Abstract
Paravalvular leak (PVL) following Bentall surgery in patients with Marfan syndrome is exceedingly rare. A 38-year-old Marfan patient underwent Bentall + Sun's procedure for Stanford type A aortic dissection with severe aortic regurgitation. On the third month after surgery, the patient was readmitted due to exertional dyspnea. Echocardiography revealed a paravalvular leak with significant left-to-right shunting, leading to symptoms including exertional dyspnea, hepatomegaly, and heart failure. After adequate preparation, the leak was successfully closed using a symmetric VSD occluder. Post-procedural imaging showed near-complete resolution of the leak, significant reduction in the pseudoaneurysm size, and improvement in heart failure symptoms. The patient was discharged in stable condition.
Introduction
The incidence of paravalvular leak after surgical aortic valve replacement ranges from 2% to 17% (1, 2). Patients with Marfan syndrome are at higher risk due to underlying connective tissue disorders (3, 4). There has been no reported case of PVL caused by valve detachment following Bentall surgery in a Marfan patient with aortic dissection. This case involves a Marfan patient with a BMI of 17.5 who developed PVL three months after Bentall surgery. Given the high risk of reoperation, transcatheter closure using a VSD occluder was carefully evaluated and successfully performed.
Case presentation
A 38-year-old male with Marfan syndrome underwent Bentall + Sun's surgery for aortic dissection. Three months later, he was readmitted with exertional dyspnea. Echocardiography confirmed a paravalvular leak with high-velocity flow across the defect into the right atrium via a surgically created shunt, resulting in heart failure. The patient declined reoperation. Multimodal imaging—including echocardiography (Figure 1, Supplementary Video S1) and computed tomography (Figure 2, Supplementary Video S2)—was used to plan transcatheter PVL closure.
Figure 1
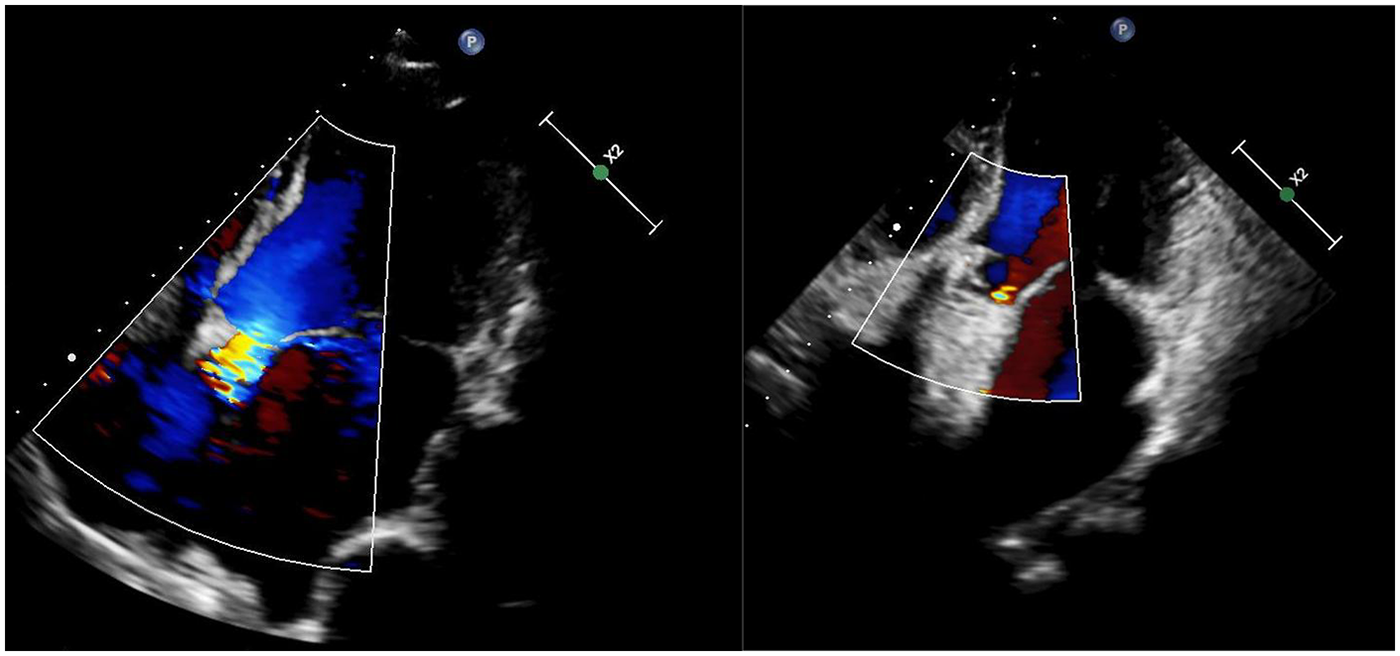
Echocardiograms before (left) and after (right) the procedure.
