Abstract
The murine hindlimb ischemia model is a classical experimental platform for studying peripheral arterial disease (PAD). It primarily includes three categories: acute ischemia models, subacute-to-chronic ischemia models, and ischemia-reperfusion models. Acute ischemia models are the most widely applied, with relatively well-established methodologies. They are typically induced by ligation or electrocauterization, but other approaches such as interventional embolization, photochemical thrombosis, physical injury, chemical injury, and embolization with traditional Chinese medicine-derived microparticles have also been employed. Subacute-to-chronic ischemia models, including the Ameroid constrictor, intravascular suture, anticoagulant silicone tube, and loop techniques, better reflect the pathophysiology of chronic PAD but have not yet been standardized for broad use. Ischemia-reperfusion models, as specialized interventional variants, are increasingly studied in the context of revascularization-related injuries. This review summarizes the methodologies, advantages, and limitations of the above models, and highlights factors influencing model establishment and experimental outcomes, aiming to provide a comprehensive reference for future research using murine hindlimb ischemia models.
1 Introduction
Peripheral arterial disease (PAD) is an ischemic disorder characterized by arterial stenosis or occlusion secondary to atherosclerosis, involving extracranial carotid, upper limb, renal, mesenteric, and lower limb arteries (1). In most of the literature, PAD refers specifically to lower limb arterial disease, which is also the focus of this review. Globally, over 200 million individuals are affected by PAD, presenting clinically with limb pain, pallor, coldness, numbness, intermittent claudication, rest pain, or even gangrene. These symptoms severely impair quality of life and threaten survival. Current therapeutic strategies mainly include pharmacologic interventions and revascularization procedures (2).
Preclinical validation in animal models is essential prior to clinical application of new therapeutic approaches. Establishing a reliable and appropriate PAD model provides the foundation for translational studies. While rabbits, pigs, and sheep have all been employed in hindlimb ischemia research (3), murine models are most widely used (4). These models can be categorized into acute ischemia, subacute-to-chronic ischemia, and ischemia-reperfusion models. Acute models are usually induced by ligation or electrocauterization, interventional or photochemical embolization, physical or chemical injury, or embolization with traditional Chinese medicine (TCM)-derived microparticles. They are technically mature, rapidly induce ischemia, and are associated with relatively low operative risk (5). However, the ischemic period is often short, differing substantially from the progressive nature of chronic occlusive PAD in humans. Subacute-to-chronic ischemia models, such as the Ameroid constrictor, intravascular suture, anticoagulant silicone tube, and loop methods, provide a more accurate simulation of chronic ischemia, though none has yet become widely adopted. With the advancement of interventional therapies—including balloon angioplasty, drug-coated balloons, stent implantation, and debulking techniques—the need to study ischemia-reperfusion (I/R) injury has also emerged. In these models, careful attention must be paid to the duration of ischemia and the associated reperfusion injury.
A comprehensive literature search was conducted using the electronic databases PubMed, Web of Science, and Google Scholar for relevant articles published up to March 2025. The search terms included: (“murine” OR “mouse” OR “rat”) AND (“hindlimb ischemia” OR “hind limb ischemia”) AND (“model” OR “modeling”) AND (“peripheral arterial disease”). The reference lists of retrieved articles were also manually screened to identify additional relevant studies. Inclusion criteria focused on original research articles and reviews that detailed the establishment, application, or evaluation of murine hindlimb ischemia models. Studies involving other animal species or not primarily focused on modeling methodology were excluded. The objective of this review is to summarize current methodologies for establishing murine hindlimb ischemia models, compare their advantages and disadvantages, and analyze critical factors that may affect model validity and experimental outcomes. These insights may help guide researchers in selecting or optimizing models for future studies.
2 Anatomy of the murine hindlimb arteries
The abdominal aorta in mice gives rise to the left and right common iliac arteries as well as the median sacral artery. Distally, the common iliac artery branches sequentially into the cranial gluteal artery, caudal gluteal artery, iliacofemoral artery, external iliac artery and internal iliac artery. The iliacofemoral artery originates from the distal segment of the common iliac artery and lies dorsal to the internal iliac artery. The external iliac artery serves as the major arterial supply to the hindlimb; after passing beneath the inguinal ligament, it continues as the femoral artery. Distally, the femoral artery gives rise to the lateral circumflex femoral artery (anterior femoral artery) and the proximal caudal femoral artery. At the proximal level of the knee joint and distal to the superficial epigastric artery, the femoral artery bifurcates into the popliteal artery and the saphenous artery. The popliteal artery traverses transversely through the popliteal fossa between the quadriceps femoris and the adductor muscles. Near its origin, the popliteal artery gives off the proximal medial genicular artery, which supplies the distal quadriceps femoris. The cranial and caudal gluteal arteries supply the biceps femoris muscle, while the iliacofemoral artery, lateral circumflex femoral artery, and proximal medial genicular artery supply the quadriceps femoris muscle. The deep femoral artery, proximal caudal femoral artery, and small branches from the saphenous and popliteal arteries supply the deep layer of the adductor muscles. The saphenous artery and the distal branches of the popliteal artery repeatedly branch to provide arterial supply to the foot (Figure 1) (6).
Figure 1
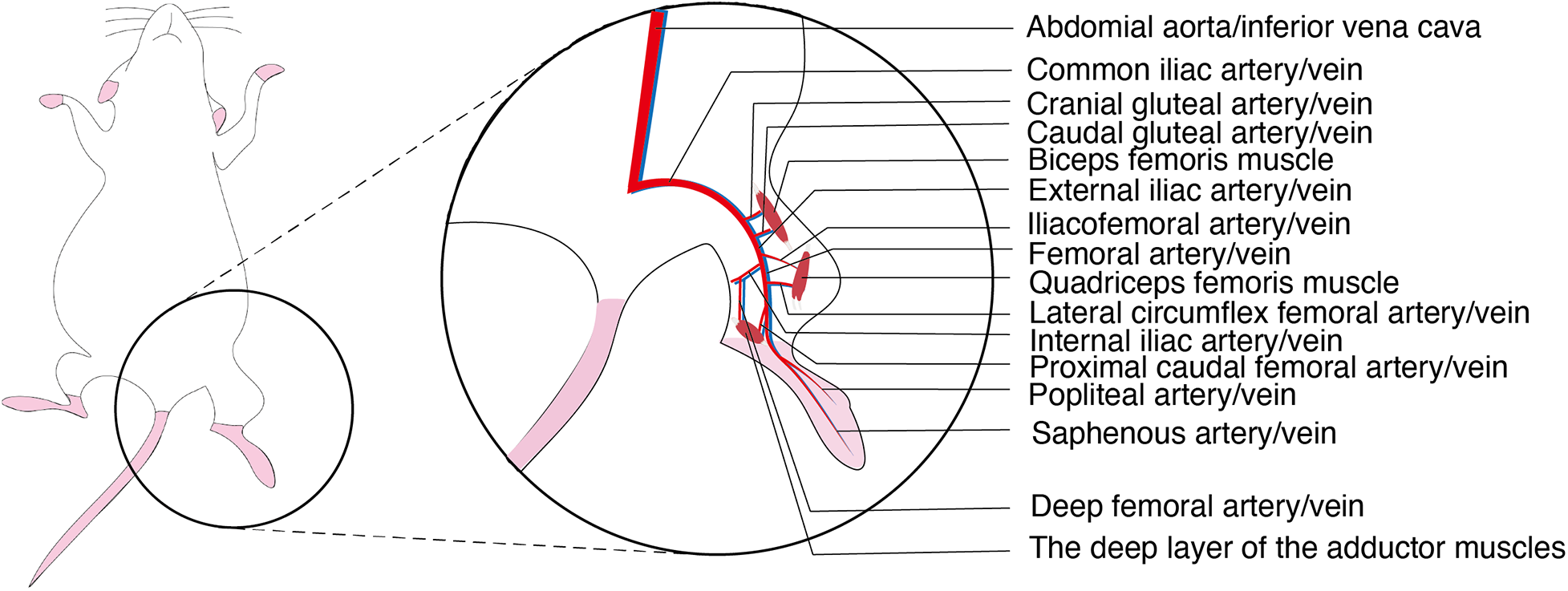
Anatomical diagram of the arteries and veins of the hindlimbs of murine.
3 Acute ischemia model
The acute hindlimb ischemia model is primarily established by rapidly interrupting arterial blood flow in the hindlimb through approaches such as ligation, electrocoagulation, interventional embolization, photochemical thrombosis, physical injury, chemical injury, or embolization with TCM microparticles (Table 1). Considerable variability often arises from the use of different modeling techniques, as well as from variations within the same technique. Therefore, it is essential to clearly define the specific procedures for each method to avoid misinterpretation and unnecessary waste of resources.
