Abstract
We report a case of severe H1N1 influenza pneumonia complicated by intermediate-high risk pulmonary embolism (PE) in a 70-year-old male presenting with dyspnea and fever. Initial chest CT demonstrated bilateral interstitial infiltrates and a throat swab was positive for H1N1 on PCR. Despite aggressive antiviral, antibiotic, and respiratory support, the patient developed refractory hypoxemia with progressively elevated D-dimer levels. Subsequent CT pulmonary angiography confirmed the diagnosis of pulmonary embolism. As a rescue therapy, catheter-directed thrombolysis (CDT) was initiated under veno-arterial extracorporeal membrane oxygenation (ECMO) support. This intervention led to immediate hemodynamic and respiratory improvement, culminating in the patient's full recovery and discharge. This case highlights the critical need to suspect concomitant pulmonary embolism in severe pneumonia and demonstrates the therapeutic potential of ECMO-assisted CDT.
Introduction
H1N1 influenza is a well-established cause of severe viral pneumonia and acute respiratory distress syndrome (ARDS) (1). Its association with intermediate- to high-risk PE has been less frequently documented in the literature. The significant overlap in clinical manifestations often results in the under-recognition of PE in these patients, potentially delaying life-saving interventions. The coexistence of severe pneumonia and PE carries a substantially elevated mortality risk and presents considerable therapeutic challenges (2). This report analyses the clinical features and therapeutic difficulties in patients with severe pneumonia complicated by PE and assess ECMO-facilitated CDT as a salvage strategy.
Case presentation
A 70-year-old male was admitted to our hospital with a 10-day history of dyspnea and fever, without accompanying hemoptysis, syncope, chest pain, or abdominal pain. His medical history was notable for coronary artery stent implantation 7 years prior, and he was currently receiving regular pharmacotherapy including aspirin, beta-blocker, and rosuvastatin. The patient had no history of pulmonary disease, hypertension, or diabetes mellitus. On physical examination, his vital signs were as follows: temperature 36.8°C, heart rate 101 beats per minute, respiratory rate 30 breaths per minute, blood pressure 100/55 mmHg, and oxygen saturation 74%–82% under dual-channel oxygen supplementation. Clinical manifestations included cyanosis of the lips, tachypnea, labored breathing and moist rales.
Blood gas analysis showed PH 7.38 (Reference Range 7.35–7.45), PaCO2 30.08 (Reference Range 35–45) mmHg, PaO2 47.84 (Reference Range 80–100) mmHg, lactate 5.66 (Reference Range 1–1.7) mmol/L, and an oxygenation index of 78.4 mmHg (FiO2 0.61). Troponin was 0.125 (Reference Range 0–0.04) ng/mL, NT-ProBNP was 3,735.2 (Reference Range 0–900) pg/mL, and a throat swab PCR test was positive for influenza A H1N1 nucleic acid. Pulmonary CT revealed interstitial infection in both lungs (Figure 1A). Lower extremity ultrasound showed hypoechoic areas within the intramuscular veins of both lower legs, and echocardiography revealed right ventricular enlargement (Figure 1B). Left ventricular systolic function was preserved.
