Abstract
Cardiovascular diseases (CVDs) continue to be the leading cause of mortality worldwide, highlighting the need for enhanced diagnostic tools to enable early intervention. However, the complexity of these diseases poses significant challenges to their diagnosis and management. Therefore, a deeper understanding of CVD mechanisms and the development of novel diagnostic and therapeutic strategies are of critical importance. Retinol-binding protein 4 (RBP4), a member of the lipocalin family, is mainly secreted by the liver and adipose tissue and is widely recognized for its role in transporting retinol (vitamin A). Beyond functioning as a selective retinol carrier, growing evidence suggests that RBP4 is intricately involved in the pathogenesis of CVDs and their associated risk factors. Although numerous studies have established a link between RBP4 and the onset and progression of CVDs, the underlying mechanisms remain incompletely elucidated. This review summarizes the biological characteristics and multifunctional roles of RBP4 in CVD pathophysiology, examines its potential as a biomarker for diagnosis and prognosis, and explores its implications for developing new strategies to prevent and treat cardiovascular disorders.
1 Introduction
Cardiovascular diseases (CVDs) remain the foremost cause of global morbidity and mortality (1). Among these, coronary artery disease (CAD) and stroke represent the main contributors to this burden (2). Ischemic heart disease (IHD), most commonly resulting from coronary artery stenosis or occlusion due to atherosclerosis (AS) or thrombosis, is a major manifestation of CVDs (3). Stroke, which encompasses both ischemic and hemorrhagic types, is predominantly ischemic in nature, accounting for approximately 85% of all cases (4). Established traditional risk factors for CVDs include hypertension, dyslipidemia, diabetes, and obesity (5). Nevertheless, a notable proportion of patients experiencing cardiovascular events do not exhibit any of these conventional risk factors (6, 7), indicating that other underlying mechanisms may influence the onset and progression of CVDs. Therefore, exploring personalized risk factors to accurately identify individuals at high risk of cardiovascular events is an important challenge in current CVDs management.
Retinol binding protein 4 (RBP4), first isolated from human serum by Kahn et al. in 1968, belongs to the lipocalin family. It is primarily secreted by the liver and adipose tissue, with a molecular weight of approximately 21 kDa. The primary role of RBP4 is to transport retinol (vitamin A) from the liver to peripheral tissues, where it is metabolized into retinoic acid (8, 9). Beyond the liver and adipose tissue, RBP4 mRNA is also detectable in the kidney, brain, and lungs (10). RBP4 exists in two forms: apo-RBP4, which is unbound to retinol, and holo-RBP4, which binds to retinol and also associates with transthyretin (TTR) in plasma to prevent renal filtration and excretion (11). Notably, urinary RBP4 has been proposed as a potential biomarker for assessing kidney function (12, 13). The biological actions of RBP4 are mediated mainly through two receptors: Toll-like receptor 4 (TLR4) and the cell-membrane receptor STRA6 (stimulated by retinoic acid 6). Binding to TLR4 activates the c-Jun N-terminal kinase (JNK) pathway, whereas interaction with STRA6 triggers the Janus kinase 2/signal transducer and activator of transcription 5 (JAK2/STAT5) signaling cascade (14–18). Circulating RBP4 is predominantly derived from hepatocytes, with a minor contribution from adipocytes and other cell types (19). In humans, the physiological circulating concentration of RBP4 typically ranges from 2 to 3 µmol/L, whereas in mice it is generally between 0 and 1 µmol/L (20). Elevated levels of RBP4 have clinical significance: serum concentrations exceeding 55 µg/mL are associated with a nearly twofold increase (1.97-fold) in the risk of developing type 2 diabetes mellitus (T2DM) (21). Similarly, epidemiological evidence indicates that a 25% rise in circulating RBP4 corresponds to an approximately 2.5-fold higher risk of cardiovascular diseases (13).
Initially, research primarily linked RBP4 to obesity, insulin resistance (IR), and type 2 diabetes mellitus (T2DM) (22). In contrast, numerous subsequent studies have associated elevated serum RBP4 levels with various CVDs, including CAD (23), heart failure (24), and ischemic stroke (IS) (25–28). Furthermore, increased RBP4 levels are correlated with several established risk factors for CVDs, such as inflammation (16, 29, 30), hypertension (25, 31), dyslipidemia (28, 32, 33), and AS (34–36), all of which contribute to the initiation and progression of cardiovascular pathology. Additionally, elevated RBP4 expression in epicardial adipose tissue has been reported in association with left ventricular diastolic dysfunction (37). Notably, critically ill patients present with significantly lower serum RBP4 levels compared to healthy controls, and low RBP4 levels have been identified as an unfavorable predictor of short-term mortality in the intensive care unit (ICU) (38). In this review, we synthesize and update recent evidence on the multifaceted role of RBP4 in CVDs and their risk factors, offering insights into its potential translational and clinical applications.
2 RBP4 structure and function
2.1 RBP4 structural characteristics
RBP4 is a single-polypeptide protein composed of 201 amino acid residues, with a molecular weight of approximately 21 kDa (39). Its core structure consists of a characteristic β-barrel, which binds specifically to one molecule of all-trans retinol—the active form of vitamin A. This binding enables retinol to remain soluble in aqueous environments and facilitates its transport in the bloodstream. The overall RBP4 molecule contains an N-terminal ring, an α-helix, and a C-terminal ring, which collectively maintain protein stability and function. In circulation, RBP4 forms a 1:1:1 complex with retinol and TTR, which enhances the stability and solubility of retinol while preventing its oxidation and toxic accumulation (10, 40). These structural properties establish RBP4 as an essential mediator of vitamin A transport in vivo and link it closely to metabolic regulation and disease pathophysiology.
2.2 RBP4 ligand interactions
The interaction between RBP4 and ligands mainly involves the following aspects (Table 1):
Table 1
| Ligand | Binding site | Affinity | Experimental Validation (in vitro/in vivo) | Function | References |
|---|---|---|---|---|---|
| Retinol | β-barrel core | High affinity | Both in vitro & in vivo | Delivers retinol to tissues (e.g., retina, liver). | (10) |
| STRA6 | Unknown | High affinity | In vitro |
|
(42, 43) |
| RBPR2 | Unknown | High affinity | In vitro |
|
(44) |
| TLRs | Unknown | Unknown | Both in vitro & in vivo (animal) |
|
(45) |
| RAR/ RXR | No (indirect interaction) | No (indirect interaction) | Both in vitro & in vivo (animal) |
|
(47–49) |
Known ligands and interaction partners of RBP4.
Binding to Retinol: (1) The primary function of RBP4 is to specifically bind and transport retinol (the active form of vitamin A) from the liver to target tissues. Before leaving hepatocytes, RBP4 binds retinol to form holo-RBP4, which then assembles in the endoplasmic reticulum into a 1:1:1 complex with TTR. This complex prevents renal filtration and degradation of RBP4 during circulation (41). Retinol binding to RBP4 is reversible; upon reaching target cells, the vitamin is internalized via membrane receptors such as STRA6 (also known as RBP4 receptor 1, RBPR2), thereby completing vitamin A uptake and metabolic regulation (10).
Interaction with the STRA6 Receptor: When the circulating RBP4-retinol-TTR complex reaches target cell, TTR dissociates to allow RBP4-retinol to bind STRA6, facilitating retinol entry into the cell (42). Beyond its transport role, STRA6 also functions as a signaling receptor (43). Its activation can modulate intracellular pathways, including the JAK/STAT cascade, thereby influencing insulin sensitivity and lipid metabolism.
Interaction with the RBPR2 Receptor: Identified in 2013, RBPR2 (stimulated by retinoic acid 6-like, STRA6L) shares about 20% homology with STRA6. It is predominantly expressed in the liver, intestine, and adipose tissue under obese conditions. RBPR2 participates in retinol uptake and has been implicated in the regulation of insulin resistance (44).
Interaction with TLR Receptors: In adipose tissue, RBP4 can bind to and activate Toll-like receptors—specifically TLR2 and the TLR4/MD-2 complex—on the surface of macrophages. This triggers the release of inflammatory cytokines, such as TNF-α and IL-1β, and contributes to the pathogenesis of insulin resistance and metabolic dysregulation (45).
Indirect Interaction with Nuclear Receptors: Under pathological conditions, elevated circulating RBP4 enhances retinol delivery to tissues. In the liver, retinol is metabolized into retinoic acid (RA) isomers, which act as ligands for nuclear receptors including the retinoic acid receptor (RAR) and retinoid X receptor (RXR) (46). Activation of these receptors regulates gene transcription and can promote processes such as lipid synthesis (47–49).
In summary, RBP4 participates in various physiological and pathological processes including vitamin A metabolism, insulin resistance, and inflammatory response through interactions with ligands such as retinol, receptors, and nuclear receptors.
2.3 RBP4-related signal transduction
The signaling pathways associated with RBP4 and their potential physiological effects are summarized as follows (Figure 1).
