Abstract
Background:
Exosomes play multifaceted roles in atherosclerosis, contributing to vascular inflammation, immune regulation, and tissue repair. This duality has drawn attention to their potential clinical utility, both as diagnostic indicators and as novel therapeutic avenues. However, despite the rapid expansion of research, a comprehensive scientometric overview of this field remains lacking.
Methods:
From 2004 to July 29, 2025, publications related to exosomes and atherosclerosis were screened in the Web of Science Core Collection (WoSCC) and Scopus. After deduplication, 1,179 records (964 articles, 215 reviews) were included. Bibliometric analyses were conducted using CiteSpace, VOSviewer, and the bibliometrix R package. Indicators examined included publication trends, global distribution, institutional and author contributions, co-citation patterns, and keyword evolution.
Results:
Publications on exosomes in atherosclerosis have risen sharply since 2015, consistent with Price's Law. China contributes the largest volume, while the United States shows stronger international collaboration. Central South University, Capital Medical University, and Harvard University rank among the most productive institutions. Key contributors include Elena Aikawa and Lijun Yuan. Major publication venues are the International Journal of Molecular Sciences, Frontiers in Cardiovascular Medicine, and Frontiers in Immunology. Keyword and co-citation mapping revealed three major research axes: RNA-mediated regulation, immune and endothelial interactions, and emerging translational applications such as engineered exosomes and diagnostic biomarkers.
Conclusion:
This bibliometric analysis characterizes global research development and shows a shift from descriptive studies toward mechanistic insight and translational innovation. These trends may facilitate future efforts in biomarker discovery, standardized exosome workflows, and therapeutic development.
1 Introduction
Atherosclerosis (AS) remains the primary cause of cardiovascular morbidity and mortality, despite significant advancements in the management of risk factors and improvements in revascularization techniques (1). Historically, AS was understood as the result of lipid deposition within the arterial walls (2); however, recent studies have revealed that it is a multifaceted, chronic immune-inflammatory disorder (3). This condition involves complex interactions between vascular and immune cells in the arterial wall, which contribute to endothelial dysfunction, vascular remodeling, and plaque formation (2).
Exosomes are small vesicles secreted by various cell types, and they play an essential role in intercellular communication by transporting microRNAs (miRs), proteins, lipids, and other molecular cargo to recipient cells (4). In the context of AS, exosomes modulate a range of pathological processes, including endothelial dysfunction, smooth muscle cell phenotypic alterations, foam cell formation in macrophages, vascular calcification, and thrombo-inflammatory responses (5, 6).
However, although research output in this field is growing rapidly, the literature lacks an integrated scientometric evaluation that maps its evolution, collaborative structure, and emerging thematic hotspots (5).
To address these gaps, this study conducted a bibliometric and scientometric analysis of global publications concerning exosomes in AS, with the aim of mapping the intellectual landscape, identifying emerging research trends, and highlighting key areas for future investigation. Using data from the Web of Science Core Collection and Scopus (2004–2025) and applying CiteSpace, VOSviewer, and Bibliometrix tools, we provide a structured overview of research development and thematic evolution. We offer a detailed assessment of global research activity in this field. This work aims to support future mechanistic studies, promote methodological standardization, and advance the clinical translation of exosome-based diagnostics and therapeutics in cardiovascular medicine.
2 Methods
2.1 Database and search strategy
While WoSCC is recognized for its stringent journal evaluation procedures and dependable citation tracking system, Scopus is valued for its wide disciplinary scope and sophisticated citation-analysis features that support interdisciplinary inquiry. Employing the two databases together allows researchers to obtain a more balanced and comprehensive evidence base, thereby enhancing the accuracy of bibliometric assessments and enabling deeper exploration of evolving research patterns and scholarly developments.
Figure 1 outlines the procedures for data retrieval and the criteria for excluding records. Bibliometric information was first collected by applying designated search terms in WoSCC and Scopus for the period from January 1, 2004, to July 29, 2025. A preliminary screening was then conducted using the publication titles, abstracts, and keywords. To define the search vocabulary, we consulted a range of earlier studies in the literature. Atherosclerosis-related terms = (“Atherosclerosis” OR “atherosclerosis” OR “atherogenesis”). Exosome-related terms = (“Exosome” OR “exosomal”). Finally, the two combined datasets yielded 588 documents from WoSCC and 1,189 documents from Scopus (Figure 1).
Figure 1
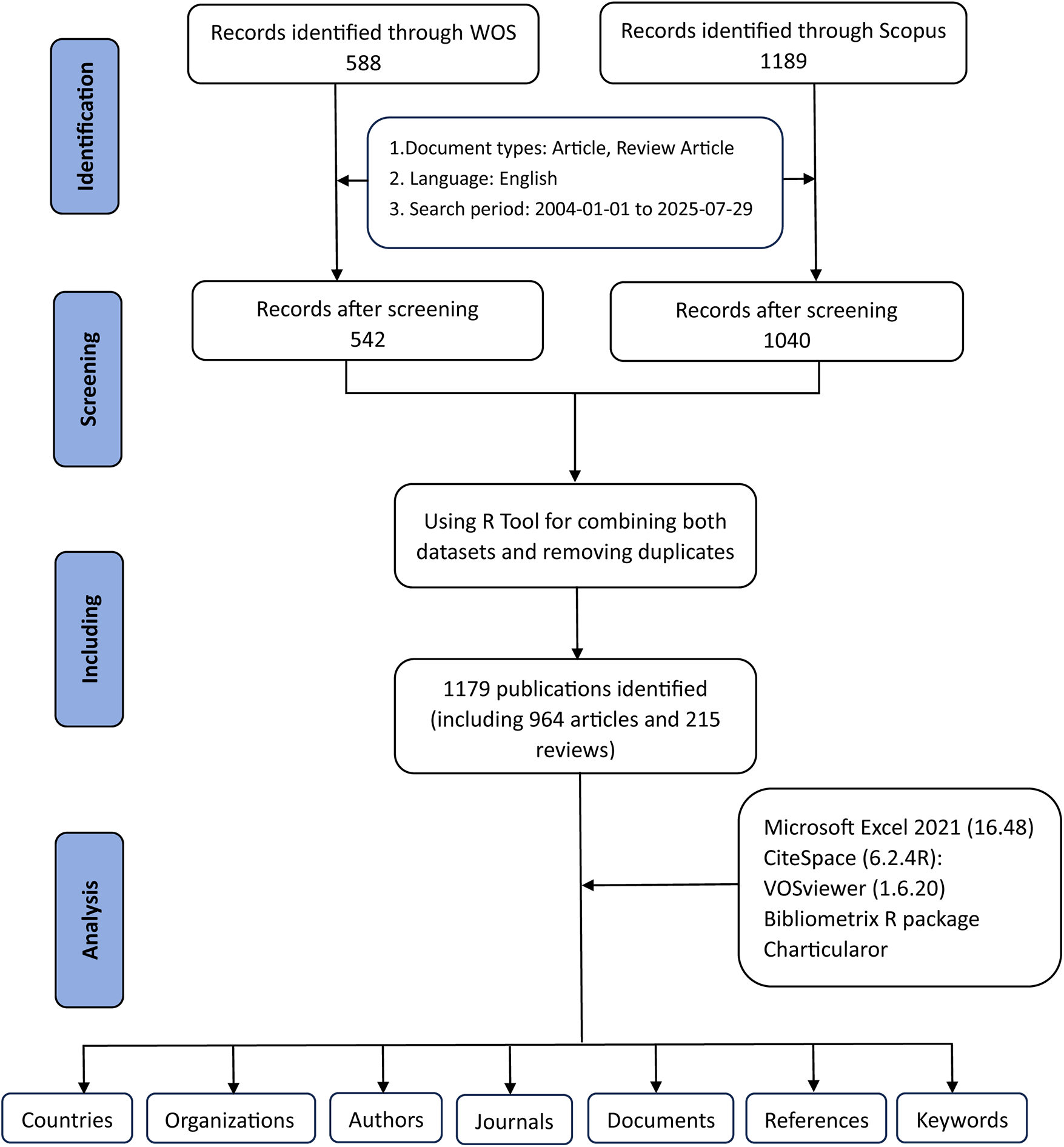
The flowchart for literature search, selection and analysis.
Exclusions were applied to remove Proceeding Papers, Corrections, Early Access articles, News Items, Book Chapters, Retractions, Reprints, Biographical Items, Book Reviews, Meeting Abstracts, Editorial Materials, and Letters. Only articles and reviews in English were retained. Additionally, the R tool was used to combine both datasets and removing duplicates. Ultimately, a total of 1,179 studies were included for further analysis, including 964 articles and 215 reviews, comprising 542 studies from WoSCC and 637 studies from Scopus (Figure 1).
2.2 Data analysis procedures
2.2.1 Citespace
CiteSpace (6.2.4R, 64-bit Advanced Edition) was employed for the data analysis (7). The data collection period spanned from January 2004 to July 2025. The nodes analyzed included authors, institutions, and keywords. Only core analytical steps were retained in the main text, while detailed software parameters were streamlined for clarity. All records retrieved from WoSCC were saved as “full records and cited references” in plain text format.
2.2.2 VOSviewer
VOSviewer (1.6.20), developed by CWTS at Leiden University, was utilized for processing the data. Full counting was used, with thresholds established based on analytical items to produce visual representations of collaborative networks (8).
2.2.3 Bibliometrix
The bibliometrix R package (https://www.bibliometrix.org) (9), developed by Dr. Massimo Aria and Corrado Cuccurullo, was employed for historiographic analysis, tracking journal and author trends, and calculating bibliometric indicators such as g-index (10), h-index (11), number of citations (NC), and number of publications (NP).
2.2.4 Other tools
Microsoft Excel 2021 (Version 16.48) was employed to organize the initial dataset. Bibliometric analyses of citation information were then conducted using the Online Analysis Platform of Literature Metrology (https://bibliometric.com/), which offers a straightforward and user-friendly analytical interface.
3 Results
3.1 Annual publication trends
Our bibliometric analysis, spanning from January 2004 to July 2025, revealed a substantial increase in both the number of publications and citations in the field of exosomes and AS. A total of 1,179 documents were retrieved from the Scopus and WoSCC databases, with an annual growth rate of 24.4%, indicating a rapidly expanding research domain over the past two decades (Supplementary Table S1). Figure 2A shows that publication output remained low before 2015 but rose sharply thereafter, reflecting growing scientific interest. Citations exhibited a similar pattern, supporting the expanding influence of this field.