Figure 2
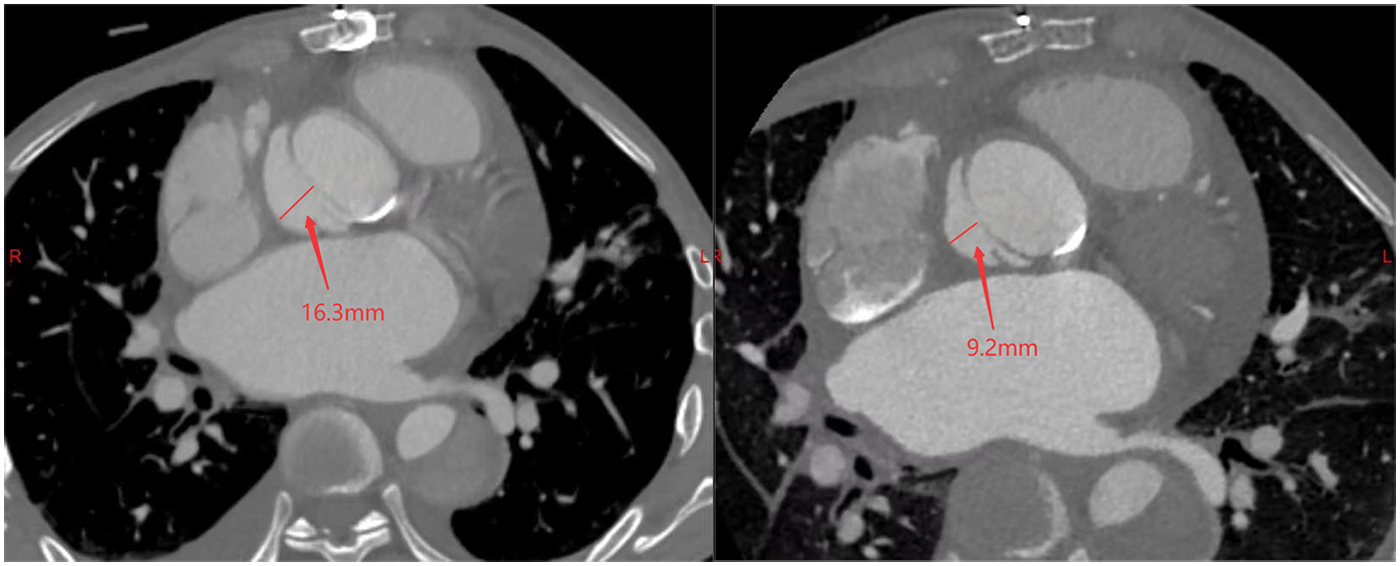
Pre-procedural (left) and post-procedural (right) CT scans demonstrating the pseudoaneurysm at its maximum width.
Unlike typical post-aortic valve replacement PVL, this case involved separation between the graft and native annulus (Figure 3), forming a pseudoaneurysm (A surgically-created autologous aortic adventitial chamber, communicating with the right atrium, serves to reduce surgical bleeding and is subsequently thrombosed for closure). The femoral arterial approach allowed only antegrade access to the defect. Left ventricular angiography was attempted to locate the leak, but it interfered with mechanical valve function, causing progressive bradycardia and hypotension, necessitating abandonment of the femoral approach. Subsequently, a right atrial approach was used to access the pseudoaneurysm via the Cabrol shunt from the initial surgery, followed by retrograde crossing into the left ventricle to establish a working pathway (Figure 4).
