Abstract
Background and aim:
Transcatheter edge-to-edge repair (TEER) is a minimally invasive approach to reduce secondary mitral regurgitation (SMR) in patients with heart failure. However, there is limited evidence on its effectiveness in achieving reverse remodeling. Our aim was to assess the effects of TEER over time and to compare the effects of TEER plus GDMT vs. GDMT alone on echocardiographic parameters.
Methods:
A systematic search of MEDLINE, EMBASE, and CENTRAL was conducted from inception to November 16, 2023. Eligible studies included patients with SMR treated with TEER and echocardiographic follow-ups. We evaluated changes in left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left atrial volume (LAV), left ventricular ejection fraction (LVEF) and NTproBNP levels.
Results:
Of 9,290 identified studies, 38 met inclusion criteria. After TEER, statistically significant reductions were observed in LVEDD (–1.63 mm), LVESD (–1.20 mm), LVEDV (–14.21 mL), and LVESV (–9.24 mL). Changes in LAV (–5.70 mL) and LVEF (+1.10%) were not statistically or clinically meaningful. NT-proBNP decreased substantially (–1,340 pg/mL). In comparative analyses, TEER plus GDMT did not show statistically significant differences vs. GDMT alone for any parameter, including LVEDD (–1.02 mm), LVEDV (–11.98 mL), LVEF (–0.14%), and LVESV (–5.29 mL). TEER reduced grade 3–4 MR from 99% to 9%.
Systematic Review Registration:
identifier CRD42023483404.
Conclusion:
TEER results in statistically significant but clinically small changes in echocardiographic parameters, and no clear advantage over GDMT alone. These findings should be interpreted with caution given the high heterogeneity and low certainty of evidence. Further studies are needed to define which SMR patient subgroups may derive meaningful reverse-remodeling benefit from TEER.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=483404, PROSPERO CRD42023483404.
1 Introduction
Heart Failure (HF) affects more than 64 million people worldwide (1). It encompasses changes in cardiac structure, such as myocardial composition, myocyte deformation, and numerous biochemical and molecular alterations affecting heart function and reserve capacity (2). These collective changes are known as cardiac remodeling. A major objective in the treatment of HF is to stop or reverse this process, the latter called reverse remodeling (3). Reverse remodeling is characterized by a reduction in chamber volumes and mass, recovery of ventricular shape, as well as improvement in ejection fraction and is often accompanied by enhanced β-adrenergic and heart-rate responsiveness (4, 5).
Mitral regurgitation (MR) is the most common valvular heart disease in HF patients. MR affects almost 65% of patients with chronic HF and 50% of those with acute HF (6). MR has primary and secondary forms. Primary MR occurs due to structural abnormalities of the mitral valve apparatus itself. In contrast, secondary mitral regurgitation (SMR) occurs due to alterations in the anatomy of left ventricle (ischemic etiology or cardiomyopathy) or left atrium (most commonly due to atrial fibrillation), causing displacement of the papillary muscles and alteration of the geometry of the mitral annulus, leading to leaflet tethering and malcoaptation (7). Severe SMR in heart failure leads to disease progression, increasing mortality, and hospitalization rates (8). This is due to volume overload in the left atrium and ventricle, elevated pulmonary pressures, reduced forward cardiac output, and subsequent deterioration of left ventricular function. These hemodynamic disturbances contribute to pulmonary congestion, decreased perfusion of vital organs, arrhythmias, and further structural remodeling of the heart, perpetuating a cycle of deteriorating cardiac performance and clinical decline (9). Managing severe SMR in HF patients requires a tailored approach by the heart team that balances the benefits and risks of surgical vs. transcatheter interventions (10). Traditional surgical methods, such as mitral valve repair, are recommended for patients undergoing coronary artery bypass grafting (CABG) or other cardiac surgeries (11).
Transcatheter edge-to-edge repair (TEER) with the MitraClip and Pascal systems has emerged as a minimally invasive alternative for patients with severe SMR (12).
Although several studies have shown that TEER can alleviate symptoms and increase functional capacity, thereby improving the quality of life of these patients, data on its effectiveness in achieving reverse remodeling are lacking (13). Therefore, this study aims to investigate the effectiveness of reverse remodeling of the TEER technique compared to guideline-directed medical therapy (GDMT). Understanding the impact of TEER on cardiac reverse remodeling may lead to optimized treatment strategies and improved outcomes for patients suffering from secondary mitral regurgitation and heart failure.
2 Methods
2.1 Study design
In this systematic review and meta-analysis, we evaluated the effects of reverse remodeling of transcatheter edge-to-edge repair (TEER) of the mitral valve. The study was divided into two parts. First, we quantified reverse remodeling by assessing changes in echocardiographic parameters, including left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left atrial volume (LAV), and ejection fraction (EF), measured from baseline to follow-up. Second, we compared these changes between patients receiving guideline-directed medical therapy alone and those receiving GDMT plus TEER.
2.2 Protocol and search strategy
The study protocol was registered with PROSPERO (CRD42023483404) with no deviations. We adhered to the PRISMA guidelines in reporting our results. We searched MEDLINE (via PubMed), EMBASE, and Cochrane CENTRAL for eligible articles without restrictions up to November 16, 2023. The search strategy focused on mitral transcatheter edge-to edge repair and mitral regurgitation.
2.3 Eligibility criteria
We included randomized controlled trials (RCTs) and cohort studies, excluding conference abstracts, editorials, case reports, case series, non-peer-reviewed articles, and animal experiments. Studies were selected to align with our predefined PICO frameworks, assessing echocardiographic parameters before and after follow-up in one part and comparing TEER + GDMT with GDMT alone in the other. Subgroup analyses were conducted by follow-up period, including post-discharge, one-month, three-month, six-month, twelve-month, and mixed follow-up periods.
2.4 Study selection and data extraction
The search results were imported into EndNote 21 (Clarivate Analytics) for duplicate removal. Two independent authors (AL, NG) screened the remaining articles by title, abstract, and full text using Rayyan (14). In addition, we performed backward citation chasing. Cohen's kappa coefficient (κ) was calculated to assess inter-reviewer reliability, and disagreements were resolved by a third reviewer (DG). Data were extracted independently by two investigators (AL, NG) into a Microsoft Excel table, focusing on LVEDD, LVESD, LVEDV, LVESV, LAV, EF, NT-proBNP levels, and mitral regurgitation grade. In addition, we collected data on study details, patient demographics, and relevant echocardiographic outcomes.
2.5 Statistical analysis
A random-effects model was used to pool effect sizes, with three studies as a minimum. The following effect size measures were pooled: mean difference (MD) of changes from baseline for LVEDD, LVESD, LVEDV, LVESV, LAV, EF, and NT-proBNP levels when comparing medical therapy with combined therapy; single means when assessing the change in echocardiographic parameters before and after surgical therapy; and proportions for the changes in mitral regurgitation grades. Pooled effect sizes were expressed as point estimates and 95% confidence interval. The inverse variance weighting method was used to calculate the pooled MD. To estimate the heterogeneity variance measure (τ2), the restricted maximum-likelihood estimator was used with the Q profile method for confidence intervals (15, 16). The t-distribution-based method was used to calculate CI of MD of individual studies. A random intercept logistic regression model was used to pool outcomes [as recommended by Schwarzer et al. (17) and Stijnen et al. (18)]. The maximum likelihood method was used to estimate the heterogeneity variance measure (τ2). In forest plots, the Clopper-Pearson method (19) was used to calculate the CI of proportion of individual studies. We used a Hartung-Knapp adjustment for Cis (20, 21). In addition, between-study heterogeneity was described by the Higgins & Thompson's I2 statistics (22).
For subgroup analysis, we used a fixed-effects “plural” model (aka. mixed-effects model). To assess the difference between the subgroups, we used a “Cochrane Q” test (an omnibus test) (16). All statistical analyses were performed with R (R Core Team 2021, v4.1.2) using the meta (Schwarzer 2022, v6.1.0 28) (23) package for basic meta-analysis calculations and plots, and dmetar (Cuijpers, Furukawa, and Ebert 2023, v0.0.9000) (24) package for additional influential analysis calculations and plots. For additional details on calculations, data synthesis and publication bias assessment, see the Supplementary Material.
2.6 Risk of bias assessment
On the basis of the recommendation of the Cochrane Collaboration, two investigators (AL, NG) independently assessed the risk of bias for each outcome using the ROBINS-I tool for cohort studies with seven domains, and the ROB2 tool for randomized control studies with five domains (25, 26). Disagreements were resolved by consensus. Risk of bias was assessed independently for each outcome, in accordance with ROBINS-I guidance and as prespecified in the PROSPERO protocol.
2.7 Quality of evidence
Certainty of evidence was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) recommendation (27). Two independent investigators (AL, NG) evaluated all criteria for all outcomes, and disagreements were resolved by consensus.
3 Results
3.1 Literature search and study selection
Altogether, 9290 studies were identified with our search key in the three main online databases. The search key resulted in 2569 hits on MEDLINE (via Pubmed), 6418 on EMBASE, and 303 on CENTRAL (via Cochrane Library). After duplicate removal, 6626 records remained for title and abstract selection. A total of 667 studies were collected for full-text selection, of which 3 records were not found. After full-text selection, 34 eligible studies remained. After full-text selection, we performed backward citation chasing and found another four studies (Figure 1).
