- 1Department of Cardiac Surgery, RWTH University Hospital, Aachen, Germany
- 2Faculty of Medicine, RWTH University, Aachen, Germany
- 3Department of Cardiothoracic Surgery, Faculty of Medicine, South Valley University, Qena, Egypt
- 4Faculty of Medicine, Witten/Herdecke University, Witten, Germany
- 5Department of Cardiovascular Surgery, Klinikum Dortmund GGmbH, Dortmund, Germany
Background: Prosthesis-patient mismatch (PPM) is linked to a poor prognosis following surgical aortic valve replacement (SAVR). The exploration of sex differences in PPM outcomes is currently limited. This study seeks to assess the sex-specific effects of PPM following SAVR was rapid deployment AV (RDAVR) prosthesis the Edwards Intuity.
Methods: From 2018 to 2023, a total of 256 patients (60 females and 196 males) who received isolated or combined RDAVR at our institution were included. The definition of PPM was established through the use of the indexed effective orifice area (EOAi) in accordance with the Valve Academic Research Consortium-3 (VARC-3) criteria. A Multivariate logistic regression was performed to identify predictors of any degree PPM.
Results: Female had higher left ventricular ejection fraction preoperatively (p = 0.018). The incidence of any PPM-degree for patients with BMI <30 kg/cm2 was significantly higher in female than in male [33 (55%) vs. 26(13.3%), p < 0.001]. The same was noted for the incidence of PPM in patients with BMI ≥30 kg/cm2 [7 (11.7%) vs. 4 (2.0%), p = 0.004]. And the incidence of severe PPM (EOAi ≤0.65 cm2/m2) for patients with BMI <30 kg/cm2 was 16.7% in females vs. 0 in males (p < 0.001). The in-hospital mortality did not differ between males and females. In the multivariate logistic regression, we could not identify independent predictors of PPM.
Conclusions: In Patients receiving RDAVR, the incidence of PPM was significantly higher in female than in male. However, we did not find a correlation with early clinical outcomes. The incidence of severe PPM after RDAVR was low in both females and males. Due to differences in geometry and function of the LV in women, further studies are necessary to indicate whether the definition of PPM in men may adhere to elevated EOAi thresholds compared to women.
1 Introduction
Aortic valve disease, specifically aortic stenosis (AS), is a significant cardiovascular disease burden, with surgical aortic valve replacement (SAVR) still being the mainstay of treatment even as transcatheter modalities are increasingly being used (1). The Edwards Intuity valve system, a rapid deployment prosthesis including conventional surgical valve implantation with innovative deployment technology, represents a significant advancement in SAVR, potentially decreasing cross-clamp and cardiopulmonary bypass times while maintaining excellent hemodynamic performance (2, 3). However, a critical consideration in valve replacement surgeries is prosthesis-patient mismatch (PPM), which occurs when the effective orifice area (EOA) of the implanted valve prosthesis is too small relative to the patient's body surface area (BSA) and cardiovascular requirements (4, 5).
There is increasing evidence that sex differences are highly significant in the manifestation, course, and treatment outcomes of cardiovascular disease (6). In the context of aortic valve replacement, female patients have distinct anatomical and physiological features which could affect procedural management and results. Females have generally smaller aortic annulus and body surface areas than males and may be at greater risk of PPM following valve replacement (7, 8).
However, the relations between the incidence of PPM and sex with the rapid deployment valves (RDV) for AVR (RDAVR) under investigated. This research aims to study the sex-related differences in clinical outcome and PPM following RDAVR using Edwards Intuity valve system.
2 Material and method
2.1 Patients' population
A retrospective analysis of prospectively collected data was conducted on all consecutive patients (n = 277) who underwent RDAVR with the Intuity prosthesis for symptomatic AS at our hospital from March 2018 to September 2023. The general exclusion criteria for RDAVR at our institution included individuals under 65 years of age or acute endocarditis and for the purpose of this study 21 patients, who already had a cardiac surgery were excluded. RWTH University Ethic committee (EK 151/09) accepted the study procedure, and informed patient consent was obtained.
This study collected patient demographics and assessed perioperative data, including procedural times and rates of procedural and technical success. Discharge evaluations encompassed echocardiography studies, hospital length of stay, ICU length of stay, and in-hospital mortality rates. The rates of early adverse events were analyzed. The adverse events were assessed in accordance with The Society of Thoracic Surgeons Guidelines for reporting mortality and morbidity after cardiac valve interventions (9).
2.2 Aortic valve replacement with the intuity prosthesis
According to the surgeon's discretion RDAVR with the EDWARDS INTUITY Valve System was carried out through upper hemi-sternotomy or full sternotomy, as previously delineated by Kocher et al. (3).
