- 1Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
- 2Center of Excellence in Biotechnology Research (CEBR), King Saud University, Riyadh, Saudi Arabia
Background: Hypertension prevalence is high in Saudi Arabia. Both lifestyle behaviors and biological intermediates influence blood pressure (BP); however, there is limited evidence from the Middle East on whether composite lifestyle scores that integrate both domains would capture BP variation better than behavioral metrics alone. This study aimed to examine associations of an overall lifestyle risk score (behavioral + biological) and a behavioral-only score with BP outcomes.
Methods: In a cross-sectional study of Saudi adults (n = 1,041), we constructed (i) an overall lifestyle score (BioBeh score), including waist circumference, total cholesterol, fasting glucose, smoking, sleep, physical activity, perceived stress, and diet quality, and (ii) a behavioral-only score (Beh score), including smoking, sleep, physical activity, perceived stress, and diet. We examined the association with BP [systolic/diastolic BP (SBP/DBP)], elevated BP, and hypertension using multivariable linear and logistic regression, adjusted for various confounders.
Results: Each 1-point higher BioBeh score was associated with lower SBP (−1.68 mmHg; 95% CI: −2.58, −0.79), lower DBP (−1.67 mmHg; 95% CI: −2.66, −0.68), and reduced odds of hypertension (OR 0.73; 95% CI: 0.63, 0.85), but not with elevated BP. No associations were found with the Beh score. Waist circumference was the strongest individual component [SBP −3.80 (−4.70, −2.91); DBP −3.31 (−4.30, −2.32) mmHg; hypertension OR 0.57; 95% CI: 0.49, 0.66].
Conclusions: In Saudi adults, a biologically enriched lifestyle score captures BP variation and hypertension risk more robustly than behavior-only metrics. These findings support risk stratification and prevention strategies that emphasize central adiposity alongside basic lipid and glucose profiles.
1 Introduction
Hypertension remains one of the leading preventable causes of premature morbidity and mortality worldwide (1). The number of people living with hypertension worldwide [blood pressure (BP) of ≥140 mmHg systolic or ≥90 mmHg diastolic or on medication (2)] increased between 1990 and 2019, rising from 650 million to 1.3 billion individuals (3). A substantial burden also exists in Saudi Arabia, mirroring this global trend (4, 5). A systematic review and meta-analysis of 29 studies reported hypertension prevalence ranging from 15% to 33% in national community samples from Saudi Arabia (4). National household survey data suggest that 9% of individuals aged 15 years and older had hypertension, with prevalence ranging from 6% to 10% across Saudi regions and increasing drastically with age (6). Likewise, the World Health Organization (WHO) 2023 hypertension country profile for Saudi Arabia reported gaps in diagnosis and management (5), which were echoed in the systematic review and meta-analysis showing generally low rates of awareness, treatment, and control (4). These findings highlight the need for improved national hypertension prevention and screening strategies (5). The WHO Global Report on Hypertension reiterated the consequences of missed opportunities for prevention, detection, and treatment and called for population-level strategies that target modifiable risk factors (7). As such, comprehensive lifestyle modification is now emphasized as a cornerstone of BP prevention and management across the life course in current guidelines (8).
Evidence has shown that lifestyle behaviors related to diet quality, physical activity, tobacco smoking, sleep, and psychosocial stress are associated with BP levels and hypertension risk (9–15). This is particularly evident in Saudi Arabia, which is undergoing rapid urbanization accompanied by lifestyle and dietary transitions (16), including adapting Western-style dietary habits, with an increased availability and intake of energy-dense processed foods and sugar-sweetened beverages (17). This is combined with a general trend toward physical inactivity, with recent national and policy analyses indicating that only about 30% of Saudi adults meet the recommended physical activity levels (18).
Researchers have combined behavioral and biological factors to develop composite lifestyle scores rather than relying on single exposures to reflect the joint effects across correlated behaviors and biological factors. These lifestyle scores provide a single summary metric of cardiovascular health and improve risk stratification relevant for prevention and clinical screening (19). Frameworks such as the American Heart Association (AHA) Life's Essential 8 score have formalized the joint contributions of behaviors (diet, physical activity, nicotine exposure, sleep) and biological factors (adiposity, lipids, glucose, BP) to cardiovascular health, with higher scores associated with lower incident hypertension and better cardiovascular outcomes (19, 20). Building on that concept, in prior work, we developed a BP-specific composite that began with a “basic” score [waist circumference (WC), smoking, total cholesterol] and then added additional lifestyle components sequentially to test whether broader coverage improved prediction performance (21). We applied this tool to the UK Airwave cohort of >40,000 adults and demonstrated that higher lifestyle scores were associated with lower systolic and diastolic BP and lower odds of hypertension (21). Although the tool showed promise in the UK cohort, its applicability in other regions has not been examined (21). Region-specific investigation is warranted in Saudi Arabia, given rapid urbanization, dietary transition, physical inactivity, and high central adiposity, in addition to persistent gaps in awareness, treatment, and control. Findings from such investigations can also guide the development of low-cost screening tools (behavior only) as compared to enhanced screeners (combining anthropometry and biological indices) to inform targeted prevention strategies in the Saudi context.