Table 1
| Modeling method | Induction approach | Strain | Age (weeks) | Sex | Ischemia recovery time | Complexity | Technical demand | Reference |
|---|---|---|---|---|---|---|---|---|
| Ligation/Electrocoagulation | Single femoral artery electrocoagulation/ligation | C57BL/6 mice | 10–12 | Male | 7 days | Simple | Moderate | (7, 8) |
| Single common iliac artery electrocoagulation/ligation | C57BL/6 mice | 10–12 | Male | 7 days | Simple | Moderate | (7) | |
| Double electrocoagulation of femoral and common iliac arteries | C57BL/6 mice | 10–12 | Male | 28 days, recovery to 54% of baseline | Simple | Moderate | (7, 9) | |
| Femoral artery double electrocoagulation/ligation with transection | C57BL/6 mice | 10–12 | Male | 21–28 days | Simple | Moderate | (7, 10) | |
| Femoral artery and vein double electrocoagulation/ligation with transection | C57BL/6 mice | 12 | Male | 14 days, recovery to 48% of baseline | Simple | Moderate | (11, 12) | |
| Common iliac artery and vein ligation with transection | C57BL/6 mice | Young (12 weeks) vs. Aged (72 weeks) | Male | Young: 14 days, recovery to 60% of baseline; Aged: 14 days, recovery to 25% of baseline | Simple | Moderate | (13) | |
| Interventional embolization | Embolic agent (hydrogel filament) at the aorto-popliteal/saphenous bifurcation | Lewis rats | 10–12 | Male | 14 days, recovery to 88.6% of baseline | Complex | High | (4) |
| Embolic agent (polyvinyl alcohol particles) in external iliac and distal arteries | Sprague–Dawley rats | 10–12 | Male | 7 days: infarction rate of thigh muscle: 17.3%; calf muscle: 33.7% | Complex | High | (14) | |
| Embolic agent (N-butyl cyanoacrylate) in external iliac and distal arteries | Sprague–Dawley rats | 10–12 | Male | 7 days: infarction rate of thigh muscle: 43.6%; calf muscle: 50.3% | Complex | High | (14) | |
| Photochemical embolization | Photosensitizers (Erythrosin B, Rose Bengal) | ICR mice, Wistar rats, Sprague–Dawley rats | 8–12 | Male | Not reported | Complex | High | (16–18) |
| Chemical injury | Ferric chloride | C57BL/6 mice, P2Y12-deficient mice | Not reported | Not reported | 21 days | Complex | High | (20, 21) |
| Physical injury | Guidewire-induced endothelial denudation | C57BL/6 mice | 18–22 | Male | Not reported | Complex | High | (22–24) |
| TCM microparticle embolization | Bletilla striata microparticles | Wistar rats | 10–12 | Both sexes | 21 days | Simple | Moderate | (27–29) |
Comparison of different acute ischemia modeling methods.
3.1 Modeling methods
3.1.1 Ligation/electrocoagulation/resection
Arterial ligation is currently the most widely used approach for PAD model establishment. Based on the ligation technique, it can be categorized into nylon suture ligation and electrocoagulation occlusion. According to the number of ligations, it can be further divided into single ligation and double ligation, namely simple femoral artery ligation, or combined femoral and common iliac artery ligation. After ligation, the procedure may or may not involve transection of the targeted vessel; transection is more suitable for evaluating angiogenesis in distal ischemic tissues.
Considering these variations, murine hindlimb arterial ligation models can be classified into the following types: single femoral artery electrocoagulation/ligation (7, 8); single common iliac artery electrocoagulation/ligation (7); combined femoral artery and common iliac artery double electrocoagulation (7, 9); femoral artery double electrocoagulation/ligation with transection (7, 10); femoral artery and vein double electrocoagulation/ligation with transection (11, 12); and external iliac artery and vein double electrocoagulation/ligation with transection (13) (Figure 2).
Figure 2
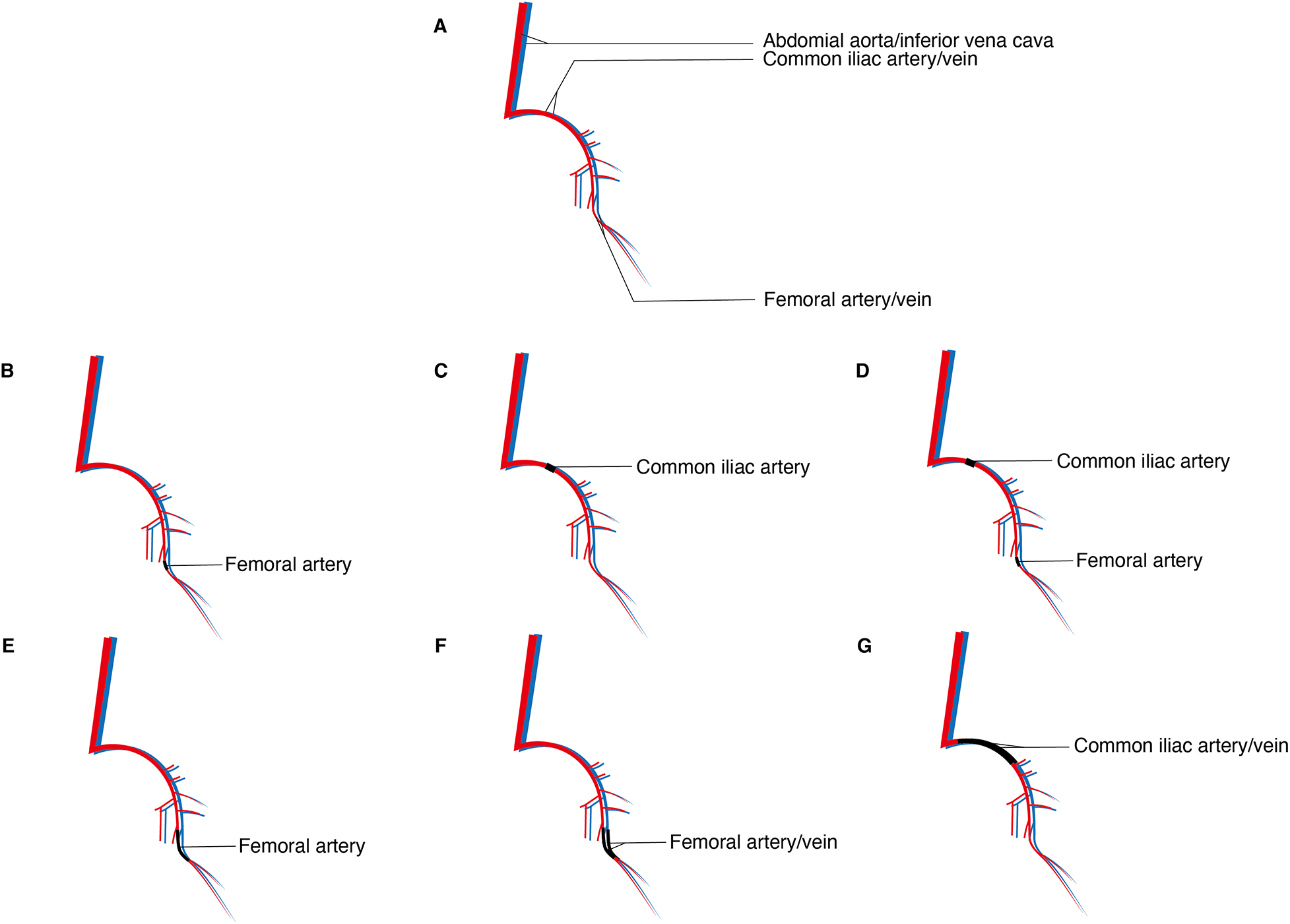
Different ligation/electrocoagulation methods; (A) sketch of the arteries and veins of the hindlimbs of the murine; (B) single femoral artery electrocoagulation/ligation; (C) single common iliac artery electrocoagulation/ligation; (D) combined femoral artery and common iliac artery double electrocoagulation; (E) femoral artery double electrocoagulation/ligation with transection; (F) femoral artery and vein double electrocoagulation/ligation with transection; (G) external iliac artery and vein double electrocoagulation/ligation with transection.
The surgical procedures among these methods are generally similar. Following standard disinfection and anesthesia, the abdominal cavity or a unilateral inguinal region is incised to expose the target vessel for the designated procedure, while the contralateral side is used as a control. Observation periods, treatment durations, and reperfusion times differ across modeling techniques. Therefore, investigators should design their protocols according to specific experimental objectives, conduct pilot studies for validation, and thereby minimize unnecessary waste of time and experimental resources.
3.1.2 Interventional embolization
During the ligation procedure, exposure, dissection, and ligation of the target artery often cause collateral damage to adjacent veins and neural networks, along with increased infiltration of inflammatory cells, which may compromise experimental outcomes. In contrast, interventional techniques exert relatively minor local effects and thereby expand the available approaches for establishing hindlimb ischemia models.
The interventional embolization method involves advancing a catheter through the common carotid artery of the rat/mice into the region between the iliac artery and the superficial caudal epigastric artery under angiographic guidance, followed by injection of embolic agents to occlude the common iliac artery, internal iliac artery, external iliac artery, femoral artery, popliteal artery, or saphenous artery, thereby inducing tissue ischemia (14). Currently used embolic agents include hydrogel filaments (4), polyvinyl alcohol (PVA) particles, and N-butyl cyanoacrylate (NBCA) (14), among which hydrogel filaments are the most widely applied.
3.1.3 Photochemical embolization
Photochemical embolization is a method of inducing hindlimb ischemia through vascular endothelial dysfunction and localized thrombosis triggered by a photochemical reaction between a photosensitizer and focused illumination (15). Commonly used photosensitizers include Rose Bengal and Erythrosin B. After intravenous administration of the photosensitizer, the target artery is irradiated with filtered green light or cold white light for 30–40 min to induce thrombus formation (16–18). This technique represents a noninvasive approach to vascular occlusion, as it does not require surgical incision to generate intravascular thrombosis. Moreover, no significant bleeding, infarction, or widespread pro-apoptotic stimulation has been observed, indicating that this method allows for precise, region-specific occlusion without major side effects (18).
3.1.4 Chemical injury
Kurz et al. (19) first established and validated a rat model of arterial injury and thrombosis induced by topical application of ferric chloride solution, and demonstrated that the resulting thrombus consisted of platelets and red blood cells interwoven within a fibrin network, resembling the characteristics of arterial thrombosis. Ohno et al. (20) subsequently applied the ferric chloride model to murine hindlimb arteries. Under a dissecting microscope, filter paper (approximately 1 × 2 mm) soaked in 10%–50% ferric chloride solution was placed directly on the target artery for 3–10 min, followed by removal, leading to gradual thrombus formation and eventual vascular occlusion. Previous studies have primarily employed P2Y12 knockout mice (20) and leukocyte-associated immunoglobulin-like receptor knockout mice (21) in this model.
3.1.5 Physical injury
The physical injury method induces ischemia by mechanically damaging the arterial wall, thereby triggering platelet adhesion and mural thrombus formation. This is achieved by rotating a flexible guidewire within the vessel lumen to disrupt the endothelium, which subsequently promotes thrombus development (22, 23). Loss and necrosis of vascular smooth muscle cells in the arterial media occur as early as day 1 after injury. Over the following 14 days, thrombus formation and platelet deposition decline progressively, from nearly 100% on day 1 to approximately 25%. This model is commonly applied in transgenic mice deficient in protease-activated receptor-1, and is particularly useful for studying cell proliferation and medial thickening (24).
