventricular diastolic function by echocardiography and for heart failure with preserved ejection fraction diagnosis: an update from the American Society of Echocardiography. J Am Soc Echocardiogr. (2025) 38:537–69. 10.1016/j.echo.2025.03.011
18.
Lang RM Badano LP Mor-Avi V Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. 10.1016/j.echo.2014.10.003
19.
Selvaraj S Martinez EE Aguilar FG Kim K-YA Peng J Sha J et al Association of central adiposity with adverse cardiac mechanics: findings from the HyperGEN study. Circ Cardiovasc Imaging. (2016) 9:e004396. 10.1161/CIRCIMAGING.115.004396
20.
Geraci G Ferrara P Pallotti F Le Moli R Calabrese V Paternò V et al Correlations between novel adiposity indices and electrocardiographic evidence of left ventricular hypertrophy in individuals with arterial hypertension. J Pers Med. (2025) 15:229. 10.3390/jpm15060229
21.
Oikonomou EK Antoniades C . The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. (2019) 16:83–99. 10.1038/s41569-018-0097-6
22.
AlRahimi J Aboud A AlQuhaibi AS Almaghrabi Y Alghamdi YS Mufti HN . Effect of isolated obesity on left ventricular function and structure: a single-center experience. Cureus. (2021) 13:e13988. 10.7759/cureus.13988
23.
Stritzke J Markus MRP Duderstadt S Lieb W Luchner A Döring A et al The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. (2009) 54:1982–9. 10.1016/j.jacc.2009.07.034
24.
Movahed MR Saito Y . Obesity is associated with left atrial enlargement, E/A reversal and left ventricular hypertrophy. Exp Clin Cardiol. (2008) 13:89–91.
25.
Bhaskaran K Dos-Santos-Silva I Leon DA Douglas IJ Smeeth L . Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. (2018) 6:944–53. 10.1016/S2213-8587(18)30288-2
26.
Min Y-I Gao Y Anugu P Anugu A Correa A . Obesity and overall mortality: findings from the Jackson heart study. BMC Public Health. (2021) 21:50. 10.1186/s12889-020-10040-9
Summary
Keywords
adiposity, Body Roundness Index, cardiovascular disease, diastolic dysfunction, echocardiography
Citation
Xie Y, Wang J, He B, Bai J, Wang N, Liu D, Wang Q, Wang H and Xie Q (2026) Association between Body Roundness Index and diastolic function in middle-aged and older adults: a cross-sectional study. Front. Cardiovasc. Med. 12:1659587. doi: 10.3389/fcvm.2025.1659587
Received
04 July 2025
Revised
18 December 2025
Accepted
19 December 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Wei Dong Gao, Johns Hopkins Medicine, Baltimore, United States
Reviewed by
Changjiang Yu, Harbin Medical University Cancer Hospital, China
Xiaomei Yang, Johns Hopkins Medicine, Baltimore, United States
Giancarlo Suffredini, Johns Hopkins University, Baltimore, United States
Updates
Copyright
© 2026 Xie, Wang, He, Bai, Wang, Liu, Wang, Wang and Xie.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Haoran Wang washingtonhr@163.com Qiaotao Xie 18639998899@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.