Similar a priori lifestyle scores that combine behavioral and biological components are evolving in other Middle Eastern settings for related outcomes (e.g., obesity phenotypes). These studies demonstrate that the components can be operationalized using routinely collected data in the region and that the resulting scores yield gradients across clinically meaningful phenotypes, feasible to implement and straightforward to interpret (22). Despite the high cardiometabolic burden in the region (23), no research has yet applied our BP lifestyle risk score or any comparable score in Saudi Arabia. Most local studies have focused on prevalence related to a single risk factor, rather than identifying a combined lifestyle/biological phenotype (4–6). The objective of the present study is to investigate the association of a behavioral-only lifestyle risk score [(Beh score) including smoking, physical activity, sleep, diet, and stress] and a combined score of behavioral and biological indices [(BioBeh score) including WC, fasting blood glucose, and total cholesterol] with systolic and diastolic BP and the odds of hypertension among Saudi adults. This design allows quantification of the incremental value of biological intermediates beyond behaviors, clarifying the relative and joint contributions of lifestyle behaviors and their biological correlates to BP profiles in a Middle Eastern population. Risk-stratified results can be operationalized locally by prioritizing individuals with unfavorable metabolic phenotypes for intensive lifestyle counseling, closer BP monitoring (including ambulatory BP), and timely pharmacologic evaluation, while those with favorable profiles receive routine follow-up.
2 Methods
2.1 Study design
This cross-sectional study was conducted in Riyadh and Almadinah, Saudi Arabia to examine the lifestyle factors associated with BP in Saudi adults. The study was approved by the KSU Institutional Review Board (KSU-IRB-21-314). All participants provided written informed consent before enrollment.
The present work used data to construct two lifestyle scores: lifestyle score-BioBeh and lifestyle score-Beh, following previously published lifestyle-score concepts, including the one developed by our team (20, 21). The scores were then examined in terms of their relationship to BP and prevalent hypertension.
2.2 Participants
Recruitment invitations were distributed via social media platforms, local primary care clinics, and King Saud University (KSU) email lists to invite staff, students, and their family members to participate. Eligible participants were Saudi nationals aged ≥18 years. Exclusion criteria included pregnancy, lactation, malignancy, chronic renal or hepatic failure, malabsorption syndromes, incomplete data, and current use of glucocorticoids or weight loss medications. Interviewers were trained to review each questionnaire item in real time and prompt participants to complete missing answers. Participants with missing data on blood pressure, any lifestyle score component, or key covariates were excluded. All analyses used complete cases only (Supplementary Figure S1).
Participants completed two encounters. First, screening and on-site assessment occurred at KSU portable clinics set up in shopping malls and at the study labs in the cities of Riyadh and Almadinah. After consent was obtained, trained interviewers collected standardized anthropometric information. In addition, interviewer-administered questionnaires captured sociodemographic data, health history, diet, physical activity, sleep, and perceived stress. Second, participants attended scheduled fasting laboratory visits at private medical laboratories for blood collection.
2.3 Sample size calculation
Using Saudi estimates of hypertension prevalence of approximately 14% and targeting a detectable odds ratio (OR) of 1.30 (6) per 1-SD higher lifestyle risk score, with 80% power (β = 0.20) and two-sided α = 0.05, the standard formula for a continuous predictor in logistic regression indicated n = 900 participants. Allowing for 10% dropout for incomplete data yielded a target of n = 1,000.
2.4 Lifestyle score development
Data were collected using structured interviewer-administered questionnaires, dietary recalls, clinical blood sampling, BP measurements, and standardized anthropometry measures, briefly described in the following.
BioBeh score variables included WC, total serum cholesterol, fasting blood glucose, smoking, sleep duration, physical activity, perceived stress, and diet quality. Beh score variables included only smoking, physical activity, sleep, diet, and perceived stress.
2.4.1 Data collection: lifestyle (behavioral) measures
2.4.1.1 Physical activity, sleep, and stress
Physical activity was assessed using the Arabic Global Physical Activity Questionnaire (GPAQ-v2), with total activity calculated as minutes per week (24). Sleep (hours/24 h) was recorded using the validated Arabic version of the Pittsburgh instrument (25). Perceived stress was assessed using the validated Arabic version of the Cohen Perceived Stress Scale (PSS-10) (26), with higher scores indicating elevated stress.
2.4.1.2 Dietary intake and quality
Usual dietary intake was assessed using a Saudi-specific food frequency questionnaire (FFQ) of 133 foods and beverages, including traditional Saudi dishes (27). Participants also completed two non-consecutive 24-h dietary recalls: one at recruitment and one by telephone using the multiple-pass method and standard food models (at recruitment) to estimate portion sizes (28). Trained dietitians administered the 24-h recalls. Dietary data from both the FFQ and recalls were analyzed using ESHA Food Processor SQL Software (ESHA Research, Salem, OR, USA). When traditional Saudi recipes were not available in the database, the team added them from a classic Saudi cookbook and, when needed, reached consensus on the most appropriate classification for items without a direct match in the food composition tables. Correlations between dietary macronutrients (e.g., protein and fat) from the FFQ and the mean of the two 24-h recalls were high (r = 0.52 and 0.61, respectively), indicating good agreement between the two dietary assessment methods in assessing participants' intakes.
Dietary data from the two 24-h recalls were used to assess diet quality as quantified by the Nutrient-Rich Food 9.3 (NRF9.3) index (29). The NRF9.3 score is a validated nutrient profiling score that evaluates overall nutritional quality of foods or diets by summing nine “nutrients to encourage” (i.e., protein, fiber, vitamins A, C, and E, calcium, iron, potassium, and magnesium) and subtracting three “nutrients to limit” (i.e., saturated fat, added sugars, and sodium) per 100 kcal. A higher NRF9.3 score indicates better nutritional quality per 100 kcal.