Figure 1
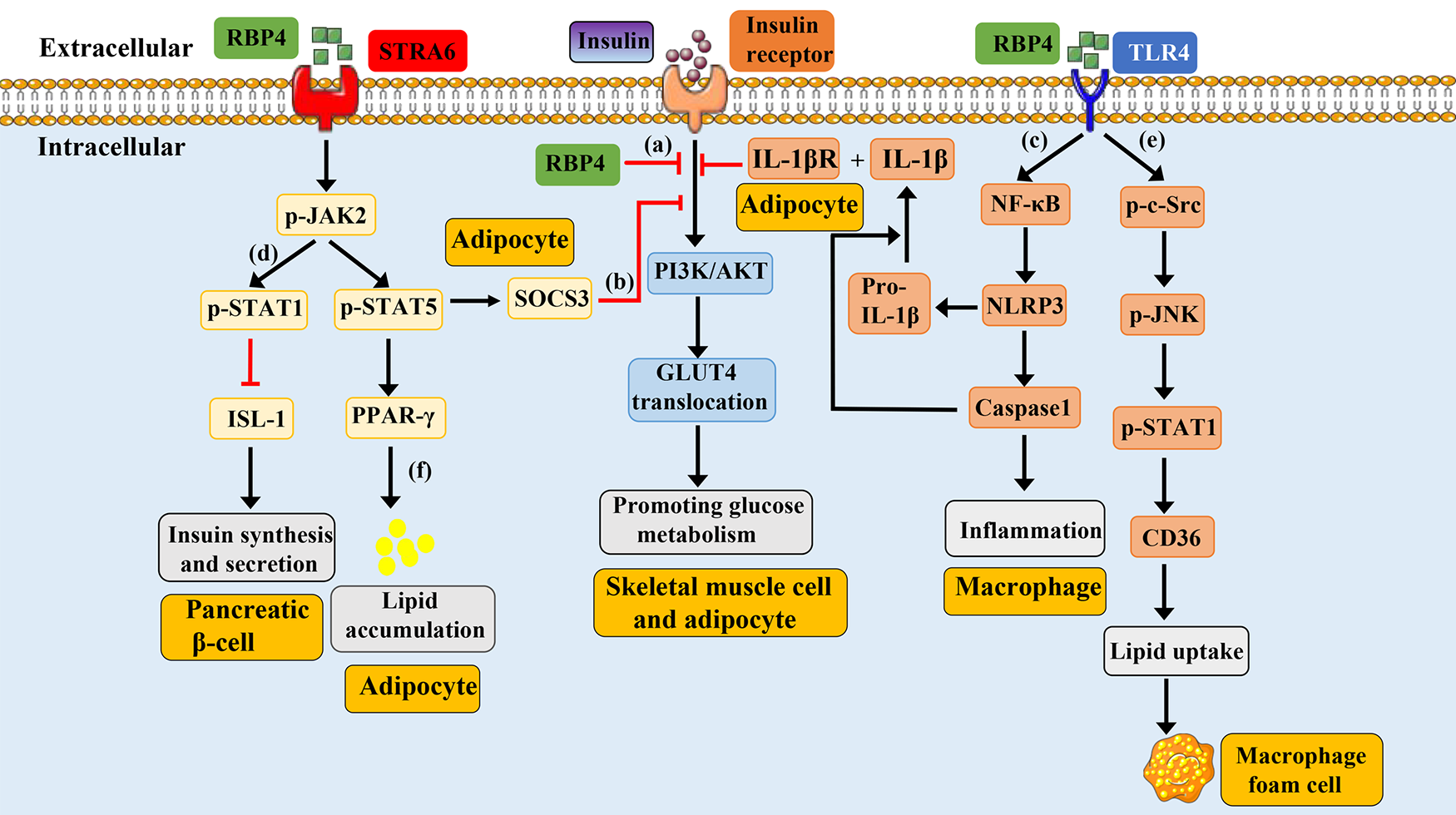
Schematic representation of RBP4-mediated signaling pathways. Under physiological conditions, the binding of insulin-to-insulin receptors promotes the activation of PI3K/AKT, leading to the translocation of GLUT4 and promoting glucose metabolism. (a): Overexpression of mouse RBP4 or injection of purified human RBP4 recombinant protein into mice can inhibit insulin induced activation of PI3K/AKT. (b): Circulating RBP4 can recruit and activate JAK2 by binding to the adipocyte surface receptor STRA6, thereby promoting phosphorylation of STAT5. Subsequently, phosphorylated STAT5 enters the nucleus, causing the expression of target genes SOCS3 and PPAR-γ, which respectively inhibit the insulin signaling pathway and promote lipid aggregation. (c): RBP4 can promote the phosphorylation of NF-κB by binding to TLR4 receptors on the surface of macrophages, thereby promoting the formation of NLRP3 inflammasome, leading to the synthesis and release of IL-1β. IL-1β binds to IL-1βR on the surface of adipocytes and induce the inhibition of the insulin signaling pathway. (d): RBP4 binds to the pancreatic cell surface receptor STRA6, causing phosphorylation of JAK, which in turn leads to phosphorylation of STAT1. Phosphorylated STAT1 enters the nucleus and inhibits the expression of ISL1 gene, resulting in reduced insulin synthesis and secretion. (e): RBP4 binds to the TLR4 receptor on the surface of macrophages, causing phosphorylation of c-src and JNK. The phosphorylation of JNK can promote the phosphorylation and nuclear translocation of STAT1. The phosphorylated STAT1 binds to the promoter of CD36 to promote its expression, which then leads to increased cholesterol uptake and the formation of foam cells. (f): RBP4 can recruit and activate JAK2 by binding to the adipocyte surface receptor STRA6, thereby promoting phosphorylation of STAT5. Subsequently, phosphorylated STAT5 enters the nucleus, causing the expression of target genes PPAR-γ, leading to lipid aggregation. GLUT4, glucose transport protein 4; JAK2, Janus kinase 2; STAT5, signal transducer and activator of transcription 5; SOCS3, cytokine signaling 3; PI3K/AKT, phosphatidylinositol 3-kinase/protein kinase B; TLR4, toll-like receptor 4; NLRP3, nucleotide-binding domain and leucine-rich repeat containing protein 3; IL-1β, interleukin-1 beta; NFκB, nuclear factor kappa B; STRA6, stimulated by retinoic acid 6; STAT1, signal transducer and activator of transcription 1; ISL-1, insulin gene enhancer binding protein 1; JNK, jun N-terminal kinase; PPARγ, peroxisome proliferator-activated receptor gamma.
Insulin Resistance-Related Signaling: RBP4 directly inhibits the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway in skeletal muscle cells, reduces glucose transport protein 4 (GLUT4) translocation, and impairs glucose uptake (9). Additionally, by binding to the STRA6 receptor on adipocytes, RBP4 activates the JAK2/STAT5/SOCS3 cascade, contributing to insulin resistance (43). Furthermore, through the TLR4/NF-κB/NLRP3 pathway, RBP4 promotes the release of pro-inflammatory cytokines such as TNF-α and IL-1β from macrophages in adipose tissue, thereby inducing insulin resistance in adipocytes (45).
Insulin Synthesis-Related Signaling: In pancreatic islet cells, RBP4 binding to STRA6 activates the JAK2/STAT1 pathway, which suppresses transcription factors involved in insulin synthesis (e.g., ISL-1), ultimately inhibiting insulin production (50).
Inflammatory Signaling: RBP4 can enhance the expression of pro-inflammatory markers in CD206+ macrophages via a JNK-dependent mechanism and promote the polarization of CD4+ T cells toward a TH1 phenotype, thereby triggering adipose tissue inflammation. This inflammatory response may further activate TLR4 and JNK signaling, increase the release of pro-inflammatory cytokines, and exacerbate insulin resistance and metabolic dysregulation (51).
Retinol Metabolism and Transport: RBP4 binds retinol to form a complex that is stabilized in circulation by association with TTR, ensuring efficient delivery to target tissues (41). At target cells, RBP4 interacts with the STRA6 receptor to mediate retinol endocytosis and intracellular release, thereby participating in retinol metabolism and physiological regulation. Retinol can further activate nuclear receptors such as RAR and RXR, modulating gene expression (47, 48).
CVDs-Related signaling: Elevated RBP4 levels are linked to CVDs including AS, CAD, and stroke (28, 52). Mechanistically, RBP4 may activate TLR4/JNK and TLR4/NF-κB/NLRP3 pathways, promoting inflammatory cell activation and cytokine release, which drives vascular inflammation (15, 45, 51). It may also enhance endothelial oxidative stress (53), facilitate foam cell formation (36), and induce lipid accumulation (43), collectively accelerating the progression of atherosclerotic plaques.
Dysregulation of these RBP4-associated signaling pathways is closely implicated in the pathogenesis of various diseases, such as diabetes, AS, stroke, and CAD. This underscores the potential of RBP4 as a therapeutic target for related disorders.
3 RBP4 in CVDs
CVDs encompass a range of disorders, among which CAD and stroke represent leading global causes of disability and mortality. AS underlies the majority of these conditions (54). Accordingly, this section focuses on the associations between RBP4 and CAD, stroke, and AS.
3.1 RBP4 in CAD
CAD, also referred to as coronary heart disease (CHD) or IHD, is the most frequent cause of myocardial infarction and imposes a substantial burden on individuals and society (55). In 2013, Sun et al. reported that elevated circulating RBP4 levels were associated with a threefold increase in CHD risk (56). Subsequent work by Li et al. indicated that serum RBP4 correlates with cardiovascular risk factors and may serve as a marker for CAD (57). Similarly, Dong et al. compared patients with and without carotid atherosclerotic plaques, as well as with and without CHD, and found significantly higher serum RBP4 levels in both plaque-positive and CHD groups. They proposed RBP4 as an independent risk factor for carotid plaque formation and CHD (58), a conclusion consistent with that of Lambadiari et al. (59). Another study by Liu et al. also observed higher serum RBP4 levels in CAD patients compared to controls (60). Together, these studies support the potential of RBP4 as a predictive and diagnostic marker for CAD. Beyond diagnosis, RBP4 has been linked to CAD complexity and severity (23, 59, 61, 62). Moreover, increased circulating RBP4 is associated with a higher risk of major adverse cardiovascular events (MACEs)—including acute coronary syndrome (ACS), heart failure, stroke, peripheral vascular disease, and cardiovascular death—in patients with stable CAD (63). A prospective study further reported that in patients with ACS, higher serum RBP4 at onset predicted a greater risk of MACEs during follow-up, suggesting its utility as a prognostic indicator in ACS (64) (Table 2). These findings collectively highlight RBP4 as a promising biomarker for the diagnosis and prognosis of CHD. Early monitoring and management of serum RBP4 levels could therefore aid in the prevention, timely diagnosis, and treatment of CHD.
Table 2
| Date | Group A | Group B | Main finding | Reference |
|---|---|---|---|---|
| In 2013 | 468 individuals with CAD | 472 matched controls | High RBP4 levels were associated with a 3-fold increased risk of incident CHD in women. | (55) |
| In 2014 | 30 with CAD and 30 with CAD and hyperinsulinemia (CAD/HIns) | 29 healthy subjects | The serum RBP4 concentrations were significantly higher in the CAD/HIns than in the CAD and control groups and associated with cardiovascular risk factors. | (56) |
| In 2015 | 50 cases with CHD | 160 cases without CHD | The serum RBP4 level was significantly higher in CHD patients than in non-CHD patients (45.94 ± 7.85 mg/L vs. 42.21 ± 6.42 mg/L). | (57) |
| In 2014 | 305 individuals with CAD | 91 individuals without CAD | Serum RBP4 levels were significantly elevated in patients with CAD compared to non-CAD patients (39.29 ± 11.72 mg/L vs. 24.83 ± 11.27 mg/L). | (58) |
| In 2019 | CAD group (n = 180) | Control group (n = 79) | The serum RBP4 level was significantly higher in CAD group than in control group (5.79 ng/mL vs. 3.6 ng/mL). | (59) |
| In 2021 | 55 patients with presenting acute coronary syndrome (ACS) | 43 control subjects | Serum RBP4 levels were significantly higher in patients with ACS compared to the without ACS (68.40 ± 47.94 mg/L vs. 49.46 ± 13.64 mg/L). | (61) |
RBP4 and CAD.
3.2 RBP4 in IS
IS is characterized by a sudden disruption of cerebral blood flow due to thrombosis or embolism, and remains a leading cause of mortality and morbidity worldwide (65, 66). Elevated serum RBP4 levels have been associated with an increased risk of cerebrovascular disease in elderly men (25). Consistently, Wang et al. reported significantly higher serum RBP4 levels in elderly patients with cerebral infarction (67). In a study by Sasaki et al., plasma RBP4 was found to be elevated in 58 IS patients compared with 53 healthy controls when measured on the morning after admission (28). Chao et al. further confirmed these findings and demonstrated that RBP4 levels correlated with both infarct size on MRI and stroke severity assessed by the National Institutes of Health Stroke Scale (NIHSS) on admission (26). Beyond severity, higher plasma RBP4 has also been linked to poorer 3-month functional outcomes after stroke (27, 52). Notably, RBP4 levels are reported to be higher in IS than in intracerebral hemorrhage (ICH), suggesting its potential utility in differentiating between these stroke subtypes (68) (Table 3). Regarding the underlying mechanisms, another study indicated that the RBP4/ Lipoprotein-associated phospholipase A2 (Lp-PLA2)/Netrin-1 pathway is involved in the onset and progression of diabetic nephropathy complicated with silent cerebral infarction and cognitive decline (69). Lp-PLA2 promotes inflammatory responses, contributes to the initiation and progression of atherosclerosis, and may facilitate plaque rupture (70–72). Netrin-1, originally identified as an axonal guidance molecule, also exerts anti-inflammatory, anti-apoptotic, and vascular protective effects (73, 74). It is hypothesized that RBP4 may upregulate Lp-PLA2 and downregulate netrin-1, thereby amplifying vascular inflammation and impairing endogenous protective responses, which could collectively promote AS and increase susceptibility to cerebral infarction. While this represents one plausible mechanistic link between RBP4 and IS, the exact molecular pathways remain incompletely understood and warrant further investigation through cellular and animal studies. In summary, RBP4 shows promise as a diagnostic biomarker for IS and a predictor of stroke prognosis. Furthermore, RBP4 antagonists may hold therapeutic potential as neuroprotective agents, and early intervention targeting RBP4 could potentially improve outcomes in stroke patients.
