Abstract
Background and objectives:
High-sensitivity troponin T (hsTnT) is a standard biomarker for myocardial injury detection, but its prognostic value may differ by renal function status. This study evaluated how renal function modifies the prognostic significance of hsTnT in ACS patients undergoing PCI.
Methods:
This study examined 14,208 acute coronary syndrome (ACS) patients who underwent percutaneous coronary intervention (PCI), stratified by renal function [estimated glomerular filtration rate (eGFR) < 60 vs. ≥60 mL/min/1.73 m2] and peak hsTnT levels [<5× vs. ≥5× upper reference limit (URL)]. Primary outcomes included one-year all-cause mortality and one-year incidence of ischemic events following the index PCI procedure.
Results:
In patients with impaired renal function, elevated hsTnT was associated with significantly increased mortality [12.84% vs. 4.29%; adjusted hazard ratio [HR] 3.63, 95% confidence interval [CI] 2.04–6.49, P < 0.0001] and ischemic events (10.66% vs. 4.88%; adjusted HR: 2.72, 95% CI: 1.51–4.88, P = 0.0008). In patients with preserved renal function, the mortality association was attenuated (1.42% vs. 0.98%; adjusted HR: 1.40, 95% CI: 0.85–2.29, P = 0.1864), although ischemic events remained significantly increased (2.93% vs. 1.54%; adjusted HR: 2.06, 95% CI: 1.41–2.97, P = 0.0002). Restricted cubic spline analysis revealed a significant non-linear relationship between hsTnT levels and mortality specifically in impaired renal function cohort (P for non-linearity = 0.0004), whereas a predominantly linear association was observed in patients with preserved renal function. A significant interaction was observed between renal function and hsTnT for mortality prediction (P for interaction = 0.0089).
Conclusions:
These findings indicate that renal function substantially modifies the prognostic significance of hsTnT among ACS patients post-PCI. Integrating renal function and peak hsTnT into risk assessment may help identify high-risk subgroups requiring intensified follow-up and management.
Introduction
Acute coronary syndrome (ACS) remains a leading cause of morbidity and mortality worldwide despite continuous advances in diagnosis and management (1–3). High-sensitivity troponin assays, including high-sensitivity cardiac troponin T (hsTnT) and I (hsTnI), are widely used biomarkers for the detection of myocardial injury, with current guidelines recommending the use of high-sensitivity cardiac troponin, without specifying I or T, for the diagnosis and risk stratification of myocardial infarction (4). The adoption of high-sensitivity assays has enabled earlier and more accurate detection of myocardial injury, transforming hsTnT from a binary diagnostic marker into a multidimensional quantitative tool for risk stratification in ACS.
However, interpreting hsTnT levels in clinical practice is complex, particularly in patients with impaired renal function (5–8). Patients with chronic kidney disease (CKD) frequently exhibit chronically elevated troponin concentrations even in the absence of acute myocardial injury. This elevation is multifactorial, resulting not only from reduced renal clearance but also from chronic inflammation induced by uraemic toxins, subclinical myocardial injury, left ventricular hypertrophy, oxidative stress, and accelerated cardiovascular remodeling within the cardiorenal syndrome (9–13). This physiological overlap complicates the distinction between chronic elevation and acute myocardial injury, contributing to potential risk misclassification.
Current risk assessment models may inadequately account for the potential interaction between renal function and troponin levels (14, 15). While elevated troponin consistently predicts poor outcomes in patients with preserved renal function, its prognostic significance in the setting of renal impairment remains incompletely characterized. Most prognostic studies either exclude patients with significant renal dysfunction or fail to analyze them as a separate cohort. The relationship between hsTnT levels and clinical outcomes may not be uniform across the spectrum of renal function, potentially following non-linear patterns that current risk models fail to capture. This study therefore examines the differential prognostic implications of hsTnT levels in 14,208 ACS patients undergoing PCI, with specific focus on how renal function modifies this relationship, to develop more sophisticated risk stratification approaches for this complex patient population.
Methods
Study design and patients
This study was a post-hoc analysis of a single-center, all-comer, prospective, real-world PCI registry in the General Hospital of Northern Theater Command. From March 2016 to March 2019, consecutive ACS patients who underwent PCI were enrolled. Inclusion criteria were ACS patients aged 18 years or older who received PCI with at least one stent implanted. Patients were excluded if they underwent diagnostic angiography only, were referred for coronary artery bypass grafting (CABG) without stent placement during the index procedure, or if data necessary for calculating hsTnT or eGFR were unavailable. The study was approved by the hospital's Research Ethics Committee (K2018-35). The study complied with the provisions of the Declaration of Helsinki. Data collection was conducted using a standard web-based platform (CV-NET system of Crealife Technology, Beijing, China).
Assessment of renal function and high-sensitivity cardiac troponin T
Baseline clinical characteristics and serum biochemistry results were collected using the electronic patient record. Serum creatinine at presentation was used to calculate the eGFR using the Modification of Diet in Renal Disease study equation: eGFR (mL/min/1.73 m2) = 175 × (serum creatinine)−1.154 (mg/dL) × age−0.203 × 0.742 (if female) (16). Based on this, patients were classified as having normal (eGFR ≥60 mL/min/1.73 m2) or impaired renal function (eGFR <60 mL/min/1.73 m2) (17).
Blood samples for hs-cTnT were collected at admission and at 6–12 h post-PCI. The peak value from serial measurements was used for outcome analysis, while admission levels served as baseline reference. Hs-cTnT was quantified by electrochemiluminescence immunoassay using the Elecsys high-sensitivity Troponin T assay (Roche Diagnostics) on a Cobas e 411 analyzer. The lower detection limit was ≤0.003 ng/mL, with a 99th percentile upper reference limit of 0.1 ng/mL.