Figure 1
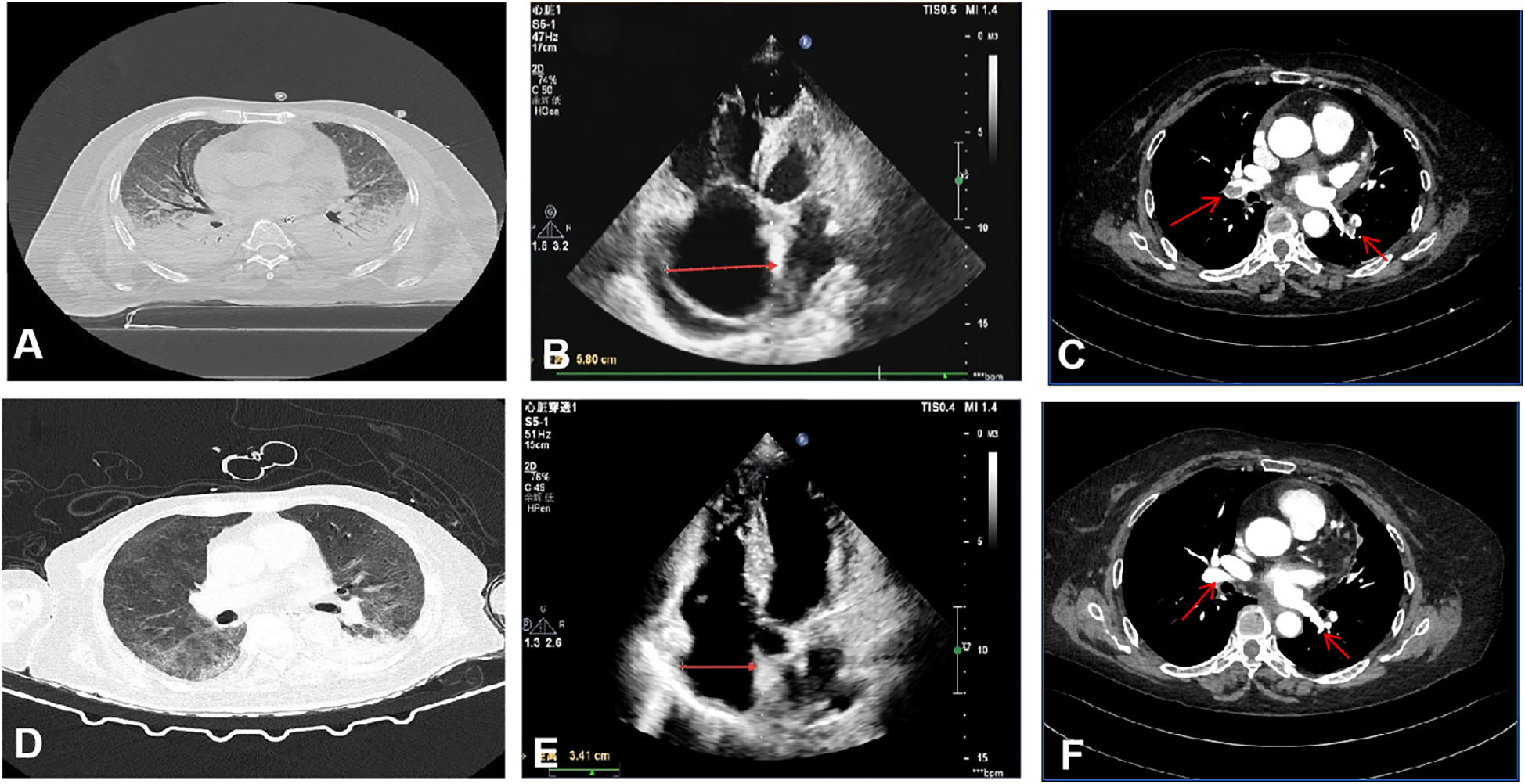
Imaging and echocardiographic findings before and after treatment. (A–C) Pre-treatment: (A) Chest CT shows bilateral pulmonary consolidation and interstitial infiltrates. (B) Transthoracic echocardiography reveals right atrial enlargement (transverse diameter 5.8 cm). (C) CT pulmonary angiography demonstrates extensive bilateral pulmonary embolism. (D–F) Post-treatment: (D) Chest CT indicates resolution of pulmonary consolidation and marked improvement of interstitial opacities. (E) Echocardiography shows significant reduction in right atrial size (transverse diameter 3.41 cm). (F) CT pulmonary angiography confirms substantial reduction in thrombus burden.