3.1.6 Embolization with TCM microparticles
Bletilla striata embolic agents are prepared from the dried tubers of Bletilla striata. Their active component, mucilage, is a high-molecular-weight compound composed of four mannose molecules and one glucose molecule, and is known to exert anti-inflammatory, antitumor, and pro-coagulant effects (25, 26). In 1998, it was first developed as a vascular embolic agent for hepatic artery embolization studies (26); in 2005, Bletilla microparticle embolization was for the first time applied in establishing a rat hindlimb ischemia model (27). The procedure is performed under standard disinfection and adequate anesthesia. After isolating the femoral artery, the main trunk is clamped, and sterilized syringes are used to inject Bletilla microparticles into the artery. The clamp is then released, hemostasis is achieved by compression, and the skin is sutured (28, 29). Previous studies have demonstrated that Bletilla microparticle embolization achieves uniform and complete occlusion of hindlimb vessels in rats, effectively preventing the development of collateral circulation. In addition, it promotes erythrocyte aggregation, shortens coagulation time, and leads to secondary thrombus formation (27).
3.2 Comparison of modeling methods
The murine hindlimb arterial ligation model is the most widely used model of PAD and has numerous advantages. The procedure is simple, cost-effective, and technically well-established. Detailed experimental protocols can be found in previously published studies. Compared with coronary artery models, PAD surgery involves distal limb vessels, resulting in lower mortality and relatively safer operations for animals (5). However, as an invasive approach, local inflammatory responses are inevitable. Avoiding injury to surrounding nerves and veins requires considerable surgical skill. Surgical trauma induces the release of large amounts of growth factors and recruitment of monocytes, which further stimulate arteriogenesis via pro- and anti-inflammatory cytokines. This may mediate endothelial cell activation, migration, proliferation, and apoptosis, thereby influencing experimental accuracy (30). Nerves are a major source of vascular endothelial growth factors (VEGF), and their injury during surgery can impair angiogenesis. Indeed, experimental evidence suggests that nerves actively promote angiogenesis and arteriogenesis (31). Minor bleeding from small vessels during surgery can usually be controlled by compression, whereas injury to large vessels often necessitates proximal ligation, which alters circulation in the ischemic limb and compromises data accuracy. In some procedures, arteries and veins are ligated together, which not only prevents inadvertent venous injury but also enhances ischemic severity and reproducibility (8).
Interventional embolization and photochemical embolization avoid local inflammation and collateral tissue injury, thereby reducing the risk of infection. However, they require stricter laboratory conditions, greater technical expertise, and higher financial costs. Interventional embolization presents a steep technical barrier: researchers must be familiar with rat/mice vascular anatomy, the procedure is time-consuming, and radiation exposure poses additional risks. Photochemically induced thrombosis closely resembles lower limb arterial embolization. In ligation models, hindlimb circulation typically improves within 7 days (7); moreover, inflammation-driven arteriogenesis introduces confounding factors when studying angiogenesis and collateral circulation. In contrast, photochemical embolization produces more severe ischemia, leading to tissue discoloration, gangrene, and limb loss, closely mimicking the clinical disease course. This model allows fine-tuned control of variables—light intensity, exposure duration, irradiation site, and photosensitizer concentration—offering high flexibility. Furthermore, photosensitizers have no toxic effects on target organs, and animal survival rates are high (16). Nevertheless, the method can cause irreversible thrombotic occlusion and free radical generation, damaging vascular walls at the irradiation site. As no standardized parameters exist for light intensity or exposure time, researchers must determine optimal conditions empirically.
The chemical injury model induces arterial thrombosis via ferric chloride application, thereby avoiding vessel transection or ligation and the associated collateral tissue injury. This method does not require specialized equipment to induce thrombosis. The thrombi formed are platelet-rich and, under a dissecting microscope, closely resemble human arterial thrombi (32).
The physical injury method is currently more commonly used in carotid artery ischemia models (24). Its application to hindlimb ischemia requires further evaluation and the establishment of standardized protocols to improve reproducibility.
TCM microparticle embolization combines both physical and chemical approaches. Bletilla striata particles induce thrombosis pharmacologically while simultaneously blocking blood flow mechanically, thereby producing limb ischemia. The particles distribute evenly within vessels; in larger arteries, they may “migrate” under hemodynamic force into smaller branches, achieving uniform and complete embolization and effectively preventing collateral circulation. The particles slowly swell in blood, and their fibrous components resist absorption and degradation, exerting a sustained mechanical occlusive effect (27–29). Previous studies have shown that ischemia induced by Bletilla embolization can last 5 weeks to ∼2 months, offering long-lasting, stable, and reproducible outcomes (26, 27). However, the degree of embolization is difficult to quantify, and the method remains rarely applied. Moreover, the amount of granulation tissue available from this model is limited, which complicates studies on angiogenesis-targeted therapies (29).
4 Subacute–chronic ischemia models
PAD is a long-term chronic ischemic condition of the lower extremities, and acute ischemia models fail to fully recapitulate its pathological course. Subacute ischemia models prolong the duration of ischemia, thereby better simulating the chronic ischemic process in humans. Moreover, in subacute–chronic ischemia, the increase in shear stress occurs gradually rather than abruptly, providing more time for the adaptation and development of collateral arteries. Currently, modeling approaches include the Ameroid constrictor method, suture ligation, high-fat diet combined with suture ligation, anticoagulant silicone tube method, and the loop ligature technique (Table 2).
Table 2
| Modeling method | Induction approach | Strain | Age (weeks) | Sex | Ischemia recovery time | Complexity | Technical demand | Reference |
|---|---|---|---|---|---|---|---|---|
| Ameroid constrictor | Ameroid constrictor | C57BL/6 mice, SD rats | 10–12 | Male | 4–5 weeks | Relatively complex | High | (35–37) |
| Ligation method | Prolene suture | Lewis rats | 4 | Male | 49 days to 70% of contralateral limb | Simple | Moderate | (38, 39) |
| High-fat Diet + ligation | High-fat diet + Prolene suture | SD rats | 2 | Male | 42 days to 60% of contralateral limb | Simple | Moderate | (40–42) |
| Anticoagulant silicone tube | Anticoagulant silicone tube | SD rats | 12 | Male | 28 days to 67% of baseline | Simple | Moderate | (43) |
| Ring method | Infusion tube ring | SD rats | Not specified | Both sexes | Not specified | Simple | Moderate | (44) |
Comparison of different subacute ischemia modeling methods.
4.1 Modeling methods
4.1.1 Ameroid constrictor method
The Ameroid constrictor has been used in the coronary arteries of pigs and dogs to model atherosclerotic coronary artery disease (33, 34). Gale et al. (35) were the first to apply the Ameroid constrictor to a rat hindlimb ischemia model. The Ameroid constrictor consists of a stainless steel outer casing that encases an inner ring of casein, a hygroscopic material. Upon absorbing body fluids, the casein gradually swells, and the rigid stainless steel shell forces the casein to expand inward, ultimately occluding blood flow in the target artery (35, 36). The modeling procedure involves exposing and dissecting the target artery, selecting an appropriately sized Ameroid constrictor, and securing it around the vessel to complete the model (Figure 3) (37).
Figure 3
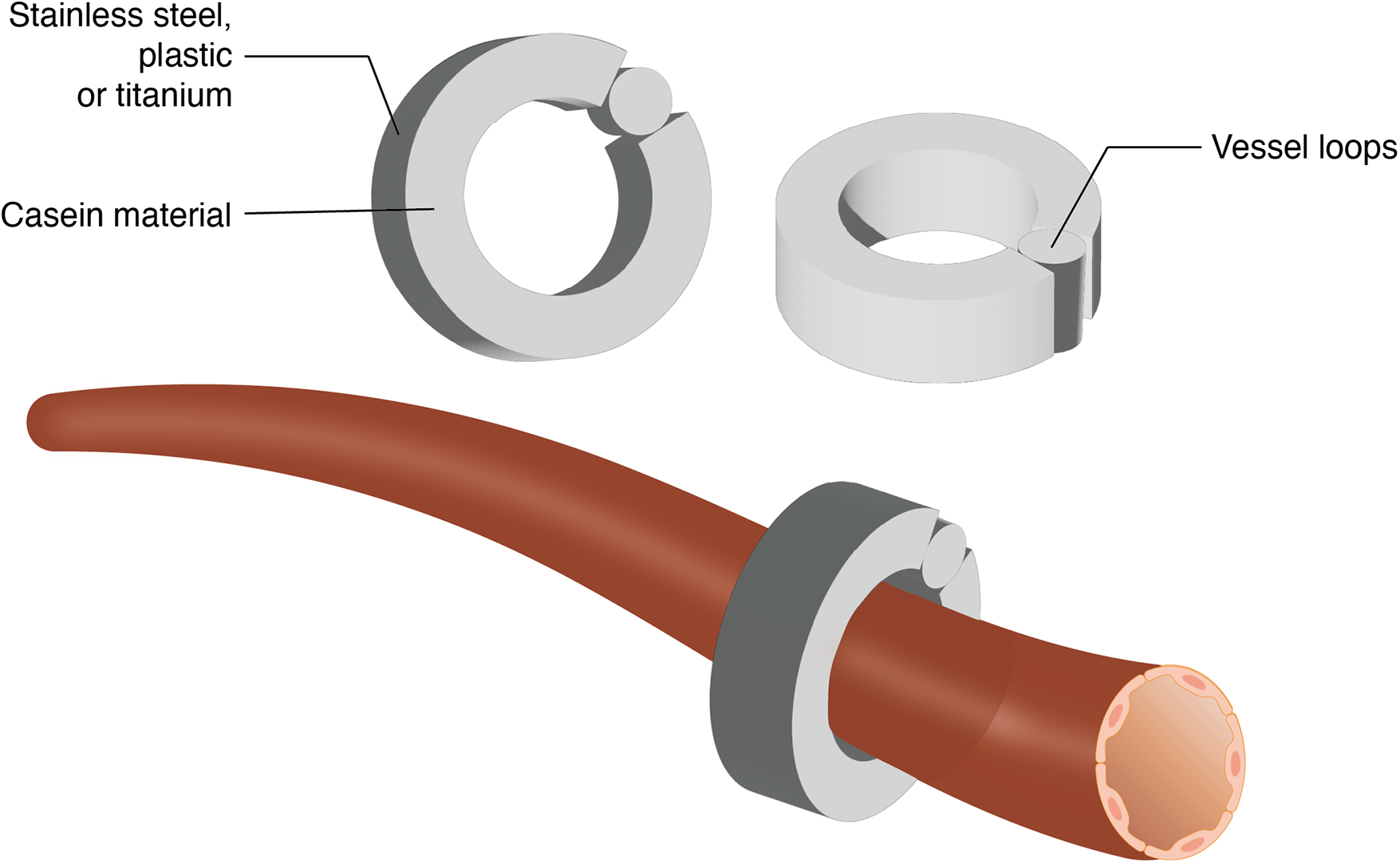
Schematic representation of ameroid constrictor placement on artery.