Figure 1
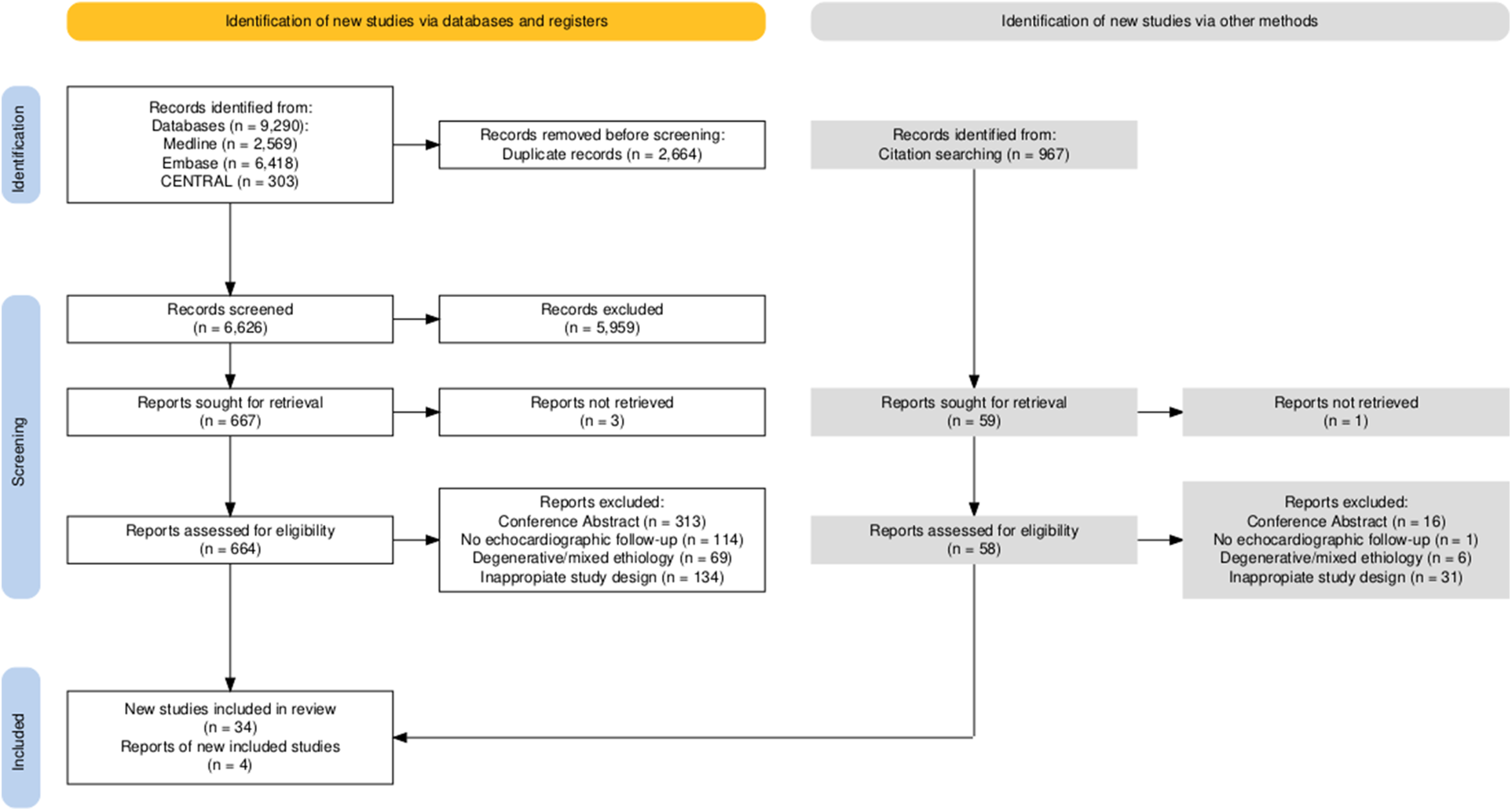
Details of the search and selection are illustrated in the PRISMA flow chart.
3.2 Study characteristics
There was one article that was an RCT (MitraFR trial) (12) and one was a post hoc analysis of the COAPT RCT trial (28), the rest of the studies were retrospective cohort studies. The main follow-up periods of the investigations were 1, 6, and 12 months. The studies included were conducted in different countries, with widely varied sample sizes. Between-study heterogeneity varied according to different follow-up durations, baseline patient characteristics, and study design. Additional clinically relevant baseline parameters, including MR severity, GDMT optimization, and CRT use, are presented in Supplementary Supplementary Tables S5 and S6.
Baseline characteristics of studies evaluating baseline-to-follow-up changes are presented in Table 1, while studies comparing TEER plus GDMT with GDMT alone are summarized in Table 2.
Table 1
| First author | Intervention age | Men intervention (%) | Diabetes mellitus intervention (%) | History of atrial fibrillation intervention (%) | Patient number at baseline | Patient number at follow-up |
|---|---|---|---|---|---|---|
| Ailawadi et al. (45) | 73.3 ± 10.5 | 352 (59.1%) | 232 (38.9%) | 388 (65%) | 597 | 402 |
| Albini et al. (46) | 79.7 ± 7.5 | 12 (92%) | 5 (38%) | 9 (69%) | 13 | 13 |
| Altiok et al. (47) | 73 ± 9 | 24 (62%) | — | — | 36 | 36 |
| Barth (LVEF < 20) et al. (7) | 68.9 ± 8.0 | 12 (100%) | 6 (50%) | 10 (83%) | 12 | 11 |
| Barth (LVEF > 20) et al. (7) | 74.7 ± 7.9 | 44 (70%) | 20 (32%) | 44 (70%) | 63 | 53 |
| Barth (PASCAL) et al. (48) | 78.8 ± 8 | 40 (63.5%) | 13 (20.6%) | 46 (73.0%) | 38 | 38 |
| Berardini et al. (49) | 67 ± 11 | 58 (77%) | 29 (39%) | — | 68 | 68 |
| Buck et al. (50) | 71.7 ± 11.1 | 34 (75.5%) | — | — | 45 | 45 |
| Chan et al. (51) | 71 ± 14 | 9 (75%) | — | — | 12 | 10 |
| Cimino et al. (52) | 73 ± 7.7 | 22 (48%) | 11 (24%) | 10 (22%) | 41 | 41 |
| Citro et al. (53) | 72.5 ± 9.6 | 26 (63.4%) | 14 (34.1%) | 20 (48.7%) | 41 | 41 |
| Demir et al. (54) | — | — | — | — | 122 | 122 |
| El Shurafa et al. (55) | 67 (56.5–72.5) | 54 (61.36%) | 57 (64.77%) | 17 (19.32%) | 88 | 69 |
| Giaimo et al. (56) | 70.3 ± 8.4 | 23 (73.6%) | 6 (20%) | 14 (46.7%) | 29 | 21 |
| Giannini et al. (57) | 75 (63–81) | 23 (65.7%) | 9 (27.5%) | 18 (51.4%) | 35 | 35 |
| Godino et al. (58) | 73 ± 8 | 50 (83%) | 18 (30%) | 21 (35%) | 53 | 53 |
| González et al. (59) | 68.2 ± 10.9 | 82 (88.2%) | 26 (28.0%) | 49 (52.7%) | 93 | 93 |
| Hagnas (decreased LVEF) et al. (60) | 72.7 ± 8.4 | 190 (60%) | 133 (42%) | 141 (44%) | 399 | 399 |
| Hagnas (improved LVEF) et al. (60) | 72.9 ± 11.8 | 23 (56%) | 11 (27%) | 29 (71%) | 399 | 399 |
| Hagnas (unchanged LVEF) et al. (60) | 71.9 ± 8.9 | 26 (65%) | 10 (25%) | 15 (38%) | 399 | 399 |
| Han Yoon (a-FMR) et al. (61) | 78.8 ± 9.7 | 51 (44%) | 35 (30.2%) | 84 (72.4%) | 116 | 116 |
| Han Yoon (v-FMR) et al. (61) | 72.1 ± 12.8 | 313 (62%) | 169 (33.5%) | 265 (52.5%) | 505 | 505 |
| Kamperidis et al. (62) | 72 ± 10 | 11 (50%) | 9 (43%) | 10 (48%) | 22 | 22 |
| Nickenig et al. (63) | 72.8 ± 9.8 | 179 (67.7%) | 87 (33.1%) | — | 264 | 264 |
| Nita (LVRR) et al. (64) | 78.2 ± 6.4 | 49 (60.5%) | 18 (22.2%) | 47 (58.0%) | 53 | 53 |
| Nita (no LVRR) et al. (64) | 75.6 ± 10.2 | 62 (74.7%) | 25 (30.1%) | 58 (69.9%) | 58 | 58 |
| Ohno (Moderate/Severe TR) et al. (65) | 73.2 ± 6.4 | 27 (57.4%) | 22 (46.8%) | 22 (46.8%) | 26 | 26 |
| Ohno (None/Mild TR) et al. (65) | 70.8 ± 9.9 | 66 (66.7%) | 35 (35.4%) | 34 (34.3%) | 73 | 73 |
| Orban et al. (66) | 74.7 ± 10.1 | 121 (58.4%) | 61 (29.5%) | — | 207 | 207 |
| Öztürk et al. (67) | 77.6 ± 9.1 | 29 (58%) | 14 (28%) | — | 50 | 43 |
| Palmiero et al. (68) | 67.8 ± 9.0 | — | — | — | 25 | 25 |
| Perl et al. (69) | 69.3 ± 15.9 | 9 (90%) | 4 (40%) | — | 10 | 9 |
| Scandura et al. (70) | 73.0 ± 6.1 | 25 (83.3%) | 14 (46.6%) | 12 (40%) | 30 | 30 |
| Shechter et al. (71) | 69 (55–76) | 64 (66.7%) | 32 (33.3%) | 42 (43.8%) | 96 | 43 |
| Taramasso et al. (72) | 68.4 ± 9.2 | 43 (82%) | 14 (26.9%) | 37 (17.3%) | 52 | 50 |
| Tay et al. (73) | 70.4 (10.1) | 60 (68.2%) | 33 (37.5%) | 42 (47.7%) | 78 | 78 |
| Toprak et al. (74) | 58.2 ± 11.96 | 21 (75%) | 5 (18%) | 4 (14%) | 19 | 19 |
| Vitarelli et al. (75) | 79.4 ± 5.5 | 18 (56.25%) | 13 (40.6%) | 6 (18.7%) | 32 | 32 |
Baseline characteristics of studies investigating TEER + GDMT effect overtime.