The patient was diagnosed with severe H1N1 viral pneumonia complicated by type I respiratory failure and heart failure, based on integrated clinical, radiological, and laboratory findings. The patient was treated with antibiotics (Empirically administered Cefoperazone-Sulbactam 3 g q8h combined with Moxifloxacin 0.4 g qd), antiviral therapy (Oseltamivir 75 mg bid), anti-inflammatory treatment (Methylprednisolone 40 mg bid), anticoagulation (6000IU QN), non-invasive ventilator-assisted ventilation, and high-flow nasal cannula oxygen therapy (HFNC). On January 15, 2024, the patient's condition deteriorated, with the oxygenation index falling to 48 mmHg, necessitating endotracheal intubation, invasive mechanical ventilation, and prone positioning. Despite these interventions, hypoxemia persisted. Elevated D-dimer levels (6.79 mg/L, Reference Range <0.5) prompted computed tomography pulmonary angiography, which revealed extensive bilateral pulmonary emboli (Figure 1C). Given the critical clinical status, a multidisciplinary team recommended veno-arterial extracorporeal membrane oxygenation (VA-ECMO) combined with percutaneous thrombectomy. On January 16, VA-ECMO was initiated percutaneously via the right femoral vein (21 Fr) and left femoral artery (17 Fr) using a Maquet HLS Set Advanced circuit. Initial settings included a pump speed of 2,500–3,000 rpm, titrated to maintain a blood flow of 2.0–3.0 L/min, and a sweep gas flow of 3.0 L/min with an FiO2 of 0.8. A standardized anticoagulation protocol with unfractionated heparin (3,000-unit bolus followed by 750 units/hour infusion) was used, targeting an ACT of 180–200 s. Meticulous catheter site care and daily surveillance for circuit thrombosis or limb ischemia were performed, enabling an uneventful ECMO course. Percutaneous mechanical thrombectomy was performed subsequently. Angiography revealed extensive filling defects involving the LA1, RA8, RA7, RA4, RA5, RA1 and RA2 segmental arteries. Following thrombus aspiration using an Indigo aspiration system, repeat pulmonary arteriography demonstrated restored patency with robust bilateral pulmonary arterial opacification, confirming successful reperfusion. The patient's oxygenation index immediately rose to 90 mmHg postoperatively. On January 23, after the above treatments and supportive care, the patient's oxygenation level significantly improved, reaching 206.5 mmHg. With pulmonary CTA showing a reduction in thrombi and subsequent echocardiography confirming a diminished right atrial size (Figures 1E,F), the patient was successfully weaned from ECMO support. On January 31, Bronchoalveolar lavage fluid cultures later identified multidrug-resistant Acinetobacter baumannii (Multidrug-Resistant), leading to targeted antibiotic therapy with polymyxin 150 mg q12h combined with Tigecycline 50 mg q12h (with an initial loading dose) until discharge. By February 13, follow-up imaging demonstrated significant resolution of pulmonary infiltrates (Figure 1D), and the patient was discharged in stable condition. (See timeline of key interventions and clinical milestones in Table 1, change of biomarker in Table 2).
Table 1
| Time | Clinical events and interventions |
|---|---|
| Jan 13, 2024 | Hospital admission & diagnosis of severe H1N1 pneumonia |
| Jan 15, 2024 | Endotracheal intubation due to worsening respiratory failure |
| Jan 16, 2024 | VA-ECMO initiation and subsequent percutaneous pulmonary artery thrombectomy |
| Jan 23, 2024 | Successful weaning from VA-ECMO |
| Feb 07, 2024 | Discharge from the Intensive Care Unit (ICU) |
| Feb 13, 2024 | Discharge from the hospital |
Timeline of key clinical events and interventions.
Table 2
| Biomarker/Date | 1–13 | 1–15 | 1–16 | 1–23 |
|---|---|---|---|---|
| NT-proBNP (pg/mL) | 3,735.2 | 13,885.8 | 5,728.6 | 1,749.8 |
| cTnI (ng/mL) | 0.125 | 0.118 | 0.933 | 0.126 |
| SaO2 (%) | 74 | 68 | 96 | 98 |
| HR (bpm) | 101 | 105 | 70 | 66 |
| D-dimer (mg/L) | 2.48 | 6.79 | 3.99 | 2.75 |
| PLT (109/L) | 147 | 115 | 145 | 139 |
| PT (S) | 13.6 | 16.4 | 15.5 | 13.4 |
| Fibrinogen (g/L) | 4.23 | 4.11 | 3.37 | 3.45 |
| APTT (S) | 34.10 | 84.10 | 31.70 | 40.08 |
| PaO2/FiO2 | 78.4 | 48 | 90 | 234.8 |
The change of NT-proBNP, cTnI, SaO2 (%), HR, D-dimer, platelet count, PT, fibrinogen, APTT and PaO2/FiO2.
NT-proBNP, N-terminal pro-B-type natriuretic peptide; cTnI, cardiac troponin I; PLT, platelet count; PT, prothrombin time; APTT, activated partial thromboplastin time.
NT-proBNP (Reference Range 0–900) pg/mL; cTnI (Reference Range 0–0.04) ng/mL; D-dimer (Reference Range 0–0.5) mg/L; PLT (Reference Range 125–350) 109/L; PT (Reference Range 9.2–13.9) S; Fibrinogen (Reference Range 2–4) g/L; APTT (Reference Range 21.2–34.8) S.