Outcomes and definitions
The primary endpoint was all-cause death at 12 months. Secondary endpoints included ischemic events at 12 months, defined as a composite of cardiac death, myocardial infarction (MI), or stroke and each component of ischemic events. Cardiovascular death was defined according to the Academic Research Consortium-2 (ARC-2) consensus criteria (18), encompassing death caused by acute myocardial infarction, sudden cardiac death including unwitnessed death, heart failure, stroke, cardiovascular procedures, cardiovascular hemorrhage, and other cardiovascular causes. All deaths were adjudicated by a clinical events committee based on hospital records, death certificates. Deaths were classified as cardiovascular unless a definite non-cardiovascular cause was established; deaths of undetermined cause were classified as cardiovascular. MI was defined according to the Third Universal Definition of Myocardial Infarction Guidelines (19). Stroke was defined as a loss of neurologic function induced by an ischemic episode that lasted at least 24 h or resulted in mortality, as determined by clinicians or imaging investigations. Unstable angina (UA) was diagnosed in patients presenting with ischemic symptoms, no ST-segment elevation on ECG, and hs-cTnT levels below the 99th percentile upper reference limit. Non-ST-segment elevation myocardial infarction (NSTEMI) was defined as elevated hs-cTnT above the 99th percentile URL without persistent ST-segment elevation. ST-segment elevation myocardial infarction (STEMI) was defined as elevated hs-cTnT above the 99th percentile URL with ST-segment elevation ≥0.1 mV in ≥2 contiguous leads or new left bundle branch block. ACS classification was recorded in the electronic medical records and CV-NET registry system at the time of presentation (20). Left ventricular ejection fraction (LVEF) was assessed by transthoracic echocardiography during the index hospitalization, typically within 24–48 h of admission, or within 24 h post-procedure for patients undergoing primary PCI for STEMI. All patients were followed-up by telephone or email at 1, 6, and 12 months.
Statistical analysis
Continuous variables were assessed for normality using the Shapiro–Wilk test (n < 5,000) or Kolmogorov–Smirnov test (n ≥ 5,000) (Supplementary Table S1 and Supplementary Figures S1–S7). Variables with normal distribution are presented as mean ± SD; non-normally distributed variables are presented as median (Q1–Q3). Categorical variables are displayed as counts and percentages. Baseline characteristics were compared between eGFR groups using chi-squared tests for categorical variables and Student's t-test or Mann–Whitney U-test for continuous variables, as appropriate. These comparisons served a descriptive purpose to characterize group differences rather than for confirmatory hypothesis testing, therefore, correction for multiple comparisons was not applied. The proportion of missing data for each variable is presented in Supplementary Table S2. Time-to-event data with estimated event rates measured with the Kaplan–Meier method were compared using the log-rank test. The proportional hazards assumption was tested using Schoenfeld residuals. A 30-day landmark analysis was performed as sensitivity analysis. Multivariable Cox regression models were adjusted for age, sex, hypertension, previous myocardial infarction, previous PCI, previous stroke, smoking status, type of ACS, anemia, arterial access, coronary arteries treated, and number of stents. The non-linear association between hsTnT concentration and 12-month outcomes was evaluated using restricted cubic spline (RCS) regression within the Cox proportional hazards framework, with 4 knots placed at the 5th, 35th, 65th, and 95th percentiles. Unless otherwise specified, a 2-sided P value <0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
Overview of the study population
This investigation encompassed 14,208 ACS patients undergoing PCI, all with documented serum creatinine measurements and post-procedural high-sensitivity troponin T (hsTnT) assessments. Baseline characteristics of included and excluded patients are presented in Supplementary Table S3. Based on renal function parameters, patients were stratified into two cohorts: impaired renal function (eGFR <60 mL/min/1.73 m2, n = 1,206) and preserved renal function (eGFR ≥60 mL/min/1.73 m2, n = 13,002). Further stratification by hsTnT elevation revealed that among patients with impaired renal function, 366 subjects (30.3%) exhibited hsTnT elevations exceeding five times the 99th percentile upper reference limit (URL), while 840 (69.7%) remained below this threshold. In the preserved renal function cohort, 2,320 patients (17.8%) demonstrated hsTnT elevation ≥5 URL, with 10,682 subjects below this cutoff. The patient selection algorithm is illustrated in Figure 1.
Figure 1
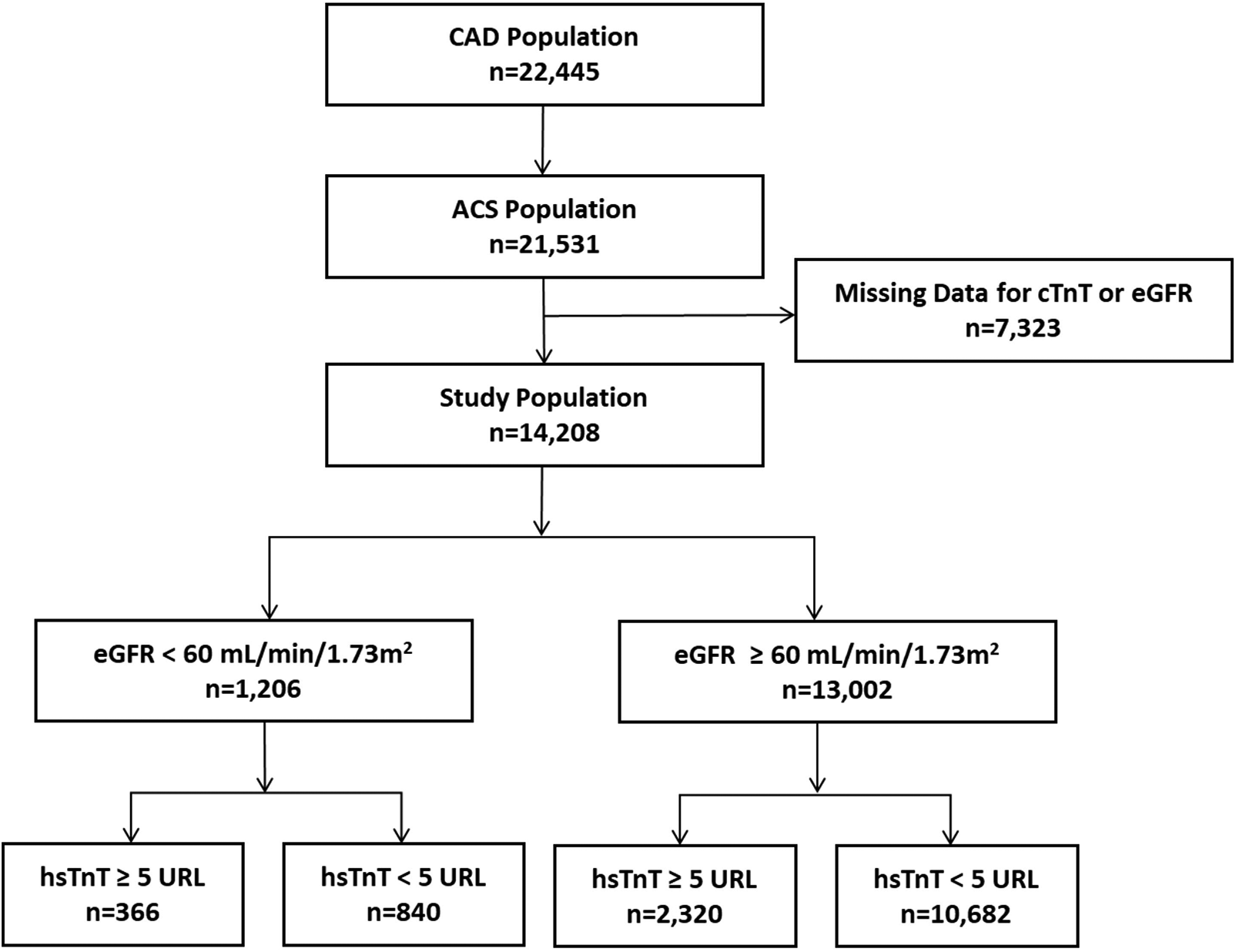
Study population flow chart. CAD, coronary artery disease; ACS, acute coronary syndrome; hsTnT, high-sensitivity troponin-T.