Table 3
| Date | Group A | Group B | Main findings | Reference |
|---|---|---|---|---|
| In 2019 | 323 patients with AIS | 323 healthy people |
|
(26) |
| In 2018 | 299 first-ever AIS | 150 age and gender-matched healthy volunteers |
|
(27) |
| In 2010 | 58 subjects with cerebral infarction | 53 control subjects | Plasma RBP4 was 16.4 ± 2.8 μg/mL in the subjects with cerebral infarction, a value significantly greater than that of 10.1 ± 1.2 μg/mL in the controls. | (28) |
| In 2022 | 136 patients with cerebral infarction | 40 age- and sex-matched control participants |
|
(66) |
RBP4 and IS.
3.3 RBP4 in AS
AS remains the primary pathological basis of CVDs worldwide and is recognized as a chronic inflammatory condition. Characterized by high incidence, disability, and mortality rates, AS poses a serious threat to human life and health (75–78). CVDs resulting from AS are collectively termed atherosclerotic cardiovascular disease (ASCVD), which primarily includes IHD and IS (79). Elevated levels of RBP4 have been reported to correlate positively with carotid intima–media thickness (IMT) (35). IMT is not only one of the most well-established markers of AS but also an important predictor of CVD risk, holding significant diagnostic and monitoring value in clinical practice (80). Furthermore, one study indicated that circulating RBP4 levels are higher in patients with carotid AS compared to healthy controls (34). Wan et al. demonstrated that RBP4 participates in the initiation and progression of AS in diabetic rats via the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway (81). These findings collectively suggest that RBP4 is involved in the pathogenesis and progression of AS. However, the potential mechanisms of RBP4 in atherosclerotic development remain insufficiently studied. Current treatment for AS largely relies on lipid-lowering statins. Nevertheless, the efficacy of statins is limited, with only about 21% of treatments associated with reduced plaque progression (82). In this context, we summarize the role of RBP4 in endothelial cells (ECs), vascular smooth muscle cells (VSMCs), and macrophages, aiming to provide new insights for the diagnosis and treatment of AS.
3.3.1 RBP4 in ECs
ECs serve as the primary interface susceptible to damage across various blood vessel types. ECs dysfunction is widely regarded as the initial step in the development of AS (83). In 2008, Park et al. conducted a study involving 50 patients with type 2 diabetes mellitus (T2DM), measuring serum levels of RBP4, soluble intracellular adhesion molecule-1 (sICAM-1), and soluble E-selectin (sE-selectin). The latter two markers are elevated upon ECs injury (84, 85), and their results demonstrated a positive correlation between RBP4 and both sICAM-1 and sE-selectin (86). Another study further supports the association between RBP4 and ECs dysfunction (87), consistent with the findings of Park et al. Similarly, in vitro experiments indicate that RBP4 promotes the secretion of various proinflammatory factors from ECs, including vascular cell adhesion molecule-1 (VCAM-1), sICAM-1, sE-selectin, monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6), via activation of NADPH oxidase and NF-κB. Notably, both apo-RBP4 (retinol-free) and holo-RBP4 (retinol-bound) exerted comparable pro-inflammatory effects, suggesting that the activity of RBP4 is independent of retinol (16). Moreover, multiple studies have reported positive correlations between RBP4 and oxidative stress biomarkers such as urinary 8-isoprostane (53), 8-iso-prostaglandin F2α (8-isoPGF2α) (88), 13-(S)-hydroxy octadecadienoic acid (88), and malondialdehyde (87), along with a negative correlation with the antioxidant glutathione (53). Given that cellular oxidative stress can directly trigger ECs inflammation (89, 90), RBP4 may not only directly stimulate the release of inflammatory factors from ECs but also promote ECs inflammation through oxidative stress pathways. However, further research is needed to clarify the precise mechanisms involved. Beyond its pro-inflammatory role, RBP4 has also been shown to promote ECs apoptosis by inducing mitochondrial dysfunction and vascular oxidative damage, mediated via suppression of the PI3K/AKT signaling pathway, both in vivo and in vitro (91). Collectively, these findings suggest that RBP4 likely contributes to the initiation and progression of AS by inducing ECs inflammation. Based on the evidence summarized above, serum RBP4 levels may reflect ECs injury and serve as an independent marker of ECs dysfunction. This could facilitate early detection of endothelial damage and enable timely interventions to prevent the onset of vascular diseases.
3.3.2 RBP4 in VSMC
VSMCs are integral components of the vascular wall and participate in nearly the entire process of AS. The proliferation, migration, and phenotypic transformation of VSMCs significantly contribute to the progression of AS pathogenesis (92–94). Both in vivo and in vitro studies have demonstrated that RBP4 promotes VSMC proliferation and migration through the JAK2/STAT3 signaling pathway, an effect that can be inhibited by vitamin D (95). Furthermore, Fei Li et al. reported that high insulin levels promote VSMC proliferation via activation of the mitogen-activated protein kinase (MAPK) signaling pathway, and RBP4 enhances this effect (96, 97). Under physiological conditions, VSMCs maintain a contractile phenotype, expressing various contractile proteins essential for vascular elasticity and integrity. Under pathological conditions, however, VSMCs can undergo a transition to a synthetic/secretory phenotype. This shift leads to increased secretion of extracellular matrix components, lipid accumulation, and ultimately promotes both the progression of AS and plaque instability (98). Importantly, phenotypic transformation of VSMCs is considered an initial step preceding their proliferation and migration (99). In this context, Wan Zhou et al. showed that RBP4 can promote the phenotypic switch of VSMCs by activating the RhoA (ras homolog family member A)/ROCK1 (Rho associated coiled-coil containing protein kinases) signaling pathway (100). Although research on RBP4 and VSMCs remains relatively limited, current evidence indicates that RBP4 likely promotes AS by stimulating VSMC proliferation, migration, and phenotypic switching. The specific molecular mechanisms underlying these effects, however, are not yet fully understood and warrant further investigation.
3.3.3 RBP4 in macrophages
Macrophages play crucial roles in the initiation and progression of aAS by phagocytosing oxidized low-density lipoprotein (Ox-LDL) to form foam cells, participating in inflammatory responses, altering their phenotypes, and secreting a variety of cytokines (101–103). In 2008, M. Broch et al. first reported that RBP4 is expressed in differentiated macrophages but not in undifferentiated monocytes, and its expression can be modulated by inflammatory stimuli (104). This finding marked an important milestone and opened new avenues for investigating the role of macrophages in AS. Studies have demonstrated that RBP4 promotes atherosclerotic progression by enhancing macrophage foam cell formation. This is achieved through activation of the c-Src–JNK–STAT1 pathway, which upregulates CD36 expression and increases cellular cholesterol uptake (36). Beyond facilitating foam cell formation, RBP4 is also implicated in macrophage-driven inflammation. Research by Julie Norseen et al. revealed that RBP4 can induce the secretion of pro-inflammatory cytokines from macrophages via the TLR4/JNK signaling pathway, an effect that is independent of retinol (15). Thus, within macrophages, RBP4 promotes AS through two key mechanisms: augmenting foam cell formation and amplifying inflammatory activation.
In summary, RBP4 contributes to AS through a tripartite mechanism: (a) inducing ECs inflammation and dysfunction, (b) stimulating VSMC proliferation, migration, and phenotypic switching, and (c) enhancing macrophage foam cell formation and pro-inflammatory responses within plaques. Although research on the relationship between RBP4 and AS continues to evolve, current evidence strongly supports its role in facilitating AS onset and progression. These findings highlight the potential of RBP4 as a dual-purpose diagnostic marker and therapeutic target, which may inform novel clinical strategies. Nevertheless, the precise molecular mechanisms and downstream signaling pathways mediated by RBP4 in AS remain incompletely understood. Future studies should therefore prioritize elucidating these mechanisms to fully clarify the pathogenic role of RBP4 in AS.
4 RBP4 and risk factors for CVDs
4.1 RBP4 and obesity, IR and T2DM
Elevated circulating levels of RBP4 have been reported in obese individuals compared with lean subjects, paralleling an increased prevalence of IR—a core pathological feature of T2DM (22, 105–107). One study further indicated that when circulating RBP4 exceeds 55 μg/mL, the risk of T2DM rises by 1.97-fold (21). Interestingly, Graham et al. demonstrated that a 4-week exercise intervention can lower RBP4 levels and improve IR (22). IR denotes diminished tissue sensitivity to insulin, resulting in reduced glucose uptake and utilization and consequent hyperglycemia (107). Notably, IR is an independent risk factor for CVD and early mortality even in the absence of diabetes (108, 109). It can promote endothelial dysfunction by inducing oxidative stress, stimulating cytokine production, and disrupting the renin–angiotensin–aldosterone system (110). Moreover, IR accelerates the progression of atherosclerotic plaques toward a vulnerable phenotype (111), whose rupture may trigger thrombosis and lead to stroke, myocardial infarction, or other acute vascular events (112). Compensatory hyperinsulinemia often arises because of reduced insulin sensitivity (113–116). Elevated insulin levels can drive AS through multiple mechanisms, including stimulation of very-low-density lipoprotein (VLDL) synthesis and secretion (117, 118), promotion of VSMC proliferation and growth (119, 120), activation of inflammation-related genes (121, 122), increased collagen synthesis (123, 124), and enhanced low-density lipoprotein (LDL) transport into smooth muscle cells (SMCs) (125, 126). In rats, insulin infusion under euglycemic conditions induces hypertension—another independent CVD risk factor (127, 128). Clinically, hyperinsulinemia is also associated with elevated risk and progression of several cancers, including those of the pancreas, breast, and colon (129).
Skeletal muscle and adipose tissue are primary sites of insulin-mediated glucose metabolism. Physiologically, insulin binding to its receptor activates the PI3K/AKT pathway, leading to GLUT4 translocation and enhanced glucose uptake. Under pathological conditions, however, key proteins in this pathway are suppressed, resulting in IR (130). Accumulating evidence links RBP4 to IR (9, 61, 131). Qin Yang et al. showed that either transgenic overexpression of RBP4 or intraperitoneal injection of recombinant human RBP4 in mice reduced insulin-stimulated PI3K activity in muscle by 30% and 34%, respectively, inducing IR (9). Beyond TLR4, the cell-surface receptor STRA6 also mediates RBP4-induced insulin resistance. Daniel C. et al. reported that holo-RBP4 binding to STRA6 recruits and activates JAK2, leading to STAT5 phosphorylation, nuclear translocation, and subsequent upregulation of cytokine signaling 3 (SOCS3) and peroxisome proliferator-activated receptor gamma (PPARγ). These changes inhibit insulin signaling and promote lipid accumulation in adipocytes (43), a finding corroborated by later work (132). Additionally, RBP4 binding to TLR4 on macrophages promotes NF-κB phosphorylation and NLRP3 inflammasome assembly, enhancing IL-1β expression and release. IL-1β then acts on adipocytes via its receptor to impair insulin-induced AKT phosphorylation, further exacerbating IR (45). RBP4 can also modulate crosstalk between innate and adaptive immune cells to promote IR. In RBP4-overexpressing mice, adipose-tissue macrophages secrete inflammatory factors that activate CD4+ T cells, largely through JNK-dependent activation of antigen-presenting cells (APCs). Transfer of these activated APCs into normal mice is sufficient to induce IR (51, 133).