2.3 Echocardiography and clinical outcomes
Before surgery and upon hospital discharge, two board-certified physicians conducted thorough transthoracic echocardiograms (TTEs) on patients positioned in the left lateral decubitus posture utilizing commercially available ultrasound machines (GE Vivid E90, GE Vingmed Ultrasound, Horten, Norway) in compliance with established guidelines (10). Pressure gradients, paravalvular leakage, and valve functionality were documented, among other characteristics. Doppler flow velocities were measured from the apical three-chamber perspective utilizing pulsed and continuous wave Doppler modes to evaluate peak and mean gradients. The preoperative aortic valve area (AVA) is calculated by dividing the left ventricular outflow tract (LVOT) stroke volume, determined from the LVOT diameter and velocity time integral, by the aortic valve's velocity time integral (VTI); AVA = (LVOT area × LVOT VTI)/Aortic Valve VTI. Postoperatively, the effective orifice area indexed (EOAi; cm2/m2) to the body surface area (BSA) of the aortic valve prosthesis was calculated by dividing the EOA by the patient's BSA.
Rapid deployment valve-related complications were characterized as those directly linked to the implantation of the RDV. Valve-related mortality was assessed due to complications arising from RDV implantation, as well as structural or nonstructural valve dysfunction, categorized by paravalvular leakage (PVL) grading (1 = mild; 2 = moderate; 3 = severe) during echocardiography, and the necessity for postoperative pacemaker implantation. Stroke is diagnosed by a neurologist and is defined as a new neurologic deficit confirmed by neuroimaging, such as computed tomography or magnetic resonance imaging.
2.4 Prosthesis-patient mismatch definition
We used the VARC-3 criteria to define PPM (11). The absence of PPM is defined independently of sex as EOAi >0.85 cm2/m2 in patients with a BMI <30 kg/m2 or EOAi >0.70 cm2/m2 in patients with a BMI ≥30 kg/m2. Moderate PPM is classified as EOAi between 0.85 and 0.66 cm2/m2 (BMI <30 kg/m2) or EOAi between 0.70 and 0.56 cm2/m2 (BMI ≥30 kg/m2). Severe PPM is defined as EOAi ≤0.65 cm2/m2 in patients with a BMI <30 kg/m2 or EOAi ≤0.55 cm2/m2 in patients with a BMI ≥30 kg/m2.
2.5 Statistical analysis
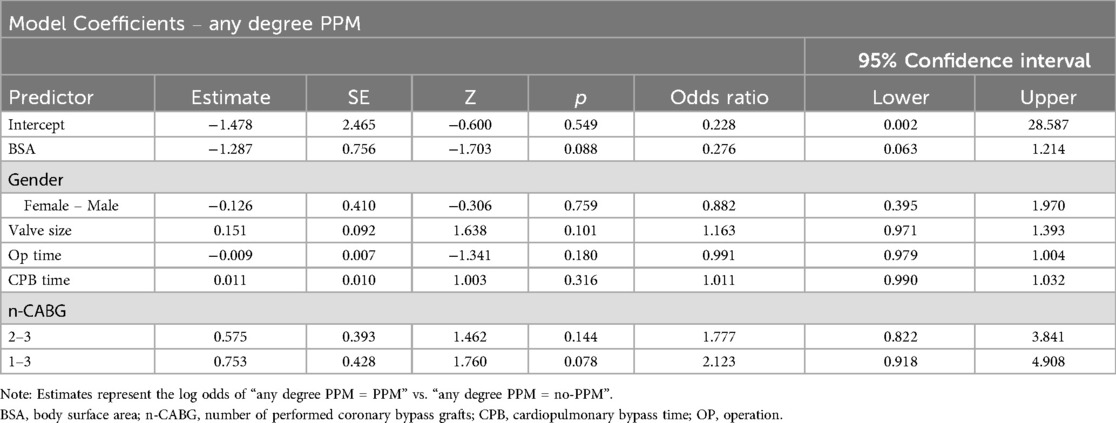
Relevant pre-, peri-, postoperative and echocardiography data were extracted from our institutional database, and statistical analysis was conducted using STATA (StataCorp. 2019. Stata Statistical Software: Release 16) and the jamovi project (2020) (jamovi software, version 2.6.3; https://www.JAMovi.org). Data were presented as mean ± SD or median (range) for continuous variables, as applicable. Categorical variables were presented as absolute counts and proportions (percentages). Continuous variable differences were assessed using unpaired Student's t-tests or Mann–Whitney U-tests, based on the normality of distribution. Fisher's exact test was employed for categorical variables due to the sample size constraints. Continuous repeating variables were examined using two-way ANOVA to compare both between and within groups. A multivariate binominal logistic regression with the variable any degree of PPM as the dependent variable and alle variables with a p-value <0.05 in the univariate analysis [gender, body surface area (BSA), valve size, cardiopulmonary bypass (CPB) time, operation time and the number of performed coronary bypass grafts] as the independent variables, was performed to identify possible factors predicting PPM. A p-value below 0.05 was deemed statistically significant.