2.4.2 Data collection: clinical (biological) measures
2.4.2.1 Biological samples
Fasting blood samples were collected for fasting plasma glucose (FPG) and total cholesterol using standard enzymatic methods (30) at the study laboratories. About 10 mL of blood was drawn after an 8–10 h fast and immediately processed (centrifuged at 1,200 × g; plasma/serum aliquoted and stored at −80 °C). All biochemistry analyses were completed in a single lab visit, and results were provided to participants.
2.4.2.2 Anthropometric measures
Anthropometry was measured by trained staff following a standard study protocol. Participants were asked to remove shoes and heavy clothing. Weight was recorded to the nearest 0.1 kg (InBody 570, South Korea) and height to the nearest 0.5 cm (Seca 274, Germany). The body mass index (BMI) was then calculated by dividing an individual's weight in kilograms by the square of their height in meters. WC was measured at the midpoint between the lowest rib and the iliac crest using a non-stretch fiberglass tape (nearest 0.5 cm) (31). All measures were collected twice, and if readings differed by >0.5 kg (weight) or >0.5 cm, a third reading was taken, and the mean of the two closest values was used.
2.4.2.3 Blood pressure
BP was measured by trained staff in a quiet room. Participants were asked to avoid smoking, caffeine, and exercise for 30 min, to empty the bladder, and to sit quietly for 3–5 min with feet flat on the floor (2). Using the Omron M6 Comfort device, three consecutive readings were collected (2), and the mean of the readings was used in the analysis. Following the guidelines of the American College of Cardiology/American Heart Association (ACC/AHA), elevated BP was defined as a systolic BP (SBP) ≥ 120 mm Hg and a diastolic BP (DBP) < 80 mmHg, while hypertension was defined as SBP ≥130 mmHg or DBP ≥80 mmHg (2).
2.4.3 Scoring framework
Building on previous work for score development (21), we classified each component as poor, intermediate, or ideal and assigned scores of 0, 1, or 2 points for risk, respectively, so that higher totals indicated lower risk. Here, “poor, intermediate, and ideal” refer to a priori health levels for each component defined from established clinical and public health thresholds: behaviors follow AHA Impact Goals; WC uses sex/ethnicity-specific cut-offs; physical activity uses AHA assessment guidance; sleep uses the American Academy of Sleep Medicine consensus recommendations; stress follows the PSS-10 questionnaire; and diet quality uses published NRF9.3 categories from a comparable UK sample. Exact thresholds for each component are provided in Table 1.
2.5 Statistical analysis
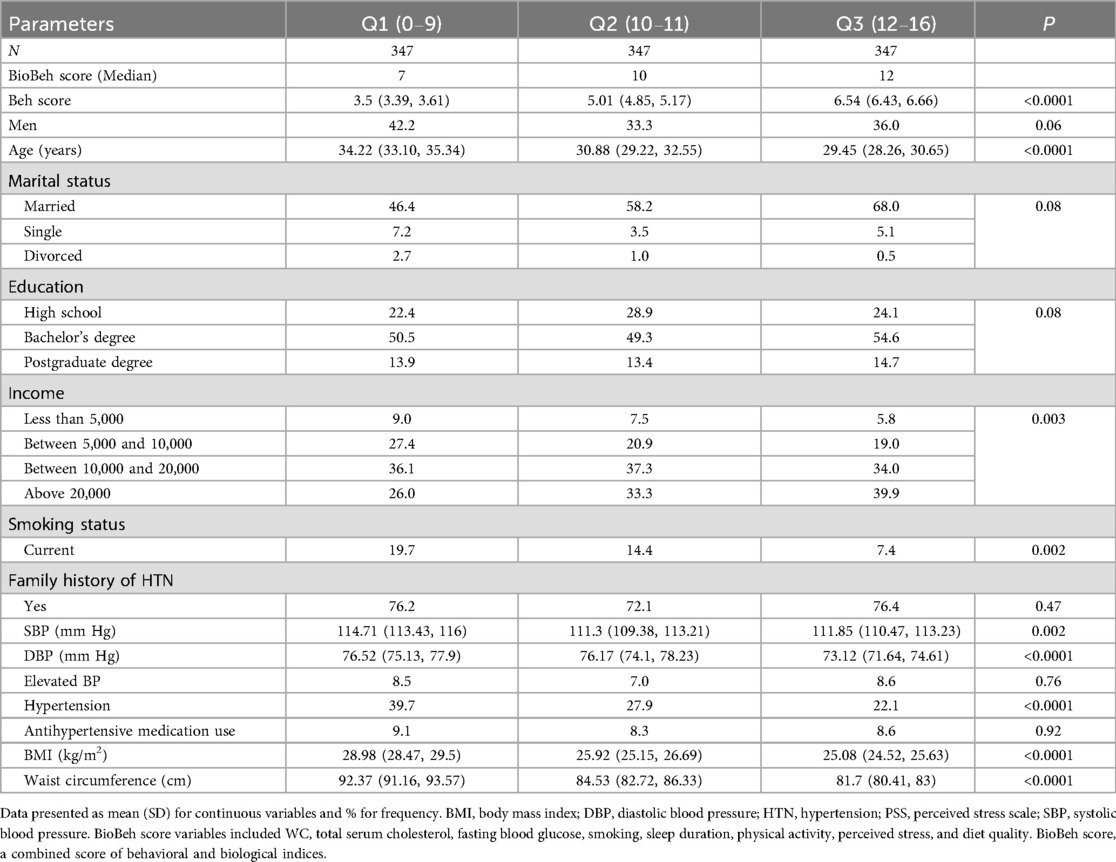
Continuous variables are presented as mean (SD) and categorical variables as percentages. Participants were stratified into tertiles of the BioBeh score using the observed distribution (Q1: 0–9, Q2: 10–11, Q3: 12–16). These tertiles are empirical and were used for descriptive purposes, consistent with common epidemiologic practice when no validated clinical cut points exist for a continuous or composite exposure. Differences across tertiles were evaluated with generalized linear models (PROC GLM) for continuous variables and χ2 tests for categorical variables.