Beyond IR, pancreatic β-cell dysfunction constitutes another major pathophysiological basis of T2DM. Clinical studies by Rong Huang et al. and others have shown an inverse correlation between circulating RBP4 levels and β-cell function (134–136). Mechanistically, basic research indicates that RBP4, via STRA6, suppresses the transcription factor insulin gene enhancer binding protein 1 (ISL-1)—a key regulator of insulin synthesis and β-cell homeostasis—thereby reducing insulin secretion (50, 137, 138).
Collectively, these findings suggest that pharmacological inhibition of RBP4 may simultaneously improve insulin sensitivity and preserve β-cell function, offering a promising novel strategy for the treatment of T2DM.
4.2 RBP4 and hypertension
Hypertension is a well-established risk factor and a leading cause of disability and mortality among patients with CVD (139, 140). Over the past five decades, the prevalence of hypertension in China has risen significantly, currently affecting approximately one-quarter of the adult population (141). Moreover, hypertension contributes to an estimated 43% of cardiovascular events in China (142, 143). A meta-analysis indicated that patients with preeclampsia, characterized by elevated blood pressure and proteinuria during pregnancy, exhibit higher plasma RBP4 levels compared with normotensive pregnant women, indirectly associating increased RBP4 levels with hypertension (144, 145). In individuals with prehypertension (defined as systolic blood pressure between 120 and 139 mmHg and/or diastolic blood pressure between 80 and 89 mmHg) serum RBP4 levels are elevated relative to those with normal blood pressure (146). Li et al. compared plasma RBP4 levels in 74 hypertensive patients and 46 healthy volunteers, reporting significantly higher RBP4 levels in the hypertensive group (147). This finding is consistent with studies by Majerczyk M et al. and Anna Solini et al. (31, 148). Animal studies further support this association: RBP4-knockout (RBP4-KO) mice demonstrated lower blood pressure than wild-type (WT) mice, whereas RBP4-overexpressing (RBP4-TG) mice exhibited higher blood pressure. Additionally, phosphorylation levels of endothelial nitric oxide synthase (eNOS) in arterial tissues were 150% in RBP4-KO mice and 80% in RBP4-TG mice compared with WT mice (149). Since eNOS regulates nitric oxide (NO) production, which is a key mediator of endothelium-dependent vasodilation and blood pressure control (150, 151), it can be inferred that RBP4 influences blood pressure by modulating NO production. Furthermore, research indicates that RBP4-induced IR may also be linked to hypertension (152, 153), offering another perspective on the relationship between RBP4 and hypertensive mechanisms. Collectively, these findings suggest that RBP4 could represent a potential therapeutic target for hypertension. However, further experimental studies are needed to clarify the precise role of RBP4 in the pathogenesis and progression of hypertension.
4.3 RBP4 and dyslipidemia
The lipids commonly assessed in clinical practice include plasma total cholesterol (TC), LDL, high-density lipoprotein (HDL), and triglycerides (TGs). Dyslipidemia is characterized by elevated levels of TC, TG, and LDL, along with reduced levels of HDL (154, 155). Elevated plasma LDL represents a significant risk factor for CVD (156), and the use of statins has been shown to effectively lower cholesterol levels and reduce CVD-related mortality worldwide (157). Studies have demonstrated a positive correlation between circulating RBP4 levels and TGs (158). Elevated RBP4 is associated not only with higher TG but also with lower HDL levels (86, 159). Research by Wessel et al. further indicated that both RBP4 and retinol are positively correlated with TG, TC, and LDL (160). Consistently, another study reported that changes in circulating RBP4 levels positively correlated with changes in LDL, small dense LDL-C, and apoB in patients (161). In summary, RBP4 is closely linked to lipid metabolism, although the specific mechanisms underlying this relationship remain unclear. Further research is needed to elucidate how RBP4 influences lipid metabolic pathways.
5 The potential of RBP4 as a therapeutic target for CVDs
Identifying disease-related proteins as targets for diagnosis and treatment represents a prominent research direction across numerous human diseases. Consequently, RBP4 holds promise as a potential target for the early diagnosis and intervention of CVDs. Its application could help shift the window for prevention and treatment forward, thereby contributing to improved public health outcomes. Numerous RBP4 antagonists have demonstrated safety and efficacy in preclinical studies. Fenretinide, a synthetic retinoid, binds to RBP4 and inhibits its interaction with TTR, promoting renal clearance of RBP4 (162, 163). Furthermore, non-retinoid ligands such as A1120 and BPN-14136 have also been shown to reduce serum RBP4 levels in animal models (164–168). Given their efficacy and safety profiles, non-retinoid RBP4 antagonists may emerge as viable therapeutic candidates. Notably, two non-retinoid antagonists, tinlarebant (BPN-14967) and STG-001 (Stargazer), are currently under clinical evaluation. In the global Phase 3 DRAGON trial, tinlarebant exhibited a well-tolerated and consistent safety profile (169), while STG-001 has completed Phase II clinical trials (170).
Beyond receptor antagonism, directly inhibiting RBP4 gene expression offers another viable strategy for reducing its serum levels. Research indicates that several antidiabetic agents, including pioglitazone (171, 172), rosiglitazone (173), and sitagliptin (174, 175), can downregulate RBP4 expression. Additionally, the lipid-lowering drug fenofibrate has been reported to inhibit RBP4 transcription in adipocytes. In a clinical study involving 15 non-diabetic patients with IR, daily administration of 200 mg fenofibrate for eight weeks resulted in a reduction of serum RBP4 levels by approximately 29.8% (176). With growing public interest in health maintenance, dietary and nutraceutical approaches have gained considerable attention. Anthocyanins, a class of water-soluble natural pigments widely present in plants, have been shown to significantly suppress RBP4 expression and lower its circulating levels (177–179). Similarly, resveratrol—a natural polyphenol found in grapes, berries, and peanuts—can reduce RBP4 expression in adipocytes (180, 181). Preclinical studies further indicate that RBP4-targeting oligonucleotides effectively decrease RBP4 expression in hepatic and adipose tissues, lower circulating RBP4 levels, and improve IR and hyperglycemia in high-fat-diet animal models (182) (Table 4).
Table 4
| Agent | Type | Mechanism | Current Stage | Pros | Cons | References |
|---|---|---|---|---|---|---|
| Fenretinide | Synthesized retinol | Promote the clearance of RBP4 from the kidneys | Phase II clinical trial | Unknown | Low bioavailability; causes night blindness | (162, 163) |
| A1120 | Nonretinol ligands | Reduce serum RBP4 levels | Animal experiment | May avoid the retinoids-associated side effects, such as nyctalopia and delayed dark adaptation | Poor human liver microsomal stability | (164–166, 168) |
| BPN-14136 | Nonretinol ligands | Reduce serum RBP4 levels | Animal experiment | Without affecting the visual cycles | Unknown | (167) |
| Tinlarebant (BPN-14967) | Nonretinol ligands | Reduce serum RBP4 levels | Phase III clinical trials | Well-tolerated and consistent safety profile | Unknown | (169) |
| STG-001 | Nonretinol ligands | Reduce serum RBP4 levels | Phase II clinical trials | Without affecting the visual cycles | Unknown | (170) |
| Antidiabetic drugs | A hypoglycemic drug | Inhibiting RBP4 gene expression | In clinical use for T2DM, but RBP4 effect is observational. | Unknown | Unknown | (171–175) |
| Fenofibrate | A lipid-lowering drug | Inhibit the transcription of RBP4 | In vitro experiment and clinical study | Unknown | Unknown | (176) |
| Anthocyanins | Natural pigment | Reduce the expression of RBP4 | Animal experiment | Unknown | Unknown | (177–179) |
| Resveratrol | Nutrient | Reduce the expression of RBP4 | Animal experiment | Unknown | Unknown | (180, 181) |
| RBP4-targeting oligonucleotide | RNA oligonucleotide | Inhibit the expression of RBP4 | In vitro and in vivo (animal) experiment | High genetic specificity; direct reduction of synthesis | Unknown | (182) |
Overview of therapeutic strategies targeting RBP4.
Collectively, these findings underscore the significant clinical potential of RBP4-targeted strategies for CVD treatment. The translation of such approaches into clinical practice appears promising. However, further investigation is necessary to fully understand the implications of RBP4 inhibition on retinoid metabolism and long-term physiological outcomes.
6 Conclusion
In summary, elevated RBP4 levels are significantly associated with an increased risk of major CVDs, such as myocardial infarction and stroke. This relationship appears to operate through and interact with key cardiometabolic risk factors, particularly IR, T2DM, hypertension, and dyslipidemia [Figure 2; created with BioGDP.com (183)]. Available evidence indicates that RBP4 may function not only as a biomarker but also as an active contributor to cardiovascular pathology. Further studies are warranted to fully clarify its underlying molecular mechanisms, which may ultimately inform the development of novel preventive and therapeutic strategies for cardiovascular disorders.
Figure 2
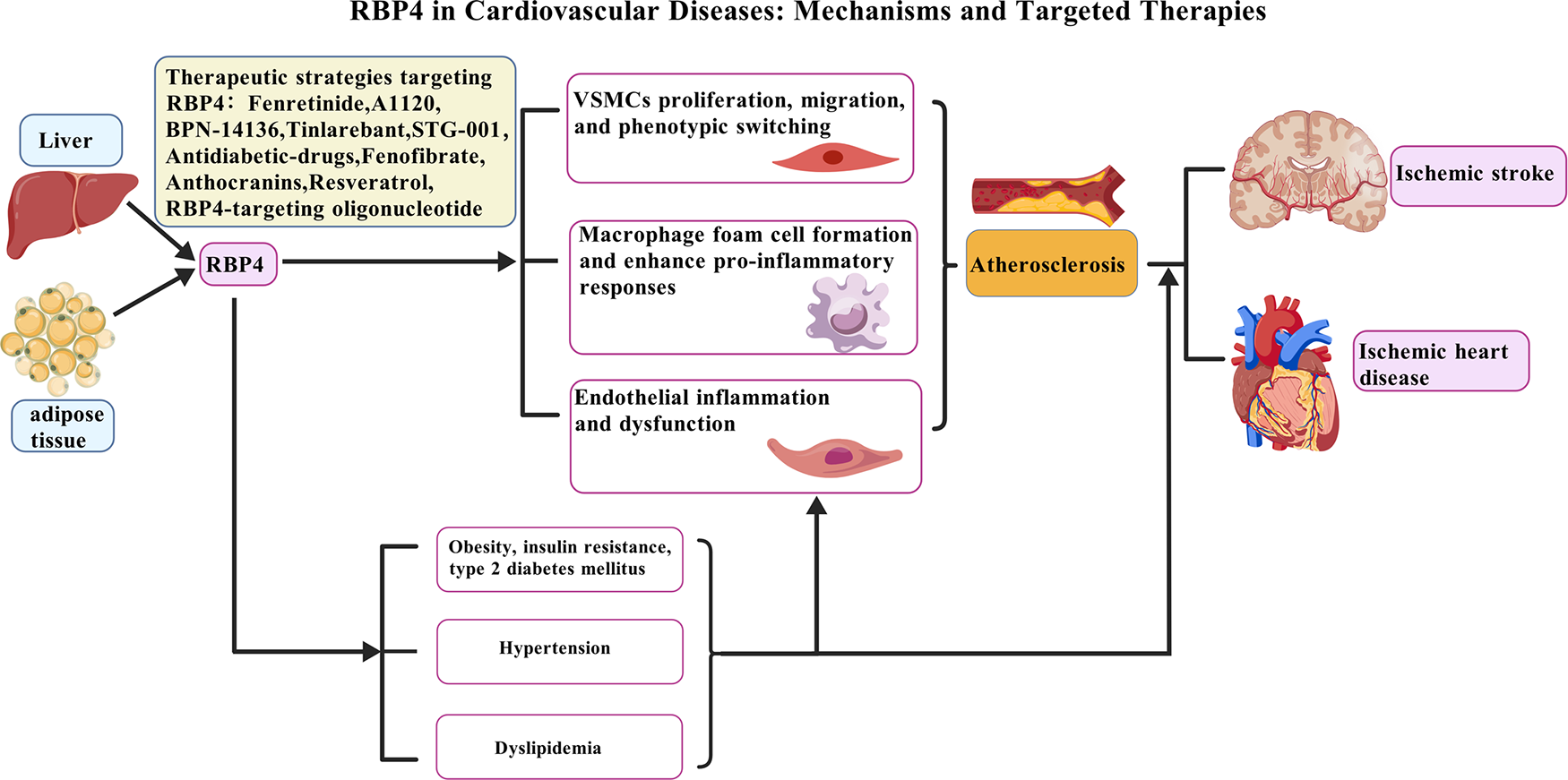
Pathogenic role of RBP4 in CVDs and potential therapeutic strategies. The increase of RBP4 level is not only related to CVDs risk factors such as obesity, insulin resistance, T2DM, hypertension and dyslipidemia, but also directly leads to the occurrence and development of atherosclerosis by acting on multiple cell types: (1) promoting VSMCs proliferation, migration, and phenotypic switching; (2) enhancing macrophage foam cell formation and pro-inflammatory responses; and (3) inducing endothelial inflammation and dysfunction. These mechanisms collectively drive the progression of CVDs, such as ischemic heart disease and ischemic stroke. Various therapeutic agents under investigation target RBP4, such as Fenretinide, BPN-14136, Tiniarebant, an RBP4-targeting oligonucleotide, as well as metabolic regulators including Resveratrol and Fenofibrate. Created with BioGDP.com.
