Abstract
Nitric oxide (NO) is a central regulator of vascular homeostasis and a key determinant of saphenous vein graft (SVG) outcomes in coronary artery bypass grafting (CABG). Endothelial dysfunction, driven by altered shear stress, oxidative stress, and cardiovascular risk factors, impairs NO production and release, contributing to SVG thrombosis, intimal hyperplasia, and atherosclerosis. SVG harvesting technique, storage, and intraoperative handling affects endothelial integrity, inflammatory response, and vascular remodeling, influencing arterialization, long-term patency, and clinical outcomes. Preservation of perivascular adipose tissue (PVAT) during vein harvesting enhances NO bioavailability, reduces inflammation and oxidative stress, and supports graft adaptation. Internal thoracic artery (ITA) grafts provide durable patency, survival benefit, and NO-mediated vasoprotection, improving SVG function and mitigating maladaptive remodeling. Graft configuration further determines SVG adaptation. ITA-composite SVGs confer continuous NO exposure, promote arterial-like remodeling, and attenuate low shear stress. Optimal secondary prevention, including antiplatelet therapy, statins and lifestyle modifications further preserves endothelial function and reduces SVG failure. Targeting NO through surgical technique, graft configuration, and pharmacologic intervention represents a unifying strategy to enhance SVG performance, arterialization, and long-term outcomes, addressing the current limitation of SVG in CABG.
Historical perspective on nitric compounds in cardiovascular medicine
Nitroglycerin (NG) was first discovered in 1847 by Ascanio Sobrero, who firstly noted that it caused severe headaches. Alfred Nobel, who visited Sobrero's laboratory in Paris in 1850, recognized NG potential and began experimenting with it, manufacturing dynamite (1).
The first medical use occurred in 1858 when Alfred Field became the first physician to use NG clinically, treating a woman with severe angina (1). Thomas Lauder Brunton, in 1871, was among the first to understand the biological mechanisms of NG, particularly its vasodilatory effects on the vessel's walls and the nitrate tolerance concept. His work culminated in the 1,885 publication in the “Textbook of Pharmacology and Therapeutics” detailing NG's effects and mechanisms (2).
The early 20th century saw further discoveries, such as Francois-Franck's identification of amyl nitrite as a coronary vasodilator in 1903, and a decade later, Cow's observations on the vasodilatory potency of sodium nitrite and amyl nitrite in sheep coronary vessels (3, 4).
Interest in nitric compounds diminished during the rest of the century, until in the 1970s pharmacologist Ferid Murad and his colleagues showed that nitrite-containing compounds stimulated guanylate cyclase, increasing cellular cyclic guanosine monophosphate (cGMP) and causing vascular relaxation (2). During the same period and independently, Robert Furchgott and John Zawadzki found that endothelial cells (ECs) play a role in arterial smooth muscle relaxation, linking this effect to the activation of guanylate cyclase by nitric oxide (NO) and nitrite-containing vasodilators. They discovered a molecule responsible for this phenomenon, which they named endothelial-derived relaxing factor (EDRF) (5).
In the 1980s, Louis Ignarro's team identified EDRF as NO, demonstrating that NG and amyl nitrite formed unstable S-nitrosothiols, stimulating cGMP and causing vascular relaxation. Ignarro's experiments confirmed that EDRF and NO activate guanylate cyclase via the same mechanisms (6). Similar findings were made by Salvador Moncada, although his contributions were overlooked when the 1998 Nobel Prize in Physiology and Medicine was awarded to Furchgott, Ignarro, and Murad (7).
Since then, scientific interest around NO increased and it has been recognized as a crucial signaling molecule, involved in vascular homeostasis but also in neurotransmission, immune response, and cellular respiration. In the cardiovascular system, NO is responsible for vasodilation, inhibition of vascular cell growth, monocyte adhesion and release of endothelin-1, prevention of platelet adhesion and aggregation, stimulation of platelet cGMP production and suppression of adhesion molecules and chemokines (3).
In cardiac surgery, NO has been considered mostly as a cardiac and vascular protector. In fact, inhaled NO has been used in perioperative management during cardiac surgery under cardiopulmonary bypass (4, 8). Nevertheless, NO plays a crucial role in coronary artery bypass graft (CABG) surgery and specifically, in conduits atherosclerosis, long-term patency, and graft failure.
The greater saphenous vein (SV) is the most frequently used conduit in CABG (9). In this review, we outline the central role of NO in the pathophysiology of saphenous vein graft (SVG) failure and provide NO-based insights into the endothelial mechanisms, clinical outcomes, and preventive strategies to reduce SVG failure and to enhance long-term patency. Critically, we appraise surgical and pharmacologic approaches aimed at enhancing NO bioavailability and prevent SVG endothelial dysfunction. Eventually, we propose NO-centered strategies for the SVG, which may guide clinical practice and future research.
Endothelial cells dysfunction and the role of nitric oxide
The vascular endothelium lines the interior walls of blood vessels. ECs and the basal lamina form a hemocompatible surface, namely, the vascular intima. Healthy vascular endothelium regulates vascular tone, cellular adhesion, thromboresistance, smooth muscle cell proliferation, and vessel wall inflammation by producing and releasing vasoactive molecules. These molecules, including bradykinin, thrombin, prostacyclin, and other prostanoids, modulate ECs function locally and drive arterial remodeling systemically. Among them, endothelium-derived NO is the most potent regulator of vascular tone and vascular homeostasis (10).
NO is one of the ten smallest molecules in nature and it is synthesized from L-arginine by endothelial nitric oxide synthase (eNOS) in the presence of cofactors (11). Once formed, NO is rapidly converted to nitrate and nitrite in the blood within 1.8 milliseconds (12). There are three isoforms of nitric oxide synthase (NOS) that produce NO: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS). Each NOS isoform has a distinct role in regulating vascular tone. eNOS and nNOS are typically present in healthy endothelial cells, and are known as constitutive nitric oxide synthase (cNOS), while iNOS is primarily expressed during inflammation and/or infection (13, 14). cNOS isoforms require elevations in intracellular calcium for activation and therefore generate low amounts of NO for short periods (seconds to minutes), whereas iNOS functions independently of calcium and sustain NO production for longer periods (hours to days) (15, 16). Thus, iNOS can generate NO at concentrations up to 1000-fold higher than cNOS, with eNOS typically producing 0.1–130 nM and iNOS often exceeding 1,000 nM (17).
In order to regulate vascular function, ECs have developed a sophisticated mechanotransduction system that converts physical forces into biochemical signals. Under normal conditions, the endothelium maintains vascular homeostasis slightly favoring vasodilation. To achieve this, NO produced by ECs is released and diffuses into vascular smooth muscle cells, activating guanylate cyclase and leading to cGMP-mediated vasodilation (18). In this biochemical process, physical forces represent crucial signals for the ECs. In fact, ECs sense hemodynamic changes through a mechanosensory network composed by ion channels, glycocalyx, cell extracellular matrix (ECM) adhesions, intercellular junctions, and the cytoskeleton. The mechanosensors activate signaling pathways to release NO and other vasoactive molecules such as bradykinin, adenosine, vascular endothelial growth factor, and serotonin in order to adjust vascular resistance and normalize blood flow. ECs are exposed to several mechanical forces: radial forces from intravascular pressure, tangential forces from cell-cell interactions and vessel vasomotion, and axial shear forces from blood flow friction against the vessel wall. Axial shear stress is the most significant of these forces responsible for NO release. Higher shear stress leads to increasing NO release from ECs (19–21).
Mechanical forces with a defined direction, such as high shear stress, induce only transient vascular signaling of pro-inflammatory and proliferative pathways, which become downregulated as these forces are sustained. In contrast, mechanical forces with undefined direction, such as low flow or oscillatory flow, cause sustained signaling of pro-inflammatory, pro-thrombotic, and proliferative cellular pathways (22, 23). This leads to vascular structural remodeling to minimize intracellular stress/strain changes and adaptive signaling, forming a feedback mechanism to maintain vascular homeostasis and try to provide atheroprotection. Thus, flow patterns can either inhibit or promote endothelial dysfunction and consequent atherosclerosis (19, 20).
Endothelial dysfunction marks the initial stage of atherosclerosis, and it is associated with numerous cardiovascular conditions. Indeed, endothelial dysfunction is commonly observed in the presence of cardiovascular risk factors (13, 24). According to Raddino et al., the most influential cardiovascular risk factors for endothelial dysfunction are age, hypertension, dyslipidemia, diabetes, cigarette smoking, obesity, hyperhomocysteinemia, and asymmetric dimethylarginine (ADMA) (24). Aging impairs vasodilation, promotes vascular remodeling, and increases oxidative stress (24). Hypertension elevates vasoconstrictor mediators, angiotensin II, and endothelin, while reducing NO bioavailability (24). Dyslipidemia disrupts NO production through oxidative stress and peroxynitrite formation (24, 25). Diabetes impairs NO synthesis and heightens oxidative stress due to hyperglycemia and insulin resistance (24, 25). Smoking reduces NO production and endothelial function through oxidative stress (24). Obesity leads to endothelial dysfunction via insulin resistance and pro-inflammatory adipokines (24). Hyperhomocysteinemia increases oxidative stress and reduces NO bioavailability, promoting inflammation (24). ADMA inhibits NO synthesis and increases oxidative stress, contributing to cardiovascular risk and endothelial dysfunction (24).
In addition, Li et al. has shown that these risk factors are associated with an increased eNOS expression rather than a decrease (26). The electron flow within NOS is tightly controlled, and any disruption can lead to the generation of superoxide (O2-) instead of NO, a phenomenon known as NOS uncoupling. The produced NO reacting with dioxygen molecules O2−, and , forms cytotoxic compounds such as hydrogen peroxide (H2O2). The excess H2O2 production enhances eNOS expression, while accelerated NO degradation through its reaction with O2− forms ONOO−, which perpetuates eNOS uncoupling and enzyme dysfunction (27). Consequently, these processes result in impaired endothelium-dependent vascular relaxation and, in the long-term, lead to endothelial dysfunction (24, 25, 28).
Thus, endothelial dysfunction arises from the interplay of hemodynamic forces, oxidative stress, cardiovascular risk factors, leading to impaired NO signaling and vascular remodeling. Understanding these mechanisms is essential to contextualize the role of NO on the SVG in CABG.
Saphenous vein graft and acting forces
Coronary artery disease (CAD), also known as ischemic heart disease, is a leading cause of mortality worldwide and is associated with 17.8 million deaths annually (29). In the United States only, 610,000 deaths annually (29). The management of CAD encompasses preventive measures, including lifestyle modifications and pharmacological treatments, as well as revascularization procedures. Revascularization is performed through percutaneous coronary intervention (PCI) and/or CABG (30). Despite the less invasive nature of PCI, CABG remains the gold standard for patients with complex multivessel coronary artery disease, left main disease, diabetes, or reduced left ventricular function, in accordance with American and European guidelines (31, 32). The two most utilized conduits for CABG are the left internal thoracic artery (LITA) and the SV (33).
In 1946, Vineberg pioneered the implantation of the LITA through a tunnel within the anterior myocardial wall. Since then, significant advancements have established LITA as the preferred graft for bypassing the left anterior descending (LAD) artery, due to its superior 10-year patency rate (>90%), improved survival rates, and higher freedom from cardiac events compared to other conduits (34). In 1962, Sabiston was the first to use a SVG as a sutured aortocoronary anastomosis to the right coronary artery (RCA) (35). Subsequent years saw a marked increase in the use of SVG, which remains the most employed conduit globally in CABG for all non-LAD coronary territories, used in 80%–90% of patients (36).
SVGs are favored for their availability, ease of harvesting, and perceived resistance during manipulation and anastomosis, as well as their reduced susceptibility to vasospasm. However, up to 10%–25% of all the SVGs occlude within the first year post-CABG surgery (37). The rate of SVG failure, defined as the complete occlusion of the graft, can reach 40%–50% at 10 years post-surgery (38–40). This high failure rate adversely affects the long-term outcomes of surgical revascularization, leading to increased episodes of angina or myocardial infarction, and necessitating repeat revascularization. Consequently, SVGs are associated with poorer long-term patency compared to arterial grafts, primarily due to the development of intimal hyperplasia and accelerated atherosclerosis, collectively referred to as vein graft disease, which ultimately leads to SVG failure (41).
SVG failure pathophysiology encompasses three distinct phases. The first phase, which leads to primary graft failure, is characterized by thrombosis occurring within hours to 12 months post SV grafting. Both intrinsic and extrinsic technical factors influence early SVG thrombosis. The grafted vein undergoes early stresses, including ischemia-reperfusion injury during harvest and storage, disruption of the vasa vasorum and nerves within the adventitia and perivascular adipose tissue (PVAT), and stresses before anastomosis, such as uncontrolled mechanical over-distension to assess for potential leaks and extensive graft handling during implantation. These exacerbate endothelial damage, negatively impacting the entire SVG (9, 42, 43).
The combination of these primary stress factors induces inflammation and the formation of reactive oxygen species (ROS), reduce eNOS expression due to endothelial denudation, and damage ECs and smooth muscle cells (SMCs). In addition, the exposure of ECM proteins on the luminal surface leads to the accumulation of fibrin and platelets, while the release of prothrombotic and pro-inflammatory mediators trigger thrombin formation and promotes leukocyte adhesion and infiltration into the vessel wall. This disruption in local hemostatic balance and the resulting hypercoagulable state are sustained by the expression and release of prothrombotic mediators, such as platelet-derived growth factor, transforming growth factor-β, fibrinogen, fibronectin, and von Willebrand factor. Consequently, thrombus formation occurs, potentially resulting in early and acute thrombosis of the graft (44).
The second phase and significant stressor for the SVG is the formation of intimal hyperplasia, which is the primary cause of intermediate-phase SVG stenosis and occlusion. ECs damage triggers the expression of various growth factors and cytokines, promoting excessive ECM deposition in the neointimal compartment. SMCs from the media of the vein and the grafted artery, along with fibroblasts from the adventitia, begin to proliferate and migrate towards the intima of the vein graft. These processes initially occur predominantly at the anastomosis sites but eventually spread throughout the entire vein graft (9, 42, 43).
Accelerated atherosclerosis and subsequent plaque rupture are the third and main causes of late-phase SVG failure. The formation of atheromatous plaques is facilitated by predisposing factors for atherosclerosis, such as high blood pressure, diabetes, and obesity, along with damage and pathophysiological alterations to the vein wall induced by highly proliferative SMCs and the expression and secretion of pro-inflammatory cytokines. Local cytokines and inflammatory mediators stimulate monocytes to infiltrate the neointimal layer and differentiate into macrophages. The uptake of circulating low-density lipoprotein (LDL) particles by macrophages leads to the formation of foam cells. The progressive growth of these atherosclerotic plaques results in the development of necrotic cores, caused by dying foam cells and increased cholesterol deposition, as well as intraplaque hemorrhage from leaky angiogenic neovessels. These factors together lead to plaque rupture and subsequent SVG occlusion (9, 42, 43).
Another critical factor for SVG failure is the exposure of the grafted vein to increased pressures within the arterial environment, known as arterialization. This process begins hours after implantation and exacerbates vein injury, promoting conduit remodeling (45, 46). Shear stress represents a fundamental variable in arterialization, influencing vein vascular morphology, remodeling, and endothelial function. In situ, venous shear stress typically ranges from 1 to 6 dyn/cm2, also previously reported as 0.2 dyn/cm2 by Lemson et al., and it is inversely proportional to vessel diameter (47, 48). SVG shows only a modest rise in wall shear stress when implanted into the arterial circulation. This is due to the SV larger diameter relative to the host artery, which impairs reaching shear stress levels observed in arterial conduits (49). Low shear stress under physiological conditions may be protective for venous ECs. Furthermore, in situ, venous ECs experience alterations in pressure (from ∼7 ± 1 mmHg supine to ∼76 ± 2 mmHg standing) and flow patterns due to orthostatic stress and temperature (50, 51). However, acute high shear stress in veins (12 dynes/cm2, consistent with arterial levels) can lead to formation of pro-inflammatory molecules by the ECs. Contrarily, prolonged high shear stress (10–15 dyn/cm2) on arterial ECs fosters an anti-inflammatory and anti-atherosclerotic environment, increasing the expression of eNOS and NO, facilitating vasorelaxation in response to elevated blood pressure and shear (Figure 1) (47, 52).