4.1.2 Suture-Induced occlusion method
The suture-induced occlusion model, developed by the research team of Shengjia Yang (38, 39) is a chronic ischemia model that uses a foreign-body reaction caused by an intravascular suture to gradually induce vascular occlusion. The procedure is as follows: after adequate anesthesia, the femoral artery is isolated and incised. A 4-0 Prolene suture is introduced into the distal segment of the femoral artery up to the knee joint under a surgical fiberscope, after which the arterial incision is closed. The intraluminal suture causes mechanical narrowing of the artery without immediate interruption of blood flow. As a foreign-body reaction develops, intimal hyperplasia ensues, leading to gradual occlusion and sustained hindlimb ischemia. This method maintains ischemia in the rat hindlimb for more than 6–7 weeks, with relatively low incidence of acute muscle necrosis and fibrosis (38).
4.1.3 High-fat diet combined with suture-induced occlusion
Chen Lei et al. (40) noted that although the suture-induced occlusion method provides a robust chronic ischemia model, surgical trauma and disease burden may shorten the survival of experimental animals, resulting in insufficient sample size. To develop a model of chronic limb ischemia with a shorter induction period, they modified Yang's method by feeding the animals a high-fat diet for two weeks before surgery. This dietary intervention significantly elevated serum lipid levels in the experimental group compared to controls, enhancing the foreign-body response to the intravascular suture, promoting intimal hyperplasia, and accelerating model establishment. Similarly, Emma Fletcher et al. (41, 42) successfully induced chronic hindlimb ischemia in C57BL/6J mice as a PAD model using a 16-week high-fat/high-sucrose diet combined with left femoral artery ligation.
4.1.4 Anticoagulant silicone tube method
Yingbin Jia's research group (43) developed a chronic ischemia model using an anticoagulant silicone tube. The procedure is as follows: after adequate anesthesia, the iliacofemoral artery is exposed, and side branches including the superficial iliac circumflex, femoral profound, external pudendal, and descending genicular arteries are ligated and transected with 7-0 atraumatic sutures. After temporarily clamping the bifurcation of the external iliac, saphenous, and popliteal arteries, one-third of the anterior wall of the femoral artery is incised. The lumen is flushed with heparinized saline, and an 8 mm-long silicone tube (inner diameter 0.6 mm) is inserted via the femoral incision. The tube is positioned to cover the arterial opening and secured with 7-0 atraumatic sutures. This setup mimics the process of chronic thrombus formation within the tube, simulating clinical peripheral arterial occlusive disease.
4.1.5 Perivascular cuff method
Yimin Fan's research group (44) established a chronic ischemia model using a perivascular cuff made from an intravenous infusion tube. The procedure is as follows: after adequate anesthesia, the skin is incised along the femoral sheath, and the femoral artery is isolated. A 5 mm-long plastic infusion tube (inner diameter 1 mm) is longitudinally cut to form a cuff, which is placed around the femoral artery. The cuff is secured in place with sutures, with tension adjusted according to vessel diameter, thereby creating a chronic hindlimb ischemia model.
4.2 Comparison of modeling methods
The Ameroid constrictor method induces chronic hindlimb ischemia through progressive compression of the target vessel, as the casein layer within the constrictor absorbs interstitial fluid and gradually expands. Ameroid constrictor-induced occlusion in mice results in relatively low shear stress–dependent and inflammatory gene expression, with limited blood flow recovery at 4–5 weeks postoperatively and minimal muscle necrosis (45). This method requires only surgical exposure and isolation of the target artery before placement of the constrictor, thereby minimizing local injury and inflammatory interference with angiogenesis, and is considered a reliable approach for establishing subacute ischemia models. However, several limitations exist. First, Ameroid constrictors are more costly compared to conventional arterial ligation. Second, if the groove of the constrictor is too shallow, the artery may slip out during constriction, leading to modeling failure. Third, uneven casein distribution within the constrictor may accelerate occlusion, negating the intended chronic process (37). Finally, the gradual occlusion reduces shear stress and prolongs collateral and distal angiogenesis, posing significant challenges to animal survival. Therefore, further refinement and optimization of this model are still required.
The suture-embolization method can maintain hindlimb ischemia for an extended period, while the pathological changes and dynamic recovery of blood flow better resemble the clinical course of chronic ischemia, making it a solid platform for evaluating therapeutic interventions. Neointimal hyperplasia appears as early as postoperative day 14, and by day 42, the artery is nearly occluded due to extensive neointimal proliferation. Interestingly, acute ischemic manifestations such as pallor, pulselessness, paresthesia, paralysis, and pain (“5 Ps”) are rarely observed; instead, muscle atrophy is evident during the late phase (42–49 days), consistent with clinical findings (38). However, since most PAD patients present with hyperlipidemia, standard rats may not fully replicate the chronic ischemia seen in humans with elevated lipid levels. Furthermore, as the method requires femoral arteriotomy, how to minimize local inflammatory infiltration and shear stress remains an issue for further investigation.
The high-fat diet combined ligation method allows for more rapid establishment of an ischemic model. Decreases in muscle strength occur earlier compared to controls, and muscle function, Doppler, and histological findings confirm more severe and sustained ischemia in the experimental group (40). Nonetheless, as chronic ischemia is inherently a long-term process, the accelerated onset of ischemia raises concerns regarding whether it induces artificially elevated shear stress. Moreover, earlier inflammatory cell infiltration observed in the experimental group may be detrimental to the fidelity of chronic ischemia modeling.
In the anticoagulant silicone tube model, branches of the external iliac artery—including the superficial circumflex iliac artery, deep femoral artery, and other collaterals—are ligated or divided, along with all branches of the femoral artery segment. This effectively blocks collateral flow and sustains hindlimb ischemia. Results show that blood flow in the ischemic limb reaches its lowest point at 7 days postoperatively. Although this does not replicate the years-long course of severe chronic ischemia seen clinically, it provides a significantly improved simulation compared to acute ischemia models (43). This method is technically simple, reproducible, associated with low mortality, and yields relatively stable ischemic severity. However, excessive interruption of collaterals, combined with femoral artery ischemia, may deviate from clinical reality.
The ring method is straightforward, low-cost, and technically feasible. However, displacement of the infusion tube ring may occur due to insufficient suture fixation, and the plastic material itself may cause local tissue injury, potentially confounding experimental outcomes. Therefore, the practical applicability of this method requires further validation.
5 I/R model
Acute lower limb ischemia is a common vascular emergency in clinical practice, characterized by a sudden reduction in blood supply to the limb, leading to intermittent claudication, tissue necrosis, and ulceration, which severely impair patients' quality of life (46, 47). Timely reperfusion is the cornerstone of treatment; however, it is often complicated by I/R injury, which causes vascular and muscular damage in the limb as well as multiple organ dysfunction syndromes involving the heart, lungs, liver, kidneys, and brain (48, 49). This complication is a major contributor to the high mortality associated with acute lower limb ischemia (47). To mitigate I/R injury, it is essential to establish and investigate ischemia/reperfusion and preconditioning models.
5.1 Modeling methods
The limb I/R model is typically established by first inducing acute ischemia by a tourniquet (50–52), pneumatic tourniquet (53), rubber band (54), orthodontic elastic band (55), aneurysm clip (56), non-traumatic microvascular clamp (51, 54, 57, 58), combined collateral cauterization and transection (58), or a tension cable system (59). These interventions are usually applied to the femoral or abdominal aorta and femoral artery ligation, thereby occluding blood flow to the lower limb for approximately 2–6 h (most commonly 3 h). Reperfusion is then achieved by removing the tourniquet or clamp, generally lasting 2–6 h.
Since I/R can cause severe injury to both the limb and internal organs, preconditioning strategies are often employed to mitigate reperfusion injury. For example, dexmedetomidine preconditioning has been shown to protect the liver and lungs against lower limb I/R injury (54, 60); local administration of DNase I and intravenous administration of a moderate dose of seviprotim (15 mg/kg bw/day) effectively eliminate neutrophil extracellular traps generated during reperfusion injury, thereby improving perfusion, promoting angiogenesis, preserving hindlimb function, and preventing muscle fibrosis (53); meclofenamate significantly alleviates skeletal muscle injury caused by lower limb I/R (51).
5.2 Evaluation
The modeling methods for I/R are relatively standardized, with the main point of debate concerning the duration of “ischemia” and “reperfusion” during the process. Previous studies have reported ischemia and reperfusion times ranging mostly from 2 to 6 h (54, 57, 61). Evidence indicates that ischemia lasting 3 h causes significant, yet localized and reversible injury, suggesting that “3 h of ischemia followed by 3 h of reperfusion” more closely mimics clinical scenarios and can therefore serve as a representative I/R model (50, 59). Although the I/R model is technically straightforward, strategies to mitigate reperfusion injury warrant further investigation. While numerous studies have demonstrated the protective role of preconditioning against I/R-induced injury in target organs, clinical translation remains limited, highlighting a critical direction for future research. Additionally, studies have shown that the severity of reperfusion injury does not differ among tissues examined after lower-limb I/R (62); thus, changes in each vital organ should be carefully monitored.