Statements
Author contributions
JC: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Writing – original draft. GC: Methodology, Writing – original draft. XX: Investigation, Writing – original draft. JZ: Conceptualization, Supervision, Writing – review & editing. FL: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Natural Science Foundation of Henan (No. 252300421602 to JC) and the Medical Science and Technology Research Projects of Henan Province Health Commission (LHGJ20240007 to FL, and LHGJ20240084 to JC).
Acknowledgments
The authors acknowledge the language editing and polishing provided for this manuscript by a scholar within the medical field who is proficient in English. No AI-based writing tools were used in the drafting, development, or substantive editing of this research.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Townsend N Kazakiewicz D Lucy Wright F Timmis A Huculeci R Torbica A et al Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. (2021) 19(2):133–43. 10.1038/s41569-021-00607-3
2.
Kim SH Cho YK Kim YJ Jung CH Lee WJ Park JY et al Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol. (2022) 21(1):81. 10.1186/s12933-022-01522-8
3.
Yin Z Zou Y Wang D Huang X Xiong S Cao L et al Regulation of the Tec family of non-receptor tyrosine kinases in cardiovascular disease. Cell Death Discov. (2022) 8(1):119. 10.1038/s41420-022-00927-4
4.
Du H Xu Y Zhu L . Role of semaphorins in ischemic stroke. Front Mol Neurosci. (2022) 15:848506. 10.3389/fnmol.2022.848506
5.
Rychter AM Skrzypczak-Zielińska M Zielińska A Eder P Souto EB Zawada A et al Is the retinol-binding protein 4 a possible risk factor for cardiovascular diseases in obesity? Int J Mol Sci. (2020) 21(15):5229. 10.3390/ijms21155229
6.
Khot UN Khot MB Bajzer CT Sapp SK Ohman EM Brener SJ et al Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. (2003) 290(7):898–904. 10.1001/jama.290.7.898
7.
Matsis K Holley A Al-Sinan A Matsis P Larsen PD Harding SA . Differing clinical characteristics between young and older patients presenting with myocardial infarction. Heart Lung Circ. (2017) 26(6):566–71. 10.1016/j.hlc.2016.09.007
8.
Blaner WS . Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev. (1989) 10(3):308–16. 10.1210/edrv-10-3-308
9.
Yang Q Graham TE Mody N Preitner F Peroni OD Zabolotny JM et al Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. (2005) 436(7049):356–62. 10.1038/nature03711
10.
Steinhoff JS Lass A Schupp M . Biological functions of RBP4 and its relevance for human diseases. Front Physiol. (2021) 12:659977. 10.3389/fphys.2021.659977
11.
Goodman AB . Retinoid receptors, transporters, and metabolizers as therapeutic targets in late onset Alzheimer disease. J Cell Physiol. (2006) 209(3):598–603. 10.1002/jcp.20784
12.
Lee JW Lee HR Shim JY Im JA Lee DC . Abdominal visceral fat reduction is associated with favorable changes of serum retinol binding protein-4 in nondiabetic subjects. Endocr J. (2008) 55(5):811–8. 10.1507/endocrj.k08e-030
13.
Cabré A Lázaro I Girona J Manzanares J Marimón F Plana N et al Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med. (2007) 262(4):496–503. 10.1111/j.1365-2796.2007.01849.x
14.
Kawaguchi R Yu J Honda J Hu J Whitelegge J Ping P et al A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science (New York, NY). (2007) 315(5813):820–5. 10.1126/science.1136244
15.
Norseen J Hosooka T Hammarstedt A Yore MM Kant S Aryal P et al Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. (2012) 32(10):2010–9. 10.1128/mcb.06193-11
16.
Farjo KM Farjo RA Halsey S Moiseyev G Ma JX . Retinol-binding protein 4 induces inflammation in human endothelial cells by an NADPH oxidase- and nuclear factor kappa B-dependent and retinol-independent mechanism. Mol Cell Biol. (2012) 32(24):5103–15. 10.1128/mcb.00820-12
17.
Gao W Wang H Zhang L Cao Y Bao JZ Liu ZX et al Retinol-binding protein 4 induces cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway. Endocrinology. (2016) 157(6):2282–93. 10.1210/en.2015-2022
18.
Cione E Caroleo MC Cannataro R Perri M Pingitore A Genchi G . Vitamin A and diabesity: new insight for drug discovery. Mini Rev Med Chem. (2016) 16(9):738–42. 10.2174/1389557515666150709112822
19.
Thompson SJ Sargsyan A Lee SA Yuen JJ Cai J Smalling R et al Hepatocytes are the principal source of circulating RBP4 in mice. Diabetes. (2017) 66(1):58–63. 10.2337/db16-0286
20.
Shirakami Y Lee SA Clugston RD Blaner WS . Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta. (2012) 1821(1):124–36. 10.1016/j.bbalip.2011.06.023
21.
Fan J Yin S Lin D Liu Y Chen N Bai X et al Association of serum retinol-binding protein 4 levels and the risk of incident type 2 diabetes in subjects with prediabetes. Diabetes Care. (2019) 42(8):1574–81. 10.2337/dc19-0265
22.
Graham TE Yang Q Blüher M Hammarstedt A Ciaraldi TP Henry RR et al Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. (2006) 354(24):2552–63. 10.1056/NEJMoa054862
23.
Liu Y Wang D Chen H Xia M . Circulating retinol binding protein 4 is associated with coronary lesion severity of patients with coronary artery disease. Atherosclerosis. (2015) 238(1):45–51. 10.1016/j.atherosclerosis.2014.11.016
24.
Chavarria N Kato TS Khan R Chokshi A Collado E Akashi H et al Increased levels of retinol binding protein 4 in patients with advanced heart failure correct after hemodynamic improvement through ventricular assist device placement. Circ J. (2012) 76(9):2148–52. 10.1253/circj.cj-12-0350
25.
Ingelsson E Sundström J Melhus H Michaëlsson K Berne C Vasan RS et al Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. (2009) 206(1):239–44. 10.1016/j.atherosclerosis.2009.02.029
26.
Liu C Che Y . Retinol-binding protein 4 predicts lesion volume (determined by MRI) and severity of acute ischemic stroke. Neurotox Res. (2019) 35(1):92–9. 10.1007/s12640-018-9933-z
27.
Zhu YY Zhang JL Liu L Han Y Ge X Zhao S . Evaluation of serum retinol-binding protein-4 levels as a biomarker of poor short-term prognosis in ischemic stroke. Biosci Rep. (2018) 38(5):BSR20180786. 10.1042/bsr20180786
28.
Sasaki M Otani T Kawakami M Ishikawa SE . Elevation of plasma retinol-binding protein 4 and reduction of plasma adiponectin in subjects with cerebral infarction. Metab Clin Exp. (2010) 59(4):527–32. 10.1016/j.metabol.2009.08.015
29.
Barazzoni R Zanetti M Semolic A Pirulli A Cattin MR Biolo G et al High plasma retinol binding protein 4 (RBP4) is associated with systemic inflammation independently of low RBP4 adipose expression and is normalized by transplantation in nonobese, nondiabetic patients with chronic kidney disease. Clin Endocrinol (Oxf). (2011) 75(1):56–63. 10.1111/j.1365-2265.2011.03990.x
30.
Yao-Borengasser A Varma V Bodles AM Rasouli N Phanavanh B Lee MJ et al Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. (2007) 92(7):2590–7. 10.1210/jc.2006-0816
31.
Solini A Santini E Madec S Rossi C Muscelli E . Retinol-binding protein-4 in women with untreated essential hypertension. Am J Hypertens. (2009) 22(9):1001–6. 10.1038/ajh.2009.116
32.
Liu C Gu J Yao Y . Longitudinal change of plasma retinol-binding protein 4 and its relation to neurological-function recovery, relapse, and death in acute ischemic stroke patients. Tohoku J Exp Med. (2023) 260(4):293–300. 10.1620/tjem.2023.J036
33.
Ng TW Watts GF Barrett PH Rye KA Chan DC . Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care. (2007) 30(11):2945–50. 10.2337/dc07-0768
34.
Kadoglou NP Lambadiari V Gastounioti A Gkekas C Giannakopoulos TG Koulia K et al The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis. (2014) 235(2):606–12. 10.1016/j.atherosclerosis.2014.05.957
35.
Bobbert T Raila J Schwarz F Mai K Henze A Pfeiffer AF et al Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. (2010) 213(2):549–51. 10.1016/j.atherosclerosis.2010.07.063
36.
Liu Y Zhong Y Chen H Wang D Wang M Ou JS et al Retinol-binding protein-dependent cholesterol uptake regulates macrophage foam cell formation and promotes atherosclerosis. Circulation. (2017) 135(14):1339–54. 10.1161/circulationaha.116.024503
37.
Christou GA Andriopoulou CE Liakopoulou A Tsape E Apostolakis E Tselepis AD et al Unraveling the role of resistin, retinol-binding protein 4 and adiponectin produced by epicardial adipose tissue in cardiac structure and function: evidence of a paracrine effect. Hormones (Athens, Greece). (2023) 22(2):321–30. 10.1007/s42000-023-00447-5
38.
Koch A Weiskirchen R Sanson E Zimmermann HW Voigt S Dückers H et al Circulating retinol binding protein 4 in critically ill patients before specific treatment: prognostic impact and correlation with organ function, metabolism and inflammation. Crit Care (London, England). (2010) 14(5):R179. 10.1186/cc9285
39.
Rask L Anundi H Böhme J Eriksson U Fredriksson A Nilsson SF et al The retinol-binding protein. Scand J Clin Lab Invest Suppl. (1980) 154:45–61.
40.
von Eynatten M Humpert PM . Retinol-binding protein-4 in experimental and clinical metabolic disease. Expert Rev Mol Diagn. (2008) 8(3):289–99. 10.1586/14737159.8.3.289
41.
Bellovino D Morimoto T Tosetti F Gaetani S . Retinol binding protein and transthyretin are secreted as a complex formed in the endoplasmic reticulum in HepG2 human hepatocarcinoma cells. Exp Cell Res. (1996) 222(1):77–83. 10.1006/excr.1996.0010
42.