Figure 2
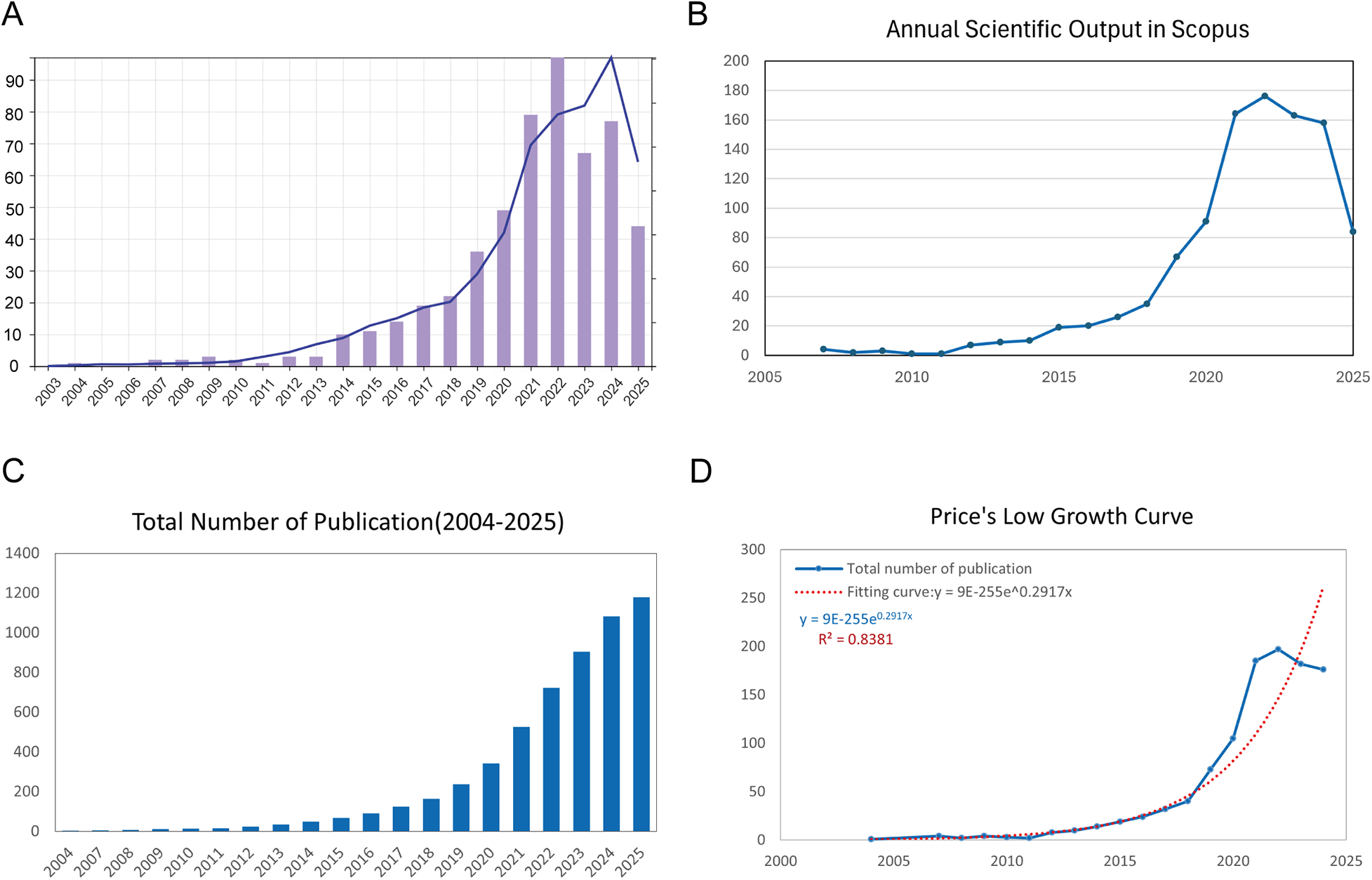
Overview of publications and citations on exosomes in atherosclerosis (2004–2025). (A) Annual trends in publication count (bars) and citation frequency (line) sourced from the WoSCC database. (B) Annual trends in publication count (bars) and citation frequency (line) sourced from the Scopus database. (C) Cumulative publication count over time, highlighting the overall expansion of the field. (D) Price's Law curve fitting analysis of cumulative publications, illustrating the exponential growth of exosome-related atherosclerosis research.
Figure 2B, sourced from Scopus, confirms the pattern observed in Figure 2A, showing a sharp increase in the number of publications after 2015, peaking in 2022. Although the number of publications in these two databases declined in 2025, it is important to note that this result is based on data up to July 29, 2025, and may not fully capture the trend for the entire year. We will monitor the full-year trends in subsequent updates. Figure 2C illustrates the cumulative publication count over time, demonstrating a clear exponential trajectory.
Importantly, Figure 2D confirms that the growth pattern aligns with Price's Law. The fitted model equation is y = 9E-255e0.2917x, with an R2 value of 0.8381, supporting the conclusion that exosome-related atherosclerosis research has entered a phase of accelerated scientific development.
3.2 Distributions of countries/regions
Figures 3A,B,D illustrate the global research network, with darker blue shades indicating stronger collaborative ties. In these visualizations, the core quantitative indicators in Figure 3A are calculated based on the number of co-published papers and weighted by the number of citations. The collaboration intensity in Figures 3B,D is measured using VOSviewer's total link strength, representing the volume of collaborative publications. The United States and China exhibit the most robust collaborative connections, forming dominant networks across North America, East Asia, and Europe. Other regions show comparatively limited participation.
Figure 3
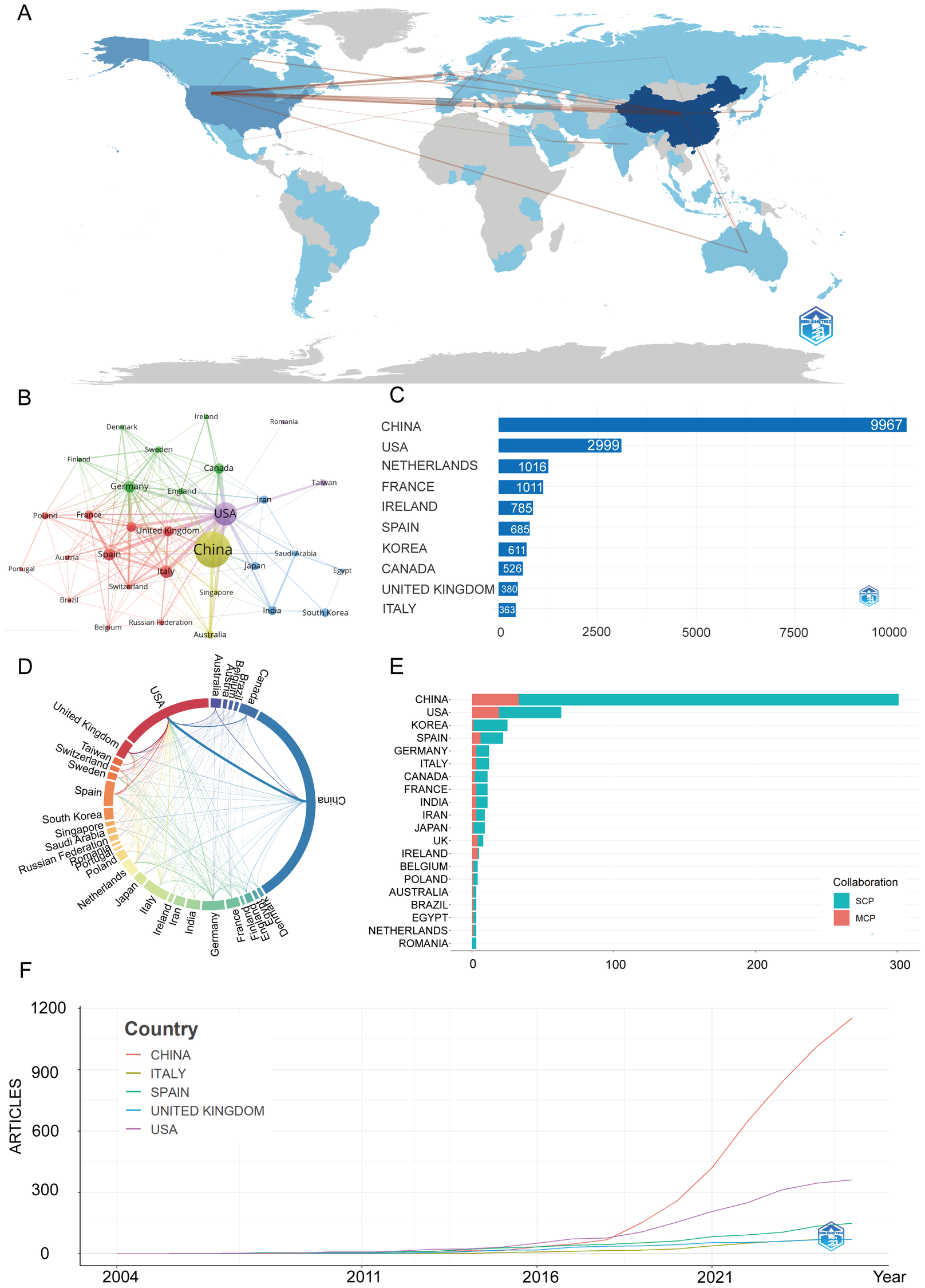
Global trends and collaboration networks in scientific publications on exosomes in atherosclerosis (2004–2025). (A) Country/Region Collaboration Map. Color depth represents the overall national collaboration intensity (the darker the color, the more active the collaboration), and line thickness represents the strength of bilateral collaboration (the thicker the line, the closer the cooperation between the two countries). (B) Country Clustering Analysis (network). Each node represents a country/region, with size proportional to publication volume. Edge thickness reflects co-authorship strength, and node color indicates collaboration clusters. Countries in the same color cluster collaborate more frequently, with spatial proximity indicating stronger links. (C) Top 10 Countries/Regions by Citation Impact. Horizontal bars display citation counts for each country/region's publications (labels indicate the count). (D) Network Map of National Research Output and Collaboration (chord diagram). Outer arcs represent countries/regions, with arc length showing overall collaborative output. Ribbons connect country pairs, with width indicating collaboration strength (number of co-authored papers). Ribbon colors match the originating arc. (E) Leading Countries by Publication Count and Collaboration Type. Stacked bars show the number of publications, split into single-country publications (SCP, teal) and multi-country publications (MCP, Salmon/red). The MCP proportion reflects international collaboration extent. (F) Top 5 Countries/Regions by Publications. Lines represent cumulative publications from 2004 to 2025, with year on the x-axis and cumulative count on the y-axis.