Two exposure constructs were examined: (i) the overall lifestyle risk score that includes biological and behavioral factors [BioBeh score, range (0–16)] and (ii) the behavioral-only health score [Beh score, range (0–10)]. For all scores and components, coding was consistent so that higher values reflected healthier levels.
Associations of lifestyle scores (BioBeh, Beh, and each component) with SBP and DBP were estimated using multivariable linear regression and expressed as mean differences (95% CI) per 1-point higher score. Associations of the scores with elevated BP and hypertension were estimated using logistic regression and expressed as odds ratios (ORs, 95% CI) per 1-point higher score. The lifestyle scores were entered as continuous variables on a per 1-point scale, assuming an approximately linear association over the observed range. We report two models. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for marital status, income, educational level, family history of hypertension, and antihypertensive medication use. These covariates were selected a priori based on plausible confounding reported in the literature. We treated the lifestyle score components themselves (WC, FPG, total cholesterol, smoking, physical activity, diet, sleep, and perceived stress) as exposures rather than adjustment variables to avoid overadjustment for intermediates on the causal pathway (35–39). Statistical significance was defined as a two-sided p < 0.05. Analyses were performed using SAS 9.3.
3 Results
3.1 Demographic and lifestyle characteristics
In total, n = 1,041 Saudi adults [31.8 (12.2) years] were included in the analyses, 38.1% of whom were males. The mean SBP/DBP was 111.5 (15.7)/75.0 (15.3) mmHg (Supplementary Table S1). The BioBeh score averaged 10.0 (2.0) points, whereas the Beh score averaged 5.0 (2.0) points.
Participants with higher BioBeh scores displayed a more favorable cardiometabolic profile, including lower BMI and WC, lower BP, and lower prevalence of hypertension compared to participants with lower scores (Table 1). In contrast, the prevalence of elevated BP was similar across score groups.
3.2 Association between lifestyle scores and BP
A 1-unit higher BioBeh score was significantly associated with lower SBP/DBP (Table 2). In fully adjusted models (Model 2), SBP was lower by 1.68 mmHg (95% CI: −2.58, −0.79) and DBP by 1.67 mmHg (95% CI: −2.66, −0.68).
In contrast, the Beh score was not significantly associated with either SBP or DBP in adjusted models. Component-wise, WC score showed the largest inverse association (lower WC score = higher WC measure) with both SBP [−3.80 (95% CI: −4.70, −2.91)] and DBP [−3.31 (95% CI: −4.30, −2.32)] mm Hg; fasting glucose and total cholesterol (both reverse-coded) were related inversely to SBP at −1.82 (95% CI: −2.67, −0.97) and −1.07 (95% CI: −0.22, −1.91), respectively. Sleep, physical activity, perceived stress, smoking, and diet quality were not associated with BP after adjustment.
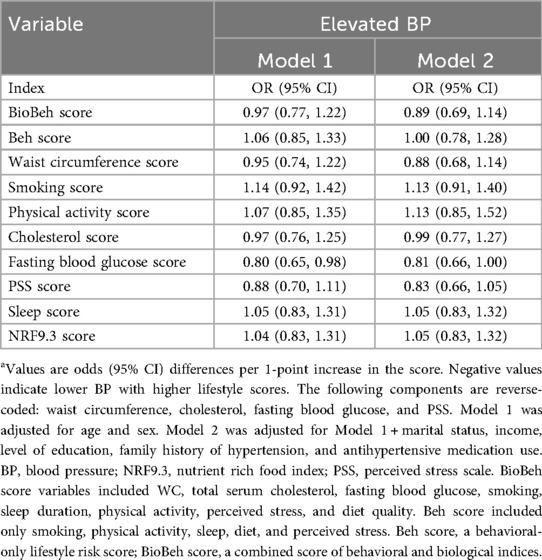
Neither the BioBeh score nor the Beh score showed a statistically significant association with elevated BP in either model (Table 3). Only borderline 19% lower odds of elevated BP were observed for higher fasting glucose score.