Although I/R causes tissue damage, appropriately applied remote ischemic preconditioning may have beneficial effects. Remote ischemic preconditioning refers to transient, non-lethal cycles of ischemia and reperfusion in the arm or leg that can protect against endothelial dysfunction induced by sustained ischemia and reperfusion (63, 64). For example, reversible and short-term lower-limb I/R (four cycles of 5 min of ischemia followed by 5 min of reperfusion) can provide substantial cardioprotection, significantly reducing myocardial IR injury (65, 66). Therefore, the dual-edged role of I/R warrants in-depth investigation.
6 Influencing factors
Different modeling methods may affect experimental outcomes; however, factors such as animal strain, genetics, sex, age, and anesthetic approach should also be carefully considered.
6.1 Strain
Helisch et al. (67) investigated C57BL/6, BALB/c, and 129S2/Sv mice following femoral artery ligation, assessing blood perfusion, collateral formation, and exercise tolerance on days 0, 7, 14, 21, and 28. Their results demonstrated that genetic differences in pre-existing collateral vascular systems influence collateral artery growth and the tissue environment after femoral artery occlusion. Other studies similarly found that, compared with BALB/c mice, C57BL/6 mice possess a more developed pre-existing collateral network and exhibit greater arteriogenesis in hindlimb ischemia models (68, 69). These findings suggest that different strains exhibit distinct blood perfusion recovery patterns, warranting stratified comparisons.
6.2 Genetics
Eugenio et al. (70) compared C57BL/6 mice with CD4-deficient mice after left femoral artery ligation. C57BL/6 mice exhibited superior hindlimb perfusion and collateral circulation compared with CD4-deficient mice. When CD4T cells from the spleen were transfused into CD4-deficient mice, the exogenous CD4 cells selectively homed to ischemic infiltrated regions within 24 h, accompanied by enhanced macrophage infiltration and VEGF expression. Consequently, blood flow recovery in the CD4 group resembled that of C57BL/6 mice. The same group further demonstrated that, after femoral artery ligation, CD8+ T cells infiltrated collateral growth sites and recruited CD4+ monocytes via IL-16 expression, underscoring the crucial role of the immune system in modulating collateral development in response to peripheral ischemia (71). Therefore, immune deficiencies in mice may confound experimental results.
6.3 Sex
Peng et al. (72) reported that female C57BL/6J mice exhibited slower perfusion recovery than males 7 days after femoral artery ligation, likely due to reduced collateral remodeling, impaired angiogenesis, and endothelial dysfunction. Sieveking et al. (73) further showed that androgens exert sex-specific effects on angiogenesis, with endogenous androgens promoting neovascularization in ischemic injury. Thus, sex differences should be considered as an influencing factor in model outcomes.
6.4 Age
Zhuo et al. (74) found that juvenile (6–8 weeks) mice demonstrated faster and more complete perfusion recovery than aged (60–64 weeks) mice. Accordingly, aged mice may be preferable for studies on pro-angiogenic interventions, as they allow clearer observation of treatment effects, whereas juvenile mice may be better suited for studies targeting anti-angiogenic processes. The inclusion of animals of different ages adds diversity and depth to experimental design.
6.5 Anesthetic
The choice of anesthesia can also influence modeling. Studies have shown that α-adrenergic agonists may induce early peripheral vasoconstriction, thereby interfering with experimental outcomes (75). Furthermore, the combined use of ketamine and α-agonists is not suitable for studies involving vascular systems rich in smooth muscle cells (76).
7 Murine hindlimb ischemia models in the context of atherosclerosis
The standard hindlimb ischemia models are typically established in healthy, young mice. However, clinical PAD predominantly occurs in aged individuals with underlying comorbidities, most notably atherosclerosis. To better simulate this clinical context, researchers have increasingly employed genetically modified mice, such as the apolipoprotein E knockout (ApoE−/−) and the low-density lipoprotein receptor knockout (LDLR−/−) mice.
When fed a high-fat diet, these mice develop systemic hypercholesterolemia and atherosclerosis. The use of such models in hindlimb ischemia studies allows for the investigation of PAD in a metabolically and vasculopathically compromised environment (77). Evidence suggests that the presence of atherosclerosis can significantly impair post-ischemic angiogenesis and arteriogenesis (78), leading to delayed perfusion recovery and more severe functional impairment compared to wild-type controls (79). Plasma hypercholesterolemia is antiangiogenic due to elevated levels of low-density lipoprotein (80).
While these models do not directly simulate “cardiac involvement” in the sense of primary myocardial pathology, the systemic atherosclerotic burden they develop does affect the coronary arteries, making them excellent models for studying the common co-morbidity of PAD and coronary artery disease. Therefore, for studies aiming to test therapeutic interventions for PAD in a clinically relevant setting, the ApoE−/− and LDLR−/− models, particularly when combined with a high-fat diet, offer a superior platform that recapitulates the complex pathophysiology of the human disease.
8 Conclusion and perspectives
Overall, murine hindlimb ischemia models exhibit considerable diversity, allowing researchers to tailor their choice of model according to specific experimental goals and conditions. Acute ischemia models are the most widely studied, with relatively mature protocols that meet most current research needs. By contrast, subacute and chronic ischemia models remain less developed, requiring further refinement and innovation. While I/R models are relatively easy to establish, strategies to minimize reperfusion injury continue to represent a research hotspot. Importantly, leveraging the beneficial aspects of I/R in preventing or treating other diseases holds significant potential for future clinical applications (Table 3).
Table 3
| Modeling type | Advantages | Limitations | Sample size | Mortality/Key safety notes | Reference |
|---|---|---|---|---|---|
| Single femoral artery electrocoagulation/ligation | (1) The surgical procedure is simple and rapid; (2) The model exhibits high standardization and excellent reproducibility; (3) The inflammatory response following ischemia is relatively mild; (4) Blood flow recovers quickly (within 7–14 days) in strains such as C57BL/6, making it suitable for studying spontaneous recovery mechanisms. |
(1) The treatment window is short, making it difficult to observe the therapeutic effects of interventions in strains with rapid recovery; (2) The degree of ischemia is strain-dependent and may be insufficient to induce a robust angiogenic response in certain strains. |
10–15 per group | Not reported | (7, 8) |
| Single common iliac artery electrocoagulation/ligation | (1) Relatively simple procedure, performed via retroperitoneal approach; (2) Controllable ischemia level, effectively reducing distal blood flow. |
(1) Compared to single femoral artery electrocoagulation, no significant advantage was demonstrated in blood flow recovery speed or pattern; (2) The therapeutic window remains similarly brief (rapid recovery within 7 days). |
9 per group (in comparative method study) | Not reported | (7) |
| Double electrocoagulation of femoral and common iliac arteries | (1) Simulates clinically relevant multi-segmental lesions with more severe and prolonged ischemia; (2) Creates a significantly extended therapeutic window (e.g., only 54% recovery after 28 days), making it ideal for evaluating the efficacy of pro-angiogenic therapies or cell therapies; (3) Equally effective in immunodeficient mice, suitable for studies using human cells. |
(1) More severe ischemia increases the risk of tissue necrosis (e.g., toe necrosis); (2) Surgery involves two sites and is slightly longer; (3) Not suitable for studying arterialization of specific pre-existing collaterals originating from the deep femoral branch, as proximal iliac artery flow is also blocked. |
9–22 per group | Approximately 30% (3/10) of C57BL/6 mice developed one or more cases of nail necrosis; in immunodeficient mice, the incidence of nail necrosis reached up to 90% (9/10). No deaths were reported. | (7, 9) |
| Femoral artery double electrocoagulation/ligation with transection | (1) Induces severe and stable ischemia, eliminating the possibility of vascular recanalization; (2) Effectively stimulates a robust angiogenesis response, serving as a classic model for studying this process. |
(1) It involves significant surgical trauma and is more complex and time-consuming to perform; (2) Blood flow restoration relies on extensive connections within the newly formed capillary network rather than typical pre-existing collateral enlargement (arteriogenesis). Angiographic patterns appear disorganized, lacking the characteristic “spiral” collaterals; (3) Not suitable for studies specifically investigating arteriogenesis. |
Each group of animals used for analysis at different time points must include ≥3 animals. | Approximately 10% of mice exhibited toe necrosis during the first postoperative week, which subsequently healed. No mortality was reported. | (10) |
| Femoral artery and vein double electrocoagulation/ligation with transection | Recommended Models: A-strip group (arterial stripping): Produces relatively severe and stable ischemia (9/10 cases with toe necrosis), suitable as a stable severe ischemia model. Group A (Arterial Transection): Simple procedure, mild and chronic ischemia (7/10 without necrosis, 3/10 with toe necrosis). Suitable as a chronic mild ischemia model, more closely resembling the clinical presentation in most patients. |
(1) Uneven Ischemia Severity: Even within the same method group, ischemic injury severity (ranging from no necrosis to knee necrosis) exhibits individual variability and instability, particularly pronounced in the Prox-A and AV-strip groups; (2) A-strip surgery is more complex: Requires dissection of an arterial segment, making the procedure more time-consuming than simple ligation or transection; (3) Interference from venous ligation: The AV group introduces venous reflux obstruction, whose pathological changes do not correspond to pure arterial disease. |
6–10 per group | Not reported | (11, 12) |
| Common iliac artery and vein ligation with transection | (1) Non-ischemic limb incision: The surgical incision is made in the abdomen, avoiding the creation of a surgical wound on the ischemic limb. This eliminates interference from wound healing on local inflammation and stem cell homing, allowing for a purer study of the effects of ischemia itself; (2) Simulates severe ischemia: The ligation site is positioned closer to the cardiac end (common iliac artery), blocking more collateral sources and potentially inducing more severe ischemia than simple femoral artery ligation alone; (3) Reveals age differences: Successfully used to compare differences in blood flow recovery, function, and angiogenesis/arteriogenesis responses between young and aged mice following severe ischemia. |
(1) Simultaneous venous ligation: Ligation of the iliac vein to enhance technical success rates and ischemia severity distinguishes this model from clinically common arterial occlusive diseases; (2) Distinct blood flow recovery pattern: Young mice exhibit 60% baseline recovery by day 14, with recovery kinetics potentially differing from other models; (3) Survival limitations in aged mice: Poor postoperative survival in aged mice restricts long-term (>2 weeks) observation. |