Discussion
Influenza is an acute respiratory infection with an insidious onset. Early symptoms resemble those of common colds and lung infections, making it highly prone to misdiagnosis as a common cold or pneumonia. The disease progresses rapidly, often developing into severe pneumonia within a short period, leading to respiratory failure, multiple organ failure, and death. The mortality rate for influenza is 5.5%, with the highest rate observed in individuals aged 85 or older at 12.2%. Among all fatal cases, 76.4% involve elderly individuals aged 65 or older (3). Approximately 13%–45% of cases require admission to the intensive care unit (ICU) (4). Influenza can increase the risk of pulmonary embolism.The development of pulmonary embolism in viral infections is multifactorial (5). Viral infection of endothelial cells triggers their activation and injury, initiating a pro-inflammatory response characterized by the release of cytokines and upregulation of von Willebrand factor (VWF) and adhesion molecules. This cascade leads to immunothrombosis, a process amplified by neutrophil extracellular traps (NETs), platelet-derived polyphosphate (PolyP), and complement activation, which synergistically drive the intrinsic coagulation pathway. The outcome is uncontrolled thrombin generation and thrombus formation. Studies indicate that patients with influenza are considerably less likely to develop in situ pulmonary thrombosis than those with COVID-19 (6). However, in patients with severe pneumonia, prolonged immobilization can lead to lower extremity deep vein thrombosis (DVT), which may subsequently cause pulmonary embolism (PE). A study showed that the incidence of influenza complicated by pulmonary embolism is 3.3%, which is lower than the 10.95% observed in COVID-19 (7). The incidence of thrombotic events in critically ill influenza ICU patients is significantly higher than in critically ill community-acquired pneumonia (CAP) patients (21.3% vs. 5.7%; p < 0.05). Compared to critically ill influenza patients without thrombosis, those with thrombosis exhibited significantly higher rates of mechanical ventilation use, longer duration of mechanical ventilation, longer ICU stay, and increased 90-day mortality (8). The PE in this patient was likely the result of a combination of embolization from a lower extremity DVT and in situ thrombosis formation within the pulmonary vasculature.
Extracorporeal membrane oxygenation (ECMO) is an extracorporeal life support technique used to treat severe cardiopulmonary failure. In recent years, the application of ECMO in intensive care and emergency medicine has become increasingly widespread, demonstrating significant efficacy, particularly in treating acute respiratory distress syndrome (ARDS), cardiogenic shock, and high-risk pulmonary embolism (9). According to the latest research and clinical practice, ECMO not only plays a crucial role in supporting vital signs during the acute phase of the disease but also provides necessary stabilization for subsequent surgical interventions (10, 11). A systematic review and meta-analysis, including eight studies and 266 patients treated with ECMO, found that the mortality rate decreased to 28% after ECMO treatment for severe H1N1 infection, suggesting that ECMO is effective for influenza-related ARDS (12–19).
Catheter-directed therapy (CDT) can be used to rapidly reduce the pulmonary arterial thrombus burden (20, 21). According to the 2022 Joint Clinical Consensus Statement of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function and the European Society of Percutaneous Cardiovascular Interventions on the Percutaneous Treatment of Acute Pulmonary Embolism (22), for high-risk pulmonary embolism patients, CDT may be considered if there are contraindications to thrombolysis or if thrombolysis fails (i.e., no hemodynamic improvement 2–4 h after thrombolysis or after completing local thrombolysis). For intermediate-high risk pulmonary embolism patients, CDT may also be considered if no improvement is observed 24–48 h after initial anticoagulation therapy. Current clinical research has also explored the efficacy of CDT in intermediate-risk PE. The ULTIMA randomized controlled trial showed that compared to standard anticoagulation therapy, the EKOS system could more significantly reduce the right ventricular-to-left ventricular diameter ratio in intermediate-risk pulmonary embolism patients within 48 h without increasing the risk of major bleeding. The 2023 FLASH registry study (23), involving 800 intermediate or high-risk pulmonary embolism patients from 50 centers in the United States, found that patients treated with the FlowTriever thrombectomy system experienced rapid clinical improvement, with an in-hospital mortality rate as low as 0.3% and no device-related serious adverse events, indicating good safety. This case demonstrates that for patients with severe ARDS and pulmonary embolism, CDT can be a life-saving intervention by rapidly improving oxygenation and hemodynamics.