Table 2
| First author (Publication year) | Intervention age | Control age | Men intervention (%) | Men control (%) | History of atrial fibrillation intervention (%) | History of atrial fibrillation control (%) | Number of patients at baseline intervention | Number of patients at follow-up intervention | Number of patients at baseline control | Number of patients at follow-up control |
|---|---|---|---|---|---|---|---|---|---|---|
| Asch et al. (28) | — | — | — | — | — | — | 281 | 281 | 295 | 295 |
| Freixa et al. (76) | 72.1+−7 | 67.2 ± 6 | 13 (81%) | 12 (80%) | 9 (56%) | 5 (33%) | 15 | 15 | 15 | 15 |
| Hubert et al. (77) | 70.0 ± 10.6 | 74.3 ± 9.7 | — | — | 14 (37.8%) | 27 (47.4%) | 37 | 32 | 19 | 16 |
| Krawczyk-Ożóg et al. (78) | 71.8 ± 7.8 | 73.0 ± 11.5 | — | — | 16 (60%) | 16 (60.9%) | 8 | 8 | 19 | 19 |
| Obadia et al. (12) | 70.1 ± 10.1 | 70.6 ± 9.9 | 120 (78.9%) | 107 (70.4%) | 52 (34.5%) | 48 (32.7%) | 152 | 86 | 152 | 76 |
| Papadopoulos et al. (79) | 72 ± 10 | 71 ± 11 | 42 (72.4%) | 25 (86.2%) | 28 (49.1%) | 32 (37.5%) | 58 | 58 | 28 | 28 |
Baseline characteristics of studies comparing TEER + GDMT to GDMT alone.
3.3 Baseline to follow-up changes in echocardiographic parameters (TEER plus GDMT)
In the first part of our analysis, we assessed changes in echocardiographic parameters from baseline to follow-up in patients who underwent TEER procedure in addition to GDMT. We analyzed important echocardiographic parameters such as LVEDD, LVESD, LVEDV, LVESV, LAV and EF.
LVEDD showed a statistically significant reduction of −1.63 mm (95% CI: −2.41 to −0.85, I2 = 87%). At discharge/1 month, the change was −1.49 mm (95% CI: −2.40 to −0.58, I2 = 0%); at 6 months, where the most considerable change was observed, it was −1.94 mm (95% CI: −3.04 to −0.84, I2 = 0%), and at 12 months, the change was −1.67 mm (95% CI: −3.33 to −0.01, I2 = 94%) (Figure 2).
Figure 2
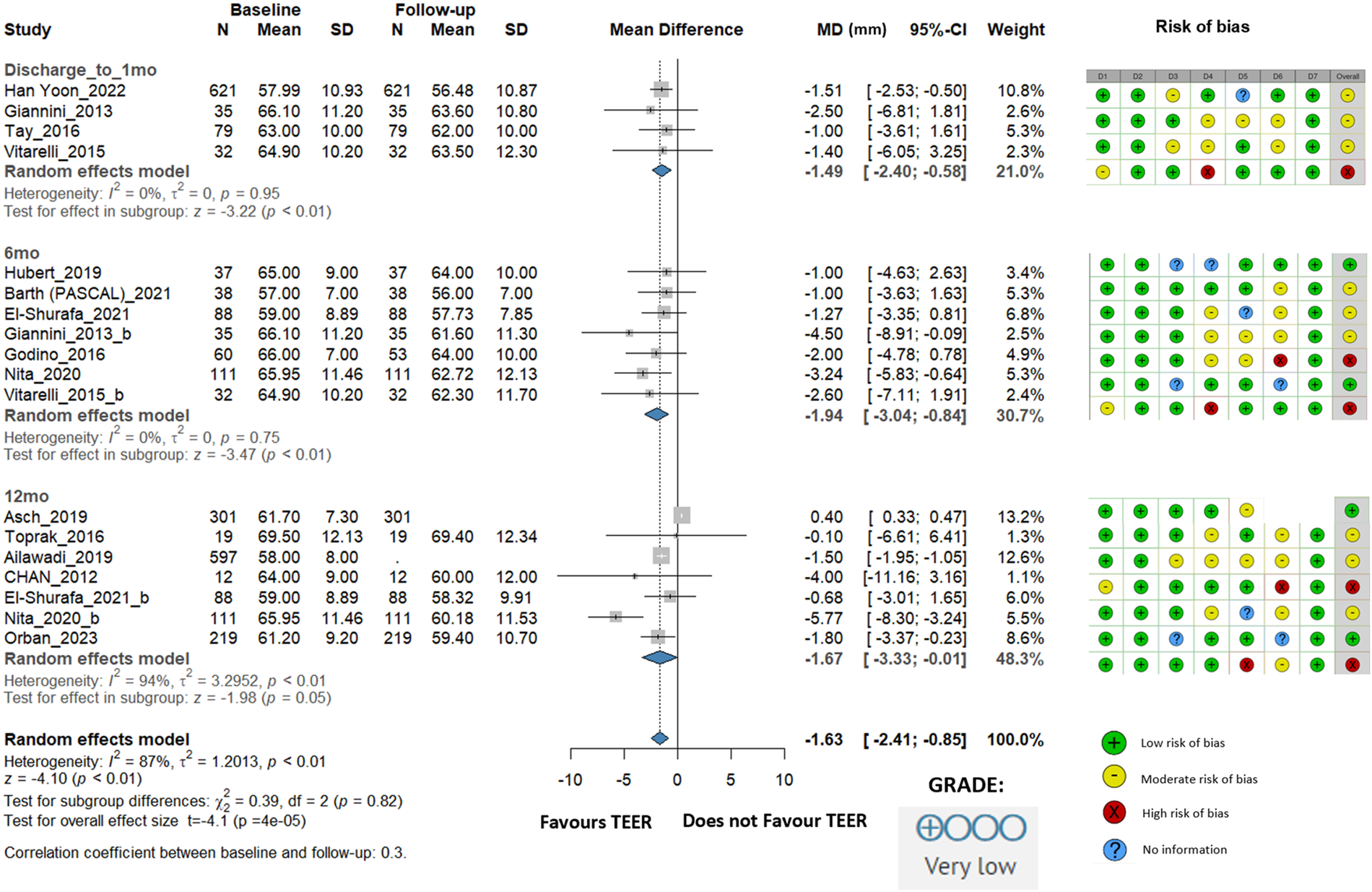
Effect of TEER + GDMT on left ventricular end diastolic diameter—1 month, 6 month, 12 month follow-up. MD, mean difference; CI, confidence interval; SD, standard deviation; D1, bias due to confounding; D2, bias in selection of participants; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurement of outcomes; D7, bias in selection of reported result.
We also found a significant overall change in the mean LVESD of −1.20 mm (95% CI: −1.97 to −0.43, I2 = 33%). At discharge/1 month, the change was −0.56 mm (95% CI: −1.65 to 0.53, I2 = 0%). At 6 months, the change was more pronounced at −2.39 mm (95% CI: −3.77 to −1.00, I2 = 0%), whereas at 12 months, the change was −0.86 mm (95% CI: −2.26 to 0.54, I2 = 70%). The mixed-length follow-up showed a change of −1.89 mm (95% CI: −3.90 to 0.13, I2 = 0%) (Supplementary Figure S1).
LVEDV changed statistically significant, the overall change was −14.21 mL (95% CI: −19.42 to −9.00, I2 = 60%). At discharge/1 month, the change was −14.01 mL (95% CI: −25.48 to −2.54, I2 = 46%), at 6 months, it was −21.09 mL (95% CI: −34.77 to −7.42, I2 = 79%), and at 12 months, it was −12.42 mL (95% CI: −20.25 to −4.60, I2 = 65%). The mixed-length follow-up subgroup showed a change of −5.74 mL (95% CI: −18.83 to 7.36, I2 = 0%), indicating no statistically significant difference (Figure 3).