15(Young)/20(Aged) per group | Not reported (poor postoperative survival in aged mice was mentioned) | (13) |
| Embolic agent (hydrogel filament) at the aortopopliteal/saphenous bifurcation | (1) Minimally invasive: Performed via catheterization, eliminating the need for incisions on the ischemic limb and maximally preserving tissue integrity (nerves, vessels, muscle bundles) in the surgical area; (2) Mild inflammatory response: Compared to open surgery, significantly reduced early inflammatory cell infiltration and release of inflammatory factors (e.g., IL-1α, IL-18), minimizing surgical trauma interference with angiogenesis studies; (3) Clinical simulation: More closely mimics the pathophysiological processes of human atherosclerotic occlusion or embolism; (4) High reproducibility: Enables controlled, uniform, and repeatable vascular occlusion. |
(1) Technically complex and demanding: Requires specialized interventional radiology skills, microsurgical equipment (binocular loupes), and real-time imaging guidance (C-arm); (2) Expensive equipment and materials: Requires specific microcatheters, guidewires, and embolization materials (e.g., hydrogel coils); (3) Risk of procedural failure: Risks include misembolization and vasospasm, leading to animal exclusion; (4) Slow early blood flow recovery: At 3 days post-procedure, blood flow recovery may be slower than in surgical models, potentially due to reduced inflammatory stimulation. |
24 per group | Not reported | (4) |
| Embolic agent (polyvinyl alcohol particles/N-butyl cyanoacrylates) in external iliac and distal arteries | (1) Avoid surgical trauma: Unlike surgical resection, it does not create incision wounds, eliminating interference from the wound healing process; (2) Induce muscle infarction: Embolization with NBCA or PVA can cause distinct acute muscle infarction, suitable for studying infarction and subsequent changes; (3) No magnetic susceptibility artifacts: Unlike platinum coils, NBCA and PVA materials produce no magnetic susceptibility artifacts during MRI examinations, facilitating imaging assessment; (4) Simulate diverse clinical scenarios: Different embolization materials (dense NBCA distal embolization vs. PVA proximal embolization) can mimic distinct clinical ischemia patterns. |
(1) High technical difficulty: Requires interventional expertise and dedicated imaging equipment; (2) Material properties influence outcomes: Different embolization materials (NBCA, PVA) produce varying degrees of ischemia and infarct size, necessitating careful selection based on study objectives; (3) Inflammatory factors not evaluated: The chemical and mechanical properties of different materials may trigger distinct cytokine releases, which were not assessed in this study. |
4 per group | Not reported | (14) |
| Photosensitizers | (1) Non-invasive, non-mechanical: Induces thrombosis within blood vessels without surgical incisions, minimizing surgical trauma and inflammatory interference; (2) Highly reproducible and controllable: Precisely regulates ischemia location and severity by adjusting light intensity, exposure duration, spot position, and photosensitizer concentration; (3) Closer to human pathology: Simulates ischemia caused by thrombotic vascular occlusion, more closely resembling the mechanism of clinical peripheral arterial disease; (4) Suitable for molecular imaging: The absence of surgical incisions avoids background signal interference from incision inflammation, facilitating continuous and repeated molecular imaging monitoring. |
(1) Requires specialized equipment: Depends on specific wavelength light sources (e.g., cool white light, green laser) and photosensitizers, presenting higher technical barriers and costs; (2) Relatively complex operation: Involves intravenous injection of photosensitizers, precise light positioning, and temporal control; (3) Potential phototoxicity: May cause free radical damage to vascular walls in irradiated areas; (4) Limited research history: Less extensively applied in limb ischemia models compared to traditional ligation methods; its long-term pathological progression and drug responses require further validation for human relevance. |
8–15 per group | All survived until the end of the experiment. No tissue damage related to the photosensitizer or light exposure was observed. | (16–18) |
| Ferric chloride | (1) Effectively induces acute thrombosis: Rapidly and reliably forms occlusive thrombi in local arteries; (2) Simulates mild limb ischemia: Induces moderate ischemia typically without severe limb necrosis, closely mimicking the intermittent claudication experienced by most PAD patients; (3) Suitable for functional assessment: Post-ischemic gait abnormalities can be quantified using high-sensitivity systems (e.g., CatWalk). |
(1) Invasive procedure: Requires surgical exposure of the vessel and placement of FeCl₃-impregnated filter paper; (2) Nonspecific chemical injury: FeCl₃ may cause direct chemical damage to perivascular tissues (including nerves), potentially confounding functional outcomes. |
10 per group | Not reported | (20) |
| Guidewire induced endothelial denudation | (1) Closest to clinical balloon angioplasty, simulating the entire process including endothelial stripping, vascular wall stretching, platelet aggregation, and inflammatory cell infiltration; (2) Induces stable and reproducible neointimal hyperplasia, suitable for studying restenosis mechanisms; (3) With standardized techniques, skilled operators can complete the procedure within 20 min. |
(1) Extremely high technical difficulty: Requires inserting a 0.38 mm diameter wire into a femoral artery branch approximately 0.1 mm in diameter, demanding meticulous manipulation; (2) Prone to intraoperative complications such as arterial perforation and major hemorrhage, which may result in animal mortality. |
Not reported | The risk of intraoperative mortality is high, primarily due to massive hemorrhage caused by arterial perforation. While specific percentages are not reported in the literature, it is emphasized that “forceful insertion could result in loss of the mouse”. | (22–24) |
| Bletilla striata microparticles | (1) Effectively establishes a chronic persistent ischemia model with significant and sustained ischemic effects (lasting up to 2 months or longer); (2) Simulates the chronic arterial occlusion process, more closely approximating the pathological state of clinical ischemic diseases (compared to acute ligation); (3) The small particle size (∼47 μm) enables embolization of peripheral small arteries and arterioles, effectively blocking the establishment of collateral circulation. |
(1) Complex preparation process for the embolization agent: Requires ultrafine grinding technology to produce Bletilla striata particles of a specific diameter (e.g., 47 μm); (2) Uneven degree of ischemia: Due to variations in the ability to establish collateral circulation among individual animals, tissue necrosis may occur to differing extents, affecting the standardization of tissue sampling; (3) Primarily validated in rat models; fewer reports on its application and optimization in mice. |
5–8 per group | No animal deaths occurred during or after the procedure. | (27–29) |
| Ameroid constrictor | (1) Simulates chronic ischemia processes closer to clinical conditions; (2) Allows controlled occlusion rates to accommodate diverse research requirements; (3) Reduces acute inflammation and necrosis. |
(1) High equipment costs and complex operational techniques; (2) Occlusion rates are influenced by device quality and environmental humidity; (3) Significant variability in responses across different mouse strains. |
8–13 per group | No significant surgical mortality. | (35–37) |
| Ligation method | (1) Simple operation and low cost; (2) Can simulate chronic ischemia with prolonged ischemic duration. |
(1) Mild ischemia with rapid recovery; (2) Limited collateral vessel opening and weak arterialization; (3) Primarily applicable to rats, with limited use in mice. |
4–10 per group | No significant surgical mortality. | (38, 39) |
| High-fat diet + Ligation | (1) Simulates common risk factors for human PAD (high-fat diet, alcohol); (2) Ischemic manifestations appear early and recover slowly, making it suitable for chronic ischemia studies. |
(1) Long experimental cycle (requires several weeks of feeding); (2) Dietary factors may introduce metabolic confounding variables; (3) Ischemia severity may vary due to individual differences. |
12–20 per group | No significant surgical mortality. | (40–42) |
| Anticoagulant silicone tube | (1) Simulates the progressive thrombosis process, closely mimicking clinical chronic occlusion; (2) Allows controlled ischemia severity with gradual blood flow restoration; (3) Relatively simple to perform with high reproducibility. |
(1) Requires prevention of silicone tube displacement or premature thrombus detachment; (2) Suitable for rats; challenging to perform in mice. |
20 per group | Not reported | (43) |
| Ring method | (1) Simple to operate, capable of simulating chronic ischemia and applicable for pain research; (2) Suitable for drug intervention studies (e.g., nerve block). |
(1) Degree of ischemia depends on ligature tightness, making standardization challenging; (2) May induce perivascular fibrosis rather than true occlusion; (3) Suitable for rats; difficult to perform on mice due to their smaller vessels. |
13 per group | Not reported | (44) |
Summary of advantages and limitations of major murine hindlimb ischemia models.
Based on the reviewed literature, the acute ischemia model induced by simple femoral artery ligation appears to be the safest and most reproducible technique, with minimal reported mortality and low operational risk, making it suitable for most angiogenesis studies. In contrast, models that induce severe ischemia, such as double ligation with transection or photochemical embolism, are associated with higher rates of limb necrosis and auto-amputation, and should be employed with caution. The subacute-to-chronic models, while better mimicking human PAD, often involve more complex surgeries and longer ischemic durations, which can pose significant challenges to animal survival and welfare, necessitating careful perioperative management. The frequent underreporting of mortality data in the literature highlights an area for future improvement, and we strongly advocate for the standardized reporting of survival rates and perioperative complications in all future studies to facilitate better model selection.
Statements
Author contributions
YZ: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. HD: Writing – original draft. QR: Investigation, Visualization, Writing – original draft. JX: Methodology, Visualization, Writing – original draft. XT: Investigation, Software, Writing – review & editing. YW: Conceptualization, Writing – review & editing. JD: Conceptualization, Writing – review & editing. YC: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the China Postdoctoral Science Foundation (No. 2025M773983) and the National Natural Science Foundation of China (No. 82174382).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PAD, peripheral arterial disease; TCM, traditional Chinese medicine; I/R, ischemia and reperfusion; VEGF, vascular endothelial growth factor; ApoE−/−, apolipoprotein E knockout; LDLR−/−, low-density lipoprotein receptor knockout.
References
1.
Aboyans V Björck M Brodmann M Collet JP Czerny M De Carlo M et al Questions and answers on diagnosis and management of patients with peripheral arterial diseases: a companion document of the 2017 ESC guidelines for the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): endorsed by: the European stroke organisation (ESO)The task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J. (2018) 39(9):e35–41. 10.1093/eurheartj/ehx499
2.