3 Results
3.1 Patient demographics and preoperative characteristics
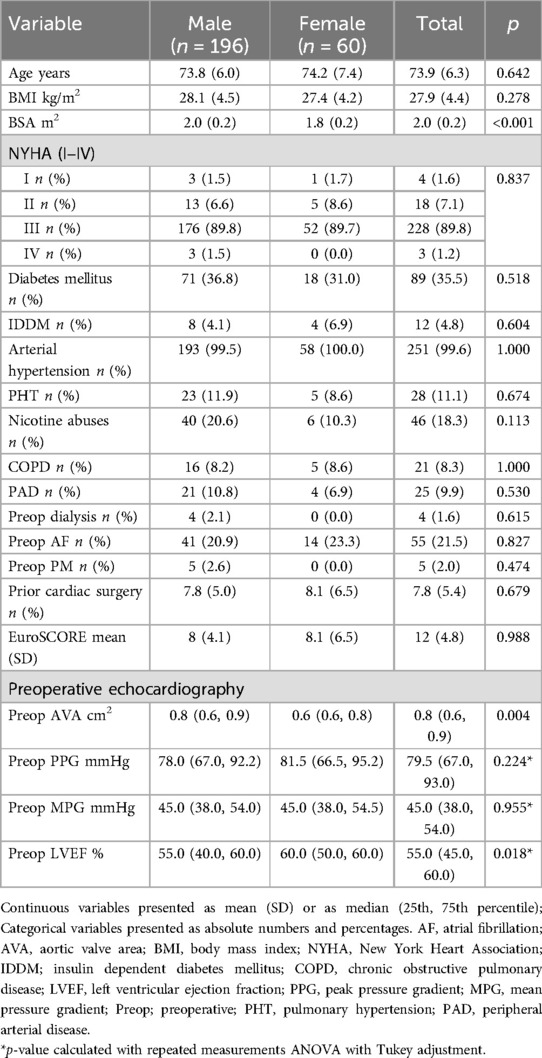
A total of 256 patients who underwent isolated or combined SAVR with the Edwards Intuity valve system were included in this analysis, comprising 196 males (76.6%) and 60 females (23.4%). With exhibition to body BSA (male vs. female: 2.0 ± 0.2 m2 vs. 1.8 ± 0.2 m2, p < 0.001) preoperative demographic data, clinical variables and comorbidities did not significantly differ between males and females Table 1. Despite these differences, the preoperative risk as assessed by European System for Cardiac Operative Risk Evaluation (EuroSCORE) was comparable between males (8% ± 4.1%) and females (8.1% ± 6.5%) (p = 0.988) (Table 1).
Preoperative echocardiographic assessment revealed similar aortic valve gradients between genders (Table 1). While the preoperative left ventricular ejection fraction (LVEF) was significantly lower in males compared to females [55% (40, 60) vs. 60% (50, 60), p = 0.018, respectively]. Moreover, the preoperative AVA, was significantly smaller in females than in males [0.6 cm2 (0.6, 0.8) vs. 0.8 cm2 (0.6, 0.9), p = 0.004, respectively] (Table 1).
3.2 Operative characteristics and valve size distribution
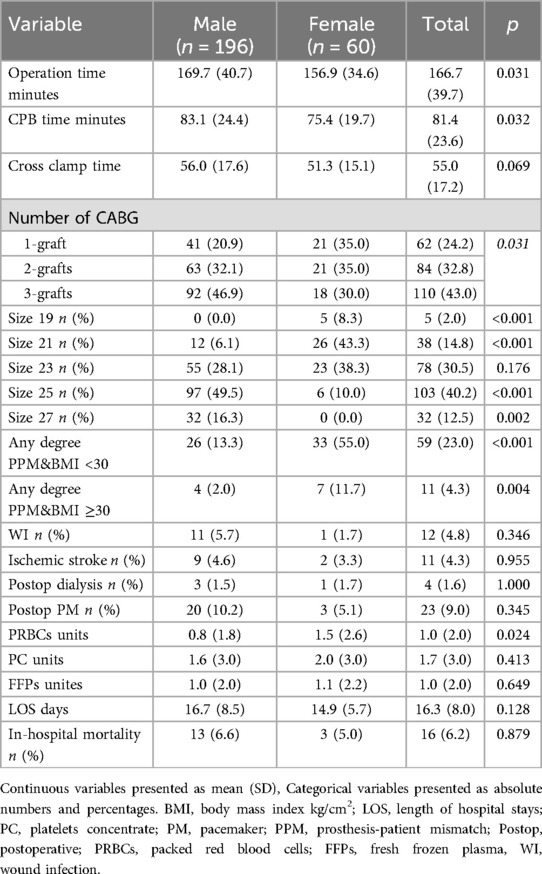
Intraoperative data revealed significant differences between sexes (Table 2). Operation time was significantly longer for males (169.7 ± 40.7 min) compared to females (156.9 ± 34.6 min) (p = 0.031). Similarly, CPB time was significantly longer in males (83.1 ± 24.4 min) than in females (75.4 ± 19.7 min) (p = 0.032). Cross-clamp time showed a trend towards longer duration in males (56.0 ± 17.6 min) vs. females (51.3 ± 15.1 min), though this difference did not reach statistical significance (p = 0.069). The number of performed coronary artery bypass grafts was significantly higher in males than in females (p = 0.031) (Table 2).