In Figure 3C, citation performance mirrors production: China accrues the highest cumulative citations, signaling broad visibility and uptake. The United States ranks second, while European countries such as the Netherlands, France, Spain, and the United Kingdom contribute meaningfully.
Figure 3E shows China's leadership is driven mainly by single-country publications, reflecting strong domestic capacity. In contrast, the United States demonstrates a higher proportion of multi-country collaborations, consistent with a more globally integrated network.
Supplementary Table S2 and Figure 3F further illustrate that China shows the steepest growth curve, surpassing all other countries after 2018 and contributing the largest share of publications in recent years. The United States ranks second, with steady gains but a more moderate slope, while European contributors—particularly the United Kingdom, Spain, and Italy—exhibit smaller yet consistent increases.
3.3 Distribution by institutions
Figures 4A,B are the institutional co-occurrence diagram and the institutional cooperation network, respectively. Capital Medical University occupies the central position with the largest node size, indicating both high publication volume and frequent collaborations. Other major hubs include the Chinese Academy of Medical Sciences/Peking Union Medical College, Central South University, and Fudan University. These institutions form tightly connected clusters, reflecting strong domestic collaboration within China.
Figure 4
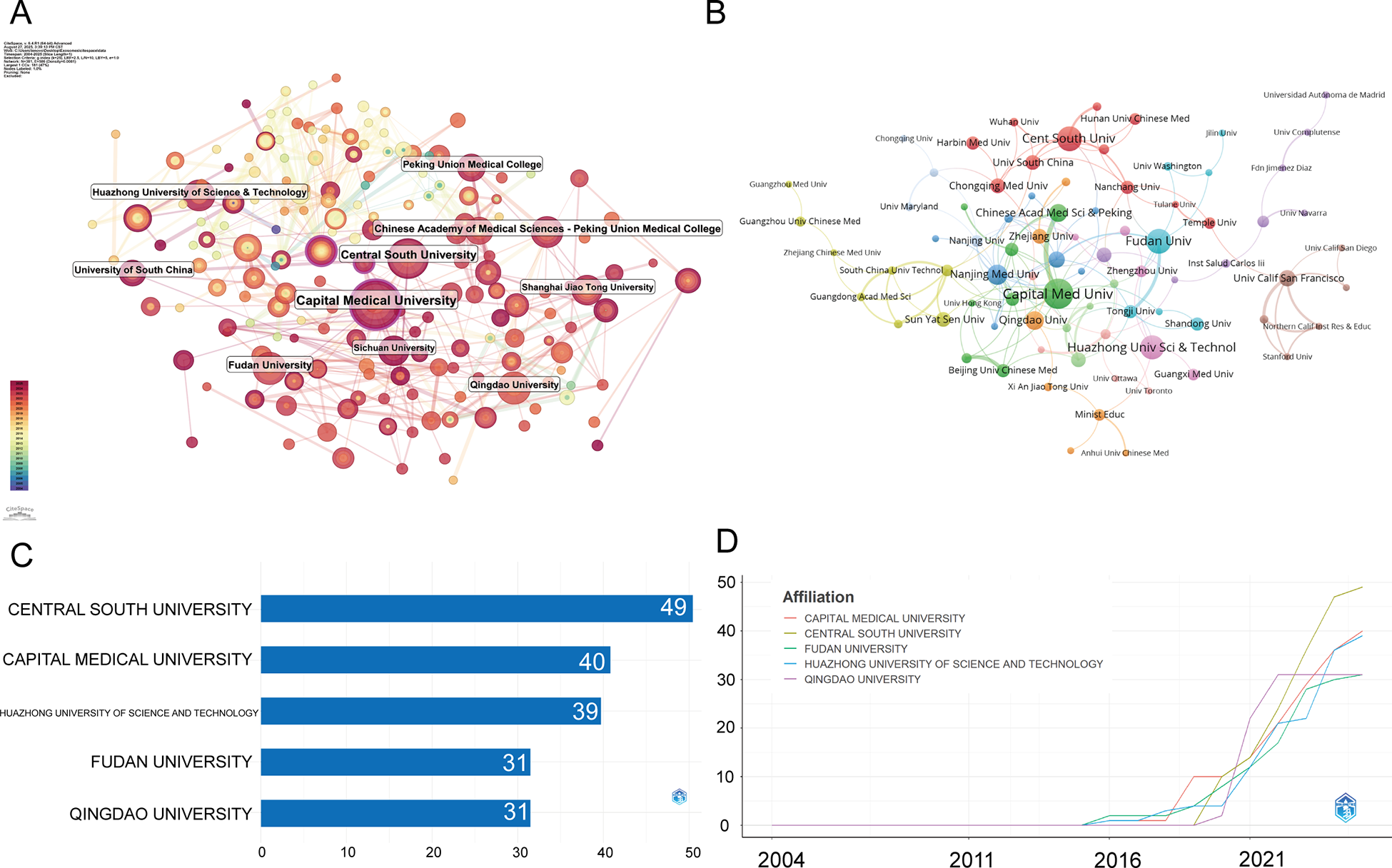
Institutional contributions and collaboration networks on exosomes in atherosclerosis (2004–2025). (A) Research institution network. Nodes represent institutions, with size indicating publication volume and line thickness showing collaboration strength. (B) Institutional collaboration network. Node size reflects collaboration extent, with colors indicating clusters based on region or affiliation. Line thickness represents cooperation intensity based on shared publications. (C) Top 5 Institutions by Citation Impact. (D) Institutional publication trends. The x-axis represents years, and the y-axis shows publication counts.
Figures 4C,D present a detailed overview of institutional contributions from January 2004 to July 2025. The top five institutions by publication output are Central South University (49 publications, 4.16%), Capital Medical University (40, 3.39%), Huazhong University of Science and Technology (39, 3.31%), Fudan University (31, 2.63%), and Qingdao University (31, 2.63%). Overall, institutional output is dominated by Chinese universities, with rapid growth observed after 2018.
3.4 Distributions of authors and co-cited authors
From 2004 to July 2025, a total of 6,836 authors contributed to 1,179 publications in the field of exosomes and AS, with an average of 6.94 co-authors per article, reflecting a highly collaborative research environment. Only 27 single-authored papers were identified, underscoring the dominance of teamwork in advancing this research area. The international co-authorship rate was only 8.74%, indicating limited cross-border collaboration (Supplementary Table S1). This outcome aligns with the patterns identified in the country-level analysis: although China contributes the largest number of publications, most are single-country studies, reflecting strong domestic research networks but relatively fewer international partnerships. The United States shows a higher proportion of multi-country publications, yet its smaller overall output does not offset the global imbalance, resulting in a modest international collaboration rate.
Such limited international cooperation may slow the integration of knowledge across regions and hinder the harmonization of research practices, including exosome-related technical methodologies. It may also affect the global influence of the field, because internationally co-authored studies typically achieve broader visibility and citation impact. Overall, the low rate suggests that research teams rely predominantly on national networks, which may restrict the diversity of perspectives and the pace of scientific convergence in this rapidly growing area.
In Figure 5A, the co-author network map reveals several distinct clusters, with Chinese scholars occupying the most prominent positions. Central nodes include researchers affiliated with leading institutions such as Central South University, Capital Medical University, and Fudan University. U.S. and European authors are often integrated through international partnerships.
Figure 5
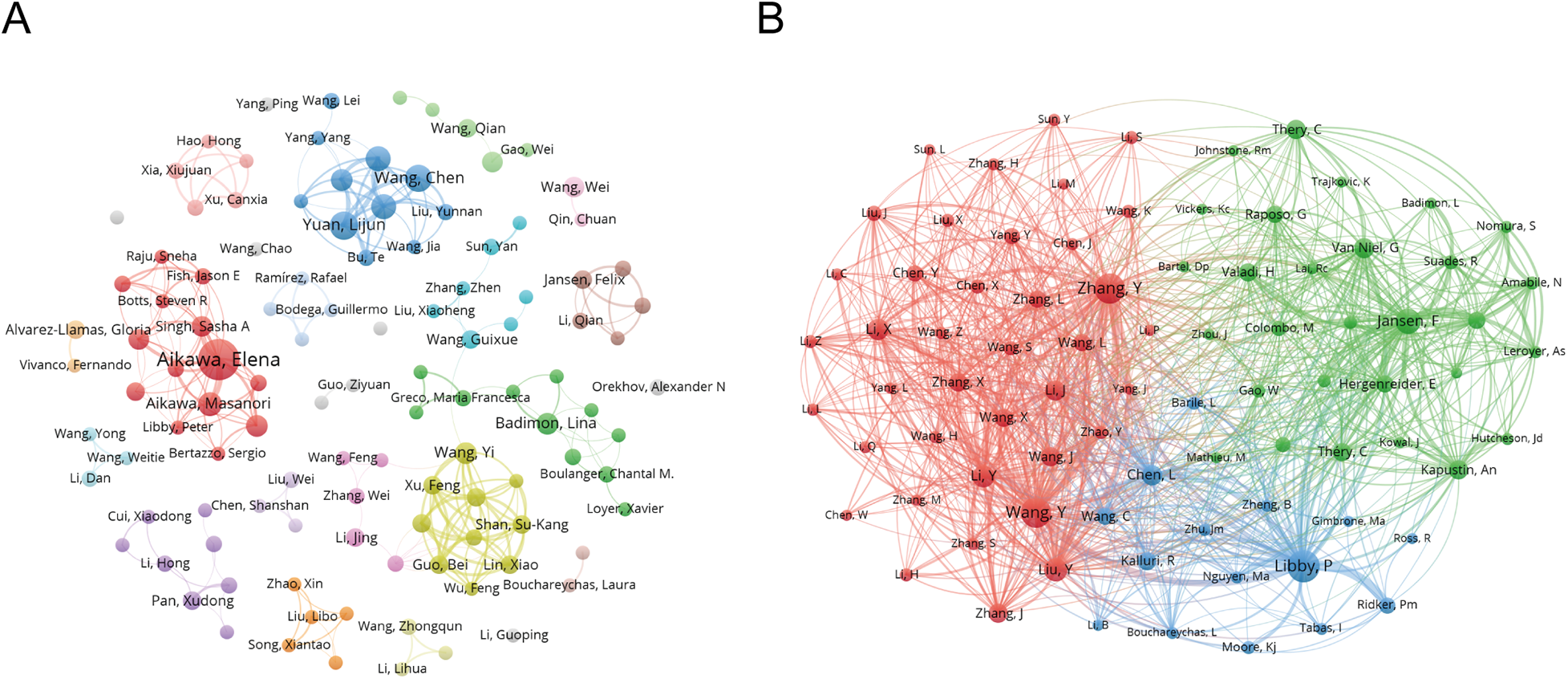
Co-authorship and Co-cited authorship networks on exosomes in atherosclerosis (2004–2025). (A) Co-authorship network: Nodes represent authors, with size reflecting publication count. Colors denote collaboration clusters. (B) Co-cited authorship network: Nodes, linked by joint publications, have edge thickness proportional to collaboration intensity. Clusters, indicated by color, reveal research groups.