Bahlool AZ Grant C Cryan SA Keane J O’Sullivan MP . All trans retinoic acid as a host-directed immunotherapy for tuberculosis. Curr Res Immunol. (2022) 3:54–72. 10.1016/j.crimmu.2022.03.003
43.
Berry DC Jin H Majumdar A Noy N . Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci U S A. (2011) 108(11):4340–5. 10.1073/pnas.1011115108
44.
Alapatt P Guo F Komanetsky SM Wang S Cai J Sargsyan A et al Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J Biol Chem. (2013) 288(2):1250–65. 10.1074/jbc.M112.369132
45.
Moraes-Vieira PM Yore MM Sontheimer-Phelps A Castoldi A Norseen J Aryal P et al Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through toll-like receptors 2 and 4. Proc Natl Acad Sci U S A. (2020) 117(49):31309–18. 10.1073/pnas.2013877117
46.
Flores-Cortez YA Barragán-Bonilla MI Mendoza-Bello JM González-Calixto C Flores-Alfaro E Espinoza-Rojo M . Interplay of retinol binding protein 4 with obesity and associated chronic alterations (review). Mol Med Rep. (2022) 26(1):244. 10.3892/mmr.2022.12760
47.
Huang W Yu J Liu T Tudor G Defnet AE Zalesak S et al Proteomic evaluation of the natural history of the acute radiation syndrome of the gastrointestinal tract in a non-human primate model of partial-body irradiation with minimal bone marrow sparing includes dysregulation of the retinoid pathway. Health Phys. (2020) 119(5):604–20. 10.1097/hp.0000000000001351
48.
Manna D Sarkar D . Multifunctional role of astrocyte elevated gene-1 (AEG-1) in cancer: focus on drug resistance. Cancers (Basel). (2021) 13(8):1792. 10.3390/cancers13081792
49.
Sun L Zheng M Gao Y Brigstock DR Gao R . Retinoic acid signaling pathway in pancreatic stellate cells: insight into the anti-fibrotic effect and mechanism. Eur J Pharmacol. (2024) 967:176374. 10.1016/j.ejphar.2024.176374
50.
Huang R Bai X Li X Wang X Zhao L . Retinol-binding protein 4 activates STRA6, provoking pancreatic β-cell dysfunction in type 2 diabetes. Diabetes. (2021) 70(2):449–63. 10.2337/db19-1241
51.
Moraes-Vieira PM Castoldi A Aryal P Wellenstein K Peroni OD Kahn BB . Antigen presentation and T-cell activation are critical for RBP4-induced insulin resistance. Diabetes. (2016) 65(5):1317–27. 10.2337/db15-1696
52.
Liu C Gu J Yao Y Guo P Hu H . Correlation between retinol binding protein 4 and CT perfusion imaging and prognosis in patients with acute ischemic stroke based on cell imaging data analysis. Contrast Media Mol Imaging. (2022) 2022:3055712. 10.1155/2022/3055712
53.
Codoñer-Franch P Mora-Herranz A Simó-Jordá R Pérez-Rambla C Boix-García L Faus-Pérez A . Retinol-binding protein 4 levels are associated with measures of liver and renal function and oxidant/antioxidant status in obese children. J Pediatr. (2013) 163(2):593–5. 10.1016/j.jpeds.2013.03.060
54.
Lu Y Zhang M Zhao P Jia M Liu B Jia Q et al Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol Med Rep. (2017) 16(1):647–53. 10.3892/mmr.2017.6646
55.
Yuan R Shi WL Xin QQ Chen KJ Cong WH . Holistic regulation of angiogenesis with Chinese herbal medicines as a new option for coronary artery disease. Evid Based Complement Altern Med. (2018) 2018:3725962. 10.1155/2018/3725962
56.
Sun Q Kiernan UA Shi L Phillips DA Kahn BB Hu FB et al Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses’ health study. Circulation. (2013) 127(19):1938–47. 10.1161/circulationaha.113.002073
57.
Li F Xia K Li C Yang T . Retinol-binding protein 4 as a novel risk factor for cardiovascular disease in patients with coronary artery disease and hyperinsulinemia. Am J Med Sci. (2014) 348(6):474–9. 10.1097/maj.0000000000000347
58.
Dong H Li X Tang Y . Serum retinol-binding protein-4 level is a high risk factor for coronary heart disease in Chinese. Clin Lab. (2015) 61(11):1675–8. 10.7754/clin.lab.2015.141216
59.
Lambadiari V Kadoglou NP Stasinos V Maratou E Antoniadis A Kolokathis F et al Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc Diabetol. (2014) 13:121. 10.1186/s12933-014-0121-z
60.
Liu T Han C Sun L Ding Z Shi F Wang R et al Association between new circulating proinflammatory and anti-inflammatory adipocytokines with coronary artery disease. Coron Artery Dis. (2019) 30(7):528–35. 10.1097/mca.0000000000000778
61.
Perumalsamy S Ahmad WAW Huri HZ . Retinol-binding protein-4-A predictor of insulin resistance and the severity of coronary artery disease in type 2 diabetes patients with coronary artery disease. Biology (Basel). (2021) 10(9):858. 10.3390/biology10090858
62.
Nar G Sanlialp SC Nar R . Retinol binding protein 4 levels relate to the presence and severity of coronary artery disease. J Med Biochem. (2021) 40(4):384–9. 10.5937/jomb0-28846
63.
Qian K Yan X Xu C Fang Y Ma M . Association between circulating retinol-binding protein 4 and adverse cardiovascular events in stable coronary artery disease. Front Cardiovasc Med. (2022) 9:829347. 10.3389/fcvm.2022.829347
64.
Ye B Zhao Q Fan J Li X Shan C Liu F et al RBP4-based multimarker score: a prognostic tool for adverse cardiovascular events in acute coronary syndrome patients. J Clin Endocrinol Metab. (2023) 108(12):3111–21. 10.1210/clinem/dgad389
65.
Fisher M Saver JL . Future directions of acute ischaemic stroke therapy. Lancet Neurol. (2015) 14(7):758–67. 10.1016/s1474-4422(15)00054-x
66.
Feigin VL Forouzanfar MH Krishnamurthi R Mensah GA Connor M Bennett DA et al Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet (London, England). (2014) 383(9913):245–54. 10.1016/s0140-6736(13)61953-4
67.
Wang Q Tian S Xiao D Zhao R Zhang X Dou Z et al Correlation of serum RBP4 level with oxidative stress and unstable carotid plaque in patients with cerebral infarction. Transl Neurosci. (2022) 13(1):354–60. 10.1515/tnsci-2022-0252
68.
Llombart V García-Berrocoso T Bustamante A Giralt D Rodriguez-Luna D Muchada M et al Plasmatic retinol-binding protein 4 and glial fibrillary acidic protein as biomarkers to differentiate ischemic stroke and intracerebral hemorrhage. J Neurochem. (2016) 136(2):416–24. 10.1111/jnc.13419
69.
Chen D Huang X Lu S Deng H Gan H Du X et al RBP4/Lp-PLA2/Netrin-1 signaling regulation of cognitive dysfunction in diabetic nephropathy complicated with silent cerebral infarction. Exp Clin Endocrinol Diabetes. (2017) 125(8):547–53. 10.1055/s-0043-109099
70.
Katan M Moon YP Paik MC Wolfert RL Sacco RL Elkind MS . Lipoprotein-associated phospholipase A2 is associated with atherosclerotic stroke risk: the Northern Manhattan study. PLoS One. (2014) 9(1):e83393. 10.1371/journal.pone.0083393
71.
Fortunato J Bláha V Bis J Št'ásek J Andrýs C Vojáček J et al Lipoprotein-associated phospholipase A₂ mass level is increased in elderly subjects with type 2 diabetes mellitus. J Diabetes Res. (2014) 2014:278063. 10.1155/2014/278063
72.
Zheng H Cui D Quan X Yang W Li Y Zhang L et al Lp-PLA2 silencing protects against ox-LDL-induced oxidative stress and cell apoptosis via Akt/mTOR signaling pathway in human THP1 macrophages. Biochem Biophys Res Commun. (2016) 477(4):1017–23. 10.1016/j.bbrc.2016.07.022
73.
Jiang J Shi K Huang YH Hsu CY Hettie KS Kung WM . Editorial: translational advances in Alzheimer’s, Parkinson’s, and other dementia: molecular mechanisms, biomarkers, diagnosis, and therapies, volume II. Front Aging Neurosci. (2022) 14:1045828. 10.3389/fnagi.2022.1045828
74.
Okutucu M Fındık H Aslan MG Arpa M . Increased serum concentration of netrin-1 after intravitreal bevacizumab injection: is it a compensatory mechanism to counteract drug side effects?BMC Ophthalmol. (2021) 21(1):243. 10.1186/s12886-021-01989-1
75.
Dou M Chen Y Hu J Ma D Xing Y . Recent advancements in CD47 signal transduction pathways involved in vascular diseases. BioMed Res Int. (2020) 2020:4749135. 10.1155/2020/4749135
76.
Fuster JJ MacLauchlan S Zuriaga MA Polackal MN Ostriker AC Chakraborty R et al Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science (New York, NY). (2017) 355(6327):842–7. 10.1126/science.aag1381
77.
Pant S Deshmukh A Gurumurthy GS Pothineni NV Watts TE Romeo F et al Inflammation and atherosclerosis–revisited. J Cardiovasc Pharmacol Ther. (2014) 19(2):170–8. 10.1177/1074248413504994
78.
Wu MY Li CJ Hou MF Chu PY . New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. (2017) 18(10):2034. 10.3390/ijms18102034
79.
Lei J Duan W Yao X Hu Z Fan H Liu Y et al Association of glucose to lymphocyte ratio with the risk of death in patients with atherosclerotic cardiovascular disease. Sci Rep. (2025) 15(1):3861. 10.1038/s41598-025-87260-9
80.
O'Leary DH Polak JF . Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol. (2002) 90(10c):18l–21L. 10.1016/s0002-9149(02)02957-0
81.
Zhou W Ye SD Wang W . Elevated retinol binding protein 4 levels are associated with atherosclerosis in diabetic rats via JAK2/STAT3 signaling pathway. World J Diabetes. (2021) 12(4):466–79. 10.4239/wjd.v12.i4.466
82.
Lee SE Chang HJ Sung JM Park HB Heo R Rizvi A et al Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. (2018) 11(10):1475–84. 10.1016/j.jcmg.2018.04.015
83.
Davignon J Ganz P . Role of endothelial dysfunction in atherosclerosis. Circulation. (2004) 109(23 Suppl 1):Iii27–32. 10.1161/01.CIR.0000131515.03336.f8
84.
Adams DH Shaw S . Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet (London, England). (1994) 343(8901):831–6. 10.1016/s0140-6736(94)92029-x
85.
Springer TA . Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. (1994) 76(2):301–14. 10.1016/0092-8674(94)90337-9
86.
Park SE Kim DH Lee JH Park JS Kang ES Ahn CW et al Retinol-binding protein-4 is associated with endothelial dysfunction in adults with newly diagnosed type 2 diabetes mellitus. Atherosclerosis. (2009) 204(1):23–5. 10.1016/j.atherosclerosis.2008.08.012
87.
Solini A Stea F Santini E Bruno RM Duranti E Taddei S et al Adipocytokine levels mark endothelial function in normotensive individuals. Cardiovasc Diabetol. (2012) 11:103. 10.1186/1475-2840-11-103
88.
Liu Y Wang D Li D Sun R Xia M . Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol Metab Syndr. (2014) 6(1):25. 10.1186/1758-5996-6-25
89.