Vascular interventional pulmonary arterial thrombectomy under ECMO support is an advanced and highly challenging treatment method. In this procedure, ECMO first provides continuous cardiopulmonary support, ensuring adequate blood supply and oxygenation to vital organs during thrombectomy. Its complex management and potential complications require close collaboration and meticulous management by a multidisciplinary team (24). Early identification and management of complications are crucial for improving patient outcomes (25). In a single-center study of 15 patients with high-risk pulmonary embolism who received combined CDT and ECMO, ECMO was successfully discontinued after a mean of 5.4 days, with only one mortality.This study provides preliminary evidence for the feasibility of percutaneous large-bore aspiration embolectomy in combination with VA-ECMO support in patients with high-risk PE (26). Our case highlights the potential benefit of early ECMO-supported CDT for PE in patients with ARDS, indicating a promising direction for future management.
Conclusion
ARDS with concomitant PE, particularly of viral etiology, may represent a fundamentally different pathophysiological entity requiring a combined cardiopulmonary support strategy. Future comparative studies of ECMO with vs. without CDT in ARDS/PE overlap syndrome are imperative to validate this approach and refine patient selection criteria for this aggressive intervention.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans in accordance with institutional policies and international guidelines. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JW: Writing – original draft, Writing – review & editing. BH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. CZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. YC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. CJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. FL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Hubei Provincial Natural Science Foundation Project. (Grant number: 2025AFC049).
Acknowledgments
We are grateful to all volunteers and staffs who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Herold S Becker C Ridge KM Budinger GR . Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J. (2015) 45(5):1463–78. 10.1183/09031936.00186214
2.
Obi AT Tignanelli CJ Jacobs BN Arya S Park PK Wakefield TW et al Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. (2019) 7(3):317–24. 10.1016/j.jvsv.2018.08.010
3.
O’Halloran AC Millman AJ Holstein R Olsen SJ Cummings CN Chai SJ et al The burden of all-cause mortality following influenza-associated hospitalizations: influenza hospitalization surveillance network, 2010–2019. Clin Infect Dis. (2025) 80(3):e43–5. 10.1093/cid/ciae547
4.
Valenzuela-Sánchez F Valenzuela-Méndez B Rodríguez-Gutiérrez JF Estella Á . Latest developments in early diagnosis and specific treatment of severe influenza infection. J Intensive Med. (2023) 4(2):160–74. 10.1016/j.jointm.2023.09.006
5.
Babkina AS Pisarev MV Grechko AV Golubev AM . Arterial thrombosis in acute respiratory infections: an underestimated but clinically relevant problem. J Clin Med. (2024) 13(19):6007. 10.3390/jcm13196007
6.
Burkhard-Koren NM Haberecker M Maccio U Ruschitzka F Schuepbach RA Zinkernagel AS et al Higher prevalence of pulmonary macrothrombi in SARS-CoV-2 than in influenza A: autopsy results from ‘Spanish flu’ 1918/1919 in Switzerland to coronavirus disease 2019. J Pathol Clin Res. (2021) 7(2):135–43. 10.1002/cjp2.189
7.
Boyd S Loh KS Lynch J Alrashed D Muzzammil S Marsh H et al The incidence of venous thromboembolism in critically ill patients with SARS-CoV-2 infection compared with critically ill influenza and community-acquired pneumonia patients: a retrospective chart review. Med Sci (Basel). (2022) 10(2):30. 10.3390/medsci10020030
8.
Lee W-C Chang C-C Ho M-C Lin C-K Lin C-M Fang Y-H et al Associations between severe influenza-complicated thromboembolism events, intensive care unit stays and mortality, and associated risk factors: a retrospective cohort study. Influenza Other Respir Viruses. (2024) 18(9):e13354. 10.1111/irv.13354
9.
Badulak J Antonini MV Stead CM Shekerdemian L Raman L Paden ML et al Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. (2021) 67(5):485–95. 10.1097/MAT.0000000000001422
10.
Abrams D Lorusso R Vincent JL Brodie D . ECMO During the COVID-19 pandemic: when is it unjustified?Crit Care. (2020) 24(1):507. 10.1186/s13054-020-03230-9
11.
Wang R Tang X Li X Li Y Liu Y Li T et al Early reapplication of prone position during venovenous ECMO for acute respiratory distress syndrome: a prospective observational study and propensity-matched analysis. Ann Intensive Care. (2024) 14(1):127. 10.1186/s13613-024-01365-4
12.
Zangrillo A Biondi-Zoccai G Landoni G Frati G Patroniti N Pesenti A et al Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. (2013) 17(1):R30. 10.1186/cc12512
13.