Table 1 quantifies author influence, showcasing metrics like the g-index (10), h-index (11), and total citation counts (TC). Elena Aikawa and Masanori Aikawa remain leading contributors, while emerging scholars such as Lijun Yuan and Zhelong Li have shown rapid growth since 2021. Together, these authors shape both the historical foundation and recent expansion of the field.
Table 1
| Rank | Author | h_index | g_index | m_index | TC | NP | PY_start | Articles | Articles fractionalized |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AIKAWA ELENA | 17 | 21 | 1.308 | 1,731 | 21 | 2013 | 21 | 4.32 |
| 2 | YUAN LIJUN | 10 | 12 | 2 | 673 | 12 | 2021 | 12 | 1.59 |
| 3 | LI ZHELONG | 9 | 10 | 1.8 | 645 | 10 | 2021 | 10 | 1.40 |
| 4 | WANG CHEN | 9 | 11 | 1.8 | 505 | 11 | 2021 | 11 | 1.56 |
| 5 | YANG GUODONG | 8 | 10 | 1.6 | 414 | 10 | 2021 | 10 | 1.09 |
| 6 | AIKAWA MASANORI | 7 | 9 | 0.538 | 1,069 | 9 | 2013 | 9 | 0.68 |
| 7 | BADIMON LINA | 7 | 8 | 0.636 | 359 | 8 | 2015 | 8 | 1.70 |
| 8 | GE JUNBO | 7 | 7 | 0.7 | 473 | 7 | 2016 | 7 | 0.87 |
| 9 | HUTCHESON JOSHUA D | 7 | 8 | 0.538 | 737 | 8 | 2013 | 8 | 1.23 |
| 10 | WANG YI | 7 | 8 | 1 | 581 | 8 | 2019 | 8 | 0.70 |
Top 10 authors on exosomes in atherosclerosis research (2004–2025).
TC, Total Citations; NP, Number of Publications; PY_start, Year of First Publication.
In Figure 5B, the co-citation network demonstrates clusters centered on influential scholars such as Elena Aikawa and Masanori Aikawa. Their studies form core intellectual nodes that are frequently co-cited. Additional co-cited authors, including Badimon and Junbo Ge, further reflect the field's mechanistic and translational research focus.
3.5 Journals and co-journals
Figure 6A shows that the International Journal of Molecular Sciences is the most influential source in exosome and atherosclerosis research, with 57 publications underscoring its central role in disseminating high-impact studies. It is closely followed by Frontiers in Cardiovascular Medicine (55 articles) and Frontiers in Immunology (38 articles). These journals account for a substantial proportion of the total research output in this area.
Figure 6
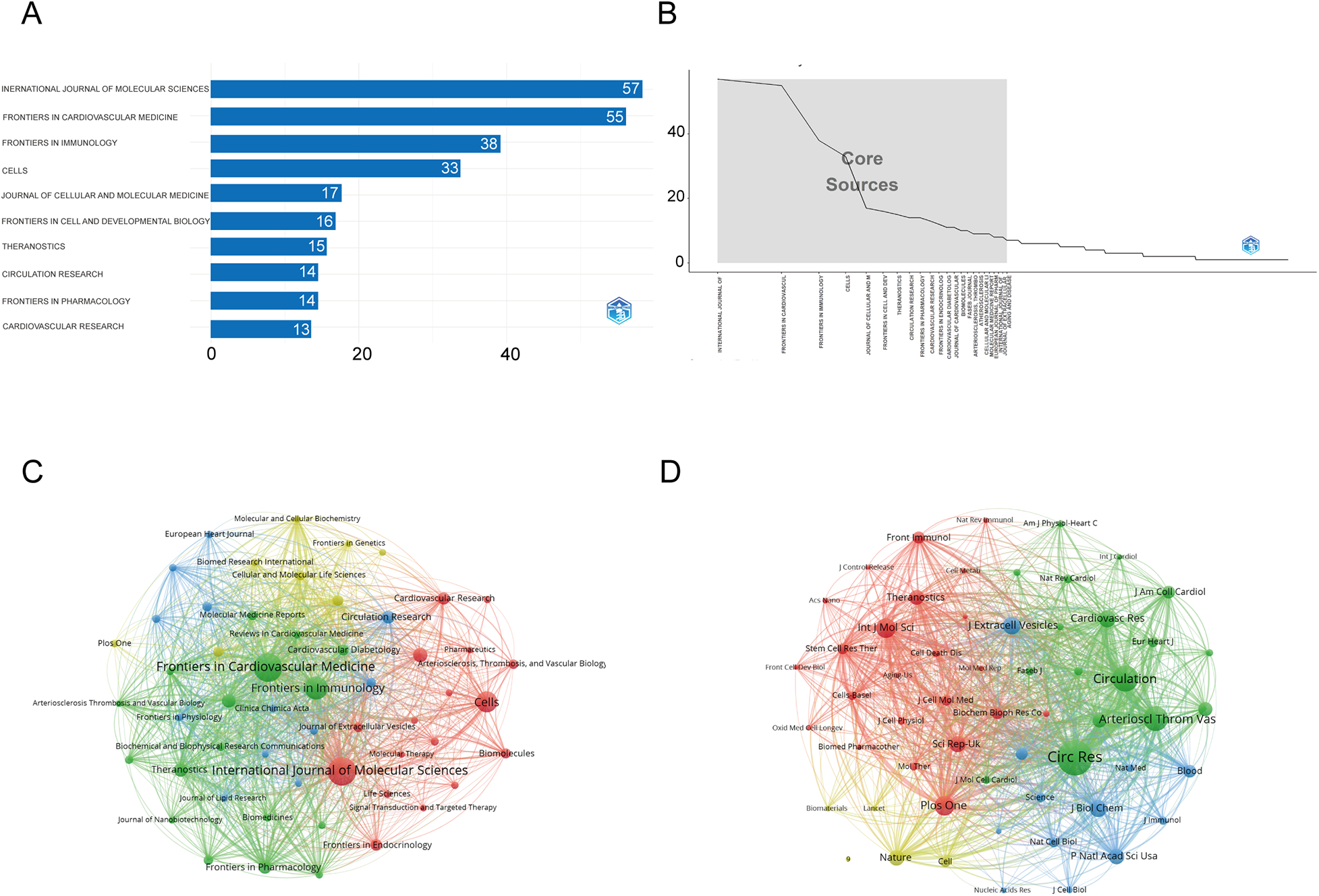
Comprehensive analysis of journals and citation networks related to exosomes in atherosclerosis (2004–2025). (A) Top journals by publication count: X-axis shows article numbers; y-axis lists journals. (B) Bradford's Law analysis: Identifies core journals based on publication distribution. (C) Journal citation network. Each node represents a journal. Node size is proportional to the number of publications, while links indicate citation relationships between journals. Line thickness reflects the strength of citation connections. Distinct colors (green, blue, red) denote clusters of journals that frequently cite each other, corresponding to thematic areas of research. (D) Journal co-citation network: Each node represents a journal, with size determined by co-citation frequency. Links indicate co-citation ties, with thicker lines reflecting stronger co-citation strength. Colors identify clusters of journals that are commonly co-cited.
Table 2 further supports their academic importance: Frontiers in Immunology holds the highest h-index of 25 with more than 2,000 citations, while International Journal of Molecular Sciences demonstrates strong citation performance (h-index 22, 1,451 citations) and a Q1 JCR ranking. Likewise, Theranostics, though publishing only 15 articles, exhibits a high impact factor (13.3, 2023 JCR), reflecting its significant influence relative to output.
Table 2
| Rank | Source | h_index | g_index | m_index | TC | NP | PY_start | IF | JCR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | FRONTIERS IN IMMUNOLOGY | 25 | 38 | 2.083 | 2,056 | 38 | 2014 | 5.9 | Q1 |
| 2 | INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES | 22 | 37 | 2.75 | 1,451 | 57 | 2018 | 4.9 | Q1 |
| 3 | FRONTIERS IN CARDIOVASCULAR MEDICINE | 18 | 29 | 2 | 1,013 | 55 | 2017 | 2.9 | Q1 |
| 4 | CELLS | 17 | 33 | 2.429 | 1,134 | 33 | 2019 | 5.1 | Q2 |
| 5 | THERANOSTICS | 13 | 15 | 1.857 | 1,311 | 15 | 2019 | 13.3 | Q1 |
| 6 | FRONTIERS IN CELL AND DEVELOPMENTAL BIOLOGY | 12 | 16 | 2 | 533 | 16 | 2020 | 4.3 | Q1 |
| 7 | FRONTIERS IN PHARMACOLOGY | 11 | 14 | 1.222 | 456 | 14 | 2017 | 4.8 | Q1 |
| 8 | JOURNAL OF CELLULAR AND MOLECULAR MEDICINE | 11 | 17 | 1.1 | 544 | 17 | 2016 | 4.2 | Q2 |
| 9 | CARDIOVASCULAR RESEARCH | 10 | 13 | 0.714 | 1,699 | 13 | 2012 | 13.3 | Q1 |
| 10 | CIRCULATION RESEARCH | 10 | 14 | 0.714 | 1,941 | 14 | 2012 | 20.8 | Q1 |
Top 10 influential journals on exosomes in atherosclerosis research (2004–2025).
IF, Impact Factor; JCR, Journal Citation Reports.
Figure 6B, in line with Bradford's Law, illustrates an uneven distribution of publications, where a limited number of journals—such as the International Journal of Molecular Sciences, Frontiers in Cardiovascular Medicine, and Frontiers in Immunology—dominate the majority of research output. This indicates that research in this field is concentrated within a limited number of leading journals. Figure 6C, the journal co-occurrence map, demonstrates that publications on exosomes and atherosclerosis are concentrated in a limited number of core journals, with International Journal of Molecular Sciences, Frontiers in Cardiovascular Medicine, and Frontiers in Immunology forming the central nodes of scholarly communication. Figure 6D further shows that foundational journals such as Circulation Research, Cardiovascular Research, and Theranostics serve as major co-citation hubs, underscoring their influence on the development of exosome-related cardiovascular research.