Study population characteristics
The eGFR was significantly higher in the preserved renal function group compared to the impaired renal function group (96.39 ± 20.11 vs. 46.03 ± 12.02 mL/min/1.73 m2; P < 0.001). Patients with impaired renal function were characterized by advanced age (68.12 ± 9.98 vs. 60.36 ± 10.15 years; P < 0.001) and higher female prevalence (38.14% vs. 25.65%; P < 0.0001). This cohort also demonstrated significantly higher rates of cardiovascular risk factors, including diabetes mellitus and hypertension, as well as greater prevalence of prior PCI, myocardial infarction, and cerebrovascular events (all P < 0.01). Regarding angiographic and procedural characteristics, patients with renal impairment exhibited more frequent left main coronary artery involvement (7.88% vs. 5.98%; P = 0.0088) and required longer stent lengths (47.17 ± 26.02 vs. 44.95 ± 26.04 mm; P = 0.006). Post-discharge pharmacotherapy patterns revealed higher clopidogrel utilization in the renal impairment group (76.63% vs. 67.04%; P < 0.0001), whereas statin (90.22% vs. 93.25%; P < 0.0001) and aspirin (94.20% vs. 98.21%; P < 0.0001) prescription rates were higher in patients with preserved renal function. Notably, post-procedural peak hsTnT values were significantly elevated in the renal impairment cohort [0.12 (0.03–0.74) vs. 0.04 (0.02–0.23) ng/L; P < 0.001] (Table 1). Peak hsTnT values also differed significantly across ACS subtypes: median (IQR) 0.02 (0.01–0.05) ng/L for UA, 0.14 (0.04–0.46) ng/L for NSTEMI, and 0.84 (0.15–2.43) ng/L for STEMI (P < 0.0001) (Supplementary Table S4). Comprehensive patient characteristics, discharge medication profiles, procedural parameters and clinical outcomes stratified by hsTnT elevation are presented in Supplementary Tables S5, S6.
Table 1
| Variable | Total cohort (N = 14,208) | eGFR < 60 (N = 1,206) | eGFR ≥ 60 (N = 13,002) | P value |
|---|---|---|---|---|
| Age, years | 61.02 ± 10.36 | 68.12 ± 9.98 | 60.36 ± 10.15 | <0.0001 |
| Male | 10,413 (73.29%) | 746 (61.86%) | 9,667 (74.35%) | <0.0001 |
| Medical history | ||||
| Hypertension | 8,839 (62.32%) | 965 (80.08%) | 7,874 (60.67%) | <0.0001 |
| Diabetes | 4,376 (30.89%) | 511 (42.55%) | 3,865 (29.81%) | <0.0001 |
| Previous MI | 2,674 (18.89%) | 329 (27.46%) | 2,345 (18.10%) | <0.0001 |
| Previous PCI | 3,695 (26.05%) | 358 (29.73%) | 3,337 (25.71%) | 0.0024 |
| Previous stroke | 2,103 (14.84%) | 274 (22.78%) | 1,829 (14.11%) | <0.0001 |
| Smoking | <.0001 | |||
| Never | 6,063 (42.84%) | 627 (52.16%) | 5,436 (41.97%) | |
| Active | 6,025 (42.57%) | 381 (31.70%) | 5,644 (43.58%) | |
| Former | 2,066 (14.60%) | 194 (16.14%) | 1,872 (14.45%) | |
| Anemia* | 2,255 (15.87%) | 555 (46.02%) | 1,700 (13.07%) | <0.0001 |
| eGFR, mL/min per 1.73 m2 | 92.18 (77.67–106.91) | 49.22 (39.97–55.36) | 94.51 (82.14–108.56) | <0.0001 |
| LVEF, % | 58.44 ± 8.60 | 54.02 ± 10.14 | 58.85 ± 8.32 | <0.0001 |
| hsTnT at peak, ng/L | 0.05 (0.02–0.26) | 0.12 (0.03–0.74) | 0.04 (0.02–0.23) | <0.0001 |
| PCI presentation | ||||
| Type of ACS | <0.0001 | |||
| UA | 8,555 (60.21%) | 589 (48.84%) | 7,966 (61.27%) | |
| NSTEMI | 2,477 (17.43%) | 262 (21.72%) | 2,215 (17.04%) | |
| STEMI | 3,176 (22.35%) | 355 (29.44%) | 2,821 (21.70%) | |
| Procedural information | ||||
| Transradial access | 12,998 (91.48%) | 1,020 (84.58%) | 11,978 (92.12%) | <0.0001 |
| Coronary arteries treated | ||||
| Left main artery | 873 (6.14%) | 95 (7.88%) | 778 (5.98%) | 0.0088 |
| Left anterior descending artery | 7,765 (54.65%) | 630 (52.24%) | 7,135 (54.88%) | 0.0784 |
| Left circumflex artery | 3,521 (24.78%) | 273 (22.64%) | 3,248 (24.98%) | 0.0713 |
| Right coronary artery | 5,251 (36.96%) | 482 (39.97%) | 4,769 (36.68%) | 0.0236 |
| Number of stents | 1.00 (1.00–2.00) | 1.00 (1.00–2.00) | 1.00 (1.00–2.00) | 0.7372 |
| Total length of stents, mm | 38.00 (24.00–60.00) | 40.50 (26.50–62.00) | 38.00 (24.00–59.00) | 0.001 |
| Average stent diameters, mm | 3.04 ± 0.75 | 3.01 ± 1.02 | 3.05 ± 0.72 | 0.2734 |
| Discharge prescription | ||||
| Aspirin | 13,905 (97.87%) | 1,136 (94.20%) | 12,769 (98.21%) | <0.0001 |
| P2Y12 inhibitors | <0.0001 | |||
| Clopidogrel | 9,592 (67.85%) | 915 (76.63%) | 8,677 (67.04%) | |
| Ticagrelor | 4,545 (32.15%) | 279 (23.37%) | 4,266 (32.96%) | |
| Statins | 13,213 (93.00%) | 1,088 (90.22%) | 12,125 (93.25%) | <0.0001 |
| ACEI/ARB | 9,421 (66.31%) | 769 (63.76%) | 8,652 (66.54%) | 0.0508 |
| βblockers | 9,799 (68.97%) | 856 (70.98%) | 8,943 (68.78%) | 0.1147 |
Baseline clinical characteristics, procedural characteristics, and medication after discharge in all patients and stratified by renal function.