Females required significantly more red blood cells packs than males (1.5 ± 2.6 units vs. 0.8 ± 1.8 units, p = 0.024).
The distribution of valve sizes differed significantly between genders (Table 2). Smaller valve sizes were predominantly implanted in females, while larger sizes were more common in males. Size 19 valves were exclusively implanted in females (8.3% vs. 0.0% in males, p < 0.001). Size 21 valves were used in 43.3% of females compared to only 6.1% of males (p < 0.001). Size 23 valves were used in 38.3% of females and 28.1% of males, though this difference did not reach statistical significance (p = 0.176). In contrast, larger valve sizes were predominantly implanted in males: size 25 valves in 49.5% of males vs. 10.0% of females (p < 0.001), and size 27 valves exclusively in males (16.7% vs. 0.0% in females, p = 0.002) (Table 2).
3.3 Postoperative outcomes
The incidence of postoperative complication like the need of pacemaker implantation, ischemic stroke, the need of dialysis and wound infection did not differ between females and males (Table 2).
The hospital length of stay was similar in both males and females (16.7 ± 8.5 days vs. 14.9 ± 5.7, p = 0.128, respectively). The rate of in-hospital mortality did not differ between males and females [13 men (6.6%) vs. 3 women (5.0%), p = 0.879] (Table 2).
3.4 Gender-based variation in valve sizing and postoperative hemodynamics
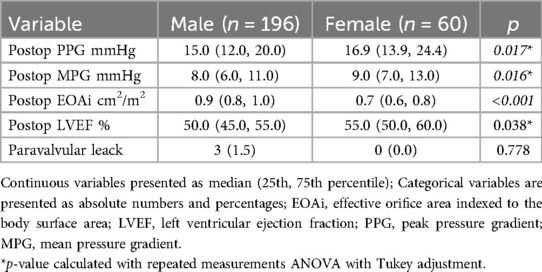
Postoperatively, LVEF remained lower in males than in females [50% (45, 55) vs. 55% (50, 60), p = 0.038, respectively]. The PPG and MPG were significantly higher in females compared to males (p = 0.017 and p = 0.016, respectively) Table 3. The EOAi measured postoperatively was smaller in female than in male [0.9 (0.8, 1.0) cm2 vs. 0.7 (0.6, 0.8) cm2, p < 0.001, respectively] (Table 3). The incidence of paravalvular leak did not differ between males and females [3 men (1.5%) vs. 0 women, p = 0.778].
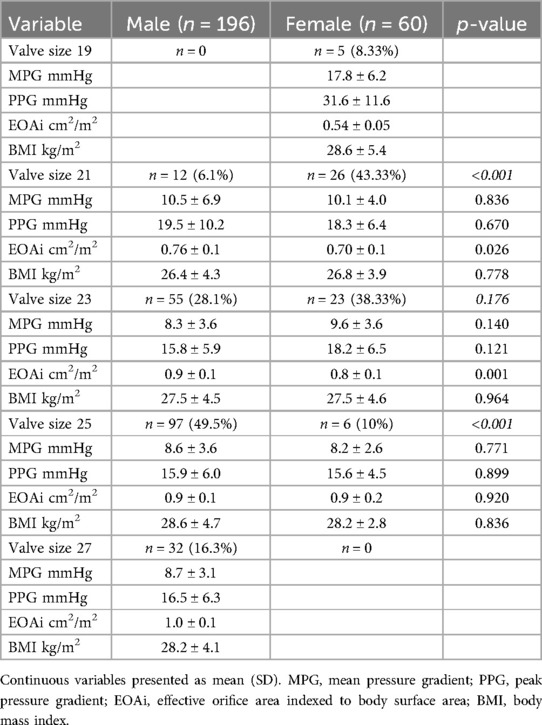
Hemodynamic performance also showed differences between males and females. For valve size 21 mm, the PPG and MPG did not differ between females and males but females had a significantly smaller EOAi compared to males (0.70 ± 0.1 cm2/m2 vs. 0.76 ± 0.1 cm2/m2, p = 0.026). Similarly, for valve size 23 mm, females demonstrated a smaller EOAi (0.8 ± 0.1 cm2/m2 vs. 0.9 ± 0.1 cm2/m2, p = 0.001), whereas the PPG and MPG did not differ between males and females (Table 4). For the valve size 25 mm we did not detect any differences in the EOAi or the pressure gradients between males and females (Table 4).