3.6 References and articles
The field of exosome research in AS has grown rapidly over the past two decades, as shown in Supplementary Table S1. Between 2004 and July 2025, a total of 1,179 documents were published, drawing on 92,243 references. On average, documents are relatively recent, with a mean age of 3.75 years, and each receives 42.3 citations, reflecting both the novelty and impact of current research.
In Table 3, the most locally cited publications are led by Zhu JM (12) in Theranostics with 68 local citations and Li JB (13) in Biochemical and Biophysical Research Communications with 51 citations. These studies highlight key mechanistic insights into macrophage- and MSC-derived exosomes in AS. Other highly cited contributions, such as Boulanger (14) and Nguyen (15) further reinforce the central role of Extracellular vesicles in this field. Collectively, these publications represent key intellectual landmarks, anchoring the citation landscape of exosome and AS research.
Table 3
| RANK | First Author | Year | Journal | Paper | DOI | Local Citations | Global Citations | LC/GC Ratio (%) | Normalized Local Citations | Normalized Global Citations |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ZHU JM | 2019 | THERANOSTICS | Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation | 10.7150/thno.37357 | 68 | 314 | 21.66 | 12.32 | 3.56 |
| 2 | LI JB | 2019 | BIOCHEM BIOPH RES CO | Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE−/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage | 10.1016/j.bbrc.2019.02.005 | 51 | 179 | 28.49 | 9.24 | 2.03 |
| 3 | BOULANGER CM | 2017 | NATURE REVIEW CARDIOLOGY | Extracellular vesicles in coronary artery disease | 10.1038/nrcardio.2017.7 | 50 | 421 | 11.88 | 7.88 | 4.47 |
| 4 | NGUYEN MA | 2018 | ARTERIOSCL THROM VAS | Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration | 10.1161/ATVBAHA.117.309795 | 50 | 189 | 26.46 | 7.52 | 2.14 |
| 5 | GAO W | 2016 | J CELL MOL MED | Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappa B pathway | 10.1111/jcmm.12923 | 47 | 237 | 19.83 | 4.39 | 2.40 |
| 6 | BOUCHAREYCHAS L | 2020 | CELL REP | Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo | 10.1016/j.celrep.2020.107881 | 44 | 171 | 25.73 | 11.38 | 2.73 |
| 7 | CHEN L | 2017 | PLOS ONE | Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis | 10.1371/journal.pone.0185406 | 40 | 231 | 17.32 | 6.31 | 2.45 |
| 8 | WANG C | 2021 | THERANOSTICS | Exosomes in atherosclerosis: Performers, bystanders, biomarkers, and therapeutic targets | 10.7150/thno.56035 | 39 | 132 | 29.55 | 17.10 | 3.42 |
| 9 | NIU CG | 2016 | J AM HEART ASSOC | Macrophage Foam Cell–Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion | 10.1161/JAHA.116.004099 | 37 | 138 | 26.81 | 3.46 | 1.40 |
| 10 | LI JN | 2017 | THROMB RES | Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response | 10.1016/j.thromres.2017.04.016 | 35 | 156 | 22.44 | 5.52 | 1.66 |
| 11 | XIE ZL | 2018 | J AM HEART ASSOC | Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization | 10.1161/JAHA.117.007442 | 33 | 151 | 21.85 | 4.96 | 1.71 |
| 12 | ZHANG YJ | 2010 | MOL CELL | Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration | 10.1016/j.molcel.2010.06.010 | 32 | 1013 | 3.16 | 2.13 | 1.39 |
| 13 | ZHANG YG | 2019 | CELL CYCLE | Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis | 10.1080/15384101.2019.1654797 | 32 | 97 | 32.99 | 5.80 | 1.10 |
| 14 | XING XH | 2020 | AGING-US | Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis | 10.18632/aging.102857 | 32 | 93 | 34.41 | 8.28 | 1.49 |
| 15 | TANG N | 2016 | FASEB J | Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells | 10.1096/fj.201600368RR | 30 | 141 | 21.28 | 2.80 | 1.43 |
| 16 | HULSMANS M | 2013 | CARDIOVASC RES | MicroRNA-21 controls neointimal lesion formation by regulating smooth muscle cell proliferation and apoptosis | 10.1093/cvr/cvt161 | 28 | 291 | 9.62 | 4.24 | 2.49 |
| 17 | NEW SE | 2013 | CIRC RES | Macrophage-Derived Matrix Vesicles: An Alternative Novel Mechanism for Microcalcification in Atherosclerotic Plaques | 10.1161/CIRCRESAHA.113.301036 | 28 | 366 | 7.65 | 4.24 | 3.13 |
| 18 | HE S | 2018 | SCAND J IMMUNOL | Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis | 10.1111/sji.12648 | 28 | 107 | 26.17 | 4.21 | 1.21 |
| 19 | WU GH | 2020 | ANGEW CHEM INT EDIT | Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment | 10.1002/anie.201913700 | 28 | 212 | 13.21 | 7.24 | 3.39 |
| 20 | HUANG CY | 2018 | MOL MED REP | Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization | 10.3892/mmr.2018.8982 | 27 | 77 | 35.06 | 4.06 | 0.87 |
Top 20 most local cited publications based on bibliometrix analysis (2004–2025).
DOI, Digital Object Identifier.
Figure 7A depicts a well-structured co-citation landscape centered on Cluster 0 (“microvesicles”), which forms the core hub for foundational work on extracellular vesicles. From this core, several tightly connected clusters extend outward. Among them, Cluster 4 (“microRNA”) and Cluster 2 (“circular RNA”) highlight regulatory RNAs as major mechanistic focuses and link directly to Cluster 11 (“diagnostic”), underscoring the translational route from cargo characterization to biomarker development. Within this context, Cluster 1 (“miR-21-3p”) marks the convergence on specific effector miRNAs, bridging the “microRNA,” “microvesicles,” and “cardiovascular diseases” clusters and thus connecting mechanism with disease relevance. Along the historical axis, Cluster 5 (“apoptotic bodies”) captures early, frequently cited work that leads conceptually toward the current RNA-centered frameworks. Finally, Cluster 8 (“biomimicry”) represents the engineering and therapeutic strand, where insights from the mechanistic and diagnostic clusters are repurposed into exosome-mimetic, drug-loading, and targeted-delivery strategies.
Figure 7
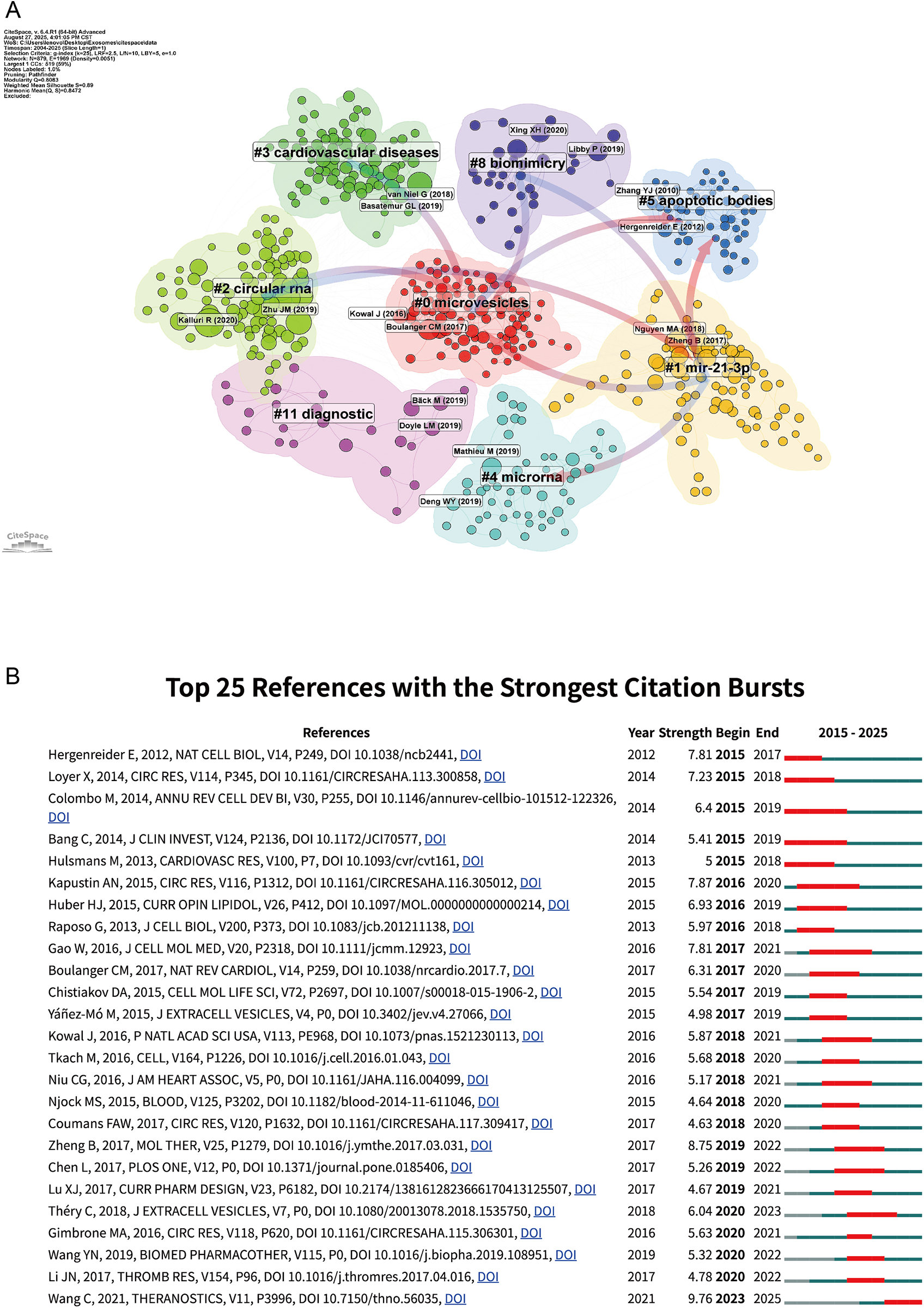
Network visualization of references and Top 25 references with strongest citation bursts on exosomes in atherosclerosis (2004–2025). (A) Clustered co-citation network based on keywords: Nodes, sized by citation frequency, are grouped by modularity with keyword labels for major clusters. Edge thickness reflects co-citation strength; colors highlight thematic diversity. (B) Top 25 References with Strongest Citation Bursts on Exosomes in Atherosclerosis: The burst timeline shows the periods during which these bursts occurred, with red segments indicating the time intervals when each reference received heightened attention.