Values are mean ± SD or No. (%). MI, myocardial infarction; PCI, percutaneous coronary intervention; hsTnT, high-sensitivity troponin-T; ACS, acute coronary syndrome; UA, unstable angina; STEMI, ST-segment-elevation myocardial infarction; NSTEMI, non-ST-segment-elevation myocardial infarction; LVEF, left ventricular ejection fraction; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker.
Anemia was defined as hemoglobin <13 g/dL for men or <12 g/dL for women.
Clinical outcomes according to eGFR
Compared to the patients with normal renal function low-risk group (eGFR ≥ 60 mL/min/1.73 m2), all-cause mortality (6.88% vs. 1.06%, P < 0.0001) within one year after PCI was significantly higher in patients with abnormal renal function (eGFR < 60 mL/min/1.73 m2). Significant differences were also found in the incidence of the risk for ischemic events (6.63% vs. 1.78%, P < 0.0001), which was mainly for the risk of cardiac death (5.31% vs. 0.74%, P < 0.0001). There were no significant differences in the incidence of MI (0.75% vs. 0.56%, P = 0.4176) and stroke (0.91% vs. 0.56%, P = 0.1286) between the groups (Table 2).
Table 2
| Outcome | Total cohort (N = 14,208) | eGFR < 60 (N = 1,206) | eGFR ≥ 60 (N = 13,002) | P value |
|---|---|---|---|---|
| All-cause death | 221 (1.56%) | 83 (6.88%) | 138 (1.06%) | <0.0001 |
| Ischemic events | 312 (2.20%) | 80 (6.63%) | 232 (1.78%) | <0.0001 |
| Cardiac death | 160 (1.13%) | 64 (5.31%) | 96 (0.74%) | <0.0001 |
| MI | 82 (0.58%) | 9 (0.75%) | 73 (0.56%) | 0.4176 |
| Stroke | 84 (0.59%) | 11 (0.91%) | 73 (0.56%) | 0.1286 |
Clinical outcomes at 1 year stratified by renal function.
Values are No. (%). MI, myocardial infarction. Ischemic events were defined as a composite of cardiac death, all MI, and/or stroke.
Within the impaired renal function group (eGFR <60 mL/min/1.73 m2), the majority of patients (63.3%) had eGFR 45–59 mL/min/1.73 m2, while only 28 patients (2.3%) had eGFR <15 mL/min/1.73 m2. One-year all-cause mortality rates increased with declining eGFR: 4.59% for eGFR 45–59, 9.18% for eGFR 30–44, 14.55% for eGFR 15–29, and 14.29% for eGFR <15 mL/min/1.73 m2. Similar patterns were observed for ischemic events (Supplementary Table S7).
Clinical outcomes according to hsTnT and eGFR
The analysis revealed a significant non-linear relationship between high-sensitivity troponin T levels and clinical outcomes, with distinct patterns emerging across different renal function categories. One-year cumulative incidence of all-cause mortality was highest in patients with eGFR <60 mL/min/1.73 m2 and hsTnT ≥5 URL (12.84%), followed by eGFR <60 mL/min/1.73 m2 and hsTnT <5 URL (4.29%), eGFR ≥60 mL/min/1.73 m2 and hsTnT ≥5 URL (1.42%), and eGFR ≥60 mL/min/1.73 m2 and hsTnT <5 URL (0.98%; log-rank P < 0.001). Similar patterns were observed for 1-year ischemic events (10.66%, 4.88%, 2.93%, and 1.54%, respectively; log-rank P < 0.001) (Figure 2).
Figure 2
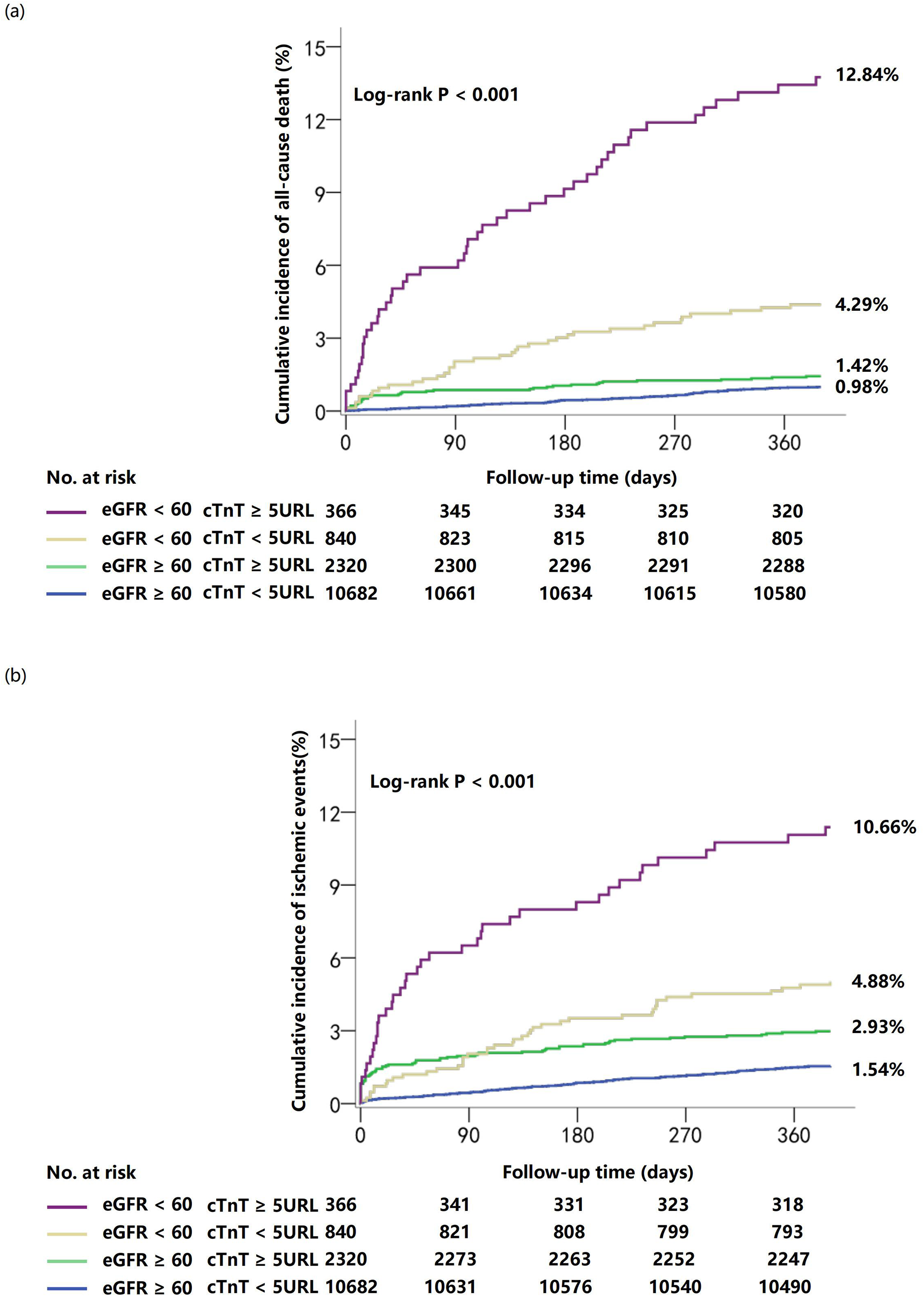
Cumulative incidence of clinical outcomes at 1 year stratified by troponin T and renal function. Curves depict cumulative incidence of all-cause death (a) and ischemic events (b).