Shamaki GR Markson F Soji-Ayoade D Agwuegbo CC Bamgbose MO Tamunoinemi BM . Peripheral artery disease: a comprehensive updated review. Curr Probl Cardiol. (2022) 47(11):101082. 10.1016/j.cpcardiol.2021.101082
3.
Lovasova V Bem R Chlupac J Dubsky M Husakova J Nemcova A et al Animal experimental models of ischemic limbs—a systematic review. Vascul Pharmacol. (2023) 153:107237. 10.1016/j.vph.2023.107237
4.
Zhuang ZW Shi J Rhodes JM Tsapakos MJ Simons M . Challenging the surgical rodent hindlimb ischemia model with the miniinterventional technique. J Vasc Interv Radiol. (2011) 22(10):1437–46. 10.1016/j.jvir.2010.12.039
5.
Niiyama H Huang NF Rollins MD Cooke JP . Murine model of hindlimb ischemia. J Vis Exp. (2009) 21(23):1035. 10.3791/1035
6.
Kochi T Imai Y Takeda A Watanabe Y Mori S Tachi M et al Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PLoS One. (2013) 8(12):e84047. 10.1371/journal.pone.0084047
7.
Hellingman AA Bastiaansen AJ de Vries MR Seghers L Lijkwan MA Lowik CW et al Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. (2010) 40(6):796–803. 10.1016/j.ejvs.2010.07.009
8.
Limbourg A Korff T Napp LC Schaper W Drexler H Limbourg FP . Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. (2009) 4(12):1737–46. 10.1038/nprot.2009.185
9.
Hellingman AA van der Vlugt LE Lijkwan MA Bastiaansen AJ Sparwasser T Smits HH et al A limited role for regulatory T cells in post-ischemic neovascularization. J Cell Mol Med. (2012) 16(2):328–36. 10.1111/j.1582-4934.2011.01300.x
10.
Couffinhal T Silver M Zheng LP Kearney M Witzenbichler B Isner JM . Mouse model of angiogenesis. Am J Pathol. (1998) 152(6):1667–79.
11.
Goto T Fukuyama N Aki A Kanabuchi K Kimura K Taira H et al Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. Tokai J Exp Clin Med. (2006) 31(3):128–32.
12.
Gomez-Rodriguez V Orbe J Martinez-Aguilar E Rodriguez JA Fernandez-Alonso L Serneels J et al Functional MMP-10 is required for efficient tissue repair after experimental hind limb ischemia. FASEB J. (2015) 29(3):960–72. 10.1096/fj.14-259689
13.
Westvik TS Fitzgerald TN Muto A Maloney SP Pimiento JM Fancher TT et al Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. (2009) 49(2):464–73. 10.1016/j.jvs.2008.08.077
14.
Shin CI Kim HC Song YS Cho HR Lee KB Lee W et al Rat model of hindlimb ischemia induced via embolization with polyvinyl alcohol and N-butyl cyanoacrylate. Korean J Radiol. (2013) 14(6):923–30. 10.3348/kjr.2013.14.6.923
15.
Przyklenk K Whittaker P . Adaptation of a photochemical method to initiate recurrent platelet-mediated thrombosis in small animals. Lasers Med Sci. (2007) 22(1):42–5. 10.1007/s10103-006-0410-1
16.
Han SS Jin Z Lee BS Han JS Choi JJ Park SJ et al Reproducible hindlimb ischemia model based on photochemically induced thrombosis to evaluate angiogenic effects. Microvasc Res. (2019) 126:103912. 10.1016/j.mvr.2019.103912
17.
Takiguchi Y Asai F Wada K Hayashi H Nakashima M . Antithrombotic effect of a novel recombinant hirudin analogue, CX-397, in a rat arterial thrombosis model. Br J Pharmacol. (1995) 116(7):3056–60. 10.1111/j.1476-5381.1995.tb15963.x
18.
Kikuchi S Umemura K Kondo K Saniabadi AR Nakashima M . Photochemically induced endothelial injury in the mouse as a screening model for inhibitors of vascular intimal thickening. Arterioscler Thromb Vasc Biol. (1998) 18(7):1069–78. 10.1161/01.ATV.18.7.1069
19.
Kurz KD Main BW Sandusky GE . Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. (1990) 60(4):269–80. 10.1016/0049-3848(90)90106-M
20.
Ohno K Tomizawa A Mizuno M Jakubowski JA Sugidachi A . Prasugrel, a platelet P2Y12 receptor antagonist, improves abnormal gait in a novel murine model of thrombotic hindlimb ischemia. J Am Heart Assoc. (2016) 5(4):e002889. 10.1161/JAHA.115.002889
21.
Smith CW Thomas SG Raslan Z Patel P Byrne M Lordkipanidze M et al Mice lacking the inhibitory collagen receptor LAIR-1 exhibit a mild thrombocytosis and hyperactive platelets. Arterioscler Thromb Vasc Biol. (2017) 37(5):823–35. 10.1161/ATVBAHA.117.309253
22.
Takayama T Shi X Wang B Franco S Zhou Y DiRenzo D et al A murine model of arterial restenosis: technical aspects of femoral wire injury. J Vis Exp. (2015) 10(97):52561. 10.3791/52561-v
23.
Pamulapati V Cuda CM Smith TL Jung J Xiong L Swaminathan S et al Inflammatory cell dynamics after murine femoral artery wire injury: a multi-parameter flow cytometry-based analysis. Cells. (2023) 12(5):689. 10.3390/cells12050689
24.
Cheung WM D'Andrea MR Andrade-Gordon P Damiano BP . Altered vascular injury responses in mice deficient in protease-activated receptor-1. Arterioscler Thromb Vasc Biol. (1999) 19(12):3014–24. 10.1161/01.ATV.19.12.3014
25.
Zheng C Feng G Zhou R Liang B . New use of bletilla striata as embolizing agent in the intervention treatment of hepatic carcinoma. Chin J Oncol. (1996) 18(04):67–9.
26.
Zheng C Feng G Zhang Y . Bletilla colloid as a vascular embolization agent: experimental studies. Chin J Oncol. (1998) 32(03):43–6.
27.
Liang C . The experimental studies of wistar rat hindlimb ischemia model. (Master degree). (2005).
28.
Liang C Tian H Xu Y Li G Tian G Song T . A comparison of a rat hind-limb ischemia model established by ligation and embolization. J Shandong Univ (Health Sci). (2007) 45(10):1008–10+ 15.
29.
Shi W Su X Zhuang S Hu Y Gao D Song J . Angiogenesis study of XFZYD on limb ischemic model of rat. J Basic Chin Med. (2014) 20(04):465–7. 10.19945/j.cnki.issn.1006-3250.2014.04.019
30.
Lingen MW . Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med. (2001) 125(1):67–71. 10.5858/2001-125-0067-ROLAEC
31.
Cen Y Liu J Qin Y Liu R Wang H Zhou Y et al Denervation in femoral artery-ligated hindlimbs diminishes ischemic recovery primarily via impaired arteriogenesis. PLoS One. (2016) 11(5):e0154941. 10.1371/journal.pone.0154941
32.
Farrehi PM Ozaki CK Carmeliet P Fay WP . Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. (1998) 97(10):1002–8. 10.1161/01.CIR.97.10.1002
33.
Tio RA Tkebuchava T Scheuermann TH Lebherz C Magner M Kearny M et al Intramyocardial gene therapy with naked DNA encoding vascular endothelial growth factor improves collateral flow to ischemic myocardium. Hum Gene Ther. (1999) 10(18):2953–60. 10.1089/10430349950016366
34.
Litvak J Siderides LE Vineberg AM . The experimental production of coronary artery insufficiency and occlusion. Am Heart J. (1957) 53(4):505–18. 10.1016/0002-8703(57)90359-9
35.
Tang GL Chang DS Sarkar R Wang R Messina LM . The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. (2005) 41(2):312–20. 10.1016/j.jvs.2004.11.012
36.
McClung JM McCord TJ Southerland K Schmidt CA Padgett ME Ryan TE et al Subacute limb ischemia induces skeletal muscle injury in genetically susceptible mice independent of vascular density. J Vasc Surg. (2016) 64(4):1101–11.e2. 10.1016/j.jvs.2015.06.139
37.
Padgett ME McCord TJ McClung JM Kontos CD . Methods for acute and subacute murine hindlimb ischemia. J Vis Exp. (2016) 21(112):54166. 10.3791/54166
38.
Yang S Chen B Luo T Tong Z Zhang S Shi X et al Preparation and evaluation of chronic hindlimb ischemia of Lewis rat. Chin J Bases Clin Gen Surg. (2012) 19(12):1291–7.
39.
Yang S Chen B Tong Z Zhang S Shi X Xu Q et al Mechanism of mesenchymal stem cells transplantation for chronic hindlimb ischemia in Lewis rat. Chin J Bases Clin Gen Surg. (2013) 20(06):608–14.
40.
Chen L Ding M Zhang Q Gao J Guo F Yang Z et al Making chronic ischemia rat models by high-fat diet and thread embolism technique: evaluation and comparison of modeling methods. Chin J Tissue Eng Res. (2019) 23(07):1063–7.
41.
Fletcher E Miserlis D Papoutsi E Steiner JL Gordon B Haynatzki G et al Chronic alcohol consumption exacerbates ischemia-associated skeletal muscle mitochondrial dysfunction in a murine model of peripheral artery disease. Biochim Biophys Acta Mol Basis Dis. (2025) 1871(2):167584. 10.1016/j.bbadis.2024.167584
42.
Fletcher E Miserlis D Sorokolet K Wilburn D Bradley C Papoutsi E et al Diet-induced obesity augments ischemic myopathy and functional decline in a murine model of peripheral artery disease. Transl Res. (2023) 260:17–31. 10.1016/j.trsl.2023.05.002
43.
Jia Y Li J Pan H Su Y Guan X Zhang B . Establishment of chronic limb ischemia model in rats and its comparison with acute chronic limb ischemia model. China J Gen Surg. (2011) 20(12):1347–50.
44.