Figure 7B lists the top 25 references with the strongest citation bursts. For example, Wang (5) show the most recent and intense influence, highlighting its role in shaping current research. In contrast, early bursts from Colombo (16) and Yáñez-Mó (17) exhibit sustained impact, reflecting their foundational contributions to extracellular vesicle biology. Théry (4) continues to guide the field's expansion toward immunological and mechanistic directions. Together, these bursts highlight both the historical foundations and evolving research hotspots in exosome–atherosclerosis studies.
3.7 Keywords analysis
The keyword analysis provides valuable insights into the thematic evolution of exosome research in AS. In total, 9,713 Keywords Plus and 2,510 author keywords were identified (Supplementary Table S1). This broad keyword base reflects the diversity and expansion of research topics in the field.
As illustrated in Figures 8A–D, the dominant research theme is clearly centered on “atherosclerosis” (382 occurrences, total link strength = 924), which appears as the most frequent and strongly linked keyword, reflecting its centrality in the field. Closely associated terms such as “extracellular vesicles” (263 occurrences, total link strength = 633) and “exosomes” (363 occurrences, total link strength = 843) underscore the pivotal role of vesicle biology in cardiovascular research. Mechanistic keywords including “inflammation” (129 occurrences, total link strength = 324) and “endothelial cells” (65 occurrences, total link strength = 177) highlight the growing attention to molecular pathways, particularly RNA-mediated regulation and endothelial dysfunction in disease progression (18, 19). The prominence of “biomarkers” (108 occurrences, total link strength = 273) and “oxidative stress” (24 occurrences, total link strength = 73) further demonstrates the translational orientation of the field, linking mechanistic studies with potential diagnostic and therapeutic applications (5). Keyword analysis shows that atherosclerosis continues to serve as the central focus, yet the field is expanding into mechanistic discovery, biomarker development, and translational applications, establishing exosome research as an emerging frontier in cardiovascular medicine.
Figure 8
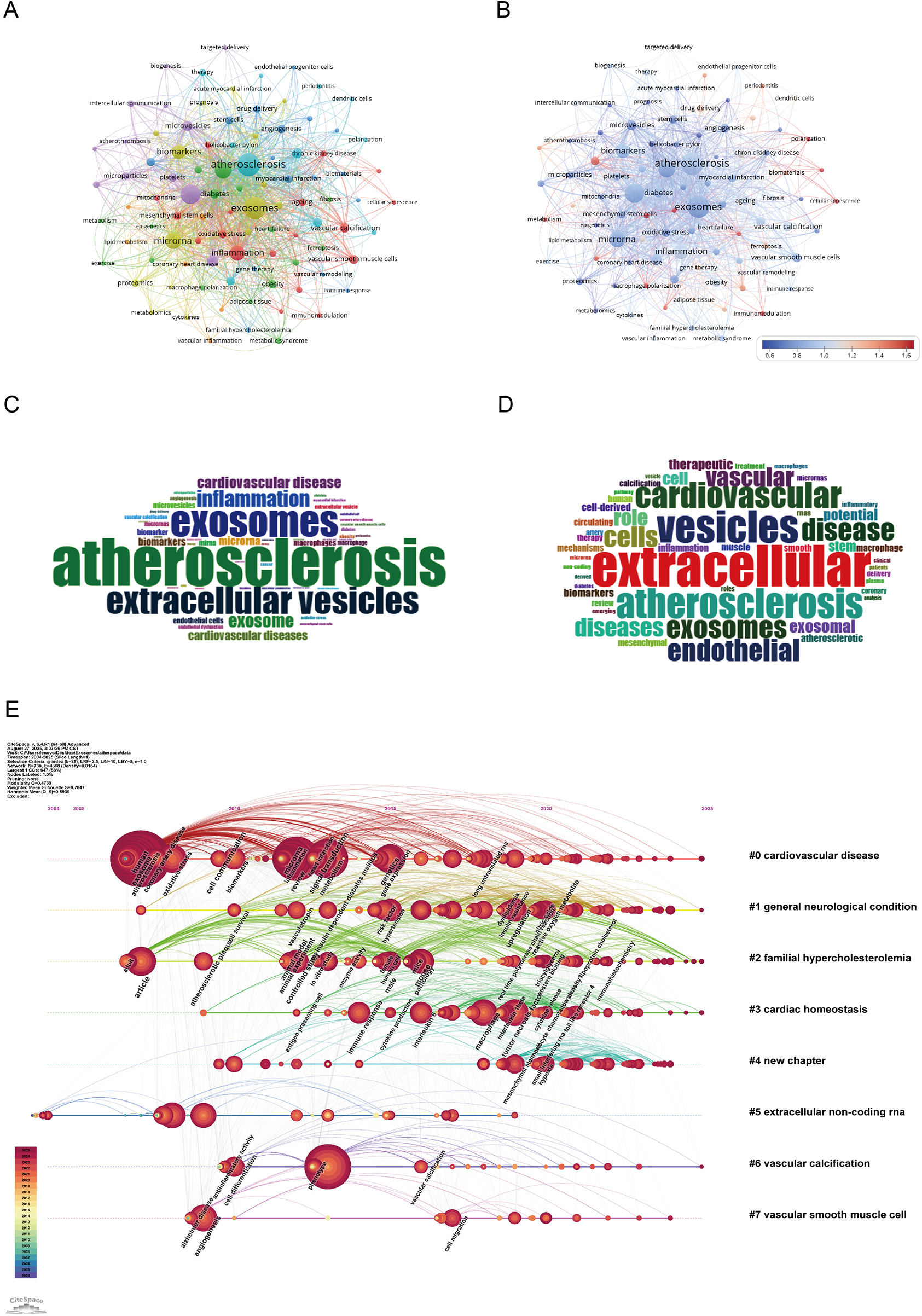
Keywords mapping of exosomes in atherosclerosis. (A) Keywords co-occurrence network: Each node represents a keyword, with size proportional to its frequency. Edges show co-occurrence relationships, with line thickness indicating co-occurrence strength. Colors represent thematic clusters identified by modularity analysis. (B) Keyword centrality network: Nodes represent keywords, sized by frequency and colored by betweenness centrality (redder nodes indicate higher centrality). Edges indicate co-occurrence relationships, highlighting pivotal keywords bridging clusters. (C) Author keywords word cloud: Word size reflects frequency, highlighting common author-assigned terms. (D) Title keywords word cloud: Word size indicates frequency, emphasizing key themes from titles. (E) Keyword timeline visualization: The x-axis shows publication years. Each horizontal line corresponds to a thematic cluster, labeled with a representative keyword. Nodes, sized by frequency, are positioned according to the year the keyword first appeared, visualizing topic emergence and evolution over time.
As shown in the keyword timeline (Figure 8E), the evolution of research on exosomes in AS demonstrates clear thematic and temporal progression from 2004 to July 2025. The earliest cluster, “cardiovascular disease” (Cluster 0), emerged as a foundational theme and has remained consistently relevant, reflecting the central role of exosome-mediated mechanisms in cardiovascular pathology. Around the mid-2010s, attention expanded to more specific disease contexts such as “general neurological condition” (Cluster 1) and “familial hypercholesterolemia” (Cluster 2), illustrating the diversification of research into comorbidities and genetic predispositions that influence AS progression.
Subsequently, clusters such as “cardiac homeostasis” (Cluster 3) and “extracellular non-coding RNA” (Cluster 5) became increasingly prominent after 2018, highlighting the mechanistic focus on immune regulation, endothelial dysfunction, and RNA-based signaling pathways. These terms emphasize the growing interest in RNA-mediated regulation of gene expression and its implications for vascular remodeling and inflammatory control.
Overall, keyword evolution demonstrates a transition from broad cardiovascular concepts to mechanistic pathways and translational applications, underscoring the growing depth and clinical relevance of exosome research in AS.
4 Discussion
Exosomes interact with various vascular processes, influencing immune cell behavior, endothelial function, smooth muscle cell growth, and blood vessel remodeling. By carrying microRNAs and proteins, exosomes can either promote inflammation or aid in repair, depending on the disease stage. Additionally, exosomes show promise as drug delivery systems, offering targeted treatments and regenerative potential. As we learn more about their biological functions, bioengineered exosomes could lead to more effective, personalized therapies for cardiovascular diseases like AS.
4.1 Research highlights
Keyword analysis and co-citation mapping jointly reveal the five most prominent research directions in the current study of exosomes in AS: exosomes in immune cell interactions, exosomal impact on endothelial dysfunction, exosomes in vascular smooth muscle cell proliferation and migration, platelet exosomes in atherosclerosis, exosomes in atherosclerosis: role in vascular repair and remodeling, and exosomal therapeutic potential in atherosclerosis. The following subsection provides a focused synthesis and integrated discussion of these hotspots.
4.1.1 Emerging roles of exosomes in immune cell interactions
Immune regulation represents one of the central mechanisms by which exosomes participate in AS.
Accumulating evidence indicates that macrophage-derived exosomes serve as pivotal signaling vectors that sculpt the local inflammatory milieu; key miRNAs—most notably miR-146a and miR-155—modulate NF-κB and JAK/STAT pathway activity, thereby governing inflammatory amplitude, cellular motility, and chemotactic responses within atherosclerotic lesions (15).
Exosomes released by macrophages often carry specific microRNAs, such as miR-146a and miR-155-5p, each exerting distinct effects on immune regulation (20). MiR-146a alleviates inflammation and slows AS progression by dampening NF-κB signaling and down-regulating TNF-α, IL-1β and other pro-inflammatory mediators (21). However, under pathological conditions such as impaired endothelial repair or sustained macrophage retention, miR-146a can switch to a pro-atherogenic role by suppressing endothelial cell migration and promoting macrophage persistence within the plaque (15).
In contrast, miR-155-5p facilitates immune activation by inhibiting negative regulators of the immune response, such as suppressor of cytokine signaling 1(SOCS1) (22). Conversely, miR-155-5p targets SOCS1 to activate the JAK2/STAT3 axis, thereby amplifying the production of IL-6, IL-12 and other inflammatory mediators, precipitating endothelial senescence and exacerbating vascular inflammation (23, 24). This sustained immunoinflammatory response accelerates atherosclerotic progression and destabilizes plaques, while circulating miR-155-5p is increasingly recognized as a diagnostic and prognostic biomarker for cardiovascular disease (25, 26).
Although the detailed molecular mechanisms remain partially unresolved, it is now established that exosomes constitute an intercellular communication network among immune cells and operate critically during the initiation, amplification, and maintenance phases of atherosclerosis. Consequently, their immunomodulatory properties are increasingly recognized as promising therapeutic targets.