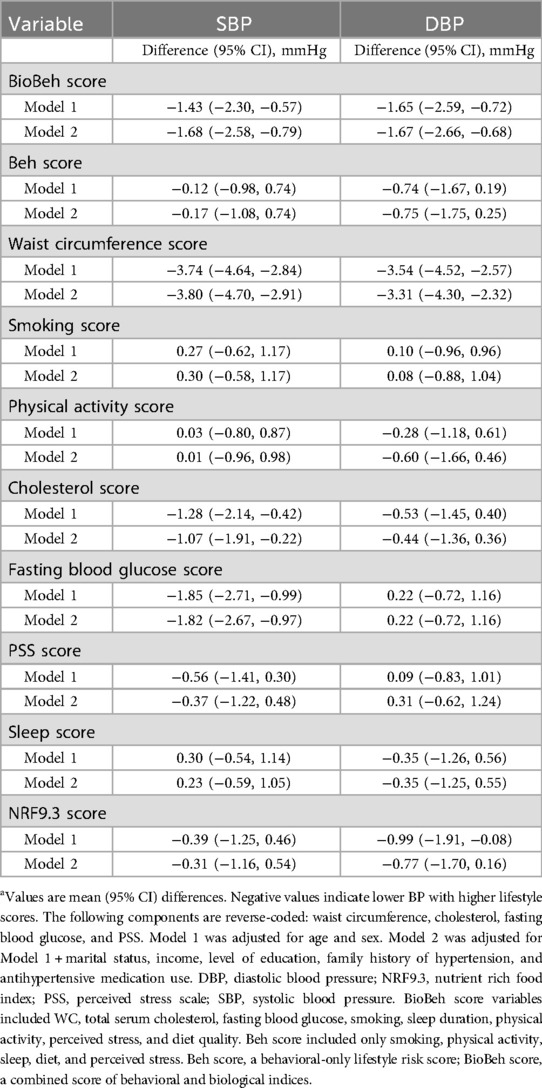
Table 3. Associations of lifestyle risk scores and components with systolic and diastolic blood pressure (n = 1,041).a
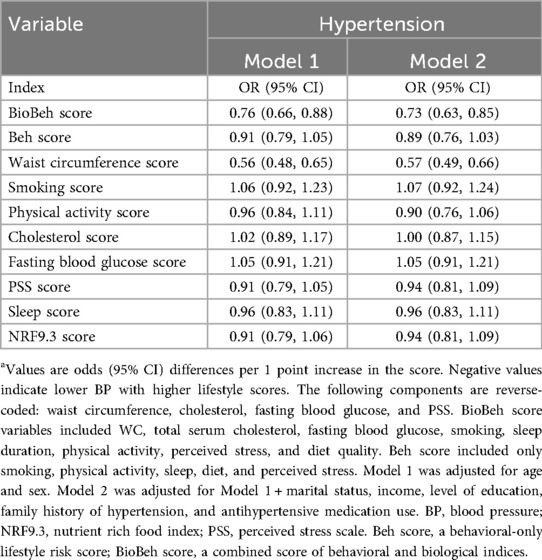
In fully adjusted models (Model 2), each one-point increase in the BioBeh score was associated with a 27% lower odds of hypertension (OR: 0.73; 95% CI: 0.63, 0.85). In contrast, the behavioral-only lifestyle risk score was not significantly associated with hypertension (OR: 0.89; 95% CI: 0.76, 1.03) (Table 4). Among individual components, the waist circumference score was associated with 43% lower odds of hypertension [OR 0.57 (95% CI: 0.49, 0.66)].
4 Discussion
4.1 Principal findings
In this sample of Saudi adults, a higher overall lifestyle score that includes behavioral and biological factors was associated with lower SBP and DBP and lower odds of hypertension. In contrast, a behavior-only score showed no significant relationship with any BP outcomes. Among individual components, WC, a measure of central adiposity (10), showed the strongest association with BP outcomes. This pattern is expected in cross-sectional analyses because biological intermediates (central adiposity, lipids, and fasting glucose) are more proximal to BP pathophysiology and are measured more objectively than self-reported behaviors, which carry greater measurement error. Our study demonstrates this gradient in a Middle Eastern cohort and shows that a biologically enriched composite captures BP and hypertension more strongly than a behavior-only score, extending prior UK Airwave findings (21) to a new population and highlighting central adiposity as a principal driver.
4.2 Comparison with prior literature
Our findings align with and add geographic diversity to evidence that biologically enriched composite lifestyle phenotypes relate meaningfully to BP. In our data, a behavior-only score was not associated with SBP/DBP or hypertension, while a score that includes biological intermediates was inversely related to both outcomes. In the UK Airwave cohort (>40,000 adults), each 1-point increase in a “basic” lifestyle score (WC, smoking, total cholesterol) was associated with ∼2 mmHg lower SBP and DBP and ∼28% lower odds of hypertension. Adding sleep, physical activity, and diet modestly attenuated but did not abolish the associations (21). Our study demonstrates a similar pattern with stronger associations for a score that includes biological intermediates, and specifically highlights central adiposity as a major driver, consistent with the Airwave study. Differences in the magnitude of effects between the current and previous study are expected, given population composition (age 31.8 vs. 40.5 years, respectively), lifestyle score definitions, and hypertension threshold (we used ACC/AHA ≥130/80 mmHg; the UK Airwave study used ≥140/90 mmHg or diagnosis/antihypertensive medication) (21). The Airwave “basic” score comprised WC, smoking, and total cholesterol with sleep, physical activity, diet, and alcohol added sequentially. Our BioBeh score included WC, total cholesterol, fasting glucose, smoking, sleep, physical activity, perceived stress, and diet. We also examined a behavior-only score (smoking, sleep, physical activity, perceived stress, and diet).