Figure 1
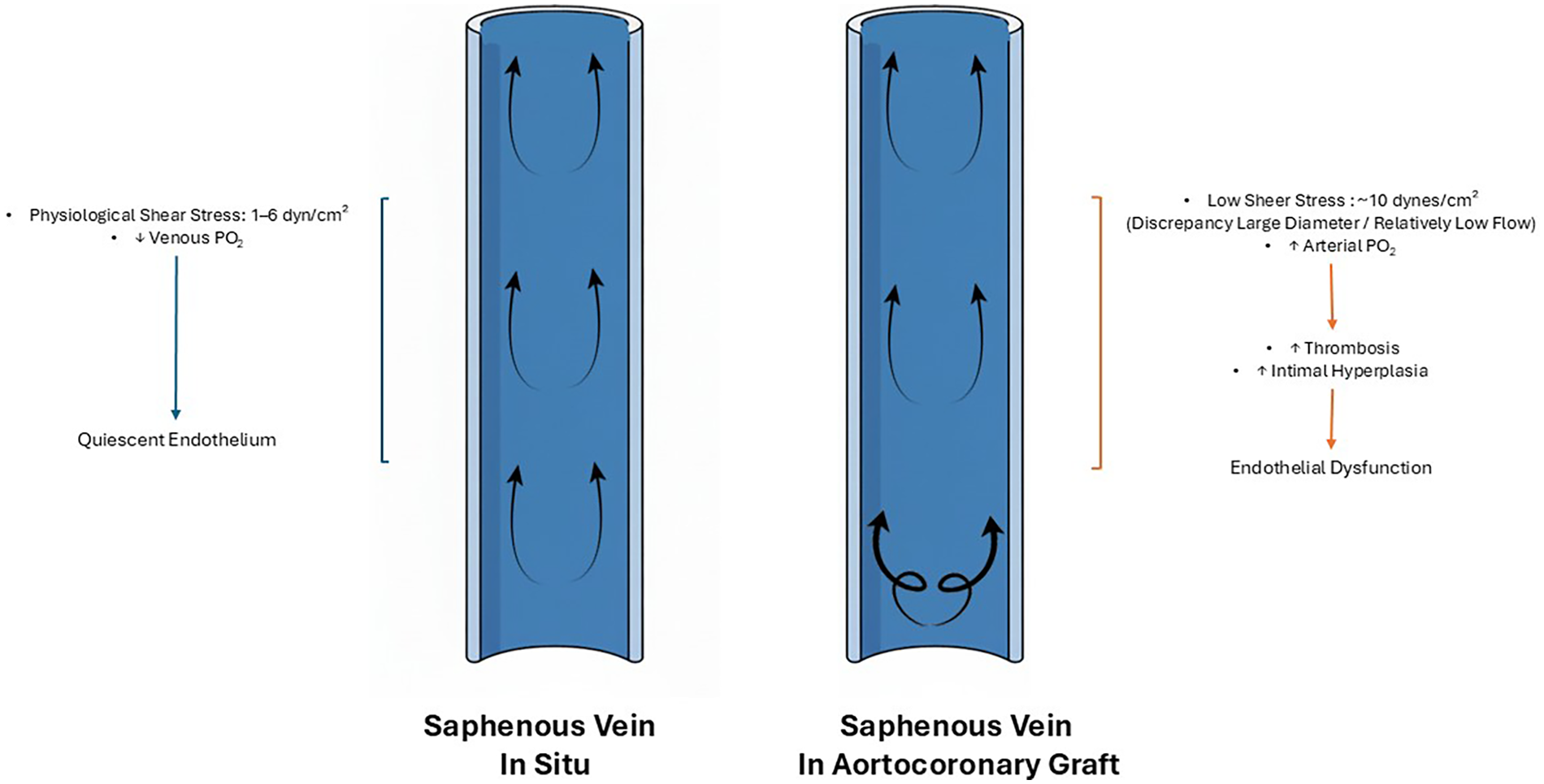
Saphenous vein in situ and aortocoronary graft. Hemodynamic changes in the saphenous vein in situ (left) versus the saphenous vein used as a aortocoronary graft (right). In situ, the saphenous vein experiences physiological shear stress (1–6 dyn/cm2), preserving endothelial integrity. In contrast, when used as aortocoronary grafts, the saphenous vein, due to the discrepancy between large diameter and relatively low flow, is exposed to pulsatile anterograde flow, proximal turbulent flow, and low shear stress (∼10 dynes/cm2) which lead to reduced nitric oxide production by the venous endothelial cells. This leads to increased risk of thrombosis and intimal hyperplasia, and eventually, to endothelial dysfunction of the saphenous vein.
A proposed explanation for the differential shear stress response between arteries and veins is the selective activation of p38 in venous ECs. This occurs because shear stress triggers p38 signaling in veins but not in arteries, owing to absent mitogen-activated protein kinase phosphatase-1 (MKP-1) regulation (47). Upregulating MKP-1 in vein grafts could reduce pro-inflammatory signaling and facilitate the arterialization process under high shear stress (47).
A potential explanation for the differential response to shear stress by arterial and venous ECs may originate from embryonic vasculogenesis, resulting in two ECs types, with distinct morphologies, physiological responses, and phenotypes adapted to their specific vascular regions and functions (21, 47, 53).
Early research on ECs heterogeneity revealed that venous ECs, isolated from bovine tissue, were consistently larger, thinner, and more pleomorphic than arterial ECs, with notable differences in growth rate and protein biosynthesis. Protein markers specific to arterial and venous ECs are defined during vasculogenesis and remain as identifiers of vessel phenotype throughout life. Several studies have suggested that SV implantation into the arterial circulation results in a loss of venous ECs identity, but not a gain in arterial identity, suggesting an incomplete adaptation to the new hemodynamic environment (54–56). Furthermore, under arterial pO₂, SV showed a 2.8 fold higher intimal hyperplasia, 5.8 fold higher cell proliferation and 4 fold increase in ROS compared to fresh or venous pO2 (57). Veins perfused at pressures of 70 mmHg for 7 days showed significant neointima formation compared to those at 7 mm Hg (58). This endothelial change was linked to altered expression of molecular markers, such as matrix metalloproteinases (MMP-2 and MMP-9). This highlights the impact of hemodynamic forces on SVGs, demonstrating that arterial perfusion induces intimal hyperplasia without necessarily causing arterialization (58). However, further evidence is required to validate this assumption.
In summary, SVG failure requires three distinct phases: thrombosis, intimal hyperplasia, and accelerated atherosclerosis. All phases are amplified by hemodynamic stress of arterialization and the incomplete adaptation of venous ECs to the arterial environment. These concepts are fundamental to develop targeted strategies to enhance SVG patency and durability.
Saphenous vein harvesting, endothelial damage and nitric oxide
SV harvesting is the first surgical step in preparing the vessel for use as a graft in CABG. The harvesting procedure inherently induces trauma, the extend of which varies according to the technique employed and may result in different degrees of vein dysfunction. Endothelial injury during harvesting initiates an inflammatory cascade, diminishes vasoactive capacity, and increases oxidative stress (59). Following graft implantation, inflammatory responses cause ECs apoptosis, which peaks approximately twenty-four hours postoperatively (60). This is followed by intimal hyperplasia and progressive wall thickening developing over weeks, with vascular remodeling persisting for up to six months. Therefore, preservation of endothelial integrity during harvesting is critical for the arterialization of SVGs, a process essential for venous adaptation to the coronary circulation and for achieving long-term graft patency.
Four techniques are employed for SVG harvesting: open conventional (OC), open no-touch (ONT), bridge, and endoscopic (61). The OC technique, widely used worldwide but less common in the United States, requires a longitudinal incision along the medial malleolus with complete dissection of the vein from surrounding tissues (62). In contrast, the ONT technique involves harvesting the vein as a pedicle through an open incision, preserving the vasa vasorum and perivascular nerves (63, 64). The bridge technique employs multiple small incisions along the vein course, minimizing wound size, bleeding, and infection risk (65). Finally, endoscopic vein harvesting is performed through a 2–3 cm incision, using systems such as Vasoview or VirtuoSaph and CO2 to create a dissection tunnel. The vein is then mobilized and harvested using electrocautery (66).
Several studies have investigated optimal strategies for SV harvesting in CABG, aiming at maximizing graft patency while reducing endothelial trauma thus, improving clinical outcomes.
In the PATENCY randomized controlled trial of 2,655 patients (mean age 61 ± 8 years; 22% women), Tian et al. demonstrated that the ONT technique significantly reduced graft occlusion compared with the OC method, both at 3 months [2.8% vs. 4.8%; odds ratio (OR) 0.57, 95% CI 0.41–0.80; p < 0.001] and 12 months (3.7% vs. 6.5%; OR 0.56, 95% CI 0.41–0.76; p < 0.001). Recurrence of angina was also lower in the ONT group at 12 months (2.3% vs. 4.1%; OR 0.55, 95% CI 0.35–0.85; p < 0.01), although rates of major adverse cardiac and cerebrovascular events (MACE) were similar. Notably, leg wound complications requiring surgical intervention were more frequent in the ONT group (10.3% vs. 4.3%; OR 2.55, 95% CI 1.85–3.52; p < 0.001) (67). The three-year follow-up confirmed the durability of these early benefits. Among the 2,655 randomized patients, 2,621 (99.4%) completed follow-up and 2,281 (86.5%) underwent CT angiography. The ONT technique remained superior, with significantly lower three-year SVG occlusion (5.7% vs. 9.0%; OR 0.62, 95% CI 0.48–0.80; absolute risk difference −3.2%) and consistent intention-to-treat results (6.1% vs. 9.3%; OR 0.63, 95% CI 0.51–0.81) (68). This trial showed that ONT technique reduced graft failure and related cardiac events by approximately one third to one half over three years, reinforcing its long-term advantage over the OC approach.
Zenati et al., in a randomized trial of 1,150 patients, part of the REGROUP trial, reported no significant differences in MACE between open and endoscopic vein harvesting at a median follow-up of 2.8 years [hazard ratio (HR) 1.12, 95% CI 0.83–1.51; p = 0.47] and at 4.7 years (23.5% vs. 21.9%; HR 0.92, 95% CI 0.72–1.18; p = 0.52). However, leg wound complications were less frequent in the endoscopic group [relative risk (RR) 2.26, 95% CI 0.99–5.15] (69, 70).
Operator experience has also been associated with SVG outcomes. Desai et al. reported that SV extracted by experienced harvesters (>30/month, >900 lifetime cases) had significantly less endothelial injury, particularly with open harvesting technique (71). Endoscopic techniques were associated with conduit injuries, including adventitial disruption, intimal tears, and intimal/medial dissections, although incidence was reduced with experienced operators. Patency resulted significantly lower in grafts with multiple dissections (67% vs. 96%, p = 0.05) (71). Similarly, Rousou et al. observed endothelial dysfunction in endoscopically harvested veins, attributed to caveolin displacement and reduced esterase activity, whereas Milutinović et al. reported better endothelial preservation with endoscopic harvesting at implantation, though no differences in clinical outcomes were observed at 1–2 years (72, 73).
Beyond harvesting technique, intraoperative graft handling is critical. The impact of storage solutions on SV's endothelium integrity is currently debated. Clinically, heparinized 0.9% saline or autologous whole blood (AWB) have been used (74). Both tend to damage the conduit's endothelium. Saline's pH is 5.5 while AWB becomes alkaline with a pH around 8.0, impairing endothelial and smooth muscle viability (75–77). Previous studies reported a less drastic injury with AWB than saline, though others found no difference between the two (76, 77). Functionally, AWB preserved conduits' contraction and relaxation better than saline (76, 78, 79). Buffered solutions provide physiologic pH and ionic balance, yielding superior endothelial preservation vs. AWB or saline (80). In PREVENT-IV, buffered solutions were associated with lower SVG failure and potentially improved outcomes compared to saline and AWB solutions (81). Endothelial damage inhibitor (EDI) is a buffered solution enriched with antioxidative, and eNOS-supporting components derived from the GALA formulation (glutathione, ascorbic acid, arginine) (82). In a small RCT to test EDI DuraGraft, SVGs stored in this solution showed significantly reduced wall thickness at 12 months compared with saline (83). Ex vivo studies on SVGs demonstrated that EDI decreases endothelial injury, lowers ROS and preserves eNOS and caveolin-1 expression, compared to saline, AWB, and standard buffered solutions (84–86). No comparative clinical studies have evaluated EDI vs. other buffer solutions for graft patency and clinical outcomes. Storage temperature may also influence SVG endothelial preservation, with limited evidence suggesting optimal protection at room temperature or 37 °C, while cooling to 4 °C may induce basal membrane separation and cellular deformation (87). Further studies on storage temperature are necessary.
Manual distension with a syringe to overcome spasm exposes the SV to pressures exceeding 300–400 mmHg. The usual exposure pressure for the SV is under 10 mmHg. Such supraphysiologic stress induces endothelial injury, medial apoptosis, and structural damage, with peak pressures up to 480 mmHg shown to disrupt all vessel wall layers (88–90). Intraluminal pressures exceeding 600 mmHg, causing endothelial denudation, smooth muscle apoptosis, and promoting intimal hyperplasia (91). Viaro et al. showed that pressures up to 200 mmHg preserved NOS activity, whereas fifteen seconds exposure to 300 mmHg led to endothelial loss and reduced eNOS expression (92). Pressure-controlled syringes may help in reducing this risk.
Currently, there is not enough evidence to demonstrate superiority in harvesting methods in terms of short and long-term cardiac outcomes. The priority remains ensuring conduit quality and long-term patency. As suggested by Sandner et al. (93), endoscopic harvesting is preferable in patients at high risk of leg wound complications, provided harvester experience is ensured. In contrast, open harvesting, ideally with the no-touch technique, remains optimal in patients at low risk of wound complications. SV should be stored in a buffer solution, high pressure distension should be avoided, and pressure controlled syringes should be used (93).
Perivascular adipose tissue and nitric oxide-mediated endothelial protection
PVAT surrounds blood vessels and has emerged as an active vascular component, often referred to as a fourth vascular layer, the tunica adiposa (94). Previously regarded as a passive support and routinely removed to facilitate SV branches identification, PVAT has since been shown to exert important biological effects (94). SV graft patency is markedly improved when the vein is harvested atraumatically with PVAT preserved using the ONT technique. Long-term data confirm its superiority over conventional harvesting, with 16-year patency rates approaching those of the LITA (Patency ONT group 83% vs. Patency LITA group 88%) (95, 96). Randomized evidence by Dreifaldt et al. further supported this advantage, as ONT SV grafts show higher overall patency (91% vs. 81%, p = 0.046) and particularly favorable results in moderate stenoses (95% vs. 74%, p = 0.017) compared to RA grafts (97).
Mechanistically, the technique obviates the need for high pressure saline distension, prevents spasm by minimizing direct manipulation, and preserves a cushion of PVAT that provides both structural support and vasoprotective paracrine signaling (98, 99). Collectively, these findings indicate that PVAT preservation is a key determinant of long-term SV graft performance.
PVAT is phenotypically distinct from other adipose deposits. While adipocytes constitute most of its volume, they account for only one third of its cellular population. In fact, PVAT also contains nerve cells, endothelial precursor cells, fibroblasts, pericytes, macrophages, T and B lymphocytes and mesenchymal stem cells (94). Adipocytes within PVAT exhibit both white and brown adiposity features, highlighting its heterogeneity and adaptive potential. Importantly, PVAT varies by anatomical location, adiposity, and local pathophysiological environment. For instance, coronary PVAT promotes vascular injury through inflammation, oxidative stress, angiogenesis, and thrombosis, thereby accelerating atherosclerosis. Conversely, SV PVAT appears protective, especially when preserved with the ONT technique, and is associated with increased graft patency in patients undergoing CABG. Notably, the phenotype of SV PVAT resembles that of PVAT surrounding the ITA than that of coronary or aortic PVAT, a finding that may underlie the favorable outcomes of SV grafts harvested with intact PVAT (94, 100, 101).
In his original article from 1968, one of the pioneer of the SVG, Favaloro advised dissecting the vein and removing the surrounding pedicle for an aortocoronary bypass (102, 103). However, through the years, PVAT demonstrated to function as a mechanical protector for the SV (Figure 2) (104). This role is linked to reduced metabolic inflammation and attenuated tissue remodeling (99). There is no mechanical barrier between the vessel wall and the PVAT, rather a fibrous layer separates adipocytes from adventitial cells. This arrangement facilitates direct communication among substances secreted by the PVAT and those released by the vessel wall (105). This leads to a complex, bidirectional communication between the vascular wall and its surrounding PVAT. Antonopoulos et al. demonstrated that vascular oxidative products such as 4-hydroxynonenal upregulate adiponectin expression in PVAT through peroxisome proliferator-activated receptor gamma (PPAR- γ) activation. In turn, PVAT-derived adiponectin enhances NO bioavailability by stimulating eNOS activation via PI3K/Akt phosphorylation and improving coupling through increased tetrahydrobiopterin (BH4) availability, while simultaneously reducing superoxide production (106–108). These findings suggest that oxidation products from the vascular wall may act as “rescue signals,” driving PVAT toward a protective phenotype as a local counter regulatory mechanism.