4.1.2 Exosomal impact on endothelial dysfunction
Recent studies highlight endothelial-derived exosomes as important mediators of cell-to-cell communication, particularly through miRNAs such as miR-126 and miR-210 (18). MiR-126 has been closely linked to endothelial stability, in part through the regulation of pro-inflammatory mediators like the CXCL12-CXCR4 axis, which influences immune cell recruitment and vascular inflammation (27). It is also thought to strengthen barrier properties, stabilize intercellular junctions, and modulate vascular permeability, thereby safeguarding endothelial integrity. In contrast, miR-210 is predominantly induced under hypoxic conditions, a feature of atherosclerotic lesions. By modulating pathways such as vascular endothelial growth factor (VEGF), it promotes angiogenesis and reduces apoptosis, while also influencing smooth muscle cell migration and differentiation, changes that support fibrous cap formation and enhance plaque stability (28).
Endothelial-derived exosomal miRNAs exert context-specific effects: although they frequently support endothelial integrity and facilitate vascular repair, under pathological conditions they can also drive maladaptive remodeling and accelerate plaque development (12, 29, 30). In our analysis, the frequent clustering of the keywords reinforces this line of research, suggesting a decisive role for endothelial dysfunction in the early phases of AS.
4.1.3 Exosomes in vascular smooth muscle cell proliferation and migration
Recent studies indicate that Vascular Smooth Muscle Cell-derived exosomes (VSMC-derived exosomes) represent a central signaling hub in atherosclerosis, linking calcification, angiogenic activity, and phenotypic modulation within a coherent pathogenic network. Rather than acting through isolated mechanisms, these exosomes coordinate VSMC transition, extracellular matrix remodeling, and endothelial responses through their microRNA cargo, including miR-155-5p (31–34). Their involvement in calcium deposition and neovascularization aligns with features of plaque growth and vulnerability, positioning VSMC-derived exosomes as key contributors to the evolution of unstable lesions (30, 33). Overall, the emerging literature highlights these vesicles not only as mediators of VSMC proliferation and migration but as integrators of multiple processes that shape plaque development across different disease stages.
4.1.4 Platelet-derived exosomes in atherosclerosis
Platelet-derived exosomes have gained increasing attention in AS research because they sit at the interface of thrombosis and vascular inflammation. These vesicles participate in early plaque formation and contribute to the modulation of plaque stability by delivering microRNAs implicated in vascular injury and endothelial responses (35). Among them, miR-223 plays a representative role by altering platelet–endothelial communication through ICAM-1 regulation (36), illustrating how platelet-derived exosomes integrate inflammatory signaling with thrombotic activity. Their functional effects vary with the local microenvironment, exhibiting either protective or pro-atherogenic patterns across different disease stages (37). In addition, accumulating evidence suggests that these exosomes support endothelial repair and attenuate vascular injury, underscoring their potential therapeutic relevance (35). Overall, platelet-derived exosomes are emerging as key mediators at the inflammation–thrombosis intersection, linking platelet activation with vascular dysfunction and shaping atherosclerotic disease progression.
4.1.5 Exosomes as regulators of vascular repair and remodeling
In AS, exosomes play a key role: they can exacerbate inflammation and disease progression, while also contributing to vascular repair and remodeling.
Within the progression of AS, exosomes participate not only in inflammatory amplification but also in vascular repair, forming a dual regulatory axis that becomes particularly relevant in advanced lesions. Among the reparative vesicles, endothelial progenitor cell–derived exosomes (EPC-exosomes) have received considerable attention because they promote endothelial resilience through coordinated effects on survival pathways, cytoprotection, and functional recovery (38–40). Rather than acting through isolated mechanisms, EPC-exosomes integrate pro-repair signals—including ACE2/Ang-(1–7)/Mas activation and microRNA transfer—into a broader response that supports endothelial restoration and vascular elasticity. Their efficacy, however, varies with the local inflammatory and hemodynamic milieu, highlighting the context dependence of exosome-mediated repair (41). Overall, current findings suggest that exosome-driven vascular remodeling represents an emerging translational direction, consolidating diverse reparative signals into a potential therapeutic strategy aimed at stabilizing the atherosclerotic vasculature.
4.1.6 Therapeutic potential and translational prospects of exosomes in atherosclerosis
Exosomes are emerging as promising therapeutic platforms in AS, where their lipid bilayer and low immunogenicity enable targeted drug delivery to diseased vascular sites (42). In addition to this delivery capacity, they naturally carry proteins, lipids, and RNAs that regulate cellular behavior and disease progression, underscoring their value as crucial mediators in the treatment of AS, owing to their ability to support intercellular communication and to transport bioactive molecules that shape disease progression (43). Exosomal X26nt derived from vascular smooth muscle cells regulates phenotypic switching and oxidative stress by reducing ER stress and enhancing SOD1 expression, indicating that exosomal RNAs such as X26nt may represent promising therapeutic targets (43). They further exhibit a dual role in AS by amplifying inflammation through pro-inflammatory signaling and oxidative stress while simultaneously promoting anti-inflammatory responses, a complexity that highlights their central involvement in disease progression and regulation (44). Additionally, plasma exosomal miR-30e and miR-92a, by targeting ATP-binding cassette transporter A1 (ABCA1), are upregulated in AS, providing new biomarkers for the clinical diagnosis and treatment of coronary AS (45).
In recent years, bioengineered exosomes have shown significant potential in the treatment of AS. For example, macrophage-derived exosomes carrying 5-aminoacetylpropionyl hexyl ester have demonstrated significant therapeutic effects on AS (46). In addition, regulating the release of exosomal microRNAs has been proposed to dampen immune activation and limit foam cell formation. Advances in bioengineering further expand the therapeutic landscape of exosomes, allowing surface functionalization with targeting ligands and the development of more efficient drug-loading technologies (42). Through the artificial modification of exosomes, systems such as thermosensitive liposomal mixed nanocarriers and membrane-coated nanoparticles are expected to overcome challenges such as low drug loading capacity and suboptimal delivery efficiency of exosomes (47). Compared with synthetic nanoparticles, exosomes combine inherent biocompatibility with superior barrier penetration, underscoring their promise as next-generation candidates for cardiovascular therapy (48).
Exosomes are positioned as a multifaceted therapeutic platform, functioning both as carriers of bioactive agents and as modulators of the vascular microenvironment, with the potential to attenuate atherosclerotic progression. In the future, exosome-like nanoparticle therapy is expected to provide a novel and effective treatment option for patients with AS.
4.2 Limitations
Several limitations of this study should be acknowledged. To begin with, the analysis relied mainly on the Web of Science Core Collection and Scopus. Although these are widely used sources, the omission of databases such as PubMed or Embase means that some relevant work might not have been captured, raising the possibility of database-related bias. It should also be acknowledged that we confined our search to English-language articles and reviews, which inevitably narrows the global perspective by overlooking non-English contributions. In addition, bibliometric indicators, including citation counts and the h-index, are shaped by factors such as database coverage, self-citation practices, and the passage of time; these influences need to be borne in mind when interpreting research impact. The software tools applied in this work, namely CiteSpace, VOSviewer, and Bibliometrix, are valuable for mapping and visualization but cannot by themselves reflect the quality or rigor of individual studies, which calls for complementary domain expertise. Finally, our analysis emphasized quantitative mapping rather than detailed biological or clinical interpretation. To translate insights from exosome research into practice, further systematic reviews and experimental validation will be necessary.
While the study has certain constraints, drawing on two widely recognized databases together with several bibliometric tools lends robustness to the analysis and helps outline major global research trends. Looking ahead, a broader approach that brings in other databases, non-English literature, and evidence from clinical trials could offer a more nuanced and comprehensive understanding of how exosomes contribute to AS.
5 Conclusion
Exosomes are increasingly recognized as central mediators in the pathogenesis of AS, influencing immune activation, endothelial dysfunction, vascular smooth muscle remodeling, and plaque stability. Our bibliometric analysis shows that research in this domain has grown exponentially over the past two decades, with a pronounced surge after 2015, reflecting the shift from descriptive studies to mechanistic and translational investigations. The emergence of keywords such as inflammation, biomarkers, vascular calcification, and RNA-mediated regulation highlights how the field is converging on molecular pathways with direct implications for diagnosis and therapy.
The results of this analysis highlight both the promise and the obstacles that remain in the field. Exosomes are emerging as minimally invasive biomarkers and as therapeutic carriers with the potential to target vascular lesions in a highly specific way. Yet their broader application is still constrained by inconsistent isolation protocols, limited international collaboration, and a gap between experimental progress and clinical translation.
Research on exosomes in AS is now shifting from descriptive studies toward precision medicine and regenerative approaches. Greater use of standardized methodologies, incorporation of multi-omics technologies, and advances in bioengineering for exosome modification could help overcome existing barriers and speed progress toward clinical application. If these developments continue, exosome-based diagnostics and therapies may redefine cardiovascular care by enabling earlier diagnosis, improved risk stratification, and novel strategies for vascular repair.
Statements
Data availability statement
The raw datasets analyzed in this study were obtained from Web of Science and Scopus via institutional subscriptions. Due to copyright restrictions, these datasets are not publicly available. Processed data (e.g., extracted keywords, citation networks) are available upon reasonable request. Requests to access these datasets should be directed to Yubo Ren, yuboren2002@163.com.
Author contributions
YR: Writing – original draft, Writing – review & editing. BZ: Writing – original draft, Writing – review & editing. LL: Writing – review & editing. WS: Writing – review & editing. ZW: Writing – review & editing. ZL: Writing – review & editing. BL: Writing – review & editing. BY: Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
The authors would like to thank the editors and the anonymous reviewers for their valuable comments and suggestions to improve the quality of the paper.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1700630/full#supplementary-material
Abbreviations
ABCA1, ATP-binding cassette transporter A1; AS, atherosclerosis; miRs, microRNAs; WoSCC, web of science core collection; TC, total citation counts; SOCS1, suppressor of cytokine signaling 1; VEGF, vascular endothelial growth factor; VSMCs, vascular smooth muscle cells; EPCs, endothelial progenitor cells.
References
1.
Herrington W Lacey B Sherliker P Armitage J Lewington S . Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. (2016) 118(4):535–46. 10.1161/circresaha.115.307611
2.
Cimmino G Muscoli S De Rosa S Cesaro A Perrone MA Selvaggio S et al Evolving concepts in the pathophysiology of atherosclerosis: from endothelial dysfunction to thrombus formation through multiple shades of inflammation. J Cardiovasc Med. (2023) 24(Supplement 2):e156–67. 10.2459/jcm.0000000000001450
3.
Libby P Buring JE Badimon L Hansson GK Deanfield J Bittencourt MS et al Atherosclerosis. Nat Rev Dis Primers. (2019) 5(1):56. 10.1038/s41572-019-0106-z
4.