3.5 Sex-related disparities in PPM based on EOAi thresholds and BMI
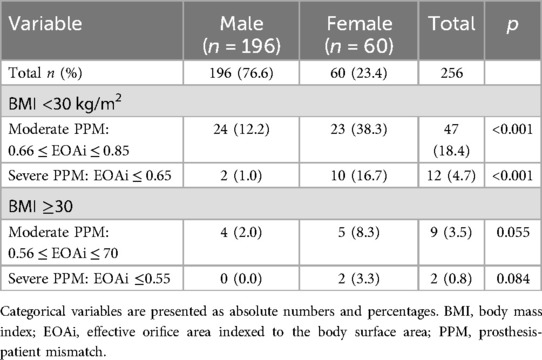
The incidence of any degree of PPM for patients with BMI <30 kg/cm2 was significantly higher in females than in males [33 (55%) vs. 26 (13.3%), p < 0.001]. The same was noted for the incidence of PPM in patients with BMI ≥30 kg/cm2 [7 (11.7%) vs. 4 (2.0%), p = 0.004].
Further analysis of PPM based on BMI and EOAi thresholds revealed markedly higher rates of PPM among females (Tables 5, 6).
3.5.1 Patients with BMI <30 kg/cm2
Overall incidence of moderate PPM (0.66 ≤ EOAi ≤ 0.85 cm2/m2) was significantly higher in females than in males (38.3% vs. 12.2%, p < 0.001, respectively) Table 5. And the incidence of severe PPM (EOAi ≤0.65 cm2/m2) for patients with BMI <30 was 16.7% in females vs. 1% in males (p < 0.001) Table 5.
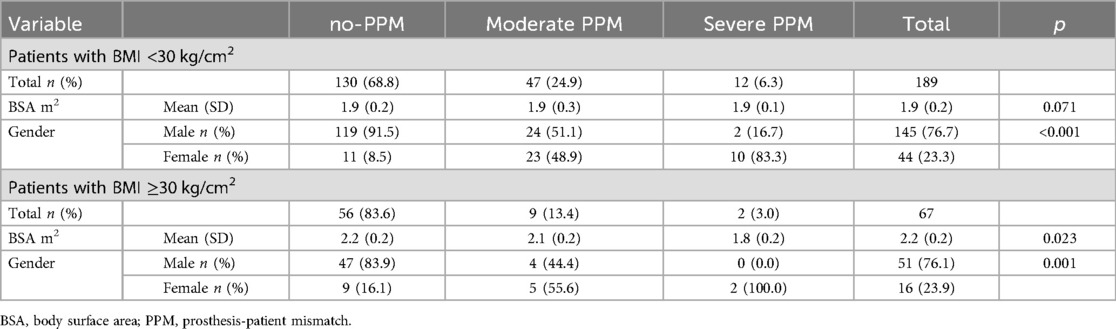
189 (73.8%) patients had a BMI <30 kg/cm2, 145 (76.7%) were male and 44 patients (23.3%) were female. 130 patients (68.8%) did not have PPM. 47 patients (24.9%) had a moderate PPM, of whom 24 patients (51.1%) were male and 23 patients (48.9%) were female. Twelve patients (6.3%) had severe PPM, of whom 10 patients (83.3%) were female (p < 0.001) Table 6. The BSA did not differ significantly between patients with severe PPM and patients without PPM in the subgroup patients with BMI <30 kg/cm2 (Table 6).
3.5.2 Patients with BMI ≥30 kg/cm2
The incidence of moderate or severe PPM in patients with BMI ≥30 kg/cm2 did not differ between females and males (Table 5).
67 (26.1%) patients had a BMI ≥30 kg/cm2, 51 (76.1%) were male and 16 patients (23.9%) were female. 56 patients (83.6%) did not have PPM. Nine patients (13.4%) had a moderate PPM, of whom 4 patients (44.4%) were male and 10 patients (55.6%) were female. Two patients (3%) had severe PPM, and both of them (100%) were female (p < 0.001) Table 6. Patients with BMI ≥30 kg/cm2 and severe PPM had significantly lower BSA compared to no-PPM and moderate PPM (1.8 ± 0.2 m2 vs. 2.2 ± 0.2 m2 vs. 2.1 ± 0.2 m2, p = 0.023, respectively) Table 6.
3.6 Logistic regression with any degree PPM as dependent variable
After entering all variables with a p-value <0.05 in the univariate analysis (gender, BSA, valve size, CPB time, operation time and the number of performed coronary bypass grafts) (Table 7) into a multivariate logistic regression with the variable any degree PPM as the dependent variable, none of the variables remained an independent predictor for PPM.
4 Discussion
This research examined sex-based variations in baseline characteristics and early postoperative outcomes related to AVR with the Intuity aortic valve prosthesis. The principal findings are as follows: (I) With the exception of a history of prior cardiac surgery, there were no differences in clinical risk profiles between male and female patients at the time of AVR; however, preoperative characteristics of female patients demonstrated a significantly higher LVEF and a smaller native AVA, with no difference in the preoperative pressure gradient; (II) Female patients demonstrated a greater propensity for smaller valve sizes; however, the EOAi was significantly lower in females compared to males, even when using the same valve size in males and females; (III) The prevalence of PPM was greater in females than in males for any EOAi threshold according to the VARC 3-criteria.