Figure 3
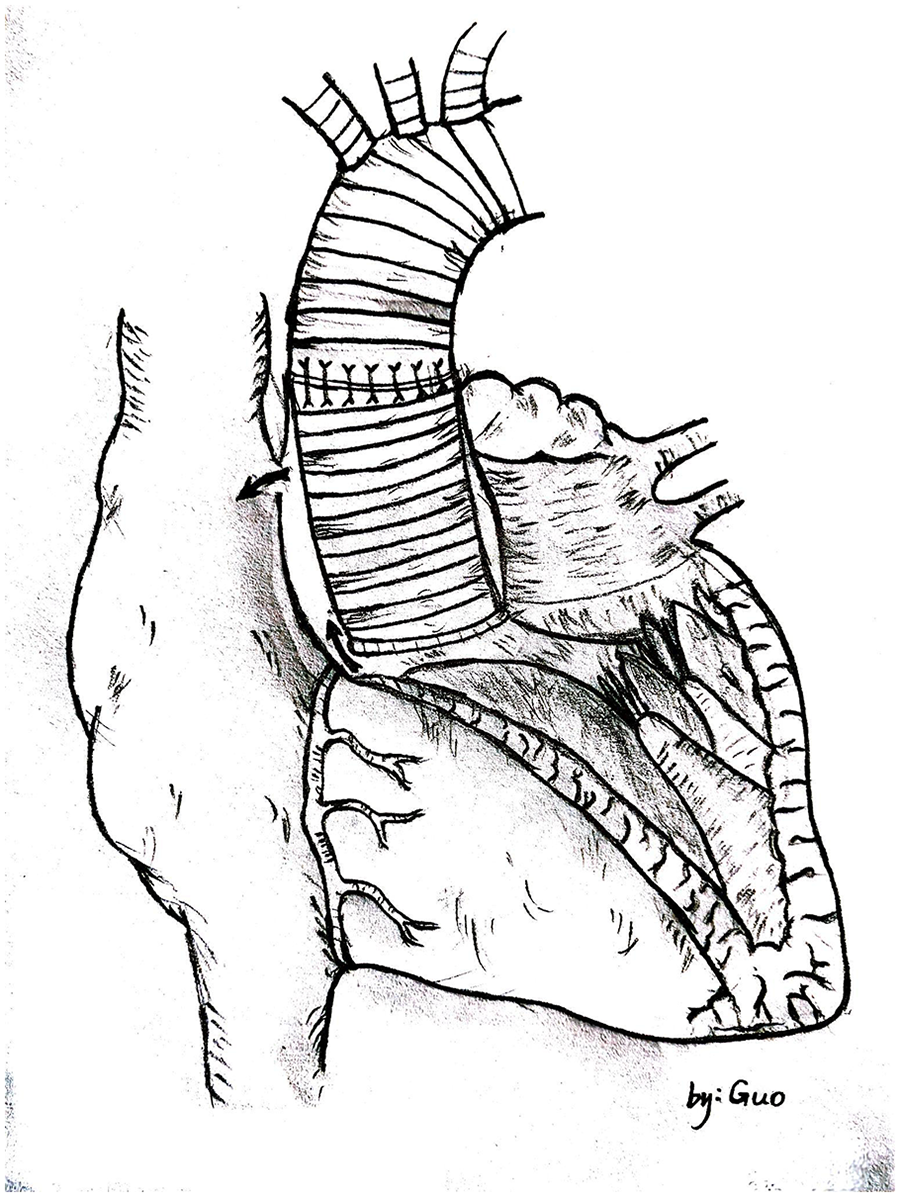
Hand-drawn diagram showing blood shunting from the paravalvular leak into the pseudoaneurysm and then to the right atrium through the cabrol shunt.