RCS regression analysis quantified this non-linear relationship, demonstrating a significant non-linear association between hsTnT levels and all-cause mortality in patients with impaired renal function (P for overall <0.0001, P for non-linearity = 0.0004). In contrast, patients with normal renal function exhibited a predominantly linear relationship (P for overall = 0.0307, P for non-linearity = 0.2647). This differential pattern indicates that mortality risk accelerates disproportionately with increasing hsTnT levels in the context of renal impairment. Notably, the relationship between hsTnT and ischemic events maintained non-linearity regardless of renal function status, though with distinct curve morphologies (impaired renal function: P for overall = 0.0014, P for non-linearity = 0.0065; preserved renal function: P for overall = 0.0001, P for non-linearity = 0.0089) (Figure 3).
Figure 3
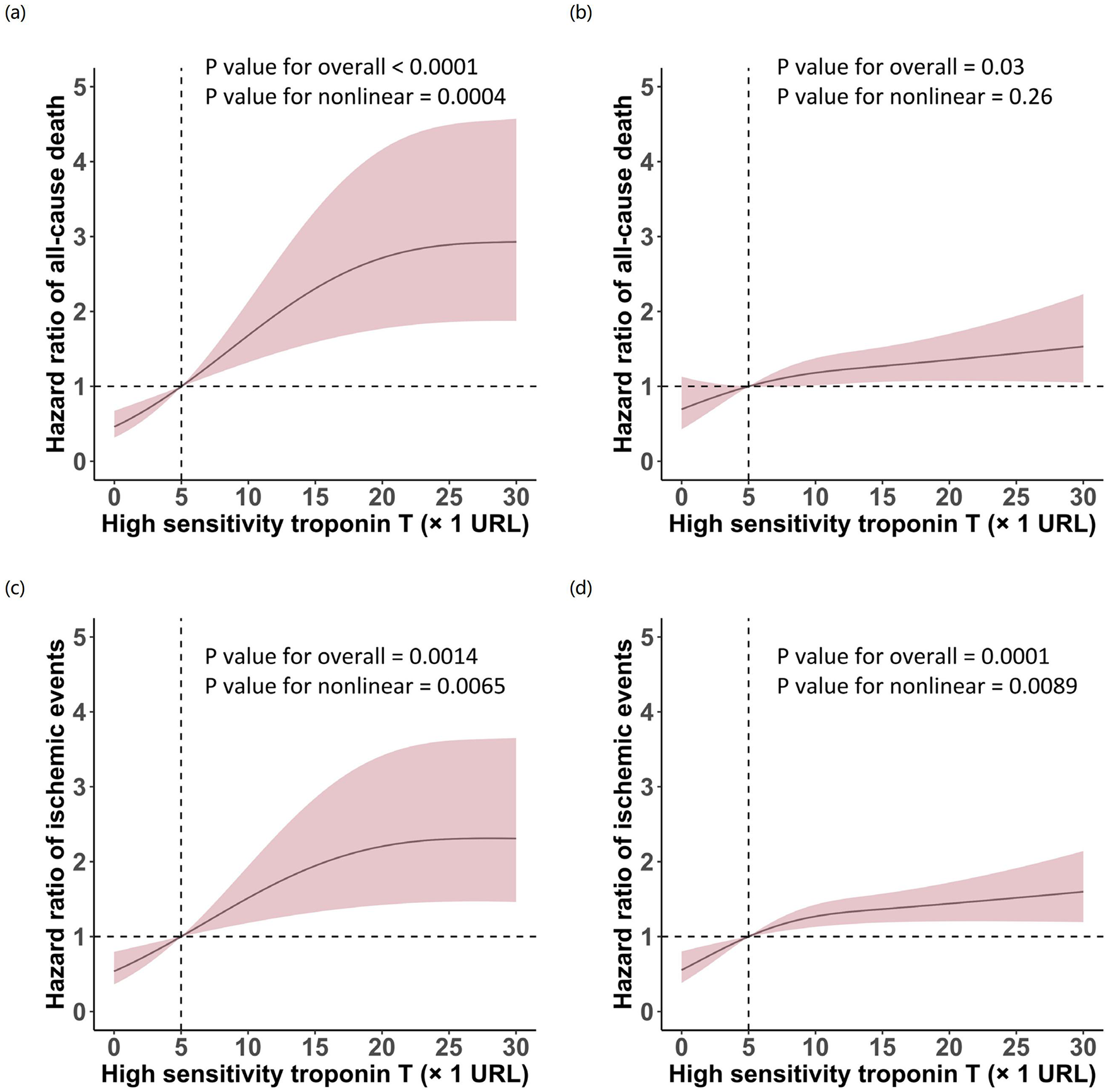
Restricted cubic spline analysis of the association between high-sensitivity troponin T levels and clinical outcomes stratified by renal function. Curves depict hazard ratios for all-cause death (a,b) and ischemic events (c,d) in patients with eGFR <60 mL/min/1.73 m2(a,c) and eGFR ≥60 mL/min/1.73 m2(b,d). Solid lines represent hazard ratios; shaded areas represent 95% confidence intervals. The vertical dashed line indicates hsTnT = 5 URL.