In our study, males had prolonged operation time and CPB time which may be attributed to the higher incidence of history of prior cardiac surgeries and the higher number of performed CABG grafts among males.
PPM was initially identified in 1978 by Dr. Rahimtoola (5), who observed that valve prostheses with a reduced EOA appeared to correlate with hemodynamic and clinical deterioration following valve replacement.
The literature presents varying conclusions regarding the relationship between PPM and survival. Numerous single-center studies have indicated no correlation between PPM and reduced survival (12–14), while an equal number have reported the opposite findings (15, 16). Numerous extensive observational meta-analyses have indicated a reduction in survival rates among patients with severe PPM, both overall and within specific subgroups; however, they did not establish a significant correlation for moderate PPM (17, 18). Our Study did not find a correlation between the incidence of PPM and the early postoperative outcome.
As of preoperative risk factors, in our study male patients compared to females are characterized by having a history of prior cardiac surgery and a lower LVEF, similarly to the findings of Steeds et al. (19) They have found that males had a larger incidence of prior cardiac surgery and a reduced LVEF; nevertheless, they also shown an elevated prevalence of renal impairment, increased surgical risk, and a more frequent occurrence of critical preoperative conditions. In contrast to Steeds et al., we did not see significant differences concerning those characteristics.
In our study we found that in patients with BMI ≥30 kg/cm2 the BSA was significantly lower in patients who developed severe PPM, these finding are partially in accordance with the large meta-analysis performed by Dayan et al. (20), who included 58 studies with a total number of patients included of 40,381 (39,568 surgical aortic valve replacement and 813 transcatheter aortic valve replacement). Dayan et al. (20), found that the effect of PPM was less significant in patients with a higher body mass index (>28 kg/m2) compared to those with a lower index. Factors associated with PPM included advanced age, female gender, hypertension, diabetes, renal failure, increased body surface area, elevated body mass index, and the use of a bioprosthesis. This finding is likely associated with the observation that, in overweight and obese patients, the cardiac output requirement does not rise proportionately with the increase in body surface area resulting from greater body weight. Consequently, the EOA indexed to BSA may exaggerate the extent of PPM in patients with higher BMI (20, 21).
It has been noted that women with comparable hemodynamic AS severity exhibit a reduced level of AV calcification in comparison to men (22). Research indicates that although men experience more favorable outcomes than women following SAVR, various studies demonstrate that women exhibit improved long-term survival rates after transcatheter aortic valve implantation (TAVI) compared to men (23, 24). Various factors have been proposed to explain the sex-related differences in outcomes following aortic valve replacement. One potential factor could be the varying response of the left ventricle to pressure overload in men compared to women (25, 26).
Left ventricular (LV) remodeling in response to pressure overload in AS can be classified into four main patterns (based on LV mass and relative wall thickness) depending on when severe AS is diagnosed: normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy (27). In cases of comparable AS severity, women exhibit a more preserved LVEF, reduced left ventricular cavity size, and lower left ventricular mass (index) in comparison to men (26, 28).
Males also were more likely to have larger valve size in comparison to females which can be explained by larger aortic anulus in males.
Interestingly for similar valve sizes (in our study valve size 21 mm, and valve size 23 mm) implanted in men and women the EOAi was lower in females. These findings could be in partly explained as follow: As mentioned above Women exhibited left ventricular concentric remodeling more frequently than men, who more often displayed eccentric left ventricular remodeling (26, 28). That's mean women have more preserved LVEF, reduced left ventricular cavity size, and lower left ventricular mass (index) in comparison to men. Leading to the fact that the measured LVOT diameter in echocardiographic is smaller and as the EOA is calculated as follow: AVA = (LVOT area × LVOT VTI)/Aortic Valve VTI. And the LVOT area is calculated as follow: LVOT Area = π * (LVOT diameter/2)2, this will lead to smaller measured EOA and smaller EOAi.
Further analysis of PPM based on BMI and EOAi thresholds revealed markedly higher rates of PPM among females. For patients with BMI <30 kg/cm2 and EOAi ≤0.85 cm2/m2, the incidence of PPM was significantly higher in females (55%) than in males (12.4%) (p < 0.001). When the threshold of EOAi ≤0.70 cm2/m2 for patients with BMI ≥30 kg/cm2 was used, PPM was present in 11.7% of females vs. 2.3% of males (p = 0.005). These results highlight a considerable sex-based discrepancy in the risk of PPM, with females being much more affected despite similar BMI. This necessitates additional evaluation during the selection and sizing of prostheses in female patients to reduce the likelihood of negative hemodynamic consequences after surgery. However, the incidence of severe PPM remained low for Patients with BMI <30 kg/cm2 [males vs. females: 2 (0.9%) vs. 10 (16.7%), p < 0.001] and for patients with BMI ≥30 kg/cm2 [males vs. females: 0 vs. 2 (3.3%), p = 0.066].