Our results are also consistent with broader frameworks, such as the AHA's Life's Essential 8, which emphasizes joint contributions of behaviors (diet, physical activity, tobacco exposure, and sleep) and biological factors (adiposity, lipids, glucose, and BP) to cardiovascular health and incident hypertension (20). Randomized controlled trials and meta-analyses further support that healthy dietary patterns and sodium reduction lower BP (12, 13). However, in cross-sectional analyses, self-reported behaviors like the ones reported in our study are measured with more error and reflect only a moment in time. Biological markers, such as WC, lipid levels, and fasting glucose, show the accumulated effects of lifestyle over time and are measured more objectively. As a result, composite scores that include these biological markers tend to show stronger and clearer associations with BP than scores based only on reported behaviors (20).
We observed no associations for elevated BP but robust associations for hypertension. Several factors may explain this finding. The ACC/AHA criteria for elevated BP (SBP 120–129 mmHg/DBP < 80 mmHg) define a narrower phenotype that is less prevalent in younger samples, limiting power to detect associations. Furthermore, behavioral and biological exposures may exert stronger effects on diastolic BP captured within the hypertension definition (≥130/80 mmHg) than on isolated systolic elevation with normal DBP (40).
4.3 Potential mechanisms
Excess visceral and central adiposity elevates BP through various pathways, including activation of the sympathetic nervous system and renin–angiotensin–aldosterone system, renal sodium retention, endothelial dysfunction, and low-grade inflammation (41, 42). Dyslipidemia may relate to higher SBP partly through arterial stiffness, with total cholesterol acting as a proxy for adverse lipid profile and vascular remodeling (9, 43). Improved fasting glucose levels are also linked to lower BP through reduced insulin resistance, improved endothelial function, and attenuation of sodium retention (43, 44). The superiority of the WC component in our findings is therefore biologically consistent and reflects large cohort evidence that central adiposity is strongly linked to BP and incident hypertension even among individuals with normal BMI.
4.4 Public health and clinical implications
Our results support risk screening strategies that emphasize central adiposity. In primary care and community settings, WC, an inexpensive, portable measure, can be used for first-level assessment. Where recent basic labs (fasting glucose and a lipid profile) are already available from routine care, these data can help prioritize individuals for intensive lifestyle counseling and early BP management (45, 46), complementing current guideline recommendations that encourage comprehensive lifestyle modification at the core of care (8, 20).
The pattern we observed (behavior-only score null; biologically enriched score associated with BP) is consistent with behaviors exerting their effects through biological intermediates. Although diet quality and physical activity were not independently related to BP in our models, we include them here as modifiable factors with trial evidence for lowering BP and central adiposity. For management, it is more precise to target central adiposity (WC) rather than “weight” alone. Effective strategies include sustained energy deficit achieved through a dietary pattern rich in fiber and minimally processed foods, with restricted intake of added sugars, refined carbohydrates; regular physical activity; and counseling on adequate sleep and stress management. Collectively, these strategies preferentially reduce visceral adiposity and WC and are feasible within routine primary care counseling.
4.5 Strengths and limitations
The cross-sectional design prevents causal inference and is subject to reverse causation. It is possible that individuals with higher BP may have altered their behaviors, which could attenuate observed associations for the behavioral-only score. Conversely, elevated or undiagnosed hypertension, or related comorbid conditions, may worsen perceived stress, sleep, and some health behaviors. Furthermore, self-reported behaviors are prone to recall error and misclassification. In addition, participants were recruited using convenience sampling through social media, university email lists, primary care clinics, and mall-based portable clinics in Riyadh and Almadinah. This approach likely selected younger, more educated, and higher-income adults with greater health awareness. Therefore, findings may not be generalizable to the broader Saudi population, particularly those living in rural areas or with lower socioeconomic status. Despite extensive multivariable adjustment for sociodemographic and other factors (e.g., age, sex, marital status, education, and income), residual confounding cannot be excluded, including unmeasured or imprecisely measured factors such as lipid-lowering therapy, sodium intake, and occupational stress (47). Because BP was recorded at a single visit, we could not capture visit-to-visit variability or white-coat effects, so some regression dilution and imprecision may remain. Finally, we did not develop or validate a prediction model, so measures such as ROC curves or AUC are beyond the scope of this analysis; future work should formally assess the discrimination and calibration of lifestyle-based scores for hypertension risk.
Strengths of this study include repeated BP measurement (three readings averaged with an automated device), use of validated tools in Arabic for physical activity, sleep, and perceived stress, and a detailed dietary assessment that combined recalls and a nutrient density index. The lifestyle scores were specified a priori from established frameworks and our prior work, but with adaptations for this setting: The behavior-only score included smoking, physical activity, sleep, diet, and perceived stress; the overall (bio + behavior) score additionally included WC, total cholesterol, and fasting glucose.
Prospective validation is needed to test whether the composite score predicts incident hypertension and BP trajectories in Saudi adults and to examine whether component weighting (e.g., giving central adiposity greater weight) improves discrimination. Implementation studies should assess whether including this phenotype in screening programs improves detection and BP control, as highlighted by the WHO's 2023 report (7).