Figure 4
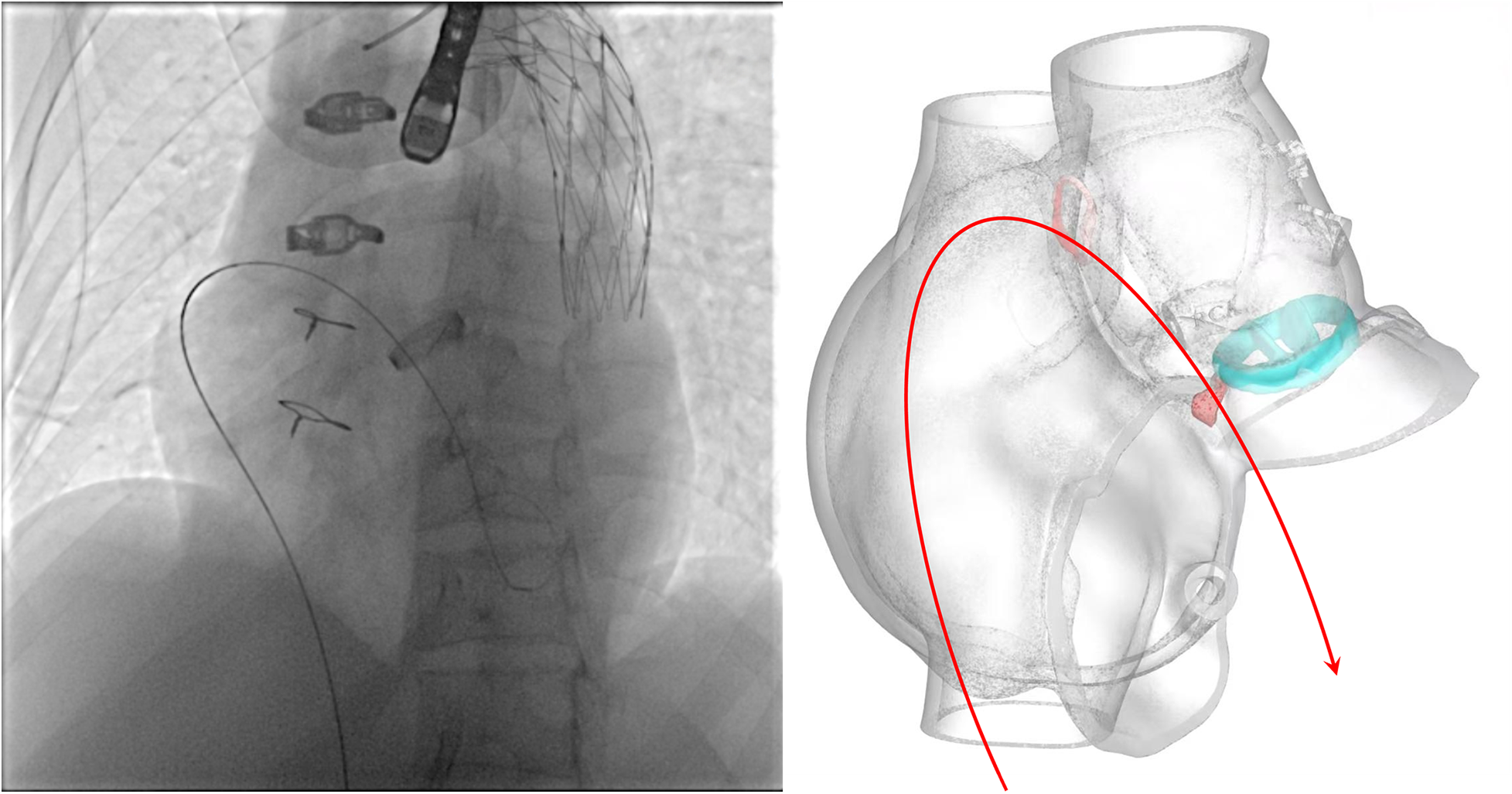
Schematic illustrating the pathway of the guidewire.
Initial attempt with a 6# VSD occluder (10 mm symmetric discs, 4 mm waist height, 6 mm waist diameter) failed due to device dislodgement. A 10# VSD occluder (14 mm symmetric discs, 4.5 mm waist height, 10 mm waist diameter) was then successfully deployed.
Post-procedural echocardiography (Figure 1, Supplementary Video S3) showed minimal residual leak. Follow-up CT (Figure 2) demonstrated significant reduction in pseudoaneurysm size. No complications—such as hemolysis, device migration, new PVL, or recurrent heart failure—were observed. The outcome was satisfactory.
At the one-month follow-up (completed after surgery), the patient was asymptomatic. Echocardiography showed significantly improved ejection fraction, normalized right heart dimensions, and only a trivial residual leak around the device. Notably, the previously observed left-to-right shunt via the Cabrol shunt was no longer detectable, indicating excellent hemodynamic results.