These finding are in accordance with Springhetti et al. (29), who analyzed 7,319 patients who underwent SAVR. Springhetti et al. (29) found that any-degree PPM was observed to be more common in women than in men (31.9% vs. 19.7%, P < .0001), with a low incidence of severe PPM (2.4% vs. 0.6%, P < .0001) as defined by VARC-3 (29). In the study by Springhetti et al. (29), PPM was more common in women than in males and was independently linked to a heightened risk of long-term mortality only in women, as per the VARC-3 classification (11). Springhetti et al. (29) found that after modifying the definition of PPM based on spline-derived EOAi thresholds categorized by sex, PPM demonstrated an independent association with outcomes in all genders.
4.1 Limitations
It is important to recognize several limitations. This analysis is retrospective in nature, which introduces certain limitations associated with the study design. Secondly, the study exclusively involved patients with severe AS who were undergoing SAVR with Intuity prosthesis. Consequently, these data may not accurately reflect the characteristics of patients with moderate AS or those undergoing TAVR or SAVR with other prosthesis. Our study suffer the lack of long-term follow-up, and as the PPM correlated with many long-term endpoints, this limit the interpretation of our study.
5 Conclusions
In Patients receiving RDAVR, the incidence of PPM was significantly higher in female than in male. However, we did not find a correlation with early clinical outcomes. The incidence of severe PPM after RDAVR was low in both females and males. Due to differences in geometry and function of the LV in women, further studies are necessary to indicate whether the definition of PPM in men may adhere to elevated EOAi thresholds compared to women.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by RWTH University Hospital Ethic Committee EK (EK151/09). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MJ: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. RZ: Data curation, Formal analysis, Investigation, Writing – review & editing. LD: Resources, Writing – review & editing. YS: Methodology, Supervision, Writing – review & editing. AM: Conceptualization, Resources, Supervision, Writing – review & editing. LT: Conceptualization, Methodology, Resources, Writing – review & editing. SL: Visualization, Writing – review & editing. MK: Conceptualization, Resources, Writing – review & editing. AA: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. AFAM: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. (2021) 60(4):727–800. doi: 10.1093/ejcts/ezab389
2. Haverich A, Wahlers TC, Borger MA, Shrestha M, Kocher AA, Walther T, et al. Three-year hemodynamic performance, left ventricular mass regression, and prosthetic-patient mismatch after rapid deployment aortic valve replacement in 287 patients. J Thorac Cardiovasc Surg. (2014) 148(6):2854–60. doi: 10.1016/j.jtcvs.2014.07.049
3. Kocher AA, Laufer G, Haverich A, Shrestha M, Walther T, Misfeld M, et al. One-year outcomes of the surgical treatment of aortic stenosis with a next generation surgical aortic valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY valve system. J Thorac Cardiovasc Surg. (2013) 145(1):110–5; discussion 5–6. doi: 10.1016/j.jtcvs.2012.07.108
4. Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. (2006) 92(8):1022–9. doi: 10.1136/hrt.2005.067363
5. Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. (1978) 58(1):20–4. doi: 10.1161/01.CIR.58.1.20
6. Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. (2017) 97(1):1–37. doi: 10.1152/physrev.00021.2015
7. Matkovic M, Zivkovic I, Micovic S, Bilbija I, Milacic P, Aleksic N, et al. Sex-related differences in patient characteristics, practice patterns and outcomes after bioprosthetic and mechanical aortic valve replacement. Interdiscip Cardiovasc Thorac Surg. (2025) 40(5):ivaf110. doi: 10.1093/icvts/ivaf110
8. Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR, et al. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. (2017) 6(9):1–13. doi: 10.1161/JAHA.117.006370
9. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg. (2008) 85(4):1490–5. doi: 10.1016/j.athoracsur.2007.12.082
10. Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European association of cardiovascular imaging endorsed by the Chinese society of echocardiography, the inter-American society of echocardiography, and the Brazilian department of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2016) 17(6):589–90. doi: 10.1093/ehjci/jew025
11. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. (2021) 42(19):1825–57. doi: 10.1093/eurheartj/ehaa799
12. Jamieson WR, Ye J, Higgins J, Cheung A, Fradet GJ, Skarsgard P, et al. Effect of prosthesis-patient mismatch on long-term survival with aortic valve replacement: assessment to 15 years. Ann Thorac Surg. (2010) 89(1):51–8; discussion 9. doi: 10.1016/j.athoracsur.2009.08.070