5 Conclusion
A lifestyle risk score that integrates biological intermediates, particularly WC, captures meaningful variation in BP and hypertension in Saudi adults, whereas behavior-only scores show weaker associations. These findings underscore the role of central adiposity and related metabolic factors as key targets for prevention and risk stratification in this population. Policies and clinical programs that directly reduce central adiposity and improve lipid and glucose profiles may yield the greatest population-level reductions in BP.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of King Saud University (KSU-IRB-21-314). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing – original draft. SA-M: Funding acquisition, Methodology, Resources, Writing – review & editing. FA: Data curation, Formal analysis, Investigation, Writing – review & editing. TM: Conceptualization, Investigation, Methodology, Writing – review & editing. MAb: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. MAl: Data curation, Formal analysis, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. We acknowledge the Ongoing Research Funding Program number (ORF-2025-559), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1713086/full#supplementary-material
References
1. Brauer M, Roth GA, Aravkin AY, Zheng P, Abate KH, Abate YH, et al. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403(10440):2162–203. doi: 10.1016/S0140-6736(24)00933-4
2. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. (2019) 73(5):e35–66. doi: 10.1161/HYP.0000000000000087
3. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/S0140-6736(21)01330-1
4. Alshammari SA, Alshammari AS, Alshammari HS, Ahamed SS. Overview of hypertension in Saudi Arabia: a systematic review and meta-analysis. Saudi Med J. (2023) 44(10):951–64. doi: 10.15537/smj.2023.44.10.20230178
6. Alenazi AM, Alqahtani BA. National and regional prevalence rates of hypertension in Saudi Arabia: a descriptive analysis using the national survey data. Front Public Health. (2023) 11:1092905. doi: 10.3389/fpubh.2023.1092905
7. WHO. Global Report on Hypertension: The Race Against a Silent Killer. Geneva: World Health Organization (2023).
8. Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. (2023) 41(12):1874–2071. doi: 10.1097/HJH.0000000000003480
9. Chen H, Chen Y, Wu W, Cai Z, Chen Z, Yan X, et al. Total cholesterol, arterial stiffness, and systolic blood pressure: a mediation analysis. Sci Rep. (2021) 11(1):1330. doi: 10.1038/s41598-020-79368-x
10. Cheng C, Sun J-Y, Zhou Y, Xie Q-Y, Wang L-Y, Kong X-Q, et al. High waist circumference is a risk factor for hypertension in normal-weight or overweight individuals with normal metabolic profiles. J Clin Hypertens (Greenwich). (2022) 24(7):908–17. doi: 10.1111/jch.14528
11. Liu M-Y, Li N, Li WA, Khan H. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol Res. (2017) 39(6):573–80. doi: 10.1080/01616412.2017.1317904
12. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. (2001) 344(1):3–10. doi: 10.1056/NEJM200101043440101
13. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of dietary approaches to stop hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. (2014) 24(12):1253–61. doi: 10.1016/j.numecd.2014.06.008
14. St-Onge M-P, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. (2016) 134(18):e367–e86. doi: 10.1161/CIR.0000000000000444
15. Li J, Matthews TA, Clausen T, Rugulies R. Workplace discrimination and risk of hypertension: findings from a prospective cohort study in the United States. J Am Heart Assoc. (2023) 12(9):e027374. doi: 10.1161/JAHA.122.027374
16. United Nations Interagency Task Force on the Prevention and Control of Noncommunicable Diseases. The Investment Case for Noncommunicable Disease Prevention and Control In the Kingdom of Saudi Arabia: Return on Investment Analysis & Institutional and Context Analysis, August 2017. Geneva: World Health Organization (2018). (WHO/NMH/NMA/17.55). License CC BY-NC-SA 3.0 IGO
17. Al-Hanawi MK, Ahmed MU, Alshareef N, Qattan AMN, Pulok MH. Determinants of sugar-sweetened beverage consumption among the Saudi adults: findings from a nationally representative survey. Front Nutr. (2022) 9:744116. doi: 10.3389/fnut.2022.744116
18. Alzahrani AA, Gelius P, Bauman AE, Gebel K. Physical activity policies in Saudi Arabia and Oman: a qualitative study using stakeholder interviews. Health Res Policy Syst. (2024) 22(1):111. doi: 10.1186/s12961-024-01192-w
19. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146(5):e18–43. doi: 10.1161/CIR.0000000000001078
20. Plante TB, Koh I, Judd SE, Howard G, Howard VJ, Zakai NA, et al. Life’s simple 7 and incident hypertension: the REGARDS study. J Am Heart Assoc. (2020) 9(19):e016482. doi: 10.1161/JAHA.120.016482
21. Aljuraiban GS, Gibson R, Chan DSM, Elliott P, Chan Q, Griep LMO. Lifestyle score and risk of hypertension in the airwave health monitoring study of British police force employees. Int J Environ Res Public Health. (2023) 20(5):4029. doi: 10.3390/ijerph20054029
22. Ebrahimi S, Shiraseb F, Ladaninezhad M, Izadi A, Navaei N, Mirzaei K. The association between the lifestyle risk score and metabolically healthy and unhealthy obesity phenotype in Iranian women with overweight and obesity: a cross-sectional study. Front Public Health. (2025) 13:1490937. doi: 10.3389/fpubh.2025.1490937
23. Alhuneafat L, Ta'ani OA, Jabri A, Tarawneh T, ElHamdan A, Naser A, et al. Cardiovascular disease burden in the Middle East and North Africa region. Curr Probl Cardiol. (2024) 49(3):102341. doi: 10.1016/j.cpcardiol.2023.102341
24. Doyle C, Khan A, Burton N. Reliability and validity of a self-administered Arabic version of the Global Physical Activity Questionnaire (GPAQ-A). J Sports Med Phys Fitness. (2019) 59(7):1221–8. doi: 10.23736/S0022-4707.18.09186-7
25. Suleiman KH, Yates BC, Berger AM, Pozehl B, Meza J. Translating the Pittsburgh sleep quality index into Arabic. West J Nurs Res. (2010) 32(2):250–68. doi: 10.1177/0193945909348230
26. Chaaya M, Osman H, Naassan G, Mahfoud Z. Validation of the Arabic version of the Cohen perceived stress scale (PSS-10) among pregnant and postpartum women. BMC Psychiatry. (2010) 10(1):111. doi: 10.1186/1471-244X-10-111
27. Samman S, Herbert J, Petocz P, Lyons-Wall PM. Development and validation of a short questionnaire for estimating the intake of zinc. Biol Trace Elem Res. (2010) 134(2):226–34. doi: 10.1007/s12011-009-8466-y
28. Rhodes DG, Murayi T, Clemens JC, Baer DJ, Sebastian RS, Moshfegh AJ. The USDA automated multiple-pass method accurately assesses population sodium intakes. Am J Clin Nutr. (2013) 97(5):958–64. doi: 10.3945/ajcn.112.044982
29. Fulgoni VL, Keast DR, Drewnowski A. Development and validation of the nutrient-rich foods index: a tool to measure nutritional quality of foods. J Nutr. (2009) 139(8):1549–54. doi: 10.3945/jn.108.101360
30. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. (2023) 69(8):808–68. doi: 10.1093/clinchem/hvad080
31. WHO. Waist Circumference and Waist-Hip Ratio Report of a WHO Expert Consultation. Geneva: World Health Organization (2008).
32. Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. (2010) 64(1):42–61. doi: 10.1038/ejcn.2009.70
33. Panel CC. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. (2015) 38(6):843–4. doi: 10.5665/sleep.4716
34. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54(24):1451–62. doi: 10.1136/bjsports-2020-102955
35. Metlock FE, Hinneh T, Benjasirisan C, Alharthi A, Ogungbe O, Turkson-Ocran R-AN, et al. Impact of social determinants of health on hypertension outcomes: a systematic review. Hypertension. (2024) 81(8):1675–700. doi: 10.1161/HYPERTENSIONAHA.123.22571
36. Ramezankhani A, Azizi F, Hadaegh F. Associations of marital status with diabetes, hypertension, cardiovascular disease and all-cause mortality: a long term follow-up study. PLoS One. (2019) 14(4):e0215593. doi: 10.1371/journal.pone.0215593
37. Rasooly D, Moonesinghe R, Littrell K, Hull L, Khoury MJ. Association between a first-degree family history and self-reported personal history of obesity, diabetes, and heart and blood conditions: results from the all of us research program. J Am Heart Assoc. (2023) 12(22):e030779. doi: 10.1161/JAHA.123.030779
38. Shariful Islam M, Fardousi A, Sizear MI, Rabbani MG, Islam R, Saif-Ur-Rahman KM. Effect of leisure-time physical activity on blood pressure in people with hypertension: a systematic review and meta-analysis. Sci Rep. (2023) 13(1):10639. doi: 10.1038/s41598-023-37149-2
39. Choudhry NK, Kronish IM, Vongpatanasin W, Ferdinand KC, Pavlik VN, Egan BM, et al. Medication adherence and blood pressure control: a scientific statement from the American Heart Association. Hypertension. (2022) 79(1):e1–e14. doi: 10.1161/HYP.0000000000000203
40. Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA. (2018) 320(17):1774–82. doi: 10.1001/jama.2018.13551
41. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. (2019) 15(6):367–85. doi: 10.1038/s41581-019-0145-4
42. Shariq OA, McKenzie TJ. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. (2020) 9(1):80–93. doi: 10.21037/gs.2019.12.03
43. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler, Thromb, Vasc Biol. (2005) 25(5):932–43. doi: 10.1161/01.ATV.0000160548.78317.29
44. Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. (2012) 380(9841):601–10. doi: 10.1016/S0140-6736(12)60987-8
45. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. (2003) 289(16):2083–93. doi: 10.1001/jama.289.16.2083
46. McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. (2018) 391(10124):949–59. doi: 10.1016/S0140-6736(18)30309-X
Keywords: blood pressure, central adiposity, hypertension, lifestyle score, risk stratification, waist circumference
Citation: Aljuraiban GS, Al-Musharaf S, Almadani FA, Mazi TA, Abulmeaty MM and Aldhwayan M (2026) Beyond behaviors: do biological intermediates improve lifestyle scoring for blood pressure? Front. Cardiovasc. Med. 12:1713086. doi: 10.3389/fcvm.2025.1713086
Received: 25 September 2025; Revised: 7 December 2025;
Accepted: 24 December 2025;
Published: 13 January 2026.
Edited by:
Eddie-Williams Owiredu, University of Alabama at Birmingham, Birmingham, United StatesReviewed by:
Mehdi Bazrafshan, Shiraz University of Medical Sciences, IranNunaya Polycarp, University of Alabama at Birmingham, Birmingham, United States
Copyright: © 2026 Aljuraiban, Al-Musharaf, Almadani, Mazi, Abulmeaty and Aldhwayan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghadeer S. Aljuraiban, Z2FsanVyYWliYW5Aa3N1LmVkdS5zYQ==
 Ghadeer S. Aljuraiban
Ghadeer S. Aljuraiban Sara Al-Musharaf
Sara Al-Musharaf Fatima A. Almadani
Fatima A. Almadani Tagreed A. Mazi
Tagreed A. Mazi Mahmoud M. Abulmeaty
Mahmoud M. Abulmeaty Madhawi Aldhwayan
Madhawi Aldhwayan