Multivariable Cox regression models confirmed a significant interaction between renal function and hsTnT levels for 1-year mortality. In patients with impaired renal function, elevated troponin conferred a 3.63-fold increase in adjusted 1-year mortality risk (aHR: 3.63; 95% CI: 2.04–6.49, P < 0.001), while in those with preserved renal function, this association was attenuated and did not reach statistical significance (aHR: 1.40; 95% CI: 0.85–2.29, P = 0.1864; P for interaction = 0.0089). This interaction effect was not observed for composite ischemic events (Pinteraction = 0.4041), but reemerged for cardiac death specifically, where elevated hsTnT demonstrated significant prognostic value only in patients with renal impairment (Table 3). These non-linear relationships and their differential patterns across renal function strata provide important insights for clinical risk assessment strategies.
Table 3
| Outcomes | hsTnT | Hazard ratio (95% CI) | P value | Adjusted hazard ratio (95% CI) | P value | P value for interaction | |
|---|---|---|---|---|---|---|---|
| ≥5 URL | <5 URL | ||||||
| All-cause death | 0.0089 | ||||||
| eGFR < 60 | 12.84% (47/366) | 4.29% (36/840) | 3.14 (2.04–4.85) | <0.0001 | 3.63 (2.04–6.49) | <0.0001 | |
| eGFR ≥ 60 | 1.42% (33/2,320) | 0.98% (105/10,682) | 1.46 (0.98–2.15) | 0.0599 | 1.40 (0.85–2.29) | 0.1864 | |
| Ischemic events | 0.4041 | ||||||
| eGFR < 60 | 10.66% (39/366) | 4.88% (41/840) | 2.29 (1.48–3.56) | 0.0002 | 2.72 (1.51–4.88) | 0.0008 | |
| eGFR ≥ 60 | 2.93% (68/2,320) | 1.54% (164/10,682) | 1.93 (1.46–2.57) | <0.0001 | 2.06 (1.41–2.97) | 0.0002 | |
| Cardiac death | 0.1031 | ||||||
| eGFR < 60 | 9.84% (36/366) | 3.33% (28/840) | 3.09 (1.88–5.06) | <0.0001 | 3.26 (1.69–6.29) | 0.0004 | |
| eGFR ≥ 60 | 1.16% (27/2,320) | 0.65% (69/10,682) | 1.81 (1.16–2.83) | 0.0089 | 1.55 (0.88–2.75) | 0.1323 | |
| MI | 0.0821 | ||||||
| eGFR < 60 | 0.55% (2/366) | 0.83% (7/840) | 0.66 (0.14–3.17) | 0.6022 | 1.04 (0.15–7.35) | 0.9680 | |
| eGFR ≥ 60 | 1.34% (31/2,320) | 0.39% (42/10,682) | 3.42 (2.15–5.44) | <0.0001 | 3.03 (1.60–5.73) | 0.0007 | |
| Stroke | 0.3475 | ||||||
| eGFR < 60 | 1.09% (4/366) | 0.83% (7/840) | 1.32 (0.39–4.50) | 0.6592 | 1.76 (0.34–9.02) | 0.4999 | |
| eGFR ≥ 60 | 0.43% (10/2,320) | 0.59% (63/10,682) | 0.73 (0.38–1.43) | 0.3576 | 1.13 (0.49–2.60) | 0.7798 | |
Adjusted associations between peak cardiac troponin T concentration and clinical outcomes at 1 year stratified by renal function.
Values are No. (%). hsTnT, high-sensitivity troponin-T; MI, myocardial infarction. Ischemic events were defined as a composite of cardiac death, all MI, and/or stroke. Adjusted for age, sex, hypertension, previous myocardial infarction, previous PCI, previous stroke, smoking status, type of ACS, anemia, arterial access, coronary arteries treated, and number of stents.
A 30-day landmark analysis was performed, excluding 45 deaths and 87 ischemic events occurring within 30 days. After landmark, the proportional hazards assumption was satisfied (all-cause death: P = 0.086; ischemic events: P = 0.19). For all-cause mortality, event rates were 0.92% (98/10,675) in the eGFR ≥60/hsTnT <5 URL group, 0.78% (18/2,305) in the eGFR ≥60/hsTnT ≥5 URL group (HR = 0.85, 95% CI: 0.51–1.41, P = 0.528), 3.37% (28/832) in the eGFR <60/hsTnT <5 URL group (HR = 3.72, 95% CI: 2.45–5.67, P < 0.001), and 9.12% (32/351) in the eGFR <60/hsTnT ≥5 URL group (HR = 10.43, 95% CI: 7.00–15.54, P < 0.001). For ischemic events, event rates were 1.30% (139/10,657), 1.36% (31/2,283; HR = 1.04, 95% CI: 0.71–1.54, P = 0.834), 3.85% (32/831; HR = 3.01, 95% CI: 2.05–4.42, P < 0.001), and 6.57% (23/350; HR = 5.29, 95% CI: 3.40–8.22, P < 0.001), respectively (Supplementary Table S8).
Discussion
In this retrospective study, we evaluated the prognostic value of hsTnT in ACS patients undergoing PCI, stratified by renal function. The main findings were as follows: (1) patients with impaired renal function (eGFR < 60 mL/min/1.73 m2) exhibited significantly higher one-year all-cause mortality and incidence of ischemic events compared to those with normal renal function (eGFR ≥ 60 mL/min/1.73 m2); (2) elevated hsTnT levels (≥5 URL) were more prevalent in patients with impaired renal function and were strongly associated with adverse outcomes; (3) RCS regression analysis indicated a nonlinear relationship between hsTnT levels and one-year all-cause mortality in patients with impaired renal function, while the relationship was linear in those with normal renal function. These results highlight the importance of considering renal function when interpreting hsTnT levels in ACS patients post-PCI.