Figure 3
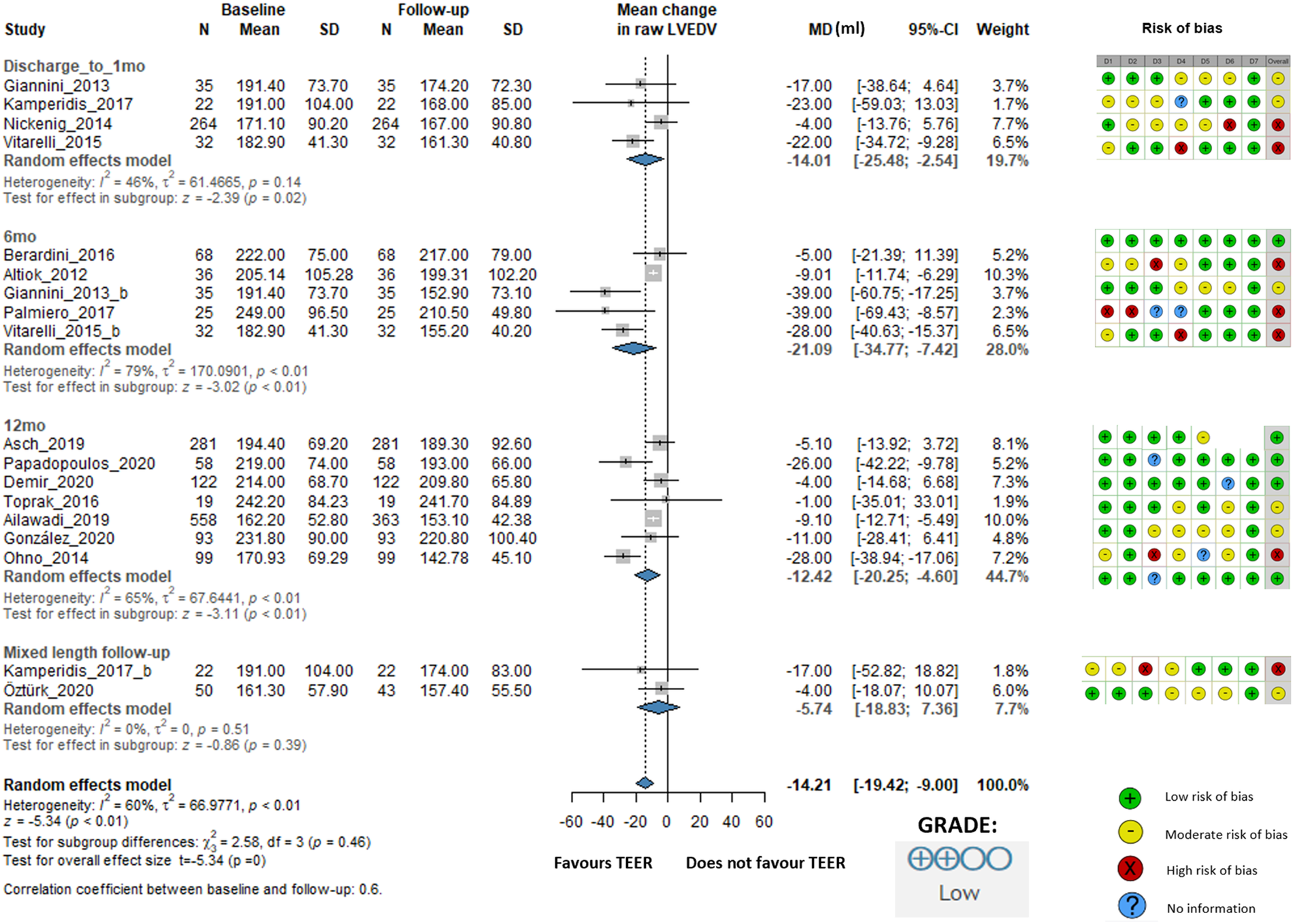
Effect of TEER + GDMT on left ventricular end-diastolic volume—1 months, 6 months, 12 months, and mixed-length follow-up. MD, mean difference; CI, confidence interval; SD, standard deviation; D1, bias due to confounding; D2, bias in selection of participants; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurement of outcomes; D7, bias in selection of reported result.
Studies providing LVEDVi showed an overall change of −4.08 mL/m2 (95% CI: −10.26 to 2.10, I2 = 74%), indicating no statistically significant difference. At discharge/1 month, the change was −2.12 mL/m2 (95% CI: −17.68 to 13.43, I2 = 62%). At 6 months, the change was −7.96 mL/m2 (95% CI: −16.10 to 0.17, I2 = 73%). However, at 12 months, there was an increase of 8.95 mL/m2 (95% CI: 2.26 to 15.64, I2 = 0%). Only two studies were available in the mixed-length follow-up subgroup, which showed a change of −11.60 mL/m2 (95% CI: −23.41 to 0.20, I2 = 0%) (Supplementary Figure S2).
LVESV showed an overall statistically significant change of −9.24 mL (95% CI: −14.00 to −4.48, I2 = 69%). At discharge/1 month, the change was −8.31 mL (95% CI: −16.61 to −0.02, I2 = 22%). At 6 months, the change was −13.25 mL (95% CI: −23.82 to −2.69, I2 = 73%), whereas at 12 months, the change was −8.42 mL (95% CI: −16.11 to −0.73, I2 = 78%). The mixed-length follow-up subgroup showed a change of −0.89 mL (95% CI: −13.92 to 12.15, I2 = 0%) (Supplementary Figure S3).
For LAV, the overall change was −5.70 mL (95% CI: −15.75 to 4.35, I2 =98%) demonstrating no statistically significant difference. At discharge/1 month, the change was −14.36 mL (95% CI: −25.23 to −3.50, I2 = 77%); at 6 months, it was −13.23 mL (95% CI: −20.00 to −6.47, I2 = 68%). At 12 months, it was −2.11 mL (95% CI: −14.56 to 10.34, I2 = 96%). These changes suggest a more beneficial effect on reversing left atrial remodeling during short-term follow-up (Supplementary Figure S4).
We also looked at LAVi, which showed an overall decrease of −6.86 mL/m2 (95% CI: −12.79 to −0.94, I2 = 98%), which, despite the statistical significance, did not reach clinical relevance (Supplementary Figure S5).
There was no improvement in ejection fraction either statistically or clinically. The overall change was 1.10% (95% CI: 0.06–2.14, I2 = 84%). At 6 months, EF improved by 2.29% (95% CI: 0.75–3.84, I2 = 57%). At 12 months, the EF improvement was 1.55% (95% CI: −0.69 to 3.78, I2 = 89%). In the mixed-length follow-up subgroup, EF slightly decreased by −0.43% (95% CI: −2.10 to 1.24, I2 = 0%) (Figure 4).
Figure 4
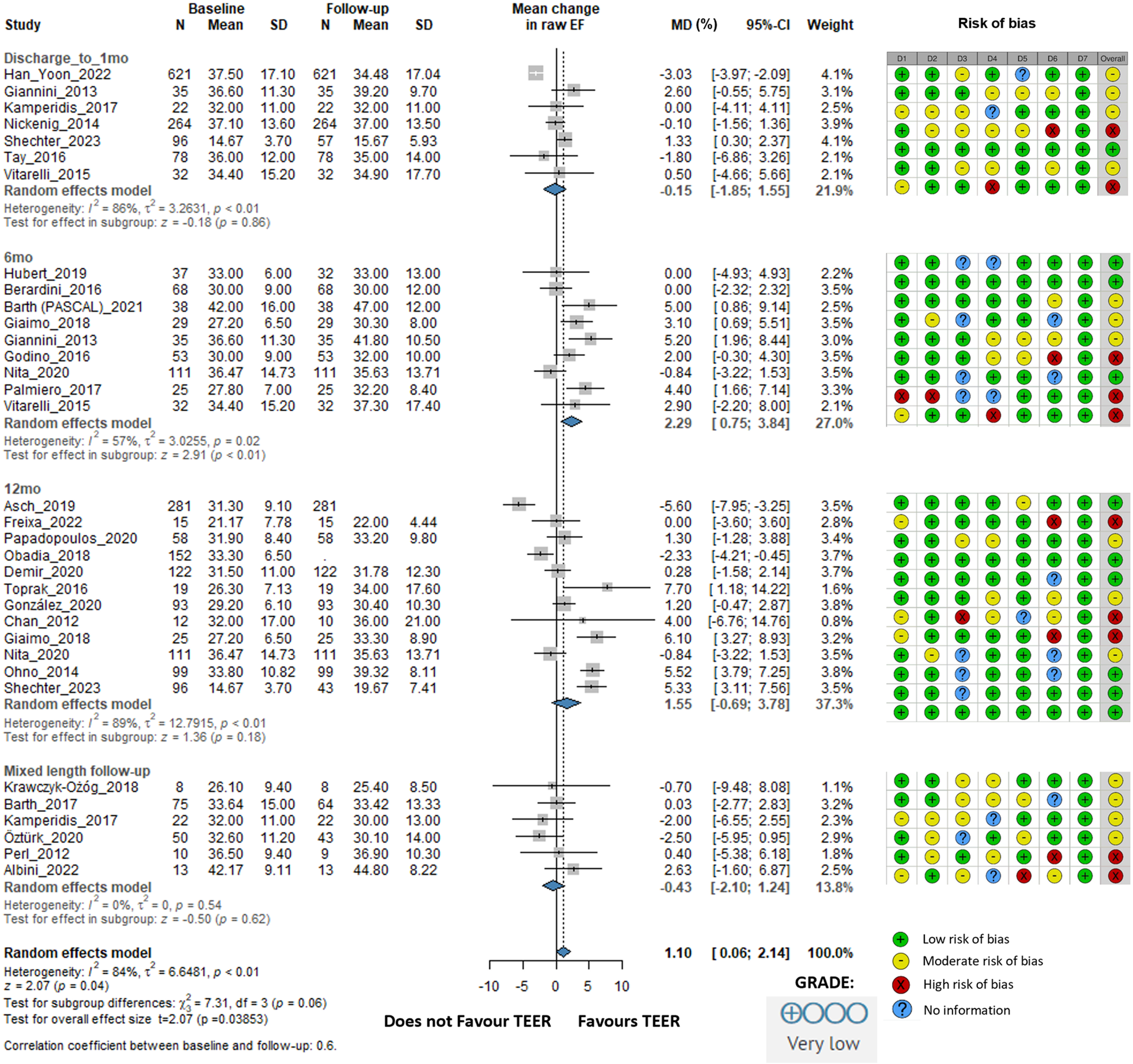
Effect of TEER + GDMT on ejection fraction—1 month, 6 months, 12 months, and mixed-length follow-up. MD, mean difference; CI, confidence interval; SD, standard deviation; D1, bias due to confounding; D2, bias in selection of participants; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurement of outcomes; D7, bias in selection of reported result.