Discussion
Surgical reintervention was once the definitive treatment for symptomatic moderate-to-severe PVL. However, reoperation carries significantly higher morbidity and mortality (up to 10%–15%) due to pericardial adhesions and altered anatomy (5–8). Transcatheter PVL closure has emerged as a viable alternative, receiving a Class IIa recommendation in the 2021 ESC Valvular Heart Disease Guidelines for high-risk surgical candidates (9). Studies demonstrate comparable efficacy to surgical repair, with lower mortality and shorter hospital stays (10–12).
Not all PVLs require intervention. Accepted indications for transcatheter closure include: hemolysis, symptomatic heart failure, mild-to-moderate PVL with declining left ventricular ejection fraction (LVEF), or progressive left ventricular enlargement. Relative indications include asymptomatic mild-to-moderate PVL, risk of infective endocarditis, and post-TAVI PVL (13). This patient met absolute criteria due to heart failure, moderate PVL, reduced LVEF, and left ventricular enlargement.
Route selection is critical and depends on anatomical specifics and technical feasibility. Options include retrograde femoral arterial, antegrade transseptal mitral, and transapical approaches (13, 14). Device selection must ensure stable closure without interfering with valve function or coronary flow. Commonly used devices include PDA, VSD occluders. Multiple devices may be required for complex or multifocal leaks (11, 13, 14). In this case, both the approach and device were selected under challenging constraints, yet proved successful.
Despite promising outcomes, limitations remain. PVLs often exhibit crescentic or irregular morphology, and the lack of dedicated devices may result in residual regurgitation. Hemolysis—caused by high-velocity flows shear stress—requires careful device selection. Other risks include device embolization, thromboembolism, and coronary obstruction (13).
Future advancements in 3D printing and computational modeling may improve preoperative planning and outcomes (15–17). Dedicated devices designed for PVL anatomy are also eagerly anticipated.
Conclusion
Transcatheter closure is a safe, effective, and minimally invasive option for high-risk patients with symptomatic PVL after aortic valve replacement. Success depends on appropriate patient selection, meticulous multimodal imaging, individualized procedural planning, and collaborative decision-making within a heart team comprising cardiologists, cardiac surgeons, and imaging specialists. This case adds to the growing evidence supporting transcatheter therapy in complex scenarios.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by The First Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SG: Writing – original draft. ZW: Writing – original draft. NZ: Writing – review & editing. YD: Writing – review & editing. HZ: Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The work was funded by the Scientific Research Fund Project of the Health Commission of Hebei Province (Project No. 20241832).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1734565/full#supplementary-material
Supplementary Video S1Preoperative echocardiography in the three-chamber view shows a significant shunt across the paravalvular leak (indicated by the arrow).
Supplementary Video S2Preoperative contrast-enhanced CT of the aortic root shows the paravalvular leak (indicated by the arrow).
Supplementary Video S3Postoperative echocardiography in the five-chamber view shows the occluder (indicated by the arrow) with a trivial shunt across the device.
Abbreviations
PVL, paravalvular leak; VSD, ventricular septal defect; PDA, patent ductus arteriosus; TAVI, transcatheter aortic valve implantation.
References
1.
Hammermeister K Sethi GK Henderson WG Grover FL Oprian C Rahimtoola SH . Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the veterans affairs randomized trial. J Am Coll Cardiol. (2000) 36(4):1152–8. 10.1016/S0735-1097(00)00834-2
2.
O'Rourke DJ Palac RT Malenka DJ Marrin CA Arbuckle BE Plehn JF . Outcome of mild periprosthetic regurgitation detected by intraoperative transesophageal echocardiography. J Am Coll Cardiol. (2001) 38(1):163–6. 10.1016/S0735-1097(01)01361-4
3.
Tsunekawa T Ogino H Matsuda H Minatoya K Sasaki H Kobayashi J et al Composite valve graft replacement of the aortic root: twenty-seven years of experience at one Japanese center. Ann Thorac Surg. (2008) 86(5):1510–7. 10.1016/j.athoracsur.2008.07.051
4.
Taniguchi K Nakano S Matsuda H Shirakura R Sakai K Okubo N et al Long-term survival and complications after composite graft replacement for ascending aortic aneurysm associated with aortic regurgitation. Circulation. (1991) 84(5 Suppl):III31–9.
5.