Théry C Witwer KW Aikawa E Alcaraz MJ Anderson JD Andriantsitohaina R et al Minimal information for studies of extracellular vesicles 2018 (Misev2018): a position statement of the international society for extracellular vesicles and update of the Misev2014 guidelines. J Extracell Vesicles. (2018) 7(1):1535750. 10.1080/20013078.2018.1535750
5.
Wang C Li Z Liu Y Yuan L . Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. (2021) 11(8):3996–4010. 10.7150/thno.56035
6.
Chen YT Yuan HX Ou ZJ Ou JS . Microparticles (Exosomes) and atherosclerosis. Curr Atheroscler Rep. (2020) 22(6):23. 10.1007/s11883-020-00841-z
7.
Chen C . Citespace ii: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57(3):359–77. 10.1002/asi.20317
8.
Van Eck N Waltman L . Software survey: vosviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84(2):523–38. 10.1007/s11192-009-0146-3
9.
Aria M Cuccurullo C . Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2018) 11(4):959–75. 10.1016/j.joi.2017.08.007
10.
Egghe L . Theory and practise of the G-Index. Scientometrics. (2006) 69(1):131–52. 10.1007/s11192-006-0144-7
11.
Hirsch JE . An Index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. (2005) 102(46):16569–72. 10.1073/pnas.0507655102
12.
Zhu J Liu B Wang Z Wang D Ni H Zhang L et al Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through mir-21-3p/pten-mediated vsmc migration and proliferation. Theranostics. (2019) 9(23):6901–19. 10.7150/thno.37357
13.
Li J Xue H Li T Chu X Xin D Xiong Y et al Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in apoe(-/-) mice via mir-Let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. (2019) 510(4):565–72. 10.1016/j.bbrc.2019.02.005
14.
Boulanger CM Loyer X Rautou PE Amabile N . Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. (2017) 14(5):259–72. 10.1038/nrcardio.2017.7
15.
Nguyen MA Karunakaran D Geoffrion M Cheng HS Tandoc K Perisic Matic L et al Extracellular vesicles secreted by atherogenic macrophages transfer microrna to inhibit cell migration. Arterioscler Thromb Vasc Biol. (2018) 38(1):49–63. 10.1161/atvbaha.117.309795
16.
Colombo M Raposo GA Théry C . Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. (2014) 30(1):255–89. 10.1146/annurev-cellbio-101512-122326
17.
Yáñez-Mó M . Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. (2015) 4:27066. 10.3402/jev.v4.27066eCollection 2015.
18.
Nikdoust F Pazoki M Mohammadtaghizadeh M Aghaali MK Amrovani M . Exosomes: potential player in endothelial dysfunction in cardiovascular disease. Cardiovasc Toxicol. (2022) 22(3):225–35. 10.1007/s12012-021-09700-y
19.
Xu L Geng T Zang G Bo L Liang Y Zhou H et al Exosome derived from Cd137-modified endothelial cells regulates the Th17 responses in atherosclerosis. J Cell Mol Med. (2020) 24(8):4659–67. 10.1111/jcmm.15130
20.
Bauer KM Round JL O'Connell RM . No small matter: emerging roles for exosomal mirnas in the immune system. Febs j. (2022) 289(14):4021–37. 10.1111/febs.16052
21.
Tao T Chen L Lin X Fan Z Zhu C Mao L . Deregulated Mir-146a-3p alleviates disease progression in atherosclerosis through inactivating Nf-Κb: an experimental study. Medicine (Baltimore). (2024) 103(20):e38061. 10.1097/md.0000000000038061
22.
Du F Yu F Wang Y Hui Y Carnevale K Fu M et al Microrna-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. (2014) 34(4):759–67. 10.1161/atvbaha.113.302701
23.
He J Zhang B Zhang H Tu Q Chen X Qiu Y et al M1 macrophage is a novel potential trigger for endothelial senescence: role of exosomal Mir-155 targeting Socs1 signal. Hum Mutat. (2025) 2025:6771390. 10.1155/humu/6771390
24.
Ma YL Ma ZJ Wang M Liao MY Yao R Liao YH . Microrna-155 induces differentiation of Raw264.7 cells into dendritic-like cells. Int J Clin Exp Pathol. (2015) 8(11):14050–62.
25.
Feinberg MW Moore KJ . Microrna regulation of atherosclerosis. Circ Res. (2016) 118(4):703–20. 10.1161/circresaha.115.306300
26.
Qi L Luo DZ Li H Yan J He W . Macrophage-Driven exosomes regulate the progression of cardiovascular disease. Front Pharmacol. (2025) 16:1563800. 10.3389/fphar.2025.1563800
27.
Bassand K Metzinger L Naïm M Mouhoubi N Haddad O Assoun V et al Mir-126-3p is essential for Cxcl12-induced angiogenesis. J Cell Mol Med. (2021) 25(13):6032–45. 10.1111/jcmm.16460
28.
Zaccagnini G Greco S Longo M Maimone B Voellenkle C Fuschi P et al Hypoxia-Induced Mir-210 modulates the inflammatory response and fibrosis upon acute ischemia. Cell Death Dis. (2021) 12(5):435. 10.1038/s41419-021-03713-9
29.
Ke X Liao Z Luo X Chen JQ Deng M Huang Y et al Endothelial colony-forming cell-derived exosomal Mir-21-5p regulates autophagic flux to promote vascular endothelial repair by inhibiting Sipl1a2 in atherosclerosis. Cell Commun Signal. (2022) 20(1):30. 10.1186/s12964-022-00828-0
30.
Ren Y Zhang H . Emerging role of exosomes in vascular diseases. Front Cardiovasc Med. (2023) 10:1090909. 10.3389/fcvm.2023.1090909
31.
Fontaine M Herkenne S Ek O Paquot A Boeckx A Paques C et al Extracellular vesicles mediate communication between endothelial and vascular smooth muscle cells. Int J Mol Sci. (2021) 23(1):331. 10.3390/ijms23010331
32.
Kapustin AN Chatrou ML Drozdov I Zheng Y Davidson SM Soong D et al Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. (2015) 116(8):1312–23. 10.1161/circresaha.116.305012
33.
Kapustin AN Shanahan CM . Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J Physiol. (2016) 594(11):2905–14. 10.1113/jp271340
34.
Zheng D Huo M Li B Wang W Piao H Wang Y et al The role of exosomes and exosomal microrna in cardiovascular disease. Front Cell Dev Biol. (2020) 8:616161. 10.3389/fcell.2020.616161
35.
Gao XF Wang ZM Wang F Gu Y Zhang JJ Chen SL . Exosomes in coronary artery disease. Int J Biol Sci. (2019) 15(11):2461–70. 10.7150/ijbs.36427
36.
Li J Tan M Xiang Q Zhou Z Yan H . Thrombin-Activated platelet-derived exosomes regulate endothelial cell expression of Icam-1 via Microrna-223 during the thrombosis-inflammation response. Thromb Res. (2017) 154:96–105. 10.1016/j.thromres.2017.04.016
37.
Wei K Huang H Liu M Shi D Ma X . Platelet-Derived exosomes and atherothrombosis. Front Cardiovasc Med. (2022) 9:886132. 10.3389/fcvm.2022.886132
38.
Wang J Li J Cheng C Liu S . Angiotensin-Converting enzyme 2 augments the effects of endothelial progenitor cells-exosomes on vascular smooth muscle cell phenotype transition. Cell Tissue Res. (2020) 382(3):509–18. 10.1007/s00441-020-03259-w
39.
Wang J Chen S Bihl J . Exosome-Mediated transfer of Ace2 (angiotensin-converting enzyme 2) from endothelial progenitor cells promotes survival and function of endothelial cell. Oxid Med Cell Longev. (2020) 2020:4213541. 10.1155/2020/4213541
40.
Hu H Wang B Jiang C Li R Zhao J . Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling mir-21-5p to modulate thrombospondin-1 expression. Clin Sci (Lond). (2019) 133(14):1629–44. 10.1042/cs20190188
41.
Heo J Kang H . Exosome-Based treatment for atherosclerosis. Int J Mol Sci. (2022) 23(2):1002. 10.3390/ijms23021002
42.
Liang Y Duan L Lu J Xia J . Engineering exosomes for targeted drug delivery. Theranostics. (2021) 11(7):3183–95. 10.7150/thno.52570
43.
Zhang Z Liu D Zheng Y Liu Y Cheng X Chang Y et al Vascular smooth muscle-secreted exosomal X26nt impedes atherosclerosis progression via the C-Fos/Xbp1/Sod1 axis. Immun Inflamm Dis. (2025) 13(8):e70251. 10.1002/iid3.70251
44.
Yang ZP Lu SH Pan YH Liao ZF Xie YT Li H et al Umbilical cord mesenchymal stem cell exosomal Mir-143-3p delays endothelial cell senescence through targeting Cox-2. PLoS One. (2025) 20(7):e0327173. 10.1371/journal.pone.0327173
45.
Wang Z Zhang J Zhang S Yan S Wang Z Wang C et al Mir-30e and Mir-92a are related to atherosclerosis by targeting Abca1. Mol Med Rep. (2019) 19(4):3298–304. 10.3892/mmr.2019.9983
46.
Wu G Zhang J Zhao Q Zhuang W Ding J Zhang C et al Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem Int Ed Engl. (2020) 59(10):4068–74. 10.1002/anie.201913700
47.
Lu M Huang Y . Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. (2020) 242:119925. 10.1016/j.biomaterials.2020.119925
48.
Chen H Wang L Zeng X Schwarz H Nanda HS Peng X et al Exosomes, a new star for targeted delivery. Front Cell Dev Biol. (2021) 9:751079. 10.3389/fcell.2021.751079
Summary
Keywords
atherosclerosis, bibliometrics, exosomes, microRNAs, therapeutic potential
Citation
Ren Y, Zhao B, Lv L, Song W, Wang Z, Lu Z, Li B and Yang B (2026) Global research trends on exosomes in atherosclerosis: a bibliometric and scientometric analysis (2004–2025). Front. Cardiovasc. Med. 12:1700630. doi: 10.3389/fcvm.2025.1700630
Received
07 September 2025
Revised
05 December 2025
Accepted
12 December 2025
Published
07 January 2026
Volume
12 - 2025
Edited by
Nhat Tu Le, Houston Methodist Research Institute, Houston, United States
Reviewed by
Dwijendra K. Gupta, Allahabad University, India
Fengwei Zhang, Shanghai University of Traditional Chinese Medicine, China
Updates
Copyright
© 2026 Ren, Zhao, Lv, Song, Wang, Lu, Li and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Bao Li libaoxys@163.com Bin Yang yangbxys@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.