Figure 2
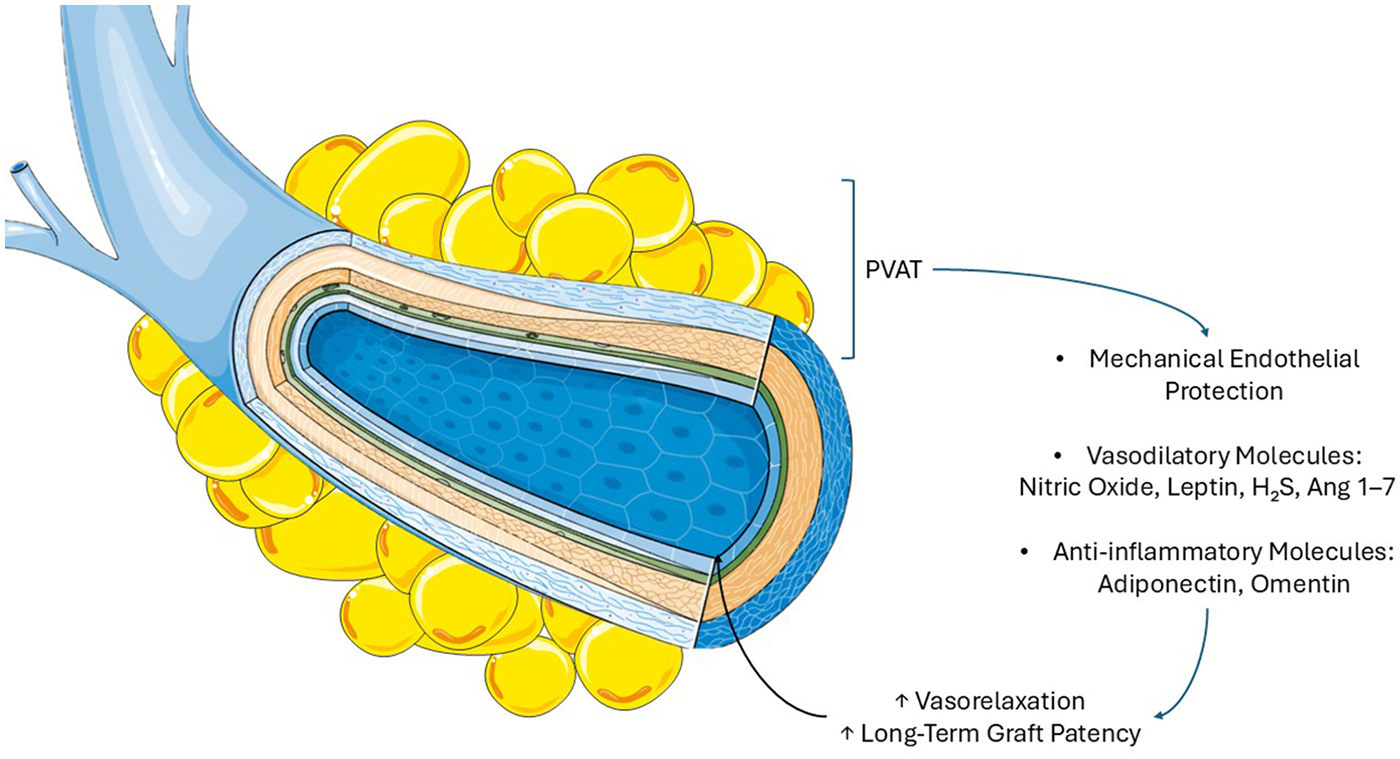
Saphenous vein graft and perivascular adipose tissue. Perivascular adipose tissue provides mechanical protection against pressure and distension injuries and secretes endothelial-protective molecules. Adiponectin reduces inflammation and enhances nitric oxide-mediated vasodilation, leptin promotes vasodilation and counteracts angiotensin II, omentin attenuates vascular inflammation, hydrogen sulfide relaxes smooth muscle by lowering intracellular calcium, and angiotensin 1–7 increases nitric oxide bioavailability and supports vasodilation. These molecules contribute to improve SVG long-term patency.
Recent evidence by Saito et al. indicates that PVAT expresses high levels of argininosuccinate synthase 1, sustaining continuous substrate availability for NO synthesis, together with expression of eNOS (109). ONT SVGs exhibited superior eNOS production compared with OC harvested grafts, largely attributable to PVAT (109). Compared with ONT harvesting, OC SVGs without PVAT, demonstrate greater vascular SMC injury and iNOS induction, although it is not clear if this rapid iNOS stimulation has harmful or protective effect on the graft (110). Segments with preserved PVAT contained higher eNOS protein and activity, were resistant to pressure induced injury, and maintained adventitial structures such as the vasa vasorum and perivascular nerves, key sources of NO (109). By contrast, OC harvesting disrupts the adventitia and vasa vasorum, promoting medial ischemia, vascular smooth muscle cell abnormalities, and neointimal hyperplasia (109, 110). Importantly, restoration of retrograde flow after anastomosis may reestablish vasa vasorum perfusion in ONT grafts. Preservation of PVAT and adventitial eNOS activity likely underlies the protective effect of the ONT technique, with downstream benefits including inhibition of platelet aggregation, reduction of early thrombosis, and facilitation of reendothelialization (109).
Besides NO, PVAT secretes other important adipokines and gaseous mediators with anti-contractile, vasodilatory, and anti-inflammatory properties. Adiponectin, abundantly secreted by PVAT, exerts vasoprotective effects by suppressing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)–mediated inflammation, reducing interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), and promoting NO bioavailability via AMP-activated protein kinase (AMPK)—dependent eNOS activation and BH4 synthesis (108, 111–113). Leptin, localized in the PVAT of SV, similarly induces vasodilation through eNOS phosphorylation, AMPK activation, and angiotensin II antagonism, supporting the clinical superiority of ONT SVGs (98, 99, 114, 115). Omentin attenuates vascular inflammation by inhibiting thioredoxin-interacting protein/nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (TXNIP/NLRP3) signaling and oxidative stress, while circulating levels correlate with endothelial health (116–118). Hydrogen sulfide (H2S), another PVAT derived gasotransmitter, induces hyperpolarization of vascular SMCs by opening big-potassium (BK) channels and inhibiting L-type Calcium (Ca2+) entry, thereby lowering intracellular Ca2+ (94, 119). Finally, PVAT-derived angiotensin 1–7 enhances NO bioavailability via Mas receptor–dependent eNOS activation and BK channel stimulation (119, 120). Together, these mediators provide a biochemical and mechanistic rationale for the superior patency of ONT SVGs, where preserved PVAT actively contributes to long-term vascular homeostasis.
PVAT plays a fundamental role also in manual intraluminal distension. In the ONT technique introduced by Souza, PVAT, abolishes spasm without antispastic solutions, and avoids high pressure distension (121). PVAT provides mechanical protection against pressure injury and secretes paracrine anticontractile factors, thereby reducing vascular damage and improving long-term patency to levels comparable with the internal thoracic artery (99, 122–124). Removal of PVAT and reliance on high pressure distension, by contrast, result in immediate endothelial trauma and reduced long-term graft survival.
PVAT exhibits remarkable metabolic plasticity, rapidly adapting to systemic and local conditions. Nutritional status and adiposity strongly influence its phenotype (125). In both Asian and European cohorts, the protective effect of no-touch harvesting was lost in patients with elevated BMI (>27–30 kg/m2), suggesting an obesity driven phenotypic switch (67, 126). Mechanistically, excess PVAT may exert a restraint on vessel dilation and, through pro-inflammatory signaling, impair adaptive remodeling (127). Experimental studies further support this concept. In lean individuals, PVAT acquires a brown adipose tissue phenotype that mitigates post-surgical inflammation by releasing neuregulin-4 (NRG4), promoting macrophage polarization toward an anti-inflammatory phenotype, and increasing NO availability. By contrast, in obesity, PVAT shifts toward a pro-inflammatory state characterized by white adipocyte predominance, elevated IL-6, chemokine ligand 2 (CCL2), TNF-α, and leptin, and increased infiltration of pro-inflammatory macrophages (128–130). This environment suppresses PVAT browning, amplifies oxidative stress, and drives maladaptive vascular remodeling through enhanced vascular smooth muscle cell and mesenchymal stem cell proliferation and migration (125).
Despite these advances, the role of PVAT in postinterventional SV remodeling remains incompletely understood. Future work integrating single cell and spatial multiomics technologies is essential to dissect the heterogeneity of PVAT, delineate its molecular interactions with the vascular wall, and ultimately clarify its impact on long term SVGs outcomes.
Nitric oxide from internal thoracic arteries benefits the saphenous vein
In CABG, the combination of an ITA and SVGs has represented the benchmark surgical strategy, although, after years, SVG failure remains a major limitation. Loop et al. in 1986 (131), demonstrated that patients receiving only SVGs had significantly worse long-term survival than those with an ITA graft, a finding subsequently confirmed in independent studies by Cameron et al. (132) and Boylan et al. (133). Long-term patency rates exceed 90% for both LITA and right internal thoracic artery (RITA) at 15 years (134), whereas SVGs exhibit failure rates of 15%–25% at 5 years and 40%–45% at 10 years (40, 135).
The ITAs can be harvested as a pedicled, including PVAT and endothoracic fascia, or skeletonized graft. Skeletonization provides greater conduit length, facilitates composite grafting, improves flow, and reduces sternal devascularization (136–138), though concerns about endothelial injury and long-term patency remain (137, 139, 140). A semi-skeletonized approach may offer a compromise, but data remain limited (141). Technical factors are also critical. Monopolar electrocautery may cause endothelial injury and vasospasm through heat transmission (>300 °C), whereas bipolar and harmonic devices minimize thermal damage (<80 °C), vasospasm and tissue charring, reducing need for clips (142, 143). Urso et al. in a randomized control trial and Keiser et al. in a large observational study suggest that harmonic skeletonization yields similar intraoperative flow and perioperative outcomes compared with electrocautery, without increasing risks of bleeding, conduit injury, sternal wound complications, or perioperative myocardial infarction (144, 145). Despite different techniques and instruments, ITAs' endothelium integrity is generally preserved during harvesting and manipulation.
The ITA exhibits unique endothelial properties that confer remarkable resistance to atherosclerosis and thrombosis. In patients with CAD, eNOS expression and NO release are markedly higher in the ITA endothelium than in carotid and radial arteries, impairing processes of atherogenesis such as lipoprotein oxidation, leukocyte trafficking, and vascular SMCs proliferation (14, 146–149). The antioxidant paraoxonase 2 (PON-2) is also abundantly expressed in the ITA, whereas its levels decline in carotids as atherosclerosis progresses (150). Platelet aggregates promote NO-mediated relaxation in the ITA, whereas they induce vasoconstriction in the SV. This underscores a protective role of platelet derived adenine nucleotides and purinergic signaling against thrombotic events after CABG in the ITA (151). Although NO bioavailability declines with aging due to impaired eNOS function, this can be partly restored by telomerase reverse transcriptase expression. Importantly, the ITA displays low telomere loss and absence of senescence associated β-galactosidase activity, suggesting resistance to age related endothelial changes that favor atherogenesis (152–157).
Shear stress profoundly influences ITA endothelium. Acute increases in flow enhance shear stress, inducing NO, prostacyclin, and endothelin-1 release to trigger vasodilation and augment blood supply (158). Sustained flow elevations remodel the artery, dilating it and increasing its diameter (159, 160). The native ITA environment is characterized by predominantly anterograde flow with minimal retrograde components, a pattern that activates cytoprotective and anti-inflammatory pathways (161). Consistently, endothelial- and flow- mediated responses are preserved in the ITA also in patients suffering from CAD and independent of age (162). By contrast, SVGs exposed to abrupt mechanical shear stress undergo inflammation and injury, whereas arterial endothelium resists this response, partly via shear induced MKP-1 expression that suppresses MAPK signaling (161, 163). ITA grafts also adapt to chronic flow changes: early post CABG they exhibit higher flow velocities than SVGs, which normalize at 1 year due to remodeling, while hyperemic flow reserve improves (164). Long-term imaging studies confirm that ITAs grafted for >10 years undergo structural adaptation, yet retain endothelial vasodilation (165). ITA's native and grafted hemodynamic environment elicits adaptive, anti-inflammatory, and cytoprotective responses that preserve vascular integrity and likely underlie its superior patency (166).
These ITAs responses may contribute to protect SVG. For instance, using the SV as a Y- or I- composite graft based on the in situ LITA offers several advantages over an aortocoronary SVG and provides outcomes comparable to the RITA composite graft.
The SV attached to the ITA is exposed to lower pulse pressure than when connected to the ascending aorta, as the ITA has lower diastolic and mean pressures (167). This reduces intimal hyperplasia, atherosclerosis, and endothelial injury. In addition, the SV conduit benefits from exposure to ITA derived vasoprotective mediators, such as NO (168, 169).
A composite SV graft also requires less conduit length than an aortocoronary graft, particularly when sequential anastomoses are used, enabling the use of SV segments with fewer valves. In fact, vein valves contribute to the development of stasis, flow disturbance, and pressure traps in the SV conduit segment and distal to the valve may further accelerate vein atherosclerosis (170). The SV has valves that are more frequently located right below the knee. Harvesting the SV from the upper or lower leg reduces this problem 171, 172).
Avoiding proximal anastomoses to the aorta also eliminates the need for aortic clamping, reducing the risk of embolic stroke and aortic dissection (171, 173).
In a randomized trial of CABG patients, SAVE RITA, Kim et al. showed that SV composite grafts were non-inferior to the right ITA in 1-year patency (97.1% vs. 97.1%, p = 0.958) and in MACE free survival at 1 and 4 years (97.3% and 94.4% vs. 98.2% and 91.1%, p = 0.597) when the SV was harvested using a minimal manipulation technique (174). A retrospective propensity matched CABG study by Hwang et al. further demonstrated comparable 5 year patency rates between SV and arterial composite grafts (93.9% vs. 89.1%, p = 0.246) (175). Compared to bilateral ITA grafting, composite SVGs preserve the RITA for future reinterventions and reduce the risk of perioperative morbidity, including deep sternal wound infection (174).
Nevertheless, studies evaluating SV Y-composite grafts based on the LITA have reported mixed results. Gaudino et al. analyzed 25 patients who received a composite SVG based on the LITA and recommended against the use of composite SVGs because they may steal flow from the LITA, leading to suboptimal LITA patency (72% at 2.5 years). Nevertheless, SVs of the composite grafts demonstrated a 96% patency. Issues were reported in the ITA used to revascularize a coronary artery with <70% stenosis (176).
Lobo Filho et al., using transit time flowmetry at baseline and during dobutamine stress, showed that both LITA and SV adapted appropriately to myocardial demand, and the presence of SV did not alter the physiological blood flow dynamics in the distal segment of the LITA (177).
Similarly, Glineur et al. demonstrated at 6 month follow-up that SV and RITA composite grafts had comparable hemodynamics in terms of pressure gradients at baseline, hyperemia and fractional flow reserve (178).
A propensity matched observational study by Hwang et al. validated the safety of composite SVGs. A total of 483 off-pump CABG patients were included, and 103 composite SVGs were matched with 103 RITA or right gastroepiploic composite grafts. No differences was found in survival, cardiac death, or graft patency up to 12 years, with SV harvested by the ONT technique (179).
The SAVE RITA trial corroborated these results. In 224 patients, SV and RITA Y-composites showed similar 10 year occlusion rates (6.9% vs. 3.4%, p = 0.21), equivalent survival (p = 0.75) and freedom from cardiac death (p = 0.16) (180).
Paterson et al. also reported favorable outcomes in 92 patients with ITA-based SV I-composites, with 89% 10 year freedom from SV occlusion and improved patency in grafts supplying multiple coronary targets (181).
Finally, Katsavrias et al., studied retrospectively 928 patients undergoing SVG to the RCA, inflow was provided either by the RITA (I-graft, n = 546) or the ascending aorta (Ao-graft, n = 382). After propensity matching of 306 patients per group and a median follow-up of 8 years, early outcomes were comparable, but the I-graft group showed superior 10-year survival (90.0% vs. 80.6%, p = 0.016) and freedom from MACE (81.3% vs. 64.7%, p = 0.021). Coronary CTA in a subset (n = 132) demonstrated higher 10-year patency with RITA inflow (82.8% vs. 58.8%, p = 0.003) and smaller SVG lumen diameter (2.7 vs. 3.4 mm, p < 0.0001). These findings suggest that continuous NO delivery from the RITA may enhance SVG performance, allowing it to function more like an arterial conduit.
These results were confirmed by Prapas et al. who studied 699 patients ≤75 years between 2000 and 2018 who underwent CABG with a single SVG directed to the RCA, using either the RITA stump as inflow (I-graft, n = 358, 51.2%) or the ascending aorta (Ao-graft, n = 341, 48.8%). After propensity matching, 272 patients were analyzed per group, with a median follow-up of 88 months (IQR 65–93). Early outcomes were comparable, but long-term survival favored the I-graft strategy (10-year survival: 90.6% vs. 78.2%, p = 0.0266), as did freedom from MACE (85.2% vs. 69.9%, p = 0.0179). At follow-up, SVG patency was significantly higher with I-graft inflow (81.6% vs. 50.7%, p < 0.0001), albeit with a smaller lumen diameter (2.7 ± 0.4 vs. 3.4 ± 0.6 mm, p < 0.0001). These findings suggest that SVG to the RCA shows superior patency and outcomes when supplied by the RITA rather than the ascending aorta (182). In summary, the ITAs demonstrates superior biological and hemodynamic properties in CABG, ensuring long-term patency and survival benefit, conferring NO-based vasoprotective effect when used as the inflow source for SVGs.