Several studies have elucidated the prognostic significance of hsTnT across diverse clinical scenarios. Pareek et al. (21) demonstrated that serial hsTnT measurements during hospitalization for suspected ACS could predict both short-term and long-term mortality outcomes. However, the specific implications of hsTnT levels in ACS patients undergoing PCI, particularly when stratified by renal function, remain partially explored. These results corroborate previous findings that hsTnT levels are elevated in CKD patients and associated with increased cardiovascular events and mortality. While prior studies examined ambulatory CKD populations without acute coronary events or longitudinal cTnT in advanced CKD, the present study extends these observations to ACS patients undergoing PCI, demonstrating that the prognostic value of post-procedural hsTnT is significantly modified by renal function status (5–7). Moreover, our study reveals the differential impact of elevated hsTnT on mortality and ischemic events between patients with normal and impaired renal function. While elevated hsTnT levels were associated with increased one-year all-cause mortality and ischemic events in both groups, a significant non-linear association was observed in patients with impaired renal function. This underscores the importance of personalized risk assessment in managing ACS patients after PCI, highlighting the pronounced impact of renal health on prognosis.
The enhanced prognostic significance of elevated hsTnT in patients with impaired renal function likely reflects the capacity of troponin to integrate both chronic and acute components of cardiovascular injury in this population. Chronic kidney disease is characterized by a constellation of pathophysiological alterations, including left ventricular hypertrophy, diffuse myocardial fibrosis, microvascular ischemia, volume and pressure overload, systemic inflammation, and reduced renal clearance of troponin degradation products, all of which contribute to persistent troponin release and elevated baseline hsTnT concentrations (5–7, 12–15). Within this context, an elevated peak hsTnT level during ACS-related PCI admission may represent the cumulative burden of chronic cardiorenal stress superimposed upon acute ischemic insult, thereby identifying patients with diffuse structural heart disease and advanced vascular pathology who face particularly elevated mortality risk. Conversely, in patients with preserved renal function, baseline hsTnT concentrations are characteristically low, and elevations predominantly reflect the acute ischemic burden of the index event and periprocedural myocardial injury. After adjustment for clinical risk factors and procedural characteristics, the residual prognostic gradient across peak hsTnT levels becomes more modest in this population, consistent with the largely linear association and attenuated adjusted hazard ratios observed in our analysis. This mechanistic framework provides a coherent explanation for why the relationship between hsTnT and mortality exhibits a steeper, non-linear pattern in the presence of renal impairment, whereas it remains flatter and less discriminative when renal function is preserved.
The renal function-dependent prognostic heterogeneity of hsTnT observed in our study supports a more individualized approach to risk stratification, whereby high-risk patients with concurrent renal impairment and elevated troponin may be prioritized for intensified surveillance and early intervention. Our findings also underscore the importance of optimizing post-PCI pharmacotherapy in patients with impaired renal function. The higher clopidogrel utilization coupled with lower statin and aspirin prescription rates observed in this population suggests potential opportunities for pharmacological optimization to mitigate the elevated risk of adverse outcomes. Notably, elevated hsTnT levels (≥5 URL) were more prevalent in patients with impaired renal function compared to those with preserved renal function, consistent with the established pathophysiological impact of renal insufficiency on cardiac biomarker kinetics. Beyond the mechanisms discussed above, recent Mendelian randomization evidence has demonstrated a causal relationship between urinary sodium-potassium ratio and myocardial infarction risk (22). Given that renal dysfunction substantially alters urinary electrolyte homeostasis, this finding provides an additional mechanistic pathway linking impaired renal function to adverse cardiovascular outcomes and may partly explain the amplified prognostic significance of elevated troponin in this population.
Several clinical implications emerge from our findings. First, current risk stratification tools such as the GRACE and TIMI scores do not incorporate renal function-specific interpretation of troponin values; our data suggest that the prognostic weight of a given hsTnT elevation differs substantially depending on baseline eGFR, and future iterations of these scores may benefit from including an interaction term or renal function-stratified coefficients for troponin. Second, rather than applying uniform hsTnT thresholds across all patients, clinicians might consider lower thresholds to identify high-risk individuals among those with eGFR <60 mL/min/1.73 m2, given the steeper mortality gradient observed in this subgroup. Third, patients presenting with the combination of impaired renal function and elevated hsTnT may warrant intensified post-discharge management, including earlier follow-up (within 2 weeks rather than 4–6 weeks), aggressive optimization of guideline-directed medical therapy with renal-appropriate dosing, and lower thresholds for referral to cardiac rehabilitation or advanced heart failure services. Future prospective studies should validate these findings and evaluate whether incorporation of renal function-adjusted troponin thresholds into clinical decision support tools improves risk discrimination and patient outcomes.
Limitations
Several limitations must be acknowledged. First, the retrospective nature of the study introduces potential biases, and the single-center design may limit generalizability. Second, both serum creatinine and hs-cTnT measurements have timing-related limitations: creatinine was measured at presentation, which may be affected by acute cardiorenal syndrome and may not accurately reflect baseline renal function, although hs-cTnT was routinely measured at admission and 6–12 h post-PCI per institutional protocol, precise data on time from symptom onset or balloon inflation to blood sampling were not recorded, and individual variability in sampling timing may influence peak troponin values. Third, patients with eGFR <60 mL/min/1.73 m2 represent a heterogeneous group, only 2.32% had eGFR <15 mL/min/1.73 m2, and data on renal replacement therapy were unavailable, limiting generalizability to patients with end-stage renal disease. Fourth, patients with impaired renal function had a higher burden of cardiovascular comorbidities, and unstable angina was more prevalent in patients with preserved renal function, which may influence troponin distribution. Although multivariable Cox regression was adjusted for these factors and the significant interaction between renal function and hsTnT persisted (P for interaction = 0.0089), residual confounding from unmeasured comorbidities cannot be entirely excluded. Finally, the proportional hazards assumption showed deviation in the primary analysis due to higher early event rates in high-risk patients; landmark sensitivity analysis confirmed consistent findings (Supplementary Table S8). Future prospective studies are needed to confirm these findings and explore the underlying mechanisms driving the interaction between hsTnT levels and renal function.
Conclusion
Our findings demonstrate that renal function fundamentally transforms the prognostic significance of elevated troponin T in ACS patients undergoing PCI. This critical interaction, where elevated troponin carries three-fold greater mortality risk in renal impairment but minimal impact with normal function, establishes the necessity for integrated risk assessment incorporating both parameters to identify patients requiring intensified surveillance and intervention.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request. Requests to access the datasets should be directed to Yaling Han (hanyaling@163.net).