We also evaluated NT-proBNP levels, although data were only available from four studies with 237 individual patients, our analysis showed a remarkable decrease with an overall change of −1,340.21 pg/mL (95% CI: −2,197.63 to −482.79, I2 = 47%) (Figure 5).
Figure 5
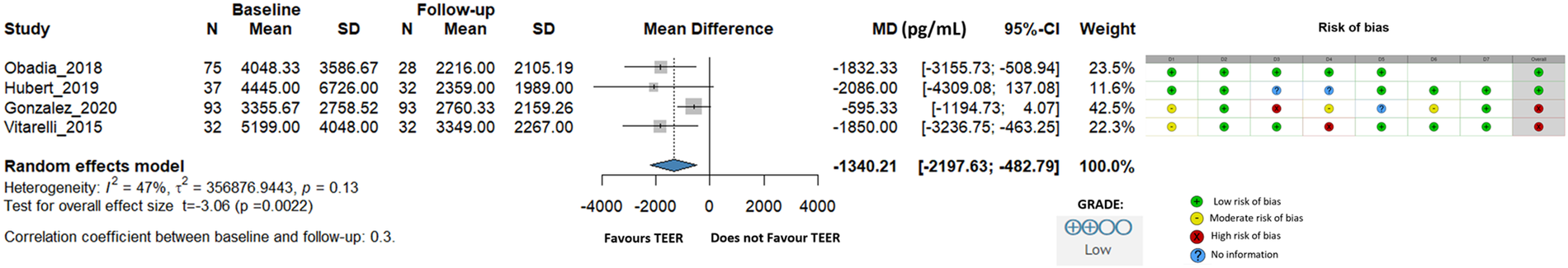
Effect of TEER + GDMT on NT-proBNP. MD, mean difference; CI, confidence interval; SD, standard deviation; D1, bias due to confounding; D2, bias in selection of participants; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurement of outcomes; D7, bias in selection of reported result.
The grade 3 mitral regurgitation proportion was 99% before TEER and 9% after the procedure showing the technical success of the procedure (Supplementary Figures S6a,b).
3.4 Comparison of GDMT to TEER plus GDMT
In the second part of our analysis, we compared changes in echocardiographic parameters between patients who received GDMT alone and those who underwent the TEER procedure in addition to GDMT.
When LVEDD was examined, the difference was −1.02 mm (95% CI: −2.81 to 0.78, I2 = 0%), indicating no statistically significant difference between groups (Figure 6).
Figure 6
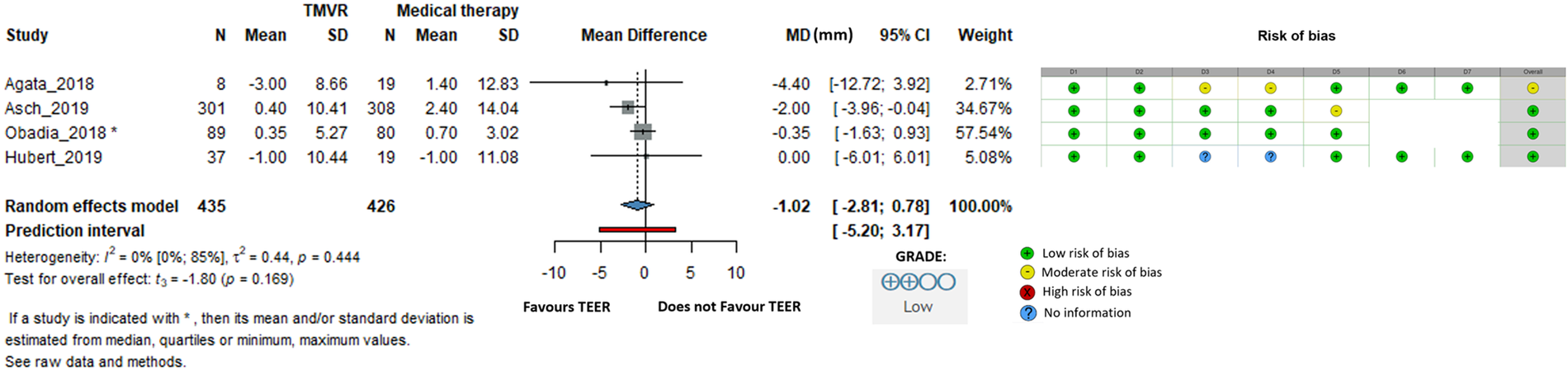
Effect of TEER + GDMT compared to GDMT alone on left ventricular end diastolic diameter. MD, mean difference; CI, confidence interval; SD, standard deviation; D1, bias due to confounding; D2, bias in selection of participants; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurement of outcomes; D7, bias in selection of reported result.
The difference in ejection fraction was −0.14% (95% CI: −3.07 to 2.79, I2 = 63%), suggesting that additional TEER to GDMT did not improve ejection fraction (Supplementary Figure S7).
For the LVEDV, the difference was −11.98 mL (95% CI: −54.22 to 30.26, I2 = 80%), that was not statistically significant (Supplementary Figure S8). The LVESV difference was −5.29 mL (95% CI: −28.55 to 17.98, I2 = 44%), supporting TEER, but not in a statistically significant manner (Supplementary Figure S9).
3.5 Risk of bias and level of evidence certainty assessments
The risk of bias evaluation for the studies included is described in the accompanying figures (Figures 2–6; Supplementary Figures S1–S8), which show a low to moderate risk of bias across the majority of studies. Although several studies revealed possible issues, such as missing outcome data or unclear blinding procedures, the overall evaluation indicates that the risk of bias was not significant enough to undermine the reliability of the results. The results of the GRADE assessment of the level of evidence certainty are presented in Supplementary Tables S2 and S4.
3.6 Heterogeneity and publication bias
Across the main outcomes, between-study heterogeneity was substantial, with wide confidence intervals indicating considerable uncertainty. The largest contributors to heterogeneity were differences in sample size, variability in effect sizes, and the markedly different confidence-interval widths across studies. Formal assessment of publication bias was limited because only a few outcomes were informed by more than ten studies, which restricts the reliability and interpretability of funnel-plot-based methods according to Cochrane recommendations. Therefore, although no obvious directional pattern suggesting bias was observed during visual inspection of the available data, publication-bias evaluation remains inherently constrained by the small number of contributing studies.
4 Discussion
Transcatheter edge-to-edge repair is a percutaneous procedure designed to reduce mitral regurgitation. To our knowledge, this is the first meta-analysis in heart failure patients with secondary mitral regurgitation since the COAPT (13), MITRA-FR (12) and Reshape-HF2 (29) trials to comprehensively investigate echocardiographic parameters to assess reverse remodeling, hypothesizing that the beneficial effects of TEER might be mediated through this mechanism (5). Although cardiac remodeling predicts a poor prognosis, its reversal has been associated with improved survival. For instance, one study reported a 3% mortality rate at 17 months in patients with reverse cardiac remodeling compared to 22% in patients without such changes (30). This can be effectively achieved through coronary revascularization, optimal medical therapy such as renin–angiotensin–aldosterone system inhibitors (31), β-blockers (4, 32), mineralocorticoid receptor antagonists, SGLT-2 inhibitors and device therapies such as cardiac resynchronization therapy (33) or ventricular assist devices (34, 35). In isolated SMR surgical intervention is limited due to significant procedural risks, high rates of recurrent MR, and lack of proven survival benefits (11, 36, 37).
4.1 Patient selection
Three large RCTs, the COAPT (13), MITRA-FR (12), and Reshape-HF2 (29) trials, evaluated the safety and efficacy of TEER in patients with symptomatic HF and severe SMR. COAPT demonstrated that TEER significantly reduced hospitalizations for heart failure and all-cause mortality compared to GDMT alone, whereas the recently published Reshape-HF2 showed lower rates of first or recurrent hospitalizations for heart failure or cardiovascular mortality compared to GDMT alone. In contrast, MITRA-FR found no significant impact on mortality or heart failure hospitalizations (38).