Graft configuration using the saphenous vein graft
Graft configuration is a major determinant of patency and long-term outcomes. Traditionally, SVGs are anastomosed to the ascending aorta, where they are exposed to higher pressure, pulsatile flow, and circumferential strain of 10%–15%. In this setting, shear stress increases modestly (10–24 dynes/cm2) and long-term values typically remain <10 dynes/cm2 (183, 184). Isobe et al. reported shear stress values of 2.1 ± 0.3 dynes/cm2 in posterolateral branches and 3.6 ± 0.6 dynes/cm2 in the LAD for SVGs, compared with 13.8 ± 1.1 dynes/cm2 for LITA-LAD grafts (185). Similar findings were confirmed by Shimizu et al., who observed shear stress of 5 ± 2 dynes/cm2 at 1.5 years after surgery, irrespective of coronary stenosis severity (186). Most shear stress values registered remain below the 10 dynes/cm2 threshold (53, 187, 188). Thus, SVGs exposed to the aortic circulation experience similar shear stress to their native venous environment. These shear stress values remain in the atheroprone range.
Low shear stress downregulates eNOS expression, impairing endothelial-dependent vasodilation and reducing NO bioavailability (189–193). This deficiency promotes vasospasm and maladaptive remodeling in aortocoronary SVGs. By contrast, when the SVG is connected to an ITA, this adverse process is attenuated. Continuous NO release from the ITA may stabilize the SVG endothelium and protect the distal coronary territory from atherosclerosis (194). In this graft configuration SVG benefits from ITA derived NO, which stabilizes ECs and mitigates the adverse effects of the arterial environment. Furthermore, ITA would buffer the aortic pressure received directly by the SVG in an aortocoronary bypass. Collectively, these mechanisms support structural integrity and long-term adaptation of the graft.
Nevertheless, composite arterial-venous grafts are not without drawbacks. Mismatches in caliber and endothelial phenotype may predispose to flow competition and steal phenomena. Gaudino and colleagues reported reduced LITA patency distal to the LITA–SV junction, whereas Hwang et al. emphasized benefits of this strategy, including continuous exposure of the SVG to arterial NO, reduced conduit length, and avoidance of direct aortic anastomosis (176, 179). In the latter study, clinical outcomes were excellent, with 96.1% survival at a median follow-up of 128 months, although complete 10-year angiographic data were available for only 47.2% of patients, limiting the strength of conclusions (179). Furthermore, SVGs used as Y-grafts from the LITA have demonstrated comparable 10 year patency to RITA grafts (179). Angiographic studies confirmed that when connected to the LITA, SVGs undergo early lumen reduction and adaptive increases in shear stress within 1 year, without abnormal intima/media thickening. This remodeling shifts shear stress toward arterial values, promoting a more arterialized phenotype and long-term patency (179). Lobo Filho et al. similarly reported preserved patency at 94 ± 49 months with LITA–SVG Y-composites, compared with lower patency in aortocoronary SVGs used for RCA revascularization (195).
Alternative strategies include attaching the SVG to the RITA, particularly in cases of a calcified ascending aorta, where the SVG becomes indistinguishable from the RITA, adapting to its caliber (182, 196). These results support the concept that continuous NO release from arterial inflow enhances SVG adaptation, allowing it to function more like an arterial conduit and achieving outcomes comparable to complete arterial revascularization (197).
Taken together, these data suggest that NO supplementation, whether derived from the LITA, RITA and/or PVAT represents a common mechanistic pathway to improve SVG patency. This unifying hypothesis may explain why SVGs harvested with the ONT technique also demonstrate improved outcomes (197, 198).
Current evidence indicates that maximizing NO bioavailability represents a unifying principle for improving SVG performance. Preservation of PVAT provides a local source of NO in aortocoronary grafts, while composite configurations with the ITA ensure continuous distal NO delivery (Figure 3). The combination of these two complementary procedures creates a synergistic vasoprotective environment that stabilizes the endothelium, attenuates maladaptive remodeling, and likely explains the superior patency and clinical outcomes observed with these strategies.
Figure 3
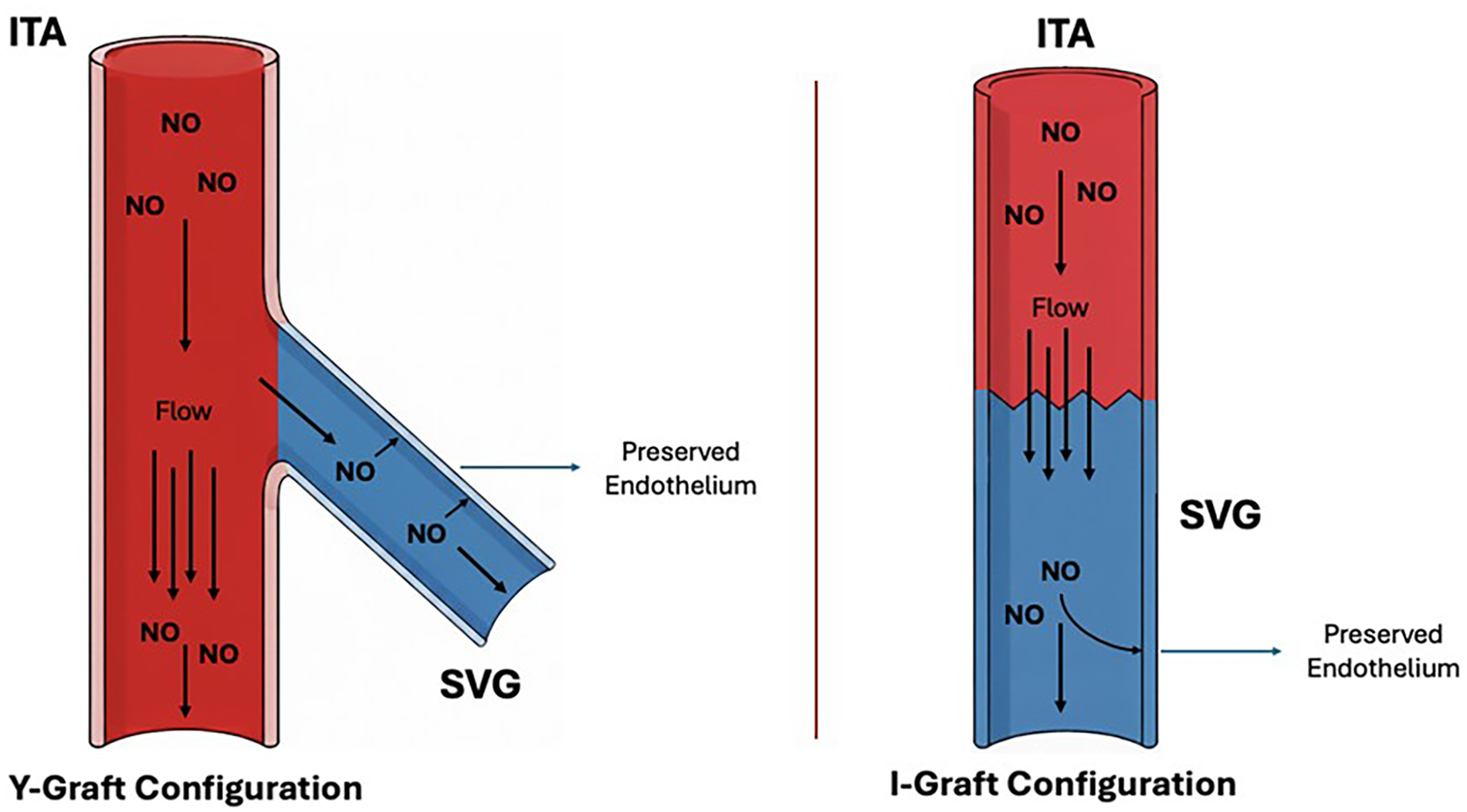
Saphenous vein graft and coronary bypass configurations. Y- and I-graft configurations using the internal thoracic artery and saphenous vein graft. In both configurations, inflow from the internal thoracic artery provides a continuous and superior source of nitric oxide and lower pulse pressure compared to an aortocoronary saphenous vein graft. These conditions preserve the endothelial integrity of saphenous vein graft, ensuring nitric oxide supply to the coronaries. Both configurations support vascular protection and long-term graft patency.
When PVAT is removed, as in OC or endoscopic SV harvesting, external support devices have been proposed to reduce SVG intimal hyperplasia. In their preclinical study, Jeremy et al. have shown that loose-fitting sheaths enhanced SVG remodeling and reduced neointimal hyperplasia in porcine models (199). In a phase I pilot study of 20 patients undergoing elective on-pump CABG, paired SVGs were tested with or without a Dacron stent (Extent). At six months, all 17 Extent-supported grafts were thrombosed, whereas all ITA-supported grafts and untreated SVGs stayed patent. Thrombosis was attributed to the rigidity, oversizing, or incomplete design of the Extent stent, which most likely caused postoperative kinking or distortion. These complications were not observed in previous porcine models. Despite the results, the authors sustained that flexible, nonrestrictive, biodegradable external support devices could have offered a safer option for future clinical use (200). This prompted the first clinical trial of the venous external support (VEST) device by Taggart et al., which showed that external stenting with a cobalt-chromium alloy modestly reduced diffuse intimal hyperplasia and improved lumen uniformity at 1 year, without altering SVG failure rates (201). Further studies found that the VEST does not lower rates of repeat revascularization or SVG occlusion, but it reliably reduces intimal hyperplasia and better preserves lumen geometry (202–204). The VEST trial follow-up is ongoing to elucidate the impact of external stenting on long-term SVG patency and clinical outcomes after CABG.
In summary, graft configuration strongly influences SVG adaptation and long-term patency. Aortocoronary SVGs are exposed to low, atheroprone shear stress and impaired NO bioavailability. Connecting SVGs to the ITA enhances NO-mediated endothelial stabilization, promotes arterial-like remodeling, and improves long-term outcomes. The VEST reduces SVG intimal hyperplasia and preserves lumen geometry, although follow-up data is necessary to evaluate long-term SVG patency and clinical outcomes.
Secondary prevention of saphenous vein graft failure
Postoperatively, the prevention of secondary SVG failure is largely attributable to acute thrombosis and late atherosclerosis. The protective role of antiplatelet and lipid lowering agents in maintaining graft patency following CABG has been evaluated.
Early randomized clinical trials established aspirin as the cornerstone of therapy against SVG failure. In the Veterans Administration Cooperative Study, aspirin significantly reduced SVG occlusion compared with placebo at one year (15.8% vs. 22.6%, p = 0.029) (205). A subsequent meta-analysis of 17 randomized trials confirmed that low to medium daily doses of aspirin (100–325 mg), initiated within six hours after CABG, achieved maximal efficacy without increasing bleeding complications (206). By contrast, delayed aspirin initiation (≥24 h) conferred no benefit (207). For long-term maintenance, low dose aspirin (75–100 mg daily) is sufficient to suppress thromboxane A2 and address interindividual variability in response, though twice daily administration may be considered in the immediate postoperative setting due to increased platelet turnover following cardiopulmonary bypass (208). Although most individual trials were not powered for mortality outcomes, pooled data from 4413 patients and a total of 13,163 grafts, demonstrated that graft failure is a strong predictor of adverse cardiac events (OR 3.98, 95% CI 3.54–4.47) and mortality (OR 2.79, 95% CI 2.01–3.89), thereby indirectly underscoring aspirin's clinical benefit (209). Compared to aspirin, ticagrelor monotherapy did not improve SVG patency (210–212). In addition, factor Xa inhibitor rivaroxaban, either alone or in combination with aspirin, did not reduce graft failure as demonstrated in the COMPASS trial (213). By contrast, dual antiplatelet therapy (DAPT) has consistently shown superior efficacy over aspirin alone. A meta-analysis of 11 studies including 25,728 patients showed that aspirin combined with clopidogrel reduced SVG occlusion (RR 0.59, 95% CI 0.43–0.82), albeit at the cost of increased bleeding (RR 1.17, 95% CI 1.00–1.37) (214). A network meta-analysis of 20 randomized trials with a total of 4,803 patients confirmed improved graft patency with aspirin plus ticagrelor (OR 0.50, 95% CI 0.31–0.79) or aspirin plus clopidogrel (OR 0.60, 95% CI 0.42–0.86) compared to aspirin alone (215). Moreover, an individual patient level meta-analysis of four randomized trials (1,316 patients) demonstrated that aspirin plus ticagrelor reduced SVG failure rates (11.2% vs. 20%; OR 0.51, 95% CI 0.35–0.74), though at the expense of increased clinically relevant bleeding (22.1% vs. 8.7%) (216). To date, no head to head randomized comparisons between clopidogrel and ticagrelor exist, though ticagrelor is associated with more potent and consistent platelet inhibition (217, 218). Ongoing studies (ODIN, TOP-CABG) are expected to clarify whether short term or de-escalation DAPT strategies may optimize the balance between ischemic protection and bleeding risk (219, 220).
Lipid lowering therapy represents another cornerstone of secondary VGF prevention after CABG. The Post-CABG trial demonstrated that aggressive LDL-C reduction (<85 mg/dL) with lovastatin significantly decreased graft disease progression by 31% over a four year follow up period (221). Observational studies further suggest that achieving LDL-C < 100 mg/dL is associated with higher graft patency rates (222). The ACTIVE trial did not demonstrate superiority of high dose vs. moderate dose atorvastatin for SVG occlusion, although its interpretation was limited by small sample size (223). Beyond lipid lowering, statins exert pleiotropic effects including enhancement of endothelial function, increased NO bioavailability, and anti-inflammatory actions (224). More recently, proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a potential therapeutic target, with elevated circulating levels associated with SVG disease (225). The NEWTON-CABG CardioLink-5 trial randomized 782 post CABG patients with ≥2 SVGs to evolocumab or placebo on top of statin therapy. Despite achieving a 48.4% placebo-adjusted LDL-C reduction at 24 months, SVG disease rates were similar between groups (21.7% vs. 19.7%, p = 0.44). These results suggest that LDL-C lowering does not significantly impact early SVG failure mechanisms (226). Elevated HDL levels may help mitigate the development of SVG disease and lower the likelihood of adverse events after CABG. Jerzewski et al. showed that HDL <40 mg/dL was associated with higher SVG occlusion (6.8% vs. 4.0%, OR 3.2; p = 0.12) and more intimal hyperplasia (p = 0.10). HDL >60 mg/dL was associated with the least intimal hyperplasia (p = 0.01) (227).
Recent studies have explored strategies involving NO pharmacological strategy against SVG failure. In isolated SV segments from 30 patients, three NO donating aspirin adducts induced dose dependent relaxation, stimulated cGMP formation, and inhibited vascular SMCs proliferation, similar to sodium nitroprusside, whereas aspirin alone was inactive. This suggests that NO pharmacological therapy has potential to prevent thrombosis, vasospasm, and neointimal hyperplasia (228). In patients with type 2 diabetes mellitus, NO releasing aspirin restored impaired endothelium dependent vasodilation in SVGs, achieving maximal relaxation comparable to non-diabetic controls (56 ± 12% vs. 61 ± 11%), despite histological abnormalities such as intimal hyperplasia and endothelial degeneration (229). Complementing these findings, S-nitrosoglutathione induced vasorelaxation in SVs predominantly via cGMP dependent mechanisms, highlighting its potential to prevent graft spasm (230). Gene therapy also represents another innovative and viable approach. SVs transduced ex vivo with adenovirus vector encoding bovine endothelial eNOS showed endothelial and adventitial expression of recombinant eNOS, whereas control veins expressed only endogenous enzyme. Nitrite generation increased from 130.3 ± 19.9 to 1,420.0 ± 298.2 nM/mg (n = 3; p < 0.05), with maximal relaxation to calcium ionophore rising from 17.4 ± 7.4% to 32 ± 4.5% (n = 6; p < 0.05). Adenovirus-mediated eNOS transfer produced functional NO, suggesting potential to reduce early SVG thrombosis (231) Collectively, these studies support the concept that NO based therapies may enhance SVG function and reduce complications following CABG.
Lifestyle and behavioral factors are strongly associated with the risk of SVG failure. Active smoking nearly doubles the risk of graft occlusion at 1 year (HR 1.96, 95% CI 1.40–2.75) and accelerates progression of graft atherosclerosis (9, 232, 233). In 40 CABG patients (20 with type II diabetes, 20 without), diabetic SVG showed greater intimal fibrosis (1.95 ± 0.99 vs. 1.3 ± 0.8; p = 0.04) and ultrastructural changes including endothelial vacuolization and collagen accumulation. eNOS expression was significantly reduced in diabetics (endothelium: 2.10 ± 0.64 vs. 1.55 ± 0.68, p = 0.01; tunica media: 1.75 ± 0.63 vs. 1.2 ± 0.52, p = 0.007). These findings suggest diabetes promotes adverse structural and molecular alterations that may predispose to early graft failure (234).