Ethics statement
The studies involving humans were approved by The study was approved by the General Hospital of Northern Theater Command's Research Ethics Committee (K2018-35). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KN: Writing – review & editing, Data curation, Formal analysis, Investigation, Project administration, Writing – original draft. XY: Data curation, Investigation, Writing – original draft, Writing – review & editing, Conceptualization, Methodology. MQ: Conceptualization, Writing – original draft, Writing – review & editing, Formal analysis. XZ: Writing – review & editing, Supervision, Validation. YL: Writing – review & editing, Conceptualization, Investigation, Project administration. YH: Conceptualization, Writing – review & editing, Funding acquisition, Resources, Supervision.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the National Key Research and Development Program of China (2024ZD0537904).
Acknowledgments
The authors appreciate the dedicated efforts of clinical research collaborators in this study.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1689234/full#supplementary-material
References
1.
Wanamaker BL Seth MM Sukul D Dixon SR Bhatt DL Madder RD et al Relationship between troponin on presentation and in-hospital mortality in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Am Heart Assoc. (2019) 8(19):e013551. 10.1161/JAHA.119.013551
2.
Collet J-P Thiele H Barbato E Barthélémy O Bauersachs J Bhatt DL et al 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. 10.1093/eurheartj/ehaa575
3.
Ibanez B James S Agewall S Antunes MJ Bucciarelli-Ducci C Bueno H et al 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. 10.1093/eurheartj/ehx393
4.
Byrne RA Rossello X Coughlan JJ Barbato E Berry C Chieffo A et al 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. (2023) 44(38):3720–826. 10.1093/eurheartj/ehad191
5.
Hti Lar Seng NS Zeratsion G Pena Zapata OY Tufail MU Jim B . Utility of cardiac troponins in patients with chronic kidney disease. Cardiol Rev. (2024) 32(1):62–70. 10.1097/CRD.0000000000000461
6.
Dubin RF Li Y He J Jaar BG Kallem R Lash JP et al Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. (2013) 14:229. 10.1186/1471-2369-14-229
7.
Chesnaye NC Al-Sodany E Szummer K Barany P Heimbürger O Almquist T et al Association of longitudinal high-sensitivity troponin T with mortality in patients with chronic kidney disease. J Am Coll Cardiol. (2022) 79(4):327–36. 10.1016/j.jacc.2021.11.023
8.
Go AS Chertow GM Fan D McCulloch CE Hsu C-y . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351(13):1296–305. 10.1056/NEJMoa041031
9.
Murphy D Banerjee D . Cardiac troponins in kidney disease. Eur Cardiol. (2025) 20:e22. 10.15420/ecr.2024.51
10.
Shoji T Emoto M Nishizawa Y Inaba M . Endocrine and metabolic changes affecting cardiovascular disease in dialysis patients. J Ren Nutr. (2015) 25(2):223–5. 10.1053/j.jrn.2014.10.018
11.
Nagami GT Kraut JA . The role of the endocrine system in the regulation of acid-base balance by the kidney and the progression of chronic kidney disease. Int J Mol Sci. (2024) 25(4):2420. 10.3390/ijms25042420
12.
Pugliese NR Fabiani I Conte L Nesti L Masi S Natali A et al Persistent congestion, renal dysfunction and inflammatory cytokines in acute heart failure: a prognosis study. J Cardiovasc Med. (2020) 21(7):494–502. 10.2459/JCM.0000000000000974
13.
Arvunescu AM Ionescu RF Cretoiu SM Dumitrescu SI Zaharia O Nanea IT . Inflammation in heart failure-future perspectives. J Clin Med. (2023) 12(24):7738. 10.3390/jcm12247738
14.
Miller-Hodges E Anand A Shah ASV Chapman AR Gallacher P Lee KK et al High-sensitivity cardiac troponin and the risk stratification of patients with renal impairment presenting with suspected acute coronary syndrome. Circulation. (2018) 137(5):425–35. 10.1161/CIRCULATIONAHA.117.030320
15.
Chuang A(-y Nguyen MT Kung W-M Lehman S Chew DP . High-sensitivity troponin in chronic kidney disease: considerations in myocardial infarction and beyond. Rev Cardiovasc Med. (2020) 21(2):191–203. 10.31083/j.rcm.2020.02.17
16.
Levey AS Coresh J Greene T Stevens LA Zhang Y( Hendriksen S et al Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. (2006) 145(4):247–54. 10.7326/0003-4819-145-4-200608150-00004
17.
Stevens LA Coresh J Feldman HI Greene T Lash JP Nelson RG et al Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. (2007) 18(10):2749–57. 10.1681/ASN.2007020199
18.
Garcia-Garcia HM McFadden EP Farb A Mehran R Stone GW Spertus J et al Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. (2018) 137(24):2635–50. 10.1161/CIRCULATIONAHA.117.029289
19.
Thygesen K Alpert JS Jaffe AS Simoons ML Chaitman BR White HD et al Third universal definition of myocardial infarction. J Am Coll Cardiol. (2012) 60(16):1581–98. 10.1016/j.jacc.2012.08.001
20.
American Diabetes Association Professional Practice C. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45(1):S17–38. 10.2337/dc22-S002
21.
Pareek M Kragholm KH Kristensen AMD Vaduganathan M Pallisgaard JL Byrne C et al Serial troponin-T and long-term outcomes in suspected acute coronary syndrome. Eur Heart J. (2023) 44(6):502–12. 10.1093/eurheartj/ehac629
22.
Wu Z Wang D Tang C . Urinary sodium-potassium ratio as a genetic predictor of myocardial infarction. Coron Artery Dis. (2025) 36(7):561–8. 10.1097/MCA.0000000000001532
Summary
Keywords
acute coronary syndrome, high-sensitivity troponin T, long-term prognosis, percutaneous coronary intervention, renal function
Citation
Na K, Yang X, Qiu M, Zhang X, Li Y and Han Y (2025) Differential prognostic value of high-sensitivity troponin T based on renal function status: insights from 14,208 ACS patients undergoing PCI. Front. Cardiovasc. Med. 12:1689234. doi: 10.3389/fcvm.2025.1689234
Received
20 August 2025
Revised
01 December 2025
Accepted
08 December 2025
Published
19 December 2025
Volume
12 - 2025
Edited by
Antonio De Vita, Agostino Gemelli University Polyclinic (IRCCS), Italy
Reviewed by
Dong Wang, Southeast University, China
Daniel Murphy, Royal Free Hospital, United Kingdom
Updates
Copyright
© 2025 Na, Yang, Qiu, Zhang, Li and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yi Li doctorliyi@126.com Yaling Han hanyaling@163.net
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.