The conflicting results between these trials may be due to differences in patient selection and trial design, echocardiographic methodology and follow-up and the use of GDMT. Multiple studies have shown that extensive LV dilation (LV end-diastolic diameter >65 mm) and LV dysfunction (LVEF <20%, LV end-systolic diameter >55 mm) are associated with less reverse LV remodeling (39). Differences in baseline characteristics between RCTs highlight this issue. In the COAPT and Reshape-HF2 trials, patients had more severe SMR, had smaller LV end-diastolic volumes and higher LVEF compared to those in the MITRA-FR trial that included patients with less severe SMR and more significant LV dilation. These differences highlight the importance of correct patient selection for optimal results with the TEER procedure (39). According to the 2025 ESC guidelines on valvular heart disease TEER is recommended in symptomatic ventricular SMR patients with specific clinical and echocardiographic criteria. In patients with advanced heart failure (when not suitable for LVAD or heart transplantation), or not entirely fulfilling all criteria or with atrial SMR TEER may be considered. to improve symptoms, functional capacity and quality of life (40).
4.2 Baseline to follow-up changes
In accordance with other authors, we defined clinically relevant reverse remodeling as a minimum 10% improvement in echocardiographic parameters (41). Although most echocardiographic parameter changes were statistically significant, thus favouring TEER, such as LVEDD (−1.63 mm), LVESD (−1.20 mm), LVEDV (−14.21 mL), LVESV (−9.24 mL), and LAVi (−5.70 mL), their clinical relevance is questionable, none of these changes reached the criteria of reverse remodeling.
We observed that the most significant changes occurred during the 6-month follow-up. During this period, changes in LVEDD (−1.94 mm), LVESD (−2.39 mm), LVEDV (−21.09 mL), and LVESV (−13.25 mL) were more pronounced compared to other follow-up periods. We believe that the potential favorable effects of TEER on cardiac remodeling may occur at some latency and diminish after one year, given that heart failure is a progressive disease. Although some meta-analyses reported significant improvements in parameters such as LVEF, LVESV, and LVEDV, others, including our own, did not find such marked changes. For example, D'Ascenzo et al. reported improvements in LVEF (4%), LVESV (−22 mL), and LVEDV (−25 mL) (42), whereas Megaly et al. found reductions in LVEDV (−14.24 mL), LVESV (−7.67 mL), LVEDD (−2.92 mm), and LVESD (−1.92 mm) (43). The variation in findings across studies may be due to differences in study populations, methodologies, follow-up durations, and baseline patient characteristics, such as the severity of heart failure and mitral regurgitation.
4.3 GDMT vs. TEER + GDMT effect
Although TEER effectively reduced MR severity, it did not demonstrate superiority over GDMT alone in promoting reverse remodeling. This finding likely reflects a combination of clinical and mechanistic factors. First, many patients undergoing TEER had advanced LV dilation or impaired contractile reserve, where the myocardium has limited ability to recover structural geometry despite reduced regurgitant volume. Second, reverse remodeling is a progressive process that may require longer follow-up than reported by most cohorts (typically ≤12 months). Third, contemporary GDMT—including ARNI, β-blockers, MRAs, and SGLT-2 inhibitors, frequently combined with CRT—can independently induce reverse remodeling, thereby reducing the measurable incremental effect of TEER. Lastly, TEER targets the regurgitant mechanism but does not modify the cardiomyopathic substrate that drives disease progression, which may explain the observed dissociation between improved hemodynamics (e.g., NT-proBNP reduction) and limited structural response. The neutral comparison between TEER + GDMT and GDMT alone likely reflects the underlying pathophysiology of functional MR rather than insufficient procedural efficacy. In patients with severely dilated ventricles, markedly elevated (indexed) LVEDV, or long-standing cardiomyopathic remodeling, the myocardium often has limited capacity for structural recovery even when regurgitant volume is reduced. Conversely, patients with more favorable ventricular geometry and preserved right ventricular function may retain a greater potential for reverse remodeling. Differences in patient selection between cohorts resembling COAPT (less dilation, fewer concomitant right-sided abnormalities, more “proportionate” MR) and MITRA-FR (larger ventricles, more advanced disease, higher rates of RV dysfunction and TR) likely contributed to the heterogeneity observed across studies. These mechanistic considerations suggest that TEER's structural impact is constrained by the underlying myocardial substrate, and that ventricular geometry—not only MR reduction—plays a central role in determining responsiveness to therapy.
4.4 Technical success
The primary goal of the operation was achieved with great success, because the proportion of patients with grade 3–4 mitral regurgitation was reduced from 99% to 9% after the procedure. This underscores the efficacy of the TEER procedure in reducing the severity of mitral regurgitation.
4.5 NT-proBNP
However only a few studies reported NT-proBNP levels, but they were decreased by almost half, due to reduced wall stretching and improved hemodynamic functions. This significant reduction in NT-proBNP levels indicates that the TEER procedure helps alleviate the burden on the cardiac muscle, resulting in a positive impact on overall cardiac function and symptom relief for patients.
4.6 Strengths and limitations
Our study has a unique design that, to the best of our knowledge, has not been previously used in meta-analyses on this topic, providing new insights. The relatively large number of patients across the studies included increases the impact and generalizability of our analysis. In addition, we examined different follow-up periods, allowing for a more detailed assessment of the effects of the TEER procedure over various timeframes. Finally, we rigorously adhered to all Cochrane Collaboration guidelines, ensuring the highest level of quality, transparency, and reproducibility of the results (44).
However, several important limitations must be acknowledged. First, as this meta-analysis is based predominantly on observational studies, the certainty of evidence was rated as low according to the GRADE framework (27). Although several included studies were judged to have high risk of bias, we did not observe a consistent directional distortion of effect estimates; rather, these limitations introduce random uncertainty into the results. Second, substantial between-study heterogeneity was present for multiple outcomes, driven by variations in sample size, effect size, and confidence interval widths, which further reduces confidence in the pooled estimates. Third, reporting across studies was inconsistent—particularly regarding MR severity, GDMT optimization, CRT use, and other clinically relevant characteristics—which prevented meaningful subgroup analyses or meta-regression despite reviewer suggestions. Fourth, publication bias could not be reliably assessed for most outcomes, as fewer than ten studies contributed data per endpoint, limiting interpretability of funnel plots. Finally, the evidence base remains constrained by the lack of prospective randomized trials directly comparing TEER with GDMT; therefore, the clinical implications of the observed statistically significant effects should be interpreted with caution. Future research should focus on standardized reporting, phenotype-specific analyses, and adequately powered randomized studies to better define which patient populations derive the greatest benefit from TEER.
5 Conclusion
While TEER effectively reduces mitral regurgitation severity, its impact on left ventricular reverse remodeling appears limited and inconsistent across studies. Considering the high heterogeneity and low certainty of available evidence, these findings should be interpreted cautiously, and carefully designed future studies are needed to clarify which patient subgroups may benefit most.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
AL: Data curation, Investigation, Conceptualization, Project administration, Writing – original draft. NG: Writing – review & editing, Investigation. DG: Methodology, Supervision, Writing – review & editing. BS: Writing – review & editing, Methodology, Formal analysis, Visualization, Conceptualization. PH: Conceptualization, Writing – review & editing, Supervision, Project administration. ZM: Conceptualization, Writing – review & editing. GD: Supervision, Writing – original draft, Conceptualization, Project administration. JP: Supervision, Writing – original draft, Project administration, Conceptualization.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that the research this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1714337/full#supplementary-material
Abbreviations
TEER, transcatheter edge-to-edge repair; GDMT, guideline-directed medical therapy; MR, mitral regurgitation; SMR, secondary mitral regurgitation; a-FMR, atrial functional mitral regurgitation; v-FMR: ventricular functional mitral regurgitation; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LAV, left atrial volume; EF, ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; MitraFR, a randomized controlled trial of mitral valve repair in patients with functional mitral regurgitation; COAPT, cardiovascular outcomes assessment of the MitraClip percutaneous therapy for heart failure patients with functional mitral regurgitation; Reshape-HF2, a study evaluating the effectiveness of MitraClip for patients with heart failure and severe MR; RCT, randomized controlled trial; PRISMA, preferred reporting items for systematic reviews and meta-analyses; GRADE, grading of recommendations, assessment, development, and evaluations; MD, mean difference; CI, confidence interval; SD, standard deviation.
References
1.
Savarese G Becher PM Lund LH Seferovic P Rosano GMC Coats AJS . Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. 10.1093/cvr/cvac013
2.
Opie LH Commerford PJ Gersh BJ Pfeffer MA . Controversies in ventricular remodelling. Lancet. (2006) 367(9507):356–67. 10.1016/S0140-6736(06)68074-4
3.
Kass DA Baughman KL Pak PH Cho PW Levin HR Gardner TJ et al Reverse remodeling from cardiomyoplasty in human heart failure. Circulation. (1995) 91(9):2314–8. 10.1161/01.CIR.91.9.2314
4.
Lowes BD Gilbert EM Abraham WT Minobe WA Larrabee P Ferguson D et al Myocardial gene expression in dilated cardiomyopathy treated with Beta-blocking agents. N Engl J Med. (2002) 346(18):1357–65. 10.1056/NEJMoa012630
5.
Koitabashi N Kass DA . Reverse remodeling in heart failure—mechanisms and therapeutic opportunities. Nat Rev Cardiol. (2012) 9(3):147–57. 10.1038/nrcardio.2011.172
6.
Pagnesi M Adamo M Sama IE Anker SD Cleland JG Dickstein K et al Impact of mitral regurgitation in patients with worsening heart failure: insights from BIOSTAT-CHF. Eur J Heart Fail. (2021) 23(10):1750–8. 10.1002/ejhf.2276
7.
Barth S Hautmann MB Kerber S Gietzen F Reents W Zacher M et al Left ventricular ejection fraction of < 20%: too bad for MitraClip©? Catheter Cardiovasc Interv. (2017) 90(6):1038–45. 10.1002/ccd.27159
8.