Endemann DH Schiffrin EL . Endothelial dysfunction. J Am Soc Nephrol. (2004) 15(8):1983–92. 10.1097/01.Asn.0000132474.50966.Da
90.
Frey RS Ushio-Fukai M Malik AB . NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signaling. (2009) 11(4):791–810. 10.1089/ars.2008.2220
91.
Wang J Chen H Liu Y Zhou W Sun R Xia M . Retinol binding protein 4 induces mitochondrial dysfunction and vascular oxidative damage. Atherosclerosis. (2015) 240(2):335–44. 10.1016/j.atherosclerosis.2015.03.036
92.
Wu FY Li CI Liao LN Liu CS Lin WY Lin CH et al Evaluation of single nucleotide polymorphisms in 6 candidate genes and carotid intima-media thickness in community-dwelling residents. PLoS One. (2020) 15(3):e0230715. 10.1371/journal.pone.0230715
93.
Bennett MR Sinha S Owens GK . Vascular smooth muscle cells in atherosclerosis. Circ Res. (2016) 118(4):692–702. 10.1161/circresaha.115.306361
94.
Yamagishi S Matsui T . Smooth muscle cell pathophysiology and advanced glycation end products (AGEs). Curr Drug Targets. (2010) 11(7):875–81. 10.2174/138945010791320827
95.
Zhou W Wang W Yuan XJ Xiao CC Xing Y Ye SD et al The effects of RBP4 and vitamin D on the proliferation and migration of vascular smooth muscle cells via the JAK2/STAT3 signaling pathway. Oxid Med Cell Longevity. (2022) 2022:3046777. 10.1155/2022/3046777
96.
Li F Xia K Sheikh MS Cheng J Li C Yang T . Retinol binding protein 4 promotes hyperinsulinism-induced proliferation of rat aortic smooth muscle cells. Mol Med Rep. (2014) 9(5):1634–40. 10.3892/mmr.2014.2028
97.
Li F Xia K Sheikh SA Cheng J Li C Yang T . Involvement of RBP4 in hyperinsulinism-induced vascular smooth muscle cell proliferation. Endocrine. (2015) 48(2):472–82. 10.1007/s12020-014-0304-0
98.
Wang R Wu W Li W Huang S Li Z Liu R et al Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis via HMGB1. J Am Heart Assoc. (2018) 7(19):e008596. 10.1161/jaha.118.008596
99.
Fang L Wang KK Zhang PF Li T Xiao ZL Yang M et al Nucleolin promotes Ang II-induced phenotypic transformation of vascular smooth muscle cells by regulating EGF and PDGF-BB. J Cell Mol Med. (2020) 24(2):1917–33. 10.1111/jcmm.14888
100.
Zhou W Yuan X Li J Wang W Ye S . Retinol binding protein 4 promotes the phenotypic transformation of vascular smooth muscle cells under high glucose condition via modulating RhoA/ROCK1 pathway. Transl Res. (2023) 259:13–27. 10.1016/j.trsl.2023.03.004
101.
Zhang L Li J Kou Y Shen L Wang H Wang Y et al Mechanisms and treatment of atherosclerosis: focus on macrophages. Front Immunol. (2024) 15:1490387. 10.3389/fimmu.2024.1490387
102.
Wu J He S Song Z Chen S Lin X Sun H et al Macrophage polarization states in atherosclerosis. Front Immunol. (2023) 14:1185587. 10.3389/fimmu.2023.1185587
103.
Hou P Fang J Liu Z Shi Y Agostini M Bernassola F et al Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. (2023) 14(10):691. 10.1038/s41419-023-06206-z
104.
Broch M Ramírez R Auguet MT Alcaide MJ Aguilar C Garcia-España A et al Macrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4). Physiol Res. (2010) 59(2):299–303. 10.33549/physiolres.931714
105.
Jonk AM Houben AJ Schaper NC de Leeuw PW Serné EH Smulders YM et al Meal-related increases in microvascular vasomotion are impaired in obese individuals: a potential mechanism in the pathogenesis of obesity-related insulin resistance. Diabetes Care. (2011) 34(Suppl 2):S342–8. 10.2337/dc11-s240
106.
Haider DG Schindler K Prager G Bohdjalian A Luger A Wolzt M et al Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. (2007) 92(3):1168–71. 10.1210/jc.2006-1839
107.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33(Supplement_1):S62–9. 10.2337/dc11-S062
108.
Després JP Lamarche B Mauriège P Cantin B Dagenais GR Moorjani S et al Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. (1996) 334(15):952–7. 10.1056/nejm199604113341504
109.
Di Pino A DeFronzo RA . Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. (2019) 40(6):1447–67. 10.1210/er.2018-00141
110.
Xu S Ilyas I Little PJ Li H Kamato D Zheng X et al Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. (2021) 73(3):924–67. 10.1124/pharmrev.120.000096
111.
Tabas I . The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. (2010) 107(7):839–50. 10.1161/circresaha.110.224766
112.
Hansson GK Robertson AK Söderberg-Nauclér C . Inflammation and atherosclerosis. Annu Rev Pathol. (2006) 1:297–329. 10.1146/annurev.pathol.1.110304.100100
113.
Defronzo RA . Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. (2009) 58(4):773–95. 10.2337/db09-9028
114.
DeFronzo RA . Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia. (2010) 53(7):1270–87. 10.1007/s00125-010-1684-1
115.
Reaven GM . Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. (1988) 37(12):1595–607. 10.2337/diab.37.12.1595
116.
Sasaoka T Ishiki M Sawa T Ishihara H Takata Y Imamura T et al Comparison of the insulin and insulin-like growth factor 1 mitogenic intracellular signaling pathways. Endocrinology. (1996) 137(10):4427–34. 10.1210/endo.137.10.8828504
117.
Koopmans SJ Kushwaha RS DeFronzo RA . Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases de novo lipogenesis but does not cause dyslipidemia in conscious normal rats. Metab Clin Exp. (1999) 48(3):330–7. 10.1016/s0026-0495(99)90081-1
118.
Winhofer Y Krssák M Jankovic D Anderwald CH Reiter G Hofer A et al Short-term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes. (2012) 61(5):1210–6. 10.2337/db11-1275
119.
Nakao J Ito H Kanayasu T Murota S . Stimulatory effect of insulin on aortic smooth muscle cell migration induced by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid and its modulation by elevated extracellular glucose levels. Diabetes. (1985) 34(2):185–91. 10.2337/diab.34.2.185
120.
Golovchenko I Goalstone ML Watson P Brownlee M Draznin B . Hyperinsulinemia enhances transcriptional activity of nuclear factor-kappaB induced by angiotensin II, hyperglycemia, and advanced glycosylation end products in vascular smooth muscle cells. Circ Res. (2000) 87(9):746–52. 10.1161/01.res.87.9.746
121.
Hajra L Evans AI Chen M Hyduk SJ Collins T Cybulsky MI . The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. (2000) 97(16):9052–7. 10.1073/pnas.97.16.9052
122.
Reyna SM Ghosh S Tantiwong P Meka CS Eagan P Jenkinson CP et al Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. (2008) 57(10):2595–602. 10.2337/db08-0038
123.
King GL Goodman AD Buzney S Moses A Kahn CR . Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. (1985) 75(3):1028–36. 10.1172/jci111764
124.
Coletta DK Balas B Chavez AO Baig M Abdul-Ghani M Kashyap SR et al Effect of acute physiological hyperinsulinemia on gene expression in human skeletal muscle in vivo. Am J Physiol Endocrinol Metab. (2008) 294(5):E910-7. 10.1152/ajpendo.00607.2007
125.
Stout RW . The effect of insulin on the incorporation of sodium (1- 14 C)-acetate into the lipids of the rat aorta. Diabetologia. (1971) 7(5):367–72. 10.1007/bf01219472
126.
Porter KE Riches K . The vascular smooth muscle cell: a therapeutic target in type 2 diabetes?Clin Sci. (2013) 125(4):167–82. 10.1042/cs20120413
127.
Moreau P Lamarche L Laflamme AK Calderone A Yamaguchi N de Champlain J . Chronic hyperinsulinaemia and hypertension: the role of the sympathetic nervous system. J Hypertens. (1995) 13(3):333–40. 10.1097/00004872-199503000-00009
128.
Juan CC Shen YW Chien Y Lin YJ Chang SF Ho LT . Insulin infusion induces endothelin-1-dependent hypertension in rats. Am J Phys Endocrinol Metab. (2004) 287(5):E948–54. 10.1152/ajpendo.00536.2003
129.
Hursting SD Berger NA . Energy balance, host-related factors, and cancer progression. J Clin Oncol. (2010) 28(26):4058–65. 10.1200/jco.2010.27.9935
130.
Kanai F Ito K Todaka M Hayashi H Kamohara S Ishii K et al Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun. (1993) 195(2):762–8. 10.1006/bbrc.1993.2111
131.
Liu C Zhou XR Ye MY Xu XQ Zhang YW Liu H et al RBP4 is associated with insulin resistance in hyperuricemia-induced rats and patients with hyperuricemia. Front Endocrinol (Lausanne). (2021) 12:653819. 10.3389/fendo.2021.653819
132.
Gliniak CM Brown JM Noy N . The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J Biol Chem. (2017) 292(36):15080–93. 10.1074/jbc.M117.782334
133.
Moraes-Vieira PM Yore MM Dwyer PM Syed I Aryal P Kahn BB . RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. (2014) 19(3):512–26. 10.1016/j.cmet.2014.01.018
134.
Huang R Yin S Ye Y Chen N Luo S Xia M et al Circulating retinol-binding protein 4 is inversely associated with pancreatic β-cell function across the spectrum of glycemia. Diabetes Care. (2020) 43(6):1258–65. 10.2337/dc19-2432
135.
Yan H Chang X Xia M Bian H Zhang L Lin H et al Serum retinol binding protein 4 is negatively related to beta cell function in Chinese women with non-alcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. (2013) 12:157. 10.1186/1476-511x-12-157
136.
Broch M Vendrell J Ricart W Richart C Fernández-Real JM . Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. (2007) 30(7):1802–6. 10.2337/dc06-2034
137.
Ediger BN Du A Liu J Hunter CS Walp ER Schug J et al Islet-1 is essential for pancreatic β-cell function. Diabetes. (2014) 63(12):4206–17. 10.2337/db14-0096
138.
Ediger BN Lim HW Juliana C Groff DN Williams LT Dominguez G et al LIM domain-binding 1 maintains the terminally differentiated state of pancreatic β cells. J Clin Invest. (2017) 127(1):215–29. 10.1172/jci88016
139.
Sun Z . Aging, arterial stiffness, and hypertension. Hypertension. (2015) 65(2):252–6. 10.1161/hypertensionaha.114.03617
140.
Li S Liu Z Joseph P Hu B Yin L Tse LA et al Modifiable risk factors associated with cardiovascular disease and mortality in China: a PURE substudy. Eur Heart J. (2022) 43(30):2852–63. 10.1093/eurheartj/ehac268
141.
Wang JG Zhang W Li Y Liu L . Hypertension in China: epidemiology and treatment initiatives. Nat Rev Cardiol. (2023) 20(8):531–45. 10.1038/s41569-022-00829-z
142.
Zhang G Yu C Zhou M Wang L Zhang Y Luo L . Burden of ischaemic heart disease and attributable risk factors in China from 1990 to 2015: findings from the global burden of disease 2015 study. BMC Cardiovasc Disord. (2018) 18(1):18. 10.1186/s12872-018-0761-0
143.
Murray CJ Barber RM Foreman KJ Abbasoglu Ozgoren A Abd-Allah F Abera SF et al Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet (London, England). (2015) 386(10009):2145–91. 10.1016/s0140-6736(15)61340-x
144.