In 276 hypertensive CABG patients followed for 3 years, higher postoperative BP (>130/80 mmHg) was strongly associated with SVG occlusion. Logistic regression confirmed BP as an independent risk factor (per patient OR 3.10, 95% CI 1.84–5.21; per graft OR 2.60, 95% CI 1.74–3.89; both p < 0.001). Use of ACEI/ARB or CCB was more common in the non-occlusion group, but no specific antihypertensive regimen correlated with graft patency (235).
Current guidelines recommend individualized, symptoms and risk-based surveillance post-CABG (236). Coronary computed tomographic angiography (CCTA) represents the primary modality for detecting graft failure, as duplex ultrasound has shown limited utility for coronary SVGs due to anatomic depth (236). CCTA offers high diagnostic accuracy, with reported sensitivity of ≈96% and specificity of ≈97% (237–239). Given the clinical importance of identifying early SVG failure, targeted follow-up should be prioritized in high-risk patients such as those with diabetes, multiple vein grafts, symptomatic ischemia, or suboptimal secondary prevention (9).
Thus, optimal secondary prevention and monitoring significantly improves graft patency and clinical outcomes, underscoring its central role in SVG management.
Practical implications: nitric oxide-based recommedations for saphenous vein graft preservation
-
Endoscopic harvesting of the saphenous vein performed by an expert harvester is recommended in patients at high risk of leg wound complications. The open no-touch harvesting technique remains optimal in patients at low risk of wound complications.
-
Perivascular adipose tissue supports saphenous vein graft by mechanically protecting the vessel, preventing graft endothelial injuries, and secreting endothelial stabilizing molecules.
-
The saphenous vein should be stored in a buffered solution and high-pressure distension (>200 mmHg) should be avoided, using pressure syringes.
-
Connecting the saphenous vein in a Y- or I-graft to the internal thoracic artery shows improved long-term patency, while the saphenous vein aortocoronary graft shows reduced long-term patency.
-
Aspirin administration (100–325 mg) within six hours after coronary artery bypass grafting showed maximal efficacy in reducing saphenous vein graft occlusion, with reduced bleeding risk.
-
Dual antiplatelet therapy, despite increased bleeding risk, has shown superior efficacy over aspirin alone in reducing saphenous vein graft occlusion.
-
Secondary prevention strategies and clinical monitoring significantly improves saphenous graft patency and clinical outcomes.
Conclusion
In surgical revascularization, the SV remains an indispensable conduit, yet SVG failure represents the Achilles' heel of CABG. Unlike arterial grafts, the SVG is a passive conduit, unable to accommodate hemodynamic stressors and prone to endothelial injury and maladaptive remodeling. NO has emerged as a unifying molecular target to prevent SVG failure, given its role in endothelial stability, vasomotor control, and long-term conduit adaptation.
Within this framework, NO-focused strategies could optimize SVG performance. Surgically, priority should be given to preserving the endothelial integrity and NO bioavailability through (1) ONT or expert-led endoscopic harvesting, (2) preservation of the PVAT derived from the native SV, (3) control of distension pressure (< 200 mmHg) through a pressure-controlled syringe (4) usage of a pH neutral (buffer) storage medium, and (5) ITA-based Y- or I-composite graft configurations. Pharmacological management should support the surgical efforts through (6) individualized antiplatelet therapy (favoring DAPT over aspirin, considering the bleeding risk) and (7) lipid-lowering therapy with statins. Secondary prevention should prioritize strict management of cardiovascular risk factors and close monitoring of individuals at increased risk for SVG failure.
Elucidating the impact of local NO bioavailability through randomized clinical trials focusing on SV harvesting, PVAT, conduit handling, grafting techniques, and pharmacologic interventions represent a crucial step in preventing SVG failure and optimizing graft patency. Future strategies to enhance NO, such as NO-donating medications or gene therapy, appear to be promising approaches to reducing SVG failure and improving long-term clinical outcomes in CABG patients.
Statements
Author contributions
MD: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SoP: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. GF: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. SA: Data curation, Investigation, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. BT: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. IC: Conceptualization, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. JN: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. OJ: Methodology, Supervision, Validation, Writing – review & editing. StP: Methodology, Supervision, Visualization, Writing – review & editing. AA: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. KK: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. AuD: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. CZ: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – review & editing, Supervision. AT: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. VL: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – review & editing, Data curation, Writing – original draft. TC: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing. HK: Conceptualization, Investigation, Writing – review & editing, Methodology. AnD: Writing – review & editing, Supervision, Validation, Visualization. JL: Validation, Visualization, Writing – review & editing. LR: Validation, Visualization, Writing – review & editing, Supervision. MR: Validation, Visualization, Writing – review & editing, Software, Supervision. DB: Supervision, Writing – review & editing, Conceptualization, Investigation, Methodology. AI: Supervision, Validation, Visualization, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Resources. FM: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. NP: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. RK: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing, Data curation, Funding acquisition, Project administration, Resources. MG: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. AC: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. Michele Dell'Aquila is supported by Rick and Alleen Duffy Fellowship in Cardiothoracic Surgery at Northwell Health.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor BW declared a past co-authorship with the author MG.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ignarro LJ . After 130 years, the molecular mechanism of action of nitroglycerin is revealed. Proc Natl Acad Sci U S A. (2002) 99(12):7816–7. 10.1073/pnas.132271799
2.
Marsh N Marsh A . A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol. (2000) 27(4):313–9. 10.1046/j.1440-1681.2000.03240.x
3.
Divakaran S Loscalzo J . The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J Am Coll Cardiol. (2017) 70(19):2393–410. 10.1016/j.jacc.2017.09.1064
4.
Nossaman VE Nossaman BD Kadowitz PJ . Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol Rev. (2010) 18(4):190–7. 10.1097/CRD.0b013e3181c8e14a
5.
Moncada S Higgs EA . The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. (2006) 147(Suppl 1):S193–201. 10.1038/sj.bjp.0706458
6.
Ignarro LJ . Nitric oxide is not just blowing in the wind. Br J Pharmacol. (2019) 176(2):131–4. 10.1111/bph.14540
7.
de Berrazueta JR . The nobel prize for nitric oxide. The unjust exclusion of Dr. Salvador moncada. Rev Esp Cardiol. (1999) 52(4):221–6. 10.1016/S0300-8932(99)74902-X
8.
Yan Y Kamenshchikov N Zheng Z Lei C . Inhaled nitric oxide and postoperative outcomes in cardiac surgery with cardiopulmonary bypass: a systematic review and meta-analysis. Nitric Oxide Biol Chem. (2024) 146:64–74. 10.1016/j.niox.2024.03.004
9.
Xenogiannis I Zenati M Bhatt DL Rao SV Rodés-Cabau J Goldman S et al Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation. (2021) 144(9):728–45. 10.1161/CIRCULATIONAHA.120.052163
10.
Krüger-Genge A Blocki A Franke RP Jung F . Vascular endothelial cell biology: an update. Int J Mol Sci. (2019) 20(18):4411. 10.3390/ijms20184411
11.
Vannini F Kashfi K Nath N . The dual role of iNOS in cancer. Redox Biol. (2015) 6:334–43. 10.1016/j.redox.2015.08.009
12.
Liu X Miller MJ Joshi MS Sadowska-Krowicka H Clark DA Lancaster JR . Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. (1998) 273(30):18709–13. 10.1074/jbc.273.30.18709
13.
Vallance P Chan N . Endothelial function and nitric oxide: clinical relevance. Heart Br Card Soc. (2001) 85(3):342–50. 10.1136/heart.85.3.342
14.
Förstermann U Sessa WC . Nitric oxide synthases: regulation and function. Eur Heart J. (2011) 33(7):829. 10.1093/eurheartj/ehr304
15.
Alderton WK Cooper CE Knowles RG . Nitric oxide synthases: structure, function and inhibition. Biochem J. (2001) 357(Pt 3):593–615. 10.1042/bj3570593
16.
Kröncke KD Fehsel K Kolb-Bachofen V . Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. (1998) 113(2):147–56. 10.1046/j.1365-2249.1998.00648.x
17.
Farahani A Farahani A Kashfi K Ghasemi A . Inducible nitric oxide synthase (iNOS): more than an inducible enzyme? Rethinking the classification of NOS isoforms. Pharmacol Res. (2025) 216:107781. 10.1016/j.phrs.2025.107781
18.
Huang KT Yin CC Wu JH Huang HH . Superoxide determines nitric oxide uptake rate by vascular smooth muscle cells. FEBS Lett. (2005) 579(20):4349–54. 10.1016/j.febslet.2005.06.071
19.
Chien S . Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. (2007) 292(3):H1209–1224. 10.1152/ajpheart.01047.2006
20.
Charbonier FW Zamani M Huang NF . Endothelial cell mechanotransduction in the dynamic vascular environment. Adv Biosyst. (2019) 3(2):1800252. 10.1002/adbi.201800252
21.
Calafiore AM Prapas S Gaudino M . Arterial conduits for coronary bypass grafting: the set-point concept. Eur Heart J. (2025) 46(10):922–5. 10.1093/eurheartj/ehae908
22.
Dong M Chen M Zhang Y He X Min J Tan Y et al Oscillatory shear stress promotes endothelial senescence and atherosclerosis via STING activation. Biochem Biophys Res Commun. (2024) 715:149979. 10.1016/j.bbrc.2024.149979
23.
Chen L Qu H Liu B Chen BC Yang Z Shi DZ et al Low or oscillatory shear stress and endothelial permeability in atherosclerosis. Front Physiol. (2024) 15:1432719. 10.3389/fphys.2024.1432719
24.
Raddino R Caretta G Teli M Bonadei I Robba D Zanini G et al Nitric oxide and cardiovascular risk factors. Heart Int. (2007) 3(1):18. 10.1177/1826186807003001-203
25.
Deanfield JE Halcox JP Rabelink TJ . Endothelial function and dysfunction: testing and clinical relevance. Circulation. (2007) 115(10):1285–95. 10.1161/CIRCULATIONAHA.106.652859
26.
Li H Wallerath T Münzel T Förstermann U . Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide Biol Chem. (2002) 7(3):149–64. 10.1016/S1089-8603(02)00111-8
27.
Förstermann U Münzel T . Endothelial nitric oxide synthase in vascular disease. Circulation. (2006) 113(13):1708–14. 10.1161/CIRCULATIONAHA.105.602532
28.
Kelm M . Nitric oxide metabolism and breakdown. Biochim Biophys Acta BBA—Bioenerg. (1999) 1411(2):273–89. 10.1016/s0005-2728(99)00020-1
29.
Brown JC Gerhardt TE Kwon E . Risk factors for coronary artery disease. In: StatPearls Publishing LLC, editors. StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK554410/(Accessed June 6, 2024).
30.
Shahjehan RD Sharma S Bhutta BS . Coronary artery disease. In: StatPearls Publishing LLC, editors. StatPearls. Treasure Island (FL): StatPearls Publishing (2025). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK564304/(Accessed August 13, 2025).
31.
Writing Committee Members, LawtonJSTamis-HollandJEBangaloreSBatesERBeckieTMBischoffJMet al2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(2):e21–129. 10.1016/j.jacc.2021.09.006
32.
Byrne RA Fremes S Capodanno D Czerny M Doenst T Emberson JR et al 2022 Joint ESC/EACTS review of the 2018 guideline recommendations on the revascularization of left main coronary artery disease in patients at low surgical risk and anatomy suitable for PCI or CABG. Eur Heart J. (2023) 44(41):4310–20. 10.1093/eurheartj/ehad476
33.
Gaudino M Taggart D Suma H Puskas JD Crea F Massetti M . The choice of conduits in coronary artery bypass surgery. J Am Coll Cardiol. (2015) 66(15):1729–37. 10.1016/j.jacc.2015.08.395
34.
Dobell AR . Arthur vineberg and the internal mammary artery implantation procedure. Ann Thorac Surg. (1992) 53(1):167–9. 10.1016/0003-4975(92)90785-3
35.
Sabiston DC . Direct surgical management of congenital and acquired lesions of the coronary circulation. Prog Cardiovasc Dis. (1963) 6:299–316. 10.1016/S0033-0620(63)80040-7
36.
Melly L Torregrossa G Lee T Jansens JL Puskas JD . Fifty years of coronary artery bypass grafting. J Thorac Dis. (2018) 10(3):1960–7. 10.21037/jtd.2018.02.43
37.
Dimagli A Soletti G Harik L Perezgrovas Olaria R Cancelli G An KR et al Angiographic outcomes for arterial and venous conduits used in CABG. J Clin Med. (2023) 12(5):2022. 10.3390/jcm12052022
38.
Caliskan E de Souza DR Böning A Liakopoulos OJ Choi YH Pepper J et al Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol. (2020) 17(3):155–69. 10.1038/s41569-019-0249-3
39.
Hall AB Brilakis ES . Saphenous vein graft failure: seeing the bigger picture. J Thorac Dis. (2019) 11(Suppl 9):S1441–4. 10.21037/jtd.2019.03.09
40.
Goldman S Zadina K Moritz T Ovitt T Sethi G Copeland JG et al Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a department of veterans affairs cooperative study. J Am Coll Cardiol. (2004) 44(11):2149–56. 10.1016/j.jacc.2004.08.064
41.
Harik L An KR Dimagli A Perezgrovas-Olaria R Soletti GJ Leith J et al Choice of conduit for coronary artery bypass grafting: technical, anatomic, and pharmacologic considerations. Vessel Plus. (2023) 7:30. 10.20517/2574-1209.2023.124
42.
Guida GA Angelini GD . Pathophysiology and mechanisms of saphenous vein graft failure. Braz J Cardiovasc Surg. (2022) 37(Spec 1):32–7. 10.21470/1678-9741-2022-0133
43.
Shukla N Jeremy JY . Pathophysiology of saphenous vein graft failure: a brief overview of interventions. Curr Opin Pharmacol. (2012) 12(2):114–20. 10.1016/j.coph.2012.01.001
44.
Dehghani T Panitch A . Endothelial cells, neutrophils and platelets: getting to the bottom of an inflammatory triangle. Open Biol. (2020) 10(10):200161. 10.1098/rsob.200161
45.
Taggart DP Gavrilov Y Krasopoulos G Rajakaruna C Zacharias J De Silva R et al External stenting and disease progression in saphenous vein grafts two years after coronary artery bypass grafting: a multicenter randomized trial. J Thorac Cardiovasc Surg. (2022) 164(5):1532–1541.e2. 10.1016/j.jtcvs.2021.03.120
46.
Girão-Silva T Fonseca-Alaniz MH Ribeiro-Silva JC Lee J Patil NP Dallan LA et al High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells. Sci Rep. (2021) 11(1):13493. 10.1038/s41598-021-93081-3
47.
McQueen LW Ladak SS Zakkar M . Acute shear stress and vein graft disease. Int J Biochem Cell Biol. (2022) 144:106173. 10.1016/j.biocel.2022.106173
48.
Lemson MS Tordoir JH Daemen MJ Kitslaar PJ . Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. (2000) 19(4):336–50. 10.1053/ejvs.1999.1040
49.
Gooch KJ Firstenberg MS Shrefler BS Scandling BW . Biomechanics and mechanobiology of saphenous vein grafts. J Biomech Eng. (2018) 140:020804. 10.1115/1.4038705
50.
Stick C Hiedl U Witzleb E . Venous pressure in the saphenous vein near the ankle during changes in posture and exercise at different ambient temperatures. Eur J Appl Physiol. (1993) 66(5):434–8. 10.1007/BF00599617
51.
Engelberger RP Keo HH Blaettler W Fahrni J Baumann F Diehm N et al The impact of orthostatic challenge on arteriovenous hemodynamics and volume changes of the lower extremity. J Vasc Surg Venous Lymphat Disord. (2013) 1(3):250–6. 10.1016/j.jvsv.2012.12.001
52.
Zakkar M Angelini GD Emanueli C . Regulation of vascular endothelium inflammatory signalling by shear stress. Curr Vasc Pharmacol. (2016) 14(2):181–6. 10.2174/1570161114666151202205139
53.
Khan MO Tran JS Zhu H Boyd J Packard RRS Karlsberg RP et al Low wall shear stress is associated with saphenous vein graft stenosis in patients with coronary artery bypass grafting. J Cardiovasc Transl Res. (2021) 14(4):770–81. 10.1007/s12265-020-09982-7
54.
Kudo FA Muto A Maloney SP Pimiento JM Bergaya S Fitzgerald TN et al Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. (2007) 27(7):1562–71. 10.1161/ATVBAHA.107.143032
55.
Aoyagi Y Schwartz AW Li Z Bai H Gonzalez L Lazcano Etchebarne C et al Changes in vascular identity during vascular remodeling. JVS-Vasc Sci. (2025) 6:100282. 10.1016/j.jvssci.2025.100282
56.