13. Tully PJ, Aty W, Rice GD, Bennetts JS, Knight JL, Baker RA. Aortic valve prosthesis-patient mismatch and long-term outcomes: 19-year single-center experience. Ann Thorac Surg. (2013) 96(3):844–50. doi: 10.1016/j.athoracsur.2013.04.075
14. Dismorr M, Glaser N, Franco-Cereceda A, Sartipy U. Effect of prosthesis-patient mismatch on long-term clinical outcomes after bioprosthetic aortic valve replacement. J Am Coll Cardiol. (2023) 81(10):964–75. doi: 10.1016/j.jacc.2022.12.023
15. Bleiziffer S, Ali A, Hettich IM, Akdere D, Laubender RP, Ruzicka D, et al. Impact of the indexed effective orifice area on mid-term cardiac-related mortality after aortic valve replacement. Heart. (2010) 96(11):865–71. doi: 10.1136/hrt.2009.177220
16. Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. (2009) 119(7):1034–48. doi: 10.1161/CIRCULATIONAHA.108.778886
17. Head SJ, Mokhles MM, Osnabrugge RLJ, Pibarot P, Mack MJ, Takkenberg JJM, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. (2012) 33(12):1518–29. doi: 10.1093/eurheartj/ehs003
18. Chen J, Lin Y, Kang B, Wang Z. Indexed effective orifice area is a significant predictor of higher mid- and long-term mortality rates following aortic valve replacement in patients with prosthesis-patient mismatch. Eur J Cardiothorac Surg. (2014) 45(2):234–40. doi: 10.1093/ejcts/ezt245
19. Steeds RP, Messika-Zeitoun D, Thambyrajah J, Serra A, Schulz E, Maly J, et al. IMPULSE: the impact of gender on the presentation and management of aortic stenosis across Europe. Open Heart. (2021) 8(1):e001443. doi: 10.1136/openhrt-2020-001443
20. Dayan V, Vignolo G, Soca G, Paganini JJ, Brusich D, Pibarot P. Predictors and outcomes of prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging. (2016) 9(8):924–33. doi: 10.1016/j.jcmg.2015.10.026
21. Mohty D, Dumesnil JG, Echahidi N, Mathieu P, Dagenais F, Voisine P, et al. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol. (2009) 53(1):39–47. doi: 10.1016/j.jacc.2008.09.022
22. Pawade T, Sheth T, Guzzetti E, Dweck MR, Clavel M-A. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. (2019) 12(9):1835–48. doi: 10.1016/j.jcmg.2019.01.045
23. Bière L, Launay M, Pinaud F, Hamel JF, Eltchaninoff H, Iung B, et al. Influence of sex on mortality and perioperative outcomes in patients undergoing TAVR: insights from the FRANCE 2 registry. J Am Coll Cardiol. (2015) 65(7):755–7. doi: 10.1016/j.jacc.2014.11.044
24. Williams M, Kodali SK, Hahn RT, Humphries KH, Nkomo VT, Cohen DJ, et al. Sex-related differences in outcomes after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis: insights from the PARTNER trial (placement of aortic transcatheter valve). J Am Coll Cardiol. (2014) 63(15):1522–8. doi: 10.1016/j.jacc.2014.01.036
25. Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. (2012) 60(19):1854–63. doi: 10.1016/j.jacc.2012.02.093
26. Stehli J, Zaman S, Stahli BE. Sex discrepancies in pathophysiology, presentation, treatment, and outcomes of severe aortic stenosis. Front Cardiovasc Med. (2023) 10:1256970. doi: 10.3389/fcvm.2023.1256970
27. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. (1975) 56(1):56–64. doi: 10.1172/JCI108079
28. Rohde LE, Zhi G, Aranki SF, Beckel NE, Lee RT, Reimold SC. Gender-associated differences in left ventricular geometry in patients with aortic valve disease and effect of distinct overload subsets. Am J Cardiol. (1997) 80(4):475–80. doi: 10.1016/s0002-9149(97)00398-6
Keywords: heart valve prosthesis implantation, aortic valve stenosis, heart valve prosthesis, women, echocardiography, prosthesis-patient mismatch, rapid deployment aortic valve replacement
Citation: Jawoosh M, Zayat R, Dogan L, Shieba Y, Moza A, Tewarie L, Lotfi S, Khattab MA, Abugameh A and Mohammed AFA (2025) Sex-related differences in prosthesis-patient mismatch following aortic valve replacement with the edwards intuity valve system. Front. Cardiovasc. Med. 12:1666443. doi: 10.3389/fcvm.2025.1666443
Received: 15 July 2025; Accepted: 18 August 2025;
Published: 4 September 2025.
Edited by:
Fabiana Isabella Gambarin, Fondazione Salvatore Maugeri, Veruno (IRCCS), ItalyReviewed by:
Natalia Pavone, Agostino Gemelli University Polyclinic (IRCCS), ItalyNorman Briffa, Sheffield Teaching Hospitals NHS Foundation Trust, United Kingdom
Copyright: © 2025 Jawoosh, Zayat, Dogan, Shieba, Moza, Tewarie, Lotfi, Khattab, Abugameh and Mohammed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rashad Zayat, cnpheWF0QHVrYWFjaGVuLmRl
†These authors have contributed equally to this work
 Muhammad Jawoosh
Muhammad Jawoosh Rashad Zayat
Rashad Zayat Leyla Dogan1,2
Leyla Dogan1,2 Yusuf Shieba
Yusuf Shieba Mohammad Amen Khattab
Mohammad Amen Khattab Ahmed F. A. Mohammed
Ahmed F. A. Mohammed