Fan Y Wang L Guo X Feng G Li X . Lumbar plexus block with ropivacaine for hind limbs pain of chronic ischemia in rats. Chin J Clin Pharmacol Ther. (2018) 23(04):400–5.
45.
Yang Y Tang G Yan J Park B Hoffman A Tie G et al Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J Vasc Surg. (2008) 48(6):1546–58. 10.1016/j.jvs.2008.07.063
46.
Rajan DK Patel NH Valji K Cardella JF Brown DB Brountzos EN et al Quality improvement guidelines for percutaneous management of acute limb ischemia. J Vasc Interv Radiol. (2009) 20(7 Suppl):S208–18. 10.1016/j.jvir.2009.04.010
47.
Henke PK . Contemporary management of acute limb ischemia: factors associated with amputation and in-hospital mortality. Semin Vasc Surg. (2009) 22(1):34–40. 10.1053/j.semvascsurg.2009.01.002
48.
Liu Z Chen Y Du Z Zhu F Huang W . Ischemic postconditioning protects against acute kidney injury after limb ischemia reperfusion by regulating HMGB1 release and autophagy. Ren Fail. (2023) 45(1):2189482. 10.1080/0886022X.2023.2189482
49.
Yassin MM Harkin DW Barros D’Sa AA Halliday MI Rowlands BJ . Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. (2002) 26(1):115–21. 10.1007/s00268-001-0169-2
50.
de Carvalho EG Corsini W Hermes TA . Severe muscle damage after a short period of ischemia and reperfusion in an animal model. Surgery. (2023) 174(2):363–8. 10.1016/j.surg.2023.04.033
51.
Erol K Sozmen EY Küçük Ü Kucuk L . Effect of pheniramine maleate on rat skeletal muscle ischemia-reperfusion injury. Ulus Travma Acil Cerrahi Derg. (2022) 28(12):1667–73. 10.14744/tjtes.2021.00312
52.
Tu H Zhang D Qian J Barksdale AN Pipinos II Patel KP et al A comparison of acute mouse hindlimb injuries between tourniquet- and femoral artery ligation-induced ischemia-reperfusion. Injury. (2021) 52(11):3217–26. 10.1016/j.injury.2021.09.002
53.
Wang CL Wang Y Jiang QL Zeng Y Yao QP Liu X et al DNase I and sivelestat ameliorate experimental hindlimb ischemia-reperfusion injury by eliminating neutrophil extracellular traps. J Inflamm Res. (2023) 16:707–21. 10.2147/JIR.S396049
54.
Xue BB Chen BH Tang YN Weng CW Lin LN . Dexmedetomidine protects against lung injury induced by limb ischemia-reperfusion via the TLR4/MyD88/NF-κB pathway. Kaohsiung J Med Sci. (2019) 35(11):672–8. 10.1002/kjm2.12115
55.
Liu Z Zhu F Li W . Effects of taohong siwu decoction on expression of LncRNA H19 and NF-κB-p65 in skeletal muscle of rats with limb ischemia-reperfusion injury. Shaanxi Jo Tradit Chin Med. (2022) 43(09):1163–9.
56.
Inan B Sonmez Ergun S Nurten A Kucukgergin C Zengin Turkmen A Seckin S et al Effects of cilostazol and diltiazem hydrochloride on ischemia-reperfusion injury in a rat hindlimb model. Heart Surg Forum. (2017) 20(2):E058–65. 10.1532/hsf.1663
57.
Tuncay A Sivgin V Ozdemirkan A Sezen SC Boyunaga H Kucuk A et al The effect of cerium oxide on lung tissue in lower extremity ischemia reperfusion injury in sevoflurane administered rats. Int J Nanomed. (2020) 15:7481–9. 10.2147/IJN.S263001
58.
Cevirme D Adademir T Kafali Basaran E Savluk OF Elibol A Erkanli Senturk G et al Comparison between iloprost and alprostadil for protection against ischemia/reperfusion injury in a rat model. Turk J Med Sci. (2018) 48(3):661–9. 10.3906/sag-1710-74
59.
Cearra I Herrero de la Parte B Moreno-Franco DI García-Alonso I . A reproducible method for biochemical, histological and functional assessment of the effects of ischaemia-reperfusion syndrome in the lower limbs. Sci Rep. (2021) 11(1):19325. 10.1038/s41598-021-98887-9
60.
Kartal S Şen A Tümkaya L Erdivanlı B Mercantepe T Yılmaz A . The effect of dexmedetomidine on liver injury secondary to lower extremity ischemia-reperfusion in a diabetic rat model. Clin Exp Hypertens. (2021) 43(7):677–82. 10.1080/10641963.2021.1937204
61.
Mi L Zhang Y Xu Y Zheng X Zhang X Wang Z et al HMGB1/RAGE pro-inflammatory axis promotes vascular endothelial cell apoptosis in limb ischemia/reperfusion injury. Biomed Pharmacother. (2019) 116:109005. 10.1016/j.biopha.2019.109005
62.
Gokalp O Eygi B Gokalp G Kiray M Besir Y Iscan S et al Which distant organ is most affected by lower extremity ischemia-reperfusion? Ann Vasc Surg. (2020) 65:271–81. 10.1016/j.avsg.2020.01.008
63.
Deanfield JE Halcox JP Rabelink TJ . Endothelial function and dysfunction: testing and clinical relevance. Circulation. (2007) 115(10):1285–95. 10.1161/CIRCULATIONAHA.106.652859
64.
Kharbanda RK Peters M Walton B Kattenhorn M Mullen M Klein N et al Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. (2001) 103(12):1624–30. 10.1161/01.CIR.103.12.1624
65.
Wang C Zuo B Wu X . The role of macrophage migration inhibitory factor in remote ischemic postconditioning. Can J Cardiol. (2019) 35(4):501–10. 10.1016/j.cjca.2018.12.040
66.
Lang JA Kim J . Remote ischaemic preconditioning—translating cardiovascular benefits to humans. J Physiol. (2022) 600(13):3053–67. 10.1113/JP282568
67.
Helisch A Wagner S Khan N Drinane M Wolfram S Heil M et al Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. (2006) 26(3):520–6. 10.1161/01.ATV.0000202677.55012.a0
68.
van Weel V Toes RE Seghers L Deckers MM de Vries MR Eilers PH et al Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. (2007) 27(11):2310–8. 10.1161/ATVBAHA.107.151407
69.
Chalothorn D Clayton JA Zhang H Pomp D Faber JE . Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. (2007) 30(2):179–91. 10.1152/physiolgenomics.00047.2007
70.
Stabile E Burnett MS Watkins C Kinnaird T Bachis A la Sala A et al Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. (2003) 108(2):205–10. 10.1161/01.CIR.0000079225.50817.71
71.
Stabile E Kinnaird T la Sala A Hanson SK Watkins C Campia U et al CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. (2006) 113(1):118–24. 10.1161/CIRCULATIONAHA.105.576702
72.
Peng X Wang J Lassance-Soares RM Najafi AH Sood S Aghili N et al Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am J Physiol Heart Circ Physiol. (2011) 300(6):H2027–34. 10.1152/ajpheart.00004.2011
73.
Sieveking DP Lim P Chow RW Dunn LL Bao S McGrath KC et al A sex-specific role for androgens in angiogenesis. J Exp Med. (2010) 207(2):345–52. 10.1084/jem.20091924
74.
Zhuo Y Li SH Chen MS Wu J Kinkaid HY Fazel S et al Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg. (2010) 139(5):1286–94, 94.e1–2. 10.1016/j.jtcvs.2009.08.052
75.
Medgett IC Ruffolo RR Jr . Alpha adrenoceptor-mediated vasoconstriction in rat hindlimb: innervated alpha-2 adrenoceptors in the saphenous arterial bed. J Pharmacol Exp Ther. (1988) 246(1):249–54. 10.1016/S0022-3565(25)21010-2
76.
Greco A Ragucci M Liuzzi R Gargiulo S Gramanzini M Coda AR et al Repeatability, reproducibility and standardisation of a laser Doppler imaging technique for the evaluation of normal mouse hindlimb perfusion. Sensors (Basel). (2012) 13(1):500–15. 10.3390/s130100500
77.
Sfyri P Matsakas A . Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: emphasis on apolipoprotein E deficiency and peripheral arterial disease. J Biomed Sci. (2017) 24(1):42. 10.1186/s12929-017-0346-8
78.
Webster KA . Translational relevance of advanced age and atherosclerosis in preclinical trials of biotherapies for peripheral artery disease. Genes (Basel). (2024) 15(1):135. 10.3390/genes15010135
79.
Couffinhal T Silver M Kearney M Sullivan A Witzenbichler B Magner M et al Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation. (1999) 99(24):3188–98. 10.1161/01.CIR.99.24.3188
80.
Bogachkov YY Chen L Le Master E Fancher IS Zhao Y Aguilar V et al LDL induces cholesterol loading and inhibits endothelial proliferation and angiogenesis in matrigels: correlation with impaired angiogenesis during wound healing. Am J Physiol Cell Physiol. (2020) 318(4):C762–76. 10.1152/ajpcell.00495.2018
Summary
Keywords
animal disease models, hindlimb ischemia, ischemia-reperfusion, murine models, peripheral arterial disease
Citation
Zhang Y, Du H, Ran Q, Xu J, Tang X, Wang Y, Deng J and Cao Y (2026) Murine hindlimb ischemia models: a narrative review. Front. Cardiovasc. Med. 12:1700963. doi: 10.3389/fcvm.2025.1700963
Received
08 September 2025
Revised
06 December 2025
Accepted
16 December 2025
Published
09 January 2026
Volume
12 - 2025
Edited by
DeLisa Fairweather, Mayo Clinic Florida, Jacksonville, United States
Reviewed by
Monica Rodríguez-Valiente, Hospital Virgen de la Arrixaca, Spain
Muhamad Taufik Ismail, Gadjah Mada University, Indonesia
Updates
Copyright
© 2026 Zhang, Du, Ran, Xu, Tang, Wang, Deng and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yuzhen Wang wangyuzhen@shutcm.edu.cn Junlin Deng dr_djl@163.com Yemin Cao caoyemin@shutcm.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.