Niklason L Dai G . Arterial venous differentiation for vascular bioengineering. Annu Rev Biomed Eng. (2018) 20:431–47. 10.1146/annurev-bioeng-062117-121231
57.
Joddar B Firstenberg MS Reen RK Varadharaj S Khan M Childers RC et al Arterial levels of oxygen stimulate intimal hyperplasia in human saphenous veins via a ROS-dependent mechanism. PLoS One. (2015) 10(3):e0120301. 10.1371/journal.pone.0120301
58.
Berard X Déglise S Alonso F Saucy F Meda P Bordenave L et al Role of hemodynamic forces in the ex vivo arterialization of human saphenous veins. J Vasc Surg. (2013) 57(5):1371–82. 10.1016/j.jvs.2012.09.041
59.
Ladak SS McQueen LW Layton GR Aujla H Adebayo A Zakkar M . The role of endothelial cells in the onset, development and modulation of vein graft disease. Cells. (2022) 11(19):3066. 10.3390/cells11193066
60.
Owens CD Gasper WJ Rahman AS Conte MS . Vein graft failure. J Vasc Surg. (2015) 61(1):203–16. 10.1016/j.jvs.2013.08.019
61.
Vuong NL Elfaituri MK Eldoadoa M Karimzadeh S Mokhtar MA Eid PS et al Saphenous vein harvesting techniques for coronary artery bypass grafting: a systematic review and meta-analysis. Coron Artery Dis. (2022) 33(2):128–36. 10.1097/MCA.0000000000001048
62.
Durko A Thuijs D Mahtab E Bekkers J . Conventional open harvesting of the great saphenous vein as a conduit for coronary artery bypass grafting. Multimed Man Cardiothorac Surg MMCTS. (2018) 2018. 10.1510/mmcts.2018.014
63.
Souza DSR Arbeus M Botelho Pinheiro B Filbey D . The no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimed Man Cardiothorac Surg MMCTS. (2009) 2009(731):mmcts.2008.003624. 10.1510/mmcts.2008.003624
64.
Samano N Dashwood M Souza D . No-touch vein grafts and the destiny of venous revascularization in coronary artery bypass grafting—a 25th anniversary perspective. Ann Cardiothorac Surg. (2018) 7(5):68185–685. 10.21037/acs.2018.05.15
65.
Cheanvechai C Groves LK Surakiatchanukul S Tanaka N Effler DB Shirey EK et al Bridge saphenous vein graft. J Thorac Cardiovasc Surg. (1975) 70(1):063–8. 10.1016/S0022-5223(19)40380-2
66.
Mahmoud SA Alahmadi MH Widrich J . Endoscopic vein harvesting. In: StatPearls Publishing LLC, editors. StatPearls. Treasure Island (FL): StatPearls Publishing (2025). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK553206/(Accessed August 14, 2025).
67.
Tian M Wang X Sun H Feng W Song Y Lu F et al No-Touch versus conventional vein harvesting techniques at 12 months after coronary artery bypass grafting surgery: multicenter randomized, controlled trial. Circulation. (2021) 144(14):1120–9. 10.1161/CIRCULATIONAHA.121.055525
68.
Tian M Wang X Feng W Wang H Liu S Liu Z et al No-touch versus conventional vein in coronary artery bypass grafting: three year follow-up of multicentre randomised PATENCY trial. Br Med J. (2025) 389:e082883. 10.1136/bmj-2024-082883
69.
Zenati MA Bhatt DL Bakaeen FG Stock EM Biswas K Gaziano JM et al Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med. (2019) 380(2):132–41. 10.1056/NEJMoa1812390
70.
Zenati MA Bhatt DL Stock EM Hattler B Wagner TH Bakaeen FG et al Intermediate-term outcomes of endoscopic or open vein harvesting for coronary artery bypass grafting. JAMA Netw Open. (2021) 4(3):e211439. 10.1001/jamanetworkopen.2021.1439
71.
Desai P Kiani S Thiruvanthan N Henkin S Kurian D Ziu P et al Impact of the learning curve for endoscopic vein harvest on conduit quality and early graft patency. Ann Thorac Surg. (2011) 91(5):1385–92. 10.1016/j.athoracsur.2011.01.079
72.
Rousou LJ Taylor KB Lu XG Healey N Crittenden MD Khuri SF et al Saphenous vein conduits harvested by endoscopic technique exhibit structural and functional damage. Ann Thorac Surg. (2009) 87(1):62–70. 10.1016/j.athoracsur.2008.08.049
73.
Milutinović A Zorc-Pleskovič R . Endothelial loss during the surgical procedure in saphenous veins harvested by open and endoscopic techniques in coronary artery bypass surgery. Bosn J Basic Med Sci. (2020) 20(4):451–8. 10.17305/bjbms.2020.4656
74.
Williams JB Harskamp RE Bose S Lawson JH Alexander JH Smith PK et al The preservation and handling of vein grafts in current surgical practice: findings of a survey among cardiovascular surgeons of top-ranked US hospitals. JAMA Surg. (2015) 150(7):681–3. 10.1001/jamasurg.2015.0404
75.
Biswas KS Thatte HS Najjar SF Rhee JH Birjiniuk V Crittenden MD et al Multi-photon microscopy in the evaluation of human saphenous vein. J Surg Res. (2001) 95(1):37–43. 10.1006/jsre.2000.6019
76.
Wilbring M Tugtekin SM Zatschler B Ebner A Reichenspurner H Matschke K et al Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur J Cardiothorac Surg. (2011) 40(4):811–5. 10.1016/j.ejcts.2011.01.02
77.
Gundry SR Jones M Ishihara T Ferrans VJ . Optimal preparation techniques for human saphenous vein grafts. Surgery. (1980) 88(6):785–94.
78.
Lawrie GM Weilbacher DE Henry PD . Endothelium-dependent relaxation in human saphenous vein grafts. Effects of preparation and clinicopathologic correlations. J Thorac Cardiovasc Surg. (1990) 100(4):612–20. 10.1016/S0022-5223(19)35507-2
79.
Zerkowski HR Knocks M Konerding MA Doetsch N Roth G Hakim K et al Endothelial damage of the venous graft in CABG. Influence of solutions used for storage and rinsing on endothelial function. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. (1993) 7(7):376–82. 10.1016/1010-7940(93)90070-R
80.
Ben Ali W Bouhout I Perrault LP . The effect of storage solutions, gene therapy, and antiproliferative agents on endothelial function and saphenous vein graft patency. J Card Surg. (2018) 33(5):235–42. 10.1111/jocs.13608
81.
Harskamp RE Alexander JH Schulte PJ Brophy CM Mack MJ Peterson ED et al Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA Surg. (2014) 149(8):798–805. 10.1001/jamasurg.2014.87
82.
Thatte HS Biswas KS Najjar SF Birjiniuk V Crittenden MD Michel T et al Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann Thorac Surg. (2003) 75(4):1145–52. 10.1016/S0003-4975(02)04705-7
83.
Perrault LP Carrier M Voisine P Olsen PS Noiseux N Jeanmart H et al Sequential multidetector computed tomography assessments after venous graft treatment solution in coronary artery bypass grafting. J Thorac Cardiovasc Surg. (2021) 161(1):96–106.e2. 10.1016/j.jtcvs.2019.10.115
84.
Nazari-Shafti TZ Thau H Zacharova E Beez CM Exarchos V Neuber S et al Endothelial damage inhibitor preserves the integrity of venous endothelial cells from patients undergoing coronary bypass surgery. Eur J Cardiothorac Surg. (2023) 64(6):ezad327. 10.1093/ejcts/ezad327
85.
Aschacher T Baranyi U Aschacher O Eichmair E Messner B Zimpfer D et al A novel endothelial damage inhibitor reduces oxidative stress and improves cellular integrity in radial artery grafts for coronary artery bypass. Front Cardiovasc Med. (2021) 8:736503. 10.3389/fcvm.2021.736503
86.
Tekin I Demir M Özdem S . Effect of different storage solutions on oxidative stress in human saphenous vein grafts. J Cardiothorac Surg. (2022) 17(1):7. 10.1186/s13019-022-01752-7
87.
Bush HL McCabe ME Nabseth DC . Functional injury of vein graft endothelium. Role of hypothermia and distention. Arch Surg Chic Ill 1960. (1984) 119(7):770–4. 10.1001/archsurg.1984.01390190014003
88.
Roubos N Rosenfeldt FL Richards SM Conyers RA Davis BB . Improved preservation of saphenous vein grafts by the use of glyceryl trinitrate-verapamil solution during harvesting. Circulation. (1995) 92(9 Suppl):II31–36. 10.1161/01.CIR.92.9.31
89.
Stigler R Steger C Schachner T Holfeld J Edlinger M Grimm M et al The impact of distension pressure on acute endothelial cell loss and neointimal proliferation in saphenous vein grafts. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. (2012) 42(4):e74–79. 10.1093/ejcts/ezs402
90.
Galea J Armstrong J Francis SE Cooper G Crossman DC Holt CM . Alterations in c-fos expression, cell proliferation and apoptosis in pressure distended human saphenous vein. Cardiovasc Res. (1999) 44(2):436–48. 10.1016/S0008-6363(99)00220-5
91.
Li FD Eagle S Brophy C Hocking KM Osgood M Komalavilas P et al Pressure control during preparation of saphenous veins. JAMA Surg. (2014) 149(7):655–62. 10.1001/jamasurg.2013.5067
92.
Viaro F Capellini VK Celotto AC Carlotti CG Rodrigues AJ Reis GS et al Immunohistochemical evaluation of three nitric oxide synthase isoforms in human saphenous vein exposed to different degrees of distension pressures. Cardiovasc Pathol. (2010) 19(6):e211–20. 10.1016/j.carpath.2009.11.002
93.
Sandner S Antoniades C Caliskan E Czerny M Dayan V Fremes SE et al Intra-operative and post-operative management of conduits for coronary artery bypass grafting: a clinical consensus statement of the European society of cardiology working group on cardiovascular surgery and the European association for cardio-thoracic surgery coronary task force. Eur Heart J. (2025) 46(1):19–34. 10.1093/eurheartj/ehae654
94.
Hillock-Watling C Gotlieb AI . The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc Pathol. (2022) 61:107459. 10.1016/j.carpath.2022.107459
95.
Samano N Geijer H Liden M Fremes S Bodin L Souza D . The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: a randomized trial. J Thorac Cardiovasc Surg. (2015) 150(4):880–8. 10.1016/j.jtcvs.2015.07.027
96.
Mikami T Dashwood MR Kawaharada N Furuhashi M . An obligatory role of perivascular adipose tissue in improved saphenous vein graft patency in coronary artery bypass grafting. Circ J. (2024) 88(6):845–52. 10.1253/circj.CJ-23-0581
97.
Dreifaldt M Mannion JD Geijer H Lidén M Bodin L Souza D . The no-touch saphenous vein is an excellent alternative conduit to the radial artery 8 years after coronary artery bypass grafting: a randomized trial. J Thorac Cardiovasc Surg. (2021) 161(2):624–30. 10.1016/j.jtcvs.2019.09.177
98.
Dashwood MR Dooley A Shi-Wen X Abraham DJ Dreifaldt M Souza DSR . Perivascular fat-derived leptin: a potential role in improved vein graft performance in coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. (2011) 12(2):170–3. 10.1510/icvts.2010.247874
99.
Fernández-Alfonso MS Gil-Ortega M Aranguez I Souza D Dreifaldt M Somoza B et al Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol. (2017) 174(20):3561–72. 10.1111/bph.13734
100.
Mikami T Furuhashi M Sakai A Numaguchi R Harada R Naraoka S et al Antiatherosclerotic phenotype of perivascular adipose tissue surrounding the saphenous vein in coronary artery bypass grafting. J Am Heart Assoc. (2021) 10(7):e018905. 10.1161/JAHA.120.018905
101.
Dashwood MR Celik Z Topal G . Reducing vasospasm of vein and arterial conduits used in coronary artery bypass surgery: are solutions the solution or is preserved perivascular fat the answer?Front Physiol. (2025) 16:1539102. 10.3389/fphys.2025.1539102
102.
Favaloro RG . Saphenous vein autograft replacement of severe segmental coronary artery occlusion: operative technique. Ann Thorac Surg. (1968) 5(4):334–9. 10.1016/S0003-4975(10)66351-5
103.
Favaloro RG . Saphenous vein graft in the surgical treatment of coronary artery disease. Operative technique. J Thorac Cardiovasc Surg. (1969) 58(2):178–85. 10.1016/S0022-5223(19)42599-3
104.
Tsui JCS Dashwood MR . Recent strategies to reduce vein graft occlusion: a need to limit the effect of vascular damage. Eur J Vasc Endovasc Surg. (2002) 23(3):202–8. 10.1053/ejvs.2002.1600
105.
Zhang YY Shi YN Zhu N Zhao TJ Guo YJ Liao DF et al PVAT Targets VSMCs to regulate vascular remodelling: angel or demon. J Drug Target. (2021) 29(5):467–75. 10.1080/1061186X.2020.1859515
106.
Antonopoulos AS Margaritis M Coutinho P Shirodaria C Psarros C Herdman L et al Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. (2014) 64(6):2207–19. 10.2337/db14-1011
107.
Antonopoulos AS Margaritis M Verheule S Recalde A Sanna F Herdman L et al Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res. (2016) 118(5):842–55. 10.1161/CIRCRESAHA.115.307856
108.
Margaritis M Antonopoulos AS Digby J Lee R Reilly S Coutinho P et al Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. (2013) 127(22):2209–21. 10.1161/CIRCULATIONAHA.112.001133
109.
Saito T Kurazumi H Suzuki R Matsunaga K Tsubone S Lv B et al Perivascular adipose tissue is a Major source of nitric oxide in saphenous vein grafts harvested via the No-touch technique. J Am Heart Assoc. (2022) 11(3):e020637. 10.1161/JAHA.120.020637
110.
Loesch A Dashwood MR Souza DSR . Does the method of harvesting the saphenous vein for coronary artery bypass surgery affect venous smooth muscle cells? iNOS immunolabelling and ultrastructural findings. Int J Surg Lond Engl. (2006) 4(1):20–9. 10.1016/j.ijsu.2005.11.002
111.
Sowka A Dobrzyn P . Role of perivascular adipose tissue-derived adiponectin in vascular homeostasis. Cells. (2021) 10(6):1485. 10.3390/cells10061485
112.
Feijóo-Bandín S Aragón-Herrera A Moraña-Fernández S Anido-Varela L Tarazón E Roselló-Lletí E et al Adipokines and inflammation: focus on cardiovascular diseases. Int J Mol Sci. (2020) 21(20):7711. 10.3390/ijms21207711
113.
Hou Y Wang XF Lang ZQ Jin YC Fu JR Xv XM et al Adiponectin is protective against endoplasmic reticulum stress-induced apoptosis of endothelial cells in sepsis. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. (2018) 51(12):e7747. 10.1590/1414-431x20187747
114.
Tong Y Zuo Z Li X Li M Wang Z Guo X et al Protective role of perivascular adipose tissue in the cardiovascular system. Front Endocrinol. (2023) 14:1296778. 10.3389/fendo.2023.1296778
115.
Bełtowski J . Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol. (2012) 39(2):168–78. 10.1111/j.1440-1681.2011.05623.x
116.
Çimen AR Cerit ET Iyidir OT Karakus R Uyar BB Toruner FB et al Serum omentin-1 levels and endothelial dysfunction in obesity. Acta Endocrinol Buchar Rom 2005. (2017) 13(2):138–43. 10.4183/aeb.2017.138
117.
Wang J Gao Y Lin F Han K Wang X . Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem Biophys. (2020) 679:108187. 10.1016/j.abb.2019.108187
118.
Zhou H Zhang Z Qian G Zhou J . Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice. Fundam Clin Pharmacol. (2020) 34(6):721–35. 10.1111/fcp.12575
119.
Wang R . Shared signaling pathways among gasotransmitters. Proc Natl Acad Sci U S A. (2012) 109(23):8801–2. 10.1073/pnas.1206646109
120.
Lee RMKW Lu C Su LY Gao YJ . Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. (2009) 27(4):782–90. 10.1097/HJH.0b013e328324ed86
121.
Souza D . A new no-touch preparation technique: technical notes. Scand J Thorac Cardiovasc Surg. (1996) 30:41–4. 10.3109/14017439609107239
122.
Souza DSR Johansson B Bojö L Karlsson R Geijer H Filbey D et al Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg. (2006) 132(2):373–8. 10.1016/j.jtcvs.2006.04.002
123.
Dashwood MR Savage K Tsui JCS Dooley A Shaw SG Fernández Alfonso MS et al Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension-induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg. (2009) 138(2):334–40. 10.1016/j.jtcvs.2008.11.060
124.
Samano N Souza D Pinheiro BB Kopjar T Dashwood M . Twenty-Five years of No-touch saphenous vein harvesting for coronary artery bypass grafting: structural observations and impact on graft performance. Braz J Cardiovasc Surg. (2020) 35(1):91–9. 10.21470/1678-9741-2019-0238
125.