Otto CM Nishimura RA Bonow RO Carabello BA Erwin JP 3rd Gentile F et al 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143(5):e35–71. 10.1161/CIR.0000000000000932
6.
Kaneko T Vassileva CM Englum B Kim S Yammine M Brennan M et al Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve-in-valve procedures. Ann Thorac Surg. (2015) 100(4):1298–304; discussion 1304. 10.1016/j.athoracsur.2015.04.062
7.
François K De Backer L Martens T Philipsen T Van Belleghem Y Bové T . Repeat aortic valve surgery: contemporary outcomes and risk stratification. Interact Cardiovasc Thorac Surg. (2021) 32(2):213–21. 10.1093/icvts/ivaa257
8.
Shah S Alashi A Pettersson GB Rodriguez LL Gillinov AM Grimm RA et al Characteristics and longer-term outcomes of paravalvular leak after aortic and mitral valve surgery. J Thorac Cardiovasc Surg. (2019) 157(5):1785–92.e1. 10.1016/j.jtcvs.2018.08.096
9.
Vahanian A Beyersdorf F Praz F Milojevic M Baldus S Bauersachs J et al 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. 10.1093/eurheartj/ehab395
10.
Wells JA 4th Condado JF Kamioka N Dong A Ritter A Lerakis S et al Outcomes after paravalvular leak closure: transcatheter versus surgical approaches. JACC Cardiovasc Interv. (2017) 10(5):500–7. 10.1016/j.jcin.2016.11.043
11.
Güner A Kirma C Ertürk M Türkmen M Alici G Karabay CY et al Transcatheter closure or surgery for symptomatic paravalvular leaks: the multicenter KISS registry. J Am Heart Assoc. (2024) 13(1):e032262. 10.1161/JAHA.123.032262
12.
Millán X Skaf S Joseph L Ruiz C García E Smolka G et al Transcatheter reduction of paravalvular leaks: a systematic review and meta-analysis. Can J Cardiol. (2015) 31(3):260–9. 10.1016/j.cjca.2014.12.012
13.
Nietlispach F Maisano F Sorajja P Leon MB Rihal C Feldman T . Percutaneous paravalvular leak closure: chasing the chameleon. Eur Heart J. (2016) 37(47):3495–502. 10.1093/eurheartj/ehw165
14.
Krishnaswamy A Kapadia SR Tuzcu EM . Percutaneous paravalvular leak closure- imaging, techniques and outcomes. Circ J. (2013) 77(1):19–27. 10.1253/circj.CJ-12-1433
15.
Xie Y Fang L Li H Xie M . Multimodality cardiac imaging for guidance of transapical transcatheter aortic valve implantation and mitral paravalvular leak closure. Eur Heart J. (2022) 43(9):924. 10.1093/eurheartj/ehab402
16.
Carlson S Habib G Chen T Leipsic J Enriquez-Sarano M Cavalcante JL . Multimodality imaging for prosthetic valves evaluation: current understanding and future directions. Prog Cardiovasc Dis. (2022) 72:66–77. 10.1016/j.pcad.2022.02.002
17.
Ciobotaru V Tadros VX Batistella M Maupas E Gallet R Decante B et al 3D-Printing To plan Complex transcatheter paravalvular leaks closure. J Clin Med. (2022) 11(16):4758. 10.3390/jcm11164758
Summary
Keywords
Bentall procedure, Marfan syndrome, paravalvular leak, pseudoaneurysm, transcatheter closure
Citation
Guo S, Wang Z, Zhang N, Dong Y, Zhang H and Zhao Y (2026) Transcatheter closure of paravalvular leak after Bentall surgery in a Marfan patient: a rare case report. Front. Cardiovasc. Med. 12:1734565. doi: 10.3389/fcvm.2025.1734565
Received
28 October 2025
Revised
24 December 2025
Accepted
26 December 2025
Published
15 January 2026
Volume
12 - 2025
Edited by
Giuseppe Gatti, Azienda Sanitaria Universitaria Giuliano Isontina, Italy
Reviewed by
Stiljan Hoxha, University of Verona, Italy
Ender Ödemiş, Koç University Hospital, Türkiye
Updates
Copyright
© 2026 Guo, Wang, Zhang, Dong, Zhang and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Youwei Zhao sjzgsc163@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.