Sud S Fan E Adhikari NKJ Friedrich JO Ferguson ND Combes A et al Comparison of venovenous extracorporeal membrane oxygenation, prone position and supine mechanical ventilation for severely hypoxemic acute respiratory distress syndrome: a network meta-analysis. Intensive Care Med. (2024) 50(7):1021–34. 10.1007/s00134-024-07492-7
14.
Shekar K Gregory SD Fraser JF . Mechanical circulatory support in the new era: an overview. Crit Care. (2016) 20:66. 10.1186/s13054-016-1235-3
15.
Noah MA Peek GJ Finney SJ Griffiths MJ Harrison DA Grieve R et al Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. (2011) 306(15):1659–68. 10.1001/jama.2011.1471
16.
Burrell A Kim J Alliegro P Romero L Neto AS Mariajoseph F et al Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. (2023) 9(9):CD010381. 10.1002/14651858.CD010381.pub3
17.
Peek GJ Mugford M Tiruvoipati R Wilson A Allen E Thalanany MM et al Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. (2009) 374(9698):1351–63. 10.1016/S0140-6736(09)61069-2
18.
Schmidt M Zogheib E Rozé H Repesse X Lebreton G Luyt C-E et al The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. (2013) 39(10):1704–13. 10.1007/s00134-013-3037-2
19.
MacLaren G Combes A Bartlett RH . Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. (2012) 38(2):210–20. 10.1007/s00134-011-2439-2
20.
Götzinger F Lauder L Sharp ASP Lang IM Rosenkranz S Konstantinides S et al Interventional therapies for pulmonary embolism. Nat Rev Cardiol. (2023) 20(10):670–84. 10.1038/s41569-023-00876-0
21.
Pruszczyk P Klok FK Kucher N Roik M Meneveau N Sharp AS et al Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC working group on pulmonary circulation and right ventricular function and the European association of percutaneous cardiovascular interventions. EuroIntervention. (2022) 18(8):e623–38. 10.4244/EIJ-D-22-00246
22.
Kucher N Boekstegers P Müller OJ Kupatt C Beyer-Westendorf J Heitzer T et al Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. (2014) 129(4):479–86. 10.1161/CIRCULATIONAHA.113.005544
23.
Bangalore S Horowitz JM Beam D Jaber WA Khandhar S Toma C et al Prevalence and predictors of cardiogenic shock in intermediate-risk pulmonary embolism: insights from the FLASH registry. JACC Cardiovasc Interv. (2023) 16(8):958–72. 10.1016/j.jcin.2023.02.004
24.
Yan LL Jin XX Yan XD Peng JB Li ZY He BL . Combined use of extracorporeal membrane oxygenation with interventional surgery for acute pancreatitis with pulmonary embolism: a case report. World J Clin Cases. (2022) 10(12):3899–906. 10.12998/wjcc.v10.i12.3899
25.
Combes A Hajage D Capellier G Demoule A Lavoué S Guervilly C et al Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. (2018) 378(21):1965–75. 10.1056/NEJMoa1800385
26.
Kucher N Ouda A Voci D Barco S Micieli E Münger M et al Percutaneous large-bore aspiration embolectomy with veno-arterial extracorporal membrane oxygenation support or standby in patients with high-risk pulmonary embolism and contraindications to thrombolysis: a preliminary single centre experience. Eur Heart J Acute Cardiovasc Care. (2023) 12(4):232–6. 10.1093/ehjacc/zuad014
Summary
Keywords
severe pneumonia, extracorporeal membrane oxygenation, pulmonaryembolism, interventional thrombectomy, case report
Citation
Wang J, Hu B, Zhang C, Chen Y, Chen S, Jiang C and Li F (2025) Case Report: A case of severe viral pneumonia complicated by pulmonary embolism treated with extracorporeal membrane oxygenation combined with interventional thrombectomy. Front. Cardiovasc. Med. 12:1680758. doi: 10.3389/fcvm.2025.1680758
Received
06 August 2025
Revised
19 November 2025
Accepted
24 November 2025
Published
04 December 2025
Volume
12 - 2025
Edited by
Luca Spiezia, University of Padua, Italy
Reviewed by
Carlos Jerjes-Sanchez, Escuela de Medicina y Ciencias de la Salud Tec Salud, Tecnológico de Monterrey, Mexico
Bekir Kocazeybek, Istanbul University-Cerrahpasa, Türkiye
Updates
Copyright
© 2025 Wang, Hu, Zhang, Chen, Chen, Jiang and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Cheng Jiang Solidwind@126.com Fajiu Li m18627933943@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.