Kruit N Sluiter TJ de Vries MR . Role of perivascular adipose tissue in vein remodeling. Arterioscler Thromb Vasc Biol. (2025) 45(5):576–84. 10.1161/ATVBAHA.124.321692
126.
Deb S Singh SK de Souza D Chu MWA Whitlock R Meyer SR et al SUPERIOR SVG: no touch saphenous harvesting to improve patency following coronary bypass grafting (a multi-centre randomized control trial, NCT01047449). J Cardiothorac Surg. (2019) 14(1):85. 10.1186/s13019-019-0887-x
127.
Lee HS Park MJ Yoon SY Joo N Song YR Kim HJ et al Role of peribrachial fat as a key determinant of brachial artery dilatation for successful arteriovenous fistula maturation in hemodialysis patients. Sci Rep. (2020) 10(1):3841. 10.1038/s41598-020-60734-8
128.
Mauro CR Ding K Xue H Tao M Longchamp A Belkin M et al Adipose phenotype predicts early human autogenous arteriovenous hemodialysis remodeling. J Vasc Surg. (2016) 63(1):171–176.e1. 10.1016/j.jvs.2014.06.110
129.
Sharma G Kuppler C He Y Tao M Ding K Longchamp A et al Local adipose-associated mediators and adaptations following arteriovenous Fistula creation. Kidney Int Rep. (2018) 3(4):970–8. 10.1016/j.ekir.2018.02.008
130.
Wang Z Lu H Garcia-Barrio M Guo Y Zhang J Chen YE et al RNA Sequencing reveals perivascular adipose tissue plasticity in response to angiotensin II. Pharmacol Res. (2022) 178:106183. 10.1016/j.phrs.2022.106183
131.
Loop FD Lytle BW Cosgrove DM Stewart RW Goormastic M Williams GW et al Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. (1986) 314(1):1–6. 10.1056/NEJM198601023140101
132.
Cameron A Davis KB Green GE Myers WO Pettinger M . Clinical implications of internal mammary artery bypass grafts: the coronary artery surgery study experience. Circulation. (1988) 77(4):815–9. 10.1161/01.CIR.77.4.815
133.
Boylan MJ Lytle BW Loop FD Taylor PC Borsh JA Goormastic M et al Surgical treatment of isolated left anterior descending coronary stenosis. Comparison of left internal mammary artery and venous autograft at 18 to 20 years of follow-up. J Thorac Cardiovasc Surg. (1994) 107(3):657–62. 10.1016/S0022-5223(94)70320-5
134.
Tatoulis J Buxton BF Fuller JA . The right internal thoracic artery: is it underutilized?Curr Opin Cardiol. (2011) 26(6):528–35. 10.1097/HCO.0b013e32834b9f87
135.
Fitzgibbon GM Kafka HP Leach AJ Keon WJ Hooper GD Burton JR . Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. (1996) 28(3):616–26. 10.1016/0735-1097(96)00206-9
136.
Calafiore AM Vitolla G Iaco AL Fino C Giammarco GD Marchesani F et al Bilateral internal mammary artery grafting: midterm results of pedicled versus skeletonized conduits. Ann Thorac Surg. (1999) 67(6):1637–42. 10.1016/S0003-4975(99)00282-9
137.
Gaudino M Audisio K Rahouma M Chadow D Cancelli G Soletti GJ et al Comparison of long-term clinical outcomes of skeletonized vs pedicled internal thoracic artery harvesting techniques in the Arterial Revascularization Trial. JAMA Cardiol. (2021) 6(12):1380–6. 10.1001/jamacardio.2021.3866
138.
Benedetto U Altman DG Gerry S Gray A Lees B Pawlaczyk R et al Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: insights from the arterial revascularization trial. J Thorac Cardiovasc Surg. (2016) 152(1):270–6. 10.1016/j.jtcvs.2016.03.056
139.
Lamy A Browne A Sheth T Zheng Z Dagenais F Noiseux N et al Skeletonized vs pedicled internal mammary artery graft harvesting in coronary artery bypass surgery: aPost Hoc Analysis from the COMPASS trial. JAMA Cardiol. (2021) 6(9):1042–9. 10.1001/jamacardio.2021.1686
140.
Dimagli A Gemelli M Kumar N Mitra M Sinha S Fudulu D et al A systematic review and meta-analysis of internal thoracic artery harvesting techniques: skeletonized vs pedicled. Int J Cardiol. (2024) 395:131577. 10.1016/j.ijcard.2023.131577
141.
Horii T Suma H . Semiskeletonization of internal thoracic artery: alternative harvest technique. Ann Thorac Surg. (1997) 63(3):867–8. 10.1016/S0003-4975(96)01123-X
142.
Isomura T Suma H Sato T Horii T . Use of the harmonic scalpel for harvesting arterial conduits in coronary artery bypass. Eur J Cardiothorac Surg. (1998) 14(1):101–3. 10.1016/S1010-7940(98)00146-8
143.
Lamm P Juchem G Weyrich P Schütz A Reichart B . The harmonic scalpel: optimizing the quality of mammary artery bypass grafts. Ann Thorac Surg. (2000) 69(6):1833–5. 10.1016/S0003-4975(00)01288-1
144.
Urso S Alvarez L Sádaba R Greco E . Skeletonization of the internal thoracic artery: a randomized comparison of harvesting methods. Interact Cardiovasc Thorac Surg. (2008) 7(1):23–6. 10.1510/icvts.2007.168831
145.
Kieser TM Rose MS Aluthman U Narine K . Quicker yet safe: skeletonization of 1640 internal mammary arteries with harmonic technology in 965 patients. Eur J Cardiothorac Surg. (2014) 45(5):e142–50. 10.1093/ejcts/ezu024
146.
Oemar BS Tschudi MR Godoy N Brovkovich V Malinski T Lüscher TF . Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. (1998) 97(25):2494–8. 10.1161/01.CIR.97.25.2494
147.
Garg UC Hassid A . Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. (1989) 83(5):1774–7. 10.1172/JCI114081
148.
Dubey RK Jackson EK Lüscher TF . Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. (1995) 96(1):141–9. 10.1172/JCI118014
149.
He GW Fan L Grove KL Furnary A Yang Q . Expression and function of endothelial nitric oxide synthase messenger RNA and protein are higher in internal mammary than in radial arteries. Ann Thorac Surg. (2011) 92(3):845–50. 10.1016/j.athoracsur.2011.04.063
150.
Fortunato G Taranto D Bracale MD M U Del Guercio L Carbone F et al Decreased paraoxonase-2 expression in human carotids during the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. (2008) 28(3):594–600. 10.1161/ATVBAHA.107.154658
151.
Yang ZH Stulz P von Segesser L Bauer E Turina M Lüscher TF . Different interactions of platelets with arterial and venous coronary bypass vessels. Lancet Lond Engl. (1991) 337(8747):939–43. 10.1016/0140-6736(91)91571-B
152.
Chang E Harley CB . Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. (1995) 92(24):11190–4. 10.1073/pnas.92.24.11190
153.
Camici GG Savarese G Akhmedov A Lüscher TF . Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J. (2015) 36(48):3392–403. 10.1093/eurheartj/ehv587
154.
Yang YM Huang A Kaley G Sun D . eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. (2009) 297(5):H1829–1836. 10.1152/ajpheart.00230.2009
155.
Liberale L Kraler S Camici GG Lüscher TF . Ageing and longevity genes in cardiovascular diseases. Basic Clin Pharmacol Toxicol. (2020) 127(2):120–31. 10.1111/bcpt.13426
156.
Matsushita H Chang E Glassford AJ Cooke JP Chiu CP Tsao PS . eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res. (2001) 89(9):793–8. 10.1161/hh2101.098443
157.
Minamino T Miyauchi H Yoshida T Ishida Y Yoshida H Komuro I . Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. (2002) 105(13):1541–4. 10.1161/01.CIR.0000013836.85741.17
158.
Alexander Y Osto E Schmidt-Trucksäss A Shechter M Trifunovic D Duncker DJ et al Endothelial function in cardiovascular medicine: a consensus paper of the European society of cardiology working groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc Res. (2021) 117(1):29–42. 10.1093/cvr/cvaa085
159.
Kouchi Y Onuki Y Wu MH Shi Q Sauvage LR . Effect of altered blood flow on the caliber and morphology of the internal thoracic artery in the dog. J Thorac Cardiovasc Surg. (1997) 113(1):114–20. 10.1016/S0022-5223(97)70406-9
160.
Masuda H Zhuang YJ Singh TM Kawamura K Murakami M Zarins CK et al Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. (1999) 19(10):2298–307. 10.1161/01.ATV.19.10.2298
161.
Nikodemska I De Bono DP Spyt TJ Wiechowski S Nikodemski T . Preoperative and early postoperative assessment of the internal thoracic artery by transcutaneous duplex ultrasound in coronary artery bypass grafting. Int J Cardiol. (1998) 66(1):39–44. 10.1016/S0167-5273(98)00188-0
162.
van Son JA Skotnicki SH Peters MB Pijls NH Noyez L van Asten WN . Noninvasive hemodynamic assessment of the internal mammary artery in myocardial revascularization. Ann Thorac Surg. (1993) 55(2):404–9. 10.1016/0003-4975(93)91011-B
163.
Zakkar M Luong LA Chaudhury H Ruud O Punjabi PP Anderson JR et al Dexamethasone arterializes venous endothelial cells by inducing mitogen-activated protein kinase phosphatase-1: a novel antiinflammatory treatment for vein grafts? Circulation. (2011) 123(5):524–32. 10.1161/CIRCULATIONAHA.110.979542
164.
Akasaka T Yoshikawa J Yoshida K Maeda K Hozumi T Nasu M et al Flow capacity of internal mammary artery grafts: early restriction and later improvement assessed by Doppler guide wire. Comparison with saphenous vein grafts. J Am Coll Cardiol. (1995) 25(3):640–7. 10.1016/0735-1097(94)00448-Y
165.
Porto I Gaudino M De Maria GL Di Vito L Vergallo R Bruno P et al Long-term morphofunctional remodeling of internal thoracic artery grafts: a frequency-domain optical coherence tomography study. Circ Cardiovasc Interv. (2013) 6(3):269–76. 10.1161/CIRCINTERVENTIONS.113.000200
166.
Kraler S Libby P Evans PC Akhmedov A Schmiady MO Reinehr M et al Resilience of the internal mammary artery to atherogenesis: shifting from risk to resistance to address unmet needs. Arterioscler Thromb Vasc Biol. (2021) 41(8):2237–51. 10.1161/ATVBAHA.121.316256
167.
Tedoriya T Kawasuji M Sakakibara N Ueyama K Watanabe Y . Pressure characteristics in arterial grafts for coronary bypass surgery. Cardiovasc Surg Lond Engl. (1995) 3(4):381–5. 10.1177/096721099500300405
168.
Hwang HY Kim KB . Saphenous vein as a composite graft from the internal thoracic artery. Ann Cardiothorac Surg. (2018) 7(5):68689. 10.21037/acs.2018.06.08
169.
Filho JGL Leitão MCA Forte AJV Filho HGL Silva AA Bastos ES et al Flow analysis of left internal thoracic artery in myocardial revascularization surgery using Y graft. Tex Heart Inst J. (2006) 33(4):430–6.
170.
Thubrikar MJ Robicsek F Fowler BL . Pressure trap created by vein valve closure and its role in graft stenosis. J Thorac Cardiovasc Surg. (1994) 107(3):707–16. 10.1016/S0022-5223(94)70326-4
171.
Kim MS Hwang SW Kim KB . Composite graft with saphenous vein conduit. J Coron Artery Dis. (2025) 31(1):11–4. 10.7793/jcad.31.002
172.
Portugal IB Ribeiro ID Sousa-Rodrigues CF Monte-Bispo RF Rocha AC . Distribution of saphenous vein valves and its practical importance. Rev Bras Cir Cardiovasc Orgao of Soc Bras Cir Cardiovasc. 2014;29(4):564–8. 10.5935/1678-9741.20140038
173.
Kim KB Hwang SW Kim MS . Techniques and outcomes of the No-touch vein conduit as a Y-composite graft. Braz J Cardiovasc Surg. (2022) 37(Spec 1):38–41. 10.21470/1678-9741-2022-0119
174.
Kim KB Hwang HY Hahn S Kim JS Oh SJ . A randomized comparison of the saphenous vein versus right internal thoracic artery as a Y-composite graft (SAVE RITA) trial: one-year angiographic results and mid-term clinical outcomes. J Thorac Cardiovasc Surg. (2014) 148(3):901–7; discussion 907-908. 10.1016/j.jtcvs.2014.03.057
175.
Hwang HY Lee KH Han JW Kim KB . Equivalency of saphenous vein and arterial composite grafts: 5-year angiography and midterm clinical follow-up. Ann Thorac Surg. (2016) 102(2):580–8. 10.1016/j.athoracsur.2016.02.017
176.
Gaudino M Alessandrini F Pragliola C Luciani N Trani C Burzotta F et al Composite Y internal thoracic artery-saphenous vein grafts: short-term angiographic results and vasoreactive profile. J Thorac Cardiovasc Surg. (2004) 127(4):1139–44. 10.1016/j.jtcvs.2003.07.051
177.
Lobo HG Lobo JG Pimentel MD Silva BGB de Souza CS Montenegro ML et al Intraoperative analysis of flow dynamics in arteriovenous composite Y grafts. Braz J Cardiovasc Surg. (2016) 31(5):351–7. 10.5935/1678-9741.20160053
178.
Glineur D Boodhwani M Poncelet A De Kerchove L Etienne PY Noirhomme P et al Comparison of fractional flow reserve of composite Y-grafts with saphenous vein or right internal thoracic arteries. J Thorac Cardiovasc Surg. (2010) 140(3):639–45. 10.1016/j.jtcvs.2009.11.013
179.
Hwang HY Lee Y Sohn SH Choi JW Kim KB . Equivalent 10-year angiographic and long-term clinical outcomes with saphenous vein composite grafts and arterial composite grafts. J Thorac Cardiovasc Surg. (2021) 162(5):1535–1543.e4. 10.1016/j.jtcvs.2020.01.109
180.
Kim MS Kim KB . Saphenous vein versus right internal thoracic artery as a Y-composite graft: ten-year angiographic and long-term clinical results of the SAVE RITA trial. Circulation. (2021) 144(14):1186–8. 10.1161/CIRCULATIONAHA.121.056438
181.
Paterson HS Thakkar J Byth K Denniss AR . Saphenous vein to internal mammary artery end-to-end composite grafts for coronary artery bypass. Late Follow-up. Heart Lung Circ. (2015) 24(2):200–5. 10.1016/j.hlc.2014.09.014
182.
Prapas S Katsavrias K Gaudino M Puskas JD Di Mauro M Zografos P et al Saphenous vein to the right coronary system from the right thoracic artery or the aorta. Long-term propensity-matched results of 2 groups. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. (2024) 65(3):ezae060. 10.1093/ejcts/ezae060
183.
Bouten CVC Dankers PYW Driessen-Mol A Pedron S Brizard AMA Baaijens FPT . Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev. (2011) 63(4–5):221–41. 10.1016/j.addr.2011.01.007
184.
Samady H Eshtehardi P McDaniel MC Suo J Dhawan SS Maynard C et al Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. (2011) 124(7):779–88. 10.1161/CIRCULATIONAHA.111.021824
185.
Isobe N Kaneko T Taniguchi K Oshima S . Comparison of the rheologic parameters in left internal thoracic artery grafts with those in saphenous vein grafts. Circ J. (2005) 69(6):700–6. 10.1253/circj.69.700
186.
Shimizu T Ito S Kikuchi Y Misaka M Hirayama T Ishimaru S et al Arterial conduit shear stress following bypass grafting for intermediate coronary artery stenosis: a comparative study with saphenous vein grafts. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. (2004) 25(4):578–84. 10.1016/j.ejcts.2003.12.039
187.
Ramachandra AB Kahn AM Marsden AL . Patient-Specific simulations reveal significant differences in mechanical stimuli in venous and arterial coronary grafts. J Cardiovasc Transl Res. (2016) 9(4):279–90. 10.1007/s12265-016-9706-0
188.
Meirson T Orion E Mario D Webb C Patel C Channon N et al Flow patterns in externally stented saphenous vein grafts and development of intimal hyperplasia. J Thorac Cardiovasc Surg. (2015) 150(4):871–8. 10.1016/j.jtcvs.2015.04.061
189.
Chatzizisis YS Baker AB Sukhova GK Koskinas KC Papafaklis MI Beigel R et al Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation. (2011) 123(6):621–30. 10.1161/CIRCULATIONAHA.110.970038
190.
Kumar A Hung OY Piccinelli M Eshtehardi P Corban MT Sternheim D et al Low coronary wall shear stress is associated with severe endothelial dysfunction in patients with nonobstructive coronary artery disease. JACC Cardiovasc Interv. (2018) 11(20):2072–80. 10.1016/j.jcin.2018.07.004
191.