Goliasch G Bartko PE Pavo N Neuhold S Wurm R Mascherbauer J et al Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J. (2018) 39(1):39–46. 10.1093/eurheartj/ehx402
9.
Grigioni F Enriquez-Sarano M Zehr KJ Bailey KR Tajik AJ . Ischemic mitral regurgitation. Circulation. (2001) 103(13):1759–64. 10.1161/01.CIR.103.13.1759
10.
Ponikowski P Voors AA Anker SD Bueno H Cleland JGF Coats AJS et al 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol Pol. (2016) 74(10):1037–147. 10.5603/KP.2016.0141
11.
Acker MA Parides MK Perrault LP Moskowitz AJ Gelijns AC Voisine P et al Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. (2014) 370(1):23–32. 10.1056/NEJMoa1312808
12.
Jean-François O David MZ Guillaume L Bernard I Guillaume B Nicolas P et al Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. (2018) 379(24):2297–306. 10.1056/NEJMoa1805374
13.
Stone GW Lindenfeld J Abraham WT Kar S Lim DS Mishell JM et al Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. (2018) 379(24):2307–18. 10.1056/NEJMoa1806640
14.
Ouzzani M Hammady H Fedorowicz Z Elmagarmid A . Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5(1):210. 10.1186/s13643-016-0384-4
15.
Veroniki AA Jackson D Viechtbauer W Bender R Bowden J Knapp G et al Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. (2016) 7(1):55–79. 10.1002/jrsm.1164
16.
Harrer M Cuijpers P Furukawa TA Ebert DD . Doing Meta-Analysis with R: A Hands-on Guide. 1st ed.Boca Raton, FL, London: Chapman & Hall/CRC Press (2021).
17.
Schwarzer G Chemaitelly H Abu-Raddad LJ Rücker G . Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. (2019) 10(3):476–83. 10.1002/jrsm.1348
18.
Stijnen T Hamza TH Özdemir P . Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. (2010) 29(29):3046–67. 10.1002/sim.4040
19.
Clopper CJ Pearson ES . The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. (1934) 26(4):404–13. 10.1093/biomet/26.4.404
20.
IntHout J Ioannidis JP Borm GF . The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. (2014) 14(1):25. 10.1186/1471-2288-14-25
21.
Knapp G Hartung J . Improved tests for a random effects meta-regression with a single covariate. Stat Med. (2003) 22(17):2693–710. 10.1002/sim.1482
22.
Higgins JPT Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. 10.1002/sim.1186
23.
Schwarzer Guido. Meta: general package for meta-analysis (2022). Available online at:https://cran.r-project.org/package=meta(Accessed November 16, 2023).
24.
Harrer M Cuijpers P Furukawa TA Ebert DD . Dmetar: companion R package for the guide doing meta-analysis in R (2023). Available online at:https://dmetar.protectlab.org.(Accessed November 16, 2023)
25.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. 10.1136/bmj.l4898
26.
Sterne JA Hernán MA Reeves BC Savović J Berkman ND Viswanathan M et al ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. 10.1136/bmj.i4919
27.
Andrews J Guyatt G Oxman AD Alderson P Dahm P Falck-Ytter Y et al GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. (2013) 66(7):719–25. 10.1016/j.jclinepi.2012.03.013
28.
Asch FM Grayburn PA Siegel RJ Kar S Lim DS Zaroff JG et al Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation. J Am Coll Cardiol. (2019) 74(24):2969–79. 10.1016/j.jacc.2019.09.017
29.
Anker SD Friede T von Bardeleben RS Butler J Khan MS Diek M et al Transcatheter valve repair in heart failure with moderate to severe mitral regurgitation. N Engl J Med. (2024) 391:1799–809. 10.1056/NEJMoa2314328
30.
Cioffi G Stefenelli C Tarantini L Opasich C . Prevalence, predictors, and prognostic implications of improvement in left ventricular systolic function and clinical status in patients >70 years of age with recently diagnosed systolic heart failure. Am J Cardiol. (2003) 92(2):166–72. 10.1016/S0002-9149(03)00532-0
31.
Sharpe N Smith H Murphy J Greaves S Hart H Gamble G . Early prevention of left ventricular dysfunction after myocardial infarction with angiotensin-converting-enzyme inhibition. Lancet. (1991) 337(8746):872–6. 10.1016/0140-6736(91)90202-Z
32.
Reiken S Wehrens XHT Vest JA Barbone A Klotz S Mancini D et al β-Blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. (2003) 107(19):2459–66. 10.1161/01.CIR.0000068316.53218.49
33.
Chakir K Kass DA . Rethinking resynch: exploring mechanisms of cardiac resynchronization beyond wall motion control. Drug Discov Today Dis Mech. (2010) 7(2):e103–7. 10.1016/j.ddmec.2010.07.003
34.
Birks EJ Tansley PD Hardy J George RS Bowles CT Burke M et al Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. (2006) 355(18):1873–84. 10.1056/NEJMoa053063
35.
Birks EJ George RS Hedger M Bahrami T Wilton P Bowles CT et al Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy. Circulation. (2011) 123(4):381–90. 10.1161/CIRCULATIONAHA.109.933960
36.
Tomislav M Buu-Khanh L Jeevanantham R Masami T Lauer MS Marc GA et al Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. (2007) 49(22):2191–201. 10.1016/j.jacc.2007.02.043
37.
Wu AH Aaronson KD Bolling SF Pagani FD Welch K Koelling TM . Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. (2005) 45(3):381–7. 10.1016/j.jacc.2004.09.073
38.
Iung B Armoiry X Vahanian A Boutitie F Mewton N Trochu J et al Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail. (2019) 21(12):1619–27. 10.1002/ejhf.1616
39.
Pibarot P Delgado V Bax JJ . MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. (2019) 20(6):620–4. 10.1093/ehjci/jez073
40.
Praz F Borger MA Lanz J Marin-Cuartas M Abreu A Adamo M et al 2025 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2025) 67:4635. 10.1093/eurheartj/ehaf194
41.
Boulet J Mehra MR . Left ventricular reverse remodeling in heart failure: remission to recovery. Struct Heart. (2021) 5(5):466–81. 10.1080/24748706.2021.1954275
42.
D’ascenzo F Moretti C Marra WG Montefusco A Omede P Taha S et al Meta-Analysis of the usefulness of mitraclip in patients with functional mitral regurgitation. Am J Cardiol. (2015) 116(2):325–31. 10.1016/j.amjcard.2015.04.025
43.
Megaly M Khalil C Abraham B Saad M Tawadros M Stanberry L et al Impact of transcatheter mitral valve repair on left ventricular remodeling in secondary mitral regurgitation: a meta-analysis. Struct Heart. (2018) 2(6):541–7. 10.1080/24748706.2018.1516912
44.
Higgins JPT Thomas J Chandler J Cumpston M Li T Page MJ et al , editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). London: Cochrane (2023). Available online at:www.training.cochrane.org/handbook
45.
Ailawadi G Lim DS Mack MJ Trento A Kar S Grayburn PA et al One-Year outcomes after MitraClip for functional mitral regurgitation. Circulation. (2019) 139(1):37–47. 10.1161/CIRCULATIONAHA.117.031733
46.
Albini A Passiatore M Imberti JF Valenti AC Leo G Vitolo M et al Ventricular and atrial remodeling after transcatheter edge-to-edge repair: a pilot study. J Pers Med. (2022) 12(11):ezaf276. 10.3390/jpm12111916
47.
Altiok E Hamada S Brehmer K Kuhr K Reith S Becker M et al Analysis of procedural effects of percutaneous edge-to-edge mitral valve repair by 2d and 3d echocardiography. Circ Cardiovasc Imaging. (2012) 5(6):748–55. 10.1161/CIRCIMAGING.112.974691
48.
Barth S Shalla A Kikec J Kerber S Zacher M Reents W et al Functional and hemodynamic results after transcatheter mitral valve leaflet repair with the PASCAL device depending on etiology in a real-world cohort. J Cardiol. (2021) 78(6):577–85. 10.1016/j.jjcc.2021.08.008
49.
Berardini A Biagini E Saia F Stolfo D Previtali M Grigioni F et al Percutaneous mitral valve repair: the last chance for symptoms improvement in advanced refractory chronic heart failure? Int J Cardiol. (2017) 228:191–7. 10.1016/j.ijcard.2016.11.241
50.
Buck T Eiswirth N Farah A Kahlert H Patsalis PC Kahlert P et al Recurrence of functional versus organic mitral regurgitation after transcatheter mitral valve repair: implications from three-dimensional echocardiographic analysis of mitral valve geometry and left ventricular dilation for a point of No return. J Am Soc Echocardiogr. (2021) 34(7):744–56. 10.1016/j.echo.2021.02.017
51.
Chan PH She HL Alegria-Barrero E Moat N di Mario C Franzen O . Real-World experience of MitraClip for treatment of severe mitral regurgitation—compromise between mitral regurgitation reduction and maintenance of adequate opening area. Circ J. (2012) 76(10):2488–93. 10.1253/circj.CJ-12-0379
52.
Cimino S Maestrini V Cantisani D Petronilli V Filomena D Mancone M et al 2D/3D Echocardiographic determinants of left ventricular reverse remodelling after MitraClip implantation. Eur Heart J Cardiovasc Imaging. (2019) 20(5):558–64. 10.1093/ehjci/jey157
53.