Abalos E Cuesta C Grosso AL Chou D Say L . Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. (2013) 170(1):1–7. 10.1016/j.ejogrb.2013.05.005
145.
Hamdan HZ Ali T Adam I . Association between retinol-binding protein 4 levels and preeclampsia: a systematic review and meta-analysis. Nutrients. (2022) 14(24):S62–9. 10.3390/nu14245201
146.
Zhang JX Zhu GP Zhang BL Cheng YY . Elevated serum retinol-binding protein 4 levels are correlated with blood pressure in prehypertensive Chinese. J Hum Hypertens. (2017) 31(10):611–5. 10.1038/jhh.2017.44
147.
Li X Zhu S Song G Zhang K Gao W Huang J et al Retinol-binding protein 4 is closely correlated to blood pressure level and E/A in untreated essential hypertension patients. Ann Palliat Med. (2019) 8(5):645–50. 10.21037/apm.2019.11.07
148.
Majerczyk M Choręza P Bożentowicz-Wikarek M Brzozowska A Arabzada H Owczarek A et al Increased plasma RBP4 concentration in older hypertensives is related to the decreased kidney function and the number of antihypertensive drugs-results from the PolSenior substudy. J Am Soc Hypertens. (2017) 11(2):71–80. 10.1016/j.jash.2016.11.009
149.
Kraus BJ Sartoretto JL Polak P Hosooka T Shiroto T Eskurza I et al Novel role for retinol-binding protein 4 in the regulation of blood pressure. FASEB J. (2015) 29(8):3133–40. 10.1096/fj.14-266064
150.
Li H Yang Z Liang W Nie H Guan Y Yang N et al DHCR24 insufficiency promotes vascular endothelial cell senescence and endothelial dysfunction via inhibition of caveolin-1/ERK signaling. The journals of gerontology series A. Biol Sci Med Sci. (2024) 79(4):glae059. 10.1093/gerona/glae059
151.
Kubis N Besnard S Silvestre JS Feletou M Huang PL Lévy BI et al Decreased arteriolar density in endothelial nitric oxide synthase knockout mice is due to hypertension, not to the constitutive defect in endothelial nitric oxide synthase enzyme. J Hypertens. (2002) 20(2):273–80. 10.1097/00004872-200202000-00017
152.
Manicardi V Camellini L Bellodi G Coscelli C Ferrannini E . Evidence for an association of high blood pressure and hyperinsulinemia in obese man. J Clin Endocrinol Metab. (1986) 62(6):1302–4. 10.1210/jcem-62-6-1302
153.
Modan M Halkin H Almog S Lusky A Eshkol A Shefi M et al Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. (1985) 75(3):809–17. 10.1172/jci111776
154.
Carrier B Wen S Zigouras S Browne RW Li Z Patel MS et al Alpha-lipoic acid reduces LDL-particle number and PCSK9 concentrations in high-fat fed obese Zucker rats. PLoS One. (2014) 9(3):e90863. 10.1371/journal.pone.0090863
155.
Wang Y Yu H He J . Role of dyslipidemia in accelerating inflammation, autoimmunity, and atherosclerosis in systemic lupus erythematosus and other autoimmune diseases. Discov Med. (2020) 30(159):49–56.
156.
Pirillo A Casula M Olmastroni E Norata GD Catapano AL . Global epidemiology of dyslipidaemias. Nat Rev Cardiol. (2021) 18(10):689–700. 10.1038/s41569-021-00541-4
157.
Vancheri F Backlund L Strender LE Godman B Wettermark B . Time trends in statin utilisation and coronary mortality in Western European countries. BMJ Open. (2016) 6(3):e010500. 10.1136/bmjopen-2015-010500
158.
Rocha M Bañuls C Bellod L Rovira-Llopis S Morillas C Solá E et al Association of serum retinol binding protein 4 with atherogenic dyslipidemia in morbid obese patients. PLoS One. (2013) 8(11):e78670. 10.1371/journal.pone.0078670
159.
Majerczyk M Kocełak P Choręza P Arabzada H Owczarek AJ Bożentowicz-Wikarek M et al Components of metabolic syndrome in relation to plasma levels of retinol binding protein 4 (RBP4) in a cohort of people aged 65 years and older. J Endocrinol Investig. (2018) 41(10):1211–9. 10.1007/s40618-018-0856-6
160.
Wessel H Saeed A Heegsma J Connelly MA Faber KN Dullaart RPF . Plasma levels of retinol binding protein 4 relate to large VLDL and small LDL particles in subjects with and without type 2 diabetes. J Clin Med. (2019) 8(11):1792. 10.3390/jcm8111792
161.
Christou GA Tellis CC Elisaf MS Tselepis AD Kiortsis DN . The changes in plasma retinol-binding protein 4 levels are associated with those of the apolipoprotein B-containing lipoproteins during dietary and drug treatment. Angiology. (2012) 63(1):67–75. 10.1177/0003319711407628
162.
Campos-Sandoval JA Redondo C Kinsella GK Pal A Jones G Eyre GS et al Fenretinide derivatives act as disrupters of interactions of serum retinol binding protein (sRBP) with transthyretin and the sRBP receptor. J Med Chem. (2011) 54(13):4378–87. 10.1021/jm200256g
163.
Mata NL Lichter JB Vogel R Han Y Bui TV Singerman LJ . Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. (2013) 33(3):498–507. 10.1097/IAE.0b013e318265801d
164.
Motani A Wang Z Conn M Siegler K Zhang Y Liu Q et al Identification and characterization of a non-retinoid ligand for retinol-binding protein 4 which lowers serum retinol-binding protein 4 levels in vivo. J Biol Chem. (2009) 284(12):7673–80. 10.1074/jbc.M809654200
165.
Dobri N Qin Q Kong J Yamamoto K Liu Z Moiseyev G et al A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Invest Ophthalmol Visual Sci. (2013) 54(1):85–95. 10.1167/iovs.12-10050
166.
Cioffi CL Racz B Freeman EE Conlon MP Chen P Stafford DG et al Bicyclic [3.3.0]-octahydrocyclopenta[c]pyrrolo antagonists of retinol binding protein 4: potential treatment of atrophic age-related macular degeneration and stargardt disease. J Med Chem. (2015) 58(15):5863–88. 10.1021/acs.jmedchem.5b00423
167.
Racz B Varadi A Kong J Allikmets R Pearson PG Johnson G et al A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle. J Biol Chem. (2018) 293(29):11574–88. 10.1074/jbc.RA118.002062
168.
Zhang KZ Li JW Xu JS Shen ZK Lin YS Zhao C et al RBP4 Promotes denervation-induced muscle atrophy through STRA6-dependent pathway. J Cachexia Sarcopenia Muscle. (2024) 15(4):1601–15. 10.1002/jcsm.13518
169.
Belite Bio. Belite Bio Announces Interim Analysis Results from the Pivotal Global Phase 3 DRAGON Trial of Tinlarebant in Adolescent Stargardt Disease Subjects (February 27, 2025).
170.
ClinicalTrials.gov. A Phase 2a Study of the Safety, Pharmacokinetics and Pharmacodynamics of STG-001 in Subjects With Stargardt Disease (STGD1) Caused by Autosomal Recessive Mutation in ATP Binding Cassette Subfamily A Member 4 (ABCA4) Gene (April 27, 2021).
171.
Lin KD Chang YH Wang CL Yang YH Hsiao PJ Li TH et al Thiazolidinedione addition reduces the serum retinol-binding protein 4 in type 2 diabetic patients treated with metformin and sulfonylurea. Transl Res. (2008) 151(6):309–14. 10.1016/j.trsl.2008.04.003
172.
Zhu C Xiao Y Liu X Han J Zhang J Wei L et al Pioglitazone lowers serum retinol binding protein 4 by suppressing its expression in adipose tissue of obese rats. Cell Physiol Biochem. (2015) 35(2):778–88. 10.1159/000369737
173.
Haider DG Schindler K Mittermayer F Müller M Nowotny P Rieger A et al Effect of rosiglitazone on visfatin and retinol-binding protein-4 plasma concentrations in HIV-positive patients. Clin Pharmacol Ther. (2007) 81(4):580–5. 10.1038/sj.clpt.6100047
174.
Hu H Xu M Qi R Wang Y Wang C Liu J et al Sitagliptin downregulates retinol-binding protein 4 and upregulates glucose transporter type 4 expression in a type 2 diabetes mellitus rat model. Int J Clin Exp Med. (2015) 8(10):17902–11.
175.
Sun X Zhang Z Ning H Sun H Ji X . Sitagliptin down-regulates retinol-binding protein 4 and reduces insulin resistance in gestational diabetes mellitus: a randomized and double-blind trial. Metab Brain Dis. (2017) 32(3):773–8. 10.1007/s11011-017-9958-7
176.
Wu H Wei L Bao Y Lu J Huang P Liu Y et al Fenofibrate reduces serum retinol-binding protein-4 by suppressing its expression in adipose tissue. Am J Physiol Endocrinol Metab. (2009) 296(4):E628–34. 10.1152/ajpendo.90526.2008
177.
Sasaki R Nishimura N Hoshino H Isa Y Kadowaki M Ichi T et al Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. (2007) 74(11):1619–27. 10.1016/j.bcp.2007.08.008
178.
Różańska D Regulska-Ilow B . The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv Clin Exp Med. (2018) 27(1):135–42. 10.17219/acem/64983
179.
Danielewski M Rapak A Kruszyńska A Małodobra-Mazur M Oleszkiewicz P Dzimira S et al Cornelian cherry (Cornus mas L.) fruit extract lowers SREBP-1c and C/EBPα in liver and alters various PPAR-α, PPAR-γ, LXR-α target genes in cholesterol-rich diet rabbit model. Int J Mol Sci. (2024) 25(2):1199. 10.3390/ijms25021199
180.
Mercader J Palou A Bonet ML . Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J Nutr Biochem. (2011) 22(9):828–34. 10.1016/j.jnutbio.2010.07.007
181.
Cui J Bai Y Wu L Sun M Lin C Zhang H et al Effects of exercise and resveratrol on retinol binding protein 4, blood glucose and insulin sensitivity in aged obese rats. Wei Sheng Yan Jiu. (2017) 46(4):602–9.
182.
Tan Y Sun LQ Kamal MA Wang X Seale JP Qu X . Suppression of retinol-binding protein 4 with RNA oligonucleotide prevents high-fat diet-induced metabolic syndrome and non-alcoholic fatty liver disease in mice. Biochim Biophys Acta. (2011) 1811(12):1045–53. 10.1016/j.bbalip.2011.09.011
183.
Jiang S Li H Zhang L Mu W Zhang Y Chen T et al Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. (2025) 53(D1):D1670–d6. 10.1093/nar/gkae973
Summary
Keywords
biomarker, CVDs, RBP4, risk factor, therapeutic target
Citation
Chen J, Chen G, Xu X, Zhang J and Liu F (2026) Retinol-binding protein 4 in cardiovascular diseases: mechanisms and therapeutic perspectives. Front. Cardiovasc. Med. 13:1703840. doi: 10.3389/fcvm.2026.1703840
Received
21 October 2025
Revised
13 January 2026
Accepted
13 January 2026
Published
29 January 2026
Volume
13 - 2026
Edited by
Tatsuya Sato, Sapporo Medical University, Japan
Reviewed by
Georgios A. Christou, University of Ioannina, Greece
Virginia Actis Dato, University of California San Diego, La Jolla, United States
Updates
Copyright
© 2026 Chen, Chen, Xu, Zhang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jiewen Zhang zhangjiewen9900@126.com Feng Liu liufenguro@163.com
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.