Raja SG Sarang Z . Endoscopic vein harvesting: technique, outcomes, concerns & controversies. J Thorac Dis. (2013) 5(Suppl 6):S630–7. 10.3978/j.issn.2072-1439.2013.10.01
192.
Won D Zhu SN Chen M Teichert AM Fish JE Matouk CC et al Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. (2007) 171(5):1691–704. 10.2353/ajpath.2007.060860
193.
Uematsu M Ohara Y Navas JP Nishida K Murphy TJ Alexander RW et al Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. (1995) 269(6 Pt 1):C1371–1378. 10.1152/ajpcell.1995.269.6.C1371
194.
Dimitrova KR Hoffman DM Geller CM Dincheva G Ko W Tranbaugh RF . Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg. (2012) 94(2):475–81. 10.1016/j.athoracsur.2012.04.035
195.
Lobo Filho JG Pimentel MD Lobo Filho HG . Composite graft and remodeling of the saphenous vein in coronary artery bypass graft. JTCVS Open. (2022) 11:129–30. 10.1016/j.xjon.2022.01.028
196.
Katsavrias K Prapas S Calafiore AM Taggart D Angouras D Iliopoulos D et al Improvement of the outcome of the saphenous vein graft when connected to the internal thoracic artery. Front Cardiovasc Med. (2024) 11:1478166. 10.3389/fcvm.2024.1478166
197.
Calafiore AM Prapas S Condello I Katsavrias K Nasso G Gaudino M . The saphenous vein graft: can a frog become a princess?Medicina (Mex). (2024) 60(12):1915. 10.3390/medicina60121915
198.
Gaudino M Sandner S Calafiore AM . Increased nitric oxide availability: the explanation for recent improvements in saphenous vein graft patency?Circulation. (2024) 150(20):1567–9. 10.1161/CIRCULATIONAHA.124.071157
199.
Jeremy JY Gadsdon P Shukla N Vijayan V Wyatt M Newby AC et al On the biology of saphenous vein grafts fitted with external synthetic sheaths and stents. Biomaterials. (2007) 28(6):895–908. 10.1016/j.biomaterials.2006.10.023
200.
Murphy GJ Newby AC Jeremy JY Baumbach A Angelini GD . A randomized trial of an external Dacron sheath for the prevention of vein graft disease: the extent study. J Thorac Cardiovasc Surg. (2007) 134(2):504–5. 10.1016/j.jtcvs.2007.01.092
201.
Taggart DP Ben Gal Y Lees B Patel N Webb C Rehman SM et al A randomized trial of external stenting for saphenous vein grafts in coronary artery bypass grafting. Ann Thorac Surg. (2015) 99(6):2039–45. 10.1016/j.athoracsur.2015.01.060
202.
Goldstein DJ Chang HL Mack MJ Voisine P Gammie JS Marks ME et al Intimal hyperplasia, saphenous vein graft disease, and clinical outcomes: insights from the CTSN VEST randomized trial. J Thorac Cardiovasc Surg. (2024) 167(5):1782–1792.e5. 10.1016/j.jtcvs.2022.10.034
203.
Soletti G Jr. Dell’Aquila M Harik L Cancelli G Alzghari T Perezgrovas-Olaria R et al The VEST external support for saphenous vein grafts in coronary surgery: a review of randomized clinical trials. J Cardiovasc Dev Dis. (2023) 10(11):453. 10.3390/jcdd10110453
204.
Soletti GJ Dimagli A Harik L Cancelli G Perezgrovas-Olaria R Alzghari T et al External stenting for saphenous vein grafts in coronary surgery: a systematic review and meta-analysis. J Clin Med. (2023) 12(23):7395. 10.3390/jcm12237395
205.
Goldman S Copeland J Moritz T Henderson W Zadina K Ovitt T et al Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a veterans administration cooperative study. Circulation. (1988) 77(6):1324–32. 10.1161/01.CIR.77.6.1324
206.
Fremes SE Levinton C Naylor CD Chen E Christakis GT Goldman BS . Optimal antithrombotic therapy following aortocoronary bypass: a meta-analysis. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. (1993) 7(4):169–80. 10.1016/1010-7940(93)90155-5
207.
Musleh G Dunning J . Does aspirin 6h after coronary artery bypass grafting optimise graft patency?Interact Cardiovasc Thorac Surg. (2003) 2(4):413–5. 10.1016/S1569-9293(03)00181-6
208.
Patrono C Rodríguez LAG Landolfi R Baigent C . Low-Dose aspirin for the prevention of atherothrombosis. N Engl J Med. (2005) 353(22):2373–83. 10.1056/NEJMra052717
209.
Gaudino M Sandner S An KR Dimagli A Di Franco A Audisio K et al Graft failure after coronary artery bypass grafting and its association with patient characteristics and clinical events: a pooled individual patient data analysis of clinical trials with imaging follow-up. Circulation. (2023) 148(17):1305–15. 10.1161/CIRCULATIONAHA.123.064090
210.
Kulik A Abreu AM Boronat V Kouchoukos NT Ruel M . Ticagrelor versus aspirin and vein graft patency after coronary bypass: a randomized trial. J Card Surg. (2022) 37(3):563–70. 10.1111/jocs.16189
211.
Zhao Q Zhu Y Xu Z Cheng Z Mei J Chen X et al Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA. (2018) 319(16):1677–86. 10.1001/jama.2018.3197
212.
Gao C Ren C Li D Li L . Clopidogrel and aspirin versus clopidogrel alone on graft patency after coronary artery bypass grafting. Ann Thorac Surg. (2009) 88(1):59–62. 10.1016/j.athoracsur.2009.04.024
213.
Lamy A Eikelboom J Sheth T Connolly S Bosch J Fox KAA et al Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: the COMPASS-CABG study. J Am Coll Cardiol. (2019) 73(2):121–30. 10.1016/j.jacc.2018.10.048
214.
Deo SV Dunlay SM Shah IK Altarabsheh SE Erwin PJ Boilson BA et al Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg. (2013) 28(2):109–16. 10.1111/jocs.12074
215.
Solo K Lavi S Kabali C Levine GN Kulik A John-Baptiste AA et al Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. Br Med J. (2019) 367:l5476. 10.1136/bmj.l5476
216.
Sandner S Redfors B Angiolillo DJ Audisio K Fremes SE Janssen PWA et al Association of dual antiplatelet therapy with ticagrelor with vein graft failure after coronary artery bypass graft surgery: a systematic review and meta-analysis. JAMA. (2022) 328(6):554–62. 10.1001/jama.2022.11966
217.
Sibbing D Aradi D Alexopoulos D ten Berg J Bhatt DL Bonello L et al Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. (2019) 12(16):1521–37. 10.1016/j.jcin.2019.03.034
218.
Calderone D Capodanno D Angiolillo DJ . An updated drug profile of ticagrelor with considerations on the treatment of patients with coronary artery disease and diabetes mellitus. Expert Rev Cardiovasc Ther. (2020) 18(8):449–64. 10.1080/14779072.2020.1792293
219.
Yuan X Chu Q Chen K Wang Y Zhang L Zheng Y et al Multicentre, randomised, double-blind, parallel controlled trial to investigate timing of platelet inhibition after coronary artery bypass grafting: tOP-CABG trial study. BMJ Open. (2023) 13(6):e070823. 10.1136/bmjopen-2022-070823
220.
Sandner S Gaudino M Redfors B Angiolillo DJ Ben-Yehuda O Bhatt DL et al One-month DAPT with ticagrelor and aspirin for patients undergoing coronary artery bypass grafting: rationale and design of the randomised, multicentre, double-blind, placebo-controlled ODIN trial. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. (2024) 20(5):e322–8. 10.4244/EIJ-D-23-00699
221.
Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. (1997) 336(3):153–62. 10.1056/NEJM199701163360301
222.
Kulik A Voisine P Mathieu P Masters RG Mesana TG May L et al Statin therapy and saphenous vein graft disease after coronary bypass surgery: analysis from the CASCADE randomized trial. Ann Thorac Surg. (2011) 92(4):1284–91. 10.1016/j.athoracsur.2011.04.107
223.
Kulik A Abreu AM Boronat V Ruel M . Intensive versus moderate statin therapy and early graft occlusion after coronary bypass surgery: the aggressive cholesterol therapy to inhibit vein graft events randomized clinical trial. J Thorac Cardiovasc Surg. (2019) 157(1):151–161.e1. 10.1016/j.jtcvs.2018.05.123
224.
Ference BA Ginsberg HN Graham I Ray KK Packard CJ Bruckert E et al Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2017) 38:2459–72. 10.1093/eurheartj/ehx144
225.
Katsuki S Jha K Lupieri P Nakano A Passos T Rogers LSA et al Proprotein convertase subtilisin/kexin 9 (PCSK9) promotes macrophage activation via LDL receptor-independent mechanisms. Circ Res. (2022) 131(11):873–89. 10.1161/CIRCRESAHA.121.320056
226.
Verma S Leiter LA Teoh H Mancini GBJ Quan A Elituv R et al Effect of evolocumab on saphenous vein graft patency after coronary artery bypass surgery (NEWTON-CABG CardioLink-5): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2025406(10509):1223–34. 10.1016/S0140-6736(25)01633-2
227.
Jerzewski K Ruel M Voisine P Le May MR Kulik A . Does high-density lipoprotein influence the development of saphenous vein graft disease after coronary bypass surgery?: exploratory analysis from the CASCADE trial. J Cardiothorac Surg. (2013) 8:172. 10.1186/1749-8090-8-172
228.
Shukla N Angelini GD Ascione R Talpahewa S Capoun R Jeremy JY . Nitric oxide donating aspirins: novel drugs for the treatment of saphenous vein graft failure. Ann Thorac Surg. (2003) 75(5):1437–42. 10.1016/s0003-4975(02)04892-0
229.
Lorusso R De Cicco G Beghi C Gherli T Poli E Corradi D et al Functional effects of nitric oxide-releasing aspirin on vein conduits of diabetic patients undergoing CABG. Int J Cardiol. (2007) 118(2):164–9. 10.1016/j.ijcard.2006.07.014
230.
Kaleli Durman D Dağtekin N Civelek E İyigün T Teskin Ö Uydeş Doğan BS . GSNO As a modulator of vascular tone in human saphenous veins. Potential implications for graft spasm. Life. (2025) 15(7):1139. 10.3390/life15071139
231.
Cable DG O’Brien T Schaff HV Pompili VJ . Recombinant endothelial nitric oxide synthase-transduced human saphenous veins: gene therapy to augment nitric oxide production in bypass conduits. Circulation. (1997) 96(9 Suppl):II-173–8.
232.
Gao J Liu Y Li YM . Review of risk factors, treatment, and prevention of saphenous vein graft disease after coronary artery bypass grafting. J Int Med Res. (2018) 46(12):4907–19. 10.1177/0300060518792445
233.
Harskamp RE Lopes RD Baisden CE de Winter RJ Alexander JH . Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. (2013) 257(5):824–33. 10.1097/SLA.0b013e318288c38d
234.
Ak E Ak K Midi A Kervancıoğlu-Demirci E Arsan S Çetinel Ş et al Histopathologic evaluation of saphenous vein grafts in patients with type II diabetes mellitus undergoing coronary artery bypass grafting. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. (2021) 52:107328. 10.1016/j.carpath.2021.107328
235.
Deng M Wen D Song Y Zhao L Cui Y Xin J et al Correlation between blood pressure control levels and long-term patency of saphenous vein grafts after coronary artery bypass grafting: a retrospective case-control study. Int J Cardiol Cardiovasc Risk Prev. (2025) 25:200414. 10.1016/j.ijcrp.2025.200414
236.
Narula J Chandrashekhar Y Ahmadi A Abbara S Berman DS Blankstein R et al SCCT 2021 Expert consensus document on coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. (2021) 15(3):192–217. 10.1016/j.jcct.2020.11.001
237.
Mantaj P Hirofuji A Dell’Aquila M Demetres M Gregg A Krieger K et al Meta-analysis of coronary bypass graft patency assessment with invasive vs computed tomographic angiography. Ann Thorac Surg. (2025) 120(6):1016–27. 10.1016/j.athoracsur.2025.06.014
238.
Burgstahler C Beck T Kuettner A Drosch T Kopp AF Heuschmid M et al Non-invasive evaluation of coronary artery bypass grafts using 16-row multi-slice computed tomography with 188 ms temporal resolution. Int J Cardiol. (2006) 106(2):244–9. 10.1016/j.ijcard.2005.02.017
239.
Schlosser T Konorza T Hunold P Kühl H Schmermund A Barkhausen J . Noninvasive visualization of coronary artery bypass grafts using 16-detector row computed tomography. J Am Coll Cardiol. (2004) 44(6):1224–9. 10.1016/j.jacc.2003.09.075
Glossary
ADMA asymmetric dimethylarginine AMPK AMP-activated protein kinase Ao ascending aorta AWB autologous whole blood BH4 tetrahydrobiopterin BK Big potassium (channel) BMI body mass index CABG coronary artery bypass graft CAD coronary artery disease Ca2+ calcium ion CCL2 chemokine ligand 2 cGMP cyclic guanosine monophosphate CI confidence interval cNOS constitutive nitric oxide synthase CO2 carbon dioxide CCTA coronary computed tomography angiography EC endothelial cell ECM extracellular matrix EDI endothelial damage inhibitor EDRF endothelial-derived relaxing factor eNOS endothelial nitric oxide synthase HR hazard ratio H2O2 hydrogen peroxide H2S hydrogen sulfide IL-6 interleukin-6 iNOS inducible nitric oxide synthase ITA internal thoracic artery L-arginine levorotatory arginine LAD left anterior descending (artery) LDL low-density lipoprotein LITA left internal thoracic artery MACE major adverse cardiac and cerebrovascular events MAPK mitogen-activated protein kinase MKP-1 mitogen-activated protein kinase phosphatase-1 MMP matrix metalloproteinase NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells NG nitroglycerin NLRP3 Nucleotide-binding domain, leucine-rich repeat-containing family, pyrin domain-containing 3 nM Nanomolar nNOS neuronal nitric oxide synthase NO nitric oxide NOS nitric oxide synthase NRG4 Neuregulin-4 OC open conventional (vein harvesting technique) ONOO− peroxynitrite ONT open no-touch (vein harvesting technique) OR odds ratio Superoxide PCI percutaneous coronary intervention PI3K phosphoinositide 3-kinase PI3K/Akt phosphatidylinositol 3-kinase/protein kinase B PKB/Akt protein kinase B PON-2 paraoxonase 2 PP pulse pressure PPAR-γ peroxisome proliferator-activated receptor gamma PREVENT-IV project of ex-vivo vein graft engineering via novel Technologies-IV trial PVAT perivascular adipose tissue RA radial artery RCA right coronary artery RCT randomized controlled trial REGROUP randomized endo-vein graft prospective trial RITA right internal thoracic artery ROS reactive oxygen species RR relative risk SAVE RITA saphenous vein vs. right internal thoracic artery SMC smooth muscle cell SV greater saphenous vein (native) SVG saphenous vein graft TNF-α tumor necrosis factor-alpha TXNIP thioredoxin-interacting protein VEST venous external support (device).
Summary
Keywords
composite graft, coronary artery bypass graft (CABG), endothelial dysfunction, graft failure, graft patency, nitric oxide (NO), perivascular adipose tissue (PVAT), saphenous vein graft (SVG)
Citation
Dell’Aquila M, Prapas S, Falco G, Abdalla S, Tejada B, Challagalla M, Condello I, Newman J, Jarral O, Pupovac S, Ali A, Katsavrias K, D’Onofrio A, Zebele C, Totaro A, Labriola V, Caldonazo T, Kirov H, Di Franco A, Leith J, Rong L, Rahouma M, Brinster D, Iribarne A, Manetta F, Patel N, Kalimi R, Gaudino M and Calafiore AM (2026) Saphenous vein graft and nitric oxide: strategies to prevent graft failure and enhance patency in coronary artery bypass grafting. Front. Cardiovasc. Med. 12:1745260. doi: 10.3389/fcvm.2025.1745260
Received
12 November 2025
Revised
02 December 2025
Accepted
09 December 2025
Published
08 January 2026
Volume
12 - 2025
Edited by
Bernhard Winkler, Vienna Health Association, Austria
Reviewed by
Michael Richard Dashwood, University College London, United Kingdom
Emre Melik Faideci, Bilecik Training and Research Hospital, Türkiye
Updates
Copyright
© 2026 Dell’Aquila, Prapas, Falco, Abdalla, Tejada, Challagalla, Condello, Newman, Jarral, Pupovac, Ali, Katsavrias, D’Onofrio, Zebele, Totaro, Labriola, Caldonazo, Kirov, Di Franco, Leith, Rong, Rahouma, Brinster, Iribarne, Manetta, Patel, Kalimi, Gaudino and Calafiore.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Michele Dell’Aquila mdellaquila@northwell.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.