Citro R Baldi C Lancellotti P Silverio A Provenza G Bellino M et al Global longitudinal strain predicts outcome after MitraClip implantation for secondary mitral regurgitation. J Cardiovasc Med. (2017) 18(9):669–78. 10.2459/JCM.0000000000000526
54.
Demir OM Ruffo MM Godino C Ancona M Ancona F Stella S et al Mid-Term clinical outcomes following percutaneous mitral valve edge-to-edge repair. J Invasive Cardiol. (2020) 32(12):E313–20. 10.25270/jic/20.00121
55.
El-Shurafa H Albabtain M Arafat A Abdulsalam W Alfonso J Ashmeik K et al Outcomes after transcatheter mitral valve edge to edge repair; a comparison of two pathologies. Acta Cardiol Sin. (2021) 37(3):286–95. 10.6515/ACS.202105_37(3).20201020A
56.
Giaimo VL Zappulla P Cirasa A Tempio D Sanfilippo M Rapisarda G et al Long-term clinical and echocardiographic outcomes of mitraclip therapy in patients nonresponders to cardiac resynchronization. Pacing Clin Electrophysiol. (2018) 41(1):65–72. 10.1111/pace.13241
57.
Giannini C Petronio AS De Carlo M Guarracino F Conte L Fiorelli F et al Integrated reverse left and right ventricular remodelling after MitraClip implantation in functional mitral regurgitation: an echocardiographic study. Eur Heart J Cardiovasc Imaging. (2014) 15(1):95–103. 10.1093/ehjci/jet141
58.
Godino C Salerno A Cera M Agricola E Fragasso G Rosa I et al Impact and evolution of right ventricular dysfunction after successful MitraClip implantation in patients with functional mitral regurgitation. IJC Heart Vasculature. (2016) 11:90–8. 10.1016/j.ijcha.2016.05.017
59.
Benito-González T Freixa X Godino C Taramasso M Estévez-Loureiro R Hernandez-Vaquero D et al Ventricular arrhythmias in patients with functional mitral regurgitation and implantable cardiac devices: implications of mitral valve repair with Mitraclip®. Ann Transl Med. (2020) 8(15):956. 10.21037/atm.2020.02.45
60.
Hagnäs MJ Grasso C Di Salvo ME Caggegi A Barbanti M Scandura S et al Impact of post-procedural change in left ventricle systolic function on survival after percutaneous edge-to-edge mitral valve repair. J Clin Med. (2021) 10(20):4748. 10.3390/jcm10204748
61.
Yoon SH Makar M Kar S Chakravarty T Oakley L Sekhon N et al Outcomes after transcatheter edge-to-edge mitral valve repair according to mitral regurgitation etiology and cardiac remodeling. JACC Cardiovasc Interv. (2022) 15(17):1711–22. 10.1016/j.jcin.2022.07.004
62.
Kamperidis V Van Wijngaarden SE Van Rosendael PJ Kong WKF Regeer MV Van Der Kley F et al Mitral valve repair for secondary mitral regurgitation in non-ischaemic dilated cardiomyopathy is associated with left ventricular reverse remodelling and increase of forward flow. Eur Heart J Cardiovasc Imaging. (2018) 19(2):208–15. 10.1093/ehjci/jex011
63.
Nickenig G Estevez-Loureiro R Franzen O Tamburino C Vanderheyden M Lüscher TF et al Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011–2012 pilot European sentinel registry. J Am Coll Cardiol. (2014) 64(9):875–84. 10.1016/j.jacc.2014.06.1166
64.
Nita N Scharnbeck D Schneider LM Seeger J Wöhrle J Rottbauer W et al Predictors of left ventricular reverse remodeling after percutaneous therapy for mitral regurgitation with the MitraClip system. Catheter Cardiovasc Interv. (2020) 96(3):687–97. 10.1002/ccd.28779
65.
Ohno Y Attizzani GF Capodanno D Cannata S Dipasqua F Immé S et al Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip system: 30-day and 12-month follow-up from the GRASP registry. Eur Heart J Cardiovasc Imaging. (2014) 15(11):1246–55. 10.1093/ehjci/jeu114
66.
Orban M Rottbauer W Williams M Mahoney P von Bardeleben RS Price MJ et al Transcatheter edge-to-edge repair for secondary mitral regurgitation with third-generation devices in heart failure patients – results from the global EXPAND post-market study. Eur J Heart Fail. (2023) 25(3):411–21. 10.1002/ejhf.2770
67.
Öztürk C Friederich M Werner N Nickenig G Hammerstingl C Schueler R . Single-center five-year outcomes after interventional edge-to-edge repair of the mitral valve. Cardiol J. (2021) 28(2):215–22. 10.5603/CJ.a2019.0071
68.
Palmiero G Ascione L Briguori C Carlomagno G Sordelli C Ascione R et al The mitral-to-aortic flow-velocity integral ratio in the real world echocardiographic evaluation of functional mitral regurgitation before and after percutaneous repair. J Interv Cardiol. (2017) 30(4):368–73. 10.1111/joic.12401
69.
Perl L Vaturi M Assali A Shapira Y Bruckheimer E Ben-Gal T et al Preliminary experience using the transcatheter mitral valve leaflet repair procedure. Israel Med Assoc J. (2013) 15(10):608–12.
70.
Scandura S Ussia GP Capranzano P Caggegi A Sarkar K Cammalleri V et al Left cardiac chambers reverse remodeling after percutaneous mitral valve repair with the mitraclip system. J Am Soc Echocardiogr. (2012) 25(10):1099–105. 10.1016/j.echo.2012.07.015
71.
Shechter A Koren O Skaf S Makar M Chakravarty T Koseki K et al Transcatheter edge-to-edge repair for chronic functional mitral regurgitation in patients with very severe left ventricular dysfunction. Am Heart J. (2023) 264:59–71. 10.1016/j.ahj.2023.05.020
72.
Taramasso M Denti P Buzzatti N De bonis M La canna G Colombo A et al Mitraclip therapy and surgical mitral repair in patients with moderate to severe left ventricular failure causing functional mitral regurgitation: a single-centre experience. Eur J Cardiothorac Surg. (2012) 42(6):920–6. 10.1093/ejcts/ezs294
73.
Tay E Muda N Yap J Muller DWM Santoso T Walters DL et al The MitraClip Asia-Pacific registry: differences in outcomes between functional and degenerative mitral regurgitation. Catheter Cardiovasc Interv. (2016) 87(7):275–81. 10.1002/ccd.26289
74.
Toprak C . Single center experience of percutaneous mitral valve repair with the mitraclip in a high-risk series in Turkey. Turk Kardiyoloji Dernegi Arsivi Arch Turkish Soc Cardiol. (2016) 44:561–9. 10.5543/tkda.2016.77177
75.
Vitarelli A Mangieri E Capotosto L Tanzilli G D’Angeli I Viceconte N et al Assessment of biventricular function by three-dimensional speckle-tracking echocardiography in secondary mitral regurgitation after repair with the MitraClip system. J Am Soc Echocardiogr. (2015) 28(9):1070–82. 10.1016/j.echo.2015.04.005
76.
Freixa X Tolosana JM Cepas-Guillen PL Hernández-Enríquez M Sanchis L Flores-Umanzor E et al Edge-to-Edge transcatheter mitral valve repair versus optimal medical treatment in nonresponders to cardiac resynchronization therapy: the MITRA-CRT trial. Circ Heart Fail. (2022) 15(12):e009501. 10.1161/CIRCHEARTFAILURE.121.009501
77.
Hubert A Galli E Leurent G Corbineau H Auriane B Guillaume L et al Left ventricular function after correction of mitral regurgitation: impact of the clipping approach. Echocardiography. (2019) 36(11):2010–8. 10.1111/echo.14523
78.
Krawczyk-Ozóg A Siudak Z Sorysz D Holda MK Plotek A Dziewierz A et al Comparison of clinical and echocardiographic outcomes and quality of life in patients with severe mitral regurgitation treated by MitraClip implantation or treated conservatively. Postepy Kardiol Interwencyjnej. (2018) 14(3):291–8. 10.5114/aic.2018.78334
79.
Papadopoulos K Ikonomidis I Chrissoheris M Chalapas A Kourkoveli P Parissis J et al Mitraclip and left ventricular reverse remodelling: a strain imaging study. ESC Heart Fail. (2020) 7(4):1409–18. 10.1002/ehf2.12750
Summary
Keywords
echocardiagraphy, edge to edge repair, heart faiIure, reverse remodeling, secondary mitral regurgitation (SMR)
Citation
Lichtfusz A, Galdzytska N, Gergő D, Szabó B, Hegyi P, Molnár Z, Duray G and Papp J (2026) Assessing the impact of transcatheter edge-to-edge repair on reverse remodeling in secondary mitral regurgitation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1714337. doi: 10.3389/fcvm.2025.1714337
Received
27 September 2025
Revised
03 December 2025
Accepted
29 December 2025
Published
30 January 2026
Volume
12 - 2025
Edited by
Christos Iliadis, University Hospital of Cologne, Germany
Reviewed by
Emídio Mata, Unidade Local de Saude do Alto Ave, Portugal
Krzysztof Smarz, Medical Centre for Postgraduate Education, Poland
Updates
Copyright
© 2026 Lichtfusz, Galdzytska, Gergő, Szabó, Hegyi, Molnár, Duray and Papp.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Gábor Z. Duray gabor.duray@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.